Abstract
Background and Objectives
The association between vascular risk factors and dementia varies with age, making generalizability of dementia risk prediction rules to individuals of different ages challenging. We determined the most important vascular risk factors for inclusion in age-specific dementia risk scores.
Methods
Framingham Heart Study Original and Offspring cohort participants with available data on the Framingham Stroke Risk Profile (FSRP) at midlife (age 55; n = 4,899, 57% women), late life (ages 65 or 70), or later life (ages 75 or 80 [n = 2,386, 62% women]) were followed for 10-year incident dementia risk from ages 65, 70, 75, and 80.
Results
Age- and sex-adjusted midlife risk factors associated with 10-year risk of dementia from age 65 included FSRP (hazard ratio [HR] 1.16, 95% CI 1.06–1.26, per 1 SD increment in log-transformed score), diabetes mellitus (DM; HR 4.31, 95% CI 1.97–9.43), and systolic blood pressure (SBP; HR 1.12, 95% CI 1.02–1.24, per 10 mm Hg increment). Late-life risk factors associated with 10-year incident dementia from ages 65 or 70 included FSRP (age 65 only: HR 1.06, 95% CI 1.02–1.10), antihypertensive use (age 65 reported: HR 1.66, 95% CI 1.12–2.46), DM (age 65 reported: HR 1.96, 95% CI 1.09–3.52), atrial fibrillation (age 65 reported: HR 2.30, 95% CI 1.00–5.27), nonstroke cardiovascular disease (nsCVD; age 65 reported: HR 1.95, 95% CI 1.24–3.07), and stroke (age 70 only: HR 3.61, 95% CI 2.21–5.92). Later-life risk factors associated with 10-year incident dementia from ages 75 or 80 included antihypertensive use (age 80 only: HR 0.74, 95% CI 0.62–0.89), DM (age 80 reported: HR 1.40, 95% CI 1.04–1.89), atrial fibrillation (age 80 reported: HR 1.43, 95% CI 1.07–1.92), and stroke (age 80 reported: HR 1.63, 95% CI 1.13–2.35). In stepwise models, SBP and DM at age 55, nsCVD at age 65, DM and stroke at ages 70 and 75, and DM, stroke, and use of antihypertensives (protective) at age 80 were the most important vascular risk factors for dementia.
Discussion
Our findings support the use of age-specific dementia risk scores, which should prioritize including, at age 55, SBP and DM; at age 65, nsCVD; at ages 70 and 75, DM and stroke; and at age 80, DM, stroke, and antihypertensive use.
Accurately predicting an individual's future risk of dementia can inform individual approaches to risk factor and lifestyle modification to help reduce disease risk. Vascular risk factors, such as hypertension and diabetes mellitus (DM), are important modifiable determinants of dementia and a core component of dementia risk prediction rules.1-4 However, the association between vascular risk factors and dementia is often complex, nonlinear, and can vary with age, making the development of generalizable dementia risk prediction rules for individuals of differing ages challenging. For example, although early to midlife obesity has been associated with increased late-life dementia risk, lower body mass index in later life is a marker of increased dementia risk, likely due to reverse causality.5 Moreover, elevated blood pressure in mid- and early late life has been associated with increased dementia risk, but lower blood pressures in later life appear to be harmful to cognition.6 Current dementia risk prediction rules1-3 typically have been developed within narrowly defined age ranges and resultant risk estimates may not be generalizable to individuals outside of these ranges. In order to develop simple and accurate age-specific clinical prediction rules for dementia, we require a better understanding of the association between vascular risk factors and dementia across mid- to later life.
The Framingham Stroke Risk Profile (FSRP) was developed and validated for predicting 10-year stroke risk among individuals ≥55 years of age.7 The FSRP has also been associated with brain atrophy and cognitive decline in a community-based setting.8-10 However, it is unknown whether the FSRP is predictive of dementia risk across mid- to later life or if the association between component vascular risk factors and incident dementia varies with age. We determined the association between the FSRP and its components at 5 time points across mid- to later life and 10-year risk of incident dementia and identified the most important vascular predictors for inclusion in age-specific dementia risk scores.
