This cohort study evaluates data from a 14-hospital integrated health care system to determine the association between time to source control and patient outcomes in community-acquired sepsis.
Key Points
Question
In addition to guideline recommendations that sepsis care should include rapid source control, what is known about the association between the timing of source control and patient outcome?
Findings
In this cohort study of 4962 patients with sepsis undergoing source control interventions in a 14-hospital integrated health care system, source control within 6 hours of sepsis onset was associated with a 29% reduction in the risk-adjusted odds of 90-day mortality compared with delayed source control.
Meaning
Source control within 6 hours of sepsis onset was associated with a reduced risk-adjusted odds of 90-day mortality.
Abstract
Importance
Rapid source control is recommended to improve patient outcomes in sepsis. Yet there are few data to guide how rapidly source control is required.
Objective
To determine the association between time to source control and patient outcomes in community-acquired sepsis.
Design, Setting, and Particpants
Multihospital integrated health care system cohort study of hospitalized adults (January 1, 2013, to December 31, 2017) with community-acquired sepsis as defined by Sepsis-3 who underwent source control procedures. Follow-up continued through January 1, 2019, and data analyses were completed March 17, 2022.
Exposures
Early (<6 hours) compared with late (6-36 hours) source control as well as each hour of source control delay (1-36 hours) from sepsis onset.
Main Outcomes and Measures
Multivariable models were clustered at the level of hospital with adjustment for patient factors, sepsis severity, resource availability, and the physiologic stress of procedures generating adjusted odds ratios (aOR) and 95% CI.
Results
Of 4962 patients with sepsis (mean [SD] age, 62 [16] years; 52% male; 85% White; mean [SD] Sequential Organ Failure Assessment score, 3.8 [2.5]), source control occurred at a median (IQR) of 15.4 hours (5.5-21.7) after sepsis onset, with 1315 patients (27%) undergoing source control within 6 hours. The crude 90-day mortality was similar for early and late source control (n = 177 [14%] vs n = 529 [15%]; P = .35). In multivariable models, early source control was associated with decreased risk-adjusted odds of 90-day mortality (aOR, 0.71; 95% CI, 0.63-0.80). This association was greater among gastrointestinal and abdominal (aOR, 0.56; 95% CI, 0.43-0.80) and soft tissue interventions (aOR, 0.72; 95% CI, 0.55-0.95) compared with orthopedic and cranial interventions (aOR, 1.33; 95% CI, 0.96-1.83; P < .001 for interaction).
Conclusions and Relevance
Source control within 6 hours of community-acquired sepsis onset was associated with a reduced risk-adjusted odds of 90-day mortality. Prioritizing the rapid identification of septic foci and initiation of source control interventions can reduce the number of avoidable deaths among patients with sepsis.
Introduction
Sepsis is a life-threatening emergency that accounts for more than half of hospital admissions in the United States.1 The prompt recognition and treatment of sepsis decreases sepsis-related mortality.2,3 However, 1 in 5 patients do not survive hospitalization, and innovative approaches to treatment are urgently needed.4
Source control is a key step in early sepsis treatment and is defined as abscess drainage, infected or necrotic tissue debridement, ongoing microbial contamination control, or infected device removal.5 One-third of patients hospitalized with sepsis undergo source control procedures.6,7 However, due to a lack of high-quality data, controversy exists surrounding how rapidly source control should be achieved.5 As a result, prompt identification of a septic foci and source control is prioritized in international clinical practice guidelines as best practice but, because of low-quality evidence, was left without a GRADE (Grading of Recommendations Assessment, Development and Evaluation).5,8,9
To address this knowledge gap, we studied electronic health record (EHR) data from a large multihospital health care system to determine the association between the timing of source control and adjusted mortality in community-acquired sepsis. We hypothesized that early source control is associated with a reduction in adjusted postintervention mortality when compared with delayed source control.
Methods
We performed a cohort study of patients with community-acquired sepsis who underwent source control procedures. We studied patients at 14 community and academic hospitals in an integrated health care system from January 1, 2013, to December 31, 2017, with data collected through January 1, 2019. This study was approved by the University of Pittsburgh Human Research Protection Office with a waiver of informed consent and Health Insurance Portability and Accountability Act authorization. Data were abstracted January 1, 2021. Data analyses were completed March 17, 2022. All reporting was in accordance with Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines.10
Data Sources and Definitions
Data were derived from inpatient hospital EHR (Cerner), including preprocedural (eg, age, patient-reported sex and race, comorbid conditions, patient status, intrahospital transfers), procedural, and postintervention data. Race was abstracted from the registration system data and categorized in accordance with the Centers for Medicare & Medicaid Services EHR meaningful use data set. Comorbid conditions were classified by hospitalization diagnosis codes from International Classification of Diseases, Ninth Revision, Clinical Modification, and International Statistical Classification of Diseases, Tenth Revision, Clinical Modification.
We described patient status, including vital signs, laboratory results, evidence of organ dysfunction as measured by the Sequential Organ Failure Assessment (SOFA) score within 6 hours of sepsis onset,11 and time from sepsis onset to antibiotic administration. Patients discharged and readmitted within 12 hours to a hospital with a higher level of care were defined as interhospital transfers. The availability of specialty and procedural care was defined by the American College of Surgeons trauma center verification mandating surgeon availability, including (1) surgeons in-house 24 hours a day (ie, level I), (2) surgeons available 24 hours a day (ie, level II), and (3) not mandated (eTable 1 in the Supplement).12 Source control procedures were identified using Current Procedure Terminology (CPT) codes (eTable 2 in the Supplement).13 Interventions included up to 6 source control procedures. The primary procedure defined intervention characteristics. Exact intervention start times were abstracted from electronic procedure reports. Postintervention data included intensive care unit (ICU) care and hospital length of stay.
We used the unique EHR patient identifier to link inpatient records to additional data sources. The institution trauma registry identified hospitalizations after trauma activations.12,14 Date of death in the health care system was captured using (1) health care system–wide inpatient acute care, long-term care, and nursing facility discharge disposition and (2) the monthly updated Social Security Death Index linked outpatient records.15,16,17
Patient Cohort
In the primary cohort, we included the index hospitalization for adults (aged ≥18 years) with community-acquired sepsis undergoing source control procedures. We defined community-acquired sepsis as evidence of International Consensus Criteria for Sepsis-3 in the EHR,18 including evidence of suspected infection within 24 hours of hospital presentation and presence of organ dysfunction within 6 hours of suspected infection.19 A suspected infection was defined by the administration of antibiotics as well as acquisition of a culture specimen and the first occurrence of either marked sepsis onset. Organ dysfunction was defined as 2 or more SOFA points.11 We excluded patients who did not undergo a source control procedure. To minimize misclassification and the survivor treatment selection bias,20,21 we excluded patients (1) who were admitted after a trauma activation, (2) whose data were missing procedure start times, (3) who underwent procedures longer than 36 hours after sepsis onset, (4) who underwent a concurrent solid organ transplant, and (5) who died within 2 days of sepsis onset.
