Abstract
Background and Objectives
A prominent theory proposes that neuroplastic recruitment of perilesional tissue supports aphasia recovery, especially when language-capable cortex is spared by smaller lesions. This theory has rarely been tested directly and findings have been inconclusive. We tested the perilesional plasticity hypothesis using 2 fMRI tasks in 2 groups of patients with previous aphasia diagnosis.
Methods
Two cohorts totaling 82 patients with chronic left-hemisphere stroke with previous aphasia diagnosis and 82 control participants underwent fMRI using either a naming task or a reliable semantic decision task. Individualized perilesional tissue was defined by dilating anatomical lesions and language regions were defined using meta-analyses. Mixed modeling examined differences in activity between groups. Relationships with lesion size and aphasia severity were examined.
Results
Patients exhibited reduced activity in perilesional language tissue relative to controls in both tasks. Although a few cortical regions exhibited greater activity irrespective of distance from the lesion, or only when distant from the lesion, no regions exhibited increased activity only when near the lesion. Larger lesions were associated with reduced language activity irrespective of distance from the lesion. Using the reliable fMRI task, reduced language activity was related to aphasia severity independent of lesion size.
Discussion
We found no evidence for neuroplastic recruitment of perilesional tissue in aphasia beyond its typical role in language. Rather, our findings are consistent with alternative hypotheses that changes in left-hemisphere activation during recovery relate to normalization of language network dysfunction and possibly recruitment of alternate cortical processors. These findings clarify left-hemisphere neuroplastic mechanisms supporting language recovery after stroke.
Stroke is a leading cause of permanent disability and sequelae are partially determined by lesion size and location. However, an important driver of recovery is thought to be neural reorganization in residual tissue beyond the lesion boundaries.1 A mechanistic account of this plasticity is necessary to make progress in aphasia neurorehabilitation and several mechanisms have been proposed.2,3
Among the proposed mechanisms is the perilesional plasticity hypothesis, which emphasizes tissue immediately surrounding the lesion, where animal studies have revealed dysfunction4,5 and suggested that collateral axonal sprouting and synaptogenesis may support functional recovery.6,7 Motor stroke recovery, in particular, appears to rely on functional takeover by perilesional sensorimotor8 or primary motor cortices.9,10
These findings have informed models of aphasia recovery, which stipulate that when language tissue is damaged, alternative perilesional processors may become recruited to support outcomes, especially around small lesions.11,12 A recent review has framed this notion as a form of “variable neuro-displacement,” in which spare functional capacity within healthy networks becomes utilized following stroke-induced damage. Under this view, the upregulated perilesional activation reflects spare capacity that is typically downregulated under healthy conditions to save energy.2
In line with this idea, several studies have found that increased perilesional activity is associated with improved long-term outcomes in spontaneous stroke aphasia recovery.13-15 However, these studies have not rigorously considered lesion characteristics,2 so heterogeneity in effects may relate to different volume and location of available perilesional tissue.
Treatment studies also provide hints of perilesional recruitment, finding increased activity after treatment that relates to gains in performance.16,17 However, because these studies have not compared the activation directly with controls, they cannot clearly establish whether treatment-related increases in perilesional activity represent neuroplastic recruitment of new tissue for language or supranormal recruitment of typical language regions due to plasticity.
Alternatively, treatment-related increases in perilesional activity may reflect normalization of function in language tissue that is dysfunctional due to network effects of the nearby lesion. Studies of spontaneous stroke aphasia recovery have found an acute reduction in left-hemisphere language activity, followed by subacute supranormal activity and a chronic normalization of activity associated with good outcomes.18 Increased perilesional activity was associated with better performance, but the activity did not exceed that of controls.19 These findings suggest that lesions cause language network dysfunction and that recovery is supported by normalization of activity rather than by recruitment of new perilesional tissue into the language network.
We tested predictions of the perilesional plasticity hypothesis, contrasting activity elicited by 2 independent language tasks in a large cohort of patients with left-hemisphere stroke and matched controls. We predicted that neuroplastic recruitment would result in supranormal perilesional task-related activity. We tested 2 related predictions: that recruitment (1) might only occur within, or proximal to, language tissue and (2) might only be evident around small lesions. We also examine whether the effect only occurs in specific brain regions. Finally, we considered alternative hypotheses explaining left-hemisphere activity observed in prior aphasia studies, namely that this activity (1) is residual activity in the language network not resulting from recruitment of new tissue or (2) represents recruitment of alternate processors irrespective of proximity to the lesion.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided informed consent in accordance with the Georgetown University Institutional Review Board.
Study Participants
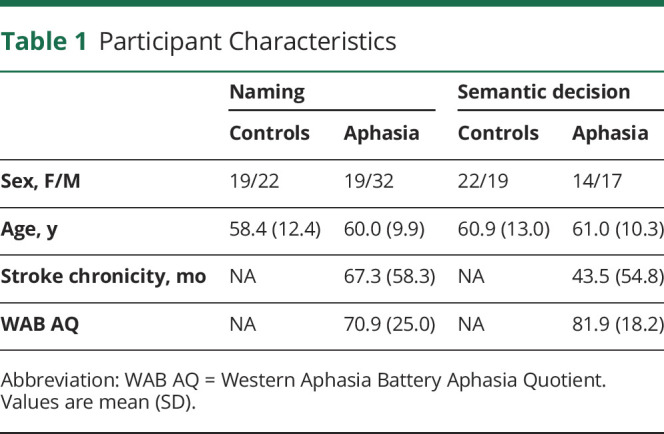
Study participants included 82 patients with left-hemisphere stroke with a prior diagnosis of aphasia and 80 controls (Table 1). Study participants were recruited in the Washington, DC area for a clinical transcranial direct current stimulation study (naming task data; Doris Duke Charitable Foundation Grant 2012062, 2013–2018) and an ongoing cross-sectional study of aphasia outcomes (semantic decision task; NIDCD R01DC014960, 2018–2020). Between the studies, the MRI scanner was upgraded (details following). Aside from stroke, patients had no other history of significant psychiatric or neurologic condition. All patients were in the chronic phase (>6 months) of recovery. With the exception of 3 small asymptomatic right-hemisphere lesions, all lesions were restricted to the left hemisphere. Additional lesion characteristics are summarized in eTables 1 and 2, links.lww.com/WNL/B980.
