Abstract
Azithromycin (AZM), a macrolide antibiotic used for the treatment of chlamydial conjunctivitis, is less effective for the treatment of this disease due to its poor bioavailability (38%). Various alternatives have been developed for improving the physicochemical properties (i.e., solubility) of the AZM without much success. To overcome the problems associated with AZM, an inclusion complex employing a modified cyclodextrin, i.e., sulfobutylether-β-cyclodextrin (SBE-β-CD), was prepared and characterized by phase solubility studies and PXRD techniques. The results portrayed the formation of an inclusion complex of AZM with SBE-β-CD in 1:2 molar stoichiometric ratios. This inclusion complex was later incorporated into a polymer matrix to prepare an in situ gel. Various combinations of Carbopol 934P and hydroxypropyl methylcellulose (HPMC K4M) polymers were used and evaluated by rheological and in vitro drug release studies. The optimized formulation (F4) containing Carbopol 934P (0.2% w/v) and HPMC K4M (0.2% w/v) was evaluated for clarity, pH, gelling capacity, drug content, rheological properties, in vitro drug release pattern, ocular irritation test, and antimicrobial efficacy. Finally, owing to the improved antimicrobial efficacy and increased residence time, the AZM:SBE-β-CD in situ gel was found to be a promising formulation for the efficient treatment of bacterial ocular disease.
1. Introduction
The eye being one of the most important parts of the body has been the subject of intense research for decades.1,2 Many forms of eye-related infections are caused by pathogenic bacteria. For instance, Bacillus cereus causes endophthalmitis and Haemophilus influenza causes conjunctivitis, yet the conventional formulations are ineffective for a complete treatment. Various topical formulations have been developed to date for ophthalmic drug delivery; these include solutions, ointments, gels, and polymeric inserts to name a few.3 Additionally, there are some dosage forms that have been reported to increase the corneal contact time. However, ointments and ocular inserts lead to poor patient compliance and cause blurred vision.4
Azithromycin (AZM) is a semisynthetic and acid-stable macrolide antibiotic possessing a wide spectrum of antibacterial activity toward Gram-negative bacilli and periodontal pathogens like Aggregatibacter actinomycetemcomitans (previously Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis). Various studies support its use for the treatment of periodontal infections,5 ocular toxoplasmosis,6 etc. It is pharmaceutically a highly lipophilic drug with a half-life t1/2 = 68 h and good tissue penetrability, which encompasses a possibility for its prolonged release.7 Despite this good penetrability of AZM, its aqueous solubility remains a major limitation for its formulation development. Thus, there are various solubility enhancement techniques, i.e., solid dispersion, inclusion complex formation, co-crystallization, etc. to solve this. With advances in the field of drug delivery, the limitations of conventional ophthalmic formulations, i.e., large dosing frequency, dose inconsistency, and instillation spillage, could be overcome. Thus, various drug delivery systems have been formulated via modifying the physicochemical properties of drugs, which increase their safety as well as efficacy, both pharmacokinetically and pharmacodynamically. In addition, Biopharmaceutical Classification system (BCS) class II drugs have always been the main targeted molecules for pharmaceutical product development. Since solubility is the only factor, which could be easily changed by modification, it was worth exploring. So far, cyclodextrin is considered to be an excellent excipient for solubility enhancements. Also, it is a Food and Drug Administration (FDA)-approved generally-regarded-as-safe (GRAS) excipient for animal and human administration.8 Structurally, cyclodextrins are truncated cones with an inner hydrophobic cavity9 and an outer surface having hydroxyl groups for forming noncovalent interactions with physiological media. The drug and CD inclusion complex formed is in a dynamic equilibrium with the medium and it dissociates, thereby releasing the drug at the desired site. Thus, it is considered to be a pharmaceutical solubilizer to improve the solubility profile of BCS class II drugs (low solubility, high permeability) and their in vivo pharmacokinetics profile.10 Nowadays, various derivatives of cyclodextrins are available, which have been investigated for pharmaceutical, biomedical, and other applications.
Ophthalmic drug delivery is proven to be effective only with the bioavailability of desired concentration of drugs at the site of action within the eye. Generally, SBE-β-CD has a degree of substitution of 6.611 and binding was fairly independent.12 It has been found that the most effective stabilization of drugs is obtained by using sulfobutylether-β-cyclodextrin (SBE-β-CD) compared to HP-βCD, which is better in increasing the solubility,13 which allows a concentration of AZM in solution of 99% up to 6 months at room temperature. The positive action of sulfobutylether-β-cyclodextrin was mainly exerted by suppressing a degradation pathway leading to the opening of the lactone ring of AZM.14 Besides AZM, other ophthalmic drugs like cyclosporin A (CsA) and moxifloxacin hydrochloride are also poorly water-soluble for the treatment of dry eye and infections, respectively. AZM has poor aqueous solubility because the dissolution of the drug is low in physiological media leading to low bioavailability. Therefore, parameters like dose requirement and dosing frequency need to be checked for improved safety and antimicrobial efficacy during formulation development. In addition, AZM usage also leads to drainage or crusting of the eye, severe burning, stinging, itching, or irritation besides causing eye pain, redness, swelling, and other signs of infections (fever, sore throat, swelling in the face or tongue, burning in the eyes, inflammation of the skin followed by a red or purple skin rash that spreads (especially in the face or upper body), blistering and peeling).15 Ointments, emulsions, oily solutions, and suspensions16 also exhibit poor bioavailability due to solubility issues and rapid washout during lachrymation.17,18 Solubilizing systems such as cyclodextrins, liposomes, and microemulsions enhance drug solubility in the aqueous tear fluid; prodrugs increase the lipophilicity of hydrophilic drugs and enhance their partition from the aqueous tear film into the lipophilic membrane barrier; mucoadhesive polymers and nanogels increase drug residence time on the eye surface, and nanoparticles are thought to enhance drug permeation into the mucus.19 For improving the antimicrobial efficacy and for prolonged action of moxifloxacin, an in situ gel was prepared using sodium alginate as the gelling agent. This resulted in gelation after instillation into the eye due to physicochemical changes (i.e., pH), thereby increasing the pre-corneal residence time and overall bioavailability of the drug in the eye.20 Hence, it was observed that the sol-to-gel phase transition of the in situ gel on the eye surface would be dependent upon the use of different polymeric compositions and their structural behavior at varied pH ranges. In addition, the sol–gel formation was proven to be effective by usage of numerous thermosensitive, ion-activated, electrosensitive, magnetic field-sensitive, ultrasonic-sensitive, and chemical material-sensitive approaches. Subsequently, many researchers have reported various systems such as (i) pH-triggered (e.g., cellulose acetate hydrogen phthalate latex), (ii) temperature-dependent (e.g. pluronics and tetronics), and (iii) ion activation (i.e., gelrite)21 for gel formation, and (iv) eye drop formulations based on γ-cyclodextrin (γCD) nanoparticle aggregates. These technologies have the potential to be used with other classes of drug molecules and to replace or complement invasive treatments, providing safer, non-invasive therapies, particularly for posterior segment conditions that can be self-administered as eye drops by patients.22 In fact, another group had prepared an in situ gel for ophthalmic preparation containing ciprofloxacin hydrochloride, which was successfully formulated as in situ gel-forming eye drops with the help of sodium alginate and hydroxypropyl methylcellulose (HPMC).23 These results demonstrated that the alginate and HPMC mixture could be used as in situ gelling carriers to improve ocular bioavailability and patient compliance. Moreover, various antimicrobial agents have poor bioavailability leading to inadequacy in the therapeutic regime of the eyes. There are some thermosensitive and mucoadhesive in situ gelling systems based on poloxamer and Carbopol 1342P or 934P exhibiting the potential to enhance ocular bioavailability.24 To date, several groups have reported the use of lipid-based systems,25−27 a niosomal gel,28 and polymeric topical mucoadhesive systems29,30 of AZM for improving its pharmaceutical attributes.