Methods
Study Sample
We included Framingham Heart Study (FHS) Original (enrolled in 1948) and Offspring cohort (enrolled in 1971) participants who have been longitudinally followed for the development of vascular risk factors, stroke, and dementia.11 Participants are examined biennially to quadrennially from the time of cohort enrollment. In the present investigation, we included participants with available data on dementia status on follow-up and risk factor data at any of 5 a priori defined timepoints across midlife (designated as age 55), late life (ages 65 or 70), and later life (ages 75 or 80 years). Individuals were eligible for inclusion at the index age if they attended an FHS examination within 2.5 years of the index age. We commenced 10-year dementia follow-up from each of the index ages, with the exception of age 55, from which we commenced follow-up from age 65 due to the negligible number of dementia cases prior to age 65 in our cohort (eFigure 1, links.lww.com/WNL/B986, participant flow diagram).
Standard Protocol Approvals, Registrations, and Patient Consents
All participants provided written informed consent before taking part in this study and the study protocols and consent forms were approved by the Boston University Medical Center Institutional Review Board (H-33395).
Outcome Measure
The primary outcome was incident all-cause dementia, diagnosed in accordance with DSM-IV criteria.12 The Original cohort participants were initially screened with a cognitive battery in 1975 and were subsequently screened for dementia at every examination visit from 1981 via the Mini-Mental State Examination (MMSE). Any participant with a score below prespecified education-based cutoffs, 3 points lower than the preceding examination, or 5 points lower than the participant's previous highest recorded score is flagged for more comprehensive neuropsychological testing. In addition to this, if concern for cognitive impairment was raised by a participant, by a participant's family member (e.g., concern for cognitive decline or functional decline potentially related to cognitive impairment), or by the FHS physician completing the examination visit, regardless of the MMSE score, the participant was referred for more comprehensive neuropsychological testing.13 The Offspring cohort participants have undergone similar monitoring at each examination visit via the MMSE since 1991, as well as undergoing a more extensive neuropsychological test battery at every examination visit since 1999. Participants with suspected cognitive impairment who do not meet diagnostic criteria for dementia undergo additional annual neuropsychological assessments between the scheduled examinations. Participants flagged for possible cognitive impairment or dementia on this testing are subsequently evaluated by a neurologist. A diagnosis of dementia is based on a review of all available neurologic examination records, neuropsychological assessments, neuroimaging investigations, hospital/nursing home/outpatient clinic records, family interviews, and autopsy results (when available) by a dementia review committee that includes a minimum of one neuropsychologist and a neurologist. If a participant dies or does not attend further follow-up, the dementia review committee reviews medical records up to the time of death/loss to follow-up, to ascertain if the participant may have developed interval cognitive impairment/dementia since the last study examination. Further details on surveillance procedures have been published.14,15
Covariates
We measured individual FSRP components, namely age, sex, systolic blood pressure (SBP), antihypertensive use, prevalent cardiovascular disease (CVD), current smoking, history of atrial fibrillation (AF), and DM, and calculated the FSRP score at each index age. We included a polygenic risk score (PGRS) for dementia based on important risk loci for Alzheimer disease dementia.16 Risk factors were measured at midlife (age 55) and at each of ages 65, 70, 75, and 80 years. SBP was defined as the mean of 2 physician-recorded measurements performed in the seated position from the participant's left arm. Antihypertensive medication use was self-reported and verified using prescription data and pill bottles when available. CVD included peripheral vascular disease (including intermittent claudication), coronary artery disease (including coronary insufficiency, angina pectoris, and myocardial infarction), cerebrovascular disease (including stroke), and congestive heart failure. We separated CVD into stroke and nonstroke CVD (nsCVD) to determine their individual contributions to dementia. Current smoking was defined as participant self-reported smoking within the previous 12 months at the time of risk factor measurement. AF was diagnosed via ECG by an FHS cardiologist. DM was defined as a fasting blood glucose ≥7 mmol/L, random blood glucose ≥11.1 mmol/L, or use of insulin or oral hypoglycemic medications.
Statistical Analysis
For our primary analysis, we included individuals with data available for any of the listed vascular risk factors at each index age and used age- and sex-adjusted Cox proportional hazards models to estimate the association between the FSRP and its individual components and 10-year risk of incident dementia starting from age 65. We completed a sensitivity analysis using a false discovery rate (FDR) correction of 35 accounting for 7 individual predictor variables for one outcome measured at 5 time points. We also completed a sensitivity analysis additionally adjusting for level of education (self-reported by participants and categorized as no high school degree, high school degree but no college degree, and college degree or higher).
