Abstract
Diseases, disorders, and insults of aging are frequently studied in otherwise healthy animal models despite rampant co-morbidities and exposures among the human population. Stressor exposures can increase neuroinflammation and augment the inflammatory response following a challenge. The impact of dietary exposure on baseline neural function and behavior has gained attention; in particular, a diet high in fructose can increase activation of the hypothalamic-pituitary-adrenal axis and alter behavior. The current study considers the implications of a diet high in fructose for neuroinflammation and outcomes following the cerebrovascular challenge of stroke. Ischemic injury may come as a “second hit” to pre-existing metabolic pathology, exacerbating inflammatory and behavioral sequelae. This study assesses the neuroinflammatory consequences of a peri-adolescent high-fructose diet model and assesses the impact of diet-induced metabolic dysfunction on behavioral and neuropathological outcomes after middle cerebral artery occlusion. We demonstrate that consumption of a high-fructose diet initiated during adolescent development increases brain complement expression, elevates plasma TNFα and serum corticosterone, and promotes depressive-like behavior. Despite these adverse effects of diet exposure, peri-adolescent fructose consumption did not exacerbate neurological behaviors or lesion volume after middle cerebral artery occlusion.
Keywords: Affective-like behaviour, Complement, Fructose, Adolescence, Stroke
1. Introduction
Environmental exposures are now appreciated as critical factors in the development of later neuroimmune responses to a secondary challenge (Bekhbat and Neigh, 2018). Given the growing youth population affected by diabetes and obesity (Ogden et al., 2014; Nadeau and Dabelea, 2008), understanding the effects of metabolic pathology on neural challenges across the lifespan is of growing importance. Diet-induced metabolic pathology has been associated with changes in endothelial cell function and neuroinflammation (Rutledge and Fructose, 2007; Glushakova et al., 2008), and reductions in hippocampal vasculature have been linked to depressive behavior in a Western-diet model in female macaques (Kalidindi et al., 2017). We have previously demonstrated that a high-fructose diet consumed during adolescence results in negative metabolic outcomes in adulthood, remodeling of the hypothalamic-pituitaryadrenal (HPA) axis, and depressive- and anxiety-like behaviors (Harrell et al., 2015a). Others have additionally recapitulated these metabolic effects (Catena et al., 2003; Hwang et al., 1987; Kasim-Karakas et al., 1996), as well as effects on the HPA axis (Brindley et al., 1981; Kinote et al., 2012). However, it has yet to be considered whether a diet high in fructose during adolescent development can induce neuroinflammation in isolation or augment neuroinflammation or behavioral impairments following an known neuroinflammatory event such as ischemia.
Given that metabolic pathology is a known risk factor for post-stroke depression (Tanislav et al., 2015), it is of particular importance to study post-stroke outcomes in metabolic contexts that are of growing prevalence in the human population. Depression affects one third of stroke survivors (Hackett et al., 2005) and results in impaired recovery (Gainotti et al., 2001) and increased mortality (House et al., 2001). This post-stroke depression causes not only human suffering but also greater burden to an already over-burdened healthcare system, increasing both inpatient and outpatient stays (Ghose et al., 2005). Research on post-stroke depression provides an opportunity to alleviate this burden and shed light on the mechanisms underlying the neurobiology of depression (Whyte et al., 2004; Alexopoulos, 2006).
The underlying neurobiology of post-stroke depression differs from that of depression not associated with stroke, and is likely related to ischemia-induced injury with associated changes in endothelial function, inflammation, and neural circuitry (Whyte et al., 2004). Ischemia is a major driver of both vascular remodeling (Liu et al., 2014) and neuroinflammation (Huang et al., 2006), and ischemia-induced depressive behavior is also associated with increased neuroinflammation (Nemeth et al., 2016; Nemeth and Neigh, 2015). Furthermore, ischemic injury may come as a “second hit” to pre-existing metabolic pathologies, such as diabetes and metabolic syndrome, that are independently linked to depression (Anderson et al., 2001; Lamers et al., 2013). In this study, we hypothesized that exposure to a high-fructose diet would promote neuroinflammation and vascular remodeling, which in turn would exacerbate anxiety- and depressive-like behavior and worsen neuropathology after an ischemic event.
2. Materials and Methods:
2.1. Animal husbandry
Timed pregnant Wistar rats (n = 12) were obtained on gestational day 12 from Charles River (Wilmington, MA). Rats were housed on a 14:10 reverse light:dark cycle in a facility controlled for humidity (60%) and temperature (20 °C–23 °C). Litters were culled on postnatal day (PND) 3 to eight pups per litter and males were selected and weaned on PND 23 (n = 97). Of these animals, one cohort was used for baseline metabolic cerebrovascular assessments and is referred to as the “Baseline Cohort” (Chow n = 23, Fructose n = 26), while another two cohorts were used for assessment of outcomes after sham surgery or middle cerebral artery occlusion (MCAO) surgery (Chow-Sham = 12, Chow-MCAO = 11, Fructose-Sham = 12, Fructose-MCAO = 10), referred to as “Surgery Cohorts.” Both Baseline and Surgery cohorts were used in the assessment of diet-induced metabolic effects prior to surgery. Details of each cohort and the studies performed on samples from each cohort are available in Supplemental Table 1. Animals were reared in-house and pair-housed until the MCAO or sham surgery, at which time animals were single-housed thereafter through the end of experimentation and euthanasia. All experiments were performed in accordance with the Institutional Animal Care and Use Committee (IACUC) of Emory University and the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
2.2. Diet
Two days post-weaning, male rats were assigned to one of two diet groups: either standard chow (Chow; n = 40; Lab Diets Rodent Diet 5001), or the high-fructose diet (Fructose; n = 44; Research Diets D05111802). The fructose diet used was 55% energy fructose and the standard chow used was 0.30% energy fructose. Both diets used contained comparable levels of vitamins and minerals deemed necessary for rodent health, and were reviewed by veterinary staff and approved by IACUC. Dietary composition of this diet is described in Supplemental Table S1. Food was provided using wire cage tops with divided spaces for food pellets and a water bottle.
In these experiments, all cohorts consumed either the standard chow diet or the high-fructose diet from PND25 throughout adolescent development and into adulthood (terminal euthanasia at PND85–100). While adolescence is difficult to define precisely in rats as in humans, it is accepted that infancy and “childhood” end at weaning (PND21–23) and that adulthood begins at PND60 (McCormick and Mathews, 2007; Spear, 2000). Surgical, behavioral, and terminal endpoints were selected to allow these experiments to be performed in adulthood, given previous data demonstrating metabolic and behavior abnormalities in adulthood (Harrell et al., 2015a).
2.3. Metabolic assessments
Metabolic assessments were taken from both the Baseline cohorts the first six weeks on the diet prior to additional testing. Blood glucose was tested weekly after an overnight fast by tail prick using a Freestyle glucometer. Animal weights were also taken concurrently with glucose readings. Post-fast body weight measurement was performed to ensure consistency between measurements by avoiding temporary post-prandial weight addition that could occur in some animals if allowed to consume ad libitum. Research assistants also measured food consumption daily. Both food in the feeder and food found on the cage floor was removed and weighed. Weights of both food in the feeder and in the cage were combined and recorded to account for food spillage.
Food consumption and animal weight were used to determine caloric efficiency. For this calculation, the body mass gained per week per animal was divided by the mean weekly caloric consumption calculated per cage (of pair-housed animals) divided by two. While less precise, this type of approximation should only serve to increase variability in caloric efficiency and thus increase probability of returning a false negative result.
2.4. Corticosterone analyses
Blood was collected via tail snip from animals in the sham and MCAO Surgery Cohorts both prior to initiating behavioral testing as well as immediately after forced swim testing (described in greater detail in Section 2.10). Tail snips were performed on all animals within two minutes of handling to allow collection prior to snip-induced elevation of corticosterone. All animals were allowed to acclimate to the testing room for two hours before handling, and all collections occurred in a separate room from the testing room.
Tail vein blood was collected in uncoated tubes and spun at 5000 rcf (Eppendorf 5415R Centrifuge) for 20 min for serum collection. Serum corticosterone was measured via enzyme-linked immunosorbent assay (ELISA; sensitivity 27 pg/mL, Enzo Life Sciences, Farmingdale, NY, USA). All samples were run in duplicate and only those samples with an intra-assay coefficient of variation < 15% were included in analysis.
2.5. Whole-Transcriptome RNA-Sequencing
Our lab has previously performed whole transcriptome RNA sequencing on peri-adolescent fructose-fed rats, indicating widespread changes in the hypothalamic transcriptome (Harrell et al., 2015a). Rats used in RNA sequencing underwent the same diet and metabolic assessments as the rest of the Baseline Cohort. Details of the collection and RNA sequencing methods are described elsewhere (Harrell et al., 2015b). In brief, the hypothalamus was dissected from a subset of rats in the Baseline Cohort that were euthanized by rapid decapitation on PND80 (Chow n = 7; Fructose n = 6). The whole hypothalamus was lysed and homogenized using Trizol RNA Extraction reagent (Life Technologies, Grand Island, NY) and QiaShredder (Qiagen, Valencia, CA). RNA was extracted with an RNEasy kit from Qiagen. RNA purity and quality was assessed using an Agilent 2100 Bioanalyzer (Agilent, Santa Clara, CA). 1 µg of total RNA was used to build mRNA sequencing libraries and a single end 100 bp sequencing reaction performed on an Illumina HiSeq 1000 (Illumina, San Diego, CA) generating ∼25 million reads per sample. Raw sequence reads were mapped to the most recent RAT assembly (RGSC5.0) using the STAR aligner (Stress, 1999). Data fragments per kilobase of transcript per million mapped reads (FPKM) normalized and differential expression were examined using the cufflinks software suite (McEwen and Reagan, 2004). All data discussed in this publication have been deposited in NCBI’s Gene Expression Omnibus (Edgar et al., 2002) and are accessible through GEO Series accession number GSE56238 at http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE56238.
