Abstract

Pyrolysis polygeneration technology has been widely applied due to its wide adaptability to coal types and mild reaction conditions. In this paper, the in situ process test combining pyrolysis and combustion of Pingshuo coal was carried out using a thermogravimetric analyzer and a fluidized-bed reactor. The influence of pyrolysis conditions on the combustion behavior and char structure was studied. The reduction of the pyrolysis temperature and the increase in pyrolysis residence time resulted in a lower burnout temperature of char, thereby improving the combustion characteristics of char. Furthermore, the maximum CO2 release rate reached 2.8 mg/(g·s) during the combustion process. The decrease in the pyrolysis temperature was conducive to the increase of CO2 release mass, suggesting a more complete burning of char. The char combustion process was mainly controlled by the chemical reaction according to the reaction kinetics study. The apparent rate constant of the combustion process increased gradually with the decrease in the pyrolysis temperature of char, and the rate of the combustion reaction increased, which contributed to the enhancement of the combustion performance of the char within the range of 850–950 °C. Meanwhile, the crystal structure inside the char changed significantly after different pyrolysis conditions. However, it had little influence on the molar content of carbon structure, which suggested that the amount of CO2 released was mainly caused by the different resistances of oxygen diffusion to the reaction interface.
1. Introduction
Direct combustion of coal is frequently utilized for power generation, resulting in low economic profits of coal resource. One of the complete exploitation methods to meet the requirements of coal development is the pyrolysis polygeneration technology.1−4 From the perspective of overall utilization, using coal as a raw material, we can improve the comprehensive utilization efficiency of coal resources in a system with a variety of production techniques. Based on the pyrolysis polygeneration technology, the separated tar can be produced by the hydrogenation process to produce other high-value chemical products, such as phenol;5,6 the residual char is eventually transported to the boiler to be burned or gasified.
However, the char produced by pyrolysis has certain defects, such as high ash content, high fixed carbon content, low volatile matter content, and low heating value that are not conducive to combustion.7,8
In recent years, many scholars have studied the effect of the char preparation process parameters. Qi et al. found that step pyrolysis favored char production relative to direct pyrolysis, which was attributed to the cross-linking reactions at low temperature.9
In addition, some researchers found that coal samples had a synergistic effect when pyrolyzed with mixtures of other substances. This phenomenon could simultaneously increase the yield of char and tar.10 Li et al. found that the pyrolysis of peat and pine branches might have a synergistic effect to facilitate the subsequent gasification of char and secondary cracking of heavy tar. Increasing the heating rate had a positive effect on the maximum weight loss rate.11 Zhao et al. investigated the availability of three natural iron ores for the catalytic reformation of volatiles from the co-pyrolysis of lignite and corn straw. Since carbon deposited contain more oxygen-containing functional groups, the continuous supply of reductants for iron ore reduction could be enhanced.12
The research on the combustion characteristics of char was also an important direction and was generally carried out by thermogravimetric analyzers. Zhang et al. found that char obtained from the smallest coal particles exhibited the worst combustion behaviors, which was because the flame-retardant minerals and inert substances tended to be dispersed in small coal particles.13 Also, the research on different types of char and their mixed combustion showed that the combustion characteristics of char were mainly affected by process factors such as pyrolysis temperature, reaction atmosphere, and heating rate during the pyrolysis process.14,15
Since the heating rate of pulverized coal combustion in the actual industrial furnace was fast, the gas–solid contact and atmosphere were different from the combustion state on the thermogravimetric analyzer. The results had limitations in predicting the char combustion in the pulverized coal furnace. Therefore, many researchers also relied on fixed-bed and fluidized-bed boiler systems to study the combustion characteristics of char. Lee et al.16 found that the good dispersion of the ash phase on the char surface was also beneficial for improving the combustion characteristics. Lorenz et al.17 proved that the combustion process of char was simpler to carry out in the large-porous surface structure of char. Zhang et al. found that the combustion characteristics of the mixed fuel could be significantly improved by blending char with biomass coal.18 Moreover, the combustion performance of char in the fluidized bed was also affected by the combustion process conditions such as the particle size, pressure, and oxygen content.19−22
Considering the above analysis, the majority of scholars only focused on the influence of different working conditions on the pyrolysis process or combustion process of char, whereas few of them considered the operation parameters from the perspective of the combined pyrolysis and combustion process.
In general, the existing research mainly first pyrolyzes coal into char and then analyzes the combustion characteristics of char. The char undergo a cooling process, which is different from the industrial application. Moreover, coal pyrolysis and char combustion are not considered as a whole, which has certain limitations. Therefore, it is urgent to research the improvement of char combustion characteristics during the combined pyrolysis and combustion experiments.
In this paper, the in situ combined pyrolysis and combustion experiments were carried out. The combustion behavior and physicochemical characteristics of Pingshuo char were investigated. Moreover, the influence of pyrolysis temperature and pyrolysis residence time on the combined pyrolysis–combustion process were obtained, thereby providing basic data for the development of technology for large-scale and efficient char combustion and promoting industrial application.
2. Experimental Section
2.1. Experimental Sample
The Pingshuo coal was provided from Shan Xi, which was crushed and ground for later use. The coal sample was ground to less than 0.2 mm. The proximate analysis, ultimate analysis, and calorific value results of the coal sample are listed in Table 1.
