Abstract
Unknown chemical releases constitute a large portion of the rapid response situations to which the U.S. Environmental Protection Agency (U.S. EPA) is called upon to respond. Workflows used to address unknown chemical releases currently involve screening for a large array of known compounds using many different targeted methods. When matches are not found, expert analytical chemistry knowledge is used to propose possible candidates from the available data, which generally includes low-resolution mass spectra and situational clues such as the location of the release, nearby industrial operations, and other field-reported facts. The past decade has witnessed dramatic improvements in capabilities for identifying unknown compounds using high-resolution mass spectrometry (HRMS) and non-targeted analysis (NTA) approaches. Complementary developments in cheminformatics tools have further enabled an increase in NTA throughput and identification confidence. Together with the expanding availability of HRMS instrumentation in monitoring laboratories, these advancements make NTA highly relevant to rapid response scenarios. In this article, we introduce the concept of NTA as it relates to rapid response needs and describe how it can be applied to address unknown chemical releases. We advocate for the consideration of HRMS-based NTA approaches to support future rapid response scenarios.
INTRODUCTION
Emergency Scenarios & the U.S. Environmental Protection Agency
Emergency scenarios involving chemicals, including chemical releases triggered by natural disasters, vehicle accidents and train derailments, terrorist attacks, major industrial incidents, or inadvertent/accidental spills, may threaten public health and ecological resources unless a concerted and rapid response to characterize, contain, and remediate contamination is undertaken. Such scenarios can include both point source releases occurring at a single point in time, like an overturned tanker truck, as well as non-point source events, such as lead leaching into drinking water supplies from aging pipes. Recent well-known scenarios include the Deepwater Horizon oil spill and subsequent cleanup efforts (U.S. EPA, 2021a) pollutant release caused by the storm surge during Hurricane Harvey (Du et al., 2020), and widespread penetration of PFAS into rivers and coastal waterways (Newton et al. 2017). In all three of the aforementioned cases, complex chemical mixtures, rather than single chemicals, were unintentionally released into surrounding ecosystems. While these reflect well-publicized, large-scale events, lesser-known small-scale releases are also common. In 2019 alone, the National Response Center (NRC) logged over 26,000 calls reporting discharges into the environment (U.S. EPA, 2021b). While facilities are required to report certain releases that are mandated by the Comprehensive Environmental Response, Compensation, and Liability Act (CERCLA; Superfund hazardous substances that exceed reportable quantities—1 – 5,000 pounds, depending on the individual chemical), anyone witnessing an oil spill, chemical release or maritime security incident is encouraged to call the National Response Center hotline to report the event. As a result, discharges contained in the NRC database range from very small quantities (drops, grams, ounces, teaspoons) to much larger amounts (tons, barrels, cubic meters). Over half (57.8%) discharges reported to the NRC in 2019 were of an unknown amount and the NRC database indicates that 37% were of an unknown composition (n= 9,685 out of 26,154) (U.S. Coast Guard, 2021). Examples of reported release materials include “unknown oil”, “unknown toxic chemicals”, “oily waste”, and “unknown green liquid”. Of these unknown substances, 70% were reported to penetrate a body of water local to the release location, magnifying the potential for environmental exposure and potential harm. Past events have also indicated that both aboveground and underground storage tanks (ASTs and USTs, respectively), often containing fuel and other regulated substances, are vulnerable to flooding and extreme weather events (Cruz & Krausmann, 2013). In fact, the incidence of industrial accidents caused by natural events (also known as natural hazard triggering technological [Natech] disasters) has increased over the past decade and is predicted to grow in the coming years (Nascimento & Alencar, 2016). Although the field of rapid response is commonly associated with natural disasters or industrial accidents, its scope also extends to broader public health decisions. Increasingly, law enforcement and hazmat teams have sought technical support from EPA in determining decontamination approaches and advising appropriate personal protective equipment (PPE) necessary for drug-contaminated locations (e.g., pill factories, illegal makeshift laboratories, and dump sites). Most commonly, ecosystems and environments contaminated by chemical releases are, in themselves, dynamic in nature, influenced by weather, tides, shipping routes, flows, and plant and animal inhabitants, and are capable of promoting biotic and abiotic chemical transformation of mixture constituents. These factors, along with the ever-increasing complexity and variety of chemical formulations in commerce, makes emergency management and risk mitigation of environmental disasters involving chemical releases exceptionally challenging.
While the responsibility for responding to disasters and emergencies is spread out across various state and federal agencies, the U.S. EPA is authorized to respond to situations involving hazardous substances via the Executive Branch. Specifically, Section 104 of CERCLA gives the President the authority to coordinate the removal of hazardous substance releases. EPA’s guidance for on-scene coordinators (U.S. EPA, 2015), federal officials responsible for directing response measures, states that CERCLA requires the EPA to “develop procurement tools for responding to emergency releases or threats of releases of hazardous substances to the environment, and releases of pollutants or contaminants that present an imminent and substantial danger to public health and welfare”. Additionally, under the Federal Emergency Management Agency’s (FEMA) National Response Framework, EPA is the Emergency Support Function (ESF) Coordinator Agency for ESF #10: Oil and Hazardous Materials Response (FEMA, 2016). At present, there is a clear mandate from FEMA and in CERCLA engaging EPA to develop tools to enhance and advance rapid response capabilities.
How Rapid Response Works
Staffed 24 hours a day by the United States Coast Guard (USCG), the NRC serves as an emergency call center by fielding initial reports of oil, chemical, radiological, biological, and etiological releases into the environment. After the NRC receives an initial report, their staff notify the appropriate federal or state agencies, which in turn may activate the National Contingency Plan (NCP) (U.S. Government Publishing Office, 2021) and federal response capabilities. The NCP was developed in 1968 to organize a national response capability, coordinate responders, and establish a National Response Team (NRT). The NRT is an organization of 15 Federal departments and agencies responsible for coordinating emergency preparedness and response to oil and hazardous substance pollution incidents. The USCG and EPA serve as Chair and Vice Chair, respectively. After receiving an initial report, NRC staff must collect any available information on the size and nature of the release, the facility or vessel involved, and responsible parties, and relay these details to the on-scene coordinator (OSC) assigned to the affected area. If the spill occurred within EPA’s jurisdiction (on inland areas or waters), an EPA OSC is responsible for rapidly evaluating potential hazards of the release and the resources required to contain it, making thorough and timely information regarding the composition of the spill critical. In addition to the NRC, EPA also has Regional and Headquarters Emergency Operations Centers that are staffed continuously to receive notifications about potential releases and to communicate with the United States Department of Homeland Security, other federal agencies, and field staff.
During a national emergency, EPA has various fixed and mobile assets that may be called upon to support the response effort. One such resource is EPA’s Portable High-throughput Integrated Laboratory Identification System (PHILIS; locations denoted by gray trucks in Sidebar 2) (U.S. EPA, 2021c), which is a suite of accredited mobile analytical chemistry laboratories capable of performing confirmatory onsite organics analysis. As of Spring 2021, the PHILIS analytical suite is comprised of 16 gas chromatograph - mass spectrometer (GC/MS) instruments, 3 Time of Flight (TOF) GC/MS instruments, 2 liquid chromatograph – tandem mass spectrometer (LC/MS/MS) instruments, and 2 thermal desorption systems for the analysis of volatile and semi-volatile organic compounds (VOC and SVOC), chemical warfare agents and their breakdown products, and toxic industrial compounds in a variety of sample matrices. Other response assets include the EPA’s Environmental Response Team (ERT) Trace Atmosphere Gas Analyzer units (TAGA; locations denoted by brown trucks in Sidebar 2), which are mobile laboratories designated for atmospheric gas analysis, as well as regional mobile laboratories (locations denoted by blue trucks in Sidebar 2). In a large-scale or highly specialized response where regional and headquarters’ analytical capabilities are exhausted, the Environmental Response Lab Network (ERLN; locations denoted by orange circles in Sidebar 2, with size reflecting the relative number of labs in each state) (U.S. EPA, 2021d), can be activated to perform additional analyses. Part of the Department of Homeland Security’s Integrated Consortium of Laboratory Networks (ICLN), the ERLN is a laboratory response network consisting of ~140 accredited public and private environmental measurement laboratories. Both PHILIS and ERLN laboratories analyze samples using validated methods to produce data of known quality assurance and quality control (QA/QC) integrity. Method selection is driven by the analyte and sample matrix of interest along with consideration of laboratory capability/capacity and the needs of risk assessors (Campisano et al., 2017). Analytical results can be rapidly submitted to decision makers, such as OSCs and Unified Command.
3. Sidebar 2.

Streamlining Non-Targeted Analysis (NTA) Workflows
A rapidly emerging area of research, NTA has garnered much attention in recent years for its use of high-resolution mass spectrometry (HRMS) to identify novel environmental contaminants. In contrast to traditional, targeted analytical chemistry methods, NTA typically does not focus on a set of specific chemicals. Rather, its focus is on assigning structures or formulae to unknown signals in HRMS data. Similarly, analytical chemists currently utilize scanning mass spectrometry methods when determining which chemicals are present in a given sample; however, in some cases, matrix interferences and sample complexity may obscure the utility of data outputs. In cases where these interferences are absent, resulting data may be non-specific and correspond to many different individual compounds. The increased resolution of HRMS instrumentation used in NTA approaches resolves many of these issues. As such, NTA is a logical tool to apply to situations where the identity of the compounds of concern are unknown, as is the case in many rapid response scenarios.