2.6. Quantitative real-time polymerase chain reaction
After nine weeks on diet at PND89-90, a subset of the Baseline Cohort animals (chow: n = 8; fructose: n = 10) was euthanized with Euthasol® and perfused with saline for two minutes, trunk blood was collected, and brains were flash frozen before storage at −80 °C. To determine whether the fructose diet had an effect on a complement factor expression, we used quantitative real-time polymerase chain reaction (qRT-PCR) to examine expression of complement factors C4b (C4 isoform, basic form, common to both the lectin-induced and classical complement pathways) and Cfb (Factor B, in the alternative complement pathway) in the hypothalamus and the hippocampus. These components were chosen based on their expression in RNAseq analysis in the hypothalamus in the peri-adolescent cohort and as representative of the classical and lectin (C4b) and alternative (Cfb) complement pathways.
The whole hypothalamus and the left hippocampus were lysed and homogenized using Trizol RNA Extraction reagent (Life Technologies, Grand Island, NY). RNA was then extracted with an RNEasy kit from Qiagen, and integrity was assessed with a NanoDrop 2000 spectrophotometer (ThermoScientific, Wilmington, DE). RNA was standardized based on the NanoDrop 2000 readings and then reverse-transcribed using the High Capacity RNA to cDNA kit (Life Technologies, Grand Island, NY). cDNA was quantified with the PicoGreen Assay (Invitrogen, Carlsbad, CA) and then standardized to 10 pg/µl. The following rat TaqMan Gene Expression Assays were purchased from Life Technologies (Grand Island, NY) with probes labeled with 6-FAM and MGB (non-fluorescent quencher) at the 5′ and 3′ ends, respectively: C4b (Rn01525746_g1) and Cfb (Rn01526084_g1). The following two-step RT-PCR cycling conditions were used on the 7900HT Sequence Detection System (Applied Biosystems): 50 °C (2 min), 95 °C (10 min), 40 cycles of 95 °C (15 s) and 60 °C (1 min). The housekeeping gene Hprt1 (Rn01527840_m1) was run as an endogenous control.
Relative gene expression of individual samples run in triplicate (with coefficient of variation cut-off set to 4%) was determined by the comparative ΔΔCT quantification method with fold change to standard chow. All TaqMan gene expression assays are guaranteed to have 90–100% amplification efficiency as determined by the genome-aided probe and primer design pipeline and reported in the “Amplification Efficiency of TaqMan Gene Expression Assays” Application Note 127AP05-03 from Life Technologies.
2.7. Tumor necrosis factor-α ELISA
Trunk blood collected from chow- and fructose-fed animals used for qRT-PCR analysis was collected at sacrifice into ethylenediaminetetracetic acid (EDTA) coated tubes and spun at 5000 rcf (Eppendorf 5415R Centrifuge) for 20 min for plasma collection, then plasma was stored at −80 °C. Plasma tumor necrosis factor-α (TNFα) was measured from undiluted samples (chow: n = 7; fructose: n = 9) via ELISA (Rat TNFα Quantikine ELISA, R&D Systems, Minneapolis, MN). Samples were run in duplicate, averaged, and compared to a standard curve to calculate concentration of plasma TNFα in pg/ml.
2.8. Vascular length
A subset of the Baseline Cohort (chow, n = 6; fructose, n = 6) were designated for immunohistochemical assessment of cerebrovascular remodeling, and these animals were euthanized on PND89 with Euthasol®. Animals were then perfused for two minutes with saline and perfused for ten minutes with 4% paraformaldehyde. Brains were removed and post-fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, and sectioned at 40 µm on a cryostat. Sections (5–6 sections per animal, section sampling frequency (ssf) = 1/12) encompassing the hippocampus from both chow (n = 6) and fructose (n = 6) were stained for rat endothelial cell antigen (mouse anti-RECA; MCA970R, ABD Serotec, Raleigh, NC). After blocking, sections were incubated in mouse-anti RECA (1:200) overnight at 4 °C, washed, incubated with biotinylated horse anti-mouse (1:200, BA2000, Vector Labs, Burlingame, CA), washed, incubated with streptavidin peroxidase (1:200, S2438, Sigma Aldrich, St. Louis, MO), and visualized with diaminobenzidine (SigmaFast 3,3′diaminobenzidine tablets, Sigma Aldrich, St. Louis, MO). Vascular length was assessed in the hippocampus and its subregions cornu ammonis 1 (CA1), CA2, CA3, and the dentate gyrus. All assessments were made by a research assistant blinded to treatment group using the Spaceballs probe from Stereoinvestigator (MBF Bioscience, Williston, VT) on a Nikon Eclipse 90i microscope (Melville, NY) fitted with MicroBrightField Stereoinvestigator Version 11 (MBF Bioscience, Williston, VT).
2.9. Blood-brain barrier permeability
To assess changes in blood-brain barrier (BBB) permeability, sections encompassing the entire rostro-caudal axis of the brain from Baseline Cohort brains (chow, n = 8 and fructose, n = 10) were stained for immunoglobulin G (IgG; 8–12 sections per animal, section sampling fraction (ssf) = 1/12). After blocking, sections were incubated in goat anti-rat IgG (1:1000, BA9400, Vector Labs, Burlingame, CA) overnight at 4 °C, washed, incubated with the Vectastain Elite ABC kit (Vector Labs, Burlingame, CA), and visualized with diaminobenzidine (SigmaFast 3,3′diaminobenzidine tablets, Sigma Aldrich, St. Louis, MO). Increased cerebral IgG presence indicates increased blood-brain-barrier (BBB) permeability, as endothelial cell tight junctions typically prevent transport of large molecules such as IgG across the BBB (Abbott et al., 2010). A Nikon Eclipse 90i microscope (Melville, NY) fitted with MicroBrightField Stereoinvestigator Version 11 (MBF Bioscience, Williston, VT) was used to visualize IgG. To assess permeability, a research assistant blinded to treatment group captured images of each brain section using Stereoinvestigator and calculated the percentage of the brain immunoreactive for IgG using the Gray Level Index to quantify mean optical density for each brain (ImageJ, NIH).
2.10. Surgery
In adulthood, at either PND 65–66 (Chow n = 12, Fructose n = 12) or PND 81–82 (Chow n = 12, Fructose n = 12), half of each diet group was submitted to transient (90 min) middle cerebral artery occlusion (MCAO; n = 5–6 per diet per time point) as previously described (Yousuf et al., 2014, 2015). The other half of each diet group in each cohort underwent sham surgery at the same time (n = 6 per diet per time point). Given that our lab has previously investigated the behavioral effects of eight to ten weeks of a high-fructose diet (Harrell et al., 2015a), we selected these time points so that one cohort underwent behavioral testing after eight weeks of dietary intervention, while the other underwent surgery after eight weeks of intervention with behavioral testing two weeks subsequently.
The MCAO surgery proceeded as follows: a midline incision was made on the ventral surface of the rat’s neck, and the right common carotid artery was isolated and ligated with 6.0 suture. The internal carotid and pterygopalatine arteries were occluded with a microvascular clip. A 4.0 filament was introduced through the external carotid artery into the internal carotid artery and advanced approximately 20 mm distal to the carotid bifurcation. Relative cerebral blood flow (CBF) was monitored by laser Doppler flowmetry for the entire 90 min of occlusion. After this time, the occluding filament was withdrawn to allow for reperfusion. Relative CBF was monitored before the wound was sutured and the rats were permitted to recover from anesthesia. The percent occlusion was calculated from CBF as Heartbeat and blood oxygen saturation levels were also monitored during surgery using a SurgiVet pulse oximeter (SurgiVetTM model V3304, Waukesha, WI, USA). Three rats died either during the MCAO surgery or in the post-MCAO period; one fructose-fed rat in cohort 1, and one fructose-fed and one chow-fed rat in cohort 2. This resulted in a final n as follows: Chow-Sham: n = 12, Chow-MCAO: n = 11; Fructose-Sham: n = 12; Fructose-MCAO n = 10.
2.11. Neurologic behavior assessment
Prior to surgery (beginning PND58 or PND68), rats were trained on four different tests to assess neurologic behavior: open field during the dark cycle to measure spontaneous locomotor activity (Whishaw IQ and Kolb B. Behavior of the Laboratory Rat: A Handbook with Tests. Oxford University Press, 2004); sticky dot to measure somatosensory neglect (Whishaw and Kolb, 2004); grip strength to assess force capacity to pull a grid assembly (Atif et al., 2013); and rotorod testing to assess motor coordination (Atif et al., 2013). Rats were tested on each assessment prior to surgery and again 72 h post-surgery as previously described (Atif et al., 2013). Light cycle behavioral testing was performed in a room illuminated with between 100 and 200 lux, while dark cycle testing was performed in a room illuminated with < 10 lux. Brief descriptions of each test follow.
