Abstract
The accurate determination of the free nicotine content in tobacco is of great importance for the quality assessment of tobacco leaves and cigarettes, as well as for the study of tar reduction and cigarette formulation. A method based on solvent extraction and gas chromatography–mass spectrometry (GC–MS) was established for direct determination of free nicotine content in tobacco. Free nicotine extraction from tobacco was optimized, using different solvents and physical techniques (shaking, ultrasonic oscillation, and static extraction). Ultimately, a 24 h static extraction with cyclohexane, followed by GC–MS analysis, gave superior results. The standard addition recovery was 98.0–104.7%, with a limit of detection of 5.3 μg/g and a relative standard deviation between 1.3 and 4.1% (n = 5). Quadratic regression of the standard curve was excellent (R2 ≥ 0.9994). The free nicotine content was determined in 67 tobacco samples, with parallel samples showing relative deviations of 0.1–3.1%. To evaluate the effect of nicotine salts present in tobacco, malic acid, citric acid, acetic acid, and nicotine citrate were spiked into samples prior to extraction and analysis. The results show no interference from bound nicotine compounds on the method. The experimental results show that direct determination of free nicotine in tobacco was achieved using a simple static cyclohexane extraction. Moreover, the high extraction efficiency of free nicotine was also achieved with ease of operation and good repeatability.
1. Introduction
Nicotine is the most important ingredient in tobacco, and it has a remarkable effect on the sensory quality of cigarettes. In terms of molecular structure, nicotine is a dibasic compound, consisting of a pyridine ring linked to a hydrogenated pyrrole ring. When in contact with acidic components, nicotine can capture up to two protons and produce a salt. Therefore, nicotine can exist in the free state, in the single-protonated state, or in the double-protonated state.1 In tobacco, nicotine mostly binds with inorganic acids or organic acids to form salts, and a small portion exists in the free state.2 Free nicotine has high lipophilicity, which facilitates absorption by the human oral mucosa, resulting in a strong physiological effect. Protonic nicotine is highly hydrophilic and has slow absorption and metabolism rates in the human body.3,4 The form of nicotine in tobacco also affects its transfer rate to cigarette smoke. Compared with bound nicotine, free nicotine is highly volatile and has a high transfer rate to cigarette smoke, imposing significant effects on the sensory qualities, such as the impact and irritancy of cigarettes.5−8 Therefore, it is essential to determine the contents of the different forms of nicotine in tobacco accurately, especially free nicotine, for quality assessments of tobacco leaves and cigarettes. This research can also be used to improve smoking products by reducing tar content.
At present, the determination of free nicotine content in tobacco is mainly based on the pH method9−12 and solvent extraction.6,13−23 Using the pH method, the total amount of the different forms of nicotine in tobacco (tobacco leaves, cut tobacco, or smoke particulate matter collected by a Cambridge filter) is determined first. Then, the total nicotine is extracted in an aqueous solution with a certain concentration, and the pH value is determined. In this way, the content of nicotine in its three forms can be calculated based on the Henderson–Hasselbach equation.24 The pH method may only provide a prediction of the relative concentration of free nicotine, which could differ markedly from the actual distribution in the sample.24
The solvent extraction method is a liquid–liquid extraction technique that is the most widely used and can be implemented in two ways. One approach is to use water (deionized water, distilled water, or neutral water with pH 7.00) to extract free nicotine from the sample, followed by extraction in an appropriate organic solvent for determination.6,14−17 The other approach is to extract free nicotine with an organic solvent, followed by extraction and purification in water for determination.18−20,25 In addition, headspace solid-phase microextraction (HS–SPME) has been used to determine the free nicotine content in tobacco leaves or cigarette smoke particulate matter.26,27 This method is based on the volatility difference between free and protonated nicotine during determination. However, the complete separation of free and protonated nicotine or the occurrence of species transformation during the determination cannot be effectively verified. Free nicotine in electronic cigarette liquids was also determined by proton nuclear magnetic resonance (1H NMR) spectroscopy.28 Although the 1H NMR method avoids the introduction of solvents in the determination, the calculation model and chemical shift interference can affect the results.
In this study, a gas chromatography–mass spectrometry (GC–MS) method was established for the direct determination of free nicotine in tobacco. In the experimental method, 24 h static extraction with cyclohexane was used to prepare the tobacco sample, followed by GC–MS analysis. Compared with the traditional liquid–liquid extraction methods, this method enables simple and direct determination of free nicotine in tobacco and avoids the loss or conversion of free nicotine via the use of an aqueous extraction or purification step. In addition, the content of free nicotine was determined in 67 tobacco samples using the proposed experimental method.
2. Materials and Methods
2.1. Reagents and Instruments
A standard nicotine solution in isopropanol (9.6 mg/mL), a standard nicotine solution in water (100 μg/mL), and nicotine citrate (CAS no. 94006-00-5, 98.6%) were purchased from Tianjin Alta Scientific (Tianjin, China). Nicotine (CAS no. 54-11-5, 99.9%) was obtained from the China National Tobacco Quality Supervision and Test Center (Zhengzhou, China). Anethole (CAS no. 4180-23-8, 98.5%) was obtained from Beijing Ballingway Technology (Beijing, China), and cyclohexane (CAS no. 110-82-7, HPLC Grade) was obtained from Thermo Fisher Scientific (Fair Lawn, New Jersey). Anhydrous sodium sulfate (CAS no. 7757-82-6, ≥99.0%), citric acid monohydrate (CAS no. 5949-29-1, 99.5%), glacial acetic acid (CAS no. 64-19-7, ≥99.5%, analytically pure), and dl-malic acid (CAS no. 6915-15-7, ≥99.0%) were purchased from Sinopharm (Shanghai, China).