In secondary analyses, we included individuals with data available for all listed vascular risk factors at the relevant index age and excluded individuals with missing data for any of the listed risk factors at that index age. To minimize the risk of sampling bias, we did not require individuals to have risk factor data available at every index age. We used multivariable adjusted Cox proportional hazards models to estimate the association between the FSRP component risk factors and 10-year risk of incident dementia starting from age 65, adjusting for (1) age, sex, and the remaining FSRP components; and (2) age, sex, the remaining FSRP components, and a PGRS for dementia in those with available genetic risk score data. We assessed model discrimination using area under the curve (AUC) and calculated the change in AUC on addition of the genetic risk score to the model. We completed additional (exploratory) analyses stratified by sex. We estimated stepwise Cox models to identify the most important risk factors for incident dementia at mid, late, and later life for inclusion in age-specific dementia risk scores. Participants were followed from the time of risk factor measurement to the time of incident dementia, death, or date last confirmed to be dementia free. Results are reported as hazard ratios (HR) with corresponding 95% CI. p < 0.05 was considered statistically significant. Analyses were completed using SAS v9.4 (SAS Institute Inc.).
Data Availability
The de-identified data used in these analyses can be obtained from the NHLBI database and the NCBI dbGaP.
Results
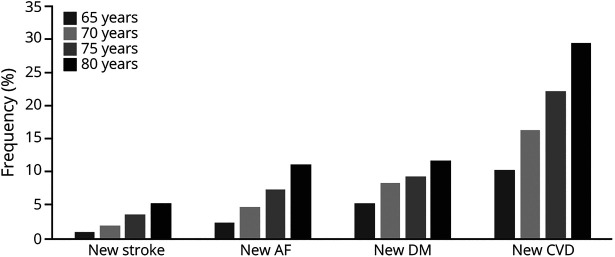
Risk factor data were available for 4,899 individuals (57.2% women) at age 55 and 2,386 (62.1% women) at age 80. The proportion of individuals with new-onset vascular risk factors progressively increased with age (Table 1 and Figure 1).
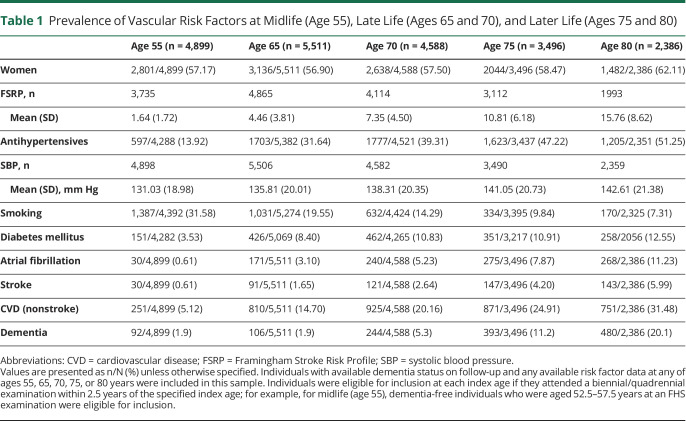
Table 1.
Prevalence of Vascular Risk Factors at Midlife (Age 55), Late Life (Ages 65 and 70), and Later Life (Ages 75 and 80)
Figure 1. Cumulative Frequency of New-Onset Vascular Risk Factors Among Dementia-Free Survivors by Age.
AF = atrial fibrillation; CVD = cardiovascular disease; DM = diabetes mellitus.
