Abstract
Background
There are many functional and aesthetic benefits to lipoabdominoplasty (combination of liposuction with abdominoplasty), including increase in core strength, reduction in urinary incontinence, and improvement in lower back pain. However, patients are still hesitant to undergo surgery due to the perceived fears of postsurgical drains, and postoperative pain.
Objectives
To propose a standardized multimodal pain protocol for patients undergoing lipoabdominoplasty procedures that aims to improve postoperative pain control.
Methods
A total of 80 patients operated on between July 2020 and December 2021 were evaluated in this study. Patients all underwent lipoabdominoplasty and were administered a standardized preoperative, intraoperative, and postoperative pain regimen. Pain scores were measured across all patients in the immediate postoperative period, and postoperative days (PODs) 1, 7, 28, and 90.
Results
Mean pain scores in the postanesthesia recovery unit were 0.46/10 (+/− 0.18). Subsequent reassessment in the postop recovery suite yielded mean pain scores of 0.34 (+/− 0.15). Mean pain scores on POD1 were 1.23 (+/− 0.15) and consistent through to POD7 at 1.24 (+/− 0.11) with patients taking an average of 6.65 total Percocet 5 mg (Endo Pharmaceuticals Inc., Malvern, PA) during the week. After POD7, 95% (76/80) of patients were only taking nonsteroidal anti-inflammatory drugs. A total of 75/80 patients (93.75%) reported zero pain at 4 to 6 weeks after surgery (mean pain score 0.10 +/− 0.08).
Conclusions
The multimodal analgesia protocol consisting of preoperative or immediate induction intravenous Tylenol (Johnson & Johnson, New Brunswick, NJ), precut local analgesia with Marcaine (Pfizer Inc., New York, NY) and lidocaine, and intraoperative use of liposomal bupivacaine can improve perioperative pain control in patients undergoing lipoabdominoplasty.
Level of Evidence: 4

Many concepts are taught within the surgical disciplines about perioperative pain control, the most consequential being the concept that anesthesia is not analgesia. Although this notion may sound simple, it is often misinterpreted by surgeons attempting to provide their patients with adequate pain control following surgery. Recent advances within the anesthesia literature have provided surgeons with a better understanding of physiologic concepts and mechanisms for pain, further uncovering an association between improved preoperative and intraoperative analgesia, and preventing long-term and chronic pain.1-3
Although there are many cosmetic benefits to lipoabdominoplasty (combination of liposuction with abdominoplasty), this procedure has been proven to provide functional benefits which include increased core strength,4 reduced urinary incontinence,5-9 diminished lower back pain,10 and improved sexual function.11-13 Despite the aforementioned benefits, the fear of pain and abdominal drain placement and management remain significant deterrents to patients seeking this type of surgery.
Multiple analgesics have been employed, either individually or in combination, to provide adequate postoperative pain control. For example, liposomal bupivacaine has been demonstrated to offer excellent pain control in several studies of surgical site pain, either as a nerve block or as a site injection into the incisional wound bed.14-17 Additionally, nonsteroidal anti-inflammatory drugs (NSAIDs), such as acetaminophen, have similarly been shown to decrease opiate dependence when given preoperatively or intraoperatively.18-21 Many protocols for Enhanced Recovery after Surgery (ERAS) utilize a combination of multimodal medications that sometimes even include goal-directed therapy and patient-controlled analgesia in conjunction with a multimodal analgesia regimen and site-specific nerve blockade.
Several interventions have already been shown to improve pain recovery after abdominoplasty to date. Specifically, studies have demonstrated that local infiltration analgesia, perioperative nutritional optimization, and liposomal bupivacaine are efficacious in reducing postoperative pain while enhancing recovery after surgery. A clinical randomized trial also demonstrated that intraoperative ketamine and magnesium therapy alone were effective at reducing pain and opioid consumption postoperatively.22-32
Despite advances in analgesic medications and postoperative pain protocols, patients considering lipoabdominoplasty procedures are often disincentivized for fear of suffering from significant postoperative pain. Given the current opioid epidemic, it has never been more important to develop a pain protocol that provides patients with effective and adequate pain control while reducing the risk of developing opioid dependence. Herein, we propose a standardized multimodal pain protocol for patients undergoing lipoabdominoplasty procedures that aims to increase the duration of postoperative pain control and improve patient outcomes while reducing narcotic medication use.
METHODS
This study was a retrospective review conducted in accordance with the Declaration of Helsinki. Written informed consent for the scientific study and data use was obtained from each patient. In total, 80 of the senior author’s consecutive patients undergoing lipoabdominoplasty between July 2020 and December 2021 were included. Data collected included patient identification number, gender, date of birth, age, surgery date, duration of surgery (minutes), anesthesia type, procedures requested by patient, procedures discussed, procedures performed, complications, and patient-reported pain scores. Complications were classified as minor, if they could be treated without reoperation, or major, if they required to return to the operating room for intervention or hospitalization. All procedures were performed by a single surgeon. Pain scores were collected by the senior surgeon at various time points postoperatively utilizing a standardized visual analog score (VAS), a patient-reported outcome instrument. Patients were asked to numerically quantify their pain on a scale from 0 to 10, 0 being no pain at all and 10 being the worst pain possible. The postoperative time points where patient’s pain was assessed included immediately after surgery in the postanesthesia recovery unit (PACU), a few hours later in the recovery unit before going home, postoperative day 1 (POD1), POD7, at their 4- to 6-week follow-up visits, and at their 12-week follow-up visits. Patients were additionally asked to record their highest pain score felt at any given time in the postoperative period. Several retrospective pain scores were elicited, in an effort to identify the patients’ perception of their greatest pain several weeks and months after surgery.
