Abstract
The fustin plant-derived bioflavonoid obtained from a common plant known as lacquer tree from family Anacardiaceae, formally known as Rhus verniciflua Stokes, is known to exert a variety of therapeutic properties. The current investigation proved the anti-ulcerative property of fustin on ethanol-induced gastric ulcers in an experimental animal model. The fustin 50 and 100 mg/kg was studied in an experimental rat model by performing an 8 day protocol. The ulcer index, pH, total acidic content, and biochemical parameters such as glutathione (GSH), superoxide dismutase (SOD), catalase activity (CAT), malondialdehyde (MDA), interleukin-1β, prostaglandin E-2, tumor necrosis factor-α (TNF-α), myeloperoxidase, and nitric oxide (NO) in serum were measured. The gastric parameter such as ulcer index, pH, and acidic content was maintained in the fustin groups compared to the ethanol control group. Clinical presentation of gastric ulcers includes a significant increase in serum levels, GSH, SOD, and CAT and decreased MDA, TNF-α, interleukin-1β, and prostaglandin E-2 parameters in contrast to normal groups. The treatment regimen with fustin has significantly restored all serum parameters in test groups. The current study helps to develop reasonable phytochemical options for the innervations of chemical-induced gastric ulcers.
1. Introduction
The clinical imbalance, which arises between physical, chemical, and psychological factors associated with gastric protective factors, leads to the occurrence of local lesions along with multiple etiologies, which is known to develop laterally if untreated in the gastric.1 Chronic alcohol consumption, tobacco chewing, excessive NSAID consumption, Helicobacter pylori infections, and excessive stress are the most common aggravating factors that adversely affect the normal GI functions.2 According to the recent research investigations, the most common cause of gastric mucosa damage is excessive uncontrolled alcohol consumption.3 As a result, the ethanol-induced experimental animal model will be the most commonly used protocol for screening anti-ulcer activity.4 Even though there are numerous synthetic agents commercially available for the innervation of various human diseases, there is a global movement to recognize the importance of alternative and traditional medicinal systems for curing human ailments.5 Medication interactions, increases in the expense of pharmacological therapy, microbial resistance, and adverse and hazardous events connected with their usage are only a few of the challenges that have plagued accessible drug therapy.6 Previous research suggested that the gastric mucosa is segmented into several layers, and any damage to this layer causes abnormal gastric acid secretion, along with nitric oxide (NO) synthase formation of free radicals, and lipid peroxidation.4 Research data suggested that Stress, alcohol and cigarette usage, sedentary lifestyles, irrational medicine use, chronic illness conditions, and bile salt reflux are the primary pathogenic variables related to the development of gastric ulcers.5 Line of research also explored that gastric cell necrosis, vascular damage, and the development of gastric ulcers altogether are molecular mechanisms related to ethanol-induced GI pathogenesis.3,4 Similarly, prolonged ethanol use activated specific biomarkers linked with inflammation, such as pro-inflammatory cytokines, as well as molecular modification of pathways such as NO.4 Trefoil factor family 2 (TFF2) is a peptide found mostly in the gastrointestinal system, especially in mucosal neck cells and epithelial cytoplasm, and is known to perform therapeutic action against stomach ulcers by stabilizing the mucin gel layers.6,7 Tumor necrosis factor (TNF-) and interleukin-6 (IL-6) have been shown to elicit a range of immunological and inflammatory responses.8 One of the transcription factors (NF-κB), which are released as proinflammatory biomarkers from ulcer tissues, results in the overproduction of various biomarkers such as cytokines, certain growth factors, as well as chemokines.9 In the current scenario, the implications of phytoconstituent as an alternative in clinical interventions for prophylaxis and treatment have been accepted globally.10,11 Recent investigations on active components (oxyresveratrol) of plant Artocarpus lakoocha revealed its efficacy in the ethanol-induced gastric acid model,12 previous studies on the plant constituent demonstrated its potential as anti-asthmatic, anti-inflammatory,13 anti-oxidant,14 neuroprotective,15,16 and ability to inhibit tyrosinase.17
For centuries, mother earth has been the initial source for researching the wide collection of agents originating from plant sources.18,19 Phytoconstituent consists of secondary metabolites with medicinal properties that are known to efficiently cure-all gastric ailments together with gastric ulcers. Recent results on plant-derived elements proposed that secondary metabolites found in medicinal plants have a gastroprotective effect. The clinical relevance of plants with an abundant number of tannins as anti-ulcerative qualities has been empirically proven; this analysis also proved to carry out molecular pathways implicated in therapeutic efficacies. The mechanism through which tannins exert antiulcer activity involves a protective layer formation to stomach tissue damages due to ulceration, which protects tissues from further damage and speeds up the healing of tissues.20−24
The common habitat plant is locally known as lacquer tree from the family Anacardiaceae formally known as Toxicodendron vernicifluum or Rhus verniciflua specifically obtained from East Asia as China and Korea are traditionally opted as an herbal local medicament or as crucial food supplements.25 The stokes R. verniciflua are known to contain certain important bio-constituents such as flavonoids, alkaloids, and polyphenol.25−27 The heartwood of the plant R. verniciflua contains an active chemical constituent from the flavone group, that is, fustin (3′,4′,7-trihydroxyflavanol). The heartwood is devoid of urshinols, which are identified as an allergic component and mainly found in the stem bark and anciently have food and medicinal properties. Recent research has also shown that the plant species R. verniciflua has anti-inflammatory and anti-tumor properties.26 Similarly, an additional number of studies look into the effect of fustin, an active component. Previous data also explored that R. verniciflua heartwood possesses antimutagenic and anti-rheumatoid properties that are mediated via the antioxidant capacity of fustin.28,29 The previously reported findings also identified a similar phenolic constituent which was preliminarily isolated from the heartwood of R. verniciflua similar to fustin that is butein (3,4,2′,4′-tetrahydroxychalcone).30,31 Similar to fustin published data also demonstrated the role of butein to exert a variety of biological activities in experimental animal model protocols which includes antimicrobial, anti-inflammatory, and antioxidant.32 Recent investigations demonstrated the presence of active phytochemicals such as flavonoids and Gallo-tannins which is responsible for exerting various biological properties. In line with previous studies, the effects of fustin were comparable to EGb761 (standard ginkgo extract) on (1-42)-induced anxiety and passive avoidance behaviors. Fustin significantly reduced (1-42)-induced drop in acetylcholine, and ChAT activity.33−36 The experiment was aimed at assessing the anti-ulcerative property of fustin on ethanol-induced gastric ulcers in the experimental animal paradigm.
2. Material and Methods
2.1. Animals
Male Wistar rats (200 ± 20 g) were randomly bred and acclimatized to standard laboratory conditions. Procured animals were analyzed for their health and approx. Two month old rats were considered for present investigations. The polypropylene cages equipped with steel stop grill and feed grill with nozzle orifice along with autoclaved husk as bedding materials were utilized for housing experimental animals. During the investigation, a 12hr/12hr light/dark cycle with room temperature range 23–28 °C and relative humidity 45–65% were maintained throughout experimentation. Experimental animals were regularly supplied with a standard pellet diet and access to water ad libitum. The experimental protocol had the approval of Institutional Ethical Committee (RKDFCP/IAEC/2020/33).
