Abstract
Background and Objectives
Individuals with cerebellar ataxia (CA) can develop impulsive behavioral symptoms, often resulting in negative interpersonal consequences, detrimentally affecting their quality of life. Limited evidence exists concerning impulsivity in CA and its associated behavioral changes. We assessed impulsive traits in CA using the Barratt Impulsivity Scale (BIS-11) and compared them with those of Parkinson disease (PD) to investigate the differences in the impulsive trait profiles between CA and PD.
Methods
We conducted a dual-center cross-sectional study with individuals with CA and PD enrolled through consecutive sampling from movement disorders clinics at Columbia University Medical Center and Vanderbilt University Medical Center, respectively. Age-matched controls were recruited at the respective institutions. Participants were excluded if they had prior or comorbid neurologic and psychiatric diseases known to be associated with impulsivity. All participants completed the BIS-11 questionnaire as a measure of impulsive traits. We used a general linear model and a least absolute shrinkage and selection operation regression to compare the total, subscale, and individual items of the BIS-11 scores between groups. Subgroup analyses were performed to isolate cerebellar contributions to impulsivity from potential effects of extracerebellar pathology and dopaminergic dysfunction or medications.
Results
A total of 190 participants—90 age-matched controls, 50 participants with CA, and 50 with PD—completed the assessments. Persons with CA reported 9.7% higher BIS-11 scores than controls (p < 0.001), while persons with PD reported 24.9% higher scores than controls (p < 0.001). In CA, the most affected domain of impulsivity was nonplanning. In contrast, persons with PD noted greater impulsivity across the nonplanning, attentional, and motor domains.
Discussion
Impulsivity in CA is uniquely driven by the nonplanning trait, unlike in PD. This suggests that the cerebellum and basal ganglia may differentially govern impulsive behaviors with the cerebellum contributing to the brain circuitry of impulsivity in a domain-specific manner.
Growing evidence suggests that the cerebellum plays a critical role in human cognition alongside its well-known function in motor coordination. Prior studies involving targeted lesions and neuroimaging have identified cerebellar involvement in complex tasks such as working memory, executive function, and planning.1-3 In patients with cerebellar dysfunction, the constellation of such cognitive and behavioral symptoms has been collectively called cerebellar cognitive affective syndrome (CCAS).4 Characteristic deficits associated with this syndrome include problems with executive function, linguistic processing, and regulation of affect.4 However, the effects of cerebellar dysfunction on other facets of higher-level cognitive processing such as impulsivity remain largely undetermined.
Impulsivity is a multidimensional construct involving a lack of behavioral inhibition or premature decision-making.5 Increased impulsivity can significantly affect personal relationships, employment, and overall quality of life. Several neurologic disorders have been known to be associated with impulsivity, including Parkinson disease (PD), frontotemporal dementia, and Huntington disease.6-8 These findings have led to the understanding that the frontobasal ganglia circuitry regulates impulsivity. Recent studies in preclinical models emphasize that the cerebellum has a critical role in modulating reward circuitry, which is closely linked to impulsivity in humans.9 Consistent with these preclinical findings, we independently identified that patients with cerebellar ataxia (CA) can often engage in impulsive behaviors.10,11 However, different sets of impulsive personality traits can drive a particular impulsive action. For example, a person may impulsively engage in gambling due to an inability to inhibit reward-based motivations (i.e., motor impulsivity) or an inability to consider the negative consequences of losing money (i.e., nonplanning impulsivity). In CA, cognitive aspects of impulsive behaviors have yet to be adequately determined and delineated from those of other neurologic disorders such as PD. Here, we explored patient-reported impulsive behaviors in CA by comparing scores on the Barratt Impulsivity Scale (BIS-11) to scores in individuals with PD and age-matched controls.
Methods
We conducted a dual-center cross-sectional study that included a total of 190 participants: 50 participants with CA, 50 participants with PD, and 90 age-matched controls. Participants with CA were recruited from the Ataxia Clinic at Columbia University Medical Center. Spinocerebellar ataxia (SCA) was diagnosed from the confirmation of variations in the respective genes. The diagnosis of multiple system atrophy–cerebellar type (MSA-C) was based on the current diagnostic criteria.12 The diagnosis of idiopathic late-onset CA (ILOCA) was made after an extensive search for autoimmune, metabolic, or paraneoplastic etiologies and genetic testing for repeat expansion-related SCAs, with the absence of a family history of ataxia, parkinsonism, or autonomic features.13 Five patients with Friedrich ataxia (FA), confirmed by genetic tests, were also recruited at Columbia University to investigate patterns of impulsivity in sensory ataxia. Individuals with PD were diagnosed from the United Kingdom Brain Bank criteria14,15 and recruited from the Parkinson Disease Center at Vanderbilt University Movement Disorders Clinic. All cases with CA and PD were adult onset. All cases of CA had structural neuroimaging studies and received comprehensive laboratory and/or genetic tests as appropriate. Age- and sex-matched controls were primarily from spouses and friends of patients: 40 controls from Columbia University to match with cases with CA and 50 controls from Vanderbilt University to match with cases with PD. Exclusion criteria were any prior or comorbid neurologic and psychiatric diseases known to be associated with impulsivity, including but not limited to dementia, attention-deficit/hyperactivity disorders, autism spectrum disorders, or bipolar disorders. Consecutive patients meeting the inclusion and exclusion criteria were enrolled at both institutions.
