Abstract

Coated diammonium phosphate (CDAP) is intended to release nutrients steadily in response to the demand of crop growth. A novel biostimulant extracted from Paecilomyces variotii has been shown to regulate gene expression in nutrient transport, enhance nitrogen (N) and phosphorus (P) uptake, and improve nutrient use efficiency. The application of CDAP combined with the Paecilomyces variotii extracts (ZNC) in maize is an efficient approach for reducing waste of resources, improving nutrient supply, and maintaining production stability. The effects of CDAP combined with ZNC on photosynthesis, enzyme activities, endogenous hormone content, maize yield, and P use efficiency (PUE) were investigated in this study. In a pot experiment, CDAP and diammonium phosphate (DAP) were tested together with P levels (1.80, 1.44 g pot–1, P2O5) and two ZNC application rates (0, 4.4 μg pot–1), which included the control treatment that had no P fertilizer added. Results showed that the key influencing elements of maize growth and yield were the soil available-P content, endogenous hormone content, and plant photosynthesis in this study. The combination of DAP and ZNC increased the soil available-P content and the auxin content in leaves at the key stage and hence increased the yield and PUE of maize, compared with DAP. The net photosynthetic rate of CDAP combined with ZNC was higher by 23.1% than that of CDAP alone, as well as by 32.0% than that of DAP combined with ZNC. Moreover, the combination of CDAP and ZNC increased the yield and PUE by 8.2% and 15.6 percentage points compared with DAP combined with ZNC while increasing the yield and PUE compared with CDAP. In conclusion, combining CDAP with ZNC as an environmentally friendly fertilizer could improve photosynthesis-related enzyme activity and enhance the net photosynthetic rate, resulting in an increase in maize yield and PUE significantly.
Introduction
Maize is one of the most important food crops as well as an essential forage and industrial raw material in China,1,2 accounting for around 1/3 of the global grain production.3 Phosphorus (P) is one of the most vital nutrients in maize growth and development; a sufficient P content of the maize root zone is a necessary condition for good maize output.4 However, with the exception of a portion of P fertilizers that are converted to the organic state through biological action, the majority of the P fertilizers are bound by calcium and magnesium in calcareous soils or chemically react with iron and aluminum in acidic soils, forming phosphate precipitation with low solubility and being fixed.5 In addition, excessive chemical fertilizer application harmed soil’s physical structure and microbial community or produced other problems. Moreover, the P use efficiency (PUE) is only 10–25% in China, causing a significant resource loss, high agricultural production costs, and serious pollution from nonpoint agricultural sources.6,7 Therefore, how to reduce P loss and boost PUE is of great significance to ensure good maize output.
A polymer-coated controlled-release phosphate fertilizer (CRP) releases nutrients in a steady manner, coordinating the release consistent with crop demand, minimizing P ineffectiveness owing to soil fixation and extending fertilizer efficacy.8 One-time fertilization of CRP could meet the nutrient supply of crops for the whole growing period,8,9 which is a novel solution to the challenges posed by current conventional fertilization practices. Lu10 made coated diammonium phosphate (CDAP) using polyolefin wax surface modification and coating polyurethane made from castor oil, which showed that the wax-modified CDAP had a better controlled-release performance with the ideal “S” shape when compared with conventional diammonium phosphate (DAP). Yaseen11 found that after coating DAP, the polymer layer surrounding fertilizer grains reduced the adsorption and precipitation of P in the soil, thus enhancing the availability of P to plants and encouraging plant growth and development. However, as a result of the long-standing fertilization habits of farmers and the influence of fertilizer prices, the development of CRP has been fraught with challenges in China. Furthermore, the perceived single function of traditional controlled-release fertilizers restricted the further promotion and application of controlled-release fertilizers.12,13
Paecilomyces variotii extract (ZNC) is a type of microbial secondary metabolite isolated and purified from the fermentation of the endophytic strain P. variotii, with small compounds and high biological activity.14 The active ingredient of ZNC could regulate crop genes like small auxin-upregulated genes to increase the auxin level of the crop root tip and promote the absorption of N and P,15,16 as well as crop growth and development, thus resulting in an increase in crop yield and PUE.17 Lu18 found that higher concentrations of ZNC (100 ng L–1) could induce a plant immune response by activating the salicylic acid pathway, stimulating the accumulation of callosum in leaves, and improving the resistance of crops to pathogenic bacteria. Wang19 also found that controlled-release urea enriched by ZNC achieved significantly higher gain yield than coated urea alone and further increased nitrogen (N) use efficiency, N partial factor productivity, and net profit. However, most of the previous studies on ZNC focused on laboratory simulation conditions, with few findings on the impact of ZNC application on the soil environment under natural growth conditions. Therefore, it is crucial to explore its effects on the nutrient supply intensity, plant growth, grain yield, and PUE of maize in combination with phosphate fertilizers.
