Abstract
Giant ovarian tumors are rare in the present day due to the early diagnosis and treatment. However, owing to the large size, it can often compress the inferior vena cava and sudden decompression of it during the removal can lead to hemodynamic instability with disastrous outcomes.
Keywords: giant ovarian neoplasm, mucinous cystadenocarcinoma
Giant ovarian tumors are rare in the present day due to the early diagnosis and treatment. However, owing to the large size, it can often compress the inferior venacava and sudden decompression of it during the removal can lead to hemodynamic instability with disastrous outcomes.
1. INTRODUCTION
Tumors in the ovary are not uncommon and can generally be classified as epithelial, germ cell tumors, sex cord‐stromal tumors, and metastatic tumors. 1 Ovarian tumors are termed “giant” if they are bigger than 20 cm and are rare these days because of early diagnosis and treatment. Serous giant tumors are more common than the mucinous subtype. 2 These giant tumors exert pressure over the adjacent organ systems and present likewise, with pressure/compression symptoms more commonly on urinary/respiratory systems. 3
Here, we report a rare case of a 30‐year‐old pre‐menopausal female patient with a giant mucinous cystadenocarcinoma of 16 kilograms who presented with huge abdominal enlargement and respiratory embarrassment.
2. CASE REPORT
A 30‐year‐old P3 + 1 L2 Tamang, non‐alcoholic and non‐smoker, without any prior surgical or any family history of breast and ovarian malignancy presented to our center with a complaint of insidious onset, gradually progressive lower abdominal distention, and amenorrhea for 6 months. Following 2 months, there was gross abdominal distention with bloating and loss of appetite. She had acute shortness of breath for a week even at rest, more in supine position along with weight loss when she presented to us. There was no history of nausea, vomiting, fever, trauma to the abdomen, or per vaginal (PV) bleed before the onset of the symptom.
On examination, she was pale and afebrile with a BP of 100/60 mmHg, pulse rate of 120 bpm, respiratory rate of 20 breaths per minute, and Sp02 of 94% in the room air. She had bilateral lower limb pitting edema. On inspection, the abdomen was grossly distended with everted umbilicus and marked venous prominences with thinned‐out shiny skin. (Figure 1) On palpation, there was a firm pelvic mass extending to the xiphisternum with an ill‐defined margin. The fluid thrill was present suggestive of ascites. Examination of the chest revealed bilateral decreased air entry with crepitations. Examination of other systems was unremarkable.
FIGURE 1.
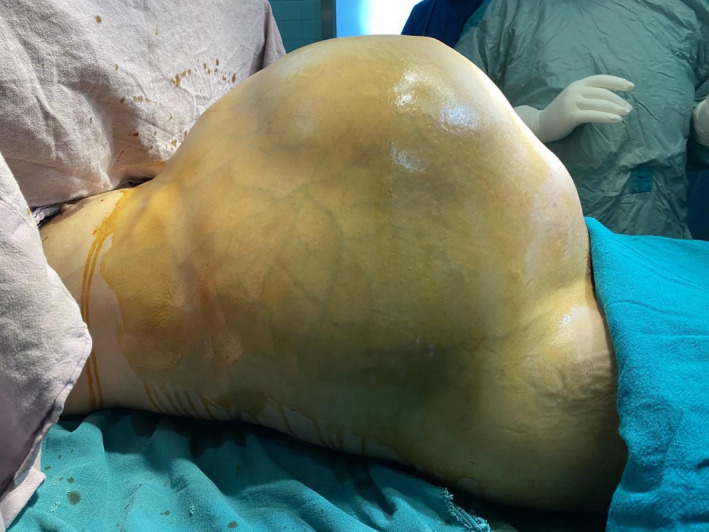
Preoperative image shows grossly distended abdomen with dilated veins
Blood counts were within normal limits; however, the patient was anemic (Hemoglobin‐9.8 gm/dl, PCV‐27gm%). Other blood investigations including tumor markers; CEA and CA‐125 were within normal limits. Beta‐HCG and alpha‐fetoprotein were within normal limits along with her urinalysis and urine culture. Transabdominal USG revealed a large heterogeneous cystic solid mass with multiple septations and mild vascularity measuring 29 cm × 16 cm × 10 cm arising from the left ovary. With the suspicion of malignant pathology, CT of abdomen and pelvis was done which revealed a large abdominopelvic complex cystic multiloculated mass measuring 35 cm × 30 cm × 25 cm with variable attenuation with mass effect on bowel and mild ascites suggesting a mucinous cystadenocarcinoma along with bilateral pleural effusion and lower lobe collapse. (Figure 2) Since a routine FNAC is contraindicated in patients with suspected ovarian tumors to prevent upstaging of disease, FNAC of the suspected ovarian mass was not performed, and hence, she was planned for a staging laparotomy by the team of treating gynecologists.
