Abstract
Gallbladder cancer is a rare but potentially fatal disease. It is often asymptomatic in early stages and is frequently found incidentally or during the workup for benign biliary disease. We present two patients who each had suspicious gallbladder imaging findings and highlight their differences on radiologic and pathologic examination.
Keywords: biliary, cholecystectomy, gallbladder, gallbladder cancer
Patients with suspicious gallbladder imaging findings should be evaluated for surgical intervention, as early surgical resection is associated with an improved survival for early gallbladder cancer.
1. INTRODUCTION
Gallbladder pathologies are common and frequently involve evaluation with radiographic imaging. Although imaging often reveals benign findings such as cholelithiasis, occasionally imaging studies demonstrate abnormalities that are suspicious for malignancy and must be further evaluated. Gallbladder cancer (GBC) is rare worldwide; however, it remains the most common type of biliary tract cancer and the sixth most common gastrointestinal malignancy. 1 It has an incidence of 1 per 100,000 persons per year in the United States. 2 GBC is usually asymptomatic in early stages. It is frequently discovered incidentally through imaging obtained for other indications or diagnosed during pathologic examination following routine cholecystectomy performed for presumed benign gallbladder disease. Five‐year survival is approximately 10%. 1 , 3 The spread of gallbladder cancer can include local invasion, regional metastasis to lymph nodes, distant metastases, and vascular encasement and invasion. 1 Prognosis of GBC is best estimated following pathologic examination, which determines tumor size, grade, depth of invasion, involvement of liver parenchyma, perineural invasion, lymphovascular invasion, and metastasis to lymph nodes and distant sites. 1
Suspicious findings for possible early GBC may present on diagnostic gallbladder imaging or discovered incidentally. We present two patients who each had suspicious gallbladder imaging findings and discuss their differences in management, pathology, and follow‐up.
2. CASE PRESENTATIONS
2.1. Case 1
A 78‐year‐old woman was admitted to the hospital with recurrent, intermittent, epigastric pain radiating to the chest and back. There were no clear exacerbating or alleviating factors, and she was otherwise asymptomatic. The patient had a history of mild aortic stenosis, gastroesophageal reflux, hyperlipidemia, and peptic ulcers. The patient's surgical history included Nissen fundoplication, and a total abdominal hysterectomy for benign disease. Her medications included low‐dose aspirin, acetaminophen, calcium, ferrous sulfate, ranitidine, and simvastatin. The patient has a five pack‐year history of smoking but quit 35 years prior to presentation. The patient does not drink alcohol or use illicit drugs. The patient's family history includes a sister and a niece with breast cancer.
The patient was afebrile and hemodynamically stable. Physical examination revealed no abdominal tenderness. Laboratory evaluation demonstrated a white blood cell count of 11.0 × 109/L (reference range: 4.0–11 × 109/L), hemoglobin of 7.4 mmol/L (reference range: 7.26–9.74 mmol/L), platelets of 303 × 109/L (reference range: 150–450 × 109/L), total bilirubin of 6.84 μmol/L (reference range: 3.42–22.23 μmol/L), alanine aminotransferase of 91 U/L (reference range 0–50 U/L), aspartate aminotransferase of 195 U/L (reference range: 0–45 U/L), and alkaline phosphatase of 109 (reference range: 40–140 U/L) (Table 1). Two‐dimensional echocardiogram documented an aortic valve area of 1.5 cm2 and a mean aortic gradient of 31 mmHg. Computed tomography (CT) of the chest, abdomen, and pelvis revealed intrahepatic and extrahepatic biliary dilatation; the common bile duct measured 2.4 cm. A right upper quadrant ultrasound demonstrated intrahepatic and extrahepatic biliary dilatation, multiple gallstones, and focal, irregular wall thickening without pericholecystic fluid (Figure 1A). Magnetic resonance cholangiopancreatography (MRCP) demonstrated a prominent gallbladder with multiple gallstones, diffuse intrahepatic and extrahepatic biliary duct dilatation, and multiple stones in the proximal and distal common bile duct (Figure 1B,C).
TABLE 1.
Laboratory evaluation
| Lab value | Reference range | Case 1 | Case 2 |
|---|---|---|---|
| Hemoglobin (mmol/L) | 7.26–9.74 | 7.4 | 6.95 |
| White cells (×109/L) | 4–11 | 11 | 4.5 |
| Platelets (×109/L) | 150–450 | 303 | 125 |
| Total bilirubin (μmol/L) | 3.42–22.23 | 6.84 | 27.36 |
| Alkaline phosphatase (U/L) | 40–150 | 109 | 91 |
| ALT (U/L) | 0–50 | 91 | 71 |
| AST (U/L) | 0–45 | 195 | 26 |
FIGURE 1.