Specialized instrumentation and equipment used in this study included a 7890B/5977B GC–MS system (Agilent, Santa Clara, CA), a Milli-Q water purification system (Millipore, Burlington, MA), an ultrasonic cleaner (8510E-DTH; Branson Ultrasonics, Sterling Heights, MI), a speed-adjusting oscillator (HY-8; Guohua Electric Appliance, Changzhou, China), and an electronic balance (AX504; Mettler Toledo, Greifensee, Switzerland) with a sensing accuracy of 0.0001 g. The study also used helium (≥99.999%, v/v, Sichuan Messer Gas Products, Chengdu, China), 0.2 μm syringe filters (Nylon 66, Tianjin Jinteng Experiment Equipment, Tianjin, China), and 100 mL SCHOTT sample bottles (with sealing ring; DURAN, Wertheim, Germany).
2.2. Methods
2.2.1. Preparation of Standard Solutions
Anethole (0.100 g, accurate to 0.1 mg) was added to a 50 mL volumetric flask and dissolved in cyclohexane to form an internal standard solution with a concentration of 2.00 mg/mL. Aliquots of 30, 60, 90, 120, and 150 μL of the standard nicotine solution (9.6 mg/mL) were, respectively, added to 50 mL volumetric flasks, and each flask was spiked with 250 μL of the anethole standard internal solution. Finally, each volumetric flask was volumetric to scale using cyclohexane to generate standard nicotine working solutions with concentrations of 5.76, 11.52, 17.28, 23.04, and 28.80 μg/mL, respectively.
2.2.2. Sample Pretreatment
Samples of tobacco leaves (or cut tobacco) to be used for analysis were dried at a temperature no higher than 40 °C, ground into a powder, and sieved over a standard 60-mesh sieve (mesh size: 0.3 mm). Sieved sample powder (0.10 g, accurate to 0.1 mg) was added to a 100 mL SCHOTT sample bottle and mixed with 50.0 mL of anethole solution (10.0 μg/mL in cyclohexane). The solution was allowed to stand for 24 ± 2 h at 22 ± 2 °C. After filtration (0.2 μm Nylon 66 syringe filter), the filtrate was analyzed by GC–MS (Figure 1).
Figure 1.
Schematic of the extraction procedure using cyclohexane. SSP and CY represent 0.1 g of sieved sample powder and 50 mL of cyclohexane, respectively. FR, filter residue; GC–MS, gas chromatography–mass spectrometry; SE, static extraction.
2.2.3. GC–MS Analysis
Chromatographic separation was carried out using an HP-5MS UI column (30 m × 0.250 mm × 0.25 μm, (5% phenyl)-methylpolysiloxane, Agilent). The analysis conditions were: column oven temperature, 45 °C; sample volume, 1 μL; sample inlet temperature, 250 °C; split ratio, 20:1; carrier gas, helium; flow rate, 1.0 mL/min; and septum purging, 3 mL/min. The oven temperature program involved an initial temperature of 45 °C, maintained for 1 min, followed by an increase to 280 °C at 20 °C/min and maintained for 2.25 min. The mass spectrometry parameters were as follows: solvent delay, 7.0 min; electron energy, 70 eV; transmission line, 280 °C; ion source, 230 °C; quadrupole, 150 °C. Selected ion monitoring (SIM) was used, and quantification was based on the internal standard method. The quantitative ion for nicotine was m/z 84, and the qualitative ions were m/z 133, 161, and 162. The quantitative ion for the internal standard, anethole, was m/z 148, and the qualitative ions were m/z 77, 117, and 147.
3. Results
3.1. Sample Pretreatment and Testing
3.1.1. Selection of Solvent Extraction Method
Initially, the traditional liquid–liquid extraction method was attempted using deionized water, followed by organic solvent. Theoretically, free nicotine and some protonated nicotine, which have good solubility in water, are extracted into water first. Subsequently, free nicotine can be selectively extracted in the organic solvent to achieve purification. However, the standard recovery rate of this experimental method was low (less than 80%), due to the pKa of free nicotine and the pH of the water used for extraction (Table 1). Some free nicotine was converted to the protonated state, resulting in a low recovery. This result is consistent with those reported in the literature.24,29,30
Table 1. Content Distribution of Three Forms of Nicotine in Aqueous Solutiona.
| pH | free nicotine (%) | single-proton nicotine (%) | double-proton nicotine (%) |
|---|---|---|---|
| 4.5 | 0.04 | 95.69 | 4.27 |
| 4.8 | 0.08 | 97.73 | 2.19 |
| 5.0 | 0.13 | 98.48 | 1.39 |
| 5.5 | 0.42 | 99.13 | 0.44 |
| 6.0 | 1.33 | 98.53 | 0.14 |
| 6.5 | 4.09 | 95.87 | 0.04 |
| 7.0 | 11.89 | 88.10 | 0.01 |
| 7.5 | 29.90 | 70.10 | 0.00 |
| 8.0 | 57.43 | 42.57 | 0.00 |
Note: pKb1 = 3.15 and pKb2 = 7.87, as used in the calculation.
