Abstract

Chalcogenide nanoparticles have become a very active field of research for their optoelectronic and biological properties. This article shows the production of tellurium dioxide nanoparticles (TeO2 NPs) by pulsed laser ablation in liquids. The produced nanoparticles were spherical with a diameter of around 70 nm. The energy band gap of those nanoparticles was determined to be around 5.2 eV. Moreover, TeO2 NPs displayed a dose-dependent antibacterial effect against antibiotic-resistant bacteria such as multidrug-resistant Escherichia coli (MDR E. coli) and methicillin-resistant Staphylococcus aureus (MR S. aureus). The “naked” nature of the nanoparticle surface helped to eradicate the antibiotic-resistant bacteria at a very low concentration, with IC50 values of ∼4.3 ± 0.9 and 3.7 ± 0.2 ppm for MDR E. coli and MR S. aureus, respectively, after just 8 h of culture. Further, the IC50 values of the naked TeO2 NPs against melanoma (skin cancer) and healthy fibroblasts were 1.6 ± 0.7 and 5.5 ± 0.2 ppm, respectively, for up to 72 h. Finally, to understand these optimal antibacterial and anticancer properties of the TeO2 NPs, the reactive oxygen species generated by the nanoparticles were measured. In summary, the present in vitro results demonstrate much promise for the presently prepared TeO2 NPs and they should be studied for a wide range of safe antibacterial and anticancer applications.
1. Introduction
Tellurium(Te) is one of the rarest chemical elements in the Earth’s crust.1−4 Its large absence on Earth finds its origin in Earth’s formation when Te is bound to hydrogen to form tellurium hydrides. Earth’s gravity was not strong enough to retain these highly volatile hydrides, and most Te escaped into space, making it rare on Earth but not in the Universe.5 Nevertheless, Te forms compounds that are of great interest to the scientific community, mainly due to their optoelectronic properties and a wide array of biological properties whose research has been gaining interest over the last few decades. For example, cadmium telluride (CdTe) is used in flexible solar cells, displaying a 16.4% efficiency,6 while copper telluride (Cu2Te) is a thermoelectric material exhibiting a figure of merit (ZT) of around 1.1.7,8 In terms of their biological properties, most alkali–metal tellurites and tellurates are useful in microbiology, while the antioxidant effects of organotellurides and diorganoditellurides and the immunomodulatory effects of the nontoxic inorganic tellurane, named AS-101, are also of great interest in research.9,10
One of the most simple Te-based compounds is its oxide, tellurium dioxide (TeO2), which is an important catalyst in oxidation, hydrogenation, and dehydrogenation processes.11 TeO2 is a polymorph material that exhibits three crystalline structures: α-TeO2 (paratellurite, gray color), β-TeO2 (tellurite, yellow color), and γ-TeO2 (metastable).12−14 α-TeO2 displays an indirect band gap of around 2.9 eV and a direct band gap of around 3.3 eV.15 β-TeO2 displays only a direct band gap of around 2.2 eV, while γ-TeO2 only exhibits an indirect band gap of around 3.1 eV.15 That is why TeO2 is also used in fiber optics and waveguide applications.16 Indeed, the visible portion of the electromagnetic spectrum ranges from 1.6 to 3.3 eV; therefore, with TeO2 nanostructures displaying energy band gaps larger than ∼3.3 eV, visible light will be easily transmitted through those structures.
One of the earliest applications of TeO2 in biomedical applications came from its use as an antibiotic.10 Indeed, in the pre-penicillin era, Te-based compounds were used by Alexander Fleming to inhibit the growth of many pathogens.17 Tellurium itself is not particularly toxic but its absence in the biological world may explain its efficacy against pathogens.10 When used in the nanometer size range, Te-based compounds, including TeO2, provide a dramatic improvement in their biomedical properties due to an increase in their surface-to-volume ratios and a sustained increase in reactivity with biological membranes. Therefore, Te-based nanoparticles (NPs) can be employed in antibacterial applications, as the sole agent,18 or in combination with bioactive glasses19 or anticancer approaches.20−23
One of the most important factors impacting the applicability and activity of any NP is how they are made and the presence of synthetic byproducts in their final form. Nowadays, TeO2 NPs are synthesized by various techniques such as biosynthesis,24 spray pyrolysis,25 thermal evaporation,26 sonochemistry,27 and pulsed laser ablation in liquids (PLAL).28,29 Among these techniques, PLAL is the one that creates NPs with a clean surface (i.e., without any surfactants or impurities attached), allowing them to interact efficiently with their environment. This advantage is particularly suitable for catalytic30−32 and antibacterial33−35 applications.
Therefore, this paper focused on the synthesis of “naked” TeO2 NPs by PLAL. The originality of our PLAL synthesis lies in the use of a high repetition rate (1 kHz) pulsed laser when irradiating a static Te target. As featured in Table 1, this study is the first to report the ablation of a pure static Te target in the kHz regime. By increasing the repetition rate from 100 Hz to 1 kHz, we noticed an increase of 36% in the production rate of TeO2 NPs. Consequently, the synthesis time can be significantly abridged to produce the same amount of NPs, as already noticed by Nikolov et al. in the case of silver NPs.36 Furthermore, this is the first time that TeO2 NPs produced by PLAL are tested against antibiotic-resistant bacteria such as multidrug-resistant Escherichia coli (MDR E. coli), methicillin-resistant Staphylococcus aureus (MR S. aureus), and a cancer cell specifically human melanoma cells.
