Abstract
Based on available evidence, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is a neuroinvasive virus. According to the centers for disease control and prevention (CDC), coronavirus disease 2019 (COVID-19) may cause epilepsy. In this line, COVID-19 can stimulate hypoxia-inducible factor-1 alpha (HIF-1α) and activate P2X7 receptor. Both HIF-1α and P2X7 receptors are linked to epileptogenesis and seizures. Therefore, in the current study, we suggested that COVID-19 may have a role in epileptogenesis and seizure through HIF-1α stimulation and P2X7 receptor activation. Consequently, pharmacological targeting of these factors could be a promising therapeutic approach for such patients.
Keywords: Epilepsy, Epileptogenesis, Seizure, COVID-19, SARS-CoV-2, HIF‐1α, P2X7 receptor
Introduction
Coronavirus Disease 2019 (COVID-19) is caused by infection with the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and affects billions of people around the world [1–7]. SARS-CoV-2 has been increasingly reported to attack not only the respiratory system and lead to respiratory complications [8] but also the central nervous system (CNS), causing neurological symptoms [9–12], and progression of multiple cancers [13, 14]. According to retrospective investigations, 36.4% of SARS-CoV-2-infected individuals presented neurological manifestations such as acute cerebrovascular diseases, disturbed consciousness, and paresthesia [15].
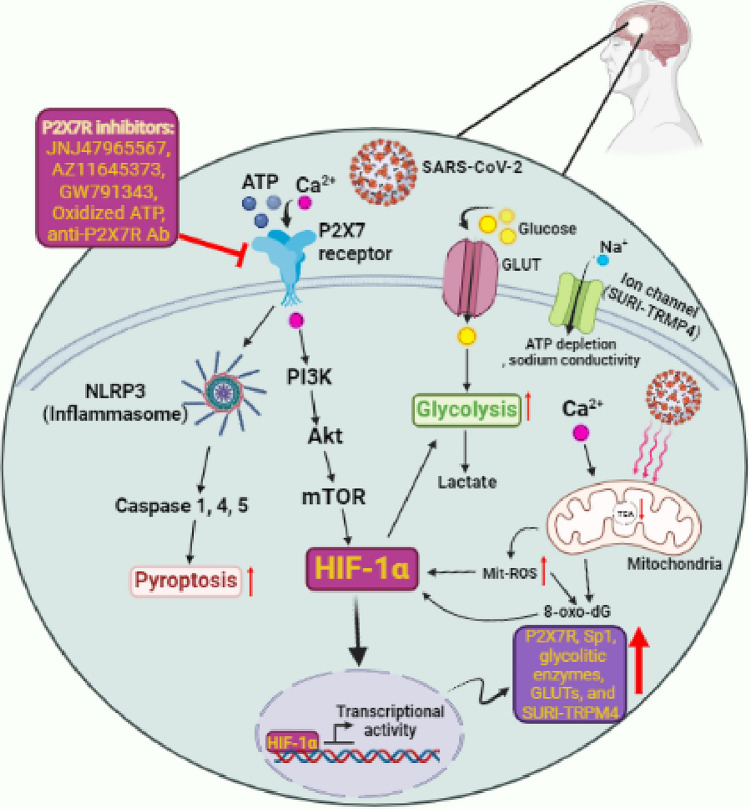
Epilepsy is one of the most common chronic neurological conditions, characterized by the spontaneous recurrence of unprovoked seizures. Approximately 0.7–1.0% of the population is affected, with the incidence being highest among elderly people and children [16]. Epilepsy can be triggered by a variety of reasons, including posttraumatic epilepsy caused by a traumatic brain injury (TBI) [17], various infections [18], and hereditary factors [19]. According to the centers for disease control and prevention (CDC), SARS-CoV-2 is one of the viruses that might induce epilepsy or worsen the condition in epileptic people [20]. In this study, we suggested that there is possibly an association between hypoxia-inducible factor-1 alpha (HIF-1α) stimulation and P2X7 receptor hyper-activation by COVID-19 and epilepsy progression that can provide new insight into targeting these factors for the treatment of epileptic patients (Fig. 2).
Fig. 2.
The potential role of SARS-CoV-2 in the progression of epilepsy by hyper-activating the P2X7 receptor. In addition to NLRP3 activation contributing to severe inflammation response and pyroptosis, SARS-CoV-2 mediated hyper-activation of P2X7 receptor leads to ectopic stimulation of HIF-1α and its down-stream targets mainly glycolytic enzymes that facilitate the virus replication and subsequently, more epilepsy complications. On the other hand, during P2X7 receptor hyperactivation, released storm Ca2 + leads to mit-ROS which itself contributes to more stimulation of HIF-1α and an increase in expression of P2X7R, Sp1, glycolytic enzymes, GLUTs, and SURI-TRPM4. P2X7R inhibitors can potentially be used to inhibit P2X7R hyperactivation in epileptic patients with COVID-19 to block these processes
Association between COVID-19 and epilepsy
An acute symptomatic seizure may result from a poor health condition, mainly a fever, caused by an infection. As one of the major concerns for neurologists and emergency physicians, infection with COVID-19 may also result in such a complication. Although various studies have looked at the incidence of acute symptomatic seizures induced by COVID-19, more comprehensive research is necessary considering the multiple pathological impacts of COVID-19 on the disease severity and other factors. The incidence of acute symptomatic seizures caused by COVID-19 has been reported to be less than 1% [21–23]. In addition, SARS and Middle East respiratory syndrome (MERS) has previously been associated with seizure rates of 2.7% and 8.6%, respectively [24, 25]. While acute seizures are symptomatic, epilepsy is a chronic condition characterized by recurrent seizures. Recently, it has been suggested that the risk of increased seizure frequency is higher in patients with tumor-related, drug-resistant epilepsy, insomnia, and financial troubles [26].