In a 3-year field trial, the findings of Chen20 showed that the application of CDAP or ZNC could increase maize yield by improving the soil P supply intensity to meet maize P demand and promote root morphological characteristics and vitality. However, there are few research on the elements that influence crop yield and PUE after applying CDAP and ZNC, particularly photosynthesis. A sufficient supply of P can provide energy for maize photosynthetic phosphorylation and accelerate the photosynthetic rate of maize, encouraging the synthesis and accumulation of organic matter while also increasing maize yield and PUE.21 Hence, maize pot experiments were conducted in this study to explore the application effects of CDAP and ZNC for photosynthesis, endogenous hormone, etc. at different levels of P fertilization and whether they can synergistically increase the efficiency after the combination of CDAP and ZNC. Moreover, the effects of CDAP and ZNC on maize yield and PUE were explored by observing soil available nutrients, photosynthetic rate, photosynthesis-related enzyme activities, and phosphatase activities.
Results
Effect of CDAP and ZNC on Maize Yield and PUE
The combination of CDAP and ZNC actively affected the maize yield and yield components in both years (Table 1). Compared with P100%, the average yields of CP100%, P100%Z, and P80%Z were significantly increased by 13.6, 11.5, and 10.4%, respectively, while the PUE was significantly increased by 24.5, 11.1, and 20.7 percentage points. In addition, CP100%Z increased yields by 8.5 and 8.2%, respectively, when compared to CP100% and P100%Z. The PUE of CP100%Z was significantly higher by 15.6 percentage points compared with P100%Z, while CP100%Z had no significant difference from CP100%. The yield of CP80%Z was 7.2% higher than that of P80%Z; meanwhile, the PUE was significantly higher by 10 percentage points. To sum up, the maize yield was significantly improved after applying ZNC, and the combination of CDAP and ZNC showed a considerable beneficial effect on yield in 2017, with a particularly noticeable effect under the condition of a 20% P reduction.
Table 1. Yield and PUE of Maize under Different Treatments.
| yield (g pot–1) |
average
yield increment vs P100% (%) |
PUE
(%) |
average
PUE increment vs P100% |
|||||
|---|---|---|---|---|---|---|---|---|
| treatment | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 | 2017 | 2018 |
| CK | 130.3e | 149.6d | –9.1 | –8.8 | ||||
| P100% | 143.4d | 164.0c | 16.7e | 15.5d | ||||
| CP100% | 166.2b | 182.6ab | 15.9 | 11.3 | 37.2c | 43.9a | 20.5 | 28.4 |
| P100%Z | 159.4c | 183.2ab | 11.2 | 11.7 | 27.3d | 27.4c | 10.3 | 11.9 |
| P80%Z | 159.6c | 179.6b | 11.3 | 9.5 | 35.0c | 38.6b | 18.3 | 23.1 |
| CP100%Z | 182.2a | 186.7a | 27.1 | 13.8 | 41.4b | 44.5a | 24.7 | 29 |
| CP80%Z | 179.7a | 182.6ab | 25.3 | 11.3 | 48.6a | 45.0a | 31.9 | 16.4 |
Note: Means followed by the same letters in each column were not significantly different at the 5% level.