In the present work, we have reported the in situ gel formulation in which an azithromycin–sulfobutylether-β-cyclodextrin (AZM-SBE-β-CD) inclusion complex has been used with enhanced solubility characteristics31 for ophthalmic delivery purposes. SBE-β-CD32 is a cyclodextrin derivative that has been widely employed for solubility enhancement via the formation of an inclusion complex. In addition to this, it is also non-irritating33 and acts as an osmotic agent.34,35 Therefore, it has been considered a suitable excipient for ocular delivery of AZM.36−38 It is based on the concept of pH-triggered in situ gelation for ophthalmic delivery of AZM with Carbopol 934P as the gelling agent. Carbopol 934P is known for its pH-dependent sol–gel phase transition; thus, the AZM:SBE-β-CD inclusion-based in situ gel39 may prove to be a feasible and viable substitute to conventional eye drops by prolonging the pre-corneal residence time and providing a sustained released characteristic for AZM-based delivery systems.40
2. Results and Discussion
2.1. Characterization of the Inclusion Complex of AZM and SBE-β-CD in the Solid Phase Using XRD
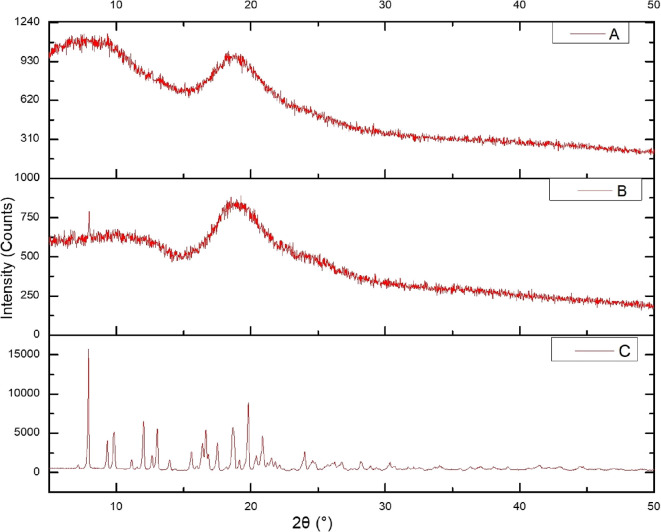
The powder X-ray diffraction (PXRD) pattern of AZM shows intense sharp peaks at 2θ at 9.30, 9.811, 18.65, 18.75, and 19.13 as shown in Figure 1. The spectrum contains various crystalline peaks for AZM. However, SBE-β-CD being amorphous in nature shows less intense peaks.41 A sharp crystalline peak was observed for AZM at 9.30 (Figure 1C); however, due to the formation of the inclusion complex of AZM and SBE-β-CD (Figure 1A,B), the peak disappeared as a result of the change in the environment. The crystalline peaks of AZM around 16.83, 17.49, and 20.36 were also reduced, indicating a reduction in its crystallinity. Further, the decline at 9.811 indicates a decrease in crystallinity of SBE-β-CD after the inclusion complex formation. Moreover, the halo pattern in XRD of the complex indicates the complete inclusion of the drug in the cyclodextrin cavity.42
Figure 1.
X-ray diffraction pattern of formulations components: (A) AZM:SBE-β-CD inclusion complex (1:2), (B) SBE−β-CD, and (C) AZM. Here, the X axis corresponds to the diffraction angle between the incident beam and transmitted beam, whereas the count on the Y axis represents intensity of the peak.
2.2. Analytical Method Validation Using UV–Visible Spectroscopy and Dose Calibration of AZM in Phosphate Buffer (pH 7.4)
The λmax of AZM was found to be 232 nm in phosphate buffer (pH 7.4); the slope was found to be 0.002, respectively, with a coefficient of determination (R2) of 0.989 as shown in Figure 2A. The accuracy of the developed UV method was 99.48, 98.96, and 100.35%, whereas the precision results were in the range of 0.45, 0.96, and 0.29%. Both accuracy and precision were within limits as per the guideline prescribed by ICH Q2 (R1). The accuracy and precision as seen by the UV spectroscopy are presented in Table 1. Robustness was calculated by varying wavelengths (±5 nm) close to the selected λmax (232 nm). The % deviation between actual and observed concentration was calculated. As presented in Table 2, the results showed that the percentage deviation at lower wavelength up to 232 nm is 0.76%. However, at a higher wavelength up to 237 nm, the variation is 0.29%. LOD was observed to be 24.75 μg/mL, and LOQ was observed to be 75 μg/mL. The method developed in the study was observed to be accurate from a precise and sensitive point of view and also is robust and cost-effective. The aforesaid validation ensures that the method used for the estimation of AZM is promising. The solubility of AZM in phosphate buffer (pH 7.4) was found to be 0.22 mg/mL. Further, it was observed that 2.27 mL of the dissolution medium was required to dissolve a single dose of AZM (0.5 mg) as shown in Table 3.
Figure 2.
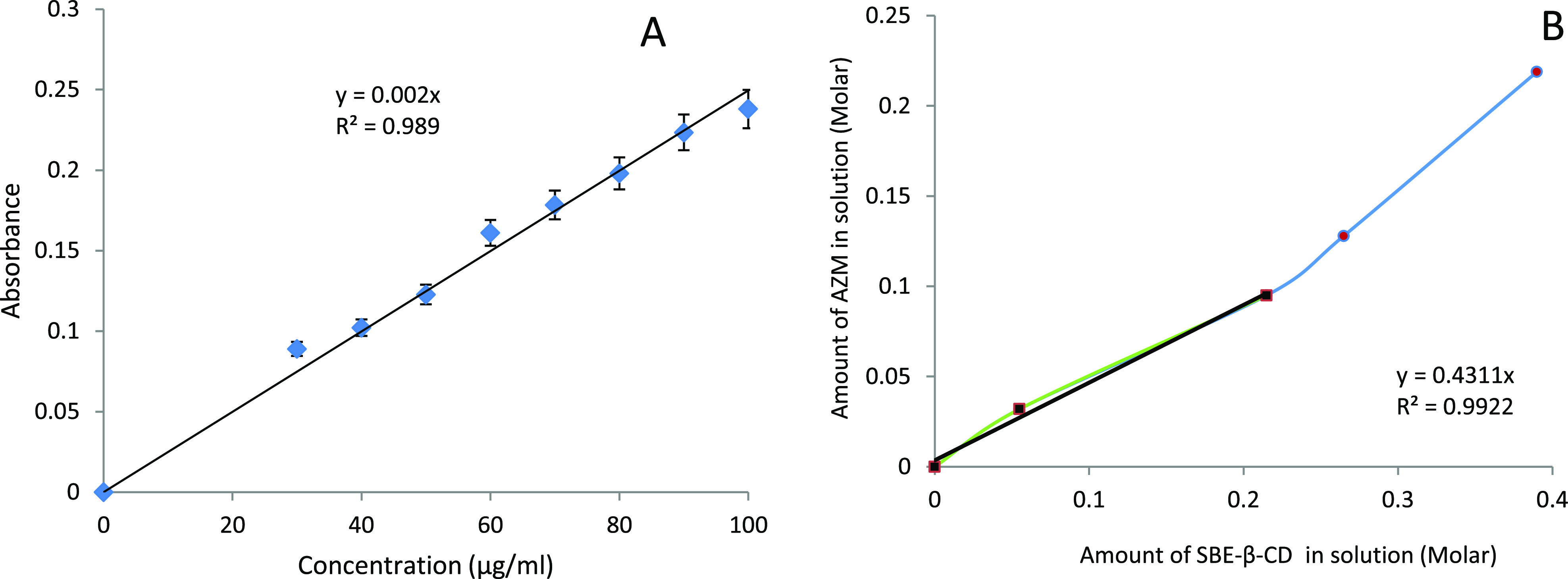
(A) Calibration plot of AZM in phosphate buffer (pH 7.4) was linear in the concentration range of 30–100 μg/mL. The R2 of the plot is 0.989. (B) Phase solubility plot depicts an increase in solubility of AZM with an increasing concentration of SBE-β-CD having R2 = 0.994.
Table 1. Accuracy and Precision Data.
| actual conc. (μg/mL) | observed conc.(μg/mL) | mean conc.(μg/mL) | standard deviation (SD; ±) | nominal % (accuracy) | (% CV) precision | ||
|---|---|---|---|---|---|---|---|
| 64 (80%) | 63.5 | 64.0 | 63.5 | 63.67 | 0.28 | 99.48 | 0.45 |
| 80 (100%) | 78.5 | 80.0 | 79.0 | 79.16 | 0.76 | 98.96 | 0.96 |
| 96 (120%) | 96.0 | 96.5 | 96.5 | 96.33 | 0.28 | 100.35 | 0.29 |
Table 2. Robustness Data for UV Spectrophotometric Method of AZM at Different Wavelengths.
| wavelength (nm) | Actual Conc. (μg/mL) | Concentration (μg/mL) | mean conc. (μg/mL) | % deviation | ||
|---|---|---|---|---|---|---|
| 237 | 80 | 80 | 79.5 | 80 | 79.83 | 0.29 |
| 232 | 80 | 79 | 80.5 | 79.5 | 79.66 | 0.76 |
| 227 | 80 | 80 | 80.5 | 79.5 | 80 | 0.50 |
Table 3. Solubility of AZMa.
| solvents | solubility (mg/mL) |
|---|---|
| phosphate buffer (pH 7.4) | 0.22 |
| distilled water | 0.0020 |
Single application of one drop (0.05 mL) containing 0.5 mg of AZM.