Study Measures
The severity of CA was evaluated with the Scale for the Assessment and Rating of Ataxia, a clinical assessment of motor function in CA.16 Neuropsychiatric impairment was measured with the CCAS scale, a cerebellar cognitive test battery, in a subgroup of patients with CA (n = 21).17 The severity of PD was measured by part III of the Movement Disorder Society–Unified Parkinson Disease Rating Scale, a clinical assessment of motor function in PD, in an off-medication condition after overnight washout of dopamine medications.18
The BIS-11 questionnaire, a 30-item instrument with responses scored on a frequency scale, was completed by all participants to assess the personality and behavioral construct of impulsiveness.19 Factor analysis of the BIS-11 indicates 3 dissociable impulsive traits, referred to as second-order factors: attentional impulsivity (inability to focus or concentrate), nonplanning impulsivity (lack of forethought), and motor impulsivity (acting without thinking).19 Each second-order factor can be further delineated into 2 first-order factors: attention and cognitive instability for attentional impulsivity, self-control and cognitive complexity for nonplanning impulsivity, and motor and perseverance for motor impulsivity.19 The total score and subscale scores for the 3 second-order factors and 6 first-order factors were determined for all participants. Higher scores in the attentional, nonplanning, and motor subscales indicate higher levels of cognitive impulsivity with a respective greater inability to concentrate, a greater lack of premeditation, and a greater tendency to act without thought.
Statistical Analysis
Primary BIS-11 Analysis of CA vs PD
Comparisons of demographic and clinical characteristics between participants and controls were performed with the Fisher exact tests for categorial variables and Student t tests for continuous variables. The Pearson correlation coefficient was computed to assess whether (1) age correlated with the BIS-11 total scores of all controls and (2) CCAS total score correlated with the BIS-11 total scores of participants with CA. The total BIS-11 and second-order factor scores of participants with CD, participants with PD, and controls were compared by use of a 1-way analysis of variance followed by the Tukey post hoc multiple-comparisons test (CA vs controls, PD vs controls, and CA vs PD) with a false discovery rate (FDR) correction at a threshold of statistical significance set to p < 0.05 for an FDR at 0.05. GraphPad Prism version 8 (San Diego, CA) was used to conduct these analyses. Further analysis of the distinct factors contributing to the primary traits was performed. To investigate impulsivity without the constraints of a priori second-order scales, we used a least absolute shrinkage and selection operation (LASSO) regression to assess the group responses to both first-order factors and individual questions of the BIS-11 with 500 bootstraps while controlling for age and sex.20 LASSO regression uses L1 regularization that results in variable selection with high prediction accuracy and specificity of interpretation. The variables with ≥80% chosen are considered significant in relation to CA/control, PD/control, or CA/PD status.21 With a threshold for significance set at p < 0.05, generalized linear models (GLMs) controlling for age and sex were then used to measure the strength of the association between first-order factors found to be predictive by LASSO regression and CA/control, PD/control, or CA/PD status. GLM and LASSO regression was performed with the glm and glmnet package, respectively, and bootstrapped in R statistical software (R Foundation for Statistical Computing, Vienna, Austria).
Secondary BIS-11 Analysis in CA Subgroups
Secondary analyses were performed to dissociate cerebellar contributions to impulsivity from that of underlying extracerebellar pathology and dopamine dysfunction or treatments. First, to account for potential contributions of extracerebellar structures to impulsivity, we divided the 50 total participants with CA into 1 group of participants with relatively isolated cerebellar disease (n = 20 SCA6/ILOCA) and 2 groups of participants with more complex cerebrocerebellar diseases with extracerebellar involvement, SCAs other than SCA6 (n = 23) and MSA-C (n = 7) (eTable 1, links.lww.com/WNL/B968). Subgroup analyses were performed by comparing the total BIS-11 and second-order factor scores of each group to those of controls. Furthermore, to investigate any differences in impulsivity between cerebellar and sensory ataxia, the total BIS-11 scores and second-order factor score of individuals with FA (n = 5) were compared with those of the individuals with CA (n = 50) by use of Student t tests. Second, to account for potential dopaminergic effects on impulsivity, we performed additional comparisons of BIS-11 scores between the participants with CA and controls after excluding the participants with CA who are on dopaminergic medication (n = 3) or those diagnosed with MSA-C (n = 7).
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided written informed consent approved by the Institutional Review Board of their respective institutions, either Columbia University Institutional Review Board or Vanderbilt University Institutional Review Board.
Data Availability
All data generated or analyzed during this study are included in this article and its supplement information files (links.lww.com/WNL/B968).
Results
Demographics
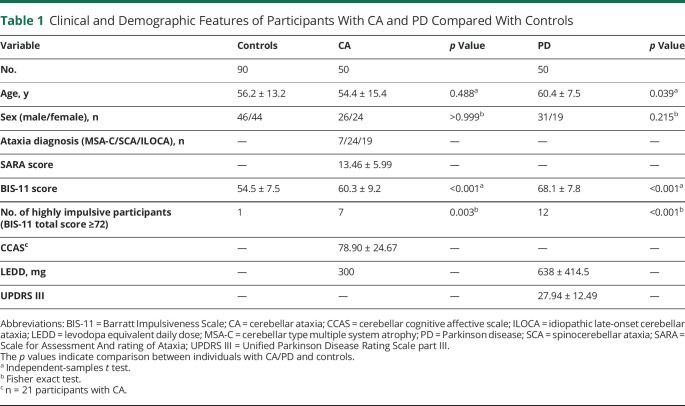
A total of 190 participants (50 with CA, 50 with PD, and 90 age-matched controls) were recruited. We first compared the 2 clinical cohorts, those with CA and participants with PD, and found that the average age of the 50 participants with CA was lower than that of the 50 participants with PD (54.4 ± 15.4 years for participants with CA, 60.4 ± 7.5 years for participants with PD, p = 0.016). Therefore, 2 control groups (40 controls for participants with CA and 50 controls for participants with PD) were separately recruited to better age-match each disease group. A correlation analysis showed that there was no association between age and BIS-11 scores among the 90 total controls (Pearson r = −0.095, p = 0.371). Furthermore, although these 2 control groups were recruited from separate institutions, their BIS-11 scores are similar (40 controls from Columbia, 53.68 ± 6.49 vs 50 controls from Vanderbilt, 55.18 ± 8.30, p = 0.350). There were no significant differences in age between the participants with CA and controls (54.4 ± 15.4 years for participants with CA, 56.2 ± 13.2 years for controls, p = 0.488) and no significant differences in sex between the 2 diseased groups and controls (52% male in CA vs 51% male in controls, p ≥ 0.999; 61% male in PD vs 51% male in controls, p = 0.215) (Table 1). Clinically, the CA group (n = 50: 24 with SCA, 19 with ILOCA, and 7 with MSA-C) had an average Scale for the Assessment and Rating of Ataxia score of 13.46 ± 5.99 with 3 patients (2 with SCA2, 1 with MSA-C) on dopaminergic medication (Table 1 and eTable 1, links.lww.com/WNL/B968). The PD group had an average Unified Parkinson Disease Rating Scale part III score of 27.94 ± 12.49 and an average levodopa equivalent daily dose of 638 ± 414.5 mg. Table 1 provides a summary of the demographic and clinical characteristics of each group.
