Abstract
Aims
To evaluate the clinical efficiency of on-table extubation (OTE) versus delayed extubation in patients aged over 60 years that underwent minimally invasive mitral or aortic valve replacement surgery and evaluate the factors associated with successful OTE implementation.
Materials
Patients over 60 years with mitral or aortic valve disease who received minimally invasive mitral or aortic valve replacement surgery from October 2020 to October 2021 were selected retrospectively. We divided patients into the on-table extubated (OTE) group (n = 71) and the delayed extubation (DE) group (n = 22). Preoperative, intraoperative, and postoperative clinical variables were compared between the two groups.
Results
Patients in the DE group underwent longer surgery time, longer aortic occlusion clamping time and longer cardiopulmonary bypass time than those in the OTE group(217.48 ± 27.83 vs 275.91 ± 77.22, p = 0.002; 76.49 ± 16.00 vs 126.55 ± 54.85, p = 0.001; 112.87 ± 18.91 vs 160.77 ± 52.17, p = 0.001). Patients in the OTE group had shorter postoperative mechanical ventilation time (min), shorter ICU time, shorter postoperative hospital length of stay and lower total cost and medication cost (p < 0.05). The AUC for aortic occlusion clamping time was 0.81 (p < 0.01), making it the most significant predictor of on-table extubation success.
Conclusions
On-table extubation following mitral or aortic valve cardiac surgery was associated with a superior clinical outcome and high cost-effectiveness.
Keywords: mitral or aortic valve disease, on-table extubation, delayed extubation, aortic occlusion clamping, cardiopulmonary bypass time
Introduction
Postoperative ventilation was traditionally thought to be required for maintaining patient stability following cardiac surgery; however, long-term mechanical ventilation may raise the risk of unfavorable outcomes in the postoperative period, particularly in elderly patients. A recent study showed that a prolonged duration of mechanical ventilation (DMV) decreases mid-term survival rates after coronary artery bypass grafting (CABG) (1). Fast-track cardiac anesthesia (FTCA) provides an alternative to standard cardiac surgical care by improving efficiency without compromising safety or clinical results (2). FTCA may result in more efficient use of resources, especially if intensive care unit (ICU) beds are scarce (3). Meanwhile, the benefits of early extubation were recognized in cardiac surgery and supported by several previous studies (4).
In our current practice, on-table extubation (OTE) was attempted even for patients who underwent complicated cardiac surgery. The factors that preclude OTE are bleeding, hypothermia, cardiovascular instability, post-operation pain, post-cardiopulmonary bypass (CPB) pulmonary injury leading to reintubation and an increase in morbidity (5, 6). This study was undertaken to evaluate the efficacy of on-table extubation versus delayed extubation in terms of postoperative complications, length of stay (LOS) in ICU, patient recovery, and hospital LOS in patients aged over 60 years that received minimally invasive mitral or aortic valve replacement surgery and to evaluate the factors associated with successful implementation of OTE.
Methods
Patients and Methods
It is a single-centre, retrospective observational study. A total of 93 patients aged over 60 years with mitral or aortic valve disease were selected from October 2020 to October 2021 in Zhejiang Provincial People’s Hospital, China. This study was approved by the ethics committees of this hospital (No. 2018KY034). Individual consent was waived owing to the retrospective nature of the study. All interventions were performed by the same surgical team.
The inclusion criteria were as follows: (1) patients above 60 years of age; (2) patients presenting with isolated mitral or aortic valve lesions; (3) Eurscore ≤5 (7). The exclusion criteria were as follows: (1) patients with potential surgical complications, such as severe chest deformity and a history of right chest surgery; (2) patients with femoral arteriovenous malformations; (3) patients with severe valvular heart disease whose cardiac structure form had seriously changed with left ventricular end-diastolic diameter >60 mm or ejection fraction (EF) <50%; (4) patients in poor general condition, accompanied by multiple organ failure, such as patients with New York Heart Association (NYHA) class grade IV heart failure (7), showing no improvement after treatment; (6) patients having emergency surgery, redo-surgery; (7) moderate pulmonary hypertension (pulmonary artery systolic pressure ≥50 mmHg); (8) patients with chronic obstructive pulmonary diseases.
