Introduction
The constant evolution of cancer treatment has led to improved outcomes in numerous malignancies1. With improved cancer survivorship and the introduction of novel therapies such as biologic agents and immunotherapeutics, the prevalence of systemic toxicities, in particular cardiotoxicity has grown2. As cardiovascular (CV) disease remains the leading non-cancer cause of death in cancer survivors, the field of cardio-oncology strives to understand and improve CV health in cancer patients3,4.
CV imaging is crucial in recognizing, understanding, monitoring, and treating cancer treatment-related cardiotoxicities (CTRCT)5. Structural and functional imaging modalities provide important information in the management of cardio-oncologic pathologies. Echocardiography continues to be highly utilized for assessment of cardiac structure and function due to its low cost, accessibility, and effectiveness. Newer echocardiographic applications, such as strain imaging, are increasingly used in evaluation of CTRCT, especially to detect subclinical disease6,7. Similarly, cardiac magnetic resonance (CMR) has become a crucial imaging modality in the field of cardio-oncology. Though less accessible, CMR offers accurate functional and structural assessment due to excellent reproducibility, high signal-to-noise ratio in addition to fine tissue characterization that is particularly helpful when echo is insufficient or suboptimal6,8. This review will discuss current evidence for use of echocardiography and CMR in cardio-oncology and practical clinical uses for each.
Traditional 2D Echocardiography
Due to availability, low cost, short acquisition time, and safety, echocardiography is the first line imaging modality for CTRCT screening by all published cardio-oncology guidelines and expert consensus statements5. Figure 1 illustrates the relative utility of echocardiography considering various parameters. While left ventricular ejection fraction (LVEF) as measured by Simpson’s biplane method is the most cited parameter in strict definitions of cardiotoxicity (definitions range from reduction in LVEF by 5–10% to absolute LVEF of <50–55%)9, LVEF assessment via 2D echocardiography lacks the sensitivity and reproducibility for primary CTRCT screening10. Thus, newer echocardiographic applications such as strain imaging, 3D echocardiography, and contrast echocardiography have growing roles in screening for CTRCT.
Figure 1:

Comparison of value of echocardiography and cardiac MRI. One filled in black circle represents minimal value, two represents limited value, three represents good value and four represents gold standard.
Echocardiographic Strain Imaging
Myocardial strain, or deformation, is the measure of percent change in the length of a myocardial segment over a given timeframe. This parameter is helpful in quantifying myocardial function directly rather than indirectly via 2D LVEF with Simpson’s biplane method. Speckle-tracking echocardiography (STE) is the preferred method of strain imaging. STE tracks the artifactual “speckles” created by reflected and scattered ultrasound beams through cardiac tissue. Strain can be calculated for the three major orientations of myocardial fibers (longitudinal, radial, and circumferential) and as global longitudinal strain (GLS) which uses data from multiple segments, all of which show promise in early detection CTRCT before changes in LVEF occur7,11. A decrease in GLS of less than or equal to 15% from baseline is considered abnormal and has been most extensively studied12.
Several studies have assessed the prognostic value of GLS in early prediction of CTRCT prior to LVEF changes. A 2019 systematic review13 evaluated twenty-one such studies of patients treated with anthracyclines with or without trastuzumab for a variety of cancers. Summary odds ratios (ORs) for prediction of CTRCT based on threshold GLS values and percent change of GLS after treatment initiation were 12.27 and 15.82, respectively. Since the publication of this systematic review, newer studies have shown similar findings14–18, some incorporating several biomarkers for additional predictive value with mixed utility19–21. GLS has been studied in pediatric populations as well. Though less robust, the studies use strain-imaging to evaluate subclinical cardiac dysfunction in varying follow up periods after anthracycline therapy or radiation22–27. As in adults, GLS in pediatric patients detects myocardial dysfunction prior to changes in LVEF. Future studies in all populations should focus on both early and long-term predictive value of strain imaging with and without biomarkers for the development of CTRCT.
Echocardiography in Anthracyclines
Anthracyclines are anti-tumor antibiotics that play a major role in the treatment of a wide range of cancers. Both anti-tumor action and cardiotoxicity are thought to be due to activation of apoptotic pathways triggered by a combination of three separate mechanisms: direct DNA damage via intercalation into DNA strands, transcription interference via inhibition of topoisomerase enzymes, and DNA and cellular damage via generation of free radicals28,29. Cardiotoxicity manifests primarily in the form of myocardial dysfunction, usually within one year30 of therapy initiation, and it can progress to irreversible heart failure, characterized by diffuse fibrosis31. Risk factors for cardiotoxicity include cumulative anthracycline exposure, age >65 years or <18 years, female sex, pre-existing cardiac risk factors, kidney disease, and exposure to other cardiotoxic therapies32. Patients can be categorized into low, intermediate and high risk for cardiotoxicity based on these risk factors (Table 1). Echocardiography is the primary screening tool for anthracycline cardiotoxicity, with GLS emerging as the most sensitive measure of early myocardial dysfunction13.
Table 1:
Low, intermediate, and high-risk features for development of CTRCT. A patient should be grouped with their highest individual risk factor.
| Risk Factor Categories for CTRCT | |
|---|---|
| Low Risk |
|
| Intermediate Risk |
|
| High Risk |
|
CVD risk factors include, but are not limited to, hypertension, insulin resistance, diabetes mellitus, smoking, obesity, and dyslipidemia.
Abbreviations: CAD, coronary artery disease; CTRCT, cancer therapy-related cardiotoxicity; CVD, cardiovascular disease; Gy, Gray unit(s); HF, heart failure; PAD, peripheral artery disease; RT, radiation therapy
Echocardiography in Her2/Neu Therapy
Trastuzumab is a monoclonal antibody used mostly in the treatment of HER2 positive breast cancer that targets HER2/neu receptors, epidermal growth factor receptor tyrosine kinases important in cell proliferation. Cardiotoxicity is the major adverse effect attributed to trastuzumab, primarily manifesting as potentially severe, reversible left ventricular (LV) dysfunction while on therapy33 as well as right ventricular (RV) dysfunction34. Myocardial dysfunction has been reported in up to 30% of patients19 with a 3% risk of severe cardiotoxicity35. Women treated with both anthracyclines and trastuzumab are at higher risk of cardiotoxicity than those treated with either alone36. Toxicity is thought to be due to inhibition of cardioprotective mechanisms (sarcomere maintenance, pro-apoptotic molecule scavenging) initiated by neoreguline-1 mediated activation of HER2. Subsequent oxidative stress and upregulation of angiotensin II is thought to further promote toxicity33. As with anthracyclines, GLS detects subclinical myocardial dysfunction prior to LVEF changes and should be implemented in any echocardiographic assessment of patients treated with trastuzumab13,18.
Echocardiography in Targeted Therapies and Immunotherapy
While anthracyclines and trastuzumab represent the classic culprits of CTRCT, emerging therapies also cause cardiotoxicities that can be assessed via echocardiography and strain imaging. These include targeted therapies (e.g. proteasome inhibitors [PIs], vascular endothelial growth factor inhibitors [VEGF-Is], tyrosine kinase inhibitors [TKIs]) and immunotherapies (e.g. immune checkpoint inhibitors [ICIs], chimeric antigen receptor T cell [CAR-T] therapy).
Echocardiography in Proteasome Inhibitors
PIs, such as bortezomib and carfilzomib, are used in the treatment of multiple myeloma and are known to cause cardiotoxicity in the form of myocardial dysfunction and heart failure37. Proposed mechanisms include interference of production of nitric oxide in the endothelium leading to vasoconstriction and inhibition of the ubiquitin-proteasome system leading to protein misfolding and cell death. Cardiomyocytes are particularly vulnerable to this second mechanism as they are non-proliferative and express elevated proteasome levels38. Meta-analyses of carfilzomib and bortezomib showed ORs of 2.03 and 1.74, respectively, for development of cardiotoxicity and a cumulative incidence of cardiotoxicity in nearly 10% of those on carfilzomib39,40. PI use is associated with decreased GLS without change in LVEF, indicating subclinical cardiac dysfunction41 and early detection of CTRCT38, but the amount of published data is minimal.
Echocardiography in Vascular Endothelial Growth Factor Inhibitors
VEGF-Is are used to treat a variety of malignancies by inhibiting tumor angiogenesis. Numerous VEGF-Is are cardiotoxic, causing hypertension and myocardial dysfunction42. Hypertension is thought to be driven via inhibition of endothelial nitric oxide production. The myocardial dysfunction is less well understood but thought to include a multitude of effects leading to inhibition of cardioprotective cellular mechanisms, coronary microvasculature destabilization, and decreased density of myocardial capillaries43. Although LVEF effects have been inconsistent, STE monitoring in patients receiving various VEGF-Is showed significant decrease in GLS from baseline both during treatment44 and up to six months following treatment45,46.
Echocardiography in Tyrosine Kinase Inhibitors
TKIs are a diverse group of agents that target tyrosine kinase (TK) molecules in various cellular functions. By targeting specific pathways, TKIs can be tailored for specific types of malignancies. Because TKs are so widely utilized in cellular biology, there is much cross-reactivity with other TKs leading to “off target” effects, including cardiotoxicity in the form of cardiomyopathy and heart failure47,48. Sunitinib and pazopanib, VEGF-Is acting via TKI mechanism, are associated with myocardial dysfunction and demonstrate significant decrease in GLS45. Newer generation Bcr-abl-targeting TKIs cause significantly lower GLS compared to first generation, but the absolute value difference is minimal with no comparison to baseline, limiting the clinical relevance of this finding49. Another subset of TKIs, BRAF and MEK inhibitors, are used in the treatment of melanoma and are known to be cardiotoxic, most commonly in the form of LVEF reduction (seen in 13% of patients50) and hypertension. Combination BRAF/MEK therapy poses higher risk for LVEF reduction, hypertension, and thromboembolic phenomena compared to BRAF therapy alone51,52. In TKI CTRCT, heart failure tends to present in the first six months of therapy. However, no published studies address predictive value of GLS or other parameters.
Echocardiography in Immunotherapies
Immunotherapies such as ICIs and CAR-T therapy are used in a wide range of cancers. ICIs work by targeting molecules that inhibit immune destruction by T-cells. Not surprisingly, this general approach can lead to unintended inflammatory cascade throughout the body, including cardiac manifestations53 including myocarditis, pericarditis, arrhythmia, cardiomyopathy, or myocardial ischemia. The most apparent risk factor for ICI-cardiotoxicity is combination therapy with another cardiotoxic agent (including another ICI)54. With increasing recognition of the immune phenomena associated with immunotherapy, retrospective studies estimate myocarditis occurs in up to 1% of patients taking ICIs55–57. In patients that develop ICI myocarditis, GLS is reduced even if LVEF is not and is associated with future major adverse cardiac events (MACE)58. There is evidence of STE-detected RV dysfunction that correlates with duration of ICI use, suggesting a role of CTRCT monitoring59.
In contrast to ICIs, CAR-T therapy works by using T cells that have been harvested from the host, reprogramming them to target tumor cells, and reintroducing them into the host. CAR-T cardiotoxicity manifests primarily as arrhythmia, cardiomyopathy, or ischemic events. LVEF reduction has been demonstrated in 5–10% of patients on CAR-T therapy60–62. To our knowledge, there are no published studies evaluating strain imaging in patients receiving CAR-T therapy. Thus, the rate of cardiac toxicity is likely underestimated.
Echocardiography in Radiation Therapy
Radiation has long been used as a cancer therapy. Cardiotoxicity arises from direct cellular damage of the myocardium and endothelial tissue of the heart and vasculature within the field of radiation. Cardiotoxicity can manifest as hypertension, coronary artery disease (CAD), valvular disease, pericarditis, myocarditis, cardiomyopathy, or arrhythmia63. Cardiotoxicity risk is increased in patients with total exposure of >15Gy and increases with increasing doses64. Strain imaging, GLS mostly, has been used to evaluate CTRCT following radiotherapy (RT) to the chest. Numerous studies show subclinical cardiac dysfunction after initiation of chest RT (in the absence of other cardiotoxic therapies) for treatment of breast cancer in both acute and follow up periods of up to three years65–71. Higher radiation doses66–68,70,71 and left-sided breast radiation67,68 are associated with larger decreases in GLS. The apical region72 and subendocardial region of the heart receiving the most radiation65 have demonstrated earlier detection of strain reduction, suggesting higher sensitivity for detection of CTRCT by assessing these regions.
3D Echocardiography
3D echocardiography is a modality that has emerging potential in the field of cardio-oncology. While not as ubiquitous as 2D echocardiography, it offers superior capability in assessment of LVEF4,73, providing values that agree with CMR74. Compared to 2D echocardiography, 3D echocardiography offers more reproducible LVEF10, faster and earlier strain assessment75,76, and more detailed structural and anatomic characteristics of cardiac masses.
Echocardiography and Cardiac Masses
Echocardiography plays a pivotal role in the detection and diagnosis of cardiac masses. Although rare, neoplastic cardiac masses carry significant morbidity and mortality77. Benign cardiac tumors are more common than malignant ones. Metastatic cardiac tumors, seen in 10–12% of patients with a known primary cancer78, are more common than primary cardiac malignancy. 2D echocardiography can delineate cardiac masses and certain characteristics (size, shape, mobility, relative density, associated effusions) quite well for initial evaluation (Figure 2, Panel A). However, there is limited ability to distinguish right-sided masses, left atrial appendage masses, extra-cardiac masses, tissue characteristics, and type of mass79. 3D echocardiography better evaluates size and anatomical associations of cardiac masses, adding benefit in surgical planning76. Perfusion contrast with 2D echocardiography enhances diagnostic utility and has sensitivity and specificity of 93–100% in differentiating thrombi vs. benign tumor vs. malignant tumor80–82. Transesophageal echocardiography (TEE) has shown high diagnostic accuracy82 with better anatomic resolution of right-sided and posterior structures83, but it is an invasive procedure associated with risk including that of sedation and expense.