FIGURE 2.
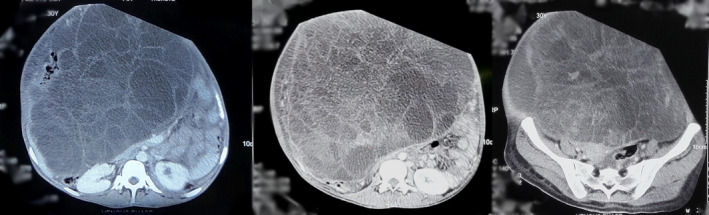
Axial CT scans show a large abdominopelvic cystic multiloculated mass with mass effect on the bowel suggestive of mucinous cystadenocarcinoma
A midline vertical incision extending 5 cm above the umbilicus was given. About 500 ml straw‐colored ascites with huge mass were seen occupying the whole abdomen from xiphisternum to pelvis arising from the left ovary. The posterior wall of the mass was adherent to the omentum with multiple large feeding vessels and the anterior wall with a transverse colon. The uterus was stretched and flat. Cyst decompression was done from few locations. Adhesiolysis was done to dissect mass from the omentum and bowel. The giant ovarian mass was exteriorized, the left salpingo‐oophorectomy was done, and the hemostasis was secured.
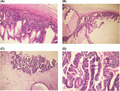
Grossly, the mass measured 42 cm × 36 cm and weighed approximately 16 kgs with irregular surface and congested blood vessels. Cut sections showed multiloculated solid and cystic areas with a smooth inner surface and mucinous fluid. (Figure 3) Histopathological examination showed multiple cystic spaces lined by tumor cells arranged in tubules, papillae, and cribriform patterns. These cells showed mucin‐filled cells with nuclear stratification and vesicular to hyperchromatic nuclei with prominent nucleoli. Moderate nuclear pleomorphism with mitotic count constituting 3 per HPF in mitotically active areas was observed. (Figure 4).
FIGURE 3.
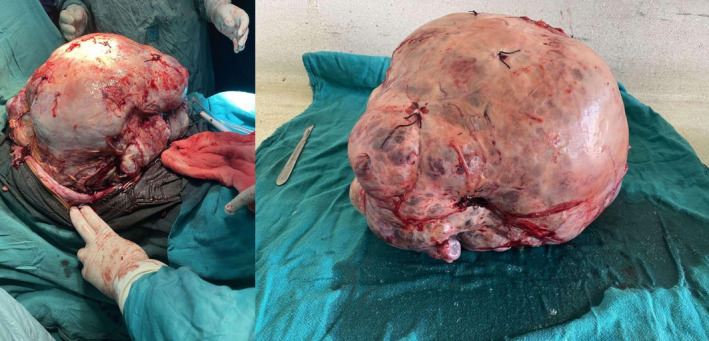
Intra‐operative image shows a huge mass arising from the ovary and excised en‐mass
FIGURE 4.