Anesthesia
Anesthesia was administered by the same team during the perioperative period. Our department’s protocol for anesthesia was as follows: etomidate 0.2 mg/kg, propofol (1–2 mg/kg), sufentanil (0.5–1 µg/kg) and rocuronium bromide (0.6 mg/kg) were used to induce anesthesia. Continuous venous infusion of remifentanil (0.2 µg/kg/min), rocuronium bromide (7 µg/kg/min) and propofol (3–8 mg/kg/h) was used for the maintenance of anesthesia during surgery. Before chest closure, sufentanil (40–60 µg) was administered. The bispectral index (BIS) was utilized as an indicator of anesthesia level (depth) and was kept between 40 and 60. After induction, a single-lumen endotracheal tube was used to intubate the patient in the supine position, with the patient’s arm abducted at an angle of 90°. The blocks were performed as originally described by Blanco and colleagues (8), serratus anterior plane block (SAPB) was performed with a 22-gauge, 50-mm needle (Pajunk Medical Systems, Jiangsu, China) under ultrasound in-plane guidance. The target site was the fascial plane between the serratus anterior muscle posterior surface and lateral periosteum of the fifth right rib on the midaxillary line; ropivacaine 0.375% 150 mg was injected into the serratus anterior muscle under continuous ultrasound guidance. Then the patient was placed in the supine position and tilted (30°) to the left with a slight elevation of the right side using a pad. Transoesophageal echocardiography was used during the operation. All of the TTE was performed by 2 sonographers with at least 20 years of experience. Rocuronium bromide was stopped when the primary intervention was over.
Thoracoscopy-Assisted Minimally Invasive Mitral or Aortic Valve Replacement
A 3-cm longitudinal incision was made in the right groin area to expose the femoral artery and vein (incision 1) for cardiopulmonary bypass (CPB). Additionally, a 4–6 cm curved incision was made along the lower margin of the right breast to gain access to the thoracic cavity via the fourth intercostal space (incision 2). A rib spreader was used to expand the intercostal space. The pericardium was incised, and the aortic root was exposed. Systemic heparinization was achieved, and the femoral artery was cannulated using a femoral artery cannula, and a vena cava cannula was cannulated from the femoral vein to the superior vena cava, respectively. The cardioplegia cannula was inserted into the aortic root following the complete bypass. When the body temperature dropped to 34°C, the ascending aorta was clamped. For mitral valve replacement, the left atrial was opened. A draw hook was used to accending the anterior wall of the left atrial through a 0.5-cm incision (incision 3). The mitral valve was then visualized. For aortic valve replacement, the aortic root was selected. The lesion valve was excised, and the valve replacement was performed using intermittent sutures. A chest tube was placed via incision 1 for closed-chest drainage.
During CPB, goal-directed perfusion was implemented to maintain an oxygen delivery (DO2) level above the identified critical value of 272 mL/min/m2 (9), Blood gas analysis was performed every half hour, oxygen delivery (DO2) was calculated using the following equation: DO2 (mL/min/m2) = 10 × pump flow (L/min/m2) × arterial oxygen content (mL/100 mL), where arterial oxygen content was calculated as follows: Arterial oxygen content (mL/100 mL) = Hb (mg/dL) × 1.34 × Hb saturation (%) + 0.003 × O2 tension (mmHg). (10) Close attention was paid to the hematocrit, which was sustained at around 25%. If the hematocrit went below 25%, perfusion flow or ultrafiltration was utilized to adjust the perfusion rate. Red blood cells (RBCs) were transfused if the hematocrit stayed below 23%.
Ventilator Setting, Blood Gas Analyses, and Extubation Criteria
Mechanical ventilation was initiated immediately after the induction of anesthesia. The volume-control mode with the following settings was used: tidal volume, 6–10 mL/kg; respiration rate, 8–12/min; and FiO2, 0.4–0.6. Ventilator settings were changed to maintain PaO2 at ≥100 mmHg, partial CO2 pressure (PaCO2) at 35–45 mmHg, and pH at 7.35–7.45. During CPB, tidal volume was reduced to a small tidal volume (1–1.5 mL/kg). After CPB, the tidal volume and respiration rate were the same as described above after full reexpansion of the lungs by several manual hyperinflations (peak pressure at 20–30 mmHg), and thorough intratracheal suctions.