In the most common interventions cohort, we included patients undergoing intervention families that accounted for more than 10% of interventions and excluded those with CPT codes that had fewer than 10 occurrences in the primary cohort (eTable 3 in the Supplement).
Source Control
We used a modified Delphi consensus method to define CPT codes specific to the identification of source control procedures.13 We used a panel of 6 surgical experts to generate a list of CPT codes for specific source control procedures and clinically adjudicate the selected codes. The agreed-on CPT codes were grouped into 6 anatomic categories: gastrointestinal and abdominal, hepatopancreatobiliary, thoracic, urologic and gynecologic, orthopedic and cranial, and soft tissue. The low or high categorization of the physiologic stress of a procedure was guided by the validated Operative Stress Score22,23 to account for the association between procedural stress and adverse outcomes. We randomly selected 1% of patients who met study criteria for independent clinical adjudication by 2 experts, recording the procedural start time and indication. Expert review confirmed agreement between clinical adjudication and intervention start times (100% accurate) as well as CPT code–identified source control procedures (>90% overall and >85% for each anatomic category).
The time to source control was defined as the time from sepsis onset to the intervention start time of the first source control procedure.7 As described above, the time of sepsis onset was defined according to EHR criteria in Sepsis-3.18,19 As recommended by the Surviving Sepsis Campaign 2021 guidelines,5 the time to source control was dichotomized at 6 hours of sepsis to define early (ie, <6 hours) and late (ie, 6-36 hours) source control.5
Outcomes
The primary outcome was 90-day postintervention mortality. Secondary outcomes included postintervention ICU admission, hospital length of stay, and in-hospital as well as 365-day mortality.
Statistical Analysis
Categorical data were presented as number (%) and continuous data as mean (SD) or median (IQR). Comparisons were performed with χ2, t test, or Kruskal-Wallis testing as appropriate. Missing preintervention vital signs and laboratory results were quantified and imputed using multivariable random forest regression (eTable 4 in the Supplement) in R version 4.1.2 (R Core Team, 2021; missRanger package [version 2.1.3; Mayer, 2021]).
We used multivariable logistic regression to quantify the association between early source control and in-hospital, 90-day, and 365-day mortality. The adjusted 90-day mortality by each hour of source control delay was calculated in postprediction modeling following fractional polynomial assessment for nonlinear associations (eMethods in the Supplement).3,24
For all models, covariates were chosen a priori and included demographics (ie, age, sex, race, body mass index, Elixhauser Comorbidity Index), sepsis characteristics (ie, SOFA), intervention data (ie, physiologic stress, anatomic category25), and hospital resources (ie, transfers, mandated surgeon availability, hospital admission year) and were clustered at the level of hospital.26,27 We tested for a priori subgroup effects in the primary cohorts (ie, age, race, sex, SOFA, transfer status, surgeon availability, intervention physiologic stress, anatomic category) and most common intervention cohort (ie, intervention families). A P value for interaction <.05 defined significance and effect modification.28,29 Statistical analyses and figures were completed in Stata version 17.0 (StataCorp) and PRISM version 9.0 (GraphPad Software).
Sensitivity Analysis
We completed 5 sensitivity analyses. First, we quantified the strength required for hypothetical unmeasured confounding to negate the primary analysis association between early source control and improved 90-day mortality using the E-value.30,31 Second, using our primary model, we evaluated the association between very early (ie, <3 hours) source control and 90-day mortality. Third, we expanded our cohort to include patients who died within 2 days of sepsis onset and evaluated the association between early source control and both 2-day and 90-day mortality. Fourth, we created 3 additional cohorts, limiting our primary cohort to exclude hospital transfers, patients with a SOFA score less than 3, or patients with time from sepsis onset to source control of longer than 30 hours or longer than 24 hours. Fifth, we expanded our primary model to include additional features of sepsis (ie, values for preprocedural serum lactate and time to antibiotic administration2,3), surgical procedure (ie, Operative Stress Score23,25), and disease-specific mortality (ie, values for preprocedural creatine, bilirubin, and international normalized ratio).
Results
Study Population and Source Control Procedures
Among 4962 patients with sepsis at 14 hospitals, source control interventions occurred a median (IQR) of 15.4 hours (5.5-21.7) after sepsis onset (Figure 1 and eFigure 1 in the Supplement). Interventions for 1 in 3 patients (n = 1486) undergoing source control included multiple source control procedures. The mean age (range, 57-67 years), median SOFA score (range, 3.2-4.5), frequency of highly stressful interventions (range, 5%-95%), and median time to source control (range, 11.0-19.5 hours) varied across anatomic categories (eTable 5 in the Supplement and Figure 1). The overall mortality rate was 14% (n = 706) at 90 days.
Figure 1. Time to Source Control in Community-Acquired Sepsis.
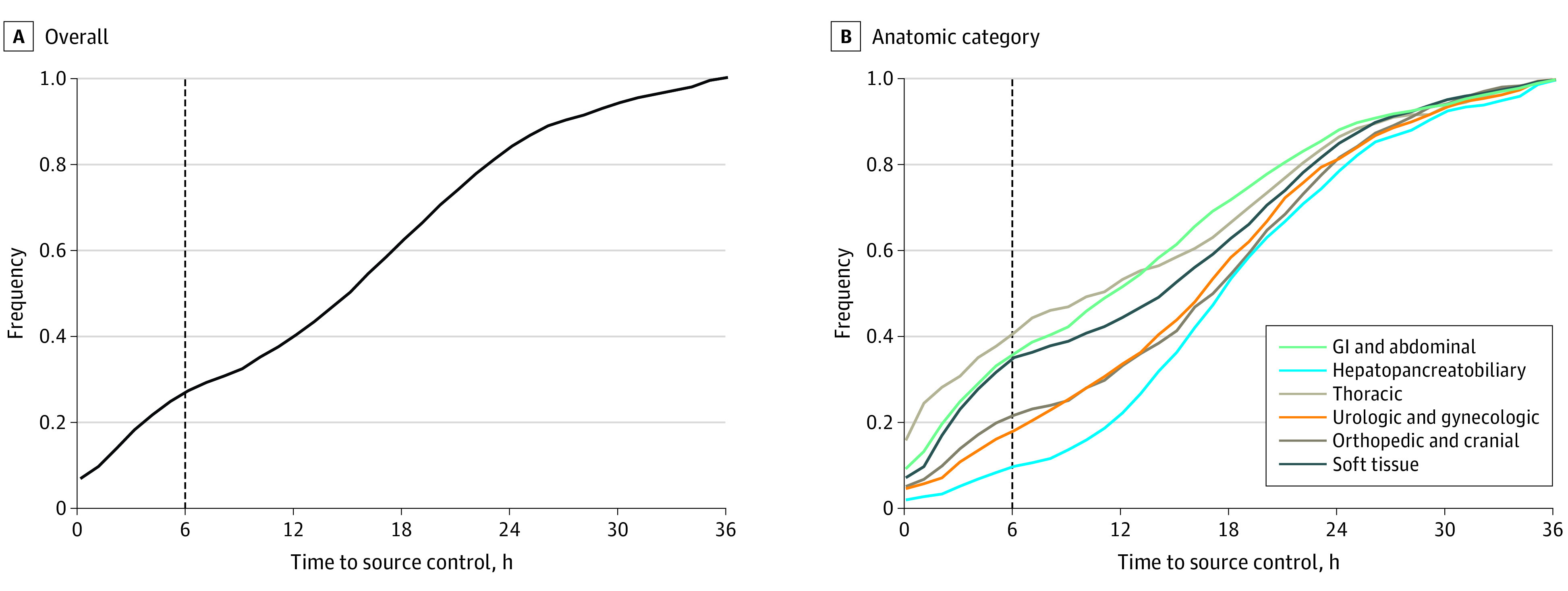
Cumulative distribution frequency graph of time to source control overall (A) and by anatomic category (B). The dashed line indicates the 6-hour time point delineating early and late source control. GI indicates gastrointestinal.