Midlife (Age 55) Risk Factors
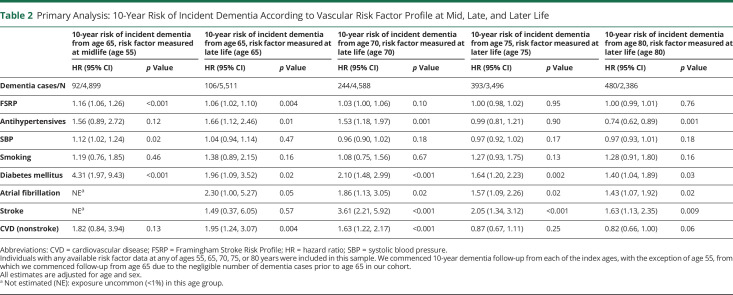
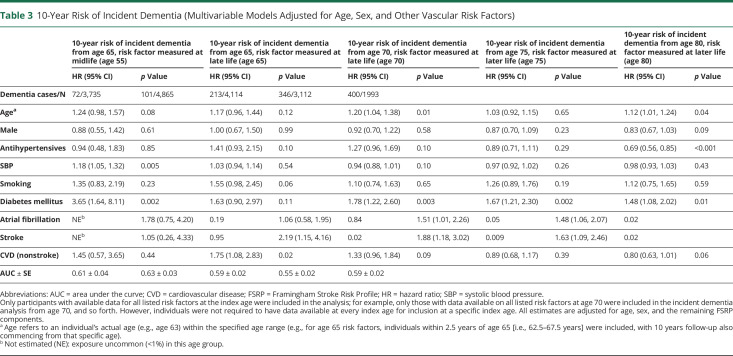
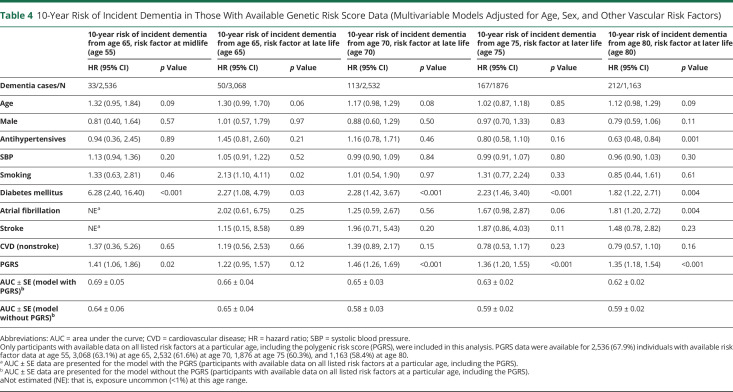
Following adjustment for age and sex, midlife FSRP score (HR 1.16, 95% CI 1.06–1.26; p < 0.001, per SDU increment in log-transformed FSRP score), SBP (HR 1.12, 95% CI 1.02–1.24; p = 0.02, per 10 mm Hg increment), and prevalent DM (HR 4.31, 95% CI 1.97–9.43; p < 0.001) were associated with an increased 10-year risk of incident dementia from age 65 (Table 2). A multivariable Cox model incorporating all midlife risk factors predicted 10-year incident dementia risk from age 65 with an AUC ± SE of 0.61 ± 0.04 (Table 3). Addition of a PGRS marginally improved model discriminative performance (AUC increased from 0.64 ± 0.06 to 0.69 ± 0.05 in those with available genetic risk score data) (Table 4).
Table 2.
Primary Analysis: 10-Year Risk of Incident Dementia According to Vascular Risk Factor Profile at Mid, Late, and Later Life
Table 3.
10-Year Risk of Incident Dementia (Multivariable Models Adjusted for Age, Sex, and Other Vascular Risk Factors)
Table 4.
10-Year Risk of Incident Dementia in Those With Available Genetic Risk Score Data (Multivariable Models Adjusted for Age, Sex, and Other Vascular Risk Factors)
Late Life (Ages 65 and 70) Risk Factors
A higher FSRP score at age 65 (but not 70) (HR 1.06, 95% CI 1.02–1.10; p = 0.004, per SDU increment in log-transformed FSRP score) and antihypertensive medication use at ages 65 (HR 1.66, 95% CI 1.12–2.46; p = 0.01) and 70 (HR 1.53, 95% CI 1.18–1.97; p = 0.001) were associated with an increased 10-year incident dementia risk. A history of DM at ages 65 (HR 1.96, 95% CI 1.09–3.52; p = 0.02) and 70 (HR 2.10, 95% CI 1.48–2.99; p < 0.001), AF at ages 65 (HR 2.30, 95% CI 1.00–5.27; p = 0.05) and 70 (HR 1.86, 95% CI 1.13–3.05; p = 0.02), nsCVD at ages 65 (HR 1.95, 95% CI 1.24–3.07; p = 0.004) and 70 (HR 1.63, 95% CI 1.22–2.17; p < 0.001) and stroke at age 70 only (HR 3.61, 95% CI 2.21–5.92; p < 0.001), were each associated with an increased 10-year risk of incident dementia (Table 2). A multivariable Cox model incorporating all 9 risk factors predicted 10-year risk of incident dementia from ages 65 and 70 years with AUCs of 0.63 ± 0.03 and 0.59 ± 0.02, respectively (Table 3). Addition of the PGRS improved model discriminative performance at age 70 (AUC increased from 0.58 ± 0.03–0.65 ± 0.03), with negligible change at age 65 (the mean age of the population in whom the genetic risk score was developed was 70) (Table 4).