Protocol
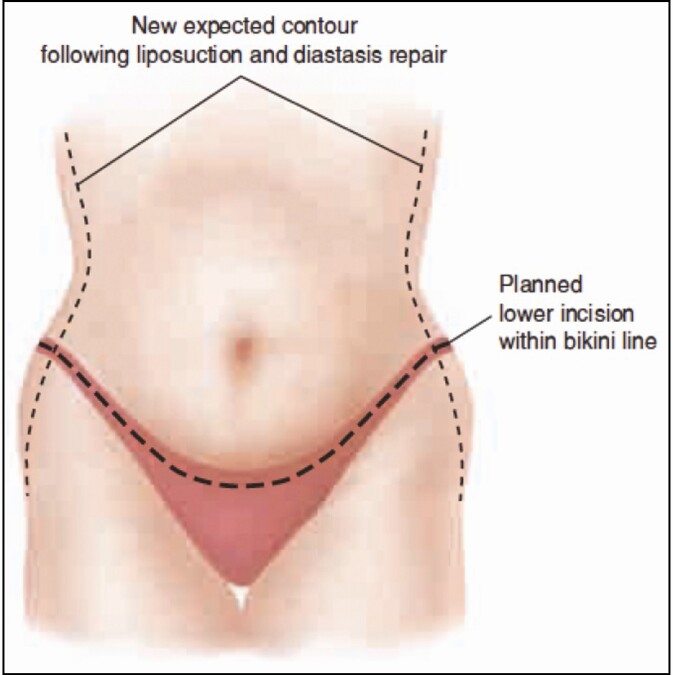
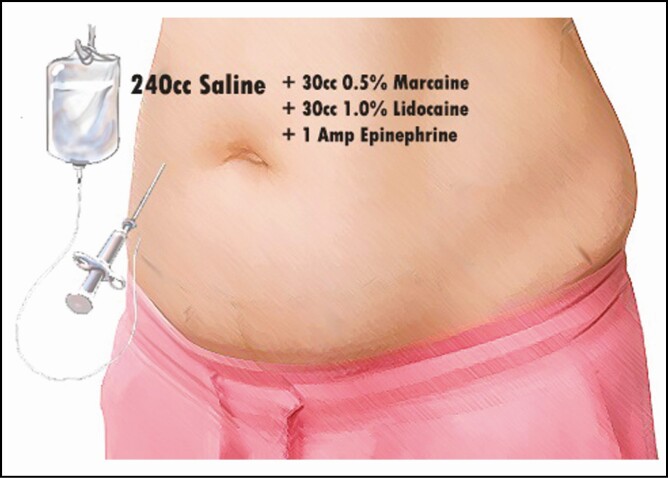
This protocol consists of a combination of medications administered intraoperatively, perioperatively, and additionally during the postoperative period. Initially, all patients were given preoperative or immediate induction IV Tylenol (Johnson & Johnson, New Brunswick, NJ). Before the first cut, a standard mixture of Marcaine 30 mL 0.5% (Pfizer Inc., New York, NY) in 240 mL saline and 30 mL lidocaine with 1 amp epinephrine was injected, with a 20-minute waiting period. These injections were performed by the surgeon with a pragmatic and systematic approach to standardize the dosage and total volume given, with approximately 100 mL delivered along the incision lines of the abdomen (Figure 1), 25 mL given along each flank, 50 mL given along the linea alba, and an additional 50 mL injected below the breast and along the costal margins. Intraoperatively, a 20-mL solution of EXPAREL (bupivacaine liposome injectable suspension, Pacira Pharmaceuticals, Inc., Tampa, FL) diluted into 280 mL saline was injected as a transversus abdominis plane (TAP) block (Figure 2) with care to treat the entire field and incision lines as well. TAP blocks were performed as multiple injections under direct visualization, without the use of ultrasound. The needle was advanced using haptic feedback to determine when it pierced the fascia and entered the appropriate plane. Patients were additionally injected with a standardized tumescent solution33 utilizing 20 mL of 1% lidocaine and 1 amp of epinephrine per liter of normal saline when undergoing concomitant liposuction. The median liposuction tumescent volume injected was 3 Liters; however, this volume varied from patient to patient based on the liposuction required in each case. Lipoabdominoplasty was performed utilizing a drainless technique with progressive tension sutures placed in the midline and laterally.
Figure 1.
Site of local anesthetic injection along planned lipoabdominoplasty incisions. Published with permission from William M. Winn.