2.2. Chemicals
Ethanol was obtained from Merk Pvt. Ltd. India. Fustin and other chemicals (MRL, India) were acquired from local sources and were of standard grade.
2.3. Experimental Protocol
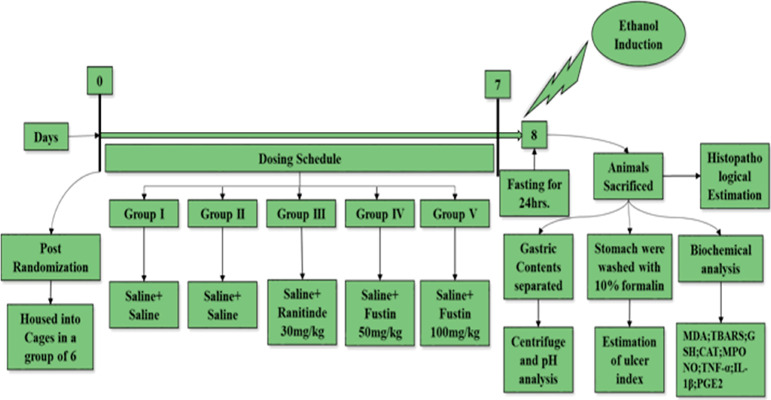
The experimental design for the current investigation is based on the earlier reported studies with some alterations in a well-connected manner. According to a previously reported study, gastric ulcers in rats were induced by administrating a single injection of ethanol (1.5 mL/rat) after 24 h of fasting through gastric gavage on day 8.4 The rats were segregated into five groups (n = 6) such as group 1 (normal control); group 2 (ethanol control); group 3 (standard treated ranitidine 30 mg/kg); group 4 (fustin 50 mg/kg); and group 5 (fustin 100 mg/kg).
Before induction of gastric ulcer on day 8 by acute administration of 1.5 mL ethanol to all experimental groups, 2, 3, 4, and 5 all animals were administered the perse and scheduled dose, group-wise before ethanol administration for days 0 to 7 (Figure 1). The standard drug-treated group receive (30 mg/kg p.o.) ranitidine; whereas the treatment group received (50 and 100 mg/kg/day p.o.) fustin, the control group received vehicle only. The rats were fasted overnight and supplied orally with absolute ethanol (1 mL). The rats were sacrificed, and stomachs of each group of animals were excised and gastric contents were collected for further analysis. The gastric contents were subjected to centrifugation and checked for pH using a pH meter. Tissue was further put for estimation of ulcer index followed by histopathological studies and biochemical estimations were also performed to confirm clinical abnormalities associated with gastric ulcers.
Figure 1.
Experimental design.
2.4. Biochemical Analysis
2.4.1. Ulcer Index
On the 8th day of the protocol, animals were euthanized and subjected to the assessment of ulcer index by using the formula
where X = Indicated total area under ulceration or total mucosal area. The ulcer index was scaled up by using ulcer scorings which present as follows 0 = absence of ulcer, 1 = shallow mucosal erosion, 2 = profound ulcer or transmural necrosis, 3 = penetrated or perforated ulcer.
2.4.2. Measurement of pH
The gastric content from all animal groups was taken and further subjected to centrifugation, and the gastric juices from supernatant liquid were analyzed by using a digital pH meter as per standard protocols.
2.4.3. Determination of Total Acidity
Total acidic content was measured as per previously reported methods,37 in which the addition of 1 mL water with 1 mL centrifuged gastric content in a round conical flask (50 mL) was carried out. In the flask, later on, a few drops of phenolphthalein indicator were added, and the mixture was put for titration by using NaOH (0.01 N) until the solution developed with pink coloration. The volume of titar (NaOH 0.01 N) required was measured. For the assessment of total acidity following formula is used
In the abovementioned formula, n is denoted as the consumed volume of NaOH, 0.01 is denoted NaOH normality, and 36.45 is indicated as NaOH Mol. Weight 1000 is the equation factor (expressed in liter).
2.4.4. Determination of Pepsin Activity
The stop-point bioassay of the denatured hemoglobin hydrolysis method was performed for the measurement of pepsin activity based on previously reported methods. In 2 mL of acetonitrile, 10 mg of p-nitrophenyl sulfite was dissolved. The solution was kept on ice for 3 h. To carry out the assay, 100 IL l of the stock solution was pipetted into a buffer containing 0.01 M glycine hydrochloride, pH 1.9 at 26 °C, resulting in a final concentration of 1.5 X 0- 4 M. A pH value between 1.8 and 2.0 optimally stimulated enzymatic hydrolysis. pH 1.9 was selected for this study. Slight turbidity suggested that the substrate has not yet been hydrolyzed, so it can be utilized for the assay. The sample cuvette is subsequently added to the reference cuvette, and 3 mL of the same substrate solution was added quickly to the reference cuvette. Complete hydrolysis of this working substrate solution was seen in 8 min. In addition to pepsin, a sample cuvette is immediately placed in a dual-beam spectrophotometer at 25 °C. From 1 to 10 units of pepsin (5 to 100 units of the gastric specimen) are added to the sample cuvette. (It was performed using a Beckman DBG and a Hake temperature-controlled water bath). Using the rate of change in absorbance at 320 nm, Anson (1938) measured p-nitrophenol liberated during substrate degradation only caused by enzymatic hydrolysis.
2.4.5. Estimation of Biochemical Indicators of Gastric Ulcers
The biochemical estimation of samples collected from all animals was carried out according to previously reported studies. According to data from recent investigations, excised stomach tissues were stored at −80 °C in all experimental groups. The prepared powdered tissue (50 mg approx.) was homogenized by using PBS buffer (500 μL) and subjected to centrifugation. The supernatants obtained from stomach homogenate were subjected to various biochemical analyses by employing a microplate reader and various commercially available ELISA kits such as glutathione (GSH);38 malondialdehyde (MDA) by TBARS;39 catalase activity (CAT);40 and superoxide dismutase (SOD).41
2.4.6. Estimation of Myeloperoxidase Activity in Gastric Tissue
In a recent investigation by modified methods, Bradley et al. postulated the presence of a marker of neutrophil infiltration, known as myeloperoxidase (MPO). Precisely, the method involves the resuspension of pellets that are obtained from gastric tissue homogenate in PBS (50 mM) having pH maintained at 6.0 incorporated along with hexadecyl trimethyl ammonium bromide (0.5%). The finally prepared mixture was subjected to a freeze cycle along with sonication by employing a sonicator. The final obtained suspension solution was once again put for centrifugation (4 °C/10 min/25000 rpm), and finally, the obtained supernatant was analyzed at 460 nm for estimation of MPO activity using o-dianisidine dihydrochloride and 0.005% hydrogen peroxide.42
2.4.7. Estimation of NO
The assay of NO is based on a previously reported study on diazotization reaction (Griess), in which by measuring nitrite as a result of formation from NO oxidation, the concentration was measured.43
2.4.8. Estimation of Inflammatory Cytokines
The ethanol and other treatment group’s responses were taken as an expression of biomarkers for proinflammatory mediators such as IL-1β (interleukin-1β); IL-6; IL-10; PGE2 (prostaglandin E-2); and TNF-α. The commercially available ELISA kits were employed for the estimation of levels of different proinflammatory biomarkers from homogenate obtained from rat stomach tissues.