Table 1. List of Studies Discussing the Synthesis of Te and TeO2 NPs by PLALa,b.
| authors | Khalef37 | Liu et al.28,38 | Guisbiers et al.29 | Saraeva et al.39 | Khalef et al.40 | this work |
|---|---|---|---|---|---|---|
| publication year | 2014 | 2016 | 2017 | 2020 | 2021 | 2022 |
| type of laser | Nd:YAG | Nd:YAG | Nd:YAG | Yb-doped | Nd:YAG | Nd:YAG |
| wavelength (nm) | 1064 | 1064 | 1064 | 1040 | 1064 | 1064 |
| repetition rate (Hz) | 1 | 20 | 20 | 20,000 | 1 | 1,000 |
| pulse duration (ns) | ∼9 | ∼10 | ∼4 | ∼120 | ∼9 | ∼100 |
| irradiation time (s) | 20 | 2, 10, 180 | 900 | 50 | 300 | |
| solvent | deionized (DI) water | DI water, methanol, ethanol, acetone, dichloromethane | DI water, acetone | DI water | DI water | DI water |
| target | static | static | static | dynamic | static | static |
| fluence (J/cm2) | ∼11 | ∼2 | ∼284 | |||
| product | TeO2 NPs | TeO2 NPs, Te NPs, | TeO2 NPs, Te NPs | TeO2 NPs | TeO2 NPs | TeO2 NPs |
| application | none | none | none | antibacterial: S. aureus biofilms | antibacterial: E. coli, S. aureus | • antibacterial: MDR E. coli, MR S. aureus |
| • anticancer: human melanoma cells |
Nd:YAG, neodymium-doped:yttrium aluminum garnet.
NPs, nanoparticles.
2. Materials and Methods
2.1. PLAL Synthesis
TeO2 NPs were synthesized by utilizing a nanosecond Nd:YAG laser (Electro Scientific Industries) operating at 1064 nm. The pulsed laser beam was reflected off a gold-coated mirror oriented at a 45° angle with respect to the laser rail (Figure 1a). A biconvex lens (focal length = 83 mm) was placed on the laser beam path between the mirror and the target to focus the beam on the target’s surface. The laser beam’s spot size on the target’s surface was measured at around ∼45 μm. Consequently, the fluence of the laser was determined to be around ∼346 J cm–2. Indeed, the laser’s pulse repetition rate was fixed at 1 kHz with an energy output per pulse of around 5.5 mJ. However, as liquid water absorbs the 1064 nm radiation, it is important to consider the effect of the liquid height on the fluence. Here, the liquid height on top of the target was set at 8 mm. Therefore, according to Hamad et al.,41 the fluence was reduced by ∼18%, giving a value of around ∼284 J cm–2. The target consisted of bulk Te pellets (99.99% from Sigma-Aldrich 263303-25G), ∼2 mm in diameter, and the pellets were sitting immobile at the bottom of a 50 mL rounded single-neck glass flask. The flask was then filled with 5 mL of deionized (DI) water. The NPs were produced by irradiating the static target for 5 min.
Figure 1.
(a) Sketch showing the PLAL synthesis protocol. (b) Tyndall effect observed on the colloid synthesized by PLAL at 1000 Hz. The left solution is the solvent, i.e., DI water, while the right solution is the colloid containing the TeO2 NPs. (c) Scanning electron microscopy (SEM) image of the TeO2 NPs contained in the colloid synthesized by PLAL at 1000 Hz. (d) Energy-dispersive X-ray (EDX) line scan through one TeO2 particle.
2.2. Physicochemical Characterization
After synthesis, the samples were characterized by ultraviolet (UV)–visible spectroscopy (Cary 5000 from Agilent), Raman spectroscopy (EZRaman-I Series from TSI), atomic emission spectroscopy (AES, 4210 MP-AES from Agilent), dynamic light scattering (DLS, NanoBrook 90Plus DLS from Brookhaven Instruments Corporation), scanning electron microscopy (SEM, JEOL JSM 7000F SEM, operating at 15 kV), differential scanning calorimetry (DSC, Mettler Toledo), X-ray photoelectron spectroscopy (XPS, Thermo Fisher Kα), and X-ray diffraction (XRD, Rigaku Miniflex 600). The Raman, DSC, XPS, and XRD spectra were collected from dried sedimentation present after centrifugation of the colloid. For SEM analysis, a droplet of the colloid was deposited onto a silicon wafer, which was then dried in an environmentally controlled glovebox.
2.3. Biological Characterization
Strains of one Gram-negative, multidrug-resistant E. coli (MDR E. coli) (ATCC BAA-2471; ATCC, Manassas, VA) bacteria, and one Gram-positive, methicillin-resistant S. aureus (MR S. aureus) (ATCC 4330; ATCC, Manassas, VA) bacteria, were used in this study to determine the antibacterial activity of the TeO2 NPs after 8 h of culture. Both bacteria were cultured according to the ATCC instructions. The entire protocol is described in refs (42, 43). All experiments were repeated in triplicate (N = 3) unless otherwise indicated to ensure the reliability of the results. Statistical significance was assessed using Student’s t-tests, setting an α value of less than 0.05 as statistically significant compared to the controls. Results were displayed as the mean ± standard deviation using Prism 9 software, 2021 version. Relevant parameters to the biomedical use of the NPs were calculated following modeling methods in the same software.
Reactive oxygen species (ROS) were quantified using 2′,7′-dichlorodihydrofluorescein diacetate (H2DCFDA) following the instructions of the kit. Briefly, human melanoma cells were seeded in a 96-well plate in the presence of different concentrations of nanoparticles with the appropriate positive and negative controls. The ROS indicator was reconstituted in anhydrous dimethyl sulfoxide (DMSO), the cell medium was then removed, and the cells were washed twice with buffer. Afterward, a fixed volume of the indicator in phosphate-buffered saline (PBS) was added to each one of the wells at a final concentration of 10 μM. The cells were incubated for 30 min, and fresh medium was added, and the cells were allowed to recover for a short time. Positive controls were included, stimulating the oxidative activity with hydrogen peroxide to a final concentration of 50 μM. The intensity of fluorescence was then observed by flow cytometry at 530 nm when the sample was excited at 485 nm.
3. Results and Discussion
3.1. Physicochemical Tests
To be classified as a colloid, the liquid-containing structures should scatter the laser light and make the laser beam visible when shining a laser pointer through the colloid. This effect is known as the Tyndall effect.44,45 In Figure 1b, two cuvettes are displayed: the left one is filled with DI water and serves as a reference, while the right one is our sample. By looking at the reference cuvette, the laser beam is not visible as the size of the water molecules was too small compared to the pointer laser wavelength (∼650 nm). However, the laser beam becomes visible when going through the sample, consequently, confirming the presence of NPs within the liquid (Figure 1b). The gray color of our colloidal solution is similar to that observed by Khalef et al.37 who also synthesized TeO2 NPs by PLAL. The NPs were then observed by SEM, as shown in Figure 1c, where the shape of NPs was identified as being spherical. The EDX line scan across one spherical NP confirmed that TeO2 was formed uniformly across the NP (Figure 1d).
