Abstract
Objective
The objective of this study was to evaluate photochemical crosslinking using Al(III) phthalocyanine chloride tetrasulfonic acid (CASPc) and light with a wavelength of 670 nm as a potential therapy to strengthen articular cartilage and prevent tissue degradation.
Design
Changes in viscoelastic properties with indentation were used to identify 2 crosslinking protocols for further testing. Crosslinked cartilage was subjected to an in vitro, accelerated wear test. The ability of the crosslinked tissue to resist biochemical degradation via collagenase was also measured. To better understand how photochemical crosslinking with CASPc varies through the depth of the tissue, the distribution of photo-initiator and penetration of light through the tissue depth was characterized. Finally, the effect of CASPc on chondrocyte viability and of co-treatment with an antioxidant was evaluated.
Results
The equilibrium modulus was the most sensitive viscoelastic measure of crosslinking. Crosslinking decreased both mechanical wear and collagenase digestion compared with control cartilage. These beneficial effects were realized despite the fact that crosslinking appeared to be localized to a region near the articular surface. In addition, chondrocyte viability was maintained in crosslinked tissue treated with antioxidants.
Conclusion
These results suggest that photochemical crosslinking with CASPc and 670 nm light holds promise as a potential therapy to prevent cartilage degeneration by protecting cartilage from mechanical wear and biochemical degradation. Limitations were also evident, however, as an antioxidant treatment was necessary to maintain chondrocyte viability in crosslinked tissue.
Keywords: photochemical crosslinking, cartilage mechanics, CASPc, wear
Introduction
Osteoarthritis (OA), a leading cause of disability worldwide, 1 is characterized by cartilage degeneration and the eventual loss of cartilage tissue. 2 In addition, OA cartilage has reduced mechanical properties, including decreased resistance to swelling and diminished elastic modulus in tension, compression, and shear deformations.3-6 These inferior properties limit the ability of the diseased cartilage tissue to fulfill its mechanical functions of distributing joint loads and providing a low-friction surface that sustains minimal wear.
One method to restore the stability and strength of cartilage is to increase the crosslinks in the collagen network. Collagen has an inherently crosslinked structure, and can obtain additional, non-native crosslinks through exposure to exogenous crosslinking agents. Collagen crosslinking significantly enhances the modulus and strength of collagenous tissues, and improves their resistance to enzymatic degradation. 7 Initial studies of cartilage crosslinking used formaldehyde or glutaraldehyde to improve wear properties.8-10 However, the crosslinks formed by these chemical agents are toxic to cells. We previously showed that crosslinking with genipin, a natural crosslinking agent with favorable biocompatibility, protects healthy and impact loaded cartilage from mechanical wear and enzymatic degradation.11,12 Genipin has also been shown to stabilize decellularized cartilage tissue.13,14 Other crosslinking agents, such as lysyl oxidase like-2 and epigallocatechin gallate, also enhance the failure strength and modulus of cartilage.15,16 However, despite these potential benefits, it would be challenging to apply any chemical crosslinking agent to damaged or degraded articular cartilage in a patient suffering from OA. Directing the crosslinking agent to a particular tissue or region of tissue would be difficult, and chemical crosslinking agents that are introduced to an articular joint would crosslink not only cartilage but also adjacent tissue within the joint capsule.
A potential alternative crosslinking mechanism is photochemical crosslinking, in which a chemical photosensitizer is excited by light and generates a reaction that covalently bonds collagen fibrils. One advantage of photo-initiated crosslinking is that crosslinking can be localized to a particular region of tissue, such as tissue that has been damaged, by controlling the location of light exposure. Photochemical crosslinking has been demonstrated to crosslink the interface between 2 cartilage surfaces, and may enhance the integration of osteochondral grafts to native cartilage.17-19 In particular, Al(III) phthalocyanine chloride tetrasulfonic acid (CASPc), a photosensitizer from the phthalocyanine group that is activated by 670 nm light, was reported to produce functional bonds between 2 cartilage surfaces without significant loss of cell viability.17,19 However, whether photochemical crosslinking with CASPc enhances the mechanical properties and resistance to collagenase degradation of cartilage tissue has not been previously investigated.
The objective of this study was to evaluate photochemical crosslinking using CASPc and 670 nm light as a potential therapy to strengthen articular cartilage and prevent tissue degradation. First, changes in viscoelastic properties with indentation were determined for 2 different incubation times in CASPc solution and a wide range of light exposure duration. From these data, 2 different crosslinking protocols were selected for further testing. Crosslinked cartilage was subjected to an in vitro, accelerated wear test to assess the effect of crosslinking on cartilage wear and coefficient of friction (COF). The ability of the crosslinked tissue to resist biochemical degradation via collagenase was also measured. To better understand how photochemical crosslinking with CASPc may vary through the depth of the tissue, the distribution of photo-initiator in the tissue and penetration of light through the depth of the tissue was determined. Finally, the interaction between CASPc and 670 nm light generates singlet oxygen, an oxidant that promotes oxidative damage. Therefore, chondrocyte viability and the effect of an antioxidant treatment with photochemical crosslinking was evaluated.
Methods
Sample Preparation and Photochemical Crosslinking
Osteochondral specimens were harvested from the condyles of thawed bovine stifles. Cylindrical specimens with 9.5 mm diameter were acquired by coring the condyles such that the articular surface was perpendicular to the coring axis. No more than 3 specimens were obtained per stifle, from locations that provided the flattest articular surfaces. Specimens were stored in gauze dampened with phosphate buffered saline (PBS) at −20 °C until the day of testing.