Table 1.
Clinical and Demographic Features of Participants With CA and PD Compared With Controls
Primary BIS-11 Analysis of CA vs PD
Total Scores
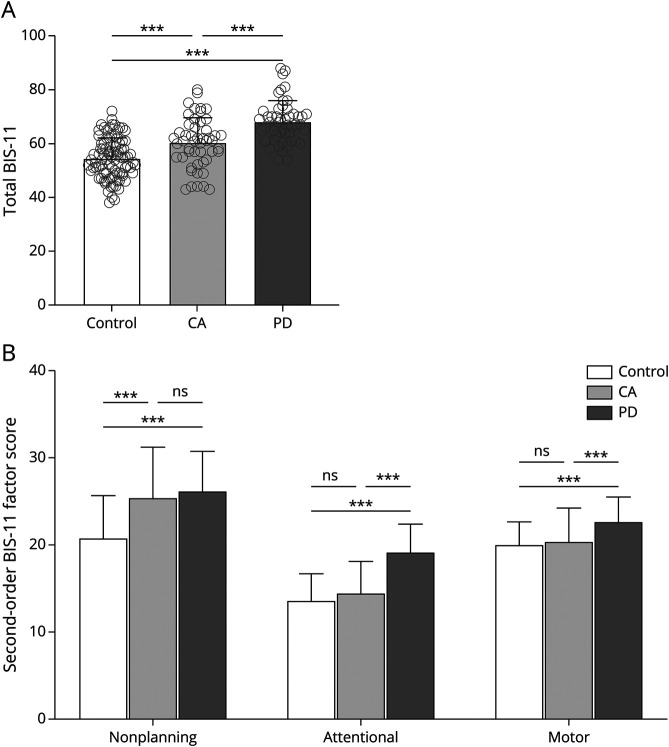
Overall, impulsivity was found to be significantly different between persons with CA, persons with PD, and controls (Table 1). The total BIS-11 score of the participants with CA was 10.8% higher than that of controls (60.30 ± 9.24 for participants with CA vs 54.41 ± 7.55 for controls, p < 0.001), while that of participants with PD was 24.9% higher than the score of controls (68.06 ± 7.84 for participants with PD vs 54.41 ± 7.55 for controls, p < 0.001) (Figure 1A). When we compared persons with CA and PD, the total BIS-11 score of participants with CA was significantly lower than that of participants with PD (60.30 ± 9.24 for participants with CA vs 68.06 ± 7.84 for participants with PD, p < 0.001) (Figure 1A). Furthermore, the number of participants deemed highly impulsive on the basis of the standard cutoff BIS-11 score of 72 points, for clinically significant impulsivity,5 was significantly greater in both participants with CA and those with PD compared to controls (14% in participants with CA vs 1% in controls, p = 0.003; 24% in participants with PD vs 1% in controls, p < 0.001) (Figure 1A). Full characterization of the highly impulsive participants with CA is shown in eTable 1 (links.lww.com/WNL/B968). These data indicate that while both participants with CA and those with PD are more impulsive than controls, participants with PD have higher impulsivity than participants with CA.
Figure 1. Comparison of BIS-11 Scores Between Diseased Groups and Controls.
Mean group responses of controls (n = 90), participants with cerebellar ataxia (CA) (n = 50), and participants with Parkinson disease (PD) (n = 50) for (A) total Barratt Impulsivity Scale (BIS-11) score and (B) second-order factors. Individual scores are shown with gray circles. Multiple-comparisons post hoc test p values after a false discovery rate correction are shown. ns = not significant. *p < 0.05, **p <0.01, ***p < 0.001.
Second-Order Factors
We next asked whether participants with CA and participants with PD have different types of impulsivity by comparing 3 second-order BIS-11 factors (attentional impulsivity, nonplanning impulsivity, and motor impulsivity) between groups (eFigure 1, links.lww.com/WNL/B968). Persons with CA noted higher nonplanning impulsivity (25.42 ± 5.90 for participants with CA vs 20.80 ± 4.87 for controls, p < 0.001) but not attentional impulsivity (14.48 ± 3.64 for participants with CA vs 13.64 ± 3.06 for controls, p = 0.151) or motor impulsivity (20.40 ± 3.88 for participants with CA vs 20.07 ± 2.58 for controls, p = 0.533) (Figure 1B). In contrast, participants with PD had higher scores across all domains of impulsivity compared to controls: nonplanning impulsivity (26.20 ± 4.57 for participants with PD vs 20.80 ± 4.87 for controls, p < 0.001), attentional impulsivity (19.16 ± 3.30 for participants with PD vs 13.64 ± 3.06 for controls, p < 0.001), and motor impulsivity (22.70 ± 2.80 for participants with PD vs 20.07 ± 2.58 for controls, p < 0.001) (Figure 1B). Compared to participants with CA, participants with PD reported higher attentional impulsivity (19.16 ± 3.30 for participants with PD vs 14.48 ± 3.64 for participants with CA, p < 0.001) and motor impulsivity (22.70 ± 2.80 for participants with PD vs 20.40 ± 3.88 for participants with CA, p < 0.001) but not nonplanning impulsivity (26.20 ± 4.57 for participants with PD vs 25.42 ± 5.90 for participants with CA, p = 0.444) (Figure 1B). This result supports the finding that participants with CA exhibit nonplanning impulsivity only, while those with PD exhibit nonplanning impulsivity in addition to attentional and motor impulsivity. From second-order factor analysis, impulsivity in CA appears specific to the nonplanning domain, whereas impulsivity in PD occurs across all domains. Overall, this demonstrates that impulsivity in CA is distinctly domain specific.