Further investigation by XRD (Figure 2a) confirmed that the colloid was made of α-TeO2 (89.5 ± 4.7%) and Te (10.5 ± 4.7%). The crystalline phase of α-TeO2 was then further identified by Raman spectroscopy by displaying peaks at 116 cm–1 (E), 136 cm–1 (A1), 262 cm–1 (B2), 386 cm–1 (A1), and 644 cm–1 (E)13,46 (Figure 2b). Clearly, the phonon states in TeO2 can be distinguished into two groups: librational and deformational.47 The two modes at frequencies lower than 150 cm–1 correspond to the librational modes of TeO4 units.48 Indeed, the Te atoms in the α-TeO2 paratellurite structure have four neighboring O atoms so that the elementary structural unit is a TeO4 disphenoid, and from such TeO4 units sharing corners, the α-TeO2 paratellurite structure is built. The three modes at frequencies above 150 cm–1 correspond to the deformational modes of the TeO4 units. Specifically, the B2 mode at 262 cm–1 is a stretching mode of the Te–O chemical bond; the A1 mode at 386 cm–1 corresponds to the bending mode of O–Te–O, and the E mode at 644 cm–1 corresponds to the stretching mode of the Te–O chemical bond.
Figure 2.
(a) XRD spectra. The peak positions of Te and α-TeO2 were obtained from the crystallography open database entries 1011098 and 1530871, respectively. (b) Raman spectra.
XPS was performed to determine the surface state of the TeO2 NPs (Figure 3). The software Avantage from Thermo Scientific was used for the acquisition and analysis. Avantage uses a mixture of Gaussian and Lorentzian to fit the peaks; the mixture ratio can be fixed or be a variable in the fitting routine. The O 1s peak found around ∼530 eV is assigned to the existence of bridging oxygen atoms Te–O–Te49 (Figure 3a). Furthermore, it is possible to identify the Te oxidation states using the satellite peak features of Te 3d (Figure 3b). There are strong satellite peaks around ∼576 and ∼586 eV indicating the presence of TeO2 at the surface, while there are two weak peaks around ∼573 and ∼583 eV indicating the presence of Te.50 Based on the surface area of the peaks corresponding to TeO2 and Te, the TeO2/Te ratio is around ∼3, meaning that there is 3 times more TeO2 in the colloid than in Te, i.e., ∼75% of the colloid is made of TeO2, while ∼25% is made of Te (Figure 3b). Remember that XPS is a surface analysis technique, which is why there is a slight discrepancy concerning the TeO2/Te ratio with the XRD measurements as XRD also measures the core of the NPs and not only the surface. It means that TeO2 is not only found at the surface of the NPs but also located at the very core.
Figure 3.
(a) XPS spectra focusing on the O 1s orbitals. (b) XPS spectra focusing on the Te 3d orbitals. For Te 3d spectrum fitting, the best fit Lorentzian–Gaussian ratio was 28.05% for TeO2 and 71.44% for the metallic Te.
The colloid was then analyzed by differential scanning calorimetry (DSC). The reason for performing DSC (Figure 4a) is to demonstrate that there are no Te nanoparticles within the colloid and clearly identify that the origin of Te detected by XRD comes from some chunks or dust of the Te target that got detached during the irradiation. Indeed, the first peak popping up at 451 °C in Figure 4a corresponds to the bulk melting temperature of Te, but no peaks appear below 451 °C, consequently confirming the absence of Te nanoparticles. There are also two other peaks appearing at 630 and 665 °C, which corresponds to two populations of TeO2 NPs. Indeed, those peaks appeared above 451 °C and below 732 °C, which is the bulk melting temperature of TeO2; therefore, those two populations cannot be made out of Te but should be made of TeO2 displaying nanometer size dimensions, as the melting temperature of the nanoparticles decreased with the size of the nanoparticle.51,52Figure 4b shows the TeO2 NPs and some Te ablation debris coming from the target.
Figure 4.
(a) Differential scanning calorimetry curve. (b) SEM image showing TeO2 NPs and Te ablation debris from the target.
Further investigation was performed by dynamic light scattering (DLS) to determine the size distribution of the NPs. Based on Figure 5a, there seem to be two main populations of NPs within the colloid, one around ∼70 nm and another around ∼800 nm. By converting the intensity size distribution into a number size distribution, there was only one population around ∼70 nm, suggesting that the second population (∼800 nm in size) observed in the intensity size distribution comes from the agglomeration of the NPs belonging to the first population (∼70 nm in size) or from microscopic chunks/dust of the target being ejected upon the impact of the laser beam. To confirm the possible agglomeration of NPs, the ζ-potential of the NPs was determined and found to be around −8 ± 1 mV, which is well below the threshold value of 30 mV corresponding to a stable colloid (Figure 5b). Consequently, the value of ζ-potential confirmed the instability of TeO2 NPs with time. To be complete, the pH of the colloid was measured at 5.2 ± 0.1.
Figure 5.
(a) Intensity size distribution as measured by DLS on the colloid synthesized at 1000 Hz. Inset: number size distribution measured by DLS on the colloid synthesized at 1000 Hz. The number size distribution is centered around ∼70 nm. (b) The ζ-potential was measured to be −8 ± 1 mV, meaning that the colloid was not stable with time.
By using UV–visible spectroscopy (Figure 6a), the direct energy band gap of the TeO2 NPs was measured at around ∼5.2 eV (Figure 6b). This value is in excellent agreement with the value reported by Khalef et al.40 who measured a value of ∼5 eV for TeO2 NPs having sizes around ∼55 nm. The strong absorption band in the UV region of the absorbance spectra (Figure 6a) is due to the transition from the valence band (p-nonbonding triplet) to the conduction band (p-antibonding triplet) of TeO2.
