The new coronavirus pandemic has promoted the new development of mobile and wearable computing in unprecedented ways. We discuss how on-body devices can help to fight the pandemic and may stay as a toolset to effectively deal with infectious diseases in the future.
WHY WEARABLES?
Researchers and health policy managers turned to smartphones and on-body devices mostly for their ubiquity, i.e., to offer health and safety-related information to a large share of the population or gather patient responses. Yet, smartphones and wearables enable fast data and information flow, which is particularly relevant for the rapid infectious character of SARS-CoV-2. We observe that continuous sensor and behavior data of smartphones and on-body devices are important as virus testing is associated with effort, cost, and provides only one-time information, and global immunization is still far away.
We focus here on already existing and newly created wearable devices and smartphone apps for everyday use, but we exclude clinical and laboratory measurement systems, e.g., for heart and respiratory assessment. For researchers and public health authorities, symptom screening and tracking based on continuous sensor data from wearables and smartphones can provide insight into a possible infection of individuals. Also important are methods for social distancing and digital contact tracing, devices and apps for managing quarantine/self-isolation, and clinical management. To round-off this overview, we summarize lessons learnt from selected initiatives.
SYMPTOM SCREENING and TRACKING
Virus spreading occurs in waves and varies in speed, creating regional differences in infection risk. To track spreading, early data of evolving symptoms is vital. The symptom indicators could be a basis to create outbreak maps and select regional safety measures. Manual symptom reporting, either structured through questionnaires or unstructured through social network reporting, has been used as a data source for more than a decade.8,9,15 The wearable sensors used in health management become interesting platforms for infectious disease tracking.1,14 Data that can deliver markers of influenza-like symptoms is readily available from wearables, including sleep duration, activity types and intensities, and daily routine changes. Fever, as a core influenza symptom, could be detected from an increase in resting heart rate (RHR), which is known to raise by 8.5 beats per minute per additional 1°K body temperature.5
One of the early initiatives into wearable sensor-based symptom tracking motivated by SARS-CoV-2 is Germany's Robert Koch Institute (RKI) “Corona Datenspende” (https://corona-datenspende.de). The RKI app development effort started in January 2020 by implementing interfaces for a wide variety of commercially available wearable sensor devices. Besides the data filtering necessary to retrieve daily averages of RHR, sleep times, and step count, substantial effort was made to comply with Germany's strict data privacy regulations. With more than 540k users providing sensor data to date, the RKI's Corona Datenspende is one of the largest initiatives in wearable-based epidemiological symptom tracking. The user density ranges widely across German districts from 0.2% to almost 1%, e.g., between a few 100's and more than 25k users. To analyze RHR increase as an indicator of fever, step count has been used to correct for physical activity. While activity-corrected average RHR increases may be an influenza indicator, there could be other explanations too. By summing the detected events over a (regional) population, the detected event counts anticipate respiratory disease reports created by physician offices. RKI regularly updates on the data and insights (https://corona-datenspende.de/science/en/reports).
While RKI's Corona Datenspende marks a milestone in coverage and device integration, it is limited to Germany only. A variety of further initiatives started deploying wearables and activity trackers worldwide, driven by manufacturers and research institutes. Examples are the DETECT study of the Scripps Research Institute (https://detectstudy.org) and Stanford's Smartwatch Study.10 Results from the DETECT study showed that 54 positive tested and 279 negative tested participants could be recognized with an 80% area-under-curve by combining reported symptom and sensor data.13 The smartwatch data analysis of Mishra et al. highlights that the parameters RHR, sleep times, and steps count show changes even before symptom onset.
COVID-19 primarily affects the respiratory system, suggesting that coughing or breathing patterns could reveal complementary symptom information to the data described so far. Jeong et al.6 investigated cough and body temperature sensing based on an accelerometer patch prototype. The patch is worn on the skin overlying the suprasternal notch (lower throat position). Initial data shows that the patch could capture several body signals beside the cough moments, including heart beat (seismocardiography), respiration rate, and movement. In addition, several body temperature monitoring patches have been specifically developed for symptom tracking. All of these new devices need to be validated for everyday use, especially on the robustness of their estimates, practicality (e.g., runtime), and data integration options.