Photochemical crosslinking was performed by first incubating thawed specimens in 0.5 mM CASPc (Frontier Scientific, Logan, UT) solution in PBS for 20 or 60 seconds at room temperature. Then, the samples were removed from CASPc solution and exposed to 670 nm light from a fiber-coupled laser (B&W Tech Inc, Newark, DE) for 0, 15, 30, 60, 120, 300, or 600 seconds. During light exposure, clear plastic wrap was placed over the cartilage surface to prevent dehydration. The laser was equipped with a fiber collimator (Edmund Optics, Barrington, NJ) with 0.25 numerical aperture and produced a 4.6 mm diameter beam spot size. To enhance the spatial uniformity of the light intensity, the total fiber length was extended to 180 cm. The power was set such that it was 273.5 ± 0.50 mW after passing through both the fibers and the collimator.
Viscoelastic Properties from Indentation
Stress-relaxation indentation tests were conducted on osteochondral specimens with a Mach-1 mechanical tester (Biomometum; Montreal, QC) equipped with a load cell with ±150 g range (AL312AL, Honeywell Industries Inc., Columbus, OH) and a 3.0 mm diameter flat punch indenter with 0.2 mm edge radius. During indentations, specimens were submerged in a hydrating solution consisting of PBS with protease inhibitors (1 mM ethylenediaminetetraacetic acid, 5 mM benzamadine, and 10 mM n-ethylmaleimide). Stress-relaxation tests started with a load threshold of 0.0875 gm force to find the contact at the cartilage surface and then consisted of a 20-second linear ramp to reach 50 µm peak displacement, followed by a 20-second hold at peak displacement. Five locations on each specimen were indented, with the first location near the center of the specimen and the other 4 locations 1 mm from the center. The stress-relaxation indentation was performed in quadruplicate at each location, then specimens were crosslinked with CASPc, as described above, and the indentation tests were repeated at the same 5 locations. The load-time data during stress relaxation was utilized to find 3-dimensional best-fit parameters E1, E2, and η of a standard linear solid (SLS) model, which has a parallel spring and dashpot in series with a free spring, using MATLAB (Mathworks, Natick, MA) optimization toolbox.11,20 Instantaneous (E0) and equilibrium (Einf) modulus and time constant (τ) due to crosslinking were calculated from the best-fit parameters. Values for the quadruplicate indentations at each location before and after crosslinking were averaged. The effect of crosslinking was evaluated at each location from the ratio of SLS parameters after crosslinking normalized by the value of the same parameter before crosslinking (n = 10-15 locations). From these results, 2 crosslinking protocols were selected for further study: (1) 20 seconds in CASPc solution and 600 seconds light exposure, denoted 20C600L, and (2) 60 seconds in CASPc solution and 30 seconds light exposure, denoted 60C30L.
Wear and Friction
Thawed osteochondral cores were crosslinked using one of the two selected protocols (n = 7 for each protocol). Untreated specimens were exposed to neither CASPc nor light as controls (n = 10). We previously reported differences in wear performed perpendicular or parallel to the preferential fiber direction. 21 Therefore, the preferential direction of the collagen fibers was determined with a split line analysis. The cartilage surface was pierced with a pin at 10 to 12 locations on the periphery. India ink was applied to the surface of the specimen and cleaned immediately with gauze. The split lines revealed the preferential direction of collagen fibers at the articular surface. 22
An accelerated wear test was performed with a reciprocal motion against a T316 stainless steel plate (#8 mirror finish) using a UMT Tribolab (Bruker, San Jose, CA) equipped with a 2-axis load cell. The specimen was manually oriented such that the split lines were aligned with the direction of wear. The specimen was loaded to a constant force of 160 N. This load was maintained for 14,000 reciprocating cycles of 18 mm length in each direction at a speed of 4 mm/second with 1 second pause at each end. 21 In addition, untreated specimens that were exposed to the reciprocating motion of the wear test without loading were included to better understand the effect of the mechanical load (n = 3). The 43.5 hours wear test was conducted at room temperature in PBS containing protease inhibitors.
Wear was measured by biochemically quantifying the amount of sulfated glycosaminoglycans (sGAGs) and hydroxyproline (HYP) that were released to the solution bath during the wear test. The solution bath was collected at the end of each experiment, lyophilized, and rehydrated with water. The solution was filtered using ultra centrifugal filter (Amicon®, 3000 MWCO, Millipore Corporation, Billerica, MA) at 4,000 g acceleration for 90 minutes. Preliminary studies found that removing salts and protease inhibitors from the solution via filtering provided more repeatable biochemical measurements, and that matrix components were not detected in the filtrate. The filtered wear particles were digested with papain, and sGAG content was measured using the dimethyl-methylene blue (DMMB) assay.21,23,24 Separate aliquots were assessed for HYP content with a chloramine-T assay.23-26 The amount of collagen in the solution was calculated based on a HYP: collagen ratio of 1:7.69. 27 The quantities of matrix components that were released from the specimens that were not loaded were subtracted from the respective values from the wear test to determine the matrix components released due to mechanical wear only.