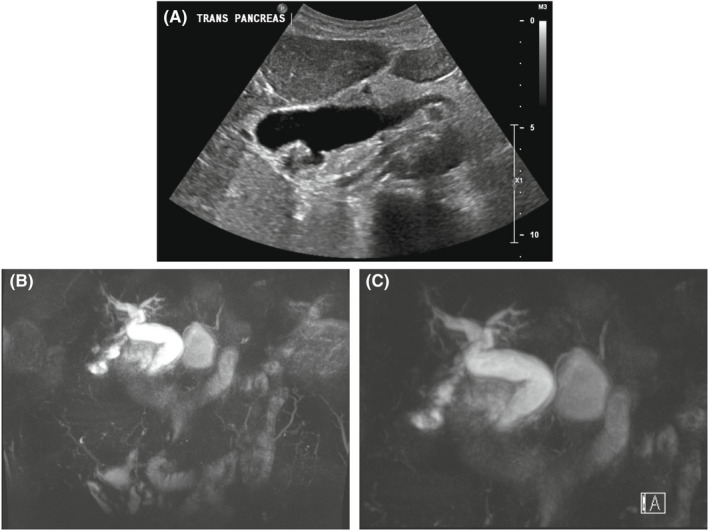
Radiographic imaging of Case 1. (A) Transabdominal ultrasound from Case 1 demonstrating intrahepatic and extrahepatic biliary dilatation, multiple gallstones, and focal, irregular wall thickening without pericholecystic fluid. (B, C) Magnetic resonance cholangiopancreatography from Case 1 that demonstrates a prominent gallbladder and diffuse intrahepatic and extrahepatic biliary ductal dilatation
Endoscopic retrograde cholangiopancreatography was performed on hospital day four. Choledocholithiasis and extrahepatic dilatation were confirmed and sphincterotomy with stone removal was performed. The patient recovered well and was discharged on hospital day five. The patient decline cholecystectomy at the time of hospitalization; however, as the patient's imaging findings were suspicious for possible early gallbladder cancer, outpatient laparoscopic cholecystectomy was performed. The patient's post‐operative course was uncomplicated.
Pathologic examination revealed invasive adenocarcinoma of the gallbladder arising from an intra‐cholecystic papillary neoplasm with high‐grade dysplasia (Figure 2). The papillary neoplasm and gallbladder cancer were restricted to the peritoneal side of the gallbladder. The tumor measured 6.1 cm in its greatest dimension with a few scattered foci of malignant glands and approached 2 mm of the peritoneal serosal margin. Tumor was not present at the hepatic bed margin. The cystic duct margin was negative for both dysplasia and invasive carcinoma. Three lymph nodes were negative for metastatic adenocarcinoma. There was a single focus of tumor suspicious for lymphovascular invasion. There was also evidence of acute and chronic cholecystitis. The final diagnosis was moderately differentiated gallbladder adenocarcinoma, American Joint Committee on Cancer pathologic stage T2aN0Mx and clinical stage II (Table 2).
FIGURE 2.
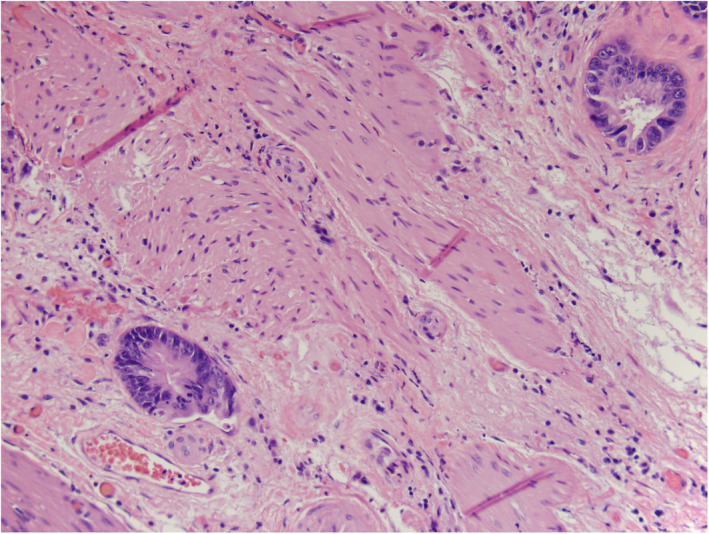
Microscopic imaging of Case 1. Malignant glands invading the muscular layer of the gallbladder (H&E stain, 20×)
TABLE 2.