Table 1. Proximate Analysis, Ultimate Analysis, and Calorific Value of Coal Sample.
| proximate analysis (wt/%) |
ultimate
analysis (wt/%) |
calorific value (kJ/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| coal sample | Mad | Vad | FCad | Aad | Cad | Had | Oad | Nad | Sad | Qad,net |
| pingshuo | 1.47 | 20.12 | 22.91 | 55.50 | 29.52 | 2.53 | 7.84 | 0.48 | 2.66 | 11 882 |
2.2. Experimental System and Research Method
2.2.1. TG System
The equipment used for thermogravimetric analysis is a PE Pyris 1 thermogravimetric analyzer, which was mainly composed of three parts: temperature control system, detection system, and recording system. The thermogravimetric analysis could reflect the loss of mass of the sample during the heating reaction, helping us understand the weight loss characteristics of the sample. The maximum temperature that could be reached by a thermogravimetric analyzer was 1000 °C, and the heating rate range was 10–50 °C/min. Before each thermogravimetric experiment, the crucible was first cleaned and placed for 1 h after evaporating the water before use. Then, high-purity argon was introduced to purge the experimental pipeline for 20 min to remove the air in the device so that the thermal reaction was not affected, which might cause errors in the experimental results.
2.2.2. Schematic of Pyrolysis and Combustion Combined Test
The pyrolysis and combustion coupling test system and device are shown in Figure 1. Pyrolysis and combustion combined tests were carried out in a fluidized-bed reactor.
Figure 1.
Schematic of pyrolysis and combustion combined test.
Multiphase pyrolysis–combustion fluidized-bed reactor was designed. Specifically, the reactor was designed in two stages: the middle of the reactor was connected by a diameter tapered tube and the bottom sieve plate of each stage could be placed with different types of samples or introduced with different gases, whose temperature could be controlled by a temperature controller of tube type heating furnace. In addition, the combined reaction of pyrolysis and combustion of the sample was achieved on the sieve plate. Moreover, some dry zirconia balls, as the heat carrier of the rapid pyrolysis process, were added to increase the contact area with pulverized coal.
For each combined pyrolysis and combustion experiment, 3 g of samples were first placed in the quartz glass tube of coal storage when the reactor reached the preset pyrolysis temperature. Then, the pulverized coal was carried to the bottom of the pyrolysis reactor at a certain flow rate of Ar gas for the reaction by adjusting the valve switch. After that, the exhausted gas flowed through the reduced tube and entered the upper gas combustion reactor for combustion. At last, the exhausted flue gas was collected using a GR-3028 UV flue gas analyzer through the filter and the flow meter, which could be used to detect CO2 concentration in the flue gas in real-time.
2.2.3. XPS Analysis
XPS spectra were measured on a Thermo ESCALAB 250 instrument in the United States. A monochromatic Al K″ (hv = 1486.6 eV) ray of 150 W was used as the excitation source. The charge correction was performed with the binding energy of C 1s = 284.80 eV as the energy standard.
2.2.4. XRD Analysis
The XRD phase test of the samples was performed using a Mini Flex II X-ray diffractometer from Rigaku Company, Japan, with Cu Kα ray, tube current of 15 mA, tube voltage of 30 kV, 5–50° scan, and a scan rate of 4°/min.
2.3. Design of Experimental Conditions
The impacts of varied pyrolysis temperatures and residence time on the combustion characteristics were investigated through the combined pyrolysis and combustion test.
During the coal pyrolysis at the bottom of the pyrolytic combustion reactor, a positive drift of O2 was first brought into the upper part of the fuel combustion reactor to burn the flue gas, and the temperature of the fuel burner was maintained steady at 900 °C. Then, when the pyrolysis process was completed and the set combustion temperature was reached, oxygen was fed into the pyrolytic combustion reactor to burn char at the flow rate of 1.8 L/min of mixed gas (Ar1: 15.8%, Ar2: 63.2%, O2: 21%) using a conversion three-way valve. Meanwhile, the temperature at the bottom of the pyrolytic combustion reactor was first increased from ambient temperature to the set pyrolysis temperature, which was within the range of 500–700 °C. The sample was rapidly introduced into the reactor and came into contact with the heat carrier. Then, it was pyrolyzed at this temperature for 1 or 10 min. After that, the temperature of the pyrolytic combustion reactor was heated up to 900 °C in 20 min. Then, O2 was sent into the reactor to burn the char. When the CO2 concentration in the flue gas monitored by the flue gas analyzer dropped to 0, it indicated that the combustion reaction was over. The test conditions are summarized in Table 2.
Table 2. Test Conditions.
| working conditions | pyrolysis temperature (°C) | pyrolysis residence time (min) | |
|---|---|---|---|
| working condition 1 | 1.1 | 500 | 1 |
| 1.2 | 600 | ||
| 1.3 | 700 | ||
| working condition 2 | 2.1 | 500 | 10 |
| 2.2 | 600 | ||
| 2.3 | 700 |
3. Results and Analysis
3.1. Thermogravimetric Analysis
To study the combustion characteristics of char, the quartz reactor was taken out from the furnace and cooled in the air for 20 min after the pyrolysis process of the sample was completed. Also, argon was continuously introduced to ensure that the char sample was not oxidized. Moreover, the proximate and ultimate analyses were carried out on the char samples prepared under different working conditions. The results are listed in Table 3.