The challenge in applying NTA to rapid response scenarios is mostly an issue of expediency. NTA has had great success in identifying unknown compounds but has had difficulty doing so accurately and reproducibly when response time is an essential consideration. However, developments from the U.S. EPA and other NTA community members, largely related to data processing and informatics tools, have streamlined NTA workflows and now allow confident compound identification to take place in a timeframe relevant to rapid response scenarios.
NTA in Relevant Scenarios
Although instances of NTA being utilized in past rapid response scenarios are scarce, European researchers have already applied this technology as a precautionary measure for the early detection of accidental spills. For example, scientists affiliated with the international Rhine monitoring station in Basel, Switzerland used a daily LC-HRMS screening strategy to monitor river water quality. Because the Rhine river is a source of drinking water for a large European population, HRMS monitoring can be used to detect upstream chemical spills and warn downstream water suppliers when spills and resultant water contamination occur. Analytical approaches for this project included targeted screening of 320 compounds, suspect screening of 1500 compounds based on reported usage in the area, and NTA to detect unanticipated chemicals. This effort resulted in the detection of 10 major spill events involving previously undetected compounds in 2014 (Hollender et al., 2017). In cases where individual chemical concentrations were determined to reach the μg/L-range, drinking water production was shutdown to preempt potential risk to consumers. In another case study (Bader et al., 2016), river water that is directly used to produce potable water was screened daily for a 1-year period using HRMS. Temporal patterns in feature intensities indicated a potential spill during week 33 of sample collection. Exact mass, isotope pattern, product ion spectrum and database matching allowed for tentative identification of the feature as the beta blocker acebutolol, which was subsequently confirmed by reference standard. A different survey of wastewater effluents collected from 10 municipal wastewater treatment plants in Switzerland via targeted, suspect screening, and NTA approaches (Schymanski et al., 2014a) showed that, of the 30 most abundant peaks in electrospray ionization (ESI) negative mode, only 4 were identified with the targeted approach. An additional 7 peaks were identified in the suspect screen and 1 peak was identified by NTA, thereby providing a 3-fold improvement in chemical coverage with the use of these HRMS techniques. Another European-led pilot study, referred to as the NORMAN Early Warning System (NormaNEWS) (Norman, 2021), utilized archived HRMS data to retrospectively assess temporal and spatial occurrence of contaminants of emerging concern in aqueous environmental samples from 14 countries (Alygizakis et al., 2018). The presence of several surfactants, pharmaceutical transformation products, and industrial chemicals in wastewater influents, effluents, and surface water was noted. Following the demonstrated success of NTA as an early warning tool for emerging contaminants, European scientists have advocated for its use in chemical monitoring under the European Water Framework Directive (Brack et al., 2019).
Likewise, the utility of NTA has been demonstrated in the long-term chemical characterization of and response to large-scale oil spills in the United States. Using Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR/MS), McKenna et al. (2013) created a library of ~30,000 chemical features from unadulterated Macondo well crude oil released in the Deepwater Horizon disaster, enabling comparisons to weathered petroleum for assessments of environmental fate and transport, and facilitating rapid suspect screening analyses in future oil release events. Similarly, other studies have used HRMS to track and identify degradation/transformation products of dispersed oil following the Deepwater Horizon oil spill (Chen et al., 2016). At the U. S. Geological Survey (USGS) Bemidji oil spill site in Minnesota, Fourier-transform ion cyclotron resonance mass spectrometry (FT-ICR/MS) was used to examine temporal changes in impacted groundwater, and mass spectra indicated a steady state of contamination across the 12-year sampling period (Islam et al., 2016). Finally, GC × GC/HRMS has proven effective in identifying source-spill pairs in a number of different diesel oil spill cases using fingerprinting techniques (Alexandrino et al., 2018). Although these studies highlight the use of HRMS and NTA to characterize oil spills in the long-term, similar approaches could also be applied in a rapid response scenario.
Suspect screening and NTA approaches are also being leveraged during emergency scenarios in clinical and forensic settings. For example, in case studies of women who became unresponsive shortly after recreational use of street “fentanyl”, suspect screening by liquid chromatography quadrupole time of flight mass spectrometry (LC-QTOF/MS) identified two fentanyl analogs, furanylfentanyl and ß-hydroxyfentanyl, in the product and the users’ serum (Hendrickson et al., 2019). Rapid identification of these analogs informed the use and titration of naloxone for opioid toxicity reversal by responding clinicians. Furthermore, in a recent analysis of 100 hair samples taken from opiate users, NTA approaches also identified fentanyl analogs, β-hydroxyfentanyl and methoxyacetylfentanyl, which were not on the panel of target analytes (Salomone et al., 2021). Finally, an assessment of serum from agitated patients in an emergency department setting identified seven different novel psychoactive substances in six patient samples (Lung et al., 2016). These studies demonstrate the applicability of NTA and suspect screening approaches to medical settings and monitoring the illegal market of drugs of abuse, where continuous modification of illicit drugs occur, and new compounds often abruptly appear.
NTA can also prove useful in rapid response scenarios even when chemical identifications are not made. For example, the successful tracking of unknown compounds was demonstrated at a wastewater treatment plant where conventional treatment was compared to treatment with an additional cleaning step of granulated activated carbon (Schymanski et al., 2014a). Thousands of unknown compounds were aligned between the influent and effluent with both the traditional treatment and the additional treatment. Without identifying any of the unknown compounds, the researchers were still able to track compounds through the treatment processes. NTA revealed that the extra purification step resulted in greater elimination of influent features (a reduction that was 42% higher by count and 3-fold higher by intensity), and ultimately, allowed researchers to differentiate between treatment types when considering a whole host of water contaminants. As such, NTA could be used to track unknowns spatially or temporally, with or without assigning chemical structures, and provide quick results regarding the dispersion of an unknown compound before too much time and effort is invested into its identification. In addition, even if the release material could not be identified with NTA, the distinct chemical profile obtained could be used for source tracking. When a source is determined, reference source samples could be collected and analyzed for chemical profile matching to the pollution site. In these ways, NTA is not only a tool for unknowns identification, but is also beneficial for intelligence gathering.
RECENT TECHNOLOGICAL ADVANCES
HRMS Instrumentation
HRMS instruments have greater resolving power compared to their low-resolution counterparts. Used as a “performance” parameter, the resolving power of an instrument relates to mass resolution, or the ability to distinguish two narrow mass spectral peaks (Table 1). Higher resolving power leads to narrower ion peaks, better mass resolution, and a more accurate measurement of exact mass. A function of increased resolution, HRMS allows researchers to accurately measure the exact masses of many chemicals in a mixture or sample. Exact mass refers to the mass of a compound composed of specific isotopes of each element and is typically reported to at least four decimal places. Monoisotopic mass is a specific exact mass calculated using the most abundant elemental isotopes in a molecule. For example, using the monoisotopic masses (in Da) for C (12.0000), H (1.0078), N (15.0001), and O (15.9949), the monoisotopic mass for fentanyl (C22H28N2O) would be 336.2202, which is distinguished from its nominal mass (the sum of all protons and neutrons) of 336 Da. HRMS instruments can typically measure exact masses with a mass error less than 5 parts per million (ppm). HRMS mass error is conventionally measured in ppm, equal to one millionth of the mass of a compound, rather than in Daltons because error is not static across a mass range, but instead scales with the mass of a compound. Some HRMS instruments, such as Orbitraps, are capable of measuring the mass of compounds to within 1 ppm, which would correspond to ± 0.0005 Da of the exact mass for a compound with a mass of 500 Da.
Table 1.
Resolving Power for Various Mass Spectrometers
| Instrument Type | Resolving Power (FWHM)1 |
|---|---|
| Fourier transform ion cyclotron resonance mass spectrometer (FT-ICR-MS) | > 1,000,000 |
| Orbitrap | 100,000 – 1,000,000 |
| High resolution time-of-flight (TOF) | 50,000 – 100,000 |
| Time-of-flight (TOF) | 10,000 |
| Quadrupole/ion trap | 1,000 – 5,000 |
Full width at half maximum
By virtue of being “high resolution”, HRMS can “resolve” compounds which have the same nominal mass but different exact masses. For example, fentanyl, a common synthetic drug that can cause fatality at low exposure levels, and acebutolol, which was recently discovered in a European river following a chemical spill (Bader et al., 2016), both have nominal masses of 336. However, fentanyl has a molecular formula of C22H28N2O (exact mass = 336.2202 Da), while acebutolol has a formula of C18H28N2O4 (exact mass = 336.2049 Da) (Figure 1). In the event of an environmental spill of one of these compounds in which the identity of the spilled compound was unknown, low resolution instrumentation would not be able to distinguish between the two molecular ions. An authentic standard or fragmentation using MS/MS or electron ionization (EI) and matching to a spectral library would be needed. However, because the exact masses differ by approximately 0.0153 Da (~ 45 ppm), HRMS (specifically, an instrument with resolving power ≥ 22,037) would be able to rapidly eliminate one of them as a possibility using only the molecular ion.
