Abstract
Hypoxia is a major regulator of tumor aggressiveness and metastasis in cancer progression. Exosomes (exos) play an important role in the communication between lung cancer and hypoxic microenvironment. However, the underlying mechanisms are largely undefined. Exos were isolated from A549 cells under hypoxia conditions. Transmission electron microscopy and nanoparticle tracking analysis were carried out to characterize exos. CCK-8 assay, flow cytometry, Western blot, wound healing, and transwell assays were performed to assess the proliferation, apoptosis, migration, and invasion of A549 cells, respectively. The M2 polarization of macrophages was evaluated by RT-qPCR and Western blot analysis. In vivo nude mice model was established to determine the regulatory effect of hypoxia/exos on the progression of lung cancer. Hypoxic A549 cell-derived exos (hypoxia/exos) promoted the proliferation and migration, and inhibited the apoptosis in A549 cells. The expression of PKM2 was significantly upregulated in hypoxia/exos. Hypoxic exosomal PKM2 induced M2 polarization of macrophages by activating AMPK pathway. Co-culture with hypoxia/exos-treated macrophages enhanced the migration, invasion, and epithelial-mesenchymal transition (EMT) in A549 cells. Moreover, treatment with hypoxia/exos facilitated the tumor growth and lung metastasis of A549 cells. Our findings reveal that hypoxic exosomal PKM2 induces M2 macrophage polarization via AMPK pathway, and thus exerts a simulative effect on the growth and metastasis of lung carcinoma.
Keywords: lung cancer, hypoxia, exosomes, macrophage, metastasis, PKM2/AMPK
Introduction
Lung cancer is the most common malignancy worldwide and its mortality ranks first 1 . Despite the advance in the current therapies, the 5-year survival of patients with lung cancer remains unsatisfactory 2 . Therefore, identification of novel diagnostic/therapeutic targets is urgently needed.
Intertumoral hypoxia is a dynamic and heterogeneous feature of most solid tumors, which drives multiple cancer processes, such as cell proliferation, epithelial-mesenchymal transition (EMT), invasion, and metastasis 3 . Hypoxia regulates the cellular communication and crosstalk between tumor cells and their microenvironment via diverse secretory factors, including exosomes (exos) 4 . Exos are vesicles of endocytic origin ranging 30–150 nm in diameter and carry packages of molecular information, including proteins, nucleic acids, lipids, and metabolites wrapped in their lipid bilayer 5 . Accumulated evidence demonstrates that exos derived from hypoxic tumor cells contribute to cancer progression by promoting tumor cell proliferation, migration, and invasion and conferring chemo-resistance6–8.
Macrophages are one of the most abundant cells with noteworthy plasticity in tumor microenvironment 9 . Macrophages could be polarized to classically activated M1 and alternatively activated M2 macrophages, depending on the microenvironmental signals 10 . M1 macrophages exert pro-inflammatory phenotype and tumor cytotoxicity, while M2 macrophages suppress the inflammation, contributing to angiogenesis and favoring tumor progression11,12. However, the association and mechanism of macrophages polarization involved in lung cancer progression remain to be elucidated.
In this study, we investigated the effect of hypoxic A549 cells-derived exos on the biological behavior of A549 cells and found that hypoxia/exos could promote the proliferation and migration of lung cancer cells both in vitro and in vivo. Moreover, we found that hypoxic exosomal PKM2 induced M2 polarization of macrophages through AMPK/p38 pathway, thus contributing to the promotion of tumor progression.
Methods
Cell Culture and Hypoxia Treatment
A549 and RAW264.7 cells were obtained from the American Type Culture Collection (Manassas, VA, USA) and cultured in Dulbecco’s Modified Eagle Medium (DMEM) medium with 10% fetal calf serum (FBS) and 1% penicillin/streptomycin at 37°C in an atmosphere containing 5% CO2. Hypoxia environment was established with a hypoxia cell incubator of 1% O2.
Exos Isolation and Identification
A549 cells were cultured under normoxic (21% CO2) or hypoxic (1% O2) conditions for 3 days. The medium was replaced with 10% exos-depleted FBS at 80-90% confluence. Then the medium was collected and centrifuged at 300 g for 10 min; 3000 g for 20 min; 10,000 g for 30 min; and ultra-centrifuged at 100,000 × g for 2 h at 4°C to precipitate exos pellets. After the pellets were resuspended in phosphate-buffered saline (PBS) and stained with the phosphotungstic acid for 10 min, transmission electron microscopy experiment was performed for exos identification 13 .
Nanoparticle Tracking Analysis
The nanoparticle relative concentrations were analyzed using the Nanosight (Malvern, Worcestershire, UK) and NTA analytical software (version 2.3, Nanosight) 14 .
