Abstract
Background
Childhood tuberculosis (TB) remains underdiagnosed. The novel lateral flow FujiLAM assay detects lipoarabinomannan (LAM) in urine, but data on performance in children remain limited.
Methods
We conducted a systematic review assessing the diagnostic performance of FujiLAM for diagnosing paediatric TB. The last search was conducted in November 2021.
Results
We included three studies with data from 698 children for FujiLAM. For FujiLAM, sensitivity using a microbiological reference standard were 60% (95% CI 15 to 95), 42% (95% CI 31 to 53) and 63% (95% CI 50 to 75), respectively. Specificity was 93% (95% CI 85 to 98), 92% (95% CI 85 to 96) and 84% (95% CI 80 to 88). Using a composite reference standard, sensitivity was 11% (95% CI 4 to 22), 27% (95% CI 20 to 34) and 33% (95% CI 26 to 40), and specificity was 92% (95% CI 73 to 99), 97% (95% CI 87 to 100) and 85% (95% CI 79 to 89). Subgroup analyses for sensitivity of FujiLAM in children living with HIV (CLHIV) compared with those who were negative for HIV infection were inconsistent across studies. Among CLHIV, sensitivity appeared higher in those with greater immunosuppression, although wide CIs limit the interpretation of observed differences. Meta-analysis was not performed due to considerable study heterogeneity.
Conclusion
The high specificity of FujiLAM demonstrates its potential as a point-of-care (POC) rule-in test for diagnosing paediatric TB. As an instrument-free POC test that uses an easy-to-obtain specimen, FujiLAM could significantly improve TB diagnosis in children in low-resource settings, however the small number of studies available highlight that further data are needed. Key priorities to be addressed in forthcoming paediatric evaluations include prospective head-to-head comparisons with AlereLAM using fresh specimens, specific subgroup analysis in CLHIV and extrapulmonary disease and studies in different geographical locations.
CRD42021270761.
Keywords: epidemiology, statistics, microbiology
What is already known on this topic
Despite recent advances, paediatric tuberculosis (TB) remains difficult to diagnose and accurate point-of-care tests that use easily obtainable non-sputum specimens are urgently needed.
Lateral flow tests detecting urine lipoarabinomannan (LAM), including the original AlereLAM and the recently developed FujiLAM, could improve diagnosis in children in low-resource settings.
FujiLAM’s analytic sensitivity for the diagnosis of pulmonary TB has been observed to be higher compared to AlereLAM in adults.
What this study adds
Using a microbiological reference standard, the sensitivity of FujiLAM for diagnosing paediatric TB ranged from 42% to 63%, whereas specificity was higher, ranging from 84% to 93%.
Gaps in studies to be prioritised in forthcoming evaluations include prospective testing of fresh specimens, subgroup analyses for children living with HIV and direct comparison with AlereLAM.
How this study might affect research, practice or policy
While more paediatric studies are needed, high specificity and use of an easy-to-obtain specimen indicates that FujiLAM could be a useful rule-in test for TB.
Introduction
Childhood tuberculosis (TB) is a major contributor to morbidity and mortality worldwide.1 Children below 5 years are disproportionally affected in case load and mortality, contributing to approximately 50% of all paediatric TB cases2 and 80% of deaths.2 3 The burden and mortality of paediatric TB is likely underestimated, as confirmation of disease remains challenging. There is an unmet need for accurate and easy-to-use diagnostic tests for children.
The WHO has defined target product profiles (TPP) for new non-sputum-based point-of-care (POC) diagnostics for TB and their use in children.4 Promising candidates include lateral flow assays detecting lipoarabinomannan (LAM), a glycolipid found in the mycobacterial cell-wall, secreted in urine. The first commercially available test was the Alere Determine TB LAM Ag (AlereLAM; Abbott, Palatine, IL, USA), which is the only instrument-free POC LAM test recommended by the WHO.5 6 According to a systematic review, pooled sensitivity of the AlereLAM is 42% in adults,7 increasing to 54% in PLHIV with CD4 ≤100 cells/µL.7 8 Recently, Fujifilm developed the Fujifilm SILVAMP TB LAM assay (FujiLAM; Fujifilm, Tokyo, Japan), a novel test detecting LAM in urine using high affinity monoclonal antibodies and silver amplification.9 10 Initial studies in hospitalised adults with HIV showed a higher diagnostic sensitivity of 70% for TB compared with AlereLAM.9 A recent modelling study also suggested that conducting FujiLAM in adults presenting with TB symptoms averted 30% of TB deaths and 18% of incident cases between 2020 and 2035.11
Compared with the increasing number of publications in adults,5 8 12–15 few studies have explored the performance of FujiLAM in children. In children, diagnostic yield, which represents both the diagnostic accuracy of a test and feasibility of obtaining a specimen,16 is improved by the availability of a specimen such as urine, compared with sputum. FujiLAM could therefore have a positive impact to reduce the burden of childhood TB. Here, we conducted a systematic review on the diagnostic performance of FujiLAM in children for diagnosing TB.