Figure 2.
Solution of bupivacaine liposome injectable suspension injected as transversus abdominis plane block. Published with permission from William M. Winn.
Postoperatively, patients were offered a single Percocet 5 mg tablet (Endo Pharmaceuticals Inc., Malvern, PA) or a Demerol 25 mg injection (Pfizer Inc., New York, NY) based on postop pain scores. Those patients reporting mild pain were offered Percocet, while those with moderate pain were given a Demerol injection. The goal was a rapid wean protocol for opiates; in the postop care facility, all patients had access to Demerol and Percocet and were offered Percocet the night of surgery independent of their pain scale. Other medications were made available for sleep (lorazepam) as well as for muscle spasm (cyclobenzaprine) on an as-needed basis, though they were rarely utilized. Patients with a history of pain, either chronic or following surgical procedures, were offered a single dose of preoperative gabapentin 100 mg oral to be taken the night before surgery and Celebrex 100 mg (Pfizer Inc., New York, NY) postoperatively scheduled around the clock for 1 week (Table 1).
Table 1.
Pain Protocol
| Preoperative (night before procedure) |
|---|
| 1. Gabapentin 100 mg × 1 (for patients with history of chronic pain) |
| Immediate induction |
| 1. Tylenol (Johnson & Johnson, New Brunswick, NJ) IV |
| Intraoperative |
| 1. 0.5% Marcaine (30 mL) (Pfizer Inc., New York, NY) in normal saline (240 mL) and 1% lidocaine (30 mL) with epinephrine (1 amp) |
| 2. Liposomal Bupivacaine (20 mL) diluted in normal saline (280 mL) tap block |
| 3. Tumescent solution 1% lidocaine (20 mL) and epinephrine (1 amp) per 1 L of normal saline—average of 3 L injected per patient |
| Immediate postoperative (PACU before discharge) |
| 1. Percocet 5 mg tablet (mild pain) (Endo Pharmaceuticals Inc., Malvern, PA) vs Demerol 25 mg injection (moderate pain) (Pfizer Inc., New York, NY) |
| Postoperative |
| 1. Percocet 5 mg tablet PRN |
| 2. Ativan (Pfizer Inc., New York, NY) PRN |
| 3. Cyclobenzaprine PRN |
| 4. Celebrex 100 mg BID ×7 d (for patients with history of chronic pain) (Pfizer Inc., New York, NY) |
BID, twice a day; PACU, postanesthesia recovery unit; PRN, as needed.
Patients were made aware of the importance of care in dosing these medications together; however, they were not counseled with regard to self-administrative behaviors and limiting their pain medications. They were prescribed medications and were not told to actively restrict or reduce them. Patients were encouraged to walk and were given deep venous thrombosis prophylaxis with Lovenox 40 mg (Sanofi-Aventis, Bridgewater, NJ) administered subcutaneously once daily for 5 days.34 Sequential compression devices were additionally applied to the lower extremities in the aftercare facility and continued in the postoperative period.
RESULTS
A total of 80 patients reported pain scores between July 23, 2020, and December 15, 2021. The mean age of patients was 42.88 years (+/− 2.40; range, 25-68 years), with a majority (78/80) being female (Table 2). The 2 male patients underwent lipoabdominoplasty procedures. The mean duration of surgery across all patients was 230.69 minutes (+/− 14.32) or less than 4 hours (Table 2). This represents the time from the first cut to closure. A total of 140 procedures were performed across all 80 patients herein. All patients underwent lipoabdominoplasty (80/80), with 45 of these patients undergoing one or more additional procedures, including breast augmentation, mastopexy augmentation, thigh lift, brachioplasty, mastopexy, additional fat grafting, neck lift, circumferential body lift, blepharoplasty, scar revision, and reduction mammaplasty (Table 3).
Table 2.
Patient Demographics
| Patients, n | 80 |
|---|---|
| Sex | |
| Female, n (%) | 78 (97.5%) |
| Male, n (%) | 2 (2.5%) |
| Average age, y (95% CI) | 42.88 (2.40) |
| Average surgical time, min (95% CI) | 230.69 (14.32) |
Table 3.
Surgical Procedures
| Procedure | Total patients, n (%) |
|---|---|
| Lipoabdominoplasty | 80 (100.0) |
| Fat grafting | 12 (15.0) |
| Mastopexy | 12 (15.0) |
| Mastopexy augmentation | 9 (11.3) |
| Breast reduction | 6 (11.3) |
| Breast augmentation | 5 (6.3) |
| Scar revision | 4 (5.0) |
| Blepharoplasty | 3 (3.8) |
| Brachioplasty | 3 (3.8) |
| Hernia repair | 2 (2.5) |
| Thigh lift | 2 (2.5) |
| Neck lift | 1 (1.3) |
| Body lift | 1 (1.3) |
| Total | 140 |
There were a total of 13 reported minor complications (16.25%), including 3 patients with delayed wound healing (3.75%), 3 patients with cellulitis (3.75%), 1 patient with swelling with no seroma (1.25%), 1 patient with breast T-junction dehiscence (1.25%), 1 patient with skin necrosis of the lower abdomen (1.25%), 1 patient with lateral femoral cutaneous pain (1.25%), and 3 patients with breast wounds requiring wound care (3.75%) (Table 4). There was one major complication (1.25%) requiring hospital admission. One of the patients with reported delayed wound healing was taking phentermine at the time of the complication.