Among the 1315 patients who underwent early source control, mean (SD) age was younger (age, 59 [17] vs 63 [17] years; P < .001), mean (SD) SOFA score was higher (4.8 [2.8] vs 3.4 [2.2]; P < .001), and antibiotics were received earlier (median [IQR], 0.4 hours [−0.4 to 1.7] vs 1.9 hours [0.5 to 4.5]; P < .001) (Table and eFigure 2 in the Supplement). Patients undergoing early source control were more frequently mechanically ventilated (626 patients [48%] vs 867 patients [24%]; P < .001), received vasoactive medications (393 [30%] vs 617 [17%]; P < .001), and were cared for at hospitals with 24-hour in-house surgeons (833 [63%] vs 1670 [46%]; P < .001) compared with patients undergoing late source control. Early source control interventions were more frequently gastrointestinal and abdominal (482 [37%] vs 890 [24%]), thoracic (139 [11%] vs 208 [6%]), or soft tissue (351 [27%] vs 679 [19%]; P < .001) as well as high stress (958 [73%] vs 1694 [46%]; P < .001) compared with late source control interventions. The crude 90-day mortality was 14% (177 patients) for early source control and 15% (529 patients) for late source control.
Table. Patient, Hospital, and Source Control Characteristics and Outcomes.
| Characteristic | No. (%) | ||
|---|---|---|---|
| All patients | Early source control (<6 h) | Late source control (6-36 h) | |
| Patient characteristics | |||
| No. of patients (%) | 4962 | 1315 (27) | 3647 (73) |
| Age, mean (SD), y | 62 (16) | 59 (17) | 63 (16) |
| Sex | |||
| Male | 2604 (52) | 670 (51) | 1934 (53) |
| Female | 2358 (48) | 645 (49) | 1713 (47) |
| Race | |||
| Black | 422 (9) | 116 (9) | 306 (8) |
| White | 4226 (85) | 1087 (83) | 3139 (86) |
| Othera | 314 (6) | 112 (9) | 202 (6) |
| Body mass index, mean (SD) | 30.9 (9.6) | 31.5 (11.0) | 30.7 (9.0) |
| SOFA score, mean (SD)b | 3.8 (2.5) | 4.8 (2.8) | 3.4 (2.2) |
| Elixhauser Comorbidity Index, mean (SD)c | 4.0 (2.4) | 3.9 (2.5) | 4.0 (2.3) |
| Selected vital signs and laboratory valuesd | |||
| Heart rate, mean (SD), /min | 98 (21) | 102 (21) | 96 (21) |
| Respiratory rate, mean (SD), /min | 21 (6) | 21 (6) | 20 (6) |
| Serum lactate level, mean (SD), mg/dL | 24.3 (24.3) | 24.3 (25.2) | 24.3 (23.4) |
| INR, mean (SD) | 1.5 (1.1) | 1.4 (0.9) | 1.6 (1.2) |
| Creatinine level, mean (SD), mg/dL | 1.6 (1.6) | 1.5 (1.5) | 1.7 (1.7) |
| Total bilirubin level, mean (SD), mg/dL | 1.9 (3.4) | 1.2 (1.9) | 2.2 (3.9) |
| Hospital and source control characteristics | |||
| Mandated surgeon availability | |||
| In-house 24 h | 2503 (50.4) | 833 (63.3) | 1670 (45.8) |
| Not mandated | 2207 (44.5) | 430 (32.7) | 1777 (48.7) |
| Available 24 h | 252 (5.1) | 52 (4.0) | 200 (5.5) |
| Intrahospital transfer status | |||
| Not transferred | 4219 (85.0) | 1141 (86.8) | 3078 (84.4) |
| Transferred | 743 (15.0) | 174 (13.2) | 569 (15.6) |
| Time to antibiotics, median (IQR), h | 1.5 (0.2 to 3.7) | 0.4 (−0.1 to 1.7) | 1.9 (0.5 to 4.5) |
| Time to source control, median (IQR), h | 15.4 (5.5 to 21.7) | 2.3 (0.4 to 3.9) | 18.7 (13.9 to 23.6) |
| Anatomic category of source control | |||
| Gastrointestinal and abdominal | 1372 (27.7) | 482 (36.7) | 890 (24.4) |
| Soft tissue | 1030 (20.8) | 351 (26.7) | 679 (18.6) |
| Hepatopancreatobiliary | 883 (17.8) | 87 (6.6) | 796 (21.8) |
| Urologic and gynecologic | 714 (14.4) | 127 (9.7) | 587 (16.1) |
| Orthopedic and cranial | 616 (12.4) | 129 (9.8) | 487 (13.4) |
| Thoracic | 347 (7.0) | 139 (10.6) | 208 (5.7) |
| Procedural physiologic stresse | |||
| More stress | 2652 (53) | 958 (73) | 1694 (46) |
| Less stress | 2310 (47) | 357 (7) | 1953 (54) |
| Hospital utilization and crude outcomes | |||
| Mechanical ventilation, any | 1493 (30) | 626 (48) | 867 (24) |
| Vasopressors, any | 1010 (20) | 393 (30) | 617 (17) |
| Admitted to ICU | 2219 (45) | 802 (61) | 1417 (39) |
| Hospital LOS, mean (SD), d | 11.8 (12.3) | 14.8 (16.1) | 10.7 (10.4) |
| Mortality | |||
| In-hospital | 287 (5.8) | 90 (6.8) | 197 (5.4) |
| 90-d | 706 (14.2) | 177 (13.5) | 529 (14.5) |
| 365-d | 1196 (24.1) | 928 (25.5) | 268 (20.4) |
Abbreviations: ICU, intensive care unit; INR, international normalized ratio; LOS, length of stay; SOFA, Sequential Organ Failure Assessment score.