2.11.1. Dark cycle open field
Both pre- and post-injury, rats underwent a five-minute open field test under red light during their dark cycle, in which overall locomotor activity in a 75 cm × 75 cm box was measured. Behavior was recorded by a video camera that was connected to an automated behavior analysis system (Capture Star, CleverSys, Inc, Reston, VA, USA). A research assistant blinded to treatment group subsequently analyzed behavior on related automatic behavioral analysis systems (TopScan and Forced Swim, CleverSys, Inc, Reston, VA, USA).
2.11.2. Sticky dot
To determine somatosensory asymmetry, each rat received adhesive stimuli (one hemisphere of a round sticky dot of 12 mm in diameter) both pre- and post-injury attached to the distal radial aspect of the left forelimb during the animal’s dark cycle. The time taken for the animal to remove the label was recorded during a 60-sec observation period. Three trials per animal at each time-point were averaged for analysis at both time points (Whishaw and Kolb, 2004; Atif et al., 2013) .
2.11.3. Grip strength
Animals were evaluated for the degree of force necessary to make the animal release a pull grid assembly by the forepaws using a grip strength meter. Rats were acclimated to the grip strength meter pre-injury for two days, pulling the grid assembly three times each day. On the third day, rats again pulled the assembly three times, and these measures were averaged and used as baseline measures. The test was repeated three days after surgery.
2.11.4. Rotorod
Pre-injury, rats were trained on the rotorod apparatus to stay on the accelerating rod for 120 s. The animals were first habituated to the stationary rod. After habituation they were exposed to the rotating rod. The rod was started at 2 rpm and accelerated linearly to 20 rpm within 300 sec. The rats were trained for three to five days pre-surgery depending on their individual latency to stay on the accelerating rod for 120 s. A test trial was then performed, in which latency to fall off the rotorod was calculated for analysis. The test trial was repeated three times per animal. This same test was also performed three days postsurgery. The two best (largest) fall latency values a rat achieved were then averaged and used for data analysis. Rats not falling off within 5 min were given a maximum score of 300 s (Atif et al., 2013).
2.12. Affective-like behavior assessment
Affective behavior assessment was performed two weeks after surgery (beginning PND79 or PND95). This time point was selected due to prior evidence from our lab indicating expression of depressive- and affective-type manifests two-weeks following microembolism-induced ischemia (Nemeth et al., 2012). The tests used in this assessment were, in order of testing, open field during the light cycle to test locomotor activity and anxiety-like behavior (Prut and Belzung, 2003); social interaction to examine anxiety-like and anhedonic behavior (File and Hyde, 1978); the elevated plus maze to examine anxiety-like behavior (Pellow et al., 1985); and the forced swim test, originally designed to measure antidepressant efficacy, which can be used to assess depressive-like behavior (Porsolt et al., 1977).
Behavioral assessments were performed as previously described (Harrell et al., 2015a; Nemeth et al., 2014). As with neurologic testing, light cycle behavioral testing was performed in a room illuminated with between 100 and 200 lux, while dark cycle testing was performed in a room illuminated with < 10 lux. All behaviors were recorded by a video camera that was connected to an automated behavior analysis system (Capture Star, CleverSys, Inc, Reston, VA, USA). A research assistant blinded to treatment condition subsequently analyzed behavior on related automatic behavioral analysis systems (TopScan and Forced Swim, CleverSys, Inc, Reston, VA, USA). Details of individual assessments are briefly described here.
2.12.1. Light cycle open field
Rats underwent a ten-minute open field test during their light cycle, in which overall locomotor activity, activity in the periphery versus the center, and grooming behavior in a 75 cm × 75 cm box was measured over a ten-minute period. Due to video failure, the behaviors of two chow-sham animals were not analyzed for this test.
2.12.2. Social interaction
Rats next underwent social interaction testing during the light cycle. In this test, they were placed into the same arena used for open field testing, now containing a novel younger male animal, and allowed to explore for ten minutes. Latency to interact, direction of interaction (test animal with experimental animal or vice versa), and total time interacting were recorded. Due to video failure, the behaviors of one chow-sham, one chow-MCAO, and two fructose-sham animals were not analyzed for this test.
2.12.3. Elevated plus maze
An elevated plus maze test performed during the dark cycle was used to model anxiety-like behavior in the rats by measuring the time spent in open arms vs. the time spent in the closed arms (Pellow et al., 1985). The specifications for the San Diego Instruments elevated plus maze used were as follows: 43 ½″ long, 4″ wide (arm width), 19 ½″ high (open arms), and 31 ½″ high (closed arms). During testing, animals were able to freely move from open to closed arms for 5 min.
2.12.4. Forced swim test
This test, originally designed to measure antidepressant efficacy, can be used to measure depressive-like behavior and coping strategies (Porsolt et al., 1977). During the light cycle, rats were placed in a clear acrylic tank (40 cm high × 18 cm diameter) filled with room temperature water. Struggling, latency to float, and immobility were analyzed. Immobility was defined as the animal’s limbs remaining motionless for at least two seconds, and struggling was defined as the animal’s limbs in motion and its head above the surface. Immediately after the end of the 10 min test, rats were removed from the tank and tail blood was collected by tail snip of less than two mm of the tail within two minutes and without anesthesia for corticosterone analysis, described below.
2.13. Lesion volume assessment
One day after the end of behavioral testing (PND84 or PND100), animals in the surgical cohort were euthanized with Euthasol®, perfused for two minutes with saline, and then perfused for ten minutes with 4% paraformaldehyde for ten minutes. Brains were removed and post-fixed in 4% paraformaldehyde, cryoprotected in 30% sucrose, and sectioned at 40 µm on a cryostat. To assess lesion damage, sections (n = 12 per animal, ssf = 1/12) from sham (Chow, n = 6; Fructose, n = 6) and MCAO animals (Chow, n = 9, Fructose, n = 9) were stained with 0.1% cresyl violet and coverslipped with Permount (Fisher Scientific) before stereological assessment.
The Cavalieri method was used to estimate mean hemispheric and lesion volumes of both the ipsilateral (right) hemisphere as well as volume of the contralateral (left) hemisphere, considering only cystic spaces as lesions for analysis. The right and left hemispheric volumes of sham animals were calculated by the same method. All stereological assessment was performed by a blinded research assistant using the Cavalieri probe from Stereoinvestigator (MBF Bioscience, Williston, VT) on the same Nikon Eclipse 90i microscope (Melville, NY) described above.
2.14. Statistical analysis
All statistics and graphing were performed using RStudio Version 0.98.1049 and GraphPad Prism Version 6.0. Data were assessed for normality using the D’Agostino and Pearson normality test. For metabolic, RT-PCR, behavioral, and immunohistochemical analyses, student’s t-tests, Analysis of Variance (ANOVA) type II tests, and Pearson’s correlations were performed with α = 0.05. T-tests are two-tailed unless otherwise noted; a Welch’s correction was used with parametric data with non-equal variances. After ANOVA, secondary analyses for planned contrasts or Holm-Sidak post-hoc tests were performed when appropriate and are noted in the text.
For whole transcriptome RNA-sequencing, differential expression was identified as transcripts either exceeding a 1.25 or inferior to a 0.75 fold change in expression in standard-chow vs. fructose-fed rats, as described in O’Conner et al. (Stress, 1999). No statistical testing was applied at the level of individual genes in RNA-seq as this study had no a priori single-gene hypothesis and was not designed to detect statistically significant associations between single gene transcripts and diet. The list of differentially expressed transcripts was used to serve as an intermediary input for higher-order bioinformatics using a False Discovery Rate (FDR) adjusted p values (q values) < 0.05, and it was therefore imported into the Thompson Reuters MetaCore Genego software for pathway analysis. Significant enrichment in pathway map folders and pathway maps was set at a FDR of q < 0.05.
3. Results
3.1. Periadolescent high-fructose diet increases caloric efficiency and fasting blood glucose
As previously demonstrated (Harrell et al., 2015a), fructose-fed rats not exposed to surgery weighed more by nine weeks on the diet than chow-fed rats (t34 = 2.17, p = 0.04; Supplemental Fig. 1a). Caloric efficiency was calculated as mg of weight gained per kCal consumed averaged over a week for the first five weeks of the diet. Fructose-fed rats had a higher caloric efficiency than chow fed rats (F1,21 = 6.43; p = 0.02; Supplemental Fig. 1b). Fasting blood glucose was also assessed during this period, and fructose-fed rats similarly had elevated fasting blood glucose (F1,20 = 14.46; p < 0.01; Supplemental Fig. 1c).
