Abstract
Background
Male reproductive health has deteriorated in recent years as a result of industrialization, which has led to the use of desirable chemicals, like Bisphenol A (BPA), of underlying toxicity. Cucumeropsis mannii seed is a common soup thickener that produces vegetable oil as well as essential nutrients making it a source of nutraceuticals enlisted with a wide range of therapeutic effects.
Methods
A total of 48 adult male Wistar rats (120 ± 200g) were used in this study. They were completely randomized and divided into six groups: A (1ml olive oil) irrespective of the weight, B [BPA 100 mg/kg body weight (bw)], C (CMSO 7.5 ml/kg bw), D (CMSO 7.5 ml/kg bw + BPA 100 mg/kg bw), E (CMSO 5.0 ml/kg bw + BPA 100 mg/kg bw), and E (CMSO 2.5 ml/kg bw + BPA 100 mg/kg bw). At the end of the administration via oral routes, rats were sacrificed and testes were collected for biochemistry and histological analysis.
Results
BPA significantly (P < 0.05) decreased total testicular protein, epididymal sperm parameters (count, volume, and motility), Mitochondrial Membrane Potential (MMP), body weight, testicular volume; and significantly (P < 0.05) increased testicular enzymes (alkaline phosphatase and lactate dehydrogenase), testicular index; plus histological damages. Interestingly, co-administration of BPA and CMSO significantly (P < 0.05) reversed the biochemical and histological changes.
Conclusions
CMSO prevented the biochemistry and histological alterations hence reducing the testicular toxicity. Therefore, CMSO has the potential to be a promising novel nutraceutical for the treatment and management of BPA-induced testicular toxicity.
Keywords: Bisphenol A, Cucumeropsis mannii, Testicular toxicity, Nutraceuticals
Bisphenol A; Cucumeropsis mannii; Testicular toxicity; Nutraceuticals.
1. Introduction
In the past few decades, male reproductive health has declined significantly. Potentially hazardous chemicals are being released into the environment at an alarming rate thereby leading to causative factors in the high incidence of various pathological conditions, including reproductive dysfunction, cardiovascular diseases, and cancers (Irigaray et al., 2007; Clapp et al., 2008; Meeker, 2010) to both humans and wildlife. There has been a declining trend in male reproductive health in industrialized nations (Sinawat, 2000; Colborn et al., 1993) and worldwide (Cimmino et al., 2020).
Global statistics show that 72.4 million couples in the world suffer from infertility problems (Gurunath et al., 2011) and about 90 % of male infertility is due to low sperm count, a decrease in sperm quality, or both (Gurunath et al., 2011). In the southeast (Nnewi and Awka) Nigerian clinics, three hundred and fourteen couples married for an average of 5 years, were evaluated for the course of infertility and a positive male factor alone account for 42.2 % with azoospermia and oligospermia (Ikechebelu et al., 2003) being the major reported pathological conditions. A meta-analysis report of a 50 % worldwide decline in sperm density from 1940 to 1990 raised considerable scientific and public concern regarding the imminent threat of synthetic chemicals to male reproductive health (Carlsen et al., 1992). Since that report, several studies have demonstrated the negative impact that synthetic chemicals have on male reproductive health (Fisher et al., 1999; Toppari et al., 1996).
Bisphenol A ([2,2-bis(4-hydroxyphenyl)propane]; BPA), invented by Dianin in 1891, is an environmental contaminant, resulting from dental composites, epoxy resin linings in food and beverage cans, plastics, and degradation of industrial plastic-related wastes (Cabaton et al., 2011; Vandenberg et al., 2009). BPA's first synthesis from phenol and acetone was reported in 1905 by Zincke (Huang et al., 2012) and has become one of the highest volume chemicals produced worldwide, with over 6 billion pounds produced each year and over 100 tons released into the atmosphere per annual production (Vandenberg et al., 2009). Because of this large production, BPA is widely spread in environmental matrices, including air, water, sewage sludge, soil, house dust, paper currencies, and even foodstuffs (Heinala et al., 2017; Ignatius et al., 2010; Liao and Kannan, 2011; Molina-Garcı´a et al., 2012; Rudel et al., 2011). It enters human systems mainly through food ingestion, with estimated daily intakes of 5.19, 1.61, and 0.0369 mg/day in Kuwait, Japan, and the United States respectively (Zhang et al., 2011). Although already regulated in some of these countries, the toxic effect of BPA has received little or no attention in Africa and Nigeria in particular. Only imported plastic products can be seen with inscription-Bisphenol free. BPA has been established as a food contact chemical that induces organ toxicity. Assessment of human exposure has estimated its global intake of more than 30 ng/kg body weight per day (Ohore and Songhe, 2019). More so, the rate of use of plastic products as cooking utensils in Africa is quite alarming which shows a potential exposure to extremely high concentrations. BPA disrupts the endocrine system by inhibiting the binding of estrogen thereby arresting spermatogenesis (Alboghobeish et al., 2019). However, plant products with essential nutrients as well as nutraceuticals have a wide range of therapeutic effects.
Cucumeropsis mannii (African Melon) popularly called “egusi” in West Africa is the true indigenous egusi of West Africa (Burkill, 1985) and its common names include egusi in Igbo, '''Elegushi''' in Yoruba, and agushi in Hausa, and "Ashi" in Izzi dialect of Ebonyi State. In English, it is known as Mann's cucumeropsis and white-seed melon. African Melon is a cucurbit crop that belongs to the Cucurbitaceae family with a fibrous and shallow root system. It is a tendril climber or crawling annual crop, mostly grown as a subsidiary crop interplanted with early maize and yam in some savanna belt of Nigeria (Mabalaha et al., 2007).
Dehulled seeds from Cucumeropsis mannii mainly consist of fats 44.4 %, protein 36.1 %, and carbohydrate 13.2 % (Badifu and Ogunsua, 1991). Minerals and water amount to 3.7 and 5.9 % respectively. From this content, a caloric value of 2190 kJ/100g has been reported (Mbuli-Lingundi et al., 1983). Generally, compositional studies have demonstrated the Cucurbitaceae family seed’s mineral, amino acid, fatty acid (Giwa et al., 2010), and phospholipid profile (Igwenyi, 2014). High levels of linoleic acid, a polyunsaturated fatty acid (PUFA) have been found in Egusi melon seed (Essien et al., 2012) and it has also been shown to contain phospholipids; phosphatidylcholine, and lysophosphosphatidylcholine (Igwenyi, 2014). Additional studies showed a favorable saturated to an unsaturated fatty acid ratio (Giwa et al., 2010), good quantities of glutamic acid, aspartic acid, arginine, isoleucine, leucine, phenylalanine, phosphorous, potassium, magnesium, zinc, and iron (Igwenyi, 2014).
C. manni has an important value in the African traditional society (Jacks et al., 1972). In Ghana the flesh fruit juice mixed with other ingredients is applied to the navel of newborn babies to accelerate the healing process until the cord relics drops off, likewise, macerated leaves are used in Gabon for purging constipated suckling babies (Ezema, 2012). In Sierra Leone cattle boys traditionally use the dried fruit-shell of C. manni as a warning horn (Hamid et al., 2011). Despite its agronomic, cultural, and nutritional importance, the plant lacks attention from research and development so it is categorized under the orphan crops of Africa (Hamid et al., 2011).
The essential oil which is the major component of C. manni seed is an important source of nutritional oils, industrial and pharmaceutical importance (Oderinde et al., 2009). The application of essential oil has spread evenly throughout the whole world as well as its analysis, which had led to the tremendous increase in the yield and quality of essential oil production. Also in aromatherapy and medicaments, disinfectants, and insect repellent, all of which are directly or indirectly applied to human life to suit people's desires and demands. The general usefulness of essential oil cannot be overemphasized as it is more beneficial than synthetic drugs (Hamid et al., 2011). Further research is inevitable, especially using an animal model to evaluate the role of seed oil in treatments of metabolic syndrome. Therefore Cucumeropsis mannii seed which is rich in essential oil is being explored for its therapeutic potentials in the management of testicular damages enlisted as the predisposing factor to male infertility.