Western Blot Analysis
Cells were harvested and ultra-centrifuged at 100,000 × g for 30 min at 4°C to collect proteins. The concentration of proteins was determined with the bicinchoninic acid (BCA) protein assay kit (Seyotin, China). Twenty microgram of protein was loaded on a sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE), separated by electrophoresis, and transferred to PVDF membrane. The membranes were blocked with 5% skimmed milk for 1 h and incubated with specific antibodies overnight at 4°C subsequently. Monoclonal antibodies (Abcam; Cambridge, UK) used in this study were as follows: CD9 (1:1000), CD81 (1:1000), TSG101 (1:1000), HSP70 (1:1000), ALIX (1:1000), Cleaved-caspase3 (1:1000), bcl-2 (1:1000),bax (1:1000), PKM2 (1:1000), p-P38 (1:1000), p38 (1:1000), p-AMPK (1:1000), AMPK (1:1000), CD206 (1:1000), Arginase-1 (1:1000), E-cadherin (1:1000), N-cadherin (1:1000), Vimentin (1:1000) and GAPDH (1:10,000). The membranes were incubated with HRP-conjugated secondary antibodies before being visualized using a Chemiluminescent HRP Substrate Kit (Seyotin,China).
CCK-8 Assay
A549 cells (1 × 104/well) were seeded into 96-well plates and treated with normoxia/exos or hypoxia/exos for 24 h, 48 h, and 72 h. Then 10 μl CCK-8 solution (Seyotin,China) was added to each well and incubated at 37°C for 2 h. The optical density (OD) value at 450 nm was measured with an Epoch microplate spectrophotometer (BioTek; Winooski, VT, USA) and cell viability was calculated.
Wound-Healing Assay
A549 cells were seeded into 6-well plates. Then cells were scratched with a 200-µl sterile pipette tip at the confluence of 90%. The wounded monolayers were washed with PBS to remove cell debris and A549 cells were cultured with fresh serum-free medium with normoxia/exos or hypoxia/exos. The distance between the two edges of the wound was calculated at 0 h, 24 h, and 48 h to evaluate the migration capacity.
Cell Apoptosis Analysis
Cell apoptosis was examined with the Annexin V-FITC apoptosis detection kit (BD Biosciences; NJ, USA). After treatment with normoxia/exos or hypoxia/exos, A549 cells were collected and resuspended in 1×binding buffer, followed by staining with Annexin V-FITC and PI in the dark at room temperature. Then the percentage of apoptotic cells was immediately analyzed by a flow cytometer (BD, Biosciences; New Jersey, USA).
Exo Uptake Assay
After being labeled with the PKH67 Green Fluorescent Cell Linker Kit (Sigma-Aldrich; St. Louis, MO, USA), exos were added to RAW264.7 cells. Then, RAW264.7 cells were fixed with 4% paraformaldehyde and stained with 4′,6-diamidino-2-phenylindole (DAPI) prior to its observation by a fluorescence microscopy.
Real-Time Quantitative PCR (RT-qPCR)
Total RNA was isolated with the TRIzol reagent (Invitrogen; Carlsbad, CA, USA) according to the manufacturer’s instructions. Reverse transcription reaction was performed by using reverse transcriptase kit (Seyotin, China). Subsequently, PCR was performed with a standard SYBR Green PCR kit (Seyotin, China). GAPDH expression was regarded as the normalization control.
Migration and Invasion Assays
Migration and invasion assays were performed using transwell chambers (24 wells) (Corning; NY, USA) coated without or with Matrigel (BD Biosciences), respectively. To perform the asssays, 5 × 104 A549 cells were re-suspended in serum-free medium and placed in the top chamber. The lower chamber was filled with 1x104 macrophages induced by hypoxic/exos and normoxia/exos in 600 μl medium containing 10% FBS. After incubation for 24 h, the migrating or invading cells were fixed with methyl alcohol and subsequently stained with 0.1% crystal violet. The number of migrating or invading cells was counted using an Inverted microscope.
Hematoxylin-Eosin (HE) Staining
Suspicious lung metastasis tissues were assessed by HE staining. The tissues were placed in 10% formalin overnight, and then dehydrated and embedded in paraffin. Then the tissue samples were sliced to 4 μm thick sections, fixed on a glass slide, dried and stained. The sections were successively immersed in xylene, ethanol with gradient concentrations, and hematoxylin. After being mounted with resin, the sections were dried naturally, and observed and photographed with a light microscope.
Animal Experiments
All animal experiments were approved by the Guangzhou Medical University. Nude mice (male, 4–6 weeks of age, 18–20 g) were purchased from Guangdong Animal Experiment Center. Luciferase-labeled A549 cells were subcutaneously injected with into the oxter of nude mice. After the tumor grew to 50-100 mm3, mice were randomly divided into 3 groups: control, normoxia/exos, and hypoxia/exos (n = 5). Exos (10 μg/ml) derived from A549 cells under normoxia or hypoxia conditions were injected into mice via the tail vein for the treatment. In vivo fluorescence imaging was performed to visualize the tumor growth. Tumors size was measured using a caliper weekly. Four weeks after injection, mice were sacrificed under general anesthesia. For metastasis, A549 cells were injected into nude mice via the tail vein. 24 h after injection, mice were treated with exos via tail vein injection. Suspicious lung metastasis tissues were analyzed by HE staining.