3.2. Hypothalamic expression of classical, lectin-induced, and alternative complement pathways is significantly enriched with transcripts altered by fructose consumption
Genome-wide transcriptional profiling of hypothalamic RNA from rats fed either standard chow or a high-fructose diet throughout the peri-adolescent period identified 17,366 transcripts mapping to distinct named genes. Of these, 2,639 transcripts or 15.2% showed a ≥25% difference in fold change of average expression level. Among the differentially expressed transcripts, 908 transcripts were downregulated and 1731 transcripts were upregulated. Pathway enrichment by differentially expressed transcripts was determined using GeneGo MetaCore software suite (Thompson Reuters) with q < 0.05. Fortythree pathways were significantly enriched by differentially expressed transcripts. While multiple immune and inflammatory related pathways were significantly enriched, it was notable that the “Classical Complement Pathway”, the “Lectin Induced Complement Pathway”, and the “Alternative Complement Pathway” were significantly enriched (q < 0.05), with at least 25% of the genes in each pathway affected. Relative expression of the gene transcripts in these pathways are depicted in Fig. 1.
Fig. 1.
Hypothalamic expression of classical, lectin-induced, and alternative complement pathways is significantly enriched with transcripts altered by fructose consumption. Genome-wide transcriptional profiling of hypothalamic RNA from chow and fructose-fed rats identified forty-three pathways that were significantly enriched by differentially expressed transcripts were significantly enriched (q < 0.05), with at least 25% of the genes in each pathway affected. These pathways included the “Classical Complement Pathway”, the “Lectin Induced Complement Pathway”, and the “Alternative Complement Pathway”. Relative expression of the gene transcripts (log2(FMPK)) in these pathways are depicted.
3.3. Fructose increases expression of hippocampal and hypothalamic complement components
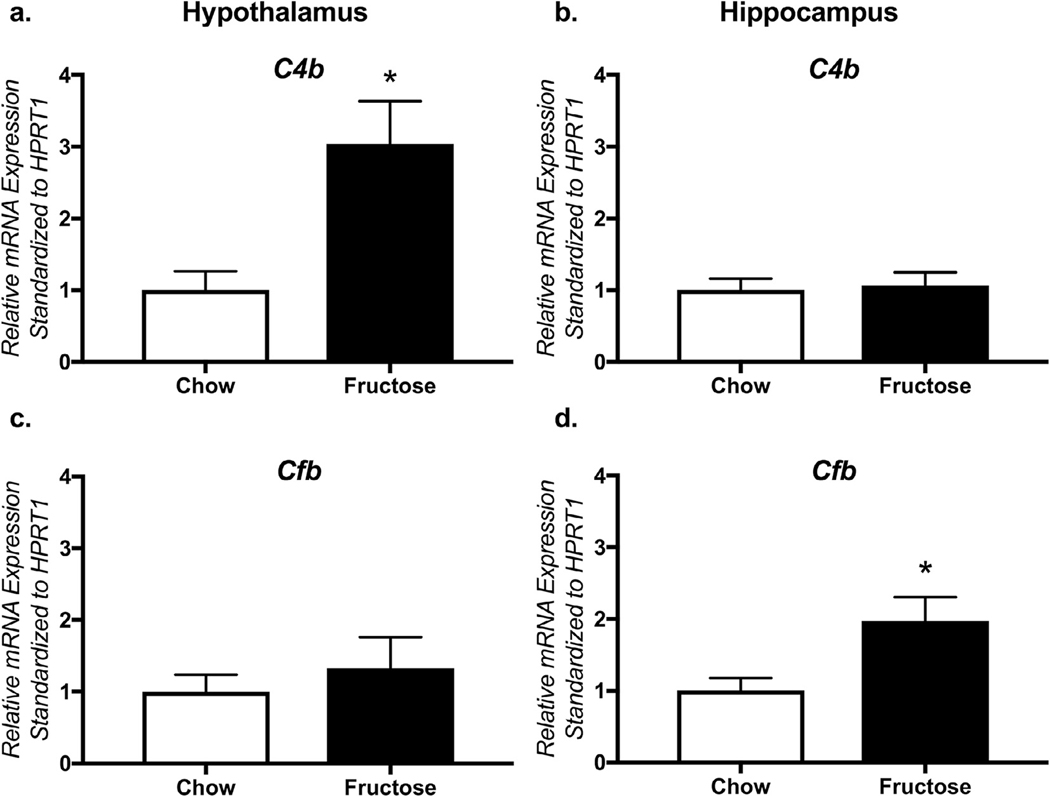
To further evaluate expression of complement components, qRT-PCR was performed in the hippocampus and hypothalamus of animals in the Baseline Cohort using primers for C4b (involved in the classical and lectin-binding pathways) and Cfb (alternative pathway). In the hypothalamus, C4b was significantly elevated in fructose-fed animals (Fig. 2a, t14 = 3.16; p = 0.01) without changes in Cfb (Fig. 2c, t14 = 0.66; p = 0.52). Hippocampal Cfb was significantly increased in fructose-fed animals (Fig. 2b, t14 = 2.37; p = 0.03). Expression of C4b (Fig. 2d, t14 = 0.28; p = 0.78) was not altered in the hippocampus.
Fig. 2.
Fructose increases expression of hippocampal and hypothalamic complement components. In the hypothalamus, C4b was significantly elevated in fructose-fed animals (a) without changes in Cfb (c). Hippocampal Cfb (d) was significantly increased in fructose-fed animals without changes in expression of C4b (b). Data shown are mean ± SEM, normalized to chow-fed animals; asterisks indicate a main effect at p < 0.05.
3.4. Fructose increases expression of plasma tumor necrosis factor-α
Plasma TNFα was assessed using the Rat TNFα Quantikine ELISA (R&D Biosystems, Minneapolis, MN) to evaluate whether fructose-induced inflammation extended outside the central nervous system. Plasma TNFα concentration was significantly increased in fructose-fed animals (Fig. 3a, t14 = 3.08; p < 0.01).
Fig. 3.
Fructose increases expression of plasma TNFα and serum corticosterone. a) Baseline plasma TNFα expression was significantly elevated in fructose-fed animals relative to chow-fed animals. b) Fructose increased serum corticosterone levels relative to chow-fed animals both at baseline and after forced swim without an effect of surgery or any interactions. Data shown are mean ± SEM; asterisks indicate a main effect at p < 0.05. BSL =Baseline; FST =Forced Swim Test; MCAO = middle cerebral artery occlusion.
3.5. Fructose does not alter vascular length of the hippocampus
Vascular length was assessed using the Stereoinvestigator Spaceballs probe (MBF Biosciences, Williston, VT), with RECA as a marker of endothelial cells. In this analysis, vascular length was not affected either when analyzing the hippocampus as a whole (Supplemental Fig. S2a,c; t12 = 0.57, p = 0.58), nor when analyzing by hippocampal subregion (all p > 0.05).
3.6. Fructose does not alter blood brain barrier permeability
For BBB permeability, the percentage of the brain immunoreactive for IgG was calculated using the Gray Level Index to quantify mean optical density for each brain (ImageJ, NIH). Fructose-fed and chow-fed brains did not differ in mean optical density when stained for IgG (Supplemental Fig. S2b,d; t16 = 0.78, p = 0.45), indicating no difference in blood brain barrier permeability.
3.7. Fructose and acute stress increase corticosterone irrespective of surgery
Corticosterone levels in chow- and fructose-fed sham and MCAO animals were assessed at baseline and after a ten-minute forced swim. Corticosterone levels of Surgery Cohorts 1 and 2 did not differ when compared in three-way repeated measures ANOVA (F1,32 = 0.91, p = 0.35), so the two Surgery Cohorts were combined for subsequent analysis. Using a three-way repeated-measures ANOVA type II, the forced swim test (Fig. 3b, F1,36 = 199.68, p < 0.01) increased serum corticosterone levels. Fructose had a strong trend to increase serum corticosterone (F1,36 = 4.06, p = 0.05), but there was no effect of surgery (F1,36 = 0.14, p = 0.70) or any interactions (all p > 0.05).
3.8. MCAO-induced impairments in neurologic behavior are not exacerbated by a high-fructose diet
The surgical cohort animals were assessed for differences in neurologic behavior three days after surgery at PND68-69 and PND84-85. Three-way repeated measures ANOVAs assessing each of these measures did not indicate any effect of cohort or date of testing (all p > 0.05), so both time-points were combined for subsequent analyses.
Total distance traveled was assessed in the open field during the dark cycle and percent change in distance traveled before and after surgery was calculated. Two-way ANOVA indicated no significant effect of surgery (Fig. 4a, sham vs MCAO, F1,42 = 0.01, p = 0.92), diet type (chow vs fructose, F1,42 = 1.44, p = 0.24), or an interaction thereof (F1,42 = 0.81, p = 0.37).
Fig. 4.
High-fructose diet did not exacerbate MCAO-induced impairments in neurologic behavior. a) In post-surgery assessment, neither surgery nor diet altered total distance traveled. b) In the post-test, MCAO surgery increased removal time in the MCAO cohorts without any effect of diet or an interaction between diet and surgery. c) In the post-test, surgery altered latency to fall from the rotorod without an effect of diet. Data shown are mean ± SEM; asterisks indicate a main effect at p < 0.05. C = Chow, F = Fructose, MCAO = middle cerebral artery occlusion.
In the sticky dot test for removal of a dot from the contralateral (left) paw, percent change in latency to remove the dot pre- or post-surgery was calculated. MCAO surgery significantly increased time to remove the sticky dot (Fig. 4b, F1,42 = 9.96, p < 0.01). However, neither diet type (F1,42 = 1.56, p = 0.22) nor an interaction between diet and surgery (F1,42 < 0.00, p = 0.98) affected latency to remove the dot.