Later Life (Ages 75 and 80) Risk Factors
In later life, FSRP score, SBP, and prevalent nsCVD were no longer associated with 10-year incident dementia risk. Antihypertensive use at age 80 was associated with a reduced 10-year risk of incident dementia (HR 0.74, 95% CI 0.62–0.89; p = 0.001). A history of DM at ages 75 (HR 1.64, 95% CI 1.20–2.23; p = 0.002) and 80 (HR 1.40, 95% CI 1.04–1.89; p = 0.03), AF at ages 75 (HR 1.57, 95% CI 1.09–2.26; p = 0.02) and 80 (HR 1.43, 95% CI 1.07–1.92; p = 0.02), and stroke at ages 75 (HR 2.05, 95% CI 1.34–3.12; p < 0.001) and 80 (HR 1.63, 95% CI 1.13–2.35; p = 0.009) were each associated with an increased 10-year incident dementia risk (Table 2). In sensitivity analyses with FDR correction and accounting for education status, results were not materially altered (eTable 1 and eTable 2, links.lww.com/WNL/B986).
Vascular Risk Factors for Inclusion in Age-Specific Dementia Risk Scores
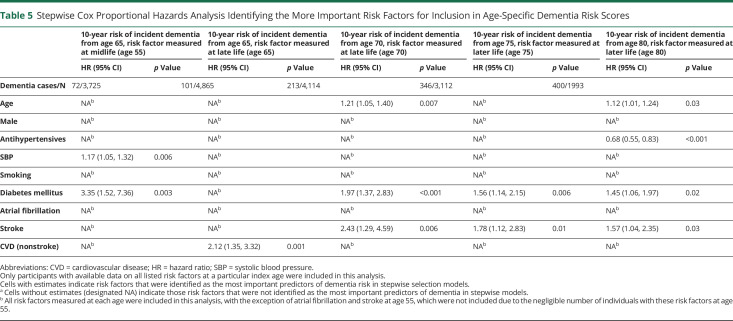
In stepwise Cox models, elevated SBP (HR 1.17, 95% CI 1.05–1.32; p = 0.006) and DM (HR 3.35, 95% CI 1.52–7.36; p = 0.003) were identified as the 2 most important midlife vascular predictors of 10-year incident dementia risk. At age 65, nsCVD was the most important predictor (HR 2.12, 95% CI 1.35–3.32; p = 0.001); at ages 70 and 75, DM and stroke were the 2 most important predictors; at age 80, DM (HR 1.45, 95% CI 1.06–1.97; p = 0.02), stroke (HR 1.57, 95% CI 1.04–2.35; p = 0.03), and antihypertensive use (HR 0.68, 95% CI 0.55–0.83; p < 0.001) were the most important vascular predictors of 10-year incident dementia risk (Figure 2 and Table 5).
Figure 2. Most Important Vascular Risk Factors for Dementia Across Mid- to Later Life.
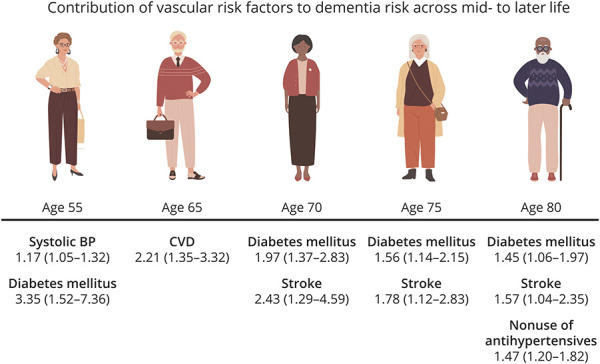
BP = blood pressure; CVD = cardiovascular disease.
Table 5.
Stepwise Cox Proportional Hazards Analysis Identifying the More Important Risk Factors for Inclusion in Age-Specific Dementia Risk Scores
Exploratory Analysis: Vascular Risk Factors Stratified by Sex
At age 65, current smoking (HR 2.28, 95% CI 1.29–4.04; p = 0.005), use of antihypertensives (HR 1.82, 95% CI 1.04–3.18; p = 0.04), and prevalent DM (HR 2.90, 95% CI 1.39–6.03; p = 0.004) were associated with incident dementia risk in women but not in men. No significant risk factors were identified in men at either ages 65 or 70. At age 70, DM (HR 2.32, 95% CI 1.41–3.82; p = 0.001), and at age 75, DM (HR 1.86 95% CI 1.23–2.83; p = 0.004) and stroke (HR 2.08, 95% CI 1.12–3.86; p = 0.02) were associated with 10-year risk of incident dementia in women; AF at age 75 was the only significant risk factor identified in men (HR 1.91, 95% CI 1.15–3.18; p = 0.01). At age 80, prevalent DM (HR 1.51, 95% CI 1.02–2.24; p = 0.04), AF (HR 1.67, 95% CI 1.08–2.58; p = 0.02), and use of antihypertensives (HR 0.64, 95% CI 0.50–0.82; p < 0.001) were associated with risk of dementia in women only; SBP (HR 0.90, 95% CI 0.82–0.99; p = 0.04), stroke (HR 1.89, 95% CI 1.04–3.44; p = 0.04), and nsCVD (HR 0.65, 95% CI 0.43–0.96; p = 0.03) were significant in men only (eTable 3, links.lww.com/WNL/B986).