Clearly, wearable symptom tracking is at an early stage and further development and analyses are necessary to better understand whether the data can reveal actionable information on virus spreading. However, the indicators obtained so far—including large initiatives and studies using COVID-19 test results—confirmed the potential of established consumer-grade wearables to deliver data on the virus spreading. The early infectious period of COVID-19 without recognizable symptoms is assumed to be 1–3 days. Early infection indicators from wearables are therefore valuable information that could help 1) individuals to enter self-quarantine and schedule testing, 2) local authorities to manage regional containment measures and screening, and 3) hospitals to allocate clinical capacity. Furthermore, for patients, wearable tracking can help to assess symptom severity, e.g., by adding the smartwatch's blood oxygen estimation to the set of home monitoring routines, just like measuring body temperature. Beyond the individual initiatives on wearable symptom tracking launched so far, an integrative global approach that provides open research data and connects with registries, including Influenzanet and FluNet, will be indispensable.
DIGITAL CONTACT TRACING
The infection probability is greatly reduced when individuals stay away from each other by a distance sufficient to avoid virus transfer by exhaled aerosols or skin contact. If someone has been diagnosed with the infection, it is imperative to know (and quarantine) anyone whom he/she might have infected too. Contact tracing aims to identify physical interactions occurring below a critical distance of 1.5–2 m (6 feet) and for a sufficient amount of time during the known incubation period; for COVID-19, the incubation period is assumed to be two weeks. Contact tracing has been traditionally done through interviews with patients, which is highly time and labor intensive, as well as error prone (do you accurately recall everyone who sat close to you last week?). Singapore's TraceTogether app (https://www.healthhub.sg/apps/38/tracetogether-app) paved the way for “Corona tracing apps” that leverage the ubiquity of Bluetooth (BT)-enabled smartphones for proximity tracking. Subsequently, Google and Apple jointly enhanced their operating systems to locally log anonymous IDs of other phones as a platform for distributed BT-based contact tracing solutions.
BT received signal strength indication (RSSI) has been extensively studied for proximity estimation in the pervasive computing community, including limitations resulting from the multipath transmission, absorption, and refraction, e.g., see Liu et al.7 While estimating if two individuals are close to each other (e.g., below 5 m) or further away (e.g., 5–10 m) works well with BT, disambiguating the signal between 1 m and 2 m is much less reliable [see Figure 1(C)]. Dedicated BT beacons tend to be more reliable than built-in BT in consumer devices.11 Singapore and Luxembourg are thus going beyond smartphone-based contact tracing by deploying dedicated wearable devices. A highly accurate alternative technology is ultra wideband positioning (www.kpdistance.com), which comes at the price of being more complex and requiring base stations in addition to the wearable devices. Further improvements can be achieved by combining radio frequency-based RSSI analysis with other sensing modalities, including ambient sound, ultrasound, ultra wideband, magnetic field, and other sensing types. Earlier research showed that “sound fingerprints” recorded at the same time by two devices is a good indication of proximity.16 We are currently working on combining ambient sound analysis with BT-based proximity estimation and have observed a very promising 10%–20% accuracy increase on social distance detection in everyday settings. Beyond passive sensing of ambient sound, active ultrasound localization (which is another established pervasive/wearable positioning technology) can be used to enhance the quality of smartphone-based proximity detection too. For example, Novid (https://www.novid.org) emits near-ultrasound signals from modern smartphone speakers and listens to other Novid apps.
Figure 1.