In the rotorod test, percent change in latency to fall pre- or post-surgery was calculated. MCAO surgery significantly decreased time to fall from the rotorod (Fig. 4c, F1,42 = 4.36, p = 0.04). However, neither diet type (F1,42 = 1.38, p = 0.25) nor an interaction between diet and surgery (F1,42 = 2.11, p = 0.15) affected latency to fall from the rotorod.
Grip strength was tested using a grip strength meter, and percent change in grip strength before and after surgery was calculated. Two-way ANOVA indicated no significant effect of surgery (Fig. 4d, F1,42 = 0.53, p = 0.47), diet type (F1,42 = 1.97, p = 0.17), or an interaction thereof (F1,42 = 0.51, p = 0.48).
3.9. Fructose decreases grooming behavior in the light-cycle open field in both sham and MCAO-affected animals
Behavior in the open field during the light-cycle can be measured to assess anxiety- and depressive-like behavior. Specifically, central tendency, or percent time spent in the center of the open field, is an index of anxiety-like behavior than typically decreases with stress exposure (Prut and Belzung, 2003). We assessed central tendency and total distance traveled in the open field during the light cycle. There was also no significant effect of diet (Fig. 5a, F1,40 = 0.77, p = 0.38), surgery (F1,40 = 1.46, p = 0.23) or an interaction (F1,40 = 0.11, p = 0.75) between diet and surgery on central tendency. There was no significant effect of diet (Fig. 5b, F1,40 = 3.82, p = 0.06), surgery (F1,40 = 3.07, p = 0.09) or an interaction between diet and surgery (F1,40 = 0.04, p = 0.84) on total distance traveled in the open field during the light cycle.
Fig. 5.
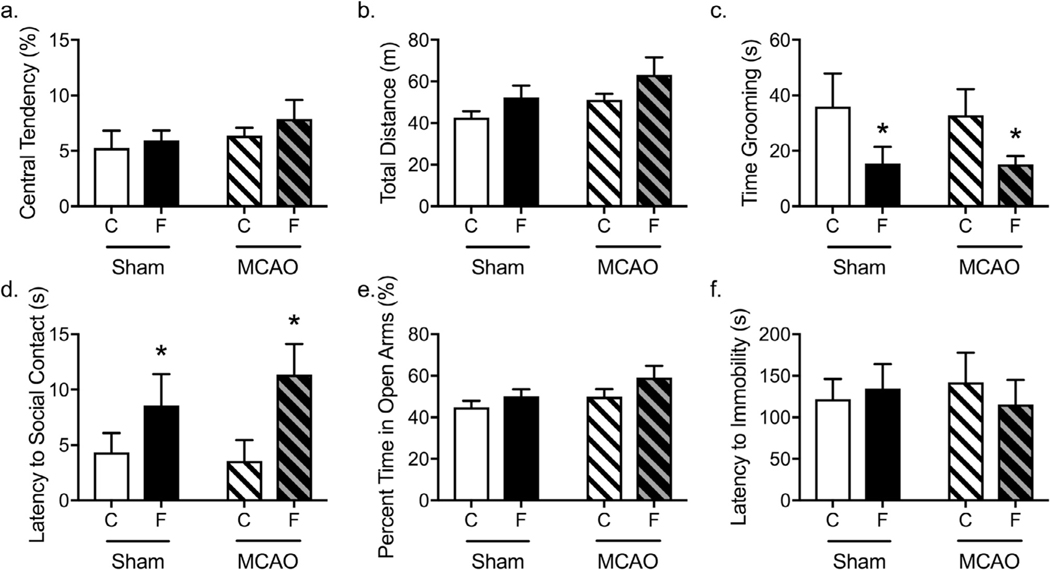
High-fructose diet and MCAO surgery promote depressive-like behavior without synergistic or interactive effects. a) High-fructose diet exposure did not alter central tendency in the open field. b) High-fructose diet exposure did not alter total distance traveled in the open field when tested during the light cycle. c) High-fructose diet exposure significantly reduced time grooming regardless of surgery. d) Fructose-fed animals had significantly increased latency to social contact, but neither surgery alone nor in interaction with diet altered this latency. e) Neither diet or surgery altered percent time in the open arms. f) Latency to immobility in the forced swim test was not altered by diet or surgery. Data shown are mean ± SEM; asterisks indicate a main effect at p < 0.05. C = Chow, F = Fructose, MCAO = middle cerebral artery occlusion. Chow-Sham: n = 10–12; Chow-MCAO: n = 10–11; Fructose-Sham: n = 10–12; Fructose-MCAO n = 10.
Grooming is an innate rodent behavior that has been studied in models of neuropsychiatric disease (Kalueff et al., 2016). High-levels of repetitive self-grooming is associated with anxiety-like behavior (Kalueff et al., 2016), while low self-grooming occurs with exposure to aversive stimuli (Estanislau et al., 2013) and has been linked to depressive-like behavior (Kalueff et al., 2016). High-fructose diet exposure significantly reduced time grooming when in the open field (Fig. 5c, F1,40 = 5.74, p = 0.02). There was no significant effect of surgery (F1,40 = 0.05, p = 0.83) or an interaction between diet and surgery (F1,40 = 0.03, p = 0.86) on grooming behavior.
3.10. Fructose increases latency to social interaction in both sham- and MCAO-affected animals
The social interaction test is a test of both anxiety-like behavior as well as anhedonia (File and Hyde, 1978). Fructose-fed animals had significantly increased latency to social contact (Fig. 5d, F1,38 = 6.60, p = 0.01), but neither surgery alone (F1,38 = 0.18, p = 0.68) nor in interaction with diet (F1,38 = 0.18, p = 0.68) altered latency to social contact.
3.11. Neither fructose nor MCAO surgery affect behavior in the elevated plus maze or the forced swim test
In the elevated plus maze, decreased time or entries into the open arms of the maze indicates anxiety-like behavior (Pellow et al., 1985). Neither surgery (F1,42 = 3.16, p = 0.08) nor diet (F1,42 = 3.40, p = 0.07), nor an interaction thereof (F1,42 = 0.23, p = 0.63) significantly affected percent time in the open arms (Fig. 5e). Moreover, neither surgery (F1,42 = 0.29, p = 0.59) nor diet (F1,42 = 0.49, p = 0.49), nor an interaction thereof (F1,42 = 0.14, p = 0.71) significantly altered the number of entries into the open arms.
The forced swim was assessed during behavioral testing two weeks post-injury test to assess depressive-like behavior. Latency to immobility in the forced swim test was not affected by surgery (Fig. 5f, F1,42 < 0.00, p = 0.99), diet (F1,42 = 0.06, p = 0.81), or an interaction between diet and surgery (F1,42 = 0.44, p = 0.51). In addition, total time immobile was not affected by surgery (F1,42 = 0.06, p = 0.81), diet (F1,42 = 0.22, p = 0.64), or an interaction between the two (F1,42 = 2.56, p = 0.12).
3.12. Fructose does not change lesion volume after MCAO
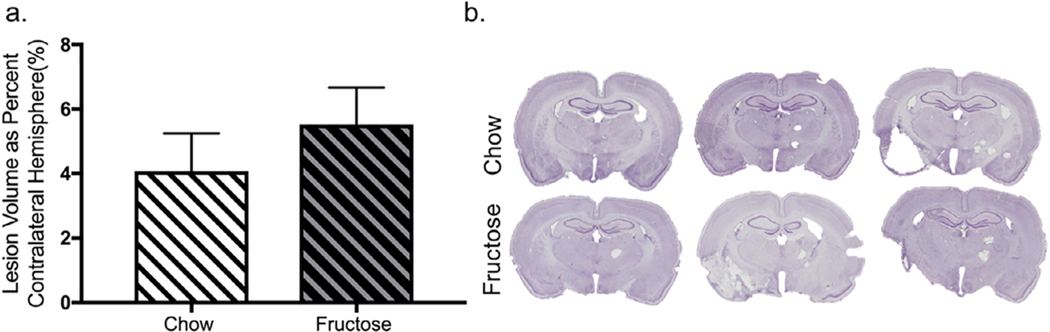
Lesion and hemispheric volumes were assessed by the Cavalieri method counting only cystic spaces as lesion, and the volume of the contralateral hemisphere minus the lesion was calculated. Direct analysis of lesion volume in MCAO animals indicated no difference between chow-fed and fructose-fed animals (Fig. 6a; t17 = 0.24; p = 0.81). Significant variability was evident in lesion volume, and representative images from chow-fed and fructose-fed sham and MCAO animals are shown in Fig. 6b. Percent occlusion and percent reperfusion were also analyzed between chow- and fructose-fed MCAO animals. There was no effect of either diet on percent occlusion (t19 = 0.70, p = 0.49) or reperfusion (t19 = 0.79, p = 0.44), nor was there a relationship between percent reperfusion and lesion-free volume (r = −0.15, p = 0.54) or percent occlusion and lesion-free volume (r = 0.37, p = 0.12).
Fig. 6.