Discussion
In the current investigation, midlife FSRP score, SBP, and DM were each associated with an increased 10-year incident dementia risk from age 65. In late life, FSRP score (age 65 only), DM, AF, nsCVD, stroke (age 70 only), and antihypertensive use, and in later life, DM, AF, stroke, and nonuse of antihypertensives (age 80 only), were each associated with increased 10-year incident dementia risk. The most important risk factors identified for inclusion in age-specific dementia risk scores were, at age 55, SBP and DM; at age 65, nsCVD; at ages 70 and 75, DM and stroke; and at age 80, DM, stroke and use of antihypertensives (protective).
For every SD unit increase in age 55 FSRP score (a metric of cumulative vascular risk), there was an approximate 1.2-fold increase in subsequent 10-year dementia risk from age 65. Our findings highlight the detrimental effects of a high midlife vascular risk factor burden on a person's future risk of developing dementia commencing from the average age of retirement (65 years). A recent study reported a greater white matter hyperintensity burden on MRI brain in those with higher vascular risk scores in early adulthood compared with in later life.17
DM was the strongest midlife vascular predictor of 10-year incident dementia risk, associated with a more than 4-fold increase in risk. DM in late and later life remained important for future dementia risk, with a 2-fold increased risk of dementia from ages 65 and 70, and a 1.5-fold increased risk from ages 75 and 80. The decreasing magnitude of association with increasing age may also reflect selective attrition of those with chronic, severe DM from our sample at younger ages (i.e., competing risk of mortality). Our results are consistent with other studies18 that suggest that presence of DM at an earlier, compared with later, age may be a key factor in the strength of its association with dementia. A recent study reported progressively increasing dementia risk for every 5-year reduction in age at onset of DM.19 A longer duration of disease likely predisposes to greater end-organ damage (e.g., stroke, nsCVD, chronic renal insufficiency, and AF) and subsequently increased dementia risk. In our stepwise models, DM was consistently identified as a key risk factor for inclusion in age-specific dementia risk scores across midlife into later life. DM has been identified as 1 of 7 risk factors responsible for up to one-third of cases of Alzheimer disease dementia20 and represents an important modifiable target for dementia prevention at a population level.
In our study, elevated midlife SBP was associated with an increased 10-year dementia risk from age 65, and was identified as 1 of 2 important risk factors for inclusion in midlife dementia risk scores. Previous studies have reported similar associations between elevated SBP at midlife and late life and increased dementia risk.6,21,22 Later-life antihypertensive use was associated with a reduced 10-year dementia risk in our sample, consistent with findings from a recent meta-analysis of antihypertensive trials.23 Antihypertensive therapy may exert protective effects on cognition in older patients by reducing the risk of large and small vessel disease.24 Alternatively, the beneficial effect of antihypertensive therapy in this age group may be due to confounding by indication, given that lower blood pressures in later life (≥75 years) have been associated with increased dementia risk25,26 and those prescribed antihypertensives at this age presumably have more robust (elevated) blood pressures, which may be protective for dementia.
With increasing age, the end-organ sequelae of cumulative vascular risk, namely stroke, AF, and nsCVD, became increasingly important predictors of future dementia risk. Stroke at age 70 was associated with a >3.5-fold increased 10-year risk of dementia, with the association persisting into later life. A history of stroke was identified as a key risk factor for inclusion in dementia risk scores specific to individuals aged 70–80.
Diagnosis of AF at ages 65 and older was associated with an increased 10-year incident dementia risk, with earlier, rather than later, onset of AF showing stronger associations. Our findings highlight the potential for modifying future dementia risk through early detection and treatment of AF. In late life, nsCVD was associated with an increased 10-year incident dementia risk and was identified as the most important risk factor for inclusion in dementia risk scores specific to age 65. We found no association between nsCVD at age 75 and incident dementia, and a trend towards a reduced risk of dementia with nsCVD at age 80, likely due to selective attrition of those with more severe nsCVD from the sample at younger ages (i.e., effect of nsCVD on dementia risk likely occurs early on).