Figure 1.
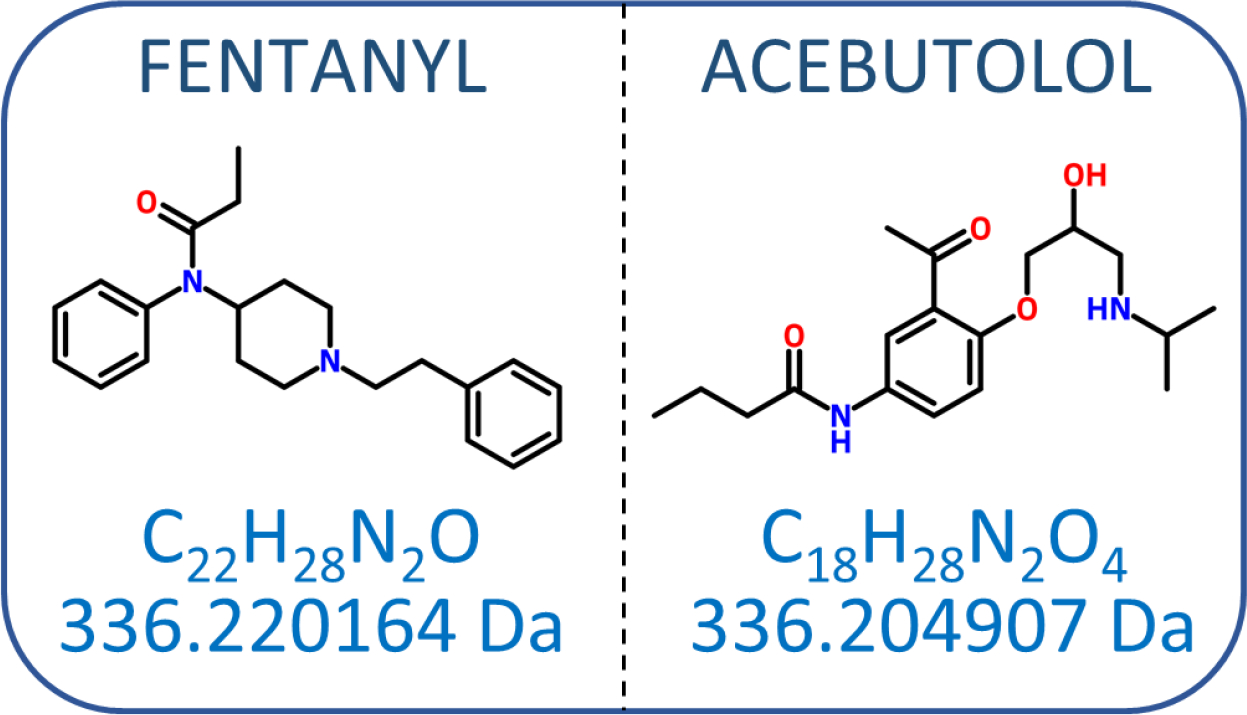
Chemical structures, formulae, and monoisotopic masses for fentanyl and acebutolol. While fentanyl and acebutolol cannot be differentiated based on unit mass (336), their chemical formulae and exact masses are distinct. Resolving power (R) = mass1 (m1)/[m1 – mass2 (m2)]. To resolve fentanyl from acebutolol, R = 336.220164/(336.220164 – 336.204907) = 22,037 is needed.
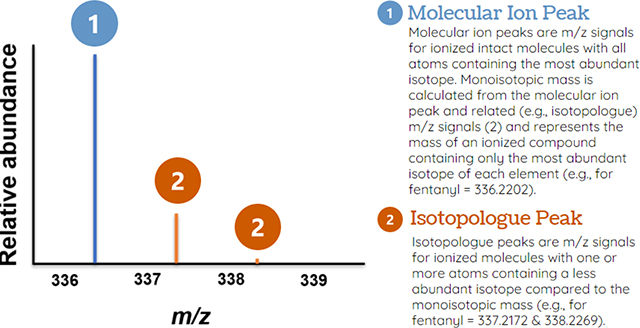
Another benefit of the increased resolution of HRMS is the ability to distinguish the monoisotopic molecular ion from isotopologues, which allows for improved formula prediction. For example, if one of the eighteen carbon atoms on a fentanyl molecule is a 13C atom instead of the predominant 12C isotope, the exact mass of this isotopologue would be 337.2235 Da instead of 336.2202 Da. Given the natural abundance of all elements in fentanyl, the isotopologue peak of fentanyl at 337.2235 will be 24% of the height of its molecular ion peak at 336.2202. Because isotopic abundances of elements are well established, most HRMS vendor software and other open software, like SIRIUS, allow for the prediction of a formula for unidentified ions using the relative abundances and, to a lesser extent, mass differences of isotopologues.
Improved resolution, which enables accurate mass measurement and isotopologue detection, results in enhanced capabilities to identify unknown compounds of interest with increased confidence. HRMS instruments have grown in popularity in the past decade because of their increasing affordability and advantages they can provide for myriad applications. Several software tools, including instrument/vendor software and third-party data processing tools, can extract useful data from large collections (thousands) of mass spectra. As methods for HRMS coupled with either gas or liquid chromatography are developed further and workflows for processing the large HRMS data files advance, potential uses for these instruments continue to grow.
Non-targeted Analysis
The process of assigning chemical identities to the unknown features from an HRMS experiment is known as NTA. Researchers use many techniques to accomplish identification including matching these features to chemical databases or spectral libraries (often referred to as “Suspect Screening”), or proposing structures based on the fragmentation pattern of the features (requires MS/MS data) and mass spectrometry first principles. A full review of NTA methods is outside the scope of this article but a few key pieces of information are relevant: 1) common chemicals are often easily and rapidly identified through suspect screening using chemical libraries; 2) these common chemicals make up only a small percentage of features that can be extracted from an environmental sample; 3) NTA can more successfully annotate molecular formulae than it can identify an unknown chemical’s structural identity; 4) identifying truly unknown chemicals is difficult and time consuming but not impossible; and 5) having some information about unknown chemicals of interest, such as source, starting material or similar chemicals can help narrow down a possible chemical identity. Such information is commonly associated as metadata in databases. Notably, NTA approaches for identifying unknowns and tracking many signals within a single sample hold promise to enhance rapid response capabilities.
NTA as a Tool for Rapid Response
Rapid Screening of Common Suspects.
In rapid response scenarios, laboratories often receive samples that have been characterized to some extent in the field. Examples of this include: reports of the composition of the chemical release, physical evidence at the site invoking a specific contaminant, and clinical evidence of the disease-causing contaminant. However, in most cases, a specific chemical contaminant is not identified with adequate reliability by those methods alone and it is necessary to conduct analytical screening. In the current paradigm for rapid response scenarios, chemical screening is typically broken into two tiers, one called the “basic screen” and the other referred to as the “expanded screen”. The basic screen is designed to capture a relatively small number of priority contaminants and uses only standardized targeted methods. The basic screen tests for some VOCs, SVOCs, carbamate pesticides, quaternary nitrogen compounds, trace metals, total mercury, cyanides, and radionuclides, and encompasses 18 individual EPA-validated methods for analyte coverage (Table 2). However, these established techniques focus mainly on regulated substances and do not provide complete coverage for all potentially harmful analytes. For instance, EPA routinely publishes the Selected Analytical Methods for Environmental Remediation and Recovery (SAM), which contains guidance on the selection of analytical methods for use by ERLN and other labs tasked with analyzing environmental samples in response to homeland security-related contamination incidents. In the 2017 SAM document, EPA acknowledged that a priori method selection may not be appropriate when responders did not anticipate having to analyze for a particular analyte (Campisano et al., 2017).
Table 2.