SI conversion factors: To convert bilirubin to μmol/L, multiply by 17.104; creatinine to μmol/L, multiply by 88.4; serum lactate to mmol/L, multiply by 0.111.
Other race corresponds to American Indian/Alaska Native, Asian, Chinese, Filipino, Hawaiian/Other Pacific Islander, Middle Eastern, or Native American.
SOFA score corresponds to the severity of organ dysfunction, reflecting 6 organ systems each with a score range of 0-4 points (cardiovascular, hepatic, hematologic, respiratory, neurological, renal), with a total score range of 0-24 points.
Elixhauser is a method of categorizing comorbidities of patients based on International Classification of Diseases diagnosis codes in administrative data, ranging from 0 to 31.
Missing data were imputed via random forest models (eTable 4 in the Supplement).
Physiologic stress was guided by Operative Stress Score (low <1, high 2-5). Procedures without an Operative Stress Score were assigned a physiologic stress level based on the rating of similar procedures (eTable 2 in the Supplement).
Primary Analysis
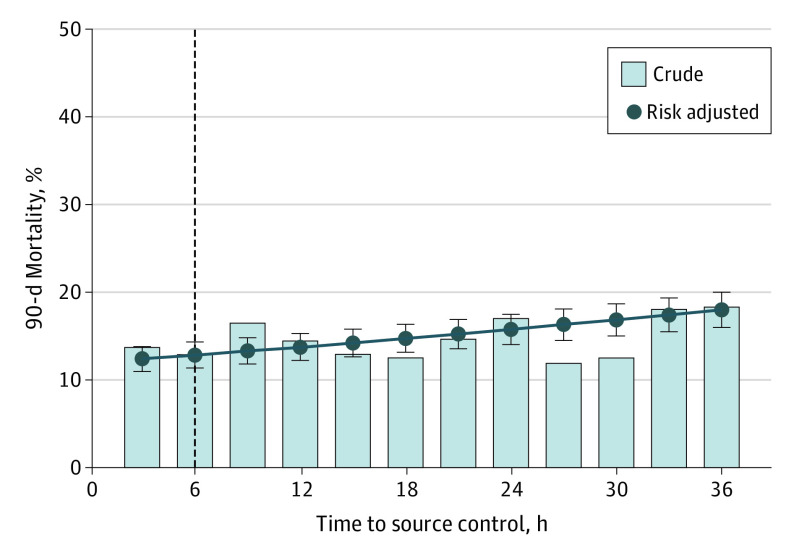
In multivariable models, early source control had a 29% reduced risk-adjusted odds of 90-day mortality compared with late source control (aOR, 0.71; 95% CI, 0.63-0.80; P < .001) (eTable 6 in the Supplement). Early source control was also associated with a 24% and 34% reduced risk-adjusted odds of in-hospital mortality (aOR, 0.76; 95% CI, 0.63-0.90; P = .002) and 365-day mortality (aOR, 0.66; 95% CI, 0.61-0.71; P < .001), respectively (eTables 7 and 8 in the Supplement). Each hour of source control delay was associated with increased 90-day mortality (aOR, 1.02; 95% CI, 1.01-1.02; P < .001). When compared with source control at 6 hours (risk-adjusted 90-day mortality, 12.9%; 95% CI, 11.4%-14.3%), the risk-adjusted absolute risk difference of 90-day mortality was reduced by approximately 0.5% at 3 hours and increased by 1% at 12 hours, 3% at 24 hours, and 5% at 36 hours (Figure 2).
Figure 2. Observed and Risk-Adjusted 90-Day Mortality for the Primary Cohort.
Error bars indicate 95% CIs; dashed line, the 6-hour time point delineating early and late source control.
The association between early source control and reduced 90-day mortality was observed across a priori subgroups, with effect modification by age, sex, SOFA score, and anatomic categories (P < .05 for each interaction) (Figure 3). For example, early source control was associated with a 41% and 38% reduction in the risk-adjusted odds of 90-day mortality for gastrointestinal and abdominal and soft tissue interventions, respectively, but failed to reach significance for hepatopancreatobiliary, thoracic, urologic and gynecologic, or orthopedic and cranial interventions (eFigure 3 in the Supplement).
Figure 3. Association Between Early Source Control and 90-Day Mortality Across Prespecified Subgroups.
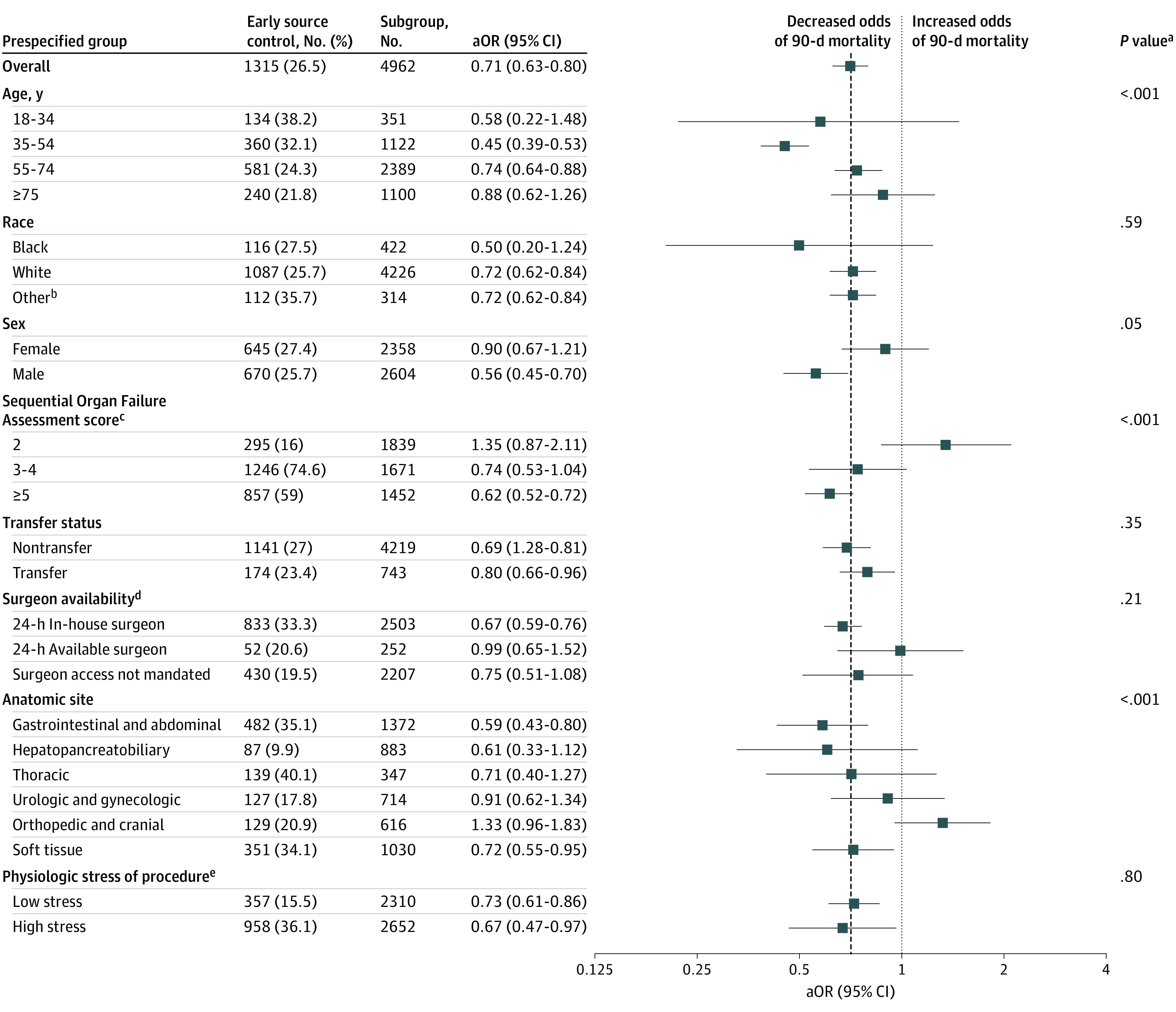
Risk-adjusted odds ratios (aORs) of 90-day mortality among prespecified subgroups. No difference in mortality among early or late source control is indicated by an aOR of 1.0, represented by the dotted line. The overall aOR of 90-day mortality associated with early source control is indicated by the dashed line.