2.3. Solubility Enhancement of AZM Using Phase Solubility Study
The phase solubility diagram was obtained at 25 °C by plotting the apparent equilibrium concentrations of the drug against SBE-β-CD concentrations (Figure 2B). The apparent solubility of AZM increased linearly as a function of SBE-β-CD concentration (R2 = 0.994) up to 0.1 M, corresponding to the aqueous solubility of SBE-β-CD. A positive deviation from the linear relation between AZM solubility and SBE-β-CD43 concentration indicates an AP type of phase solubility graph exhibiting a linear relationship,44 defined by Saokham et al.(10) The molar ratio determined by earlier equations was found to be 1:2, and the complexation efficiency was found to be 0.758. Additionally, this value explains the successful formation of a 1:2 complex and suggested that a water-soluble complex was formed in solution. The slope of the curve (m) was found to be 0.4311 along with a correlation coefficient of 0.9922, as shown in Figure 2B. The phase solubility studies showed that SBE-β-CD increases the solubility of the drug linearly.45 The S0 value was calculated from Figure 2B and was found to be 0.0020 mg/mL.
2.4. Study of AZM in the Presence of SBE-β-CD and Its Antimicrobial Efficacy
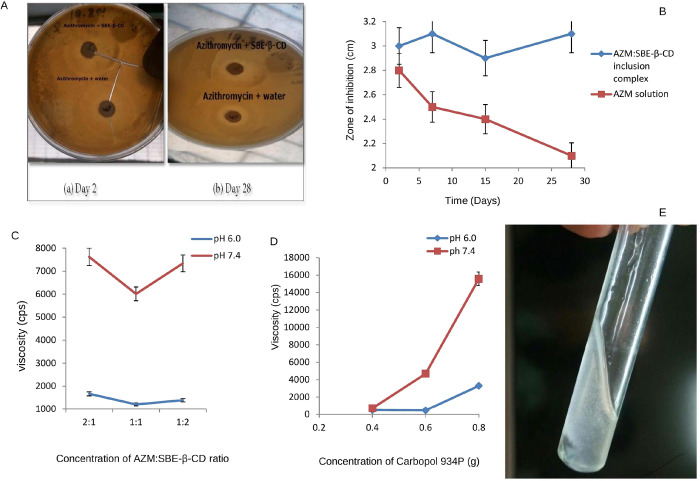
The physical mixture showed similar inhibitory activity to that observed for free AZM while the isolated SBE-β-CD showed no antibacterial effect (Figure 3A). Further, the antimicrobial study depicted that the AZM:SBE-β-CD inclusion complexes had the same inhibitory effect as the free AZM, indicating that the drug stability was maintained throughout the preparation methods in this study. The antimicrobial study indicates no significant difference the in zone of inhibition of plain AZM aqueous solution and its aqueous solution with SBE-β-CD (Figure 3A). Moreover, zones of inhibition of AZM aqueous solution decreased significantly with time (t test, p < 0.05), whereas the zones of inhibition of AZM aqueous solution with SBE-β-CD remained unchanged. This indicates the stabilizing effect of SBE-β-CD on AZM as shown in Figure 3B. Thus, from the above study it was observed that the inclusion of AZM in SBE-β-CD improved the storage stability and had antimicrobial activity when compared to AZM aqueous solution.
Figure 3.
(A) Photographic image of the nutrient agar plate portrays the zone of inhibition for (a) azithromycin solution and a physical mixture of AZM and SBE-β-CD and (b) AZM:SBE-β-CD inclusion complex and azithromycin solution after day 2 and day 28. (B) Graphical representation for zone of inhibition vs time of the AZM:SBE-β-CD inclusion complex (black color filled square dots) and AZM solution (red color filled circles). (C) Graph showing the average effect of concentration of AZM:SBE-β-CD on viscosity at 20 rpm at pH 6.0 (solution phase; blue color) and pH 7.4 (gel phase; brown color). (D) Graph showing the average effect of concentration of Carbopol 934P on viscosity at 20 rpm at pH 6.0 (solution phase; blue color) and pH 7.4 (gel phase; brown color). (E) Formation of the AZM ophthalmic in situ gel.
2.5. Measurement of Gelling Capacity and Determination of Drug Content of the AZM In Situ Gel
The AZM content of all formulations was found to range between 85.9 ± 8.192 and 95.2 ± 4.25% (Table 4). The drug content of all formulations was found within the compendia specification limit of 85–115%. This is a remarkable observation for these formulations of in situ gel. The gelling capacity data of the prepared formulations presented in Table 4 show that the formulation F2 underwent prompt gelation and rapid dissolution. On the contrary, the formulation F8 had immediate gelation but existed only for 1 h. This connoted that the transition time of the formed gel was reduced, whereas the erosion time was increased on increasing the polymer concentration.
Table 4. Gelling Capacity and Percentage Drug Content of Prepared Formulations of AZM In Situ Gel.
| formulation code | gelling capacity | result | % drug content |
|---|---|---|---|
| F1 | – | no gelation | 95.2 ± 4.25 |
| F2 | + | gel formation after a few minutes, dissolves rapidly | 89.7 ± 2.532 |
| F3 | ++ | instant gelation, remains for a few hours | 92.2 ± 3.619 |
| F4 | +++ | immediate gel formation and stable for longer period | 93.1 ± 3.05 |
| F5 | ++ | instant gelation, remains for a few hours | 94.3 ± 3.883 |
| F6 | ++ | instant gelation, remains for a few hours | 93.6 ± 6.686 |
| F7 | ++ | instant gelation, remains for a few hours | 85.9 ± 8.192 |
| F8 | +++ | immediate gel formation and stable for a longer period | 94.2 ± 3.758 |
| F9 | + | gel after a few minutes, dissolves rapidly | 91.7 ± 2.91 |
2.6. Rheological Study of the AZM In Situ Gel
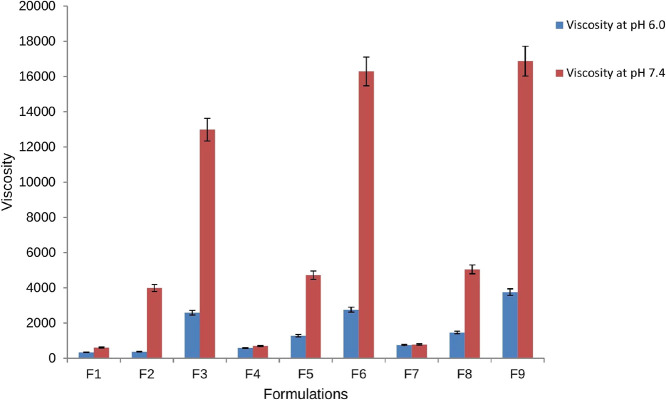
Newtonian flow was exhibited by all the formulations on rheological evaluation before gelling while pseudoplastic flow was seen for these formulations after gelling in the eye. There was an increase in the viscosity after gelling as seen in our case. Additionally, the gel formed in situ retained its integrity for an extended period without dissolving or eroding. The results of rheological studies of the in situ gel showed that the viscosity decreased as the shear stress (rpm) increased. With this increase in shear stress, the normally disarranged molecules of the gelling material aligned their long axis in the direction of the flow (Figure 4). Such orientation reduced the internal resistance of the material and hence decreased the viscosity. Figure 3C shows the average effect of the AZM:SBE-β-CD ratio on the viscosity of the formulation. This graph shows that the viscosity of the in situ gel decreases with the increasing percentage of SBE-β-CD in respect of the drug (1:2). Further, an increase in the percentage of SBE-β-CD elevates the viscosity of the formulation.
Figure 4.
Graphical representation of the viscosity of the prepared formulations at pH 6.0 (solution phase in blue color bars) and pH 7.4 (gel phase in brown color bars) of the in situ gel.
Statistical analysis was carried out to study the average effect of concentration of Carbopol 934P on the viscosity of the formulation, which is shown in Figure 3D. At pH 6, there was practically no significant effect of the concentration of Carbopol 934P (up to 0.6%), but a further increase in its concentration leads to a large increase in viscosity of the formulation. At pH 7.4, the viscosity was increased with an increase in the amount of Carbopol 934P (from 0.4 to 0.8 g). The addition of Carbopol 934P led to the formation of an in situ viscous gel that minimized the leakage of solution from the eye during instillation.46 Thus, it was observed that at pH 7.4 (physiological pH the of eye), all the formulations transitioned to the gel form, thereby confirming the pseudoplastic nature of the in situ gel. Further, the viscosity of the prepared formulations is presented in Figure 4.
