Abstract
Brain disorders are characterized by the progressive loss of structure and function of the brain as a consequence of progressive degeneration and/or death of nerve cells. Aging is a major risk factor for brain disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), and stroke. Various cellular and molecular events have been shown to play a role in the progress of neurodegenerative diseases. Emerging studies suggest that primary cilia could be a key regulator in brain diseases. The primary cilium is a singular cellular organelle expressed on the surface of many cell types, such as astrocytes and neurons in the mature brain. Primary cilia detect extracellular cues, such as Sonic Hedgehog (SHH) protein, and transduce these signals into cells to regulate various signaling pathways. Abnormalities in ciliary length and frequency (ratio of ciliated cells) have been implicated in various human diseases, including brain disorders. This review summarizes current findings and thoughts on the role of primary cilia and ciliary signaling pathways in aging and age-related brain disorders.
Keywords: Aging, Alzheimer’s disease, Parkinson’s disease, Amyotrophic lateral sclerosis, Primary cilia, Hedgehog signaling, Wnt signaling, Notch signaling
1. Introduction
Aging is a sum of the dynamic changes occurring in biological, physiological, environmental, psychological, behavioral, and social processes in an organism. An aging brain manifests changes in its structure and functioning. At the cellular level, there is an extensive loss of functioning in brain cells due to phenomena such as cellular senescence and stem cell exhaustion (Bartzokis et al., 2003; Hedden and Gabrieli, 2004; Lopez-Otin et al., 2013; Yankner et al., 2008).
Aging is a major risk factor for various age-associated brain disorders such as Alzheimer’s disease (AD), Parkinson’s disease (PD), amyotrophic lateral sclerosis (ALS), stroke, etc. (Park et al., 2020). AD is a progressive loss of brain cell connections, whereby their degeneration and death severely impact memory and brain functioning (King et al., 2020). PD is a severe decrease in dopamine levels due to neuronal degeneration and gliosis in the substantia nigra pars compacta (SNpc), affecting movement coordination (Chai and Kohyama, 2019). ALS is characterized by progressive muscle weakness that is caused by nerve cell breakdown and loss of physical functioning (Rojas et al., 2020). Stroke is caused by impaired blood supply resulting in damage to the brain, which often leads to incoordination in walking, speaking, and understanding and numbness of the face, arm, and leg (Abdullahi et al., 2018). Studies have demonstrated a strong correlation of age with the development of brain disorders, including AD and PD (Aarsland et al., 2017; Cao et al., 2019; Kalia and Lang, 2016; Mattson et al., 2004; Scheltens et al., 2016) and stroke (Krishnamurthi et al., 2013).
Emerging studies have demonstrated that primary cilia play a pivotal role in brain development and brain disorders (Park et al., 2019). Primary cilia are essential for synapses of newborn neurons and defects in primary cilia lead to the shortening of dendrites of neurons and their failure of integration into the adult brain (Kumamoto et al., 2012). In addition, PD and ALS are also linked to defective primary cilia (Gazea et al., 2016). Primary ciliary signaling pathways such as Sonic Hedgehog (SHH) and Wnt pathways have been shown to contribute to the progress of these disorders (Gazea et al., 2016). The current review will summarize the profound role of primary cilia and ciliary signaling pathways in AD, PD, ALS, and stroke.
2. Primary cilia and ciliary signaling
Primary cilia are present in various mammalian cells, including stem, epithelial, endothelial, muscle, and various brain cells (Venkatesh, 2017). Primary cilia are non-motile tubular structures composed of nine microtubules with 9 + 0 axoneme arrangement, while motile cilia are tubular structures of two central microtubules surrounded by nine microtubules with 9 + 2 axoneme arrangement (Satir and Christensen, 2007). Primary cilia harbor various G protein-coupled receptors (GPCRs) that can sense extracellular chemical, osmotic, and mechanical stimuli. Primary cilia thus play a crucial role in signal transduction (Dummer et al., 2016; Hilgendorf et al., 2016). Defects in primary ciliary length and morphology often result in dysregulation of signaling transduction and cellular functionality, which in turn contribute to the development of various diseases termed ciliopathies (Ocbina et al., 2011).
Primary cilia are essential organelles for the transduction of the Hedgehog (Hh) signaling pathway (Fig. 1). In the absence of Hh ligand, PTCH (a 12 transmembrane domain receptor of Hedgehog ligand) interacts with Smoothened (SMO) and inhibits translocation of SMO into the primary cilia. This leads to phosphorylation and cleavage of full-length glioma-associated oncogene (GliFL) to Gli repressor (GliR). Following nuclear translocation, GliR binds to the promoter regions of Hh target genes and represses transcription of these genes (Jenks et al., 2018). In the presence of extracellular Hh, it binds to PTCH and relieves SMO inhibition. The released SMO enters the tip of the primary cilium, where it represses the Suppressor of Fused (SuFu). This leads to the modification of GliFL to Gli activator form (GliA) and nuclear translocation of GliA and activation of the Hh target genes (Jenks et al., 2018). (See Table 1.)
Fig. 1.

Hedgehog signaling at the primary cilium.
(A) In the absence of Hh ligand, PTCH (a 12 transmembrane domain receptor of Hedgehog ligand) interacts with Smoothened (SMO) at the base of primary cilia and inhibits translocation of SMO into the primary cilia. This leads to phosphorylation and cleavage of full-length glioma-associated oncogene (GliFL) to Gli repressor (GliR). Following nuclear translocation, GliR binds to the promoter regions of Hh target genes and represses transcription of these genes.
(B) In the presence of extracellular Hh, it binds to PTCH and relieves SMO inhibition. The released SMO subsequently enters the tip of the primary cilium where represses Suppressor of Fused (SuFu). This leads to the modification of GliFL to Gli activator form (GliA) and nuclear translocation of GliA and activation of the Hh target genes.
Table 1.
Major observations of primary cilia and ciliary signaling in age-related diseases.
| Disease | Model | Alteration of primary cilia (length, frequency, fragment) | Methods (Marker) | Ciliary Signaling | Therapeutic attempt | Reference |
|---|---|---|---|---|---|---|
| AD | 3xAD-transgenic mice | Decreased in the number of HH3 positive cells with age and in AD mice | Immunostaining for HH3 | NA | NA | (Rodriguez et al.,2008) |
| 3xAD-transgenic mice | Reduction in length of primary cilia in the hippocampal dentate gyral cells | Immunostaining for SSTR3 and p75NTR | NA | NA | (Chakravarthy et al., 2012) | |
| hfNBMs | NGF treatment increased the percentage of cells exhibiting primary cilium | Immunostaining for acetylated α-tubulin | NA | Intravenous administration of hfNBMs improved memory functions in AD rats | (Morelli et al., 2017) | |
| Mouse NIH3T3 and human HeLa cells | Amyloid-β decreased primary cilia length and frequency | Immunostaining for acetylated α-tubulin | SHH | Inhibition of Aβ production rescued the cilia morphological changes | (Vorobyeva and Saunders, 2018) | |
| APP/PS1 mice | Elongated in the hippocampus of APP/PS1 mice | Immunostaining for AC3 and ARL13B | NA | 5-HT6 antagonist SB271046 rescued the cognitive impairment in APP/PS1 mice | (Hu et al., 2017) | |
| J20-APP transgenic mice | NA | NA | Wnt signaling | NA | (Tapia-Rojas and Inestrosa, 2018) | |
| APP/PS1 mice | NA | NA | Wnt/β-catenin signaling | intra-hippocampal administration of WASP-1 rescues hippocampal synaptic impairments | (Vargas et al., 2015) | |
| SAMP8 mice | NA | NA | Downregulation of Wnt signaling in the hippocampus | NA | (Bayod et al., 2015) | |
| APP/PS1 mice | NA | NA | Notch | SP600125 treatment reverses AD Phenotypes | (Rahman et al., 2012; Zhou et al., 2015) | |
| APP/PS1 mice | NA | NA | Notch | Intragastrical administration of curcumin promoted proliferation of adult neural stem cells and birth of neurons and ameliorated cognitive impairment | (Li et al., 2019) | |
| AD patients | NA | NA | Elevated levels of TGFβ in the CSF and serum | NA | (Chao et al., 1994b) | |
| Human glioblastoma cells | NA | NA | TGFβ bound to βAPP | NA | (Bodmer et al., 1990) | |
| Human-APP transgenic mice | NA | NA | Overexpression of TGFβ accelerated the deposition of Aβ | TGFβ could promote amyloidogenesis | (Wyss-Coray et al., 1997) | |
| Human-APP transgenic mice | NA | NA | TGFβ | TGFβ reduces amyloid plaque burden | (Wyss-Coray et al., 2001) | |
| AβOs administered mice | NA | NA | TGFβ | Astrocyte-derived TGFβ protects synapses against AβOs | (Diniz et al., 2017) | |
| PD | 6-OHDA-lesioned rats | Elongated primary cilia in striatal neurons | Immunostaining for AC3 | NA | Bromocriptine administration abrogates the elongation of striatal neuronal cilia in lesioned sides of hemiparkinsonian rats. | (Miyoshi et al., 2014) |
| LRRK2-R1441C MEFs and LRRK2-G2019S expressing 3 T3 fibroblasts | Hyperactive LRRK2 decreased primary ciliogenesis | Immunostaining for ARL13B | NA | NA | (Steger et al., 2017) | |
| Human LRRK2 G2019S iPS cells and LRRK2 R1441C mice | LRRK2 kinase interferes with primary ciliogenesis | Immunostaining for ARL13B in vitro and AC3 & SSTR3 in vivo. | SHH | NA | (Dhekne et al., 2018) | |