aAll P values were calculated from the interaction between the treatment group and subgroups in the primary logistic regression model.
bOther race corresponds to American Indian/Alaska Native, Asian, Chinese, Filipino, Hawaiian/Other Pacific Islander, Middle Eastern, or Native American.
cScore corresponds to severity of organ dysfunction, reflecting 6 organ systems each with a score range of 0 to 4 points (cardiovascular, hepatic, hematologic, respiratory, neurological, renal), with a total score range of 0 to 24 points.
dSurgeon availability determined by the hospital-specific American College of Surgeons surgeon availability mandate (eTable 1 in the Supplement).
ePhysiologic stress was determined by Operative Stress Score (low <1; high 2-5). Procedures without an Operative Stress Score were assigned a physiologic stress level based on the rating of similar procedures (eTable 2 in the Supplement).
Most Common Interventions
When models were restricted to the 5 most common interventions (67%), the association between early source control and 90-day mortality persisted (aOR, 0.61; 95% CI, 0.46-0.80; P < .001), as well as the association between each hour of source control delay and 90-day mortality (aOR, 1.02; 95% CI, 1.01-1.03; P < .001) (eTable 9 in the Supplement). The association between early source control and reduced 90-day mortality remained different by intervention type (P = .01 for interaction) (Figure 4 and eTable 10 and eFigure 4 in the Supplement).
Figure 4. Observed and Adjusted Risk of 90-Day Mortality Among the Most Common Interventions (n = 3309).
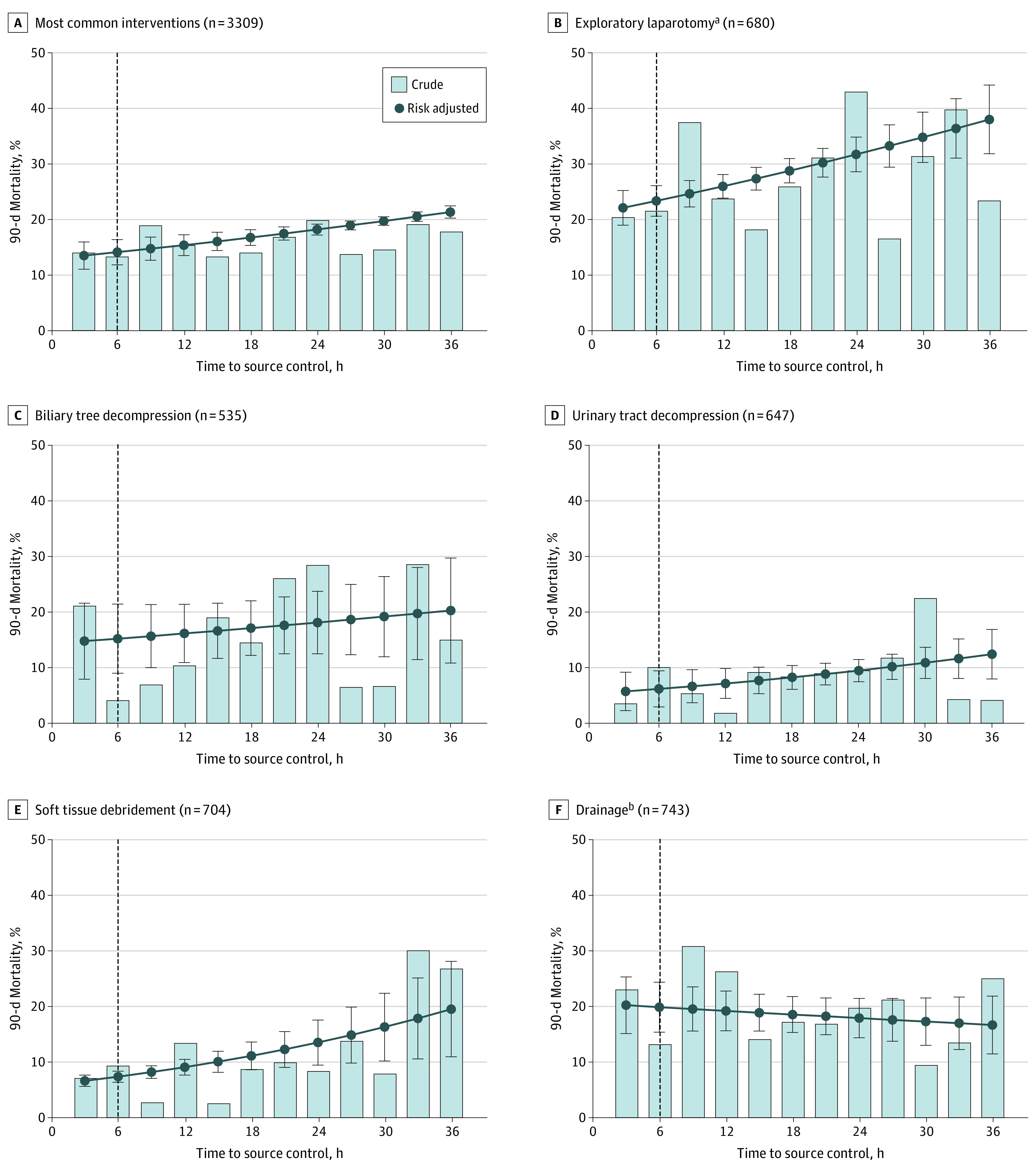
The most common intervention families were determined by similar Current Procedural Terminology codes, defined in eTable 3 in the Supplement. Error bars indicate 95% CIs; dashed line, the 6-hour time point delineating early and late source control.
aIncludes both exploratory laparotomy and laparoscopy procedures.
bPercutaneous and incisional drainage.