Figure 2:

(A) Mass identified in the RV outflow tract on echocardiography. Suspected to be a metastatic lesion from an unknown primary source. (B) Two masses identified on CMR (horizontal long axis view). The LV mass is a metastatic melanoma. The RA mass is a thrombus. (C) One RV mass on CMR (short axis view), diagnosed as metastatic melanoma with surrounding thrombus. (D) One LV mass identified on CMR (vertical long axis view), diagnosed as a poorly differentiated synovial sarcoma.
Echocardiography in Tamponade and Pericardiocentesis
Pericardial effusions can be due to infection, autoimmune inflammation, direct effect of malignancy, adverse effect of cancer therapy (radiation-induced pericardial disease, ICI inflammation, volume overload), post-surgical, or idiopathic. Pericardial effusions are readily identified on traditional 2D echocardiography, though may require multiple views if the effusion is loculated. If posterior, TEE may be required to adequately view the effusion.
Pericardiocentesis, drainage of a pericardial effusion, is generally indicated for asymptomatic and large effusions, hemodynamically significant effusions (i.e. impending or active tamponade), or fluid biopsy to aid diagnosis. While overt tamponade implies active hemodynamic consequences such as hypotension, tachycardia, dyspnea, or pulses paradoxus from compression of the heart, effusions at high risk for developing tamponade cannot be readily identified on physical exam alone. Echocardiography, viewed as the gold-standard for diagnosis, can identify signs of compression before the presence of symptoms. These include RA or RV collapse during diastole, abnormal septal motion indicative of interventricular dependence, dilated inferior vena cava without inspiratory collapse, swinging heart, or decreased mitral early filling velocity on doppler84. Echocardiography-guided pericardiocentesis, which has been used for decades, is safe and effective in cancer patients, and should be the utilized before surgical drainage when possible85–87.
Echocardiography in Cardiac Amyloidosis
Cardiac amyloidosis is a group of conditions that result in the infiltration and expansion of myocardial extracellular space with amyloid protein deposits. Though various proteins can misfold and deposit as amyloid, transthyretin (ATTR) and immunoglobulin light chains (AL) account for 95% of cases. Cardiac amyloidosis usually manifests clinically as heart failure (preserved ejection fraction more often than reduced ejection fraction), restrictive cardiomyopathy, and dysrhythmias. Typical 2D echocardiographic findings include ventricular wall hypertrophy (concentric more common in AL, asymmetric more common in ATTR), diastolic and/or systolic dysfunction, restrictive LV filling, bi-atrial enlargement, valvular thickening, and a “sparkling” texture of the myocardium9,88. Two-dimensional STE often reveals a characteristic pattern of reduced GLS with apical sparing, due to segmental differences in total amyloid mass distribution89, with a reported sensitivity of 93% and specificity of 82% for cardiac amyloid when compared to LV hypertrophy controls90. The combination of GLS values with apical sparing and serum T-troponin offers better sensitivity and specificity91. One recent study92 found a GLS/EF ratio >4.1 to be a strong predictor of cardiac amyloidosis, with an OR of 35.57. While echocardiography is the universal initial imaging modality when suspicious for cardiac amyloid, it cannot reliably distinguish between amyloid and other causes of hypertrophy. Thus, echocardiography alone is not sufficient for diagnosis.
Practical Use of Echocardiography in Everyday Clinical Practice
Each institution and case will have varying protocols for which views and data are obtained on echocardiogram. The following has been suggested for comprehensive initial echocardiographic assessment in screening for CTRCT: 2D LVEF via Simpson’s biplane method or 3D LVEF, 2D or 3D GLS, 2D or 3D LV systolic volume, RV function markers (such as tricuspid annular plane systolic excursion (TAPSE), RV fractional area change, RVEF, RF free wall strain), velocity of tricuspid regurgitation9. Suggested echocardiographic surveillance protocols for patients on anthracycline therapy and trastuzumab therapy can be found in Figures 2 and 3, respectively. Suggested echocardiographic surveillance protocols for patients on radiation therapy, targeted therapy, or immunotherapy can be found in Table 2.
Figure 3:

Suggested echocardiographic surveillance protocol of cardiotoxicity in patients on anthracycline therapy. Low risk features include age 18–50 years, cumulative dose <200 mg/m2 doxorubicin (or equivalent), no pre-existing or new CVD risk factors. Intermediate features include age 50–65 years, cumulative dose 200–400 mg/m2 doxorubicin (or equivalent), 1–2 pre-existing or new CVD risk factors. High risk features include age <18 or >65 years, cumulative dose >400 mg/m2 doxorubicin (or equivalent), any combination of cardiotoxic cancer therapies, underlying CVD, history of CTRCT.
Abbreviations: CAD coronary artery disease; CMR, cardiovascular magnetic resonance imaging; CTRCT, cancer therapy-related cardiotoxicity; CVD, cardiovascular disease; Echo, echocardiography.
Table 2:
Suggested echocardiographic and CMR surveillance protocols of cardiotoxicity for patients undergoing targeted therapy, immunotherapy, or radiation therapy.
| Suggested Clinical Imaging Surveillance Protocols for CTRCT in Various Cancer Therapies | ||
|---|---|---|
| Class | Echocardiography | Cardiac MRI |
| All Classes |
|
|
| Proteasome inhibitors |
|
|
| VEGF-I and TKI (including BRAF, MEK, and VEGF inhibitors with TKI mechanism) |
|
|
| Immunotherapies (ex. ICIs, CAR-T cell therapies, allogeneic stem transplantation) |
|
|
| Radiotherapy |
|
|
Abbreviations: BRAF, B-Raf proto-oncogene; CAD, coronary artery disease; CAR-T, chimeric antigen T-cell therapy; CTRCT, cancer therapy-related cardiotoxicity; CMR, cardiovascular magnetic resonance imaging; CVD, cardiovascular disease; ECG, electrocardiogram; Echo, echocardiography; ICI, immune checkpoint inhibitor; LV, left ventricular; MEK, mitogenactivated protein kinase kinase; RV, right ventricular; TKI, tyrosine kinase inhibitor; VEGF-I, vascular endothelial growth factor inhibitor.
Recommendations for targeted therapies and immunotherapy are less defined due to the relative dearth of data in novel agents. Individual risk assessment and joint decision making is paramount in developing a patient-centered screening plan. Regardless of therapy, baseline echocardiography prior to initiation of therapy should be obtained in all intermediate and high-risk patients and can be considered in low-risk patients. Additionally, any time there are signs or symptoms of cardiotoxicity, echocardiography should be obtained and referral to a cardio-oncologist should be considered.
Cardiac MRI
CMR and LVEF
Although CMR is not deployed for routine screening in cardio-oncology, it offers superior imaging in tissue characterization, volume assessments, spatial resolution, and potentially strain imaging. Table 1 illustrates the relative utility of CMR in various parameters. CMR can investigate most adverse cardiac effects from cancer therapies and allows the specific assessment of RV and LV function, ventricular and atrial volumes, ventricular and left atrial deformation, myocardial mass, pericardial disease, fibrosis, infiltrative tissue, edema, and inflammation9,93. Measurement of LVEF is considered the gold standard due to excellent accuracy and precision6,94. CMR is utilized for LVEF measurement when there are poor echocardiographic windows or echocardiography is equivocal or unreliable. Given the poor agreement of 2D echocardiography with CMR-derived LVEF, CMR should be used any time highly accurate LVEF quantification is needed, especially if 3D echocardiography is not available5,95.
CMR Strain Imaging
Like echocardiography, CMR-derived LVEF lacks the sensitivity to detect early myocardial dysfunction in CTRCT. Cardiac deformation quantification via strain imaging techniques is available in some clinical CMR labs and useful in detection of subclinical myocardial dysfunction. Techniques include CMR reference tagging, phase velocity mapping, displacement encoding with stimulated echoes (DENSE), strain encoded (SENC) imaging (Figure 4), and feature tracking (FT). CMR reference tagging is the most validated and considered gold standard for CMR strain imaging, however CMR-FT is gaining traction due to ease of clinical use93,96,97. Analogous to STE but with better resolution98, CMR-FT is a post-acquisition processing method that can be applied to images obtained for LVEF assessment, requiring no extra scanner time96. CMR-FT shows good reproducibility in global strain measurements, provided the same software package is used99,100. The potential for early detection and prognostication in CTRCT by CMR strain has been demonstrated in patients receiving various cardiotoxic chemotherapies101,102, and is discussed further below. CMR-FT has even shown promise in evaluation of left atrial (LA) strain, which, along with MRI measured LA volume, could represent an important marker for potential development of atrial arrhythmias and clot formation98,103.
Figure 4:
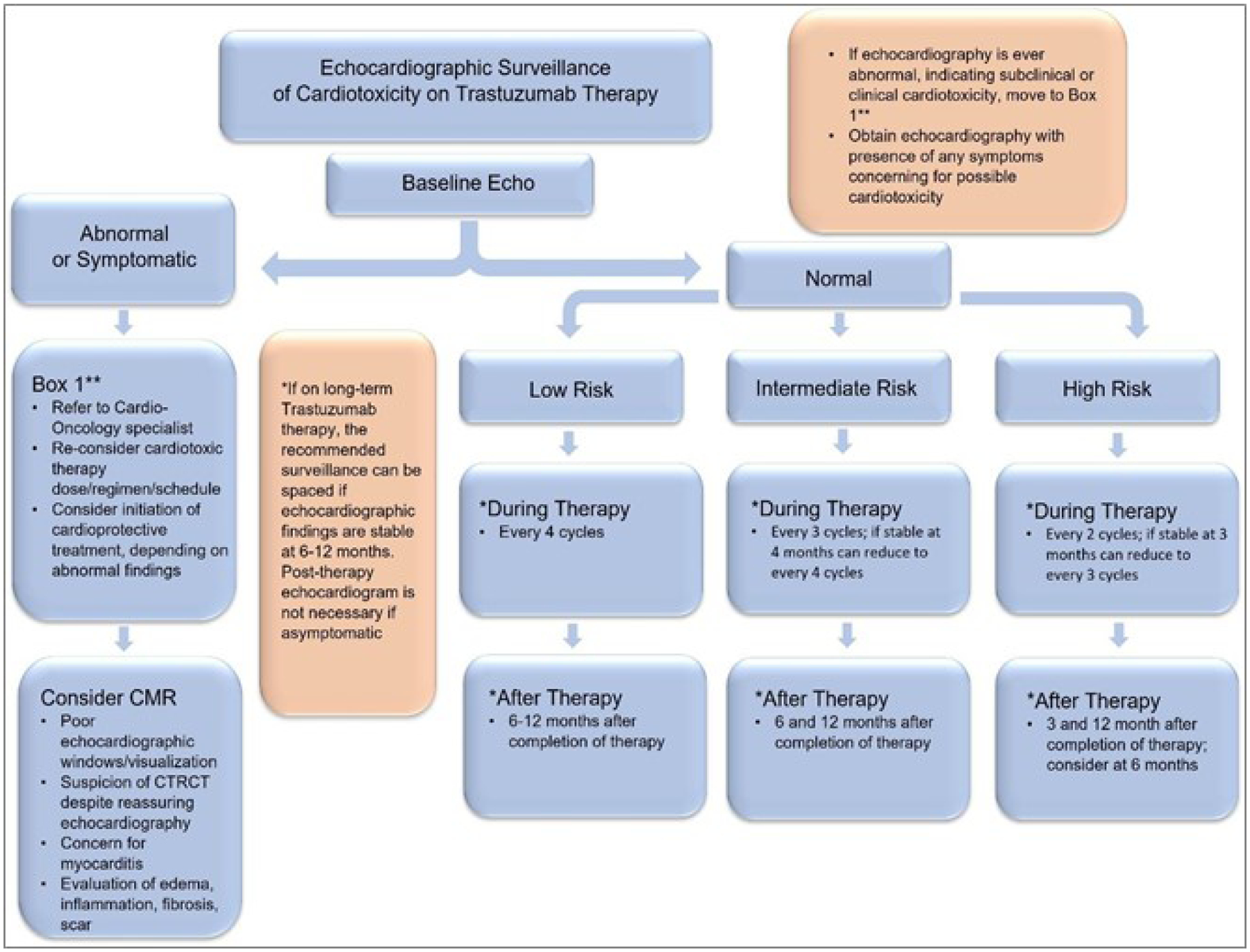
Suggested echocardiographic surveillance protocol of cardiotoxicity in patients on trastuzumab therapy. Low risk features include age 18–50 years, no pre-existing or new CVD risk factors. Intermediate features include age 50–65 years, 1–2 pre-existing or new CVD risk factors. High risk features include age <18 or >65 years, any combination of cardiotoxic cancer therapies, underlying CVD, history of CTRCT.
Abbreviations: CAD coronary artery disease; CMR, cardiovascular magnetic resonance imaging; CTRCT, cancer therapy-related cardiotoxicity; CVD, cardiovascular disease; Echo, echocardiography.
CMR T1 and T2 Mapping
T1-weighted imaging (Figure 5) reflects the inherent intracellular and extracellular makeup of tissue. Unitless values of intensity (compared to a reference “region of interest” within the same image) are assigned to each pixel but are only comparable within the same image. T1 is lengthened (brightened) by water, edema, and inflammation; it is shortened (darkened) by iron, fat, and contrast104. T1 mapping is a relatively new CMR application that allows for creation of color maps, where each colored pixel represents a quantified, parametric, tissue-specific T1 value of the corresponding voxel that is comparable across images105. This allows sensitive detection of subtle T1 changes within the myocardium, often representing early stages of disease. T1 mapping reliably distinguishes regional and diffuse fibrosis (scar)106, edema107, and infarction108. It has shown promise in detecting numerous cardiac disease states98,109,110. Late gadolinium enhancement (LGE) (Figure 5), conversely, only visualizes regional fibrosis well98. T1 mapping solves this limitation while providing a contrast-free alternative for fibrosis detection. T1 mapping is primarily utilized for evaluation of the LV, but there are sequences that can evaluate fibrosis of the LA, with possible application in therapies causing atrial arrhythmias111. Contrast T1 imaging is useful, however, in quantifying extracellular volume (ECV), which correlates histologically112 with the excess collagen deposition of fibrosis.