| Mice model of MPTP-induced PD | Enhanced ciliogenesis in the substantia nigra dopamine neurons | Immunostaining for AC3 | NA | MPTP-induced primary ciliogenesis was significantly reduced in IFT88 shRNA-expressing neurons | (Bae et al., 2019) | |
| 6-OHDA-induced rat model of PD | NA | NA | SHH | Intrastriatal administration of SHH rescues behavioral impairment | (Tsuboi and Shults, 2002) | |
| 6-OHDA-induced rat model of PD | NA | NA | SHH | gene transfer of SHH and GLI1protect dopaminergic nigrostriatal neuronal cell bodies from a specific neurotoxic insult | (Hurtado-Lorenzo et al., 2004) | |
| 6-OHDA-induced rat model of PD | NA | NA | Wnt antagonist - Dkk1 is upregulated | LiCl, an inhibitor of GSK-3β, rescues the Wnt signaling pathway in the ventral midbrain of 6-OHDA PD rats | (Dun et al., 2012) | |
| 6-OHDA-induced rat model of PD | NA | NA | Wnt | LiCl administration restored motor function and memory | (Qi et al., 2017) | |
| RenVm cells | NA | NA | Wnt | LiCl facilitates dopaminergic differentiation | (Soleimani and Ghasemi, 2017) | |
| α-syn Tg mice | NA | NA | Notch | NA | (Crews et al., 2008) | |
| Mouse embryos e transfected with LRRK2 plasmid | NA | NA | Suppression of Notch signaling accelerated neuronal differentiation | NA | (Imai et al., 2015) | |
| MPTP-induced mouse model of PD | NA | NA | Notch | Administration of osthole attenuates motor deficits | (Wang et al., 2019) | |
| αSO injected mice (intracerebroventricular) | NA | NA | TGFβ | TGFβ promotes glutamatergic synapse formation in the striatum of parkinsonian animals | (Diniz et al., 2019) | |
| ALS | G93A SOD1 (mSOD) mice | The proportion of ciliated neurons is reduced | Immunostaining for AC3 | NA | NA | (Ma et al., 2011) |
| HT22 cells transfected with wtSOD, mSOD, or empty vector | NA | NA | SHH | SHH or the SHH agonist PUR reduces cell death | (Peterson and Turnbull, 2012) | |
| Cell cultures derived from wild type or mSOD1 mice | SHH increased the number of ciliated motor neurons in culture | Immunostaining for AC3 | SHH | SHH increased the percentage of ciliated motor neurons, especially in mSOD1 culture | (Ma et al., 2013) | |
| mSOD mice and Shh Light II cells | NA | NA | Notch (reduced in motor neurons and increased in astroglia) | NA | (Ma et al., 2017) | |
| SOD1* mice | NA | NA | Notch | AGT251 increases survival of SOD1* mice | (von Grabowiecki et al., 2015) | |
| mSOD mice | NA | NA | Wnt is upregulated in the spinal cord astrocytes | NA | (Chen et al., 2012a) | |
| NSC34hSOD1G93A cells | NA | NA | Wnt is downregulated neuron-like cells | NA | (Pinto et al., 2013) | |
| Human ALS spinal cord | NA | NA | Wnt | NA | (Gonzalez-Fernandez et al., 2019) | |
| SOD1(G93A) mice | NA | NA | Astrocyte-derived TGFβ1 accelerates disease progression | TGFβ signaling inhibitor extends the survival time of ALS mice | (Endo et al., 2015) | |
| SOD1(G93A) mice | NA | NA | TGFβ | ActRIIB.mFc increases muscle mass and myofiber diameter | (Morrison et al.,2009) | |
| Stroke | Endothelial cells | NA | Immunostaining for acetylated α-tubulin | NA | ECs lacking primary cilia show increased mineralization in response to BMP-6 | (Sanchez-Duffhues et al., 2015) |
| Apoe−/− mice | NA | Immunostaining for ARL13B | NA | Loss of primary cilia in ECs induces atherosclerosis | (Dinsmore and Reiter, 2016) | |
| MCAO stroke mice | NA | NA | SHH | Intravenous PUR attenuates neuroinflammation and promotes regeneration | (Chechneva et al., 2014) | |
| Hypoxic-ischemic mice | NA | NA | SHH | PUR protects against neuronal damage and brain injury in acute experimental ischemic stroke | (Liu et al., 2020b) | |
| MCAO stroke rats | NA | NA | SHH | BMSC treatment improves neurological outcomes via activating the Shh/Gli1 signaling pathway | (Zhang et al.,2009) | |
| MCAO stroke rats | NA | NA | SHH | Intrathecal SHH protein improves neurological recovery and stimulates neural progenitor cell proliferation | (Bambakidiset al., 2012) | |
| MCAO stroke rats | NA | NA | SHH | Intracerebroventricular SHH protein promotes angiogenesis | (Chen et al., 2017a) | |
| MCAO and doubleridge mice | NA | NA | Wnt | Inhibition of the Wnt antagonist protects neurons | (Mastroiacovo et al., 2009) | |
| ET-1 induced focal ischemic injury in mice | NA | NA | Wnt | Overexpression of Wnt3a enhances neurogenesis and improves neurological function after focal ischemic injury | (Shruster et al., 2012) | |
| MCAO | NA | NA | Notch | Inhibition of Notch signaling promotes neuronal regeneration after stroke | (Arumugam et al., 2006) | |
| VAM and Notch4*-Tet mice | NA | NA | Endothelial Notch-1 signaling is upregulated | NA | (Murphy et al., 2008) | |
| I/R rats | NA | NA | TGFβ | Administration of Sb505124, an ALK5 inhibitor, protected rats against I/R injury | (Lou et al., 2018) | |
| Ast-Tbr2DN MCAO mice | NA | NA | Ast-Tbr2DN mice exhibit a global reduction in TGFβ signaling after stroke | Inhibiting astrocytic TGFβ signaling worsens motor outcomes during the first week after stroke | (Cekanaviciute et al., 2014) |
NA: Not available; HH3: phosphorylated Histone H3, a proliferating mitotic marker; SSTR3: somatostatin receptor 3; p75NTR: p75 neurotrophin receptor; AC3: Adenylyl Cyclase type 3; hfNBMs: human fetal nucleus basalis of Meynert cells; NGF: nerve growth factor; APP/PS1 mice: double transgenic APPswe/PS1dE9 mice; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mSOD: G93A SOD1; wtSOD: human wild type SOD1; SOD1* mice: FVB transgenic mice expressing the missense mutation G86R (human G85R equivalent) in the SOD1 gene; NSC34hSOD1G93A cells: motor neuron-like NSC34 cells that stably express human mutant SOD1; ECs: Endothelial cells; BMP-6: bone morphogenetic protein-6; PUR: Purmorphamine, an agonist of the Smoothened (Smo) receptor; MCAO: middle cerebral artery occlusion; BMSC: bone marrow stromal cell; ET-1: endothelin-1; MEFs: mouse embryonic fibroblasts; 6-OHDA: 6-hydroxydopamine; Dkk1: Dickkopf-1; α-syn Tg mice: Transgenic mice expressing human A53T mutant α-syn; VAM: Brain arteriovenous malformations; I/R: ischemia-reperfusion.
The basal body of primary cilia harbors several components of the Wnt signaling pathway, such as Wnt receptors – FRIZZLED transmembrane proteins, Dishevelled (DVL), β-catenin, GSK3B, AXIN, and adenomatosis polyposis coli (APC) (Rimkus et al., 2016). In the absence of canonical Wnt stimulation, the GSK3B-AXIN-APC destruction complex triggers the phosphorylation, ubiquitination, and degradation of β-catenin. In the presence of extracellular Wnt ligands, they bind to FRIZZLED and a single-pass transmembrane coreceptor – low-density lipoprotein receptor-related protein (LRP) – resulting in the activation of DVL, which in turn recruits the destruction complex to the cell membrane and inhibits the phosphorylation and degradation of β-catenin (Fig. 2). This ultimately promotes nuclear translocation of β-catenin and transcription of the Wnt target genes (Bisgrove and Yost, 2006). The primary cilium can fine-regulate the activation of Wnt target genes by controlling the degradation of DVL through cilia proteins INVS, NPHP3, and KIF3A (Veland et al., 2009; Wheway et al., 2018).
Fig. 2.

Wnt signaling at the primary cilium.
(A) In the absence of Wnt ligands, the AXIN-APC-GSK3B destruction complex triggers the phosphorylation, ubiquitination, and degradation of β-catenin.
(B) Wnt ligands bind to FRIZZLED and LRP receptors resulting in the activation of Dishevelled (DVL), which in turn recruits the destruction complex to the plasma membrane and inhibits the phosphorylation and degradation of β-catenin and promotes nuclear β-catenin accumulation.
(C) In the presence of a cilium, cilia proteins INVS, NPHP3 and KIF3A control the activity of Wnt signaling via inhibiting the phosphorylation of DVL.
The Notch receptor is located on the primary ciliary membrane; upon binding of its extracellular domain to a Notch ligand, such as JAGGED or DELTA ligand on the membrane of an adjacent cell, the Notch receptor is cleaved and the interaction leads to the release of the Notch intracellular domain (NICD) (Rohatgi et al., 2007) (Fig. 3). As a result, NICD translocates to the nucleus, where it forms a complex with the transcription factor CSL and, in turn, activates the transcription of Notch target genes (Rohatgi et al., 2007). Notch target genes such as Hes genes can regulate primary ciliary translocation of PTCH and SMO (Corbit et al., 2005; Huangfu et al., 2003), suggesting the existence of crosstalk between ciliary signaling pathways.
Fig. 3.

Notch signaling at the primary cilium.
(A) A Notch ligand, such as JAGGED or DELTA on the membrane of an adjacent cell, binds to the Notch receptors resulting in the cleavage of the Notch receptor and release of Notch intracellular domain (NICD). As a result, NICD translocates to the nucleus, where it forms a complex with the transcription factor CSL and, in turn, activates the transcription of Notch target genes.
The TGFβ signaling in primary cilia operates by activating receptor R-SMADs transcription factors (SMAD2/3 and SMAD1/5/8) via internalization of active receptors by clathrin-mediated endocytosis (CME) at the ciliary pocket (Garcia-Gonzalo and Reiter, 2017). The R-SMADs form a trimeric complex with the co-SMAD, SMAD4. This leads to nuclear translocation of the complex and activates the transcription of the target genes (Fig. 4). TGFβ can also activate extracellular signal-regulated kinase 1/2 (ERK1/2) in the primary cilium (Garcia-Gonzalo and Reiter, 2017; Oh and Katsanis, 2013). Activation of TGFβ receptors promotes the SHH signaling pathway (Garcia-Gonzalo and Reiter, 2017), adding another layer of crosstalk between ciliary signaling pathways. (See Fig. 5.)
Fig. 4.
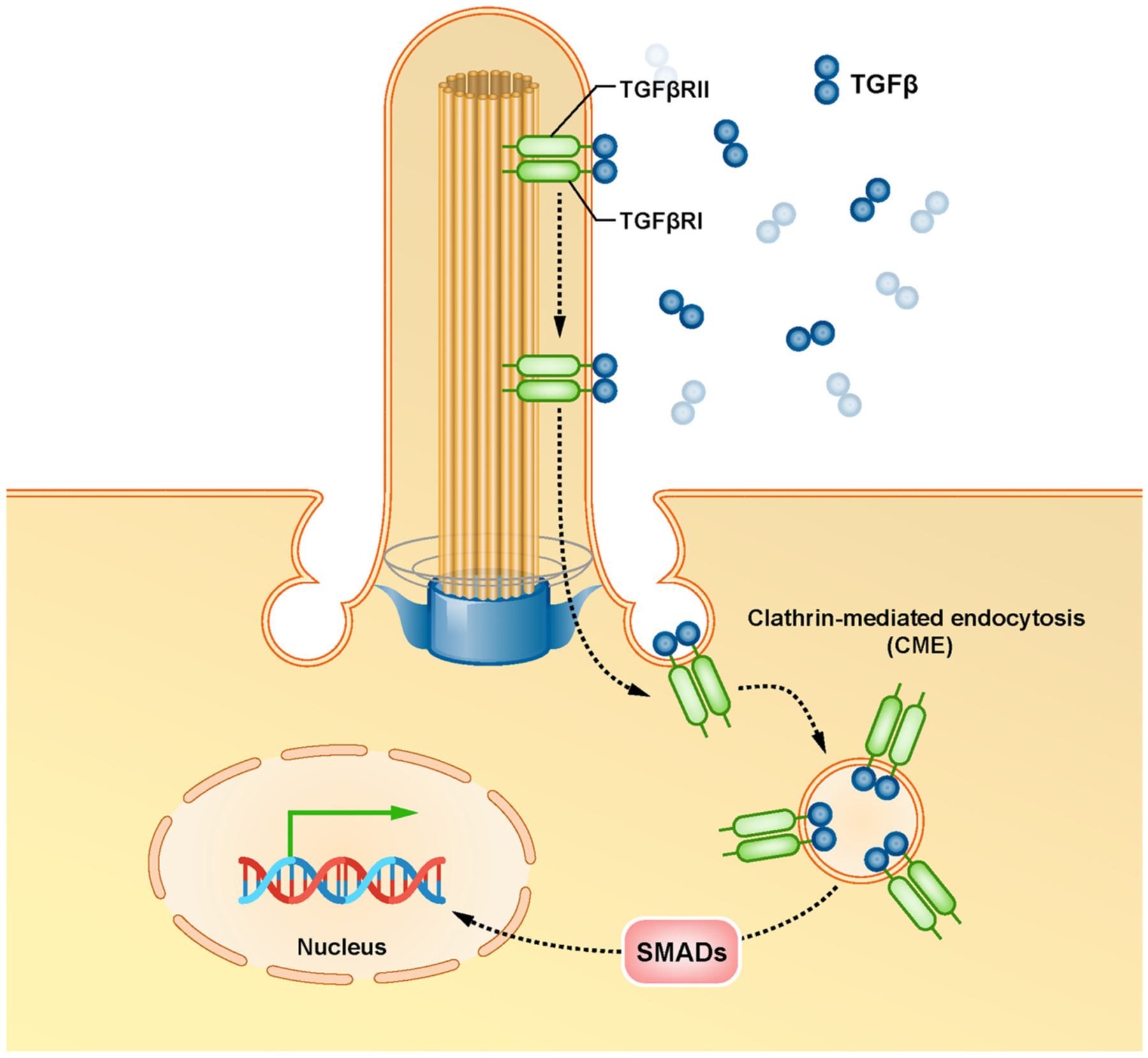
TGFβ signaling at the primary cilium.