Table 1.
Participant Characteristics
Behavioral Testing
All patients underwent a battery of behavioral testing including administration of the Western Aphasia Battery–Revised.20 All patients were also evaluated for the presence of apraxia of speech, either by the Apraxia of Speech Rating Scale, 3rd edition21 (semantic decision cohort) or the Apraxia Battery for Adults, 2nd edition22 (naming cohort). Group information including aphasia type diagnosis is tabulated in eTable 3, links.lww.com/WNL/B980, and apraxia of speech presence and severity is tabulated in eTable 4, links.lww.com/WNL/B980.
Image Acquisition
Sessions for the naming task were conducted on a Siemens 3T Trio. Sequences included a high-resolution T1-weighted scan (repetition time [TR] 1900, echo time [TE] 2.52, 176 0.9-mm sagittal slices, field of view [FOV] 240, matrix 256 × 256, fractional anisotropy [FA] 9°), a T2-weighted scan (TR 3,200, TE 45, 176 1.25-mm sagittal slices, FOV 240, matrix 192 × 192, FA 120°), and the blood oxygenation level–dependent (BOLD) T2*-weighted scan (TR 2,500, TE 30, 47 3.2-mm axial slices, FOV 204, matrix 64 × 64, FA 90°) consisting of 168 volumes and lasting 6:00.
Sessions for the semantic decision task were conducted on a Siemens 3T Trio. Sequences included a high-resolution T1-weighted scan (TR 1900, TE 2.98, 176 1-mm sagittal slices, FOV 256, matrix 256 × 256, FA 9°, SMS 4), a T2-weighted fluid-attenuated inversion recovery (FLAIR) scan (TR 5,000, TE 38.2, 192 1-mm sagittal slices, FOV 256, matrix 256 × 256, FA 120°), and a BOLD T2*-weighted scan (TR 794 ms, 48 2.6-mm slices with 10% gap, 2.9 mm voxels, FOV 211 mm, matrix 74 × 74, FA 50°, SMS 4) consisting of 504 volumes lasting 6:40.
Lesion Segmentation and Coregistration
Lesion masks were manually segmented on each patient's magnetization-prepared rapid gradient echo (MPRAGE) and T2/FLAIR images using ITK-SNAP software23 by P.E.T. (Figure 1).
Figure 1. Serial Sagittal Slices Through the Left Hemisphere of Both Cohorts Showing the Overlap of Anatomical Lesion Tracings.
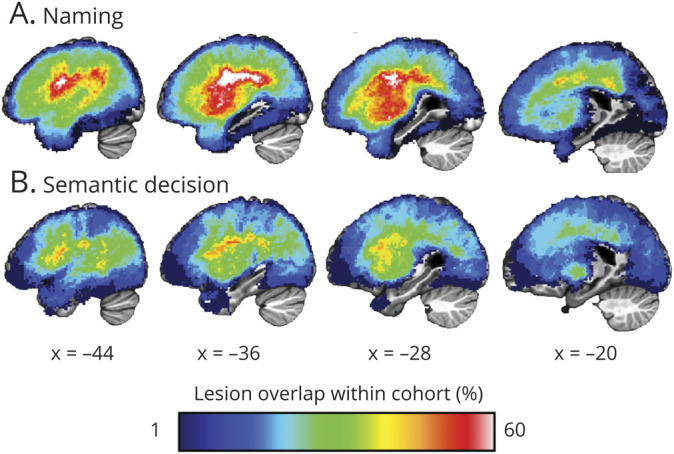
Lesion overlap for the cohort who underwent the naming task (A) and the semantic decision task (B). Percent of lesion overlap within each cohort is indicated by the spectrum color.
Perilesional Tissue Definition
We utilized a dilation model of perilesional tissue16 in which perilesional tissue is defined as a shell falling outside of each individual's anatomical lesion tracing, implemented in MATLAB 2020b using the imdilate function (MathWorks). For brainwide questions, we evaluated 4-mm-thick shells spanning 0–4 mm, 4–8 mm, 8–12 mm, and 12–16 mm from the lesion boundary. We examined a range of distances from the lesion boundary based on prior work demonstrating reduced perfusion, which may affect task-related BOLD fMRI signal, up to 8 mm from the lesion boundary.24 Our further analyses considered perilesional tissue as a single slab between 4 and 16 mm from the lesion boundary. For these slab-based analyses, we discounted voxels immediately neighboring the lesion due to possible partial volume effects.19 All analyses were restricted to left-hemisphere tissue falling within a standard SPM 12 gray matter tissue probability mask thresholded at >10% likelihood.
Functional Language Mapping Procedures
Each study participant underwent functional language mapping using 1 of 2 fMRI tasks. The first task was a common spoken picture-naming task, described in detail previously.25 The second task was an adaptive semantic decision task validated in patients with aphasia, described previously in detail.26 We chose this task because it has been shown to produce activation maps with good test–retest reproducibility and good validity in that it is known to activate language regions (c.f. other tasks27). Briefly, study participants viewed word pairs and indicated via button press if they are related in meaning (e.g., shark–whale). During a control condition, study participants indicated via button press if pseudofont pairs (e.g., ƋΔƱƩΔ–ƋƟƱƧΔ) were identical. This task is adaptive so that stimuli and presentation rate become more demanding with more correct responses (see Wilson et al. for details). All study participants performed greater than chance in the semantic decision task language condition (one-sided binomial test, p < 0.05).