2. Materials and methods
Analytical grades of Bisphenol A ([2, 2-bis (4-hydroxyphenyl) propane] in the form of pure pellets were obtained from Sigma Aldrich Company, the UK through Bristol Scientific. The reagents used were commercial kits and mostly products of Randox Laboratories (UK).
2.1. Plant material
The plant material used in this study, Cucumeropsis mannii Seeds, were purchased from market women in the Iboko market, Izzi L.G.A., Ebonyi State, Nigeria. The Cucumeropsis mannii seed oil was extracted using the methods of Oti and Eze–Ilochi (2017) with modifications. During extraction, warm water was added in drops at intervals to enhance the release of the oil since water will help to rupture the cells. The water is then bound to hydrocolloids (gum and mucilage) released by the ruptured cells (Dror et al., 2006) and sediment with time. The extract was left to stand undisturbed to sediment in a corked bottle for 5–7days before separation by decantation method to obtain a purer form of the oil which was stored in a separate bottle.
2.2. Animal handling
The animals were used according to the Departmental Ethical Review Committee guidelines (Approval Number: EBSU/BCH/ET/20/002) and per the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80-23, revised in 1996).
2.3. Acute toxicity of CMSO
According to OECD/OCDE Guidelines no. 425, the acute toxicity study was carried out using the limit dose up and down method. Male albino Wistar rats (aged 2 months) were used in the experiment, and they were acclimatized to the laboratory condition for seven days before starting. A male rat was given 50 ml/kg of CSMO orally after an overnight fast. Following CSMO administration, the animal was closely monitored for the first 30 min for physical or behavioral changes, then for the next 24 h, and then every day for the next 14 days. Food was given after 3 h of CMSO administration. Since the first rat survived, four more male rats were recruited and fasted for 4 h. They were then given the same dosage of CMSO and subjected to the same stringent monitoring for the next 14 days for any signs of toxicity (Eleazu et al., 2021; Tadesse et al., 2014; Saleem et al., 2017). Within the 24 h and 14 day testing periods, the rats did not exhibit any signs of gross physical or behavioral modifications such as hair erection, decrease in eating, or motor movements at the limit test dose of 50 ml/kg. For this reason, based on OECD guideline No 425, 10 % of the limit dose (5 ml/kg) was chosen as the middle/intermediate dose, half of it (2.5 ml/kg) as the lower dose, and 1.5 times the middle dose (7.5 ml/kg) as the higher dose (OECD, 2008).
2.4. Experimental design
A total of forty-eight (48) male albino Wistar rats were randomly assigned into six (6) experimental groups of A, B, C, D, E, and F with eight (8) rats in each group. Groups A, B, and C are control groups while groups D, E, and F are the treatment groups. BPA in form pellet was dissolved into a solution of 5 g/100ml olive oil.
Group A: Normal control received 1 ml of olive oil
Group B: Negative control (BPA intoxicated group) received 100 mg/kg body weight
Group C: Positive control (CSMO control) received 7.5 ml/kg body weight
Group D: Treatment group 1 pre-administered 100 mg/kg body weight of BPA and treated with 7.5 ml/kg body weight of CMSO.
Group E: Treatment group 1 pre-administered 100 mg/kg body weight of BPA and treated with 5 ml/kg body weight of CMSO.
Group F: Treatment group 1 pre-administered 100 mg/kg body weight of BPA and treated with 2.5 ml/kg body weight of CMSO.
Administration of both BPA and CMSO were concurrently by oral intubation once every day and the weight of the animals across the group was also measured every seven (7) days for six weeks.
2.5. Tissue sample collection
Death of 2 rats in group B, 1 rat in group F, and 1 weak rat in group E was observed a few days to the end of the 42 days of trial. We, therefore, ensured to have six (6) healthy rats in each group of the study. The animals across the groups were sacrificed by cervical dislocation under mild anesthesia. Blood samples were collected via the femoral vein. Scrotal sacs were dissected to recover the testicles and their morphology (length, width, and volume) and indices examined in each group. In this stage, 0.1 mol/L hydrochloric acid buffer (pH = 7.4) was added to the right testis and mixed with a homogenizer and centrifuged at 3000 rpm for 15 min and the testicular length and width will be determined with a pair of dividers and a meter rule. The testicular volume will be calculated using the following equation as adopted by Alboghobeish et al. (2019):
Calculations:
| Testicular Volume (TV) = (D2/4 × π) L × K | eqn 1 |
Where;
L = Testicular length; D = Testicular width; K = constant (0.9), π = 3.14
| eqn 2 |
-
a.
Biochemical Parameters
-
i.
Determination of rats' epididymal sperm count, volume, motility, and morphology in BPA-induced testicular toxicity in rats
The sample preparation was by the Swim-up technique and the epididymal sperm count, volume, motility, and morphology are by use of hemocytometer as adopted by Muhammad (2015). The sperm motility was calculated by multiplying the sperm volume by the concentration of percentage moving sperm. Further, the morphology was measured from a total count of spermatozoa in smears staining techniques of Wells and Awa (1970).
-
ii.
Determination of total testicular proteins
The total testicular protein was determined using the Lowry method (1951) and using Bovine serum albumin (BSA), a globular protein (1 mg/ml) as the standard.
-
iii.
Determination of alkaline phosphatase and lactate dehydrogenase activities in rat testicles
Diagnostic kits using a spectrophotometer as adopted by Ahangarpour et al. (2017) were applied to determine the alkaline phosphatase (ALP) and lactate dehydrogenase (LDH) activities in testicular homogenates.
-
iv.
Determination of Mitochondrial membrane potential (MMP) in rat testicles
MMP was determined using the mitochondrial uptake of the cationic fluorescent dye, Rhodamine (Rh123). For incubation of the mitochondrial suspensions (0.5 mg protein/ml), the tubes were mildly shaken at 37 °C with 1.5 μM Rh 123 for 10 min followed by an estimation of extinction wavelength using luminescence fluorescence spectrophotometer as adopted by Baracca et al. (2003).
-
b.
Histopathological examination of rat testicles
After the right testicle sampling, the left testicles of the animals were separated and placed in the Bouin's fluid for histopathological examination according to the methods of Singh (2003) and Nandini et al. (2018). Stained sections were studied and photographed at 400X magnifications. The factors such as testicular necrosis within interstitial cells of the Leydig and Sertoli compartments, spermatogenic arrest, hemorrhage, and necrosis of seminiferous tubules were evaluated using the semiquantitative method.
-
c.
Statistical Analysis
All results were analyzed using Graph-pad Prism 5. Data were expressed as mean ± standard deviation. The means of the parameters were compared using one-way ANOVA and P < 0.05 was considered statistically significant levels in the Turkey analysis.