Methods
Reporting was according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.17
Search strategy and study selection
We identified studies via PubMed and EMBASE and registration of past and ongoing studies (clinicaltrials.gov, WHO trial registry). Additionally, we consulted experts in TB diagnostics to identify relevant publications. There were no restrictions on language or time of publication. The full-search strategy incorporated terms (text words, keywords and medical subject headings) related to LAM, TB and children, and is presented in the online supplemental material. The last search was conducted on 10 November 2021.
bmjpo-2022-001447supp001.pdf (42KB, pdf)
Original studies that reported diagnostic accuracy estimations on the performance of FujiLAM in children (defined as less than 18 years) for TB were included. We excluded animal studies, conference proceedings, editorials and reviews. The eligibility assessment was performed by two investigators (LO and NK), who independently screened titles and abstracts followed by full-text review. Any disagreement was resolved through discussion with a third reviewer.
Risk of bias assessment
Two independent investigators (LO and NK) assessed the quality of included studies using the Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) framework,18 all standard items were applied.19 Consensus was achieved through discussion and consultation with a third reviewer if necessary. RevMan (V.5, The Cochrane Collaboration, 2020)20 was used for visualisation.
Data collection
The following information was extracted from the original publications by LO and NK independently with any discrepancies discussed with a third reviewer:
Characteristics of cohort (including age, clinical presentation, country of origin and HIV status).
Inclusion and exclusion criteria.
Reference standards
Diagnostic accuracy measures
Summary measures and data analysis
The outcome measures were sensitivity and specificity of FujiLAM to diagnose active TB in children, using a microbiological reference standard (MRS; culture and/or WHO-endorsed nucleic acid amplification tests—NAAT) or a composite reference standard (CRS). Sensitivity was defined as probability of a positive test in diseased children. Specificity represented the probability of a negative test result when the disease was absent. Point estimates and CIs were calculated using the raw data provided by the original publication with the statistical software of RevMan (V.5, The Cochrane Collaboration, 2020).20
Patient and public involvement
Being a systematic review, this research was done without patient or public involvement. As a secondary analysis, no ethical approval was sought.
Results
Study results
One hundred and forty-nine unique records were identified from which 24 full texts were reviewed for eligibility and three studies met inclusion criteria (online supplemental figure 1). No further registered trials or publications on preprint servers were identified. Table 1 shows the study characteristics. The clinical settings differed; two studies were conducted in sub-Saharan Africa and one in Haiti. Studies also varied in the healthcare level for recruitment and proportion of children with microbiologically confirmed TB. In all studies, enrolment was prospective, but FujiLAM was evaluated on biobanked samples. Due to study heterogeneity and the small number of studies, a meta-analysis was not done.
Table 1.
Cohort characteristics
| Nicol et al | Nkereuwem et al | Barrio et al | ||
| Cohort size | 241 | 415 | 79 | |
| Study design | Prospective enrolment Plus enrichment of CLHIV |
Prospective enrolment | Prospective enrolment Plus control cohort |
|
| Index test | Comparator | AlereLAM | AlereLAM | None |
| Sample storage | Yes, −80°C | Yes, −80°C | Yes, −20°C | |
| Country | South Africa | Gambia, Mali, Nigeria, Tanzania | Haiti | |
| Healthcare level of recruitment of study participants | Tertiary hospital | Mixed (community, tertiary hospital, urban comprehensive healthcare) | Reference hospital | |
| Age in months (median) Median (IQR) |
45.2 (21.2–88.8) |
67.2 (27.6–111.6) |
76 (58–121) |
|
| Age categories | <5 years | 118 (58%) | 194 (47%) | 24 (30%) |
| ≥5 years | 86 (42%) | 221 (53%) | 55 (70%) | |
| Male sex | 111 (54%) | 225 (54%) | 51 (65%) | |
| TB status | Confirmed TB | 84 (41%) | 63 (15%) | 5 |
| Unconfirmed TB | 81 (40%) | 113 (27%) | 50 | |
| Unlikely TB | 39 (19%) | 239 (58%) | 24 | |
| HIV status | HIV infected | 40 (20%) | 61 (15%) | Excluded |
| CD4 cells/uL median (IQR) | 552 (206–849) | – | – | |
| Malnutrition | Stunted | 73 (40%) | 134 (32%) | 12 (21%) |
CLHIV, children living with HIV; LAM, lipoarabinomannan; TB, tuberculosis.