Fructose does not change lesion volume after MCAO a) Lesion volume in MCAO animals did not differ between chow-fed and fructose-fed. b) Significant variability was evident in lesion volume, and representative images from chow-fed and fructose-fed sham and MCAO animals are shown. Data shown are mean ± SEM; asterisks indicate a main effect at p < 0.05. MCAO = middle cerebral artery occlusion.
4. Discussion
Metabolic dysfunction can promote depressive symptoms (Anderson et al., 2001; Lamers et al., 2013), increase stroke risk (Reilly and Kelly, 2011), and increase risk for development of post-stroke depression (Tanislav et al., 2015). We hypothesized that a high-fructose diet initiated in adolescence would create a metabolic and cerebrovascular environment that would be vulnerable to the “second hit” of an ischemic event, exacerbating post-stroke outcomes. Contributing to this hypothesis, we present data demonstrating that periadolescent fructose-fed rats had increased expression of cerebral complement components and plasma TNFα, without changes in vascular density or blood-brain-barrier permeability. Consistent with previous data, exposure to a high-fructose diet also increased baseline corticosterone and promoted depressive-like behavior in both sham animals and after MCAO surgery. However, fructose did not alter neurologic motor behavior or lesion volume after ischemia. Collectively, the data presented herein indicate that a high-fructose diet consumed during adolescence promotes a pro-inflammatory state while altering HPA axis output and affective-like behaviors, but it does not exacerbate outcomes after MCAO in male rats.
In the present study, the hypothalamus was selected as a region of interest for gene expression changes as it plays an essential role in food intake and energy homeostasis (Marty et al., 2007). As the primary locus of response for alterations in metabolic homeostasis, the hypothalamus is expected to undergo changes in gene expression with changes in metabolism. We also sought to establish whether the effects of fructose consumption on inflammatory gene expression extended beyond the hypothalamus. The hippocampus was selected as a secondary region of interest as it is a key modulator of the stress response and is susceptible to diet-induced inflammatory changes (Bilbo and Tsang, 2010; Marissal-Arvy et al., 2013).
RNA-sequencing analysis revealed that all three complement pathways (classical, lectin, and alternative; Fig. 1) were significantly enriched with differentially expressed gene transcripts in the hypothalamus of the fructose-fed animals. To validate the RNA-sequencing findings, we examined expression of the gene C4b (C4 isoform, basic form, common to both the lectin-induced and classical complement pathways) and Cfb (Factor B, in the alternative complement pathway). C4b showed increased expression in the hypothalamus of the fructose-fed rats, while Cfb was elevated in the hippocampus. Other studies have noted fructose-induced increases in hippocampal expression of additional inflammatory factors, including NFκB, IL-6, IL-1β, TNF-α, and myeloperoxidase (Cigliano et al., 2018; Djordjevic et al., 2015), but this is the first demonstration of fructose-induced increased central nervous system complement expression.
While the data here suggest that a periadolescent high-fructose diet promotes complement gene expression in a brain-region-specific manner, it also indicates that the pro-inflammatory effects of fructose are not limited to the central nervous system. Plasma TNFα expression was more than 40% higher in fructose-fed rats than in chow-fed rats. This is consistent with studies showing that a high-fructose diet increases other peripheral markers of inflammation, including in serum (Kelany et al., 2017), aortic tissue (Nyby et al., 2007), and liver (Collino et al., 2010). However, the effects of a high-fructose diet on peripheral complement remain to be determined. Future studies will be necessary to assess whether fructose-induced changes in peripheral inflammatory markers extend to the complement cascade, or whether changes in complement specifically are limited to the central nervous system.
The complement system plays an essential role in our innate immune response, primarily promoting cell lysis, generation of pro-inflammatory mediators, and opsonization (Levinson and Levinson, 2012). Once thought to be immune privileged, the central nervous system is now known to contain many features of the immune system, including complement proteins (Bonifati and Kishore, 2007). Complement activation has deleterious effects on ischemia induced injury, both through direct tissue damage by cleaved anaphylaxotoxins and through subsequent upregulation of endothelial cell adhesion molecules and neutrophil accumulation (D’Ambrosio et al., 2001).
Given both the increased hypothalamic and hippocampal complement expression and increases in basal corticosteroid levels, we had hypothesized that fructose-fed animals would suffer greater impairment from MCAO. However, while fructose-fed animals were susceptible to metabolic and affective-like behavioral changes, their neurologic response to MCAO was not impaired. Moveover, lesion volume after MCAO was also not affected. Though CNS complement activation has been shown to exacerbate ischemic injury in several rodent models (D’Ambrosio et al., 2001), one of the primary roles of complement is clearance of debris and damaged neurons (Alexander et al., 2008). It is possible that basal elevation in complement expression may have led to a conditioned local immune response that prevented greater ischemic injury in the fructose-fed animals. Future studies examining the degree of activation of different complement pathways and components may shed light on the role of diet-induced increases in complement and associated changes in behavior and recovery from ischemic injury. Such studies should also examine the relationship between central expression of complement, fructose-induced peripheral inflammation, and behavioral change.
Furthermore, the fructose diet in this study did not alter either hippocampal vascular length. The hippocampus was selected for vascular length analysis given that it has been shown to undergo vascular changes in response to dietary and psychosocial stress (Kalidindi et al., 2017; Neigh et al., 2017; Neigh et al., 2010). However, we cannot rule out vascular changes in brain regions outside the hippocampus. Given the absence of changes observed in whole-brain BBB permeability, we do not anticipate widespread vascular changes associated with the high fructose diet. It should be noted that the method used for this assay (IgG staining) will only assess changes in BBB permeability to large molecules. Future studies may wish to assess diet-induced changes in the BBB using assays that evaluate permeability to smaller molecules (such as sodium fluorescein).
While the fructose diet did not change neurologic behavior or lesion volume, it did promote some negative-valence behaviors after both sham and MCAO surgeries. The tests used in this study, namely the open field test, the social interaction test, the elevated plus maze, and the forced swim test, were selected to evaluate for anxiety-and depressive-like features. Negative-valence behaviors in these tests are associated with response to stress paradigms and can be modified by anxiolytics or antidepressants (Prut and Belzung, 2003; File and Hyde, 1978; Pellow et al., 1985; Porsolt et al., 1977). Here, fructose-fed animals tested after either eight or ten weeks of dietary exposure displayed reduced grooming in the open field and increased latency to interact in the social interaction test, behaviors which have been linked to anxiety-and depressive-like characteristics (Whishaw and Kolb, 2004). No effects of fructose or MCAO surgery were identified in the elevated-plus maze or in the forced swim test. While a fructose-related increase in negative-valence behaviors is consistent with previous findings (Harrell et al., 2015a), the specific tests in which these changes in behavior were evident differ in the current study. Prior data indicated increases in anxiety-and depressive-like behavior in the elevated plus maze and the forced swim test (Harrell et al., 2015a). Notably, behavioral outcomes are highly sensitive to multiple variables including light cycle (Steinman et al., 2011; Silva et al., 2010) , bedding selection (Sakhai et al., 2013), and nearby construction (Dallman et al., 1999). Although every effort was made to recapitulate conditions in our previously published study (Harrell et al., 2015a), the animals in this study were run in a different location than the previous study and slight environmental modifications, even beyond our perception, could have contributed to the shift in behavioral outcomes. Despite this shift, physiological effects of the diet are robust and the domains of behavioral alterations are consistent between the two studies.
In addition to the behavioral and inflammatory changes associated with the high-fructose diet, this study demonstrates some of the diet’s metabolic effects. Fructose-fed rats had higher terminal weights, greater caloric efficiency, and elevated fasting blood glucose than their chow-fed counterparts. This is consistent with previous data from our lab, which also showed increased fat pad mass and an enhanced blood glucose response to a glucose tolerance test. Other high-fructose animal models have demonstrated that such a diet increases blood pressure, insulin resistance, and steatosis (Hwang et al., 1987; Kawasaki et al., 2009) . Future studies should expand the metabolic characterization of the high-fructose diet and consider potential methods for reversal in order to better understand which specific metabolic effects are most linked to behavioral, inflammatory, and neurophysiological changes.
While stroke and post-stroke depression occur in young populations (Tanislav et al., 2015), their prevalence increases in older populations (Whyte et al., 2004). This study was not designed to compare the effect of age or the effect of time post-stroke on affective and neurological outcomes; however, both factors have the potential to influence findings. At least one study using a MCAO model indicated no difference in post-stroke depressive behavior between rats aged 20 weeks compared to 24 months (Boyko et al., 2013). However, others have shown that older animals that undergo MCAO surgery have greater neurologic behavioral impairment relative to younger animals while also being more susceptible to blood-brain-barrier damage and pro-inflammatory signaling (DiNapoli et al., 2008; Dinapoli et al., 2010). In addition, several studies have noted that the timing of studies after cerebral ischemia can impact results (DeVries et al., 2001; Kronenberg et al., 2014). For example, in contrast to unchanged open-field locomotor activity observed in the present study, others have shown post-stroke hyperactivity presenting several weeks after the initial surgery (Winter et al., 2005). With respect to affective behavior, microembolism can induce anxiety-and depressive-like behavior at 14–17 days post-surgery, but not at 4–6 days post-surgery (Nemeth et al., 2012). Future studies will be necessary to better determine the effects of diet on MCAO behavioral outcomes both across the life span and over time after ischemia.