Suspicious radiologic findings of benign versus possible early gallbladder cancer
| Gallbladder pathology | Transabdominal US or EUS 8 | CT | MRCP 27 |
|---|---|---|---|
| Hyperplastic, inflammatory, fibrous, granulomatous polyps | Low echogenicity | Small, rounded intraluminal lesions 28 | Homogenously low T1 and T2 signals Homogenous T1 postcontrast enhancement |
| Cholesterol polyps | Highly echogenic punctate foci | Hounsfield unit similar to bile 28 | Homogenously low T1 and T2 signals |
| Adenoma | Solid echogenicity Multiple microcystic spaces | Small enhancing polypoid lesion, homogenous texture 28 | Homogenous T1 postcontrast enhancement |
| Adenomyomatosis | Cystic anechoic foci and comet enhancements | Gallbladder wall thickening Rokitansky‐Aschoff sinuses 29 | Focal intramural T1 and T2 signals with late postcontrast homogenous enhancement |
| Xanthogranulomatous inflammation | Combination of hyperechoic and hypoechoic intramural signals | Intact mucosal lining associated with diffuse wall thickening and pericholecystic fat stranding 9 | Diffuse gallbladder wall thickening with contrast enhancement and with or without intramural abscesses |
| Gallbladder carcinoma | Heterogenous, irregular echogenicity | Discontinuous mucosa with irregular masses and wall thickening with or without hepatic involvement 9 | Protruding mass or wall thickening that is hyperintense on T2‐ but not T1‐weighted signals |
Endoscopic ultrasound imaging (EUS) was completed for staging and showed no evidence of nodal disease. Surveillance CT scans and serum tumor markers, including cancer embryonic antigen (CEA) and CA 19–9 levels, have all been negative for recurrence at 21 months following cholecystectomy.
In summary, the patient underwent an R0 resection of a pT2aN0 gallbladder cancer with simple cholecystectomy and sampling of three portal lymph nodes. The patient was considered to be at high risk for cancer recurrence and a candidate for re‐operative surgery. However, because of her advanced age, multiple medical comorbidities, negative EUS examination, negative lymph nodes, and the fact that her tumor was restricted to the peritoneal side of the gallbladder, no additional surgical resection was performed. After consideration of risks and benefits, the patient and her surgical oncologist selected close surveillance with serum tumor markers and serial CT scans as an alternative to chemotherarpy. Surveillance studies have been performed every 3 months for the first year, every 6 months for the second year, followed by annual surveillance studies. Proceeding without adjuvant chemotherapy is supported by the findings from several clinical trials including BCAT, PRODIGE 12, and BILCAP. 4 , 5 , 6 Surgical intervention in the future might be considered in the event of local‐regional recurrence.
2.2. Case 2
An 82‐year‐old man presented to an emergency department for evaluation after a fall. CT scan of the abdomen demonstrated T8 fracture and incidentally demonstrated gallstones and focal gallbladder wall thickening. The patient's medical history included atrial fibrillation, coronary artery disease, low‐grade prostate cancer, and pulmonary hypertension. His surgical history included appendectomy, inguinal herniorrhaphy, coronary artery bypass graft, and prostatectomy. His family history was significant for gastric cancer in his mother. The patient was a former smoker.
On examination, the patient was afebrile with normal vital signs. Physical examination was significant for mild focal tenderness on palpation of the spine. Abdominal examination was unremarkable. Laboratory evaluation included white blood cell count of 4.5 × 109/L (reference range: 4.0–11.0 × 109/L), hemoglobin of 6.95 mmol/L (reference range: 8.25–10.98 mmol/L), platelet count of 125 × 109/L (reference range: 150–450 × 109/L), direct bilirubin of 8.55 μmol/L (reference range 0–3.42 μmol/L), total bilirubin of 27.36 μmol/L (reference range: 3.42–22.23 μmol/L), alanine transaminase of 15 U/L (reference range: 0–70 U/L), aspartate aminotransferase of 22 U/L (reference range: 0–45 U/L), and alkaline phosphatase of 181 U/L (reference range: 40–150 U/L) (Table 1). A right upper quadrant ultrasound revealed wall thickening of the gallbladder fundus and one small gallstone (Figure 3A). The patient's CT of the chest, abdomen, and pelvis demonstrated multiple gallstones and focal wall thickening of the gallbladder fundus (Figure 3B). The patient was referred to general surgery clinic following hospital discharge.
FIGURE 3.
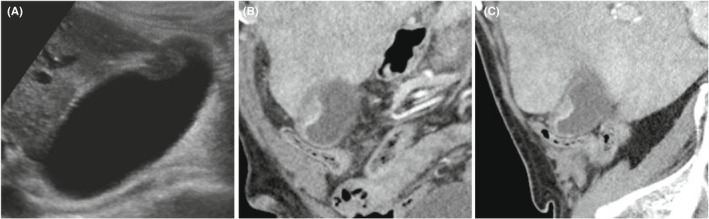
Radiographic imaging of Case 2. (A) Abdominal ultrasound demonstrating wall thickening of the gallbladder fundus. (B) Coronal and (C) sagittal CT images of chest, abdomen, and pelvis of Case 2, demonstrating multiple small gallstones and focal wall thickening of the gallbladder fundus
Clinic evaluation included both general surgery and surgical oncology evaluations. Although the imaging findings were suspicious for possible early gallbladder cancer, simple cholecystectomy with a frozen section diagnosis was considered the best option for this patient. A conventional laparoscopic cholecystectomy was performed. Intraoperatively, the patient had mild fibrosis of the liver and multiple bile duct hamartomas present along the surface of the liver. The gallbladder was sent for frozen section, and pathologic examination demonstrated a 1.2 × 0.8 × 0.6 cm yellow‐tan mass within the submucosa of the gallbladder fundus (Figure 4). The gallbladder had a wall thickness ranging from 0.2–0.4 cm. Histologic examination demonstrated an adenomyomatosis, an intramural abscess, xanthogranulomatous inflammation, and chronic cholecystitis (Figure 5). Pathologic examination was negative for malignancy and dysplasia.