Next, an alternative solvent extraction approach was employed, in which the powderized tobacco sample was extracted in organic solvent first, followed by purification with water. In principle, this technique extracts free nicotine and some nicotine salts into the organic phase. Subsequently, washing with distilled water (or deionized water or neutral water with pH 7.00) removes the protonic nicotine. The free nicotine remains in the organic phase for analysis. However, in application, the blank standard addition recovery of this method was very low. The recovery was determined by washing 25 mL aliquots of standard solutions (solution A, 5.76 μg/mL in cyclohexane, derived from commercial standard solution; solution B, 5.87 μg/mL in cyclohexane, prepared from pure nicotine) with 25 mL of freshly prepared deionized water with shaking for 1 h. After separation of the phases using a separatory funnel, anethole was added into the organic fraction, and the organic fractions were dried over anhydrous sodium sulfate (∼0.2 g) to give solutions C and D, respectively. Solutions A, B, C, and D were then analyzed by GC–MS analysis after filtration (0.2 μm Nylon 66 syringe filter). The results in Table 2 show that the blank standard addition recovery of standard nicotine solutions A and B was between 19.9 and 27.6% after washing with deionized water. Although the solubility of free nicotine in deionized water is quite low (see Table 1), it appears to migrate into the aqueous phase when both phases exist, where it becomes protonic. This process solubilizes the nicotine in the aqueous phase and in doing so drives the reaction further toward the protonic state. As a result, most of the free nicotine dissolved in cyclohexane was transferred to water and removed as protonic nicotine.
Table 2. Blank Standard Addition Recovery of Experimental Methoda.
| no. | prepared concentration (μg/mL) | analyzed concentration (μg/mL) | no. | analyzed concentration (μg/mL) | recovery (%) |
|---|---|---|---|---|---|
| A | 5.76 | 5.88 | C | 1.54 | 26.7 |
| 5.88 | 1.59 | 27.6 | |||
| 5.83 | 1.56 | 27.1 | |||
| B | 5.87 | 5.89 | D | 1.21 | 20.6 |
| 5.91 | 1.20 | 20.4 | |||
| 5.79 | 1.17 | 19.9 |
Note: A, B, C, and D represent commercial standard solution of free nicotine dissolved in cyclohexane, pure nicotine dissolved in cyclohexane, solution A washed by deionized water, and solution B washed by deionized water, respectively.
Based on these results, it became apparent that it was better to avoid the use of water as a solvent for extraction or purification during the determination of free nicotine content in tobacco. As a result, cyclohexane was used as the extraction solvent to directly analyze the free nicotine content in tobacco without a purification step. Using this strategy, we aimed to avoid changing the original distribution of the nicotine forms in the sample.
3.1.2. Effect of Organic Acids and Nicotine Salts in Tobacco on the Determination
Malic acid and citric acid are two organic acids with the highest content in tobacco; acetic acid also has a high content in tobacco leaves. These acids form a number of nicotine salts when they bind with nicotine in tobacco.1,2,31 If the analysis interferes with the bound forms of nicotine, the nicotine salts formed from these acids will have a substantial effect on the determination. Thus, the three acids were selected as representative organic acids to verify the experimental method.
Four 0.5 mL aliquots of standard nicotine solution (100 μg/mL, prepared from 99.9% pure nicotine in water) were respectively spiked with 0.1 g of malic acid (no. 1), 0.1 g of citric acid monohydrate (no. 2), 0.1 mL of glacial acetic acid (no. 3), and 0.1 mL of freshly prepared deionized water (no. 4). Each sample was shaken well and then allowed to stand for 24 h. Cyclohexane (50 mL) was added to each sample, and the mixture was shaken for 1 h. Next, 25 mL of the organic phase was removed using a separatory funnel. Each organic fraction was dried over anhydrous sodium sulfate (0.25 g) and filtered with a 0.2 μm Nylon 66 filter, prior to GC–MS analysis. The results show that no nicotine was detected in sample solutions nos. 1–3, and the percent recovery of nicotine in sample solution no. 4 was 85.4%.
Nicotine citrate (bis[(S)-nicotine], (C10H14N2)2·C6H8O7) was used as a standard for pure bound nicotine. The tobacco powder was first well mixed and sieved. Four portions (nos. 1–4) of sieved tobacco powder were accurately weighed (0.100 g) and each was combined with 50.0 mL of cyclohexane containing the internal standard. Aliquots of nicotine citrate liquid (0.5 mL, 2000 μg/mL in cyclohexane) were added into samples nos. 3 and 4. In parallel, four 50 mL aliquots of cyclohexane containing the internal standard (nos. 5–8), and 0.5 mL of nicotine citrate liquid (2 000 μg/mL in cyclohexane) were added to samples nos. 7 and 8. Samples 1–8 were shaken with a velocity-modulated oscillator for 2 h and then filtered (0.2 μm syringe filter) before GC–MS analysis. The determined values for samples 1–8 were 24.62, 24.61, 24.71, 24.66, 00109, 0.0062, 0.0185, and 0.0176 μg/mL, respectively. Because the signal-to-noise ratios of samples 5–8 were very low, although the determined value was listed, it was only noise. The results show there was no notable change in the nicotine content in the blank solvents or tobacco samples after the addition of the nicotine citrate standard solution.
These results indicate the presence of bound nicotine in the sample had little influence on the determination. In addition, cyclohexane is an inert aprotic solvent32 and has little effect on the distribution of the different forms of nicotine in the sample. Therefore, cyclohexane was considered suitable as the extraction solvent for the analysis of free nicotine in tobacco.
3.1.3. Comparison of Extraction Methods
The effects of the three physical extraction methods (shaking, ultrasonic oscillation, and static extraction) on the analysis of free nicotine in tobacco were investigated. Portions of sieved sample powder (0.10 g, accurate to 0.1 mg) were placed in 100 mL SCHOTT sample bottles and 50.0 mL aliquots of anethole solution (10.0 μg/mL in cyclohexane) were added. Extractions were then conducted via shaking, ultrasonic oscillation, and static extraction, respectively, for the durations given in Table 3. The extracts were immediately analyzed via GC–MS. The results in Table 3 show that 3 h of shaking and 2 h of ultrasonic oscillation were insufficient to completely extract free nicotine from the tobacco. This is possibly because cyclohexane is an organic solvent with a weak polarity, and has poor penetrability through plant cells. Thus, it was unable to completely dissolve free nicotine from tobacco cells in a short period.33 For the extraction with ultrasonic oscillation, the long-term operation increased the temperature of the extract, which likely decreased the determination accuracy and reproducibility. In addition, the cavitation effect during ultrasonic oscillation likely produced a strong transient high temperature and high pressure in parts of the solution,34 which could convert bound nicotine into free nicotine. As a result, the experiments were not conducted with extended extraction times. For static extraction over 24 h, although the extraction time is long, a high extraction efficiency of free nicotine was achieved with ease of operation, good repeatability, and without degradation of bound nicotine. As a result, the static extraction method was adopted for the remainder of the study.