We identified some notable differences in the association of vascular risk factors with dementia between women and men. At age 65, smoking, use of antihypertensives, and prevalent DM were associated with incident dementia risk in women, but not in men. At age 75, DM and prevalent stroke were associated with incident dementia risk in women; only AF was significantly associated with dementia risk in men. At age 80, DM, AF, and antihypertensive use (protective) were associated with risk of dementia in women; SBP (protective), stroke, and nsCVD (protective) were significant in men. These observed differences highlight a potential need for sex-specific as well as age-specific dementia risk scores. However, given the small sample sizes in our sex-stratified analyses, we caution that these results are purely exploratory in nature and require further investigation in larger cohorts.
Our study has a number of limitations. Our sample was predominantly White, limiting generalizability of our findings to other ethnicities. We included dementia-free survivors at 5-yearly intervals in our analyses; however, individuals with more severe vascular risk factor profiles between examinations may have died prior to a diagnosis of dementia, underestimating the strength of association between vascular risk factors and dementia. For some variables, data were missing in a substantial proportion of participants; for example, PGRS data were available for only 68% of individuals at age 55 and 58% at age 80. Our analysis did not include neuroimaging markers of dementia pathology, such as white matter hyperintensity volume, free water, or hippocampal or total brain volume. We also did not include intermediary markers of vascular disease, such as carotid intima–media thickness, left ventricular hypertrophy, or renal function. However, our aim was to identify important vascular risk factors for inclusion in age-specific dementia risk scores that can be easily measured in routine clinical practice and do not require additional investigations or neuroimaging data. Dementia was diagnosed using clinical rather than biomarker-based (CSF or PET β-amyloid and tau) research criteria27 and we did not have sufficient power to explore dementia subtypes in our study. Notable strengths include our sample of clinically confirmed dementia-free individuals at baseline for each index age, meticulous surveillance procedures for incident dementia, in-depth phenotyping and measurement of vascular risk factors across multiple lifespan points, and our relatively long duration of follow-up spanning from midlife into later life. FDR correction of our primary analysis did not materially alter our findings, with the exception of AF at age 65 and DM at age 80, which were no longer significant, likely due to sample size limitations.
Dementia is a heterogeneous disease and risk prediction scores for dementia need to be tailored towards the individual, taking age, sex, vascular risk burden, and end-organ damage into account. Our findings support the use of age-specific dementia risk scores in preference to a one-size-fits-all approach to dementia risk prediction. We suggest that age-specific dementia risk scores should prioritize including, at age 55, SBP and DM; at age 65, nsCVD; at ages 70 and 75, DM and stroke; and at age 80, DM, stroke, and use of antihypertensives. However, we acknowledge the utility of a single, broadly generalizable dementia risk prediction score at a population level, for example, in selecting participants for enrollment in large, simple trials for dementia prevention,28 in informing public health policy, and in developing population-level interventions for optimizing and preserving brain health.
Acknowledgment
The authors thank the Framingham Heart Study participants.
Glossary
- AF
atrial fibrillation
- AUC
area under the curve
- CVD
cardiovascular disease
- DM
diabetes mellitus
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders, 4th edition
- FDR
false discovery rate
- FHS
Framingham Heart Study
- FSRP
Framingham Stroke Risk Profile
- HR
hazard ratio
- MMSE
Mini-Mental State Examination
- nsCVD
nonstroke cardiovascular disease
- PGRS
polygenic risk score
- SBP
systolic blood pressure
Appendix. Authors
Footnotes
CME Course: NPub.org/cmelist
Study Funding
This research was supported by the Health Research Board of Ireland (CSF-2020-011) and the Alzheimer's Association (AACSF-18-566570). The Framingham Heart Study is supported by the National Heart, Lung, and Blood Institute (NHLBI) (contracts N01-HC-25195, HHSN268201500001, and 75N92019D00031-0-75). This research was also supported by NHLBI grants R01 HL60040 and R01 HL70100, grants from the National Institute on Aging (R01 AG054076, R01 AG049607, R01 AG033193, R01 AG059421, R01 AG066524, U01 AG049505, U01 AG052409), and grants from the National Institute of Neurologic Disorders and Stroke (R01 NS017950 and UH2 NS100605). None of the funding entities had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication. The authors were independent from all funding agencies. All authors had full access to all of the data (including statistical reports and tables) in the study and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Disclosures