2.7. In Vitro Drug Dissolution Study of the AZM In Situ Gel
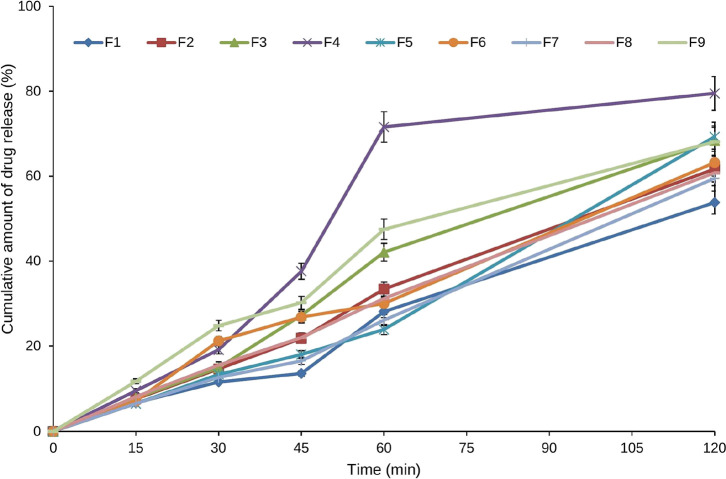
The in vitro drug release of the AZM in situ gel was carried out in dissolution testing apparatus. Formulation F4 showed the highest drug release of 79.5 ± 6.2% as shown in Table 5 and Figure 5. The highest drug release in formulation F4 may be due to the higher amount of SBE-β-CD and lower amount of Carbopol 934P. Here, a lower amount of Carbopol 934P led to the formation of a less viscous gel and showed better drug release. Hence, a higher amount of SBE-β-CD in AZM solution (F4) would permit the highest drug release in 120 min.
Table 5. In Vitro Drug Release from In Situ Gel along with the Theoretical Release Profile.
| cumulative
amount of drug release (%) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| time (min) | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
| 15 | 6.7 ± 0.5 | 7.6 ± 0.6 | 7.8 ± 0.5 | 9.5 ± 0.8 | 6.5 ± 0.5 | 7.1 ± 0.65 | 6.5 ± 0.6 | 8.1 ± 0.7 | 11.7 ± 0.1 |
| 30 | 11.56 ± 0.3 | 14.6 ± 1.3 | 14.9 ± 1.4 | 19.1 ± 1.6 | 13.3 ± 1.1 | 21.2 ± 1.9 | 12.6 ± 1.1 | 15.5 ± 1.2 | 24.8 ± 2.1 |
| 45 | 13.55 ± 1.1 | 21.8 ± 1.8 | 27.2 ± 2.5 | 37.6 ± 3.3 | 18.0 ± 1.5 | 26.8 ± 2.4 | 16.5 ± 1.5 | 22 ± 1.8 | 30.2 ± 2.8 |
| 60 | 28.1 ± 2.5 | 33.4 ± 3.2 | 42.1 ± 3.8 | 71.6 ± 6.4 | 23.9 ± 2.2 | 30.0 ± 2.8 | 26.1 ± 2.4 | 31.2 ± 2.5 | 47.5 ± 4.5 |
| 120 | 53.8 ± 6.1 | 61.7 ± 7.8 | 68.4 ± 8.1 | 79.5 ± 6.2 | 69.3 ± 7.9 | 63.2 ± 6.1 | 59.5 ± 5.9 | 60.8 ± 6.3 | 68.1 ± 6.6 |
Figure 5.
Graph portrays cumulative in vitro release profile of the prepared AZM in situ gel formulations in phosphate buffer (pH 7.4) at 37 °C.
2.8. In Vitro Stability Study of the Optimized In Situ Gel
The optimized sterilized formulation showed good physical stability, and no discoloration or physical changes were observed after storage as shown in Table 6A,B and Figure 6.
Table 6. (A) Stability Parameters of the Formulation F4 In Situ Ophthalmic Gel of AZM and (B) Formulation F4 In Vitro Release of the In Situ Ophthalmic Gel of AZM from the Optimized Checkpoint Formulation Stored under Accelerated Stability Conditions.
| (A) | sampling
intervals |
||||
|---|---|---|---|---|---|
| quality attribute | 0 weeks | 1st week | 2nd week | 3rd week | 4th week |
| pH | 6.3 | 6.3 | 6.3 | 6.3 | 6.3 |
| clarity | clear | clear | clear | clear | clear |
| drug content (%) | 92.1 ± 3.05 | 91.4 ± 3.02 | 90.4 ± 3.02 | 89.8 ± 2.04 | 88.9 ± 2.03 |
| gelling capacity | +++ | +++ | +++ | +++ | +++ |
| (B) | cumulative
amount of drug release (%) |
|
|---|---|---|
| time (min) | 0 weeks | 4th week |
| 15 | 9.5 ± 0.8 | 9.2 ± 0.6 |
| 30 | 19.1 ± 1.6 | 18.68 ± 1.2 |
| 45 | 37.6 ± 3.3 | 32.5 ± 2.1 |
| 60 | 71.6 ± 6.4 | 69.8 ± 5.3 |
| 120 | 79.5 ± 6.0 | 74.5 ± 9.2 |
Figure 6.
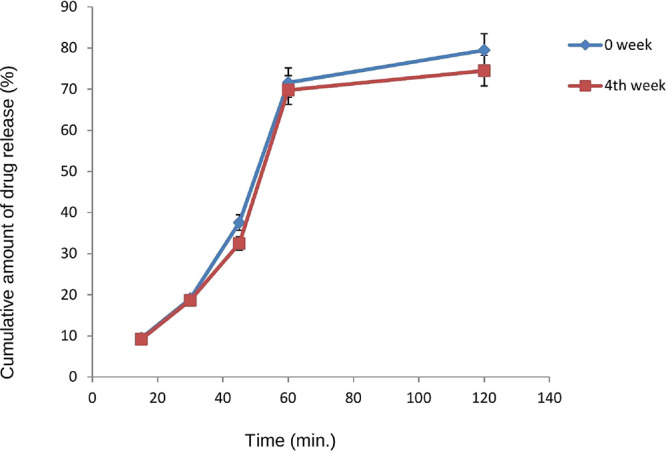
Graph showing in vitro release of optimized formulation F4 stored under accelerated stability conditions.
2.9. Ocular Irritancy Test of the AZM In Situ Gel
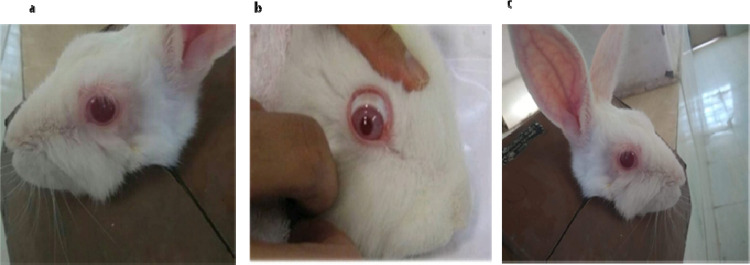
The eye irritation studies were carried out to evaluate the tolerability of the in situ gel. The eye study conducted on rabbits by instilling the gel for 3 days did not show any sign of irritation, i.e., 0 score for all grades, implying that the rabbits tolerated the optimized checkpoint formulation very well (Figure 7). The grading responses for the ocular irritation test on a rabbit eye such as redness of the eye, itching of the eye, uveitis, and epiphora at different time intervals, i.e., 1, 4, 24, 48, and 72 h, were found to be zero.
Figure 7.
Photographs of rabbits used for ocular irritation test (a) for the control group, (b) after 1 h, and (c) after 72 h with no signs of redness and irritation.
