Summary:
Aerobic exercise is associated with decreased cancer incidence and cancer-associated mortality. However, little is known about the effects of exercise on pancreatic ductal adenocarcinoma (PDA), a disease for which current therapeutic options are limited. Herein, we show that aerobic exercise reduces PDA tumor growth, modulating systemic and intra-tumoral immunity via CD8 T cells. In clinical samples, exercise similarly enhances CD8 T cell infiltration into human PDA tumors. Mechanistically, exercise promotes immune mobilization and accumulation of tumor-infiltrating IL15Rα+ CD8 T cells, which are responsible for the tumor protective effects. Underscoring the translational potential of the IL-15/IL-15Rα axis, IL-15 super-agonist (NIZ985) treatment attenuates tumor growth, prolongs survival, and enhances sensitivity to chemotherapy. Finally, exercise or NIZ985 both sensitize pancreatic tumors to αPD-1, with improved anti-tumor and survival benefits. Collectively, our findings highlight the therapeutic potential of an exercise-oncology axis and identify IL-15 activation as a promising treatment strategy for this deadly disease.
Graphical Abstract
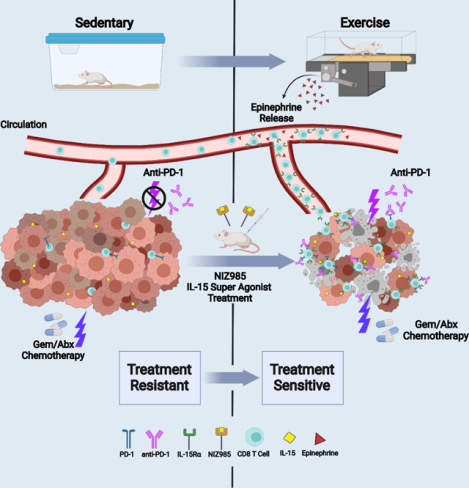
eTOC Blurb:
Kurz. et. al discover that aerobic exercise slows pancreatic cancer growth in mice through activation of the immune system, in particular CD8+ T cells. The beneficial effects of exercise can also be mimicked by treatment with IL-15 super-agonist, NIZ985. Importantly, both exercise and NIZ985 increase the therapeutic sensitivity of intractable murine pancreatic tumors.
INTRODUCTION:
As the 3rd leading cause of cancer-related death in the United States, the public health burden of pancreatic ductal adenocarcinoma (PDA) is vast (Rawla et al., 2019). There are currently no effective means to cure advanced disease. The advent of immune-checkpoint (ICB) therapy has ushered in new options for patients diagnosed with multiple tumor types that until recently were largely untreatable at advanced stages (Larkin et al., 2019). However, PDA remains recalcitrant to these immunotherapies (O’Reilly et al., 2019), likely due to the modest T cell infiltrate and highly immune-suppressive tumor microenvironment (TME) (Balachandran et al., 2019; Bear et al., 2020). Furthering our understanding of the factors that can abrogate immune-suppression in PDA, therefore, remains crucial.
Beyond local mediators in the TME, there is growing appreciation for the contribution of systemic factors in shaping anti-tumor immunity (Hiam-Galvez et al., 2021). For example, tumors promote dysregulation of hematopoiesis, resulting in the systemic expansion and accumulation of immunosuppressive myeloid populations, with contraction of anti-tumor lymphoid populations (Allen et al., 2020; Wu et al., 2014). Specifically, the frequency of bone marrow granulocyte macrophage progenitors increases throughout tumor development, skewing marrow toward immature myeloid cells at the expense of mature antigen-presenting dendritic cells (DCs), which develop from a common progenitor (Wu et al., 2014). In addition, both regulatory-T (T-regs) and -B cells expand peripherally and infiltrate the tumor (Murakami et al., 2019; Wang et al., 2019). While the role of systemic immunity in PDA is still poorly understood, studies report a decrease in circulating DCs in PDA patients and mice, suggesting the involvement of a systemic defect (Lin et al., 2020; Meyer et al., 2018).
These immune perturbations that accompany tumor development can be triggered by multiple mechanisms. Patient fitness, viral infection, diet, and composition of the gut microbiome, have all been shown to influence the organization of the peripheral immune system in cancer, and therefore the anti-tumor immune response (Barnstorf et al., 2019; Gopalakrishnan et al., 2018). Of relevance to our study is evidence supporting a role for aerobic exercise in modulating peripheral immunity and promoting immune cell mobilization (Idorn and Hojman, 2016; Kruger et al., 2008). For example, an exercise-induced increase in circulating NK (Natural Killer)-cells promotes anti-tumor immunity in mouse models of liver cancer and melanoma (Pedersen et al., 2016). Alternatively, modulation of serum metabolites during exercise can reprogram CD8 T cells to attack murine breast tumors (Rundqvist et al., 2020). Others have shown that exercise increases intra-tumoral CD8 T cell infiltration in murine breast cancer via CXCR3 signaling and improves responses to immunotherapy (Gomes-Santos et al., 2021; Wennerberg et al., 2020)
In this work, we find aerobic exercise enhances anti-tumor immunity in both murine and human PDA. We document a dependence on IL-15 signaling for the anti-tumor and immune activating effects of exercise. Furthermore, we demonstrate that pharmacological activation of IL-15 promotes survival benefits and increases sensitivity of pancreatic tumors to immunotherapy and chemotherapy. These findings identify an exercise/immune modality that could be leveraged clinically for the treatment of pancreatic cancer.
RESULTS
Aerobic exercise restricts pancreatic tumor growth
To assess the impact of aerobic exercise on pancreatic tumor growth, we employed a low intensity treadmill-running exercise regimen [5x/week; 30 mins; 15 cm/sec] [(Ashcraft et al., 2016; Wennerberg et al., 2020) (Figure 1a) in multiple murine models of PDA. In a slow-progressing autochthonous genetic model [p48Cre/WT;LSL-KRasG12D/WT (KC)], exercise led to a pronounced delay in oncogenesis and reduction in desmoplasia (Figure 1b, Supp. Figure 1a). To determine whether exercise modulates the growth of aggressive or established PDA tumors, we orthotopically implanted mutant p53R172H/WT;KRASG12D/WT;pdx-1Cre/+ (KPC) cells into the pancreata of wild type C57BL/6 mice and assessed tumor weight 3–4 weeks post-operation (post-op) (Das et al., 2020). In this model, exercise resulted in a 20–30% reduction in tumor weight when initiated on post-op Day 1 using multiple KPC cell lines (Figure 1c, Supp. Figure 1b), and when initiated on post-op Day 12 in an established tumor model (Figure 1d). Environmental stressors can impact tumor growth dynamics and anti-tumor immunity (Liu et al., 2021). We therefore placed mice on the treadmill for the same duration and frequency as exercised mice, but at a speed of 0 cm/second (“treadmill sedentary”) and found that treadmill exposure had no independent effect on tumor weight (Supp. Figure 1c). Furthermore, as weight loss and sarcopenia can impact tumor growth, we assessed body weights of control and exercised mice and found no significant differences (Supp. Figure 1d). These results indicate that changes to environment or body weight are not likely driving the observed anti-tumor effects of exercise in our model. Overall, these findings suggest that exercise provides tumor protective benefits in the setting of PDA tumor initiation and progression.
Figure 1: Exercise restricts pancreatic cancer growth and reprograms tumor immunity.
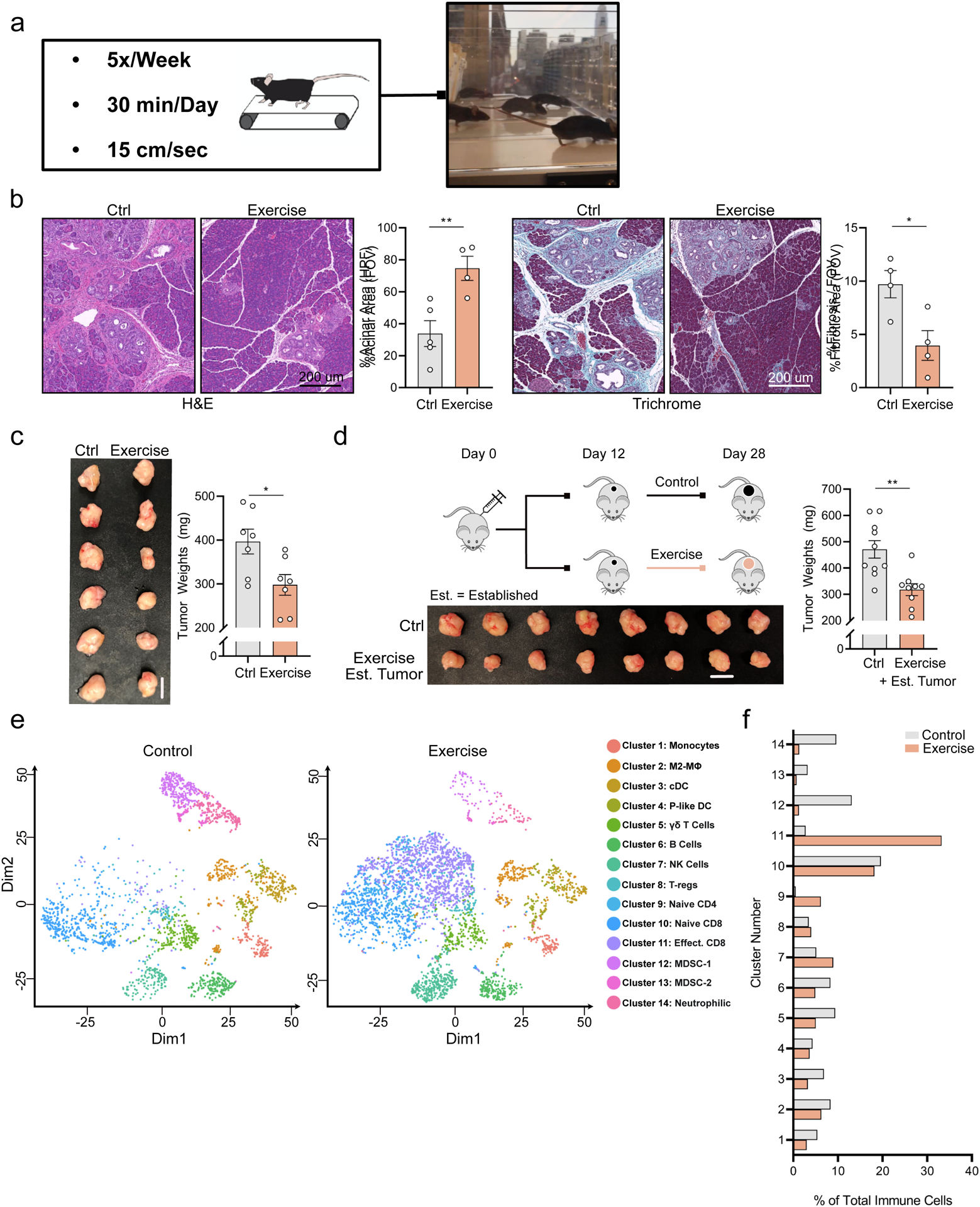
a. The forced treadmill running model (exercise) is shown. b. 8-week old KC mice were exercised for 6 weeks or remained sedentary. Pancreatic tissue sections stained with Hematoxylin and Eosin (left) and Trichrome and Gomori (right) at 14 weeks of age are shown, with quantification of % acinar and %fibrotic area/field of view (FOV). Each dot = one mouse; n = 4–5. c. 8-week old female C57BL/6J wild type (WT) mice [hereafter “WT mice”] were injected orthotopically with 1×10^5 KPC 4662 cells into the pancreas at Day 0 [hereafter “orthotopically injected”] and exercise was started at Day 1. Day 21 tumor weights and images are shown. Each dot = one tumor (n = 7). Experiment was repeated ten times. d. WT mice were injected orthotopically with 5×10^4 KPC cells and began exercise at Day 12. Schema, tumor images, and weights are shown. Each dot = one tumor (n = 9–10). Experiment was repeated three times. (e-f) WT mice were injected orthotopically and exercise was started at Day 1. e. Single cell RNASeq (sc-RNAseq) was performed on all live leukocytes (PI-, CD45+) from Day 21 tumors. tSNE plots show distribution of clusters, identified by distinct colors (n = 3 tumors pooled / group). f. Relative fraction of each cluster in control and exercise. (p < 0.05 = *, p < 0.01 = **). See Supp. 1
Exercise modulates the intra-tumoral immune milieu
We next sought to uncover the mechanisms that govern exercise-induced tumor protection in PDA. Exercise-induced reductions in tumor growth have been shown to be immune-dependent (Ashcraft et al., 2016; Estruel-Amades et al., 2020; Gomes-Santos et al., 2021; Pedersen et al., 2016; Rundqvist et al., 2020; Wennerberg et al., 2020). We therefore performed unbiased analysis of the immune milieu using 10x single cell RNA sequencing (sc-RNAseq) of control and exercise tumors. tSNE algorithms were used to determine the distribution of cellular clusters and their identities (Figure 1e, Supp. Figure 1e). We observed a significant expansion of lymphocyte clusters, particularly CD8 T cells, as well as a contraction of myeloid derived suppressor cells (MDSCs) and neutrophilic-cell clusters in exercise tumors compared to controls (Figure 1f).
Exercise diminishes immune-suppressive myeloid cells in PDA
We first focused on MDSCs, a dominant immune cell type in human pancreatic tumors that promote immune evasion and therapeutic resistance (Thyagarajan et al., 2019). We performed re-clustering on myeloid cells only (Supp. Figure 1f&g) and observed three distinct cell ‘states’ in the tumor (Figure 2a, Supp. Figure 1h). Using RNA velocity, we also predicted the future ‘state’ of each myeloid cell based on its transcriptome (La Manno et al., 2018). These analyses uncovered a population of cells transitioning from State 3 toward State 1 in control PDA tumors, that was largely absent from exercise (Figure 2a, vector arrows). To determine cell properties, we assessed the expression of characteristic markers of monocytic, granulocytic, or antigen presenting lineages (Yanez et al., 2017) (Supp. Figure 2a–e), and quantified their expression across states (Supp. Figure 2f). We found the contraction of myeloid cells in exercise tumors was driven largely by reductions in the MDSC lineage (State 1) or cells transitioning toward the MDSC state (from State 3) (Figure 2b, Supp. Figure 2g).
Figure 2: Exercise rewires MDSCs and effector lymphocytes.