Upon binding to TGFβ, receptors of the transforming growth factor-β (TGFβRI and TGFβRII) translocate from the ciliary membrane to the ciliary pocket where they undergo internalization by clathrin-mediated endocytosis (CME), resulting in activation and nuclear translocation of receptor R-SMADs transcription factors (SMAD2/3 and SMAD1/5/8) and ultimately activates the transcription of the target genes.
Fig. 5.

Primary cilia and ciliary signaling in age-related diseases.
3. Primary cilia and ciliary signaling in aging and age-related brain disorders
Emerging studies have demonstrated that primary cilia and ciliary signaling pathways are involved in aging and various brain disease-related cellular processes, including cell cycle control (Abdelhamed et al., 2013), cell proliferation (Willaredt et al., 2008), differentiation (Wheway et al., 2018), migration (Wheway et al., 2018), as well as neuronal stem cell (NSC) maintenance (Stasiulewicz et al., 2015). For example, disruption of cortical and hippocampal primary cilia impacts cognitive function through alterations in behavior, learning, memory, and new object recognition (Mohapatra et al., 2013; Schmid et al., 2018; Schneider et al., 2005). Moreover, primary cilia have a profound role in degeneration of cognitive impairment present in AD patients by impacting the maturation of cholinergic neurons in the forebrain (Guadiana et al., 2013). Therefore, defective primary cilia are strongly associated with aging and many neurodegenerative disorders incldung PD and ALS by abnormal functioning of ciliary signaling pathways (Gazea et al., 2016).
3.1. Primary cilia in normal brain aging
During aging, brain cells undergo various morphological and functional changes. Neural stem cell (NSC) exhaustion is one of the hallmarks of brain aging (Alvarez-Satta et al., 2019). Primary cilia and SHH signaling are required to form adult NSCs (Han et al., 2008). Further study suggests that primary cilia control the cell cycle of neural progenitors (Li et al., 2011). These findings suggest that primary cilia and ciliary signaling could regulate the aging process by controlling NSC proliferation and quiescence. The length of primary cilia is increased with age in the CA1 and CA3 but not in the dentate gyrus (DG) of rats (Guadiana et al., 2016). Moreover, a recent meta-analysis study suggests that the expression of primary cilia structure and function associated genes shows age-dependent patterns across the human lifespan (Chen et al., 2021). However, how primary cilia are regulated and function during brain aging is still under-studied.
The deficiency of SHH signaling in hypothalamus astrocytes prevents high-fat diet-induced obesity and insulin resistance, suggesting that astrocytic SHH signaling is a pivotal regulator in metabolic alterations associated with aging (Tirou et al., 2021). A study in the Octodon degus demonstrated that Wnt signaling activity is decreased in the brain during aging (Inestrosa et al., 2020). Notably, administration of ANDRO, an activator of the Wnt signaling, rescued the Wnt signaling loss in the brain of adult O. degus (Inestrosa et al., 2020). Previous studies showed that ANDRO treatment recovers synaptic protein loss and cognitive impairment (Rivera et al., 2016; Serrano et al., 2014), indicating that Wnt signaling plays a crucial role in aging. Cellular quiescence is another hallmark of aging. Notch signaling activity is significantly reduced in the aged brain (Sun et al., 2013). Administration of Notch1 activator increases the numbers of proliferating cells in aged rat brain, suggesting Notch signaling is a key player in regulating neurogenesis (Sun et al., 2013). Activation of TGFβ signaling pathway can promote cellular quiescence of NSCs in adult neurogenic niches (Kandasamy et al., 2014). These studies suggest that primary cilia and cilia signaling pathways are involved in aging. However, the detailed mechanisms underlying primary cilia-mediated age-associated cognitive decline remain elusive. Crosstalk between primary cilia pathways plays a crucial role in the process of aging (Wu et al., 2021); whether interactions between primary cilia and the pathways play a role during aging remains largely unknown and warrants further investigation.
3.2. Primary cilia and ciliary signaling pathways in Alzheimer’s disease
The dysregulation of primary ciliogenesis and ciliary signaling pathways have been shown to reduce cell proliferation and neurogenesis associated with aging and age-related diseases, including AD. Using the triple transgenic AD model mice (3xTg-AD) that produce both Aβ1–42 and the mutant human tau protein tauP301L, Rodríguez et al. found an age-dependent decrease in neurogenesis (Rodriguez et al., 2008). Moreover, male 3xTg-AD mice exhibited a further reduction in the production of new neurons in the subventricular zone (SVZ) and the subgranular zone (SGZ) of the DG in the hippocampus at 9 months of age and a complete depletion at 12 months (Rodriguez et al., 2008). Interestingly, female 3xTg-AD mice presented an earlier decrease in neurogenesis at 4 months with the maximum reduction at 12 months compared to controls (Rodriguez et al., 2008). These findings, therefore, indicate that deficiency of primary cilium-mediated cell proliferation contributes to the reduced neurogenesis in AD Tg mice accumulating both Aβ42 and tau protein and plays an important role in the progress of AD (Armato et al., 2013; Chakravarthy et al., 2012; Morelli et al., 2017).
Chakravarthy et al. demonstrated that the length of primary cilia is significantly reduced in the hippocampal dentate granule cells in 3xTg-AD mice (Chakravarthy et al., 2012). To investigate whether it was one or both accumulation of Aβ1–42 and mutant tau that decreased the length of primary cilia in the granular cells of 3xTg-AD mice, the investigators compared the primary cilia length in the hippocampal tissues from 2xTg-AD mice and tauopathy mice that produce only large amounts of Aβ1–42 and only a mutant human tau protein, respectively. Intriguingly, no significant difference in the cilial length or gross morphology was observed in the granule cells between 2xTg-AD mice and C57/BL6 wild-type mice at 6–8 months of age and between tau-Tg mice and wild-type B6.C3H mice aged 3–4 months (Chakravarthy et al., 2012). These findings thus suggest that AD-associated reduction in the length of primary cilia requires both accumulation of Aβ1–42 and mutant tau in mice. However, in an in vitro study, Armato et al. demonstrated that Aβ1–42 decreases primary cilia length and frequency, in turn disrupting SHH signaling in NIH3T3 cells (Vorobyeva and Saunders, 2018). Notably, He et al. demonstrated that SHH signal components – SHH, SMO, GLI1, and GLI2 – were upregulated in the hippocampus of APP23 mice and AD patients compared with the controls. At the same time, PTCH1, PTCH2, and GLI3 were downregulated in APP23 mice and AD patients (He et al., 2014). The authors further demonstrated that exposure to a high dose of Aβ1–42 significantly decreased the expression of PTCH and GLI but increased the expression of SHH compared with exposure to the low dose of Aβ1–42 in isolated hippocampal glial precursor cells (GPCs) (He et al., 2014). These findings suggest that dysregulation of primary cilia and ciliary signaling could vary depending on the amount of Aβ1–42 accumulation in AD patients’ brains.
The length of primary cilia in the hippocampus of APP/PS1 mice has been shown to be significantly increased compared with control mice (Hu et al., 2017). Both gain-of-function and loss-of-function studies have demonstrated that serotonin receptor 5-HT6 plays a pivotal role in mediating the elongation of primary cilia and may contribute to AD development (Hu et al., 2017). Importantly, intraperitoneal administration of 5-HT6 antagonist SB271046 decreased cilia length and rescued the cognitive impairment in APP/PS1 mice (Hu et al., 2017).
The degeneration of cholinergic neurons of the nucleus basalis of Meynert (NBM) in the basal forebrain (BF) has been linked to cognitive decline in AD (Kilimann et al., 2014). Morelli et al. demonstrated that intravenous administration of cholinergic neurons from the human fetal NBM (hfNBMs) significantly improved memory functions in AD rats treated with quisqualic acid (QA) (Morelli et al., 2017). Studies showed that the nerve growth factor (NGF) plays an essential role in maintaining the functions of the brain cholinergic neurons (Aloe et al., 2015). An in vitro study suggests that NGF can synergize the effect of SHH in promoting the proliferation of cholinergic neurons (Reilly et al., 2002). Interestingly, NGF treatment significantly increased the percentage of hfNBMs exhibiting primary cilium (Morelli et al., 2017), indicating that primary cilia and ciliary signaling could be the therapeutic targets for treating AD. The SHH inhibitory receptor PTCH1 has been shown to be upregulated by the amyloid precursor protein intracellular domain (AICD) derived from gamma-secretase-mediated cleavage of beta-amyloid precursor protein (APP) (Roncarati et al., 2002; Trazzi et al., 2011). In the Ts65Dn mouse Down syndrome model, Giacomini et al. demonstrated that subcutaneous administration of ELND006 – an inhibitor of APP γ-secretase – decreased the production of AICD and the expression of PTCH1 and restored neurogenesis in Ts65Dn mice (Giacomini et al., 2015).
Increasing amounts of evidence have shown that the Wnt signaling pathway plays an important role in AD pathogenesis. Downregulation of Wnt signaling activity has been observed in the human AD brain (Folke et al., 2019; Riise et al., 2015) as well as the brain of various AD rodent models such as J20 Tg (Tapia-Rojas and Inestrosa, 2018), double transgenic APPswe/PS1dE9 (Vargas et al., 2015), and SAMP8 (Bayod et al., 2015) mice. These results thus suggest that activation of Wnt signaling is a promising treatment for AD (Jia et al., 2019). Intriguingly, Vargas et al. have demonstrated that intrahippocampal administration of WASP-1 (Wnt-activating small molecule potentiator-1) was able to activate Wnt/β-catenin signaling in hippocampal neurons and rescue hippocampal synaptic impairments in APP/PS1 mice (Vargas et al., 2015).
The role of the Notch signaling pathway has been implicated in the memory process and adult neurogenesis (Alberi et al., 2013). Notch aberrant activation has been shown to contribute to AD pathology (Kopan and Goate, 2000; Woo et al., 2009). Loss of function of Notch results in similar phenotypes observed in AD, such as neuronal dysfunction and spatial memory deficits (Costa et al., 2003; Marathe et al., 2017). Rahman et al. found that intraperitoneal injection of c-Jun N-terminal kinase (JNK) specific inhibitor SP600125 decreased Notch1 signaling in 3-month-old C57BL/6 mouse brains without induction of apoptosis (Rahman et al., 2012). In an AD mouse model, Zhou et al. demonstrated that chronic treatment of SP600125 decreases amyloid plaque burden, β-amyloid production, and tau hyperphosphorylation and reversed cognitive deficits in APP/PS1 AD mice (Zhou et al., 2015). At the same time, intragastrical administration of curcumin promotes proliferation of adult neural stem cells and the birth of neurons through activation of Notch signaling pathway in 9-month-old APP/PS1 AD mice (Li et al., 2019). These findings, therefore, suggest that the Notch signaling pathway could be one of the therapeutic targets for treating AD.