Figure 6.
(a) UV–visible spectra of the colloid shown in Figure 1b. (b) Tauc plot displaying an energy band gap of around ∼5.2 eV.
3.2. Biological Tests
Finally, within those synthesis conditions, TeO2 spherical NPs with a “naked” surface and a size distribution of around ∼70 nm were obtained, which is in the optimal size range to interact with biological cells.53,54 Consequently, the spherical TeO2 NPs were tested against MDR E. coli and MR S. aureus, two harmful pathogens, one Gram-negative and one Gram-positive, that developed a resistance to antibiotics (Figure 7). The NPs were active against both pathogens at a range of concentrations between 2 and 10 ppm, showing a clear dose-dependent inhibition that was more lineal and substantial in MR S. aureus. Indeed, the cell wall of Gram-positive bacteria such as MR S. aureus includes a layer of peptidoglycan as well as teichoic acid and abundant pores that allow foreign nanoparticles to penetrate, resulting in cell membrane damage and cell death, while the cell wall of Gram-negative bacteria such as MDR E. coli is composed of lipopolysaccharides, lipoproteins, and phospholipids, which form a penetration barrier to nanoparticles.55 Therefore, the TeO2 NPs showed an effective bacterial inhibition at concentrations of ∼10 ppm, which was much less than the concentration of ∼25 ppm of selenium (Se) NPs prepared also by PLAL required to fully inhibit the growth of MR S. aureus and MDR E. coli in a previous study.33 The size distribution of those Se NPs was centered around 43 ± 20 nm. Consequently, the TeO2 NPs were more effective than the Se NPs synthesized by the same technique in terms of antimicrobial effectiveness. As TeO2 (oxidation state +4) and Se (oxidation state 0) are both chalcogenide compounds, bacteria are using the same metabolic machinery associated with sulfur (S) in the production of amino acids; therefore, the difference in their antibacterial efficiency could come from their oxidation state being different. Another possible cause of TeO2’s higher efficiency is its ability to interact with Se present in some selenoproteins and enzymes, which could disturb the vital functions of the bacteria.3,10
Figure 7.
Colony counting assay of (a) MDR E. coli and (b) MR S. aureus for 8 h in the presence of different concentrations of TeO2 NPs. All values represent the mean ± standard deviation. *p < 0.05, **p < 0.01 (compared to controls).
The minimum inhibitory concentration (MIC) values were calculated to quantify the static effects of the NPs in the studied bacterial strains. MIC values were 4.3 ± 0.9 and 3.7 ± 0.2 ppm for MDR E. coli and MR S. aureus, respectively. These values are in correlation with similar nanostructures of Te, such as Te/Te oxide NPs,42 Te nanorods,56 and composites of gold and silver with Te.57
To then assess the cytocompatibility of the NPs, cell studies were performed with HDF and human melanoma cells as in vitro models for potential skin treatments (Figure 8). The half-maximal inhibitory concentration (IC50) values were calculated with the aim to study the potency of the TeO2 NPs to inhibit the growth of both HDF and human melanoma cells. These values were found at 5.5 ± 0.2 and 1.6 ± 0.7 ppm for HDF and human melanoma cells, respectively. These IC50 values are in concordance with similar nanostructures based on Te found in the literature and show that the NPs can be safely used in the presence of HDF cells and cause a remarkable cytotoxic behavior when exposed to human melanoma cells.21,42,58
Figure 8.
(a) Human dermal fibroblast (HDF) cells and (b) human melanoma cells in the presence of different concentrations of the NPs. N = 3. Data are represented as mean ± SD; *p < 0.05, **p < 0.01 compared to the control.
SEM microscopy of human melanoma cells exposed to a concentration of 4 ppm of NPs showed signs of necrosis and apoptosis all over the cells. Visual cues, such as smoothing, loss of microvillous structures, blebbing, and shrinking, are often markers of apoptosis (Figure 9a),59 while necrosis can be observed in those cells that partially disintegrate, leaving granular particles (Figure 9b).60
Figure 9.
(a) Representative electron microscopy image of human melanoma cells before their interaction with the TeO2 NPs. (b) Representative electron microscopy image of human melanoma cells after being exposed to a fixed concentration of 4 ppm of TeO2 NPs; image is taken after 24 h of contact.
Lastly, to elucidate the potential mechanism of action, a reactive oxygen species (ROS) test was conducted in the presence of human melanoma cells. The four ROS types are the superoxide radical (O2–), the hydroxyl radical (•OH), hydrogen peroxide (H2O2), and singlet oxygen (O2).55 Results indicated that there is a significant release of ROS from the NPs to the cell media even at the lowest concentrations compared with the controls and that it linearly increases with larger amounts of NPs present in the media (Figure 10). These findings are in concordance with previously published studies by Gupta et al.,61 who showed that the antimicrobial effects of TeO2 NPs were attributed to the generation of ROS inside the bacterial cells.
Figure 10.
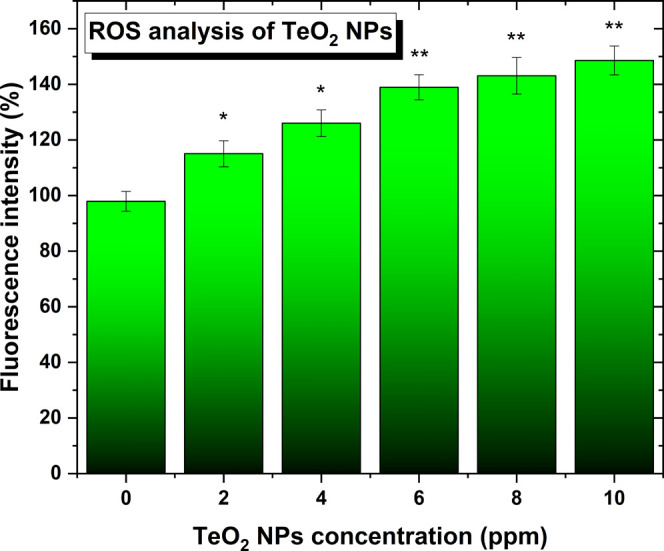
Reactive oxygen species (ROS) induced by the NPs in human melanoma cell experiments. A trend of the release of the species with the increase in NP concentration for the same time frame is seen. N = 3. Data is represented as mean ± SD; *p < 0.05, **p < 0.01 (compared to 0 concentration).