Table 4.
Complications
| Variable | Total |
|---|---|
| Minor complication | |
| Cellulitis | 3 |
| Delayed wound healinga | 3 |
| Breast wound | 3 |
| Breast T-junction dehiscence | 1 |
| Skin necrosis—lower abdomen | 1 |
| Abdominal swelling (no seroma) | 1 |
| Lateral femoral cutaneous nerve pain | 1 |
| Major complication | |
| Hospitalization | 1 |
| Total complications, n (%) | 14 (17.5%) |
aOne patient taking phentermine at the time of complication.
Pain scores were collected in the PACU immediately after surgery, postop day zero a few hours after surgery in the recovery suite, POD 1, POD 7, 4 to 6 weeks after surgery, and 12 weeks after surgery (Table 5). A total of 9 patients required Demerol 25 mg in the PACU for postoperative pain control, with the remaining requiring no additional pain medications. In the postsurgery recovery suite, a total of 6 patients (7.5%) required additional Demerol 25 mg for adequate pain control, whereas the vast majority of patients (68 patients, 85%) required a single Percocet 5 mg. There were 6 patients who did not require any additional postoperative pain medications. Mean pain scores were 0.46/10 (+/− 0.18) and 0.34 (+/− 0.15) in the postoperative recovery suite. Mean pain scores on POD1 were 1.23 (+/− 0.15), and remained consistent through POD7 at which time average pain scores were reported to be 1.24 (+/− 0.11). Patients took on average 6.65 total Percocet 5 mg tabs during the first postoperative week. After POD7, 95% (76/80) of patients were only taking NSAID medications. Patient pain complaints included soreness, aching, burning, and sharp pains.
Table 5.
Average Pain Scores
| Interval | Average pain score, 1-10 (95% CI) |
|---|---|
| PACU | 0.46 (+/− 0.18) |
| Recovery (POD0) | 0.34 (+/− 0.15) |
| POD1 | 1.23 (+/− 0.15) |
| POD7 | 1.24 (+/− 0.11) |
| 4-6 wk postoperatively | 0.10 (+/− 0.08) |
| 12 wk postoperatively | 0.10 (+/− 0.08) |
PACU, postanesthesia recovery unit; POD, postoperative day.
The most common location for the pain patients described was in the flanks and at the back where liposuction had been performed. These areas were not treated intraoperatively with liposomal bupivacaine. Therefore, this reflects an internal control, as patients uniformly denied abdominal pain in areas treated well with injections and EXPAREL.
Of 80 patients, a total of 75 (93.75%) reported zero pain at 4 to 6 weeks after surgery (mean pain score 0.10 (+/− 0.08). This was consistent through to 3 months after surgery. The 5 patients reporting pain at 4 to 6 weeks and 12 weeks after surgery characterized the pain as 1/10 or 2/10 most of the time. The mean maximum pain experienced at 12 weeks after surgery was 3.10 (+/− 0.28). The majority of these pain scores were reported before 4 weeks postop. All patients in this study reported that they experienced less pain than a cesarean section and that undergoing surgery was worth it to them. The majority of patients in this study (68/80, 85%) were opiate naive. Three patients (3.75%) had chronic pain before the procedure, one of which was being treated with Celebrex.
Seven patients used cannabidiol (CBD) products throughout the postoperative period in addition to, or in place of, NSAID medications. These patients reported maximum pain scores of 3.29 (+/− 0.30) at 12 weeks postoperatively. This was not statistically significantly different from those patients who did not use CBD products (p = 0.96).
Discussion
This study attempts to demonstrate the effectiveness of the senior author’s multimodal pain protocol utilized in patients undergoing abdominoplasty. Classically, there are 2 main deterrents to lipoabdominoplasty, the first being postoperative pain, as many patients fear that they may suffer from pain similar to that of a previous cesarean section or they have heard of other patients suffering extreme pain following the previous lipoabdominoplasty.35 If pain is more appropriately and comprehensively treated before, during, and after surgery, the patient’s postoperative experience can be significantly improved, and thus their perceived outcomes can be enhanced. The second biggest concern is the use and maintenance of internal drains. The senior author routinely performs a drainless abdominoplasty technique, not simply to avoid burdensome drain care for patients but also to better control and improve the anatomic results of the procedure. However, the absence of drains may additionally decrease the instances of traction pain, physical irritation, and drain site pain. This coupled with internal progression sutures offloads tension at the site of the skin closure, which reduces pain with upright posture and pulling. All of these interventions work in concert synergistically to improve the patient experience, reducing pain by removing various classic causes of additional pain and by improving postoperative mobility which reduces downtime, thereby further promoting recovery.