Sensitivity Analysis
Model results were robust in sensitivity analyses (eTable 11 in the Supplement). First, the E-value indicated an unmeasured confounder would require an aOR of at least 2.17 (lower limit CI, 1.81) to negate the association between early source control and reduced 90-day mortality. Second, compared with early source control, very early source control was associated with a similar reduced risk-adjusted odds of 90-day mortality (aOR, 0.69; 95% CI, 0.58-0.81). Third, on including patients who died within 2 days of sepsis onset (n = 5054), early source control did not have an association with 2-day mortality (aOR, 1.04; 95% CI, 0.77-1.40) but continued to have an association with reduced 90-day mortality (aOR, 0.75; 95% CI, 0.65-0.86). Fourth, models were consistent when excluding hospital transfers (aOR, 0.64; 95% CI, 0.54-0.77), SOFA score less than 3 (aOR, 0.60; 95% CI, 0.49-0.75), or source control procedures occurring after 30 hours (aOR, 0.74; 95% CI, 0.62-0.85) or 24 hours (aOR, 0.73; 95% CI, 0.62-0.89) from sepsis onset. Fifth, association between early source control and reduced mortality persisted after including individual variables (aOR range, 0.66-0.75) or all (aOR, 0.72; 95% CI, 0.59-0.86) sepsis, procedural, and disease-specific variables in the primary model. For example, the association between early source control and mortality was robust (aOR, 0.70; 95% CI, 0.63-0.79) when including time to antibiotics (aOR for 1-hour delay, 1.01; 95% CI, 1.00-1.02).
Discussion
In an integrated health care system of 14 community and academic hospitals, source control within 6 hours of community-acquired sepsis onset was associated with a reduced risk-adjusted odds of 90-day mortality. This association was consistent across a range of age, sex, and illness severity factors as well as among the most common interventions.
Our inclusive investigation of sepsis and source control procedures was consistent with prior work demonstrating shorter time to source control was associated with reduced mortality overall7 and among patients with necrotizing fasciitis,32,33,34,35 cholangitis,36 and intraabdominal perforations.37,38,39 However, investigation of the optimal source control timing in sepsis has yielded discordant results.40,41,42 Most studies were small, investigated time to source control in secondary analysis, and had a limited set of potential confounders. We extend these data in multiple ways. First, we used International Consensus Criteria to identify patients with sepsis (ie, Sepsis-318) undergoing source control, including procedures defined by modified Delphi consensus methodology and validated through clinical adjudication.13 Second, we used multiple EHR-defined features to adjust for patient factors, sepsis severity, resource availability, and the physiologic stress of procedures in the models. Third, the association between early source control and reduced mortality was robust in sensitivity analyses. Further, given that the E-value is larger in magnitude than other aOR for model covariates, it is therefore unlikely that a confounder or a set of confounders could negate our findings.
Early source control was associated with the greatest risk reduction among middle-aged patients (ie, 35-54 years), not older adults. Older patients lack physiologic reserve. Among patients with sepsis43 and those undergoing surgical interventions,22,25 frailty is independently associated with adverse outcomes. Early source control may not overcome the high underlying risk of adverse events among older patients. Early source control was also associated with a greater risk reduction for men compared with women. Although underlying biological differences may contribute,44,45 critically ill women are less likely to receive prompt care and undergo interventions when compared with men.46,47,48 Therefore, a combination of sex-specific patient preferences and implicit clinician bias may contribute to these sex-based differences. Rapid time to source control was more frequently observed in sicker patients, and early source control was associated with a greater reduction in risk-adjusted mortality compared with patients with less organ dysfunction. These data support the clinical bias of health care professionals to promptly achieve source control among patients presenting in extremis.
Notably, transfer status, surgeon availability, and the physiologic stress of an intervention did not significantly moderate the association between early source control and adjusted risk-reduced mortality. These data suggest that perhaps early source control may be key to improving sepsis outcomes independent of available resources required for source control. Furthermore, early source control did not have a significant association with reduced risk-adjusted mortality in patients undergoing hepatopancreatobiliary, thoracic, urologic and gynecologic, or orthopedic and cranial interventions. The subgroup size and proportion of early source control procedures limit our ability to identify an association in these subgroups. Notably, only the risk-adjusted odds for orthopedic and cranial interventions were greater than 1. These interventions were predominately amputations and hardware removal, which are often preceded by prolonged attempts at limb salvage, including revascularization, wound care, and antibiotic therapy.
From a clinical standpoint, these data support the Surviving Sepsis Campaign guidelines that recommend rapid identification of septic foci with prompt source control as logistically feasible.5 However, the lack of prior data left these guidelines without an evidence GRADE.5,8,9 Conducting a clinical trial to obtain data to support these guidelines would not be feasible. Although the current data were not randomized, they were robust, they were adjusted for confounders, and they overcome indication bias of the earliest care provided to patients with sepsis in extremis. Therefore, these data support prioritizing rapid identification of septic foci and initiation of source control interventions within 6 hours of sepsis onset.
Limitations
These results have limitations. First, data were generated as a part of routine clinical care and evaluated in retrospect. Second, as with all observational studies, residual confounding may exist because these data do not measure source control–specific pathology (ie, bowel resection for diverticulitis vs ischemia) or specialty-specific practice patterns (ie, proclivity within and between both proceduralists and surgeons to act immediately). Third, CPT code–identified source control procedures prioritized specificity over sensitivity, and some patients undergoing source control procedures may be misclassified in these analyses. Fourth, the quality and completeness of source control after procedures is unknown. Fifth, the multihospital health care system provides care within a specific geographic region in the United States, and our analysis included only community-acquired sepsis, which may limit the external validity of the results. Sixth, the evaluation of subgroup effects was exploratory, limited by the sample sizes, and must be interpreted with caution.