In a healthy young man without any epileptic seizures, seizures with lymphocytosis during SARS-CoV-2 infection have been observed [27]. In the mornings, a patient without altered consciousness presented to Klinikum Altmühlfranken Weißenburg Hospital, Germany, with painful muscle spasms in the left upper and lower limbs. A full physical exam, radiological imaging, electroencephalography, lumbar puncture, and autoimmune profile are either normal or inconsistent with the patient's symptoms. The patient's follow-up revealed fever and severe cough on day 4 and a diagnosis of focal epilepsy [28]. In another case, an immunocompromised woman in her 78 s experienced seizure-like symptoms during infection with COVID-19. Her cerebrospinal fluid (CSF) showed inflammation through increased cytokines such as interleukin-6 (IL-6), interleukin-8 (IL-8), and interferon-gamma-induced protein-10 (IP-10) without any indication of a viral infection [29]. Moreover, other studies revealed that many COVID-19 patients had epileptiform discharges or seizures in their electroencephalograms (EEGs) [22, 30, 31]. COVID-19-positive patients had nearly 35% higher new onset encephalopathy than the COVID-19-negative patients [31]. In individuals with COVID-19, seizures may occur as a result of hypoxia, metabolic disturbances, organ failure, medications, or brain damage [32].
Possible link between COVID-19 hyper-activated P2X7 receptor and epilepsy
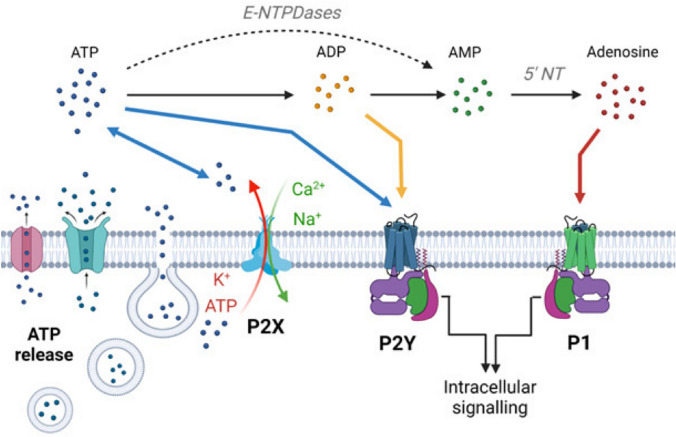
The purinergic receptors are divided into adenosine-sensitive P1 receptors (A1, A2A, A2B, A3) which are activated by extracellular adenosine, and adenine-receptor-like P2 receptors (P2X and P2Y) which are activated by extracellular adenine and uridine nucleotides (e.g., ATP). There are seven mammalian P2X receptor subtypes (P2X1 to P2X7), which all respond to ATP. In neurons and glial cells, including microglia and astrocytes, purines such as ATP and adenosine are released actively or passively through exocytotic and non-exocytotic mechanisms. During the exocytotic mechanism, nucleotides must be stored in secretory/synaptic vesicles via the vesicular nucleotide transporter (VNUT), while different types of channels, such as pannexins and connexin, can release nucleotides through the non-exocytotic mechanism. Unlike ATP, adenosine can also be released into the extracellular space via two different processes: Concentrative Nucleoside Transporters (CNTs) and Equilibrative Nucleoside Transporters (ENTS). The released nucleotides act on the P2X (ligand-gated) and P2Y (G protein-coupled) receptors located on neuronal or glial membranes and activate them. In turn, adenosine generated by the ectonucleotidases, such as NTPDases, NPPases, and alkaline, phosphatase activate P1 (G protein-coupled) receptors as a result of the nucleotide hydrolysis (Fig. 1) [33–36].
Fig. 1.
The illustration shows the purinergic system and related signaling overview: from ATP release mechanisms to ATP receptors. (A) Neurons and glia release ATP via transporters, membrane channels, and exocytosis. P2X7 channels (P2X7R) can also release ATP. Once released, ATP will be converted into adenosine through the intermediates ADP and AMP via ectoenzymes including alkaline phosphatase, NPPases, and NTPDases. Extracellular ATP receptors are P2X (ligand-gated) and P2Y (G protein-coupled) receptors. ADP receptors are subtypes of P2Y receptors. Adenosine can activate P1 (G protein-coupled) receptors [35]
In the CNS, P2X7 receptors, as ATP-gated ion channels, are also activated by viral infections and lead to molecular (mainly activation of the neuroimmune response, formation of reactive oxygen species (ROS), and glutamate release) behavioral and mental disorders. In 2002, Vianna et al. explored the expression of P2X7 receptors during epilepsy using pilocarpine-induced chronic epileptic rats. They found that the expression of P2X7 receptors was elevated in the hippocampus, specifically in mossy fibers and the dentate gyrus in chronic epileptic rats [37]. Furthermore, a subsequent rodent study indicated that immuno-reactivity of the P2X7 receptor and ATP responsiveness in microglia were enhanced after status epilepticus [38]. When compared to age-matched controls, samples with hypoxic/ischemic encephalopathy (HIE) or seizures had higher transcript levels of the P2X7 receptors [39]. According to Dona et al. study, a higher level of P2X7 receptor immunoreactivity was found in epileptic rats during acute and chronic phases of the condition [40]. Intracerebroventricular injection of P2X7 receptor agonists increased the severity of seizures during status epilepticus triggered by intra-amygdala kainic acid in mice [41]. Therefore, activation of P2X7 receptors may aggravate seizures. Accordingly, in response to P2X7 receptor antagonists, the expression of interleukin (IL)-1β and damage to the hippocampus following seizures were also reduced. Moreover, pre-or early post-treatment of mice with P2X7 receptor antagonists significantly decreased the severity of seizures [42]. It has been revealed that inhibition of the P2X7 receptor by the antagonist A-438079 prevents seizures and neocortical damage [43]. Other P2X7 receptor inhibitors have been suggested for therapeutic approaches such as JNJ47965567, AZ11645373, GW791343, oxidized ATP, and anti-P2X7 receptor monoclonal antibodies (mAb) (Fig. 2) [44–48].