In this author’s first contribution to this topic, it was demonstrated that the technique discussed herein can be performed safely with reduced risk of seroma.36 In a follow-up article by the same authors, it was shown that liposuction did not increase the risk of seroma.36,37 Several other articles focused on the ideal shape of the abdomen,38 and systematic reviews demonstrate that liposuction does not increase the risk of skin necrosis complications.39 This article seeks to contribute to the idea that pain can be better treated and prevented when a systematic approach, combining state-of-the-art technologies and medications, is employed in a pragmatic fashion.
Lipoabdominoplasty is a procedure that is extremely common; however, there is a paucity of literature more recently describing methods to improve patient outcomes with multiple studies contending the efficacy of injectable analgesic medications. In any case, they do agree that either medication does reduce pain postoperatively.40-43 This study did not evaluate liposomal vs traditional bupivacaine.
Compared with other studies describing pain protocols in patients undergoing abdominoplasty, patients in this series demonstrated lower pain scores at all time points. For example, in a study of 46 patients undergoing Fleur-de-Lys abdominoplasty who were randomized to receive a pain regimen consisting of locally infiltrated ropivacaine or levobupivacaine, pain scores were found to be 1.07 +/− 0.59 and 1.00 +/− 0.53, respectively, 2 hours after surgery, 2.33 +/− 0.49 and 1.07 +/− 0.80 4 hours after surgery, and 3.73 +/− 0.70 and 1.67 +/− 0.90 on POD1.44 Compared with both the group of patients receiving ropivacaine and the group receiving levobupivacaine, patient’s receiving the senior author’s pain protocol seemed to experience less pain in the immediate postoperative period, reporting lower average pain scores in the PACU (0.46), in recovery (0.34), and at POD1 (1.23).
When considering studies utilizing more targeted analgesic techniques, average pain scores were again found to be lower in our patient population. In a study of 23 patients undergoing abdominoplasty with pain control administered through rib blocks, the average pain scores were found to be 2 in the recovery room (compared with 0.34 in our series) with no long-term pain scores recorded.45 An additional study of 13 consecutive patients who received TAP blocks during abdominoplasty found average pain scores using VAS to be 2.5 on POD1 (compared with our patients’ average pain score of 1.23 at the same time point).45 This study additionally found the average oxycodone use among these patients to be 7.5 tabs or 75 mg of oxycodone by POD3 compared with the senior author’s patients who required less opiates postoperatively with an average of 6.65 Percocet 5 mg tablets or 33.25 mg of oxycodone during the first postoperative week.46
The study with the most comprehensive pain regimen consisting of 209 patients undergoing abdominoplasty still failed to provide comparable average pain scores. Of the 209 patients, 77 received the following pain protocol: local anesthesia in the skin, intercostal blocks before incision, and pararectus blocks before plication which contained 0.25% Marcaine with 1:200,000 epinephrine, Pontocaine (Hospira, Inc., Lake Forest, IL), and Depo-Medrol (Pfizer, New York, NY) and were found to have average pain scores, assessed by surveys at the sixth week postoperatively, of 1.00 in recovery, 2.00 on POD2, 2.00 on POD3-7, and 1.00 after the first week.47
Our pain protocol is most similar to that described by Bartlett et al who described a narcotic-sparing ERAS protocol in 10 patients undergoing various cosmetic procedures.48 One significant difference is the standardized preoperative preloading and scheduled postoperative use of celecoxib and gabapentin. This practice has additionally been described by Nguyen et al who demonstrated the effectiveness of a non-opioid pain regimen that utilized scheduled celecoxib and gabapentin preoperatively and as part of the postoperative regimen in 187 patients undergoing various aesthetic procedures.49 The protocol described by the senior author of this paper does not utilize scheduled gabapentin and only offers these medications to patients with a history of chronic pain or difficulty with postoperative pain control. Additionally, the protocols described by Bartlett et al and Nguyen et al are both strictly narcotic-sparing ERAS protocols, while the one described here offers minimal narcotic medications as part of the multimodal protocol.48,49 Despite these differences, the patients in this study achieved comparable pain scores. Therefore, including gabapentin and celecoxib in our multimodal pain protocol for all patients undergoing lipoabdominoplasty may serve as a valuable contribution to further reducing pain scores in our patient population. Despite these differences, this recently published literature further demonstrates the utility of ERAS protocols in reducing pain scores and decreasing narcotic use in patients undergoing a cosmetic procedure such as lipoabdominoplasty.
Finally, there was no statistically significant difference in pain scores between patients taking Tetrahydrocannabinol (THC) products and those who did not. THC is not included in the standardized pain protocol, and its use was patient driven. These results may be limited by the study population size, with further investigation required to make definitive conclusions regarding its efficacy. Therefore, despite these findings, patients who would firmly like to take THC products postoperatively are not discouraged to do so.
The regimen proposed here provides a standardized pain protocol that can be administered safely and effectively by any surgeon performing abdominoplasty in either an inpatient or outpatient setting. Compared with most of the available literature, this series represents one of the largest and most extensive series with long-term pain outcomes measures to date and appears to provide patients with superior levels of pain control for a longer duration of time, while reducing postoperative opiate use.