The authors report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. Lancet Neurol. 2006;5:735-741. [DOI] [PubMed] [Google Scholar]
- 2.Barnes DE, Covinsky KE, Whitmer RA, Kuller LH, Lopez OL, Yaffe K. Predicting risk of dementia in older adults: the late-life dementia risk index. Neurology. 2009;73:173-179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barnes DE, Beiser AS, Lee A, et al. Development and validation of a brief dementia screening indicator for primary care. Alzheimers Dement. 2014;10:656-665.e651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:2672-2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Floud S, Simpson RF, Balkwill A, et al. Body mass index, diet, physical inactivity, and the incidence of dementia in 1 million UK women. Neurology. 2020;94:e123–e132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology. 2017;89:2447-2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dufouil C, Beiser A, McLure LA, et al. Revised Framingham stroke risk profile to reflect temporal trends. Circulation. 2017;135:1145-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Seshadri S, Wolf P, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function the Framingham Offspring Study. Neurology. 2004;63:1591-1599. [DOI] [PubMed] [Google Scholar]
- 9.Kaffashian S, Dugravot A, Elbaz A, et al. Predicting cognitive decline: a dementia risk score vs. the Framingham vascular risk scores. Neurology. 2013;80:1300-1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Elias MF, Sullivan LM, D'Agostino RB, et al. Framingham stroke risk profile and lowered cognitive performance. Stroke. 2004;35:404-409. [DOI] [PubMed] [Google Scholar]
- 11.Feinleib M, Kannel WB, Garrison RJ, McNamara PM, Castelli WP. The Framingham Offspring Study: design and preliminary data. Prev Med. 1975;4:518-525. [DOI] [PubMed] [Google Scholar]
- 12.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders; 1994. [Google Scholar]
- 13.Seshadri S, Wolf P, Beiser A, et al. Lifetime risk of dementia and Alzheimer's disease the impact of mortality on risk estimates in the Framingham Study. Neurology. 1997;49:1498-1504. [DOI] [PubMed] [Google Scholar]
- 14.Satizabal C, Beiser AS, Seshadri S. Incidence of dementia over three decades in the Framingham heart study. N Engl J Med. 2016;375:93-94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Au R, Seshadri S, Knox K, et al. The Framingham brain donation program: neuropathology along the cognitive continuum. Curr Alzheimer Res. 2012;9:673-686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kunkle BW, Grenier-Boley B, Sims R, et al. Genetic meta-analysis of diagnosed Alzheimer's disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat Genet. 2019;51:414-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lane CA, Barnes J, Nicholas JM, et al. Associations between vascular risk across adulthood and brain pathology in late life: evidence from a British birth cohort. JAMA Neurol. 2019:1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitmer RA, Sidney S, Selby J, Johnston SC, Yaffe K. Midlife cardiovascular risk factors and risk of dementia in late life. Neurology 2005;64:277-281. [DOI] [PubMed] [Google Scholar]
- 19.Barbiellini Amidei C, Fayosse A, Dumurgier J, et al. Association between age at diabetes onset and subsequent risk of dementia. JAMA. 2021;325:1640-1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol. 2014;13:788-794. [DOI] [PubMed] [Google Scholar]
- 21.Walker KA, Sharrett AR, Wu A, et al. Association of midlife to late-life blood pressure patterns with incident dementia. JAMA. 2019;322:535-545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39:3119-3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hughes D, Judge C, Murphy R, et al. Association of blood pressure lowering with incident dementia or cognitive impairment: a systematic review and meta-analysis. JAMA. 2020;323:1934-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White WB, Wakefield DB, Moscufo N, et al. Effects of intensive versus standard ambulatory blood pressure control on cerebrovascular outcomes in older people (INFINITY). Circulation. 2019;140:1626-1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ. Low blood pressure and the risk of dementia in very old individuals. Neurology. 2003;61:1667-1672. [DOI] [PubMed] [Google Scholar]
- 26.Qiu C, von Strauss E, Winblad B, Fratiglioni L. Decline in blood pressure over time and risk of dementia: a longitudinal study from the Kungsholmen project. Stroke. 2004;35:1810-1815. [DOI] [PubMed] [Google Scholar]
- 27.Jack CR Jr., Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimer's Dement. 2018;14:535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whiteley WN, Anand S, Bangdiwala SI, et al. Are large simple trials for dementia prevention possible? Age Ageing. 2020;49:154-160. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The de-identified data used in these analyses can be obtained from the NHLBI database and the NCBI dbGaP.