3. Results
3.1. Effect of CMSO on epididymal sperm count, volume, motility, and morphology in BPA-induced testicular toxicity in rats
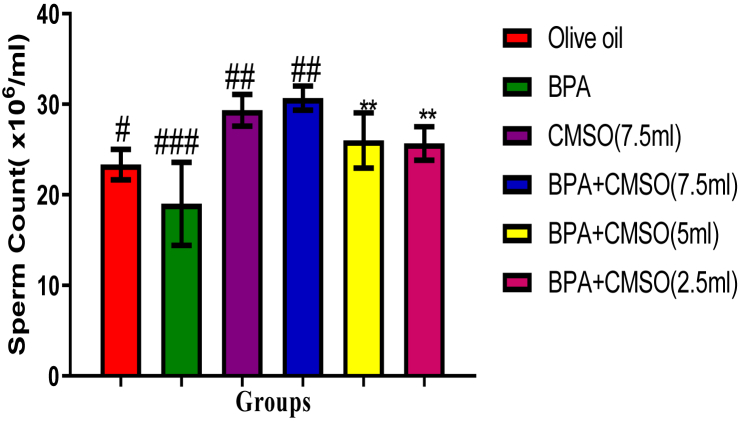
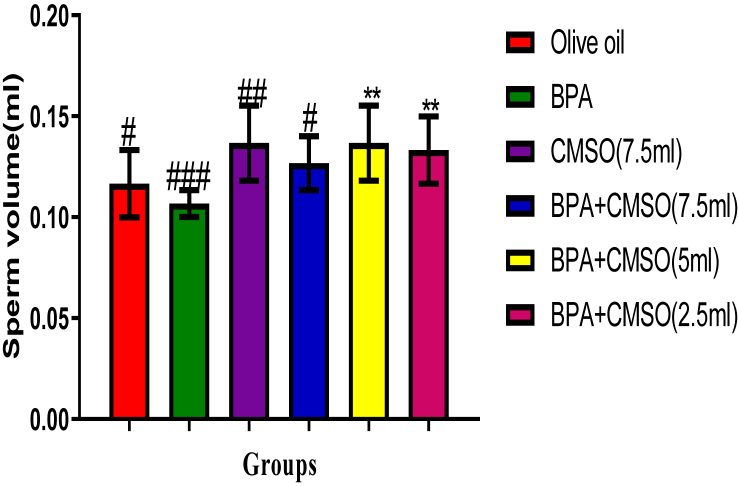
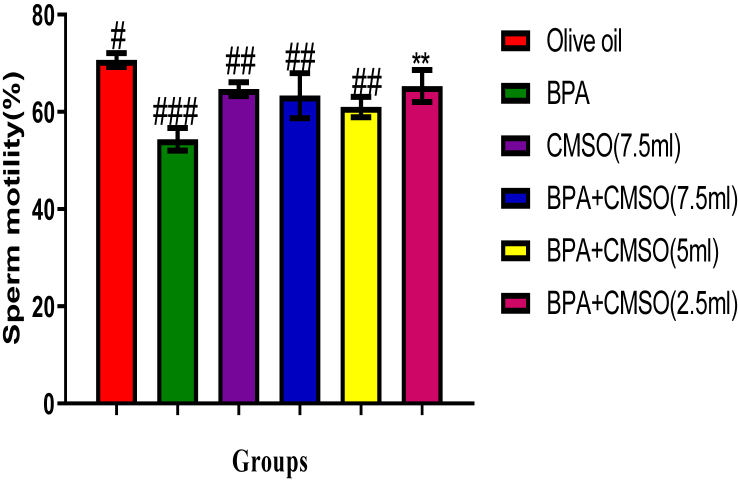
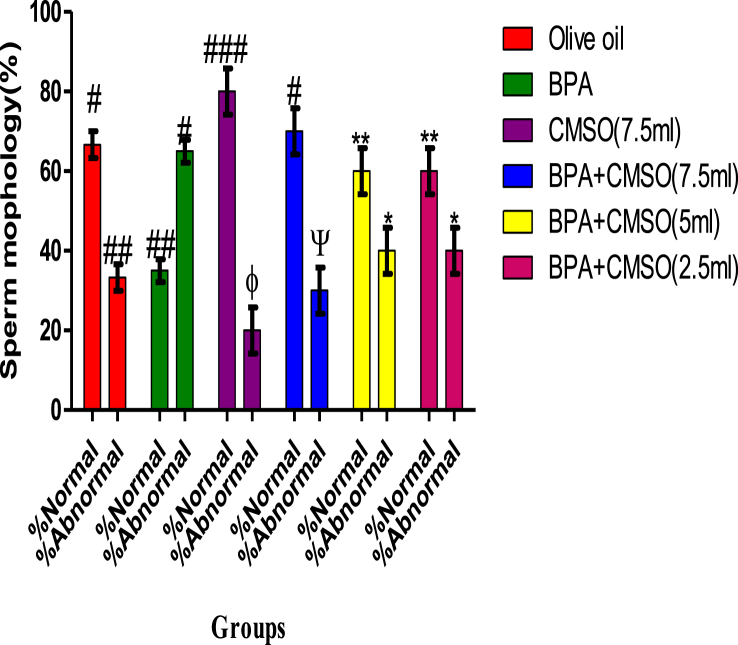
BPA administration in rats significantly (P < 0.05) lowered the epididymal sperm count, volume, and motility with elevated abnormal sperm (Figures 1, 2, 3, 4). However, co-administration of CMSO with BPA in rats significantly (P < 0.05) elevated the sperm count, volume, mobility and reduced the abnormal sperm in a dose-dependent manner (Figures 1, 2, 3, 4).
Figure 1.
Effect of CMSO on epididymal sperm count in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 2.
Effect of CMSO on Sperm volume in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 3.
Effect of CMSO on Sperm motility in BPA-induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 4.
Effect of CMSO on Sperm morphology in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
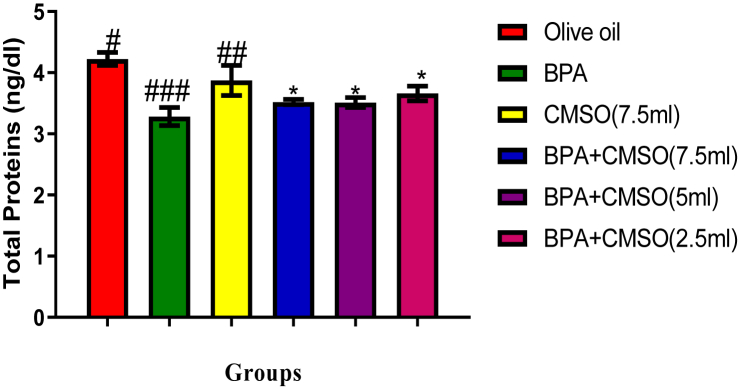
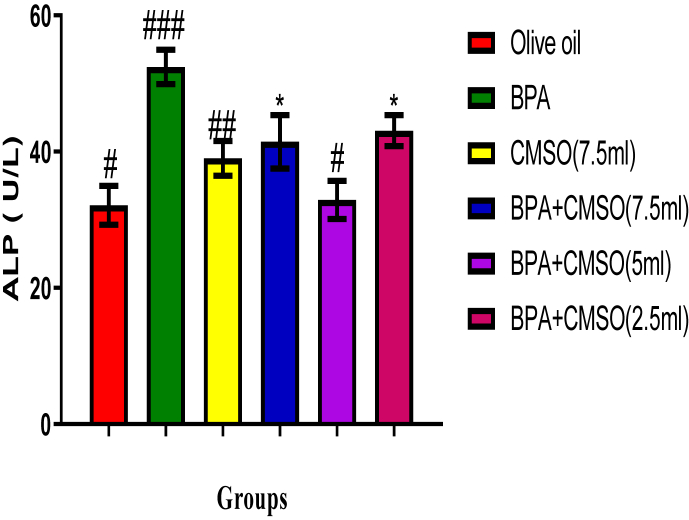
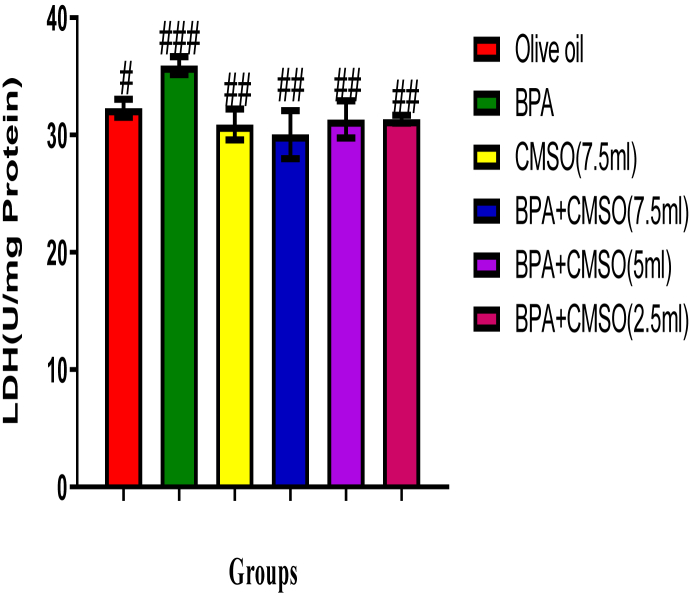
3.2. Effect of CMSO on testicular proteins, alkaline phosphatase (ALP), and lactate dehydrogenase (LDH) activities in BPA–induced testicular toxicity in rats
Results showed that BPA administration significantly (P < 0.05) lowered the total protein level but increased the activities of ALP and LDH whereas co-administration of CMSO with BPA significantly (P < 0.05) increase the level of total protein but lowered the activities of ALP and LDH in testicular homogenate in BPA-induced testicular toxicity in rats (Figure 5, 6, 7).