Table 3. Nicotine Analysis Using Different Physical Extraction Methodsa.
| shaking* |
ultrasonic oscillation |
static extraction |
|||
|---|---|---|---|---|---|
| time (min) | free nicotine | time (min) | free nicotine | time (h) | free nicotine |
| 30 | 2.28 | 20 | 2.25 | 22 | 5.75 |
| 60 | 2.77 | 40 | 2.70 | 24 | 5.70 |
| 90 | 3.05 | 60 | 2.98 | 26 | 5.86 |
| 120 | 3.20 | 80 | 3.26 | ||
| 150 | 3.46 | 100 | 3.41 | ||
| 180 | 3.63 | 120 | 3.60 | ||
Note: Data given in units of mg/g. * Rotation speed ∼150 rpm.
3.1.4. Sample Inlet Temperature Investigation
In establishing the GC–MS method, tests were performed to identify the optimum sample inlet temperature on the gas chromatograph. Samples of flue-cured tobacco (0.1057 g) and cigar tobacco leaves (0.1069 g) were added to 100 mL SCHOTT sample bottles. Sample pretreatment and GC–MS analysis were conducted, as described in the Methods section. The results are given in Table 4. When the sample inlet temperature was 200 to 270 °C, the peak area of nicotine increased and then decreased slightly, with increased volatilization temperature. From 240 to 260 °C, the nicotine signal was the highest. With the precision and other factors taken into account, 250 °C was selected as the sample inlet temperature.
Table 4. Sample Inlet Temperature Optimization for GC–MS Analysisa.
| flue-cured tobacco |
cigar |
|||
|---|---|---|---|---|
| temperature (°C) | peak area of nicotine | relative deviation of peak area (%) | peak area of nicotine | relative deviation of peak area (%) |
| 200 | 241 084 | 0.27 | 957 941 | 3.02 |
| 210 | 274 033 | 1.92 | 1 046 144 | 2.18 |
| 220 | 278 573 | 4.86 | 1 049 427 | 2.12 |
| 230 | 289 612 | 0.13 | 1 056 319 | 1.85 |
| 240 | 296 387 | 0.97 | 1 076 930 | 0.85 |
| 250 | 304 335 | 0.64 | 1 077 927 | 1.91 |
| 260 | 307 411 | 1.77 | 1 061 349 | 2.87 |
| 270 | 292 470 | 0.12 | 1 074 440 | 1.77 |
Note: GC–MS, gas chromatography–mass spectrometry.
3.2. Method Validation
3.2.1. Standard Curves and Limit of Detection
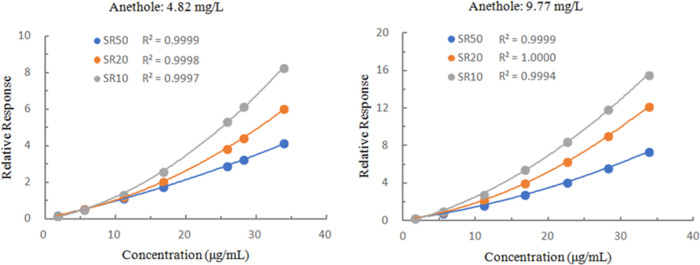
Fitting of the standard curve was achieved using first-order (linear) and second-order (quadratic) regression models. While the correlation coefficient of the linear regression was high (R2 ≥ 0.9995), that of the quadratic regression was slightly higher. To this end, standard solutions with a series of concentrations were prepared, and experiments were carried out using internal standards with different concentrations and different split ratios at the sample inlet. The quadratic regression equation of the standard curve was determined for different experimental conditions. As indicated in Figure 2, the correlation of the standard curve was very good under different experimental conditions and within the concentration range of the experiment (all R2 ≥ 0.9994). The reason may be that as the concentration of the standard solution increases or the split ratio decreases, the amount of nicotine entering the detector increases, and the relationship between the chromatographic peak area and the concentration of the standard solution is not always linear. At low concentration and large split ratio, the peak area response factors of different concentrations of nicotine have little difference. In contrast, the peak area response factors of different concentrations of nicotine differed greatly at high concentration and small split ratio. Specifically, in a narrow concentration range, a linear regression may provide the best fit. For a wider concentration range, only quadratic fitting can be used.35 According to the comprehensive analysis of various conditions, the experiments were carried out with a split ratio of 20:1, and the concentration of the internal standard was 10.0 μg/mL. The determination was conducted with the step-by-step dilution, and the limit of detection (LOD; signal-to-noise ratio = 3) of the experimental method was 5.3 μg/g, fully meeting the requirements for the determination of free nicotine in tobacco. Chromatograms of a standard solution and a sample are shown in Figure 3. In each case, the total ion chromatogram was easily interpreted, and no interfering components were observed. The peak shapes of nicotine and anethole were excellent, and the baseline separation was complete.
Figure 2.
Quadratic regression equations fitted to a standard curve under different experimental conditions. SR50, SR20, and SR10 represent the split ratios of 50:1, 20:1, and 10:1 respectively.
Figure 3.