First-Order Factors
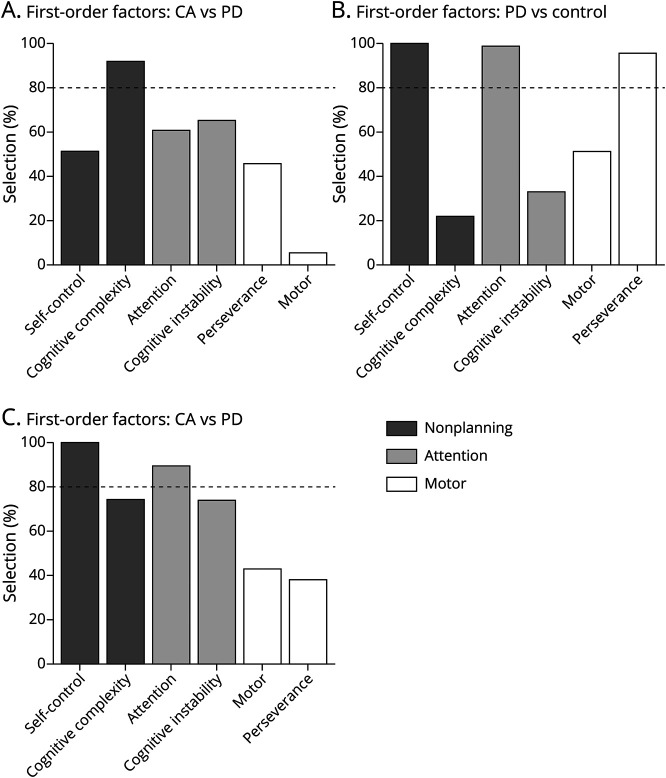
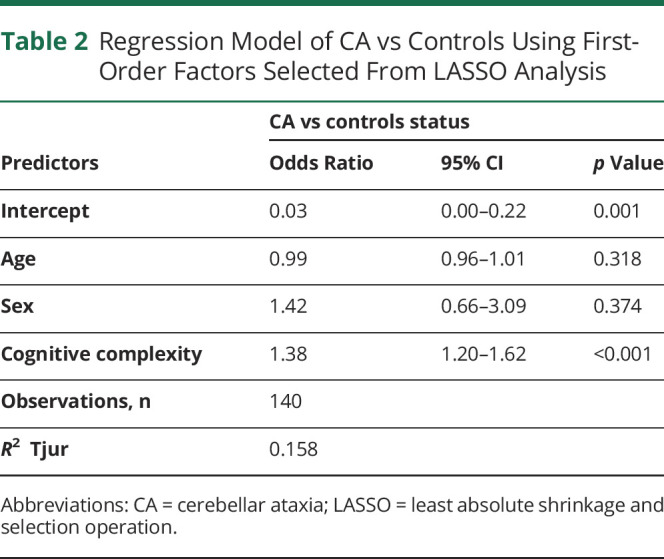
To further explore the differences between the subfactors of impulsivity between participants with CA and controls, we used LASSO analysis and identified 1 of the 6 first-order factors that were chosen with a frequency of ≥80%. Cognitive complexity, a subfactor of nonplanning impulsivity, was selected as the only first-order factor differentiating between participants with CA and controls (Figure 2A). A GLM controlling for age and sex showed that cognitive complexity remained significant between participants with CA and controls with an associated odds ratio of 1.38 (95% CI 1.20–1.62, p < 0.001) (Table 2). This result suggests that the nonplanning impulsivity in CA is driven mainly by the lack of cognitive complexity.
Figure 2. LASSO Selection of First-Order BIS-11 Factors.
Frequency at which each first-order factor is selected from least absolute shrinkage and selection operation (LASSO) regression for (A) individuals with cerebellar ataxia (CA) vs controls, (B) individuals with Parkinson disease (PD) vs controls, and (C) individuals with CA vs those with PD. The 80% threshold for significant predictive power is shown with the dotted line.
Table 2.