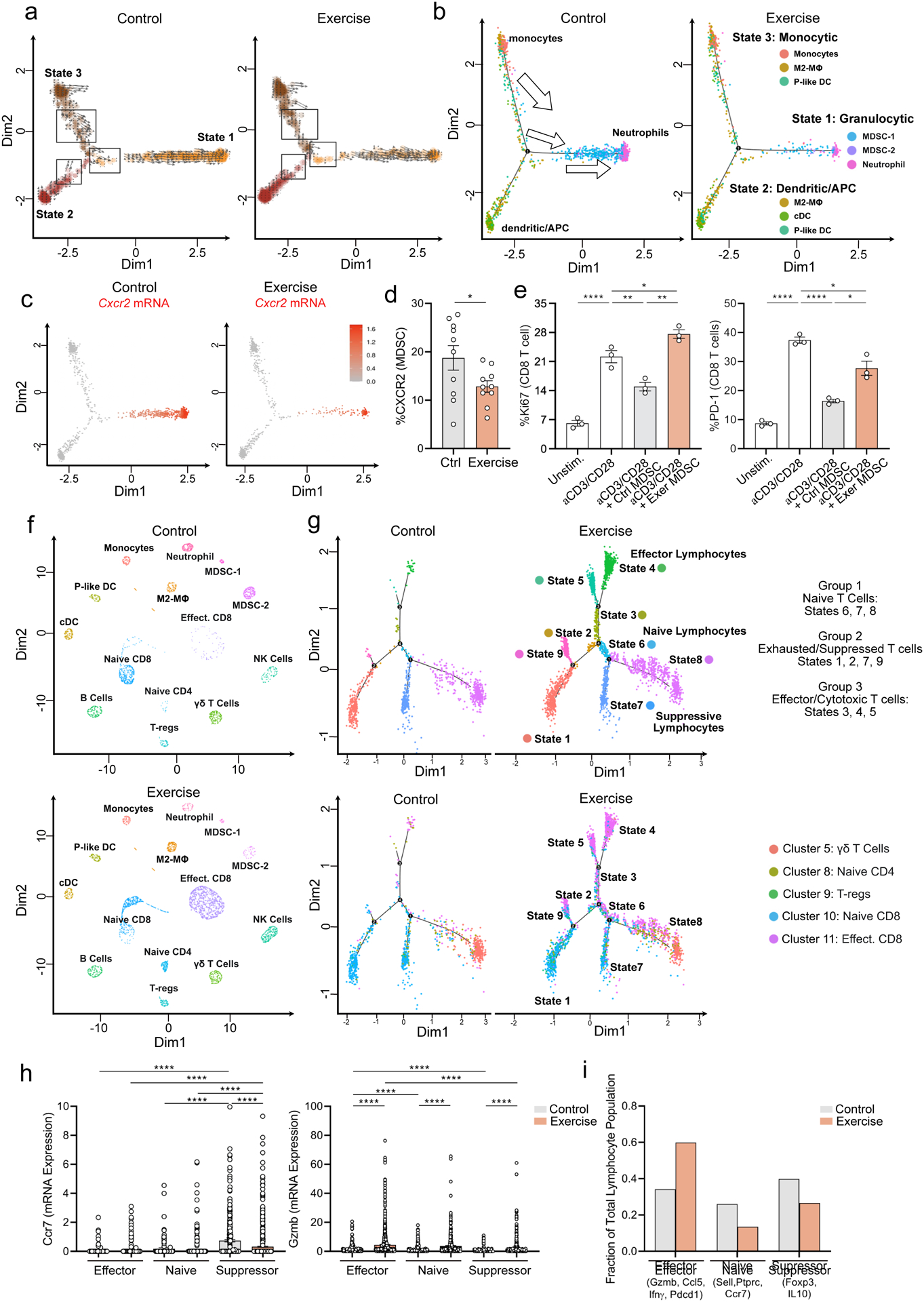
WT mice were injected orthotopically and exercise was started at Day 1. (a-c) Live leukocytes (PI−, CD45+) from Day 21 tumors were analyzed by scRNAseq a. RNA Velocity analysis on all myeloid cells with cell states shown on uMAP plots. Vector arrows represent predicted direction of future transcriptome b. Overlay of phenotypic clusters on the 3 states of myeloid cells. Arrows indicate directionality of transcriptomic predictions. c. Cxcr2 mRNA expression uMAP plots for control and exercise tumors. d. Expression of CXCR2 on Cd11b+ Gr1+ cells by flow cytometry (FC) from Day 21 control and exercise tumors (n =9–10 mice). Experiment was repeated three times e. T cell inhibition assay with MDSC from control or exercise tumors showing Ki67 and PD-1 levels at 72 hours by FC. Each dot = one mouse; n=3. Experiment was repeated twice. f-i. scRNAseq: KNetL plots show distribution of clusters in control and exercise (f). Trajectory analysis shows T cell states (top) with overlay of KNetL clusters (bottom) (g). mRNA expression of Ccr7 and Gzmb across T cell states in control and exercise, each dot = one cell (h). Fraction of total lymphocytes assigned to effector, naïve, and suppressive states (i). (p < 0.05 = *, p < 0.01 = **, p < .0001****). See Supp. 2–3
To better understand how exercise could drive the contraction of MDSCs, we probed expression levels of receptors on myeloid cells known to drive MDSC formation (Han et al., 2019). Of these, exercise reduced mRNA levels of Cxcr2 (and Csf3r) on myeloid cells (Figure 2c, Supp. Figure 2b–e), and lowered CXCR2 expression on MDSCs in vivo (Figure 2d). We therefore assessed the functional properties of MDSCs, through their capacity for immune-suppression, and found MDSCs isolated from exercise tumors were significantly less inhibitory to T cell activation ex vivo (Figure 2e, Supp. Figure 2h). These data indicate that exercise remodels the myeloid landscape in PDA to reduce MDSCs and, in part, to reverse their T-cell suppressing function.
Exercise rewires T lymphocytes toward effector and cytotoxic phenotypes
We next assessed the effects of exercise on intra-tumoral lymphocytes, employing K-nearest-neighbor-based Network Layout (KNetL) analysis to display clearer separation of T cell populations (Kim et al., 2021) (Figure 2f). After re-clustering on T cells only (Supp. Figure 3a), 3 distinct phenotypic groups were identified based on gene signatures: naïve, exhausted/suppressed, and effector/cytotoxic (Figure 2g, Supp. Figure 3b). Of note, the dramatic increase in T cell proportion in exercise tumors was driven almost entirely by cells with an effector CD8 T cell phenotype (Figure 2h&i).
Using immune-fluorescence, we corroborated an increase in the raw number of tumor-infiltrating CD8 T cells in exercise tumors (Figure 3a). No similar increase in CD8 T cell number was observed in the tumor draining lymph node or spleen (Supp. Figure 3c&d), suggesting a tumor-site-specific phenomenon. In CD8 T cells from exercise tumors, KEGG analysis revealed significant enrichment for an ‘effector CD8’ phenotype (Figure 3b). We also observed increased markers of activation and effector function, with enhanced cytotoxic canonical signaling at the transcriptomic level (Figure 3c&d). We, therefore, confirmed an enhanced cytotoxic and activated phenotype of CD8 T cells in vivo in exercise tumors using flow cytometry (FC) (Figure 3e, Supp. Figure 4a). Together, these data suggest that exercise rewires the phenotype of tumor-infiltrating lymphocytes in PDA and promotes activation of CD8 T cells.
Figure 3: Exercise-induced tumor protection requires CD8 T cells.
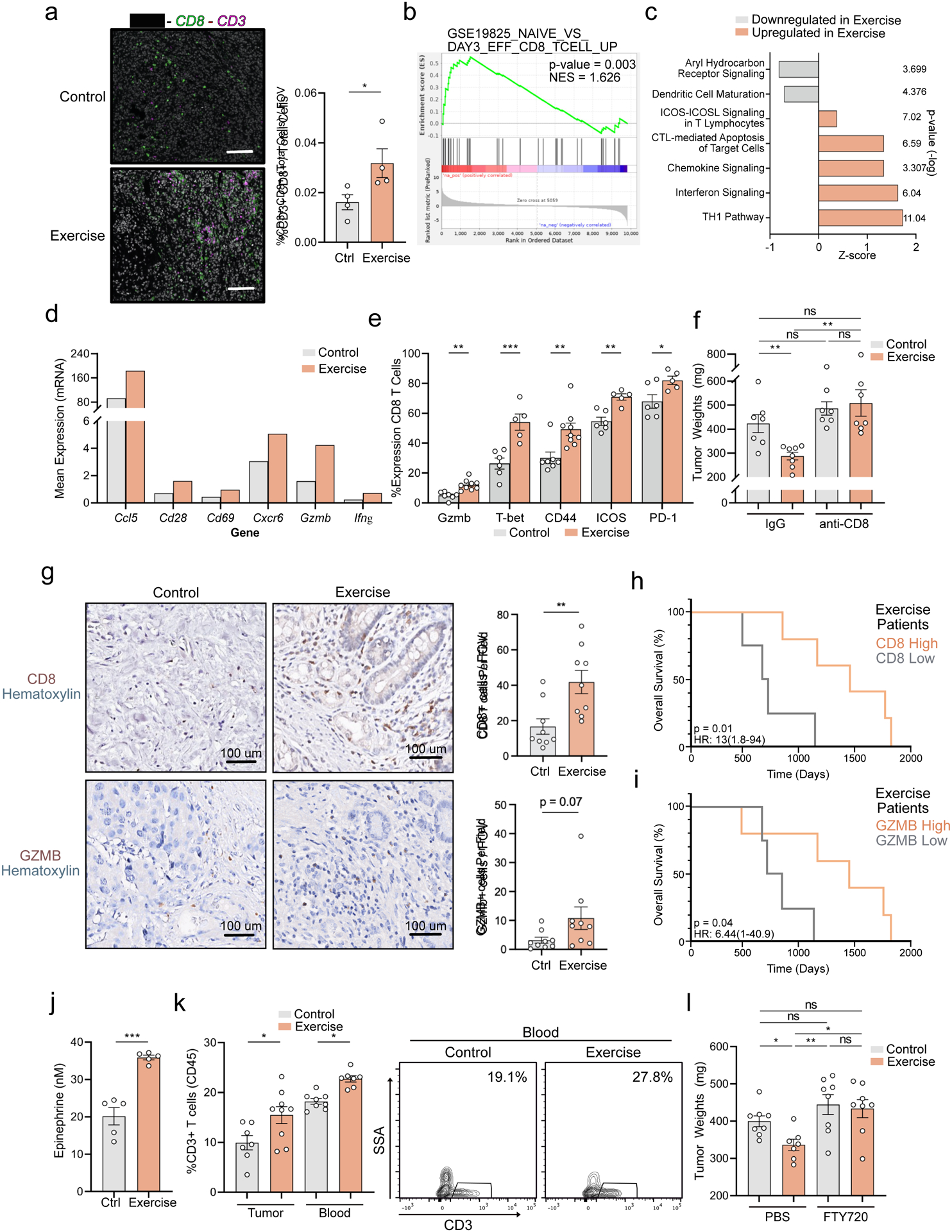
(a-f) WT mice were injected orthotopically and exercise was started at Day 1. a. Day 21 FFPE tumor sections were stained by IF for CD8, CD3, and DAPI. The % of CD8+ CD3+ cells was quantified. Each dot = one mouse; n=4. (b-d) scRNAseq of Day 21 tumors: b. KEGG analysis of Cluster 11 CD8 T cells. c. Canonical pathway perturbations in IPA (Ingenuity Pathway Analysis) in exercise CD8 T cells. d. Relative mRNA expression of select markers on CD8 T cells, all FDR p-adjusted values < 0.05. e. FC for GZMB, T-bet, CD44, ICOS, and PD-1 as a fraction of CD8. Each dot = one mouse (n = 5–9). Experiment was repeated ten times. f. Control and exercise mice were treated with IgG or anti-CD8. Day 21 tumor weights are shown, each dot = one mouse (n=7–8). Experiment was repeated three times. g. IHC for GZMB+ and CD8+ cells on FFPE tumor sections from exercised pancreatic cancer patients or historical controls; positive cells/FOV quantified. Each dot = one patient (n = 9). h-i. Kaplan-Meier survival curve of exercise patients stratified by CD8 high [top 50%] (n =5), and CD8 low [bottom 50%] (n = 4) (h), or GZMB high (n =5), GZMB low (n = 4) (i), based on levels in (g). j. ELISA for epi on sera isolated 20 minutes after completion of exercise from WT mice. Each dot = one mouse (n = 5), experiment was repeated twice. (k-l) WT mice were injected orthotopically and exercise was started at Day 1. k. At Day 21, CD3+ T cells in tumor and blood were assessed by FC 20 minutes post-exercise. Each dot = one mouse (n= 7–9). Representative contour plots are shown. Experiment was repeated three times. (l) Mice were treated with PBS or FTY720. Day 21 tumor weights are shown, each dot = one tumor (n =7–8). Experiment was repeated three times. (p > 0.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***). See Supp. 3–6
CD8 T cells are required for exercise-induced reductions in PDA tumor growth
Based on our observations, we hypothesized that exercise-induced changes to MDSCs may be responsible for reductions in tumor growth. To test this, we orthotopically co-implanted MDSCs isolated from control and exercise tumors with KPC cells and growth was assessed (Marigo et al., 2010). No significant differences in tumor weight were observed, suggesting that exercise-induced reductions in the immune-suppressive properties of MDSCs, alone, may not be sufficient (Supp. Figure 4b). Furthermore, we observed that the tumor protective effects of exercise were lost in both Rag1KO and athymic nude mice (Supp. Figure 4c&d), two immune-deficient models that lack mature lymphocyte populations, but maintain MDSCs. Overall, these data suggest that MDSC rewiring or contraction are not sufficient to mediate exercise-induced anti-tumor benefits in PDA.
We next sought to investigate whether the expansion of effector CD8 T cells was required for exercise-mediated tumor protection. We depleted CD8 T cells in control and exercise mice (Supp. Figure 4e) and found exercise was unable to reduce tumor weight in the absence of CD8 T cells (Figure 3f, Supp. Figure 4f). Furthermore, we observed that apoptotic tumor cells were increased in exercise tumors compared to controls (Supp. Figure 4g), consistent with cytotoxic immune-mediated killing of target cancer cells (Martinez-Lostao et al., 2015). This increase in tumor cell apoptosis was reversed in the context of CD8-T cell depletion, corroborating a CD8-dependent tumoricidal effect (Supp. Figure 4g). Collectively, these data indicate that the protective effects of exercise in PDA are dependent on CD8 T cells.
Exercise Intervention Increases Intra-tumoral CD8 T cells in Human PDA Patients
Interventions to improve the composition of immune infiltrates in human PDA tumors are desperately needed (Foucher et al., 2018). Given our data, we asked whether an exercise intervention could modulate anti-tumor immunity in human disease. We analyzed tumor specimens from PDA patients who underwent exercise prior to surgical resection in a prospective clinical trial. Consistent with our murine data, patients who participated in pre-operative exercise exhibited a significantly higher number of infiltrating CD8 T cells and a trend toward higher expression of GZMB, compared to matched historical controls (Figure 3g). Furthermore, we observed a significant increase in the median overall survival of patients with high levels of intra-tumoral CD8 or GZMB in the exercise cohort (Figure 3h&i), while no detectable difference associated with CD8 or GZMB status was seen in the control cohort (Supp. Figure 5a&b). These results demonstrate that exercise training can induce increased CD8 T cell infiltration into human PDA tumors and potentially enhance their functional capacity.
Exercise-induced increase in intra-tumoral CD8 T cells requires peripheral mobilization
During exercise, an epinephrine-mediated sympathetic spike can mobilize immune cells into circulation in a phenomenon termed Exercise-Induced Leukocytosis (EIL) (Dimitrov et al., 2010; Kruger et al., 2008; Risoy et al., 2003; Sand et al., 2013; Schedlowski et al., 1996). To explore the potential contribution of EIL to our model, we first validated that serum epinephrine (epi) levels were elevated with exercise (Figure 3j). This spike in epi was accompanied by a transient increase in circulating CD3+ T cells (Figure 3k). Consistent with EIL, we found that propranolol, the non-selective β-adrenergic blocker, abrogated exercise-induced tumor protection and intra-tumoral CD8 T cell activation, suggesting a role for adrenergic signaling in exercise-mediated effects in PDA (Supp. Figure 5c&d). In agreement with previous work showing an immune stimulatory effect of β-adrenergic blockade in sedentary conditions (Daher et al., 2019), propranolol treatment of control mice led to enhanced CD8 T cell activation (Supp. Figure 5c). However, similar to earlier reports (Evans et al., 2016; Winograd et al., 2015), this improvement in T-cell mediated immunity alone was not sufficient to confer reduction in pancreatic tumor-growth (Supp. Figure 5d). These data indicate that EIL contributes to the observed anti-tumor effect of exercise, and suggest that adrenergic regulation of immune cell function varies depending on physiological context.