GC–MS analysis chromatogram of the internal standard and sample. (a) Standard solution; (b) sample solution: anethole (1) and nicotine (2). EI, electron ionization; GC–MS, gas chromatography–mass spectrometry; TIC, total ion current; SIM, selected ion monitoring.
3.2.2. Standard Addition Recovery
Five portions of sieved sample powder (100 mg each, accurate to 0.1 mg) were analyzed according to the solvent extraction and GC–MS procedures described in the Methodssection. The relative standard deviation (RSD) from the experimental method was between 1.3 and 4.1% (Table 5), indicating the experimental method has high precision. The accuracy of the method was verified by determining the standard addition recovery. The standard addition recovery values corresponding to low, medium, and high concentrations were 98.0, 100.9, and 104.7%, respectively (Table 5), indicating that the method has high accuracy.
Table 5. Standard Addition Recovery and Precision of Experimental Methoda.
| base value (mg/g) | amount added (mg/g) | determined value (mg/g) | recovery (%) | RSD (%, n = 5) |
|---|---|---|---|---|
| 6.83 | 1.9 | |||
| 6.83 | 2.73 | 9.51 | 98.0 | 1.3 |
| 6.83 | 5.37 | 12.25 | 100.9 | 1.5 |
| 6.83 | 8.17 | 15.38 | 104.7 | 4.1 |
Note: RSD, relative standard deviation.
3.2.3. Testing of Real Tobacco Samples
The contents of free nicotine and total nicotine in 67 tobacco samples were determined via the experimental method and the standard method.33 In the standard method, sieved sample powder was sonicated in a hydrochloric acid solution. Then, the acidic and neutral compounds were extracted with dichloromethane. The remaining aqueous solution was alkalized by sodium hydroxide to release total nicotine. Finally, the total nicotine was extracted by dichloromethane and determined by GC–MS analysis. The determined free nicotine content in the samples was between 1.41 and 26.03 mg/g, with an average of 11.25 mg/g. The relative deviation of parallel samples during the determination was between 0.1 and 3.1%, indicating the experimental method also has good repeatability (Table 6). In the tested samples, the ratio of free nicotine to total nicotine was 14.2–78.1%, with an average of 40.9%.
Table 6. Contents of Free Nicotine and Total Nicotine in 67 Tobacco Samplesa.
| free nicotine |
free nicotine |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| no. | type | conc. (mg/g) | RD (%) | total nicotine (mg/g) | FN/TN (%) | no. | type | conc. (mg/g) | RD (%) | total nicotine (mg/g) | FN/TN (%) |
| 1 | CigarT | 14.05 | 1.3 | 23.51 | 59.8 | 35 | filler | 17.39 | 1.0 | 50.16 | 34.7 |
| 2 | CigarT | 15.08 | 2.2 | 19.74 | 76.4 | 36 | filler | 8.53 | 1.0 | 34.33 | 24.9 |
| 3 | CigarT | 14.33 | 0.1 | 18.34 | 78.1 | 37 | filler | 8.54 | 2.4 | 30.55 | 28.0 |
| 4 | CigarT | 11.46 | 0.1 | 23.81 | 48.1 | 38 | filler | 23.07 | 1.1 | 67.45 | 34.2 |
| 5 | CigarT | 26.03 | 0.1 | 51.90 | 50.1 | 39 | filler | 18.15 | 0.1 | 53.51 | 33.9 |
| 6 | wrapper | 17.87 | 1.2 | 52.28 | 34.2 | 40 | filler | 25.96 | 1.8 | 76.37 | 34.0 |
| 7 | filler | 22.09 | 1.1 | 49.16 | 44.9 | 41 | filler | 20.28 | 1.4 | 65.19 | 31.1 |
| 8 | cigar | 9.73 | 3.0 | 23.15 | 42.0 | 42 | filler | 10.17 | 2.4 | 15.83 | 64.3 |
| 9 | cigar | 11.01 | 0.3 | 24.44 | 45.1 | 43 | filler | 12.58 | 0.1 | 19.21 | 65.5 |
| 10 | cigar | 7.93 | 0.5 | 17.08 | 46.4 | 44 | filler | 9.50 | 1.6 | 13.60 | 69.9 |
| 11 | cigar | 9.43 | 3.1 | 16.84 | 56.0 | 45 | filler | 15.74 | 2.7 | 41.94 | 37.5 |
| 12 | cigar | 11.68 | 0.5 | 22.84 | 51.1 | 46 | filler | 7.14 | 0.6 | 11.14 | 64.0 |
| 13 | cigar | 11.51 | 1.4 | 22.28 | 51.7 | 47 | binder | 2.47 | 2.0 | 12.10 | 20.4 |
| 14 | cigar | 14.17 | 1.3 | 25.49 | 55.6 | 48 | filler | 2.55 | 1.2 | 14.19 | 17.9 |
| 15 | cigar | 7.63 | 0.8 | 16.25 | 47.0 | 49 | filler | 1.41 | 1.6 | 8.44 | 16.7 |
| 16 | cigar | 10.51 | 0.9 | 20.66 | 50.9 | 50 | binder | 10.89 | 0.4 | 31.20 | 34.9 |
| 17 | cigar | 13.73 | 2.0 | 26.75 | 51.3 | 51 | filler | 11.51 | 1.3 | 30.04 | 38.3 |
| 18 | cigar | 5.28 | 0.6 | 27.21 | 19.4 | 52 | filler | 6.96 | 1.6 | 18.36 | 37.9 |
| 19 | cigar | 8.37 | 0.6 | 15.10 | 55.4 | 53 | filler | 10.24 | 2.0 | 21.49 | 47.7 |
| 20 | cigar | 9.59 | 1.1 | 17.24 | 55.6 | 54 | filler | 10.79 | 2.7 | 22.81 | 47.3 |
| 21 | cigar | 13.40 | 0.4 | 22.97 | 58.3 | 55 | CutT | 4.18 | 0.8 | 19.39 | 21.5 |
| 22 | cigar | 9.02 | 1.7 | 18.15 | 49.7 | 56 | CutT | 3.96 | 1.7 | 22.27 | 17.8 |
| 23 | cigar | 8.13 | 2.7 | 17.49 | 46.5 | 57 | CutT | 4.66 | 1.8 | 24.81 | 18.8 |
| 24 | CigarT | 8.61 | 0.4 | 20.32 | 42.4 | 58 | CutT | 4.39 | 1.5 | 25.98 | 16.9 |
| 25 | CigarT | 25.47 | 0.8 | 36.09 | 70.6 | 59 | CutT | 5.27 | 2.4 | 25.95 | 20.3 |
| 26 | CigarT | 18.05 | 0.6 | 39.77 | 45.4 | 60 | FCT | 2.88 | 1.0 | 19.14 | 15.0 |
| 27 | CigarT | 23.61 | 0.6 | 60.58 | 39.0 | 61 | FCT | 2.70 | 1.4 | 18.96 | 14.2 |
| 28 | CigarT | 23.49 | 0.1 | 48.72 | 48.2 | 62 | FCT | 4.45 | 1.1 | 22.89 | 19.5 |
| 29 | CigarT | 17.63 | 1.7 | 31.98 | 55.1 | 63 | FCT | 6.24 | 1.2 | 25.46 | 24.5 |
| 30 | CigarT | 20.01 | 1.8 | 27.37 | 73.1 | 64 | FCT | 5.96 | 1.5 | 22.74 | 26.2 |