Figure 5:

Representative images of (A) late gadolinium enhancement, and elevated (B) myocardial native T1, and (C) myocardial T2 in a patient with suspected targeted cancer therapy (TKI) cardiotoxicity, as visualized by cardiac magnetic resonance imaging. Red arrows in panels (B) and (C) indicate areas of elevated T1 and T2, respectively; the pink outline in panel (B) denotes a region of interest (ROI) in the septum.
T2-weighted imaging (Figure 5), like T1, is reflective of the intracellular and extracellular makeup of tissue. T2 time is increased by free water and most helpful in distinguishing edema and inflammation, proving to be comparable to Lake Louise criteria for diagnosis of myocarditis113. T2 mapping can now be created, just as T1 mapping can104, with good reproducibility114. However, it has not been as thoroughly studied in CTRCT as has T1 mapping.
CMR in Anthracyclines
Anthracycline cardiotoxicity is characterized by myocardial dysfunction that in its most severe presentation manifests as heart failure with reduced ejection28. Although CMR is more sensitive and accurate in LVEF assessment than echocardiography, it is not sensitive enough for CTRCT screening109. CMR strain imaging, while not as extensively studied or as readily available as echocardiographic strain imaging, shows a similar promise in early detection and prediction of CTRCT96 via both systolic115–119 and diastolic120 measurements. Because anthracycline CTRCT is characterized by diffuse fibrosis31, LGE is seen in just 6% of patients121. Numerous studies have demonstrated that T1 mapping and ECV quantification detect fibrosis after anthracycline therapy, representing an avenue for early diagnosis of CTRCT118,122–125. One prospective study118 showed elevated T1 and T2 mapping values in patients on anthracycline therapy compared to controls three months after therapy initiation, but only elevated T1 more than 12 months after therapy initiation. This suggests that initial edema/inflammation eventually progresses to fibrosis. The authors created criteria for detection of cardiotoxicity in these patients which led to diagnostic accuracy of 84% for detection of CTRCT, outperforming both GLS and troponin alone. More studies are needed to elucidate ideal threshold values for optimum diagnostic and prognostic ability. Published data on T2 mapping in anthracyclines, though minimal, shows early edema and inflammation118,126 after administration indicating early signs of CTRCT with excellent sensitivty127. There is encouraging data for the utility of T2 mapping in CTRCT in various animal models128–130, but human studies remain relatively sparse. Other CMR-derived measurements have been studied in anthracycline use. LA enlargement has been associated with increasing anthracycline doses, potentially offering a marker of diastolic dysfunction131. Decrease in myocardial mass after anthracycline therapy has been repeatedly demonstrated95,121,132,133, which is associated with increased risk of MACE121 and HF symptoms133.
CMR in HER2/Neu Therapy
CMR strain parameters have shown promising ability to predict the development of CTRCT in patients on Trastuzumab therapy. One prospective study demonstrated that a 15% relative decrease in tagged-CMR GLS, tagged-CMR GCS, and CMR-FT correspond to increased odds of CTRCT of 47%, 50%, and 87% respectively134. Multiple other studies have shown a decrease in various CMR-measured strain parameters, often with persistence up to twelve months. Recovery by eighteen months is frequently seen, highlighting the reversibility of trastuzumab cardiotoxicity135–137. Diastolic function has been assessed with both strain and LV diastolic filling rates, but these data are inconsistent137,138. CMR evaluation of LGE highlights a key difference in the pathogenesis of trastuzumab from anthracyclines. While LGE is uncommon in anthracycline cardiotoxicity due to diffuse fibrosis, LV lateral wall LGE enhancement is seen in 94–100%139,140 of patients treated with trastuzumab who had reduced LVEF. This parallels the knowledge that trastuzumab cardiotoxicity primarily affects the LV. There is CMR evidence to add to echocardiographic evidence34 that RV myocardial dysfunction is also seen, though more subtle than LV dysfunction141. Animal studies show increased T1 and T2 values in subjects receiving trastuzumab with eventual recovery142–144. Human data show similar findings145 but clinical utility in early detection and prediction of CTRCT is limited due to temporal variability101,146.
CMR in Proteasome Inhibitors
Case reports represent the bulk of literature covering the heterogeneous CMR findings in PI cardiotoxicity. Fibrosis indicated by LGE is the most common finding, seen mostly at the basal and inferior portions of the septum147–149. Conversely, two patients receiving both carfilzomib and bortezomib developed significant but reversible LVEF reduction with wall motion abnormalities without LGE37. The only prospective study we are aware of studied eleven patients with cardiovascular risk factors but no pre-existing cardiovascular disease for up to six months after bortezomib therapy with echocardiography and CMR. All imaging parameters, including echocardiographic GLS and CMR LGE, were normal150. More data is needed for proper understanding of CMR findings in PI-associated CTRCT.
CMR in TKIs and VEGF-Is
There is scant published data on CMR findings in VEGF-Is and TKIs, mostly in the form of case reports. Many overlap as they involve TKIs that inhibit VEGF pathways. One prospective study evaluated cardiotoxicity of 90 patients in phase I trials of a VEGF-I monoclonal antibody, VEGF-I TKI, or a kinesin inhibitor151. Out of 90 patients, 10 developed cardiotoxicity. Of these ten, two were taking the VEGF-I TKI, and six were taking the VEGF-I monoclonal antibody. The only patient with a reduced EF was taking the VEGF-I TKI. None of the 10 patients had LGE on CMR. One case report of sunitinib-induced cardiomyopathy included CMR evaluation that showed no LGE or resting perfusion defects152. We are not aware of any published data pertaining to CMR strain, T1 mapping, or T2 mapping in relation to cardiotoxicity of TKIs or VEGF-Is. Because of the propensity of some TKIs to invoke atrial arrhythmias, such as ibrutinib, LA evaluation with LGE, T1 and T2 mapping, and CMR strain could be beneficial and warrants further study. Hypertension and vascular effects of therapies that inhibit VEGF pathways make CAD and myocardial ischemia an important adverse effect to monitor for. Along with stress and perfusion imaging, CMR offers techniques with ability to quantify myocardial viability6,98.
CMR in Immunotherapy
CMR is the gold-standard non-invasive diagnostic tool for myocarditis, the most important cardiotoxic effect of immunotherapy. The Lake Louise Criteria (LLC), introduced in 2009 for the diagnosis of non-ICI myocarditis, requires clinical suspicion of myocarditis plus two of the following: regional or global myocardial increase in T2-weighted images, increased global myocardial early T1 gadolinium enhancement, or ≥ 1 focal lesion with non-ischemic regional LGE153. The emergence of T1 and T2 mapping and ECV quantification has outdated the original criteria, leading some groups to use modified LLC to incorporate these parameters to increase diagnostic accuracy55,154,155. One study found 100% of ICI myocarditis cases met original LLC or had abnormal T1 or T2 imaging155. A meta-analysis of non-ICI myocarditis patients found native T1 and T2 mapping and ECV provide comparable diagnostic performance to LLC with native T1 having higher sensitivity than LLC for non-ICI myocarditis. It is important to note the underlying inflammatory mechanisms of ICI myocarditis are not necessarily comparable to non-ICI myocarditis. Additionally, diagnostic specificity is challenging in ICI myocarditis due to inherent heterogeneity, stemming from varying levels of inflammation in varying cardiac structures156. This heterogeneity is reflected in the literature in both clinical presentations and imaging findings157–159.
Reduced LVEF on CMR is not a sensitive finding for ICI myocarditis, reported in just 39%−50% of patients155,158–160. Strain imaging via CMR-FT has demonstrated significant reduction of LV-GLS in all ICI myocarditis patients, with even larger reduction in those with reduced LVEF158. While LA strain has demonstrated additional prognostic value when combined with LV strain and LLC161 in non-ICI myocarditis, this was not seen in ICI myocarditis. In ICI myocarditis, LGE has been reported in 48–80% of patients56,157–159. LGE occurs in non-coronary distributions, but location varies by case159. Presence of LGE is an independent predictor of mortality in ICI myocarditis158 but has not been associated with MACE159. Interestingly, in one study159, LGE was seen in 21.6% of patients when CMR was obtained prior to day four of admission and 72% when obtained on or after that. Thus, LGE prognostic value might differ depending on the timing of CMR acquisition.
Qualitative T1 and T2 lack adequate sensitivity compared to quantitative mapping155,158,162. A retrospective155 study that evaluated T1 and T2 mapping in patients with ICI myocarditis found elevated values compared to controls in 78% and 43% of the patients, respectively. Elevated T1 values were independently associated with MACE and lower MACE-free survival. No patients with normal T1 values had MACE, suggesting a clinically important negative predictive value. While abnormal T2-mapping has been associated with MACE in non-ICI myocarditis163, this is not reflected in ICI myocarditis155,159. Increased ECV is also associated with MACE and death in non-ICI myocarditis164,165, though no published data addresses this in ICI myocarditis.
CMR in Radiation Therapy
Cardiotoxicity from RT has a wide spectrum of manifestations, all stemming from direct macrovascular and microvascular damage. Literature surrounding radiation can be difficult to interpret, as most studies involve patients who in addition to radiotherapy have received anthracyclines and/or trastuzumab. Radiation cardiotoxicity can lead to reduction in LVEF detectable by CMR. This is seen transiently at 6 months with resolution at 2 years166, and, in survivors, many years after therapy167. LA volume assessment reveals an association between LA size and radiation dose, suggesting a possible early marker of diastolic dysfunction131. CMR strain imaging for the detection of subclinical cardiac dysfunction in patients who received radiation is not as well studied. In one study there is a transient decrease in CMR-derived strain at 6 and 12 months that recovers by 24 months166. Other studies in humans and rats have similar findings, but it is unclear if there is a correlation between GLS reduction and radiation dose168,169. Fibrosis indicated by LGE has been well-documented following radiotherapy, however, studies are vastly heterogeneous, reporting LGE incidence of 0%−100%168,170–173. Multiple studies demonstrate LGE presence in areas that received the highest radiation doses170,172,173. Studies of T1 mapping post-RT are sparse and inconsistent. Some show elevated T1 mapping values in areas of higher radiation172,174, while others found either no elevation of T1 values168 or no correlation with radiation dose171. There is minimal published data on T2 mapping post-RT.
CMR offers sensitive and detailed structural and tissue characterization of pericardial toxicity and its associated hemodynamic consequences. LGE of the pericardium is indicative of active pericarditis, while chronic constrictive pericarditis will not enhance. CMR tagging can identify pericardial adhesions. Real-time cine imaging during respiration can reveal hemodynamic consequences of constrictive pericarditis, such as ventricular interdependence. Real-time phase contrast imaging can be useful in detecting effects of respiration on flow through the mitral and tricuspid valves6,63,104. CMR can also be helpful in evaluation of valvular pathology induced by radiation toxicity, generally not seen for 10–20 years after therapy. CMR is particularly useful in visualizing the pulmonary valve, which can be challenging via echocardiography. CAD and ischemic heart disease secondary to radiation therapy are also assessable by CMR via perfusion imaging and coronary artery visualization6,63,98,104. CMR can even play a role in prevention of cardiotoxicity. Through various techniques under investigation, CMR shows promise in preparatory and real-time imaging to improve safety and precision of radiotherapy175 and radiosurgery176, reducing total radiation exposure.
Potential Role of Stress Perfusion and Other Cardiac MRI Imaging Techniques
Numerous cancer therapies increase risk of vascular disease, as does malignancy itself. Contrast-enhanced myocardial stress perfusion has excellent diagnostic performance in detection of ischemic disease177. However, screening via stress-perfusion CMR without symptoms does not provide additional benefit in cardiotoxicity diagnosis or management178. Pulse wave velocity, an MRI method of quantifying arterial stiffness, is elevated during and after breast cancer treatment with various anti-cancer therapies179,180 and could represent a method of risk stratification. 4-dimensional flow MRI can provide flow and pressure parameters of the vasculature. Its role in risk stratification is still under exploration181. Real-time CMR has shown benefit in enhancing precision of radiation targeting to reduce total radiation exposure175,176 and increasing diagnostic yield of endomyocardial biopsy182.
CMR and Cardiac Masses
The superior tissue characterization and spatial resolution of CMR makes it the preferred method of imaging for intra-cardiac and pericardial masses (Figure 2, Panels B–D). CMR is particularly helpful in confirmation and further characterization when echocardiography is not sufficient183. Balanced steady-state free precession (SSFP) is the primary method for detailed anatomic description. T1- or T2-weighted double inversion recovery (DIR) imaging with or without fat saturation is useful in tissue characterization of masses. Gadolinium enhancement is helpful in assessing tumor vascularity and associated myocardial fibrosis184. CMR performs well at distinguishing non-tumors from tumors, and benign tumors from malignant ones185,186. With a wider field of view, CMR can catch pericardial and extra-cardiac masses missed by echocardiography. Additionally, CMR outperforms echocardiography in predicting ultimate tumor diagnosis as confirmed by pathology. CMR has fewer false positives and can eliminate the need for biopsy by ruling out a mass seen on echocardiography183.
CMR in Tamponade and Pericardiocentesis
While echocardiography is generally sufficient to diagnose pericardial effusion and tamponade, CMR can offer further details in the assessment of pericardial disease. CMR can identify loculated or posterior effusions, pericardial thickening, and pericardial masses where echocardiography often cannot. As described above, CMR is certainly capable of detecting signs of tamponade6,63,104, however, it is generally unnecessary unless echocardiography is unavailable or CMR is to be obtained for another reason. One of the more helpful applications of CMR in pericardial disease is the ability to distinguish constrictive pericarditis (thickened pericardium, interventricular dependence) from restrictive cardiomyopathy (diastolic dysfunction, large but normally contoured atria, thickened myocardium, normal pericardium)187,188. CMR does not play a role in percutaneous pericardiocentesis but can aid in planning for surgical drainage of a pericardial effusion.