Statistical Analysis
All in vitro results were derived from at least three independent experiments. Statistical analyses, histograms drawing, and scatter plots were performed with SPSS 22.0 software (SPSS Inc. USA) and GraphPad Prism 6.0 software (GraphPad Software, Inc.) The significance of differences between two groups was determined using Student’s t-test. Comparations among more than two groups were performed with one-way analysis of variance (ANOVA). P < 0.05 was considered to indicate a statistically significant difference.
Results
Characterization of Exos From A549 Cells Under Normoxia and Hypoxia
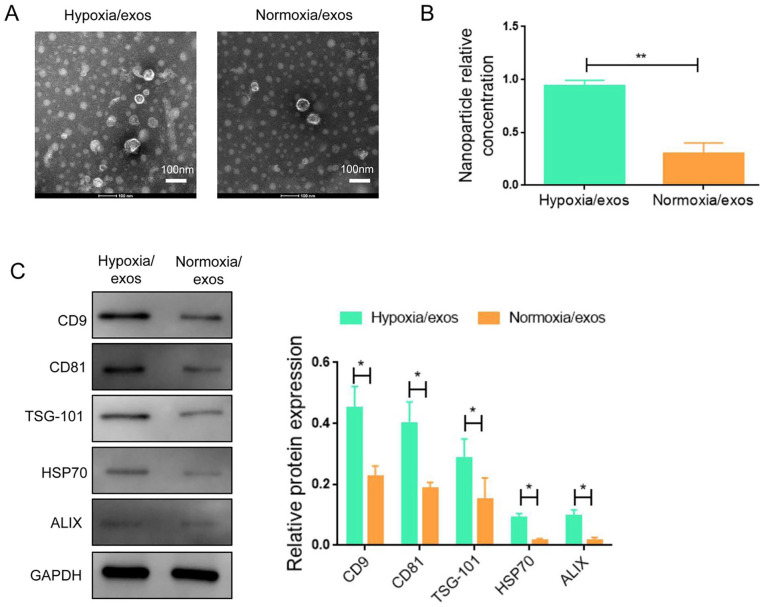
A549 cells were placed under normoxia and hypoxia for 48 h and exos were isolated from the conditioned media. The exo morphology was identified by transmission electron microscope assay. The exos were presented as cup-shaped double-layer membrane structure ranging from 30 to150 nm in diameter (Fig. 1A). Nanoparticle tracking analysis showed that the nanoparticle relative concentration of exos derived from hypoxic A549 cells was significantly higher than that from normoxic condition (Fig. 1B). Hypoxia exos exhibited the elevated expression of exo specific markers (CD9, CD81, TSG-101, HSP70, and ALIX), as characterized by Western blot (Fig. 1C and Supplemental Fig. S1).
Figure 1.
Characterization of exos from A549 cells under normoxia and hypoxia conditions. (A) Exos were identified by transmission electron microscope. (B) Identical concentrations of A549 cells were seeded under normoxia and hypoxia conditions and the nanoparticle relative concentration was determined. (C) Western blot analysis of marker proteins of exos (CD9, CD81, TSG101, HSP70, ALIX). Data represent the mean ± SD of three separate experiments; comparison was performed with Student’s t-test. Scale bar: 100 nm. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05; **P < 0.01.
Hypoxia/Exos Promote the Proliferation and Migration, and Inhibit Apoptosis in A549 Cells
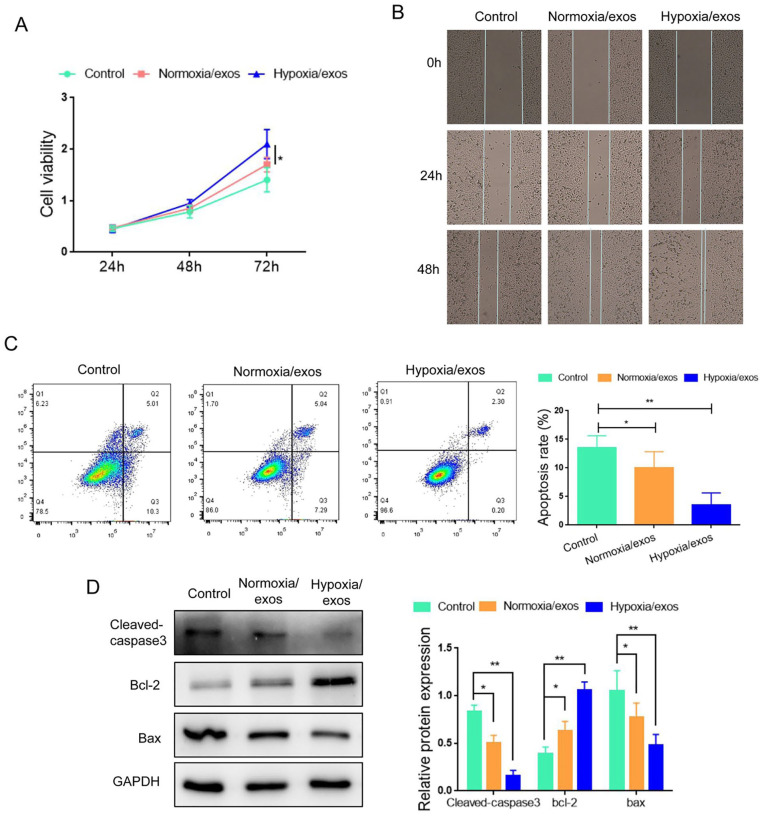
Next, we explored the effect of hypoxia/exos on A549 cells. As shown in Fig. 2A, A549 cells cultured with hypoxia/exos exhibited higher proliferation capacity than those cultured with normoxia/exos. Wound healing assay demonstrated that hypoxia/exos enhanced the migration ability of A549 cells (Fig. 2B). Moreover, flow cytometry analysis showed that administration of hypoxia/exos inhibited the apoptosis of A549 cells, compared with normoxia/exos (Fig. 2C). In line with these observations, the expression of cleaved-caspase3 and Bax was lower in the hypoxia/exo group than the normoxia/exo group (Fig. 2D), while the level of Bcl2 was increased in the hypoxia/exo group compared with the normoxia/exo group. These data indicated that hypoxia/exos could facilitated the proliferation and migration, and antagonized the apoptosis in A549 cells.