Limitations to this study include its retrospective design, which has limited ability to determine causation. Furthermore, this study represents a single-surgeon experience, which may limit the ability to generalize our results and pain scores. Therefore, the patients’ pain scores may represent the senior author’s comfort and experience with the protocol technique.
Conclusions
This protocol, which consists of a combination of multimodal analgesia and appropriate injected medications, can improve perioperative pain control in patients undergoing lipoabdominoplasty. When ERAS protocols are correctly delivered, this can dramatically improve the patient experience and can shorten the patient’s recovery phase and satisfaction with surgical results.
Acknowledgment
The authors acknowledge the work of William M. Winn for his illustrations included in Figures 1 and 2.
Contributor Information
Orr Shauly, Department of Surgery, Division of Plastic and Reconstructive Surgery, Emory University School of Medicine, Atlanta, GA, USA.
Pedram Goel, Department of Plastic Surgery, University of California, Irvine, Orange, CA, USA.
Daniel J Gould, Division of Plastic and Reconstructive Surgery, Keck School of Medicine of USC, Los Angeles, CA, USA.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
References
- 1. Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155(2):210-216. doi: 10.1016/j.pain.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Verrill P. Does the gate theory of pain supplant all others? Br J Hosp Med. 1990;43(5):325. [PubMed] [Google Scholar]
- 3. Moayedi M, Davis KD. Theories of pain: from specificity to gate control. J Neurophysiol. 2013;109(1):5-12. doi: 10.1152/jn.00457.2012 [DOI] [PubMed] [Google Scholar]
- 4. Olsson A, Kiwanuka O, Sandblom G, Stackelberg O. Evaluation of functional outcomes following rectus diastasis repair-an up-to-date literature review. Hernia. 2021;25(4):905-914. doi: 10.1007/s10029-021-02462-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Carruthers KH, Kocak E, Hulsen JH, McMahan JD. Improvement in stress urinary incontinence after abdominoplasty. Aesthet Surg J. 2014;34(7):1091- 1098. doi: 10.1177/1090820X14544023 [DOI] [PubMed] [Google Scholar]
- 6. Solanki NS, Duffield JA, Dean NR, Morgan RG. The effect of abdominoplasty on urinary incontinence in women. Plast Reconstr Surg. 2010;126(4):206e- 209e. doi: 10.1097/PRS.0b013e3181ea9372 [DOI] [PubMed] [Google Scholar]
- 7. Güneren E, Eroğlu L, Koçak I, Uysal OA. Urinary incontinence was improved after abdominoplasty using a very low incision. Plast Reconstr Surg. 1999;104(5):1582-1584. doi: 10.1097/00006534-199910000-00076 [DOI] [PubMed] [Google Scholar]
- 8. Mast BA. Alleviation of urinary incontinence after abdominoplasty. Ann Plast Surg. 1999;42(4):456-457. doi: 10.1097/00000637-199904000-00021 [DOI] [PubMed] [Google Scholar]
- 9. Mushin OP, Kraenzlin FS, Fazili A, Ghazi A, Bossert RP. The impact of body contouring procedures on urologic outcomes in massive weight loss patients. Plast Reconstr Surg. 2017;139(5):1086e- 1092e. doi: 10.1097/PRS.0000000000003251 [DOI] [PubMed] [Google Scholar]
- 10. Hoffmeister E. Abdominoplasty improves low back pain and urinary incontinence in postpartum women. Lippincott Bone Joint Newsl. 2018;24(7):73-77. doi: 10.1097/01.BONEJ.0000541311.09971.67 [DOI] [Google Scholar]
- 11. Bykowski MR, Rubin JP, Gusenoff JA. The impact of abdominal contouring with monsplasty on sexual function and urogenital distress in women following massive weight loss. Aesthet Surg J. 2017;37(1):63-70. doi: 10.1093/asj/sjw144 [DOI] [PubMed] [Google Scholar]
- 12. Uimonen M, Repo JP, Homsy P, et al. Health-related quality of life in patients having undergone abdominoplasty after massive weight loss. J Plast Reconstr Aesthet Surg. 2021;74(9):2296-2302. doi: 10.1016/j.bjps.2020.12.056 [DOI] [PubMed] [Google Scholar]
- 13. de Brito MJA, Nahas FX, Bussolaro RA, Shinmyo LM, Barbosa MVJ, Ferreira LM. Effects of abdominoplasty on female sexuality: a pilot study. J Sex Med. 2012;9(3):918- 926. doi: 10.1111/j.1743-6109.2011.02583.x [DOI] [PubMed] [Google Scholar]