Table 3. Proximate Analysis and Ultimate Analysis of Char.
| proximate analysis (wt/%) |
ultimate analysis (wt/%) |
|||||||
|---|---|---|---|---|---|---|---|---|
| char samples | Mad | Vdaf | FCd | Ad | Cd | Hd | Nd | Sa,d |
| 1.1 | 1.61 | 20.12 | 26.35 | 56.44 | 29.52 | 2.53 | 0.48 | 2.66 |
| 1.2 | 0.86 | 39.50 | 27.78 | 60.73 | 31.52 | 2.22 | 0.48 | 2.91 |
| 1.3 | 1.16 | 29.26 | 24.91 | 67.34 | 29.22 | 1.52 | 0.44 | 2.67 |
| 2.1 | 1.54 | 23.73 | 33.34 | 59.12 | 24.23 | 1.32 | 0.30 | 2.49 |
| 2.2 | 1.05 | 17.48 | 29.58 | 65.93 | 33.54 | 1.46 | 0.54 | 2.24 |
| 2.3 | 0.95 | 12.66 | 28.24 | 67.64 | 30.74 | 1.23 | 0.53 | 2.13 |
The combustion characteristics of different char samples were determined using a thermogravimetric analyzer. The flow rate was 40 mL/min in a nitrogen argon atmosphere. The settings of the remaining parameters were implemented following the standard GB/T 33304-2016. The heating rate was 20 °C/min, and the temperature range was 30–1000 °C.
Figure 2 depicts char combustion thermogravimetry curves with different pyrolysis temperatures and residence times. As shown in Figure 2a, pyrolysis temperature and pyrolysis residence time had a significant impact on the combustion characteristics of the char. The curve moved to the high-temperature zone with the increase in the pyrolysis temperature, which illustrated that the initial temperature of the char weight loss was gradually delayed. In addition, when the pyrolysis temperature was higher, the burnout temperature and the weight of solid residue were also higher. On the other hand, with the increase in the pyrolysis residence time, the burnout temperature gradually decreased at the end of the combustion reaction. Meanwhile, from the DTG curve shown in Figure 2b, a significant weight loss peak could be observed in the temperature range of 380–545 °C, which may be caused by the burning of volatile matter and fixed carbon.23−25 Therefore, it was inferred that the decrease in the pyrolysis temperature and the increase in pyrolysis residence time could reduce the burnout temperature of char, which could contribute to the efficient use of char.
Figure 2.
Char combustion thermogravimetry curves of the char sample: (a) TG and (b) DTG.
3.2. Effects of Pyrolysis Conditions on Combustion Characteristic Parameters
It could be seen from the combustion parameters in Tables 3 and 4 that the ignition temperature Ti for working situation 1 was significantly lower than that for working condition 2. Under working condition 1, less substances were precipitated in the process of preparing char, so it had a larger specific surface area, which also meant that the char in working condition 1 was more likely to catch fire.26 The highest ignition temperature of the working situations of 2.3 was 435 °C. In addition, the maximum burning rate temperature Tp for working condition 1 was also lower than that for working situation 2. The effects indicated that reducing the pyrolysis temperature and pyrolysis residence time was also helpful for the ignition of the char whose combustion characteristics were extremely improved.
Table 4. Combustion Characteristic Parameters of the Sample.
| working conditions | Ti (°C) | Tf (°C) | (dw/dt)max (%/min) | Tp (°C) |
|---|---|---|---|---|
| 1.1 | 380 | 531 | –6.3 | 402 |
| 1.2 | 384 | 539 | –5.2 | 415 |
| 1.3 | 414 | 543 | –4.8 | 429 |
| 2.1 | 400 | 514 | –9.1 | 439 |
| 2.2 | 421 | 521 | –8.5 | 461 |
| 2.3 | 435 | 540 | –7.1 | 472 |
3.3. Release Characteristics of CO2
The releasing characteristics of CO2 could also be used as an indicator of the intensity of the coal reaction process. Through the combined pyrolysis and combustion test on the circulating fluidized-bed test bench, the CO2 emission of the char in the reaction process was more precisely obtained.
Figure 3 illustrates the CO2 release rate curve during the combined pyrolysis–combustion process. It could be seen from the figure that there was an apparent CO2 release peak in the pyrolysis process and the combustion process, indicating that the pyrolysis process and the combustion process release CO2. With the prolongation of the combustion process time, the releasing rate of CO2 showed a trend of first increasing and then reducing. With the increase in the pyrolysis temperature, the increase in the maximum CO2 release rate in the pyrolysis stage was significantly lower than that in the combustion stage. The maximum release rate of CO2 increased during the combustion process, indicating that the combustion of char was improved.
Figure 3.

Effect of pyrolysis conditions on the CO2 release rate.