Figure 5.
Effect of CMSO on Total protein in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 6.
Effect of CMSO on Alkaline phosphatase activity in BPA-induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 7.
Effect of CMSO on Lactate Dehydrogenase activity in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
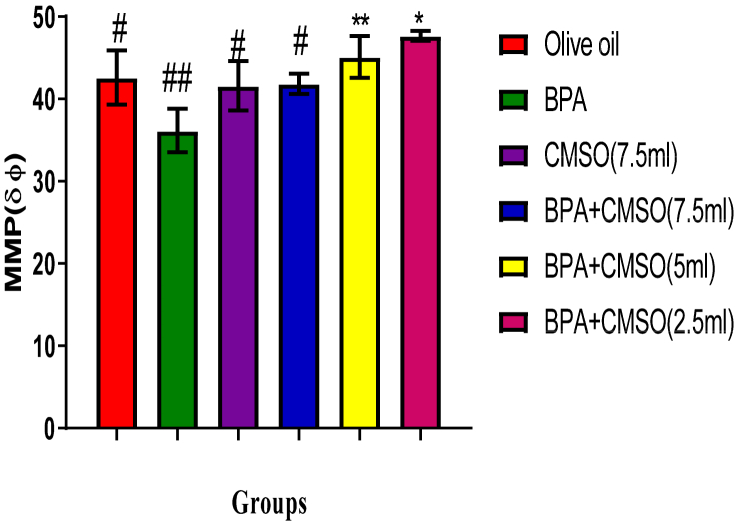
3.3. Effect of CMSO on mitochondrial membrane potential (MMP) in testicular homogenate in BPA –induced testicular toxicity in rats
BPA administration in rats significantly (P < 0.05) decreased the MMP in testicular homogenate in rats (Figure 8). However, the co-administration of BPA + CSMO significantly (P < 0.05) increased the MMP in CMSO dose-dependent manner (Figure 8).
Figure 8.
Effect of CMSO on Mitochondria membrane potential level in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
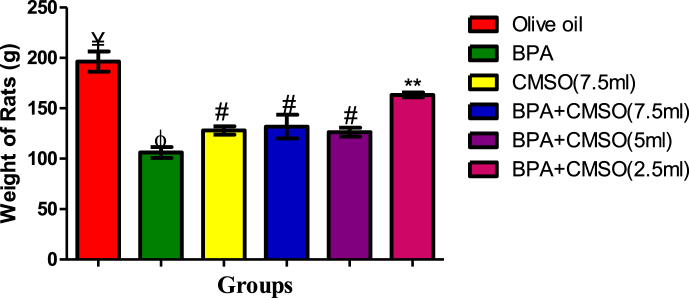
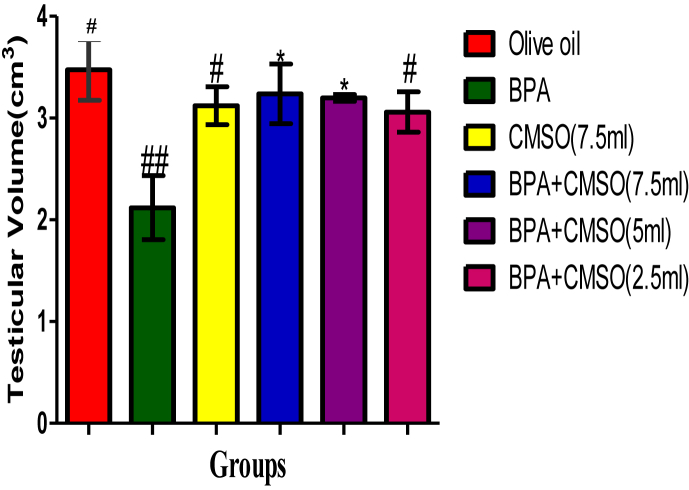
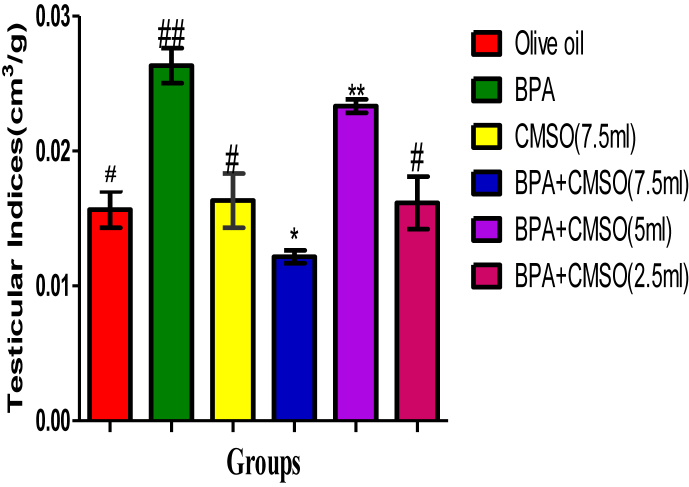
3.4. Effect of CMSO on bodyweight, testicular volume, and testicular index of the rats in BPA –induced testicular toxicity in rats
BPA administration in rats significantly (P < 0.05) decreased the testicular volume and the weights of the rats respectively, but significantly (P < 0.05) increase the testicular index in rats (Figures 9, 10, 11). However, the testicular volume and the rats’ weights significantly (P < 0.05) increased with a significant (P < 0.05) decrease in the testicular indices after co-administration of BPA + CSMO in the treatment groups ((Figures 9, 10, 11).
Figure 9.
Effect of CMSO on Bodyweight in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 10.
Effect of CMSO on Testis volume in testicular homogenate in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
Figure 11.
Effect of CMSO on Testis index in testicular homogenate in BPA induced testicular toxicity in albino rats. Data are shown as mean ± S.D (n = 6). BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil). Mean values with the different signs are significantly different at P < 0.05. BPA (Bisphenol A), CMSO (Cucumeropsis mannii Seed Oil).
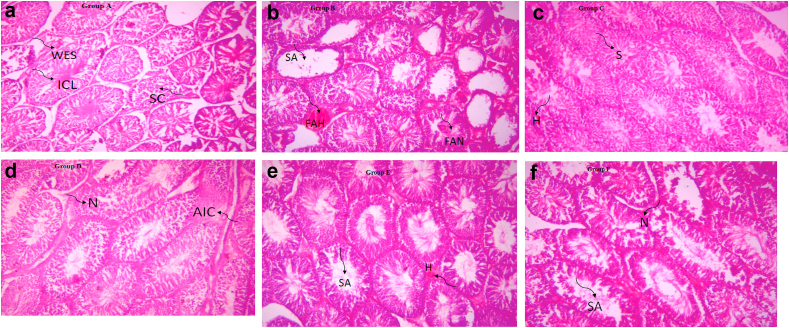
3.5. Effect of CMSO on histopathology of testis in BPA –induced testicular toxicity in rats
Figures 12a-f show the photomicrographs of testicular tissues. For the control groups, rats in Group A (Figure 12a) had normal testicular architecture with seminiferous tubules that are lined with interstitial cells of the Leydig (ICL), Sertoli cell (SC), and well-enhanced spermatogenesis (WES). Rats in Group B (Figure 12b) had moderate to severe effects on the testicular tissue with severe spermatogenic arrest (SA), moderate focal area of hemorrhage (FAH), and moderate necrotic (N) seminiferous tubules. Rats in Group C (Figure 12c) had mild areas of hemorrhage (H) otherwise normal with enhanced spermatogenesis (S). For the test groups (Figure 12d-f), however, photomicrographs of the testicular tissues showed that rats in Group D (Figure 12d) had moderate healing with a mild aggregate of an inflammatory cell (AIC) and mild necrosis (N) of the interstitial cell of the Leydig and the Sertoli cell. Rats in Group E (Figure 12e) had moderate healing with mild spermatogenic arrest (SA) and moderate focal area of hemorrhage (FAH). Rats in Group F (Figure 12f) had mild healing with moderate spermatogenic arrest (SA), focal area of hemorrhage (FAH), and moderate necrotic (N) appearance of the Sertoli cell and interstitial cells of the Leydig.