FIGURE 4.
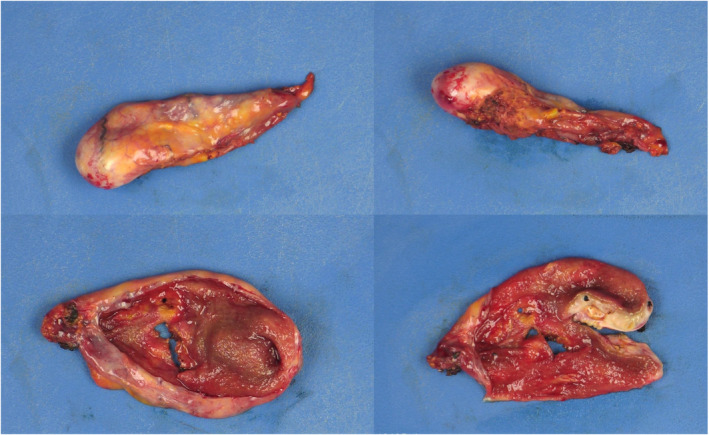
Gross specimen of Case 2. Post‐fixation gross cholecystectomy specimen
FIGURE 5.
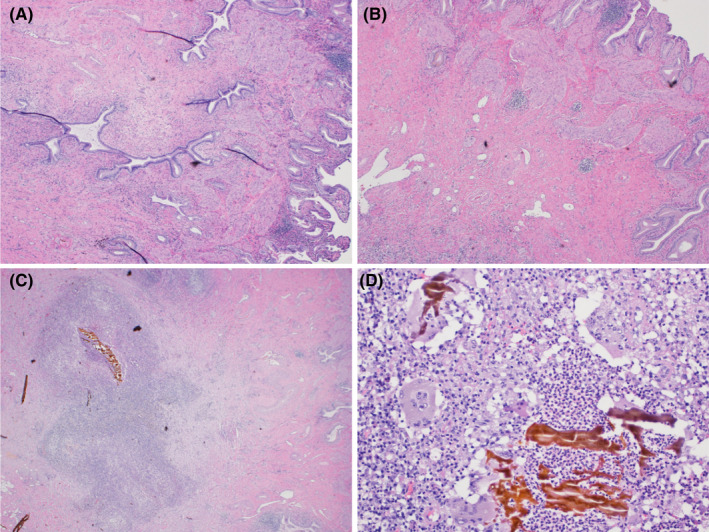
Microscopic imaging of Case 2. H&E‐stained sections demonstrating adenomyomatosis of the gallbladder with benign glands and associated smooth muscle bundle at 2× magnification (A) and 4× magnification (B). Also seen within the same specimen, an intramural abscess at 4× magnification (C) and associated xanthogranulomatous inflammation with foamy histiocytes and multinucleated giant cells surrounding inspissated bile (D) shown at 20× magnification
Anticoagulation with apixaban was resumed on post‐operative day one. The patient re‐presented on post‐operative day six with contact dermatitis and a 3.9 cm subcutaneous hematoma confirmed by CT scan. Apixaban was held for 4 days, the hematoma resolved without surgical intervention, and triamcinolone cream was applied to the rash with rapid improvement. The patient reported no other complications.
3. DISCUSSION
Suspicious findings for possible GBC may be identified by diagnostic imaging, as in Case 1, or discovered incidentally, as in Case 2. It is critical to fully evaluate all abnormal imaging findings of the gallbladder that are suspicious for malignancy (Table 3). Our two patients illustrate both the benign and malignant pathologies related to suspicious radiologic findings: the patient in Case 1 was ultimately diagnosed with invasive adenocarcinomatosis of the gallbladder, and the patient in Case 2 was diagnosed with a benign adenomyoma and xanthogranulomatous inflammation. We discuss the evaluation of patients with suspicious imaging findings and the surgical management of early gallbladder cancer.
TABLE 3.