bmjpo-2022-001447supp002.pdf (40.8KB, pdf)
Quality
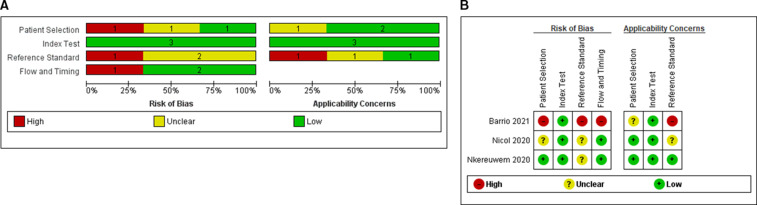
Figure 1 summarises the risk of bias and applicability of included studies, there were no disagreements between reviewers. Regarding patient selection, one study enriched their cohort by specifically including known microbiologically confirmed children living with HIV (CLHIV); therefore, risk of bias was deemed unclear.21 Another study had a high risk of bias because the authors did not explicitly state whether samples were taken consecutively and also recruited healthy controls, which can overestimate diagnostic performance.22 The index test domain was at low risk of bias, with all studies reporting blinded interpretation by two readers. Due to the inherent challenges of microbiological investigations in confirming TB disease in children,23–25 two studies had an unclear risk of bias for correctly classifying the target condition, despite including culture.21 26 One study was judged as having a high risk of bias as the MRS only included Xpert MTB/RIF and not culture.22 Risk of bias was low for most studies regarding patient flow except for one study in which only certain patients received the MRS. Full details of the QUADAS-2 assessment are included in online supplemental table 1.
Figure 1.
Assessment of study quality of FujiLAM paediatric studies using the QUADAS-2 framework. Risk of bias and applicability concerns graph (A) and summary (B) review authors’ judgements about each domain presented as percentages across included studies. LAM, lipoarabinomannan; QUADAS-2, Quality Assessment of Diagnostic Accuracy Studies 2.
bmjpo-2022-001447supp003.pdf (52KB, pdf)
Test accuracy
Table 2 outlines the inclusion and exclusion criteria, microbiological investigations (specimen collected and tests performed), reference standards and case definitions of studies. All studies applied an MRS, being roughly equivalent to the National Institutes of Health (NIH) case definition of ‘confirmed TB’,25 requiring microbiological confirmation of MTB, although underlying tests and testing algorithms varied between the studies. CRS were also used, with both microbiologically confirmed and clinically diagnosed TB defined as CRS positive, however, underlying clinical information varied between studies.
Table 2.
Definitions of reference standards and diagnostic classifications
| Nicol et al | Nkereuwem et al | Barrio et al | ||
| Enrolment criteria | Inclusion criteria |
|
|
|
| Exclusion criteria |
|
|
|
|
| Symptoms of TB |
|
Symptoms suggestive of pulmonary tuberculosis:
|
Not specified | |
| TB sampling and microbiological investigations | At least one induced sputum Xpert MTB/RIF or Xpert MTB/RIF Ultra and MGIT | At least one induced sputum Xpert MTB/Rif Ultra (all sites) and MGIT/LJ (not Nigerian site) | Three consecutive respiratory samples (induced or nasopharyngeal/nasogastric aspiration) Smear microscopy, Xpert MTB/RIF if positive smear microscopy OR abnormal X-ray | |
| TB case classification | Confirmed TB | Any induced sputum culture or Xpert MTB/RIF positive for Mycobacterium tuberculosis | Bacteriological confirmation of M. tuberculosis (culture, Xpert MTB/RIF assay or both) from at least one respiratory specimen | Any sputum Xpert MTB/RIF positive for M. tuberculosis |
| Unconfirmed TB | All children not defined as confirmed or unlikely TB | Bacteriological confirmation not obtained, and at least one (if TST/QFT-GIT pos) or two (if TST/QFT-GIT neg) of the following:
|
Bacteriological confirmation not obtained And positive TST/QFT-GIT and at least one of the following:
OR if TST/QFT-GIT negative at least
|
|
| Unlikely TB | All of the following:
|
Bacteriological confirmation not obtained and criteria for unconfirmed tuberculosis not met | Only evidence of M. tuberculosis infection or presented only one clinical criterion compatible with TB Controls: negative TST and QFT-GIT, and no signs or symptoms of TB | |
| Definition of reference standards | Microbiological reference standard (MRS) | Positive=confirmed TB Negative=unconfirmed and unlikely TB | Positive=confirmed TB Negative=unconfirmed and unlikely TB | Positive=confirmed TB Negative=unconfirmed and unlikely TB+controls |
| Composite reference standard (CRS) | Positive=confirmed and unconfirmed TB Negative=unlikely TB | Positive=confirmed and unconfirmed TB Negative=unlikely TB | Positive=confirmed and unconfirmed TB Negative=unlikely TB+controls |
|
FN, false negative; FP, false positives; QFT-GIT, QuantiFERON-TB Gold In-Tube; TB, tuberculosis; TN, true negatives; TP, true positives.
In total, 698 children were included in this analysis. The sensitivity and specificity of index tests across the studies are shown in table 3, table 4 and figure 2. Two studies reported on invalid results, which were excluded from final analyses. One study reported n=1 invalid result,26 another stated n=22 invalid results,21 which reduced to n=4 after re-testing.
Table 3.