Image Preprocessing and Statistical Analysis
For both tasks, standard preprocessing was performed in AFNI,28 including slice timing correction, realignment for head motion, despiking, smoothing with a 5-mm full width at half maximum kernel, temporal high-pass filtering at 0.01 Hz, and detrending. A whole-brain general linear model was estimated using the fmrilm function from FMRISTAT,29 with covariates including the time course of a white matter and CSF seed, and the 6 head-motion parameters not convolved with the hemodynamic response function (HRF). Each of the 32 naming task trials was modeled using 3 event types, convolved with the HRF (covert speech period [7.5–9 seconds], overt speech period [5.5 seconds], and fixation [15 seconds]). The contrast of interest was an average of covert and overt greater than fixation (0.5 0.5 –1). The semantic decision task was modeled using 2 alternating boxcar functions (corresponding to the language and control conditions), convolved with the HRF. The contrast of interest was semantic greater than control (1 –1). Resulting SPMs were then warped to MNI space based on the transformation estimated from the MPRAGE. Finally, images were resliced to 2-mm isotropic voxels.
Independent Task-Specific Functional Definition of Brain Tissue
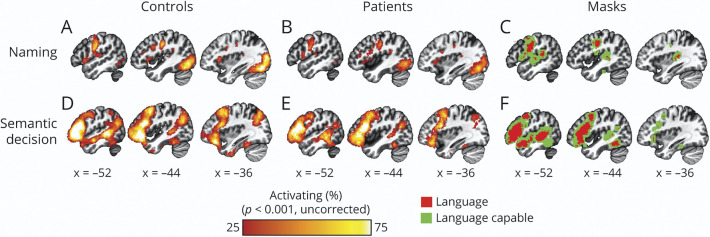
To avoid circularity in selecting regions for analyses,30 we independently defined language cortex based on meta-analytic results from task-relevant Neurosynth queries.31 For the naming task, we operationalized language cortex (Figure 2, C, F, red) using results of the search term “speech production” (association test Z > 6.0, false discovery rate [FDR] 0.01). We applied the same procedure for the semantic decision task using the search term “language.”
Figure 2. Group Task-Activation Maps for Controls and Patients for Naming and Semantic Decision Tasks.
For both tasks, maps were similar for controls (A, D) and patients (B, E) and exhibited a high degree of consistency with expected areas based on the respective meta-analytic mask. Percent of each cohort activating is shown as a conjunction of the individuals in each cohort thresholded voxelwise p < 0.001, uncorrected. C and F show the extent of independently defined meta-analytic masks defining task-specific language cortex (red) and language-capable cortex (green).
We operationalized “language-capable” cortex (Figure 2, C and F, green) as an 8-mm shell dilated around each task-specific language mask. Finally, we operationalized nonlanguage cortex as voxels falling outside of both language and language-capable masks. Regional analyses were conducted in parcels of a 134 segment atlas.32
Is Perilesional Activity Different in Patients With Aphasia Than in Controls?
For each patient, nonlesioned tissue was first characterized brainwide for each functional tissue category (language, language-capable, nonlanguage) at each of 5 distances from the lesion boundary (0–4 mm, 4–8 mm, 8–12 mm, 12–16 mm, >16 mm). For each patient's masks, we calculated average activation for both that patient and for each control, applying the patient's mask to each control in order to compare equivalent tissue. In this way, we generated a set of individualized control values, specific to each patient, which excludes any contribution from tissue lesioned in that patient.
Then linear mixed effects modeling was used to compare activity in patients to the controls' activity while accounting for lesion differences across patients. The model was repeated for each functional tissue category at each shell distance. The model was specified with a fixed effect of group (patient vs control) and random effects of study participant and the lesion mask applied to the data (to account for random effects associated with the lesion masks applied to both groups).
For the regional analysis, we calculated a voxelwise intersection of each mask with the atlas to obtain the relevant voxels falling within each region. We then consider effects separately for when a region is near a lesion boundary (4–16 mm) and far from the lesion (>16 mm). Regions were only examined if at least 5 patients had perilesional tissue within its mask. The regional analyses were corrected for multiple comparisons based on FDR at p < 0.05.33
Does Perilesional Recruitment Depend on Lesion Size?
To measure the relationship between perilesional activity and lesion size, we correlated activity and lesion size within each of the 3 functional tissue types, both in the vicinity of the lesion (4–16 mm from the lesion boundary) and distant from the lesion (>16 mm from the lesion boundary). For language tissue, we used the linear mixed effects model to test whether patients with small lesions (<50 mL) or large lesions (>100 mL) exhibited abnormal activity, relative to controls, in the vicinity of the lesion (4–16 mm) or distant from the lesion (>16 mm).
Does Activation Predict Behavioral Impairment, Independent of Lesion Size?
We used a semi-partial Spearman correlation (2-tailed) to test whether activity in language regions related to degree of behavioral impairment, independent of lesion size. We focused on a general measure of aphasia severity, the Aphasia Quotient (AQ) from the Western Aphasia Battery (WAB).20 For the naming task, we also examined the relationship between activity and the Naming & Word Finding Subtest from the WAB.
Data Availability
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.
Results
Task Activation and Convergence With Tissue Masks
Within the left hemisphere, the naming task reliably activated ventral premotor and motor cortex, as well as superior temporal cortex, which is highly consistent with the meta-analysis results (Figure 2, A–C). The task also reliably activated inferior occipital cortex, likely relating to the visual presentation of the picture stimuli.
We also observed a high degree of consistency between the activation for the semantic decision task and the meta-analytic mask (Figure 2, D–F). Specifically, both patients and controls most reliably activated left inferior frontal cortex, premotor cortex, both anterior superior temporal gyrus and posterior superior temporal lobe, and fusiform gyrus. These results are also highly consistent with previously reported activation using this task.26
Perilesional Tissue Exhibits Reduced Activity
We first tested whether patients exhibit perilesional recruitment at various distances from the lesion boundary and within different functional tissue types. In the naming task (Figure 3, A–C, and eTable 5, links.lww.com/WNL/B980), language cortex, language-capable cortex, and nonlanguage cortex all exhibited a significant reduction in task-related activation relative to controls, immediately adjacent (0–4 mm) to the lesion boundary. The reduction was evident in language and language-capable cortex out to 12 mm from the lesion boundary (p < 0.01). In the semantic decision task (Figure 3, D–F, and eTable 5, links.lww.com/WNL/B980), both language and language-capable cortex exhibited reduced activation out to 8 mm from the lesion boundary (p < 0.01). Activation in nonlanguage cortex was no different from controls.