- 14. Discepola P, Bouhara M, Kwon M, et al. EXPAREL® (Long-Acting Liposomal Bupivacaine) use for popliteal nerve block in postoperative pain control after ankle fracture fixation. Pain Res Manag. 2020;2020:5982567. doi: 10.1155/2020/5982567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sadeq F, DePamphilis MA, Dabek RJ, Bojovic B, Fuzaylov G, Driscoll DN. Evaluation of liposomal bupivacaine infiltration at reconstructive skin graft donor sites in adolescent and young adult burn patients: a retrospective analysis. Burns. 2021;S0305-4179(21)00246-1. doi: 10.1016/j.burns.2021.08.025 [DOI] [PubMed] [Google Scholar]
- 16. Jacobus D, Mehr S, Ziccardi V. Liposomal bupivacaine use in exploratory lingual nerve microsurgery: does liposomal bupivacaine use decrease postoperative pain and opioid consumption compared to bupivacaine hydrochloride? A pilot study. Quintessence Int. 2021;52(9):812- 818. doi: 10.3290/j.qi.b1763651 [DOI] [PubMed] [Google Scholar]
- 17. Kaye AD, Armstead-Williams C, Hyatali F, et al. Exparel for postoperative pain management: a comprehensive review. Curr Pain Headache Rep. 2020;24(11):73. doi: 10.1007/s11916-020-00905-4 [DOI] [PubMed] [Google Scholar]
- 18. Singh AM, Kirsch JM, Patel MS, et al. Effect of perioperative acetaminophen on pain management in patients undergoing rotator cuff repair: a prospective randomized study. J Shoulder Elbow Surg. 2021;30(9):2014- 2021. doi: 10.1016/j.jse.2021.03.132 [DOI] [PubMed] [Google Scholar]
- 19. Choi M, Wang L, Coroneos CJ, Voineskos SH, Paul J. Managing postoperative pain in adult outpatients: a systematic review and meta-analysis comparing codeine with NSAIDs. CMAJ 2021;193(24):E895-E905. doi: 10.1503/cmaj.201915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cao Q, Fan C, Yuan R, Dong H, Zhang S, Meng H. Comparison of intravenous and oral administration of acetaminophen in adults undergoing general anesthesia. Pain Pract. 2021;22(3):405-413. doi: 10.1111/papr.13092 [DOI] [PubMed] [Google Scholar]
- 21. Phillips SJ, Peck CJ, Pourtaheri N, et al. Decreasing inpatient opioid use following orthognathic surgery. J Craniofac Surg. 2021;32(8):2808-2811. doi: 10.1097/SCS.0000000000008001 [DOI] [PubMed] [Google Scholar]
- 22. Mansour RF, Afifi MA, Abdelghany MS. Transversus Abdominis Plane (TAP) block: a comparative study between levobupivacaine versus levobupivacaine plus ketamine in abdominoplasty. Pain Res Manag. 2021;2021:1762853. doi: 10.1155/2021/1762853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ali H, Ismail AA, Wahdan AS. Low-dose ketamine infusion versus morphine infusion during abdominoplasty to change the postoperative pain profile. Anesth Pain Med. 2020;10(6):e108469. doi: 10.5812/aapm.108469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Meouchy MG, Awaida CJ, Jabbour HJ, Rayess YA, Jabbour SF, Nasr MW. Ultrasound-guided quadratus lumborum block for postoperative pain in abdominoplasty: a randomized controlled study. Plast Reconstr Surg. 2021;147(4):851- 859. doi: 10.1097/PRS.0000000000007767 [DOI] [PubMed] [Google Scholar]
- 25. Ræder J. Quadratus lumborum block for the benefit of patients after full abdominoplasty? Scand J Pain. 2019;19(4):637-638. doi: 10.1515/sjpain-2019-2018 [DOI] [PubMed] [Google Scholar]
- 26. Bresnick S. Efficacy of a local anesthetic pain pump in abdominoplasty. Plast Reconstr Surg. 2008;121(3):1065. doi: 10.1097/01.prs.0000299642.67257.3d [DOI] [PubMed] [Google Scholar]
- 27. Paul M. Breast augmentation and abdominoplasty: postoperative management with pain pumps. Aesthet Surg J. 2005;25(1):69- 71. doi: 10.1016/j.asj.2005.01.001 [DOI] [PubMed] [Google Scholar]
- 28. Vivozon, Inc. A Multicenter, Randomized, Double-Blind, Parallel Group, Placebo- Controlled Trial to Evaluate the Efficacy and Safety of VVZ-149 Injections for the Treatment of Post-Operative Pain Following Abdominoplasty. ClinicalTrials.gov Identifier: NCT03997838. Updated August 25, 2020. https://clinicaltrials.gov/ct2/show/NCT03997838. Accessed January 14, 2022. [Google Scholar]
- 29. Bagatin D. Influence of local infiltration analgesia on postoperative pain in abdominoplasty patients. Acta Clin Croat. 2019;58(Suppl 1):23-28. doi: 10.20471/acc.2019.58.s1.03 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sherif RD, Lisiecki J, Waljee J, Gilman RH. Opioid prescribing habits and pain management among aesthetic plastic surgeons. Aesthetic Plast Surg. 2021;46(2):965-971. doi: 10.1007/s00266-021-02494-y [DOI] [PubMed] [Google Scholar]