3.3. Mechanism of Tellurium Dioxide Formation
The solvent in PLAL (DI water in our case) confines the plasma plume and also provides a reactive medium to generate a compound based on the target’s chemical element,62 in this case, Te. When the laser beam hits the Te target, it starts releasing Te into the solvent and it also breaks down water molecules located on the beam path according to the water splitting reaction63
| 1 |
When the plasma cools down (the laser beam is off), Te, H2, and O2 start reacting together to form Te-based compounds according to the following chemical reactions
| 2 |
| 3 |
Based on the species present during the irradiation, Te will react preferentially with O2 because the enthalpy of formation of TeO2 is more negative than that of tellurium hydride (TeH2), meaning that TeO2 is expected to form, as observed experimentally in Section 3.1. Furthermore, the diatomic bond enthalpy of Te–O (∼376.1 ± 20.9 kJ/mol) is larger than that of Te–Te (∼259.8 ± 5.0 kJ/mol), meaning that Te prefers to bind with oxygen than Te.64
The oxidation of tellurium during the PLAL synthesis is caused by reactive oxygen species due to the decomposition of water molecules during irradiation. Indeed, the breakdown of the water molecules occurs because of the high-temperature plasma plume generated by the laser–target interactions. This phenomenon has already been observed in the formation of nanoparticles by PLAL.63,65,66
4. Conclusions
Here, an infrared nanosecond pulsed laser emitting at 1064 nm was used to irradiate a pure Te target immersed in DI water. The irradiation lasted only 5 min at 1 kHz, and spherical α-TeO2 NPs were successfully synthesized by PLAL. The presence of Te chunks/dust in the colloid comes directly from the target by being mechanically ejected when the laser beam hits the Te target. Due to the presence of two chalcogenide elements, O and Te, TeO2 NPs were found to be toxic for microorganisms such as MDR E. coli and MR S. aureus at very low concentrations of ∼10 ppm. This can be understood by the similarity of the Te chemistry to S, as they both belong to the chalcogen family (O, S, Se, and Te). Indeed, Te can be incorporated into S-containing amino acids (such as cysteine and methionine), which are semi-essential and essential amino acids for bacterial function, respectively. Then, those amino acids, which comprise proteins and enzymes, can consequently disrupt the metabolism of the bacteria. Moreover, TeO2 NPs displayed a greater cytotoxic effect against human melanoma cells than human dermal fibroblasts. More work is currently underway to design other Te-based nanodrugs by PLAL and to further elucidate the mechanism by which these novel TeO2 NPs kill antibiotic-resistant bacteria and cancer cells.
Acknowledgments
G.G. would like to thank Arkansas IDeA Network for Biomedical Research Excellence (INBRE) grant #P20GM103429-20 for financial support. T.H. and E.H. would like to thank the McNair Scholars Research Program for financial support. D.M. and T.J.W. would like to thank Northeastern University for funding. T.H., E.H., and G.G. would like to thank the Center for Integrative Nanotechnology Sciences (CINS) of UA Little Rock for the use of their UV–vis, SEM, DSC, and XPS.
Author Contributions
T.H. (txhesabizade@ualr.edu): investigation (synthesis, UV–visible spectroscopy, Raman spectroscopy, DLS, ζ-potential, SEM, and EDX) and formal analysis; E.H. (edhicks@ualr.edu): investigation (synthesis, DLS, and ζ-potential); D.M.-C. (davidmedinacrz@gmail.com): investigation (antibacterial and anticancer properties, SEM) and formal analysis; S.E.B. (sxbourdo@ualr.edu): investigation (DSC), formal analysis, and validation; F.W. (fxwatanabe@ualr.edu): investigation (XPS), formal analysis, and validation; M.B. (mmbonney@ualr.edu): investigation (XRD) and formal analysis; J.N. (jxnichols@ualr.edu): investigation (XRD), formal analysis, and validation; T.J.W. (websterthomas02@gmail.com): validation and writing—reviewing and editing; and G.G. (gxguisbiers@ualr.edu): conceptualization, resources, visualization, validation, writing—reviewing and editing, formal analysis, funding acquisition, and project administration.
The authors declare the following competing financial interest(s): The authors declare that the University of Arkansas at Little Rock has filled a provisional US patent on those tellurium dioxide nanoparticles.
References
- Ibers J. Tellurium in a twist. Nat. Chem. 2009, 1, 508 10.1038/nchem.350. [DOI] [PubMed] [Google Scholar]
- Goldfarb R. J.Tellurium: Providing a Bright Future for Solar Energy, U. S. Geological Survey, Fact Sheet 2014-3077, 2015.