Diagnostic accuracy estimates as reported by the original publications, with 95% CI
| Barrio et al | Nicol et al | Nkereuwem et al | |||||||||||||||||||||||||
| Total | TP | FP | FN | TN | Sens | Spec | Total | TP | FP | FN | TN | Sens | Spec | Total | TP | FP | FN | TN | Sens | Spec | |||||||
| (n) | (n) | (n) | (n) | (n) | % | 95% CI | % | 95% CI | (n) | (n) | (n) | (n) | (n) | % | 95% CI | % | 95% CI | (n) | (n) | (n) | (n) | (n) | % | 95% CI | % | 95% CI | |
| Overall | |||||||||||||||||||||||||||
| MRS | 79 | 3 | 5 | 2 | 69 | 60 | 17 to 93 | 95 | 73 to 100 | 204 | 35 | 10 | 49 | 110 | 41 | 32 to 52 | 92 | 85 to 95 | 415 | 40 | 55 | 23 | 297 | 65 | 44 to 85 | 84 | 77 to 89 |
| CRS | 73 | 6 | 2 | 49 | 22 | 11 | 5 to 23 | 92 | 72 to 99 | 204 | 44 | 1 | 121 | 38 | 26 | 21 to 34 | 97 | 87 to 100 | 415 | 58 | 37 | 118 | 202 | 33 | 25 to 42 | 83 | 72 to 92 |
| HIV positive | |||||||||||||||||||||||||||
| MRS | – | – | – | – | – | – | – | – | – | 40 | 15 | 1 | 10 | 14 | 60 | 41 to 77 | 93 | 70 to 99 | 61 | 8 | 11 | 7 | 35 | 55 | 29 to 82 | 76 | 62 to 87 |
| CRS | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 61 | 14 | 5 | 30 | 12 | 32 | 18.9 to 47.0 | 71 | 47 to 92 |
| HIV negative | |||||||||||||||||||||||||||
| MRS | – | – | – | – | – | – | – | – | – | 164 | 20 | 9 | 39 | 96 | 34 | 23 to 47 | 91 | 85 to 95 | 344 | 31 | 40 | 15 | 258 | 68 | 42 to 88 | 86 | 79 to 91 |
| CRS | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 344 | 43 | 28 | 86 | 187 | 33 | 24 to 44 | 86 | 76 to 92 |
| Age | <2 years | <5 years | |||||||||||||||||||||||||
| MRS | – | – | – | – | – | – | – | – | – | 59 | 9 | 7 | 13 | 30 | 41 | 23 to 61 | 81 | 66 to 91 | 194 | 16 | 35 | 10 | 133 | 62 | 37 to 86 | 79 | 69 to 86 |
| CRS | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 194 | 28 | 23 | 55 | 88 | 33 | 20 to 48 | 78 | 67 to 87 |
| Age | >2 years | <5 years | |||||||||||||||||||||||||
| MRS | – | – | – | – | – | – | – | – | – | 145 | 26 | 3 | 36 | 80 | 42 | 30 to 54 | 96 | 90 to 99 | 221 | 24 | 20 | 13 | 164 | 67 | 40 to 90 | 89 | 82 to 94 |
| CRS | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | – | 221 | 30 | 14 | 63 | 114 | 33 | 22 to 44 | 88 | 76 to 96 |
CRS, composite reference standard; MRS, microbiological reference standard.
Table 4.
Diagnostic test performance stratified by clinical case definition
| FujiLAM result | All | Confirmed TB n/N (%) | Unconfirmed TB | Unlikely TB | Controls | ||||||||||||
| Pos | Neg | Pos | Neg | Pos | Neg | Pos | Neg | ||||||||||
| Barrio | 79 | 3/5 | 60% | 2/5 | 40% | 3/50 | 6% | 47/50 | 94% | 1/4 | 25% | 3/4 | 75% | 1/20 | 5% | 19/20 | 95% |
| Nicol | 204 | 35/84 | 42% | 49/84 | 58% | 9/81 | 11% | 72/81 | 89% | 1/39 | 3% | 38/39 | 97% | – | – | – | – |
| Nkeurewen | 415 | 40/63 | 63% | 23/63 | 37% | 18/113 | 16% | 95/113 | 84% | 37/239 | 15% | 202/239 | 85% | – | – | – | – |
LAM, lipoarabinomannan; TB, tuberculosis.
Figure 2.