Effect of CDAP Combined with ZNC on Soil Available-P Supply Intensity
P is an essential component of many agricultural components of crops and participates in a variety of metabolic processes that promote crop stress resistance. The application of CDAP and ZNC had a significant effect on the change in the soil available-P content during the maize growing period (Figure 1). At the V12 stage, the soil available-P content of P100%Z was 25.6% higher than that of P100% and that of CP100%Z was 77.7 and 14.3% higher than those of CP100% and P100%Z, respectively. Compared with P100%, the soil available-P content of CP100% and P100%Z significantly increased by 31.1 and 51.9%, respectively, during the silking period. After a 20% P reduction, CDAP increased the available-P content by 7.8% compared with P100%. At the maturity stage, the available-P content of CP100% was improved by 14.2% in comparison with P100%.
Figure 1.

Soil available-P content of treatments at different growth stages. Error bars represent ±SE. V3: the seedling stage, V6: the six-leaf stage, V12: the 12-leaf stage, R1: the silking stage, and R6: the maturity stage.
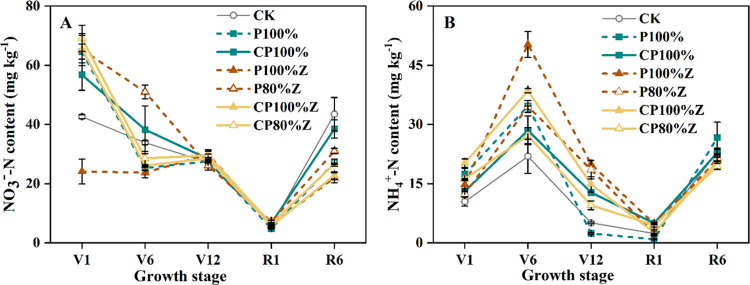
Throughout the whole growth period of maize, the soil inorganic nitrogen contents (NO3–-N, NH4+-N) of different treatments followed a similar trend in soil (Figure 2). There was no significant difference in the NO3–-N content among different treatments at the seedling stage. Compared with P100%, the NO3–-N content of CP100% and P100%Z were significantly increased by 51.1 and 80.8%, respectively, and that of P80%Z was obviously increased by 113.4% under the 20% P reduction condition at the jointing stage. In comparison with CP100% and P100%Z, CP100%Z significantly improved the NO3–-N content by 34.4 and 12.3%, respectively. At the silking stage, the NO3–-N content of each treatment reached the minimum value, and the content of P100%Z was significantly increased by 55.3% compared with conventional P100% treatment, while CP80% was obviously increased by 75%. In addition, the NH4+-N content of CP100%Z was 16.1% higher than that of P80%Z during the seedling stage. At the V12 stage, CP100% and P100%Z compared with P100% significantly improved the NH4+-N content by 416.2 and 707.7%, respectively; meanwhile, P80%Z significantly increased the NH4+-N content by 651.4%.
Figure 2.
Soil NO3–-N (A) and NH4+-N (B) contents of treatments at different growth stages. Error bars represent ±SE. V3: the seedling stage, V6: the six-leaf stage, V12: the 12-leaf stage, R1: the silking stage, and R6: the maturity stage.
Effect of CDAP Combined with ZNC on Photosynthesis in Maize
Photosynthesis is a crucial mechanism for the generation and accumulation of organic matter in crops. At the V12 stage, the maize net photosynthesis rate of CP100% was increased by 8.1% compared with P100%, while that of P80%Z was increased by 13.2% (Figure 3). The net photosynthetic rate of CP100%Z was 23.1 and 32.0% higher than those of CP100% and P100%Z, respectively. Besides, CP80%Z significantly increased the rate by 17.9% in comparison with P80%Z. PEPC and ATP synthase can promote photosynthetic assimilation and phosphorylation to provide energy for photosynthesis, while AGPase and PPDK can use the energy produced by photosynthesis to synthesize starch and other substances to promote the growth and development of plants (Table 2). Compared with P100%, CP100%Z and P100%Z increased the PEPC enzyme activity by 17.7 and 5.9%, respectively. In comparison with CP100%, the AGPase activity of CP80%Z was significantly increased by 31.2%, while the activity of CP100%Z was significantly increased by 37.3%. There was no significant difference between P100% and CP100%Z in AGPase activity.