Figure 2.
Hypoxia/exos promotes the proliferation and inhibited apoptosis in A549 cells. (A) The proliferation of A549 cells after treatment with hypoxia/exos and normoxia/exos was determined by CCK-8 assay. (B) The migration of A549 cells was assessed by wound healing assay. (C) The apoptosis of A549 cells was detected by flow cytometry analysis. (D) The protein levels of cleaved-caspase 3, Bcl-2 and Bax were determined by Western blot. Data represent the mean ± SD of three separate experiments; comparison was performed with Student’s t-test. Scale bar: 100 μm. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05; **P < 0.01.
Hypoxia/Exos Induced the Polarization of M2 Macrophages
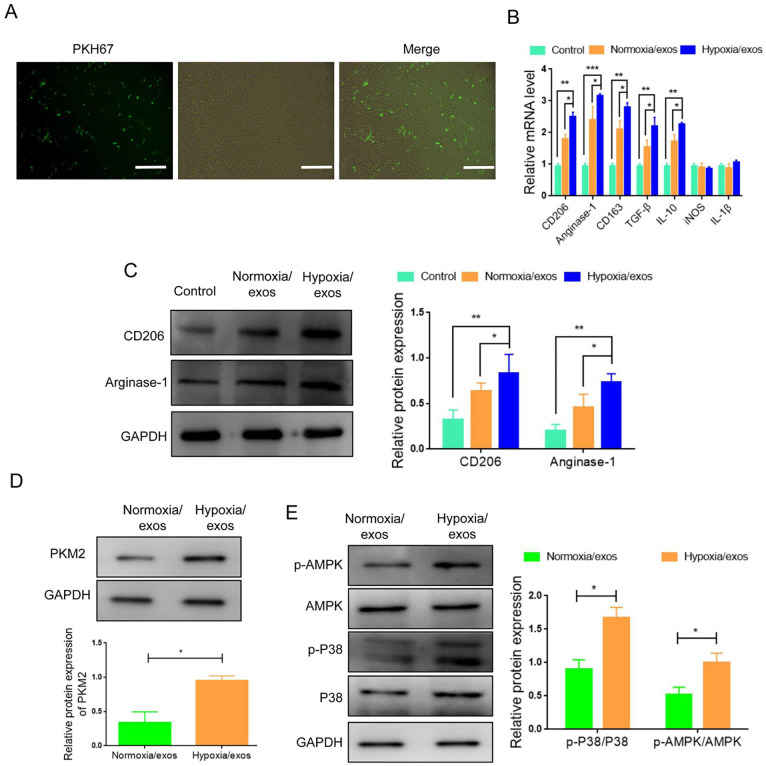
M2 macrophages are beneficial for cancer progression. We then investigated whether hypoxia/exos had an effect on macrophage polarization. The uptake assay showed that PKH67-labeled exos could be internalized by RAW264.7 cells (Fig. 3A). M1 macrophages are characterized by the expression of iNOS and IL-1β, while M2 macrophages are characterized by the expression of CD206, CD163, TGF-β, IL-10, and Arginase-1. The results of RT-qPCR showed that the mRNA expression of CD206, CD163, TGF-β, IL-10, Arginase-1 was significantly higher in macrophages incubated with hypoxia/exos than those incubated with normoxia/exos (Fig. 3B). Consistently, the protein levels of M2 markers (CD206 and Arginase-1) were increased following the treatment with hypoxia/exos (Fig. 3C). Together, these results indicated that hypoxia/exos could induce macrophage toward to the M2 phenotype.
Figure 3.