COVID-19 patients develop an inflammatory condition caused by a cytokine storm syndrome [49]. The inflammatory processes are closely associated with hyper-activation of P2X7 receptors, which are stimulated by released ATP from distressed cells and in turn lead to activation of inflammasomes [50, 51]. Following the studies that have indicated the potent effects of P2X7 receptors-targeted drugs on the modulation of seizures, researchers have become increasingly interested in the role of P2X7 receptors in the pathophysiology of epilepsy [42]. As a result of recent studies, it has been hypothesized that neuroinvasion through the BBB and stimulation of neuro-inflammatory responses observed during COVID-19 infection can be mediated by P2X7 receptors hyper-activation that leads to stimulating NLR family pyrin domain containing 3 (NLRP3) (NACHT, LRR, and PYD domain-containing protein 3) inflammasome and subsequently, cause the release of several proinflammatory cytokines such as IL-1β, IL-18, IL-1α, IL-36α [43, 49, 52]. It has been also thought that neurodegenerative diseases and psychiatric disorders caused by the COVID-19 virus are possible consequences of this cascade [50]. Moreover, the P2X7/NLRP3 axis is involved in pyroptosis (osmotic lysis and release of proinflammatory content), which is a type of cell death characterized by caspase activation such as caspase-1 and caspase-11 in mice as well as caspase-1, caspase-4, and caspase-5 in humans [53]. In addition, P2X7 receptor stimulation promotes the release of other cytokines and chemokines, including IL-6, tumor necrosis factor-alpha (TNF-α), IL-8, chemokine (C–C motif) ligand (CCL) 2, CCL3, and CXCL2 as well as pro-fibrotic factors such as TGF-β, and extracellular matrix remodeling factors, such as metalloproteinase-9 and tissue inhibitor of metalloproteinase (TIMP)-1 [54, 55]. In this line, according to the evidence presented and discussed now, infection with SARS-CoV-2 may result in an immense release of ATP, the earliest and most ubiquitous damage-associated molecular pattern (DAMP) released at all inflammatory sites, in the cellular microenvironment that is high enough to activate the P2X7 receptor [53] (Fig. 2). Therefore, using data derived from clinical observations related to patients with COVID-19 and other human beta-coronavirus infections, we suggest a possible role of the P2X7 receptor/NLRP3 inflammasome pathway of SARS-CoV-2 infection in the immunopathogenesis of epilepsy and seizures.
Possible link between HIF-1α stimulated by COVID-19 and epilepsy
Seizure is a non-linear process, with slow accumulation and an immediate release process of energy flux, as when earthquakes occur [56]. Ion channels open during seizures, causing an unequal balance between inhibitory and stimulatory neurotransmitters, which in turn increases energy consumption and neuronal excitability. According to functional Magnetic resonance imaging (MRI), glucose metabolism and blood flow increase, as does oxygen consumption, while the levels of deoxyhemoglobin and blood oxygen decrease [57, 58]. The evidence indicates that seizures increase energy consumption and the energy supply is limited in epileptic seizures. To compensate for this shortfall, the body increases ATP synthesis via glycolysis and aerobic metabolism (Krebs cycle). In seizures, the brain is forced into a relatively hypoxic environment, resulting in a decline in aerobic metabolism. So far, there is evidence that the activity of the main enzymes in the tricarboxylic acid cycle (TCA cycle) decreases with epileptic seizures [59, 60] as well as mitochondrial oxidative stress, resulting in electron transport chain (respiratory chain) dysfunction and reduced ATP production, as a barrier in the supply of energy to the brain [61]. Furthermore, earlier research has shown that during epileptic seizures, the CNS's energy consumption increases, while impediments to aerobic metabolism reduce the CNS's energy supply [59–61]. HIF-1α plays a key role in the cellular responses to hypoxic conditions [62, 63] (Fig. 2). HIF-1α regulates a variety of physiological processes, including metabolism, angiogenesis, and cell proliferation [64–66]. HIF-1α was recently observed to be increased in the hippocampus of patients with temporal lobe epilepsy as well as in animal models [67, 68], showing that HIF-1α plays a crucial role in changing hippocampal structure during epilepsy. Furthermore, it revealed that HIF-1α promotes apoptosis of hippocampal neurons and expression of TNF-α during epilepsy [69, 70], as well as apoptosis in other cells [62]. Jiang et al. found that mRNA and protein levels of HIF-1α in epileptic brain tissues were significantly greater than in control subjects [71].
Besides that, to replicate and spread quickly and efficiently, viruses alter the metabolism of host cells. A good example would be the enhanced uptake of nutrients such as glucose in order to maintain metabolic signaling, namely aerobic glycolysis, which is the primary metabolic pathway for glucose and its byproducts for biosynthesis [42]. Krishnan et al. determined that glycolysis is crucial for the replication of the virus, and interfering with these metabolic pathways led to a substantial decrease in virus proliferation. Accordingly, they hypothesized that SARS-CoV-2 results in toxic metabolite efflux and plays a role in disease severity by utilizing and rewiring pathways governing central carbon metabolism. Their recent studies have also shown that SARS-CoV-2, like hypoxic condition, affects phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) signaling as well as HIF-1α signaling in infected cells, resulting in up-regulation of glucose transporters (GLUTs), glycolysis enzymes, and subsequently glycolysis hyper-activation [72].