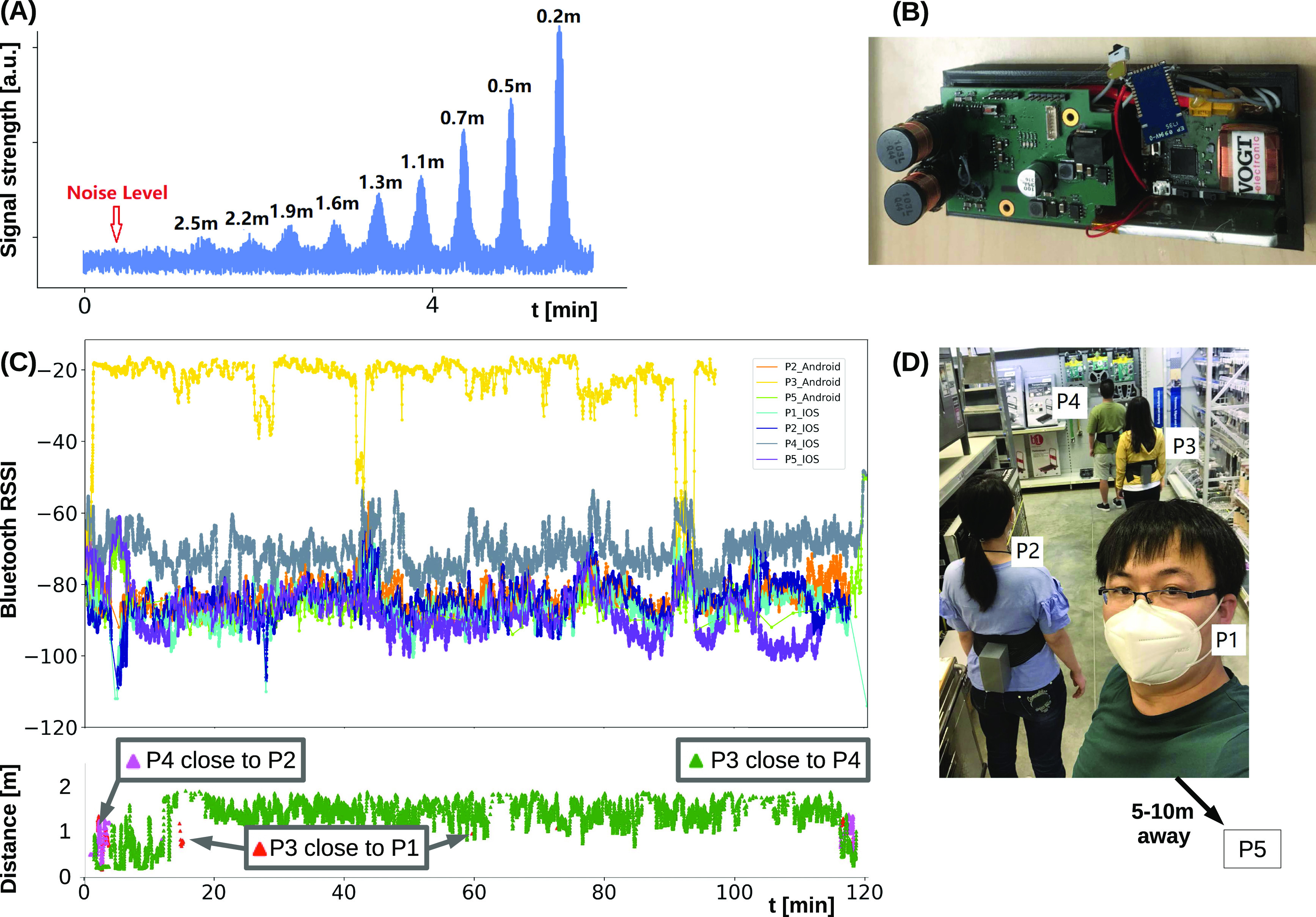
Wearable proximity estimation in the range of 0.2–2.5 m based on oscillating magnetic fields. (A) Distance versus received signal strength analysis. (B) Wearable prototype hardware. (C) Study data analysing BT and magnetic field based proximity estimation in a shop scene with five participants (P1-5) in groups (P1, P2) and (P3, P4), while P5 is further away. Top panel: BT signals are weakly separable. Bottom panel: Example magnetic field-based distance estimates for P3 showing rare events of getting below 2 m to others than P4. (D) Analysis scene from a workshop test of the proximity estimation methods.
Wearable applications that require reliability with respect to environmental interference will benefit from sensing methods that produce distinguishable signals just at the 2 m social distancing range. A promising candidate technology is sensing an oscillating magnetic field.3 The approach of Bian et al. is based on induction, much like wireless smartphone chargers work. The wearable device includes a transmitter coil operated by a periodically changing current, resulting in an expanding and retracting magnetic field. When another coil (another device worn by a second user) is moving into the changing magnetic field, a current is induced. The strength of the receiver signal is proportional to the transmitter current, coil sizes, and distance to the third power. Consequently, the proximity effect is highly localized. Practical wearable systems can be made with 2 cm coil diameter and ∼1 A current, to estimate proximity up to 2 m [see Figure 1(B) and (D)]. Besides its accuracy, magnetic field sensing is unaffected by issues including multipath propagation, refraction, or absorption, as the field just expands and retracts.