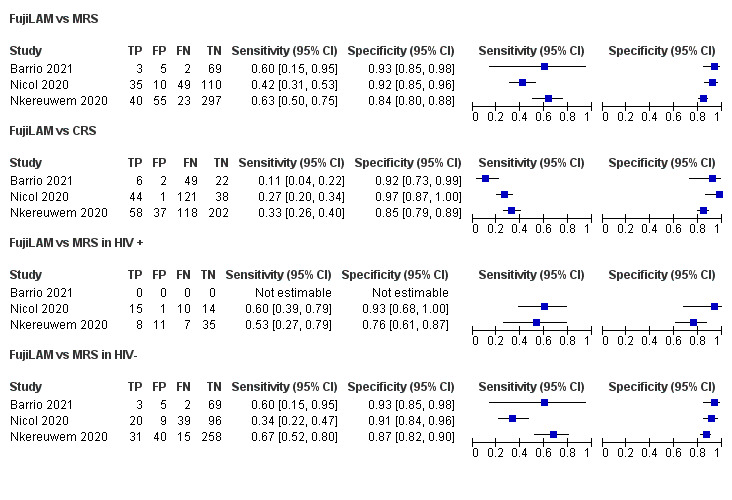
Forest plots of performance of lateral flow LAM assays against MRS and CRS. Performance estimates were calculated using the raw numbers provided in the studies and visualised using RevMan.20 CRS, composite reference standard; LAM, lipoarabinomannan; MRS, microbiological reference standard.
Microbiological reference standard (MRS)
When applying the MRS, sensitivity of FujiLAM was estimated at 60% (95% CI 15 to 95),22 42% (95% CI 31 to 53)21 and 63% (95% CI 50 to 75)26 for the three studies, respectively. CIs were wide and overlapped for all three studies. In contrast, specificity estimations were more consistent across studies, with 93% (95% CI 85 to 98),22 92% (95% CI 85 to 96)21 and 84% (95% CI 80 to 88)26 (figure 2). Two studies performed head-to-head comparisons with AlereLAM.21 26 Nkereuwem et al, found that FujiLAM had a sensitivity more than double of AlereLAM (31%, 95% CI 9 to 62), while maintaining a similarly high specificity (88%–95% CI 79 to 94).26 In the study by Nicol et al, sensitivity of AlereLAM was slightly higher (50%, 95% CI 40 to 61), although specificity was much lower (66%, 95% CI 57 to 74).21
Composite reference standard (CRS)
When applying the CRS, sensitivity of FujiLAM was 11% (95% CI −22),22 27% (95% CI 20 to 34)21 and 33% (95% CI 26 to 40).26 Sensitivity was pronouncedly lower than for MRS, with differences of 49%,22 15%21 and 30%.26 Specificity estimates were closer to MRS with 92% (95% CI 73 to 99),22 97% (95% CI 87 to 100)21 and 85% (95% CI 79 to 89).26
Results stratified by HIV
One study excluded CLHIV, therefore only two studies assessed performance in this subgroup. Data only allowed for comparison to the MRS (figure 2). For CLHIV, sensitivity of FujiLAM was 60% (95% CI 39 to 79)21 and 53% (95% CI 27 to 79).26 Specificity in CLHIV was 93% (95% CI 68 to 100)21 and 76% (95% CI 61 to 87).26 One study demonstrated test performance stratified by CD4-count, suggesting a higher sensitivity of 80% (95% CI 38 to 96) in children with CD4-counts<200/uL, compared with 55% (95% CI 34 to 74) in children with CD4-counts>200/uL.21 In contrast, specificity was higher in children with CD4-counts>200/uL (100%, 95% CI 74 to 100; compared with 75%, 95% CI 30 to 95)21 For both performance estimates, CIs were very wide and overlapped.
In HIV-negative children, estimates on FujiLAM performance differed considerably. While one study reported a lower sensitivity, 34% (95% CI 22 to 47) versus 60% (95% CI 39 to 79) in CLHIV,21 another stated a sensitivity of 67% (95% CI 52 to 80) compared with 53% (95% CI 27 to 79) in CLHIV,26 but again CIs overlapped. Specificity estimates for HIV negative children (91% (95% CI 8 to 96),21 and 87% (95% CI 82 to 90)26 were overall comparable to those in CLHIV.