Exercise has also been shown to promote vascular remodeling in murine and human PDA, enhancing chemotherapeutic drug delivery (Florez Bedoya et al., 2019; Schadler et al., 2016). Of note, these prior studies employed subcutaneous/immunodeficient mouse models, potentially limiting the relevance of comparison to orthotopic tumors implanted into WT mice (Zhan et al., 2017). Nevertheless, given that vascular normalization can improve intra-tumoral immune cell infiltration (Mpekris et al., 2020), we sought to analyze changes to the tumoral vascular bed with exercise. No significant differences in vessel length or lumen diameter between conditions were detected, suggesting that the exercise-induced increases in CD8 T cells in our model are mediated by alternative mechanisms (Supp. Figure 5e). We next hypothesized that intact S1P-S1PR gradients (S1PGs), which promote lymphocyte egress from blood and secondary lymphoid organs to sites of tissue injury (Pappu et al., 2007), may contribute to exercise-induced T cell expansion in blood and tumor. To test this, we treated mice with Fingolimod (FTY720), an S1PG inhibitor (Chiba, 2005), and found the exercise-induced expansion of intra-tumoral and peripheral CD8 T cells was lost (Supp. Figure 5f&g, Supp. Figure 6a&b). Moreover, FTY720 abrogated exercise-induced tumor protection, but had no impact on tumor weight in the absence of exercise (Figure 3l, Supp. Figure 6c). These data indicate that S1PG-dependence in PDA is uniquely associated with the immune modulatory effects of exercise.
IL-15/IL-15Rα axis is required for the anti-tumor effects of exercise
To investigate the mechanisms underlying the activation of CD8 T cells in exercised mice, we focused on IL-6, IL-8, and IL-15, myokines that mediate the metabolic and immune response to exercise (Pedersen et al., 2007; Pedersen et al., 2016). Of these, only IL-15 promotes CD8 T cell survival and a cytotoxic/effector phenotype (Berard et al., 2003; Lu et al., 2002; Wu et al., 2020). We identified IL-15 as an upstream regulator of tumor infiltrating CD8 T cells in exercise (Figure 4a), further evidenced by elevated expression of genes downstream of IL-15 engagement in exercise CD8 T cells (Figure 4b). We therefore hypothesized that an influx of IL-15Rα+ T cells, those specifically responsive to IL-15 signaling, may drive exercise-induced anti-tumor immunity. Corroboratively, we found a significant increase in the fraction and number of IL-15Rα+ CD8 T cells in the tumors of exercised mice, in both an initiation and established-tumor model (Figure 4c–e, Supp. Figure 6d). Phenotypic analysis of tumor-infiltrating IL-15Rα+ CD8 T cells showed a significant upregulation of proliferation and activation markers compared to their IL-15Rα-neg counterparts (Figure 4f). To assess the functional significance of the IL-15/IL-15Rα axis, we blocked IL-15 signaling in vivo and observed reversal of exercise-mediated tumor protection, anti-tumor immunity, and influx of IL15Rα+ CD8 T cells (Figure 4g–i, Supp. Figure 6e). In contrast, anti-IL-15 had no impact on the composition of intra-tumoral myeloid populations, suggesting a lymphocyte-specific phenomenon (Supp. Figure 6f). Taken together, these data support a heretofore undescribed role for the IL-15/IL15Rα+ axis in promoting anti-tumor immunity in exercise.
Figure 4: IL-15/IL-15Rα axis is required for exercise-mediated anti-tumor effects.
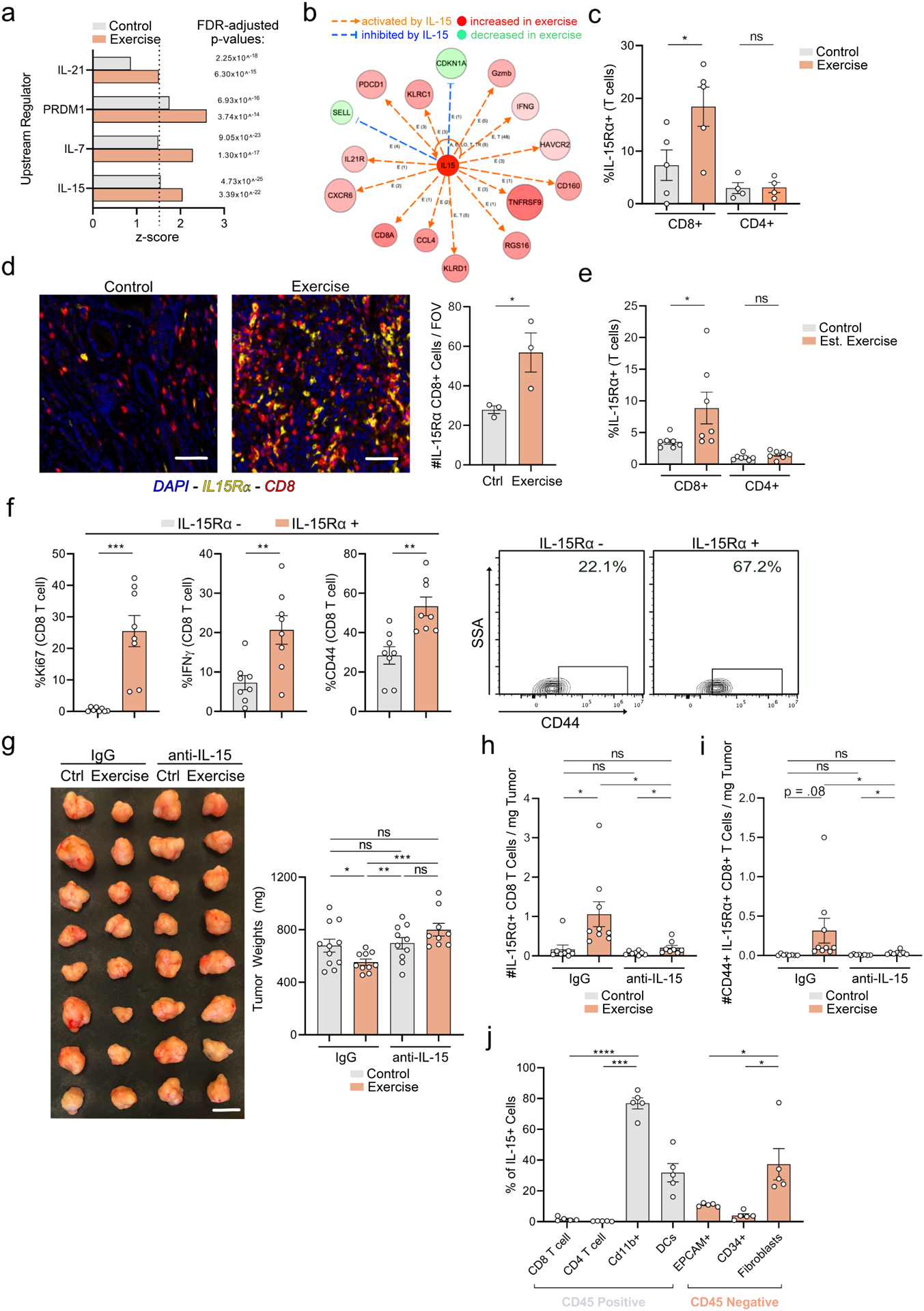
a. Predicted upstream regulators of CD8+ T cells on sc-RNA seq. in IPA. b. Transcript levels of genes downstream of IL-15 in exercise CD8 T cells in IPA. c-f. WT mice were injected orthotopically and exercise was started on Day 1 (c-d) or Day 12 (e). c. Day 21 tumors were analyzed by FC for % of IL-15Rα+ T cells. Each dot = one tumor (n=4–5). d. Day 21 tumors were stained by multiplex IF for CD8, IL-15Rα, and DAPI. The number of CD8+ IL-15Rα+ (orange) cells were quantified. Each dot = one mouse (n=3). e. Day 28 tumors were assessed by FC for % of IL-15Rα+ T cells. Each dot = one tumor (n=7). Experiment was repeated three times. f. Exercise tumors at Day 21 were analyzed by FC for Ki67, IFNγ and CD44 and in IL-15Rα+/− CD8 T cells. Each dot = one tumor (n=8). Representative plots are shown for CD44. (g-i) Orthotopically implanted control and exercise mice were treated with IgG or anti-IL-15. Day 21 tumor weights are shown (g). Tumors were assessed by FC for number of IL-15Rα+ CD8+ T cells (h) and CD44+ IL-15Rα+ CD8+ T cells (i). Each dot = one tumor (n=9–11). Experiment was repeated three times. j. WT mice were injected orthotopically. Day 21 tumors were analyzed by flow for IL-15 levels in indicated populations. Each dot = one mouse (n =5). Experiment was repeated twice. (p > 0.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***, p<0.0001 = ****). See Supp. 6–7.
We next hypothesized that adrenergic tone could affect levels of IL-15 in circulation, leading to the expansion of IL-15Rα+ CD8 T cells in exercise. However, mice that were exercised or treated with a physiological dose of epinephrine exhibited no change in circulating IL-15 levels (Supp. Figure 6g&h). Alternatively, we postulated exercise may increase the circulating pool of IL-15Rα+ CD8 T cells, which migrate to tumor and encounter high levels of IL-15 in the PDA TME. In support of this hypothesis, we detected high levels of IL-15 in various immune and non-immune cell types in murine PDA tumors in vivo (Figure 4j). We also observed that the magnitude of increase in IL-15Rα+ CD8 T cells was greater in the tumors of exercised mice compared to in peripheral blood (~4x vs 1.5x), consistent with intra-tumoral cytokine engagement (Supp. Figure 7a). In support of EIL driving the migration of IL-15Rα+ CD8 T cells, epinephrine treatment mimicked the increase in circulating IL15Rα+ CD8 T cells seen in exercise, an increase which was reversed by FTY720 treatment (Supp. Figure 7b&c). Collectively, these observations support an interplay between systemic and intra-tumoral immunity in the engagement of IL-15/IL-15Rα+ signaling in exercise.
Exercise sensitizes PDA tumors to α-PD-1 treatment
Responses to ICB therapy in pancreatic cancer patients are rare, and the identification of effective combination strategies involving multiple immune-based agents remains a clinical need (Saka et al., 2020). Of note, we observed that IL-15Rα+ CD8 T cells selectively upregulate checkpoint PD-1 in exercise (Supp. Figure 7d). We therefore hypothesized that exercise may modulate the efficacy of α-PD-1 treatment. Combination of α-PD-1 and exercise led to drastic increases in CD3 T cells, and activated/cytotoxic CD8+ T cells (Figure 5a–c). We also observed a synergistic increase in the number of IL-15Rα+ CD8 T cells in exercised mice treated with α-PD-1, suggesting these cells may be particularly responsive to checkpoint blockade (Figure 5d, Supp. Figure 7e). Consistent with previous literature (Winograd et al., 2015), α-PD-1 therapy alone had no effect on tumor size in our model. However, combination of exercise and α-PD-1 led to a more effective reduction in tumor growth compared to either monotherapy (Figure 5e). These data suggest that exercise unlocks the sensitivity of recalcitrant pancreatic tumors to α-PD-1.
Figure 5: Exercises sensitizes pancreatic cancer to α-PD1.
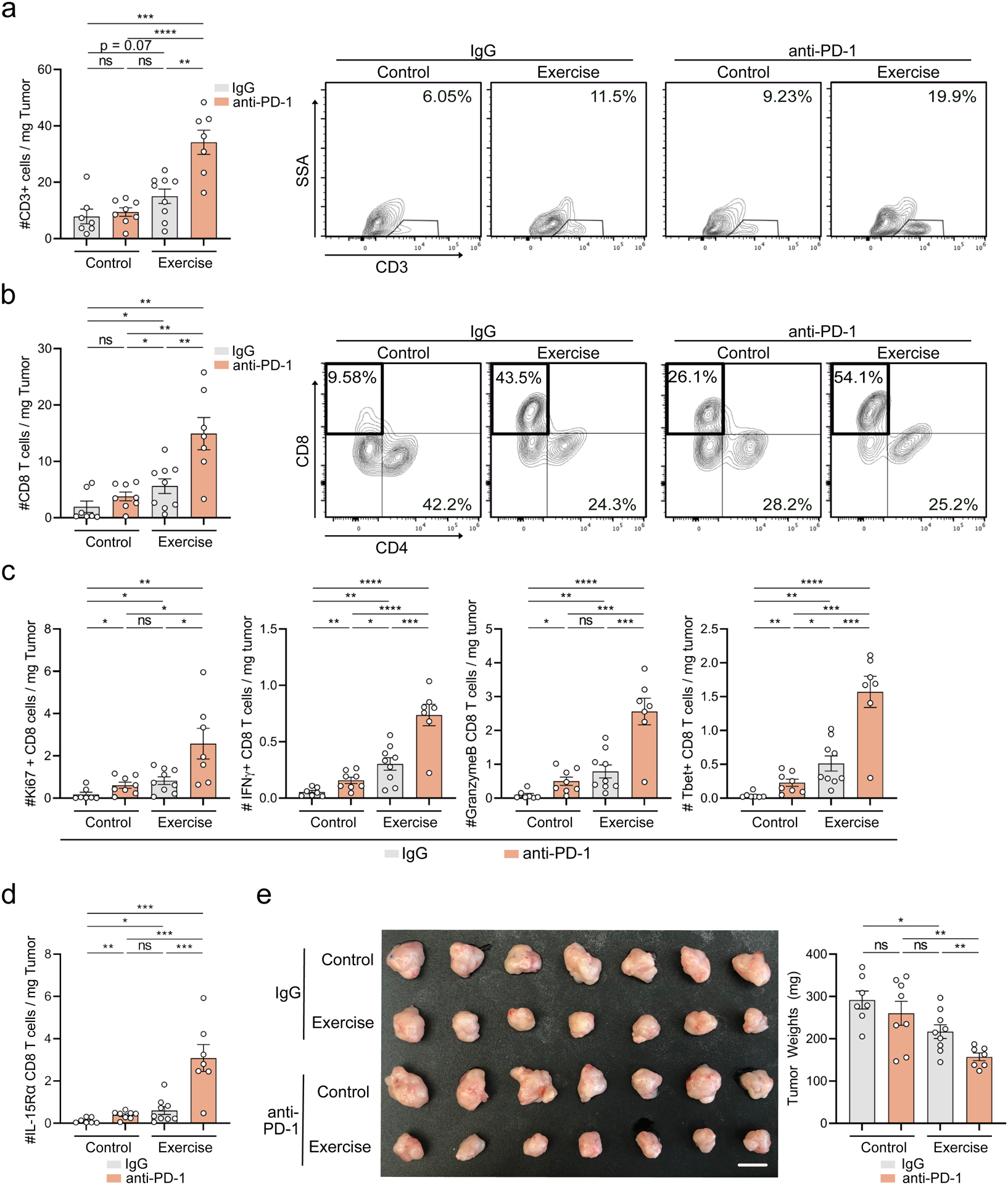
a-e. WT mice were injected orthotopically and exercise was started at Day 1. Mice were treated with IgG or α-PD-1 blocking antibody. Day 21 tumors were assessed by FC for CD3 (a), CD8/CD4 (b), Ki67, IFNγ, GZMB, and T-bet (c) and IL-15Rα (d) on CD8 T cells. Tumor images and weights are shown (e). Each dot = one tumor (n=7–9). Experiment was repeated three times. (p > 0.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***, p < 0.0001 = ****). See Supp. 7
IL-15 S.A. effectively promotes anti-tumor immunity in murine PDA
Given our findings that the tumor-protective properties of exercise are dependent on IL-15 signaling, we sought to characterize the effects of pharmacological activation of IL-15 in PDA. Treatment of mice with IL-15 super-agonist (IL-15 S.A.: heterodimer of IL-15 and IL15Rα, Novartis NIZ985) resulted in a significant reduction in tumor growth (Figure 6a). Akin to exercise, IL-15 S.A. treatment increased T cells, CD8 T cells, and GZMB+ CD8 T cells in the tumor (Figure 6b–d). IL-15 S.A. also upregulated PD-1 and IL-15Rα expression on intra-tumoral CD8 T cells (Figure 6e–f). Consistent with a T-cell specific effect, we observed no changes to myeloid cell composition associated with IL-15 S.A. therapy (Supp. Figure 7f). Furthermore, we found evidence for T cell activation and IL-15Rα+ CD8 T cell enrichment in the blood of treated mice, indicating a systemic response to therapy (Supp. Figure 7g–i). The addition of exercise to IL-15 S.A. treatment conferred no additional tumor protection or T-cell infiltrate, suggesting a mechanistic redundancy between the two approaches (Supp. Figure 7j&k). To assess the therapeutic implications of these findings, we tested the efficacy of IL-15 S.A. treatment in an established tumor model and found that IL-15 S.A. significantly slowed tumor growth (Figure 6g–h). This protection was accompanied by an overall increase in T-cells and activated CD8 T cells in the tumor (Figure 6i). Importantly, we found that IL-15 S.A. prolonged the survival of mice with established tumors by approximately 50% relative to controls (Figure 6j). Taken together, these data demonstrate that activation of the IL-15 axis, via treatment with IL-15 S.A., is sufficient to induce anti-tumor immunity.