2.5. Histopathological Investigation
The previously reported investigations obtained the procedure for histopathological investigations by performing various specimens among all animal groups. In this, procedure the glandular section of the stomach from all groups was used to prepare tissue specimens and placed in a neutral formalin buffer (10%) throughout the night. The hematoxylin and eosin stain was used for staining and preparing 3 μm tissue block sections, and after cutting blocks were subjected to the histopathological investigations.44
2.6. Statistical Analysis
The data obtained from the present investigation were assessed by using license version software, that is, 5.02 version Graph Pad Prism. The results were expressed as mean ± SEM. The level of significance was plotted by employing the statistical method of analysis of variance one way (ANOVA) between different variables among each experimental group followed by a post hoc test (Tukey’s test). In statistical analysis, the p-value indicated the level of significance, whereas a p-value < 0.05 indicated a significant value.
3. Results
3.1. Determination of Ulcer Index
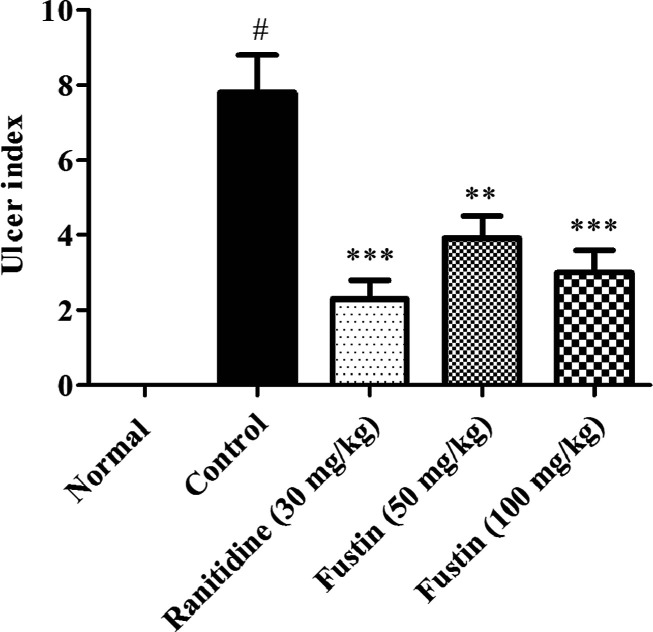
In this investigation, the perse ethanol-control group recorded a significant elevation of the ulcer index as compared to normal treatment groups (p < 0.01). The group which received ranitidine as standard drug treatment before induction of ethanol demonstrated a marked downfall in the index of ulceration (p < 0.001). The higher dose of the fustin treatment group with 100 mg/kg (p < 0.001) and lower dose 50 mg/kg (p < 0.01) significantly decrease the ulceration among respective animal groups (Figure 2).
Figure 2.
Determination of the ulcer index.
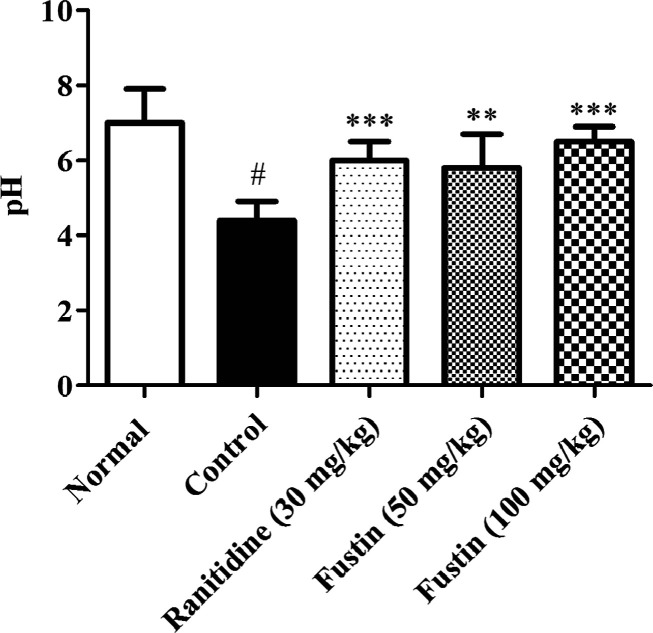
3.2. Estimation of pH
Figure 3 indicates the effect of fustin treatment on pH estimation in ethanol-induced gastric ulcers in rats. In this investigation, the control ethanol group recorded significant acidic pH on the 8th day as compared to normal treatment groups (p < 0.01). Similarly, the animal groups which received ranitidine as standard drug treatment before induction of ethanol demonstrated significance in restoring the pH in alkaline mode as compared to ethanol control groups (p < 0.001). The fustin treatment group with 100 mg/kg and lower dose 50 mg/kg (p < 0.01) demonstrated significance in restoring the alkaline pH similar to the normal treatment group (p < 0.001).
Figure 3.
Measurement of pH.
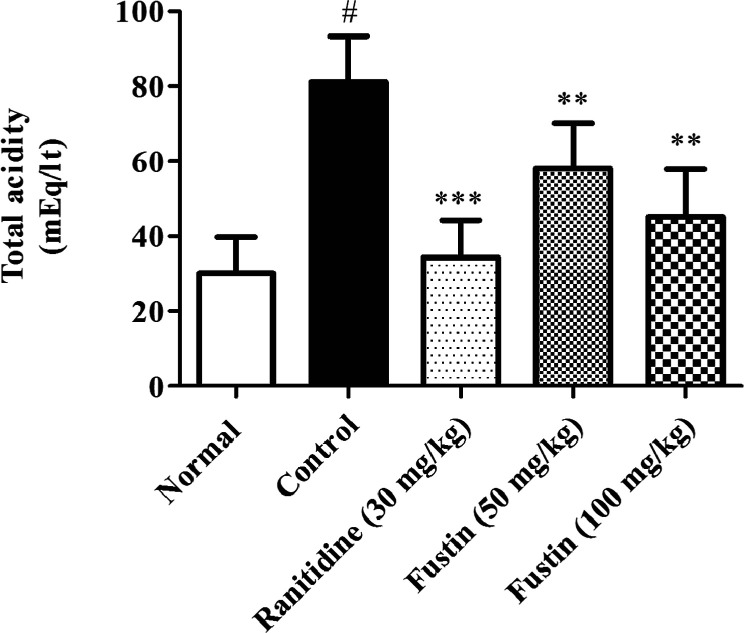
3.3. Determination of Total Acidity
3.3.1. Effect of Fustin on Total Acidity in Ethanol-Induced Gastric Ulcer in Rats
In this investigation, the ethanol-treated group recorded significantly higher levels of total acidic content on the 8th day as compared to normal treatment groups (p < 0.001). Furthermore, the group which received ranitidine as standard drug treatment before induction of ethanol demonstrated significance in restoring the total acidity as compared to ethanol-control groups (p < 0.001). Fustin (50 and 100 mg/kg) moderately lowered the total acidity among the treated group as collated to ethanol-control groups (p < 0.01) followed by a post hoc test (Figure 4).
Figure 4.
Determination of total acidity.