Discussion
We examined the accuracy of the recently developed FujiLAM to diagnose paediatric TB across the available literature. While there are numerous studies evaluating FujiLAM for diagnosing TB in adults,5 8 12–15 there are only three paediatric publications.21 22 26 The estimated sensitivities ranged from 42% to 63%, whereas specificity was higher, ranging from 84% to 93%, when applying an MRS. Although sensitivity targets for the WHO TPP for a diagnostic (>66%) or triage (>90%) test were not met, the high specificity of FujiLAM across all studies is promising, especially given the rapidity and ease of use. Urine can mostly be obtained within the first 24 hours of admission, compared with sputum where collection is difficult, and benefits for diagnostic yield are likely.27 28 FujiLAM could have particular utility when used in combination within a diagnostic algorithm to rule-in TB in children with a high pretest probability, like CLHIV or malnourished children in high endemic settings.29
The estimated sensitivity of FujiLAM here is comparable to results from a multicentre diagnostic accuracy study in HIV-negative adults (53%).5 In this study, a strong association of sensitivity with bacterial load was observed, which likely impacts performance in children, as they generally have paucibacillary disease. Diagnostic evaluations for TB in children remain difficult, as available reference standards are imperfect. While the MRS might miss TB cases, a CRS potentially includes children not ill with TB, both hampering the interpretability of sensitivity estimates. Using an MRS will underestimate the number of children with TB and therefore overestimate the number of true negatives, as MRS can misclassify paediatric TB positive cases as negative cases. How studies define their reference standards may also contribute to hetereogeneity in accuracy estimates. While all studies applied the NIH clinical case definitions for intrathoracic TB,25 underlying clinical and microbiological investigations varied. For example, one study solely performed NAAT but not culture, and only in cases with positive smear microscopy or abnormal chest X-ray, potentially underestimating sensitivity.22 This heterogeneity of classifications outlines the necessity of applying standardised diagnostic classifications rigorously to enable cross-comparisons and meta-analyses.23–25
Patient cohorts (and therefore pretest probabilities) also differed considerably between studies. Participants were recruited from different levels of healthcare, reflecting real-life variation, which is favourable for the generalisability of results.30 However, all tests were performed on biobanked specimen in research laboratory settings. Broger and colleagues compared FujiLAM read-outs of fresh versus biobanked samples from adult patients, and while categorical agreement was high, a reduction of positive percentage agreement was observed.31 Studies using FujiLAM on fresh specimens, prospectively, and in real-life settings will need to be conducted in children.
Important subgroups for diagnostics tests include CLHIV and the very young, who are at high risk of dying from TB.2 We found that the two studies reporting FujiLAM’s accuracy in CLHIV had contrasting results, with reliable conclusions difficult to draw due to small numbers and overlapping CIs. Analyses stratified by age were only performed in the African studies, but different age cut-offs were used,21 26 hence direct comparison was not possible. Estimates in the original publications suggest a similar sensitivity, but a decrease in specificity in younger children. An explanation could be contamination in nappy-wearing children, with specificity potentially compromised due to corynebacteria, dust, soil and stool.29 32 33 Future studies should follow strict collection criteria to prevent contamination and describe them in detail. Finally, data on LAM-assays in extrapulmonary cases (EPTB) remain scarce and reported sensitivities of FujiLAM range from 47% to 94% in adults.12 Extrapulmonary manifestations are more common in children, but only one study recruited those cases and did not show subgroup analysis.22
The FujiLAM assay was developed to improve the sensitivity of AlereLAM, therefore comparison between the two is scientifically and clinically relevant. Only two studies performed paired head-to-head comparisons; whereas FujiLAM sensitivity was significantly higher compared with AlereLAM in one study using an MRS,26 AlereLAM was more sensitive in another.21 Moreover, within each study, most CIs between the two tests overlapped, suggesting a lack of evidence for test superiority. The range of estimates for sensitivity and specificity for FujiLAM in CLHIV in this systematic review against an MRS (53%–60% and 76%–93%, respectively) was also similar to estimates for AlereLAM from a Cochrane review of HIV positive children (42%–56% and 80%–95%),7 the group in whom AlereLAM is currently recommended by the WHO.34 Since these indirect comparisons between different studies can be biased by differences in population and setting, more studies that directly compare AlereLAM and FujiLAM in paired analyses are needed to understand whether FujiLAM could replace AlereLAM as a POC test in children.
All included studies, and thus this review, have limitations and data gaps. The geographical distribution of cohorts included sub-Saharan Africa and Haiti and results may not be generalisable to other regions. Important subgroup analyses could not be performed due to unavailability of data and variable application of definitions, such as test performance in EPTB, CD4-count in CLHIV (except for one study) and specific age-groups. Finally, no comment could be made on the impact of FujiLAM on clinical outcomes such as mortality reduction, as has been shown for AlereLAM in hospitalised HIV-infected adults.35
This review summarises the current evidence of FujiLAM, with the high specificity demonstrating its potential as a POC rule-in test for diagnosing paediatric TB. It reflects the current state of knowledge, highlighting that more data on FujiLAM in children are needed to understand the diagnostic value of this test in different groups at scale and suggests the priorities to be addressed in forthcoming evaluations. In particular, the need for prospective assessments that directly compare FujiLAM to AlereLAM in real-life settings, recruitment from several geographical regions and subgroup analyses focusing on CLHIV and EPTB.
Supplementary Material
Footnotes
Collaborators: MR is employed by FIND, the Global Alliance for Diagnostics. FIND is a not-for-profit NGO that collaborates in partnerships to develop, evaluate and implement new diagnostics for LMIC. FIND has product evaluation agreements with FujiFilm and several other private sector companies that design diagnostics and related products for treatment of tuberculosis and other diseases. These agreements strictly define FIND’s independence and neutrality vis-à-vis the companies whose products get evaluated and describe roles and responsibilities.
Contributors: LO and NK designed the study, wrote the study protocol, performed the study screening, selection and data collection as first reviewer; performed the statistical analysis and drafted the manuscript. RS and EB supervised the research work and was the third reviewer in case of discordance between LO and NK during study screening and selection. The manuscript was revised by MR and NH. All authors revised and approved the final version of this manuscript.