CMR in Cardiac Amyloidosis
CMR, with its fine tissue characterization, plays an important role in cardiac amyloidosis screening, diagnosis, and surveillance of disease burden. LGE is almost invariably seen in cardiac amyloidosis, with the typical patterns being diffuse subendocardial (representing earlier stages) and diffuse transmural (representing later stages). RV LGE is also seen in >90% of patients. Transmural LGE is more common in ATTR than AL189 and also carries prognostic value190. Native T1 values are markedly elevated in cardiac amyloid, and T1 mapping is particularly helpful when amyloid renal disease precludes the use of gadolinium162. ECV quantification is a valuable tool given the underlying pathologic mechanisms of amyloidosis191. ECV values are markedly elevated in cardiac amyloidosis, often indicating >40% ECV. Serial measurement of ECV is reliable192, prognostic193, and indicative of disease progression or response to therapy194–196. T2 mapping, of which there is little published data in regards to amyloid, shows higher values in amyloidosis compared to controls. However, there is little difference in AL and ATTR197. Although LGE, T1 mapping, and ECV all provide valuable roles in cardiac amyloidosis, a recent meta-analysis suggests that ECV provides the highest diagnostic and prognostic capability of the three198. Regardless, all should be included in any cardiac amyloidosis evaluation.
Practical Use in Everyday Clinical Practice
Guidelines do not recommend routine screening for CTRCT via CMR unless echocardiography is not feasible due to poor windows or CMR is needed for other indications5. Additionally, if echocardiography is normal or equivocal, but clinical suspicion for cardiotoxicity remains high, CMR should be obtained. CMR protocols for CTRCT evaluation differ between institutions and individual cases. Commonly used sequences include cine balanced SSFP, strain imaging through myocardial tagging or other methods, phase contrast flow, native T1 and T2 sequences with respective parametric mapping, post contrast T1 ECV mapping, first pass arterial perfusion, inversion recovery, and 4D flow93,199. Suggested CMR surveillance protocols for patients on anthracyclines and trastuzumab can be found in Figure 3 and Figure 4, respectively. Suggested CMR surveillance protocols for patients on radiation therapy, targeted therapy, and immunotherapy can be found in Table 2. Again, individual risk assessment and joint decision making is a crucial part in developing a patient-centered surveillance plan. Patients should be referred to a cardio-oncologist any time there are signs or symptoms of cardiotoxicity.
Future Directions and Ongoing Clinical Trials
The field of cardio-oncology has seen a boom in research over the past ten years. The more we learn, the more our gaps in knowledge become evident. Strain imaging via echocardiography represents our best early imaging biomarker for CTRCT, and the addition of serum biomarkers can increase diagnostic and prognostic value. The majority of the available data, however, is in observational studies of patients exposed to anthracyclines, trastuzumab, or radiation. More prospective studies with larger cohorts are needed in patients exposed to all groups of cardiotoxic cancer therapies, especially novel therapies. The LATER CARDS study will provide a large prospective dataset of echocardiographic findings and serum biomarkers in the under-studied pediatric population200.
CMR, though offering better imaging sensitivity and specificity for most cardiac pathologies, is more difficult to clinically implement and has not been as robustly studied as echocardiography. Future research should aim for larger, prospective studies evaluating traditional and emerging CMR protocols. Patients on targeted and immunotherapies are under-studied compared to anthracyclines, trastuzumab, and radiation and should be a major focus of research as well. The CARTIER study is a prospective study of elderly patients on cardiotoxic chemotherapy who will receive serial CMR before each cycle of treatment and with follow up after treatment completion127. The CareBest prospective study, currently underway, will enroll >2000 breast cancer patients and study various CMR parameters in a short CMR protocol201. Similarly, other CMR-based trials across various cancer drug-classes are underway, which should illuminate the role of trackable subclinical disease in the development of limiting CVD.
Machine learning is a growing research tool with much potential in cardio-oncology. It allows for identification of similar groups buried within the noise of highly heterogenous populations. Machine learning has been used to identify potentially cardioprotective variants in cardiac injury pathway genes among pediatric cancer survivors with anthracycline-induced CTRCT202. It has also been used to identify unique asymptomatic diastolic dysfunction phenotypes based on echocardiographic parameters, each with distinct long-term outcomes risks203. It is natural to foresee the incredible value of machine learning to sift and organize the vast amount of hard data collected with echocardiography and CMR into clinically useful CTRCT prediction tools6. Some combination of serum biomarkers and echocardiographic strain parameters is the intuitive place to start given our current knowledge.
Finally, research should also focus on our ability to implement CTRCT screening protocols. Only 30–40% of patients receive optimal screening via echocardiography204. Implementing user-friendly guidelines, development of dedicated cardio-oncology programs and multidisciplinary clinics, improved patient and provider education of CTRCT, and development of quicker and cheaper imaging protocols could increase patient participation in CTRCT. Continued improvement of CTRCT screening protocols has the potential to positively impact survival and outcomes in the ever-growing cancer patient population.
Figure 6:

(A) Representative screenshot of CMR (SENC) image in short axis view with overlying endocardial (yellow) and epicardial (green) borders. (B) MyoStrain LV and RV longitudinal strain with corresponding heat map, and absolute values of each individual segment. (C) MyoStrain LV and RV circumferential strain with corresponding heat map, and absolute values of each individual segment.
Abbreviations: CMR, cardiovascular magnetic resonance imaging; LV, left ventricular; RV, right ventricular; SENC, strain encoded.
Key Points.
Echocardiography is the first line imaging modality for cancer therapy related cardiotoxicity (CTRCT) screening and investigation.
Echocardiographic strain imaging can detect subclinical cardiac dysfunction and is a promising tool for prediction and prognostication of CTRCT.
Cardiac MRI (CMR) should be obtained if echocardiography is insufficient or suboptimal, highly accurate volume assessments are needed, or myocarditis is suspected.
CMR is the gold standard for ventricular volume and functional assessment.
T1 mapping can identify and quantify edema, infarction, and fibrosis. T2 mapping can identify and quantify edema and inflammation. Both should be included in CMR protocols as they offer invaluable tissue characterization for the diagnosis of various CTRCT manifestations, including myocarditis.
Surveillance protocols for CTRCT via echocardiography and/or CMR vary based on the patient’s individual risk and cancer therapy.
Baseline echocardiography should be obtained prior to initiation of any cancer therapy in all intermediate and high-risk patients and considered in low-risk patients.
Echocardiography should be obtained any time there are signs or symptoms of cardiac involvement in a patient with history of exposure to cardiotoxic cancer therapy. Referral to a cardio-oncologist should also be considered at that time.
Synopsis:
Cardiovascular (CV) events are an increasingly common limitation of effective anticancer therapy. Over the last decade imaging has become essential to patients receiving contemporary cancer therapy. Herein we discuss the current state of CV imaging in cardio-oncology. We also provide a practical apparatus for the use of imaging in everyday cardiovascular care of oncology patients to improve outcomes for those at risk for cardiotoxicity, or with established cardiovascular disease. Finally, we consider future directions in the field given the wave of new anticancer therapies.
Clinics Care Points.
Echocardiography
When using echocardiography to measure LVEF, remember there is limited precision. Values can vary up to 10% between studies.
A normal EF does not rule out the possibility of CTRCT.
When interpreting deformation values, remember that threshold numbers and strict normal ranges have not been widely agreed upon. Relative change from baseline is a better datapoint to follow.
Multidisciplinary clinics can minimize patient stress, enhance communication, and increase patient access to guideline and evidence-based screening/treatment.
Echocardiography alone is not sufficient to diagnose myocarditis. CMR should be obtained anytime there is a question of myocarditis.
Normal EF and lack of LGE do not rule out myocarditis
While appropriate surveillance practices allow us to catch and treat CTRCT early, optimizing modifiable risk factors (diet, exercise, smoking, hypertension, etc.) is equally important.
Educating patients on signs and symptoms of CTRCT is important for patient empowerment and home monitoring of symptoms.
Consult with an imaging or cardio-oncology specialist prior to obtaining echocardiography and/or CMR to ensure all needed parameters/sequences are acquired. This can prevent repeated scans and optimize available clinical data.
Acknowledgements:
The manuscript’s content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding:
This work was supported in part by an NIH P50-CA140158 grant. The funder had no role in the: design and conduct; collection, management, and interpretation of the data; or in preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication. Dr. Addison is supported by NIH grant number K23-HL155890, and an American Heart Association- Robert Wood Johnson Foundation Faculty Development Program grant. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement
The Authors have nothing to disclose.
REFEFENCES
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. Jan 2020;70(1):7–30. doi: 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Shahrokni A, Wu AJ, Carter J, Lichtman SM. Long-term Toxicity of Cancer Treatment in Older Patients. Clin Geriatr Med. Feb 2016;32(1):63–80. doi: 10.1016/j.cger.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Campia U, Moslehi JJ, Amiri-Kordestani L, et al. Cardio-Oncology: Vascular and Metabolic Perspectives: A Scientific Statement From the American Heart Association. Circulation. Mar 26 2019;139(13):e579–e602. doi: 10.1161/cir.0000000000000641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: The Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. Sep 21 2016;37(36):2768–2801. doi: 10.1093/eurheartj/ehw211 [DOI] [PubMed] [Google Scholar]
- 5.Biersmith MA, Tong MS, Guha A, Simonetti OP, Addison D. Multimodality Cardiac Imaging in the Era of Emerging Cancer Therapies. J Am Heart Assoc. 01 2020;9(2):e013755. doi: 10.1161/JAHA.119.013755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seraphim A, Westwood M, Bhuva AN, et al. Advanced Imaging Modalities to Monitor for Cardiotoxicity. Curr Treat Options Oncol. Aug 8 2019;20(9):73. doi: 10.1007/s11864-019-0672-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fava AM, Meredith D, Desai MY. Clinical Applications of Echo Strain Imaging: a Current Appraisal. Curr Treat Options Cardiovasc Med. Aug 31 2019;21(10):50. doi: 10.1007/s11936-019-0761-0 [DOI] [PubMed] [Google Scholar]
- 8.Bottinor W, Trankle CR, Hundley WG. The Role of Cardiovascular MRI in Cardio-Oncology. Heart Fail Clin. Jan 2021;17(1):121–133. doi: 10.1016/j.hfc.2020.08.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Čelutkienė J, Pudil R, López-Fernández T, et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: a position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur J Heart Fail. Sep 2020;22(9):1504–1524. doi: 10.1002/ejhf.1957 [DOI] [PubMed] [Google Scholar]
- 10.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popović ZB, Marwick TH. Reproducibility of echocardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J Am Coll Cardiol. Jan 8 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035 [DOI] [PubMed] [Google Scholar]