Figure 12.
a: Photomicrograph of Group A section of the testis (400X). b: Photomicrograph of Group B section of the testis (400X). c: Photomicrograph of Group C section of the testis (400X). d: Photomicrograph of Group D section of the testis (400X). e: Photomicrograph of Group E section of the testis (400X). f: Photomicrograph of Group F section of the testis (400X).
4. Discussions
The exploitation of plant bioactive components, which are nutraceuticals, can help to control the rise of a male positive factor in couples' infertility resulting from human exposure to toxicants. In BPA-exposed rats, many changes indicating male reproductive organ damage were examined to elucidate the potential therapeutic effects of Cucumeropsis mannii seed which has been reported to have a variety of nutraceuticals.
Research has shown that consumption of 100g dehulled C. mannii seeds cover the daily requirement of essential fatty acids, vitamin E, and amino acids (Mbuli-Lingundi et al., 1983; Abiodun and Adeleke, 2010) which has enhanced tissue biogenesis and well as protections. Consistently, due to the abundance of macro-and micro-nutrients, melon has been put forth as a solution to fight malnutrition in both infants and adults as part of a high-nutrient formulation, particularly in resource-limited settings (Gurudeeban et al., 2010). Therefore, nutritional intervention can stimulate tissue repairs and protection in the events of testicular damages as well as to attenuate male infertility.
Male infertility has previously been related to being a drop in sperm count (Working and Chellman, 1993). In this study, BPA administration in rats resulted in a significant reduction in epididymal sperm count, volume, and motility, as well as an increase in abnormal sperm. In rats, however, co-administration of CMSO with BPA increased sperm count, volume, motility, and decreased aberrant sperm in a dose-dependent manner (Figures 1, 2, 3, and 4). Plant natural compounds have been found to enhance epididymal sperm parameters in BPA-exposed mice in previous research (Alboghobeish et al., 2019; Aikawa et al., 2004). BPA binding to the cell surface triggered a negative feedback loop in the hypothalamus and anterior pituitary, which reduced the production of gonadotropin-releasing hormone (GnRH). This is considered to happen in men with high testosterone levels (Santiago et al., 2021). As a result, Sertoli cells generated the hormone inhibin, which was then released into the circulation as if sperm levels were abnormally high. Meanwhile, studies have shown that Sertoli cells topographically and functionally control the spermatogenic process (Pöllänen and Cooper, 1994; Nagano et al., 2001; Franca et al., 1998) hence determining the number of sperm generated per cell. Co-administration of BPA and CMSO, on the other hand, led to an increase in sperm count, volume, mobility morphology, and a decrease in abnormal sperm. CMSO perhaps increased the lipophilicity of the testis cell membrane to prevent BPA from binding which could have led to the release of inhibins. Therefore CMSO has a potential protective effect against BPA-induced testicular toxicity.
Protein as well as testicular enzymes (ALP and LDH) is crucial metabolites of the male reproductive system. BPA intoxication caused a decreased amount of the testicular protein and incurred over-expression of the testicular enzymes whereas combined administration of BPA with CMSO increased the number of proteins and stabilized the activities of the testicular enzymes (Figures 5, 6, and 7). This is consistent with findings from previous research on the effects of natural extracts on BPA-induced testicular damage (El-Beshbishy et al., 2013; Alboghobeish et al., 2019). Previous research has revealed that ambient testicular toxicants have direct antagonistic effects on reproductive organ proteins and lipids (Revathy et al., 2017). In the present study, CMSO possibly reversed the testicular total protein level due to the presence of nutraceuticals perhaps polyunsaturated acids which promote tissue biogenesis. Therefore CSMO has a restorative effect via an increase in protein synthesis in the testicles.
Consistently, LDH and ALP over-expressions occur when tissues are impaired. Increased anaerobic respiration is induced by overexpression of LDH, which disrupts glucose homeostasis (Ainscow et al., 2000). Furthermore, overexpression of ALP in germ cells such as the testis results in the upregulation of the gene encoding germ-cell ALP, which leads to the beginning of proliferation. LDH is abundant in Sertoli and spermatogenic cells, and it plays an essential role in testis energy production, biotransformation, and hydrogen translocation from the cytoplasm to mitochondria through redox coupling α-hydroxyl acid/α-keto acid linked with spermatozoa metabolism (Revathy et al., 2017). The maturation of the germinal epithelial layer of seminiferous tubules and postmeiotic spermatogenic cells is linked to the LDH enzyme in testicles (El-Beshbishy et al., 2013; Sinha et al., 1997).
In the current study, BPA intoxication caused LDH increased activity by inducing lactic acidosis, which lowered the testicular pH, allowing ALP activation, causing ATP hydrolysis and testicular exhaustion. Besides, ATP hydrolysis triggered the expression of abnormal sperm cells within the Leydig and Sertoli compartments during spermatogenesis by producing genetic instabilities and alterations which transforms a normal cell into a malignant cell (Ashraf, 2020). Like malignant cells, the abnormal sperm cells employed LDH to increase their aerobic metabolism (glycolysis and ATP production, and lactate production) even in the presence of oxygen. However, the present study suggests that due to the high essential lipid content of CMSO, its coadministration with BPA lowered the number of damaged cells which decreased the expression of LDH and ALP via lowering the amount of oxidized NADH produced during the conversion of lactate to pyruvate (anaerobic respiration) fostering the testicular ATP hydrolysis by the ALP. In addition, since they are unsaturated, these essential fatty acids present in CMSO can take up the hydrogen ion (H+) released by the oxidation of NADH to stabilize the pH and prevent the activation of the ALP. Therefore, CMSO has a protective effect on the testicular cell membrane by increasing its lipophilicity to prevent permeation of BPA into the testicular milieu and pH regulation via reduction of NAD+. Interestingly, the ALP decrease upon coadministration of BPA with CMSO could be attributed to the high content of essential fatty acids in the oil since lipids are fundamentally known as a key antecedent of spermatogenesis (Koksal et al., 2012; Mohammad, 2015).
Mitochondrial mobility becomes depolarized when the mitochondrial membrane is injured and this causes the signaling pathway to be altered which results in a decrease in MMP (Geens et al., 2012). In the current study, BPA administration in rats significantly decreased the MMP in testicular homogenate but the co-administration of BPA + CMSO significantly increased the MMP in rats’ testis (Figure 8). These findings correlate with the previous study on the protective effects of natural extracts against BPA-induced testicular toxicity. Eruca sativa aqueous extract, as well as a low dose of medicinal plants natural products such as polyphenols (gallic acid) and flavonoids (quercetin, kaempferol, cirsilineol, and acacetin), reduced MMP to protect against BPA toxicity both in vitro and in vivo (Zhang et al., 2017; Grami et al., 2018, 2020). BPA intoxication prevented materials from entering the mitochondria, preventing phosphorylation, which causes depolarization of the mitochondrial membranes in sperm cells. This study suggests that to avoid depolarization, CMSO enhanced the lipophilicity of the testis membrane and inhibited BPA binding/permeation into the mitochondria.