According to recent studies, human cell lines infected by SARS-CoV-2 express high levels of HIF-1α and inflammatory cytokines [73]. So, COVID-19 pathogenesis may be influenced by HIF-1α as it is also involved in glycolysis and the inflammatory response [74, 75]. Recently, it has been suggested that the ORF3a protein of SARS-CoV-2 can stimulate HIF-1α production by damaging mitochondria and increasing mitochondrial reactive oxygen species (Mito-ROS). Consequently, HIF-1α promotes viral infection/replication and aggravation of inflammatory responses [73, 76]. In ROS production, Fenton and Huber-Weiss reactions play a crucial role. When ferrous iron (Fe2+) reacts with hydrogen peroxide (H2O2), Fenton's reaction causes the formation of ferric iron (Fe3+) and hydroxyl radicals. In high concentrations, O2 and H2O2 can trigger the Haber–Weiss reaction to produce highly reactive species such as hydroxyl radicals, which have a great affinity for guanine in DNA and the nucleotide pool, resulting in the formation of 8-oxo-dG [77]. In both chronic and acute epilepsy models, it has been demonstrated that the level of 8-hydroxy-2 deoxyguanosine (8-oxo-dG) increases when seizures occur [78]. On the other hand, SUR1-TRPM4 channels are upregulated in response to HIF-1α activation. This channel has also been shown to be upregulated during acute status epilepticus and may contribute to seizures by increasing sodium conductivity [79] (Fig. 2). There is an Abcc8 promoter region where Sp1, a member of a damage-activated transcription factor family, might bind and potentially upregulate this ion channel. The Sp1 can also be regulated by HIF-1α since the promoter region of Sp1 contains an HRE region [80].
In addition, in a hypoxia-independent manner, P2X7-mediated upregulation of HIF-1α and ischemic tolerance was reported after ischemic insult in astrocytes, so P2X7 modulation reduced HIF-1α [81]. In fact, activated P2X7 receptors upregulate HIF-1a via activation of PI3K/Akt/mTOR signaling [46, 82–85]. On the other hand, it has also indicated that P2X7 receptor-dependent HIF-1α upregulation has a positive effect on the expression of P2X7 receptors in the hypoxic microenvironment as a cyclic pathway [46, 86–88]. Moreover, Over-produced Sp1 mediated by HIF-1α binds to the CG-rich binding site of the P2X7R promoter in neuronal cell lines and has been related to epileptic crises [89]. So, we hypothesize that undesirable conditions, such as metabolism reprogramming, mitochondrial damaging/dysfunction, and increasing sodium conductivity, occurring in epilepsy are governed by hyper-activation of P2X7 receptors and their downstream factors, HIF-1α, that could be severed by SARS-CoV-2.
Conclusion and future directions
The evidence regarding the interactions between COVID-19 and epilepsy needs to be kept up to date daily by clinicians. Also, further investigation is needed into the molecular signaling pathways of COVID-19 on epileptogenesis. Glycolysis induced by HIF-1α up-regulation plays a critical role in epileptogenesis and virus replication. Hence, metabolic disruption of these processes can hinder SARS-CoV-2 replication and epileptogenesis/seizures associated with COVID-19. In addition, it is known that P2X7 receptor hyper-activation is associated with an increase in the severity of epilepsy and seizures. Everything considered we hypothesized that there might be a link between P2X7 receptor hyper-activation following SARS-CoV-2 infection and the occurrence of epilepsy/seizures. We also propose that the antagonists of P2X7 receptors might be considered as a promising strategy for the prevention or treatment of neurological complications in COVID-19 patients suffering from epilepsy or seizures. However, further investigations are required in order to identify the role of stimulated HIF-1α and hyper-activated P2X7 receptors in the neuropathies of patients with epilepsy during or following COVID-19 infection.
Acknowledgements
Not applicable.
Author contributions
HZ conceived and designed the study. HZ, AA, MNA, RF, ZB, FI, and MH searched and wrote the manuscript text. HZ and AA created the figures. HZ, MNA, CT, and SRM revised the manuscript. MRF and HZ supervised the study. All authors read and approved the final manuscript.
Funding
There is no fondation for this study.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Hamidreza Zalpoor, Email: hamidreza.zlpr1998@gmail.com.
Majid Reza Farrokhi, Email: farrokhimr@yahoo.com.
References
- 1.Khomari F, Nabi-Afjadi M, Yarahmadi S, Eskandari H, Bahreini E. Effects of cell proteostasis network on the survival of SARS-CoV-2. Biological Proced Online. 2021;23(1):1–10. doi: 10.1186/s12575-021-00145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khesht AMS, Karpisheh V, Saeed BQ, Zekiy AO, Yapanto LM, Afjadi MN, et al. Different T cell related immunological profiles in COVID-19 patients compared to healthy controls. Int Immunopharmacol. 2021;97:107828. doi: 10.1016/j.intimp.2021.107828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nabi-Afjadi M, Karami H, Goudarzi K, Alipourfard I, Bahreini E. The effect of vitamin D, magnesium and zinc supplements on interferon signaling pathways and their relationship to control SARS-CoV-2 infection. Clin Mol Allergy. 2021;19(1):1–10. doi: 10.1186/s12948-021-00161-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payandeh Z, Mohammadkhani N, Nabi Afjadi M, Khalili S, Rajabibazl M, Houjaghani Z, et al. The immunology of SARS-CoV-2 infection, the potential antibody based treatments and vaccination strategies. Expert Rev Anti Infect Ther. 2021;19(7):899–910. doi: 10.1080/14787210.2020.1863144. [DOI] [PubMed] [Google Scholar]