Figure 6: IL-15 S.A. monotherapy promotes anti-tumor immunity.
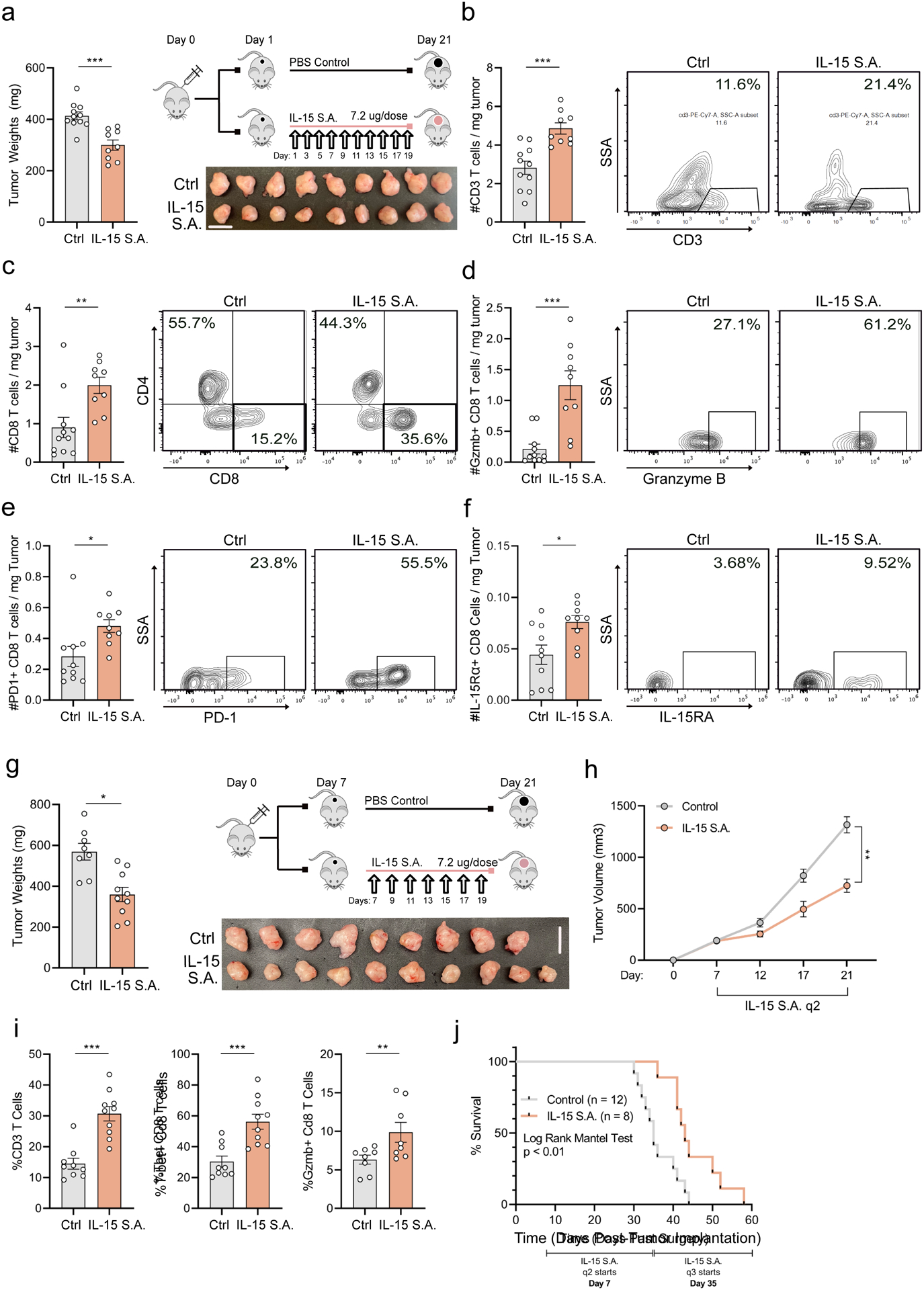
(a-f) WT mice were injected orthotopically and treated with IL-15 S.A., as shown. Day 21 tumor weights and images are shown. (a). Tumors were assessed by FC for CD3 (b), CD4 and CD8 (c), and GZMB (d), PD-1 (e), and IL-15Rα (f) on CD8 T cells. Each dot = one mouse (n = 9–11). Experiment was repeated at least three times. (g-j). WT mice were injected orthotopically and treated with IL-15 S.A., starting Day 7. Day 21 tumor weights and images are shown. Each dot = one mouse (n = 8–10) (g). Tumor volumes were monitored using ultrasound and each curve represents average of treatment group (n = 8–10 mice) (h) i. Tumors were analyzed by FC for CD3 (% of CD45), and %T-bet and GZMB on CD8 T cells. Each dot = one tumor (n=8–10). Experiment was repeated three times. j. Kaplan-Meier survival curve. (p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***). See Supp. 7
Given the improvement in survival in IL-15S.A.-treated mice and increase in IL-15Rα+ CD8 T cells, we sought to determine the relevance of this signaling axis to human disease. Using data from the Cancer Genome Atlas (TCGA), we found that levels of IL15RA uniquely correlate with markers of CD8 T cell activation in human PDAC tumors (Supp. Figure 8a). These data indicate that engagement of the IL-15 signaling axis, and the immune activation that ensues, may be translationally relevant.
IL-15 S.A. Enhances the Efficacy of PD-1 Blockade, Promoting a Durable Response
Because we observed that exercise-induced engagement of IL-15 signaling sensitized PDA tumors to α-PD-1, we next assessed the effects of combining α-PD-1 and IL-15 S.A. in vivo (Figure 7a). Compared to either monotherapy, mice treated with IL-15 S.A. + α-PD-1 showed significantly slower tumor growth (Figure 7b&c), and, importantly, a pronounced prolongation of survival (Figure 7d). Consistent with this enhanced survival, combination of α-PD-1 + IL-15 S.A. promoted a synergistic expansion of overall immune cells, CD3+ T cells, and IFNγ+ CD8 T cells (Figure 7e&f, Supp. Figure 8b) in the tumor. In fact, combination treatment induced a pan-T cell activation, marked by a reduction in terminally exhausted CD8 T cells and an expansion of TCF1+ cells, considered the ‘recoverable’ fraction of exhausted CD8 T cells (Beltra et al., 2020; Chen et al., 2019) (Figure 7g&h). Antigen-experienced CD4 T cells also significantly increased, while T-regs, a population that may portend poor prognosis human PDA (Tang et al., 2014), decreased (Figure 7i&j). In aggregate, these results identify IL-15 S.A. + α-PD-1 combination as an effective strategy for eliciting a durable anti-tumor immune response.
Figure 7: α-PD-1 + IL-15 S.A. slows tumor growth and promotes durable immune responses.
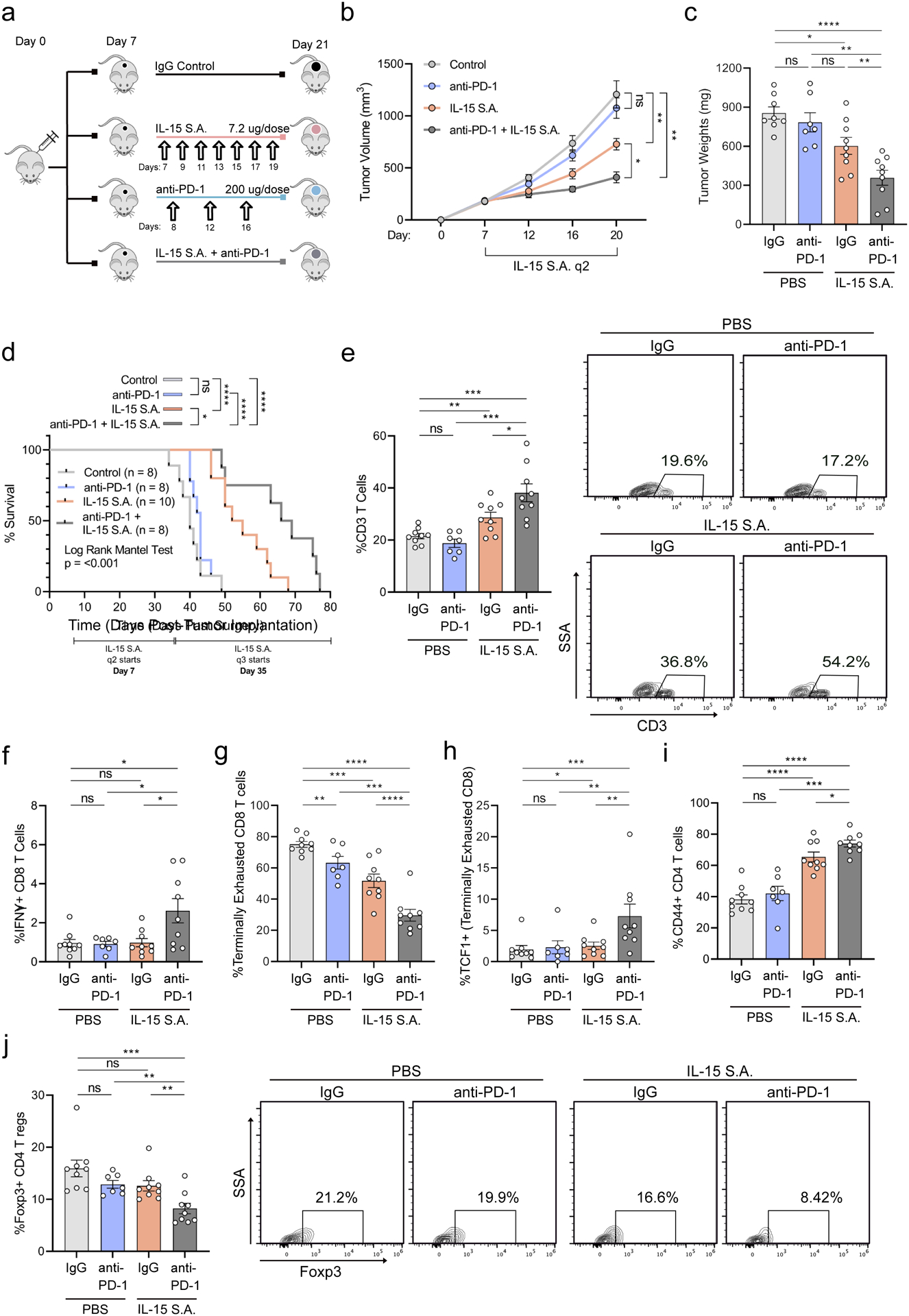
a-j. WT mice were injected orthotopically and mice were treated starting on Day 7, as shown (a). Tumor volumes were monitored using ultrasound. Each curve represents average of treatment group (n = 7–9 mice per group) (b). At Day 21 tumor weights were quantified. Each dot = one mouse (n = 7–9) (c). d. Kaplan-Meier survival curve. e-j. Tumors were analyzed by FC for CD3 as fraction of CD45 (e), IFNγ (f), CD44 and PD-1 (g) as fraction of CD8 T cells, TCF-1 as fraction of CD44+ PD-1+ [terminally exhausted] CD8 T cells (h), and CD44 (i), and FOXP3 as fraction of CD4 T cells (j). Representative plots are shown for CD3 T cells and FOXP3. Each dot = one tumor (n=7–9). Experiment was repeated three times. (p>.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***, p<0.0001 = ****). See Supp.7–8.
Chemotherapeutic Combination with IL-15 S.A. for the Treatment of Murine PDA
Given the failure of previous immunotherapy clinical trials in PDA, future treatment strategies are unlikely to be translated to the clinic in the absence of a chemotherapy backbone (Coveler et al., 2020; O’Neil et al., 2016; O’Reilly et al., 2019; Wainberg et al., 2020). Gemcitabine + Nab-paclitaxel (gem/abx) is used as a standard of care for patients with PDA (Von Hoff et al., 2013). We therefore sought to determine the efficacy of IL-15 S.A. in combination with gem/abx (Figure 8a). Treatment with gem/abx + IL-15 S.A. (G.I.) was more effective in slowing tumor growth compared to either monotherapy (Figure 8b). These results are in stark contrast to the addition of α-PD-1 to gem/abx, which results in no additional benefit for PDA patients (Wainberg et al., 2020). G.I. combination also resulted in significant increases in CD8 and CD4 T cell activation, as well as NK and NK T cell activation (Figure 8c&d, Supp. Figure 8c-e), all effects largely absent from either monotherapy. These results suggest that IL-15 S.A. promotes enhanced tumor protection when combined with gem/abx, conferring distinct immunologic effects. In addition, mice treated with Gem/Abx + α-PD-1 + IL-15 S.A. triple therapy (G.P.I., Figure 8e) exhibited an even further enhancement in tumor reduction, compared to G.I. alone (Figure 8f). Although T cells or NK Cells were not activated further in G.P.I. treated mice (Supp. Figure 8f), we did observe G.P.I. induced the largest fraction of TCF1+ CD8 T cells, compared to all other combinations (Figure 8g). In aggregate, these results demonstrate that IL-15 S.A. +/− α-PD-1 is more effective in combination with chemotherapy, underscoring the potential of this approach to be translated to the clinic.
Figure 8: Chemotherapeutic combination with IL-15 S.A. protects against murine PDA.