Analyte Coverage and Techniques Used in the Basic Chemical Screen1
| Chemical Class | Analytical Technique | EPA Method | Example Analytes |
|---|---|---|---|
| Volatile organic compounds (VOCs)2 | Purge-and-trap Tandem Photoionization Detector/Electrolytic Conductivity Detector (PID/ELCD), Purge-and-trap gas chromatography/mass spectrometry (GC/MS) | 502.2, 8021B 524.2, 8260B |
1,1,2-Trichloroethane; ethylbenzene; naphthalene; trichloroethene |
| Semi-volatile organic compounds (SVOCs)2 | Solid-phase extraction GC/MS | 525.2, 8270D/3535A | Atrazine; benzo(a)pyrene; dimethyl phthalate |
| Carbamate pesticides2 | High performance liquid chromatography (HPLC) – fluorescence detection | 531.1, 531.2 | Aldicarb; carbofuran; sevin (carbaryl) |
| Quaternary nitrogen compounds2 | HPLC – ultraviolet (UV) detection | 549.2 | Diquat; paraquat |
| Trace metals | Inductively coupled plasma atomic emission spectroscopy (ICP-AES), ICP-MS, graphite furnace atomic absorption (AA) spectroscopy | 200.7, 200.8, 200.9 | Arsenic; chromium; copper; lead |
| Total mercury | Cold vapor AA | 245.1, 245.2 with persulfate | Mercury |
| Cyanides | Wet chemistry | 335.2, 335.3, 335.4 | Free cyanide |
| Radionuclides | Gross alpha, Gross beta, Gross gamma | 900 | Cesium-137; iridium-192; cobalt-60 |
Adapted from: https://www.epa.gov/sites/production/files/2015-06/documents/module_4.pdf (Table 4-3)
Indicates analytes that are amenable to HRMS characterization
In the absence of analyte confirmation using the basic screen, the expanded screen can be applied to samples from the contaminated site. The expanded screen utilizes a suite of techniques, both established and exploratory, to achieve broad coverage of all priority chemical contaminants. Currently, the requirements for analytical confirmation using the expanded screen are generally not well defined and a wide variety of available screening techniques in federal guidance elicits uncertainty about the selection of suitable methods and equipment. In fact, EPA Regional laboratories have great latitude in assisting responders and can often develop or adapt on-demand methods based on the incident. However, results of the expanded screen are typically not considered definitive and thus must be confirmed via targeted methodologies. Nevertheless, data generated by two hypothetical HRMS methods (one GC and one LC) could be quickly queried for many of the analytes on the basic screening list using a suspect screening analysis approach. This approach could supplant the need for many different targeted analytical methods to be used in concert in the characterization of a single sample (see SI file for initial suspect list compiled of GC/ and LC/HRMS amenable analytes from the basic screen). Moreover, as contaminants are identified in spill responses, they can be added to the initial suspect screening list to facilitate an initial triage evaluation of suspected material and rapid identification in future scenarios.
Identification of Unknowns.
NTA is perhaps most recognized for its ability to identify previously unknown chemicals. Thus, its most obvious contribution to a rapid response scenario is the identification of organic compounds from chemical spills and other events involving unknown substances. As demonstrated from the available data on 2019 response scenarios, 37% of incidents involve an unknown chemical. NTA workflows can be used to select a compound from a suspect list (as described above) or identify a completely unknown compound— however the latter is much more difficult. As will be described in detail below, NTA often begins with the measurement of accurate mass values which can provide strong evidence towards identifying a suspected compound. The number of candidate molecules proposed for a given accurate mass is dependent on the molecular weight, with smaller molecules having fewer candidates. Identifying high molecular weight compounds can therefore be more challenging given the increased size of the candidate pool. To aid identification, a formula can be predicted from the measured accurate mass and related isotopologues, providing further evidence for identification or narrowing the list of candidates to a manageable number. It should be noted that a simple formula, enumerated to account for the bonding and valence rules of chemistry, can be associated with an enormous number of organic molecules. For example, the formula for fentanyl, C22H28N2O, can produce 2,147,483,646 molecules but a combination of structure database lookup and associated metadata will shrink potential hits to a manageable number (MOLGEN 5.0; Gugisch et al., 2015). Using informatics tools, further data processing ultimately yields a tentative identification, to which a confidence level can be assigned.
Processing Data Generated by HRMS Instruments
The processing of HRMS data can be customized according to specific research goals and has the potential to provide information that would be extremely useful in a rapid response situation. The first step in processing HRMS data is known as “peak picking” or “feature finding” when working with LC data, or deconvolution for GC data. In this step, the processing software (e.g., Agilent’s Profinder, Thermo Fisher’s Compound Discoverer, open source XCMS) identifies signals that appear to come from the same chemical (including components [i.e., clusters of related isotopologues], fragments, multimers, and adducts) and groups these signals. The software will also extract a chromatographic peak from this associated group of signals, thereby providing a peak area that is related to the chemical’s abundance and its corresponding chromatographic retention time (this grouping of signals and their association with a specific retention time, monoisotopic mass, and intensity is known as a “feature”).
For GC data, these features are typically matched to spectral databases and no further processing is needed. GC-HRMS data can be uniquely screened against both low-resolution mass spectral databases, which have been built up over several decades and can contain hundreds-of-thousands of spectra (e.g., NIST/EPA/NIH Mass Spectral Library), and newer HRMS databases (e.g., MassBank of North America), which, although growing, contain fewer compounds but offer a higher degree of confidence for spectral matches. For compounds that are not present in any of these databases (e.g., newly discovered compounds), in silico fragmenters can be used to predict spectra (further discussion below).
For LC data, processing software can generally determine the molecular ion (and consequently the monoisotopic mass) from the group of related mass signals mapped to an unknown feature. It is important to note that no annotation in terms of identifying a molecular formula or structure has occurred at this point in the workflow, but simply the extraction of features. If multiple samples are under examination, then alignment of features across these samples provides details regarding detection frequency, abundance as a function of time, sample, treatment, etc. The output from the alignment process is a matrix of features (including the monoisotopic mass and retention time) and samples with peak areas, indicating the relative abundance of each feature in each sample. This data matrix allows for blank subtraction, filtering based on selected thresholds and QA/QC limits, and statistical analysis. Aligning unannotated data without explicitly identifying sample composition can also yield information pertinent to rapid response decision making—for example, aligned unannotated data can be used to define a source fingerprint of released material which enables contamination tracking, both spatially and temporally.
Informatics Tools at U.S. EPA
Following initial data processing, NTA utilizes informatics approaches to facilitate the identification of chemical entities based on mass and formula searches and spectral library matching. There is an abundance of informatics approaches that have been delivered to the community in recent years, with some of these being commercial vendor software or, increasingly, free and open-source software solutions. While a listing of available tools can be found at www.nontargetedanalysis.org, we focus specifically on software applications that have been or are being developed within the EPA (see SI file for more details).
Compound identification requires a structure database to search against using both mass and formula, with formula-based searching being the preferred approach. The CompTox Chemicals Dashboard (the Dashboard) has been available since 2016 and provides access to ~883,000 substances (as of January 2021). Substances are uniquely identified by a “DTXSID”, which will be mentioned throughout this section for referencing chemicals and associated metadata. Individual substances can have many forms including single small molecules, salts and mixtures, and UVCB (Unknown or Variable Composition, Complex Reaction Products and Biological Materials) chemicals such as polymers, complex surfactants and biological substances. For the purposes of structural identification, chemical structures with associated mass and formula data are those of value to the analysis.
Many of the entries in the Dashboard are not single compounds but exist as salt forms or as a component of multi-component substances. For example, consider the chemical fentanyl (DTXSID9023049) and its associated “mixtures, components and isotopomers” (Figure 2; U.S. EPA, 2021e). These chemicals are mapped to their parent chemical using “MS-Ready mappings”, a cheminformatics approach that produces mappings and relates chemicals that could potentially be detected within a mass spectrometer. This provides a path between a mass or formula search input, a resultant set of candidate structures, and the mappings of these structures to all potential chemical substances that could contain that chemical based on removal of stereochemistry, neutralization of charge, and presence in a multi-component system. Assuming that the list of MS-Ready mappings for fentanyl represents a set of candidate structures, metadata can help prioritize candidates for identification. Metadata have been demonstrated to be valuable in the identification of “known unknowns” utilizing the ChemSpider database (Little et al., 2011), and more recently, the CompTox Chemicals Dashboard (McEachran et al., 2017). Metadata available for ranking in the Dashboard user interface include counts in the EPA’s Chemicals and Products Database (CPDat) (Dionisio et al., 2018), the number of data sources in the Dashboard and in PubChem, and the number of references in PubMed. It was demonstrated that the number of associated data sources was the best predictor of positive identification McEachran et al., 2017).
Figure 2.