Figure 3. Results From Models of Effect of Group (Patient vs Control) on Brain-wide Activation by Tissue Type and Distance From Lesion (X Axes) for the Naming Task and for the Semantic Decision Task.
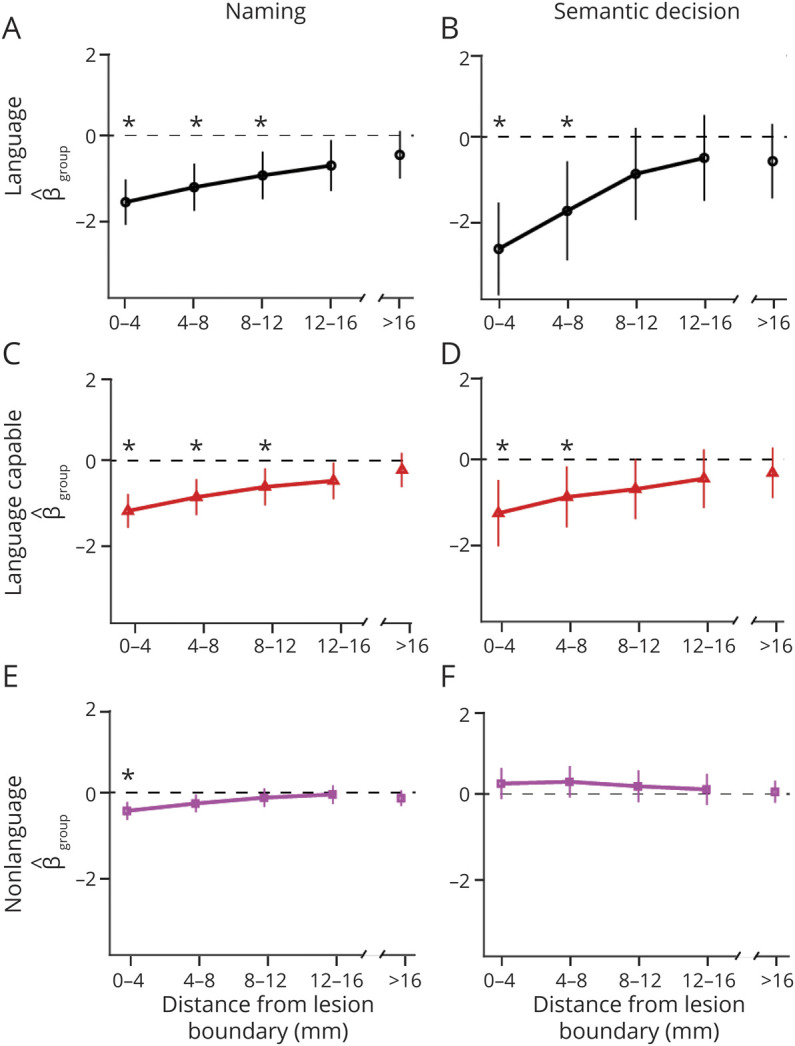
The y axis shows estimate of the effect of group status (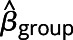 ) and 95% CI. An asterisk indicates a significant difference between patients and controls (p < 0.01). The discontinuity in the x axes indicates that the rightmost data points included all voxels beyond the perilesional shell. Results are shown for language cortex (A, B), language-capable cortex (C, D), and nonlanguage cortex (E, F). For the naming task, patient activation was reduced in language and language-capable tissue up to 12 mm from the lesion boundary and up to 4 mm for nonlanguage tissue. For the semantic decision task, activation was reduced in language and language-capable tissue up to 8 mm from the lesion boundary.
) and 95% CI. An asterisk indicates a significant difference between patients and controls (p < 0.01). The discontinuity in the x axes indicates that the rightmost data points included all voxels beyond the perilesional shell. Results are shown for language cortex (A, B), language-capable cortex (C, D), and nonlanguage cortex (E, F). For the naming task, patient activation was reduced in language and language-capable tissue up to 12 mm from the lesion boundary and up to 4 mm for nonlanguage tissue. For the semantic decision task, activation was reduced in language and language-capable tissue up to 8 mm from the lesion boundary.
No Brain Regions Exhibit Selectively Increased Activity in Perilesional Cortex
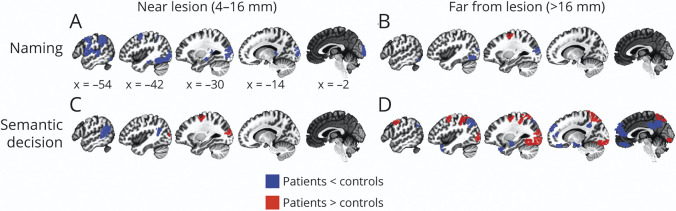
Although we found no evidence for perilesional plasticity above and beyond typical activation levels in controls when examining hemisphere-wide tissue types, it remains possible that recruitment of perilesional tissue occurs only in certain cortical areas. To assess this, we next compared perilesional activity for patients vs controls in individual brain regions defined based on a parcellation atlas.32 In the naming task, there were no brain regions in which perilesional tissue exhibited increased activity, but there were several regions with reduced activity in perilesional tissue, including the posterior frontal lobe and operculum, lateral and medial temporal lobe regions, supramarginal gyrus, and occipital cortex (Figure 4A and eTable 6, links.lww.com/WNL/B980). In tissue farther from the lesion, increased activity was observed relative to controls in posterior superior frontal sulcus, and reduced activity was observed in lateral occipital lobe and fusiform gyrus (Figure 4B and eTable 6, links.lww.com/WNL/B980).