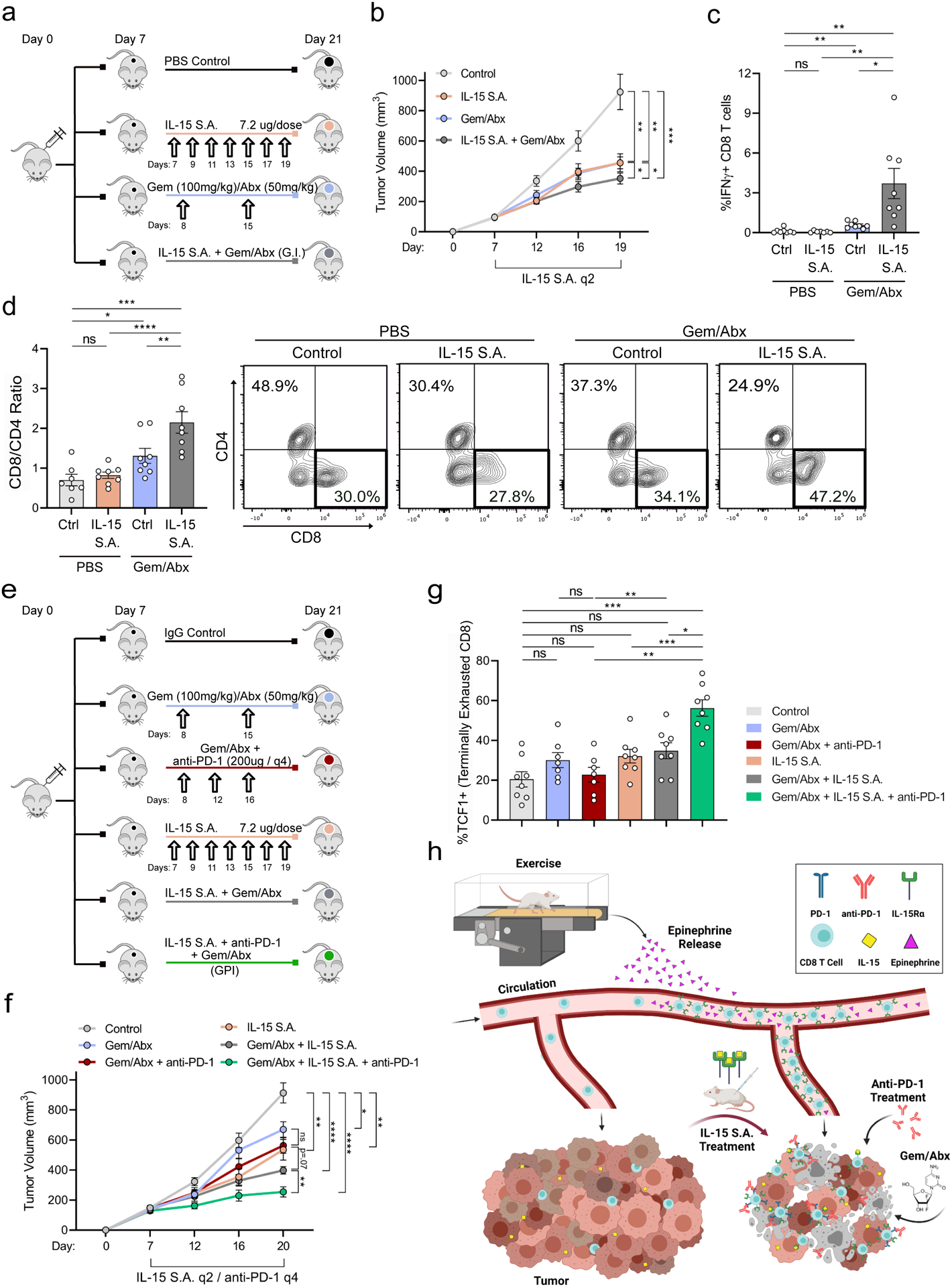
a-d. WT mice were injected orthotopically and were treated starting on Day 7, as shown (a). Tumor volumes were monitored using ultrasound. Each curve represents average of treatment group (n = 8–9 mice) (b). Day 21 tumors were analyzed by FC for CD3, CD8, and IFNγ (c) and CD4 and CD8 (d). Representative plots are shown for CD8/CD4. Each dot = one tumor (n= 8–9). Experiment was repeated three times. (e-g) WT mice were injected orthotopically and were treated starting on Day 7, as shown (e). Tumor volumes were monitored using ultrasound. Each curve represents average of treatment group (n = 7–8 mice) (f). FC of tumors assessing TCF1+ of CD44+PD-1+ CD8 T cells. Each dot = one tumor (n= 7–8) Experiment was repeated twice (g). h. Schematic of proposed mechanism. (p>.05 = ns, p < 0.05 = *, p < 0.01 = **, p < 0.001 = ***, p<0.0001 = ****). See Supp. 8
DISCUSSION:
This study identifies a heretofore undescribed role for exercise in driving an immune-mediated anti-tumor effect in pancreatic cancer through the activation of the IL-15/IL-15Rα axis. The immune modulatory effects of exercise can be harnessed pharmacologically through the utilization of an IL-15 super-agonist (Novartis, NIZ985). NIZ985 treatment prolongs mouse survival and enhances sensitivity to both ICB and standard of care chemotherapy. Collectively, these findings establish a therapeutically exploitable link between activation of the IL-15/IL-15Rα axis and the subversion of immune suppression in PDA (Schematic Figure 8h).
The effects of exercise on local and systemic immunity have thus far been characterized in select murine tumor models including breast, melanoma, liver, and lung (Gomes-Santos et al., 2021; Pedersen et al., 2016; Rundqvist et al., 2020). While in most of these studies, the observed anti-tumor effects of exercise were linked to cytotoxic immune cells, the underlying mechanisms varied significantly depending on tumor type and exercise regimen. For example, IL-6 dependent NK cells drive anti-tumor immunity in voluntary exercise in melanoma, while a similar exercise regimen promotes a T-cell dependent sensitivity to immunotherapy in murine breast cancer (Gomes-Santos et al., 2021; Pedersen et al., 2016). Two studies examining the impact of exercise on breast cancer growth, one an ‘exercise until exhaustion model’ and the other an ‘intensity-specific’ exercise regimen of pre-set duration, reported different mechanisms by which immune cells infiltrate tumors during exercise (Gomes-Santos et al., 2021; Wennerberg et al., 2020). Thus, understanding the tumor type- and regimen- specific characteristics that govern exercise-induced tumor protection is a prerequisite for assessing the therapeutic potential of exercise interventions.
Within this framework, the mild/moderate exercise regimen used on mice in this study was chosen in consideration of the high morbidity and impaired functional status of pancreatic cancer patients. The clinical samples in our study were obtained from a prospective trial assessing the effect of a structured exercise regimen in PDA patients prior to surgical resection (NCT03187951) (Florez Bedoya et al., 2019). Our findings that exercise promotes increased CD8 T cell infiltration into these human PDA tumors suggest a shared mechanism between murine models and human disease. While future work will be required to determine the clinical feasability of rigorous exercise regimens for pancreatic cancer patients on a larger-scale, our results validate the use of experimental models of exercise for the identification of new intervention strategies in a disease with few therapeutic options.
In addition to the reported effects of exercise on lymphocytes, exercise has also been shown to reduce the accumulation of MDSCs in models of breast cancer (Garritson et al., 2020), while we observed a constraining effect on signals that promote MDSC production in PDA. In cancer bearing hosts, MDSC-promoting signals typically encompass tumor-derived growth factors and inflammatory mediators that promote their expansion by disrupting normal myelopoiesis in the bone marrow (BM) (Kumar et al., 2016). Accordingly, the decrease in MDSCs in our exercise model could result from modulation of BM composition. Indeed, exercise can modulate the BM stem cell niche in models of cardiovascular disease, reducing the proliferative capacity of hematopoietic stem/progenitor cells and modulating leukocyte output into the periphery (Frodermann et al., 2019). Future investigations of the potential link between exercise, BM composition, and anti-tumor immunity in PDA are therefore warranted.
The involvement of the IL-15/IL15Rα+ axis in exercise-mounted immune responses is advantageous due to its unique biological properties. IL-15Rα can undergo trans-endosomal recycling in a complex with its ligand (IL-15) allowing for the continual reappearance of the cytokine at the cell surface (Dubois et al., 2002), prolonging activation of IL-15Rα + cells long after the original IL-15 stimulus is removed. These dynamics provide a mechanism by which transient increases in IL-15Rα+ CD8 T cells in exercise could induce a sustained boost to tumor immunity. Clinically, IL-15 super-agonists, including NIZ985, have shown promising safety data in early phase clinical trials for refractory solid tumors, with and without αPD-1 (Bessard et al., 2009; Desbois et al., 2016; Guo et al., 2020; Wrangle et al., 2018), but have not yet formally been evaluated in human PDA. Our findings suggest that trials assessing the efficacy of this approach are warranted; a notion further supported by our observation that NIZ985 efficacy is enhanced in combination with chemotherapy. While the current study focuses on exercise-mediated subversion of immune suppression by IL-15, our findings demonstrate that delineation of the mechanism of exercise-induced changes to the tumor immune milieu can lead to new approaches that improve the responsiveness of pancreatic tumors to immune and non-immune based therapeutics.
STAR Methods:
Resource Availability:
Lead Contact:
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dafna Bar-Sagi (dafna.bar-sagi@nyulangone.org)
Materials Availability:
This study did not generate new unique reagents.
Data and Code Availability:
Single-cell RNA seq data have been deposited at GEO and are publicly available as of date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table for Kurz et. al.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE anti-mouse CD125 (IL-15Rα) (clone 6B4C88) | Biolegend | Cat #: 153503 |
| IL15RA Polyclonal Antibody | Invitrogen | Cat #: PA5 – 79467 |
| In VivoMAb anti-mouse PD-1 (clone RMP1-14) | BioXcell | Cat #: BE0146 |
| In VivoMAb Anti-mouse IL-15 (clone AIO.3) | BioXcell | Cat #: BE0315 |
| In VivoMAb Anti-mouse CD8 alpha (clone 2.43) | BioXcell | Cat #: BE0061 |
| PE/Cyanine7 anti-mouse CD8 alpha (clone 53–6.7) | Biolegend | Cat #: 100721 |
| APC anti-mouse CD279 (PD-1) (clone 29F.A12) | Biolegend | Cat #: 135209 |
| Brilliant Violent 421™ anti-mouse CD3 (clone 17A2) | Biolegend | Cat #: 100227 |
| PerCP/Cyanine5.5 anti-mouse IFN-Y (clone XMG1.2) | Biolegend | Cat #: 505821 |
| Goat Anti-Rabbit IgG H&L (AF488) | Abcam | Cat #: ab150077 |
| Recombinant anti-IL-15 antibody [EPR22635–214] | Abcam | Cat #: ab273631 |
| PE/Dazzle™ 594 anti-T-bet (clone 4B10) | Biolegend | Cat #: 644827 |
| PerCP/Cyanine5.5 anti-human/mouse Granzyme B Recombinant Antibody (clone QA16A02) | Biolegend | Cat #: 372211 |
| PE/Dazzle™ 594 anti-mouse Ki67 (clone 16A8) | Biolegend | Cat #: 652427 |
| Alexa Flour 647 anti-TCF1 (TCF7) (clone 7F11A10) | Biolegend | Cat #: 655203 |
| Mouse anti-Human CD8 Ab-1 (Clone C8/144B) | Thermofisher | Cat #: MS-457-S |
| Mouse anti-Human GranzymeB (clone 11F1) | Leica Biosystems | Cat #: GRAN-B-L-CE |
| Brilliant Violent 421™ anti-mouse/human CD44 (clone IM7) | Biolegend | Cat #: 103039 |
| Bacterial and virus strains | ||
| Biological samples | ||
| Human Pancreatic Cancer Surgical Specimens from Exercised Patients | University of Texas MD Anderson Cancer Center | https://clinicaltrials.gov/ct2/show/NCT03187951 |
| Human Pancreatic Cancer Surgical Specimens from Historical Control Cohort | University of Texas MD Anderson Cancer Center | https://www.nature.com/articles/s41598-019-49582-3 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant human IL-15 Superagonist (IL-15 S.A.), hetero-dimer of human IL-15 and sIL-15Ra made in HEK293 Cells | Novartis, via MTA with NYU Langone | NIZ985 |
| Fingolimod hydrochloride (FTY720) > 98% HPLC | Sigma Aldrich/Millipore | Cat #: SML0700, CAS Number: 162359-56-0 |
| (−)-Epinephrine | Sigma Aldrich | Cat #: E4250, CAS Number: 51-43-4. EC Number: 200-098-7 |
| (±)-Propranolol hydrochloride, > 99% TLC, powder | Sigma Aldrich | Cat #: P0884, CAS Number: 318-98-9. EC Number: 206-268-7 |
| Critical commercial assays | ||
| Epinephrine ELISA Kit (Colorimetric) | Novus Biologicals | Cat #: KA1882 |
| Mouse IL-15 ELISA Kit | Sigma-Aldrich | Cat #: RAB0260 |
| Deposited data | ||
| 10x Single Cell RNA Sequencing Raw Data | This paper | GEO: GSE176311 |
| Experimental models: Cell lines | ||
| Mouse: KPC 4662 Pancreatic Cancer cell line Derived from LSL-KRASG12D/+ p53R172H/+; Pdx-1Cre/+ mice |
Gift from Robert Vonderheide, UPENN | N/A |
| Mouse: KPC 1203 Pancreatic Cancer cell line Derived from LSL-KRASG12D/+ p53R172H/+; Pdx-1Cre/+ mice |
NYU Grossman School of Medicine, Department of Cell Biology | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J wild type mice | The Jackson Laboratory | JAX: 000664 |
| Mouse: KC LSL-KRASG12D/+; p48Cre/+ | NYU Langone Medical Center, Division of Comparative Medicine mouse facility | N/A |
| Mouse: NU/J Foxn1nu athymic nude mice | The Jackson Laboratory | JAX: 002019; RRID: ISMR_JAX:002019 |
| Mouse: B6.129S7-Rag1tm1Mom/J Rag1KO mice | The Jackson Laboratory | JAX: 002216, RRID: IMSR_JAX:002216 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| GraphPad Prism 9.3.1 | Dotmatics | https://www.graphpad.com |
| iCellR R package (v1.5.5) | This paper | https://CRAN.R-project.org/package=iCellR |
| Ingenuity Pathway Analysis (IPA) | Qiagen | https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/?gclid=Cj0KCQjwpcOTBhCZARIsAEAYLuU2uBoPYEwAjfKm5S0IeMMKdT2e6A9WQDKXBNipkzh0QNE9wNgH-mQaAspZEALw_wcB |
| FlowJo Version 10.6.1 | Treestar | https://www.flowjo.com/solutions/flowjo |
| inform Tissue Analysis Software | Akoya Biosciences | https://www.akoyabio.com/phenoimager/software/inform-tissue-finder/ |
| Vectra Polaris Multispectral Imaging System | Akoya Biosciences | https://www.akoyabio.com/phenoimager/instruments/vectra-3-0/ |
| Other | ||
| Rodent Five-Lane Treadmill | Harvard Apparatus | Cat #: 76–0895 |
Experimental Model and Subject Details:
Murine exercise and in-vivo treatments
C57BL/6, Rag1KO, and FoxNude athymic mice were purchased from Jackson Labs (Bar Harbor, ME) and bred in-house. LSL-KRASG12D/+; p48Cre/+ (KC) mice were bred in the Division of Comparative Medicine mouse facility at New York University Langone Medical Center. Both male and female KC mice were used, as indicated, and animals were age matched within each experiment. For orthotopic studies, 8–10 week old female mice were administered intra-pancreatic injections of 4662 or 1203 KPC cells derived from LSL-KRASG12D/+ p53R172H/+; pdx-1Cre/+ (KPC) mice, as previously described (Yang et al., 2018). Briefly, cells were suspended in PBS with 50% Matrigel (BD Biosciences, Franklin Lakes, NJ) and either 5×104 or 1×105 KPC cells were injected into the pancreas via laparotomy. Mice were sacrificed on Day 21 for analyses, unless otherwise indicated. For experiments, unless otherwise indicated, exercised mice were involuntarily placed on a Rodent 5-lane treadmill (aerobic exercise or exercise; Harvard Apparatus, Cat No: 76–0895), for 30 minutes per day at 15 cm/second, for a minimum of 5 days/week. In the 3 days leading up to sacrifice, exercise mice were obligately exercised, regardless of previous number of consecutive days exercised. All mice were assessed for their ability to run on the treadmill and if mice were unable to perform involuntary exercise 24 hours after surgical implantation, they were removed from the experiment prior to onset. In select experiments, mice were subject to involuntary exercise starting on post-operative Day 12, instead of Day 1 (established tumor model). Treadmill “sham” control mice in matched experiments were placed on a stationary (0 cm/sec) treadmill for the same duration of time as experimental mice. Where indicated, neutralizing antibodies directed against CD8 (200 ug/dose, clone 2.43) or appropriate RatIgG2b isotype control (200ug/dose, clone LTF-2) starting Day 1 post-op, against IL-15 (200 ug/dose, clone AIO.3) or appropriate RatIgG2a isotype control (200ug/dose, clone 2A3) starting Day 3 post-op, or against PD-1 (200 ug/dose, clone RMP1–14) or appropriate RatIgG2a isotype control (200 ug/dose, clone 2A3) starting Day 3 post-op (all BioXcell, West Lebanon, NH), were utilized 3x weekly and administered i.p., as previously described (Grasselly et al., 2018; Li et al., 2017; Meyer and DeNardo, 2018). Where indicated, mice were treated every 48 hours i.p. with .350mg/kg (7.2 ug) IL-15 S.A. NIZ985 (IL-15 S.A.). NIZ985 is a complex of 2 polypeptide chains, IL-15 and sIL-15Rα. This protein is a recombinant human protein that has been produced in an engineered HEK293 cell line. Where indicated, mice were treated every day i.p. with 2mg/kg with FTY720 (Sigma Aldrich). Stocks of FTY720 were resuspended in DMSO (20 mg/mL) and stored at −80°C; for injection, FTY720 was first resuspended in 2% βhydroxypropyl-cyclodextrin in PBS and diluted at 45% volume in PBS (Sigma Aldrich) based on previous protocols (Ramos-Perez et al., 2015). Where indicated, mice were provided either normal drinking water or .5g/L propranolol (Sigma Aldrich) drinking water and bifurcated into control and exercise cohorts (Pedersen et al., 2016). Where indicated, mice underwent ultrasound-guided randomization based off tumor volume on Day 7 post-operation. Mice were then subject to treatment regimens beginning on Day 7. In these experiments, mice treated with either anti-PD-1 200 ug, (clone RMP1-14, q4), NIZ985 (7.2 ug q2), 100 mg/kg Gemcitabine (1x week) and 50mg/kg abraxane (Cellgene, 1x week), or all three drugs, i.p.. Where indicated, mice underwent ultrasound-guided randomization based off tumor volume on Day 7 post-operation , were subject to treatment regimens listed above, and were monitored for survival post-operation. 4662 and 1203 KPC cell lines have not been authenticated. All studies were approved by and were conducted in accordance with the Institutional Animal Care and Use Committee at NYU Grossman School of Medicine policies on the care, welfare, and treatment of laboratory animals. All experiments met or exceeded the standards of the Association for the Assessment and Accreditation of Laboratory Animal Care, International (AAALAC), the United States Department of Health and Human Services, and all local and federal animal welfare laws.