Blood gas analysis (BGA) was measured before surgery and within 30 min of each time point. Pulmonary function parameters were calculated as follows: PFR = PaO2/FiO2.
Assessable metrics were selected through the collaboration between anesthesiologists and cardiac surgeons, which included assessment of postoperative bleeding, stability of hemodynamic parameters, vasopressor requirement and adequacy of gas exchange. Remifentanil and propofol would be withdrawn if these requirements were met before closing the chest wall.
Patients were extubated when they met the following extubation criteria: (1) hemodynamic stability with reasonable urine output and warm peripheral extremities; (2) PaO2 ≥ 75 mmHg and PaCO2 ≤ 50 mmHg with adequate spontaneous respiration under FiO2 ≤ 0.4 and end of expiratory pressure ≤5 cm H2O; (3) awake and able to respond to commands without new neurological symptoms; (4) no active bleeding with a reasonable change in hemoglobin and no requirement for volume replacement; and (5) no reasonable fear of reintubation.
Data Collection
Trained staff collected detailed data, including the clinical cost for each patient, from the electronic medical records at our medical center. The baseline characteristics collected for each patient involved age, gender, height, weight, body mass index (BMI, calculated based on height and weight recorded by the nurse on the day of hospital admission), diabetes mellitus, hypertension, chronic obstructive pulmonary disease (COPD), left ventricular ejection fraction (LVEF), preoperative hemoglobin, hematocrit (Hct), preoperative serum creatinine (sCr); eGFR (estimated glomerular filtration rate, calculated based on the Modification of Diet in Renal Disease formula).
BGA data before surgery and the end of surgery were also recorded. BGA was measured within 30 min of each time point. Pulmonary function parameters were calculated as follows: PFR = PaO2/FiO2, alveolar-arterial oxygen gradient (AaDO2) = (760−47) × FiO2−PaCO2/0.8−PaO2.
Intraoperative data: type and duration of surgery, CPB time, aortic cross-clamp time, plasma and red blood cell infusion, dosage of sufentanil, remifentanil and rocuronium bromide.
Postoperative data: LVEF, hemoglobin, hematocrit, sCr, eGFR, extubation time, length of ICU, postoperative hospital stay.
Postoperative adverse events during the hospital stay included death, heart failure requiring intravenous medical therapy (e.g., inotropes, furosemide) beyond the fourth postoperative day, myocardial infarction, symptomatic stroke, and acute renal failure requiring hemofiltration. Assessment of delirium was performed preoperatively (baseline) and postoperatively at 12-h intervals or as needed according to the ICU patient’s condition using the confusion assessment method (CAM). When patients were discharged from the ICU to the surgical floor, delirium was also assessed using CAM.
Statistical Analyses
All statistical analyses were performed using the SPSS statistical package, version 25.0 (SPSS Inc., Chicago, IL, USA). Data are expressed as mean ± standard deviation. Differences between groups were evaluated using the Mann–Whitney U test for continuous variables and Fisher’s exact test for binary categorical variables. Univariate and multivariate logistic regression analyses were performed to identify predictors of prolonged delayed extubation. Receiver operating characteristic (ROC) curves evaluated the sensitivity and specificity of predicting successful on-table extubation. Frequencies and percentages were used for categorical variables. A p-value of <0.05 was considered statistically significant.
Result
We successfully extubated 71 (76.3%) patients within 1 h after surgery using the on-table (in operation room) extubation strategy and no patients received any reintubation, 22 patients did not meet the extubation criteria within one hour after surgery; thus, delayed extubation was performed in the intensive care unit (ICU). When the 71 on-table extubated (OTE) patients were compared to the 22 delayed extubation (DE) patients, the OTE group’s general characteristics did not differ substantially from those of the DE group (Table 1).
Table 1.