Figure 3.
Leaf photosynthetic rate of maize under different treatments. Bar heights represent means, and error bars represent ±SE. The same letters on the bars were not significantly different based on a one-way ANOVA followed by Duncan’s multiple range tests (P < 0.05).
Table 2. Activities of Photosynthesis-Related Enzymes under Different Treatments.
| PEPC | AGPase | ATP8 | PPDK | |
|---|---|---|---|---|
| treatment | U L–1 | |||
| CK | 0.24b | 3.45c | 43.07a | 52.15e |
| P100% | 0.17f | 3.67b | 33.55d | 51.62ef |
| CP100% | 0.20c | 2.36g | 43.13a | 75.83a |
| P100%Z | 0.18e | 3.10e | 32.42e | 53.54d |
| P80%Z | 0.26a | 2.92f | 39.88c | 50.55f |
| CP100%Z | 0.19d | 3.24d | 41.64b | 68.95b |
| CP80%Z | 0.19d | 3.83a | 39.49c | 57.86c |
Note: PEPC, phosphoenolpyruvate carboxylase; AGPase, ADP-glucose pyrophosphorylase; ATP8, ATP synthase; and PPDK, pyruvate phosphate dikinase. Means followed by similar lowercase letters within the same column of each item were not significant in the difference at the 5% level.
Effects of CDAP Combined with ZNC on Enzyme Activities Related to the AMP Synthesis, Glycolytic Processes, and Tricarboxylic Acid Cycle
The ATP required for photosynthesis is produced by AMP synthesis, glycolysis, and the tricarboxylic acid cycle (Table 3). During the key maize fertility period, the AMPSS activity of CP100% and P100%Z was increased by 15.4 and 38.8% compared with P100%, while that of P80%Z and CP80%Z was significantly increased by 38.8 and 19.9% under the 20% P reduction condition, respectively. The PRPP activity of CP100%Z was significantly increased by 52.6 and 29.9% compared with CP100% and P100%Z, respectively. In comparison with P100%, CP100%Z increased the PRPPAT activity by 11.5 and 17.3%, respectively. Meanwhile, the PRPPAT activity of P80%Z and CP80%Z was 15.8 and 12.2% higher than that of P100% after a 20% P reduction. There was no significant difference in SCS activity between CP100%Z and P100%Z, while the activity of CP100%Z was 21.0% higher than that of CP100%. The CP100% treatment worked best, which significantly increased the GAPDH activity by 45.7% compared with P100%, and P80%Z and CP80%Z increased the enzyme activity by 25.2 and 27.3% after 20% P reduction, respectively; the effect was generally better than conventional P treatment.
Table 3. Activities of AAMPSS, PRPP, GAPDH, and SCS Enzymes under Different Treatments.
| AMPSS | PRPP | PRPPAT | SCS | GAPDH | |
|---|---|---|---|---|---|
| treatment | U L–1 | ||||
| CK | 288.96d | 210.15c | 116e | 205.24e | 154.25b |
| P100% | 292.05d | 222.38b | 139d | 295.08c | 114.45d |
| CP100% | 336.94c | 156.46h | 155b | 258.11d | 166.76a |
| P100%Z | 405.39a | 183.65d | 163a | 301.48b | 101.51e |
| P80%Z | 405.39a | 171.41e | 161a | 301.28b | 143.29c |
| CP100%Z | 346.76b | 238.69a | 145c | 312.22a | 154.80b |
| CP80%Z | 350.12b | 223.40b | 156b | 196.56f | 145.70c |
Note: AMPSS, adenylosuccinate synthase; PRPP, phosphoribosyl pyrophosphate; SCS, succinyl coenzyme A synthetase; and GAPDH, glyceraldehyde-3-phosphate dehydrogenase. Means followed by similar lowercase letters within the same column of each item were not significant in the difference at the 5% level.