Earlier studies have demonstrated that levels of transforming growth factor-beta (TGFβ) were elevated in both cerebrospinal fluid (CSF) and serum samples of AD patients (Chao et al., 1994a; Chao et al., 1994b). Following studies demonstrated that TGFβ is involved in several aspects of AD pathogenesis, including βAPP synthesis and processing, plaque formation, glial response, and neuronal cell death (Masliah et al., 2001). TGFβ is able to bind to βAPP (Bodmer et al., 1990). Moreover, in human APP/TGFβ1 bigenic mice, Wyss-Coray et al. demonstrated that co-expression of TGFβ accelerated the deposition of amyloid-beta(Aβ) peptide (Wyss-Coray et al., 1997). A Comparative Study demonstrated that expression of TGFβ type I (RI) and type II (RII) receptors is increased in reactive glia in AD patients compared with controls (Lippa et al., 1998), indicating upregulated TGFβ could regulate glial responses in AD. Interestingly, overexpression of TGFβ in astrocytes has been shown to promote amyloidosis in mice at young ages (Lifshitz et al., 2013). These studies suggest that TGFβ signaling pathway could contribute to the degenerative process in AD. In contrast, evidence also supports that TGFβ signaling pathway could serve as a neurotrophic pathway and play protective and survival roles in neurons (Wyss-Coray, 2006). Moreover, TGFβ can promote microglial phagocytosis activity, increase amyloid-beta clearance, and reduce plaque burden in transgenic mice (Wyss-Coray et al., 2001). Furthermore, astrocyte-derived TGFβ has been shown to protect synapses against amyloid-β oligomers (AβOs) in AD models (Diniz et al., 2017).
3.3. Primary cilia and ciliary signaling pathways in Parkinson’s disease
Parkinson’s disease (PD) is a neurodegenerative disease characterized by slowness of movement, loss of muscle and balance (Kim et al., 2018). The pathological hallmarks of PD include the loss of dopamine neurons in the substantia nigra (Ferrer et al., 2011). In the 6-Hydroxydopamine-induced hemiparkinsonian rat model, Miyoshi et al. demonstrated the increased length of primary cilia in striatal neurons on the ipsilateral (operated) compared with the contralateral (not operated) sides of the dorsolateral quarter (Miyoshi et al., 2014). Interestingly, bromocriptine administration was able to abrogate the elongation of striatal neuronal cilia in lesioned sides of hemiparkinsonian rats (Miyoshi et al., 2014), suggesting primary cilia could be a target for the treatment of PD.
Recent findings in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced mouse model of PD demonstrated enhanced primary ciliogenesis in dopamine neurons (Bae et al., 2019). Motor function assessment, using the rotarod performance test, demonstrated that the average time spent on a rod was significantly reduced in MPTP-treated mice compared with saline-injected mice. Interestingly, the depletion of primary cilia in dopamine neurons led to severe motor dysfunction in MPTP-administered mice (Bae et al., 2019). These results thus suggest that primary cilia play a protective role against MPTP-induced dopamine motor disability (Bae et al., 2019).
Leucine-rich repeat kinase 2 (LRRK2) is a protein kinase that phosphorylates a subset of Rab GTPases, including Rab8, Rab10, and Rab12 (Steger et al., 2017). Mutations in LRRK2 are one of the common genetic causes of late-onset, autosomal-dominant familial PD (Nguyen et al., 2020). Affinity enrichment mass spectrometry (AE-MS) results suggest that the primary ciliogenesis regulators, RILPL-1 and -2, can interact with phosphorylated Rab8, Rab10, and Rab12 (Steger et al., 2017). Strikingly, both the number of ciliated cells and length of primary cilia were significantly decreased in LRRK2-R1441G knock-in mouse embryonic fibroblasts (MEFs), which harbor increased kinase activity, compared with LRRK2 inhibitor MLi-2 treated cells (Steger et al., 2017). Importantly, the number of ciliated choline acetyltransferase (ChAT)-positive cholinergic neurons was significantly decreased in the striatum of R1441C mice – a valid preclinical model for PD compared with wild type (Dhekne et al., 2018). Altogether, the lack of dopaminergic signaling in striatal neurons of PD patients could be caused by LRRK2-mediated protein trafficking alterations in primary cilia (Dhekne et al., 2018; Miyoshi et al., 2014; Steger et al., 2017).
The role of SHH in the induction and differentiation of nigrostriatal dopaminergic neurons has been well established (Hynes et al., 1995). Intrastriatal administration of SHH has been shown to rescue behavioral impairment in the 6-hydroxydopamine (6-OHDA)-induced rat model of PD (Tsuboi and Shults, 2002). In line with this study, gene transfers of SHH and its downstream transcription factor-GLI1 have also been shown to protect dopaminergic nigrostriatal neurons in 6-OHDA-treated rats (Hurtado-Lorenzo et al., 2004). Despite the protective role of SHH signaling reported in many studies, the underlying molecular mechanisms by which the SHH signaling pathway regulates neuronal survival and functions remain largely unknown. The potential adverse effect of the SHH signaling pathway also warrants further investigation.
Over the last two decades, a considerable amount of experimental evidence has demonstrated that dysregulation of Wnt signaling contributes to the development and progression of PD (Marchetti, 2018; Purro et al., 2014). Wnt signaling regulates multiple cellular functions such as proliferation, differentiation, and cell fate determination. Impaired Wnt signaling is associated with the degeneration of midbrain dopaminergic neurons of the substantia nigra in PD (Stephano et al., 2018). In line with this, the levels of secreted Wnt antagonist Dickkopf-1 (Dkk1) have been shown to be elevated in MPP+ stimulated neurons (a cellular model of PD) and the ventral midbrain of 6-OHDA administered rats (Dun et al., 2012; Dun et al., 2013). Interestingly, knockdown of Dkk1 has been shown to protect against MPP + -induced neurotoxicity in neurons (Dun et al., 2013). Lithium Chloride (LiCl) can inhibit GSK3β activity and stabilize β-catenin and thus serves as an agonist of the canonical Wnt signaling pathway (Hedgepeth et al., 1997). LiCl treatment induces dopaminergic differentiation both in vitro and in vivo (Qi et al., 2017; Soleimani and Ghasemi, 2017). Animal studies demonstrated that administration of LiCl rescues the activity of Wnt signaling pathway in the ventral midbrain and restores motor function and memory in PD models (Dun et al., 2012; Qi et al., 2017).
Progressive accumulation of misfolded α-synuclein (α-syn) in cortical and subcortical brain regions is another hallmark of the pathogenesis of PD (Trojanowski and Lee, 1998). Transgenic mice expressing human A53T mutant α-syn (α-syn Tg mice) have been widely used to mimic PD pathology. A previous study demonstrated a reduced expression of Notch-1 in α-syn Tg mice compared with non-Tg controls (Crews et al., 2008). The animal study also supports the accumulation of α-syn decreases NPC survival via downregulation of Notch-1 expression and its signaling (Crews et al., 2008). Of note, a study has also shown that suppression of Notch signaling by the LRRK2 complex in the mouse brain leads to accelerated neuronal differentiation (Imai et al., 2015). A recent study suggested that administration of osthole attenuates motor deficits via suppressing Notch signaling pathway in the MPTP-induced mouse PD model (Wang et al., 2019). These findings suggest that Notch is fine regulated in PD, but the molecular mechanism(s) is not fully elucidated yet.
TGFβ has been shown to be upregulated in the brain and the ventricular CSF of PD patients (Mogi et al., 1995; Vawter et al., 1996). Deficiency of either TGFβ or its downstream gene Smad3 promotes the development of neurological and motor symptoms associated with PD (Giraldez-Perez et al., 2014; Tesseur et al., 2017), while activation of TGFβ signaling plays a neuroprotective role in both mouse and rat PD models (Chen et al., 2017b; Tesseur et al., 2017). Moreover, both in vitro and in vivo studies have demonstrated that α-syn oligomers (αSO) stimulation increased levels of TGFβ, which in turn induced the astrocyte reactivity and enhanced the synaptogenic capacity of astrocytes (Diniz et al., 2019). Intriguingly, inhibition of TGFβ signaling impaired glutamatergic synapse formation mediated by astrocyte conditioned medium in vitro and striatal synapse formation in vivo (Diniz et al., 2019). Importantly, TGFβ treatment protected mesencephalic neurons against synapse loss triggered by αSO (Diniz et al., 2019).
3.4. Primary cilia and ciliary signaling pathways in Amyotrophic lateral sclerosis (ALS)
Amyotrophic lateral sclerosis (ALS) is an age-related disorder characterized by neurodegeneration of motor neurons in the brain and spinal cord (Gordon, 2013). Both in vitro and in vivo studies have demonstrated that SHH treatment promotes induction and differentiation of moto-neurons (Ericson et al., 1996; Lee et al., 2007; Lewis and Eisen, 2001; Tanabe et al., 1995). Transplantation of SHH treated human motor neurons delayed clinical onset and prolonged life in ALS mice (Lee et al., 2014). Ma and coworkers found that the proportion of primary cilia is reduced in the spinal motor neurons of G93A SOD1 (mSOD) mice, a mouse model of ALS (Ma et al., 2011). Intriguingly, Peterson and Turnbull demonstrated that SHH or the SHH agonist PUR reduces cell death in a cellular model of ALS (Peterson and Turnbull, 2012). The same group further demonstrated that treatment of SHH significantly increased the percentage of motor neurons in culture and the number of ciliated motor neurons, especially so for mSOD cells (Ma et al., 2013). These findings thus suggest that primary cilia and the associated signaling pathways merit further consideration as potential therapeutic targets for ALS.
Wnt signaling pathway is associated with ALS. Both mRNA and protein of Wnt1, Wnt2, Wnt3a, Wnt4, Wnt5a, and Wnt7a have been shown to be upregulated in the spinal cord astrocytes of mSOD mice with disease progression (Chen et al., 2012a; Chen et al., 2012b; Li et al., 2013; Wang et al., 2013; Yu et al., 2013). Additionally, results of qPCR and immunofluorescence assays demonstrated FRIZZLED-5 – a Wnt signaling component – was significantly upregulated in neurons in the spinal cord of mSOD mice at late stages (Gonzalez-Fernandez et al., 2016). The authors also demonstrated that the levels of FRIZZLED-5 were negatively correlated with NeuN signal in neurons (Gonzalez-Fernandez et al., 2016). In an in vitro model of ALS, Wnt signaling activity, however, has been shown to be decreased in motor neuron-like NSC34 cells that stably express human mutant SOD1 (NSC34hSOD1G93A cells) compared with cells expressing wild type SOD1 (NSC34hSOD1WT cells) (Pinto et al., 2013). In line with this result, McLoon et al. demonstrated that the expression of Wnt1, Wnt3a, Wnt5a, and Wnt7a is downregulated in the neuromuscular junctions at the terminal stage in mSOD mice compared with age-matched controls (McLoon et al., 2014). Notably, the activation of the Wnt signaling pathway in ALS has also been validated in ALS human spinal cords (Gonzalez-Fernandez et al., 2019). González-Fernández et al. investigated the expression of Wnt signaling components in healthy and ALS human spinal cords using qPCR and demonstrated a significant increase in the levels of Wnt3, Wnt4, Fz2, and Fz8 (Gonzalez-Fernandez et al., 2019). Immunofluorescence assays demonstrated that the amount of Fz2+ astrocytes was significantly increased with concomitant upregulation of Wnt5a in ALS human spinal cord samples compared with healthy controls (Gonzalez-Fernandez et al., 2019). These findings suggest that the Wnt signaling pathway plays an important role in human ALS pathology and could be a potential therapeutic target for the treatment or prevention of ALS (Gonzalez-Fernandez et al., 2020). The Notch signaling has been shown to crosstalk with the SHH signaling in a mouse model of ALS (Ma et al., 2017).