Friction and normal loads were recorded by UMT Tribolab load sensor at a rate of 100 Hz during the wear test and initial and equilibrium COF were extracted. For calculating COF, MATLAB (Mathworks, Natick, MA) code was developed to discard data from the acceleration and deceleration of each cycle so that only data at 4 mm/second velocity was considered. An average value of COF was calculated for each reciprocating cycle. The averaged value for the first reciprocating cycle was considered the initial COF and the average of last 8,000 cycles was considered the equilibrium COF. 21
Collagenase Digestion
The effect of photochemical crosslinking on the resistance of cartilage to degradation by collagenase was investigated. Osteochondral specimens were treated with one of the two selected crosslinking protocols or were left untreated (n = 5 for each protocol). Using a sledge microtome (HM 450 Richard Allan, Kalamazoo, MI) equipped with a freezing stage (Physitemp, Clifton, NJ), three 50 μm sections were taken starting from the articular surface of each specimen. Individual sections were incubated for 45 minutes at 37 °C in 0.5 ml of a 2 mg/ml solution of type I collagenase from Clostridium histolyticum in 50 mM Trizma buffer at pH 7.4 containing 10 mM CaCl2 (all from Sigma-Aldrich, St. Louis, MO).11,12 To quantify the digested collagen, the digest solution for all sections were collected and assayed for HYP and the amount of digested collagen was estimated, as above.
CASPc and Light Penetration
To observe CASPc penetration into the cartilage and the effect of light exposure, osteochondral specimens were incubated in CASPc for 20 seconds, with and without 600 seconds light exposure, or for 60 seconds incubation, with and without 30 seconds of light exposure. Then, specimens were cut in half with a scalpel, and the cross section was imaged with a stereomicroscope (Stemi 508, Carl Zeiss, Jena, Germany).
To study light penetration through the cartilage depth, including through cartilage that was treated with CASPc, specimens were incubated in CASPc solution for 20 or 60 seconds or were left untreated (n = 3 for each group) and then four 50-μm thick sections were cut starting from articular surface as described previously for collagenase digestion (Section “Collagenase Digestion”). Transmission of 670 nm light from a laser through each section was measured. Each hydrated section was placed in the center of a plastic shim in the shape of a ring, and both the section and shim were sandwiched between a glass slide and cover slip. The plastic ring shim had the same thickness as cartilage sections (50 μm) and acted as a spacer to prevent the tissue from being compressed. All 4 sections of both treated and control samples were exposed to 670 nm light for 600 seconds while the power of light transmitted through the tissue was measured and recorded using a light sensor (PowerMax-USB, Coherent Inc, Santa Clara, CA) at 10 Hz. The power of light transmitted through the glass slide and cover slip without cartilage was also determined.
Chondrocyte Viability and Effect of Antioxidants
Fresh metacarpophalangeal joints were obtained from a local abattoir (Kenny’s Fine Meats, Mooresville, IN). Cartilage tissue was extracted using a 6 mm biopsy punch and sterile scalpel using aseptic techniques. The cartilage explants were cultured in Dulbecco’s modified Eagle medium (DMEM) containing high glucose and L-glutamine (Corning Inc., Corning, NY) supplemented with 50 µg/ml ascorbic acid (Sigma-Aldrich) and Insulin-Transferrin-Selenium (Gibco, Grand Island, NY). For the antioxidant treatment, 20 mM N-acetyl-L-cysteine (NAC; Sigma-Aldrich) and 10 mM sodium pyruvate (Sigma-Aldrich) were added to the culture medium and CASPc solution. After 24 hours in culture, the explants were aseptically crosslinked with the 60C30L crosslinking protocol with or without antioxidants added to the CASPc solution or were incubated in CASPc solution alone for 60 seconds but were not exposed to 670 nm light as a control. After crosslinking, explants were rinsed and were cultured either with or without antioxidants as per their initial treatment for an additional 24 hours.
To determine the effect of crosslinking and antioxidants on chondrocyte viability, explants were cut in half, and incubated with 2.5 µM calcein AM to stain the live cells green and 5 µM ethidium homodimer to stain the dead cells red (LIVE/DEAD™ Viability/Cytotoxicity Kit, Invitrogen, Carlsbad, CA) for 30 minutes. After rinsing in PBS, specimens were imaged using confocal fluorescent microscopy (Olympus FV-1000 MPE). To evaluate apoptotic signaling, additional explant halves were stained with CellEvent caspase 3/7 reporter (Invitrogen) and imaged.
In addition, whether antioxidants inhibited the photochemical crosslinking reaction was determined by evaluating viscoelastic properties of cartilage that had been crosslinked with or without antioxidants. The indentation test was performed on specimens from thawed condyles before and after the 60C30L crosslinking protocol with and without the addition of antioxidants in the CASPc and PBS solutions (n = 10-15).
Statistics
Differences between crosslinking treatments were determined using one-way and two-way analyses of variance (ANOVAs) with Dunnett’s post hoc with significance set at p < 0.05 (OriginPro 2020, OriginLab Corporation, Northampton, MA). Data are presented as mean ± standard deviation.
Results
Viscoelastic Properties of Cartilage
The instantaneous stiffness, equilibrium stiffness, and relaxation time constant in untreated tissue were 3.13 ± 1.28 MPa, 0.37 ± 0.15 MPa, and 0.92 ± 0.09 seconds, respectively. The instantaneous and equilibrium modulus of cartilage was increased by photochemical crosslinking, and was dependent both on the incubation and light exposure times. In both 20 and 60 second CASPc incubation times, the average instantaneous modulus increased by at least 4.8% at higher light exposure times (120-600 seconds; Fig. 1A and D). With 20 seconds incubation in CASPc solution, the equilibrium modulus also showed increases of 14.8% to 22.3% with light exposure of more than 120 seconds ( Fig. 1B ). Equilibrium modulus with 60 seconds incubation increased for a wider range of light exposure light times, beginning at 30 seconds, with a peak increase of 30.5% ( Fig. 1E ). No changes to the relaxation time constant with crosslinking were detected (Fig. 1C and F).