- Chivers T.; Laitinen R. S. Tellurium: a maverick among the chalcogens. Chem. Soc. Rev. 2015, 44, 1725–1739. 10.1039/C4CS00434E. [DOI] [PubMed] [Google Scholar]
- Geoffrion L. D.; Guisbiers G. Physico-chemical properties of selenium–tellurium alloys across the scales. Nanoscale Adv. 2021, 3, 4254–4270. 10.1039/D1NA00087J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson D. L.Theory of the Earth; Blackwell Scientific Publications, 1989. [Google Scholar]
- Mahabaduge H. P.; Rance W. L.; Burst J. M.; Reese M. O.; Meysing D. M.; Wolden C. A.; Li J.; Beach J. D.; Gessert T. A.; Metzger W. K.; et al. High-efficiency, flexible CdTe solar cells on ultra-thin glass substrates. Appl. Phys. Lett. 2015, 106, 133501 10.1063/1.4916634. [DOI] [Google Scholar]
- He Y.; Zhang T.; Shi X.; Wei S.-H.; Chen L. High thermoelectric performance in copper telluride. NPG Asia Mater. 2015, 7, e210 10.1038/am.2015.91. [DOI] [Google Scholar]
- Snyder G. J.; Snyder A. H. Figure of merit ZT of a thermoelectric device defined from materials properties. Energy Environ. Sci. 2017, 10, 2280–2283. 10.1039/C7EE02007D. [DOI] [Google Scholar]
- Cunha R. L. O. R.; Gouvea I. E.; Juliano L. A glimpse on biological activities of tellurium compounds. An. Acad. Bras. Cienc. 2009, 81, 393–407. 10.1590/s0001-37652009000300006. [DOI] [PubMed] [Google Scholar]
- Ba L. A.; Döring M.; Jamier V.; Jacob C. Tellurium: an element with great biological potency and potential. Org. Biomol. Chem. 2010, 8, 4203–4216. 10.1039/C0OB00086H. [DOI] [PubMed] [Google Scholar]
- Knockaert G. Tellurium and Tellurium Compounds. Ullmann’s Encycl. Ind. Chem. 2011, 35, 685–695. 10.1002/14356007.a26_177.pub2. [DOI] [Google Scholar]
- Deringer V. L.; Stoffel R. P.; Dronskowski R. Thermochemical Ranking and Dynamic Stability of TeO2 Polymorphs from Ab Initio Theory. Cryst. Growth Des. 2014, 14, 871–878. 10.1021/cg401822g. [DOI] [Google Scholar]
- Ceriotti M.; Pietrucci F.; Bernasconi M. Ab initio study of the vibrational properties of crystalline TeO2: The α, β, and γ phases. Phys. Rev. B 2006, 73, 104304 10.1103/PhysRevB.73.104304. [DOI] [Google Scholar]
- Li Y.; Fan W.; Sun H.; Cheng X.; Li P.; Zhao X. Structural, electronic, and optical properties of α, β, and γ–TeO2. J. Appl. Phys. 2010, 107, 093506 10.1063/1.3406135. [DOI] [Google Scholar]
- Moufok S.; Kadi L.; Amrani B.; Khodja K. D. Electronic structure and optical properties of TeO2 polymorphs. Results Phys. 2019, 13, 102315 10.1016/j.rinp.2019.102315. [DOI] [Google Scholar]
- Jha A.; Richards B. D. O.; Jose G.; Fernandez T. T.; Hill C. J.; Lousteau J.; Joshi P. Review on structural, thermal, optical and spectroscopic properties of tellurium oxide based glasses for fibre optic and waveguide applications. Int. Mater. Rev. 2012, 57, 357–382. 10.1179/1743280412Y.0000000005. [DOI] [Google Scholar]
- Fleming A. On the specific antibacterial properties of penicillin and potassium tellurite. Incorporating a method of demonstrating some bacterial antagonisms. J. Pathol. Bacteriol. 1932, 35, 831–842. 10.1002/path.1700350603. [DOI] [Google Scholar]
- Lin Z.-H.; Lee C.-H.; Chang H.-Y.; Chang H.-T. Antibacterial Activities of Tellurium Nanomaterials. Chem. - Asian J. 2012, 7, 930–934. 10.1002/asia.201101006. [DOI] [PubMed] [Google Scholar]
- Miola M.; Massera J.; Cochis A.; Kumar A.; Rimondini L.; Vernè E. Tellurium: A new active element for innovative multifunctional bioactive glasses. Mater. Sci. Eng., C 2021, 123, 111957 10.1016/j.msec.2021.111957. [DOI] [PubMed] [Google Scholar]
- Vahidi H.; Kobarfard F.; Alizadeh A.; Saravanan M.; Barabadi H. Green nanotechnology-based tellurium nanoparticles: Exploration of their antioxidant, antibacterial, antifungal and cytotoxic potentials against cancerous and normal cells compared to potassium tellurite. Inorg. Chem. Commun. 2021, 124, 108385 10.1016/j.inoche.2020.108385. [DOI] [Google Scholar]
- Medina-Cruz D.; Tien-Street W.; Zhang B.; Huang X.; Vernet-Crua A.; Nieto-Arguello A.; Cholula-Diaz J. L.; Martinez L.; Huttel Y.; Ujue Gonzalez M.; et al. Citric juice-mediated synthesis of tellurium nanoparticles with antimicrobial and anticancer properties. Green Chem. 2019, 21, 1982–1998. 10.1039/C9GC00131J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. D.; Cruz D. M.; Roy A. K.; Webster T. J. Synthesis and characterization of PVP-coated tellurium nanorods and their antibacterial and anticancer properties. J. Nanopart. Res. 2018, 20, 254 10.1007/s11051-018-4354-8. [DOI] [Google Scholar]
- Vernet-Crua A.; Medina D.; Zhang B.; Gonzalez M. U.; Huttel Y.; Garcia-Martin J. M.; Diaz J. L. C.; Webster T. J. Comparison of cytocompatibility and anticancer properties of traditional and green chemistry-synthesized tellurium nanowires. Int. J. Nanomed. 2019, 14, 3155–3176. 10.2147/IJN.S175640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayyad G. S.; Mosallam F. M.; El-Sayed S. S.; El-Batal A. I. Facile Biosynthesis of Tellurium Dioxide Nanoparticles by Streptomyces cyaneus Melanin Pigment and Gamma Radiation for Repressing Some Aspergillus Pathogens and Bacterial Wound Cultures. J. Cluster Sci. 2020, 31, 147–159. 10.1007/s10876-019-01629-1. [DOI] [Google Scholar]