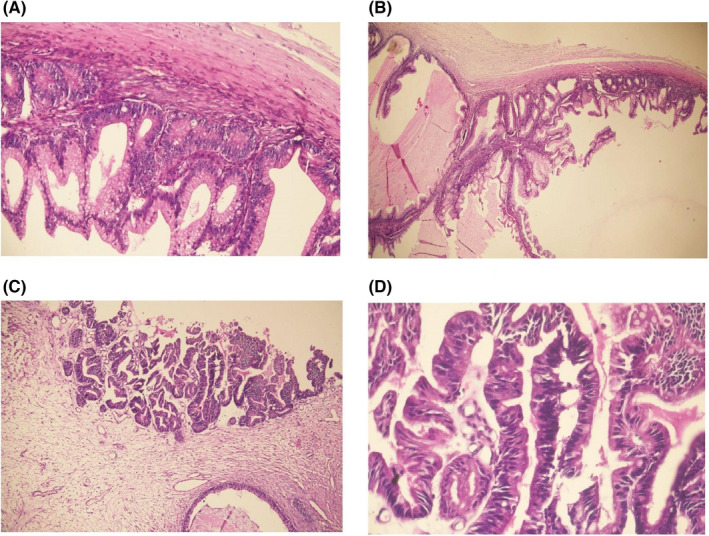
Infiltrating tumor cells arranged in tubules and cribriform pattern (A, B). These mucin‐filled cells showed nuclear stratification and vesicular to hyperchromatic nuclei (C) with a moderate degree of nuclear pleomorphism (D)
The patient became hemodynamically unstable following rapid decompression. Massive transfusion of seven units of packed cells, six units of fresh frozen plasma, and four liters of succinylated gelatin with seven liters of Ringer's lactate during surgery. After 4 h of surgery, she was admitted to the intensive care unit (ICU) for cardiopulmonary support. She was critically ill with inotropic support and ventilation. She died after 4 days of admission in the ICU due to multiorgan failure.
3. DISCUSSION
Most ovarian tumors are epithelial tumors. Mucinous ovarian carcinoma (MOC) is a rare histotype of epithelial ovarian cancer (EOC), with <5% of all EOC cases, and serous histotype is the more common. 4 Ovarian cysts with diameters between 10 and 20 cm are considered large. 5
Ovarian neoplasms and cysts may present vaguely, and with non‐specific complaints such as difficulty breathing, decreased appetite, heartburn, abdominal fullness, bloating, and/or pain abdomen, due to the compression effects of the same. Our patient presented with increased urinary frequency, probably owing to its mechanism of urinary bladder compression. The complications of ovarian neoplasms range from torsion, rupture of an adnexal mass, hemorrhage locally to pleural effusion, small bowel obstruction, venous thromboembolism, and even death. 6 Our patient was no exception presenting with an adnexal mass, but without ascites. The abdomen was hard on palpation, with no shifting dullness but a fluid thrill was present.
If a patient presents with large abdominal masses, a wide range of diagnoses should be thought of, from normal physiological intrauterine pregnancy to pathological fibromyomatosis, pelvic endometriosis, urinary retention, abdominal cysts, abdominal pregnancy, intestinal tumors, hydronephrotic kidney, retroperitoneal tumors, obesity, localized ascites, urachal cyst, mesenteric cyst, abdominal cocoon, echinococcosis among many others. 7 If cystic cavities from the ovary also delineate inner nodules or masses, the findings indicate malignancy. In our case too, the tumor was multilocular with diverse inner solid masses, indicative of malignant lesion, and histopathological examination confirmed the diagnosis of cystadenocarcinoma. Other malignant tumors (anaplastic carcinoma, carcinosarcoma, fibrosarcoma, rhabdomyosarcoma, undifferentiated sarcoma), mixed nodules, and leiomyoma among others should also be ruled out. 1
Pelvic ultrasonography is the imaging modality of choice in the initial evaluation of adnexal mass, and serum CA‐125 is used very frequently as a tumor marker to evaluate the same. But benign conditions such as endometriosis, adenomyosis, and PID can also cause elevation of CA‐125. 8 Nonetheless, tumor markers play a vital role in the workup of an adnexal mass, with carcinoembryonic antigen (CEA), CA‐125, and CA19‐9 being more likely elevated in MOC. CEA is expected to be elevated more in MOC than in non‐mucinous ovarian carcinoma. 9 However, all the tumor markers were normal in our case.
Computed tomography (CT) scan is preferred to pelvic ultrasonography for preoperative assessment as it also aids in evaluating the extent of the disease. Primary mucinous tumors of the ovary are expected to be unilateral and larger (5–48 cm). In T2 weighted MRI, cystic lesions appear multilocular with some solid parts (if present) appearing with intermediate intensity, hyperintensity on DWI, and with a type 3 enhancement curve. 10 CT scans in our case showed multilocular huge homogenous mass occupying the entire abdomen with the size described earlier.