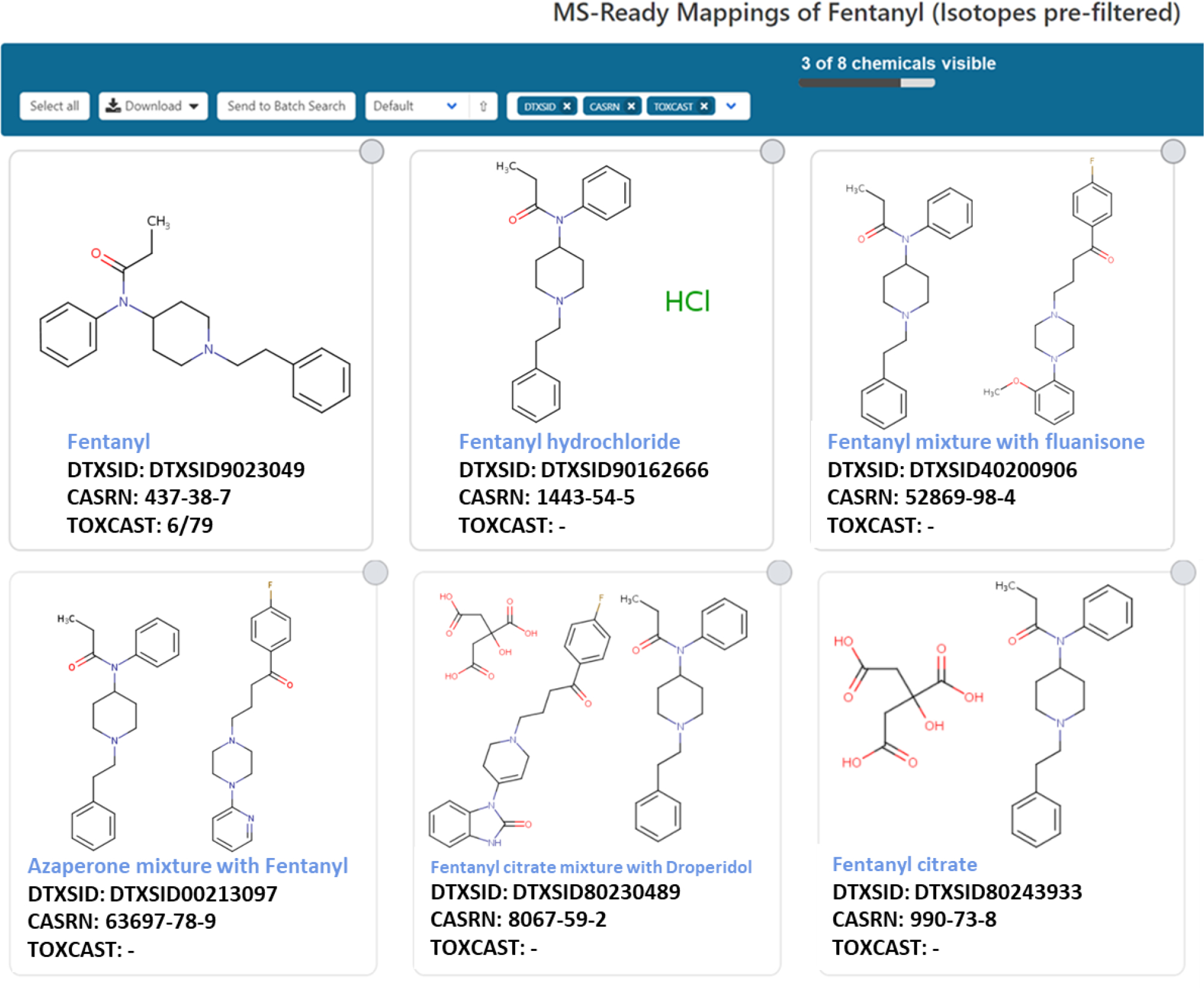
The MS-ready mappings for fentanyl. Note that MS-Ready mappings have isotopically labeled compounds (n =2) filtered out by default.
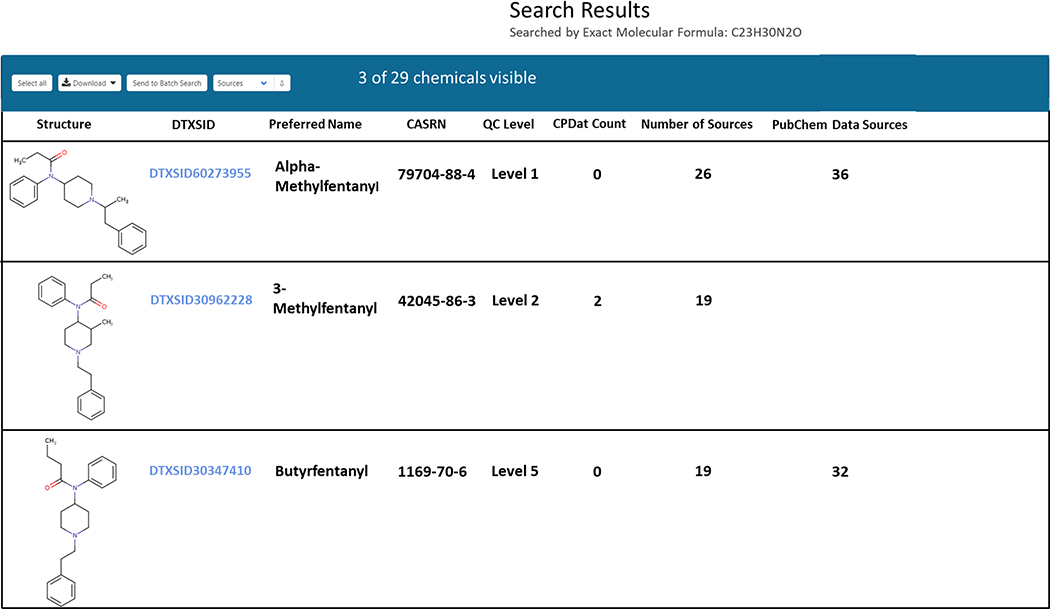
It is noteworthy that additional metadata for ranking become available by pushing a result set through the Dashboard’s batch search (Lowe et al., 2021), including availability of in vivo hazard data, in vitro bioactivity data and presence in specific chemical lists. The structure identification workflow through the Dashboard generally utilizes the input of a mass or formula through the advanced search interface and allows for both MS-Ready and Exact Formula searches. However, batch searching is possible based on exact formulae, MS-Ready formulae or mass values, and the results set is exported into a format for review or incorporation into other software (i.e., Excel, tab or comma-separated files). An example of chemical candidate ranking by metadata following molecular formula search (input = C23H30N2O) is shown in Figure 3, with fentanyl analog isomers ranked in the top three of 29 candidates.
Figure 3.
Candidate ranking using available metadata includes data source counts in the CompTox Chemicals Dashboard and PubChem and PubMed article counts.
The potential utility of in silico predicted mass spectral data to include with metadata for candidate ranking has also been investigated. Using the Competitive Fragmentation Modeling-ID (CFM-ID) open source prediction software, spectra were generated for >700,000 MS-ready structures. The combination of both spectral matching and metadata ranking was applied to all Critical Assessment of Small Molecule Identification (CASMI) contest datasets and demonstrated excellent performance relative to the contest winners (McEachran et al., 2020). The approach has also been applied to study a number of mixtures associated with the EPA’s Non-Targeted Analysis Collaborative Trial (ENTACT) project using an expanded database of >765,000 substances (Chao et al., 2020). A prototype web-based tool for searching an experimental spectrum against the in silico spectral database has been developed and is undergoing testing. This will allow users to see both the candidate results returned for the spectrum as well as visualizations of the in silico spectra for identifying unknowns. In addition, a library containing approximately 400 fentanyl analogues has been assembled and will be released on the Dashboard in the near future. At present, these data are available by request.
While the approaches above describe the application of the Dashboard to support searching (using mass or formula), introducing raw data from an HRMS instrument into this workflow represents an existing and significant gap. At present, our research has extended to produce a web-based application that takes extracted and aligned feature data from a mass spectrometer and uses the data from the Dashboard to perform a more highly automated analysis. The WebApp includes feature removal (e.g., duplicates, non-reproducible features, blank features), feature flagging (e.g., adducts, neutral losses, multimers, negative mass defect) and utilization of Dashboard data (e.g., data source and publication counts, presence in specific lists on the Dashboard, and consumer product data). These informatics tools, most of which are already publicly available, could aid an emergency response team equipped with HRMS instrumentation using an NTA approach to rapidly identify hazards.
REDUCING SUSPECTS & INCREASING IDENTIFICATION CONFIDENCE
Following NTA convention, unknown compounds are identified at five levels of confidence (1–5), with 1 being the most confident identification and requiring confirmation with an authentic standard (Schymanski et al., 2014b). All unknown features begin at a Level 5, the level of least confidence, where they are merely a mass of interest. To understand how NTA may be used in a rapid response scenario, it is useful to examine each confidence level (Figure 4).
Figure 4.