Flow Cytometry (FC)
Single cell suspensions of PDA tumors were prepared for FC as described previously with slight modifications (Das et al., 2020). Briefly, pancreata were placed in cold 2% FACS (cold PBS with 2% FBS) with Collagenase IV (1 mg/mL; Worthington Biochemical, Lakewood, NJ), Trypsin inhibitor (1mg/mL; EMD Millipore, Billerica, MA) and DNase I (2 U/mL; Promega, Madison, WI), and minced with scissors to sub-millimeter pieces. Tissues were then incubated at 37°C for 20 minutes with gentle shaking every 5 minutes and then passed through a 70μm mesh and centrifuged at 350g for 5 minutes. Cell pellets were re-suspended in PBS and stained with Zombie Yellow Fixable viability kit (Biolegend). After wash, cell pellets were re-suspended in FACS buffer. After blocking FcγRIII/II with an anti-CD16/CD32 mAb (eBiosciences, San Diego, CA), cells were labeled by incubating 1×106 cells with fluorescently conjugated mAbs directed against mouse CD44 (IM7), PD-1 (29F.1A12), CD3 (17A2), CD4 (RM4–5), CD8 (53–6.7), CD45 (30-F11), CD11b (M1/70), Gr1 (RB6–8C5), MHC II (M5/114.15.2), ICOS (15F9), CD69 (H1.2F3), NK.1. (PK136), IL-15ralpha (6B4C88), CD140a (APA5), EPCAM (G8.8), CD34 (MEC14.7), (all BioLegend, San Diego, CA). If applicable, cells were subjected to fixation and permeabilization (Biolegend, Cat# 420801 and 421002) protocols and then subjected to staining for the following intra-cellular antibodies: IL-15 [(Abcam, ab273631) with secondary AF488 Goat anti-Rabbit IgG (ab150077)] , T-bet (Biolegend, 4B10), TCF-1 (Biolegend, 7F11A10), FOXP3 (MF-14), IFNγ (XMG1.2), Ki67 (16A8), and Granzyme B (QA16A02). For FC of whole blood, PBMC were isolated by overlaying whole blood diluted 1:1 in PBS over an equal amount of Ficoll (GE Healthcare, Princeton, NJ). Cells were then spun at 2100 RPM and the buffy coat harvested as described (Jaatinen and Laine, 2007). FC was performed on the Attune NxT Flow Cytometer (ThermoFisher, Waltham, MA). FACS-sorting was performed on the SY3200 (Sony, Tokyo, Japan). Data were analyzed using FlowJo Version 10.6.1 (Treestar, Ashland, OR).
Patient Samples and Cohort Acquisition
Seventy patients with a diagnosis of pancreatic ductal adenocarcinoma with planned surgical resection were enrolled in a single arm exercise intervention (Institutional Review Board of MD Anderson Cancer Center protocol #2014–0702 and PA16–0249, and ClinicalTrials.gov NCT02295956; registration date 11/20/2014) in accordance with informed consent. Details of the clinical characteristics (including age, gender, and stage) from the intervention and historical control cohorts have been previously described (Ngo-Huang et al., 2019; Ngo-Huang et al., 2017). Exercise occurred prior to surgery, concurrent with neoadjuvant chemotherapy or chemoradiation. Patients were prescribed 60 minutes per week of unsupervised aerobic activity and 60 minutes per week of unsupervised strength training. Exercise minutes were tracked by patient-completed exercise logs, and by wrist worn accelerometers in a subset of patients. Histology sections obtained from surgically resected tumors of patients in the exercise trial were compared to historical controls with similar clinical demographics, as described previously (Florez Bedoya et al., 2019). Due to limited number of human FFPE samples, no gender-specific analysis was performed.
Immunohistochemistry on Human Samples
Immunohistochemistry (IHC) was performed as previously described (Riquelme et al., 2019). Briefly, 5 um sections of FFPE tumor tissue were used for IHC analysis by using anti-human antibodies against the following: CD8 (CD8 T cells; Thermo Fisher Scientific Cat#MS-457-S) and Granzyme B (GzmB; Leica Biosystems Cat#PA0291). The densities of cells expressing CD8 and GzmB were measured by counting positively stained cells in ten random 20X fields in the tumor. The average total number of cells positive for each marker in the ten random fields was expressed in cells per field.
Murine Immunofluorescence (IF) and Microscopy
Tissues were fixed for 48 hours in 10% buffered formalin at 4°C and embedded into paraffin in a Leica Peloris automated processor. Five-micron sections of the paraffin-embedded tissues were stained with hematoxylin and eosin (H&E) or Gomori Trichrome, where appropriate. Histology was analyzed on a Zeiss LSM700 confocal microscope. The percentage of acinar area (fibrosis) in each slide was calculated on Adobe Photoshop software by dividing the number of pancreatic acinar pixels (or Trichrome Gomori blue stained pixels) by the total number of tissue pixels present in each field of view (FOV). Where appropriate, FFPE samples were stained with Akoya Biosciences® Opal™ multiplex automation kit (Akoya Biosciences, Menlo Park, CA). Automated staining was performed on Leica BondRX® autostainer (Leica Microsystems, Inc., Buffalo Grove, IL). The protocol was performed according to manufacturers’ instructions. Primary antibodies included CD3 (Biorad, Clone CD3–12), CD8 (Cell Signaling Tech, Clone D4W2Z), IL15rα (ThermoFisher; PA5–79467), CK8 (TROMA-I) [TROMA-I, deposited to the DSHB by Brulet, P. / Kemler, R. (DSHB Hybridoma Product TROMA-I)], CK19 (Millipore, TROMA-3), CC3 (CST, D3E9), and CD31 (CST, D8V9E). Briefly, all slides underwent sequential epitope retrieval, antibody incubation and tyramide signal amplification (TSA). Primary and secondary antibodies were removed during epitope retrieval steps while fluorophores remain covalently attached to the epitope. Multispectral Image Acquisition and Analysis was performed on a Vectra® Polaris multispectral imaging system (Akoya Biosciences, Menlo Park, CA) and the fluorophores spectrally unmixed using either Phenochart (for whole slide scans) or InForm Automated Image Analysis (for selected MSI fields, Akoya Biosciences) software.
Epinephrine Treatment and Epinephrine ELISA
In indicated experiments, wild type mice were injected i.p. with 20 μg of Epinephrine dissolved in 200 μl of PBS and sacrificed 20 minutes after injection. 100 μl of whole blood was collected, processed, and assessed by FC. In separate experiments, mice were subjected to 30 minutes of exercise, then sacrificed 20 minutes after completion of exercise, and 200 μl of whole blood was collected. Sera was isolated from whole blood using a previously described centrifugation isolation (Tuck et al., 2009), and subjected to Epinephrine ELISA detection kit (Novus Biologicals, CO).
IL-15 ELISA
In indicated experiments, wild type mice were injected i.p. with 20 μg of Epinephrine dissolved in 200 μl of PBS and sacrificed 20 minutes after injection. 200 μl of whole blood was collected. Sera was isolated from whole blood using centrifugation (Tuck et al., 2009) and subjected to mouse IL-15 ELISA detection kit (Sigma Aldrich, RAB0260). In separate experiments, mice were subjected to 30 minutes of exercise, then sacrificed 20 minutes after completion of exercise, and 200 μl of whole blood was collected. Sera was isolated and subjected to mouse IL-15 ELISA detection kit (Sigma Aldrich, RAB0260).
MDSC Adoptive Transfer
WT 8–10-week-old female mice were administered intra-pancreatic orthotopic injections of 1×10^5 4662 KPC pancreatic cancer cells admixed with either 5×10^5 CD11b+ Gr1+ cells FACS sort-isolated from control tumors or 5×10^5 CD11b+ Gr1+ cells FACS-isolated from exercise tumors. Mice were sacrificed at day 21 post implantation and tumor weights were measured.
T cell inhibition Assay
T cell inhibition assays were done in technical replicates of 6 in a v-bottom 96-well plate. Sorted splenic CD3+ T cells (5 × 104/well) were either unstimulated or stimulated with anti-CD3/CD28 magnetic beads (each at 2 μg/mL). Stimulated splenic T cells were plated alone or co-cultured with sorted CD11b+ Gr1+ MDSCs (1.2 × 105/well) isolated from control and exercise tumors. T cells in all conditions remained in culture for 72 hours, were isolated at harvest from stimulation beads using magnetic separation, and were analyzed by FC for markers of activation and proliferation.
Single Cell RNAseq Processing and Analysis
The cell concentration, singularity, and viability of single cell suspensions of live leukocytes (PI-, CD45+) isolated from control and exercise tumors by FACS (FC and cell sorting) were confirmed before scRNA-Seq (10x Genomics). scRNA-Seq was performed by the Genome Technology Center (GTC) and data processing and analysis was performed by the Applied Bioinformatics Laboratory (ABL). Sequencing results were demultiplexed and converted to FASTQ format using Illumina bcl2fastq software. The Cell Ranger Single-Cell Software Suite (https://support.10xgenomics.com/single-cell-gene-expression/software/pipelines/latest/what-is-cell-ranger) was used to perform sample demultiplexing and single-cell 3’ gene counting. The cDNA insert was aligned to the mm10/GRCm38 reference genome. Only confidently mapped non-PCR duplicates with valid UMIs were used to generate the gene-barcode matrix. Further analysis including the identification of highly variable genes, dimensionality reduction, standard unsupervised clustering algorithms, and the discovery of differentially expressed genes was performed using the iCellR R package (v1.5.5) (https://CRAN.R-project.org/package=iCellR). To exclude low quality cells and cells that were extreme outliers in terms of library complexity, we calculated the distribution of genes detected per cell, UMIs, and the proportion of mitochondrial genes. Cells with more than 10% of the transcripts coming from mitochondrial genes or low number of covered genes (gene-count <500) were removed, and the matrix was normalized based on library size accordingly.
Gene dispersion, base mean, and coverage were calculated and genes with high coverage (top 500) and high dispersion (dispersion >1.5) were utilized to run principal component analysis (PCA) (total 2000 genes). The final dataset included 6,915 (4,241 Exercise, 2,674 No Exercise) total cells with a median of 1,203 detected genes. For dimensionality reduction, T-distributed Stochastic Neighbor Embedding (t-SNE), Uniform Manifold Approximation and Projection (UMAP), and K-nearest-neighbor-based Network graph drawing Layout (KNetL) were then performed (zoom=300). As previously described, zoom option dictates the force in the network system for the layout used in KNetL map. PhenoGraph clustering was then performed, and the results were shown on both the t-SNE and KNetL map, and marker genes were found for each cluster and visualized on heatmaps. Marker genes were then used to assign cell types. Imputation was used for some data visualizations only and not for the analysis. For imputation we used KNN (k-nearest-neighbor) to average the expression of 10 neighboring cells per cell, using iCellR’s “run.impute” function.
DDRTree method implemented in the Monocle R package (v2) (Qiu et al., 2017) was used to draw the trajectory branches and cell fate directionality was predicted using RNA velocity generalized through dynamical modeling implemented in the scVelo Python package (v0.2.3) (Bergen et al., 2020).
Clustering of Single Cell RNAseq Data
Differential expression analysis yielded 14 clusters. We assigned cell type identities based on the expression of known population markers using Immgen Gene Skyline as a reference as follows: Cluster 1: Inflammatory Monocytes – Cd68+, Clec4a1+, Ifitm3+, F13a1+, Ms4a4c+, Vcan hi Cd3e neg ; Cluster 2: M2-Macrophages – Cd68+ Clec4a1 lo, F13a1 lo, Mrc1 hi Atp6v0d2 hi, Cluster 3: Classical Dendritic Cells– Batf3+, Cd68 lo, Clec9a hi, H2Aa hi, Itgae hi, Vwf hi; Cluster 4: Plasmacytoid-like Dendritic Cells – Batf3 hi, Cd209a+, Clec9a lo, Epcam hi, Ifitm3/1 hi ; Cluster 5: Gamma Delta T cells – Cxcr4 hi, Ccr7 hi, Ly6c2 med, Cd4 lo, Cd8 lo, Cd3e med; Cluster 6: B Cells – Ccr7 hi, Cd19+, Cxcr4 hi, Ms4a1+, Ly6d+, Cd3e neg; Cluster 7: NK Cells – Gzma hi, Ly6c2 hi, Ncr1+, Nkg7 med, Cd3e neg; Cluster 8: T-regs – Cd3e hi, Cd4+, Cd69 med, Foxp3+, Ltb hi; Cluster 9: Naïve CD4 T cells – Cd4+ Cd3e+, Gcg hi, ccl5 med, CD69 lo, Ptprc hi; Cluster 10: Naïve CD8 T cells – Cd3e hi, CD8a hi, Gzma med, Ltb med, Ccl5 neg; Cluster 11: Effector CD8 T cells – CD3e+, CD8a hi, Ccl5+, Nkg7 hi, CD69 hi, ; Cluster 12: Myeloid Derived Suppressor Cells 1 –Acta2 hi, Cd14 lo, Cd68 neg, Fcgr3 hi, Ly6c lo, S100a8/9 med, Ly6g lo, Itgam+, Cxcr2 hi, Gcsf3r med, Atp6v0d2 lo ; Cluster 13: Myeloid Derived Suppressor Cells 2 - Acta2 hi, Cd14 lo, Cd68 neg, Fcgr3 hi, Ly6c lo, S100a8/9 med, Ly6g lo, Itgam+, Cxcr2 hi, Csf3r med; Atp6v0d2 hi; Cluster 14: Neutrophils – Mmp9 hi Cxcr2 hi CD14 hi S100a9/S100a8+, Ptgs2 hi, Spp1 hi . The resulting genes with adjusted p-value <0.05 were considered significant. To identify the signaling pathways in which genes are enriched, Ingenuity Pathway Analysis (IPA, Qiagen) was carried out for genes that were considered significant. The canonical pathways analyzed in IPA are represented as bar-plots or bubble plots for upstream regulators.