Conclusions
Source control within 6 hours of community-acquired sepsis onset was associated with a reduced risk-adjusted odds of 90-day mortality. Prioritizing the rapid identification of septic foci and initiation of source control interventions can reduce the number of avoidable deaths among patients with sepsis.
eMethods. Fractional polynomial model assessment
eTable 1. Hospital specific American College of Surgeons surgeon availability mandate
eTable 2. Source control procedures, anatomic category, and procedural stress
eTable 3. Most common source control intervention families
eTable 4. Feature summary statistics before and after imputation
eTable 5. Cohort characteristics by anatomic location of source control
eTable 6. Logistic regression - primary model with all-cause 90-day mortality as outcome
eTable 7. Logistic regression - primary model with all-cause in-hospital mortality as outcome
eTable 8. Logistic regression - primary model with all-cause 365-day mortality as outcome
eTable 9. Cohort characteristics by most common intervention
eTable 10. Adjusted absolute risk difference of 90-day mortality when comparted to source control at six hours, by most common interventions
eTable 11. Sensitivity analyses - modifications to covariate set
eFigure 1. Cohort accrual
eFigure 2. Correlation between variables and time to source control
eFigure 3. Observed and adjusted risk of 90-day mortality by source control anatomic categories
eFigure 4. Association between early source control and 90-day mortality among the most common interventions
References
- 1.Rhee C, Jones TM, Hamad Y, et al. ; Centers for Disease Control and Prevention (CDC) Prevention Epicenters Program . Prevalence, underlying causes, and preventability of sepsis-associated mortality in US acute care hospitals. JAMA Netw Open. 2019;2(2):e187571. doi: 10.1001/jamanetworkopen.2018.7571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kahn JM, Davis BS, Yabes JG, et al. Association between state-mandated protocolized sepsis care and in-hospital mortality among adults with sepsis. JAMA. 2019;322(3):240-250. doi: 10.1001/jama.2019.9021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour CW, Gesten F, Prescott HC, et al. Time to treatment and mortality during mandated emergency care for sepsis. N Engl J Med. 2017;376(23):2235-2244. doi: 10.1056/NEJMoa1703058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program . Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Evans L, Rhodes A, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock 2021. Intensive Care Med. 2021;47(11):1181-1247. doi: 10.1007/s00134-021-06506-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent JL, Rello J, Marshall J, et al. ; EPIC II Group of Investigators . International study of the prevalence and outcomes of infection in intensive care units. JAMA. 2009;302(21):2323-2329. doi: 10.1001/jama.2009.1754 [DOI] [PubMed] [Google Scholar]
- 7.Bloos F, Rüddel H, Thomas-Rüddel D, et al. ; MEDUSA study group . Effect of a multifaceted educational intervention for anti-infectious measures on sepsis mortality: a cluster randomized trial. Intensive Care Med. 2017;43(11):1602-1612. doi: 10.1007/s00134-017-4782-4 [DOI] [PubMed] [Google Scholar]
- 8.Guyatt GH, Schünemann HJ, Djulbegovic B, Akl EA. Guideline panels should not GRADE good practice statements. J Clin Epidemiol. 2015;68(5):597-600. doi: 10.1016/j.jclinepi.2014.12.011 [DOI] [PubMed] [Google Scholar]
- 9.Schünemann HJ, Wiercioch W, Brozek J, et al. GRADE Evidence to Decision (EtD) frameworks for adoption, adaptation, and de novo development of trustworthy recommendations: GRADE-ADOLOPMENT. J Clin Epidemiol. 2017;81(81):101-110. doi: 10.1016/j.jclinepi.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg. 2014;12(12):1495-1499. doi: 10.1016/j.ijsu.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 11.Vincent J, Moreno R, Takala J, et al. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 12.Pennsylvania Trauma Systems Foundation . Standards of accreditation adult levels I-III. Accessed June 12, 2022. https://www.ptsf.org/become-a-trauma-center/center-standards/
- 13.Li SR, Handzel RM, Tonetti D, et al. Consensus Current Procedural Terminology code definition of source control for sepsis. J Surg Res. 2022;275:327-335. doi:10.1016/j.jss.2022.02.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashmi ZG, Kaji AH, Nathens AB. Practical guide to surgical data sets: National Trauma Data Bank (NTDB). JAMA Surg. 2018;153(9):852-853. doi: 10.1001/jamasurg.2018.0483 [DOI] [PubMed] [Google Scholar]
- 15.Hill ME, Rosenwaike I. The Social Security Administration’s Death Master File: the completeness of death reporting at older ages. Soc Secur Bull. 2001-2002;64(1):45-51. [PubMed] [Google Scholar]
- 16.Navar AM, Peterson ED, Steen DL, et al. Evaluation of mortality data from the Social Security Administration Death Master File for clinical research. JAMA Cardiol. 2019;4(4):375-379. doi: 10.1001/jamacardio.2019.0198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reitz KM, Marroquin OC, Zenati MS, et al. Association between preoperative metformin exposure and postoperative outcomes in adults with type 2 diabetes. JAMA Surg. 2020;155(6):e200416. doi: 10.1001/jamasurg.2020.0416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seymour CW, Kennedy JN, Wang S, et al. Derivation, validation, and potential treatment implications of novel clinical phenotypes for sepsis. JAMA. 2019;321(20):2003-2017. doi: 10.1001/jama.2019.5791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yadav K, Lewis RJ. Immortal time bias in observational studies. JAMA. 2021;325(7):686-687. doi: 10.1001/jama.2020.9151 [DOI] [PubMed] [Google Scholar]
- 21.Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ. 2010;340(7752):b5087. doi: 10.1136/bmj.b5087 [DOI] [PubMed] [Google Scholar]
- 22.Shinall MC Jr, Arya S, Youk A, et al. Association of preoperative patient frailty and operative stress with postoperative mortality. JAMA Surg. 2020;155(1):e194620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Q, Kim J, Hall DE, et al. Association of frailty and the expanded Operative Stress Score with preoperative acute serious conditions, complications and mortality in males compared to females: a retrospective observational study. Ann Surg. Published online June 25, 2021. doi: 10.1097/SLA.0000000000005027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Royston P, Sauerbrei W. Building multivariable regression models with continuous covariates in clinical epidemiology–with an emphasis on fractional polynomials. Methods Inf Med. 2005;44(4):561-571. doi: 10.1055/s-0038-1634008 [DOI] [PubMed] [Google Scholar]