- 5.Nabi-Afjadi M, Heydari M, Zalpoor H, Arman I, Sadoughi A, Sahami P, et al. Lectins and lectibodies: potential promising antiviral agents. Cell Mol Biol Lett. 2022;27(1):1–25. doi: 10.1186/s11658-022-00338-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Samidoust P, Delshad ME, Talemi RN, Mojtahedi K, Samidoust A, Jahangiri S, et al. Incidence, characteristics, and outcome of COVID-19 in patients on liver transplant program: a retrospective study in the north of Iran. New Microbes New Infect. 2021;44:100935. doi: 10.1016/j.nmni.2021.100935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sedighimehr N, Fathi J, Hadi N, Rezaeian ZS. Rehabilitation, a necessity in hospitalized and discharged people infected with COVID-19: a narrative review. Phys Ther Rev. 2021;26(3):202–210. doi: 10.1080/10833196.2021.1899472. [DOI] [Google Scholar]
- 8.Aghajanzadeh M, Haghighi M, Rimaz S, Fomani AA, Tangestaninejad A, Ashoobi MT, et al. Pneumomediastinum, pneumopericardium pneumothorax and subcutaneous emphysema in Iranian COVID-19 patients. J Curr Biomed Rep. 2021;2(4):201–205. doi: 10.52547/JCBioR.2.4.201. [DOI] [Google Scholar]
- 9.Asadi-Pooya AA, Simani L, Shahisavandi M, Barzegar Z. COVID-19, de novo seizures, and epilepsy: a systematic review. Neurol Sci. 2021;42(2):415–431. doi: 10.1007/s10072-020-04932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zalpoor H, Akbari A, Samei A, Forghaniesfidvajani R, Kamali M, Afzalnia A, et al. The roles of Eph receptors, neuropilin-1, P2X7, and CD147 in COVID-19-associated neurodegenerative diseases: inflammasome and JaK inhibitors as potential promising therapies. Cell Mol Biol Lett. 2022;27(1):1–21. doi: 10.1186/s11658-022-00311-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zalpoor H, Akbari A, Nabi-Afjadi M. Ephrin (Eph) receptor and downstream signaling pathways: a promising potential targeted therapy for COVID-19 and associated cancers and diseases. Human Cell. 2022;35:952–954. doi: 10.1007/s13577-022-00697-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zalpoor H, Shapourian H, Akbari A, Shahveh S, Haghshenas L. Increased neuropilin-1 expression by COVID-19: a possible cause of long-term neurological complications and progression of primary brain tumors. Human Cell. 2022;35:1301–1303. doi: 10.1007/s13577-022-00716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zalpoor H, Rezaei M, Yahyazadeh S, Ganjalikhani-Hakemi M. Flt3-ITD mutated acute myeloid leukemia patients and COVID-19: potential roles of autophagy and HIF-1α in leukemia progression and mortality. Human Cell. 2022;35:1304–1305. doi: 10.1007/s13577-022-00718-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zalpoor H, Bakhtiyari M, Liaghat M, Nabi-Afjadi M, Ganjalikhani-Hakemi M. Quercetin potential effects against SARS-CoV-2 infection and COVID-19-associated cancer progression by inhibiting mTOR and hypoxia-inducible factor-1α (HIF-1α) Phytother Res. 2022 doi: 10.1002/ptr.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan. China JAMA neurology. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiest KM, Sauro KM, Wiebe S, Patten SB, Kwon C-S, Dykeman J, et al. Prevalence and incidence of epilepsy: a systematic review and meta-analysis of international studies. Neurology. 2017;88(3):296–303. doi: 10.1212/WNL.0000000000003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aarabi B, Taghipour M, Haghnegahdar A, Farokhi M, Mobley L. Prognostic factors in the occurrence of posttraumatic epilepsy after penetrating head injury suffered during military service. Neurosurg Focus. 2000;8(1):1–6. doi: 10.3171/foc.2000.8.1.155. [DOI] [PubMed] [Google Scholar]
- 18.Vezzani A, Fujinami RS, White HS, Preux P-M, Blümcke I, Sander JW, et al. Infections, inflammation and epilepsy. Acta Neuropathol. 2016;131(2):211–234. doi: 10.1007/s00401-015-1481-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pandolfo M, editor. Genetics of epilepsy. Seminars in neurology, vol. 31, no. 05. Thieme Medical Publishers; 2011. p. 506–18. [DOI] [PubMed]
- 20.Kuroda N. Epilepsy and COVID-19: associations and important considerations. Epilepsy Behav. 2020;108:1. doi: 10.1016/j.yebeh.2020.107122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Granata T, Bisulli F, Arzimanoglou A, Rocamora R. Did the COVID-19 pandemic silence the needs of people with epilepsy? Epileptic Disord. 2020;22(4):439–442. doi: 10.1684/epd.2020.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anand P, Al-Faraj A, Sader E, Dashkoff J, Abdennadher M, Murugesan R, et al. Seizure as the presenting symptom of COVID-19: a retrospective case series. Epilepsy Behav. 2020;112:107335. doi: 10.1016/j.yebeh.2020.107335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lu L, Xiong W, Liu D, Liu J, Yang D, Li N, et al. New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. 2020;61(6):e49–e53. doi: 10.1111/epi.16524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saad M, Omrani AS, Baig K, Bahloul A, Elzein F, Matin MA, et al. Clinical aspects and outcomes of 70 patients with Middle East respiratory syndrome coronavirus infection: a single-center experience in Saudi Arabia. Int J Infect Dis. 2014;29:301–306. doi: 10.1016/j.ijid.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Li H, Fan R, Wen B, Zhang J, Cao X, et al. Coronavirus infections in the central nervous system and respiratory tract show distinct features in hospitalized children. Intervirology. 