Figure 4. Regional Differences in Patient vs Control Activity on 2 Language Mapping Tasks, Including Naming and Semantic Decision.
Results are shown separately for perilesional tissue (4–16 mm of lesion boundary; top) and for tissue distant from the lesion (>16 mm from lesion boundary; bottom). In the naming task, (A) no regions near the lesion exhibited increased activation, but decreased activation was evident in frontal, parietal, and occipital lobes. (B) Far from the lesion, the decreased occipital lobe activation persisted, and 1 frontal lobe parcel exhibited increased activation. In the semantic decision task, (C) perilesional superior frontal sulcus and lateral occipital cortex exhibited increased activation, but (D) this increased activation was also evident in tissue distant from the lesion along with increased activity in other regions. Blue parcels, controls > people with aphasia; red parcels, people with aphasia > controls; p < 0.05, Benjamini–Hochberg false discovery rate.
In the semantic decision task, patients exhibited greater activation than controls in perilesional tissue within lateral occipital cortex and posterior superior frontal sulcus, and decreased perilesional activation was observed in posterior superior temporal gyrus and supramarginal gyrus (Figure 4C and eTable 7, links.lww.com/WNL/B980). In tissue farther from the lesion, increased activity was also observed in lateral occipital cortex and posterior superior frontal sulcus (Figure 4D and eTable 7, links.lww.com/WNL/B980), demonstrating that stroke-related increases in activity in these regions occurred irrespective of proximity to the lesion. Increased activity was also observed in nonperilesional tissue within the superior parietal lobule, intraparietal sulcus, and much of the inferior occipital lobe. Decreased activation was observed in tissue distant from the lesion within midline structures such as ventromedial prefrontal and retrosplenial cortices, areas of lateral and medial temporal lobe, and angular gyrus.
Perilesional Recruitment Is Not Observed in Patients With Small Lesions
Models of aphasia recovery suggest that perilesional recruitment may be particularly evident around smaller lesions. We hypothesized that if smaller lesions were predisposed to perilesional recruitment, then lesion size would correlate with activation in patients with aphasia, and individuals with the smallest lesions (<50 mL) would exhibit perilesional activation that exceeds the control cohort in the same location.
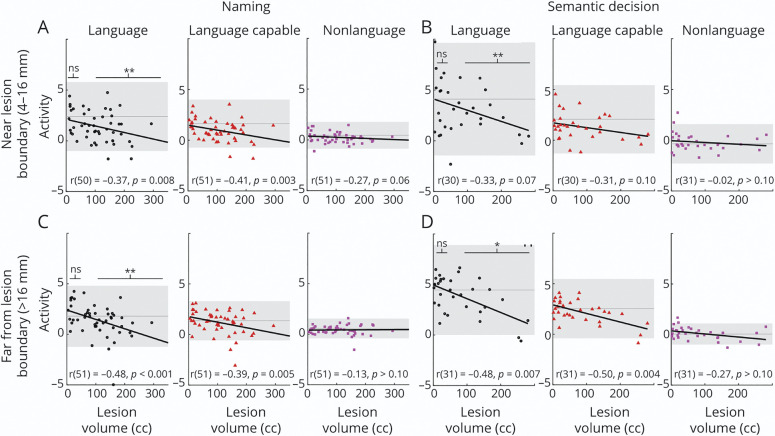
In the naming task, lesion volume and activity were significantly inversely related for language cortex both in perilesional tissue (Figure 5A) and in tissue far from the lesion (Figure 5C). The same pattern was observed in language-capable cortex both in perilesional tissue (Figure 5A) and in tissue far from the lesion (Figure 5C). There was no significant relationship evident in nonlanguage cortex, whether in perilesional tissue (Figure 5A) or in tissue far from the lesion (Figure 5C).
Figure 5. Scatterplots of, Lines of Best Fit for, and Correlation Coefficients Between Activation and Lesion Volume in Perilesional Cortex and Cortex far From the Lesion for the Naming Task and Semantic Decision Task.
(A, B) Perilesional cortex (4–12 mm from lesion boundary); (C, D) cortex far from the lesion (>16 mm from lesion boundary); (A, C) naming task; (B, D) semantic decision task. Scatterplots are shown for language cortex (black circles), language-capable cortex (red triangles), and nonlanguage cortex (magenta squares). The y axis is the average t statistic for the task contrast within the relevant mask, with each marker representing a single participant with aphasia. The x axis represents lesion volume in cubic centimeters (cc). Mean activation for controls is shown as a dark gray line, with 95% CI as a gray band. Results of linear mixed effects models of the effect of group (aphasia vs control) on activity in language tissue are also shown for small lesions (<50 cc) and large lesions (>100 cc). *p < 0.05, **p < 0.001. In the naming task, lesion volume was significantly inversely related to activity for both language and language-capable cortex regardless of distance from lesion. In the semantic decision task, lesion volume was inversely related to activity in language and language-capable cortex, but only far from the lesion. For both tasks, in language cortex, patients with small lesions exhibited activity no different from controls, whereas patients with large lesions exhibited reduced activity relative to controls, regardless of distance from the lesion.
In the semantic decision task, lesion volume was not related to activity in language cortex near the lesion, but was inversely related to activity far from the lesion (Figure 5D). The same pattern was observed for language-capable cortex (Figure 5, B and D). There was no significant relationship evident in nonlanguage cortex, whether in perilesional tissue (Figure 5B) or far from the lesion (Figure 5D).