Figure 1.
Changes in E0, Einf, and τ at different light exposures (from 0 to 600 seconds) following 20 seconds (A, B, and C) or 60 seconds (D, E, and F) CASPc incubation. Significant differences from no light exposure (0 seconds) indicated by * for p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001.
Wear and COF
Cartilage wear was quantified by the amount of cartilage matrix components released to the fluid bath during the wear test due to the mechanical load. The amount of collagen and sGAGs released into the solution bath for specimens that were not loaded was 69.3 ± 32.4 μg and 1.92 ± 0.47 mg, respectively. These values were subtracted from the respective values from the wear test to determine the amount of matrix components released because of mechanical wear only.
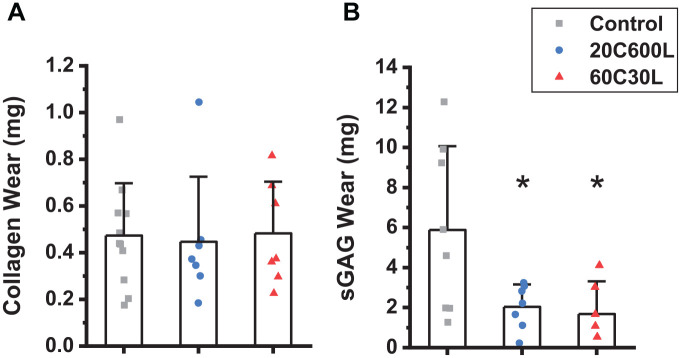
The average amount of collagen released into the fluid bath due to mechanical wear for the untreated controls was 0.54 ± 0.23 mg. For the 2 selected crosslinking protocols, 20C600L and 60C30L, 0.52 ± 0.28 mg and 0.55 ± 0.22 mg were released, respectively ( Fig. 2A ). No significant difference in collagen released during the wear test was detected between controls and crosslinked specimens. For the untreated control group, the amount of sGAGs released due to wear was 5.88 ± 4.17 mg. The sGAG wear decreased to 2.04 ± 1.12 mg and 1.69 ± 1.63 mg in the 20C600L and 60C30L crosslinked groups, respectively ( Fig. 2B ).
Figure 2.
(A) Collagen and (B) sGAGs released to the solution bath due to wear for untreated controls and specimens crosslinked with 20 seconds CASPc incubation and 600 seconds light exposure (20C600L), or 60 seconds CASPc incubation and 30 seconds light exposure (60C30L). sGAGs = sulfated glycosaminoglycans; CASPc =Al(III) phthalocyanine chloride tetrasulfonic acid. * indicates significant difference from control, p < 0.05.
For both treated and untreated samples, the COF started from a low value and increased with time, similar to previous reports.11,12,21,28-31 The initial COF of control specimens was 1.59 ± 0.63 × 10-3 and for the 20C600L and 60C30L crosslinking protocols, it was 1.42 ± 0.65 × 10-3 and 1.27 ± 0.53 × 10–3, respectively. No significant difference was detected between crosslinked and control specimens for initial COF. Equilibrium COF for controls was 0.21 ± 0.03 and for crosslinking protocols, 20C600L and 60C30L, its values were 0.26 ± 0.03 and 0.24 ± 0.05, respectively ( Fig. 3 ). The equilibrium COF of the 20C600L specimens tended to be larger than controls (p = 0.058).
Figure 3.
(A) Initial and (B) eqiulibrium COF for untreated controls and crosslinking with 20 seconds CASPc incubation and 600 seconds light exposure (20C600L) or 60 seconds CASPc incubation and 30 seconds light exposure (60C30L). COF = coefficient of friction; CASPc = Al(III) phthalocyanine chloride tetrasulfonic acid.
Collagenase Digestion
The effect of photochemical crosslinking on cartilage’s resistance to collagenase digestion was assessed. The amount of collagen digested from the most superficial section treated with 60 seconds incubation time and 30 seconds light exposure was slightly smaller than that of controls, while there was no difference in the amount of collagen released in other sections from the crosslinked groups compared with control ( Fig. 4 ).
Figure 4.

Amount of collagen digested by collagenase in 50 μm sections starting from the articular surface (0 µm) for crosslinked and untreated control specimens. * indicates significant difference from control, p < 0.05.
Light Attenuation and CASPc Penetration
The power of transmitted light through 50 µm sections of cartilage was measured for 600 seconds to study the effect of CASPc on the light transmission through cartilage. The power of transmitted light through the microscope slides without cartilage was 257.1 ± 0.3 mW. This power decreased to 199.2 ± 0.1 mW when light was transmitted through the most superficial section of the controls. The power of transmitted light was approximately constant over time in all sections of the untreated controls ( Fig. 5A ). For the most superficial section treated with 20 seconds CASPc incubation, the power of transmitted light started from a lower value of 153.6 ± 17.5 mW and increased to 186.6 ± 9.4 mW after 600 seconds (red curve in Fig. 5B ). The same behavior was observed for 60 seconds CASPc incubation time, except the transmitted light’s power started from a lower value compared with the 20 seconds CASPc incubation time (116.7 ± 35.8) before reaching its maximum value at 600 seconds (178.7 ± 6.7 mW, red curve in Fig. 5C ). For sections taken below the articular surface, the power of transmitted light was approximately constant over time and similar to the control values ( Fig. 5 ).