While being more accurate, it is clear that the wearable sensor devices for proximity estimation based on sound, ultrasound, or magnetic field cannot compete with BT-based contact tracing considering the potential user reach of BT-enabled smartphones and the support built into the operating systems. To this end, epidemiological models showed that under reasonable conditions digital contact tracing can effectively reduce the SARS-CoV-2 virus reproduction rate when at least 60% of a population participates.4 While getting the majority of a region's population to voluntarily participate may be hard, wearable digital contact tracing could well serve a local community, business, or building.
SCALING UP: SIMULATING CONTACTS
Current epidemiological models do not capture behavior variations, which can have cascading consequences for the pandemic's evolution. Deterministic models4 use average transition ratios from one state to another, which makes all individuals at any given time interchangeable. Models using social contact networks (e.g., see the article by Block et al.2) do not model the dynamic nature of the contact network. As people change location, so does the available contact network change. Therefore, deterministic and static social network models cannot observe the changes induced by individual behavior and the changes inflicted by contact tracing. In contrast, a particle behavior model (PBM) could simulate an individual's daily interaction on a minute by minute scale. We can consider each particle that gets close to an infected one or stays at home. With the PBM, we can assess the consequences of behavioral decisions.
Using digital contact tracing and virus testing to exit quarantine, Figure 2(A) illustrates how the Susceptible-Exposed-Infectious-Removed (SEIR) model fails to reveal early as well as late exposure to the virus, thus providing a misleading sense of safety. PBM instead can play different scenarios that indicate possible shortcomings of a mitigation strategy to researchers. For example, Figure 2(B) illustrates a failure to contain an exposed particle. The situation occurred as an exposed individual gets isolated per digital contact tracing, but was interacting with a household member during the three days before the infection was reported to the contact tracing app. As we assume a low 3.5-day delay between being infectious and observing symptoms or receiving positive test results, the individual became infectious and spread the virus. We observe similar effects when digital contact tracing is used with a two-week quarantine, but without testing.
Figure 2.
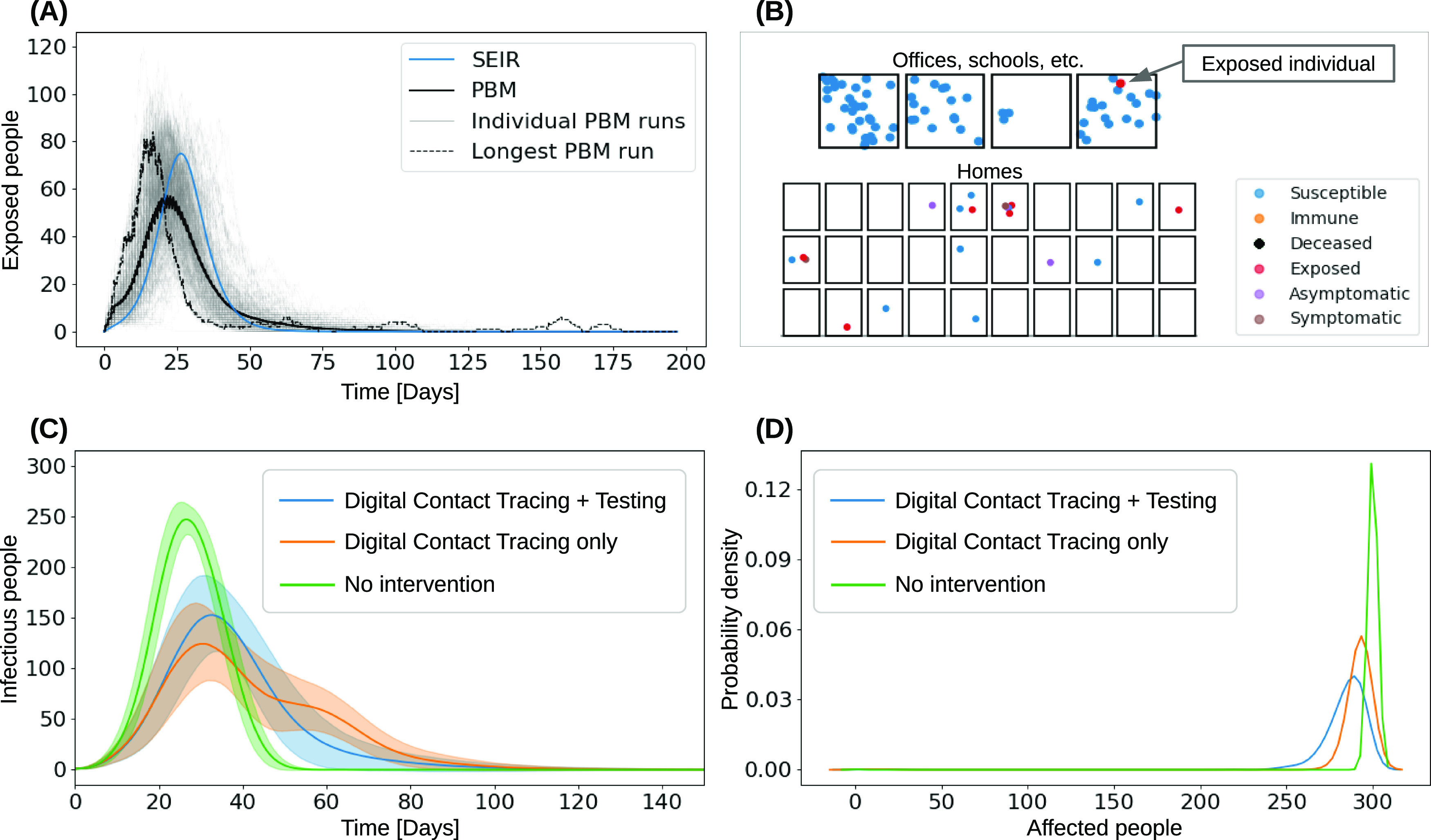
Individual-level PBM simulations of the COVID-19 pandemic in 300 individuals, unless stated otherwise. (A) Comparison of PBM and SEIR models. SEIR fails to reveal early exposure and late recurrences. (B) Example offices and homes scenario of 90 individuals. An individual got infected at home by a quarantined household member due to being on the contact list of a positive tested patient. (C,D) PBM-based estimation, showing that different tracing mitigation strategies prolong the pandemic's duration (C) and that digital contact tracing and testing have only marginal influence on the total number of individuals contracting COVID-19 (D).