Although we did not observe individuals with increased language activity compared to the control range (Figure 5, gray band), there were also not many individuals with decreased activity compared to the control range. Although there were not dramatic increases or decreases in activity in individuals, there still might be group effects on average in patients with small lesions or patients with large lesions compared to controls. To test this, we broke out a group with small lesions (<50 mL) and large lesions (>100 mL) to perform a between-group comparison with controls (see eTable 8 for group counts, links.lww.com/WNL/B980). In both tasks, individuals with small lesions (<50 mL) exhibited activity no different from controls in language cortex near or far from the lesion (eTable 8, links.lww.com/WNL/B980). In contrast, in both tasks, individuals with larger lesions (>100 mL) exhibited significantly decreased activity in language cortex irrespective of distance from the lesion.
Disrupted Language Activity Accounts for Behavioral Impairment
Although we did not find a relationship between lesion size and perilesional recruitment, we did find that large lesions cause widely disrupted language activity both near and far from the lesion. To address whether these reductions in language activity have behavioral relevance, we next asked whether language activity relates to aphasia severity. In the semantic decision task, there was a significant relationship between activity in task-specific language cortex and overall aphasia severity (WAB AQ), independent of lesion volume, regardless of whether the tissue was perilesional (4–16 mm from the lesion; r[30] = 0.45, p = 0.01) or far from the lesion (>16 mm from the lesion; r[31] = 0 .44, p = 0.01). In the naming task, no significant relationship was observed for WAB AQ (perilesional: r[49] = 0 .24, p = 0.10; far from lesion: r [50] = 0 .18, p > 0.10) or for naming (WAB Naming & Word Finding subscore; perilesional: r[50] = 0.20, p > 0.10; far from lesion: r[51] = 0 .20, p > 0.10).
Discussion
The main goal of this study was to test predictions of the perilesional plasticity hypothesis in poststroke aphasia. We predicted that recruitment of perilesional tissue through neuroplasticity would result in supranormal task-related activity around lesions. However, we found a brainwide pattern consistent with reduced perilesional activity relative to controls. Moreover, we observed no specific brain regions in which recruitment was evident only when the tissue was perilesional. When we examined whether perilesional recruitment was evident around small lesions, we found that, although larger lesions were associated with less activity, smaller lesions exhibited perilesional activity no different than controls. Overall, our results are inconsistent with the theory that perilesional plasticity results in recruitment of new brain regions into the language network, or that it results in engagement of typical language regions beyond their normal role in neurotypical individuals.
Our results support an alternative interpretation of perilesional recruitment: that strokes to the language network produce network-wide disruptions with decreased language activity and that perilesional activation is actually just normal activation of unlesioned language processors. We found that the degree of network disruption depended on lesion size, such that large lesions caused widespread disruption, but small lesions resulted in activity no different from controls. Moreover, we found that less disruption of signal in residual language tissue, when measured with a task that produces reliable single-subject maps,26 relates to better behavioral performance even after accounting for the amount of anatomical damage caused by the lesion.
These findings are consistent with previous aphasia treatment studies that found that increased activation in the left hemisphere was associated with improved naming after anomia treatment, with greater increase in activation associated with more improvement,16,17,34 and cross-sectional findings that greater activity in preserved left hemisphere, relative to controls, was associated with better picture naming performance.35 These cross-sectional chronic results also complement studies of spontaneous aphasia recovery that found a recovery trajectory in which good outcomes in the chronic phase were correlated with task-related activity returning to normal levels.18,19 More broadly, these findings are consistent with a recent review of aphasia recovery, which found that lesions caused overall reduced activation in patients with aphasia, with activity in left-hemisphere language regions relating to better language function.36
In addition to normalization of language processing, previous reports of perilesional plasticity may also reflect increased engagement of alternative left-hemisphere processors irrespective of their proximity to the lesion. This is supported by the regional analysis finding that certain processors were engaged above control levels, but that in every case, these were either regions distant from the lesion or regions that were recruited irrespective of their proximity to the lesion.
Several types of processes might underlie the recruitment we measured as increases in alternative left-hemisphere processors: for instance, the increased activation might relate to compensatory plasticity, the use of compensatory strategies relying on spared ability,37 or network-specific changes such as increased reliance on “domain general” processes.38 Our finding of increased activity in posterior superior frontal lobe and parietal lobe shows consistent localization with a domain general dorsal attention/salience network.39 Previous work has found increased left hemisphere activity in patients with aphasia during language processing, but a common region exhibiting increased activation would be unlikely to be perilesional because perilesional tissue would be in different places for different individuals.38,40 Thus, greater activation observed in these regions might relate to compensatory increased reliance on domain-general processing for language tasks. Our finding of increased activity in lateral occipital cortex, irrespective of distance from the lesion, might be explained by recruitment of additional visual processing of written stimuli in the semantic decision task.
One limitation of prior fMRI studies of aphasia is that they have typically examined only one task in a single group of patients. Much of the heterogeneity of results in the literature likely results from idiosyncrasies of individual patient samples or the tasks used to elicit language activity. Here, we compared results from the same analysis approach using 2 different tasks in 2 different patient samples. The results addressing the question of perilesional plasticity are remarkably consistent across the 2 tasks, providing very strong support for the conclusions above. However, there were some different findings between tasks. Not surprisingly, different regions were engaged by the 2 tasks, and therefore the localization of effects in the regionwise analysis was different. In addition, the behavioral relationships are stronger for the task with greater test–retest reliability (although they numerically trend in the same direction in the naming task, they do not approach significance). The stronger relationship with behavior supports the use of reliable tasks for questions related to neuroplasticity.41
In addition to addressing theoretical questions related to the neural mechanisms of aphasia recovery, our findings may also have clinical implications. The findings highlight the importance of task selection for functional mapping of eloquent cortex. Although we find the same pattern of results in both fMRI tasks, we find more reliable effects using the adaptive task with documented validity and reliability.26 This increased reliability should be considered when choosing a task for mapping eloquent cortex.
Our findings may also have implications for selecting neurostimulation treatment targets for aphasia. One approach to neurostimulation in aphasia has targeted perilesional tissue, with the goal of enhancing perilesional plasticity.42 Our findings suggest that neurostimulation might better target residual language tissue irrespective of its proximity to the lesion, with the goal of eliciting network restoration rather than perilesional plasticity.