Figure 5.
Power of transmitted light through 50 µm sections starting from the articular surface (0 µm) through the cartilage depth for: (A) Control or no CASPc incubation, (B) 20 seconds CASPc incubation, (C) 60 seconds CASPc incubation. Solid lines denote average values of sections at each depth and shaded areas represent standard deviation. CASPc = Al(III) phthalocyanine chloride tetrasulfonic acid.
As CASPc is highly pigmented, the relative amount of CASPc in the tissue is distinguishable by the intensity of color. After 20 and 60 seconds incubation in CASPc, all specimens become aqua-blue in color, with the longer incubation time in CASPc leading to a darker color ( Fig. 6 , first row). In addition, the most intense color in both incubation times was at the surface of the specimen, with very little indication of the photochemical initiator deeper in the tissue. Exposure of the tissue to 670 nm light reduced the color intensity at the location of light exposure. This was most evident with 20 seconds of CASPc incubation ( Fig. 6 , second row).
Figure 6.
Macroscopic cross-sectional and top views of representative cartilage specimens treated by crosslinking protocols 20C600L and 60C30L. Scale bar is 2 mm in length. CASPc = Al(III) phthalocyanine chloride tetrasulfonic acid.
Chondrocyte Viability and Effect of Antioxidants
Chondrocyte viability was maintained in specimens incubated in CASPc solution alone for 60 seconds, in the absence of 670 nm light exposure. In contrast, a band of dead chondrocytes (stained red) was observed at the articular surface in specimens crosslinked with the 60C30L protocol that were not treated with antioxidants ( Fig. 7 ). When antioxidants were added to the culture medium and CASPc solution, chondrocyte viability in crosslinked specimens ( Fig. 7 ) was similar to specimens that were not crosslinked. Consistent with these results, apoptotic signaling via the caspase 3/7 pathway was elevated in crosslinked specimens compared with those that were only exposed to CASPc and was rescued with the addition of antioxidants ( Fig. 7 ).
Figure 7.

Confocal images of cartilage that was incubated in CASPc solution for 60 seconds but not exposed to 670 nm light (left), crosslinked with 60C30L protocol without AO treatment (middle), or crosslinked with 60C30L protocol with AO (right). Top row: Live and dead cells are stained with green and red, respectively. Bottom row: Apoptosis via caspase 3/7 signaling is indicated with green stain. Scale bar is 200 µm in length. CASPc = Al(III) phthalocyanine chloride tetrasulfonic acid; AO = antioxidant.
The effect of the antioxidant treatment on photochemical crosslinking was evaluated. Viscoelastic properties were determined from stress-relaxation indentation before and after crosslinking with the 60C30L protocol with and without the addition of antioxidants. Moduli did not change with the antioxidant treatment ( Fig. 8 ). However, the relaxation time constant increased by an average of 10% with the addition of antioxidants.
Figure 8.

Changes in viscoelastic parameters from the standard linear solid model due to 60C30L crosslinking protocol with and without AO. AO = antioxidant. * indicates significant difference in the same parameter without AO, p < 0.05.
Discussion
This study investigated 2 photochemical crosslinking protocols using CASPc and 670 nm light to strengthen the collagen network of cartilage. The first crosslinking protocol, denoted 20C600L, consisted of 20 seconds incubation in CASPc solution and 600 minutes light exposure. This protocol was chosen because it was previously reported to adhere two cartilage surfaces to one another. 19 A longer CASPc incubation of 60 seconds was also tested, and resulted in increased CASPc in the tissue, as evidenced by increased light attenuation through sections taken from the articular surface ( Fig. 5 ) and a more intense color of the photo-initiator ( Fig. 6 ). The higher level of CASPc in the tissue resulted in a significant increase in indentation modulus with as little as 30 seconds light exposure ( Fig. 1 ). Therefore, a second protocol consisting of 60 seconds incubation in CASPc solution and 30 seconds light duration (denoted 60C30L) was also selected for further study. The 30 seconds duration of light exposure coupled with 60 seconds of CASPc treatment produced protective effects on articular cartilage that were similar to those of the much longer 600 seconds duration of light exposure following 20 seconds of CASPc treatment. This shorter duration of light exposure may be more feasible to implement in a clinical setting than a longer one. In addition, minimizing the duration of light exposure may limit exposure to oxidants and any other potential adverse effects. Results suggest that photochemical crosslinking holds promise as a potential therapy to prevent cartilage degeneration, as crosslinked tissue released less sGAGs during the in vitro wear test ( Fig. 2 ). Resistance to collagenase digestion also improved with crosslinking, although the effect was modest ( Fig. 4 ). Potential limitations were also evident, however, as an antioxidant treatment was necessary to maintain chondrocyte viability and suppress apoptotic signaling in crosslinked tissue ( Fig. 7 ).