Hypoxia/exos induce the polarization of M2 macrophages. (A) Immunofluorescence staining displayed the internalization of hypoxia/exos (green) by RAW264.7 cells. (B) Real-Time Quantitative PCR analysis for M2 markers (CD206, CD163, TGF-β, IL-10, Arginase-1) and M1 markers (iNOS, IL-1β). (C) Western blot analysis of the protein expression of M2 markers (CD206 and Arginase-1). (D) Western blot analysis of PKM2 expression in hypoxia/exos or normoxia/exo. (E) Western blot analysis of the protein and phosphorylation levels of AMPK and p38 in macrophages exposed to hypoxia/exos or normoxia/exos. Data represent the mean ± SD of three separate experiments; comparison was performed with Student’s t-test. Scale bar: 100 μm. GAPDH: glyceraldehyde-3-phosphate dehydrogenase. *P < 0.05; **P < 0.01; ***P < 0.001.
Glucose metabolism is important change in cancer cells under a hypoxic environment. PKM2 has been reported to play a critical role in glycolysis15,16. Interestingly, we found that the expression of PKM2 in hypoxia/exo was significantly increased compared with normoxia/exo (Fig. 3D). Western bolt results manifested higher levels of p-AMPK, and p-P38 in macrophages following stimulation with hypoxia/exos (Fig. 3E). Thus, these results implied that hypoxia/exos induces M2 polarization is associated with exosomal PKM2-mediated AMPK/p38 activation.
Hypoxia/Exos-Induced M2 Macrophages Favor the Migration, Invasion, and EMT in A549 Cells
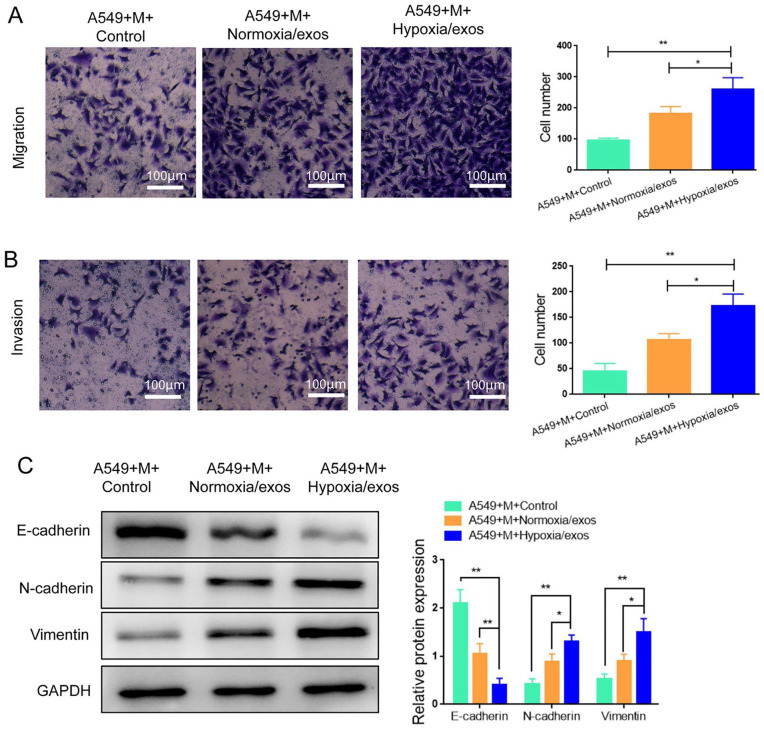
To determine the effects of M2 macrophages on the migration and invasion ability of A549 cells, we performed transwell assay. The results revealed that A549 cells co-incubated with M2 macrophages treated with hypoxia/exos exhibited superior migration and invasion abilities compared with the control cells (Fig. 4A, B). It is known that re-activation of the EMT program promotes cancer progression and enhances the metastatic phenotype 17 . Western blot analysis presented that the expression of E-cadherin, a major epithelial marker, was decreased, while the expression levels of mesenchymal cell markers N-cadherin and vimentin were enhanced in A549 co-cultured with hypoxia/exo-treated macrophages (Fig. 4C). All together, these results confirmed a more superior role of M2 macrophages induced by hypoxia/exos in promoting migration, invasion, and EMT in A549 cells.
Figure 4.
M2 macrophages polarized by hypoxia/exos promote the migration, invasion, and epithelial-mesenchymal transition in A549 cells. (A) Transwell migration assay in A549 cells co-cultured with hypoxia/exos (or normoxia/exo)-treated macrophages. (B) Transwell invasion assay in A549 cells co-cultured with hypoxia/exos (or normoxia/exo)-treated macrophages. (C) Western blot analysis of EMT related proteins (E-cadherin, N-cadherin, Vimentin). M, RAW264.7 macrophages; Data represent the mean ± SD of three separate experiments; comparison was performed with Student’s t-test. Scale bar: 100 μm. *P < 0.05; **P < 0.01.