In conclusion, a high-fructose diet consumed during adolescent development promotes a pro-inflammatory state both in the central nervous system and peripherally; elevates HPA axis output at baseline; and induces depressive-like behavior in adulthood after both sham surgery and transient ischemia induced by MCAO. In terms of neurologic behavior and lesion size after MCAO, adaptive responses to the metabolic influence of the high-fructose diet may result in equivalent outcomes in spite of the diet’s ability to act as a chronic stressor as evidenced by the elevation in basal corticosterone and depressive-like behaviors. This study paves the way for additional research assessing the impact of a high-fructose diet, such as the effect of fructose on a pro-inflammatory profile and the effect of treatments to counteract the metabolic, hormonal, and behavioral effects of fructose.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Bushra Wali, PhD, Christina Nemeth, PhD, Sean Kelly, and Leah Vaughan for their technical assistance. Funding for the studies in this manuscript was provided by the National Institutes of Health R21MH091321-01 (GNN), the American Heart Association Predoctoral Fellowship (14PRE18910002; CSH), Virginia Commonwealth Department of Anatomy and Neurobiology (GNN), and the National Institute of Health Institutional Research and Academic Career Development Award (K12GM093857; MMH).
Footnotes
Conflict of Interest Disclosure
The authors CSH, CZ, DM, DS, IS, MM, and GNN declare that they have no conflicts of interest, financial or otherwise.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at https://doi.org/10.1016/j.bbi.2018.05.018.
References
- Abbott NJ, Patabendige AA, Dolman DE, et al. , 2010. Structure and function of the blood-brain barrier. Neurobiol. Dis. 37, 13–25. 10.1016/j.nbd.2009.07.030S0969-9961(09)00208-3 [pii]. [DOI] [PubMed] [Google Scholar]
- Alexander JJ, Anderson AJ, Barnum SR, et al. , 2008. The complement cascade: YinYang in neuroinflammation–neuro-protection and -degeneration. J. Neurochem. 107, 1169–1187. 10.1111/j.1471-4159.2008.05668.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos GS, 2006. The vascular depression hypothesis: 10 years later. Biol. Psychiatry 60, 1304–1305. 10.1016/j.biopsych.2006.09.006. [DOI] [PubMed] [Google Scholar]
- Anderson RJ, Freedland KE, Clouse RE, et al. , 2001. The prevalence of comorbid depression in adults with diabetes: a meta-analysis. Diabetes Care 24, 1069–1078. [DOI] [PubMed] [Google Scholar]
- Atif F, Yousuf S, Sayeed I, et al. , 2013. Combination treatment with progesterone and vitamin D hormone is more effective than monotherapy in ischemic stroke: the role of BDNF/TrkB/Erk1/2 signaling in neuroprotection. Neuropharmacology 67, 78–87. 10.1016/j.neuropharm.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bekhbat M, Neigh GN, 2018. Sex differences in the neuro-immune consequences of stress: focus on depression and anxiety. Brain, Behav. Immun. 67, 1–12. 10.1016/j.bbi.2017.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Tsang V, 2010. Enduring consequences of maternal obesity for brain inflammation and behavior of offspring. FASEB J. 24, 2104–2115. 10.1096/fj.09-144014. [DOI] [PubMed] [Google Scholar]
- Bonifati DM, Kishore U, 2007. Role of complement in neurodegeneration and neuroinflammation. Mol. Immunol. 44, 999–1010. 10.1016/j.molimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Boyko M, Kutz R, Gruenbaum BF, et al. , 2013. The influence of aging on poststroke depression using a rat model via middle cerebral artery occlusion. Cogn. Affect Behav. Neurosci. 13, 847–859. 10.3758/s13415-013-0177-3. [DOI] [PubMed] [Google Scholar]
- Brindley DN, Cooling J, Glenny HP, et al. , 1981. Effects of chronic modification of dietary fat and carbohydrate on the insulin, corticosterone and metabolic responses of rats fed acutely with glucose, fructose or ethanol. Biochem. J. 200, 275–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena C, Giacchetti G, Novello M, et al. , 2003. Cellular mechanisms of insulin resistance in rats with fructose-induced hypertension. American journal of hypertension, 16:973–97. Research Support, Non-U.S. Gov’t 2003/10/24. [DOI] [PubMed] [Google Scholar]
- Cigliano L, Spagnuolo MS, Crescenzo R, et al. , 2018. Short-term fructose feeding induces inflammation and oxidative stress in the hippocampus of young and adult rats. Mol. Neurobiol. 55, 2869–2883. 10.1007/s12035-017-0518-2. [DOI] [PubMed] [Google Scholar]
- Collino M, Aragno M, Castiglia S, 2010. et al.Pioglitazone improves lipid and insulin levels in overweight rats on a high cholesterol and fructose diet by decreasing hepatic inflammation. Br. J. Pharmacol. 160, 1892–1902. 10.1111/j.1476-5381.2010.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dallman MF, Akana SF, Bell ME, et al. , 1999. Warning! Nearby construction can profoundly affect your experiments. Endocrine 11, 111–113. 10.1385/ENDO:11:2:111. [DOI] [PubMed] [Google Scholar]
- D’Ambrosio AL, Pinsky DJ, Connolly ES, 2001. The role of the complement cascade in ischemia/reperfusion injury: implications for neuroprotection. Mol. Med. 7, 367–382. [PMC free article] [PubMed] [Google Scholar]
- DeVries AC, Nelson RJ, Traystman RJ, et al. , 2001. Cognitive and behavioral assessment in experimental stroke research: will it prove useful? Neurosci. Biobehav. Rev. 25, 325–342. [DOI] [PubMed] [Google Scholar]
- DiNapoli VA, Huber JD, Houser K, et al. , 2008. Early disruptions of the blood-brain barrier may contribute to exacerbated neuronal damage and prolonged functional recovery following stroke in aged rats. Neurobiol. Aging 29, 753–764. 10.1016/j.neurobiolaging.2006.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinapoli VA, Benkovic SA, Li X, et al. , 2010. Age exaggerates proinflammatory cytokine signaling and truncates signal transducers and activators of transcription 3 signaling following ischemic stroke in the rat. Neuroscience 170, 633–644. 10.1016/j.neuroscience.2010.07.011S0306-4522(10)00989-9 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djordjevic A, Bursac B, Velickovic N, et al. , 2015. The impact of different fructose loads on insulin sensitivity, inflammation, and PSA-NCAM-mediated plasticity in the hippocampus of fructose-fed male rats. Nutr. Neurosci. 18, 66–75. 10.1179/1476830513Y.0000000098. [DOI] [PubMed] [Google Scholar]
- Edgar R, Domrachev M, Lash AE, 2002. Gene expression omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 30, 207–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estanislau C, Diaz-Moran S, Canete T, et al. , 2013. Context-dependent differences in grooming behavior among the NIH heterogeneous stock and the Roman high- and low-avoidance rats. Neurosci. Res. 77, 187–201. 10.1016/j.neures.2013.09.012. [DOI] [PubMed] [Google Scholar]
- File SE, Hyde JR, 1978. Can social interaction be used to measure anxiety? Br. J. Pharmacol. 62, 19–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gainotti G, Antonucci G, Marra C, et al. , 2001. Relation between depression after stroke, antidepressant therapy, and functional recovery. J. Neurol. Neurosurg. Psychiatry 71, 258–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose SS, Williams LS, Swindle RW, 2005. Depression and other mental health diagnoses after stroke increase inpatient and outpatient medical utilization three years poststroke. Med. Care 43, 1259–1264. [DOI] [PubMed] [Google Scholar]
- Glushakova O, Kosugi T, Roncal C, et al. , 2008. Fructose induces the inflammatory molecule ICAM-1 in endothelial cells. J. Am. Soc. Nephrol.: JASN 19, 1712–1720. 10.1681/ASN.2007121304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackett ML, Yapa C, Parag V, et al. , 2005. Frequency of depression after stroke: a systematic review of observational studies. Stroke; J. Cereb. Circ. 36, 1330–1340. 10.1161/01.STR.0000165928.19135.35. [DOI] [PubMed] [Google Scholar]
- Harrell CS, Burgado J, Kelly SD, et al. , 2015a. High-fructose diet during periadolescent development increases depressive-like behavior and remodels the hypothalamic transcriptome in male rats. Psychoneuroendocrinology 62, 252–264. 10.1016/j.psyneuen.2015.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrell CS, Gillespie CF, Neigh GN, 2015b. Energetic stress: the reciprocal relationship between energy availability and the stress response. Physiol. Behav. 10.1016/j.physbeh.2015.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- House A, Knapp P, Bamford J, et al. , 2001. Mortality at 12 and 24 months after stroke may be associated with depressive symptoms at 1 month. Stroke; J. Cereb. Circ. 32, 696–701. [DOI] [PubMed] [Google Scholar]
- Huang J, Upadhyay UM, Tamargo RJ, 2006. Inflammation in stroke and focal cerebral ischemia. Surg. Neurol. 66, 232–245 S0090–3019(06)00101–7 [pii] 10.1016/j.surneu.2005.12.028. [DOI] [PubMed] [Google Scholar]