Demonstrates a comprehensive list of preoperative characteristics of the participants.
| OTE group | DE group | p-value | |
|---|---|---|---|
| Number of patients | 71 | 22 | |
| Age (years) | 72.11 ± 3.16 | 73.27 ± 2.83 | 0.127 |
| Body mass index (kg/m2) | 22.39 ± 3.26 | 23.13 ± 3.25 | 0.355 |
| Male gender, n (%) | 34(47.9) | 10(45.5) | 0.842 |
| Hypertension, n (%) | 23(32.4%) | 10(45.5%) | 0.263 |
| Diabetes mellitus, n (%) | 10(14.1) | 1(4.5) | 0.660 |
| Previous stroke, n (%) | 3(4.2%) | 1(4.5%) | 1.000 |
| Atrial fibrillation, n (%) | 39(54.9%) | 12(54.5%) | 0.809 |
| Coronary artery disease, n (%) | 14(19.7%) | 6(27.3) | 0.646 |
| Current smoker, n (%) | 7(9.9%) | 3(13.6%) | 0.916 |
| Euroscore II | 3.94 ± 0.82 | 3.86 ± 0.73 | 0.661 |
| NYHA CLASS | |||
| I | 11 | 2 | 0.722 |
| II | 50 | 17 | |
| III | 10 | 3 | |
| End-diastolic length of the left ventricle (mm) | 53.04 ± 9.31 | 52.14 ± 7.82 | 0.680 |
| End-systolic length of the left atrium (mm) | 51.72 ± 10.62 | 49.45 ± 8.16 | 0.361 |
| Preoperative LVEF (%) | 61.66 ± 7.36 | 61.36 ± 6.76 | 0.866 |
| Pulmonary hypertension, n (%) | 40(56.3%) | 11(50.0%) | 0.602 |
| Preoperative hemoglobin (g/L) | 129.21 ± 16.31 | 134.27 ± 18.75 | 0.223 |
| Preoperative Hct (%) | 39.26 ± 8.00 | 39.71 ± 5.31 | 0.805 |
| Preoperative sCr (μmol/l) | 79.87 ± 15.42 | 84.61 ± 12.95 | 0.195 |
| Preoperative eGFR (mL/min·1.73 m2) | 83.69 ± 21.35 | 81.76 ± 16.96 | 0.705 |
| Preoperative PaO2/FiO2 | 387.68 ± 32.48 | 379.71 ± 11.53 | 0.274 |
| Preoperative PaCO2 | 40.82 ± 3.05 | 40.58 ± 2.90 | 0.748 |
| Preoperative PA-ao2 | 16.44 ± 5.14 | 17.51 ± 5.22 | 0.408 |
| Lactic acid | 1.31 ± 0.42 | 1.33 ± 0.39 | 0.851 |
Percentages are given as total within group; Continuous data are expressed as mean ± standard deviation.
eGFR, estimated glomerular filtration rate (calculated using the Modification of Diet in Renal Disease (MDRD) formula); sCr, serum creatinine; Hct, hematocrit; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association.
Intro-Operative and Post-Operative Data
Patients in the DE group underwent longer surgery time, longer aortic occlusion clamping time, longer cardiopulmonary bypassing time than patients in the OTE group, and significant decreases in PFR (PaO2/FiO2) were observed at the end of surgery in the DE group (p < 0.001). The dose of sufentanil, rocuronium and remifentanil was higher in the DE group, and this change was associated with the prolonged operation time in the DE group. Patients in the OTE group had shorter postoperative mechanical ventilation time, shorter intensive care unit time, and shorter postoperative hospital length of stay (p < 0.05) (Table 2).
Table 2.
Intraoperative parameters and postoperative parameters.