Other PBM applications include estimating the infection wave duration and behavioral effects in response to mitigation actions. Figure 2(C) and (D) illustrates the effect of different mitigation strategies on a pandemic's duration. By creating digital twins of local businesses, neighborhoods, towns or cities, PBM could help to explore the disease propagation and analyze optimal mitigation strategies per location and population behavior.
DEALING WITH ISOLATION AND CLINICAL NEEDS
Further topics for mobile and wearable devices arise from the deep impact that the COVID-19 pandemic has on everyone's daily life as well as clinical management needs. The indiscriminate restrictions in various regions as well as quarantine/self-isolation regulations have led to mental and social health issues, including anxiety and depression. Several smartphone apps are picking up on the topic. New York City put up a webpage suggesting digital health services across the band of mental health (https://nycwell.cityofnewyork.us/en/covid-19-digital-mental-health-resources). On the clinical side, staff training, resource allocation, and remote patient management are among the most important topics of digital health applications. A comprehensive overview is available from mHealth Hub (https://mhealth-hub.org/mhealth-solutions-against-covid-19). Yet, wearable devices are rarely integrated in the efforts mentioned above.
LESSONS LEARNT
The citizen-science projects on wearable symptom tracking (RKI Corona Datenspende, Scripps DETECT) have revealed the critical lack of data and interface standards available in today's consumer wearable devices. The devices and their web portals are often proprietary and incompatible, thus not yet prepared to support pseudonymous symptom tracking projects. Basic data, including age, gender, weight, and height, can be requested by a surveillance service in most wearable platforms, yet the response often includes unwanted profile information, causing privacy concerns. RKI's development built on existing commercial software and thus quickly created wide coverage among wearables, at the expense of data flow transparency. The RKI initiative was criticized for building on closed source code and the Scripps initiative for complex data privacy statements. The proprietary device interfaces are one reason why the initiatives struggle with transparency. To support the fight against pandemics, wearable device providers need to adopt open interfaces and standard data structures. The Corona Components Standards (http://cocos.team) is an initiative to standardize data structure and terminology for COVID-19 and specifically addresses digital health applications.