Hypoxia/Exos Facilitate Tumor Growth and Metastasis of A549 Cells in Vivo
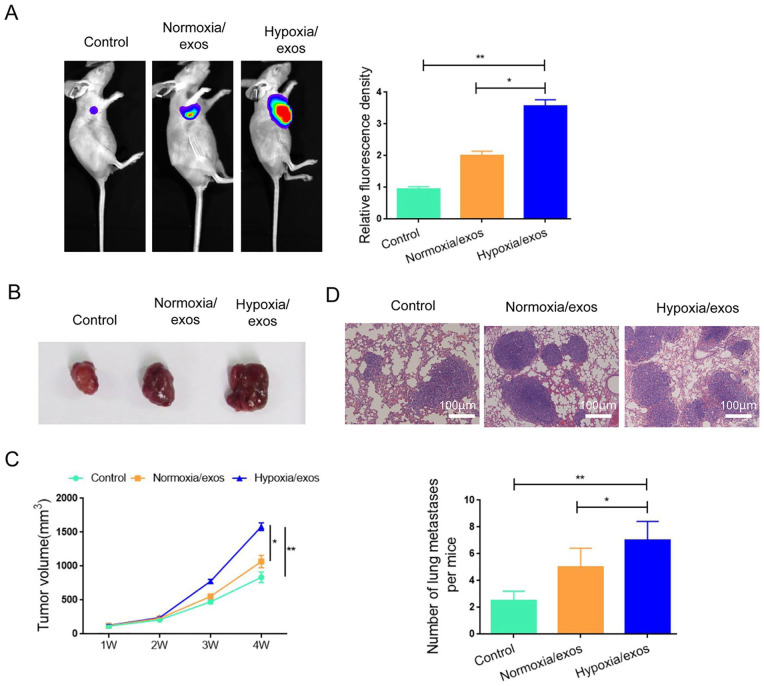
Given the important role of hypoxa/exos in the proliferation and migration of A549 cells in vitro, we further verified the function of hypoxia/exos using the xenograft model via injection with luciferase-labeled A549 cells. Higher fluorescence intensity and increased tumor volume and tumor weight were observed in the hypoxia/exos group compared with the normoxia/exos group (Fig. 5A–C), indicating that hypoxia/exos exacerbated progressive growth in vivo. In addition, more lung metastatic nodules were observed in hypoxia/exos-treated mice compared with the control mice (Fig. 5D, E). Collectively, these data suggest that hypoxia/exos have a promoting effect on lung cancer progression.
Figure 5.
Hypoxia/exos facilitate the tumor growth and metastasis of A549 cells in vivo. (A) In vivo fluorescence imaging for detecting the tumor growth of A549 cells. (B) The growth curve of tumor growth in mice after treatment with hypoxia/exos (or normoxia/exo) for 4 weeks. (C) The tumor weight of tumors isolated from nude mice treated with hypoxia/exos (or normoxia/exo). (D) Hematoxylin-Eosin staining of lung metastasis nodules. (E) The number of metastatic nodules in D. Data represent the mean ± SD of three separate experiments; comparison was performed with Student’s t-test. Scale bar: 100 μm. *P < 0.05; **P < 0.01.
Discussion
Hypoxia is a common phenomenon in the tumor microenvironment and is associated with tumor progression and drug resistance 3 . Accumulating studies have been indicated that hypoxia triggers exos release from cancer cells, thus facilitating tumor progression 18 . Herein, we isolated the exos from A549 cells under hypoxia conditions and investigated their function and mechanism in lung cancer progression. Our results showed that hypoxia/exos have an oncogenic activity in lung cancer, which is associated with exosomal PKM2-mediated induction of M2 macrophages via AMPK/p38 signaling.
Exos secreted by multiple cells have been reported to involve in tumorous biological function and exosomal concentration/cargo can be altered by hypoxia 4 . Previous studies have been demonstrated that the acceleration of tumor progression was closely associated with the exos released from hypoxia tumor cells. For example, it has been reported that ovarian cancer cells exposed to hypoxia significantly increase their exos release and treatment with hypoxia-induced exos lead to a more aggressive and chemoresistant ovarian cancer phenotype 6 . Consistently, we found that culture under hypoxic conditions favored the release of exos by lung cancer cells, as demonstrated with increased exo concentration and elevated protein expressions of exo markers (CD9, CD81, TSG-101, HSP70, ALZX). Moreover, we found that Hypoxia/exos enhanced the proliferation and migration of A549 cells in vitro and promoted the tumor growth and metastasis of A549 cells in vivo. Thus, our results revealed an oncogenic activity of hypoxia/exos in lung cancer.
It is well acknowledged that macrophages act as scavengers, modulating the immune response and maintaining tissue homeostasis 19 . Macrophages can be reprogramed by metabolites, cytokines, and other signaling mediators, which make them the key participants in tumor microenvironment and associated with enhanced tumor progression 20 . It has been reported that M2 macrophage polarization could be regulated by exos. Bardi et al. 21 have showed that exos released from melanoma cells induced a mixed M1 and M2 pro-tumor macrophages. Exos from adipose-derived stem cells polarize M2 macrophages and thus attenuate adipose inflammation and obesity 22 . In agreement with these results, our results showed that hypoxia/exos induced M2 macrophage polarization, which was contributed to the enhanced migration and invasion in A549 cells.