- 11.Bansal M, Kasliwal RR. How do I do it? Speckle-tracking echocardiography. Indian Heart J. Jan-Feb 2013;65(1):117–23. doi: 10.1016/j.ihj.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu JE, Barac A, Thavendiranathan P, Scherrer-Crosbie M. Strain Imaging in Cardio-Oncology. JACC CardioOncol. Dec 2020;2(5):677–689. doi: 10.1016/j.jaccao.2020.10.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Oikonomou EK, Kokkinidis DG, Kampaktsis PN, et al. Assessment of Prognostic Value of Left Ventricular Global Longitudinal Strain for Early Prediction of Chemotherapy-Induced Cardiotoxicity: A Systematic Review and Meta-analysis. JAMA Cardiol. Oct 1 2019;4(10):1007–1018. doi: 10.1001/jamacardio.2019.2952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cascino GJ, Voss WB, Canaani J, et al. Two-dimensional speckle-tracking strain detects subclinical cardiotoxicity in older patients treated for acute myeloid leukemia. Echocardiography. Nov 2019;36(11):2033–2040. doi: 10.1111/echo.14518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keramida K, Farmakis D, López Fernández T, Lancellotti P. Focused echocardiography in cardio-oncology. Echocardiography. Aug 2020;37(8):1149–1158. doi: 10.1111/echo.14800 [DOI] [PubMed] [Google Scholar]
- 16.Wang B, Yu Y, Zhang Y, et al. Speckle tracking echocardiography in the early detection and prediction of anthracycline cardiotoxicity in diffuse large B-cell lymphoma treated with (R)-CHOP regimen. Echocardiography. Mar 2020;37(3):421–428. doi: 10.1111/echo.14622 [DOI] [PubMed] [Google Scholar]
- 17.Laufer-Perl M, Arnold JH, Mor L, et al. The association of reduced global longitudinal strain with cancer therapy-related cardiac dysfunction among patients receiving cancer therapy. Clin Res Cardiol. Feb 2020;109(2):255–262. doi: 10.1007/s00392-019-01508-9 [DOI] [PubMed] [Google Scholar]
- 18.McGregor PC, Moura FA, Banchs J, Aragam JR. Role of myocardial strain imaging in surveillance and management of cancer therapeutics-related cardiac dysfunction: A systematic review. Echocardiography. Feb 2021;38(2):314–328. doi: 10.1111/echo.14944 [DOI] [PubMed] [Google Scholar]
- 19.El-Sherbeny WS, Sabry NM, Sharbay RM. Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J Echocardiogr. Jun 2019;17(2):76–83. doi: 10.1007/s12574-018-0394-4 [DOI] [PubMed] [Google Scholar]
- 20.Chen J, Wang L, Wu FF, Sun G. Early detection of cardiotoxicity by 3D speckle tracking imaging of area strain in breast cancer patients receiving chemotherapy. Echocardiography. Sep 2019;36(9):1682–1688. doi: 10.1111/echo.14467 [DOI] [PubMed] [Google Scholar]
- 21.Mahjoob MP, Sheikholeslami SA, Dadras M, et al. Prognostic Value of Cardiac Biomarkers Assessment in Combination with Myocardial 2D Strain Echocardiography for Early Detection of Anthracycline-Related Cardiac Toxicity. Cardiovasc Hematol Disord Drug Targets. 2020;20(1):74–83. doi: 10.2174/1871529x19666190912150942 [DOI] [PubMed] [Google Scholar]
- 22.Khairat I, Khalfallah M, Shaban A, Farag IA, Elkady A. Right ventricular 2D speckle-tracking echocardiography in children with osteosarcoma under chemotherapy. Egypt Heart J. Nov 21 2019;71(1):23. doi: 10.1186/s43044-019-0028-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slieker MG, Fackoury C, Slorach C, et al. Echocardiographic Assessment of Cardiac Function in Pediatric Survivors of Anthracycline-Treated Childhood Cancer. Circ Cardiovasc Imaging. Dec 2019;12(12):e008869. doi: 10.1161/circimaging.119.008869 [DOI] [PubMed] [Google Scholar]
- 24.Wolf CM, Reiner B, Kühn A, et al. Subclinical Cardiac Dysfunction in Childhood Cancer Survivors on 10-Years Follow-Up Correlates With Cumulative Anthracycline Dose and Is Best Detected by Cardiopulmonary Exercise Testing, Circulating Serum Biomarker, Speckle Tracking Echocardiography, and Tissue Doppler Imaging. Front Pediatr. 2020;8:123. doi: 10.3389/fped.2020.00123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez HR, Salloum R, Wright E, et al. Echocardiographic myocardial strain analysis describes subclinical cardiac dysfunction after craniospinal irradiation in pediatric and young adult patients with central nervous system tumors. Cardiooncology. Feb 2 2021;7(1):5. doi: 10.1186/s40959-021-00093-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sitte V, Burkhardt B, Weber R, et al. Advanced Imaging and New Cardiac Biomarkers in Long-term Follow-up After Childhood Cancer. J Pediatr Hematol Oncol. Apr 2021;doi: 10.1097/MPH.0000000000002156 [DOI] [PubMed] [Google Scholar]
- 27.Yu HK, Yu W, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J Am Soc Echocardiogr. Aug 2013;26(8):846–52. doi: 10.1016/j.echo.2013.04.018 [DOI] [PubMed] [Google Scholar]
- 28.Bhagat A, Kleinerman ES. Anthracycline-Induced Cardiotoxicity: Causes, Mechanisms, and Prevention. Adv Exp Med Biol. 2020;1257:181–192. doi: 10.1007/978-3-030-43032-0_15 [DOI] [PubMed] [Google Scholar]
- 29.Cappetta D, De Angelis A, Sapio L, et al. Oxidative Stress and Cellular Response to Doxorubicin: A Common Factor in the Complex Milieu of Anthracycline Cardiotoxicity. Oxid Med Cell Longev. 2017;2017:1521020. doi: 10.1155/2017/1521020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. Jun 2 2015;131(22):1981–8. doi: 10.1161/circulationaha.114.013777 [DOI] [PubMed] [Google Scholar]
- 31.Bernaba BN, Chan JB, Lai CK, Fishbein MC. Pathology of late-onset anthracycline cardiomyopathy. Cardiovasc Pathol. Sep-Oct 2010;19(5):308–11. doi: 10.1016/j.carpath.2009.07.004 [DOI] [PubMed] [Google Scholar]
- 32.Saleh Y, Abdelkarim O, Herzallah K, Abela GS. Anthracycline-induced cardiotoxicity: mechanisms of action, incidence, risk factors, prevention, and treatment. Heart Fail Rev. May 14 2020;doi: 10.1007/s10741-020-09968-2 [DOI] [PubMed] [Google Scholar]
- 33.Nicolazzi MA, Carnicelli A, Fuorlo M, et al. Anthracycline and trastuzumab-induced cardiotoxicity in breast cancer. Eur Rev Med Pharmacol Sci. Apr 2018;22(7):2175–2185. doi: 10.26355/eurrev_201804_14752 [DOI] [PubMed] [Google Scholar]
- 34.Keramida K, Farmakis D, Bingcang J, et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart Fail. Apr 2019;21(4):529–535. doi: 10.1002/ejhf.1385 [DOI] [PubMed] [Google Scholar]
- 35.Mantarro S, Rossi M, Bonifazi M, et al. Risk of severe cardiotoxicity following treatment with trastuzumab: a meta-analysis of randomized and cohort studies of 29,000 women with breast cancer. Intern Emerg Med. Feb 2016;11(1):123–40. doi: 10.1007/s11739-015-1362-x [DOI] [PubMed] [Google Scholar]
- 36.Balduzzi S, Mantarro S, Guarneri V, et al. Trastuzumab‐containing regimens for metastatic breast cancer. Cochrane Database of Systematic Reviews. 2014;(6)doi: 10.1002/14651858.CD006242.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grandin EW, Ky B, Cornell RF, Carver J, Lenihan DJ. Patterns of cardiac toxicity associated with irreversible proteasome inhibition in the treatment of multiple myeloma. J Card Fail. Feb 2015;21(2):138–44. doi: 10.1016/j.cardfail.2014.11.008 [DOI] [PubMed] [Google Scholar]
- 38.Wu P, Oren O, Gertz MA, Yang EH. Proteasome Inhibitor-Related Cardiotoxicity: Mechanisms, Diagnosis, and Management. Curr Oncol Rep. Jun 8 2020;22(7):66. doi: 10.1007/s11912-020-00931-w [DOI] [PubMed] [Google Scholar]
- 39.Shah C, Bishnoi R, Jain A, et al. Cardiotoxicity associated with carfilzomib: systematic review and meta-analysis. Leuk Lymphoma. Nov 2018;59(11):2557–2569. doi: 10.1080/10428194.2018.1437269 [DOI] [PubMed] [Google Scholar]
- 40.Scott K, Hayden PJ, Will A, Wheatley K, Coyne I. Bortezomib for the treatment of multiple myeloma. Cochrane Database of Systematic Reviews. 2016;(4)doi: 10.1002/14651858.CD010816.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Iannaccone A, Bruno G, Ravera A, et al. Evaluation of Cardiovascular Toxicity Associated with Treatments Containing Proteasome Inhibitors in Multiple Myeloma Therapy. High Blood Press Cardiovasc Prev. Jun 2018;25(2):209–218. doi: 10.1007/s40292-018-0256-1 [DOI] [PubMed] [Google Scholar]
- 42.Abdel-Qadir H, Ethier JL, Lee DS, Thavendiranathan P, Amir E. Cardiovascular toxicity of angiogenesis inhibitors in treatment of malignancy: A systematic review and meta-analysis. Cancer Treat Rev. Feb 2017;53:120–127. doi: 10.1016/j.ctrv.2016.12.002 [DOI] [PubMed] [Google Scholar]
- 43.Touyz RM, Herrmann J. Cardiotoxicity with vascular endothelial growth factor inhibitor therapy. NPJ Precis Oncol. 2018;2:13. doi: 10.1038/s41698-018-0056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moreo A, Vallerio P, Ricotta R, et al. Effects of Cancer Therapy Targeting Vascular Endothelial Growth Factor Receptor on Central Blood Pressure and Cardiovascular System. Am J Hypertens. Feb 2016;29(2):158–62. doi: 10.1093/ajh/hpv077 [DOI] [PubMed] [Google Scholar]
- 45.Nhola LF, Abdelmoneim SS, Villarraga HR, et al. Echocardiographic Assessment for the Detection of Cardiotoxicity Due to Vascular Endothelial Growth Factor Inhibitor Therapy in Metastatic Renal Cell and Colorectal Cancers. J Am Soc Echocardiogr. Feb 2019;32(2):267–276. doi: 10.1016/j.echo.2018.09.019 [DOI] [PubMed] [Google Scholar]
- 46.Sonaglioni A, Albini A, Fossile E, et al. Speckle-Tracking Echocardiography for Cardioncological Evaluation in Bevacizumab-Treated Colorectal Cancer Patients. Cardiovasc Toxicol. Dec 2020;20(6):581–592. doi: 10.1007/s12012-020-09583-5 [DOI] [PubMed] [Google Scholar]
- 47.Chaar M, Kamta J, Ait-Oudhia S. Mechanisms, monitoring, and management of tyrosine kinase inhibitors-associated cardiovascular toxicities. Onco Targets Ther. 2018;11:6227–6237. doi: 10.2147/ott.s170138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brown SA, Ray JC, Herrmann J. Precision Cardio-Oncology: a Systems-Based Perspective on Cardiotoxicity of Tyrosine Kinase Inhibitors and Immune Checkpoint Inhibitors. J Cardiovasc Transl Res. Jun 2020;13(3):402–416. doi: 10.1007/s12265-020-09992-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Novo G, Di Lisi D, Bronte E, et al. Cardiovascular Toxicity in Cancer Patients Treated with Tyrosine Kinase Inhibitors: A Real-World Single-Center Experience. Oncology. 2020;98(7):445–451. doi: 10.1159/000505486 [DOI] [PubMed] [Google Scholar]
- 50.Berger M, Amini-Adlé M, Maucort-Boulch D, et al. Left ventricular ejection fraction decrease related to BRAF and/or MEK inhibitors in metastatic melanoma patients: A retrospective analysis. Cancer Med. Apr 2020;9(8):2611–2620. doi: 10.1002/cam4.2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mincu RI, Mahabadi AA, Michel L, et al. Cardiovascular Adverse Events Associated With BRAF and MEK Inhibitors: A Systematic Review and Meta-analysis. JAMA Netw Open. Aug 2 2019;2(8):e198890. doi: 10.1001/jamanetworkopen.2019.8890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guha A, Jain P, Fradley MG, et al. Cardiovascular adverse events associated with BRAF versus BRAF/MEK inhibitor: Cross-sectional and longitudinal analysis using two large national registries. Cancer Med. Jun 2021;10(12):3862–3872. doi: 10.1002/cam4.3938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Waheed N, Fradley MG, DeRemer DL, et al. Newly diagnosed cardiovascular disease in patients treated with immune checkpoint inhibitors: a retrospective analysis of patients at an academic tertiary care center. Cardiooncology. Mar 18 2021;7(1):10. doi: 10.1186/s40959-021-00097-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lyon AR, Yousaf N, Battisti NML, Moslehi J, Larkin J. Immune checkpoint inhibitors and cardiovascular toxicity. Lancet Oncol. Sep 2018;19(9):e447–e458. doi: 10.1016/s1470-2045(18)30457-1 [DOI] [PubMed] [Google Scholar]
- 55.Bonaca MP, Olenchock BA, Salem JE, et al. Myocarditis in the Setting of Cancer Therapeutics: Proposed Case Definitions for Emerging Clinical Syndromes in Cardio-Oncology. Circulation. Jul 2 2019;140(2):80–91. doi: 10.1161/circulationaha.118.034497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mahmood SS, Fradley MG, Cohen JV, et al. Myocarditis in Patients Treated With Immune Checkpoint Inhibitors. J Am Coll Cardiol. Apr 24 2018;71(16):1755–1764. doi: 10.1016/j.jacc.2018.02.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ganatra S, Neilan TG. Immune Checkpoint Inhibitor-Associated Myocarditis. Oncologist. Aug 2018;23(8):879–886. doi: 10.1634/theoncologist.2018-0130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awadalla M, Mahmood SS, Groarke JD, et al. Global Longitudinal Strain and Cardiac Events in Patients With Immune Checkpoint Inhibitor-Related Myocarditis. J Am Coll Cardiol. Feb 11 2020;75(5):467–478. doi: 10.1016/j.jacc.2019.11.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mylvaganam R, Avery R, Goldberg I, et al. Adverse effects of immune checkpoint inhibitor therapies on right ventricular function and pulmonary arterial dilatation. Pulm Circ. Jan-Mar 2021;11(1):2045894021992236. doi: 10.1177/2045894021992236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stein-Merlob AF, Rothberg MV, Holman P, Yang EH. Immunotherapy-Associated Cardiotoxicity of Immune Checkpoint Inhibitors and Chimeric Antigen Receptor T Cell Therapy: Diagnostic and Management Challenges and Strategies. Curr Cardiol Rep. Jan 22 2021;23(3):11. doi: 10.1007/s11886-021-01440-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Alvi RM, Frigault MJ, Fradley MG, et al. Cardiovascular Events Among Adults Treated With Chimeric Antigen Receptor T-Cells (CAR-T). J Am Coll Cardiol. Dec 24 2019;74(25):3099–3108. doi: 10.1016/j.jacc.2019.10.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ganatra S, Redd R, Hayek SS, et al. Chimeric Antigen Receptor T-Cell Therapy-Associated Cardiomyopathy in Patients With Refractory or Relapsed Non-Hodgkin Lymphoma. Circulation. Oct 27 2020;142(17):1687–1690. doi: 10.1161/circulationaha.120.048100 [DOI] [PubMed] [Google Scholar]