The body weight, testicular volume, and testicular indices also reveal the integrity of male fertility. BPA administration significantly decreased the testicular volume and the body weights of the rats respectively but significantly increased the testicular index (Figures 9, 10, and 11). Findings in the present study correlated with previous reports on the effects of phytocompounds from natural extracts and seed oil on body weight in BPA-induced toxicity. The flavonoid naringin (Alboghobeish et al., 2019), Cordyceps militaris (Wang et al., 2016), Eruca Sativa (Grami et al., 2020), Trigonella foenum-graecum (Kaur and Sadwal, 2019), Lycopene (carotenoid) (Tamilselvan et al., 2013), Lespedeza cuneata (Jiang et al., 2016), and Aloe vera (Behmanesh et al., 2018) all improved the body weight and testis weights, volume, and lowered the testicular index in BPA induced testicular/reproductive toxicity in vivo and in vitro. The present study suggests that a decrease in body weight following BPA intoxication was a feedback response to the loss of the body tissues biogenesis. Disruption of the testicular tissues causes the folding of the wall along the length of the seminiferous tubules, particularly, when fluid enters into the seminiferous tubules (Sriraman et al., 2005). Also, BPA increased the fluidity of the testis cell membrane causing regression of the seminiferous tubules to incur decreased testicular volume as structural damage in the tubular compartment disrupts spermatogenesis and steroidogenesis in the Leydig and Sertoli cells. However, coadministration of BPA + CMSO stabilized the testicular volume by preventing regression of the seminiferous tubules. CMSO’s hydrophobicity prevented water from entering the tubular compartments, thus, preventing testicular regression/wrinkling. Again, Testicular Index represents the ratio of the testicular volume to the body weight, thus, comparing the amount of damage to the testicular tissues with the amount of damage to the whole-body tissues. Reduced body weight and testicular volume resulted in increased testicular index meaning high testicular damage. CMSO protected important molecules, such as proteins in the entire organism and testis respectively from being damaged. Therefore, CMSO improved the body weight, testicular volume, and testicular index perhaps by regenerative potentials of the essential lipids and other nutraceuticals present.
The histopathological examination helps to identify areas with structural damages in sections of stained biological tissues using a microscope with high resolutions. In this study, the result showed that the Normal control group maintained normal testicular architecture (Figure 12a). BPA had moderate to severe effect on the testicular tissue with severe spermatogenic arrest (SA), moderate focal area of hemorrhage (FAH), and moderate necrotic (N) seminiferous tubules (Figure 12b). CMSO only administration group caused mild areas of hemorrhage (H) otherwise normal with enhanced spermatogenesis (S) (Figure 12c). Interestingly, with the coadministration of BPA and CMSO, in the test groups (Figure 12d-f), there was a drastic reduction in the histological damages. These findings correlate with the previous report from other researchers on the effect of the natural product on the histopathology of BPA-induced toxicity in rats. For instance, melatonin (Othman et al., 2016; Olukole et al., 2019), Aloe vera extract (Behmanesh et al., 2018), Quercetin (Jahan et al., 2016), Trigonella foenum-graecum (Fenugreek), and Lespedeza cuneata (Kaur and Sadwal, 2019; Park et al., 2018), naringin (Alboghobeish et al., 2019) restored histological alterations in BPA induced toxicities and improved spermatogenesis. In the present study, therefore, the ability of CMSO to restore histological alterations may be due to the presence of bioactive compounds with the potential to protect the testis cell membrane and enhance tissue regenerations.
5. Conclusion
Male reproductive health can be impaired by the BPA underlying toxicity. Rats’ exposure to BPA led to structural and functional damages in testes and epididymis where sperm is produced. BPA intoxication disrupted biochemical activities and cause histological alterations within the testicular compartments, especially in Leydig and Sertoli cells. Interestingly, with co-administration of CMSO, these dysregulations caused by BPA were normalized. This suggests that CMSO has potential therapeutic effects. Therefore, CMSO perhaps has bioactive compounds which protected the testis cell membrane and modulate tissue regeneration to attenuate the biochemistry and histological alterations against BPA-induced testicular toxicity in the rats.
Declarations
Author contribution statement
Agu, P. C.: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Aja, P. M.: Conceived and designed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Ekpono, Ugbala Ezebuilo., Ogwoni, H. A., Ezeh, E. M.: Contributed reagents, materials, analysis tools or data.
Oscar-Amobi, P. C., Asuk, Atamgba Agbor: Performed the experiments; Wrote the paper.
Anif, O. G.: Conceived and designed the experiments; Wrote the paper.
Awoke, J. N.: Analyzed and interpreted the data; Wrote the paper.
Nwite, F. E., Ukachi, O. U., Orji, O. U., Nweke, P. C., Ekpono, Ugbala Ejike., Ewa, G. O.: Performed the experiments.
Igwenyi, I. O., Egwu, C. O., Alum, E. U., Chukwu, D. C., Famurewa, A. C.: Analyzed and interpreted the data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data will be made available on request.
Declaration of interests statement
The authors declare no competing interests.
Additional information
No additional information is available for this paper.
References
- Abiodun O.A., Adeleke R.O. Comparative studies on the nutritional composition of four melon seed varieties. Pakistan J. Nutr. 2010;9(9):905–908. [Google Scholar]
- Ahangarpour A., Zeidooni L., Rezaei M., Alboghobeish S., Samimi A., Oroojan A.A. Protective effect of metformin on the toxicity of butyric acid and arsenic in isolated liver mitochondria and Langerhans islets in male mice: an in vitro study. Iran. J. Basic Med. Sci. 2017;20:1297–1303. doi: 10.22038/IJBMS.2017.9567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aikawa H., Koyama S., Matsuda M., Nakahashi K., Akazome Y., Mori T. Relief effect of vitamin A on the decreased motility of sperm and the increased incidence of malformed sperm in mice exposed neonatally to bisphenol A. Cell Tissue Res. 2004;315:119–124. doi: 10.1007/s00441-003-0806-1. [DOI] [PubMed] [Google Scholar]
- Ainscow E.K., Zhao C., Rutter G.A. Acute overexpression of lactate dehydrogenase-A perturbs β-cell mitochondrial metabolism and insulin secretion. Diabetes. 2000;49:1149–1155. doi: 10.2337/diabetes.49.7.1149. [DOI] [PubMed] [Google Scholar]
- Alboghobeish S., Mahdavinia M., Zeidooni L., Samimi A., Oroojan A.A., Alizadeh S., Dehghani M.A., Ahangarpour A., Khorsandi L. Efficacy of naringin against reproductive toxicity and testicular damages induced by bisphenol A in rats. Iran. J. Basic Med. Sci. 2019;22:315–323. doi: 10.22038/ijbms.2019.29757.7184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf M.A. Biomedical Research International; 2020. Phytochemicals as Potential Anticancer Drugs: Time to Ponder Nature’s Bounty; pp. 1–7. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badifu G.I., Ogunsua A.O. Chemical composition of kernels from some species of Cucurbitaceae grown in Nigeria. Plant Foods Hum. Nutr. 1991;41:35–44. doi: 10.1007/BF02196380. (Dordrecht, Netherlands) [DOI] [PubMed] [Google Scholar]