Furthermore, wearable device providers should endorse data privacy measures tailored for pseudonymous applications (see previous Wearable Computing Department by Psychoula et al.12). Server-to-server APIs are preferred by various wearable vendors; however, in a closed-source application, users can only agree to a specific scope of data to be requested by the surveillance application. In contrast, client-to-server integrations, such as those offered by Apple's HealthKit, are more transparent, as data can be analyzed externally for consistency with the privacy statement.
Various wearable device efforts on symptom tracking and contact tracing are under development. Table 1 shows a snapshot highlighting the dominant principles and approaches. Whether epidemiological-scale initiative or dedicated monitoring device, none of the widely used nor specifically made wearables to track symptoms will reveal a COVID-19 infection specifically. The reason is that symptoms are too similar to other influenza-like diseases, including the yearly flu. Moreover, symptom variations among COVID-19 patients, up to fully asymptomatic ones, suggests that only tests at the molecular level could identify the virus type. Nevertheless, for both tracking and tracing, it is the speed of information flow that lets mobile and wearable devices add an important contribution to fight the pandemic.
Table 1. Selected wearable initiatives on symptom screening and tracking, as well as contact tracing related to COVID-19.
| Method type | Data sources | Platforms | Initiatives | Performance |
| Screen & track | RHR, sleep times, step count | Consumer-grade wearables & activity trackers | RKI Corona Datenspende, Scripps DETECT,13 Stanford Smartwatch Study10 | RHR and step count patterns confirm physician office influenza reports; 80% patient detection rate for sensor and symptom reporting; sensor data provides indicators before symptom reports. |
| Screen & track | Cough, heart & respiratory rate (accelerometer), body temperature | Custom stretchable patch | Jeong et al.,6 | Cough-related motion, heart beat (seismocardiogram), and respiration rate observable in the data. |
| Tracing | BT | Smartphones, BT beacons | Liu et al.,7 Montanari et al.,11 Bian et al.3 | Smartphone-based proximity estimation unreliable in <2m range. |
| Tracing | Oscillating magnetic field | Custom belt-attached wearable | Bian et al.3 | Highly localised proximity estimation up to 2.5 m. |
| Tracing | Ultra wideband positioning | Bracelet and base stations | www.kpdistance.com | Proximity accuracy down to 0.1 m. |
| Tracing | BT combined with sound fingerprints | Smartphones | Thiel et al.16 and unpublished work | Up to 20% accuracy increase on social distance detection wrt. BT. |
| Tracing | Near ultrasound | Smartphones | https://www.novid.org | Sub-meter accuracy. |
Another lesson learnt is that precise characterization of novel viruses regarding their infectiousness (i.e., dynamic reproduction rate), ratio of asymptomatic cases, etc. takes several months at least. One consequence of the characterization delay is that estimations on technology benefits, such as contact tracing and pandemic simulations, remain vague for long. In our contact tracing simulations, we deal with the missing data by modeling many variables by normal distributions and keeping key parameters free. The downside of an inclusive probabilistic modeling, however, is an increase in model and evaluation complexity, as well as reduced precision of estimates. At last, it is likely that when all the apps, wearables, and services, which are being developed now, are readily available at the outbreak of a virus, symptom tracking, contact tracing, and disease management are likely getting faster and more efficient. Moreover, the COVID-19 pandemic is likely creating a breakthrough for w`earables in regular telehealth that will stay.
Biographies
Oliver Amft is a professor and director with the Chair of Digital Health, FAU Erlangen-Nürnberg, Erlangen, Germany. Contact him at amft@computer.org
Luis Ignacio Lopera González is a postdoctoral researcher with the Chair of Digital Health, FAU Erlangen-Nürnberg, Erlangen, Germany. Contact him at luis.i.lopera.g@ieee.org
Paul Lukowicz is a professor and head with the Research Unit “Embedded Intelligence,” the German Research Center for Artificial Intelligence, Kaiserslautern, Germany. Contact him at paul.lukowicz@dfki.de
Sizhen Bian is working toward the doctoral degree at the Research Unit “Embedded Intelligence,” the German Research Center for Artificial Intelligence, Kaiserslautern, Germany. Contact him at sizhen.bian@dfki.de
Paul Burggraf is a co-founder and managing partner with mHealth Pioneers (Thryve). Contact him at paul.burggraf@thryve.de
Contributor Information
Oliver Amft, Email: amft@computer.org.