| OTE group | DE group | p-value | |
|---|---|---|---|
| Number of patients | 71 | 22 | |
| Operation type | |||
| Mitral valve surgery | 52 | 13 | 0.206 |
| Aortic valve surgery | 19 | 9 | |
| Operation time (min) | 217.48 ± 27.83 | 275.91 ± 77.22 | 0.002 |
| Aortic occlusion clamping time | 76.49 ± 16.00 | 126.55 ± 54.85 | 0.001 |
| Cardiopulmonary bypass time (time) | 112.87 ± 18.91 | 160.77 ± 52.17 | 0.001 |
| Red cell transfusions, n (%) | 8(11.3%) | 5(22.7) | 0.049 |
| Plasma transfusions, n (%) | 4 (5.6%) | 2 (9.1%) | 0.883 |
| Fluid balance (mL) | 1228.87 ± 268.42 | 1277.27 ± 209.10 | 0.382 |
| Urine output | 601.97 ± 318.80 | 620.45 ± 343.53 | 0.816 |
| Sufentanil | 51.53 ± 6.37 | 57.86 ± 8.00 | 0.002 |
| Rocuronium | 115.15 ± 25.363 | 134.86 ± 26.552 | 0.003 |
| Remifentanil | 2.38 ± 0.58 | 2.96 ± 0.74 | 0.003 |
| Postoperative PaO2/FiO2 | 334.36 ± 54.01 | 200.14 ± 44.325 | 0.001 |
| Postoperative PaCO2 | 40.19 ± 4.00 | 38.86 ± 4.66 | 0.200 |
| Postoperative lactic acid | 1.23 ± 0.37 | 1.31 ± 0.28 | 0.361 |
| Postoperative mechanical ventilation timea (min) | 22.63 ± 4.89 | 425.29 ± 155.33 | 0.001 |
| Intensive care unit time (h) | 15.44 ± 3.40 | 23.65 ± 9.50 | 0.001 |
| Postoperative hospital stay (d) | 7.90 ± 1.43 | 11.33 ± 1.56 | 0.001 |
| First Postoperative day drainage (mL) | 183.94 ± 57.26 | 206.19 ± 60.79 | 0.126 |
| Second Postoperative day drainage (mL) | 60.49 ± 19.55 | 59.52 ± 15.96 | 0.836 |
The time from the end of operation to extubation.
As our data showed that operative time, aortic occlusion clamping time, cardiopulmonary bypassing time and the dose of sufentanil, rocuronium and remifentanil were associated with on-table extubation, and significant decreases in PFR (PaO2/FiO2) were observed at the end of surgery in DE group(p < 0.001). Receiver operating characteristic (ROC) curves were used to evaluate the sensitivity and specificity of these variables regarding on-table extubation feasibility. Aortic occlusion clamping time was the most significant predictor of successful on-table extubation, with an AUC of 0.81 (p < 0.01) (Figure. 1). Notably, using a cut-off of 92 min, aortic occlusion clamping time was able to predict the success of OTE with a sensitivity of 69.56% and a specificity of 85.71%.
Figure 1.
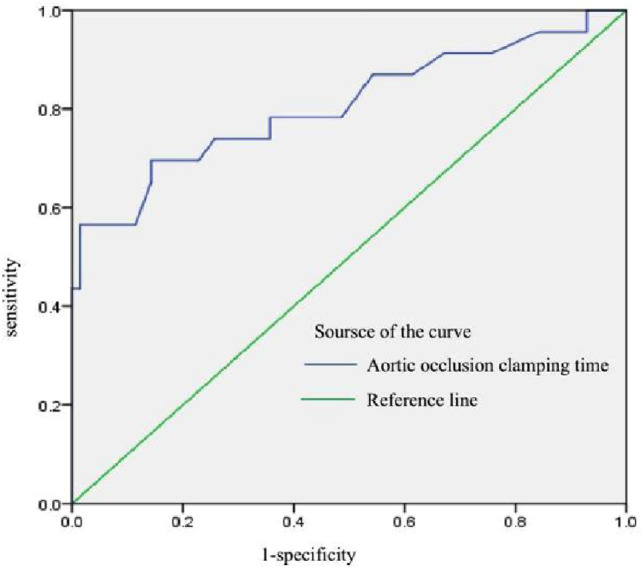
Receiver operating characteristic curve for aortic occlusion clamping time.
Postoperative Adverse Events
The rate of acute kidney injury (AKI) was higher in the DE group (2.82%, 27.27%; p = 0.001). In contrast, there was no significant difference between delirium, arrhythmia, and thoracentesis incidence.
months Follow-up Data
There were no prosthetic valve-related complications or death reported during the follow-up period. In addition, there were no significant differences between the two groups in left ventricular end-diastolic diameter, left atrial diameter, LVEF, pulmonary arterial pressure, or heart function after the operation, which illustrated that there was no significant difference in efficacy between the two groups (Table 3).
Table 3.