3. Materials and Methods
3.1. Materials
AZM was procured from M/s Sun Pharma, Paonta Sahib, India. Carbopol 934P (Lot No. 9003-01-4) (Loba Chemicals, Mumbai), HPMC K4M (Lot No. 9004-64-2) (Colorcon, UK), SBE-β-CD (Cydex Pharmaceuticals, USA), edetate disodium (Lot No. 6381-92-6) (Loba Chemicals, Mumbai), benzalkonium chloride (Ases Chemicals Works, Jodhpur), citric acid (Ases Chemicals Works, Jodhpur), disodium hydrogen phosphate (Lot No. 10028-24-7), potassium dihydrogen orthophosphate (Lot No. 7778-77-0), and sodium hydroxide (Lot No. 1310-73-2) were purchased from Loba Chemicals, Mumbai. Whatman’s filter paper-41 (Lot No. WHA1441125) (M/s Whatman Int. Ltd., England), Bacillus subtilis (Cat. No. - MCC 2010; National Centre for Microbial Resource, Pune, India), and purified water (in house laboratory) were used as is for the experiments. Albino rabbits for testing were obtained from the central animal house, Lachoo Memorial College of Science and Technology (Autonomous), Jodhpur, Rajasthan.
3.2. Methods
3.2.1. Preparation of the AZM:SBE-β-CD Inclusion Complex
A physical mixture of AZM with SBE-β-CD was prepared in a 1:2 molar ratio using a mortar and pestle to obtain a homogeneous powder blend. The inclusion complex of AZM:SBE-β-CD was prepared by a kneading method;47 appropriate amounts of AZM:SBE-β-CD in a 1:2 M ratio were kneaded to a paste-like consistency with a small amount of purified water. After grinding the paste, it was evaporated under reduced pressure and dried in a vacuum oven to obtain a dried powder of the AZM:SBE-β-CD inclusion complex.
3.2.1.1. Formulation of the AZM In Situ Gel
HPMC (K4M) was added in disodium hydrogen phosphate solution as per the formulation in Table 1 and was allowed to hydrate. Further, Carbopol 934P was sprinkled over HPMC solution and allowed to hydrate overnight (Table 7). Edetate disodium (EDTA) solution was then added to the solution with continuous stirring. Kneaded mixtures of AZM and SB-β-CD were prepared using different molar ratios and added to the Carbopol 934P/HPMC solution with continuous stirring until the contents were uniformly blended. Benzalkonium chloride (BKC) was then added, and the solution was brought up to 100 mL with the addition of purified water. All formulations were allowed to equilibrate for 24 h at room temperature prior to further evaluation, as shown in Table 7.
Table 7. Formulation Composition of Azithromycin In Situ Ophthalmic Gel.
| batch code | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| AZM:SBE-β-CD ratio | 1:1 | 1:1 | 1:1 | 1:2 | 1:2 | 1:2 | 2:1 | 2:1 | 2:1 |
| AZM:SBE-β-CD (g) | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Carbopol 934P (g) | 0.4 | 0.6 | 0.8 | 0.4 | 0.6 | 0.8 | 0.4 | 0.6 | 0.8 |
| HPMC K4M (g) | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 |
| edetate disodium (g) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| benzalkonium chloride (g) | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 |
| citric acid (g) | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 | 0.40 |
| disodium hydrogen Phosphate(g) | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 | 1.12 |
| purified water (q.s.) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
3.2.2. Physicochemical Characterization
3.2.2.1. Powder X-ray Diffraction (PXRD) Study
X-ray diffraction (X’Pert PRO MRD PANalytical, Almelo, Netherlands) is a technique used to determine the atomic and molecular structure of a crystal wherein a beam of incident X-rays diffracts into various distinct directions by the crystalline atoms. The powder X-ray diffraction (PXRD) patterns were determined for AZM, SBE-β-CD, and the AZM:SBE-β-CD physical mixture and their inclusion complex with diffraction angles 2θ from 2° to 50° for the characterization of their structural properties.48
3.2.3. Analytical Method Validation for Azithromycin Using UV–Visible Spectroscopy
For determination of absorption maxima (λmax), 10 mg of drug was dissolved in 100 mL of phosphate buffer, pH 7.4 (buffer was prepared by mixing 0.2 M potassium dihydrogen orthophosphate (25.0 mL) and 0.2 M sodium hydroxide (19.55 mL) in water to make the final volume 100 mL) (INDIAN PHARMACOPOEIA, 2007, p-241 to 242) to prepare the stock solution (100 μg/mL). Subsequently, different concentrations (30, 40, 50, 60, 70, 80, 90, and 100 μg/mL) of drug were prepared by transferring 3, 4, 5, 6, 7, 8, 9, and 10 mL of the stock solution into a 10 mL volumetric flask and the volume was made up with phosphate buffer (pH 7.4). The λmax was found to be 232 nm.49 Further on, the absorbance values of these solutions were taken at a λmax of 232 nm using a UV–visible 1400 spectrophotometer (Shimadzu, Japan).50 The analytical method was validated as per the International Conference on Harmonization (ICH) guidelines Q2 (R1),51 and various parameters like linearity, accuracy, precision and robustness, limit of quantification (LOQ), and limit of detection (LOD) were determined.
3.2.3.1. Linearity
The sample was analyzed using a UV–visible spectrophotometer, using phosphate buffer (pH 7.4) as a blank. The linearity of AZM was studied in a concentration range of 30, 40, 50, 60, 70, 80, 90, and 100 μg/mL. Each concentration was determined in triplicate.
3.2.3.2. Accuracy and Precision
The accuracy and precision were determined at three different levels of the target concentration, i.e., 64 μg/mL (80%), 80 μg/mL (100%), and 96 μg/mL (120%). A total of 3 replicates of each of those samples were prepared and analyzed. Precision is expressed as % coefficient of variation (%CV) while accuracy is measured as % nominal as per eqs 1 and 2
| 1 |
| 2 |
3.2.3.3. Robustness
The robustness of the proposed method was determined by carrying out an analysis of an 80 μg/mL solution with different wavelengths (237, 232, and 227 nm) while other working parameters of the UV spectrophotometer were kept the same. The robustness of the proposed method was evaluated by calculating the method efficiency at varying wavelengths (±5 nm).
3.2.3.4. Limit of Detection (LOD) and Limit of Quantification (LOQ)
LOD is the lowest amount of analyte in the sample that can be detected but does not necessarily quantitate as an exact value under the stated conditions of the test. LOQ is the lowest amount of analyte in the sample that can be quantitatively determined with acceptable precision and accuracy under the stated conditions of the test. The LOD and LOQ of the proposed method were determined from calibration standards by using eqs 3 and 4:
| 3 |
| 4 |
where, σ is the standard deviation of the intercepts of the calibration curve equation and S is the mean of slopes of the related calibration curve equations.
3.2.4. Solubility Determination
The AZM solubility was determined by the shake flask method. An excess quantity of AZM was added into 10 mL of phosphate buffer (pH 7.4) (supersaturated) and stirred at room temperature for 72 h until equilibrium was attained. The solution was then passed through a 0.45 μm membrane filter, and the amount of the drug dissolved was analyzed by a UV–visible spectrophotometer.52
3.2.5. Phase Solubility Studies
A stock solution of SBE-β-CD (0.185 mM) was prepared in distilled water and various dilutions of concentrations of 46, 23, 11.6, and 0 mM. In each dilution, 10 mg of AZM was added and kept stirring for 72 h. After this, the solution was filtered through a 0.45 μm membrane filter, and the amount of AZM was analyzed by a UV–visible spectrophotometer.53
Further, the complexation efficiency54 and drug–cyclodextrin molar ratio were calculated using eqs 5 and 6(55)
| 5 |
| 6 |
3.2.6. Antimicrobial Activity of AZM
The antimicrobial activity of AZM was observed to check the stability of the kneaded AZM:SBE-β-CD inclusion complex. The sterile nutrient broth was prepared by using beef extract (0.3 g), peptone (0.3 g), sodium chloride (0.1 g), and distilled water (30 mL). A master culture of B. subtilis was prepared in the nutrient broth and was incubated at 37 °C for 24 h.
3.2.7. Stability Study of the Inclusion Complex of AZM
An aqueous solution of the AZM:SBE-β-CD inclusion complex (1:2) was kept in a stability chamber at 40 °C during the entire period.
3.2.8. Preparation of Nutrient Agar Plates
The nutrient agar medium was prepared, transferred aseptically into the two sterilized Petri plates, and allowed to solidify. The B. subtilis culture was added to the nutrient agar plates by a pour plate method and incubated at 37 °C. Further, the wells were made in the Petri plates in the aseptic cabinet using a sterile borer of a diameter of 10 mm. The solutions (A) and (B) were transferred into the wells, and the plates were incubated at 37 °C. Eventually, the zone of inhibition was measured after 2, 7, 15, and 28 days.56
3.2.9. Drug Content Determination
Accurately 0.5 mL of the formulation was pipetted out and diluted with distilled water up to 100 mL. A 5 mL aliquot was withdrawn from this solution and diluted to 25 mL with distilled water.57 Finally, the AZM concentration was determined at λmax = 232 nm using eq 7(58)
| 7 |
3.2.10. Rheological Study Using the Brookfield Viscometer
The viscosity of the prepared in situ gel was determined using a Brookfield viscometer (DVII Pro digital viscometer) with spindle number 96. The formulation was added to a beaker and allowed to settle for 30 min at room temperature before the final measurement. The spindle was lowered perpendicularly into the center of the in situ gel formulation, taking care that the spindle did not touch the bottom of the beaker, and rotated at speeds of 5, 10, 20, and 50 rpm for 5 min.59 The viscosity was recorded at different time points, and the average of three readings was recorded with standard deviation and was statistically evaluated. Also, the average effect of the concentration of AZM:SBE-β-CD and Carbopol 934P on viscosity at 20 rpm for pH 6.0 (solution phase; blue color) and pH 7.4 (gel phase; brown color) was statistically evaluated.