Figure 4 depicts the total amount of CO2 released in the combustion process of char under various working conditions. The CO2 emission reduced slightly after reducing the pyrolysis residence time, and the CO2 release tended to be lessened with the increase in the pyrolysis temperature, as shown in the graph. This might be due to the similarity between the total combined reaction’s combustion mode and the dynamic combustion mode, causing the enhancement of the incomplete char combustion. In the combustion process under working condition 1, the volatile matter and the fixed carbon content in the char were relatively high, and the combustion was strengthened, which would inevitably result in a gradual lack of oxygen around the char particles and the production of carbon monoxide. CO and CO2 gas layers would be eventually built on the surface of the char in the later stages of the combustion process due to insufficient interfacial gas diffusion, preventing oxygen diffusion from entering the particles.27 CO and CO2 gas layers would gradually form on the surface of the char particles, and the thickness of the surface ash also increased in the later stage of the combustion process, which hindered the diffusion of oxygen into the particles. The increase in the pyrolysis residence time significantly reduced CO emissions and allowed the combustion process to proceed more completely, which resulted in the release of more heat.28 The pyrolysis process of the sample at a higher temperature would produce more pyrolysis products so that the interaction between the pyrolysis products was enhanced, which contributed to the generation of more CO2 and methane gas so that the released CO2 content gradually decreased in the combustion process.29 Therefore, it was further clarified that increasing the residence time of pyrolysis was beneficial to promoting the reduction of the burnout temperature of char.
Figure 4.
CO2 release quality in the combustion process.
3.4. Kinetics Analysis
Based on the experimental data, the isothermal kinetic analysis method was used to calculate the kinetic parameters of the combustion reaction of pulverized coal under different conditions to grasp the reaction characteristics. The unreacted core model was proposed by Jayaraman, et.al.30 and was commonly employed in the research of gas–solid reaction kinetics. To simplify the mathematical processing, assuming that the char particles were regularly spherical with the radius of r0, the chemical reaction occurred at the interface between the porous solid product layer and the unreacted solid reaction core. As the reaction proceeded, the unreacted solid reaction core gradually shrunk.31,32
In the study of the kinetics of the gas–solid reaction, the reaction could be divided into five successive steps: (1) outer diffusion of gaseous reactants through the gas boundary layer; (2) internal diffusion of gas reactants passing through the porous product layer to the surface of the unreacted core; (3) chemical reaction occurred at the reaction interface; (4) internal diffusion of gas products through the porous product layer; and (5) the gas product diffused through the gas boundary layer into the gas phase. The rate control of this reaction could be separated into three situations, ignoring the effect of product diffusion, external diffusion control, internal diffusion control, and interface chemical reaction control.33,34 When the response conversion rate x and the reaction time t were both individually controlled, an analysis could be used to determine the relationship between them.
| 1 |
| 2 |
| 3 |
In the above formula, x is the reaction rate, t is the reaction time, and k is the rate constant of the reaction.
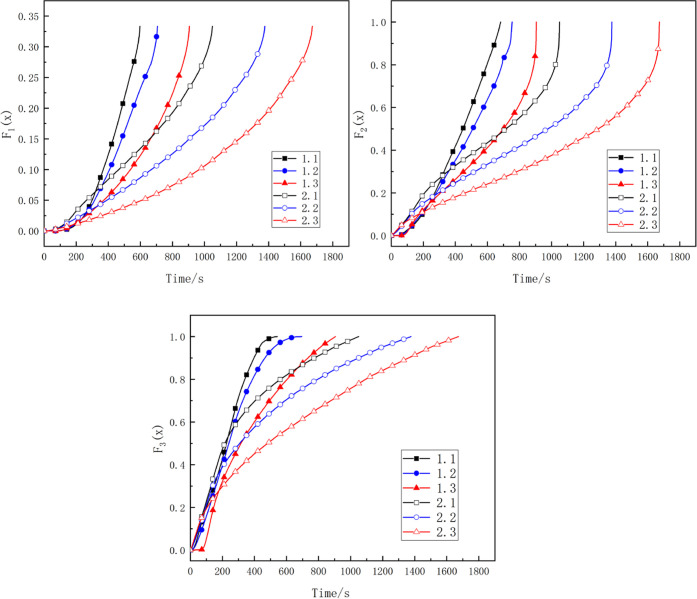
The conversion rate and the corresponding reaction time in the combustion process of char preparation under different pyrolysis conditions were substituted into the kinetic equations shown in Table 5, and the changing relationship of F(x) with reaction time t under different working conditions was obtained, as shown in Figure 5.
Table 5. Form of Combustion Kinetics Equation.
| equation codes | kinetic equations | control of the reaction |
|---|---|---|
| F1(x) |  |
internal diffusion control |
| F2(x) |  |
chemical reaction control |
| F3(x) | 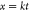 |
external diffusion control |
Figure 5.
Relationship between F(x) and reaction time t.
Through the binary linear regression analysis of the function F(x) of each control situation, the corresponding linear correlation coefficient and the residual sum of squares were compared, and it was concluded that when F2(x) was used for fitting, the absolute value of the correlation coefficient was closer to 1, the residual sum of squares was the smallest, and the linear correlation with the reaction time was the best. Therefore, the main limiting situation in the combustion process for the preparation of char under different pyrolysis conditions was the chemical reaction between gas and solid phase. The linear regression result of F2(x) is shown in Figure 6.