| 31 | filler | 3.97 | 0.5 | 10.72 | 37.1 | 65 | FCT | 7.58 | 3.1 | 35.41 | 21.4 |
| 32 | filler | 9.82 | 0.7 | 24.51 | 40.0 | 66 | FCT | 8.65 | 0.8 | 25.49 | 33.9 |
| 33 | filler | 3.64 | 2.5 | 19.62 | 18.6 | 67 | FCT | 6.40 | 2.4 | 30.14 | 21.2 |
| 34 | binder | 16.40 | 1.0 | 38.57 | 42.5 | ||||||
Note: CigarT, cigar tobacco; CutT, cut tobacco; FCT, flue-cured tobacco; FN/TN, ratio of free nicotine to total nicotine; RD, relative deviation.
4. Discussion
The objective of the experiment was to develop a method for detecting the content of free nicotine in traditional tobacco and its products, i.e., tobacco leaf and cut tobacco, that could fulfill the testing needs of the project team and the entire company. Traditionally, free nicotine in tobacco has been determined through methods, such as pH calculation, 1H NMR, HS–SPME, and liquid–liquid extraction; however, each has serious limitations that affect the accuracy of determination. Here, we optimized a solvent extraction method to improve the extraction efficiency of free nicotine, as well as simplify the pretreatment process.
First, free nicotine was extracted with deionized water and subsequently isolated using an organic solvent. The recovery was low because of the conversion of free nicotine to protonic nicotine. Next, free nicotine was extracted with an organic solvent, and subsequently, the organic extract was washed with water to remove protonic nicotine prior to analysis. This method was expected to work well. However, the recovery experiments, using two free-base state nicotine standards, produced unsatisfactory results. The low recovery was a result of the extraction of free nicotine into the aqueous phase. In an aqueous solution, the ratio of free nicotine to total nicotine only reached 57.43% at pH 8.0 and 11.89% at pH 7.0 (Table 1). After the free-base state nicotine standard was added to the organic–aqueous two-phase system, most of the free nicotine moved into the aqueous phase and became protonated. This was unexpected, as free nicotine is more soluble in the organic phase. This phenomenon explains the low recovery in the experiments and the inaccurate results during real sample determination. Therefore, the use of water as a solvent for the extraction or purification of tobacco samples was eliminated to reduce shifts in the species distribution of nicotine.
Cyclohexane is an aprotic, nonpolar solvent that does not easily dissociate during the extraction of free nicotine from tobacco. Because cyclohexane does not provide protons, it has little effect on the original species distribution of nicotine. Thus, the results were more representative of the real distribution in the sample. In this paper, a static cyclohexane extraction method for free nicotine determination in tobacco leaves was first reported. The aqueous solvent was eliminated; thus, authors reduced the loss of free nicotine due to switching the solvent environment. The use of static extraction also resulted in better recoveries than other physical extraction techniques. The method is simple and produced excellent results. Moreover, cyclohexane was used to extract free nicotine from tobacco samples, and the presence of organic acids and bound nicotine in the tobacco did not interfere with the determination process. Therefore, detailed validation experiments were performed, which yielded satisfactory results.
We attributed the success of the cyclohexane extraction method to the ability of cyclohexane to selectively dissolve nicotine in the free state, but not in the protonated state, from the plant the vacuoles. The vacuoles store metabolites, such as nicotine, organic acids, and pigments, which occupy 90% of the cell volume during plant cell maturation (Figure 4). During extraction, cyclohexane penetrates the porous middle lamella and cell wall. After penetrating the cell wall, cyclohexane disintegrates the hydrophobic phospholipid cell membrane and tonoplast successively to extract free nicotine from the vacuoles. Moreover, the protonated nicotine cannot be extracted because it is insoluble in cyclohexane. In addition, the method was not affected by the presence of nicotine salts. Although some of the bound nicotine is highly soluble in cyclohexane, these compounds could have different retention times from free nicotine in the GC–MS spectra; thus, it does not interfere with the determination. A GC–MS full scan analysis of the sample solution was also conducted. The results reveal that no bound nicotine was detected within the detection window. Researchers have speculated that the solubility difference may be the reason bound nicotine would not interfere with the determination when cyclohexane was used to extract free nicotine from tobacco.33 In this case, the free nicotine, dissolved in cyclohexane, can be easily extracted and detected, whereas bonded nicotine salts that are not soluble in cyclohexane cannot be detected. In future studies, we speculate that similar results may be obtained using aprotic, nonpolar solvents similar to cyclohexane, e.g., hexane.