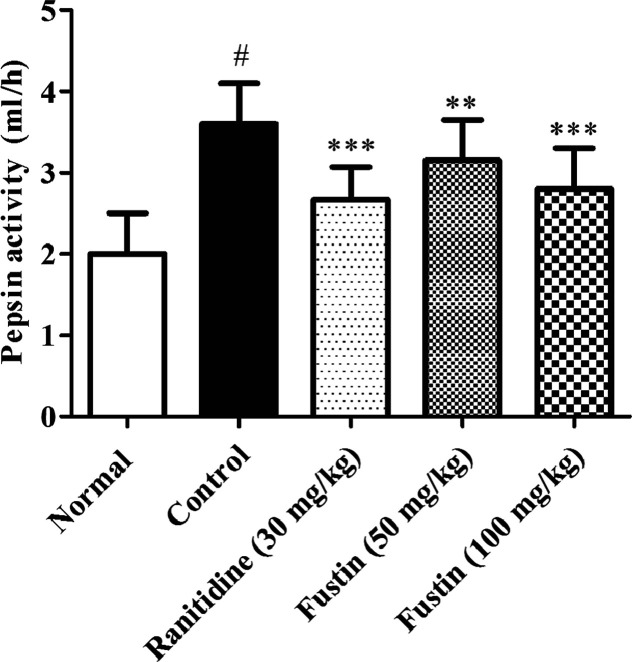
3.3.2. Effect of Fustin on Pepsin Activity in Ethanol-Induced Gastric Ulcer in Rats
The ethanol-treated group was significantly higher levels of pepsin activity as collated to normal treatment groups (p < 0.001). The ranitidine and fustin (50 and 100 mg/kg) were significant regained the pepsin level in comparison to ethanol-control groups (p < 0.001) followed by a post hoc test (Figure 5).
Figure 5.
Determination of pepsin activity.
3.4. Biochemical Indicators Assessment
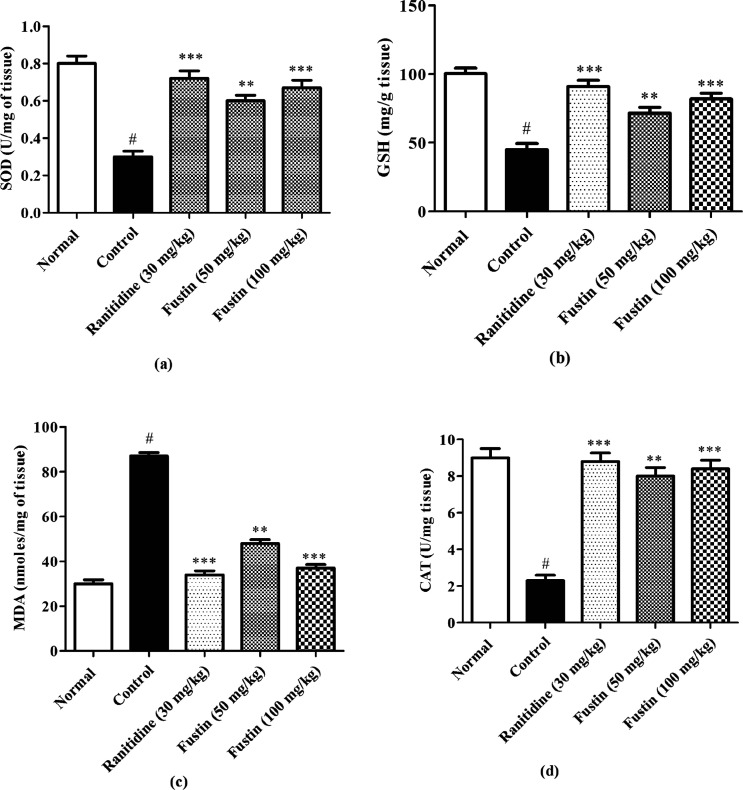
3.4.1. Effect of Fustin on Biochemical Indicators of Ulceration in Ethanol-Induced Gastric Ulcer in Rats
Figure 6a–d shows pre-clinical demonstration at the end of the experiment, which demonstrated the effect of fustin treatment on biochemical parameters of ulceration in ethanol-induced gastric ulcers in rats. The ethanol group recorded markedly higher levels of MDA (p < 0.001). Subsequently, lower levels of SOD, GSH, and CAT (p < 0.001) in the ethanol-control group as compared with normal treatment groups were observed. The ranitidine group demonstrated significant increases in the serum levels of SOD, GSH, and CAT (p < 0.001) and decreased serum levels of MDA as compared to ethanol-control groups (p < 0.001). 100 and 50 mg/kg of fustin moderately increase the serum levels of the SOD, GSH, and CAT among the treated group and moderately decrease the serum MDA level as compared to ethanol-control groups (p < 0.01) followed by post hoc test.
Figure 6.
(a–d) Effect of fustin on biochemical indicators (SOD, GSH, MDA, and CAT) assessment in ethanol-induced gastric ulcers in rats.
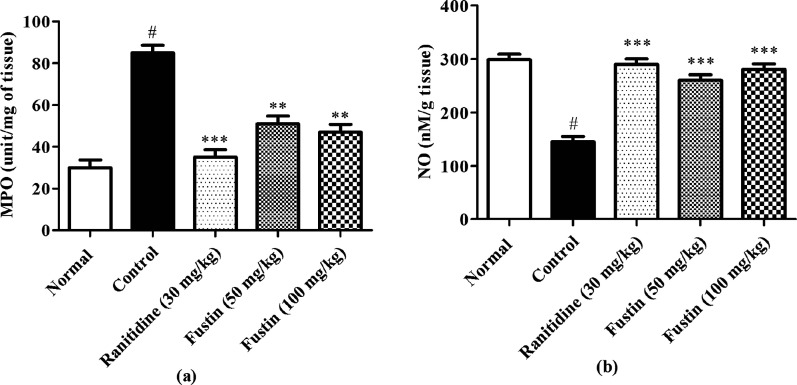
3.5. Determination of MPO Activity in Gastric Tissue
Figure 7a shows pre-clinical demonstrations at the end of the experiment, which indicated the effect of fustin treatment on MPO activity in ethanol-induced gastric ulcers in rats. The ethanol-control group recorded remarkably higher levels of MPO activity as collated with normal treatment groups (p < 0.001). Furthermore, the groups which received ranitidine as a standard drug and fustin (50 and 100 mg/kg) treatment before induction of ethanol demonstrated significant restoration of the MPO activity by decreasing its serum levels as compared to ethanol-control groups (p < 0.001).
Figure 7.
(a,b) Determination of MPO activity and NO assay in gastric tissue.
3.6. NO Assay
In this investigation, the ethanol-treated group showed a significant decrease in levels of NO in comparison to normal treatment groups (p < 0.001). The animal groups which received ranitidine and fustin (50 and 100 mg/kg) before induction of ethanol demonstrated significance in restoring the normal levels of NO as compared to ethanol-control groups (p < 0.001) [Figure 7b].