- Hwang IS, Ho H, Hoffman BB, et al. , 1987. Fructose-induced insulin resistance and hypertension in rats. Hypertension 10, 512–516. [DOI] [PubMed] [Google Scholar]
- Kalidindi A, Kelly SD, Singleton KS, et al. , 2017. Reduced marker of vascularization in the anterior hippocampus in a female monkey model of depression. Physiol. Behav. 172, 12–15. 10.1016/j.physbeh.2016.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Song C, et al. , 2016. Neurobiology of rodent self-grooming and its value for translational neuroscience. Nat. Rev. Neurosci. 17, 45–59. 10.1038/nrn.2015.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasim-Karakas SE, Vriend H, Almario R, et al. , 1996. Effects of dietary carbohydrates on glucose and lipid metabolism in golden Syrian hamsters. J. Lab. Clin. Med. 128, 208–213 S0022–2143(96)90013-X [pii]. [DOI] [PubMed] [Google Scholar]
- Kawasaki T, Igarashi K, Koeda T, et al. , 2009. Rats fed fructose-enriched diets have characteristics of nonalcoholic hepatic steatosis. J. Nutr. 139, 2067–2071. 10.3945/jn.109.105858. [DOI] [PubMed] [Google Scholar]
- Kelany ME, Hakami TM, Omar AH, 2017. Curcumin improves the metabolic syndrome in high-fructose-diet-fed rats: role of TNF-alpha, NF-kappaB, and oxidative stress. Can. J. Physiol. Pharmacol. 95, 140–150. 10.1139/cjpp-2016-0152. [DOI] [PubMed] [Google Scholar]
- Kinote A, Faria JA, Roman EA, et al. , 2012. Fructose-induced hypothalamic AMPK activation stimulates hepatic PEPCK and gluconeogenesis due to increased corticosterone levels. Endocrinology 153, 3633–3645. 10.1210/en.20121341en.2012-1341 [pii]. [DOI] [PubMed] [Google Scholar]
- Kronenberg G, Gertz K, Heinz A, et al. , 2014. Of mice and men: modelling post-stroke depression experimentally. Br. J. Pharmacol. 171, 4673–4689. 10.1111/bph.12775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamers F, Vogelzangs N, Merikangas KR, et al. , 2013. Evidence for a differential role of HPA-axis function, inflammation and metabolic syndrome in melancholic versus atypical depression. Mol. Psychiatry 18, 692–699. 10.1038/mp.2012.144. [DOI] [PubMed] [Google Scholar]
- Levinson W, 2012. Complement. In: Levinson W. (Ed.), Review of Medical Microbiology & Immunology, 12 ed. McGraw-Hill, New York. [Google Scholar]
- Liu J, Wang Y, Akamatsu Y, et al. , 2014. Vascular remodeling after ischemic stroke: mechanisms and therapeutic potentials. Prog. Neurobiol. 115, 138–156. 10.1016/j.pneurobio.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissal-Arvy N, Batandier C, Dallennes J, et al. , 2013. Effect of a high-fat-high-fructose diet, stress and cinnamon on central expression of genes related to immune system, hypothalamic-pituitary-adrenocortical axis function and cerebral plasticity in rats. Br. J. Nutr. 1–12. 10.1017/S0007114513003577. [DOI] [PubMed] [Google Scholar]
- Marty N, Dallaporta M, Thorens B, 2007. Brain glucose sensing, counterregulation, and energy homeostasis. Physiology 22, 241–251. 10.1152/physiol.00010.2007. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Mathews IZ, 2007. HPA function in adolescence: role of sex hormones in its regulation and the enduring consequences of exposure to stressors. Pharmacol. Biochem. Behav. 86, 220–233. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Reagan LP, 2004. Glucose transporter expression in the central nervous system: relationship to synaptic function. Eur. J. Pharmacol. 490, 13–24. 10.1016/j.ejphar.2004.02.041S0014299904001980 [pii]. [DOI] [PubMed] [Google Scholar]
- Nadeau K, Dabelea D, 2008. Epidemiology of type 2 diabetes in children and adolescents. Endocr. Res. 33, 35–58. 10.1080/07435800802080138793173082 [pii]. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Owens MJ, Taylor WR, et al. , 2010. Changes in the vascular area fraction of the hippocampus and amygdala are induced by prenatal dexamethasone and/or adult stress. J. Cereb. Blood Flow Metab.: Offic. J. Int. Soc. Cereb. Blood Flow Metab. 30, 1100–1104. 10.1038/jcbfm.2010.46jcbfm201046 [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neigh GN, Nemeth CL, Kelly SD, et al. , 2017. Prenatal stress-induced increases in hippocampal von Willebrand factor expression are prevented by concurrent prenatal escitalopram. Physiol. Behav. 172, 24–30. 10.1016/j.physbeh.2016.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Neigh GN, 2015. Microemboli alter the acute stress response and cause prolonged expression of MCP-1 in the hippocampus. Psychoneuroendocrinology 54, 71–77. 10.1016/j.psyneuen.2015.01.023. [DOI] [PubMed] [Google Scholar]
- Nemeth CL, Shurte MS, McTigue DM, et al. , 2012. Microembolism infarcts lead to delayed changes in affective-like behaviors followed by spatial memory impairment. Behav. Brain Res. 234, 259–266. 10.1016/j.bbr.2012.06.014. [DOI] [PubMed] [Google Scholar]
- Nemeth CL, Glasper ER, Harrell CS, et al. , 2014. Meloxicam blocks neuroinflammation, but not depressive-like behaviors, in HIV-1 transgenic female rats. PLoS One 9, e108399. 10.1371/journal.pone.0108399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth CL, Miller AH, Tansey MG, et al. , 2016. Inflammatory mechanisms contribute to microembolism-induced anxiety-like and depressive-like behaviors. Behav. Brain Res. 303, 160–167. 10.1016/j.bbr.2016.01.057. [DOI] [PubMed] [Google Scholar]
- Nyby MD, Abedi K, Smutko V, et al. , 2007. Vascular Angiotensin type 1 receptor expression is associated with vascular dysfunction, oxidative stress and inflammation in fructose-fed rats. Hypertens. Res. 30, 451–457. 10.1291/hypres.30.451. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, et al. , 2014. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA: J. Am. Med. Assoc. 311, 806–814. 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellow S, Chopin P, File SE, et al. , 1985. Validation of open:closed arm entries in an elevated plus-maze as a measure of anxiety in the rat. J. Neurosci. Methods 14, 149–167 0165–0270(85)90031–7 [pii]. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Le Pichon M, Jalfre M, 1977. Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732. [DOI] [PubMed] [Google Scholar]
- Prut L, Belzung C, 2003. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Kelly J, 2011. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int. J. Obes. (Lond) 35, 891–898. 10.1038/ijo.2010.222ijo2010222 [pii]. [DOI] [PubMed] [Google Scholar]
- Rutledge AC, Adeli K, 2007. Fructose and the metabolic syndrome: pathophysiology and molecular mechanisms. Nutr. Rev. 65, S13–23. [DOI] [PubMed] [Google Scholar]
- Sakhai SA, Preslik J, Francis DD, 2013. Influence of housing variables on the development of stress-sensitive behaviors in the rat. Physiol. Behav. 120, 156–163. 10.1016/j.physbeh.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Silva AL, Fry WH, Sweeney C, et al. , 2010. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav. Brain Res. 208, 528–534. 10.1016/j.bbr.2009.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spear LP, 2000. The adolescent brain and age-related behavioral manifestations. Neurosci. Biobehav. reviews 24, 417–463. [DOI] [PubMed] [Google Scholar]
- Steinman MQ, Crean KK, Trainor BC, 2011. Photoperiod interacts with food restriction in performance in the Barnes maze in female California mice. Eur. J. Neurosci. 33, 361–370. 10.1111/j.1460-9568.2010.07528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annual review of neuroscience 1999; 22: 105–122. Research Support, Non-U.S. Gov’t Research Support, U.S. Gov’t, P.H.S. Review 1999/04/15. DOI: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- Tanislav C, Kropp P, Grittner U, et al. , 2015. Clinically relevant depressive symptoms in young stroke patients - results of the sifap1 study. Neuroepidemiology 44, 30–38. 10.1159/000371389. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ, Kolb B, 2004. In: Behavior of the Laboratory Rat: A Handbook with Tests. Oxford: University Press, pp. 520. [Google Scholar]
- Whyte EM, Mulsant BH, Vanderbilt J, et al. , 2004. Depression after stroke: a prospective epidemiological study. J. Am. Geriatrics Soc. 52, 774–778. 10.1111/j.1532-5415.2004.52217.x. [DOI] [PubMed] [Google Scholar]
- Winter B, Juckel G, Viktorov I, et al. , 2005. Anxious and hyperactive phenotype following brief ischemic episodes in mice. Biol. Psychiatry 57, 1166–1175. 10.1016/j.biopsych.2005.02.010. [DOI] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I, et al. , 2014. Progesterone in transient ischemic stroke: a dose-response study. Psychopharmacology 231, 3313–3323. 10.1007/s00213-014-3556-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousuf S, Atif F, Sayeed I, et al. , 2015. Long-term behavioral deficits and recovery after transient ischemia in middle-aged rats: effects of behavioral testing. Restor. Neurol. Neurosci. 10.3233/RNN-140450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.