Early gallbladder cancer staging
| AJCC stage 30 | Stage grouping | Description | Surgical intervention 31 |
|---|---|---|---|
| 0 | Tis | Malignancy confined to epithelium | Laparoscopic cholecystectomy |
| I | T1a | Lamina propria invaded by malignant cells | Laparoscopic cholecystectomy |
| I | T1b | Tumor cells present in but not through the muscularis propria | Radical cholecystectomy or re‐resection (unclear survival benefits) 11 , 12 , 13 , 18 , 19 , 20 |
| IIA | T2a | Perimuscular mass present on the peritoneal side of the gallbladder | Radical cholecystectomy or re‐resection (unclear survival benefits) 18 , 19 , 20 , 32 |
| IIB | T2b | Perimuscular mass present on the hepatic side of the gallbladder | Radical cholecystectomy or re‐resection 18 , 19 , 20 , 32 |
3.1. Evaluation of suspicious imaging findings
Suspicious imaging gallbladder lesions are often first identified by transabdominal ultrasound, which can determine the dimensions and characterize the morphology of abnormal findings. Gallbladder wall abnormalities are classified as either (1) protuberant or (2) diffuse or focal wall thickening. Gallbladder polyps, which are protuberant lesions, are detected in up to 7% of healthy subjects. 7 Gallbladder polyps <5 mm are not likely to represent gallbladder carcinoma. Patients with gallbladder polyps >10 mm should be referred to a surgeon. Patients with gallbladder polyps between 6 and 10 mm should be monitored with surveillance ultrasound every 6–12 months. When gallbladder polyps of any size are identified in patients with primary sclerosing cholangitis (PSC), cholecystectomy should be performed due to high risk of malignancy for patients with PSC. All symptomatic gallbladder polyps should also be removed by cholecystectomy. 7 Certain sonographic findings support a benign diagnosis for polyps <10 mm in dimension. Cholesterol polyps have a thin attachment to the wall, are highly echogenic, and often have punctate foci representing cholesterolosis. Gallbladder adenomas often contain multiple hypoechoic microcystic spaces. 8
Suspicious findings that involve gallbladder wall thickening include adenomyomatosis, xanthogranulomatous inflammation, and gallbladder adenocarcinoma. 8 Adenomyomatosis is relatively common and has a typical appearance of cystic anechoic foci throughout the gallbladder with comet‐like enhancement. 7 , 8 Xanthogranulomatous inflammation is typically characterized by a diffusely thickened gallbladder wall but can be difficult to distinguish from early gallbladder carcinoma. 9 Gallbladder carcinoma often demonstrates heterogenous focal wall thickening, irregular echogenicity, and thinning of the outer layer of the gallbladder wall. 8 Loss of tissue planes and involvement of the liver and extrahepatic biliary ducts are features of advanced gallbladder cancer.
If there is significant preoperative concern for gallbladder cancer based on ultrasound findings, we suggest obtaining a multi‐phase, thin slice liver protocol CT of the abdomen and pelvis, which can assess the vascular relationship of the mass and gallbladder for resectability. CT also can evaluate for distant disease, peritoneal disease, and nodal disease. If there is concern for ductal or hilar biliary involvement, the addition of MRCP is helpful.
In summary, concerning radiographic findings include focal wall thickening and polypoid lesions >10 mm. The malignant potential for suspicious radiologic findings is increased in older patients with a large gallstone burden. 7 Magnetic resonance imaging may identify lymph node involvement in patients with suspicious gallbladder ultrasound findings. Patients with suspicious lesions confined to the gallbladder and without clear lymph node involvement should be assessed for either simple or radical cholecystectomy. Although not recommended by National Comprehensive Cancer Network guidelines, patients with advanced disease and prominent, indeterminate, or suspicious lymph nodes can be evaluated with EUS for tissue acquisition and lymph node staging assessment. Multiple retrospective studies have shown that lymph node‐positive biliary tract cancers, including gallbladder cancer, have poor prognosis despite radical resection. 10
3.2. Management of gallbladder cancer
We discuss the surgical management of GBC based on four distinct scenarios: (1) incidentally discovered malignancy following cholecystectomy, (2) low to intermediate suspicion for GBC based on preoperative imaging or intraoperative findings, (3) incidental intraoperative discovering of a concerning gallbladder appearance, and (4) imaging findings with an obvious gallbladder mass that are highly concerning for malignancy.
In patients with incidentally discovered malignancy following cholecystectomy, scenario 1, treatment is multimodal and depends on the stage and suitability of the patient for a particular therapy. For early‐stage tumors with local or limited regional disease, R0 surgical resection is the only curative option. T1a tumors are adequately treated with simple cholecystectomy alone in most cases. Patients with T1b disease should undergo a radical cholecystectomy that includes partial hepatectomy of segments 4b/5 and an en bloc portal lymphadenectomy. Patients who are poor surgical candidates or who have advanced or widespread disease may be treated chemotherapy with or without radiotherapy.