- 25.George EL, Hall DE, Youk A, et al. Association between patient frailty and postoperative mortality across multiple noncardiac surgical specialties. JAMA Surg. 2021;156(1):e205152. doi: 10.1001/jamasurg.2020.5152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheetz KH, Chhabra KR, Smith ME, Dimick JB, Nathan H. Association of discretionary hospital volume standards for high-risk cancer surgery with patient outcomes and access, 2005-2016. JAMA Surg. 2019;154(11):1005-1012. doi: 10.1001/jamasurg.2019.3017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Teng CY, Davis BS, Rosengart MR, Carley KM, Kahn JM. Assessment of hospital characteristics and interhospital transfer patterns of adults with emergency general surgery conditions. JAMA Netw Open. 2021;4(9):e2123389. doi: 10.1001/jamanetworkopen.2021.23389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun X, Briel M, Walter SD, Guyatt GH. Is a subgroup effect believable? updating criteria to evaluate the credibility of subgroup analyses. BMJ. 2010;340(7751):c117. doi: 10.1136/bmj.c117 [DOI] [PubMed] [Google Scholar]
- 29.Wang R, Lagakos SW, Ware JH, Hunter DJ, Drazen JM. Statistics in medicine: reporting of subgroup analyses in clinical trials. N Engl J Med. 2007;357(21):2189-2194. doi: 10.1056/NEJMsr077003 [DOI] [PubMed] [Google Scholar]
- 30.VanderWeele TJ, Ding P. Sensitivity analysis in observational research: introducing the E-value. Ann Intern Med. 2017;167(4):268-274. doi: 10.7326/M16-2607 [DOI] [PubMed] [Google Scholar]
- 31.Mathur MB, Ding P, Riddell CA, VanderWeele TJ. Web site and R package for computing E-values. Epidemiology. 2018;29(5):e45-e47. doi: 10.1097/EDE.0000000000000864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology, and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460. [PubMed] [Google Scholar]
- 33.Moss RL, Musemeche CA, Kosloske AM. Necrotizing fasciitis in children: prompt recognition and aggressive therapy improve survival. J Pediatr Surg. 1996;31(8):1142-1146. doi: 10.1016/S0022-3468(96)90104-9 [DOI] [PubMed] [Google Scholar]
- 34.Chao WN, Tsai CF, Chang HR, et al. Impact of timing of surgery on outcome of Vibrio vulnificus-related necrotizing fasciitis. Am J Surg. 2013;206(1):32-39. doi: 10.1016/j.amjsurg.2012.08.008 [DOI] [PubMed] [Google Scholar]
- 35.Boyer A, Vargas F, Coste F, et al. Influence of surgical treatment timing on mortality from necrotizing soft tissue infections requiring intensive care management. Intensive Care Med. 2009;35(5):847-853. doi: 10.1007/s00134-008-1373-4 [DOI] [PubMed] [Google Scholar]
- 36.Karvellas CJ, Abraldes JG, Zepeda-Gomez S, et al. ; Cooperative Antimicrobial Therapy of Septic Shock (CATSS) Database Research Group . The impact of delayed biliary decompression and anti-microbial therapy in 260 patients with cholangitis-associated septic shock. Aliment Pharmacol Ther. 2016;44(7):755-766. doi: 10.1111/apt.13764 [DOI] [PubMed] [Google Scholar]
- 37.Buck DL, Vester-Andersen M, Møller MH; Danish Clinical Register of Emergency Surgery . Surgical delay is a critical determinant of survival in perforated peptic ulcer. Br J Surg. 2013;100(8):1045-1049. doi: 10.1002/bjs.9175 [DOI] [PubMed] [Google Scholar]
- 38.Azuhata T, Kinoshita K, Kawano D, et al. Time from admission to initiation of surgery for source control is a critical determinant of survival in patients with gastrointestinal perforation with associated septic shock. Crit Care. 2014;18(3):R87. doi: 10.1186/cc13854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tellor B, Skrupky LP, Symons W, High E, Micek ST, Mazuski JE. Inadequate source control and inappropriate antibiotics are key determinants of mortality in patients with intra-abdominal sepsis and associated bacteremia. Surg Infect (Larchmt). 2015;16(6):785-793. doi: 10.1089/sur.2014.166 [DOI] [PubMed] [Google Scholar]
- 40.Martínez ML, Ferrer R, Torrents E, et al. ; Edusepsis Study Group . Impact of source control in patients with severe sepsis and septic shock. Crit Care Med. 2017;45(1):11-19. doi: 10.1097/CCM.0000000000002011 [DOI] [PubMed] [Google Scholar]
- 41.Bloos F, Thomas-Rüddel D, Rüddel H, et al. ; MEDUSA Study Group . Impact of compliance with infection management guidelines on outcome in patients with severe sepsis: a prospective observational multi-center study. Crit Care. 2014;18(2):R42. doi: 10.1186/cc13755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H, Chung SP, Choi SH, et al. ; Korean Shock Society (KoSS) Investigators . Impact of timing to source control in patients with septic shock: a prospective multi-center observational study. J Crit Care. 2019;53:176-182. doi: 10.1016/j.jcrc.2019.06.012 [DOI] [PubMed] [Google Scholar]
- 43.Fernando SM, McIsaac DI, Perry JJ, et al. Frailty and associated outcomes and resource utilization among older ICU patients with suspected infection. Crit Care Med. 2019;47(8):e669-e676. doi: 10.1097/CCM.0000000000003831 [DOI] [PubMed] [Google Scholar]
- 44.Knöferl MW, Angele MK, Diodato MD, et al. Female sex hormones regulate macrophage function after trauma-hemorrhage and prevent increased death rate from subsequent sepsis. Ann Surg. 2002;235(1):105-112. doi: 10.1097/00000658-200201000-00014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diodato MD, Knöferl MW, Schwacha MG, Bland KI, Chaudry IH. Gender differences in the inflammatory response and survival following haemorrhage and subsequent sepsis. Cytokine. 2001;14(3):162-169. doi: 10.1006/cyto.2001.0861 [DOI] [PubMed] [Google Scholar]
- 46.Chapman EN, Kaatz A, Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28(11):1504-1510. doi: 10.1007/s11606-013-2441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hawker GA, Wright JG, Coyte PC, et al. Differences between men and women in the rate of use of hip and knee arthroplasty. N Engl J Med. 2000;342(14):1016-1022. doi: 10.1056/NEJM200004063421405 [DOI] [PubMed] [Google Scholar]
- 48.Sunden-Cullberg J, Nilsson A, Inghammar M. Sex-based differences in ED management of critically ill patients with sepsis: a nationwide cohort study. Intensive Care Med. 2020;46(4):727-736. doi: 10.1007/s00134-019-05910-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Fractional polynomial model assessment
eTable 1. Hospital specific American College of Surgeons surgeon availability mandate
eTable 2. Source control procedures, anatomic category, and procedural stress
eTable 3. Most common source control intervention families
eTable 4. Feature summary statistics before and after imputation
eTable 5. Cohort characteristics by anatomic location of source control
eTable 6. Logistic regression - primary model with all-cause 90-day mortality as outcome
eTable 7. Logistic regression - primary model with all-cause in-hospital mortality as outcome
eTable 8. Logistic regression - primary model with all-cause 365-day mortality as outcome
eTable 9. Cohort characteristics by most common intervention
eTable 10. Adjusted absolute risk difference of 90-day mortality when comparted to source control at six hours, by most common interventions
eTable 11. Sensitivity analyses - modifications to covariate set
eFigure 1. Cohort accrual
eFigure 2. Correlation between variables and time to source control
eFigure 3. Observed and adjusted risk of 90-day mortality by source control anatomic categories
eFigure 4. Association between early source control and 90-day mortality among the most common interventions