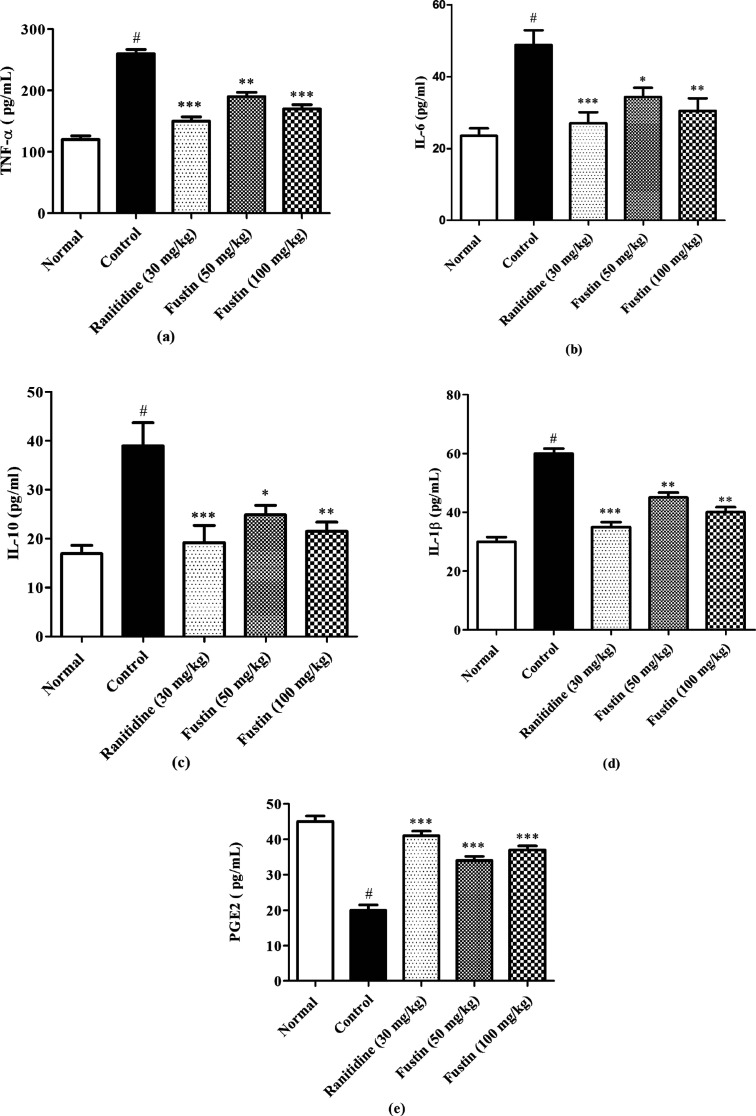
3.7. Determination of Inflammatory Cytokines
In the assessment, the ethanol group recorded markedly higher levels of TNF-α, IL-6, IL-10, and IL-1β (p < 0.001). Subsequently, lower levels of PGE2 (p < 0.001) in the ethanol-control group as compared to normal treatment groups were observed. The group which received ranitidine and fustin (50 and 100 mg/kg) before induction of ethanol induction demonstrated a marked increase in levels of PGE2 (p < 0.001) and decreased serum levels of TNF-α, IL-6, IL-10 and IL-1β as compared to ethanol-control groups (p < 0.001) [Figure 8a–e].
Figure 8.
(a–e) Determination of TNF-α, IL-6, IL-10 IL-1β, and prostaglandin E-2.
3.8. Histopathological Procedure
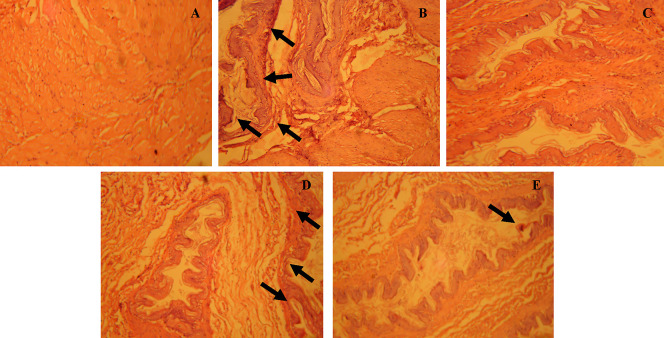
The photo-micrographic examinations of excised stomach tissues revealed the protective effects of fustin against ethanol-induced ulceration. The hematoxylin and eosin stain was used for staining. Furthermore, the normal control-treated group (Figure 9A) demonstrated the intact villi with no markings associated with exfoliations. The ethanol-control group exerts significant markings associated with lesions to villi along with signs of hemorrhages marked with a black arrow (Figure 9B). In standard drug treatment, Ranitidine offers significant protection to gastric villi by preventing cellular necrosis associated with ethanol administration (Figure 9C). A lower dose of fustin 50 mg/kg (Figure 9D) was able to restore the normal histo-morphology by preventing the further necrosis associated with ethanol administration as compared to 100 mg/kg of fustin (Figure 9E).
Figure 9.
Histopathological procedure in ethanol-induced in gastric tissue; (A) group 1 (normal control); (B) group 2 (ethanol control); (C) group 3 (standard treated ranitidine 30 mg/kg); (D) group 4 (fustin 50 mg/kg); and (E) group 5 (fustin 100 mg/kg).
4. Discussion
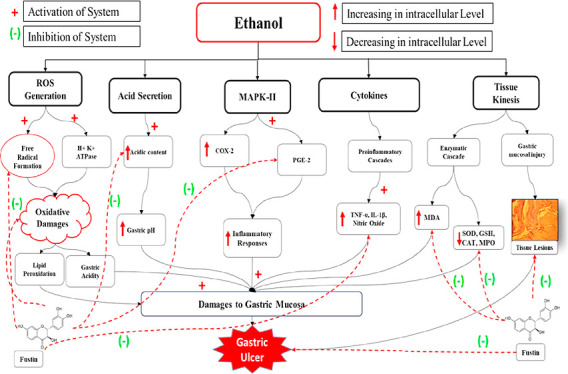
The prime cause of gastric ulceration worldwide has been considered significant because of chronic uncontrolled consumption of alcohol. Recent investigations explore the relevance in humans by performing various preclinical investigations in animal models for the assessment of gastric ulceration by induction of ethanol. Simultaneously, a group of researchers also identified the significance of evaluating the plant-derived compounds in antiulcer activities as novel therapeutic approaches.4 Ethanol induction in animal models through the oral route revealed severe damage to the stomach tissues over the period. The molecular mechanism involved in damage includes the probable involvement of gastric mucosa.45,46 The characterizations of gastric lesions have been extensively carried out by the presence of hemorrhage, mucosal loss, submucosal edema, and injuries to gastric villi in humans.47,48
The present investigation elucidated the novel application of fustin in the assessment of antiulcer activity against ethanol-induced gastric ulcers. The study offers multiple comparisons and analyses between normal control and ethanol-control groups in rats, which postulated that ethanol-treated group animals significantly exert symptoms of gastric ulcerations, such as increased numbers of the ulcer index, increased level of total acidic content, and MPO activity, and simultaneously, ulceration is also indicated through certain abnormal levels of biomarkers such as SOD, GSH, MDA, CAT, TNF-α, PGE2, IL-6, IL-10, and IL-1β from serum levels. Moreover, acute induction of absolute ethanol resulted in histopathological changes in the stomach as a result of a remarkable increase in the lesions to gastric villi. Previous findings demonstrated that ethanol toxicity to lab animals is associated with hemorrhagic cellular changes followed by interfering with the serum levels of several pro-inflammatory biomarkers.48 Furthermore, ethanol induction in animal models is also associated with necrotic coagulations of the gastric mucosa.49
Similarly, another set of experiments postulated that induction of ethanol showed a remarkable increase in the ulcer index after scarification was made at end of protocol on day 8th after acute administration of ethanol, which signifies a relative animal model for gastric ulceration. This pre-clinical elucidation helps the present study to select a proper model for the assessment of fustin against ethanol-induced gastric ulceration. Earlier investigations have identified the significance of plant-derived flavonoids in increasing the pH of gastric content which is attributed to the gastro–protective activity of plant-based flavonoids.50,51 The current study showed the significance of fustin as flavonoids in relative modification in gastric pH and its implications in gastro-protection. The results present study revealed that administration of fustin before the ethanol induction in the fustin-treated group significantly increases the pH and total acidic content similar to the effects produced by ranitidine. Moreover, the ethanol-control group has a significant elevation of pepsin activity as collated to the normal control group ethanol, similar to the previous investigation that postulated abnormal peptic activity in the presence of chronic ethanol consumption.52
In the pathogenesis of gastric ulcers, injury to gastric mucosa played a crucial role which is mediated through certain inflammatory responses characterized by aggregation of certain proinflammatory cytokines in stomach linings as a result of ingestion of ethanol.53 Some of the important contributing cytokines include TNF-α, IL-6, IL-10, PEG2, and IL-1β, which are released from macrophages during inflammation.54 Based on that finding, the present study includes the estimation of this proinflammatory cytokine in ethanol-induced rat models and investigates the role of fustin against this cytokine, where a higher dose of 100 mg/kg proved to be significant in all the abovementioned parameters.