Regression Model of CA vs Controls Using First-Order Factors Selected From LASSO Analysis
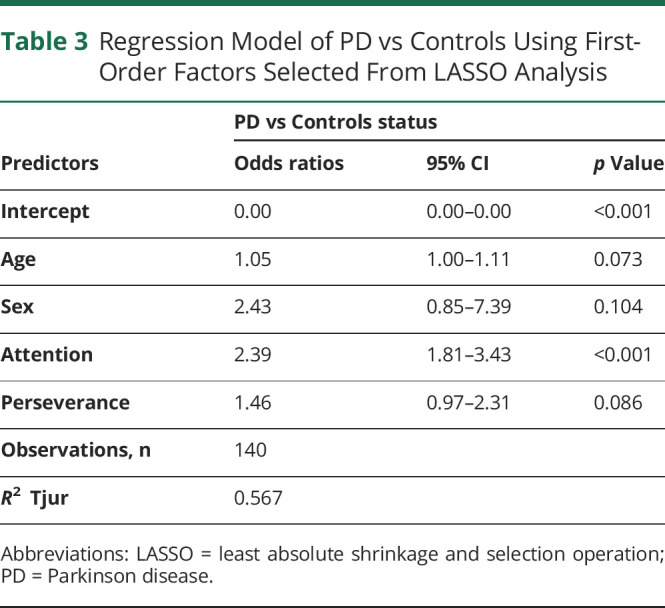
We next compared the subfactors between participants with PD and controls using LASSO analysis, and we found that attention, self-control, and perseverance were all selected with a frequency of ≥80% of 6 first-order factors (Figure 2B). Self-control, a subfactor of nonplanning impulsivity, was found to perfectly separate individuals with PD from controls with 100% selection at a threshold score of 21; all controls scored ≤21, while all participants with PD scored ≥21. To avoid masking the effects of attention and cognitive complexity, self-control was excluded from the GLM. Controlling for age and sex, the GLM found that the attention subfactor remained significant in differentiating between participants with PD and controls with an associated odds ratio of 2.39 (95% CI 1.81–3.43, p < 0.001), while perseverance, a subfactor of motor impulsivity, was no longer significant (p = 0.086) (Table 3). First-order analysis in PD demonstrates that the impulsivity comes from distinct subfactors, self-control, and attention. While both participants with PD and those with CA have nonplanning deficits according to second-order analysis, the underlying mechanism from the first-order analysis appears to be distinct: a lack of cognitive complexity in participants with CA and a deficit in self-control in participants with PD (Figure 2).
Table 3.
Regression Model of PD vs Controls Using First-Order Factors Selected From LASSO Analysis
LASSO analysis was also used to compare the subfactors between CA and participants with PD. Of the 6 first-order factors, we found that attention and self-control were selected with a frequency of ≥80% (Figure 2C). Self-control was found to perfectly separate CA from PD with 100% selection at a threshold score of 18, with all participants with CA scoring at or below the threshold and all participants with PD scoring above the threshold. After accounting for age and sex and excluding self-control to avoid masking the effects of attention, the GLM found that the attention subfactor remained significant in differentiating between individuals with CA and those with PD with an associated odds ratio of 0.63 (95% CI 0.50–0.77, p < 0.001) (eTable 2, links.lww.com/WNL/B968). In addition to highlighting differences in attention, these results support that different first-order factors may drive nonplanning impulsivity in CA and PD, considering self-control is a subfactor of nonplanning impulsivity.
Individual Questions
LASSO regression also showed how responses to the 30 individual BIS-11 questions contribute to the disease status (participants with CA vs controls and participants with CA vs those with PD). When we compared participants with CA with controls, 5 questions, predominantly in the nonplanning domain, were chosen with a frequency of ≥80%. Participants with CA were more likely to respond with “almost always,” unless a question is reverse scored (as indicated with an asterisks), in which participants with CA were more likely to select “rarely/never” (eFigure 2A, links.lww.com/WNL/B968). In a comparison of participants with PD and controls, 4 questions primarily in the attentional domain were selected with a frequency of ≥80% (eFigure 2B). For these questions, participants with PD were more likely to respond with “almost always” than those with CA, unless a question is reverse scored (as indicated with an asterisks), in which participants with PD were more likely to select “rarely/never.”
CA Subgroup Analysis
Isolated Cerebellar Diseases vs Complex Cerebrocerebellar Diseases
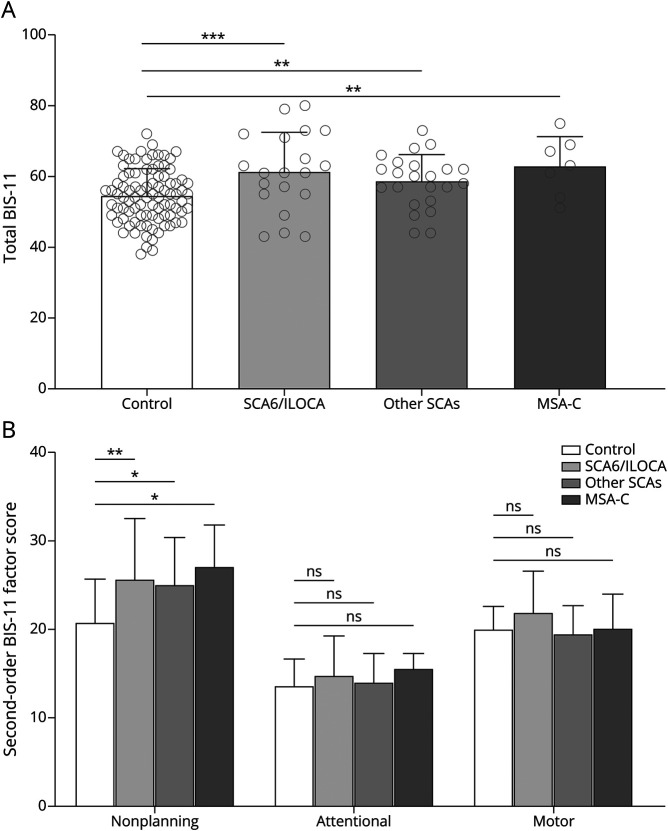
Because many patients with CA may also have extracerebellar pathology, we next separated the groups into more isolated cerebellar diseases, SCA6 and ILOCA, and more complex cerebellar diseases, including SCAs other than SCA6 and MSA-C. We found that patients with isolated cerebellar disease reported a total BIS-11 score that was 13% higher than that of controls (61.30 ± 11.23 vs 54.41 ± 7.55, p = 0.003). Similarly, participants with CA, excluding those with SCA6, reported an 8% higher total BIS-11 score than controls (58.61 ± 7.48 vs 54.41 ± 7.55, p = 0.034), and those with MSA-C reported a 16% higher total BIS-11 score than controls (62.86 ± 8.42 vs 54.41 ± 7.55, p = 0.016) (Figure 3A). Analysis of second-order factors of impulsivity indicated that, for each group, nonplanning impulsivity was heightened compared to controls but not attentional impulsivity or motor impulsivity (Figure 3B), confirming that nonplanning impulsivity occurs across different diagnoses of CA. Last, we compared levels of impulsivity in individuals with CA with that of participants with FA (n = 5), who have sensory ataxia in addition to CA involving the dentate nuclei. We found that impulsivity in participants with FA does not differ from that in individuals with other forms of CA (59.20 ± 12.24 vs 60.30 ± 9.24, p = 0.403) (eTable 3, links.lww.com/WNL/B968).