Results suggest that photochemical crosslinking with CASPc is limited to a region near the articular surface that is not more than 50 µm deep. The pigmented color of the photochemical initiator remained near the articular surface and did not appear to penetrate deep into the tissue ( Fig. 6 ). Furthermore, 670 nm light transmission was attenuated only through the 50 µm section taken from the articular surface ( Fig. 5 ), suggesting that the photochemical initiator, which absorbs light at this wavelength, did not permeate further than 50 µm. Further evidence that the crosslinking reaction was localized near the articular surface was found with collagenase digestion, which was decreased only in the most superficial 50 µm section of crosslinked tissue ( Fig. 4 ). CASPc may be concentrated at the articular surface in the current study due to short incubation times that do not permit diffusion into the tissue. However, this pattern of depth-dependent crosslinking is likely to be a feature of any crosslinking reaction that uses a photochemical initiator as light absorption by the photochemical initiator at the surface would attenuate the light intensity deeper in the tissue. Localization of crosslinking to the superficial region of cartilage may be sufficient, particularly as an early OA treatment, as this is often the location of the initial damage, and protection of the superficial zone may be effective in forestalling the disease. As new techniques to detect OA at earlier stages are developed, crosslinking may become a more effective treatment.
Crosslinking of the superficial cartilage was sufficient to increase viscoelastic modulus values identified by indentation measurements. Previous studies have shown that indentation, similar to unconfined compression and confined compression, is a sensitive technique to detect changes in stiffness due to crosslinking by genipin and ribose.11,32,33 However, a fundamental assumption of the model that was used to find viscoelastic modulus values is that the indented material is homogeneous and uniform. 20 Although this assumption is not strictly valid for cartilage in general, crosslinking that is localized to the articular surface would generate material properties that are even more heterogeneous than native cartilage. Modulus values that were determined are likely average values over a volume of tissue, with larger changes in properties in the crosslinked region near the articular surface, and little to no change in properties elsewhere. The largest and most significant change in material properties with crosslinking were found in the equilibrium modulus. This may be because crosslinking alters the properties of the cartilage matrix, and the equilibrium modulus represents tissue properties when fluid has been exuded and the solid matrix carries its maximum load. 34
Results indicate that photochemical crosslinking of articular cartilage by CASPc enhanced its resistance to wear by significantly decreasing the amount of sGAGs released to the fluid bath in both crosslinking protocols. However, no difference was detected in collagen released during the wear test between untreated control and treated specimens ( Fig. 2 ). These results are somewhat counter-intuitive, as CASPc is assumed to crosslink only the collagen network, and no direct effect on proteoglycans is known. However, large aggregating GAG-rich proteoglycans are retained in the tissue by the interconnected collagen network, and increasing the stiffness and strength of collagen may enhance the physical entrapment of proteoglycans under the mechanical loads of the wear test. In addition, the amount of sGAGs released during the wear test was nearly an order of magnitude larger than that of collagen ( Fig. 2 ). The relatively large release of sGAGs may make it a more sensitive measure of cartilage wear.
Material properties play a critical role in the tribological function of cartilage. 23 Specifically, interstitial fluid pressurization is considered the main mechanism for maintaining its low-friction response.29,35 As collagen crosslinking changes the material properties of cartilage, load distribution to the interstitial fluid and solid matrix may also be affected, as well as the corresponding COF. Initial COF of crosslinked cartilage was not significantly different from controls, while the equilibrium COF tended to be slightly larger in the 20C600L group. However, equilibrium COF was reached only after hours of constant loading and is not a physiological friction response. In addition, crosslinking increases the strength of cartilage, 36 which here resulted in decreased wear even with the slight increase in COF.
Live/dead staining of the crosslinked cartilage showed an increase in cell death following crosslinking that was reversed with an antioxidant treatment ( Fig. 7A-C ). Similarly, staining for the early apoptotic pathway caspase 3/7 also demonstrated increased apoptosis following crosslinking that was rescued with antioxidants ( Fig. 7D-F ). The 670 nm light interacts with CASPc to induce the formation of singlet oxygen, 19 which is necessary for the crosslinking reaction. Singlet oxygen is very reactive and unstable, generating hydrogen peroxide and free oxygen radicals. These molecules are implicated in damaging cellular mechanisms including apoptosis, inflammation, aging, and the injury response.37-39 Sodium pyruvate, which scavenges hydrogen peroxide,40,41 and NAC, which scavenges free oxygen radicals, 42 were added to the CASPc solution and media to protect against oxidative stress. The results of the current study indicate that an antioxidant treatment maintains cell viability and reduces apoptosis, although the minimum concentrations and durations of the antioxidant treatment have yet to be determined. Importantly, the antioxidant treatment did not interfere with the photochemical crosslinking reaction, as increases in modulus via indentation with crosslinking were maintained with the antioxidant treatment ( Fig. 8 ).
In conclusion, results of this study indicate that CASPc photo-initiator and 670 nm light strengthen articular cartilage by crosslinking collagen in the superficial zone of the tissue. Parameters of the crosslinking protocol, such as the duration of light exposure and the amount of the chemical photo-initiator in the tissue, were interrelated. These data suggest that specific combinations of photo-initiator concentration, incubation time, light intensity, and light exposure duration may be optimized in future studies to maximize desirable outcomes. The crosslinking treatment at the articular surface was sufficient to reduce the loss of sGAGs from the tissue during an accelerated in vitro wear test. Although crosslinking increased the cartilage’s resistance to biochemical degradation via collagenase, this effect was minimal and was only observed at the articular surface. In spite of these potential benefits, challenges remain before photochemical crosslinking with CASPc can be tested in vivo as a treatment to reduce cartilage degradation. In particular, antioxidants were necessary to preserve chondrocyte viability in crosslinked cartilage. More work is necessary to determine effective antioxidant treatments that maintain chondrocyte health during photochemical crosslinking procedures. In addition, the effect of crosslinking on fluid flow and the exchange of nutrients and waste products in the tissue has not been studied. Furthermore, whether the altered mechanical environment of the tissue influences chondrocyte behavior requires further understanding.