- 31. Narayan D, Kaye AD, Vadivelu N.. Perioperative Pain Management for General and Plastic Surgery. Oxford University Press; 2018. [Google Scholar]
- 32. Friedberg B. Anesthesia in Cosmetic Surgery. Cambridge University Press; 2007. [Google Scholar]
- 33. Hanke CW, Sommer B, Sattler G.. Tumescent Local Anesthesia. Springer Science & Business Media; 2012. [Google Scholar]
- 34. Swanson E. Evidence-Based Body Contouring Surgery and VTE Prevention. Springer; 2018. [Google Scholar]
- 35. Hunstad JP, Repta R.. Atlas of Abdominoplasty. Elsevier Health Sciences; 2008. [Google Scholar]
- 36. Macias LH, Kwon E, Gould DJ, Spring MA, Stevens WG. Decrease in seroma rate after adopting progressive tension sutures without drains: a single surgery center experience of 451 abdominoplasties over 7 years. Aesthet Surg J. 2016;36(9):1029-1035. doi: 10.1093/asj/sjw040 [DOI] [PubMed] [Google Scholar]
- 37. Gould DJ, Macias LH, Saeg F, Dauwe P, Hammoudeh Z, Grant Stevens W. Seroma rates are not increased when combining liposuction with progressive tension suture abdominoplasty: a retrospective cohort study of 619 patients. Aesthet Surg J. 2018;38(7):763-769. doi: 10.1093/asj/sjx235 [DOI] [PubMed] [Google Scholar]
- 38. Gould DJ, Shauly O, Qureshi AA, Stevens WG. Defining “Ideal Abs” through a crowdsourcing-based assessment. Aesthet Surg J. 2020;40(4):NP167-NP173. doi: 10.1093/asj/sjz344 [DOI] [PubMed] [Google Scholar]
- 39. Raghuram AC, Yu RP, Gould DJ. The addition of partial or circumferential liposuction to abdominoplasty is not associated with a higher risk of skin necrosis. Aesthet Surg J. 2021;41(6):NP433- NP444. doi: 10.1093/asj/sjaa251 [DOI] [PubMed] [Google Scholar]
- 40. Dilawri A, Wyman M, Shah S. Liposomal bupivacaine versus immediate-release bupivacaine for postoperative pain control. Ann Pharmacother. 2021;56(6):664-670. doi: 10.1177/10600280211043554 [DOI] [PubMed] [Google Scholar]
- 41. Ji YD, Harris JA, Gibson LE, McKinley SK, Phitayakorn R. The efficacy of liposomal bupivacaine for opioid and pain reduction: a systematic review of randomized clinical trials. J Surg Res. 2021;264:510-533. doi: 10.1016/j.jss.2021.02.024 [DOI] [PubMed] [Google Scholar]
- 42. Ilfeld BM, Eisenach JC, Gabriel RA. Clinical effectiveness of liposomal bupivacaine administered by infiltration or peripheral nerve block to treat postoperative pain. Anesthesiology. 2021;134(2):283-344. doi: 10.1097/ALN.0000000000003630 [DOI] [PubMed] [Google Scholar]
- 43. Varas V, Bertinelli P, Carrasco P, et al. Intraoperative ketamine and magnesium therapy to control postoperative pain after abdominoplasty and/or liposuction: a clinical randomized trial. J Pain Res. 2020;13: 2937-2946. doi: 10.2147/JPR.S276710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kakagia DD, Fotiadis S, Tripsiannis G, Tsoutsos D. Postoperative analgesic effect of locally infiltrated levobupivacaine in fleur-de-Lys abdominoplasty. Aesthetic Plast Surg. 2007;31(2):128-132. doi: 10.1007/s00266-006-0187-4 [DOI] [PubMed] [Google Scholar]
- 45. Michaels BM, Eko FN. Outpatient abdominoplasty facilitated by rib blocks. Plast Reconstr Surg. 2009;124(2):635- 642. doi: 10.1097/PRS.0b013e3181addbd7 [DOI] [PubMed] [Google Scholar]
- 46. Oppenheimer AJ, Fiala TGS, Oppenheimer DC. Direct transversus abdominis plane blocks with Exparel during abdominoplasty. Ann Plast Surg. 2016;77(5):499- 500. doi: 10.1097/SAP.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 47. Feng LJ. Painless abdominoplasty: the efficacy of combined intercostal and pararectus blocks in reducing postoperative pain and recovery time. Plast Reconstr Surg. 2010;126(5):1723-1732. doi: 10.1097/PRS.0b013e3181ef8fe5 [DOI] [PubMed] [Google Scholar]
- 48. Bartlett EL, Zavlin D, Friedman JD, Abdollahi A, Rappaport NH. Enhanced recovery after surgery: the plastic surgery paradigm shift. Aesthet Surg J. 2018;38(6):676-685. doi: 10.1093/asj/sjx217 [DOI] [PubMed] [Google Scholar]
- 49. Nguyen TC, Lombana NF, Zavlin D, Moliver CL. Transition to nonopioid analgesia does not impair pain control after major aesthetic plastic surgery. Aesthet Surg J. 2018;38(10):1139-1144. doi: 10.1093/asj/sjy050 [DOI] [PubMed] [Google Scholar]