Figure 3. Comparison of BIS-11 Scores Between Subgroups of CA.
Mean group responses of controls (n = 90) and patients with isolated cerebellar disease, spinocerebellar ataxia (SCA) type 6 (SCA6) and idiopathic late-onset cerebellar ataxia (ILOCA) (n = 20), and complex cerebrocerebellar diseases, SCAs other than SCA6 (n = 23) and multiple system atrophy–cerebellar type (MSA-C) (n = 7) for (A) the total Barratt Impulsivity Scale (BIS-11) score and (B) second-order factors. Individual scores are shown with gray circles. Multiple-comparisons post hoc test p values after a false discovery rate correction are shown. *p < 0.05, **p <0.01, ***p < 0.001.
Dopaminergic Effects
Because dopamine is known to be related to impulsivity, we performed additional analyses to exclude patients with CA who take dopaminergic medications or those who have a diagnosis of MSA. When patients with CA on dopaminergic medications were excluded (n = 3, eTable 1, links.lww.com/WNL/B968), the total BIS-11 scores of the remaining participants were 11% higher than those of controls (60.66 ± 9.47 vs 54.41 ± 7.55, p < 0.001), and nonplanning impulsivity remained the only second-order factor significantly different from that of controls (eFigure 3A and 3B). These findings remained consistent after the exclusion of patients with MSA-C (eFigure 3A and 3B). Overall, these data support that the impulsivity in patients with CA is not driven primarily by the use of dopaminergic medications or underlying dopaminergic dysfunction.
Correlation With Other Cerebellar Cognitive Symptoms
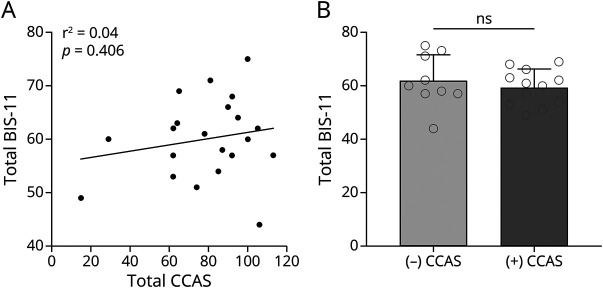
Because impulsivity could be part of a broader set of cognitive and behavioral symptoms resulting from cerebellar dysfunction, we next investigated the relationship between impulsivity and other cerebellar cognitive affective symptoms using the CCAS scale. We discovered that the total BIS-11 scores in participants with CA did not correlate with CCAS scores (n = 21, Pearson r = 0.19, p = 0.406; Figure 4A). Fifty-seven percent of the participants with CA have definite CCAS according to 3 failed tests in the CCAS assessment criteria.17 We further compared total BIS-11 scores between those with and those without definite CCAS, and we did not find any differences (59.42 ± 6.65 in those with CCAS vs 61.89 ± 9.78 without those without CCAS, p = 0.498) (Figure 4B). These data suggest that impulsivity is a distinct cognitive behavioral symptom of cerebellar diseases.
Figure 4. Correlation Between BIS-11 and CCAS Scores in Ataxic Participants.
(A) Correlation between Barratt Impulsivity Scale (BIS-11) and cerebellar cognitive affective syndrome (CCAS) total scores in individuals with cerebellar ataxia (CA) (n = 21). (B) Comparison of BIS-11 total scores between patients with CA with and without confirmed CCAS. ns = not significant.
Discussion
In this dual-center cross-sectional study, we demonstrate that persons with CA exhibit heightened levels of impulsivity, specifically with regard to nonplanning. We compared impulsivity in 2 clinical populations, individuals with CA and those with PD, to that in controls using the BIS-11 questionnaire. Although significant levels of impulsivity were seen in both clinical groups, the traits underlying these impulsive shifts differed. Participants with CA noted domain-specific nonplanning impulsivity, while participants with PD had elevated global impulsive behaviors across the attention, nonplanning, and motor domains. In addition, nonplanning impulsivity evident in participants with CA appears driven primarily by a lack of cognitive complexity. In contrast, the nonplanning impulsivity seen in PD was found to arise mainly from a lack of self-control. We further identified questions within the BIS-11 model that are specifically relevant to impulsivity for both CA and PD groups. Overall, our results show the presence of distinct cognitive deficits in CA and provide insight into the cerebellar contributions to impulsivity, which appears to be distinct from PD.
To date, most neuroanatomic models of impulsivity have not included the cerebellum. Impulsivity has instead been thought to involve primarily alterations of cortico-striatal networks.22,23 Recent data from animal models, however, suggest that the cerebellum is critical for reward processing and that cerebellar neuronal firing can encode reward signals that may have a profound impact on impulsive behaviors.9 These findings are supported by our recent clinical observations of heightened impulsivity in patients with CA; specifically, the associated behavioral changes include increased gambling, hobbyism and punding, and medication overuse.10,11 However, these impulsive behaviors can originate from distinct combinations of underlying traits.5,24 With the use of a multitrait model of impulsivity, the results of our present study indicate that impulsivity in CA arises specifically as a result of disordered planning from a lack of cognitive complexity, which differs from the profile of impulsive traits seen in PD.