3 months follow-up data comparison between two groups.
| OTE group | DE group | p-value | |
|---|---|---|---|
| End-diastolic length of the left ventricle (mm) | 49.75 ± 7.60 | 51.43 ± 7.74 | 0.377 |
| End-systolic length of the left atrium (mm) | 47.96 ± 10.33 | 43.81 ± 7.49 | 0.773 |
| LVEF (%) | 57.47 ± 9.37 | 56.81 ± 8.76 | 0.090 |
| Pulmonary artery systolic pressure | 34.50 ± 4.79 | 5.78 ± 4.76 | 0.483 |
| Perivalvular leakage | 0 | 0 |
Cost Analysis of Different Extubation Treatment
As illustrated in Table 4, in both groups, the total cost and medication cost of the DE group were higher than that of the OTE group (p < 0.001), while the surgery cost was similar between the two groups.
Table 4.
Cost analysis of different extubation treatments.
| OTE group | DE group | p-value | |
|---|---|---|---|
| Total cost (yuan) | 105809.76 ± 11519.21 | 124094.27 ± 15576.02 | 0.001 |
| Medication cost (yuan) | 10248.93 ± 1617.34 | 13940.99 ± 2257.97 | 0.001 |
| Non-medication cost (yuan) | 95560.83 ± 10970.79 | 110153.28 ± 15061.67 | 0.001 |
| Surgery cost (yuan) | 15327.44 ± 4407.39 | 15035.90 ± 2131.13 | 0.677 |
| Other medical cost (yuan) | 80233.39 ± 11924.29 | 95117.38 ± 15075.72 | 0.001 |
Discussion
Generally, complex valve surgery necessitates a more prolonged CPB, resulting in an extended duration of mechanical ventilation and ICU stay (11, 12). Delayed extubation is primarily reserved for high-risk individuals at high risk of serious cardiorespiratory complications. As a new recovery concept, early extubation of patients from mechanical ventilation within a few hours after heart surgery may minimize the length of ICU and hospital stays and enhance clinical outcomes (13). Given that the practicality of early extubation has remained limited to elective patients, it is crucial to investigate the predictive factors associated with the successful implementation of this procedure.
Our retrospective analysis demonstrated that on-table extubation is safe and feasible in people aged above 60 years undergoing mitral or aortic valve surgery via thoracic right-anterior minimal incision combined with serratus anterior plane block (SAPB). The majority (76%) of older patients could be successfully extubated in the operating room at the end of the surgery, resulting in better clinical outcomes if they meet the extubation criteria. The OTE rate of our study was lower than Ziae Totonchi’s study, whose OTE rate was 96% since the patients were younger (49.26 ± 13.26 years), most surgeries performed (83.3%) were coronary artery bypass grafting, and the CPB time was shorter (57.04 ± 23.05 min) (14). In contrast, the OTE rate of our study was higher than a previous study in pediatric patients after congenital cardiac surgery (53.1%) (15). Patients in the OTE group had shorter postoperative mechanical ventilation time, shorter intensive care unit time, and shorter postoperative hospital length of stay (p < 0.05). The rate of acute kidney injury (AKI) was higher in the DE group (2.82%, 27.27%; p = 0.001). The total cost and medication cost of the DE group were higher than that of the OTE group (p < 0.001). Based on our findings, we concluded that if patients met the OTE criteria, it could be a more cost-effective method to achieve a better clinical outcome.
Prolonged operative time, aortic occlusion clamping time, and cardiopulmonary bypassing time were inversely associated with the ability to accomplish OTE. In elderly patients, the incidence of postoperative complications and perioperative mortality is significantly increased when the CPB time is prolonged. The study showed that the longer the CPB time, the higher the perioperative mortality. Compared to patients with a CPB time shorter than 75 min, the perioperative risk of death with a CPB time longer than 75 min increased sharply (16). Impaired pulmonary gas exchange is a common complication after cardiopulmonary bypass (CPB) (6). In our study, the mean CPB time in the OTE group was 112.87 ± 18.91 min, compared to 160.77 ± 52.17 min in the DE group. Aortic occlusion clamping time was the most significant predictor of successful on-table extubation with an AUC of 0.81 (p < 0.01).