Glucose metabolism change is a hallmark of cancer under hypoxia conditions. PKM2 is a key enzyme involved in glycolysis regulation and has an important regulatory role in cancer progression/resistance under hypoxia conditions23–26. It has been reported that exosomal PKM2 secreted by hypoxic cisplatin-resistance cells transmitted cisplatin-resistance to sensitive non-small cell lung cancer (NSCLC) cells both in vitro and in vivo 27 . We hypothesized that hypoxia treatment might induced PKM2 expression. As expected, the protein level of PKM2 was significantly increased in hypoxia/exos compared with Normoxia/exos. Previous studies have been demonstrated that AMPK plays an essential role in M2 macrophage polarization28,29. Interestingly, our data showed that treatment with hypoxia/exos enhanced the phosphorylation of AMPK, as well as the phosphorylation of its downstream factor p38 in macrophages. Collectively, our results implied that hypoxic exosmal PKM2 induces M2 macrophages, which was related to activation of AMPK/p38 pathway.
Conclusions
Our findings show that hypoxia/exos induced M2 macrophage polarization via AMPK/p38 pathway and thus promotes lung tumor growth and metastasis. This study manifests hypoxia/exos as the possible targets for fighting against cancer progression and metastasis.
Supplemental Material
Supplemental material, sj-tif-1-cll-10.1177_09636897221106998 for Hypoxic Tumor-Derived Exosomes Induce M2 Macrophage Polarization via PKM2/AMPK to Promote Lung Cancer Progression by Shiyu Zhou, Yu Lan, Yuqun Li, Zhenxing Li, Jinding Pu and Liping Wei in Cell Transplantation
Acknowledgments
We thank all of the members in the lab. We are grateful to the financial support of the Guangzhou Health Science and Technology Project.
Footnotes
Author Contributions: (I) Conception and design: LY and ZSY; (II) Administrative support: LYQ; (III) Provision of study materials or patients: LZX; (IV) Collection and assembly of data: LY and ZSY; (V) Data analysis and interpretation: LY and ZSY; (VI) Manuscript writing: All authors; (VII) Final approval of manuscript: All authors.
Availability of Data and Materials: The data that support the findings of this study are available from the corresponding author [ email:weiliping2017123@163.com] upon reasonable request.
Ethical Approval: All animal experiments were approved by the Ethics Committee of Guangzhou Medical University (GZXK2020-06)
Statement of Informed Consent: There are no human subjects in this article and informed consent is not applicable.
Statement of Human and Animal Rights: All procedures in this study were conducted in accordance with the Ethics Committee of Guangzhou Medical University.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the Guangzhou Health Science and Technology Project (NO.20201A011093).
ORCID iD: Liping Wei  https://orcid.org/0000-0002-3500-8016
https://orcid.org/0000-0002-3500-8016
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. [DOI] [PubMed] [Google Scholar]
- 2. Socinski MA, Obasaju C, Gandara D, Hirsch FR, Bonomi P, Bunn PA, Jr, Kim ES, Langer CJ, Natale RB, Novello S, Paz-Ares L, et al. Current and emergent therapy options for advanced squamous cell lung cancer. J Thorac Oncol. 2018;13: 165–83. [DOI] [PubMed] [Google Scholar]
- 3. Schito L, Semenza GL. Hypoxia-inducible factors: master regulators of cancer progression. Trends Cancer. 2016;2(12):758–70. [DOI] [PubMed] [Google Scholar]
- 4. Kumar A, Deep G. Hypoxia in tumor microenvironment regulates exosome biogenesis: molecular mechanisms and translational opportunities. Cancer Lett. 2020;479:23–30. [DOI] [PubMed] [Google Scholar]
- 5. Mashouri L, Yousefi H, Aref AR, Ahadi AM, Molaei F, Alahari SK. Exosomes: composition, biogenesis, and mechanisms in cancer metastasis and drug resistance. Mol Cancer. 2019;18:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Dorayappan KDP, Wanner R, Wallbillich JJ, Saini U, Zingarelli R, Suarez AA, Cohn DE, Selvendiran K. Hypoxia-induced exosomes contribute to a more aggressive and chemoresistant ovarian cancer phenotype: a novel mechanism linking STAT3/Rab proteins. Oncogene. 2018;37:3806–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Xue M, Chen W, Xiang A, Wang R, Chen H, Pan J, Pang H, An H, Wang X, Hou H, Li X. Hypoxic exosomes facilitate bladder tumor growth and development through transferring long non-coding RNA-UCA1. Mol Cancer. 2017;16:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zhang X, Sai B, Wang F, Wang L, Wang Y, Zheng L, Li G, Tang J, Xiang J. Hypoxic BMSC-derived exosomal miRNAs promote metastasis of lung cancer cells via STAT3-induced EMT. Mol Cancer. 2019;18:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Najafi M, Hashemi Goradel N, Farhood B, Salehi E, Nashtaei MS, Khanlarkhani N, Khezri Z, Majidpoor J, Abouzaripour M, Habibi M, Kashani IR, et al. Macrophage polarity in cancer: a review. J Cell Biochem. 2019;120:2756–65. [DOI] [PubMed] [Google Scholar]