- 63.Lancellotti P, Nkomo VT, Badano LP, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur Heart J Cardiovasc Imaging. Aug 2013;14(8):721–40. doi: 10.1093/ehjci/jet123 [DOI] [PubMed] [Google Scholar]
- 64.Darby SC, Cutter DJ, Boerma M, et al. Radiation-related heart disease: current knowledge and future prospects. Int J Radiat Oncol Biol Phys. Mar 1 2010;76(3):656–65. doi: 10.1016/j.ijrobp.2009.09.064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Walker V, Lairez O, Fondard O, et al. Myocardial deformation after radiotherapy: a layer-specific and territorial longitudinal strain analysis in a cohort of left-sided breast cancer patients (BACCARAT study). Radiat Oncol. Aug 20 2020;15(1):201. doi: 10.1186/s13014-020-01635-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Bogaard VAB, van Luijk P, Hummel YM, et al. Cardiac Function After Radiation Therapy for Breast Cancer. Int J Radiat Oncol Biol Phys. Jun 1 2019;104(2):392–400. doi: 10.1016/j.ijrobp.2019.02.003 [DOI] [PubMed] [Google Scholar]
- 67.Skyttä T, Tuohinen S, Luukkaala T, Virtanen V, Raatikainen P, Kellokumpu-Lehtinen PL. Adjuvant radiotherapy-induced cardiac changes among patients with early breast cancer: a three-year follow-up study(). Acta Oncol. Sep 2019;58(9):1250–1258. doi: 10.1080/0284186x.2019.1630751 [DOI] [PubMed] [Google Scholar]
- 68.Erven K, Jurcut R, Weltens C, et al. Acute radiation effects on cardiac function detected by strain rate imaging in breast cancer patients. Int J Radiat Oncol Biol Phys. Apr 1 2011;79(5):1444–51. doi: 10.1016/j.ijrobp.2010.01.004 [DOI] [PubMed] [Google Scholar]
- 69.Tuohinen SS, Skyttä T, Poutanen T, et al. Radiotherapy-induced global and regional differences in early-stage left-sided versus right-sided breast cancer patients: speckle tracking echocardiography study. Int J Cardiovasc Imaging. Apr 2017;33(4):463–472. doi: 10.1007/s10554-016-1021-y [DOI] [PubMed] [Google Scholar]
- 70.Lo Q, Hee L, Batumalai V, et al. Strain Imaging Detects Dose-Dependent Segmental Cardiac Dysfunction in the Acute Phase After Breast Irradiation. Int J Radiat Oncol Biol Phys. Sep 1 2017;99(1):182–190. doi: 10.1016/j.ijrobp.2017.05.030 [DOI] [PubMed] [Google Scholar]
- 71.Lo Q, Hee L, Batumalai V, et al. Subclinical cardiac dysfunction detected by strain imaging during breast irradiation with persistent changes 6 weeks after treatment. Int J Radiat Oncol Biol Phys. Jun 1 2015;92(2):268–76. doi: 10.1016/j.ijrobp.2014.11.016 [DOI] [PubMed] [Google Scholar]
- 72.Tuohinen SS, Skyttä T, Huhtala H, et al. 3-Year Follow-Up of Radiation-Associated Changes in Diastolic Function by Speckle Tracking Echocardiography. JACC CardioOncol. Jun 2021;3(2):277–289. doi: 10.1016/j.jaccao.2021.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. Aug 18 2004;44(4):878–86. doi: 10.1016/j.jacc.2004.05.050 [DOI] [PubMed] [Google Scholar]
- 74.Habeeb NM, Youssef OI, Elguindy WM, Ibrahim AS, Hussein WH. Three Dimensional (3D) Echocardiography as a Tool of Left Ventricular Assessment in Children with Dilated Cardiomyopathy: Comparison to Cardiac MRI. Open Access Maced J Med Sci. Dec 20 2018;6(12):2310–2315. doi: 10.3889/oamjms.2018.270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Altman M, Bergerot C, Aussoleil A, et al. Assessment of left ventricular systolic function by deformation imaging derived from speckle tracking: a comparison between 2D and 3D echo modalities. Eur Heart J Cardiovasc Imaging. Mar 2014;15(3):316–23. doi: 10.1093/ehjci/jet103 [DOI] [PubMed] [Google Scholar]
- 76.Song FY, Shi J, Guo Y, et al. Assessment of biventricular systolic strain derived from the two-dimensional and three-dimensional speckle tracking echocardiography in lymphoma patients after anthracycline therapy. Int J Cardiovasc Imaging. Jun 2017;33(6):857–868. doi: 10.1007/s10554-017-1082-6 [DOI] [PubMed] [Google Scholar]
- 77.Poterucha TJ, Kochav J, O’Connor DS, Rosner GF. Cardiac Tumors: Clinical Presentation, Diagnosis, and Management. Curr Treat Options Oncol. Jun 27 2019;20(8):66. doi: 10.1007/s11864-019-0662-1 [DOI] [PubMed] [Google Scholar]
- 78.Abraham KP, Reddy V, Gattuso P. Neoplasms metastatic to the heart: review of 3314 consecutive autopsies. Am J Cardiovasc Pathol. 1990;3(3):195–8. [PubMed] [Google Scholar]
- 79.Patel R, Lim RP, Saric M, et al. Diagnostic Performance of Cardiac Magnetic Resonance Imaging and Echocardiography in Evaluation of Cardiac and Paracardiac Masses. Am J Cardiol. Jan 1 2016;117(1):135–40. doi: 10.1016/j.amjcard.2015.10.014 [DOI] [PubMed] [Google Scholar]
- 80.Mansencal N, Revault-d’Allonnes L, Pelage JP, Farcot JC, Lacombe P, Dubourg O. Usefulness of contrast echocardiography for assessment of intracardiac masses. Arch Cardiovasc Dis. Mar 2009;102(3):177–83. doi: 10.1016/j.acvd.2008.12.007 [DOI] [PubMed] [Google Scholar]
- 81.Tang QY, Guo LD, Wang WX, et al. Usefulness of contrast perfusion echocardiography for differential diagnosis of cardiac masses. Ultrasound Med Biol. Sep 2015;41(9):2382–90. doi: 10.1016/j.ultrasmedbio.2015.05.010 [DOI] [PubMed] [Google Scholar]
- 82.Xia H, Gan L, Jiang Y, et al. Use of transesophageal echocardiography and contrast echocardiography in the evaluation of cardiac masses. Int J Cardiol. Jun 1 2017;236:466–472. doi: 10.1016/j.ijcard.2017.01.073 [DOI] [PubMed] [Google Scholar]
- 83.Mügge A, Daniel WG, Haverich A, Lichtlen PR. Diagnosis of noninfective cardiac mass lesions by two-dimensional echocardiography. Comparison of the transthoracic and transesophageal approaches. Circulation. Jan 1991;83(1):70–8. doi: 10.1161/01.cir.83.1.70 [DOI] [PubMed] [Google Scholar]
- 84.Tsang TS, Oh JK, Seward JB, Tajik AJ. Diagnostic value of echocardiography in cardiac tamponade. Herz. Dec 2000;25(8):734–40. doi: 10.1007/pl00001991 [DOI] [PubMed] [Google Scholar]
- 85.Tsang TS, Seward JB, Barnes ME, et al. Outcomes of primary and secondary treatment of pericardial effusion in patients with malignancy. Mayo Clin Proc. Mar 2000;75(3):248–53. doi: 10.4065/75.3.248 [DOI] [PubMed] [Google Scholar]
- 86.Tsang TS. Echocardiography-Guided Pericardiocentesis for Effusions in Patients With Cancer Revisited. J Am Coll Cardiol. Sep 8 2015;66(10):1129–31. doi: 10.1016/j.jacc.2015.07.027 [DOI] [PubMed] [Google Scholar]
- 87.El Haddad D, Iliescu C, Yusuf SW, et al. Outcomes of Cancer Patients Undergoing Percutaneous Pericardiocentesis for Pericardial Effusion. J Am Coll Cardiol. Sep 8 2015;66(10):1119–28. doi: 10.1016/j.jacc.2015.06.1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Addison D, Slivnick JA, Campbell CM, Vallakati A, Jneid H, Schelbert E. Recent Advances and Current Dilemmas in the Diagnosis and Management of Transthyretin Cardiac Amyloidosis. J Am Heart Assoc. May 2021;10(9):e019840. doi: 10.1161/JAHA.120.019840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bravo PE, Fujikura K, Kijewski MF, et al. Relative Apical Sparing of Myocardial Longitudinal Strain Is Explained by Regional Differences in Total Amyloid Mass Rather Than the Proportion of Amyloid Deposits. JACC Cardiovasc Imaging. Jul 2019;12(7 Pt 1):1165–1173. doi: 10.1016/j.jcmg.2018.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Phelan D, Collier P, Thavendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. Oct 2012;98(19):1442–8. doi: 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 91.Nicol M, Baudet M, Brun S, et al. Diagnostic score of cardiac involvement in AL amyloidosis. Eur Heart J Cardiovasc Imaging. May 1 2020;21(5):542–548. doi: 10.1093/ehjci/jez180 [DOI] [PubMed] [Google Scholar]
- 92.Kyrouac D, Schiffer W, Lennep B, et al. Echocardiographic and clinical predictors of cardiac amyloidosis: limitations of apical sparing. ESC Heart Fail. Feb 2022;9(1):385–397. doi: 10.1002/ehf2.13738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Harries I, Liang K, Williams M, et al. Magnetic Resonance Imaging to Detect Cardiovascular Effects of Cancer Therapy: JACC CardioOncology State-of-the-Art Review. JACC CardioOncol. Jun 2020;2(2):270–292. doi: 10.1016/j.jaccao.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moody WE, Edwards NC, Chue CD, et al. Variability in cardiac MR measurement of left ventricular ejection fraction, volumes and mass in healthy adults: defining a significant change at 1 year. Br J Radiol. May 2015;88(1049):20140831. doi: 10.1259/bjr.20140831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. Aug 10 2012;30(23):2876–84. doi: 10.1200/jco.2011.40.3584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scatteia A, Baritussio A, Bucciarelli-Ducci C. Strain imaging using cardiac magnetic resonance. Heart Fail Rev. Jul 2017;22(4):465–476. doi: 10.1007/s10741-017-9621-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Amzulescu MS, Langet H, Saloux E, et al. Head-to-Head Comparison of Global and Regional Two-Dimensional Speckle Tracking Strain Versus Cardiac Magnetic Resonance Tagging in a Multicenter Validation Study. Circ Cardiovasc Imaging. Nov 2017;10(11)doi: 10.1161/circimaging.117.006530 [DOI] [PubMed] [Google Scholar]
- 98.Soufer A, Baldassarre LA. The Role of Cardiac Magnetic Resonance Imaging to Detect Cardiac Toxicity From Cancer Therapeutics. Curr Treat Options Cardiovasc Med. May 18 2019;21(6):28. doi: 10.1007/s11936-019-0732-5 [DOI] [PubMed] [Google Scholar]
- 99.Barreiro-Pérez M, Curione D, Symons R, Claus P, Voigt JU, Bogaert J. Left ventricular global myocardial strain assessment comparing the reproducibility of four commercially available CMR-feature tracking algorithms. Eur Radiol. Dec 2018;28(12):5137–5147. doi: 10.1007/s00330-018-5538-4 [DOI] [PubMed] [Google Scholar]
- 100.Ananthapadmanabhan S, Deng E, Femia G, et al. Intra- and inter-observer reproducibility of multilayer cardiac magnetic resonance feature tracking derived longitudinal and circumferential strain. Cardiovasc Diagn Ther. Apr 2020;10(2):173–182. doi: 10.21037/cdt.2020.01.10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lambert J, Lamacie M, Thampinathan B, et al. Variability in echocardiography and MRI for detection of cancer therapy cardiotoxicity. Heart. Jun 2020;106(11):817–823. doi: 10.1136/heartjnl-2019-316297 [DOI] [PubMed] [Google Scholar]
- 102.Suerken CK, D’Agostino RB Jr., Jordan JH, et al. Simultaneous Left Ventricular Volume and Strain Changes During Chemotherapy Associate With 2-Year Postchemotherapy Measures of Left Ventricular Ejection Fraction. J Am Heart Assoc. Jan 21 2020;9(2):e015400. doi: 10.1161/jaha.119.015400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Evin M, Cluzel P, Lamy J, et al. Assessment of left atrial function by MRI myocardial feature tracking. J Magn Reson Imaging. Aug 2015;42(2):379–89. doi: 10.1002/jmri.24851 [DOI] [PubMed] [Google Scholar]
- 104.Burrage MK, Ferreira VM. The use of cardiovascular magnetic resonance as an early non-invasive biomarker for cardiotoxicity in cardio-oncology. Cardiovasc Diagn Ther. Jun 2020;10(3):610–624. doi: 10.21037/cdt-20-165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taylor AJ, Salerno M, Dharmakumar R, Jerosch-Herold M. T1 Mapping: Basic Techniques and Clinical Applications. JACC Cardiovasc Imaging. Jan 2016;9(1):67–81. doi: 10.1016/j.jcmg.2015.11.005 [DOI] [PubMed] [Google Scholar]
- 106.Karamitsos TD, Arvanitaki A, Karvounis H, Neubauer S, Ferreira VM. Myocardial Tissue Characterization and Fibrosis by Imaging. JACC Cardiovasc Imaging. May 2020;13(5):1221–1234. doi: 10.1016/j.jcmg.2019.06.030 [DOI] [PubMed] [Google Scholar]
- 107.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. Non-contrast T1-mapping detects acute myocardial edema with high diagnostic accuracy: a comparison to T2-weighted cardiovascular magnetic resonance. J Cardiovasc Magn Reson. Jun 21 2012;14(1):42. doi: 10.1186/1532-429x-14-42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.h-Ici DO, Jeuthe S, Al-Wakeel N, et al. T1 mapping in ischaemic heart disease. Eur Heart J Cardiovasc Imaging. Jun 2014;15(6):597–602. doi: 10.1093/ehjci/jeu024 [DOI] [PubMed] [Google Scholar]