Footnotes
Acknowledgments and Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by NIH NIAMS Grant R01 AR069657.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: The authors attest that all animal tissue used in this study was collected from a local abattoir. No human tissue was used in this study.
ORCID iD: Diane R. Wagner  https://orcid.org/0000-0001-8013-0777
https://orcid.org/0000-0001-8013-0777
References
- 1. Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145-53. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Ashford S, Williard J. Osteoarthritis. Nurse Pract. 2014;39(5):1-8. doi: 10.1097/01.NPR.0000445886.71205.c4. [DOI] [PubMed] [Google Scholar]
- 3. Setton LA, Mow VC, Muller FJ, Pita JC, Howell DS. Mechanical properties of canine articular cartilage are significantly altered following transection of the anterior cruciate ligament. J Orthop Res. 1994;12(4):451-63. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=8064477. [DOI] [PubMed] [Google Scholar]
- 4. Obeid EM, Adams MA, Newman JH. Mechanical properties of articular cartilage in knees with unicompartmental osteoarthritis. J Bone Joint Surg Br. 1994;76(2):315-9. [PubMed] [Google Scholar]
- 5. Krishnan Y, Grodzinsky AJ. Cartilage diseases. Matrix Biol 2018;71-72:51-69. doi: 10.1016/j.matbio.2018.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Setton LA, Elliott DM, Mow VC. Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage. 1999;7(1):2-14. doi: 10.1053/joca.1998.0170. [DOI] [PubMed] [Google Scholar]
- 7. Gu L, Shan T, Ma YX, Tay FR, Niu L. Novel biomedical applications of crosslinked collagen. Trends Biotechnol. 2019;37(5):464-91. doi: 10.1016/j.tibtech.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 8. Lipshitz H, Etheredge R, III, Glimcher MJ. In vitro studies of the wear of articular cartilage-III. The wear characteristics of chemical modified articular cartilage when worn against a highly polished characterized stainless steel surface. J Biomech. 1980;13(5):423-36. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=6772646. [DOI] [PubMed] [Google Scholar]
- 9. Radin EL, Swann DA, Paul IL, McGrath PJ. Factors influencing articular cartilage wear in vitro. Arthritis Rheum. 1982;25(8):974-80. [DOI] [PubMed] [Google Scholar]
- 10. Oungoulian SR, Hehir KE, Zhu K, Willis CE, Marinescu AG, Merali N, et al. Effect of glutaraldehyde fixation on the frictional response of immature bovine articular cartilage explants. J Biomech. 2014;47(3):694-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McGann ME, Bonitsky CM, Jackson ML, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking of cartilage enhances resistance to biochemical degradation and mechanical wear. J Orthop Res. 2015;33(11):1571-9. doi: 10.1002/jor.22939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bonitsky CM, McGann ME, Selep MJ, Ovaert TC, Trippel SB, Wagner DR. Genipin crosslinking decreases the mechanical wear and biochemical degradation of impacted cartilage in vitro. J Orthop Res. 2017;35:558-65. doi: 10.1002/jor.23411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pinheiro A, Cooley A, Liao J, Prabhu R, Elder S. Comparison of natural crosslinking agents for the stabilization of xenogenic articular cartilage. J Orthop Res. 2016;34(6):1037-46. doi: 10.1002/jor.23121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elder S, Pinheiro A, Young C, Smith P, Wright E. Evaluation of genipin for stabilization of decellularized porcine cartilage. J Orthop Res. 2017;35(9):1949-57. doi: 10.1002/jor.23483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Makris EA, Responte DJ, Paschos NK, Hu JC, Athanasiou KA. Developing functional musculoskeletal tissues through hypoxia and lysyl oxidase-induced collagen cross-linking. Proc Natl Acad Sci U S A. 2014;111(45):E4832-41. doi: 10.1073/PNAS.1414271111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Elder S, Clune J, Walker J, Gloth P. Suitability of EGCG as a means of stabilizing a porcine osteochondral xenograft. J Funct Biomater. 2017;8(4):43. doi: 10.3390/JFB8040043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Arvayo AL, Wong IJ, Dragoo JL, Levenston ME. Enhancing integration of articular cartilage grafts via photochemical bonding. J Orthop Res. 2018;36(9):2406-15. doi: 10.1002/jor.23898. [DOI] [PubMed] [Google Scholar]
- 18. Arvayo AL, Imbrie-Moore A, Levenston ME. Rapid and durable photochemical bonding of cartilage using the porphyrin photosensitizer verteporfin. Osteoarthritis Cartilage. 2019;27(10):1537-44. doi: 10.1016/j.joca.2019.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sitterle VB, Nishimuta JF, Levenston ME. Photochemical approaches for bonding of cartilage tissues. Osteoarthritis Cartilage. 2009;17(12):1649-56. [DOI] [PubMed] [Google Scholar]
- 20. Cheng L, Xia X, Yu W, Scriven LE, Gerberich WW. Flat-punch indentation of viscoelastic material. J Polym Sci Part B Polym Phys. 2000;38(1):10-22. [Google Scholar]
- 21. Hossain MJ, Noori-Dokht H, Karnik S, Alyafei N, Joukar A, Trippel SB, et al. Anisotropic properties of articular cartilage in an accelerated in vitro wear test. J Mech Behav Biomed Mater. 2020;109:103834. doi: 10.1016/j.jmbbm.2020.103834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Below S, Arnoczky SP, Dodds J, Kooima C, Walter N. The split-line pattern of the distal femur: a consideration in the orientation of autologous cartilage grafts. Arthroscopy. 2002;18:613-7. doi: 10.1053/jars.2002.29877. [DOI] [PubMed] [Google Scholar]