- 10. Sica A, Mantovani A. Macrophage plasticity and polarization: in vivo veritas. J Clin Invest. 2012;122:787–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang W, Shi R, Kang X, Zhang X, Chen P, Zhang L, Hou A, Wang R, Zhao Y, Zhao K, Liu Y, et al. Monoacylglycerol lipase regulates cannabinoid receptor 2-dependent macrophage activation and cancer progression. Nat Commun. 2018;9:2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gordon S, Martine FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604. [DOI] [PubMed] [Google Scholar]
- 13. Wu X, Showiheen SAA, Sun AR, Crawford R, Xiao Y, Mao X, Prasadam I. Exosomes extraction and identification. Methods Mol Biol. 2019;2054:81–91. [DOI] [PubMed] [Google Scholar]
- 14. Mao Y, Wang Y, Dong L, Zhang Y, Zhang Y, Wang C, Zhang Q, Yang S, Cao L, Zhang X, Li X, et al. Hypoxic exosomes facilitate angiogenesis and metastasis in esophageal squamous cell carcinoma through altering the phenotype and transcriptome of endothelial cells. J Exp Clin Cancer Res. 2019;38:389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang YC, Chien MH, Liu HY, Chang YC, Chen CK, Lee WJ, Kuo TC, Hsiao M, Hua KT, Cheng TY. Nuclear translocation of PKM2/AMPK complex sustains cancer stem cell populations under glucose restriction stress. Cancer Lett. 2018;421:28–40. [DOI] [PubMed] [Google Scholar]
- 16. Liu M, Zhang Z, Wang H, Chen X, Jin C. Activation of AMPK by metformin promotes renal cancer cell proliferation under glucose deprivation through its interaction with PKM2. Int J Biol Sci. 2019;15:617–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chaffer CL, San Juan BP, Lim E, Weinberg RA. EMT, cell plasticity and metastasis. Cancer Metastasis Rev. 2016;35:645–54. [DOI] [PubMed] [Google Scholar]
- 18. King HW, Michael MZ, Gleadle JM. Hypoxic enhancement of exosome release by breast cancer cells. BMC Cancer. 2012; 12:421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mehla K, Singh PK. Metabolic regulation of macrophage polarization in cancer. Trends Cancer. 2019;5:822–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nielsen SR, Schmid MC. Macrophages as key drivers of cancer progression and metastasis. Mediators Inflamm. 2017;2017:9624760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bardi GT, Smith MA, Hood JL. Melanoma exosomes promote mixed M1 and M2 macrophage polarization. Cytokine. 2018; 105:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao H, Shang Q, Pan Z, Bai Y, Li Z, Zhang H, Zhang Q, Guo C, Zhang L, Wang Q. Exosomes from adipose-derived stem cells attenuate adipose inflammation and obesity through polarizing M2 macrophages and beiging in white adipose tissue. Diabetes. 2018;67:235–47. [DOI] [PubMed] [Google Scholar]
- 23. Luo W, Hu H, Chang R, Zhong J, Knabel M, O’Meally R, Cole RN, Pandey A, Semenza GL. Pyruvate kinase M2 is a PHD3-stimulated coactivator for hypoxia-inducible factor 1. Cell. 2011;145:732–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Schwartz L, Supuran CT, Alfarouk KO. The Warburg effect and the hallmarks of cancer. Anticancer Agents Med Chem. 2017;17:164–70. [DOI] [PubMed] [Google Scholar]
- 25. Wong N, Ojo D, Yan J, Tang D. PKM2 contributes to cancer metabolism. Cancer Lett. 2015;356:184–91. [DOI] [PubMed] [Google Scholar]
- 26. Li YH, Li XF, Liu JT, Wang H, Fan LL, Li J, Sun GP. PKM2, a potential target for regulating cancer. Gene. 2018;668:48–53. [DOI] [PubMed] [Google Scholar]
- 27. Wang D, Zhao C, Xu F, Zhang A, Jin M, Zhang K, Liu L, Hua Q, Zhao J, Liu J, Yang H, et al. Cisplatin-resistant NSCLC cells induced by hypoxia transmit resistance to sensitive cells through exosomal PKM2. Theranostics. 2021;11:2860–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ye W, Wang J, Lin D, Ding Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int J Biol Macromol. 2020;146:25–35. [DOI] [PubMed] [Google Scholar]
- 29. Xu F, Cui WQ, Wei Y, Cui J, Qiu J, Hu LL, Gong WY, Dong JC, Liu BJ. Astragaloside IV inhibits lung cancer progression and metastasis by modulating macrophage polarization through AMPK signaling. J Exp Clin Cancer Res. 2018;37:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-cll-10.1177_09636897221106998 for Hypoxic Tumor-Derived Exosomes Induce M2 Macrophage Polarization via PKM2/AMPK to Promote Lung Cancer Progression by Shiyu Zhou, Yu Lan, Yuqun Li, Zhenxing Li, Jinding Pu and Liping Wei in Cell Transplantation