- 109.Löffler AI, Salerno M. Cardiac MRI for the evaluation of oncologic cardiotoxicity. J Nucl Cardiol. Dec 2018;25(6):2148–2158. doi: 10.1007/s12350-018-1293-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Nakamori S, Dohi K, Ishida M, et al. Native T1 Mapping and Extracellular Volume Mapping for the Assessment of Diffuse Myocardial Fibrosis in Dilated Cardiomyopathy. JACC Cardiovasc Imaging. Jan 2018;11(1):48–59. doi: 10.1016/j.jcmg.2017.04.006 [DOI] [PubMed] [Google Scholar]
- 111.Hu C, Sinusas AJ, Huber S, et al. T1-refBlochi: high resolution 3D post-contrast T1 myocardial mapping based on a single 3D late gadolinium enhancement volume, Bloch equations, and a reference T1. J Cardiovasc Magn Reson. Aug 18 2017;19(1):63. doi: 10.1186/s12968-017-0375-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sibley CT, Noureldin RA, Gai N, et al. T1 Mapping in cardiomyopathy at cardiac MR: comparison with endomyocardial biopsy. Radiology. Dec 2012;265(3):724–32. doi: 10.1148/radiol.12112721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pan JA, Lee YJ, Salerno M. Diagnostic Performance of Extracellular Volume, Native T1, and T2 Mapping Versus Lake Louise Criteria by Cardiac Magnetic Resonance for Detection of Acute Myocarditis: A Meta-Analysis. Circ Cardiovasc Imaging. Jul 2018;11(7):e007598. doi: 10.1161/circimaging.118.007598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yu AF, Chan AT, Steingart RM. Cardiac Magnetic Resonance and Cardio-Oncology: Does T(2) Signal the End of Anthracycline Cardiotoxicity? J Am Coll Cardiol. Feb 26 2019;73(7):792–794. doi: 10.1016/j.jacc.2018.11.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lunning MA, Kutty S, Rome ET, et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol. Aug 2015;38(4):377–81. doi: 10.1097/COC.0b013e31829e19be [DOI] [PubMed] [Google Scholar]
- 116.Toro-Salazar OH, Lee JH, Zellars KN, et al. Use of integrated imaging and serum biomarker profiles to identify subclinical dysfunction in pediatric cancer patients treated with anthracyclines. Cardiooncology. 2018;4doi: 10.1186/s40959-018-0030-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jolly MP, Jordan JH, Meléndez GC, McNeal GR, D’Agostino RB Jr., Hundley WG. Automated assessments of circumferential strain from cine CMR correlate with LVEF declines in cancer patients early after receipt of cardio-toxic chemotherapy. J Cardiovasc Magn Reson. Aug 2 2017;19(1):59. doi: 10.1186/s12968-017-0373-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Haslbauer JD, Lindner S, Valbuena-Lopez S, et al. CMR imaging biosignature of cardiac involvement due to cancer-related treatment by T1 and T2 mapping. Int J Cardiol. Jan 15 2019;275:179–186. doi: 10.1016/j.ijcard.2018.10.023 [DOI] [PubMed] [Google Scholar]
- 119.Safaei AM, Kamangar TM, Asadian S, et al. Detection of the Early Cardiotoxic Effects of Doxorubicin-Containing Chemotherapy Regimens in Patients with Breast Cancer through Novel Cardiac Magnetic Resonance Imaging: A Short-term Follow-up. J Clin Imaging Sci. 2021;11:33. doi: 10.25259/jcis_58_2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barbosa MF, Fusco DR, Gaiolla RD, et al. Characterization of subclinical diastolic dysfunction by cardiac magnetic resonance feature-tracking in adult survivors of non-Hodgkin lymphoma treated with anthracyclines. BMC Cardiovasc Disord. Apr 12 2021;21(1):170. doi: 10.1186/s12872-021-01996-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am J Cardiol. Dec 1 2012;110(11):1679–86. doi: 10.1016/j.amjcard.2012.07.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am J Cardiol. Mar 1 2013;111(5):717–22. doi: 10.1016/j.amjcard.2012.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. Jun 10 2013;15(1):48. doi: 10.1186/1532-429x-15-48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Jordan JH, Vasu S, Morgan TM, et al. Anthracycline-Associated T1 Mapping Characteristics Are Elevated Independent of the Presence of Cardiovascular Comorbidities in Cancer Survivors. Circ Cardiovasc Imaging. Aug 2016;9(8)doi: 10.1161/circimaging.115.004325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am Heart J. Jun 2001;141(6):1007–13. doi: 10.1067/mhj.2001.115436 [DOI] [PubMed] [Google Scholar]
- 126.Lustberg MB, Reinbolt R, Addison D, et al. Early Detection of Anthracycline-Induced Cardiotoxicity in Breast Cancer Survivors With T2 Cardiac Magnetic Resonance. Circ Cardiovasc Imaging. 05 2019;12(5):e008777. doi: 10.1161/CIRCIMAGING.118.008777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Martin-Garcia A, Diaz-Pelaez E, Lopez-Corral L, et al. T2 Mapping Identifies Early Anthracycline-Induced Cardiotoxicity in Elderly Patients With Cancer. JACC Cardiovasc Imaging. Jul 2020;13(7):1630–1632. doi: 10.1016/j.jcmg.2020.01.017 [DOI] [PubMed] [Google Scholar]
- 128.Park HS, Hong YJ, Han K, et al. Ultrahigh-field cardiovascular magnetic resonance T1 and T2 mapping for the assessment of anthracycline-induced cardiotoxicity in rat models: validation against histopathologic changes. J Cardiovasc Magn Reson. Jun 17 2021;23(1):76. doi: 10.1186/s12968-021-00767-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Galán-Arriola C, Lobo M, Vílchez-Tschischke JP, et al. Serial Magnetic Resonance Imaging to Identify Early Stages of Anthracycline-Induced Cardiotoxicity. J Am Coll Cardiol. Feb 26 2019;73(7):779–791. doi: 10.1016/j.jacc.2018.11.046 [DOI] [PubMed] [Google Scholar]
- 130.Noel CV, Rainusso N, Robertson M, et al. Early detection of myocardial changes with and without dexrazoxane using serial magnetic resonance imaging in a pre-clinical mouse model. Cardiooncology. Jun 16 2021;7(1):23. doi: 10.1186/s40959-021-00109-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Ville de Goyet M, Brichard B, Robert A, et al. Prospective cardiac MRI for the analysis of biventricular function in children undergoing cancer treatments. Pediatr Blood Cancer. May 2015;62(5):867–74. doi: 10.1002/pbc.25381 [DOI] [PubMed] [Google Scholar]
- 132.Drafts BC, Twomley KM, D’Agostino R Jr., et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imaging. Aug 2013;6(8):877–85. doi: 10.1016/j.jcmg.2012.11.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Jordan JH, Castellino SM, Meléndez GC, et al. Left Ventricular Mass Change After Anthracycline Chemotherapy. Circ Heart Fail. Jul 2018;11(7):e004560. doi: 10.1161/circheartfailure.117.004560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Houbois CP, Nolan M, Somerset E, et al. Serial Cardiovascular Magnetic Resonance Strain Measurements to Identify Cardiotoxicity in Breast Cancer: Comparison With Echocardiography. JACC Cardiovasc Imaging. May 2021;14(5):962–974. doi: 10.1016/j.jcmg.2020.09.039 [DOI] [PubMed] [Google Scholar]
- 135.Nakano S, Takahashi M, Kimura F, et al. Cardiac magnetic resonance imaging-based myocardial strain study for evaluation of cardiotoxicity in breast cancer patients treated with trastuzumab: A pilot study to evaluate the feasibility of the method. Cardiol J. 2016;23(3):270–80. doi: 10.5603/CJ.a2016.0023 [DOI] [PubMed] [Google Scholar]
- 136.Ong G, Brezden-Masley C, Dhir V, et al. Myocardial strain imaging by cardiac magnetic resonance for detection of subclinical myocardial dysfunction in breast cancer patients receiving trastuzumab and chemotherapy. Int J Cardiol. Jun 15 2018;261:228–233. doi: 10.1016/j.ijcard.2018.03.041 [DOI] [PubMed] [Google Scholar]
- 137.Gong IY, Ong G, Brezden-Masley C, et al. Early diastolic strain rate measurements by cardiac MRI in breast cancer patients treated with trastuzumab: a longitudinal study. Int J Cardiovasc Imaging. Apr 2019;35(4):653–662. doi: 10.1007/s10554-018-1482-2 [DOI] [PubMed] [Google Scholar]
- 138.Song L, Brezden-Masley C, Ramanan V, et al. Serial Measurements of Left Ventricular Systolic and Diastolic Function by Cardiac Magnetic Resonance Imaging in Patients with Early Stage Breast Cancer on Trastuzumab. Am J Cardiol. Apr 1 2019;123(7):1173–1179. doi: 10.1016/j.amjcard.2018.12.046 [DOI] [PubMed] [Google Scholar]
- 139.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J Cardiovasc Magn Reson. Jan 22 2008;10(1):5. doi: 10.1186/1532-429x-10-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res Treat. Sep 2009;117(2):357–64. doi: 10.1007/s10549-008-0260-6 [DOI] [PubMed] [Google Scholar]
- 141.Barthur A, Brezden-Masley C, Connelly KA, et al. Longitudinal assessment of right ventricular structure and function by cardiovascular magnetic resonance in breast cancer patients treated with trastuzumab: a prospective observational study. J Cardiovasc Magn Reson. Apr 10 2017;19(1):44. doi: 10.1186/s12968-017-0356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Naresh NK, Misener S, Zhang Z, et al. Cardiac MRI Myocardial Functional and Tissue Characterization Detects Early Cardiac Dysfunction in a Mouse Model of Chemotherapy-Induced Cardiotoxicity. NMR Biomed. Sep 2020;33(9):e4327. doi: 10.1002/nbm.4327 [DOI] [PubMed] [Google Scholar]
- 143.Cottin Y, Ribuot C, Maupoil V, et al. Early incidence of adriamycin treatment on cardiac parameters in the rat. Can J Physiol Pharmacol. Feb 1994;72(2):140–5. doi: 10.1139/y94-022 [DOI] [PubMed] [Google Scholar]
- 144.Lightfoot JC, D’Agostino RB Jr., Hamilton CA, et al. Novel approach to early detection of doxorubicin cardiotoxicity by gadolinium-enhanced cardiovascular magnetic resonance imaging in an experimental model. Circ Cardiovasc Imaging. Sep 2010;3(5):550–8. doi: 10.1161/circimaging.109.918540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Thavendiranathan P, Walls M, Giri S, et al. Improved detection of myocardial involvement in acute inflammatory cardiomyopathies using T2 mapping. Circ Cardiovasc Imaging. Jan 2012;5(1):102–10. doi: 10.1161/circimaging.111.967836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Altaha MA, Nolan M, Marwick TH, et al. Can Quantitative CMR Tissue Characterization Adequately Identify Cardiotoxicity During Chemotherapy?: Impact of Temporal and Observer Variability. JACC Cardiovasc Imaging. Apr 2020;13(4):951–962. doi: 10.1016/j.jcmg.2019.10.016 [DOI] [PubMed] [Google Scholar]
- 147.Foley PW, Hamilton MS, Leyva F. Myocardial scarring following chemotherapy for multiple myeloma detected using late gadolinium hyperenhancement cardiovascular magnetic resonance. J Cardiovasc Med (Hagerstown). May 2010;11(5):386–8. doi: 10.2459/JCM.0b013e32832f3ff2 [DOI] [PubMed] [Google Scholar]
- 148.Jouni H, Aubry MC, Lacy MQ, et al. Ixazomib cardiotoxicity: A possible class effect of proteasome inhibitors. Am J Hematol. Feb 2017;92(2):220–221. doi: 10.1002/ajh.24608 [DOI] [PubMed] [Google Scholar]
- 149.Diwadkar S, Patel AA, Fradley MG. Bortezomib-Induced Complete Heart Block and Myocardial Scar: The Potential Role of Cardiac Biomarkers in Monitoring Cardiotoxicity. Case Rep Cardiol. 2016;2016:3456287. doi: 10.1155/2016/3456287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Heitner SB, Minnier J, Naher A, et al. Bortezomib-based Chemotherapy for Multiple Myeloma Patients Without Comorbid Cardiovascular Disease Shows No Cardiotoxicity. Clin Lymphoma Myeloma Leuk. Dec 2018;18(12):796–802. doi: 10.1016/j.clml.2018.08.004 [DOI] [PubMed] [Google Scholar]
- 151.Ederhy S, Massard C, Dufaitre G, et al. Frequency and management of troponin I elevation in patients treated with molecular targeted therapies in phase I trials. Invest New Drugs. Apr 2012;30(2):611–5. doi: 10.1007/s10637-010-9546-8 [DOI] [PubMed] [Google Scholar]
- 152.Wu CF, Chuang WP, Li AH, Hsiao CH. Cardiac magnetic resonance imaging in sunitinib malate-related cardiomyopathy: no late gadolinium enhancement. J Chin Med Assoc. Jun 2009;72(6):323–7. doi: 10.1016/s1726-4901(09)70379-x [DOI] [PubMed] [Google Scholar]
- 153.Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular magnetic resonance in myocarditis: A JACC White Paper. J Am Coll Cardiol. Apr 28 2009;53(17):1475–87. doi: 10.1016/j.jacc.2009.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Ferreira VM, Piechnik SK, Dall’Armellina E, et al. T(1) mapping for the diagnosis of acute myocarditis using CMR: comparison to T2-weighted and late gadolinium enhanced imaging. JACC Cardiovasc Imaging. Oct 2013;6(10):1048–1058. doi: 10.1016/j.jcmg.2013.03.008 [DOI] [PubMed] [Google Scholar]
- 155.Thavendiranathan P, Zhang L, Zafar A, et al. Myocardial T1 and T2 Mapping by Magnetic Resonance in Patients With Immune Checkpoint Inhibitor-Associated Myocarditis. J Am Coll Cardiol. Mar 30 2021;77(12):1503–1516. doi: 10.1016/j.jacc.2021.01.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Friedrich MG. Immune checkpoint inhibitor cardiotoxicity: what can we learn from real life data on CMR as a diagnostic tool? Eur Heart J. 2020:1744–1746. vol. 18. [DOI] [PubMed] [Google Scholar]
- 157.Guo CW, Alexander M, Dib Y, et al. A closer look at immune-mediated myocarditis in the era of combined checkpoint blockade and targeted therapies. Eur J Cancer. Jan 2020;124:15–24. doi: 10.1016/j.ejca.2019.09.009 [DOI] [PubMed] [Google Scholar]