The Notch signaling activity was significantly reduced in motor neurons but increased in astroglia in the spinal cord of mSOD mice compared with wild-type mice (Ma et al., 2017). Double immunofluorescence staining of spinal cord sections demonstrated reduced NICD expression in neurons and increased Notch activation in glial cells, especially astrocytes of mSOD mice (Liu et al., 2020a). Inhibition of Notch signaling decreased the SHH signaling in Shh Light II cells (Ma et al., 2017). Muscular atrophy is another hallmark of ALS. Grabowiecki et al. found that Notch and Notch target genes were upregulated in the muscles of mice treated with doxorubicin – a mouse model of muscle atrophy (von Grabowiecki et al., 2015). Administration of AGT251, a tocopherol-omega alkanol chain derivative, was able to protect muscles from doxorubicin-induced cachexia (von Grabowiecki et al., 2015). Interestingly, injection of AGT251 increased the survival of SOD1* mice – an ALS model that are FVB transgenic mice expressing the missense mutation G86R (human G85R equivalent) in the SOD1 gene (von Grabowiecki et al., 2015). However, the effect of AGT251 on Notch signaling in the motor neurons and muscles of patients with ALS remains unknown.
The levels of TGFβ1 have been shown to be increased in the serum and CSF of ALS patients compared with controls (Ilzecka et al., 2002). Moreover, the levels of CSF TGFβ1 showed a significant positive correlation with the duration of ALS (Ilzecka et al., 2002). In line with these findings, the levels of Phosphorylated Smad2/3 (pSmad2/3) were also elevated in the spinal cord of ALS patients and G93A mutant SOD1 transgenic (mSOD1 Tg) mice (Nakamura et al., 2008). Endo et al. demonstrated that TGFβ1 is upregulated in astrocytes of murine and human ALS (Endo et al., 2015). Interestingly, astrocyte-specific overproduction of TGFβ1 accelerates disease progression in SOD1G93A mice (Endo et al., 2015). Therefore, the TGFβ signaling pathways have been proposed as pathogenic factors in the development of ALS, and inhibition of TGFβ signaling pathways could offer promising targets for the treatment of ALS (Morrison et al., 2009; Peters et al., 2017). Indeed, administration of TGFβ signaling inhibitor is able to extend the survival time of ALS mice (Endo et al., 2015). Similarly, treatment with ActRIIB. mFc, a signaling receptor for TGFβ ligands and myostatin, has also been shown to increase muscle mass and myofiber diameter in SOD1G93A transgenic mice (Morrison et al., 2009).
3.5. Primary cilia and ciliary signaling pathways in stroke
Stroke is caused by the interruption or reduction of blood supply due to a blocked blood vessel or bleeding in the brain. Disruption of the blood-brain barrier (BBB) – composed of endothelial cells, pericytes, and astrocytes – is one of the hallmarks of stroke (Abdullahi et al., 2018; Campisi et al., 2018). Endothelial cells lacking primary cilia have been shown to be susceptible to bone morphogenetic protein-induced cellular calcification, which is a leading cause of blood vessel destruction (Sanchez-Duffhues et al., 2015). Loss of primary cilia in endothelial cells promotes atherosclerosis in Apoe−/−mice fed a high-fat, high-cholesterol diet, suggesting the protective role of primary cilia in atherosclerosis (Dinsmore and Reiter, 2016). However, the role of primary cilia in stroke remains largely unknown, and therefore, studies are urgently warranted.
Though the role of primary cilia in stroke is not clear, several ciliary signaling pathways have been implicated in this disease. Animal studies demonstrated that both intrathecal and intracerebroventricular administration of SHH protein improved neurological recovery and stimulated neural progenitor cell proliferation in middle cerebral artery occlusion (MCAO) stroke rats (Bambakidis et al., 2012; Chen et al., 2017a). In line with this finding, studies have demonstrated that both single intravenous administration (Chechneva et al., 2014) and repeated intraperitoneal administration (Liu et al., 2020b) of PUR could attenuate neuroinflammation and protect the brain against ischemic injury in MCAO and hypoxic-ischemic (HI) mice, respectively. Additionally, oligodendrocytes have also been shown to play a role in ischemic damage (Chen et al., 2001). Interestingly, treatment with bone marrow stromal cell (BMSC) promotes oligodendrogenesis by activating the SHH/Gli1 signaling pathway, which improves neurological outcomes in the brain of MCAO stroke rats (Zhang et al., 2009). These findings thus suggest that targeting the SHH signaling could be an effective strategy for treating stroke. The molecular mechanisms by which SHH-mediated neuroprotection functions, however, warrant further investigation.
A large body of evidence has demonstrated the neuroprotective role of Wnt signaling in stroke. Animal studies have demonstrated that activation of Wnt signaling pathway attenuates BBB dysfunction and protects neurons in stroke (Mastroiacovo et al., 2009; Zhao et al., 2020). Dickkopf-1 (DKK1) is a secreted inhibitor of canonical β-catenin-dependent Wnt signaling (Kagey and He, 2017). High levels of DKK1 in the circulating system have been associated with stroke (He et al., 2016; Seifert-Held et al., 2011). Doubleridge mice contain an insertional mutant in the transcriptional enhancer of the dkk-1 gene (Adamska et al., 2003). Doubleridge mice exhibited an attenuated reduced infarct volume in response to MCAO compared with control animals, suggesting that the upregulation of DKK-1 in stroke contributes to the pathophysiology of stroke (Mastroiacovo et al., 2009). Similarly, both stereotaxic injection of Wnt3a overexpressing lentivirus and intranasal administration of Wnt3a protein in the striatum or subventricular zone enhanced neuro-genesis and improved neurological function in mice subjected to focal ischemic stroke (Shruster et al., 2012; Wei et al., 2018). Additionally, various drugs/treatments, such as morroniside (Sun et al., 2014), cornin (Xu et al., 2016), and electro-acupuncture (Chen et al., 2015) have been shown to promote neurogenesis via Wnt/β-catenin signaling pathway in stroke animals. These findings thus support that activating the Wnt signaling pathway could be beneficial in increasing neurogenesis, reducing BBB dysfunction, and decreasing neuroinflammation in stroke.
Increasing evidence suggests that Notch signaling plays a crucial role in the neuropathology of stroke. Activation of Notch signaling worsens brain damage and functional outcome in ischemic stroke (Arumugam et al., 2006). Rodent studies have demonstrated that activating Notch signaling in the endothelium during brain development induces brain arteriovenous malformations (BAVMs), an important cause of stroke (Murphy et al., 2008). Furthermore, activation of Notch signaling pathway can increase proliferation and differentiation of adult SVZ neural progenitor cells as well as the proliferation of smooth muscle cells (SMCs), which in turn promote arteriogenesis in stroke (Chen et al., 2009; Wang et al., 2009; Zacharek et al., 2009). Under normal conditions, Notch signaling maintains ependymal cells in quiescence, while after stroke, ependymal cells are activated to generate neuroblasts and glial cells (Carlen et al., 2009). Constitutively active Notch signaling inhibited both death and differentiation of ependymal cells in MCAO stroke mice (Carlen et al., 2009). Astrocytes also play a pivotal role in stroke (Becerra-Calixto and Cardona-Gomez, 2017; Zhao and Rempe, 2010). Gamma-secretase inhibitor administration after stroke decreased the number of proliferative reactive astrocytes and immune cell invasion into the peri-infarct area (Shimada et al., 2011). Interestingly, Notch1 inhibition reduces reactive astrocyte formation in astrocyte-specific Notch1 conditional knockout mice suggesting Notch1 plays a pivotal role in reactive astrocyte formation in the peri-infarct area after stroke (Shimada et al., 2011). A recent study also demonstrated that inhibiting Notch1 signaling by overexpression of a circular RNA – circCCDC9 protected the blood-brain barrier and inhibited apoptosis in acute ischaemic stroke (Wu et al., 2020). These findings thus suggest that the Notch1 signaling pathway plays a vital role in stroke and could be a therapeutic target for stroke treatment.
TGFβ has both neuroprotective and neurotoxic roles in stroke. Lou et al. demonstrated that cerebral ischemia/reperfusion (I/R) injury-induced TGFβ signaling activation evidenced by upregulation of activin receptor-like kinase (ALK5) and phosphorylation of SMAD2/3 in rats (Lou et al., 2018). Interestingly, the authors showed that administration of Sb505124, an ALK5 inhibitor, significantly attenuated TGFβ signaling activation and protected rats against I/R injury (Lou et al., 2018), suggesting TGFβ signaling could be a target for the treatment of stroke. Meanwhile, TGFβ is also known as an anti-inflammatory and neuro-protective cytokine. To investigate the role of astrocytic TGFβ in stroke, Cekanaviciute et al. generated Ast-Tbr2DN mice where TGFβ signaling is inhibited specifically in astrocytes (Cekanaviciute et al., 2014). Ast-Tbr2DN mice showed elevated neuroinflammation during the sub-acute period after distal middle cerebral occlusion (dMCAO) stroke (Cekanaviciute et al., 2014). Importantly, Ast-Tbr2DN mice exhibited worse motor outcomes and late infarct expansion after photothrombotic motor cortex stroke (Cekanaviciute et al., 2014). These findings thus suggest that the TGFβ signaling should be fine-tuned precisely to the purpose of therapy.
3.6. Conclusions and perspectives
As mentioned above, dysregulation of neuronal primary cilia and ciliary signaling plays a key role in AD and PD. Studies have suggested that other types of cells, such as astrocytes, are also engaged in various neurological diseases, including AD and PD (Joe et al., 2018; Sanchez et al., 2021; Verkhratsky et al., 2010; Vincent et al., 2010). However, the role of primary cilia and ciliary signaling in astrocytes in these diseases remains poorly understood. The potential role of primary cilia in glial cells under neurodegenerative diseases has been elegantly discussed elsewhere (Ki et al., 2021).
Although studies have demonstrated the crucial role of primary cilia and ciliary signaling pathways in aging and age-related brain disorders, contradictory observations suggest they could play their roles in spatial- and temporal-dependent manners. Therefore, to understand the precise function of primary cilia and ciliary signaling pathways in disorders, it is necessary to combine genetic approaches, such as conditional tissue/cell-specific knockout and disease animal models. Additional tools to fine-tune primary ciliary signaling activity will allow the examination of the exact effects of primary cilia and ciliary signaling pathways in aging and age-related diseases.
Owing to the fact that primary cilia can sense and transmit extra-cellular signals into intracellular biochemical responses, they thus could play an essential role in mediating cell-cell communication under normal conditions and disease status. Recent studies have demonstrated that extracellular vesicles (EVs) are important intracellular mediators that carry a variety of cargo, including RNAs, proteins, lipids, and DNAs (Chivero et al., 2021; Hu et al., 2012; Hu et al., 2016). Interesting studies have demonstrated that SHH can be packaged on the membrane of EVs and mediate the activation of the SHH signaling pathway in neighboring cells (Liegeois et al., 2006; Vyas et al., 2014). The role of EVs in aging and age-related brain disorders has also been well documented (Hill, 2019; Robbins, 2017). It is thus possible that EVs could play a crucial role in aging and age-related diseases via mediating the activation of ciliary signaling pathways. Moreover, as reported recently, EVs, primary cilia and ciliary signaling could also be involved in intercellular communication (Ma et al., 2021). The underlying mechanism(s) of EV-mediated ciliary signaling in brain disorders warrants further investigation.
Acknowledgments
We would like to thank the support from the Nebraska Center for Substance Abuse Research (NCSAR).
Funding
This work was supported by the NIH grants DA046831, DA042704, and DA043138, MH112848. The project described was also supported by the NIH, National Institute of Mental Health, 2P30MH062261 (CHAIN). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. L.C. acknowledges the support of The National Natural Science Foundation of China (62002212) and 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (2020LKSFG07D, 2020LKSFG07A).
Footnotes
Declaration of Competing Interest
The authors declare no competing financial interests in relation to the work described.