Luis Ignacio Lopera González, Email: luis.i.lopera.g@ieee.org.
Paul Lukowicz, Email: paul.lukowicz@dfki.de.
Sizhen Bian, Email: sizhen.bian@dfki.de.
Paul Burggraf, Email: paul.burggraf@thryve.de.
Oliver Amft, Email: oliver.amft@fau.de.
Kristof Van Laerhoven, Email: kvl@eti.uni-siegen.de.
REFERENCES
- 1.Amft O., “How wearable computing is shaping digital health, IEEE Pervasive Comput., vol. 17, no. 1, pp. 92–98, Jan.–Mar. 2018. [Google Scholar]
- 2.Block P., et al. , “Social network-based distancing strategies to flatten the COVID-19 curve in a post-lockdown world,” Nature Hum. Behav., vol. 4, pp. 588–596, 2020. [DOI] [PubMed] [Google Scholar]
- 3.Bian S., Rey V. F., Bahle G., and Lukowicz P., “A wearable magnetic field based proximity sensing system for monitoring COVID-19 social distancing, to appear, ISWC, 2020.
- 4.Ferretti L., et al. , “Quantifying SARS-CoV-2 transmission suggests epidemic control with digital contact tracing,” Science, vol. 368, 2020, Art. no. 6491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karjalainen J. and Viitasalo M., “Fever and Cardiac Rhythm,” Arch Int. Med., vol. 146, pp. 1169–1171, 1986. [PubMed] [Google Scholar]
- 6.Jeong H., Rogers J. A., and Xu S., “Continuous on-body sensing for the COVID-19 pandemic: Gaps and opportunities,” Sci. Adv., vol. 6, no. 36, 2020, Art. no. eabd4794, doi: 10.1126/sciadv.abd4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu S., Jiang Y., and Striegel A., “Face-to-face proximity estimationusing bluetooth on smartphones,” IEEE Trans. Mobile Comput., vol. 13, no. 4, pp. 811–823, 2013. [Google Scholar]
- 8.Madan A., Cebrian M., Lazer D., and Pentland A., “Social sensing for epidemiological behavior change,” in Proc. Ubicomp 2010: Proc. 12th ACM Int. Conf. Ubiquitous Comput., 2010, pp. 291–300. [Google Scholar]
- 9.Menni C., et al. , “Real-time tracking of self-reported symptoms to predict potential COVID-19. Nature Medicine, vol. 26, pp. 1037–1040, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mishra T., et al. , “Early Detection Of COVID-19 Using A Smartwatch,” 2020. medRxiv 2020.07.06.20147512.
- 11.Montanari A., Nawaz S., Mascolo C., and Sailer K., “A study of bluetooth low energy performance for human proximity detection in the workplace,” in Proc. IEEE Int. Conf. Pervasive Comput. Commun., 2017, pp. 90–99. [Google Scholar]
- 12.Psychoula I., Chen L., and Amft O., “Privacy risk awareness in wearables and the internet of things,” IEEE Pervasive Comput., vol. 19, no. 3, pp. 60–66, Jul.–Sep. 2020. [Google Scholar]
- 13.Quer G., et al. , “Passive monitoring of physiological data and self-reported symptoms to detect clusters of people with COVID-19,” 2020. medRxiv 2020.07.06.20141333.
- 14.Radin J. M., Wineinger N. E., Topol E. J., and Steinhubl S. R., “Harnessing wearable device data to improve state-level real-time surveillance of influenza-like illness in the USA: A population-based study,” Lancet Digital Health, vol. 2, pp. e85–e93, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun K., Chen J., and Viboud C., “Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: A population-level observational study,” Lancet Digital Health, vol., pp. e201–e208, 2020. [DOI] [PMC free article] [PubMed]
- 16.Thiel B., Kloch K., and Lukowicz P., “Sound-based proximity detection with mobile phones,” in Proc. 3rd Int. Workshop Sens. Appl. Mobile Phones, 2012, pp. 1–4. [Google Scholar]