Statistical Analysis
Data throughout is presented as individual values with standard error of the mean. Statistical significance was determined by the student’s t test using GraphPad Prism 9, (GraphPad Software, La Jolla, CA), p-value was adjusted by FDR for multiple t-tests where indicated. Survival analyses were analyzed with Log Rank Mantel Test. ANOVA was used for multi-variate comparisons, where indicated. Human data sets were analyzed using Mann-Whitney U Test with confidence level: 95%, and p values for comparisons between groups were determined. Throughout, p-values <0.05 were considered statistically significant.
Additional Resources
For further information on the clinical trial (NCT03187951) from which human samples were used, please see: https://clinicaltrials.gov/ct2/show/NCT03187951.
Supplementary Material
Highlights:
Aerobic exercise restricts pancreatic tumor growth by enhancing anti-tumor immunity
The anti-tumor effects of aerobic exercise are driven by IL-15Rα + CD8 T cells
Pharmacological activation of IL-15/IL-15Rα promotes a durable anti-tumor response
Aerobic exercise and IL-15 activation sensitize pancreatic tumors to α-PD-1 therapy
Acknowledgements:
The KPC cell line (4662) was a kind gift from Dr. R.H. Vonderheide. Graphical Abstract created with BioRender.com We would like to thank the Genome Technology Center (GTC) and the Applied Bioinformatics Laboratories (ABL); the NYULMC FC Core, supported in part by grant UL1 TR000038 from NCATS; and the Experimental Pathology Research Laboratory, all partially supported by NIH/NCI 5 P30CA16087. Ex-path is also supported by S10 OD021747 (PerkinElmer/AkoyaBiosciences® Vectra® multispectral imaging system). NIZ985 (IL-15 S.A.) was kindly provided by Novartis to the Bar-Sagi Lab according to Material Transfer Agreement between Novartis and NYU. E.K. was supported by NIH/NCI grant F30 CA243205 and T32-GM136573. C.A.H. supported by T32CA193111. E.A. Vucic supported by Canadian Institutes of Health Research Fellowship (146792). Support also from the Wyck Knox Jr. Family Foundation; Center for Energy Balance in Cancer Prevention & Survivorship, Duncan Family Institute for Cancer Prevention and Risk Assessment; Cancer Prevention & Research Institute of Texas Training Grant/MD Anderson Cancer Prevention Research Training Program (RP170259); NIH/NCI (R21CA218732 and P30CA016672). FM supported by NCI (R37CA237384-01A1) and CPRIT (RP200173). D.B.-S. supported by NIH/NCI Grant CA210263.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Interests:
The authors have no competing interests to declare.
REFERENCES:
- Allen BM, Hiam KJ, Burnett CE, Venida A, DeBarge R, Tenvooren I, Marquez DM, Cho NW, Carmi Y, and Spitzer MH (2020). Systemic dysfunction and plasticity of the immune macroenvironment in cancer models. Nat Med 26, 1125–1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcraft KA, Peace RM, Betof AS, Dewhirst MW, and Jones LW (2016). Efficacy and Mechanisms of Aerobic Exercise on Cancer Initiation, Progression, and Metastasis: A Critical Systematic Review of In Vivo Preclinical Data. Cancer Res 76, 4032–4050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balachandran VP, Beatty GL, and Dougan SK (2019). Broadening the Impact of Immunotherapy to Pancreatic Cancer: Challenges and Opportunities. Gastroenterology 156, 2056–2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnstorf I, Borsa M, Baumann N, Pallmer K, Yermanos A, Joller N, Sporri R, Welten SPM, Krautler NJ, and Oxenius A (2019). Chronic virus infection compromises memory bystander T cell function in an IL-6/STAT1-dependent manner. J Exp Med 216, 571–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bear AS, Vonderheide RH, and O’Hara MH (2020). Challenges and Opportunities for Pancreatic Cancer Immunotherapy. Cancer Cell 38, 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltra JC, Manne S, Abdel-Hakeem MS, Kurachi M, Giles JR, Chen Z, Casella V, Ngiow SF, Khan O, Huang YJ, et al. (2020). Developmental Relationships of Four Exhausted CD8(+) T Cell Subsets Reveals Underlying Transcriptional and Epigenetic Landscape Control Mechanisms. Immunity 52, 825–841 e828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berard M, Brandt K, Bulfone-Paus S, and Tough DF (2003). IL-15 promotes the survival of naive and memory phenotype CD8+ T cells. J Immunol 170, 5018–5026. [DOI] [PubMed] [Google Scholar]
- Bergen V, Lange M, Peidli S, Wolf FA, and Theis FJ (2020). Generalizing RNA velocity to transient cell states through dynamical modeling. Nat Biotechnol 38, 1408–1414. [DOI] [PubMed] [Google Scholar]
- Bessard A, Sole V, Bouchaud G, Quemener A, and Jacques Y (2009). High antitumor activity of RLI, an interleukin-15 (IL-15)-IL-15 receptor alpha fusion protein, in metastatic melanoma and colorectal cancer. Mol Cancer Ther 8, 2736–2745. [DOI] [PubMed] [Google Scholar]
- Chen Z, Ji Z, Ngiow SF, Manne S, Cai Z, Huang AC, Johnson J, Staupe RP, Bengsch B, Xu C, et al. (2019). TCF-1-Centered Transcriptional Network Drives an Effector versus Exhausted CD8 T Cell-Fate Decision. Immunity 51, 840–855 e845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiba K (2005). FTY720, a new class of immunomodulator, inhibits lymphocyte egress from secondary lymphoid tissues and thymus by agonistic activity at sphingosine 1-phosphate receptors. Pharmacol Ther 108, 308–319. [DOI] [PubMed] [Google Scholar]
- Coveler AL, Bajor DL, Masood A, Yilmaz E, Shields AF, Javle MM, Paluri RK, Vaccaro GM, Zalupski M, Grilley-Olson JE, et al. (2020). Phase I study of SEA-CD40, gemcitabine, nab-paclitaxel, and pembrolizumab in patients with metastatic pancreatic ductal adenocarcinoma (PDAC). Journal of Clinical Oncology 38, TPS4671–TPS4671. [Google Scholar]
- Daher C, Vimeux L, Stoeva R, Peranzoni E, Bismuth G, Wieduwild E, Lucas B, Donnadieu E, Bercovici N, Trautmann A, and Feuillet V (2019). Blockade of beta-Adrenergic Receptors Improves CD8(+) T-cell Priming and Cancer Vaccine Efficacy. Cancer Immunol Res 7, 1849–1863. [DOI] [PubMed] [Google Scholar]
- Das S, Shapiro B, Vucic EA, Vogt S, and Bar-Sagi D (2020). Tumor Cell-Derived IL1beta Promotes Desmoplasia and Immune Suppression in Pancreatic Cancer. Cancer Res 80, 1088–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbois M, Le Vu P, Coutzac C, Marcheteau E, Beal C, Terme M, Gey A, Morisseau S, Teppaz G, Boselli L, et al. (2016). IL-15 Trans-Signaling with the Superagonist RLI Promotes Effector/Memory CD8+ T Cell Responses and Enhances Antitumor Activity of PD-1 Antagonists. J Immunol 197, 168–178. [DOI] [PubMed] [Google Scholar]
- Dimitrov S, Lange T, and Born J (2010). Selective mobilization of cytotoxic leukocytes by epinephrine. J Immunol 184, 503–511. [DOI] [PubMed] [Google Scholar]
- Dubois S, Mariner J, Waldmann TA, and Tagaya Y (2002). IL-15Ralpha recycles and presents IL-15 In trans to neighboring cells. Immunity 17, 537–547. [DOI] [PubMed] [Google Scholar]
- Estruel-Amades S, Camps-Bossacoma M, Massot-Cladera M, Perez-Cano FJ, and Castell M (2020). Alterations in the innate immune system due to exhausting exercise in intensively trained rats. Sci Rep 10, 967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans RA, Diamond MS, Rech AJ, Chao T, Richardson MW, Lin JH, Bajor DL, Byrne KT, Stanger BZ, Riley JL, et al. (2016). Lack of immunoediting in murine pancreatic cancer reversed with neoantigen. JCI Insight 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florez Bedoya CA, Cardoso ACF, Parker N, Ngo-Huang A, Petzel MQ, Kim MP, Fogelman D, Romero SG, Wang H, Park M, et al. (2019). Exercise during preoperative therapy increases tumor vascularity in pancreatic tumor patients. Sci Rep 9, 13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foucher ED, Ghigo C, Chouaib S, Galon J, Iovanna J, and Olive D (2018). Pancreatic Ductal Adenocarcinoma: A Strong Imbalance of Good and Bad Immunological Cops in the Tumor Microenvironment. Front Immunol 9, 1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frodermann V, Rohde D, Courties G, Severe N, Schloss MJ, Amatullah H, McAlpine CS, Cremer S, Hoyer FF, Ji F, et al. (2019). Exercise reduces inflammatory cell production and cardiovascular inflammation via instruction of hematopoietic progenitor cells. Nat Med 25, 1761–1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garritson J, Krynski L, Haverbeck L, Haughian JM, Pullen NA, and Hayward R (2020). Physical activity delays accumulation of immunosuppressive myeloid-derived suppressor cells. PLoS One 15, e0234548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes-Santos IL, Amoozgar Z, Kumar AS, Ho WW, Roh K, Talele NP, Curtis H, Kawaguchi K, Jain RK, and Fukumura D (2021). Exercise Training Improves Tumor Control by Increasing CD8(+) T-cell Infiltration via CXCR3 Signaling and Sensitizes Breast Cancer to Immune Checkpoint Blockade. Cancer Immunol Res 9, 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan V, Spencer CN, Nezi L, Reuben A, Andrews MC, Karpinets TV, Prieto PA, Vicente D, Hoffman K, Wei SC, et al. (2018). Gut microbiome modulates response to anti-PD-1 immunotherapy in melanoma patients. Science 359, 97–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasselly C, Denis M, Bourguignon A, Talhi N, Mathe D, Tourette A, Serre L, Jordheim LP, Matera EL, and Dumontet C (2018). The Antitumor Activity of Combinations of Cytotoxic Chemotherapy and Immune Checkpoint Inhibitors Is Model-Dependent. Front Immunol 9, 2100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo S, Smeltz RB, Nanajian A, and Heller R (2020). IL-15/IL-15Ralpha Heterodimeric Complex as Cancer Immunotherapy in Murine Breast Cancer Models. Front Immunol 11, 614667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han X, Shi H, Sun Y, Shang C, Luan T, Wang D, Ba X, and Zeng X (2019). CXCR2 expression on granulocyte and macrophage progenitors under tumor conditions contributes to mo-MDSC generation via SAP18/ERK/STAT3. Cell Death Dis 10, 598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiam-Galvez KJ, Allen BM, and Spitzer MH (2021). Systemic immunity in cancer. Nat Rev Cancer 21, 345–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Idorn M, and Hojman P (2016). Exercise-Dependent Regulation of NK Cells in Cancer Protection. Trends Mol Med 22, 565–577. [DOI] [PubMed] [Google Scholar]
- Jaatinen T, and Laine J (2007). Isolation of mononuclear cells from human cord blood by Ficoll-Paque density gradient. Curr Protoc Stem Cell Biol Chapter 2, Unit 2A 1. [DOI] [PubMed] [Google Scholar]
- Kim SR, Puranik AS, Jiang K, Chen X, Zhu XY, Taylor I, Khodadadi-Jamayran A, Lerman A, Hickson LJ, Childs BG, et al. (2021). Progressive Cellular Senescence Mediates Renal Dysfunction in Ischemic Nephropathy. J Am Soc Nephrol 32, 1987–2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruger K, Lechtermann A, Fobker M, Volker K, and Mooren FC (2008). Exercise-induced redistribution of T lymphocytes is regulated by adrenergic mechanisms. Brain Behav Immun 22, 324–338. [DOI] [PubMed] [Google Scholar]
- Kumar V, Patel S, Tcyganov E, and Gabrilovich DI (2016). The Nature of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. Trends Immunol 37, 208–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Manno G, Soldatov R, Zeisel A, Braun E, Hochgerner H, Petukhov V, Lidschreiber K, Kastriti ME, Lonnerberg P, Furlan A, et al. (2018). RNA velocity of single cells. Nature 560, 494–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkin J, Chiarion-Sileni V, Gonzalez R, Grob JJ, Rutkowski P, Lao CD, Cowey CL, Schadendorf D, Wagstaff J, Dummer R, et al. (2019). Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N Engl J Med 381, 1535–1546. [DOI] [PubMed] [Google Scholar]
- Li HY, McSharry M, Bullock B, Nguyen TT, Kwak J, Poczobutt JM, Sippel TR, Heasley LE, Weiser-Evans MC, Clambey ET, and Nemenoff RA (2017). The Tumor Microenvironment Regulates Sensitivity of Murine Lung Tumors to PD-1/PD-L1 Antibody Blockade. Cancer Immunol Res 5, 767–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JH, Huffman AP, Wattenberg MM, Walter DM, Carpenter EL, Feldser DM, Beatty GL, Furth EE, and Vonderheide RH (2020). Type 1 conventional dendritic cells are systemically dysregulated early in pancreatic carcinogenesis. J Exp Med 217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Yang Y, Chen C, Li L, Li J, Wang X, Chu Q, Qiu L, Ba Q, Li X, and Wang H (2021). Environmental eustress modulates beta-ARs/CCL2 axis to induce anti-tumor immunity and sensitize immunotherapy against liver cancer in mice. Nat Commun 12, 5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu J, Giuntoli RL 2nd, Omiya R, Kobayashi H, Kennedy R, and Celis E (2002). Interleukin 15 promotes antigen-independent in vitro expansion and long-term survival of antitumor cytotoxic T lymphocytes. Clin Cancer Res 8, 3877–3884. [PubMed] [Google Scholar]
- Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, et al. (2010). Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity 32, 790–802. [DOI] [PubMed] [Google Scholar]
- Martinez-Lostao L, Anel A, and Pardo J (2015). How Do Cytotoxic Lymphocytes Kill Cancer Cells? Clin Cancer Res 21, 5047–5056. [DOI] [PubMed] [Google Scholar]
- Meyer MA, Baer JM, Knolhoff BL, Nywening TM, Panni RZ, Su X, Weilbaecher KN, Hawkins WG, Ma C, Fields RC, et al. (2018). Breast and pancreatic cancer interrupt IRF8-dependent dendritic cell development to overcome immune surveillance. Nat Commun 9, 1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MA, and DeNardo DG (2018). Better Together: B7S1 Checkpoint Blockade Synergizes with anti-PD1. Immunity 48, 621–623. [DOI] [PubMed] [Google Scholar]