Figure 6.

Linear analysis result of F2(x) and reaction time.
The value of the apparent rate constant k of the combustion reaction under each working condition could also be calculated. Also, the correlation coefficient and the value of k are shown in Table 6. With the decrease in the pyrolysis temperature of the char, the apparent rate constant of the combustion reaction increased. The rate of the combustion reaction also increased, as shown in Table 6. Consequently, appropriately lowering the pyrolysis temperature and pyrolysis residence time could make the char burn more easily.35
Table 6. Linear Correlation Coefficient and the Apparent Rate Constant k.
| working conditions | 1.1 | 1.2 | 1.3 | 2.1 | 2.2 | 2.3 |
|---|---|---|---|---|---|---|
| linear correlation coefficient | 0.996 | 0.985 | 0.993 | 0.997 | 0.997 | 0.995 |
| k/min–1 | 0.073 | 0.066 | 0.045 | 0.044 | 0.033 | 0.025 |
3.5. XPS Analysis
XPS analysis could be used to determine the surface element concentration and chemical states of different char samples.36,37 The XPS analysis chart is shown in Figure 7, which is the C 1s peak fitting results of the char. The figure shows that the carbon element in coal mainly existed in five forms. The peak at 285 eV indicated that aromatics or substituted alkanes were the most important forms of carbon in the sample.38 The peak at 286.3 eV belonged to C–O.39,40 Also, the peaks at 289.1 eV were assigned to O–C=O forms.41
Figure 7.

C 1s XPS spectrum of char.
The peak splitting results of C 1s, as shown in Table 7, showed that the state of existence of C elements in the pyrolysis char with different pyrolysis temperatures and residence times was the same, and the pyrolysis process had little influence on the carbon chain. The surface chemical structures of different char were similar, but the proportions of the functional groups in different samples were slightly different. However, the CO2 release rate during the combustion of the char prepared under working condition 1 was significantly improved, suggesting that the main reason affecting the combustion process was the influence of the difficulty of oxygen diffused into the reaction interface. The thickness of the ash layer on the surface of the char particles prepared in working condition 2 increased, which increased the resistance of oxygen diffusion to the interior of the particles, making the combustion process relatively mild.
Table 7. High-Resolution Scanning Measurement Results of the C 1s Key Environment.
| molar content of carbon structure under various conditions (%) | C–C, C=C | C–H | C–O | C=O, O–C–O | COO |
|---|---|---|---|---|---|
| raw coal | 63.4 | 20.0 | 9.1 | 1.9 | 5.5 |
| 1.1 | 60.1 | 25.7 | 10.3 | 1.8 | 2.0 |
| 1.2 | 65.2 | 21.8 | 7.5 | 2.3 | 3.2 |
| 1.3 | 64.1 | 22.9 | 7.1 | 2.5 | 3.4 |
| 2.1 | 66.7 | 21.7 | 8.0 | 1.2 | 2.4 |
| 2.2 | 64.5 | 22.0 | 8.0 | 2.4 | 3.0 |
| 2.3 | 61.1 | 23.4 | 10.3 | 2.4 | 2.8 |
3.6. XRD Analysis
To obtain the effect of different pyrolysis conditions on the crystal structure of the char, the char was analyzed by the XRD technique.
The XRD patterns of chars obtained under different working conditions are shown in Figure 8. The large background intensities presented in all of the diffraction peaks revealed the existence of amorphous carbon in all char.42,43 The broad 002 band at 20–30° characterized the stacking in aromatic layers of char crystallites, and the 100 band at 40–50° represented the hexagonal ring structure in crystallites.44,45
Figure 8.

XRD patterns of char.
The microcrystalline structure parameters for all of the char samples are summarized in Table 8. The structural parameters of Lc,a, Lc,G, and N gradually grew with the increase in temperature. Meanwhile, the increase in the structural parameter N indicated that the degree of graphitization of the char was gradually deepened and the structure was gradually stabilized. From the thermogravimetric analysis results of the char, it could be concluded that as the temperature increased, the ignition temperature and the burnout temperature of the char also increased. Meanwhile, the structure of char became more stable, and its oxidative combustion process was more difficult to carry out. Therefore, it was shown that high temperature was beneficial to promote the stabilization of the char structure.
Table 8. Crystalline Structure Parameters of Char by XRD.
| sample | d002,P (Å) | Lc,P (Å) | d002,G (Å) | Lc,G (Å) | XP | XG | d002,a (Å) | Lc,a (Å) | N |
|---|---|---|---|---|---|---|---|---|---|
| 1.1 | 4.31 | 30.12 | 3.60 | 16.39 | 10.62 | 89.38 | 3.67 | 17.85 | 4.86 |
| 1.2 | 4.26 | 25.71 | 3.60 | 17.03 | 16.25 | 83.75 | 3.71 | 18.44 | 4.98 |
| 1.3 | 4.24 | 24.45 | 3.58 | 17.05 | 19.28 | 80.72 | 3.70 | 18.48 | 4.99 |
| 2.1 | 4.34 | 37.05 | 3.58 | 14.83 | 22.93 | 77.07 | 3.75 | 19.92 | 5.31 |
| 2.2 | 4.34 | 40.78 | 3.56 | 15.21 | 20.72 | 79.28 | 3.72 | 20.51 | 5.51 |
| 2.3 | 4.30 | 39.97 | 3.55 | 16.81 | 24.47 | 75.53 | 3.73 | 22.48 | 6.02 |
4. Conclusions
The combined pyrolysis and combustion processes were performed on Pingshuo coal in the present paper, and the combustion behavior and physicochemical properties of char were analyzed using a thermogravimetric analyzer and a fluidized-bed reactor. The conclusions could be summarized as follows:
-
(1)
Increasing the pyrolysis residence time properly could bring about a decrease in the char burnout temperature and allow the combustion reaction to proceed more effectively.