3.2.11. Gelling Capacity
In order to determine the gelling capacity, 100 μL of the formulation was placed in a vial containing 2 mL of freshly prepared artificial tear fluid (stock solution of NaCl (0.670 g), sodium bicarbonate (0.200 g), and CaCl2·2H2O (0.008 g) in 100 mL of purified water (q.s.)). The mixture was equilibrated at 37 °C, and gel formation was assessed visually along with recording the time.60
3.2.12. In Vitro Drug Release Study
The in vitro release of AZM from the prepared formulation was determined in freshly prepared phosphate buffer (0.1 M, pH 7.4) through the dialysis sac method. The dialysis membrane was first soaked overnight in the dissolution medium; finally, 1 mL volume of the formulation was accurately pipetted into this assembly. This membrane was fastened to the metallic drive shaft followed by its suspension in a beaker containing 150 mL of the dissolution medium with the temperature maintained at 37 ± 0.5 °C. This was done to ensure the continuous contact of the dialysis tube with the receptor medium surface. The shaft’s rotation speed was set at 50 rpm; 1 mL aliquots were withdrawn at regular time intervals and replaced by the addition of an equal volume to the receptor medium.61 The aliquots were further diluted with receptor medium, and absorbance was measured at 232 nm.
3.2.13. Stability Studies of the Optimized In Situ Gel
The stability studies of the prepared ophthalmic in situ formulations (pH 7.4) were carried out at 40 °C and 75% relative humidity (RH) for 1 month62 in a white round low-density polyethylene (LDPE) bottle with a clear LDPE dropper tip and a tan-colored high-density polyethylene (HDPE) eye dropper cap referred to as ″AzaSite (azithromycin) solution″. The effects of temperature, humidity, and time were evaluated on the gel to assess the stability of the prepared formulation.
3.2.14. Eye Irritation Test
Healthy adult male albino rabbits (n = 3) weighing 290–340 g were used in this study. The animals were put in cages in the animal house of Lachoo Memorial College of Science and Technology, Jodhpur (Autonomous), Reg. No.1719/PO/Re/S/13/CPCSEA under controlled environmental conditions (12 h light/dark cycle; temperature 20 ± 2 °C; RH 55 ± 5%) for a minimum of 5 days before the experiments. During this period, animals had free access to a standard diet of green grass and tap water. All experimental and care procedures were conducted in accordance with the Institutional Animal Ethics Committee Ref. No. LMC/PHARM/IAEC/2017-2018/222.
The OECD guideline 405 was followed for the in vivo eye irritancy test of the developed formulation, intended exclusively for albino rabbits. Initially, the test substance was applied in a single dose into the conjunctival sac of one eye of the rabbit. The other eye was left untreated and considered as a control. An in vivo eye irritancy confirmatory test was carried out based on the irritant effect in the initial test. According to the guideline, it was recommended to conduct the test sequentially in one animal at a time, rather than exposing two additional animals. Therefore, the irritant or negative response was again confirmed using two additional animals. Finally, the ocular irritation was recorded in terms of scores at 1, 24, 48, and 72 h.63
4. Conclusions
The poorly soluble drug AZM was successfully formulated as an in situ gelling ophthalmic formulation using Carbopol 934P as a pH-triggered gelling agent. The solubility and stability of AZM were improved using SBE-β-CD. The phase solubility study clearly indicates an increase in solubility of AZM on the addition of SBE-β-CD. The antimicrobial study indicated the reduction in degradation of AZM on the addition of SBE-β-CD. The gelling and viscosity studies indicated the solution to gel transition and subsequent increase in viscosity of the formulation on changing the pH of the medium. Further, in vitro release studies showed the extended release of AZM within a period of 2 h. Considering the gelling and release studies, formulation F4 was selected as the best candidate for further studies. The stability study and eye irritation study showed the stability and compatibility of the formulation with ocular tissues. Thus, the developed in situ gelling ophthalmic formulation of AZM can be a viable alternative to conventional eye drops of AZM, which suffer from demerits of poor solubility, poor stability, rapid pre-corneal elimination of the formulation, and a need for a high frequency of eye drop instillation. The AZM:SBE-β-CD in situ gel is advantageous in comparison to other similar formulations due to its sustained and prolonged release. This formulation has good efficacy and has an enhanced residence time. Moreover, no irritation is observed upon its use. Its better permeability and improved precorneal retention further add to its advantages. These results will open up further discussion for pharmacological intervention with SBE-β-CD and provide a potentially strong alternative for the treatment of ophthalmic infections.
Acknowledgments
The authors are thankful to Sun Pharma Paonta Sahib and Lachoo Memorial College of Science and Technology (Autonomous), Jodhpur, for their support throughout the research work. R.P.B. and G.S. are supported by the UGC-faculty recharge program, DST-UT grant (S&T&RE/RP/147/e-2873/(19-20)/Sanc/12/2019/1878-1885 dt 05/12/2019), and DBT (BT/PR27444/BRB/10/1645/2018). R.P.B. is also supported by DST (ECR/2017/000124). N.S. acknowledges the Department of Science and Technology [DST/INSPIRE/04/2016/001368] New Delhi, India, for financial support.
Glossary
Abbreviations
- AZM
azithromycin
- SBE-β-CD
sulfobutylether β-cyclodextrin
- HPMC
hydroxypropyl methylcellulose
Author Contributions
# A.T., S.J., A.P., and A.S. provided equal contribution.
The authors declare no competing financial interest.