Some other investigations also demonstrated a significant contribution of ROS production in the generation of neutrophil-associated gastric mucosal damage and the molecular mechanism involved in the pathophysiology of ethanol-induced antioxidant depilation.55,56 Similarly, few investigators also highlighted the role ROS in lipid peroxidation resulting in the abnormal occurrence of certain biochemical markers.57,58 Our study data postulated that the presence of biomarkers of lipid peroxidation in the ethanol-control group such as increased levels of MDA is observed in the ethanol-treated group, whereas there is a marked downfall in the SOD, GSH, and CAT levels in ethanol-induced gastric ulceration which clinically indicated the role of ethanol in lipid peroxidation. Furthermore, the treatment with fustin (50 and 100 mg/kg) demonstrated similar effects as Ranitidine where both are elucidated to decrease the MDA activity and simultaneously restored GSH, SOD, and CAT levels in experimental groups.
The recent investigation also heightened the role of enzyme MPO as a biomarker for infiltration of neutrophils; based on that, the present study data postulated that ethanol administration results in an abnormal rise in the MPO activity, which is counteracted by fustin in experimental animal models.
Gastric mucosal integrity is maintained by the endogenous mediator derived from NO synthase known as NO.59 Previous investigations explored this ability of NO because of its vitality in decreasing neutrophil infiltration.60 Our data postulated that ethanol administration leads to a decrease in the NO activity which is counteracted by fustin in the animal model.
5. Conclusions
The present study explores several clinical proofs that claim the significant gastro-protective activity of fustin and its ability to reduce acidic content, proinflammatory biomarkers, and elevated biochemical parameters. This may help to develop economical phytochemical alternatives for the innervations of chemical-induced gastric ulcers..
Acknowledgments
This research project is supported by Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Authors are thankful to Princess Nourah bint Abdulrahman University Researcher Supporting Project number (PNURSP2022R108), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this study.
The authors declare no competing financial interest.
References
- Li W.-F.; Hao D.-J.; Fan T.; Huang H.-M.; Yao H.; Niu X.-F. Protective effect of chelerythrine against ethanol-induced gastric ulcer in mice. Chem.-Biol. Interact. 2014, 208, 18–27. 10.1016/j.cbi.2013.11.011. [DOI] [PubMed] [Google Scholar]
- Heibashy M.; Mazen G.; Ibrahim M. Efficacy and safety of some medical herbs on gastric ulcer induced by aspirin in rats. J. Pharmacol. Biol. Sci. 2014, 9, 19–27. [Google Scholar]
- Franke A.; Teyssen S.; Singer M. V. Alcohol-related diseases of the esophagus and stomach. Dig. Dis. 2005, 23, 204–213. 10.1159/000090167. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Tian X.; Gou L.; Fu X.; Li S.; Lan N.; Yin X. Protective effect of l-citrulline against ethanol-induced gastric ulcer in rats. Environ. Toxicol. Pharmacol. 2012, 34, 280–287. 10.1016/j.etap.2012.04.009. [DOI] [PubMed] [Google Scholar]
- World Health Organization . WHO Traditional Medicine Strategy: 2014–2023; World Health Organization, 2013.
- Saheed S.; Olarewaju S.; Taofeeq G.; Olatunde S.; Alanamu A. Combined administration of Spondias mombin and Ficus exasperata leaf extracts stall Indomethacin-mediated gastric mucosal onslaught in rats. Afr. J. Tradit., Complementary Altern. Med. 2015, 12, 45–51. 10.4314/ajtcam.v12i1.7. [DOI] [Google Scholar]
- Padma T. V. Ayurveda. Nature 2005, 436, 486. 10.1038/436486a. [DOI] [PubMed] [Google Scholar]
- Agarwal P.; Alok S.; Verma A. An update on ayurvedic herb henna (Lawsonia inermis L.): A review. Int. J. Pharma Sci. Res. 2014, 5, 330. 10.13040/IJPSR.0975-8232.5(2).330-39. [DOI] [Google Scholar]
- Bigoniya P.; Singh K. Ulcer protective potential of standardized hesperidin, a citrus flavonoid isolated from Citrus sinensis. Rev. Bras. Farmacogn. 2014, 24, 330–340. 10.1016/j.bjp.2014.07.011. [DOI] [Google Scholar]
- Florence A.; Sukumaran S.; Joselin J.; Brintha T.; Jeeva S. Phytochemical screening of selected medicinal plants of the family Lythraceae. Biosci. Discov. 2015, 6, 73–82. [Google Scholar]
- Graham S. A.; Freudenstein J. V.; Luker M. A phylogenetic study of Cuphea (Lythraceae) based on morphology and nuclear rDNA ITS sequences. Syst. Bot. 2006, 31, 764–778. 10.1600/036364406779696004. [DOI] [Google Scholar]
- Fernandes F. R.; Santos A. L. d.; Arruda A. M. S. d.; Vasques-Pinto L. d. M. C.; Godinho R. O.; Torres L. M. B.; Lapa A. J.; Souccar C. Antinociceptive and antiinflammatory activities of the aqueous extract and isolated Cuphea carthagenensis (Jacq.) JF Macbr. Rev. Bras. Farmacogn. 2002, 12, 55–56. 10.1590/s0102-695x2002000300027. [DOI] [Google Scholar]
- Morales-Serna J. A.; García-Ríos E.; Madrigal D.; Cárdenas J.; Salmón M. Constituents of organic extracts of Cuphea hyssopifolia. J. Mex. Chem. Soc. 2011, 55, 62–64. [Google Scholar]
- Bate-Smith E. C. The phenolic constituents of plants and their taxonomic significance. I. Dicotyledons. Bot. J. Linn. Soc. 1962, 58, 95–173. 10.1111/j.1095-8339.1962.tb00890.x. [DOI] [Google Scholar]
- Hagerman A.Quantification of tannins in tree foliage: a laboratory manual for the FAO/IAEA co-ordinated research project on. The Use of Nuclear and Related Techniques to Develop Simple Tannin Assays for Predicting and Improving the Safety and Efficiency of Feeding Ruminants on Tanninferous Tree Foliage, 2000https://www.iaea.org/programmes/nafa/d3/crp/pubd31022manual-tannin.pdf.