2016;59(3):163–169. doi: 10.1159/000453066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fonseca E, Quintana M, Lallana S, Luis Restrepo J, Abraira L, Santamarina E, et al. Epilepsy in time of COVID-19: a survey-based study. Acta Neurol Scand. 2020;142(6):545–554. doi: 10.1111/ane.13335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lyons S, O’Kelly B, Woods S, Rowan C, Brady D, Sheehan G, et al. Seizure with CSF lymphocytosis as a presenting feature of COVID-19 in an otherwise healthy young man. Seizure-Eur J Epilepsy. 2020;80:113–114. doi: 10.1016/j.seizure.2020.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Elgamasy S, Kamel MG, Ghozy S, Khalil A, Morra ME, Islam SM. First case of focal epilepsy associated with SARS-coronavirus-2. J Med Virol. 2020;92(10):2238–2242. doi: 10.1002/jmv.26113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Farhadian S, Glick LR, Vogels CB, Thomas J, Chiarella J, Casanovas-Massana A, et al. Acute encephalopathy with elevated CSF inflammatory markers as the initial presentation of COVID-19. BMC Neurol. 2020;20(1):1–5. doi: 10.1186/s12883-020-01812-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pilato MS, Urban A, Alkawadri R, Barot NV, Castellano JF, Rajasekaran V, et al. EEG findings in coronavirus disease. J Clin Neurophysiol. 2022;39(2):159–165. doi: 10.1097/WNP.0000000000000752. [DOI] [PubMed] [Google Scholar]
- 31.Galanopoulou AS, Ferastraoaru V, Correa DJ, Cherian K, Duberstein S, Gursky J, et al. EEG findings in acutely ill patients investigated for SARS-CoV-2/COVID-19: a small case series preliminary report. Epilepsia Open. 2020;5(2):314–324. doi: 10.1002/epi4.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asadi-Pooya AA. Seizures associated with coronavirus infections. Seizure. 2020;79:49–52. doi: 10.1016/j.seizure.2020.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Menéndez Méndez A, Smith J, Engel T. Neonatal seizures and purinergic signalling. Int J Mol Sci. 2020;21(21):7832. doi: 10.3390/ijms21217832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beamer E, Kuchukulla M, Boison D, Engel T. ATP and adenosine—Two players in the control of seizures and epilepsy development. Prog Neurobiol. 2021;204:102105. doi: 10.1016/j.pneurobio.2021.102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gil B, Smith J, Tang Y, Illes P, Engel T. Beyond seizure control: treating comorbidities in epilepsy via targeting of the P2X7 receptor. Int J Mol Sci. 2022;23(4):2380. doi: 10.3390/ijms23042380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beamer E, Conte G, Engel T. ATP release during seizures—a critical evaluation of the evidence. Brain Res Bull. 2019;151:65–73. doi: 10.1016/j.brainresbull.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 37.Vianna EPM, Ferreira AT, Naffah-Mazzacoratti MdG, Sanabria ERG, Funke M, Cavalheiro EA, et al. Evidence That ATP participates in the pathophysiology of pilocarpine-induced temporal lobe epilepsy: a fluorimetric, immunohistochemical, and western blot studies. Epilepsia. 2002;43:227–229. doi: 10.1046/j.1528-1157.43.s.5.26.x. [DOI] [PubMed] [Google Scholar]
- 38.Rappold P, Lynd-Balta E, Joseph S. P2X7 receptor immunoreactive profile confined to resting and activated microglia in the epileptic brain. Brain Res. 2006;1089(1):171–178. doi: 10.1016/j.brainres.2006.03.040. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez-Alvarez N, Jimenez-Mateos EM, Engel T, Quinlan S, Reschke CR, Conroy RM, et al. Effects of P2X7 receptor antagonists on hypoxia-induced neonatal seizures in mice. Neuropharmacology. 2017;116:351–363. doi: 10.1016/j.neuropharm.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 40.Doná F, Ulrich H, Persike DS, Conceição IM, Blini JP, Cavalheiro EA, et al. Alteration of purinergic P2X4 and P2X7 receptor expression in rats with temporal-lobe epilepsy induced by pilocarpine. Epilepsy Res. 2009;83(2–3):157–167. doi: 10.1016/j.eplepsyres.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Engel T, Gomez-Villafuertes R, Tanaka K, Mesuret G, Sanz-Rodriguez A, Garcia-Huerta P, et al. Seizure suppression and neuroprotection by targeting the purinergic P2X7 receptor during status epilepticus in mice. FASEB J. 2012;26(4):1616–1628. doi: 10.1096/fj.11-196089. [DOI] [PubMed] [Google Scholar]
- 42.Engel T, Jimenez-Pacheco A, Miras-Portugal MT, Diaz-Hernandez M, Henshall DC. P2X7 receptor in epilepsy; role in pathophysiology and potential targeting for seizure control. Int J Physiol Pathophysiol Pharmacol. 2012;4(4):174. [PMC free article] [PubMed] [Google Scholar]
- 43.De Marchi E, Orioli E, Dal Ben D, Adinolfi E. P2X7 receptor as a therapeutic target. Adv Protein Chem Struct Biol. 2016;104:39–79. doi: 10.1016/bs.apcsb.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 44.Homerin G, Jawhara S, Dezitter X, Baudelet D, Dufrénoy P, Rigo B, et al. Pyroglutamide-based P2X7 receptor antagonists targeting inflammatory bowel disease. J Med Chem. 2019;63(5):2074–2094. doi: 10.1021/acs.jmedchem.9b00584. [DOI] [PubMed] [Google Scholar]
- 45.Calzaferri F, Ruiz-Ruiz C, de Diego AM, de Pascual R, Méndez-López I, Cano-Abad MF, et al. The purinergic P2X7 receptor as a potential drug target to combat neuroinflammation in neurodegenerative diseases. Med Res Rev. 2020;40(6):2427–2465. doi: 10.1002/med.21710. [DOI] [PubMed] [Google Scholar]