In contrast to the domain-specific impulsivity exhibited by participants with CA, impulsivity in participants with PD was found to extend globally across cognitive and behavioral BIS-11 constructs. This is consistent with previous studies that used the BIS-11 to assess impulsive traits in patients with PD, which have implicated all domains.6,25 In addition, we noted that, although participants with PD reported overall greater levels of impulsivity compared to participants with CA, the degree of nonplanning impulsivity at the second-order level was found to be similar in participants with CA and those with PD. However, this second-order nonplanning trait can be further dissociated into 2 first-order factors, self-control and cognitive complexity (eFigure 1, links.lww.com/WNL/B968). Through first-order analysis, we found that nonplanning impulsivity in participants with CA was driven by a lack of cognitive complexity, as opposed to nonplanning impulsivity in participants with PD, which arose as a lack of self-control. This suggests that the planning deficits due to cerebellar degeneration are more cognitive in origin and demonstrates that differences in impulsivity between CA and PD extend to the first-order level. The intolerance for complex cognition in CA is consistent with recent studies that showed that there was impaired working memory, or the ability to maintain information in the mind for the purpose of manipulation, after damage to the cerebellum.26 Although the exact cerebellar circuitry that governs impulsivity remains to be determined, our results suggest that both the cerebellum and basal ganglia have distinct roles in governing impulsivity and demonstrate that diverse forms of impulsivity can occur across various neurologic disorders.
Overall, the planning deficits we observed in CA could be evidence of a broader part of the universal cerebellar transform in which the cerebellum acts as a hub for general motor and nonmotor predictive processing.4 In the context of motor control, the cerebellum is involved in the prediction of sensory outcomes of a motor action through internal models that are iteratively refined to optimize behavior.27,28 Due to its relatively uniform cytoarchitecture, the cerebellum has been hypothesized to perform an analogous predictive function in the cognitive domain.29 Through connections with many regions of the cerebral cortex, the cerebellum is optimally positioned to receive and integrate information to inform predictions not only in motor realms but also in cognitive realms. In a recent fMRI study, distinct cerebellar task activations were identified for working memory, language, emotion, and social cognition, and these findings suggest that distinct triple representations of cortical information localize to different lobules of the cerebellum.30 Another fMRI study reported evidence of a hierarchical organization of the nonmotor cerebellar that mirrors that of the prefrontal cortex, suggesting that the cerebellum may support complex cognition.31 In this context, our findings of heightened nonplanning impulsivity in the participants with CA may reflect the failure of predicting the consequences of making a particular decision.
Recognizing the unique expressions of impulsivity across neurologic disorders has important clinical and personal implications. Equipping neurologists with the knowledge of the different impulsive trait profiles enables clinicians and researchers to better identify clinical presentations that may be specific to certain patient populations. Further characterization of these trait profiles can help advance the development of screening tools and tailored counseling and future interventions. Increasing patients’ and caregivers' awareness of these different impulsive patterns can also further the lifestyle modification to mitigate their impacts.
The limitations of our study are as follows. First, because the study design is cross-sectional, it remains unclear how impulsivity emerges and changes over the disease course. Second, it should be noted that even though patients with PD were clinically assessed after overnight washout of dopamine medications, this procedure may not be able to completely exclude the effect of the medication. However, these results are consistent with that reported previously.25 Third, BIS-11 is a self-reported measures which might be affected by the cognitive status, resulting in underreporting of impulsivity. While not every study participant received clinical bedside batteries such as the Mini-Mental Status Examination, we used CCAS instead to investigate the cerebellum-specific cognitive status. Future studies administering a more expanded cognitive battery can provide an in-depth neuropsychiatric profile of patients with CA. Last but not least, although we assessed patterns of impulsivity in both isolated cerebellar diseases and complex cerebrocerebellar diseases through subgroup analyses, future studies focusing on patients with discrete lesions in the cerebellum such as from tumors or infarcts will further shed light on the distinct role of the cerebellum in impulsivity. Another important future direction is neuroimaging and task-based investigation, which will further elucidate the involvement of specific cerebellar regions in impulsivity and cognition. International collaborative studies are also needed to confirm the results by using imaging techniques and additional battery to assess impulsivity. Considering that deep brain stimulation has been shown to be effective in managing impulsive behaviors in PD,32,33 methods of brain stimulation can be explored as a potential therapy for impulsivity in CA. Last, it should be noted that the BIS-11 is rated on the basis of a patient's own report. The field has not yet established a valid and reliable scale from the caregiver’s perspective to corroborate self-reported impulsive behaviors in either PD or CA. Development of caregiver report scales to objectively quantify and track symptomatic changes along the patient's disease trajectory would be beneficial in understanding the reliability of self-reported ratings of impulsivity.
This study provides evidence that patients with cerebellar dysfunction report domain-specific cognitive changes, with heightened impulsivity driven largely by poor planning. This suggests that the cerebellum may play an important role in controlling specific domains of impulsive behaviors along with the basal ganglia and prefrontal cortex.
Glossary
- BIS-11
Barratt Impulsivity Scale
- CA
cerebellar ataxia
- CCAS
cerebellar cognitive affective syndrome
- FA
Friedrich ataxia
- FDR
false discovery rate
- GLM
generalized linear model
- ILOCA
idiopathic late-onset CA
- LASSO
least absolute shrinkage and selection operation
- MSA-C
multiple system atrophy–cerebellar type
- PD
Parkinson disease
- SCA
spinocerebellar ataxia
Appendix. Authors
Study Funding
This study was supported by NIH R01NS104423, NIH R01NS118179, and NIH R01NS124854.