Approximately 7% of all ovarian tumors presenting as primary origin are metastases, almost half of them are bilateral. However, if the origin is assumed to be non‐ovarian, CA 125 (KU/L)/CEA (ng/ml) ratio is <26, and if histopathological evaluation postoperatively suggests gastrointestinal origin, GI endoscopic examination (upper/lower) is warranted. 11 Features favoring metastasis are bilaterality, surface involvement, dirty necrosis, and vascular invasion. Immunohistochemistry helps clear the doubts regarding the origin of the tumor cells. Primary MOCs are positive for Dpc4 and both CK7 and CK20, colorectal primaries for CK20 only, racemase and β‐catenin, pancreatic primaries for mesothelin, fascin, and prostate stem cell antigen (PSCA), and breast primaries for CK7 only, estrogen receptors, and gross cystic disease fluid protein (GCDFP‐15). P16 and in situ hybridization for HPV can confirm endocervical origin. 12
Staging surgery is done for early epithelial ovarian carcinomas and involves peritoneal washing for cytology, removal of the uterus, bilateral fallopian tubes, and bilateral ovaries, pelvic lymph nodes, para‐aortic lymph nodes, omentum, and obtaining multiple peritoneal biopsies. Cytoreductive surgery, done for advanced disease aims removal of all discernible disease and leaving behind just microscopic residual tumor cells. 13 , 14 Mucinous contents should not be spilled during surgery while removing the ovary, as this might increase the risk of recurrence. 15 Retroperitoneal lymph nodes assessment also falls under staging surgery, but the role of systematic lymphadenectomy in early‐stage MOC is not confirmed. 16 The patient underwent en‐mass removal of the tumor along with peritoneal washing and left salpingo‐oophorectomy.
The low prevalence of MOCs has allowed the regimen used in all EOCs including MOC to be a combination of carboplatin, platinum‐based chemotherapy, and paclitaxel. 17 But, MOCs have been shown to be less responsive to platinum‐based therapy than serous histotypes. 18
Large adnexal mass might compress the vena cava, and its removal might predispose to hemodynamic issues. Likewise, the removal of the mass can lead to pulmonary edema and splanchnic vasodilation, increasing the likelihood of further hemodynamic instability. Gentle removal of the mass is suggested for preventing disastrous outcomes. 19 In our case, the sudden decompression of vena cava might be the cause of hemodynamic instability.
More than 90% of the patients with early‐stage MOC live for 5 years. However, in metastatic disease, the survival range lies between 12 and 30 months. Prognosis also depends on the subtypes of MOCs, with infiltrative subtypes carrying a poorer prognosis. 20 Owing to multi‐organ failure, our patient passed away shortly. Thus, the disease‐free survival could not be assessed.
4. CONCLUSION
Mucinous ovarian carcinoma is a rare histotype of ovarian cancer. With this report, we emphasize on early diagnosis and management of ovarian tumors before they are huge to cause a surgical challenge and for a better postoperative prognosis.
AUTHOR CONTRIBUTION
BMS, SA, RR, SK, HC, SC, and PS were involved in counseling and treatment of the patient. SBT examined and interpreted the pathology. BMS and SS collected all the required case information, images, slides, reports, and contributed to writing manuscripts. BMS, SS, and SK reviewed the literature and contributed to both writing and editing the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST
None.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENTS
We gratefully acknowledge the work of members of our hospital and the patient.
Shrestha BM, Shrestha S, Kharel S, et al. Giant ovarian mucinous cystadenocarcinoma: A case report. Clin Case Rep. 2022;10:e06067. doi: 10.1002/ccr3.6067
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- 1. Katke RD. Giant mucinous cystadenocarcinoma of ovary: a case report and review of literature. J Midlife Health. 2016;7(1):41‐44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Molina GA, Izurieta AN, Moyon MA, et al. Giant ovarian cystadenocarcinoma in an adult patient, a rare finding in modern times. J Surg Case Rep. 2019;2019(7):rjz207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Journal of Postgraduate Gynecology & Obstetrics. http://www.jpgo.org/2014/09/giant‐borderline‐mucinous‐cystadenoma.html. Accessed May 30, 2021.