- 46.Rotondo JC, Mazziotta C, Lanzillotti C, Stefani C, Badiale G, Campione G, et al. The role of purinergic P2X7 receptor in inflammation and cancer: Novel molecular insights and clinical applications. Cancers. 2022;14(5):1116. doi: 10.3390/cancers14051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Drill M, Jones NC, Hunn M, O’Brien TJ, Monif M. Antagonism of the ATP-gated P2X7 receptor: a potential therapeutic strategy for cancer. Purinergic Signalling. 2021;17(2):215–227. doi: 10.1007/s11302-021-09776-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martínez-García JJ, Martínez-Banaclocha H, Angosto-Bazarra D, de Torre-Minguela C, Baroja-Mazo A, Alarcón-Vila C, et al. P2X7 receptor induces mitochondrial failure in monocytes and compromises NLRP3 inflammasome activation during sepsis. Nat Commun. 2019;10(1):1–14. doi: 10.1038/s41467-019-10626-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Di Virgilio F, Tang Y, Sarti AC, Rossato M. A rationale for targeting the P2X7 receptor in Coronavirus disease 19. Br J Pharmacol. 2020;177(21):4990–4994. doi: 10.1111/bph.15138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ribeiro DE, Oliveira-Giacomelli Á, Glaser T, Arnaud-Sampaio VF, Andrejew R, Dieckmann L, et al. Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology. Mol Psychiatry. 2021;26(4):1044–1059. doi: 10.1038/s41380-020-00965-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sperlágh B, Illes P. P2X7 receptor: an emerging target in central nervous system diseases. Trends Pharmacol Sci. 2014;35(10):537–547. doi: 10.1016/j.tips.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Bartlett R, Stokes L, Sluyter R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol Rev. 2014;66(3):638–675. doi: 10.1124/pr.113.008003. [DOI] [PubMed] [Google Scholar]
- 53.Pacheco PA, Faria RX. The potential involvement of P2X7 receptor in COVID-19 pathogenesis: a new therapeutic target? Scand J Immunol. 2021;93(2):e12960. doi: 10.1111/sji.12960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Riteau N, Gasse P, Fauconnier L, Gombault A, Couegnat M, Fick L, et al. Extracellular ATP is a danger signal activating P2X7 receptor in lung inflammation and fibrosis. Am J Respir Crit Care Med. 2010;182(6):774–783. doi: 10.1164/rccm.201003-0359OC. [DOI] [PubMed] [Google Scholar]
- 55.Di Virgilio F, Dal Ben D, Sarti AC, Giuliani AL, Falzoni S. The P2X7 receptor in infection and inflammation. Immunity. 2017;47(1):15–31. doi: 10.1016/j.immuni.2017.06.020. [DOI] [PubMed] [Google Scholar]
- 56.Osorio I, Frei MG, Sornette D, Milton J, Lai Y-C. Epileptic seizures: quakes of the brain? Phys Rev E. 2010;82(2):021919. doi: 10.1103/PhysRevE.82.021919. [DOI] [PubMed] [Google Scholar]
- 57.Federico P, Abbott DF, Briellmann RS, Harvey AS, Jackson GD. Functional MRI of the pre-ictal state. Brain. 2005;128(8):1811–1817. doi: 10.1093/brain/awh533. [DOI] [PubMed] [Google Scholar]
- 58.Yang H, Wu J, Guo R, Peng Y, Zheng W, Liu D, et al. Glycolysis in energy metabolism during seizures. Neural Regen Res. 2013;8(14):1316. doi: 10.4103/1673-5374.121652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Folbergrová J, Ješina P, Drahota Z, Lisý V, Haugvicová R, Vojtíšková A, et al. Mitochondrial complex I inhibition in cerebral cortex of immature rats following homocysteic acid-induced seizures. Exp Neurol. 2007;204(2):597–609. doi: 10.1016/j.expneurol.2006.12.010. [DOI] [PubMed] [Google Scholar]
- 60.Acharya MM, Katyare SS. Structural and functional alterations in mitochondrial membrane in picrotoxin-induced epileptic rat brain. Exp Neurol. 2005;192(1):79–88. doi: 10.1016/j.expneurol.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 61.Waldbaum S, Patel M. Mitochondria, oxidative stress, and temporal lobe epilepsy. Epilepsy Res. 2010;88(1):23–45. doi: 10.1016/j.eplepsyres.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greijer A, Van Der Groep P, Kemming D, Shvarts A, Semenza G, Meijer G, et al. Up-regulation of gene expression by hypoxia is mediated predominantly by hypoxia-inducible factor 1 (HIF-1) The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland. 2005;206(3):291–304. doi: 10.1002/path.1778. [DOI] [PubMed] [Google Scholar]
- 63.Semenza GL, Roth PH, Fang H-M, Wang GL. Transcriptional regulation of genes encoding glycolytic enzymes by hypoxia-inducible factor 1. J Biol Chem. 1994;269(38):23757–23763. doi: 10.1016/S0021-9258(17)31580-6. [DOI] [PubMed] [Google Scholar]
- 64.Allen SP, Seehra RS, Heath PR, Hall BP, Bates J, Garwood CJ, et al. Transcriptomic analysis of human astrocytes in vitro reveals hypoxia-induced mitochondrial dysfunction, modulation of metabolism, and dysregulation of the immune response. Int J Mol Sci. 2020;21(21):8028. doi: 10.3390/ijms21218028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ménégaut L, Thomas C, Jalil A, Julla JB, Magnani C, Ceroi A, et al. Interplay between liver X receptor and hypoxia inducible factor 1α potentiates interleukin-1β production in human macrophages. Cell Rep. 2020;31(7):107665. doi: 10.1016/j.celrep.2020.107665. [DOI] [PubMed] [Google Scholar]
- 66.Liu J, Zhang X, Chen K, Cheng Y, Liu S, Xia M, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1α-mediated glycolysis. Immunity. 2019;50(3):600–15.e15. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 67.Feast A, Martinian L, Liu J, Catarino CB, Thom M, Sisodiya SM. Investigation of hypoxia-inducible factor-1α in hippocampal sclerosis: A postmortem study. Epilepsia. 2012;53(8):1349–1359. doi: 10.1111/j.1528-1167.2012.03591.x. [DOI] [PubMed] [Google Scholar]
- 68.Gualtieri F, Marinelli C, Longo D, Pugnaghi M, Nichelli PF, Meletti S, et al. Hypoxia markers are expressed in interneurons exposed to recurrent seizures. NeuroMol Med. 2013;15(1):133–146. doi: 10.1007/s12017-012-8203-0. [DOI] [PubMed] [Google Scholar]