Disclosure
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Buckner RL. The cerebellum and cognitive function: 25 years of insight from anatomy and neuroimaging. Neuron. 2013;80(3):807-815. [DOI] [PubMed] [Google Scholar]
- 2.Kim SG, Uğurbil K, Strick PL. Activation of a cerebellar output nucleus during cognitive processing. Science. 1994;265(5174):949-951. [DOI] [PubMed] [Google Scholar]
- 3.Stoodley CJ, Schmahmann JD. Functional topography of the human cerebellum. Handb Clin Neurol. 2018;154:59-70. [DOI] [PubMed] [Google Scholar]
- 4.Schmahmann JD. Disorders of the cerebellum: ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16(3):367-378. [DOI] [PubMed] [Google Scholar]
- 5.Stanford MS, Mathias CW, Dougherty DM, Lake SL, Anderson NE, Patton JH. Fifty years of the Barratt Impulsiveness Scale: an update and review. Personal Individual Differences. 2009;47(5):385-395. [Google Scholar]
- 6.Weintraub D, Claassen DO. Impulse control and related disorders in Parkinson's disease. Int Rev Neurobiol. 2017;133:679-717. [DOI] [PubMed] [Google Scholar]
- 7.Lansdall CJ, Coyle-Gilchrist ITS, Jones PS, et al. Apathy and impulsivity in frontotemporal lobar degeneration syndromes. Brain. 2017;140(6):1792-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson PL, Potts GF, Sanchez-Ramos J, Cimino CR. Self-reported impulsivity in Huntington's disease patients and relationship to executive dysfunction and reward responsiveness. J Clin Exp Neuropsychol. 2017;39(7):694-706. [DOI] [PubMed] [Google Scholar]
- 9.Wagner MJ, Kim TH, Savall J, Schnitzer MJ, Luo L. Cerebellar granule cells encode the expectation of reward. Nature. 2017;544(7648):96-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amokrane N, Lin CR, Desai NA, Kuo SH. The impact of compulsivity and impulsivity in cerebellar ataxia: a case series. Tremor Other Hyperkinet Mov. 2020;10:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amokrane N, Viswanathan A, Freedman S, et al. Impulsivity in cerebellar ataxias: testing the cerebellar reward hypothesis in humans. Mov Disord. 2020;35(8):1491-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gilman S, Wenning GK, Low PA, et al. Second consensus statement on the diagnosis of multiple system atrophy. Neurology. 2008;71(9):670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fogel BL, Perlman S. An approach to the patient with late-onset cerebellar ataxia. Nat Clin Pract Neurol. 2006;2(11):629-635. [DOI] [PubMed] [Google Scholar]
- 14.Tolosa E, Wenning G, Poewe W. The diagnosis of Parkinson's disease. Lancet Neurol. 2006;5(1):75-86. [DOI] [PubMed] [Google Scholar]
- 15.Daniel SE, Lees AJ. Parkinson's disease society Brain Bank, London: overview and research. J Neural Transm Suppl. 1993;39:165-172. [PubMed] [Google Scholar]
- 16.Schmitz-Hübsch T, du Montcel ST, Baliko L, et al. Scale for the Assessment and Rating of Ataxia: development of a new clinal scale. Neurology. 2006;66(11):1717-1720. [DOI] [PubMed] [Google Scholar]
- 17.Hoche F, Guell X, Vangel MG, Sherman JC, Schmahmann JD. The cerebellar cognitive affective/Schmahmann syndrome scale. Brain. 2018;141(1):248-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ebersbach G, Baas H, Csoti I, Müngersdorf M, Deuschl G. Scales in Parkinson's disease. J Neurol. 2006;253(4):iv32–iv35. [DOI] [PubMed] [Google Scholar]
- 19.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt Impulsiveness Scale. J Clin Psychol. 1995;51(6):768-774. [DOI] [PubMed] [Google Scholar]
- 20.Tibshirani R. Regression shrinkage and selection via the LASSO. J R Stat Soc Ser B (Methodological). 1996;58(1):267-288. [Google Scholar]
- 21.Friedman J, Hastie T, Tibshirani R. Regularization paths for generalized linear models via coordinate descent. J Stat Softw. 2010;33(1):1-22. [PMC free article] [PubMed] [Google Scholar]
- 22.Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69(4):680-694. [DOI] [PubMed] [Google Scholar]
- 23.Robbins TW, Gillan CM, Smith DG, de Wit S, Ersche KD. Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci. 2012;16(1):81-91. [DOI] [PubMed] [Google Scholar]
- 24.Kocka A, Gagnon J. Definition of impulsivity and related terms following traumatic brain injury: a review of the different concepts and measures used to assess impulsivity, disinhibition and other related concepts. Behav Sci (Basel). 2014;4(4):352-370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aumann MA, Stark AJ, Hughes SB, et al. Self-reported rates of impulsivity in Parkinson's disease. Ann Clin Transl Neurol. 2020;7(4):437-448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravizza SM, McCormick CA, Schlerf JE, Justus T, Ivry RB, Fiez JA. Cerebellar damage produces selective deficits in verbal working memory. Brain. 2006;129(pt 2):306-320. [DOI] [PubMed] [Google Scholar]
- 27.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3):272-303. [DOI] [PubMed] [Google Scholar]
- 28.Marr D. A theory of cerebellar cortex. J Physiol. 1969;202(2):437-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ito M. Control of mental activities by internal models in the cerebellum. Nat Rev Neurosci. 2008;9(4):304-313. [DOI] [PubMed] [Google Scholar]
- 30.Guell X, Gabrieli JDE, Schmahmann JD. Triple representation of language, working memory, social and emotion processing in the cerebellum: convergent evidence from task and seed-based resting-state fMRI analyses in a single large cohort. Neuroimage. 2018;172:437-449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.D'Mello AM, Gabrieli JDE, Nee DE. Evidence for hierarchical cognitive control in the human cerebellum. Curr Biol. 2020;30(10):1881-1892.e1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisinger RS, Scott BM, Le A, et al. Pavlovian bias in Parkinson's disease: an objective marker of impulsivity that modulates with deep brain stimulation. Sci Rep. 2020;10(1):13448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossi PJ, De Jesus S, Hess CW, et al. Measures of impulsivity in Parkinson's disease decrease after DBS in the setting of stable dopamine therapy. Parkinsonism Relat Disord. 2017;44:13-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplement information files (links.lww.com/WNL/B968).