- Zhang H.; Swihart M. T. Synthesis of Tellurium Dioxide Nanoparticles by Spray Pyrolysis. Chem. Mater. 2007, 19, 1290–1301. 10.1021/cm062257n. [DOI] [Google Scholar]
- Jung T.-K.; Ryou M.; Lee J.-W.; Hyun S.-K.; Na H. G.; Jin C. Comparison of Structural and Optical Properties of TeO2 Nanostructures Synthesized Using Various Substrate Conditions. Met. Mater. Int. 2017, 23, 1133–1138. 10.1007/s12540-017-7047-4. [DOI] [Google Scholar]
- Arab F.; Mousavi-Kamazani M.; Salavati-Niasari M. Facile sonochemical synthesis of tellurium and tellurium dioxide nanoparticles: Reducing Te(IV) to Te via ultrasonic irradiation in methanol. Ultrason. Sonochem. 2017, 37, 335–343. 10.1016/j.ultsonch.2017.01.026. [DOI] [PubMed] [Google Scholar]
- Liu J.; Liang C.; Zhu X.; Lin Y.; Zhang H.; Wu S. Understanding the Solvent Molecules Induced Spontaneous Growth of Uncapped Tellurium Nanoparticles. Sci. Rep. 2016, 6, 32631 10.1038/srep32631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisbiers G.; Mimun L. C.; Mendoza-Cruz R.; Nash K. L. Synthesis of tunable tellurium nanoparticles. Semicond. Sci. Technol. 2017, 32, 04LT01 10.1088/1361-6641/aa6173. [DOI] [Google Scholar]
- Forsythe R. C.; Cox C. P.; Wilsey M. K.; Müller A. M. Pulsed Laser in Liquids Made Nanomaterials for Catalysis. Chem. Rev. 2021, 121, 7568–7637. 10.1021/acs.chemrev.0c01069. [DOI] [PubMed] [Google Scholar]
- Taylor P.; Kusper M.; Hesabizadeh T.; Geoffrion L. D.; Watanabe F.; Herth E.; Guisbiers G. Synthesis of naked vanadium pentoxide nanoparticles. Nanoscale Adv. 2021, 3, 1954–1961. 10.1039/D1NA00029B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista L. M. F.; Kunzler K.; John M. G.; Clark B.; Bullock A.; Ferri J.; Gupton B. F.; Tibbetts K. M. Laser synthesis of uncapped palladium nanocatalysts. Appl. Surf. Sci. 2021, 557, 149811 10.1016/j.apsusc.2021.149811. [DOI] [Google Scholar]
- Geoffrion L. D.; Hesabizadeh T.; Medina-Cruz D.; Kusper M.; Taylor P.; Vernet-Crua A.; Chen J.; Ajo A.; Webster T. J.; Guisbiers G. Naked selenium nanoparticles for antibacterial and anticancer treatments. ACS Omega 2020, 5, 2660–2669. 10.1021/acsomega.9b03172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geoffrion L. D.; Medina-Cruz D.; Kusper M.; Elsaidi S.; Watanabe F.; Parajuli P.; Ponce A.; Hoang T. B.; Brintlinger T. H.; Webster T. J.; Guisbiers G. Bi2O3 nano-flakes as a cost-effective antibacterial agent. Nanoscale Adv. 2021, 3, 4106–4118. 10.1039/D0NA00910E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guisbiers G.; Wang Q.; Khachatryan E.; Arellano-Jimenez M. J.; Webster T. J.; Larese-Casanova P.; Nash K. L. Anti-bacterial selenium nanoparticles produced by UV/VIS/NIR pulsed nanosecond laser ablation in liquids. Laser Phys. Lett. 2014, 12, 016003 10.1088/1612-2011/12/1/016003. [DOI] [Google Scholar]
- Nikolov A. S.; Balchev I. I.; Nedyalkov N. N.; Kostadinov I. K.; Karashanova D. B.; Atanasova G. B. Influence of the laser pulse repetition rate and scanning speed on the morphology of Ag nanostructures fabricated by pulsed laser ablation of solid target in water. Appl. Phys. A 2017, 123, 719 10.1007/s00339-017-1328-0. [DOI] [Google Scholar]
- Khalef W. K. Preparation and Characterization of Teo2 Nano particles by Pulsed Laser Ablation in Water. Eng. Technol. J. 2014, 32, 396–405. [Google Scholar]
- Private communication between Guisbiers and Liang - Email: Questions about your Scientific Reports paper published in 2016. 2021.
- Saraeva I. N.; Tolordava E. R.; Nastulyavichus A. A.; Ivanova A. K.; Kudryashov S. I.; Rudenko A. A.; Melnik N. N.; Zayarny D. A.; Ionin A. A.; Romanova Y. M.; Gonchukov S. A. A bacterial misericorde: laser-generated silicon nanorazors with embedded biotoxic nanoparticles combat the formation of durable biofilms. Laser Phys. Lett. 2020, 17, 025601 10.1088/1612-202X/ab5fca. [DOI] [Google Scholar]
- Khalef W. K.; Marzoog T. R.; Faisal A. D. Synthesis and characterization of tellurium oxide nanoparticles using pulse laser ablation and study their antibacterial activity. J. Phys.: Conf. Ser. 2021, 1795, 012049 10.1088/1742-6596/1795/1/012049. [DOI] [Google Scholar]
- Hamad A.; Li L.; Liu Z. Comparison of characteristics of selected metallic and metal oxide nanoparticles produced by picosecond laser ablation at 532 and 1064 nm wavelengths. Appl. Phys. A 2016, 122, 904 10.1007/s00339-016-0426-8. [DOI] [Google Scholar]
- Medina-Cruz D.; Vernet-Crua A.; Mostafavi E.; González M. U.; Martínez L.; Jones A.-A. D. III; Kusper M.; Sotelo E.; Gao M.; Geoffrion L. D.; et al. Aloe Vera-Mediated Te Nanostructures: Highly Potent Antibacterial Agents and Moderated Anticancer Effects. Nanomaterials 2021, 11, 514 10.3390/nano11020514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.abcam.com/cellular-ros-assay-kit-red-ab186027.html.