-
(2)
The release characteristics of CO2 showed that lowering the pyrolysis temperature enhanced the combustion rate. The maximum release rate of CO2 reached 2.8 mg/(g·s) in the process of combustion. The chemical reaction between the gas and solid phases was found to be the key limiting situation in the combustion process for the preparation of char with various pyrolysis settings according to a kinetic study. The apparent rate constant of the combustion process was negatively correlated with the pyrolysis temperature of char, and the rate of the combustion reaction also increased with the decrease in the pyrolysis temperature of char.
-
(3)
The degree of graphitization of the char internal crystal structure after different pyrolysis conditions increased significantly. However, the pyrolysis condition had little influence on the molar content of carbon structure. Therefore, the differences in the CO2 amount released during the char combustion process were mainly caused by different resistances of oxygen diffusion. As the pyrolysis temperature increased, the thickness of the ash layer on the surface of the char particles increased, which increased the resistance of oxygen diffusion to the interior of the particles, making the combustion process milder.
Acknowledgments
This work was financially supported by the National National Key Research and Development Projects of China (2018YFB0605002–03).
The authors declare no competing financial interest.
References
- Wang Q.; Li K.; Guo Z.; Fang M.; Luo Z. Effects of CO atmosphere on the pyrolysis of a typical lignite. Chem. Eng. Technol. 2021, 44, 85–94. 10.1002/ceat.202000273. [DOI] [Google Scholar]
- Wang Q.; Li K.; Guo Z.; Fang M.; Luo Z.; Cen K. Effects of CO2 atmosphere on slow pyrolysis of high-ash lignite. Carbon Resour. Convers. 2018, 1, 94–103. 10.1016/j.crcon.2018.04.002. [DOI] [Google Scholar]
- Fang M. X.; Cen J. M.; Wang Q. H.; Shi Z. L.; Luo Z. Y.; Cen K. F. 25 MW circulating fluidized bed heat-power-coal gas poly-generation installation. J. Power Eng. 2007, 27, 635–639. [Google Scholar]
- Gao Q.; Li S.; Yuan Y.; Zhang Y.; Yao Q. Ultrafine particulate matter formation in the early stage of pulverized coal combustion of high-sodium lignite. Fuel 2015, 158, 224–231. 10.1016/j.fuel.2015.05.028. [DOI] [Google Scholar]
- Zhang R. Thermodynamic and economic analysis of a coal staged conversion utilization polygeneration system. Energy Technol. 2015, 3, 646–657. 10.1002/ente.201500009. [DOI] [Google Scholar]
- Lei Z.; Shu H.; Zhang L.; Jia Y. Gas-Modified Pyrolysis Coke for in Situ Catalytic Cracking of Coal Tar. ACS Omega 2020, 5, 14911–14923. 10.1021/acsomega.0c00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K.; Li W.; Zhao W. Coal chemical industry and its sustainable development in China. Energy 2010, 35, 4349–4355. 10.1016/j.energy.2009.05.029. [DOI] [Google Scholar]
- Wang P.; Wang C.; Du Y.; Feng Q.; Wang Z.; Yao W.; Liu J.; Zhang J.; Che D. Experiments and simulation on co-combustion of char and coal in a full-scale tangentially fired utility boiler. Energy Fuels 2019, 33, 3012–3027. 10.1021/acs.energyfuels.8b04482. [DOI] [Google Scholar]
- Qi J.; Fan C.; Wu H.; Li S. Structure evolution of lignite char in step pyrolysis and its combustion reactivity. Fuel 2022, 315, 123256 10.1016/j.fuel.2022.123256. [DOI] [Google Scholar]
- Zhao H.; Song Q.; Liu S.; Li Y.; Wang X.; Shu X. Study on catalytic co-pyrolysis of physical mixture/staged pyrolysis characteristics of lignite and straw over an catalytic beds of char and its mechanism. Energy Convers. Manage. 2018, 161, 13–26. 10.1016/j.enconman.2018.01.083. [DOI] [Google Scholar]
- Li Y.; Zhao H.; Sui X.; Wang X.; Ji H. Studies on individual pyrolysis and co-pyrolysis of peat-biomass blends: Thermal decomposition behavior, possible synergism, product characteristic evaluations and kinetics. Fuel 2022, 310, 122280 10.1016/j.fuel.2021.122280. [DOI] [Google Scholar]