Finally, although indirect, our findings may also have implications for patient selection for mechanical thrombectomy in acute ischemic stroke. Mechanical thrombectomy is established as an effective treatment for occlusion of proximal vessels and evidence for intervention on distal/medium branches is emerging. Our results suggest that because perilesional tissue cannot be recruited to “take over” for damaged language tissue, then thrombectomy might be considered when eloquent cortex is at risk due to occlusion of not only large vessels, but also distal/medium middle cerebral artery branches. Prospective clinical trials would be needed to determine whether mechanical thrombectomy improves aphasia outcomes in these cases.43
Our analyses were specifically designed to test the perilesional plasticity hypothesis, so we did not examine right hemisphere tissue and cannot make claims about potential right hemisphere compensatory mechanisms or other proposed mechanisms of aphasia recovery.44 There are also some important limitations of our approach to testing the perilesional plasticity hypothesis. We focused on BOLD signal elicited from cortical tissue, and did not control for the influence of damage to subcortical white matter pathways, which is known to contribute to aphasic deficits. We also did not characterize potential perilesional hypoperfusion. However, we observed effects outside the 8 mm range of hypoperfusion measured by Richardson et al.24 We also observed reductions in activity distant from the lesions and increased activity in perilesional tissue in one region in the semantic decision task, with similar levels of increased activity when the tissue was perilesional and when it was not. This strongly suggests that perilesional hypoperfusion was not a major factor in the observed effects. In addition, our 82 patients were in the chronic stage of recovery, and we did not examine the transition from acute to chronic, or directly assess effects of treatment. Perhaps perilesional plasticity is transiently observable during recovery, or only in a behaviorally enriched treatment context. Future treatment studies should conduct analyses similar to those presented here to test if increases in perilesional activity extend beyond the typical level of activation in controls. Finally, our results do not conclusively prove that perilesional plasticity is not at play in aphasia recovery. Rather, our results show that perilesional plasticity does not result in supranormal signal magnitude using task-related BOLD fMRI. However, recruitment of perilesional tissue may be evident in other types of brain measures, or may be evident at small scales beyond the spatial resolution typically employed in fMRI.
We found no evidence for neuroplastic recruitment of perilesional tissue measured by BOLD fMRI in 2 groups of patients with chronic aphasia using different tasks. We did find evidence for lesion size–dependent language network dysfunction, suggesting that normalization of task-related activity may explain some of the findings in previous studies. These results place constraints on mechanistic accounts of chronic poststroke aphasia neuroplasticity measured with BOLD fMRI.
Acknowledgment
The authors thank Mackenzie Fama, Zainab Anbari, and Kate Spiegel for data collection and the research participants for their participation.
Glossary
- AQ
Aphasia Quotient
- BOLD
blood oxygenation level–dependent
- FA
fractional anisotropy
- FDR
false discovery rate
- FLAIR
fluid-attenuated inversion recovery
- FOV
field of view
- HRF
hemodynamic response function
- MPRAGE
magnetization-prepared rapid gradient echo
- TE
echo time
- TI
inversion time
- TR
repetition time
- WAB
Western Aphasia Battery
Appendix. Authors
Study Funding
The research was supported by the Doris Duke Charitable Foundation (grant 21012062) to P.E.T.; NIH/NCATS via GHUCCTS (KL2TR000102 and TL1TR001431) and the NIDCD (R01DC014960) to P.E.T.; and U10NS086513, K12HD093427, and K99DC018828 to A.T.D.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Grefkes C, Fink GR. Recovery from stroke: current concepts and future perspectives. Neurol Res Pract. 2020;2:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stefaniak JD, Halai AD, Lambon Ralph MA. The neural and neurocomputational bases of recovery from post-stroke aphasia. Nat Rev Neurol. 2020;16(1):43-55. [DOI] [PubMed] [Google Scholar]
- 3.Turkeltaub P. A taxonomy of brain–behavior relationships after stroke. J Speech Lang Hear Res. 2019;62(11):3907-3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Neumann-Haefelin T, Witte OW. Periinfarct and remote excitability changes after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20(1):45-52. [DOI] [PubMed] [Google Scholar]
- 5.van der Zijden JP, van der Toorn A, van der Marel K, Dijkhuizen RM. Longitudinal in vivo MRI of alterations in perilesional tissue after transient ischemic stroke in rats. Exp Neurol. 2008;212(1):207-212. [DOI] [PubMed] [Google Scholar]
- 6.Nudo RJ. Recovery after damage to motor cortical areas. Curr Opin Neurobiol. 1999;9(6):740-747. [DOI] [PubMed] [Google Scholar]
- 7.Stroemer P, Thomas K, Hulsebosch C. Neocortical neural sprouting, synaptogenesis, and behavioral recovery after neocortical infarction in rats. Stroke. 1995;26(11):2135-2144. [DOI] [PubMed] [Google Scholar]
- 8.Teasell R, Bayona NA, Bitensky J. Plasticity and reorganization of the brain post stroke. Top Stroke Rehabil. 2005;12(3):11-26. [DOI] [PubMed] [Google Scholar]
- 9.Jaillard A, Martin CD, Garambois K, Lebas JF, Hommel M. Vicarious function within the human primary motor cortex? Brain. 2005;128(5):1122-1138. [DOI] [PubMed] [Google Scholar]
- 10.Xerri C, Merzenich MM, Peterson BE, Jenkins W. Plasticity of primary somatosensory cortex paralleling sensorimotor skill recovery from stroke in adult monkeys. J Neurophysiol. 1998;79(4):2119-2148. [DOI] [PubMed] [Google Scholar]
- 11.Heiss WD, Thiel A. A proposed regional hierarchy in recovery of post-stroke aphasia. Brain Lang. 2006;98(1):118-123. [DOI] [PubMed] [Google Scholar]