- 69.Yang J, He F, Meng Q, Sun Y, Wang W, Wang C. Inhibiting HIF-1α decreases expression of TNF-α and caspase-3 in specific brain regions exposed kainic acid-induced status epilepticus. Cell Physiol Biochem. 2016;38(1):75–82. doi: 10.1159/000438610. [DOI] [PubMed] [Google Scholar]
- 70.Long Q, Fan C, Kai W, Luo Q, Xin W, Wang P, et al. Hypoxia inducible factor-1α expression is associated with hippocampal apoptosis during epileptogenesis. Brain Res. 2014;1590:20–30. doi: 10.1016/j.brainres.2014.09.028. [DOI] [PubMed] [Google Scholar]
- 71.Jiang G, Zhou R, He X, Shi Z, Huang M, Yu J, et al. Expression levels of microRNA-199 and hypoxia-inducible factor-1 alpha in brain tissue of patients with intractable epilepsy. Int J Neurosci. 2016;126(4):326–334. doi: 10.3109/00207454.2014.994209. [DOI] [PubMed] [Google Scholar]
- 72.Krishnan S, Nordqvist H, Ambikan AT, Gupta S, Sperk M, Svensson-Akusjärvi S, et al. Metabolic perturbation associated with COVID-19 disease severity and SARS-CoV-2 replication. Mol Cell Proteom. 2021 doi: 10.1016/j.mcpro.2021.100159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tian M, Liu W, Li X, Zhao P, Shereen MA, Zhu C, et al. HIF-1α promotes SARS-CoV-2 infection and aggravates inflammatory responses to COVID-19. Signal Transduct Target Ther. 2021;6(1):1–13. doi: 10.1038/s41392-021-00726-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jahani M, Dokaneheifard S, Mansouri K. Hypoxia: A key feature of COVID-19 launching activation of HIF-1 and cytokine storm. J Inflamm. 2020;17(1):1–10. doi: 10.1186/s12950-020-00263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Codo AC, Davanzo GG, de Brito ML, de Souza GF, Muraro SP, Virgilio-da-Silva JV, et al. Elevated glucose levels favor SARS-CoV-2 infection and monocyte response through a HIF-1α/glycolysis-dependent axis. Cell Metab. 2020;32(3):437–46.e5. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zalpoor H, Bakhtiyari M, Liaghat M, Nabi-Afjadi M, Ganjalikhani-Hakemi M. Quercetin potential effects against SARS-CoV-2 infection and COVID-19-associated cancer progression by inhibiting mTOR and hypoxia-inducible factor-1α (HIF-1α) Phytother Res. 2022 doi: 10.1002/ptr.7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jarrett SG, Liang L-P, Hellier JL, Staley KJ, Patel M. Mitochondrial DNA damage and impaired base excision repair during epileptogenesis. Neurobiol Dis. 2008;30(1):130–138. doi: 10.1016/j.nbd.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Patel M. Mitochondrial dysfunction and oxidative stress: cause and consequence of epileptic seizures. Free Radic Biol Med. 2004;37(12):1951–1962. doi: 10.1016/j.freeradbiomed.2004.08.021. [DOI] [PubMed] [Google Scholar]
- 79.Moyer MB, Serra R, Bachani M, Ivanova S, Gerzanich V, Ksendzovsky A, et al. SUR1-TRPM4 is upregulated in mouse model of epilepsy. 2021.
- 80.Erdemli KT. Investigation of the pathology of brain derived endothelial cells in in-vitro hypoxia models. Izmir Institute of Technology; 2021. [Google Scholar]
- 81.Hirayama Y, Koizumi S. Astrocytes and ischemic tolerance. Neurosci Res. 2018;126:53–59. doi: 10.1016/j.neures.2017.11.013. [DOI] [PubMed] [Google Scholar]
- 82.Ugur M, Ugur Ö. A mechanism-based approach to P2X7 receptor action. Mol Pharmacol. 2019;95(4):442–450. doi: 10.1124/mol.118.115022. [DOI] [PubMed] [Google Scholar]
- 83.Martínez-Cuesta MÁ, Blanch-Ruiz MA, Ortega-Luna R, Sánchez-López A, Álvarez Á. Structural and functional basis for understanding the biological significance of P2X7 receptor. Int J Mol Sci. 2020;21(22):8454. doi: 10.3390/ijms21228454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Amoroso F, Capece M, Rotondo A, Cangelosi D, Ferracin M, Franceschini A, et al. The P2X7 receptor is a key modulator of the PI3K/GSK3β/VEGF signaling network: evidence in experimental neuroblastoma. Oncogene. 2015;34(41):5240–5251. doi: 10.1038/onc.2014.444. [DOI] [PubMed] [Google Scholar]
- 85.Amoroso F, Falzoni S, Adinolfi E, Ferrari D, Di Virgilio F. The P2X7 receptor is a key modulator of aerobic glycolysis. Cell Death Dis. 2012;3(8):e370-e. doi: 10.1038/cddis.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Souza CO, Santoro GF, Figliuolo VR, Nanini HF, de Souza HS, Castelo-Branco MTL, et al. Extracellular ATP induces cell death in human intestinal epithelial cells. Biochim et Biophys Acta (BBA) Gener Subjects. 2012;1820(12):1867–1878. doi: 10.1016/j.bbagen.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 87.Zhang Y, Jiang M, Cui BW, Jin CH, Wu YL, Shang Y, et al. P2X7 receptor-targeted regulation by tetrahydroxystilbene glucoside in alcoholic hepatosteatosis: a new strategy towards macrophage–hepatocyte crosstalk. Br J Pharmacol. 2020;177(12):2793–2811. doi: 10.1111/bph.15007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Tafani M, Schito L, Pellegrini L, Villanova L, Marfe G, Anwar T, et al. Hypoxia-increased RAGE and P2X7R expression regulates tumor cell invasion through phosphorylation of Erk1/2 and Akt and nuclear translocation of NF-κB. Carcinogenesis. 2011;32(8):1167–1175. doi: 10.1093/carcin/bgr101. [DOI] [PubMed] [Google Scholar]
- 89.Engel T, Brennan GP, Sanz-Rodriguez A, Alves M, Beamer E, Watters O, et al. A calcium-sensitive feed-forward loop regulating the expression of the ATP-gated purinergic P2X7 receptor via specificity protein 1 and microRNA-22. Biochim Biophys Acta (BBA) Mol Cell Res. 2017;1864(2):255–266. doi: 10.1016/j.bbamcr.2016.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.