- Baracca A., Sgarbi G., Solaini G., Lenaz G. Rhodamine 123 as a probe of mitochondrial membrane potential: evaluation of proton flux through F0 during ATP synthesis. Biochim. Biophys. Acta. 2003;1606:137–146. doi: 10.1016/s0005-2728(03)00110-5. [DOI] [PubMed] [Google Scholar]
- Burkill H.M. Vol. 2. Royal Botanic gardens; Kew: 1985. p. 10. (The Useful Plants of West Africa). [Google Scholar]
- Cabaton N.J., Wadia P.R., Rubin B.S. Perinatal exposure to environmentally relevant levels of bisphenol-A decreases fertility and fecundity in CD-1 mice. Environ. Health Persp. 2011;119:547–552. doi: 10.1289/ehp.1002559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsen E., Giwercman A., Keiding N., Skakkebaek N.E. Evidence for decreasing quality of semen during past 50 years. Brit. Med. J. 1992;305:609–613. doi: 10.1136/bmj.305.6854.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimmino I., Fiory F., Perruolo G., Miele C., Beguinot F., Formisano P., Oriente F. Potential mechanisms of bisphenol A (BPA) contributing to human disease. Int. J. Mol. Sci. 2020;21:1561. doi: 10.3390/ijms21165761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapp R.W., Jacobs M.M., Loechler E.L. Environmental and occupational causes of cancer: new evidence 2005–2007. Rev. Environ. Health. 2008;23:1–37. doi: 10.1515/reveh.2008.23.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colborn T., vom Saal F.S., Soto A.M. Developmental effects of endocrine-disrupting chemicals in wildlife and humans. Environ. Health Persp. 1993;101:378–384. doi: 10.1289/ehp.93101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dror Y., Cohen Y., Yerushalmi-Rozen R. Structure of gum Arabic in aqueous solution. J. Polym. Sci. B Polym. Phys. 2006;44(22):3265–3271. [Google Scholar]
- El-Beshbishy H.A., Aly H.A., El-Shafey M. Lipoic acid mitigates bisphenol A-induced testicular mitochondrial toxicity in rats. Toxicol. Ind. Health. 2013;29:875–887. doi: 10.1177/0748233712446728. [DOI] [PubMed] [Google Scholar]
- Eleazu K., Aja P.M., Eleazu C.O. Cocoyam (Colocasia esculenta) modulates some parameters of testosterone propionate induced rat model of benign prostatic hyperplasia. Drug Chem. Toxicol. 2021 doi: 10.1080/01480545.2021.1892956. [DOI] [PubMed] [Google Scholar]
- Essien E.A., Umoren S.A., EEssien E., Udoh A.P. Preparation and evaluation of Cucumeropsis mannii naud. Seed oil metallic soaps as driers in gloss paint. J. Mater. Environ. Sci. 2012;3(3):477–484. [Google Scholar]
- Ezema B.O. University Of Nigeria; Nsukka: 2012. Purification and Characterization of Lipase (EC.3.1.1.3) from the Seeds of Cucumeropsis Mannii (White Melon) Masters Degree Thesis. [Google Scholar]
- Fisher J.S., Turner K.J., Brown D., Sharpe R.M. Effect of neonatal exposure to estrogenic compounds on the development of the excurrent ducts of the rat testis through puberty to adulthood. Environ. Health Persp. 1999;107:397–405. doi: 10.1289/ehp.99107397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franca L.R., Ogawa T., Avarbock M.R., Brinster R.L., Russell L.D. Germ cell genotype controls the cell cycle during spermatogenesis in the rat. Biol. Reprod. 1998;59:1371–1377. doi: 10.1095/biolreprod59.6.1371. [DOI] [PubMed] [Google Scholar]
- Geens T., Aerts D., Berthot C., Bourguignon J.-P., Goeyens L., Lecomte P. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem. Toxicol. 2012;50:3725–3740. doi: 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Giwa S., Abdullah L.C., Adam N.M. Investigating “egusi” (Citrullus colocynthis L.) seed oil as potential biodiesel feedstock. Energies. 2010;3(4):607–618. [Google Scholar]
- Grami D., Rtibi K., Hammami I., Selmi S., De Toni L., Foresta C., Marzouki L., Sebai H. Protective action of Eruca sativa leaves aqueous extracts against bisphenol A-caused in vivo testicular damages. J. Med. Food. 2020;23:600–610. doi: 10.1089/jmf.2019.0170. [DOI] [PubMed] [Google Scholar]
- Grami D., Rtibi K., Selmi S., Jridi M., Sebai H., Marzouki L., Sabovic I., Foresta C., De Toni L. Aqueous extract of Eruca Sativa protects human spermatozoa from mitochondrial failure due to bisphenol A exposure. Reprod. Toxicol. 2018;82:103–110. doi: 10.1016/j.reprotox.2018.10.008. [DOI] [PubMed] [Google Scholar]
- Gurudeeban S., Satyavani K., Ramanathan T. Bitter apple (Citrullus coloncynthis): an overview of chemical composition and biomedical potentials. Asian J. Plant Sci. 2010;9:394–401. [Google Scholar]
- Gurunath S., Pandian Z., Anderson R.A., Bhattacharya S. Defining infertility a systematic review of prevalence studies. Hum. Reprod. Update. 2011;17:575–588. doi: 10.1093/humupd/dmr015. [DOI] [PubMed] [Google Scholar]
- Hamid A.A., Aiyelaagbe O.O., Usman L.A. Essential oils: its medicinal and pharmacological uses. Int. J. Curr. Res. 2011;33(2):86–98. [Google Scholar]
- Heinala M., Ylinen K., Tuomi T. Assessment of occupational exposure to Bisphenol A in five different production companies in Finland. Annals Work Exp. Health. 2017;61:44–55. doi: 10.1093/annweh/wxw006. [DOI] [PubMed] [Google Scholar]
- Huang Y.Q., Wong C.K., Zheng J.S. Bisphenol A (BPA) in China: a review of sources, environmental levels, and potential human health impacts. Environ. Int. 2012;42:91–99. doi: 10.1016/j.envint.2011.04.010. [DOI] [PubMed] [Google Scholar]
- Ignatius C.M., Francis E.E., Emeka E.N. BPA and environmental estrogen in potable water sources in Enugu municipality, Southeast, Nigeria. Bull. Environ. Contam. Toxicol. 2010;85:534–537. doi: 10.1007/s00128-010-0111-0. [DOI] [PubMed] [Google Scholar]
- Igwenyi I.O. Phytochemical analysis and vitamin composition of Irvigna gabonesis and Citrullus colocynthis. IOSR-JPBS. 2014;9(3):37–40. [Google Scholar]
- Ikechebelu J.I., Adinma J.I.B., Orie E.F., Ikegwuonu S.O. High prevalence of male infertility in southeastern Nigerian. J. Obst. Gynecol. 2003;23(6):657–659. doi: 10.1080/01443610310001604475. [DOI] [PubMed] [Google Scholar]
- Irigaray P., Newby J.A., Clapp R., Hardell L., Howard V. Lifestyle-related factors and environmental agents causing cancer: an overview. Biomed. Pharmacother. 2007;61:640–658. doi: 10.1016/j.biopha.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Jacks T.J., Hensarling T.P., Yatsu L.Y. Cucurbit seeds: I. Characterizations and its uses of oils and proteins, a review. Econ. Bot. 1972;26:135–141. [Google Scholar]
- Jahan S., Ain Q.U., Ullah H. Therapeutic effects of quercetin against bisphenol A-induced testicular damage in male Sprague Dawley rats. Syst. Biol. Reprod. Med. 2016;62(2):114–124. doi: 10.3109/19396368.2015.1115139. [DOI] [PubMed] [Google Scholar]
- Jiang Z., Wang J., Li X., Zhang X. Echinacoside and Cistanche tubulosa (Schenk) R. wight ameliorate bisphenol A-induced testicular and sperm damage in rats through gonad axis regulated steroidogenic enzymes. J. Ethnopharmacol. 2016;193:321–328. doi: 10.1016/j.jep.2016.07.033. [DOI] [PubMed] [Google Scholar]
- Kaur S., Sadwal S. Studies on the phytomodulatory potential of fenugreek (Trigonella foenum-graecum) on bisphenol-A induced testicular damage in mice. Andrology. 2019;52 doi: 10.1111/and.13492. [DOI] [PubMed] [Google Scholar]