References
- Barnwal R. P.; Devi K. M.; Agarwal G.; Sharma Y.; Chary K. V. R. Temperature-dependent oligomerization in M-crystallin: lead or lag toward cataract, an NMR perspective. Proteins 2011, 79, 569–580. 10.1002/prot.22905. [DOI] [PubMed] [Google Scholar]
- Barnwal R. P.; Jobby M. K.; Devi K. M.; Sharma Y.; Chary K. V. R. Solution Structure and Calcium-Binding Properties of M-Crystallin, A Primordial βγ-Crystallin from Archaea. J. Mol. Biol. 2009, 386, 675–689. 10.1016/j.jmb.2008.12.058. [DOI] [PubMed] [Google Scholar]
- GGratieri T.; Gelfuso G. M.; de Freitas O.; Rocha E. M.; Lopez R. F. Enhancing and sustaining the topical ocular delivery of fluconazole using chitosan solution and poloxamer/chitosan in situ forming gel. Eur. J. Pharm. Biopharm. 2011, 79, 320–327. 10.1016/j.ejpb.2011.05.006. [DOI] [PubMed] [Google Scholar]
- Vijaya C.; Goud K. S. Ion-activated in situ gelling ophthalmic delivery systems of azithromycin. Indian J. Pharm. Sci. 2011, 73, 615. 10.4103/0250-474X.100234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradeep A. R.; Sagar S. V.; Daisy H. Clinical and microbiologic effects of subgingivally delivered 0. 5% azithromycin in the treatment of chronic periodontitis. J. Periodontol. 2008, 79, 2125–2135. 10.1902/jop.2008.070589. [DOI] [PubMed] [Google Scholar]
- Bosch-Driessen L. H.; Verbraak F. D.; Suttorp-Schulten M. S.; van Ruyven R. L.; Klok A. M.; Hoyng C. B.; Rothova A. A prospective, randomized trial of pyrimethamine and azithromycin vs pyrimethamine and sulfadiazine for the treatment of ocular toxoplasmosis. Am. J. Ophthalmol. 2002, 134, 34–40. 10.1016/S0002-9394(02)01537-4. [DOI] [PubMed] [Google Scholar]
- Escalante M. G.; Eubank T. D.; Leblebicioglu B.; Walters J. D. Comparison of Azithromycin and Amoxicillin Before Dental Implant Placement: An Exploratory Study of Bioavailability and Resolution of Postoperative Inflammation. J. Periodontol. 2015, 86, 1190–1200. 10.1902/jop.2015.150024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braga S.; Cyclodextrins S. Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. 10.3390/biom9120801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wankar J.; Bonvicini F.; Benkovics G.; Marassi V.; Malanga M.; Fenyvesi E.; Gentilomi G. A.; Reschiglian P.; Roda B.; Manet I. Widening the Therapeutic Perspectives of Clofazimine by Its Loading in Sulfobutylether β-Cyclodextrin Nanocarriers: Nanomolar IC50 Values against MDR S. epidermidis. Mol. Pharmaceutics 2018, 15, 3823–3836. 10.1021/acs.molpharmaceut.8b00321. [DOI] [PubMed] [Google Scholar]
- Saokham P.; Muankaew C.; Jansook P.; Loftsson T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. 10.3390/molecules23051161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z.; Landy D.; Sizun C.; Cézard C.; Solgadi A.; Przybylski C.; de Chaisemartin L.; Herfindal L.; Barratt G.; Legrand F. X. Cyclodextrin complexation studies as the first step for repurposing of chlorpromazine. Int. J. Pharm. 2020, 584, 119391 10.1016/j.ijpharm.2020.119391. [DOI] [PubMed] [Google Scholar]
- Stella V. J.; Rajewski R. A. Sulfobutylether-β-cyclodextrin. Int. J. Pharm. 2020, 583, 119396 10.1016/j.ijpharm.2020.119396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saita M. G.; Aleo D.; Melilli B.; Patti A. Effect of cyclodextrin additives on azithromycin in aqueous solution and insight into the stabilization mechanism by sulfobutyl ether-β-cyclodextrin. Int. J. Pharm. 2019, 566, 674–679. 10.1016/j.ijpharm.2019.06.025. [DOI] [PubMed] [Google Scholar]
- Gidwani B.; Vyas A. A comprehensive review on cyclodextrin-based carriers for delivery of chemotherapeutic cytotoxic anticancer drugs. BioMed Res. Int. 2015, 2015, 1. 10.1155/2015/198268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawade D. P.; Kasliwal R. H.; Chaple D. R. Azithromycin-a novel drug delivery system for ocular application. World J. Pharm. Res. 2018, 7, 799–813. [Google Scholar]
- Rajewski R. A.; Stella V. J. Pharmaceutical applications of cyclodextrins. 2. In vivo drug delivery. J. Pharm. Sci. 1996, 85, 1142–1169. 10.1021/js960075u. [DOI] [PubMed] [Google Scholar]
- Agrahari V.; Mandal A.; Agrahari V.; Trinh H. M.; Joseph M.; Ray A.; Hadji H.; Mitra R.; Pal D.; Mitra A. K. A comprehensive insight on ocular pharmacokinetics. Drug Deliv. Transl. Res 2016, 6, 735–754. 10.1007/s13346-016-0339-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain G. P.; Patel S.; Gandhi J.; Shah P. Development of Moxifloxacin Hydrochloride loaded in-situ gel for the treatment of periodontitis: In-vitro drug release study and antibacterial activity. J. Oral Biol. Craniofac. Res. 2019, 9, 190–200. 10.1016/j.jobcr.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T.; Stefánsson E. Cyclodextrins and topical drug delivery to the anterior and posterior segments of the eye. Int. J. Pharm. 2017, 531, 413–423. 10.1016/j.ijpharm.2017.04.010. [DOI] [PubMed] [Google Scholar]
- Mandal S.; Thimmasetty M. K.; Prabhushankar G. L.; Geetha M. S. Formulation and evaluation of an in situ gel-forming ophthalmic formulation of moxifloxacin hydrochloride. Int. J. Pharm. Investig. 2012, 2, 78. 10.4103/2230-973X.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu K.; Chen L.; Xu W.; Li H.; Zhang Y.; Xie W.; Zheng J. Preparation of a paeonol-containing temperature-sensitive in situ gel and its preliminary efficacy on allergic rhinitis. Int. J. Mol. Sci. 2013, 14, 6499–6515. 10.3390/ijms14036499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loftsson T.; Stefánsson E. Aqueous eye drops containing drug/cyclodextrin nanoparticles deliver therapeutic drug concentrations to both anterior and posterior segment. Acta Ophthalmol. 2022, 100, 7–25. 10.1111/aos.14861. [DOI] [PubMed] [Google Scholar]
- Makwana S. B.; Patel V. A.; Parmar S. J. Development and characterization of in-situ gel for ophthalmic formulation containing ciprofloxacin hydrochloride. Results Pharma. Sci. 2016, 6, 1–6. 10.1016/j.rinphs.2015.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao F.; Zhang X.; Ping Q. New method for ophthalmic delivery of azithromycin by poloxamer/carbopol-based in situ gelling system. Drug Delivery 2010, 17, 500–507. 10.3109/10717544.2010.483255. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Lin X.; Tang X. Lipid emulsions as a potential delivery system for ocular use of azithromycin. Drug Dev. Ind. Pharm. 2009, 35, 887–896. 10.1080/03639040802680271. [DOI] [PubMed] [Google Scholar]
- Ren T.; Lin X.; Zhang Q.; You D.; Liu X.; Tao X.; Gou J.; Zhang Y.; Yin T.; He H.; Tang X. Encapsulation of azithromycin ion pair in liposome for enhancing ocular delivery and therapeutic efficacy on dry eye. Mol. Pharmaceutics 2018, 15, 4862–4871. 10.1021/acs.molpharmaceut.8b00516. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S.; Reddy P. Effect of surfactant on azithromycin dihydrate loaded stearic acid solid lipid nanoparticles. Turkish J. Pharm. Sci. 2019, 16, 425. 10.4274/tjps.galenos.2018.82160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar S.; Singhal T. Preformulation Studies of Niosomal Gel of Prednisolone & Azithromycin for Topical Drug Delivery System. J. Innov. Pharm. Biol. Sci. 2015, 2, 312–321. [Google Scholar]
- Bhatia H. B.; Sachan A.; Bhandari A. Studies on thermoreversive mucoadhesive ophthalmic in situ gel of azthromycin. J. Drug Deliv. Ther. 2013, 3, 106–109. [Google Scholar]
- Bowman L. M.; Si E.; Pang J.; Archibald R.; Friedlaender M. Development of a topical polymeric mucoadhesive ocular delivery system for azithromycin. J. Ocul. Pharmacol. Ther. 2009, 25, 133–140. 10.1089/jop.2008.0066. [DOI] [PubMed] [Google Scholar]
- Jacob S.; Nair A. B. Cyclodextrin complexes: Perspective from drug delivery and formulation. Drug Dev. Res. 2018, 79, 201–217. 10.1002/ddr.21452. [DOI] [PubMed] [Google Scholar]
- Zia V.; Rajewski R. A.; Stella V. J. Effect of cyclodextrin charge on complexation of neutral and charged substrates: comparison of (SBE) 7M-β-CD to HP-β-CD. J. Pharm. Res. 2001, 18, 667–673. 10.1023/A:1011041628797. [DOI] [PubMed] [Google Scholar]