- 4. Rossato M. Giant mucinous cystadenoma of the ovary mimicking ascites: a case report. Clin Med Rev Case Rep. 2016;3(4):103. doi: 10.23937/2378-3656/1410103 [DOI] [Google Scholar]
- 5. Jones DR, Vasilakis A, Pillai L, Timberlake GA. Giant, benign, mucinous cystadenoma of the ovary: case study and literature review. Am Surg. 1992;58(7):400‐403. [PubMed] [Google Scholar]
- 6. Yeika EV, Efie DT, Tolefac PN, Fomengia JN. Giant ovarian cyst masquerading as a massive ascites: a case report. BMC Res Notes. 2017;10(1):749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cevik M, Guldur ME. An extra‐large ovarian mucinous cystadenoma in a premenarchal girl and a review of the literature. J Pediatr Adolesc Gynecol. 2013;26(1):22‐26. doi: 10.1016/j.jpag.2012.04.007 [DOI] [PubMed] [Google Scholar]
- 8. Hellstrom I, Hellstrom KE. SMRP and HE4 as biomarkers for ovarian carcinoma when used alone and in combination with CA125 and/or each other. Adv Exp Med Biol. 2008;622:15‐21. doi: 10.1007/978-0-387-68969-2_2 [DOI] [PubMed] [Google Scholar]
- 9. Tuxen MK, Sölétormos G, Dombernowsky P. Tumor markers in the management of patients with ovarian cancer. Cancer Treat Rev. 1995;21(3):215‐245. doi: 10.1016/0305-7372(95)90002-0 [DOI] [PubMed] [Google Scholar]
- 10. Brown J, Frumovitz M. Mucinous tumors of the ovary: current thoughts on diagnosis and management. Curr Oncol Rep. 2014;16(6):389. doi: 10.1007/s11912-014-0389-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Babaier A, Ghatage P. Mucinous cancer of the ovary: overview and current status. Diagnostics. 2020;10(1):52. doi: 10.3390/diagnostics10010052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Frumovitz M, Schmeler KM, Malpica A, Sood AK, Gershenson DM. Unmasking the complexities of mucinous ovarian carcinoma. Gynecol Oncol. 2010;117(3):491‐496. doi: 10.1016/j.ygyno.2010.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Xu W, Rush J, Rickett K, Coward JIG. Mucinous ovarian cancer: a therapeutic review. Crit Rev Oncol Hematol. 2016;102:26‐36. doi: 10.1016/j.critrevonc.2016.03.015 [DOI] [PubMed] [Google Scholar]
- 14. Morice P, Gouy S, Leary A. Mucinous ovarian carcinoma. N Engl J Med. 2019;380(13):1256‐1266. doi: 10.1056/nejmra1813254 [DOI] [PubMed] [Google Scholar]
- 15. Lee KR, Scully RE. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with “pseudomyxoma peritonei.”. Am J Surg Pathol. 2000;24(11):1447‐1464. [DOI] [PubMed] [Google Scholar]
- 16. Ledermann JA, Raja FA, Fotopoulou C, Gonzalez‐Martin A, Colombo N, Sessa C. Newly diagnosed and relapsed epithelial ovarian carcinoma: ESMO clinical practice guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2013;24:vi24‐vi32. doi: 10.1093/annonc/mdt333 [DOI] [PubMed] [Google Scholar]
- 17. Muggia FM, Braly PS, Brady MF, et al. Phase III randomized study of cisplatin versus paclitaxel versus cisplatin and paclitaxel in patients with suboptimal stage III or IV ovarian cancer: a gynecologic oncology group study. J Clin Oncol. 2000;18(1):106‐115. [DOI] [PubMed] [Google Scholar]
- 18. Shimada M, Kigawa J, Ohishi Y, et al. Clinicopathological characteristics of mucinous adenocarcinoma of the ovary. Gynecol Oncol. 2009;113(3):331‐334. doi: 10.1016/j.ygyno.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 19. Kobayashi T, Yokoyama K. Anesthetic management of a patient with a giant ovarian tumor complicated with cerebral palsy. Masui. 1990;39(9):1234‐1238. [PubMed] [Google Scholar]
- 20. Zaino RJ, Brady MF, Lele SM, Michael H, Greer B, Bookman MA. Advanced stage mucinous adenocarcinoma of the ovary is both rare and highly lethal. Cancer. 2011;117(3):554‐562. doi: 10.1002/cncr.25460 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