Several studies demonstrate a survival benefit for patients who undergo an extended cholecystectomy. 11 , 12 Radical resection has also been proven to be beneficial for patients with T1b/T2 cancer with significant improvement in cancer‐specific survival. 13 Lymph node dissection and pathologic evaluation are important components of radical resection. Retrospective studies have shown that lymph node dissection is associated with significant improvement in median overall survival. 14 , 15
In patients with incidentally diagnosed T1b, T2, or T3 gallbladder cancer, re‐resection is associated with improved median survival and is recommended unless contraindicated by advanced disease or if the patient is a poor surgical candidate. 16 , 17 Some recent studies suggest that simple cholecystectomy may be adequate for T1b gallbladder adenocarcinoma, with no significant survival benefit between a simple cholecystectomy and extended cholecystectomy, and limited survival advantage of re‐resection in patients with T1b and T2 disease. 18 , 19 , 20 Additionally, patients with hepatic‐sided T2 (T2b) GBC have worse outcomes than those with peritoneal‐sided GBC (T2a); however, it is currently unclear if liver resection can improve outcomes of patients with hepatic‐sided GBC. 10 Re‐resection without a clear survival benefit potentially exposes patients to unnecessary surgical risk.
Following appropriate surgical intervention, patients may elect for adjuvant chemotherapy, which has potential survival benefits; chemotherapy regimens include combinations of cisplatin, gemcitabine, mitomycin C, and 5‐fluoruracil. 21 , 22 In contrast, several randomized controlled trials, including the BILCAP trial, demonstrated no benefit of adjuvant chemotherapy, including the agents of gemcitabine alone, 6 gemcitabine and oxaliplatin, 5 and capecitabine. 4 Adjuvant chemoradiation is also an option for some patients with GBC. A systematic review of several randomized controlled trials and retrospective studies supports the use of chemoradiation in patients with GBC and microscopically positive surgical resection margins 23 ; one study also supports its use in patients with GBC regardless of margin status. 24 There is some evidence to support a role for neoadjuvant therapy; however, a recent systematic review found insufficient data for routine use of neoadjuvant chemotherapy or chemoradiotherapy in the treatment of advanced GBC 25 , highlighting the need for additional research. Pembrolizumab, a monoclonal antibody against PD‐1, is under investigation for use in patients with advanced biliary tract cancers in combination with capecitabine and oxaliplatin (NCT03111732).
The creation of novel targeted therapies may be possible as future research aimed to generate an enhanced understanding of the molecular pathogenesis of GBC. Additionally, prevention and early surgical intervention of cholelithiasis and cholecystitis may reduce rates of GBC. Populations at high risk for this lethal malignancy, including indigenous populations in North and South America. Such patients often have limited access to medical care and present a specific challenge to prevention. Continued research is warranted to understand the pathogenesis of this disease and to identify optimal interventions for patients with various clinical and pathologic stages to guide operative decisions and to increase survival for this potentially lethal malignancy.
When there is a low to intermediate suspicion for GBC based on imaging findings, scenario 2, patients should undergo a simple cholecystectomy. Further management will depend upon the final pathology, as described below.
Management for patients with an incidental intraoperative discovery concerning gallbladder cancer, scenario 3, such as a gallbladder mass, nodularity, or marked asymmetry, will depend on the experience of the surgeon. In some cases, the operation should be stopped, and the patient should be referred to an appropriate specialist. For surgeons with appropriate experience, diagnostic laparoscopy should be used to assess for and biopsy all liver lesions and/or peritoneal changes suspicious for possible metastases. If there are no signs of metastatic disease and the gallbladder is clearly resectable, the surgeon can proceed with cholecystectomy, removal of the cystic artery lymph node, and intraoperative frozen sections for diagnosis. Caution must be exercised to not rupture the gallbladder.
Patients who present with imaging findings of obvious gallbladder masses that are highly concerning for malignancy, scenario 4, should be referred to an appropriate surgical specialist and staged prior to treatment. If there is no metastatic disease and the mass is resectable, the patient can proceed with definitive resection. A recent meta‐analysis demonstrates a trend towards laparoscopic treatment of gallbladder cancer, especially when there is low suspicion for locally advanced disease based on preoperative imaging, supported by increased survival rates with similar rates of recurrence. 26
4. CONCLUSIONS
Gallbladder cancer is a rare but potentially lethal malignancy. Gallbladder cancer is asymptomatic in its early stages and often diagnosed incidentally. Abnormalities suspicious for gallbladder cancer, whether found incidentally or by imaging obtained for diagnostic purposes, should prompt a full evaluation with consideration for surgical intervention when appropriate. For patients with gallbladder cancer, surgical intervention is associated with improved survival and involves simple versus extended cholecystectomy. Re‐resection following simple cholecystectomy, which includes partial hepatectomy and regional lymphadenectomy, is recommended for patients with T1a disease or greater; however, the survival benefit for an operation for T1b and T2a lesions is likely to be minimal. As demonstrated in Case 1, patients who are poor surgical candidates can be managed with close surveillance with or without adjuvant therapy. The decision to pursue re‐resection and adjuvant chemotherapy or radiotherapy should be based on pathological stage of the tumor as well as specific patient characteristics, such as age and comorbidities, which determine the patient's operative and post‐operative risks.