- Žilić S.; Serpen A.; Akıllıoğlu G.; Janković M.; Gökmen V. Distributions of phenolic compounds, yellow pigments and oxidative enzymes in wheat grains and their relation to antioxidant capacity of bran and debranned flour. J. Cereal Sci. 2012, 56, 652–658. 10.1016/j.jcs.2012.07.014. [DOI] [Google Scholar]
- Moustafa E. S.; Swilam N.; Ghanem O.; Hashim A.; Nawwar M.; Lindequist U.; Linscheid M. A coumarin with an unusual structure from Cuphea ignea, its cytotoxicity and antioxidant activities. Pharmazie 2018, 73, 241–243. 10.1691/ph.2018.7946. [DOI] [PubMed] [Google Scholar]
- Barnes E. C.; Kumar R.; Davis R. A. The use of isolated natural products as scaffolds for the generation of chemically diverse screening libraries for drug discovery. Nat. Prod. Rep. 2016, 33, 372–381. 10.1039/c5np00121h. [DOI] [PubMed] [Google Scholar]
- Newman D. J.; Cragg G. M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbul S.; Ahmad M. A.; Mohd A.; Mohd A. Role of phenolic compounds in peptic ulcer: An overview. J. Pharm. BioAllied Sci. 2011, 3, 361. 10.4103/0975-7406.84437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharifi-Rad M.; Fokou P.; Sharopov F.; Martorell M.; Ademiluyi A.; Rajkovic J.; Salehi B.; Martins N.; Iriti M.; Sharifi-Rad J. Antiulcer agents: From plant extracts to phytochemicals in healing promotion. Molecules 2018, 23, 1751. 10.3390/molecules23071751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farzaei M. H.; Abdollahi M.; Rahimi R. Role of dietary polyphenols in the management of peptic ulcer. World J. Gastroenterol. 2015, 21, 6499. 10.3748/wjg.v21.i21.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopetuso L. R.; Scaldaferri F.; Bruno G.; Petito V.; Franceschi F.; Gasbarrini A. The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015, 19, 1068–1076. [PubMed] [Google Scholar]
- De Jesus N. Z. T.; Falcão H. d. S.; Gomes I. F.; Leite T. J. d. A.; Lima G. R. d. M.; Barbosa-Filho J. M.; Tavares J. F.; Silva M. S. d.; Athayde-Filho P. F. d.; Batista L. M. Tannins, peptic ulcers and related mechanisms. Int. J. Mol. Sci. 2012, 13, 3203–3228. 10.3390/ijms13033203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J. H.; Shin Y. C.; Ko S.-G. Integrating traditional medicine into modern inflammatory diseases care: multitargeting by Rhus verniciflua Stokes. Mediators Inflammation 2014, 2014, 154561. 10.1155/2014/154561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi W.; Jung H.; Kim K.; Lee S.; Yoon S.; Park J.; Kim S.; Cheon S.; Eo W.; Lee S. Rhus verniciflua stokes against advanced cancer: a perspective from the Korean Integrative Cancer Center. J. Biomed. Biotechnol. 2012, 2012, 874276. 10.1155/2012/874276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.-S.; Nam T.-G.; Han M.-W.; Ahn S.-M.; Choi H. S.; Kim T. Y.; Chun O. K.; Koo S. I.; Kim D.-O. Protective effect of detoxified Rhus verniciflua stokes on human keratinocytes and dermal fibroblasts against oxidative stress and identification of the bioactive phenolics. Biosci., Biotechnol., Biochem. 2013, 77, 1682–1688. 10.1271/bbb.130236. [DOI] [PubMed] [Google Scholar]
- Choi J.; Yoon B.-J.; Han Y. N.; Lee K.-T.; Ha J.; Jung H.-J.; Park H.-J. Antirheumatoid arthritis effect of Rhus verniciflua and of the active component, sulfuretin. Planta Med. 2003, 69, 899–904. 10.1055/s-2003-45097. [DOI] [PubMed] [Google Scholar]
- Park K.-Y.; Jung G.-O.; Lee K.-T.; Choi J.; Choi M.-Y.; Kim G.-T.; Jung H.-J.; Park H.-J. Antimutagenic activity of flavonoids from the heartwood of Rhus verniciflua. J. Ethnopharmacol. 2004, 90, 73–79. 10.1016/j.jep.2003.09.043. [DOI] [PubMed] [Google Scholar]
- Lim K.-T.; Hu C.; Kitts D. D. Antioxidant activity of a Rhus verniciflua Stokes ethanol extract. Food Chem. Toxicol. 2001, 39, 229–237. 10.1016/s0278-6915(00)00135-6. [DOI] [PubMed] [Google Scholar]
- Jeon W. K.; Lee J. H.; Kim H. K.; Lee A. Y.; Lee S. O.; Kim Y. S.; Ryu S. Y.; Kim S. Y.; Lee Y. J.; Ko B. S. Anti-platelet effects of bioactive compounds isolated from the bark of Rhus verniciflua Stokes. J. Ethnopharmacol. 2006, 106, 62–69. 10.1016/j.jep.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Kim M. O.; Yang J.; Kwon Y. S.; Kim M. J. Antioxidant and anticancer effects of fermented Rhus verniciflua stem bark extracts in HCT-116 cells. ScienceAsia 2015, 41, 322. 10.2306/scienceasia1513-1874.2015.41.322. [DOI] [Google Scholar]
- Lee J.-H.; Kim M.; Chang K.-H.; Hong C. Y.; Na C.-S.; Dong M.-S.; Lee D.; Lee M.-Y. Antiplatelet effects of Rhus verniciflua Stokes heartwood and its active constituents—fisetin, butein, and sulfuretin—in rats. J. Med. Food 2015, 18, 21–30. 10.1089/jmf.2013.3116. [DOI] [PubMed] [Google Scholar]
- Djakpo O.; Yao W. Rhus chinensis and Galla Chinensis–folklore to modern evidence. Phytother. Res. 2010, 24, 1739–1747. 10.1002/ptr.3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin M. J.; Kim I. S.; Park J. S.; Dong M.-S.; Na C.-S.; Yoo H. H. Pharmacokinetic profile of eight phenolic compounds and their conjugated metabolites after oral administration of Rhus verniciflua extracts in rats. J. Agric. Food Chem. 2015, 63, 5410–5416. 10.1021/acs.jafc.5b01724. [DOI] [PubMed] [Google Scholar]
- Jang J. Y.; Shin H.; Lim J.-W.; Ahn J. H.; Jo Y. H.; Lee K. Y.; Hwang B. Y.; Jung S.-J.; Kang S. Y.; Lee M. K. Comparison of antibacterial activity and phenolic constituents of bark, lignum, leaves and fruit of Rhus verniciflua. PLoS One 2018, 13, e0200257 10.1371/journal.pone.0200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santin J. R.; Lemos M.; Júnior L. C. K.; Niero R.; de Andrade S. F. Antiulcer effects of Achyrocline satureoides (Lam.) DC (Asteraceae)(Marcela), a folk medicine plant, in different experimental models. J. Ethnopharmacol. 2010, 130, 334–339. 10.1016/j.jep.2010.05.014. [DOI] [PubMed] [Google Scholar]
- Necheles T. F.; Boles T. A.; Allen D. M. Erythrocyte glutathione-peroxidase deficiencyand hemolytic disease of the newborn infant. J. Pediatr. 1968, 72, 319–324. 10.1016/s0022-3476(68)80202-1. [DOI] [Google Scholar]