Funding: FINDs work to support development and manufacturer independent evaluations in clinical trials of the FujiFilm SILVAMP TB test is made possible through a grant from the Global Health Innovative Technology (GHIT) Fund (Japan) (grant number G2015-201).
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
All data relevant to the study are included in the article or uploaded as supplementary information.
Ethics statements
Patient consent for publication
Not required.
Ethics approval
Not applicable.
References
- 1.Dodd PJ, Yuen CM, Sismanidis C, et al. The global burden of tuberculosis mortality in children: a mathematical modelling study. Lancet Glob Health 2017;5:e898–906. 10.1016/S2214-109X(17)30289-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Organization WH . Roadmap towards ending TB in children and adolescents; 2018.
- 3.Frost WH. The age selection of mortality from tuberculosis in successive decades. 1939. Am J Epidemiol 1995;141:91–6. 10.1093/oxfordjournals.aje.a117343 [DOI] [PubMed] [Google Scholar]
- 4.Organization WH . High-priority target product profi les for new tuberculosis diagnostics: report of a consensus meeting. Geneva, Switzerland; 2014. 2014. [Google Scholar]
- 5.Broger T, Nicol MP, Sigal GB, et al. Diagnostic accuracy of 3 urine lipoarabinomannan tuberculosis assays in HIV-negative outpatients. J Clin Invest 2020;130:5756–64. 10.1172/JCI140461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Organization WH . Who consolidated guidelines on tuberculosis: module 3: diagnosis–rapid diagnostics for tuberculosis detection: web annex 4: evidence synthesis and analysis; 2020.
- 7.Bjerrum S, Schiller I, Dendukuri N, et al. Lateral flow urine lipoarabinomannan assay for detecting active tuberculosis in people living with HIV. Cochrane Database Syst Rev 2019;10:CD011420. 10.1002/14651858.CD011420.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bjerrum S, Broger T, Székely R, et al. Diagnostic accuracy of a novel and rapid lipoarabinomannan test for diagnosing tuberculosis among people with human immunodeficiency virus. Open Forum Infect Dis 2020;7:ofz530. 10.1093/ofid/ofz530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broger T, Sossen B, du Toit E, et al. Novel lipoarabinomannan point-of-care tuberculosis test for people with HIV: a diagnostic accuracy study. Lancet Infect Dis 2019;19:852–61. 10.1016/S1473-3099(19)30001-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sigal GB, Pinter A, Lowary TL, et al. A novel sensitive immunoassay targeting the 5-methylthio-d-xylofuranose-lipoarabinomannan epitope meets the who's performance target for tuberculosis diagnosis. J Clin Microbiol 2018;56. 10.1128/JCM.01338-18. [Epub ahead of print: 27 11 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ricks S, Denkinger CM, Schumacher SG, et al. The potential impact of urine-LAM diagnostics on tuberculosis incidence and mortality: a modelling analysis. PLoS Med 2020;17:e1003466. 10.1371/journal.pmed.1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kerkhoff AD, Sossen B, Schutz C, et al. Diagnostic sensitivity of SILVAMP TB-LAM (FujiLAM) point-of-care urine assay for extra-pulmonary tuberculosis in people living with HIV. Eur Respir J 2020;55:1901259. 10.1183/13993003.01259-2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Broger T, Nicol MP, Székely R, et al. Diagnostic accuracy of a novel tuberculosis point-of-care urine lipoarabinomannan assay for people living with HIV: a meta-analysis of individual in- and outpatient data. PLoS Med 2020;17:e1003113. 10.1371/journal.pmed.1003113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muyoyeta M, Kerkhoff AD, Chilukutu L, et al. Diagnostic accuracy of a novel point-of-care urine lipoarabinomannan assay for the detection of tuberculosis among adult outpatients in Zambia: a prospective cross-sectional study. Eur Respir J 2021;58. 10.1183/13993003.03999-2020. [Epub ahead of print: 18 11 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ignatius EH, Cohen KA, Bishai WR. Getting to the point in point-of-care diagnostics for tuberculosis. J Clin Invest 2020;130:5671–3. 10.1172/JCI142497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lawn SD, Kerkhoff AD, Burton R, et al. Diagnostic accuracy, incremental yield and prognostic value of determine TB-LAM for routine diagnostic testing for tuberculosis in HIV-infected patients requiring acute hospital admission in South Africa: a prospective cohort. BMC Med 2017;15:67. 10.1186/s12916-017-0822-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moher D, Altman DG, Liberati A. PRISMA statement. epidemiology. 22(1). Cambridge: Mass, 2011: 128. [DOI] [PubMed] [Google Scholar]
- 18.Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med 2011;155:529–36. 10.7326/0003-4819-155-8-201110180-00009 [DOI] [PubMed] [Google Scholar]