References
- Aarsland D, et al. , 2017. Cognitive decline in Parkinson disease. Nat. Rev. Neurol 13, 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdelhamed ZA, et al. , 2013. Variable expressivity of ciliopathy neurological phenotypes that encompass Meckel-Gruber syndrome and Joubert syndrome is caused by complex de-regulated ciliogenesis, Shh and Wnt signalling defects. Hum. Mol. Genet 22, 1358–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdullahi W, et al. , 2018. Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am. J. Phys. Cell Physiol 315, C343–C356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamska M, et al. , 2003. Doubleridge, a mouse mutant with defective compaction of the apical ectodermal ridge and normal dorsal-ventral patterning of the limb. Dev. Biol 255, 350–362. [DOI] [PubMed] [Google Scholar]
- Alberi L, et al. , 2013. Notch signaling in the brain: in good and bad times. Ageing Res. Rev 12, 801–814. [DOI] [PubMed] [Google Scholar]
- Aloe L, et al. , 2015. Nerve Growth Factor: A Focus on Neuroscience and Therapy. Curr. Neuropharmacol 13, 294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Satta M, et al. , 2019. Primary cilium and brain aging: role in neural stem cells, neurodegenerative diseases and glioblastoma. Ageing Res. Rev 52, 53–63. [DOI] [PubMed] [Google Scholar]
- Armato U, et al. , 2013. Alzheimer’s disease: an update of the roles of receptors, astrocytes and primary cilia (review). Int. J. Mol. Med 31, 3–10. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, et al. , 2006. Gamma secretase-mediated Notch signaling worsens brain damage and functional outcome in ischemic stroke. Nat. Med 12, 621–623. [DOI] [PubMed] [Google Scholar]
- Bae JE, et al. , 2019. Primary cilia mediate mitochondrial stress responses to promote dopamine neuron survival in a Parkinson’s disease model. Cell Death Dis 10, 952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bambakidis NC, et al. , 2012. Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of Sonic hedgehog. J. Neurosurg 116, 1114–1120. [DOI] [PubMed] [Google Scholar]
- Bartzokis G, et al. , 2003. White matter structural integrity in healthy aging adults and patients with Alzheimer disease: a magnetic resonance imaging study. Arch. Neurol 60, 393–398. [DOI] [PubMed] [Google Scholar]
- Bayod S, et al. , 2015. Downregulation of canonical Wnt signaling in hippocampus of SAMP8 mice. Neurobiol. Aging 36, 720–729. [DOI] [PubMed] [Google Scholar]
- Becerra-Calixto A, Cardona-Gomez GP, 2017. The role of astrocytes in neuroprotection after brain stroke: potential in cell therapy. Front. Mol. Neurosci 10, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisgrove BW, Yost HJ, 2006. The roles of cilia in developmental disorders and disease. Development. 133, 4131–4143. [DOI] [PubMed] [Google Scholar]
- Bodmer S, et al. , 1990. Transforming growth factor-beta bound to soluble derivatives of the beta amyloid precursor protein of Alzheimer’s disease. Biochem. Biophys. Res. Commun 171, 890–897. [DOI] [PubMed] [Google Scholar]
- Campisi M, et al. , 2018. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials. 180, 117–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao M, et al. , 2019. Identification of age- and gender-associated long noncoding RNAs in the human brain with Alzheimer’s disease. Neurobiol. Aging 81, 116–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlen M, et al. , 2009. Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke. Nat. Neurosci 12, 259–267. [DOI] [PubMed] [Google Scholar]
- Cekanaviciute E, et al. , 2014. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 62, 1227–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai M, Kohyama J, 2019. Non-cell-autonomous neurotoxicity in Parkinson’s disease mediated by astroglial alpha-synuclein. Stem Cell Rep. 12, 183–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarthy B, et al. , 2012. Reduction of the immunostainable length of the hippocampal dentate granule cells’ primary cilia in 3xAD-transgenic mice producing human Abeta(1–42) and tau. Biochem. Biophys. Res. Commun 427, 218–222. [DOI] [PubMed] [Google Scholar]
- Chao CC, et al. , 1994a. Serum cytokine levels in patients with Alzheimer’s disease. Clin. Diagn. Lab. Immunol 1, 433–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao CC, et al. , 1994b. Transforming growth factor beta in Alzheimer’s disease. Clin. Diagn. Lab. Immunol 1, 109–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chechneva OV, et al. , 2014. A Smoothened receptor agonist is neuroprotective and promotes regeneration after ischemic brain injury. Cell Death Dis 5, e1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, et al. , 2001. Therapeutic benefit of intravenous administration of bone marrow stromal cells after cerebral ischemia in rats. Stroke. 32, 1005–1011. [DOI] [PubMed] [Google Scholar]
- Chen J, et al. , 2009. Niaspan treatment increases tumor necrosis factor-alpha-converting enzyme and promotes arteriogenesis after stroke. J. Cereb. Blood Flow Metab 29, 911–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, et al. , 2012a. Activation of the Wnt/beta-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem. Biophys. Res. Commun 420, 397–403. [DOI] [PubMed] [Google Scholar]
- Chen Y, et al. , 2012b. Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol. Res 34, 390–399. [DOI] [PubMed] [Google Scholar]
- Chen B, et al. , 2015. Electro-acupuncture exerts beneficial effects against cerebral ischemia and promotes the proliferation of neural progenitor cells in the cortical peri-infarct area through the Wnt/beta-catenin signaling pathway. Int. J. Mol. Med 36, 1215–1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SC, et al. , 2017a. Administration of sonic hedgehog protein induces angiogenesis and has therapeutic effects after stroke in rats. Neuroscience 352, 285–295. [DOI] [PubMed] [Google Scholar]
- Chen X, et al. , 2017b. TGF-beta1 neuroprotection via inhibition of microglial activation in a rat model of Parkinson’s disease. J. NeuroImmune Pharmacol 12, 433–446. [DOI] [PubMed] [Google Scholar]
- Chen S, et al. , 2021. Dynamic Changes of Brain Cilia Transcriptomes across the Human Lifespan. Int. J. Mol. Sci 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chivero ET, et al. , 2021. Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders. Cell. Mol. Life Sci 78, 4849–4865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbit KC, et al. , 2005. Vertebrate Smoothened functions at the primary cilium. Nature. 437, 1018–1021. [DOI] [PubMed] [Google Scholar]
- Costa RM, et al. , 2003. Learning and memory deficits in Notch mutant mice. Curr. Biol 13, 1348–1354. [DOI] [PubMed] [Google Scholar]
- Crews L, et al. , 2008. Alpha-synuclein alters Notch-1 expression and neurogenesis in mouse embryonic stem cells and in the hippocampus of transgenic mice. J. Neurosci 28, 4250–4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhekne HS, et al. , 2018. A pathway for Parkinson’s Disease LRRK2 kinase to block primary cilia and Sonic hedgehog signaling in the brain. Elife. 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, et al. , 2017. Astrocyte transforming growth factor beta 1 protects synapses against abeta oligomers in Alzheimer’s disease model. J. Neurosci 37, 6797–6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diniz LP, et al. , 2019. alpha-synuclein oligomers enhance astrocyte-induced synapse formation through TGF-beta1 signaling in a Parkinson’s disease model. J. Neurochem 150, 138–157. [DOI] [PubMed] [Google Scholar]
- Dinsmore C, Reiter JF, 2016. Endothelial primary cilia inhibit atherosclerosis. EMBO Rep. 17, 156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dummer A, et al. , 2016. Measuring the primary cilium length: improved method for unbiased high-throughput analysis. Cilia. 5, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dun Y, et al. , 2012. Inhibition of the canonical Wnt pathway by Dickkopf-1 contributes to the neurodegeneration in 6-OHDA-lesioned rats. Neurosci. Lett 525, 83–88. [DOI] [PubMed] [Google Scholar]
- Dun Y, et al. , 2013. Induction of Dickkopf-1 contributes to the neurotoxicity of MPP+ in PC12 cells via inhibition of the canonical Wnt signaling pathway. Neuropharmacology. 67, 168–175. [DOI] [PubMed] [Google Scholar]
- Endo F, et al. , 2015. Astrocyte-derived TGF-beta1 accelerates disease progression in ALS mice by interfering with the neuroprotective functions of microglia and T cells. Cell Rep. 11, 592–604. [DOI] [PubMed] [Google Scholar]
- Ericson J, et al. , 1996. Two critical periods of Sonic Hedgehog signaling required for the specification of motor neuron identity. Cell. 87, 661–673. [DOI] [PubMed] [Google Scholar]
- Ferrer I, et al. , 2011. Neuropathology of sporadic Parkinson disease before the appearance of parkinsonism: preclinical Parkinson disease. J. Neural Transm. (Vienna) 118, 821–839. [DOI] [PubMed] [Google Scholar]
- Folke J, et al. , 2019. Impaired Wnt signaling in the prefrontal cortex of Alzheimer’s disease. Mol. Neurobiol 56, 873–891. [DOI] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Reiter JF, 2017. Open sesame: how transition fibers and the transition zone control ciliary composition. Cold Spring Harb. Perspect. Biol 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazea M, et al. , 2016. Primary cilia are critical for Sonic hedgehog-mediated dopaminergic neurogenesis in the embryonic midbrain. Dev. Biol 409, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacomini A, et al. , 2015. Inhibition of APP gamma-secretase restores Sonic Hedgehog signaling and neurogenesis in the Ts65Dn mouse model of Down syndrome. Neurobiol. Dis 82, 385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraldez-Perez R, et al. , 2014. Models of alpha-synuclein aggregation in Parkinson’s disease. Acta Neuropathol. Commun 2, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez C, et al. , 2016. Wnt signaling alteration in the spinal cord of amyotrophic lateral sclerosis transgenic mice: special focus on frizzled-5 cellular expression pattern. PLoS One 11, e0155867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Fernandez C, et al. , 2019. Wnt Signaling Alterations in the Human Spinal Cord of Amyotrophic Lateral Sclerosis Cases: Spotlight on Fz2 and Wnt5a. Mol. Neurobiol 56, 6777–6791. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Fernandez C, et al. , 2020. New insights into Wnt signaling alterations in amyotrophic lateral sclerosis: a potential therapeutic target? Neural Regen. Res 15, 1580–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon PH, 2013. Amyotrophic lateral sclerosis: an update for 2013 clinical features, pathophysiology, management and therapeutic trials. Aging Dis. 4, 295–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadiana SM, et al. , 2013. Arborization of dendrites by developing neocortical neurons is dependent on primary cilia and type 3 adenylyl cyclase. J. Neurosci 33, 2626–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadiana SM, et al. , 2016. Type 3 adenylyl cyclase and somatostatin receptor 3 expression persists in aged rat neocortical and hippocampal neuronal cilia. Front. Aging Neurosci 8, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YG, et al. , 2008. Hedgehog signaling and primary cilia are required for the formation of adult neural stem cells. Nat. Neurosci 11, 277–284. [DOI] [PubMed] [Google Scholar]
- He P, et al. , 2014. Deficiency of patched 1-induced Gli1 signal transduction results in astrogenesis in Swedish mutated APP transgenic mice. Hum. Mol. Genet 23, 6512–6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XW, et al. , 2016. High serum levels of sclerostin and Dickkopf-1 are associated with acute ischaemic stroke. Atherosclerosis. 253, 22–28. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD, 2004. Insights into the ageing mind: a view from cognitive neuroscience. Nat. Rev. Neurosci 5, 87–96. [DOI] [PubMed] [Google Scholar]
- Hedgepeth CM, et al. , 1997. Activation of the Wnt signaling pathway: a molecular mechanism for lithium action. Dev. Biol 185, 82–91. [DOI] [PubMed] [Google Scholar]