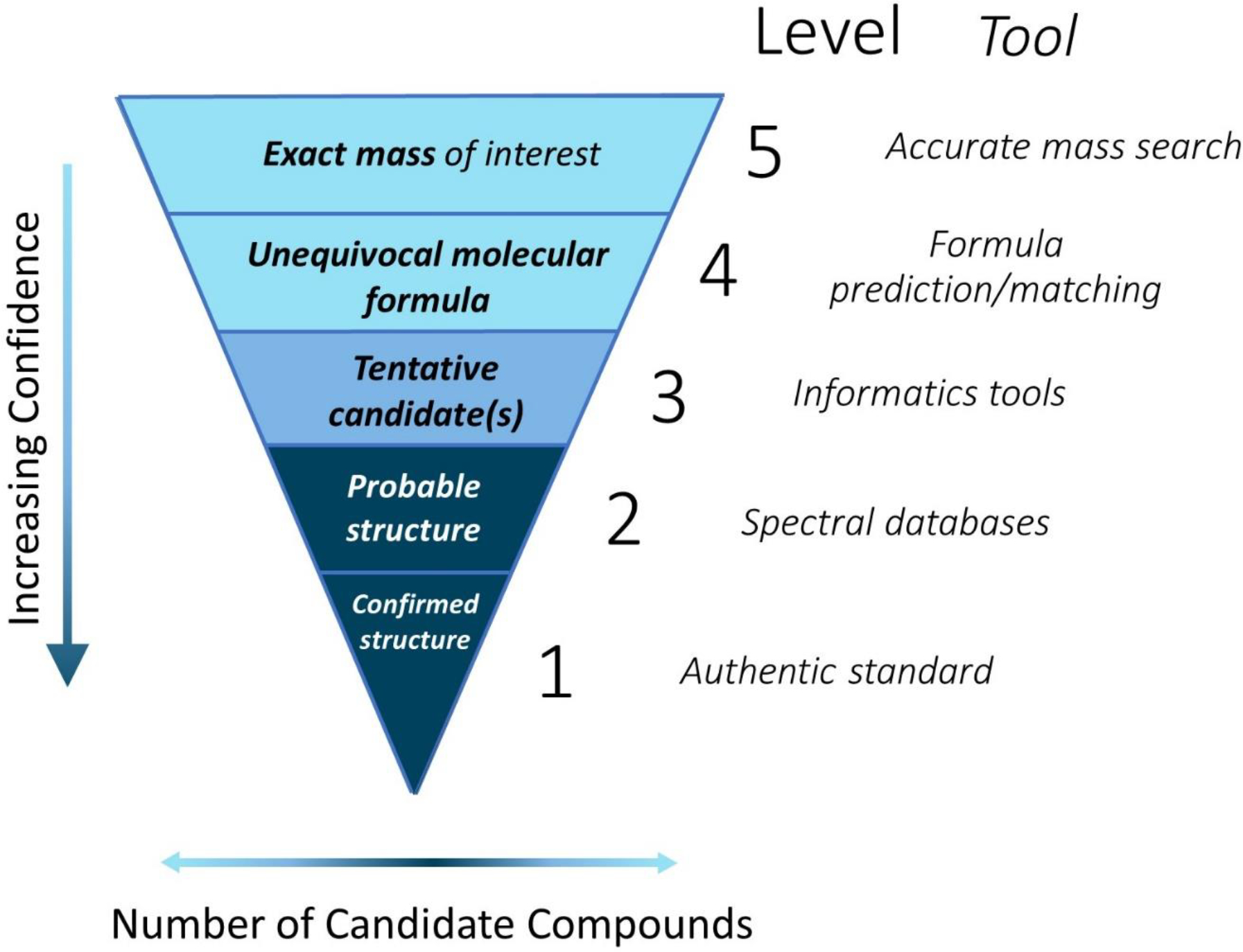
NTA workflows move unidentified compounds to higher levels of confidence whilst narrowing the field of candidate compounds [adapted from Schymanski et al., 2014b].
Level 5: A Mass of Interest
At the lowest level of confidence, only an observed accurate mass can be assigned to a feature of interest. Unknown features exist at this level when a formula cannot be assigned from MS1 (i.e., MS) data and there is no spectral match to the LC MS2 (i.e., MS/MS) spectrum or GC-EI spectrum. With HRMS LC data, NTA workflows produce a narrowed list of suspects from an accurate mass measurement of the molecular ion, which cannot be achieved with low resolution data. Using the Dashboard’s advanced search function, the database can be searched by mass within a given mass window. For typical HRMS instruments, a 5-ppm window is a reasonable setting given expected mass error. For low resolution instruments, this window would need to be expanded to ± 0.5 Da (roughly 1000–5000 ppm, depending on the mass of the compound). To illustrate the resulting number of compounds, searching the mass of butyrfentanyl, a fentanyl analog, using the Dashboard with a mass window typical of low-resolution instruments (± 0.5 Da) results in 2,061 candidate compounds, while using a high-resolution window (5 ppm) results in only 30 (Figure 5).
Figure 5.
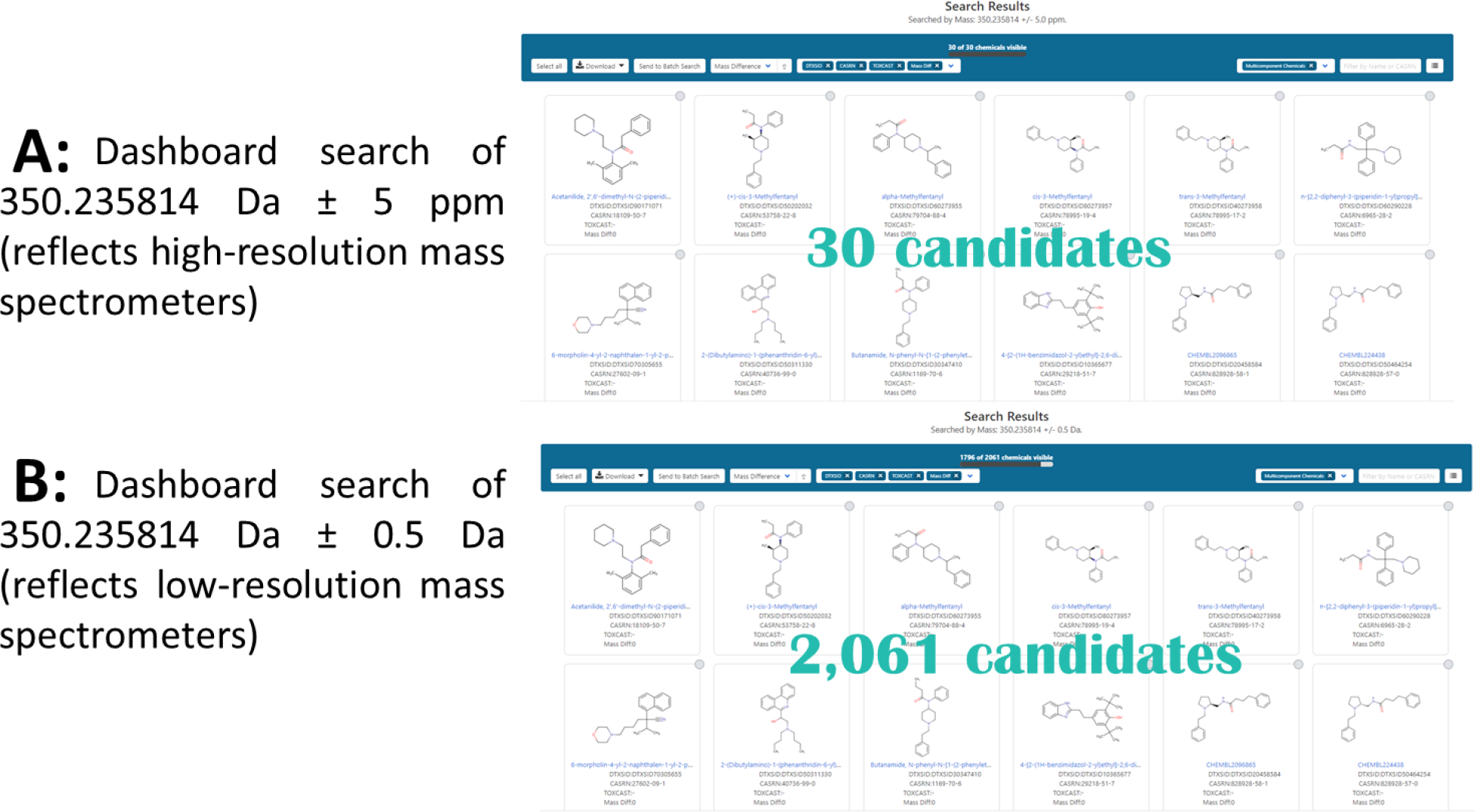
Dashboard results from a search of butyrfentanyl’s mass (350.235814) with A: a mass window of 5 ppm; B: a mass window of 0.5 Da.
Level 4: A Formula Match
A great advantage of HRMS over low-resolution instrumentation comes with its ability to clearly resolve isotopologues – spectral features that differ only in their isotopic composition. Nearly every organic molecule will exhibit at least one isotopologue on an HRMS given sufficient concentration/response. The mass difference and abundance ratios of the isotopologues are indicative of the elemental composition of the molecule, and most vendor software can predict formulas for unknown molecules using fine isotope patterns and exact mass. Thus, isotopologues can be used to determine the formula for the molecular ion, which is easily predicted using LC/HRMS but can also be predicted for GC/HRMS using chemical ionization. Additionally, isotopologues can be used to determine the formula for a fragment of the parent compound, which may be useful in determining its structure. By using isotopologues to predict formulas, an unknown compound is far more likely to be assigned a formula (Level 4) when using HRMS than when using low-resolution instrumentation, where it would likely remain as merely a mass of interest (Level 5). Armed with a formula and an accurate mass, the list of possible candidates is narrowed significantly. As described above, searching the Dashboard using a mass of 350.235814 ± 5 ppm (the exact mass of butyrfentanyl) yields 30 potential chemical candidates. However, adding C23H30N2O (butyrfentanyl’s chemical formula) as an additional input to the search narrows the chemical candidate further (Figure 3).
Level 3: Tentative Candidates
Tentative candidates can be identified using an accurate mass and/or a formula with the aid of the informatics tools previously mentioned. The Dashboard can be used to download a list of all candidates along with their corresponding data sources and relevant toxicity information. Ranking candidate compounds by number of data sources has been shown to aid in the identification of unknowns, with the correct chemical ranking in the top five candidate compounds 85–88% of the time (McEachran et al., 2017). Knowledge about the chemical release (physical appearance at the site of spill, industrial activities at the spill location, etc.) can also be used to deem candidates as tentative (Level 3) rather than just possible.