- Järvinen T.; Järvinen K.; Urtti A.; Thompson D.; Stella V. J. Sulfobutyl ether β-cyclodextrin (SBE-β-CD) in eyedrops improves the tolerability of a topically applied pilocarpine prodrug in rabbits. J. Ocul. Pharmacol. Ther. 1995, 11, 95–106. 10.1089/jop.1995.11.95. [DOI] [PubMed] [Google Scholar]
- Okimoto K.; Rajewski R. A.; Stella V. J. Release of testosterone from an osmotic pump tablet utilizing (SBE) 7m-β-cyclodextrin as both a solubilizing and an osmotic pump agent. J. Controlled Release 1999, 58, 29–38. 10.1016/S0168-3659(98)00142-4. [DOI] [PubMed] [Google Scholar]
- Anraku M.; Hiraga A.; Iohara D.; Pipkin J. D.; Uekama K.; Hirayama F. Slow-release of famotidine from tablets consisting of chitosan/sulfobutyl ether β-cyclodextrin composites. Int. J. Pharm. 2015, 487, 142–147. 10.1016/j.ijpharm.2015.04.022. [DOI] [PubMed] [Google Scholar]
- Xu J.; Zhang Y.; Li X.; Zheng Y. Inclusion complex of nateglinide with sulfobutyl ether β-cyclodextrin: Preparation, characterization and water solubility. J. Mol. Struct. 2017, 1141, 328–334. 10.1016/j.molstruc.2017.03.116. [DOI] [Google Scholar]
- Semcheddine F.; Guissi N. E. I.; Liu X.; Wu Z.; Wang B. Effects of the preparation method on the formation of true nimodipine SBE-β-CD/HP-β-CD inclusion complexes and their dissolution rates enhancement. AAPS. PharmSciTech. 2015, 16, 704–715. 10.1208/s12249-014-0257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devasari N.; Dora C. P.; Singh C.; Paidi S. R.; Kumar V.; Sobhia M. E.; Suresh S. Inclusion complex of erlotinib with sulfobutyl ether-β-cyclodextrin: Preparation, characterization, in silico, in vitro and in vivo evaluation. Carbohydr. Polym. 2015, 134, 547–556. 10.1016/j.carbpol.2015.08.012. [DOI] [PubMed] [Google Scholar]
- Deng F.; Hu W.; Chen H.; Tang Y.; Zhang L. Development of a chitosan-based nanoparticle formulation for ophthalmic delivery of honokiol. Curr. Drug Delivery 2018, 15, 594–600. 10.2174/1567201814666170419113933. [DOI] [PubMed] [Google Scholar]
- Jothi M.; Harikumar S. L.; Aggarwal G. In-situ ophthalmic gels for the treatment of eye diseases. Int. J. Pharm. Sci. 2012, 3, 1891. [Google Scholar]
- de Araújo M. V. G.; Vieira E. K. B.; Lázaro G. S.; Conegero L. S.; Almeida L. E.; Barreto L. S.; da Costa N. B. Jr.; Gimenez I. F. Sulfadiazine/hydroxypropyl-β-cyclodextrin host–guest system: characterization, phase-solubility and molecular modeling. Bioorg. Med. Chem. 2008, 16, 5788–5794. 10.1016/j.bmc.2008.03.057. [DOI] [PubMed] [Google Scholar]
- Han D.; Han Z.; Liu L.; Wang Y.; Xin S.; Zhang H.; Yu Z. Solubility enhancement of myricetin by inclusion complexation with heptakis-O-(2-hydroxypropyl)-β-cyclodextrin: A joint experimental and theoretical study. Int. J. Mol. Sci. 2020, 21, 766. 10.3390/ijms21030766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R.; Sinha V. R.; Dahiya L.; Sarwal A. Transdermal delivery of duloxetine-sulfobutylether-β-cyclodextrin complex for effective management of depression. Int. J. Pharm. 2021, 594, 120129 10.1016/j.ijpharm.2020.120129. [DOI] [PubMed] [Google Scholar]
- De Paula D.; Oliveira D. C. R.; Tedesco A. C.; Bentley M. V. L. B. Enhancing effect of modified beta-cyclodextrins on in vitro skin permeation of estradiol. Rev. Ciênc. Farm. Básica. Apl. 2007, 43, 111–120. [Google Scholar]
- Jarho P.; Urtti A.; Järvinen T. Hydroxypropyl-β-cyclodextrin increases the aqueous solubility and stability of pilocarpine prodrugs. J. Pharm. Res. 1995, 12, 1371–1375. 10.1023/A:1016290127371. [DOI] [PubMed] [Google Scholar]
- Iglesias B. R.; Lorenzo A. C.; Concheiro A. Poly (acrylic acid) micro gels (carbopol 934)/surfactant interactions in aqueous media. Part 1: nonionic surfactants. Int. J. Pharm. 2003, 258, 165–177. 10.1016/S0378-5173(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Lin S. Z.; Wouessidjewe D.; Poelman M. C.; Duchêne D. Indomethacin and cyclodextrin complexes. Int. J. Pharm. 1991, 69, 211–219. 10.1016/0378-5173(91)90363-S. [DOI] [Google Scholar]
- Bagavatula H.; Lankalapalli S.; Tenneti V. K. V. S.; Beeraka N. M. R.; Bulusu B. T. Comparative studies on solubility and dissolution enhancement of different itraconazole salts and their complexes. Adv. Pharmacol. Sci. 2014, 2, 85–95. 10.13189/app.2014.020601. [DOI] [Google Scholar]
- Nyola N.; Jeyabalan G. S. Simultaneous estimation of Azith Pharmaceutical Ingredients Spectrophotometry. Hygeia. J.D. Med. 2012, 4, 27–32. [Google Scholar]
- Bhimani S.; Sanghvi G.; Pethani T.; Dave G.; Airao V.; Sharma T.; Sheth N.; Vaishnav D. Development of the UV spectrophotometric method of azithromycin in API and stress degradation studies. Int. Lett. Chem. Phys. Astron. 2016, 68, 48–53. 10.18052/www.scipress.com/ILCPA.68.48. [DOI] [Google Scholar]
- Guideline I. H. T.Q2 (R1): Validation of analytical procedures: text and methodology. In International Conference on Harmonization, Geneva, 2005; Vol. 1( (20), ), p 05.
- Baka E.; Comer J. E. A.; Takács-Novák K. Study of equilibrium solubility measurement by saturation shake-flask method using hydrochlorothiazide as model compound. J. Pharm. Biomed. Anal. 2008, 46, 335–341. 10.1016/j.jpba.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Loh G. O. K.; Tan Y. T. F.; Peh K. K. Enhancement of norfloxacin solubility via inclusion complexation with β-cyclodextrin and its derivative hydroxypropyl-β-cyclodextrin. Asian J. Pharm. Sci. 2016, 11, 536–546. 10.1016/j.ajps.2016.02.009. [DOI] [Google Scholar]
- Loftsson T.; Hreinsdóttir D.; Másson M. The complexation efficiency. J. Inclusion Phenom. Macrocyclic Chem. 2007, 57, 545–552. 10.1007/s10847-006-9247-2. [DOI] [Google Scholar]
- Alopaeus J. F.; Göbel A.; Breitkreutz J.; Sande S. A.; Tho I. Investigation of hydroxypropyl-β-cyclodextrin inclusion complexation of two poorly soluble model drugs and their taste-sensation-Effect of electrolytes, freeze-drying and incorporation into oral film formulations. J. Drug. Deliv. Sci. Technol. 2021, 61, 102245 10.1016/j.jddst.2020.102245. [DOI] [Google Scholar]
- Moreno A. D. H.; Silva M. F. C. D.; Salgado H. R. N. Stability study of azithromycin in ophthalmic preparations. Braz. J. Pharm. Sci 2009, 45, 219–226. 10.1590/S1984-82502009000200005. [DOI] [Google Scholar]
- Rajinikanth P. S.; Balasubramaniam J.; Mishra B. Development and evaluation of a novel floating in situ gelling system of Azithromycin dihydrate. Indo. Am. J. Pharm. Res. 2013, 3, 3821–3831. [Google Scholar]
- Abdo R. W.; Saadi N.; Hijazi N. I.; Suleiman Y. A.. Quality control and testing evaluation of pharmaceutical aerosols. InDrug Deliv. Syst ., Tekade R. K. Ed.; Academic Press, 2020; pp. 579–614, 10.1016/B978-0-12-814487-9.00012-0. [DOI] [Google Scholar]
- Meenakshi P.; Hetal T.; Kasture P. V. Preparation and evaluation of thermoreversible formulations of flunarizine hydrochloride for nasal delivery. Int. J. Pharm. Pharm. Sci 2010, 2, 115–120. [Google Scholar]
- Song J.; Bi H.; Xie X.; Guo J.; Wang X.; Liu D. Preparation and evaluation of sinomenine hydrochloride in situ gel for uveitis treatment. Int. Immunopharmacol. 2013, 17, 99–107. 10.1016/j.intimp.2013.05.020. [DOI] [PubMed] [Google Scholar]
- Sun L.; Zhang W.; Liu X.; Sun J. Preparation and evaluation of sustained-release azithromycin tablets in vitro and in vivo. Asian J. Pharm. Sci. 2014, 9, 155–161. 10.1016/j.ajps.2014.03.003. [DOI] [Google Scholar]
- Srividya B.; Cardoza R. M.; Amin P. D. Sustained ophthalmic delivery of ofloxacin from a pH triggered in situ gelling system. J. Controlled Release 2001, 73, 205–211. 10.1016/S0168-3659(01)00279-6. [DOI] [PubMed] [Google Scholar]
- Yu S.; Wang Q. M.; Wang X.; Liu D.; Zhang W.; Ye T.; Yang X.; Pan W. Liposome incorporated ion sensitive in situ gels for opthalmic delivery of timolol maleate. Int. J. Pharm. 2015, 480, 128–136. 10.1016/j.ijpharm.2015.01.032. [DOI] [PubMed] [Google Scholar]