- Hilgendorf KI, et al. , 2016. The primary cilium as a cellular receiver: organizing ciliary GPCR signaling. Curr. Opin. Cell Biol 39, 84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill AF, 2019. Extracellular Vesicles and Neurodegenerative Diseases. J. Neurosci 39, 9269–9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, et al. , 2012. Exosomal miRNAs: biological properties and therapeutic potential. Front. Genet 3, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu G, et al. , 2016. Emerging roles of extracellular vesicles in neurodegenerative disorders: focus on HIV-associated neurological complications. Cell Death Dis. 7, e2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, et al. , 2017. Serotonin 5-HT6 receptors affect cognition in a mouse model of Alzheimer’s disease by regulating cilia function. Alzheimers Res. Ther 9, 76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, et al. , 2003. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature. 426, 83–87. [DOI] [PubMed] [Google Scholar]
- Hurtado-Lorenzo A, et al. , 2004. Differentiation and transcription factor gene therapy in experimental parkinson’s disease: sonic hedgehog and Gli-1, but not Nurr-1, protect nigrostriatal cell bodies from 6-OHDA-induced neurodegeneration. Mol. Ther 10, 507–524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes M, et al. , 1995. Induction of midbrain dopaminergic neurons by Sonic hedgehog. Neuron. 15, 35–44. [DOI] [PubMed] [Google Scholar]
- Ilzecka J, et al. , 2002. Transforming growth factor-Beta 1 (tgf-Beta 1) in patients with amyotrophic lateral sclerosis. Cytokine. 20, 239–243. [DOI] [PubMed] [Google Scholar]
- Imai Y, et al. , 2015. The Parkinson’s disease-associated protein kinase LRRK2 modulates notch signaling through the endosomal pathway. PLoS Genet. 11, e1005503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, et al. , 2020. Wnt signaling pathway dysregulation in the aging brain: lessons from the octodon degus. Front. Cell Dev. Biol 8, 734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenks AD, et al. , 2018. Primary cilia mediate diverse kinase inhibitor resistance mechanisms in cancer. Cell Rep. 23, 3042–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia L, et al. , 2019. Restoring Wnt/beta-catenin signaling is a promising therapeutic strategy for Alzheimer’s disease. Mol. Brain 12, 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joe EH, et al. , 2018. Astrocytes, microglia, and Parkinson’s disease. Exp Neurobiol. 27, 77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagey MH, He X, 2017. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol 174, 4637–4650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalia LV, Lang AE, 2016. Parkinson disease in 2015: evolving basic, pathological and clinical concepts in PD. Nat. Rev. Neurol 12, 65–66. [DOI] [PubMed] [Google Scholar]
- Kandasamy M, et al. , 2014. TGF-beta signalling in the adult neurogenic niche promotes stem cell quiescence as well as generation of new neurons. J. Cell. Mol. Med 18, 1444–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ki SM, et al. , 2021. Primary cilia in glial cells: an oasis in the journey to overcoming neurodegenerative diseases. Front. Neurosci 15, 736888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilimann I, et al. , 2014. Subregional basal forebrain atrophy in Alzheimer’s disease: a multicenter study. J. Alzheimers Dis 40, 687–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SD, et al. , 2018. Parkinson disease. Handb. Clin. Neurol 159, 173–193. [DOI] [PubMed] [Google Scholar]
- King A, et al. , 2020. The neuropathological diagnosis of Alzheimer’s disease-the challenges of pathological mimics and concomitant pathology. Brain Sci. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R, Goate A, 2000. A common enzyme connects notch signaling and Alzheimer’s disease. Genes Dev. 14, 2799–2806. [DOI] [PubMed] [Google Scholar]
- Krishnamurthi RV, et al. , 2013. Global and regional burden of first-ever ischaemic and haemorrhagic stroke during 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet Glob. Health 1, e259–e281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumamoto N, et al. , 2012. A role for primary cilia in glutamatergic synaptic integration of adult-born neurons. Nat. Neurosci 15 (399–405), S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, et al. , 2007. Directed differentiation and transplantation of human embryonic stem cell-derived motoneurons. Stem Cells 25, 1931–1939. [DOI] [PubMed] [Google Scholar]
- Lee HJ, et al. , 2014. Human motor neurons generated from neural stem cells delay clinical onset and prolong life in ALS mouse model. PLoS One 9, e97518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis KE, Eisen JS, 2001. Hedgehog signaling is required for primary motoneuron induction in zebrafish. Development. 128, 3485–3495. [DOI] [PubMed] [Google Scholar]
- Li A, et al. , 2011. Ciliary transition zone activation of phosphorylated Tctex-1 controls ciliary resorption, S-phase entry and fate of neural progenitors. Nat. Cell Biol 13, 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, et al. , 2013. Expression of Wnt5a and its receptor Fzd2 is changed in the spinal cord of adult amyotrophic lateral sclerosis transgenic mice. Int. J. Clin. Exp. Pathol 6, 1245–1260. [PMC free article] [PubMed] [Google Scholar]
- Li J, et al. , 2019. Curcumin promotes proliferation of adult neural stem cells and the birth of neurons in Alzheimer’s disease mice via notch signaling pathway. Cell Rep. 21, 152–161. [DOI] [PubMed] [Google Scholar]
- Liegeois S, et al. , 2006. The V0-ATPase mediates apical secretion of exosomes containing Hedgehog-related proteins in Caenorhabditis elegans. J. Cell Biol 173, 949–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifshitz V, et al. , 2013. Scavenger receptor A deficiency accelerates cerebrovascular amyloidosis in an animal model. J. Mol. Neurosci 50, 198–203. [DOI] [PubMed] [Google Scholar]
- Lippa CF, et al. , 1998. TGF-beta receptors-I and -II immunoexpression in Alzheimer’s disease: a comparison with aging and progressive supranuclear palsy. Neurobiol. Aging 19, 527–533. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. , 2020a. Activation of the notch signaling pathway and cellular localization of notch signaling molecules in the spinal cord of SOD1-G93A ALS model mice. Neuroscience. 432, 84–93. [DOI] [PubMed] [Google Scholar]
- Liu D, et al. , 2020b. Purmorphamine attenuates neuro-inflammation and synaptic impairments after hypoxic-ischemic injury in neonatal mice via Shh signaling. Front. Pharmacol 11, 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Otin C, et al. , 2013. The hallmarks of aging. Cell. 153, 1194–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou Z, et al. , 2018. Upregulation of NOX2 and NOX4 mediated by TGF-beta signaling pathway exacerbates cerebral ischemia/reperfusion oxidative stress injury. Cell. Physiol. Biochem 46, 2103–2113. [DOI] [PubMed] [Google Scholar]
- Ma X, et al. , 2011. Adenylyl cyclase type 3, a marker of primary cilia, is reduced in primary cell culture and in lumbar spinal cord in situ in G93A SOD1 mice. BMC Neurosci. 12, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, et al. , 2013. Trophic and proliferative effects of Shh on motor neurons in embryonic spinal cord culture from wildtype and G93A SOD1 mice. BMC Neurosci. 14, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, et al. , 2017. Crosstalk between Notch and Sonic hedgehog signaling in a mouse model of amyotrophic lateral sclerosis. Neuroreport. 28, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma R, et al. , 2021. Astrocyte-derived extracellular vesicle-mediated activation of primary ciliary signaling contributes to the development of morphine tolerance. Biol. Psychiatry 90 (8), P575–P585. [DOI] [PubMed] [Google Scholar]
- Marathe S, et al. , 2017. Jagged1 is altered in Alzheimer’s disease and regulates spatial memory processing. Front. Cell. Neurosci 11, 220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetti B, 2018. Wnt/beta-catenin signaling pathway governs a full program for dopaminergic neuron survival, neurorescue and regeneration in the MPTP mouse model of Parkinson’s disease. Int. J. Mol. Sci 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, et al. , 2001. Functional role of TGF beta in Alzheimer’s disease microvascular injury: lessons from transgenic mice. Neurochem. Int 39, 393–400. [DOI] [PubMed] [Google Scholar]
- Mastroiacovo F, et al. , 2009. Induction of the Wnt antagonist, Dickkopf-1, contributes to the development of neuronal death in models of brain focal ischemia. J. Cereb. Blood Flow Metab 29, 264–276. [DOI] [PubMed] [Google Scholar]
- Mattson MP, et al. , 2004. A neural signaling triumvirate that influences ageing and age-related disease: insulin/IGF-1, BDNF and serotonin. Ageing Res. Rev 3, 445–464. [DOI] [PubMed] [Google Scholar]
- McLoon LK, et al. , 2014. Wnt and extraocular muscle sparing in amyotrophic lateral sclerosis. Invest. Ophthalmol. Vis. Sci 55, 5482–5496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi K, et al. , 2014. Lack of dopaminergic inputs elongates the primary cilia of striatal neurons. PLoS One 9, e97918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M, et al. , 1995. Transforming growth factor-beta 1 levels are elevated in the striatum and in ventricular cerebrospinal fluid in Parkinson’s disease. Neurosci. Lett 193, 129–132. [DOI] [PubMed] [Google Scholar]
- Mohapatra B, et al. , 2013. Protein tyrosine kinase regulation by ubiquitination: critical roles of Cbl-family ubiquitin ligases. Biochim. Biophys. Acta 1833, 122–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morelli A, et al. , 2017. Young human cholinergic neurons respond to physiological regulators and improve cognitive symptoms in an animal model of Alzheimer’s disease. Front. Cell. Neurosci 11, 339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison BM, et al. , 2009. A soluble activin type IIB receptor improves function in a mouse model of amyotrophic lateral sclerosis. Exp. Neurol 217, 258–268. [DOI] [PubMed] [Google Scholar]
- Murphy PA, et al. , 2008. Endothelial Notch4 signaling induces hallmarks of brain arteriovenous malformations in mice. Proc. Natl. Acad. Sci. U. S. A 105, 10901–10906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, et al. , 2008. Phosphorylated Smad2/3 immunoreactivity in sporadic and familial amyotrophic lateral sclerosis and its mouse model. Acta Neuropathol. 115, 327–334. [DOI] [PubMed] [Google Scholar]
- Nguyen APT, et al. , 2020. Dopaminergic neurodegeneration induced by Parkinson’s disease-linked G2019S LRRK2 is dependent on kinase and GTPase activity. Proc. Natl. Acad. Sci. U. S. A 117, 17296–17307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ocbina PJ, et al. , 2011. Complex interactions between genes controlling trafficking in primary cilia. Nat. Genet 43, 547–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Katsanis N, 2013. Context-dependent regulation of Wnt signaling through the primary cilium. J. Am. Soc. Nephrol 24, 10–18. [DOI] [PubMed] [Google Scholar]
- Park SM, et al. , 2019. Roles of primary cilia in the developing brain. Front. Cell. Neurosci 13, 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MH, et al. , 2020. Potential therapeutic target for aging and age-related neurodegenerative diseases: the role of acid sphingomyelinase. Exp. Mol. Med 52, 380–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters S, et al. , 2017. The TGF-beta system as a potential pathogenic player in disease modulation of amyotrophic lateral sclerosis. Front. Neurol 8, 669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson R, Turnbull J, 2012. Sonic hedgehog is cytoprotective against oxidative challenge in a cellular model of amyotrophic lateral sclerosis. J. Mol. Neurosci 47, 31–41. [DOI] [PubMed] [Google Scholar]