- Zhao H.; Li Y.; Song Q.; Liu S.; Ma L.; Shu X. Catalytic reforming of volatiles from co-pyrolysis of lignite blended with corn straw over three iron ores: Effect of iron ore types on the product distribution, carbon-deposited iron ore reactivity and its mechanism. Fuel 2021, 286, 119398 10.1016/j.fuel.2020.119398. [DOI] [Google Scholar]
- Zhang J.; Wang C.; Jia X.; Wang P.; Che D. Experimental study on combustion and NO formation characteristics of char. Fuel 2019, 258, 116108 10.1016/j.fuel.2019.116108. [DOI] [Google Scholar]
- Yao Z.; Ma X.; Wang Z.; Chen L. Characteristics of co-combustion and kinetic study on hydrochar with oil shale: A thermogravimetric analysis. Appl. Therm. Eng. 2017, 110, 1420–1427. 10.1016/j.applthermaleng.2016.09.063. [DOI] [Google Scholar]
- Liu H. P.; Liang W. X.; Qin H.; Wang Q.; et al. Thermal behavior of co-combustion of oil shale char with torrefied cornstalk. Appl. Therm. Eng. 2016, 109, 413–422. 10.1016/j.applthermaleng.2016.08.084. [DOI] [Google Scholar]
- Lee D. W.; Bae J. S.; Park S. J.; Lee Y. J.; Hong J. C.; Choi Y. C. The pore structure variation of coal char during pyrolysis and its relationship with char combustion reactivity. Ind. Eng. Chem. Res. 2012, 51, 13580–13588. 10.1021/ie301927v. [DOI] [Google Scholar]
- Lorenz H.; Carrea E.; Tamura M.; Haas J. The role of char surface structure development in pulverized fuel combustion. Fuel 2000, 79, 1161–1172. 10.1016/S0016-2361(99)00259-8. [DOI] [Google Scholar]
- Zhang J.; Jia X.; Wang C.; et al. Experimental investigation on combustion and NO formation characteristics of char and bituminous coal blends. Fuel 2019, 247, 87–96. 10.1016/j.fuel.2019.03.045. [DOI] [Google Scholar]
- Liu S.; Zhu G.; Niu Y.; Wen L.; Lei Y.; Wang D.; Hui S. Characteristics of particulate emissions from coal char combustion: Char fragmentation and ash coalescence behaviors. Fuel 2022, 310, 122283 10.1016/j.fuel.2021.122283. [DOI] [Google Scholar]
- Pang L.; Shao Y.; Zhong W.; Liu H. Experimental study and modeling of oxy-char combustion in a pressurized fluidized bed combustor. Chem. Eng. J. 2021, 418, 129356 10.1016/j.cej.2021.129356. [DOI] [Google Scholar]
- Ke X.; Li Y.; Jiang L.; Zhang M.; Lu J.; Huang Z. Experimental and modeling investigation on char combustion and char-nitrogen evolution characteristics under different conditions. J. Energy Inst. 2022, 102, 256–267. 10.1016/j.joei.2022.04.001. [DOI] [Google Scholar]
- Zhang J.; Shi Z.; Zheng Y.; Tan H.; Wang X. Fragmentation and mineral transformation behavior during combustion of char produced at elevated pressure. Energy Convers. Manage. 2022, 258, 115538 10.1016/j.enconman.2022.115538. [DOI] [Google Scholar]
- Song Y.; Zhao Y.; Hu X.; Zhang L.; Sun S. Z.; Li C. Z. Destruction of tar during volatile-char interactions at low temperature. Fuel Process. Technol. 2018, 171, 215–222. 10.1016/j.fuproc.2017.11.023. [DOI] [Google Scholar]
- Zheng S.; Hu Y.; Wang Z.; Cheng X. Experimental investigation on ignition and burnout characteristics of char and bituminous coal blends. J. Energy Inst. 2020, 93, 1373–1381. 10.1016/j.joei.2019.12.007. [DOI] [Google Scholar]
- Wang C.; Wang C.; Jia X.; Gao X.; Che D.; et al. Experimental investigation on combustion characteristics and kinetics during Co-Firing bituminous coal with ultra-low volatile carbon-based solid fuels. J. Energy Inst. 2021, 95, 87–100. 10.1016/j.joei.2021.01.005. [DOI] [Google Scholar]
- Niu H.; Liu Y.; Wu K.; Wu J.; Li S.; Wang H. Study on Pore Structure Change Characteristics of Water-Immersed and Air-Dried Coal Based on SEM-BET. Combust. Sci. Technol. 2022, 1–23. 10.1080/00102202.2022.2054272. [DOI] [Google Scholar]
- Shen T.; Yan J.; Zhu X.; Wang L.; Shen L. Catalytic combustion behaviors of petroleum coke with hematitecatalyst in a micro fluidized bed thermogravimetric analysis. Chem. Eng. J. 2021, 422, 130087 10.1016/j.cej.2021.130087. [DOI] [Google Scholar]
- Su H.; Kang N.; Shi B.; Ji H.; Li Y.; Shi J. Simultaneous thermal analysis on the dynamical oxygen-lean combustion behaviors of coal in a O2/N2/CO2 atmosphere. J. Energy Inst. 2021, 96, 128–139. 10.1016/j.joei.2021.03.003. [DOI] [Google Scholar]