- 158.Higgins AY, Arbune A, Soufer A, et al. Left ventricular myocardial strain and tissue characterization by cardiac magnetic resonance imaging in immune checkpoint inhibitor associated cardiotoxicity. PLoS One. 2021;16(2):e0246764. doi: 10.1371/journal.pone.0246764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Zhang L, Awadalla M, Mahmood SS, et al. Cardiovascular magnetic resonance in immune checkpoint inhibitor-associated myocarditis. Eur Heart J. May 7 2020;41(18):1733–1743. doi: 10.1093/eurheartj/ehaa051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Touat M, Maisonobe T, Knauss S, et al. Immune checkpoint inhibitor-related myositis and myocarditis in patients with cancer. Neurology. Sep 4 2018;91(10):e985–e994. doi: 10.1212/wnl.0000000000006124 [DOI] [PubMed] [Google Scholar]
- 161.Doerner J, Bunck AC, Michels G, Maintz D, Baeßler B. Incremental value of cardiovascular magnetic resonance feature tracking derived atrial and ventricular strain parameters in a comprehensive approach for the diagnosis of acute myocarditis. Eur J Radiol. Jul 2018;104:120–128. doi: 10.1016/j.ejrad.2018.05.012 [DOI] [PubMed] [Google Scholar]
- 162.Messroghli DR, Moon JC, Ferreira VM, et al. Clinical recommendations for cardiovascular magnetic resonance mapping of T1, T2, T2* and extracellular volume: A consensus statement by the Society for Cardiovascular Magnetic Resonance (SCMR) endorsed by the European Association for Cardiovascular Imaging (EACVI). J Cardiovasc Magn Reson. Oct 9 2017;19(1):75. doi: 10.1186/s12968-017-0389-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Spieker M, Haberkorn S, Gastl M, et al. Abnormal T2 mapping cardiovascular magnetic resonance correlates with adverse clinical outcome in patients with suspected acute myocarditis. J Cardiovasc Magn Reson. Mar 29 2017;19(1):38. doi: 10.1186/s12968-017-0350-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gräni C, Bière L, Eichhorn C, et al. Incremental value of extracellular volume assessment by cardiovascular magnetic resonance imaging in risk stratifying patients with suspected myocarditis. The International Journal of Cardiovascular Imaging. 2019;35(6):1067–1078. doi: 10.1007/s10554-019-01552-6 [DOI] [PubMed] [Google Scholar]
- 165.Gräni C, Eichhorn C, Bière L, et al. Prognostic Value of Cardiac Magnetic Resonance Tissue Characterization in Risk Stratifying Patients With Suspected Myocarditis. Journal of the American College of Cardiology. 2017;70(16):1964–1976. doi: 10.1016/j.jacc.2017.08.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Heggemann F, Grotz H, Welzel G, et al. Cardiac Function After Multimodal Breast Cancer Therapy Assessed With Functional Magnetic Resonance Imaging and Echocardiography Imaging. Int J Radiat Oncol Biol Phys. Nov 15 2015;93(4):836–44. doi: 10.1016/j.ijrobp.2015.07.2287 [DOI] [PubMed] [Google Scholar]
- 167.Ylänen K, Eerola A, Vettenranta K, Poutanen T. Three-dimensional echocardiography and cardiac magnetic resonance imaging in the screening of long-term survivors of childhood cancer after cardiotoxic therapy. Am J Cardiol. Jun 1 2014;113(11):1886–92. doi: 10.1016/j.amjcard.2014.03.019 [DOI] [PubMed] [Google Scholar]
- 168.Bergom C, Rubenstein J, Wilson JF, et al. A Pilot Study of Cardiac MRI in Breast Cancer Survivors After Cardiotoxic Chemotherapy and Three-Dimensional Conformal Radiotherapy. Front Oncol. 2020;10:506739. doi: 10.3389/fonc.2020.506739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ibrahim EH, Baruah D, Croisille P, et al. Cardiac Magnetic Resonance for Early Detection of Radiation Therapy-Induced Cardiotoxicity in a Small Animal Model. JACC CardioOncol. Mar 2021;3(1):113–130. doi: 10.1016/j.jaccao.2020.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Huang YJ, Harrison A, Sarkar V, et al. Detection of late radiation damage on left atrial fibrosis using cardiac late gadolinium enhancement magnetic resonance imaging. Adv Radiat Oncol. Apr-Jun 2016;1(2):106–114. doi: 10.1016/j.adro.2016.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ricco A, Slade A, Canada JM, et al. Cardiac MRI utilizing late gadolinium enhancement (LGE) and T1 mapping in the detection of radiation induced heart disease. Cardiooncology. 2020;6:6. doi: 10.1186/s40959-020-00061-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Takagi H, Ota H, Umezawa R, et al. Left Ventricular T1 Mapping during Chemotherapy-Radiation Therapy: Serial Assessment of Participants with Esophageal Cancer. Radiology. Nov 2018;289(2):347–354. doi: 10.1148/radiol.2018172076 [DOI] [PubMed] [Google Scholar]
- 173.Umezawa R, Ota H, Takanami K, et al. MRI findings of radiation-induced myocardial damage in patients with oesophageal cancer. Clin Radiol. Dec 2014;69(12):1273–9. doi: 10.1016/j.crad.2014.08.010 [DOI] [PubMed] [Google Scholar]
- 174.Tuohinen S, Skytta T, Virtanen V, et al. 30Radiotherapy-induced changes in breast cancer patients in extra cellular volume and T1 mapping in cardiac magnetic resonance imaging and in ECG six years after radiotherapy treatment. European Heart Journal - Cardiovascular Imaging. 2019;20(Supplement_2)doi: 10.1093/ehjci/jez111.008 [DOI] [Google Scholar]
- 175.Lee SL, Mahler P, Olson S, et al. Reduction of cardiac dose using respiratory-gated MR-linac plans for gastro-esophageal junction cancer. Med Dosim. Summer 2021;46(2):152–156. doi: 10.1016/j.meddos.2020.10.002 [DOI] [PubMed] [Google Scholar]
- 176.Ipsen S, Blanck O, Oborn B, et al. Radiotherapy beyond cancer: target localization in real-time MRI and treatment planning for cardiac radiosurgery. Med Phys. Dec 2014;41(12):120702. doi: 10.1118/1.4901414 [DOI] [PubMed] [Google Scholar]
- 177.Danad I, Szymonifka J, Twisk JWR, et al. Diagnostic performance of cardiac imaging methods to diagnose ischaemia-causing coronary artery disease when directly compared with fractional flow reserve as a reference standard: a meta-analysis. Eur Heart J. Apr 1 2017;38(13):991–998. doi: 10.1093/eurheartj/ehw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kirkham AA, Virani SA, Campbell KL. The utility of cardiac stress testing for detection of cardiovascular disease in breast cancer survivors: a systematic review. Int J Womens Health. 2015;7:127–40. doi: 10.2147/ijwh.s68745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Di Lisi D, Madonna R, Zito C, et al. Anticancer therapy-induced vascular toxicity: VEGF inhibition and beyond. Int J Cardiol. Jan 15 2017;227:11–17. doi: 10.1016/j.ijcard.2016.11.174 [DOI] [PubMed] [Google Scholar]
- 180.Grover S, Lou PW, Bradbrook C, et al. Early and late changes in markers of aortic stiffness with breast cancer therapy. Intern Med J. Feb 2015;45(2):140–7. doi: 10.1111/imj.12645 [DOI] [PubMed] [Google Scholar]
- 181.Sierra-Galan LM, François CJ. Clinical Applications of MRA 4D-Flow. Curr Treat Options Cardiovasc Med. Sep 10 2019;21(10):58. doi: 10.1007/s11936-019-0758-8 [DOI] [PubMed] [Google Scholar]
- 182.Unterberg-Buchwald C, Ritter CO, Reupke V, et al. Targeted endomyocardial biopsy guided by real-time cardiovascular magnetic resonance. J Cardiovasc Magn Reson. Apr 19 2017;19(1):45. doi: 10.1186/s12968-017-0357-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Rathi VK, Czajka AT, Thompson DV, et al. Can cardiovascular MRI be used to more definitively characterize cardiac masses initially identified using echocardiography? Echocardiography. May 2018;35(5):735–742. doi: 10.1111/echo.14017 [DOI] [PubMed] [Google Scholar]
- 184.Lichtenberger JP 3rd, Dulberger AR, Gonzales PE, Bueno J, Carter BW. MR Imaging of Cardiac Masses. Top Magn Reson Imaging. Apr 2018;27(2):103–111. doi: 10.1097/rmr.0000000000000166 [DOI] [PubMed] [Google Scholar]
- 185.Tumma R, Dong W, Wang J, Litt H, Han Y. Evaluation of cardiac masses by CMR-strengths and pitfalls: a tertiary center experience. Int J Cardiovasc Imaging. Jun 2016;32(6):913–20. doi: 10.1007/s10554-016-0845-9 [DOI] [PubMed] [Google Scholar]
- 186.Slonimsky E, Konen O, Di Segni E, Konen E, Goitein O. Cardiac MRI: A Useful Tool for Differentiating Cardiac Thrombi from Tumors. Isr Med Assoc J. Aug 2018;20(8):472–475. [PubMed] [Google Scholar]
- 187.Gupta A, Singh Gulati G, Seth S, Sharma S. Cardiac MRI in restrictive cardiomyopathy. Clin Radiol. Feb 2012;67(2):95–105. doi: 10.1016/j.crad.2011.05.020 [DOI] [PubMed] [Google Scholar]
- 188.Adler Y, Charron P, Imazio M, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: The Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC)Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. Nov 7 2015;36(42):2921–2964. doi: 10.1093/eurheartj/ehv318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Martinez-Naharro A, Treibel TA, Abdel-Gadir A, et al. Magnetic Resonance in Transthyretin Cardiac Amyloidosis. J Am Coll Cardiol. Jul 25 2017;70(4):466–477. doi: 10.1016/j.jacc.2017.05.053 [DOI] [PubMed] [Google Scholar]
- 190.Fontana M, Pica S, Reant P, et al. Prognostic Value of Late Gadolinium Enhancement Cardiovascular Magnetic Resonance in Cardiac Amyloidosis. Circulation. Oct 20 2015;132(16):1570–9. doi: 10.1161/circulationaha.115.016567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Dorbala S, Cuddy S, Falk RH. How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc Imaging. Jun 2020;13(6):1368–1383. doi: 10.1016/j.jcmg.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Fontana M, White SK, Banypersad SM, et al. Comparison of T1 mapping techniques for ECV quantification. Histological validation and reproducibility of ShMOLLI versus multibreath-hold T1 quantification equilibrium contrast CMR. J Cardiovasc Magn Reson. Dec 28 2012;14(1):88. doi: 10.1186/1532-429x-14-88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Wan K, Li W, Sun J, et al. Regional amyloid distribution and impact on mortality in light-chain amyloidosis: a T1 mapping cardiac magnetic resonance study. Amyloid. Mar 2019;26(1):45–51. doi: 10.1080/13506129.2019.1578742 [DOI] [PubMed] [Google Scholar]
- 194.Martinez-Naharro A, Abdel-Gadir A, Treibel TA, et al. CMR-Verified Regression of Cardiac AL Amyloid After Chemotherapy. JACC Cardiovasc Imaging. Jan 2018;11(1):152–154. doi: 10.1016/j.jcmg.2017.02.012 [DOI] [PubMed] [Google Scholar]
- 195.Richards DB, Cookson LM, Berges AC, et al. Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. Sep 17 2015;373(12):1106–14. doi: 10.1056/NEJMoa1504942 [DOI] [PubMed] [Google Scholar]
- 196.Fontana M, Martinez-Naharro A, Chacko L, et al. Reduction in CMR Derived Extracellular Volume With Patisiran Indicates Cardiac Amyloid Regression. JACC Cardiovasc Imaging. Jan 2021;14(1):189–199. doi: 10.1016/j.jcmg.2020.07.043 [DOI] [PubMed] [Google Scholar]
- 197.Kotecha T, Martinez-Naharro A, Treibel TA, et al. Myocardial Edema and Prognosis in Amyloidosis. J Am Coll Cardiol. Jun 26 2018;71(25):2919–2931. doi: 10.1016/j.jacc.2018.03.536 [DOI] [PubMed] [Google Scholar]
- 198.Pan JA, Kerwin MJ, Salerno M. Native T1 Mapping, Extracellular Volume Mapping, and Late Gadolinium Enhancement in Cardiac Amyloidosis: A Meta-Analysis. JACC Cardiovasc Imaging. Jun 2020;13(6):1299–1310. doi: 10.1016/j.jcmg.2020.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jeong D, Gladish G, Chitiboi T, Fradley MG, Gage KL, Schiebler ML. MRI in cardio-oncology: A review of cardiac complications in oncologic care. J Magn Reson Imaging. Nov 2019;50(5):1349–1366. doi: 10.1002/jmri.26895 [DOI] [PubMed] [Google Scholar]
- 200.Leerink JM, Feijen E, van der Pal HJH, et al. Diagnostic tools for early detection of cardiac dysfunction in childhood cancer survivors: Methodological aspects of the Dutch late effects after childhood cancer (LATER) cardiology study. Am Heart J. Jan 2020;219:89–98. doi: 10.1016/j.ahj.2019.10.010 [DOI] [PubMed] [Google Scholar]
- 201.Hong YJ, Kim GM, Han K, et al. Cardiotoxicity evaluation using magnetic resonance imaging in breast Cancer patients (CareBest): study protocol for a prospective trial. BMC Cardiovasc Disord. Jun 3 2020;20(1):264. doi: 10.1186/s12872-020-01497-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Chaix MA, Parmar N, Kinnear C, et al. Machine Learning Identifies Clinical and Genetic Factors Associated With Anthracycline Cardiotoxicity in Pediatric Cancer Survivors. JACC CardioOncol. Dec 2020;2(5):690–706. doi: 10.1016/j.jaccao.2020.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Kobayashi M, Huttin O, Magnusson M, et al. Machine Learning-Derived Echocardiographic Phenotypes Predict Heart Failure Incidence in Asymptomatic Individuals. JACC Cardiovasc Imaging. Sep 8 2021;doi: 10.1016/j.jcmg.2021.07.004 [DOI] [PubMed] [Google Scholar]
- 204.McDonald JP, MacNamara JP, Zaha VG. Challenges in Implementing Optimal Echocardiographic Screening in Cardio-Oncology. Curr Treat Options Cardiovasc Med. Jul 10 2019;21(8):39. doi: 10.1007/s11936-019-0740-5 [DOI] [PubMed] [Google Scholar]