- Pinto C, et al. , 2013. Characterization of Wnt/beta-catenin and BMP/Smad signaling pathways in an in vitro model of amyotrophic lateral sclerosis. Front. Cell. Neurosci 7, 239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purro SA, et al. , 2014. Dysfunction of Wnt signaling and synaptic disassembly in neurodegenerative diseases. J. Mol. Cell Biol 6, 75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi L, et al. , 2017. Lithium chloride promotes neuronal differentiation of rat neural stem cells and enhances neural regeneration in Parkinson’s disease model. Cytotechnology. 69, 277–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M, et al. , 2012. Intraperitoneal injection of JNK-specific inhibitor SP600125 inhibits the expression of presenilin-1 and Notch signaling in mouse brain without induction of apoptosis. Brain Res. 1448, 117–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reilly JO, et al. , 2002. Cooperative effects of Sonic Hedgehog and NGF on basal forebrain cholinergic neurons. Mol. Cell. Neurosci 19, 88–96. [DOI] [PubMed] [Google Scholar]
- Riise J, et al. , 2015. Aberrant Wnt signaling pathway in medial temporal lobe structures of Alzheimer’s disease. J. Neural Transm. (Vienna) 122, 1303–1318. [DOI] [PubMed] [Google Scholar]
- Rimkus TK, et al. , 2016. Targeting the sonic hedgehog signaling pathway: review of smoothened and gli inhibitors. Cancers (Basel). 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera DS, et al. , 2016. Andrographolide recovers cognitive impairment in a natural model of Alzheimer’s disease (Octodon degus). Neurobiol. Aging 46, 204–220. [DOI] [PubMed] [Google Scholar]
- Robbins PD, 2017. Extracellular vesicles and aging. Stem Cell Investig. 4, 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez JJ, et al. , 2008. Impaired adult neurogenesis in the dentate gyrus of a triple transgenic mouse model of Alzheimer’s disease. PLoS One 3, e2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, et al. , 2007. Patched1 regulates hedgehog signaling at the primary cilium. Science. 317, 372–376. [DOI] [PubMed] [Google Scholar]
- Rojas P, et al. , 2020. Amyotrophic lateral sclerosis: a neurodegenerative motor neuron disease with ocular involvement. Front. Neurosci 14, 566858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncarati R, et al. , 2002. The gamma-secretase-generated intracellular domain of beta-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc. Natl. Acad. Sci. U. S. A 99, 7102–7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez A, et al. , 2021. Astrocytes, a promising opportunity to control the progress of Parkinson’s disease. Biomedicines 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Duffhues G, et al. , 2015. SLUG is expressed in endothelial cells lacking primary cilia to promote cellular calcification. Arterioscler. Thromb. Vasc. Biol 35, 616–627. [DOI] [PubMed] [Google Scholar]
- Satir P, Christensen ST, 2007. Overview of structure and function of mammalian cilia. Annu. Rev. Physiol 69, 377–400. [DOI] [PubMed] [Google Scholar]
- Scheltens P, et al. , 2016. Alzheimer’s disease. Lancet. 388, 505–517. [DOI] [PubMed] [Google Scholar]
- Schmid FM, et al. , 2018. IFT20 modulates ciliary PDGFRalpha signaling by regulating the stability of Cbl E3 ubiquitin ligases. J. Cell Biol 217, 151–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider L, et al. , 2005. PDGFRalphaalpha signaling is regulated through the primary cilium in fibroblasts. Curr. Biol 15, 1861–1866. [DOI] [PubMed] [Google Scholar]
- Seifert-Held T, et al. , 2011. Circulating Dickkopf-1 in acute ischemic stroke and clinically stable cerebrovascular disease. Atherosclerosis. 218, 233–237. [DOI] [PubMed] [Google Scholar]
- Serrano FG, et al. , 2014. Andrographolide reduces cognitive impairment in young and mature AbetaPPswe/PS-1 mice. Mol. Neurodegener 9, 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada IS, et al. , 2011. Proliferating reactive astrocytes are regulated by Notch-1 in the peri-infarct area after stroke. Stroke. 42, 3231–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shruster A, et al. , 2012. Wnt signaling enhances neurogenesis and improves neurological function after focal ischemic injury. PLoS One 7, e40843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soleimani M, Ghasemi N, 2017. Lithium chloride can induce differentiation of human immortalized RenVm cells into dopaminergic neurons. Avicenna J. Med. Biotechnol 9, 176–180. [PMC free article] [PubMed] [Google Scholar]
- Stasiulewicz M, et al. , 2015. A conserved role for Notch signaling in priming the cellular response to Shh through ciliary localisation of the key Shh transducer Smo. Development. 142, 2291–2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steger M, et al. , 2017. Systematic proteomic analysis of LRRK2-mediated Rab GTPase phosphorylation establishes a connection to ciliogenesis. Elife. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephano F, et al. , 2018. Impaired Wnt signaling in dopamine containing neurons is associated with pathogenesis in a rotenone triggered Drosophila Parkinson’s disease model. Sci. Rep 8, 2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun F, et al. , 2013. Notch1 signaling modulates neuronal progenitor activity in the subventricular zone in response to aging and focal ischemia. Aging Cell 12, 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FL, et al. , 2014. Promoting neurogenesis via Wnt/beta-catenin signaling pathway accounts for the neurorestorative effects of morroniside against cerebral ischemia injury. Eur. J. Pharmacol 738, 214–221. [DOI] [PubMed] [Google Scholar]
- Tanabe Y, et al. , 1995. Induction of motor neurons by Sonic hedgehog is independent of floor plate differentiation. Curr. Biol 5, 651–658. [DOI] [PubMed] [Google Scholar]
- Tapia-Rojas C, Inestrosa NC, 2018. Wnt signaling loss accelerates the appearance of neuropathological hallmarks of Alzheimer’s disease in J20-APP transgenic and wild-type mice. J. Neurochem 144, 443–465. [DOI] [PubMed] [Google Scholar]
- Tesseur I, et al. , 2017. Deficiency in neuronal TGF-beta signaling leads to nigrostriatal degeneration and activation of TGF-beta signaling protects against MPTP neurotoxicity in mice. J. Neurosci 37, 4584–4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tirou L, et al. , 2021. Sonic Hedgehog receptor Patched deficiency in astrocytes enhances glucose metabolism in mice. Mol. Metab 47, 101172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trazzi S, et al. , 2011. APP-dependent up-regulation of Ptch1 underlies proliferation impairment of neural precursors in Down syndrome. Hum. Mol. Genet 20, 1560–1573. [DOI] [PubMed] [Google Scholar]
- Trojanowski JQ, Lee VM, 1998. Aggregation of neurofilament and alpha-synuclein proteins in Lewy bodies: implications for the pathogenesis of Parkinson disease and Lewy body dementia. Arch. Neurol 55, 151–152. [DOI] [PubMed] [Google Scholar]
- Tsuboi K, Shults CW, 2002. Intrastriatal injection of sonic hedgehog reduces behavioral impairment in a rat model of Parkinson’s disease. Exp. Neurol 173, 95–104. [DOI] [PubMed] [Google Scholar]
- Vargas JY, et al. , 2015. WASP-1, a canonical Wnt signaling potentiator, rescues hippocampal synaptic impairments induced by Abeta oligomers. Exp. Neurol 264, 14–25. [DOI] [PubMed] [Google Scholar]
- Vawter MP, et al. , 1996. TGFbeta1 and TGFbeta2 concentrations are elevated in Parkinson’s disease in ventricular cerebrospinal fluid. Exp. Neurol 142, 313–322. [DOI] [PubMed] [Google Scholar]
- Veland IR, et al. , 2009. Primary cilia and signaling pathways in mammalian development, health and disease. Nephron. Physiol 111, p39–p53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh D, 2017. Primary cilia. J. Oral. Maxillofac. Pathol 21, 8–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A, et al. , 2010. Astrocytes in Alzheimer’s disease. Neurotherapeutics. 7, 399–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent AJ, et al. , 2010. Astrocytes in Alzheimer’s disease: emerging roles in calcium dysregulation and synaptic plasticity. J. Alzheimers Dis 22, 699–714. [DOI] [PubMed] [Google Scholar]
- von Grabowiecki Y, et al. , 2015. Regulation of a Notch3-Hes1 pathway and protective effect by a tocopherol-omega alkanol chain derivative in muscle atrophy. J. Pharmacol. Exp. Ther 352, 23–32. [DOI] [PubMed] [Google Scholar]
- Vorobyeva AG, Saunders AJ, 2018. Amyloid-beta interrupts canonical Sonic hedgehog signaling by distorting primary cilia structure. Cilia. 7, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas N, et al. , 2014. Vertebrate Hedgehog is secreted on two types of extracellular vesicles with different signaling properties. Sci. Rep 4, 7357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, et al. , 2009. The Notch pathway mediates expansion of a progenitor pool and neuronal differentiation in adult neural progenitor cells after stroke. Neuroscience. 158, 1356–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, et al. , 2013. Role of Wnt1 and Fzd1 in the spinal cord pathogenesis of amyotrophic lateral sclerosis-transgenic mice. Biotechnol. Lett 35, 1199–1207. [DOI] [PubMed] [Google Scholar]
- Wang Y, et al. , 2019. Osthole alleviates MPTP-induced Parkinson’s disease mice by suppressing Notch signaling pathway. Int. J. Neurosci 129, 833–841. [DOI] [PubMed] [Google Scholar]
- Wei ZZ, et al. , 2018. Neuroprotective and regenerative roles of intranasal Wnt-3a administration after focal ischemic stroke in mice. J. Cereb. Blood Flow Metab 38, 404–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway G, et al. , 2018. Signaling through the primary cilium. Front. Cell Dev. Biol 6, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willaredt MA, et al. , 2008. A crucial role for primary cilia in cortical morphogenesis. J. Neurosci 28, 12887–12900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo HN, et al. , 2009. Alzheimer’s disease and Notch signaling. Biochem. Biophys. Res. Commun 390, 1093–1097. [DOI] [PubMed] [Google Scholar]
- Wu L, et al. , 2020. Circular RNA circCCDC9 alleviates ischaemic stroke ischaemia/reperfusion injury via the Notch pathway. J. Cell. Mol. Med 24 (24), 14152–14159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, et al. , 2021. The crosstalk between the Notch, Wnt, and SHH signaling pathways in regulating the proliferation and regeneration of sensory progenitor cells in the mouse cochlea. Cell Tissue Res. 386, 281–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyss-Coray T, 2006. Tgf-Beta pathway as a potential target in neurodegeneration and Alzheimer’s. Curr. Alzheimer Res 3, 191–195. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, et al. , 1997. Amyloidogenic role of cytokine TGF-beta1 in transgenic mice and in Alzheimer’s disease. Nature. 389, 603–606. [DOI] [PubMed] [Google Scholar]
- Wyss-Coray T, et al. , 2001. TGF-beta1 promotes microglial amyloid-beta clearance and reduces plaque burden in transgenic mice. Nat. Med 7, 612–618. [DOI] [PubMed] [Google Scholar]
- Xu Y, et al. , 2016. Cornin increases angiogenesis and improves functional recovery after stroke via the Ang1/Tie2 axis and the Wnt/beta-catenin pathway. Arch. Pharm. Res 39, 133–142. [DOI] [PubMed] [Google Scholar]
- Yankner BA, et al. , 2008. The aging brain. Annu. Rev. Pathol 3, 41–66. [DOI] [PubMed] [Google Scholar]
- Yu L, et al. , 2013. Wnt Signaling is altered by spinal cord neuronal dysfunction in amyotrophic lateral sclerosis transgenic mice. Neurochem. Res 38, 1904–1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zacharek A, et al. , 2009. Simvastatin increases notch signaling activity and promotes arteriogenesis after stroke. Stroke. 40, 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, et al. , 2009. Bone marrow stromal cells increase oligodendrogenesis after stroke. J. Cereb. Blood Flow Metab 29, 1166–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Rempe DA, 2010. Targeting astrocytes for stroke therapy. Neurotherapeutics. 7, 439–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, et al. , 2020. JLX001 attenuates blood-brain barrier dysfunction in MCAO/R rats via activating the Wnt/beta-catenin signaling pathway. Life Sci. 118221. [DOI] [PubMed] [Google Scholar]
- Zhou Q, et al. , 2015. Inhibition of c-Jun N-terminal kinase activation reverses Alzheimer disease phenotypes in APPswe/PS1dE9 mice. Ann. Neurol 77, 637–654. [DOI] [PubMed] [Google Scholar]