- Jayaraman K.; Gokalp I.; Bostyn S. High ash coal pyrolysis at different heating rates to analyze its char structure, kinetics and evolved species. J. Anal. Appl. Pyrolysis 2015, 113, 426–433. 10.1016/j.jaap.2015.03.007. [DOI] [Google Scholar]
- Jayaraman K.; Gokalp I.; Bonifaci E.; Merlo N. Kinetics of steam and CO2 gasification of high ash coal-char produced under various heating rates. Fuel 2015, 154, 370–379. 10.1016/j.fuel.2015.02.091. [DOI] [Google Scholar]
- Papanastassiou D.; Bitsianes G. Mechanisms and kinetics underlying the oxidation of magnetite in the induration of iron ore pellets. Metall. Mater. Trans. B 1973, 4, 487–496. 10.1007/BF02648701. [DOI] [Google Scholar]
- Kazemi M.; Pour M. S.; Sichen D. Experimental and modeling study on reduction of hematite pellets by hydrogen gas. Metall. Mater. Trans. B 2017, 48, 1114–1122. 10.1007/s11663-016-0895-3. [DOI] [Google Scholar]
- Verhoef E. V.; Dijkema G. P. J.; Reuter M. A. Process knowledge, system dynamics, and metal ecology. J. Ind. Ecol. 2008, 8, 23–43. 10.1162/1088198041269382. [DOI] [Google Scholar]
- Liang X.; Wang Q.; Luo Z.; Eddings E.; Ring T.; Li S.; Lin J.; Xue S.; Han L.; Xie G. Experimental and numerical investigation on sulfur transformation in pressurized oxy-fuel combustion of pulverized coal. Appl. Energy 2019, 253, 113542 10.1016/j.apenergy.2019.113542. [DOI] [Google Scholar]
- Zhou M.; Zhou H.; Cheng Y.; Xing Y. Investigation on the combustion behaviors of coke and biomass char in quasi-granule with CuO-CeO2 catalysts in iron ore sintering. J. Energy Inst. 2020, 93, 1934–1941. 10.1016/j.joei.2020.04.008. [DOI] [Google Scholar]
- Liu H.; Chen T.; Fang L. Evolution of char structure during non-isothermal low temperature pyrolysis of ZhunDong coal by microwave heating: A comparative study with conventional heating. J. Energy Inst. 2020, 93, 1195–1206. 10.1016/j.joei.2019.11.003. [DOI] [Google Scholar]
- Joyce J.; Dixon T.; Diniz Da Costa J. C. Characterization of sugar cane waste biomass derived chars from pressurized gasification. Process Saf. Environ. Prot. 2006, 84, 429–439. 10.1205/psep05021. [DOI] [Google Scholar]
- Li N.; Wang Y.; Cui S.; Sun D. Investigations on NO reduction with biomass char: Char structural changes during the heat treatment in N2 and subsequent NO/O2 gasification. Fuel 2021, 287, 119564 10.1016/j.fuel.2020.119564. [DOI] [Google Scholar]
- Geng W.; Kumabe Y.; Nakajima T.; Takanashi H.; Ohki A. Analysis of hydrothermally-treated and weathered coals by X-ray photoelectron spectroscopy (XPS). Fuel 2009, 88, 644–649. 10.1016/j.fuel.2008.09.025. [DOI] [Google Scholar]
- Donadelli J. A.; Cánnevaa A.; Erra G.; Calvo A. XPS direct analysis on shale rocks: Correlation with kerogen type and maturity. Fuel 2019, 257, 116004 10.1016/j.fuel.2019.116004. [DOI] [Google Scholar]
- Pei L.; Li D.; Liu X.; Cui W.; Shao R.; Xue F.; Li W. Investigation on asphaltenes structures during low temperature coal tar hydrotreatment under various reaction temperatures. Energy Fuels 2017, 31, 4705–4713. 10.1021/acs.energyfuels.6b03180. [DOI] [Google Scholar]
- Baysal M.; Yürüm A.; Yıldız B.; Yürüm Y. Structure of some western Anatolia coals investigated by FTIR, Raman, 13C solid state NMR spectroscopy and X-ray diffraction. Int. J. Coal Geol. 2016, 163, 166–176. 10.1016/j.coal.2016.07.009. [DOI] [Google Scholar]
- Li W.; Shen Y.; Guo J.; Kong J.; Wang M.; Chang L. Effects of Additives on Coke Reactivity and Sulfur Transformation during Co-pyrolysis of Long Flame Coal and High-Sulfur Coking Coal. ACS Omega 2021, 6, 34967–34976. 10.1021/acsomega.1c05642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonibare O. O.; Haeger T.; Foley S. F. Structural characterization of Nigerian coals by X-ray diffraction, Raman and FTIR spectroscopy. Energy 2010, 35, 5347–5353. 10.1016/j.energy.2010.07.025. [DOI] [Google Scholar]
- Tong W.; Cai Z.; Liu Q.; Ren S.; Kong M. Evaluation of biochar combustion reactivity under pyrolysis temperature: microstructure characterization, kinetics and thermodynamics. J. Energy Inst. 2020, 93, 1914–1923. 10.1016/j.joei.2020.04.006. [DOI] [Google Scholar]