The dose of sufentanil, rocuronium and remifentanil was higher in the DE group, and this change corresponds to the prolonged operation time in the DE group. We believe that the anesthetic technique plays an important role too. Herein, the anesthetic protocol focused on minimizing sedating drugs, encouraging shorter-acting intraoperative opiates, and performing SAPB to decrease the sufentanil dose during operation (17). SAPB with general anesthesia (GA) can provide high-quality analgesia and quick rehabilitation (18). During CPB, mechanical ventilation was switched to a small tidal volume (1–1.5 mL/kg, the FiO2 was 50%), which not only does not affect the surgical procedure but may also reduce pulmonary injury during CPB, particularly in valve surgery via thoracic right-anterior minimal incision (19). The need for postoperative analgesia during the recovery area stay was considerably higher in the remifentanil group than in the sufentanil group (20). In our hosptal, we used sufentanil combined with remifentanil.
Nevertheless, there are some limitations to this study. This was a single-center retrospective study with a small sample size. A prospective, multicenter, randomized controlled trial is warranted in the future to support our result. Moreover, the follow-up time of our study was short, and a longer follow-up is needed to identify the long-term outcome of on-table extubation. In this retrospective study, we implemented on-table extubation under our present anesthesia protocol, but more studies are needed to determine the outcome of OTE under different anesthesia conditions.
Conclusions
On-table extubation after mitral or aortic valve cardiac surgery is a relatively new strategy. Herein, we performed OTE in accordance with our enrollment criteria and our hospital’s anesthesia protocol. Importantly, we uncovered that successful on-table extubation in the operative room was associated with a better clinical outcome and high cost-effectiveness.
Acknowledgments
We are grateful to everyone who participated in the study and helped with data analyses and manuscript preparation.
Funding
This study was supported by the Social Development Project of Public Welfare Technology Application in Zhejiang Province(Grant no. LGF19H020010), Health Science and Technology Program of Zhejiang Province (2021KY476, 2019KY299, 2021KY504). The funders had no role in the study design, data collection and analysis, decision to publish, or manuscript preparation.
Data Availability Statements
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Ethics Statement
The studies involving human participants were reviewed and approved by The studies involving human participants were reviewed and approved by the Institutional Ethics Committee of Zhejiang Provincial People’s Hospital, China (No. 2018KY034). Individual consent was waived since this is a retrospective study. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author Contributions
QW and YFG conceived and designed the study, drafted the manuscript, as well as supervised the study and critically revised the manuscript. YFG, ZBH, QX, and YC collected clinical data. QW, YFG, and HM were responsible for drafting the manuscript and statistical analyses. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Saleh HZ, Shaw M, Al-Rawi O, Yates J, Pullan DM, Chalmers JA, et al. Outcomes and predictors of prolonged ventilation in patients undergoing elective coronary surgery. Interact Cardiovasc Thorac Surg. (2012) 15(1):51–6. 10.1093/icvts/ivs076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lloyd-Donald P, Lee WS, Hooper JW, Lee DK, Moore A, Chandra N, et al. Fast-track recovery program after cardiac surgery in a teaching hospital: a quality improvement initiative. BMC Res Notes. (2021) 14(1):201. 10.1186/s13104-021-05620-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dorsa AG, Rossi AI, Thierer J, Lupiañez B, Vrancic JM, Vaccarino GN, et al. Immediate extubation after off-pump coronary artery bypass graft surgery in 1,196 consecutive patients: feasibility, safety and predictors of when not to attempt it. J Cardiothorac Vasc Anesth. (2011) 25:431–6. 10.1053/j.jvca.2010.08.013 [DOI] [PubMed] [Google Scholar]