- Koksal M., Oguz E., Baba F., Eren M.A., Ciftci H. Effects of melatonin on testis histology, oxidative stress and spermatogenesis after experimental testis ischemia-rep in rats. Eur. Rev. Med. Pharmacol. Sci. 2012;16:582–588. [PubMed] [Google Scholar]
- Liao C., Kannan K. High levels of Bisphenol A in paper currencies from several countries, and implications for dermal exposure. Environ. Sci. Technol. 2011;45:6761–6768. doi: 10.1021/es200977t. [DOI] [PubMed] [Google Scholar]
- Mabalaha M.B., Mitel Y.C., Yeboah S.O. J. Am. Oil Chem. Soci. 2007;84:31. [Google Scholar]
- Mbuli-Lingundi Y., Belitz H.D., Gerstenberg H., Kaiser K.P., Maniwa K., Mödl A., Scherz H., Weder J.K. Chemical composition of seeds from Cucumeropsis mannii Naudin and their suitability as food. Zeitschrift fur Lebensmittel-Untersuchung und –Forschung. 1983;177:37–40. doi: 10.1007/BF01042494. [DOI] [PubMed] [Google Scholar]
- Meeker J.D. Exposure to environmental endocrine-disrupting compounds and men's health. Maturitas. 2010;66:236–241. doi: 10.1016/j.maturitas.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Molina-Garcı´a L., Fernandez-de Cordova M.L., Ruiz- Medina A. Analysis of Bisphenol A in milk by using a multicommuted fluorimetric sensor. Talanta. 2012;96:195–201. doi: 10.1016/j.talanta.2012.02.021. [DOI] [PubMed] [Google Scholar]
- Muhammad J.L. A simple practical method for rat epididymal sperm count (Rattus norvegicus) Biomedica. 2015;41:1–3. [Google Scholar]
- Nagano M., McCarrey J.Y.R., Brinster R.L. Primate spermatogonial cells colonize mouse testis. Biol. Reprod. 2001;64:1409–1416. doi: 10.1095/biolreprod64.5.1409. [DOI] [PubMed] [Google Scholar]
- Nandini B., Sneha G.K., Supriya P.V. Toxic effects of different doses of cyclophosphamide on liver and kidney tissue in Swiss albino mice: a histopathological study. Ethiop. J. Sci. 2018;28(6):711. doi: 10.4314/ejhs.v28i6.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oderinde R.A., Ajiyi I.A., Adewuyi K. Characterization of seed and seed oil of huracrepitans and the kinetics of degradation of the oil during heating. Eur. J. Environ. Agric. Food Chem. 2009;8:201. [Google Scholar]
- OECD . 2008. Acute Oral Toxicity Testing Procedures.http://www.oecd.org/env/testguidelines [Google Scholar]
- Ohore O.E., Songhe Z. Endocrine-disrupting effects of bisphenol A exposure and recent advances on its removal by water treatment systems-A review. Scientific African. 2019;5 [Google Scholar]
- Olukole S.G., Lanipekun D.O., Ola-Davies E.O., Oke B.O. Maternal exposure to environmentally relevant doses of bisphenol A causes reproductive dysfunction in F1 adult male rats: protective role of melatonin. Environ. Sci. Pollut. Res. 2019;26:28940–28950. doi: 10.1007/s11356-019-06153-3. [DOI] [PubMed] [Google Scholar]
- Othman A.I., Edrees G.M., El-Missiry M.A., Ali D.A., Aboel-Nour M., Dabdoub B.R. Melatonin controlled apoptosis and protected the testes and sperm quality against bisphenol A-induced oxidative toxicity. Toxicol. Ind. Health. 2016;32:1537–1549. doi: 10.1177/0748233714561286. [DOI] [PubMed] [Google Scholar]
- Oti W.J.O., Eze–Ilochi N.O. Extraction and characterization of oil from melon and coconut seeds. Int. J. Pharmac. Sci. Inven. ISSN (Online) 2017;6(9):2319–6718. [Google Scholar]
- Pöllänen P., Cooper T.G. Immunology of the testicular excurrent ducts. J. Reprod. Immunol. 1994;26:167–216. doi: 10.1016/0165-0378(94)90019-1. [DOI] [PubMed] [Google Scholar]
- Revathy R., Langeswaran K., Ponnulakshmi R., Balasubramanian M.P., Selvaraj J. Ipomoea batatas tuber efficiency on bisphenol A-induced male reproductive toxicity in Sprague Dawley rats. J. Biol. Active Prod. Nature. 2017;7:118–130. [Google Scholar]
- Rudel R.A., Gray J.M., Engel C.L. Food packaging and Bisphenol A and bis(2- ethyhexyl) phthalate exposure: findings from a dietary intervention. Environ. Health Persp. 2011;119:914–920. doi: 10.1289/ehp.1003170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem U., Amin S., Ahmad B., Azeem H., Anwar F., Mary S. Acute oral toxicity evaluation of aqueous ethanolic extract of Saccharum munja Roxb. roots in albino mice as per OECD 425 TG. Toxicology Reports. 2017;4:580–585. doi: 10.1016/j.toxrep.2017.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santiago J., Silva J.V., Santos M.A.S., Fardilha M. Fighting bisphenol A-induced male infertility: the power of antioxidants. Antioxidants. 2021;10:289. doi: 10.3390/antiox10020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinawat S. The environmental impact on male fertility. J. Med. Assoc. Thail. 2000;83:880–885. [PubMed] [Google Scholar]
- Singh D.R. first ed. CBS Publishers and Distributors; 2003. Principles and Techniques in Histology Microscopy and Photomicrography Published; pp. 20–40. [Google Scholar]
- Sinha N., Narayan R., Saxena D. Effect of endosulfan on the testis of growing rats. Bull. Environ. Contam. Toxicol. 1997;58:79–86. doi: 10.1007/s001289900303. [DOI] [PubMed] [Google Scholar]
- Sriraman V., Anbalagan M., Rao A.J. Hormonal regulation of Leydig cell proliferation and differentiation in rodent testis: a dynamic interplay between gonadotrophins and testicular factors. Reprod. Biomed. Online. 2005;11:507–518. doi: 10.1016/s1472-6483(10)61147-9. [DOI] [PubMed] [Google Scholar]
- Tadesse W.T., Hailu A.E., Gurmu A.E., Mechesso A.F. Experimental assessment of antidiarrheal and antisecretory activity of 80% methanolic leaf extract of Zehneria scabra in mice. BMC Compl. Alternative Med. 2014;14(1):23–27. doi: 10.1186/1472-6882-14-460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamilselvan P., Bharathiraja K., Vijayaprakash S., Balasubramanian M.P. Protective role of lycopene on bisphenol A-induced changes in sperm characteristics, testicular damage, and oxidative stress in rats. Int. J. Pharm. Biol. Sci. 2013;4:131–143. [Google Scholar]
- Toppari J., Larsen J.C., Christiansen P., Giwercman A., Grandjean P. Male reproductive health and environmental xenoestrogens. Environ. Health Persp. 1996;104(4):741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg L.N., Maffini M.V., Sonnenschein C. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Chen C., Jiang Z., Wang M., Jiang H., Zhang X. Protective effect of Cordyceps militaris extracts against bisphenol A-induced reproductive damage. Syst. Biol. Reprod. Med. 2016;62:249–257. doi: 10.1080/19396368.2016.1182234. [DOI] [PubMed] [Google Scholar]
- Wells M.E., Awa O.A. New technique for assessing acrosomal characteristics of spermatozoa. J. Dairy Sci. 1970;53:227. doi: 10.3168/jds.S0022-0302(70)86184-7. [DOI] [PubMed] [Google Scholar]
- Working P., Chellman G. The testis, spermatogenesis, and the excurrent duct system. Reproduc. Toxicol. Infert. 1993;93:55–76. [Google Scholar]
- Zhang Y., Han L., Yang H., Pang J., Li P., Zhang G., Li F., Wang F. Bisphenol A affects cell viability involved in autophagy and apoptosis in goat testis Sertoli cells. Environ. Toxicol. Pharmacol. 2017;2832:13–25. doi: 10.1016/j.etap.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Zhang Z., Alomirah H., Cho H.S. Urinary Bisphenol A-concentrations and their implications for human exposure in several Asian countries. Environ. Sci. Technol. 2011;45:7044–7050. doi: 10.1021/es200976k. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be made available on request.