- 23. Ateshian G, a Mow VC, Huiskes R. Friction, lubrication, and wear of articular cartilage and diarthrodial joints. In: Mow VC, Huiskes R, editors. Basic orthop biomech mechano-biology. 2005. doi: 10.1186/1475-925X-4-28. [DOI] [Google Scholar]
- 24. Oungoulian SR, Chang S, Bortz O, Hehir KE, Zhu K, Willis CE, et al. Articular cartilage wear characterization with a particle sizing and counting analyzer. J Biomech Eng. 2013;135:0245011-4. doi: 10.1115/1.4023456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lipshitz H, Etheredge R, III, Glimcher MJ. In vitro wear of articular cartilage. J Bone Jt Surg Am. 1975;57(4):527-34. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1141265. [PubMed] [Google Scholar]
- 26. McGann ME, Vahdati A, Wagner DR. Methods to assess in vitro wear of articular cartilage. Proc Inst Mech Eng H. 2012;226(8):612-22. [DOI] [PubMed] [Google Scholar]
- 27. Ignat’eva NY, Danilov NA, Sobol EN. Determination of hydroxyproline in tissues and the evaluation of the collagen content of the tissues. J Anal Chem. 2007;62(1):51-7. doi: 10.1134/S106193480701011X. [DOI] [Google Scholar]
- 28. Forster H, Fisher J. The influence of continuous sliding and subsequent surface wear on the friction of articular cartilage. Proc Inst Mech Eng H. 1999;213(4):329-45. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=10466364. [DOI] [PubMed] [Google Scholar]
- 29. Krishnan R, Kopacz M, Ateshian GA. Experimental verification of the role of interstitial fluid pressurization in cartilage lubrication. J Orthop Res. 2004;22(3):565-70. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=15099636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lewis PR, McCutchen CW. Mechanism of animal joints: experimental evidence for weeping lubrication in Mammalian Joints. Nature. 1959;184:1285. doi: 10.1038/1841285a0. [DOI] [PubMed] [Google Scholar]
- 31. Northwood E, Fisher J. A multi-directional in vitro investigation into friction, damage and wear of innovative chondroplasty materials against articular cartilage. Clin Biomech (Bristol, Avon). 2007;22:834-42. doi: 10.1016/j.clinbiomech.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 32. McGann ME, Bonitsky CM, Ovaert TC, Wagner DR. The effect of collagen crosslinking on the biphasic poroviscoelastic cartilage properties determined from a semi-automated microindentation protocol for stress relaxation. J Mech Behav Biomed Mater. 2014;34:264-72. [DOI] [PubMed] [Google Scholar]
- 33. Korhonen RK, Wong M, Arokoski J, Lindgren R, Helminen HJ, Hunziker EB, et al. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002;24(2):99-108. doi: 10.1016/S1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 34. Soltz MA, Ateshian GA. Experimental verification and theoretical prediction of cartilage interstitial fluid pressurization at an impermeable contact interface in confined compression. J Biomech. 1998;31(10):927-34. doi: 10.1016/S0021-9290(98)00105-5. [DOI] [PubMed] [Google Scholar]
- 35. Ateshian GA. The role of interstitial fluid pressurization in articular cartilage lubrication. J Biomech. 2009;42(9):1163-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Chen AC, Temple MM, Ng DM, Verzijl N, DeGroot J, TeKoppele JM, et al. Induction of advanced glycation end products and alterations of the tensile properties of articular cartilage. Arthritis Rheum. 2002;46(12):3212-7. Available from: http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=12483725. [DOI] [PubMed] [Google Scholar]
- 37. Henrotin YE, Bruckner P, Pujol JP. The role of reactive oxygen species in homeostasis and degradation of cartilage. Osteoarthritis Cartilage. 2003;11(10):747-55. doi: 10.1016/S1063-4584(03)00150-X. [DOI] [PubMed] [Google Scholar]
- 38. Bolduc JA, Collins JA, Loeser RF. Reactive oxygen species, aging and articular cartilage homeostasis. Free Radic Biol Med. 2019;132:73-82. doi: 10.1016/j.freeradbiomed.2018.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Henrotin Y, Kurz B, Aigner T. Oxygen and reactive oxygen species in cartilage degradation: friends or foes? Osteoarthr Cartil. 2005;13(8):643-54. doi: 10.1016/j.joca.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 40. Desagher S, Glowinski J, Prémont J. Pyruvate protects neurons against hydrogen peroxide-induced toxicity. J Neurosci. 1997;17(23):9060-7. doi: 10.1523/jneurosci.17-23-09060.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wang X, Perez E, Liu R, Yan L-J, Mallet RT, Yang S-H. Pyruvate protects mitochondria from oxidative stress in human neuroblastoma SK-N-SH cells. Brain Res. 2007;1132(1):1-9. doi: 10.1016/J.BRAINRES.2006.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nakagawa S, Arai Y, Mazda O, Kishida T, Takahashi KA, Sakao K, et al. N-acetylcysteine prevents nitric oxide-induced chondrocyte apoptosis and cartilage degeneration in an experimental model of osteoarthritis. J Orthop Res. 2010;28(2):156-63. doi: 10.1002/jor.20976. [DOI] [PubMed] [Google Scholar]