- 12.Thompson CK, den Ouden DB. Neuroimaging and recovery of language in aphasia. Curr Neurol Neurosci Rep. 2008;8(6):475-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heiss WD, Kessler J, Thiel A, Ghaemi M, Karbe H. Differential capacity of left and right hemispheric areas for compensation of poststroke aphasia. Ann Neurol. 1999;45(4):430-438. [DOI] [PubMed] [Google Scholar]
- 14.Szaflarski JP, Eaton K, Ball AL, et al. Post-stroke aphasia recovery assessed with fMRI and a picture identification task. J Stroke Cerebrovasc Dis. 2011;20(4):336-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Warburton E, Price CJ, Swinburn K, Wise RJS. Mechanisms of recovery from aphasia: evidence from positron emission tomography studies. J Neurol Neurosurg Psychiatry. 1999;66(2):155-161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fridriksson J, Richardson JD, Fillmore P, Cai B. Left hemisphere plasticity and aphasia recovery. NeuroImage. 2012;60(2):854-863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meinzer M, Flaisch T, Breitenstein C, Wienbruch C, Elbert T, Rockstroh B. Functional re-recruitment of dysfunctional brain areas predicts language recovery in chronic aphasia. NeuroImage. 2008;39(4):2038-2046. [DOI] [PubMed] [Google Scholar]
- 18.Saur D. Dynamics of language reorganization after stroke. Brain. 2006;129(6):1371-1384. [DOI] [PubMed] [Google Scholar]
- 19.Stockert A, Wawrzyniak M, Klingbeil J, et al. Dynamics of language reorganization after left temporo-parietal and frontal stroke. Brain. 2020;143(3):844-861. [DOI] [PubMed] [Google Scholar]
- 20.Kertesz A. WAB-R: Western Aphasia Battery–Revised. 2007. [Google Scholar]
- 21.Strand EA, Duffy JR, Clark HM, Josephs K. The Apraxia of Speech Rating Scale: a tool for diagnosis and description of apraxia of speech. J Commun Disord. 2014;51:43-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dabul B. ABA2 Apraxia Battery for Adults. 2nd edProEd; 2000. [Google Scholar]
- 23.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. NeuroImage. 2006;31(3):1116-1128. [DOI] [PubMed] [Google Scholar]
- 24.Richardson JD, Baker JM, Morgan PS, Rorden C, Bonilha L, Fridriksson J. Cerebral perfusion in chronic stroke: implications for lesion-symptom mapping and functional MRI. Behav Neurol. 2011;24(2):117-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skipper‐Kallal LM, Lacey EH, Xing S, Turkeltaub PE. Functional activation independently contributes to naming ability and relates to lesion site in post-stroke aphasia. Hum Brain Mapp. 2017;38(4):2051-2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson SM, Yen M, Eriksson DK. An adaptive semantic matching paradigm for reliable and valid language mapping in individuals with aphasia. Hum Brain Mapp. 2018;39(8):3285-3307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wilson SM, Bautista A, Yen M, Lauderdale S, Eriksson DK. Validity and reliability of four language mapping paradigms. NeuroImage. 2017;16:399-408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162-173. [DOI] [PubMed] [Google Scholar]
- 29.Worsley KJ, Liao CH, Aston J, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15(1):1-15. [DOI] [PubMed] [Google Scholar]
- 30.Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12(5):535-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yarkoni T, Poldrack RA, Nichols TE, Van Essen DC, Wager TD. Large-scale automated synthesis of human functional neuroimaging data. Nat Methods. 2011;8(8):665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shen X, Tokoglu F, Papademetris X, Constable RT. Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage. 2013;82:403-415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Ser B (Methodol). 1995;57(1):289-300. [Google Scholar]
- 34.Fridriksson J Preservation and modulation of specific left hemisphere regions is vital for treated recovery from anomia in stroke. J Neurosci. 2010;30(35):11558-11564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fridriksson J, Bonilha L, Baker JM, Moser D, Rorden C. Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cereb Cortex. 2010;20(5):1013-1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilson SM, Schneck SM. Neuroplasticity in post-stroke aphasia: a systematic review and meta-analysis of functional imaging studies of reorganization of language processing. Neurobiol Lang. 2021;2(1):22-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bury SD, Jones TA. Unilateral sensorimotor cortex lesions in adult rats facilitate motor skill learning with the “unaffected” forelimb and training-induced dendritic structural plasticity in the motor cortex. J Neurosci. 2002;22(19):8597-8606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Geranmayeh F, Brownsett SL, Wise RJ. Task-induced brain activity in aphasic stroke patients: what is driving recovery? Brain. 2014;137(10):2632-2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fedorenko E, Duncan J, Kanwisher N. Broad domain generality in focal regions of frontal and parietal cortex. Proc Natl Acad Sci. 2013;110(41):16616-16621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brownsett SL, Warren JE, Geranmayeh F, Woodhead Z, Leech R, Wise RJ. Cognitive control and its impact on recovery from aphasic stroke. Brain. 2014;137(1):242-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson SM, Eriksson DK, Yen M, Demarco AT, Schneck SM, Lucanie JM. Language mapping in aphasia. J Speech Lang Hear Res. 2019;62(11):3937-3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Aguiar V, Paolazzi CL, Miceli G. tDCS in post-stroke aphasia: the role of stimulation parameters, behavioral treatment and patient characteristics. Cortex. 2015;63:296-316. [DOI] [PubMed] [Google Scholar]
- 43.Saver JL, Chapot R, Agid R, et al. Thrombectomy for distal, medium vessel occlusions: a consensus statement on present knowledge and promising directions. Stroke. 2020;51(9):2872-2884. [DOI] [PubMed] [Google Scholar]
- 44.Turkeltaub PE. Brain stimulation and the role of the right hemisphere in aphasia recovery. Curr Neurol Neurosci Rep. 2015;15(11):72. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not provided in the article because of space limitations may be shared (anonymized) at the request of any qualified investigator for purposes of replicating procedures and results.