AUTHOR CONTRIBUTIONS
E.J.S. and M.W reviewed patient charts and wrote the manuscript. T.M and K. A. provided pathology images and descriptions. N.A., M.H., J.A., and J.V.H. provided additional essential patient details, intellectual guidance, and assisted with manuscript preparation.
CONFLICTS OF INTEREST
The authors declare no conflicts of interest.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Ethics approval is not required for case reports at our institution.
CONSENT
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor‐in‐Chief of this journal on request.
PERMISSION TO REPRODUCE MATERIAL FROM OTHER SOURCES
The authors declare no materials from other sources.
ACKNOWLEDGEMENTS
None.
Spartz EJ, Wheelwright M, Mettler T, et al. Evaluation of abnormal gallbladder imaging findings: Surgical management and pathologic correlations in early‐stage gallbladder cancer. Clin Case Rep. 2022;10:e06037. doi: 10.1002/ccr3.6037
DATA AVAILABILITY STATEMENT
All data included in the study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Wistuba II, Gazdar AF. Gallbladder cancer: lessons from a rare tumour. Nat Rev Cancer. 2004;4(9):695‐706. doi: 10.1038/nrc1429 [DOI] [PubMed] [Google Scholar]
- 2. Henley SJ, Weir HK, Jim MA, Watson M, Richardson LC. Gallbladder cancer incidence and mortality, United States 1999‐2011. Cancer Epidemiol Biomarkers Prev. 2015;24(9):1319‐1326. doi: 10.1158/1055-9965.EPI-15-0199 [DOI] [PubMed] [Google Scholar]
- 3. Carriaga MT, Henson DE. Liver, gallbladder, extrahepatic bile ducts, and pancreas. Cancer. 1995;75(1 S):171‐190. doi: [DOI] [PubMed] [Google Scholar]
- 4. Primrose JN, Fox RP, Palmer DH, et al. Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 2019;20(5):663‐673. doi: 10.1016/S1470-2045(18)30915-X [DOI] [PubMed] [Google Scholar]
- 5. Edeline J, Benabdelghani M, Bertaut A, et al. Gemcitabine and oxaliplatin chemotherapy or surveillance in resected biliary tract cancer (Prodige 12‐accord 18‐Unicancer GI): a randomized phase III study. J Clin Oncol. 2019;37(8):658‐667. doi: 10.1200/JCO.18.00050 [DOI] [PubMed] [Google Scholar]
- 6. Ebata T, Hirano S, Konishi M, et al. Randomized clinical trial of adjuvant gemcitabine chemotherapy versus observation in resected bile duct cancer. Br J Surg. 2018;105(3):192‐202. doi: 10.1002/bjs.10776 [DOI] [PubMed] [Google Scholar]
- 7. Anderson MA, Appalaneni V, Ben‐Menachem T, et al. The role of endoscopy in the evaluation and treatment of patients with biliary neoplasia. Gastrointest Endosc. 2013;77(2):167‐174. doi: 10.1016/j.gie.2012.09.029 [DOI] [PubMed] [Google Scholar]
- 8. Tanaka K, Katanuma A, Hayashi T, Kin T, Takahashi K. Role of endoscopic ultrasound for gallbladder disease. J Med Ultrason. 2020;48(2):187‐198. doi: 10.1007/s10396-020-01030-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Arora A, Mahensaria SS. GI Surgey annual. In: Chattttopadhyay TK, Sahni P, Pal S, eds. GI Surgery Annal. Springer Nature; 1977:83‐92. doi: 10.1097/00006534-197759040-00019 [DOI] [Google Scholar]
- 10. Matsukuma S, Tokumitsu Y, Shindo Y, Matsui H, Nagano H. Essential updates to the surgical treatment of biliary tract cancer. Ann Gastroenterol Surg. 2019;3(4):378‐389. doi: 10.1002/ags3.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg. 2004;8(1):83‐89. doi: 10.1016/j.gassur.2003.09.022 [DOI] [PubMed] [Google Scholar]
- 12. Kasumova GG, Tabatabaie O, Najarian RM, et al. Surgical management of gallbladder cancer. Ann Surg. 2017;266(4):625‐631. doi: 10.1097/SLA.0000000000002385 [DOI] [PubMed] [Google Scholar]
- 13. Jensen EH, Abraham A, Habermann EB, et al. A critical analysis of the surgical management of early‐stage gallbladder cancer in the United States. J Gastrointest Surg. 2009;13(4):722‐727. doi: 10.1007/s11605-008-0772-8 [DOI] [PubMed] [Google Scholar]
- 14. Jensen EH, Abraham A, Jarosek S, et al. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery. 2009;146(4):706‐713. doi: 10.1016/j.surg.2009.06.056 [DOI] [PubMed] [Google Scholar]