- Mpekris F, Voutouri C, Baish JW, Duda DG, Munn LL, Stylianopoulos T, and Jain RK (2020). Combining microenvironment normalization strategies to improve cancer immunotherapy. Proc Natl Acad Sci U S A 117, 3728–3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakami Y, Saito H, Shimizu S, Kono Y, Shishido Y, Miyatani K, Matsunaga T, Fukumoto Y, Ashida K, Sakabe T, et al. (2019). Increased regulatory B cells are involved in immune evasion in patients with gastric cancer. Sci Rep 9, 13083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Huang A, Parker NH, Bruera E, Lee RE, Simpson R, O’Connor DP, Petzel MQB, Fontillas RC, Schadler K, Xiao L, et al. (2019). Home-Based Exercise Prehabilitation During Preoperative Treatment for Pancreatic Cancer Is Associated With Improvement in Physical Function and Quality of Life. Integr Cancer Ther 18, 1534735419894061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo-Huang A, Parker NH, Wang X, Petzel MQB, Fogelman D, Schadler KL, Bruera E, Fleming JB, Lee JE, and Katz MHG (2017). Home-based exercise during preoperative therapy for pancreatic cancer. Langenbecks Arch Surg 402, 1175–1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neil BH, Scott AJ, Ma WW, Cohen SJ, Aisner DL, Menter AR, Tejani MA, Cho JK, Granfortuna J, Coveler AL, et al. (2016). A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann Oncol 27, 1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly EM, Oh DY, Dhani N, Renouf DJ, Lee MA, Sun W, Fisher G, Hezel A, Chang SC, Vlahovic G, et al. (2019). Durvalumab With or Without Tremelimumab for Patients With Metastatic Pancreatic Ductal Adenocarcinoma: A Phase 2 Randomized Clinical Trial. JAMA Oncol 5, 1431–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pappu R, Schwab SR, Cornelissen I, Pereira JP, Regard JB, Xu Y, Camerer E, Zheng YW, Huang Y, Cyster JG, and Coughlin SR (2007). Promotion of lymphocyte egress into blood and lymph by distinct sources of sphingosine-1-phosphate. Science 316, 295–298. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Akerstrom TC, Nielsen AR, and Fischer CP (2007). Role of myokines in exercise and metabolism. J Appl Physiol (1985) 103, 1093–1098. [DOI] [PubMed] [Google Scholar]
- Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, Johannesen HH, Becker JC, Pedersen KS, Dethlefsen C, et al. (2016). Voluntary Running Suppresses Tumor Growth through Epinephrine- and IL-6-Dependent NK Cell Mobilization and Redistribution. Cell Metab 23, 554–562. [DOI] [PubMed] [Google Scholar]
- Qiu X, Hill A, Packer J, Lin D, Ma YA, and Trapnell C (2017). Single-cell mRNA quantification and differential analysis with Census. Nat Methods 14, 309–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Perez WD, Fang V, Escalante-Alcalde D, Cammer M, and Schwab SR (2015). A map of the distribution of sphingosine 1-phosphate in the spleen. Nat Immunol 16, 1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rawla P, Sunkara T, and Gaduputi V (2019). Epidemiology of Pancreatic Cancer: Global Trends, Etiology and Risk Factors. World J Oncol 10, 10–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riquelme E, Zhang Y, Zhang L, Montiel M, Zoltan M, Dong W, Quesada P, Sahin I, Chandra V, San Lucas A, et al. (2019). Tumor Microbiome Diversity and Composition Influence Pancreatic Cancer Outcomes. Cell 178, 795–806 e712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risoy BA, Raastad T, Hallen J, Lappegard KT, Baeverfjord K, Kravdal A, Siebke EM, and Benestad HB (2003). Delayed leukocytosis after hard strength and endurance exercise: aspects of regulatory mechanisms. BMC Physiol 3, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rundqvist H, Velica P, Barbieri L, Gameiro PA, Bargiela D, Gojkovic M, Mijwel S, Reitzner SM, Wulliman D, Ahlstedt E, et al. (2020). Cytotoxic T-cells mediate exercise-induced reductions in tumor growth. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saka D, Gokalp M, Piyade B, Cevik NC, Arik Sever E, Unutmaz D, Ceyhan GO, Demir IE, and Asimgil H (2020). Mechanisms of T-Cell Exhaustion in Pancreatic Cancer. Cancers (Basel) 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sand KL, Flatebo T, Andersen MB, and Maghazachi AA (2013). Effects of exercise on leukocytosis and blood hemostasis in 800 healthy young females and males. World J Exp Med 3, 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadler KL, Thomas NJ, Galie PA, Bhang DH, Roby KC, Addai P, Till JE, Sturgeon K, Zaslavsky A, Chen CS, and Ryeom S (2016). Tumor vessel normalization after aerobic exercise enhances chemotherapeutic efficacy. Oncotarget 7, 65429–65440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schedlowski M, Hosch W, Oberbeck R, Benschop RJ, Jacobs R, Raab HR, and Schmidt RE (1996). Catecholamines modulate human NK cell circulation and function via spleen-independent beta 2-adrenergic mechanisms. J Immunol 156, 93–99. [PubMed] [Google Scholar]
- Tang Y, Xu X, Guo S, Zhang C, Tang Y, Tian Y, Ni B, Lu B, and Wang H (2014). An increased abundance of tumor-infiltrating regulatory T cells is correlated with the progression and prognosis of pancreatic ductal adenocarcinoma. PLoS One 9, e91551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyagarajan A, Alshehri MSA, Miller KLR, Sherwin CM, Travers JB, and Sahu RP (2019). Myeloid-Derived Suppressor Cells and Pancreatic Cancer: Implications in Novel Therapeutic Approaches. Cancers (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuck MK, Chan DW, Chia D, Godwin AK, Grizzle WE, Krueger KE, Rom W, Sanda M, Sorbara L, Stass S, et al. (2009). Standard operating procedures for serum and plasma collection: early detection research network consensus statement standard operating procedure integration working group. J Proteome Res 8, 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. (2013). Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med 369, 1691–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wainberg ZA, Hochster HS, Kim EJ, George B, Kaylan A, Chiorean EG, Waterhouse DM, Guiterrez M, Parikh A, Jain R, et al. (2020). Open-label, Phase I Study of Nivolumab Combined with nab-Paclitaxel Plus Gemcitabine in Advanced Pancreatic Cancer. Clin Cancer Res 26, 4814–4822. [DOI] [PubMed] [Google Scholar]
- Wang L, Simons DL, Lu X, Tu TY, Solomon S, Wang R, Rosario A, Avalos C, Schmolze D, Yim J, et al. (2019). Connecting blood and intratumoral Treg cell activity in predicting future relapse in breast cancer. Nat Immunol 20, 1220–1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerberg E, Lhuillier C, Rybstein MD, Dannenberg K, Rudqvist NP, Koelwyn GJ, Jones LW, and Demaria S (2020). Exercise reduces immune suppression and breast cancer progression in a preclinical model. Oncotarget 11, 452–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winograd R, Byrne KT, Evans RA, Odorizzi PM, Meyer AR, Bajor DL, Clendenin C, Stanger BZ, Furth EE, Wherry EJ, and Vonderheide RH (2015). Induction of T-cell Immunity Overcomes Complete Resistance to PD-1 and CTLA-4 Blockade and Improves Survival in Pancreatic Carcinoma. Cancer Immunol Res 3, 399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrangle JM, Velcheti V, Patel MR, Garrett-Mayer E, Hill EG, Ravenel JG, Miller JS, Farhad M, Anderton K, Lindsey K, et al. (2018). ALT-803, an IL-15 superagonist, in combination with nivolumab in patients with metastatic non-small cell lung cancer: a non-randomised, open-label, phase 1b trial. Lancet Oncol 19, 694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Weisshaar N, Hotz-Wagenblatt A, Madi A, Ma S, Mieg A, Hering M, Mohr K, Schlimbach T, Borgers H, and Cui G (2020). Skeletal muscle antagonizes antiviral CD8(+) T cell exhaustion. Sci Adv 6, eaba3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu WC, Sun HW, Chen HT, Liang J, Yu XJ, Wu C, Wang Z, and Zheng L (2014). Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc Natl Acad Sci U S A 111, 4221–4226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanez A, Coetzee SG, Olsson A, Muench DE, Berman BP, Hazelett DJ, Salomonis N, Grimes HL, and Goodridge HS (2017). Granulocyte-Monocyte Progenitors and Monocyte-Dendritic Cell Progenitors Independently Produce Functionally Distinct Monocytes. Immunity 47, 890–902 e894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang A, Herter-Sprie G, Zhang H, Lin EY, Biancur D, Wang X, Deng J, Hai J, Yang S, Wong KK, and Kimmelman AC (2018). Autophagy Sustains Pancreatic Cancer Growth through Both Cell-Autonomous and Nonautonomous Mechanisms. Cancer Discov 8, 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan B, Wen S, Lu J, Shen G, Lin X, Feng J, and Huang H (2017). Identification and causes of metabonomic difference between orthotopic and subcutaneous xenograft of pancreatic cancer. Oncotarget 8, 61264–61281. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Single-cell RNA seq data have been deposited at GEO and are publicly available as of date of publication. Accession numbers are listed in the key resources table.
This paper does not report original code.
Any additional information required to reanalyze the data reported in this paper is available from the lead contact upon request.
Key resources table for Kurz et. al.
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Antibodies | ||
| PE anti-mouse CD125 (IL-15Rα) (clone 6B4C88) | Biolegend | Cat #: 153503 |
| IL15RA Polyclonal Antibody | Invitrogen | Cat #: PA5 – 79467 |
| In VivoMAb anti-mouse PD-1 (clone RMP1-14) | BioXcell | Cat #: BE0146 |
| In VivoMAb Anti-mouse IL-15 (clone AIO.3) | BioXcell | Cat #: BE0315 |
| In VivoMAb Anti-mouse CD8 alpha (clone 2.43) | BioXcell | Cat #: BE0061 |
| PE/Cyanine7 anti-mouse CD8 alpha (clone 53–6.7) | Biolegend | Cat #: 100721 |
| APC anti-mouse CD279 (PD-1) (clone 29F.A12) | Biolegend | Cat #: 135209 |
| Brilliant Violent 421™ anti-mouse CD3 (clone 17A2) | Biolegend | Cat #: 100227 |
| PerCP/Cyanine5.5 anti-mouse IFN-Y (clone XMG1.2) | Biolegend | Cat #: 505821 |
| Goat Anti-Rabbit IgG H&L (AF488) | Abcam | Cat #: ab150077 |
| Recombinant anti-IL-15 antibody [EPR22635–214] | Abcam | Cat #: ab273631 |
| PE/Dazzle™ 594 anti-T-bet (clone 4B10) | Biolegend | Cat #: 644827 |
| PerCP/Cyanine5.5 anti-human/mouse Granzyme B Recombinant Antibody (clone QA16A02) | Biolegend | Cat #: 372211 |
| PE/Dazzle™ 594 anti-mouse Ki67 (clone 16A8) | Biolegend | Cat #: 652427 |
| Alexa Flour 647 anti-TCF1 (TCF7) (clone 7F11A10) | Biolegend | Cat #: 655203 |
| Mouse anti-Human CD8 Ab-1 (Clone C8/144B) | Thermofisher | Cat #: MS-457-S |
| Mouse anti-Human GranzymeB (clone 11F1) | Leica Biosystems | Cat #: GRAN-B-L-CE |
| Brilliant Violent 421™ anti-mouse/human CD44 (clone IM7) | Biolegend | Cat #: 103039 |
| Bacterial and virus strains | ||
| Biological samples | ||
| Human Pancreatic Cancer Surgical Specimens from Exercised Patients | University of Texas MD Anderson Cancer Center | https://clinicaltrials.gov/ct2/show/NCT03187951 |
| Human Pancreatic Cancer Surgical Specimens from Historical Control Cohort | University of Texas MD Anderson Cancer Center | https://www.nature.com/articles/s41598-019-49582-3 |
| Chemicals, peptides, and recombinant proteins | ||
| Recombinant human IL-15 Superagonist (IL-15 S.A.), hetero-dimer of human IL-15 and sIL-15Ra made in HEK293 Cells | Novartis, via MTA with NYU Langone | NIZ985 |
| Fingolimod hydrochloride (FTY720) > 98% HPLC | Sigma Aldrich/Millipore | Cat #: SML0700, CAS Number: 162359-56-0 |
| (−)-Epinephrine | Sigma Aldrich | Cat #: E4250, CAS Number: 51-43-4. EC Number: 200-098-7 |
| (±)-Propranolol hydrochloride, > 99% TLC, powder | Sigma Aldrich | Cat #: P0884, CAS Number: 318-98-9. EC Number: 206-268-7 |
| Critical commercial assays | ||
| Epinephrine ELISA Kit (Colorimetric) | Novus Biologicals | Cat #: KA1882 |
| Mouse IL-15 ELISA Kit | Sigma-Aldrich | Cat #: RAB0260 |
| Deposited data | ||
| 10x Single Cell RNA Sequencing Raw Data | This paper | GEO: GSE176311 |
| Experimental models: Cell lines | ||
| Mouse: KPC 4662 Pancreatic Cancer cell line Derived from LSL-KRASG12D/+ p53R172H/+; Pdx-1Cre/+ mice |
Gift from Robert Vonderheide, UPENN | N/A |
| Mouse: KPC 1203 Pancreatic Cancer cell line Derived from LSL-KRASG12D/+ p53R172H/+; Pdx-1Cre/+ mice |
NYU Grossman School of Medicine, Department of Cell Biology | N/A |
| Experimental models: Organisms/strains | ||
| Mouse: C57BL/6J wild type mice | The Jackson Laboratory | JAX: 000664 |
| Mouse: KC LSL-KRASG12D/+; p48Cre/+ | NYU Langone Medical Center, Division of Comparative Medicine mouse facility | N/A |
| Mouse: NU/J Foxn1nu athymic nude mice | The Jackson Laboratory | JAX: 002019; RRID: ISMR_JAX:002019 |
| Mouse: B6.129S7-Rag1tm1Mom/J Rag1KO mice | The Jackson Laboratory | JAX: 002216, RRID: IMSR_JAX:002216 |
| Oligonucleotides | ||
| Recombinant DNA | ||
| Software and algorithms | ||
| GraphPad Prism 9.3.1 | Dotmatics | https://www.graphpad.com |
| iCellR R package (v1.5.5) | This paper | https://CRAN.R-project.org/package=iCellR |
| Ingenuity Pathway Analysis (IPA) | Qiagen | https://digitalinsights.qiagen.com/products-overview/discovery-insights-portfolio/analysis-and-visualization/qiagen-ipa/?gclid=Cj0KCQjwpcOTBhCZARIsAEAYLuU2uBoPYEwAjfKm5S0IeMMKdT2e6A9WQDKXBNipkzh0QNE9wNgH-mQaAspZEALw_wcB |
| FlowJo Version 10.6.1 | Treestar | https://www.flowjo.com/solutions/flowjo |
| inform Tissue Analysis Software | Akoya Biosciences | https://www.akoyabio.com/phenoimager/software/inform-tissue-finder/ |
| Vectra Polaris Multispectral Imaging System | Akoya Biosciences | https://www.akoyabio.com/phenoimager/instruments/vectra-3-0/ |
| Other | ||
| Rodent Five-Lane Treadmill | Harvard Apparatus | Cat #: 76–0895 |