Level 2: A Spectral Match
Millions of spectra representing tens-of-thousands of compounds have been archived in various HRMS libraries as described above. Beyond these experimental spectral libraries, an in silico library of over 750,000 compounds, generated using the CFM-ID algorithm, can be searched. Using CFM-ID, spectra can be predicted for low, medium, and high collision energy levels for any InChI or SMILES string that is input by a user. While libraries of reference spectra exist for low resolution spectra, HRMS spectral matching provides a much higher degree of confidence than low resolution matching. By defining a mass to the fourth or fifth decimal place rather than just by its integer value, fewer combinations of elemental components whose sum equal the mass are possible, thereby restricting the number of potential molecular fragments. Indeed, data acquired by HRMS may clear up accidental matches (i.e., false positives) produced using low resolution instruments and spectral libraries (Stein, 2012). Confidence increases with each matched fragment, and spectral matches are often found using matching functions in vendor software. Thus, any candidate compound from a lower level of confidence can be brought up to a Level 2 with spectral searching using experimental or in silico HRMS libraries. For example, the vast majority of GC identifications jump from Level 5 to Level 2 with spectral matching using a library.
Level 1: A Known Compound
Level 1 compounds are confirmed by a reference standard, with fragmentation and retention time matching. Following an NTA workflow, an unknown compound may be identified at a lower level of confidence, or several candidates may be suspected for identification. If a laboratory has access to standards corresponding to any of the candidates, those standards could be used to confirm or refute the identification of the candidate compound. In addition to commercial sources for chemical standards, laboratories within the U.S. EPA’s Office of Research and Development in Research Triangle Park have access to over 4,000 standards from the ToxCast library and the Office of Pesticide Programs maintains a pesticide repository in Fort Meade, Maryland. If one of these thousands of compounds is a likely candidate for an unknown compound identified at Levels 2–5, existing standards could be accessed in a rapid response scenario to support unequivocal identification. Finally, in scenarios where HRMS is used to support source tracking, a reference standard may be obtainable from the source (e.g., industrial facility near the site of the release, chemical transportation vehicle, etc.). For those cases where a reference standard is not available from laboratory repositories or commercially, but can be obtained from a source, the pollutant HRMS chemical profile can be compared to the source material to provide identification from a source determination perspective.
HAZARD AND TOXICITY TOOLS FOR RAPID EVALUATION
While chemical identification can clearly be supported by informatics tools, the prioritization of potential candidates for further verification should relate to their potential for human health and ecotoxicological effects. For emergency response situations, acute toxicity effects through oral, dermal or inhalation exposure, genotoxicity and mutagenicity effects, skin or eye irritation and sensitization, and acute aquatic toxicity represent the primary concerns for the emergency responders. Available data related to these toxicological effects would be highly beneficial for comparison across potential candidates and for further validation of identified chemicals of concern. Many of the pertinent toxicology data exist within the CompTox Chemicals Dashboard, via the Hazard tab. These data are associated with the ToxVal database, which collates publicly available toxicity values typically used in risk assessments. These include point of departure (POD) data and no-observed and lowest-observed (adverse) effect levels (NOEL, NOAEL, LOEL, LOAEL) data extracted from repeated dose toxicity studies submitted under the European Regulation on Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH). Also included are reference dose and concentration values (RfDs and RfCs) and cancer slope factors and unit risks (CSFs and CURs) from EPA’s Integrated Risk Information System (IRIS) and EPA’s Provisional Peer-Reviewed Toxicity Values (PPRTV) documents. Acute toxicity information has been extracted from a number of different sources, including: Organisation for Economic Co-operation and Development (OECD), eChemPortal, European Chemicals Agency (ECHA), National Library of Medicine (NLM) and ChemIDplus via EPA’s Toxicity Estimation Software Tool (TEST). While the database provides access to data for >50,000 chemicals and hundreds of thousands of toxicity data points, it requires considerable work to extract and assemble the data from the Dashboard in a facile manner to enable hazard comparison across a set of chemicals. This need has been addressed in a proof-of-concept application.
Vegosen and Martin (2020) have reported on the alternatives assessment dashboard. This application compares hazards for multiple endpoints across several chemicals with the intention of providing a manner for the prioritization of chemicals for further assessment. They compiled and integrated chemical hazard data for several human health and ecotoxicity endpoints from public online sources including hazardous chemical lists, Globally Harmonized System hazard codes (H-codes) or hazard categories from government health agencies, experimental quantitative toxicity values and, in contrast to ToxVal, included predicted (in addition to experimental) values from Quantitative Structure–Activity Relationship (QSAR) models obtained using EPA’s TEST software (vide supra). Scoring criteria based on the EPA’s Design for the Environment Program Alternatives Assessment Criteria for Hazard Evaluation provides hazard scores (i.e., low, medium, high, or very high) for each record. Since the original report, the application has continued to expand with both additional data (i.e., integration of ToxVal data from the CompTox Chemicals Dashboard) and additional functionality, and is now deployed inside the EPA as a proof-of-concept application now known as the Hazard Comparison Dashboard (HCD) that is expected to be made publicly accessible in the near future. The application includes a set of web services that are being investigated for integration into the Non-Targeted Analysis WebApp so that candidates can be characterized for prioritization by integration of both experimental and predicted data available from HCD. Integrating toxicity data from the HCD into an NTA workflow, especially via an automated NTA WebApp, would give rapid responders access to putative chemical candidates paired with their available toxicity information, ultimately supporting informed decision making.
CONCLUSIONS AND OUTLOOK
Rapid response scenarios involving unknown chemicals are common. While NTA approaches can aid in unknown chemicals identification, they have not been widely utilized in rapid response scenarios likely because time constraints involved in a rapid response do not allow for the rigorous data analysis previously required to perform NTA. The EPA has developed tools to streamline NTA data processing which now make NTA a more viable tool in rapid response scenarios. Therefore, it is important that EPA explore the possibility and practicality of using NTA as a companion to current use low-resolution targeted and screening methods to better characterize potential risk. Ultimately, an emergency response team equipped with state-of-the-art tools like HRMS instruments and cheminformatic platforms facilitating high-throughput NTA workflows and rapid toxicity comparison could be better prepared to assess unknown chemical releases compared to those relying on low-resolution methods alone.
An estimate of chemical concentration is critical in prioritizing candidates generated from NTA workflows based on available hazard information. For this reason, new methods for rapid and standard-free quantitative NTA are needed. In the longer term, effective use of NTA in rapid response situations will require a network of laboratories with dedicated staff trained in NTA. A growing number of labs already have HRMS instruments and data processing tools are currently available; however, many labs are likely unprepared to respond quickly in a rapid response scenario using NTA workflows. While we anticipate that an NTA workflow could generate useful data on a timeline that is relevant to rapid response situations (on the order of hours to a few days following sample receipt), this has not yet been demonstrated definitively. Indeed, time is a critical limitation in rapid response events. Furthermore, NTA requires the ability to extract an unknown chemical from a substrate, which is important for many rapid response sample collection techniques that rely on extraction from a wipe (e.g. drywall wipe) or piece of material (e.g. soil or sediment). For an unknown chemical, the proper extraction technique would also be unknown and may lead to a false negative. Thus, a test of the ideas presented here must be undertaken. Such a test could take the form of a mock scenario, where NTA analysts are blinded to the study design and release materials. It could also take the form of a collaboration in a real-world rapid response event with a response team. These efforts will better educate NTA practitioners on the data requirements and timelines governing an effective response, while also disseminating key NTA tools to chemists that support emergency responses.
Supplementary Material
1. Sidebar 1.

2. Text Box 1.

4. Text Box 2.
5. Sidebar 3.
Acknowledgments
The authors thank Christoph Steinbeck from the Friedrich-Schiller-University in Jena, Germany for his computing assistance using MOLGEN. We are grateful to Lawrence Kaelin from EPA’s Office of Emergency Management for sharing his experience and helpful insight on rapid response background and procedures. We also thank Daniel Chang and John Sloop for their critical reviews of our manuscript.
Footnotes
Disclaimer
The views expressed in this article are those of the author(s) and do not necessarily represent the views or policies of the U.S. Environmental Protection Agency.