- 15. Xu L, Tan H, Liu X, et al. Survival benefits of simple versus extended cholecystectomy and lymphadenectomy for patients with T1b gallbladder cancer: an analysis of the surveillance, epidemiology, and end results database (2004 to 2013). Cancer Med. 2019;2020:3668‐3679. doi: 10.1002/cam4.2989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Aloia TA, Járufe N, Javle M, et al. Gallbladder cancer: expert consensus statement. Hpb. 2015;17(8):681‐690. doi: 10.1111/hpb.12444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. de Savornin Lohman EAJ, van der Geest LG, de Bitter TJJ, et al. Re‐resection in incidental gallbladder cancer: survival and the incidence of residual disease. Ann Surg Oncol. 2020;27(4):1132‐1142. doi: 10.1245/s10434-019-08074-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yuza K, Sakata J, Prasoon P, et al. Long‐term outcomes of surgical resection for T1b gallbladder cancer: an institutional evaluation. BMC Cancer. 2020;20(1):1‐9. doi: 10.1186/s12885-019-6507-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang Z, Li Y, Jiang W, et al. Simple cholecystectomy is adequate for patients with t1b gallbladder adenocarcinoma < 1 cm in diameter. Front Oncologia. 2019;9:409. doi: 10.3389/fonc.2019.00409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Watson H, Dasari B, Wyatt J, et al. Does a second resection provide a survival benefit in patients diagnosed with incidental T1b/T2 gallbladder cancer following cholecystectomy? Hpb. 2017;19(2):104‐107. doi: 10.1016/j.hpb.2016.11.006 [DOI] [PubMed] [Google Scholar]
- 21. Valle J, Wasan H, Palmer DH, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2010;4(4):395‐397. doi: 10.1586/egh.10.45 [DOI] [PubMed] [Google Scholar]
- 22. Takada T, Amano H, Yasuda H, et al. Is postoperative adjuvant chemotherapy useful for gallbladder carcinoma? A phase III multicenter prospective randomized controlled trial in patients with resected pancreaticobiliary carcinoma. Cancer. 2002;95(8):1685‐1695. doi: 10.1002/cncr.10831 [DOI] [PubMed] [Google Scholar]
- 23. Shroff RT, Kennedy EB, Bachini M, et al. Adjuvant therapy for resected biliary tract cancer: ASCO clinical practice guideline. J Clin Oncol. 2019;37(12):1015‐1027. doi: 10.1200/JCO.18.02178 [DOI] [PubMed] [Google Scholar]
- 24. Cao Y, Huang H, Wang Z, Zhang G. The inflammatory CXC chemokines, GROαhigh, IP‐10low, and MIGlow, in tumor microenvironment can be used as new indicators for non‐small cell lung cancer progression. Immunol Invest. 2017;46(4):361‐374. doi: 10.1080/08820139.2017.1280052 [DOI] [PubMed] [Google Scholar]
- 25. Hakeem AR, Papoulas M, Menon KV. The role of neoadjuvant chemotherapy or chemoradiotherapy for advanced gallbladder cancer – a systematic review. Eur J Surg Oncol. 2019;45(2):83‐91. doi: 10.1016/j.ejso.2018.08.020 [DOI] [PubMed] [Google Scholar]
- 26. Nakanishi H, Miangul S, Oluwaremi TT, Sim BL, Hong SS, Than CA. Open versus laparoscopic surgery in the management of patients with gallbladder cancer: a systematic review and meta‐analysis. Am J Surg. 2022;224(1 Pt B):348‐357. doi: 10.1016/J.AMJSURG.2022.03.002 [DOI] [PubMed] [Google Scholar]
- 27. Bilgin M, Shaikh F, Semelka RC, Bilgin SS, Balci NC, Erdogan A. Magnetic resonance imaging of gallbladder and biliary system. Top Magn Reson Imaging. 2009;20(1):31‐42. doi: 10.1097/RMR.0b013e3181b48aa2 [DOI] [PubMed] [Google Scholar]
- 28. Yu MH, Kim YJ, Park HS, Il JS. Benign gallbladder diseases: imaging techniques and tips for differentiating with malignant gallbladder diseases. World J Gastroenterol. 2020;26(22):2967‐2986. doi: 10.3748/wjg.v26.i22.2967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bonatti M, Vezzali N, Lombardo F, et al. Gallbladder adenomyomatosis: imaging findings, tricks and pitfalls. Insights Imaging. 2017;8(2):243‐253. doi: 10.1007/s13244-017-0544-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. AJCC Cancer Staging Manual | Mahul B. Amin | Springer . https://www.springer.com/us/book/9783319406176. Accessed June 3, 2021.
- 31. National Comprehensive Cancer Network . Hepatobiliary Cancers. https://www.nccn.org/professionals/physician_gls/pdf/hepatobiliary.pdf. Accessed June 3, 2021.
- 32. Chen M, Cao J, Xiang Y, et al. Hepatectomy strategy for T2 gallbladder cancer between segment IVb and V resection and wedge resection: a propensity score‐matched study. Surg (United States). 2021;169(6):1304‐1311. doi: 10.1016/j.surg.2020.12.039 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included in the study are available from the corresponding author on reasonable request.