Figure 4.

Diagram of the plant cell structure.
5. Conclusions
A novel solvent extraction and GC–MS method for the direct determination of the free nicotine content in tobacco was established. The standard addition recovery of the experimental method was between 98.0 and 104.7%. The LOD was 5.3 μg/g, with a relative standard deviation between 1.3 and 4.1% (n = 5). The correlation coefficient of the quadratic regression of the standard curve was high (R2 ≥ 0.9994), demonstrating a good fit. Of the 67 tobacco samples tested in the study, the relative deviation of parallel samples was 0.1–3.1%, indicating that the experimental method had good repeatability.
The method involved static extraction for 24 h of free nicotine in tobacco samples with cyclohexane, followed by GC–MS analysis. Compared with alternative solvent extraction procedures that incorporate a water extraction or purification step, the method presented here directly determined free nicotine content in the organic cyclohexane extract with high analytical figures of merit. The method is easy to perform, and by avoiding a two-part organic–aqueous extraction system, the extraction efficiency and accuracy were remarkably improved. Therefore, the results of this method are considered to closely represent the actual distribution of free nicotine in the sample.
Acknowledgments
The authors are grateful for the financial support from the China Tobacco Sichuan Industrial Co., Ltd. (nos. ctx201901, js202212, and jc202203). They also thank LetPub (www.letpub.com) and Liwen Bianji (Edanz) (www.liwenbianji.cn) for language editing of this manuscript, and Yonghong Tian, Xixiang Zhang, Junhua Li, Guoce Liu, and De’an Lei of the Technology Center of China Tobacco Sichuan Industrial Co., Ltd. for their help with the GC–MS experiments.
The authors declare no competing financial interest.
References
- Davis D. L.; Nielsen M. T.. Tobacco – Production, Chemistry and Technology; Wang Y. T.; Yuan X. S.; Jin Z. L.; Xie J. P.; Yu M. F.; Liu G. Y.; Zhang H.; Zheng F. G.; Zheng X. Z.. et al. , Eds.; Chemical Industry Press: Beijing, 2003. [Google Scholar]
- Han F. G.; Yan K. Y.; Zhao M. Q.; Yin Q. Y.; Lu H.; Li Y. S.; Zhou Y.; Zhou X. Z.; Ji X. M.; Wang R. X.. et al. Tobacco alkaloids. In Tobacco Chemistry, 2nd ed.; China Agricultural Press: Beijing, 2010; pp 85–125. [Google Scholar]
- Armitage A. K.; Turner D. M. Absorption of nicotine in cigarette and cigar smoke through the oral mucosa. Nature 1970, 226, 1231–1232. 10.1038/2261231a0. [DOI] [PubMed] [Google Scholar]
- Schievelbein H.; Eberhardt R.; Löschenkohl K.; Rahlfs V.; Bedall F. K. Absorption of nicotine through the oral mucosa I. Measurement of nicotine concentration in the blood after application of nicotine and total particulate matter. Agents Actions 1973, 3, 254–258. 10.1007/BF01968551. [DOI] [PubMed] [Google Scholar]
- Chuanfang Y.; Binbin L.; Dingrong M. Correlation of cigarette impact with major chemical components in cut tobacco and smoke. Tob. Sci. Technol. 2006, 39, 34–37. [Google Scholar]
- Wang M. F.; Zeng X. Y.; Li X. Y.; Liao T. G.; Zhu B. K.; Xia J. J. Effects of tatal nicotine and free-base nicotine content in virginia type cigarette smoke on sensory coziness. Food Ind. 2010, 31, 27–31. [Google Scholar]
- Duell A. K.; Pankow J. F.; Peyton D. H. Nicotine in tobacco product aerosols: ‘It’s déjà vu all over again’. Tob. Control 2020, 29, 656–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H.; Liu Z. H.; Si X. X.; Fan Y.; Wang K.; Zhang F. M.; Shen Q. P.; Zhang T.; Miao M. M.; Zhu R. Z. Effects of diammonium phosphate on free nicotine release in smoke and sensory quality of cigarette. Tob. Sci. Technol. 2015, 48, 28–32. [Google Scholar]
- Lee J. M.; Jang G. C.; Hwang K. J.; Kim Y. H.; Rhee M. S. Calculation of free nicotine by determination of pH and nicotine in tobacco. J. Korean Soc. Tob. Sci. 2005, 27, 219–225. [Google Scholar]
- Mingliang S.; Ming W.; Jianping X. Advance on the forms of nicotine in tobacco and smoke. Tob. Sci. Technol. 2004, 37, 20–26. [Google Scholar]
- Binbin L.; Huimin L.; Jianping X. Review of determination of smoke pH and its relationship with different forms of nicotine. Tob. Sci. Technol. 2002, 35, 19–22. [Google Scholar]
- Wang Y. G.; Qin Y. H.; Lu S. M.; Miao M. M.; Cao Q. E. Review on the determination of free nicotine from total particulate matter of mainstream smoke. Yunnan Chem. Technol. 2007, 34, 59–62. [Google Scholar]
- Lu L. H.; Ru J. X.; Li X. L.; Gao F. H.; Xie Y. Determination and distribution of free-base and protonated nicotine in e-liquids. Tob. Sci. Technol. 2021, 54, 36–44. [Google Scholar]