- Lefevre G.; Beljean-Leymarie M.; Beyerle F.; Bonnefont-Rousselot D.; Cristol J.-P.; Therond P.; Torreilles J. Evaluation of lipid peroxidation by assaying the thiobarbituric acid-reactive substances. Ann. Biol. Clin. 1998, 56, 305–319. [PubMed] [Google Scholar]
- Aebi H. [13] Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. 10.1016/s0076-6879(84)05016-3. [DOI] [PubMed] [Google Scholar]
- Masayasu M.; Hiroshi Y. A simplified assay method of superoxide dismutase activity for clinical use. Clin. Chim. Acta 1979, 92, 337–342. 10.1016/0009-8981(79)90211-0. [DOI] [PubMed] [Google Scholar]
- Bradley P. P.; Priebat D. A.; Christensen R. D.; Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Dermatol. 1982, 78, 206–209. 10.1111/1523-1747.ep12506462. [DOI] [PubMed] [Google Scholar]
- Yucel A. A.; Gulen S.; Dincer S.; Yucel A. E.; Yetkin G. I. Comparison of two different applications of the Griess method for nitric oxide measurement. J. Exp. Integr. Med. 2012, 2, 167. 10.5455/jeim.200312.or.024. [DOI] [Google Scholar]
- Bancroft J. D.; Gamble M.. Theory and Practice of Histological Techniques; Elsevier Health Sciences, 2008. [Google Scholar]
- Mousa A. M.; El-Sammad N. M.; Hassan S. K.; Abd El Nasser A. M.; Hashim A. N.; Moustafa E. S.; Bakry S. M.; Elsayed E. A. Antiulcerogenic effect of Cuphea ignea extract against ethanol-induced gastric ulcer in rats. BMC Complementary Altern. Med. 2019, 19, 345. 10.1186/s12906-019-2760-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidahmed H. M. A.; Azizan A. H. S.; Mohan S.; Abdulla M. A.; Abdelwahab S. I.; Taha M. M. E.; Hadi A. H. A.; Ketuly K. A.; Hashim N. M.; Loke M. F. Gastroprotective effect of desmosdumotin C isolated from Mitrella kentii against ethanol-induced gastric mucosal hemorrhage in rats: possible involvement of glutathione, heat-shock protein-70, sulfhydryl compounds, nitric oxide, and anti-Helicobacter pylori activity. BMC Complementary Altern. Med. 2013, 13, 183. 10.1186/1472-6882-13-183. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Park S. W.; Oh T. Y.; Kim Y. S.; Sim H.; Park S. J.; Jang E. J.; Park J. S.; Baik H. W.; Hahm K. B. Artemisia asiatica extracts protect against ethanol-induced injury in gastric mucosa of rats. Gastroenterol. Hepatol. 2008, 23, 976–984. 10.1111/j.1440-1746.2008.05333.x. [DOI] [PubMed] [Google Scholar]
- Hu T.-M.; Lee R.-P.; Lee C.-J.; Subeq Y.-M.; Lin N.-T.; Hsu B.-G. Heavy ethanol intoxication increases proinflammatory cytokines and aggravates hemorrhagic shock-induced organ damage in rats. Mediators Inflammation 2013, 2013, 121786. 10.1155/2013/121786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W.; Huang H.; Niu X.; Fan T.; Mu Q.; Li H. Protective effect of tetrahydrocoptisine against ethanol-induced gastric ulcer in mice. Toxicol. Appl. Pharmacol. 2013, 272, 21–29. 10.1016/j.taap.2013.05.035. [DOI] [PubMed] [Google Scholar]
- Zhao X.; Zhu K.; Yi R.; Peng D.; Song J.-L. Total flavonoid from Ba lotus leaf protected the reserpine-induced gastric ulcer in mice. Biomed. Res. 2017, 28, 345. [Google Scholar]
- Liu B.; Feng X.; Zhang J.; Wei Y.; Zhao X. Preventive effect of Anji White tea flavonoids on alcohol-induced gastric injury through their antioxidant effects in kunming mice. Biomolecules 2019, 9, 137. 10.3390/biom9040137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puurunen J. Effect of ethanol on peptic activity in the rat stomach. Digestion 1982, 23, 97–103. 10.1159/000198694. [DOI] [PubMed] [Google Scholar]
- Kang J.-W.; Yun N.; Han H.-J.; Kim J.-Y.; Kim J.-Y.; Lee S.-M. Protective effect of flos lonicerae against experimental gastric ulcers in rats: mechanisms of antioxidant and anti-inflammatory action. J. Evidence-Based Complementary Altern. Med. 2014, 2014, 596920. 10.1155/2014/596920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozza A. L.; Meira de Faria F.; Souza Brito A. R.; Pellizzon C. H. The gastroprotective effect of menthol: involvement of anti-apoptotic, antioxidant and anti-inflammatory activities. PLoS One 2014, 9, e86686 10.1371/journal.pone.0086686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine L.; Takeuchi K.; Tarnawski A. Gastric mucosal defense and cytoprotection: bench to bedside. Gastroenterology 2008, 135, 41–60. 10.1053/j.gastro.2008.05.030. [DOI] [PubMed] [Google Scholar]
- AlRashdi A. S.; Salama S. M.; Alkiyumi S. S.; Abdulla M. A.; Hadi A. H. A.; Abdelwahab S. I.; Taha M. M.; Hussiani J.; Asykin N. Mechanisms of gastroprotective effects of ethanolic leaf extract of Jasminum sambac against HCl/ethanol-induced gastric mucosal injury in rats. J. Evidence-Based Complementary Altern. Med. 2012, 2012, 786426. 10.1155/2012/786426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kan J.; Hood M.; Burns C.; Scholten J.; Chuang J.; Tian F.; Pan X.; Du J.; Gui M. A novel combination of wheat peptides and fucoidan attenuates ethanol-induced gastric mucosal damage through anti-oxidant, anti-inflammatory, and pro-survival mechanisms. Nutrients 2017, 9, 978. 10.3390/nu9090978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu T.; Yang Y.; Kwak Y.-S.; Song G. G.; Kim M.-Y.; Rhee M. H.; Cho J. Y. Ginsenoside Rc from Panax ginseng exerts anti-inflammatory activity by targeting TANK-binding kinase 1/interferon regulatory factor-3 and p38/ATF-2. J. Ginseng Res. 2017, 41, 127–133. 10.1016/j.jgr.2016.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J.-W.; Seo C.-S.; Kim T.-I.; Moon O.-S.; Won Y.-S.; Son H.-Y.; Son J.-K.; Kwon H.-J. Protective Effects of Manassantin A against Ethanol-Induced Gastric Injury in Rats. Biol. Pharm. Bull. 2016, 39, 221–229. 10.1248/bpb.b15-00642. [DOI] [PubMed] [Google Scholar]
- Ohta Y.; Nishida K. Protective effect of L-arginine against stress-induced gastric mucosal lesions in rats and its relation to nitric oxide-mediated inhibition of neutrophil infiltration. Pharmacol. Res. 2001, 43, 535–541. 10.1006/phrs.2001.0812. [DOI] [PubMed] [Google Scholar]