- Smith W.; Tyndall A. J. Sci. Mon. 1920, 11, 331–340. [Google Scholar]
- Kraemer E. O.; Dexter S. T. The Light-Scattering Capacity (Tyndall Effect) and Colloidal Behavior of Gelatine Sols and Gels. J. Phys. Chem. A 1927, 31, 764–782. 10.1021/j150275a014. [DOI] [Google Scholar]
- Rodriguez V.; Couzi M.; Adamietz F.; Dussauze M.; Guery G.; Cardinal T.; Veber P.; Richardson K.; Thomas P. Hyper-Raman and Raman scattering in paratellurite TeO2. J. Raman Spectrosc. 2013, 44, 739–745. 10.1002/jrs.4251. [DOI] [Google Scholar]
- Jafari A.; Klobes B.; Sergueev I.; Moseley D. H.; Manley M. E.; Dronskowski R.; Deringer V. L.; Stoffel R. P.; Bessas D.; Chumakov A. I.; et al. Phonon Spectroscopy in Antimony and Tellurium Oxides. J. Mater. Chem. A 2020, 124, 7869–7880. 10.1021/acs.jpca.0c05060. [DOI] [PubMed] [Google Scholar]
- Gupta N.; Kaur A.; Khanna A.; Gonzàlez F.; Pesquera C.; Iordanova R.; Chen B. Structure-property correlations in TiO2-Bi2O3-B2O3-TeO2 glasses. J. Non-Cryst. Solids 2017, 470, 168–177. 10.1016/j.jnoncrysol.2017.05.021. [DOI] [Google Scholar]
- Charton P.; Gengembre L.; Armand P. TeO2-WO3 Glasses: Infrared, XPS and XANES Structural Characterizations. J. Solid State Chem. 2002, 168, 175–183. 10.1006/jssc.2002.9707. [DOI] [Google Scholar]
- https://www.thermofisher.com/us/en/home/materials-science/learning-center/periodic-table/metalloid/tellurium.html.
- Guisbiers G. Advances in thermodynamic modelling of nanoparticles. Adv. Phys.: X 2019, 4, 1668299 10.1080/23746149.2019.1668299. [DOI] [Google Scholar]
- Guisbiers G. Size-dependent materials properties toward a universal equation. Nanoscale Res. Lett. 2010, 5, 1132–1136. 10.1007/s11671-010-9614-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolai J.; Mandal K.; Jana N. R. Nanoparticle Size Effects in Biomedical Applications. ACS Appl. Nano Mater. 2021, 4, 6471–6496. 10.1021/acsanm.1c00987. [DOI] [Google Scholar]
- Zannoni D.; Borsetti F.; Harrison J. J.; Turner R. J.. The Bacterial Response to the Chalcogen Metalloids Se and Te. In Advances in Microbial Physiology; Elsevier, 2007; Vol. 53, pp 1–312. [DOI] [PubMed] [Google Scholar]
- Wang L.; Hu C.; Shao L. The antimicrobial activity of nanoparticles: present situation and prospects for the future. Int. J. Nanomed. 2017, 12, 1227–1249. 10.2147/IJN.S121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakibaie M.; Adeli-Sardou M.; Mohammadi-Khorsand T.; Zeydabadi-Nejad M.; Amirafzali E.; Amirpour-Rostami S.; Ameri A.; Forootanfar H. Antimicrobial and Antioxidant Activity of the Biologically Synthesized Tellurium Nanorods; A Preliminary In vitro Study. Iran. J. Biotechnol. 2017, 15, 268–276. 10.15171/IJB.1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang H.-Y.; Cang J.; Roy P.; Chang H.-T.; Huang Y.-C.; Huang C.-C. Synthesis and antimicrobial activity of gold/silver-tellurium nanostructures. ACS Appl. Mater. Interfaces 2014, 6, 8305–8312. 10.1021/am501134h. [DOI] [PubMed] [Google Scholar]
- Huang W.; He L.; Ouyang J.; Chen Q.; Liu C.; Tao W.; Chen T. Triangle-Shaped Tellurium Nanostars Potentiate Radiotherapy by Boosting Checkpoint Blockade Immunotherapy. Matter 2020, 3, 1725–1753. 10.1016/j.matt.2020.08.027. [DOI] [Google Scholar]
- Pesce M.; De Felici M. Apoptosis in mouse primordial germ cells: a study by transmission and scanning electron microscope. Anat. Embryol. 1994, 189, 435–440. 10.1007/BF00185438. [DOI] [PubMed] [Google Scholar]
- Cabral G. A.; Toney D. M.; Fischer-Stenger K.; Harrison M. P.; Marciano-Cabral F. Anandamide inhibits macrophage-mediated killing of tumor necrosis factor-sensitive cells. Life Sci. 1995, 56, 2065–2072. 10.1016/0024-3205(95)00190-H. [DOI] [PubMed] [Google Scholar]
- Gupta P. K.; Sharma P. P.; Sharma A.; Khan Z. H.; Solanki P. R. Electrochemical and antimicrobial activity of tellurium oxide nanoparticles. Mater. Sci. Eng.: B 2016, 211, 166–172. 10.1016/j.mseb.2016.07.002. [DOI] [Google Scholar]
- Yan Z.; Chrisey D. B. Pulsed laser ablation in liquid for micro-/nanostructure generation. J. Photochem. Photobiol., C 2012, 13, 204–223. 10.1016/j.jphotochemrev.2012.04.004. [DOI] [Google Scholar]
- Barmina E. V.; Gudkov S. V.; Simakin A. V.; Shafeev G. A. Stable Products of Laser-Induced Breakdown of Aqueous Colloidal Solutions of Nanoparticles. J. Laser Micro/Nanoeng. 2017, 12, 254–257. 10.2961/jlmn.2017.03.0014. [DOI] [Google Scholar]
- https://www.webelements.com/tellurium/compound_properties.html.
- Marzun G.; Bönnemann H.; Lehmann C.; Spliethoff B.; Weidenthaler C.; Barcikowski S. Role of Dissolved and Molecular Oxygen on Cu and PtCu Alloy Particle Structure during Laser Ablation Synthesis in Liquids. ChemPhysChem 2017, 18, 1175–1184. 10.1002/cphc.201601315. [DOI] [PubMed] [Google Scholar]
- Kalus M.-R.; Lanyumba R.; Lorenzo-Parodi N.; Jochmann M. A.; Kerpen K.; Hagemann U.; Schmidt T. C.; Barcikowski S.; Gökce B. Determining the role of redox-active materials during laser-induced water decomposition. Phys. Chem. Chem. Phys. 2019, 21, 18636–18651. 10.1039/C9CP02663K. [DOI] [PubMed] [Google Scholar]