- Lu B.; Xie J.; Liu H. Relationship between free nicotine in tobacco and its pH value. Tob. Sci. Technol. 2003, 36, 6–10. [Google Scholar]
- Lu L. J.; Zhang H. T.; Zhou H.; Hao H.; Zhang D. Y.; Zhang J. S. Determination on free nicotine in tobacco. J. Anhui Agric. Sci. 2009, 37, 17946–17947. [Google Scholar]
- Wang M. F.; Liu X. M.; Zhu B. K.; Xia J. J.; Wang Q. Z. Fast determination of nicotine in both free-and protonated form in mainstream tobacco smoke by UPLC. J. Yunnan Univ. Nat. Sci. Ed. 2010, 32, 463–468. [Google Scholar]
- Li W. L.; Ma Y. H.; Duan Y. J.; Xu J. C. Determination of free nicotine in tobacco by ultraviolet spectrophotometry. J. Anhui Agric. Sci. 2012, 40, 3600–3601. [Google Scholar]
- Lu B. B.; Xie J. P.; Liu H. M. Studies on the relationship between pH value and free nicotine in cigarette smoke. Acta Tab. Sin. 2005, 11, 7–16. [Google Scholar]
- Zhang H.; Zhu R. Z.; Meng Z. Y.; Wang K.; Mou D. R.; Zhu L. H.; Xiang N. J. Analysis of the determination and distribution of free nicotine in the mainstream smoke of cigarettes. Phys. Test Chem. Anal. B 2014, 50, 1444–1447. [Google Scholar]
- Yin Q. Y.; Yang T. Z.; Guo B. Y.; Huang J. H. Determination of free nicotine in tobacco leaves by UV spectrophotometry. Chin. Tob. Sci. 2008, 29, 20–22. [Google Scholar]
- Gholap V. V.; Heyder R. S.; Kosmider L.; Halquist M. S. An analytical perspective on determination of free base nicotine in e-liquids. J. Anal. Methods Chem. 2020, 2020, 6178570 10.1155/2020/6178570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hellani A.; El-Hage R.; Baalbaki R.; Salman R.; Talih S.; Shihadeh A.; Saliba N. A. Free-base and protonated nicotine in electronic cigarette liquids and aerosols. Chem. Res. Toxicol. 2015, 28, 1532–1537. 10.1021/acs.chemrestox.5b00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salman R.; Talih S.; El-Hage R.; Haddad C.; Karaoghlanian N.; El-Hellani A.; Saliba N. A.; Shihadeh A. Free-base and total nicotine, reactive oxygen species, and carbonyl emissions from IQOS, a heated tobacco product. Nicotine Tob. Res. 2019, 21, 1285–1288. 10.1093/ntr/nty235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie J. P. Transfer of nicotine from tobacco to smokers: A brief review of ammonia and the “pH” factors. Tob. Sci. Technol. 2001, 34, 28–31. [Google Scholar]
- Wang Z. Y.; Wei Y. L. Study on the conduct of free nicotine cigarette smoke and smoke moisture content of “Tarzan” cigarette adding by cigarette paper additives. Food Ind. 2012, 33, 50–53. [Google Scholar]
- Bao M.; Joza P.; Rickert W. S.; Lauterbach J. H. An improved headspace solid-phase microextraction method for the analysis of free-base nicotine in particulate phase of mainstream cigarette smoke. Anal. Chim. Acta 2010, 663, 49–54. 10.1016/j.aca.2010.01.036. [DOI] [PubMed] [Google Scholar]
- Wang Q. A.; Wang M. F.; Zhe W.; He L.; Zhu B. K.; Liao T. G. Determination of free-base nicotine in main-stream cigarette smoke by HS-SPME GC/MS. J. Hunan Univ. Nat. Sci. 2011, 38, 70–75. [Google Scholar]
- Duell A. K.; Pankow J. F.; Peyton D. H. Free-base nicotine determination in electronic cigarette liquids by 1H NMR spectroscopy. Chem. Res. Toxicol. 2018, 31, 431–434. 10.1021/acs.chemrestox.8b00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K. Y.; Gong Z. L.; Zhang Y. Contrastive analysis of pH values of flue-cured tobacco leaves. Tob. Sci. Technol. 2007, 40, 49–52. [Google Scholar]
- Du S.; Wu Y. C.; Yao Z. D. Study on quantitative analysis method of free nicotine in tobacco by aqueous extraction system. J. Anhui Univ. 2006, 30, 91–94. [Google Scholar]
- Yan K. Y.Tobacco Alkaloids. In Tobacco Chemistry; Zhengzhou University Press: Zhengzhou, 2002; pp 113–135. [Google Scholar]
- Sun Y. Q.; Hu Y. Z.. Classification and Optimization of Solvent Systems. In Selection and Optimization of Liquid Chromatography Solvent Systems; Chemical Industry Press: Beijing, 2008; pp 243–264. [Google Scholar]
- Ding L.Analysis and Study of Alkaloids in Tobacco and Mainstream Smoke of Cigarettes; University of Science and Technology of China: Hefei, 2004. Master’s Thesis. [Google Scholar]
- Liu X. Y.; Liao Y. C.; Wang Y.; Tian Q. Y.; Xie K. X.; Ou H. Research progress on application of ultrasound hyphenated technique in extraction of active phytochemicals. Food Ferment. Ind. 2021, 10.13995/j.cnki.11-1802/ts.029505. [DOI] [Google Scholar]
- Lavagnini I.; Magno F. A statistical overview on univariate calibration, inverse regression, and detection limits: Application to gas chromatography/mass spectrometry technique. Mass Spectrom. Rev. 2007, 26, 1–18. 10.1002/mas.20100. [DOI] [PubMed] [Google Scholar]