REFERENCES
- Alexandrino GL, Tomasi G, Kienhuis PG, Augusto F, & Christensen JH (2018). Forensic investigations of diesel oil spills in the environment using comprehensive two-dimensional gas chromatography–high resolution mass spectrometry and chemometrics: new perspectives in the absence of recalcitrant biomarkers. Environmental science & technology, 53(1), 550–559. [DOI] [PubMed] [Google Scholar]
- Alygizakis NA, Samanipour S, Hollender J, Ibáñez M, Kaserzon S, Kokkali V, … & Thomas KV (2018). Exploring the potential of a global emerging contaminant early warning network through the use of retrospective suspect screening with high-resolution mass spectrometry. Environmental science & technology, 52(9), 5135–5144. [DOI] [PubMed] [Google Scholar]
- Bader T, Schulz W, Lucke T, Seitz W, & Winzenbacher R (2016). Application of non-target analysis with LC-HRMS for the monitoring of raw and potable water: strategy and results. In Assessing Transformation Products of Chemicals by Non-Target and Suspect Screening− Strategies and Workflows Volume 2 (pp. 49–70). American Chemical Society. [Google Scholar]
- Brack W, Hollender J, de Alda ML, Müller C, Schulze T, Schymanski E, … & Krauss M (2019). High-resolution mass spectrometry to complement monitoring and track emerging chemicals and pollution trends in European water resources. Environmental Sciences Europe, 31(1), 1–6. [Google Scholar]
- Campisano R, Hall K, Griggs J, Willison S, Reimer S, Mash H, Magnuson M, Boczek L, & Rhodes E. (2017). Selected Analytical Methods for Environmental Remediation and Recovery (SAM). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-17/356, 2017. Available from: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=339252 [Google Scholar]
- Chao A, Al-Ghoul H, McEachran AD, Balabin I, Transue T, Cathey T, … & Sobus JR (2020). In silico MS/MS spectra for identifying unknowns: a critical examination using CFM-ID algorithms and ENTACT mixture samples. Analytical and bioanalytical chemistry, 412(6), 1303–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Hou A, Corilo YE, Lin Q, Lu J, Mendelssohn IA, … & McKenna AM (2016). 4 years after the Deepwater Horizon spill: molecular transformation of Macondo Well oil in Louisiana salt marsh sediments revealed by FT-ICR mass spectrometry. Environmental science & technology, 50(17), 9061–9069. [DOI] [PubMed] [Google Scholar]
- Cruz AM, & Krausmann E (2013). Vulnerability of the oil and gas sector to climate change and extreme weather events. Climatic change, 121(1), 41–53. [Google Scholar]
- Dionisio KL, Phillips K, Price PS, Grulke CM, Williams A, Biryol D, … & Isaacs KK (2018). The Chemical and Products Database, a resource for exposure-relevant data on chemicals in consumer products. Scientific data, 5(1), 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Park K, Yu X, Zhang YJ, & Ye F (2020). Massive pollutants released to Galveston Bay during Hurricane Harvey: Understanding their retention and pathway using Lagrangian numerical simulations. Science of The Total Environment, 704, 135364. [DOI] [PubMed] [Google Scholar]
- Federal Emergency Management Agency. (2016, June). Emergency Support Function #10 – Oil and Hazardous Materials Response Annex. https://www.fema.gov/sites/default/files/2020-07/fema_ESF_10_Oil-Hazardous-Materials.pdf
- Gugisch R, Kerber A, Kohnert A, Laue R, Meringer M, Rücker C, & Wassermann A (2015). MOLGEN 5.0, a molecular structure generator. In Advances in mathematical chemistry and applications (pp. 113–138). Bentham Science Publishers. [Google Scholar]
- Hendrickson RG, Akpunonu P, Hughes AR, & Gerona R (2019). Highly potent fentanyl analogs: apnea from exposure to small quantities of ß-hydroxyfentanyl and furanylfentanyl. Clinical Toxicology, 57(9), 813–815. [DOI] [PubMed] [Google Scholar]
- Hollender J, Schymanski EL, Singer HP, & Ferguson PL (2017). Nontarget screening with high resolution mass spectrometry in the environment: ready to go? Environmental science & technology, 51(20), 11505–11512 [DOI] [PubMed] [Google Scholar]
- Islam A, Ahmed A, Hur M, Thorn K, & Kim S (2016). Molecular-level evidence provided by ultrahigh resolution mass spectrometry for oil-derived doc in groundwater at Bemidji, Minnesota. Journal of Hazardous Materials, 320, 123–132. [DOI] [PubMed] [Google Scholar]
- Little JL, Williams AJ, Pshenichnov A, & Tkachenko V (2011). Identification of “known unknowns” utilizing accurate mass data and ChemSpider. Journal of the American Society for Mass Spectrometry, 23(1), 179–185. [DOI] [PubMed] [Google Scholar]
- Lowe CN, & Williams AJ (2021). Enabling High-Throughput Searches for Multiple Chemical Data Using the US-EPA CompTox Chemicals Dashboard. Journal of Chemical Information and Modeling, 61(2), 565–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lung D, Wilson N, Chatenet FT, LaCroix C, & Gerona R (2016). Non-targeted screening for novel psychoactive substances among agitated emergency department patients. Clinical Toxicology, 54(4), 319–323. [DOI] [PubMed] [Google Scholar]
- McEachran AD, Chao A, Al-Ghoul H, Lowe C, Grulke C, Sobus JR, & Williams AJ (2020). Revisiting five years of CASMI contests with EPA identification tools. Metabolites, 10(6), 260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEachran AD, Sobus JR, & Williams AJ (2017). Identifying known unknowns using the US EPA’s CompTox Chemistry Dashboard. Analytical and bioanalytical chemistry, 409(7), 1729–1735. [DOI] [PubMed] [Google Scholar]
- McKenna AM, Nelson RK, Reddy CM, Savory JJ, Kaiser NK, Fitzsimmons JE, … & Rodgers RP (2013). Expansion of the analytical window for oil spill characterization by ultrahigh resolution mass spectrometry: Beyond gas chromatography. Environmental Science & Technology, 47(13), 7530–7539. [DOI] [PubMed] [Google Scholar]
- Nascimento KRDS, & Alencar MH (2016). Management of risks in natural disasters: A systematic review of the literature on NATECH events. Journal of Loss Prevention in the Process Industries, 44, 347–359. [Google Scholar]
- Newton S, McMahen R, Stoeckel JA, Chislock M, Lindstrom A, & Strynar M (2017). Novel polyfluorinated compounds identified using high resolution mass spectrometry downstream of manufacturing facilities near Decatur, Alabama. Environmental science & technology, 51(3), 1544–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomone A, Di Corcia D, Negri P, Kolia M, Amante E, Gerace E, & Vincenti M (2021). Targeted and untargeted detection of fentanyl analogues and their metabolites in hair by means of UHPLC-QTOF-HRMS. Analytical and bioanalytical chemistry, 413(1), 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schymanski EL, Singer HP, Longrée P, Loos M, Ruff M, Stravs MA, … & Hollender J (2014a). Strategies to characterize polar organic contamination in wastewater: exploring the capability of high resolution mass spectrometry. Environmental science & technology, 48(3), 1811–1818. [DOI] [PubMed] [Google Scholar]
- Schymanski EL, Jeon J, Gulde R, Fenner K, Ruff M, Singer HP, & Hollender J (2014b). Identifying small molecules via high resolution mass spectrometry: communicating confidence. Environmental science & technology, 48(4), 2097–2098 [DOI] [PubMed] [Google Scholar]
- Stein S (2012). Mass spectral reference libraries: an ever-expanding resource for chemical identification. Analytical Chemistry, 84(17), 7274–7282. [DOI] [PubMed] [Google Scholar]
- Norman. (2021, January 25). The Norman Early Warning System (NormaNEWS). https://www.normandata.eu. [Google Scholar]
- U.S. Coast Guard. (2021, January 25). National Response Center. https://nrc.uscg.mil/. [Google Scholar]
- U.S. EPA. (2015, April). OSC Warrant Officer Training: OSC Toolbox Guide. CERCLA Education Center. https://www.epa.gov/sites/production/files/2015-08/documents/toolbox.pdf. [Google Scholar]
- U.S. EPA. (2021a, January 25). Deepwater Horizon – BP Gulf of Mexico Oil Spill. Enforcement. https://www.epa.gov/enforcement/deepwater-horizon-bp-gulf-mexico-oil-spill. [Google Scholar]
- U.S. EPA. (2021b, January 25). National Response Center. Emergency Response. https://www.epa.gov/emergency-response/national-response-center. [Google Scholar]
- U.S. EPA. (2021c, January 25). PHILIS (Portable High-Throughput Integrated Laboratory Identification System). Emergency Response. https://www.epa.gov/emergency-response/philis-portable-high-throughput-integrated-laboratory-identification-system. [Google Scholar]
- U.S. EPA. (2021d, January 25). Environmental Response Laboratory Network. Emergency Response. https://www.epa.gov/emergency-response/environmental-response-laboratory-network. [Google Scholar]
- U.S. EPA. (2021e, January 25). MS-Ready Mappings of Fentanyl (Isotopes pre-filtered). CompTox Chemicals Dashboard. https://comptox.epa.gov/dashboard/dsstoxdb/ms_ready_mixture?cid=3049. [Google Scholar]
- U.S. Government Publishing Office. (2021, January). 40 C.F.R. § 300. National Oil and Hazardous Substances Pollution Contingency Plan. Electronic Code of Federal Regulations. https://www.ecfr.gov/cgi-bin/text-idx?SID=a81fbc39b97f58cf5c0304cb28cac58a&mc=true&node=pt40.30.300&rgn=div5. [Google Scholar]
- Vegosen L, & Martin TM (2020). An automated framework for compiling and integrating chemical hazard data. Clean technologies and environmental policy, 22(2), 441–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.