- 4.Villafranca A, Thomson IA, Grocott HP, Avidan MS, Kahn S, Jacobsohn E. The impact of bispectral index versus end-tidal anesthetic concentration-guided anesthesia on time to tracheal extubation in fast-track cardiac surgery. Anesth Analg. (2013) 116(3):541–8. 10.1213/ANE.0b013e31827b117e [DOI] [PubMed] [Google Scholar]
- 5.Tanner TG, Colvin MO. Pulmonary complications of cardiac surgery. Lung. (2020) 198(6):889–96. 10.1007/s00408-020-00405-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murata T, Maeda M, Amitani R, Hiromoto A, et al. Postoperative changes in pulmonary function after valve surgery: oxygenation Index early after cardiopulmonary is a predictor of postoperative course. J Clin Med. (2021) 10(15):3262. 10.3390/jcm10153262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gogbashian A, Sedrakyan A, Treasure T. EuroSCORE: a systematic review of international performance. Eur J Cardiothorac Surg. (2004) 25(5):695–700. 10.1016/j.ejcts.2004.02.022 [DOI] [PubMed] [Google Scholar]
- 8.Blanco R, Parras T, McDonnell JG, Prats-Galino A. Serratus plane block: a novel ultrasound-guided thoracic wall nerve block. Anaesthesia. (2013) 68(11):1107–13. 10.1111/anae.12344 [DOI] [PubMed] [Google Scholar]
- 9.Ranucci M, Romitti F, Isgrò G, Cotza M, Brozzi S, Boncilli A, et al. Oxygen delivery during cardiopulmonary bypass and acute renal failure after coronary operations. Ann Thorac Surg. (2005) 80(6):2213–20. 10.1016/j.athoracsur.2005.05.069 [DOI] [PubMed] [Google Scholar]
- 10.de Somer F, Mulholland JW, Bryan MR, Aloisio T, Van Nooten GJ, Ranucci M. O2 delivery and CO2 production during cardiopulmonary bypass as determinants of acute kidney injury: time for a goal-directed perfusion management? Crit Care. (2011) 15(4):R192. 10.1186/cc10349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cislaghi F, Condemi AM, Corona A. Predictors of prolonged mechanical ventilation in a cohort of 5123 cardiac surgical patients. Eur. J. Anesthesiol. (2009) 26:396–403. 10.1097/EJA.0b013e3283232c69 [DOI] [PubMed] [Google Scholar]
- 12.London MJ, Shroyer AL, Coll JR, MaWhinney S, Fullerton DA, Hammermeister KE, et al. Early extubation following cardiac surgery in a veterans population. Anesthesiology. (1998) 88(6):1447–58. 10.1097/00000542-199806000-00006 [DOI] [PubMed] [Google Scholar]
- 13.Singh KE, Baum VC. Pro: early extubation in the operating room following cardiac surgery in adults. Semin Cardiothorac Vasc Anesth. (2012) 16(4):182–6. 10.1177/1089253212451150 [DOI] [PubMed] [Google Scholar]
- 14.Totonchi Z, Azarfarin R, Jafari L, Alizadeh Ghavidel A, Baharestani B, Alizadehasl A, et al. Feasibility of on-table extubation after cardiac surgery with cardiopulmonary bypass: a randomized clinical trial. Anesth Pain Med. (2018) 8(5):e80158. 10.5812/aapm.80158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tirotta CF, Alcos S, Lagueruela RG, Salyakina D, Wang W, Hughes J, et al. Three-year experience with immediate extubation in pediatric patients after congenital cardiac surgery. J Cardiothorac Surg. (2020) 15(1):1. 10.1186/s13019-020-1051-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson AJ, Barros Neto FX, Costa Mde A, Dantas LD, Hueb AC, Prata MF. Predictors of mortality in patients over 70 years-old undergoing CABG or valve surgery with cardiopulmonary bypass. Rev Bras Cir Cardiovasc. (2011) 26(1):69–75. 10.1590/S0102-76382011000100014 [DOI] [PubMed] [Google Scholar]
- 17.Wong WT, Lai VK, Chee YE, Lee A. Fast-track cardiac care for adult cardiac surgical patients. Cochrane Database Syst Rev. (2016) 9(9):CD003587. 10.1002/14651858.CD003587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jack JM, McLellan E, Versyck B, Englesakis MF, Chin KJ. The role of serratus anterior plane and pectoral nerves blocks in cardiac surgery, thoracic surgery and trauma: a qualitative systematic review. Anaesthesia. (2020) 75(10):1372–85. 10.1111/anae.15000 [DOI] [PubMed] [Google Scholar]
- 19.Bignami E, Guarnieri M, Saglietti F, Belletti A, Trumello C, Giambuzzi I, et al. Mechanical ventilation during cardiopulmonary bypass: a review. J Cardiothorac Vasc Anesth. (2016) 30(6):1668–75. 10.1053/j.jvca.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 20.Zakhary WZA, Turton EW, Flo Forner A, von Aspern K, Borger MA, Ender JK. A comparison of sufentanil vs. remifentanil in fast-track cardiac surgery patients. Anaesthesia. (2019) 74(5):602–8. 10.1111/anae.14572 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.