- 19.Whiting P, Rutjes AWS, Reitsma JB, et al. The development of QUADAS: a tool for the quality assessment of studies of diagnostic accuracy included in systematic reviews. BMC Med Res Methodol 2003;3:25. 10.1186/1471-2288-3-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Collaboration TC . Review manager (RevMan). Version 5.4.1, 2020. [Google Scholar]
- 21.Nicol MP, Schumacher SG, Workman L, et al. Accuracy of a novel urine test, Fujifilm SILVAMP TB Lam, for the diagnosis of pulmonary tuberculosis in children. Clin Infect Dis 2020. 10.1093/cid/ciaa1052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Comella-Del-Barrio P, Molina-Moya B, Gautier J, et al. Diagnostic performance of the Fujifilm SILVAMP TB-LAM in children with presumptive tuberculosis. J Clin Med 2021;10. 10.3390/jcm10091914. [Epub ahead of print: 28 04 2021]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cuevas LE, Browning R, Bossuyt P, et al. Evaluation of tuberculosis diagnostics in children: 2. methodological issues for conducting and reporting research evaluations of tuberculosis diagnostics for intrathoracic tuberculosis in children. consensus from an expert panel. J Infect Dis 2012;205 Suppl 2:S209–15. 10.1093/infdis/jir879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Graham SM, Ahmed T, Amanullah F, et al. Evaluation of tuberculosis diagnostics in children: 1. proposed clinical case definitions for classification of intrathoracic tuberculosis disease. consensus from an expert panel. J Infect Dis 2012;205 Suppl 2:S199–208. 10.1093/infdis/jis008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Graham SM, Cuevas LE, Jean-Philippe P, et al. Clinical case definitions for classification of intrathoracic tuberculosis in children: an update. Clin Infect Dis 2015;61Suppl 3:S179–87. 10.1093/cid/civ581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nkereuwem E, Togun T, Gomez MP, et al. Comparing accuracy of lipoarabinomannan urine tests for diagnosis of pulmonary tuberculosis in children from four African countries: a cross-sectional study. Lancet Infect Dis 2021;21:376–84. 10.1016/S1473-3099(20)30598-3 [DOI] [PubMed] [Google Scholar]
- 27.Gupta-Wright A, Corbett EL, van Oosterhout JJ, et al. Rapid urine-based screening for tuberculosis in HIV-positive patients admitted to hospital in Africa (stamp): a pragmatic, multicentre, parallel-group, double-blind, randomised controlled trial. Lancet 2018;392:292–301. 10.1016/S0140-6736(18)31267-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicol MP, Zar HJ. New specimens and laboratory diagnostics for childhood pulmonary TB: progress and prospects. Paediatr Respir Rev 2011;12:16–21. 10.1016/j.prrv.2010.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Marais BJ. Improved urine lipoarabinomannan (LAM) tests: the answer for child tuberculosis diagnosis? Clinical Infectious Diseases 2021;72:e289–90. 10.1093/cid/ciaa1058 [DOI] [PubMed] [Google Scholar]
- 30.Irwig L, Bossuyt P, Glasziou P, et al. Designing studies to ensure that estimates of test accuracy are transferable. BMJ 2002;324:669–71. 10.1136/bmj.324.7338.669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Broger T, Muyoyeta M, Kerkhoff AD, et al. Tuberculosis test results using fresh versus biobanked urine samples with FujiLAM. Lancet Infect Dis 2020;20:22–3. 10.1016/S1473-3099(19)30684-X [DOI] [PubMed] [Google Scholar]
- 32.Nicol MP, Allen V, Workman L, et al. Urine lipoarabinomannan testing for diagnosis of pulmonary tuberculosis in children: a prospective study. Lancet Glob Health 2014;2:e278–84. 10.1016/S2214-109X(14)70195-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kroidl I, Clowes P, Mwakyelu J, et al. Reasons for false-positive lipoarabinomannan ELISA results in a Tanzanian population. Scand J Infect Dis 2014;46:144-8. 10.3109/00365548.2013.853133 [DOI] [PubMed] [Google Scholar]
- 34.Organization WH . The use of lateral flow urine lipoarabinomannan assay (LF-LAM) for the diagnosis and screening of active tuberculosis in people living with HIV: policy guidance; 2015.
- 35.Peter JG, Zijenah LS, Chanda D, et al. Effect on mortality of point-of-care, urine-based lipoarabinomannan testing to guide tuberculosis treatment initiation in HIV-positive hospital inpatients: a pragmatic, parallel-group, multicountry, open-label, randomised controlled trial. Lancet 2016;387:1187–97. 10.1016/S0140-6736(15)01092-2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjpo-2022-001447supp001.pdf (42KB, pdf)
bmjpo-2022-001447supp002.pdf (40.8KB, pdf)
bmjpo-2022-001447supp003.pdf (52KB, pdf)
Data Availability Statement
All data relevant to the study are included in the article or uploaded as supplementary information.