Effect of CDAP Combined with ZNC on the Endogenous Hormone Content of Maize Leaves
Endogenous hormones such as GA and ABA can promote the growth and development of crop leaves and roots, as well as the available nutrients uptake of roots (Figure 4). Compared with P100%, the GA content of CP100% was increased by 20.0% and the enzyme activity of CP80% Z was increased by 16.9%. The P100%Z significantly increased the IAA content by 33.6%, while P80%Z and CP80%Z increased the content by 27.0 and 7.7%, respectively, compared with P100%. Compared with CP100%, the CP100%Z increased the IAA content by 7.25%. In addition, the ABA content of CP100% was significantly increased by 32.3% compared with P100%, and that of CP80%Z was significantly increased by 53%. In addition, compared with P100%Z, CP100%Z significantly increased the ABA content by 33.4%. Moreover, the CTK activity of CP100% and P80%Z was 32.3 and 44.2% higher than that of P100%, and the CTK activity of CP100%Z was 21.8% higher.
Figure 4.

Contents of IAA (A), ABA (B), CTK (C), and GA (D) under different treatments. Bar heights represent means, and error bars represent ±SE. The same letters on the bars were not significantly different based on a one-way ANOVA followed by Duncan’s multiple range tests (P < 0.05).
Effect of CDAP Combined with ZNC on Acid Phosphatase and Alkaline Phosphatase Activities of Root and Soil
To increase the P uptake of crops, phosphatase could use energy molecules such as ATP to hydrolyze organophosphorus into inorganic phosphorus. Compared with P100%, the AP activity of root in CP100% and P100%Z was increased by 11.5 and 57.3%, respectively (Table 4). The CP100%Z significantly increased the root AP activity by 47.2% compared with CP100%, and CP100%Z improved the root AP activity by 4.4% in comparison with P100%Z. Besides, the root ALP activity of CP100% and P100%Z was obviously increased by 24.7 and 65.4%, respectively, compared with that of P100%. CP100%Z increased the root ALP activity by 42.7 and 7.6%, respectively, compared with those of CP100% and P100%Z. In addition, there was no significant difference in the soil AP activity between all treatments, with CP100% having a somewhat stronger effect. Compared with P100%, the soil ALP activity of CP100% was significantly increased by 38.5%. The soil ALP activity of CP100%Z was not significantly increased.
Table 4. Acid Phosphatase and Alkaline Phosphatase Activities of Maize Root and Soil under Different Treatments.
| AP (U g–1) |
ALP (U g–1) |
|||
|---|---|---|---|---|
| treatment | root | soil | root | soil |
| CK | 0.6098c | 0.5271b | 0.0826d | 0.1004b |
| P100% | 0.5150e | 0.5774ab | 0.0736e | 0.0933bc |
| CP100% | 0.5744d | 0.5913a | 0.0918c | 0.1292a |
| P100%Z | 0.8099b | 0.5473ab | 0.1217b | 0.0848c |
| CP100%Z | 0.8456a | 0.5650ab | 0.1310a | 0.0880c |
Note: AP, acid phosphatase and ALP, alkaline phosphatase. Means followed by similar lowercase letters within the same column of each item were not significant in the difference at the 5% level.
Correlation Analysis and Principal Component Analysis of Maize Yield, PUE, and Their Related Indicators
Principal component analysis is a statistical method for aggregating information from big data sets into smaller data sets that are easier to visualize and analyze. The V12 stage is the most vigorous period of maximum nutritional and reproductive growth of maize, which is directly related to maize yield. In this study, the soil available-P content did not show an obvious correlation with yield and PUE (Figure 5A). Soil microbial activity was high due to high soil moisture and temperature, so the soil available-P content was 39.42 mg kg–1 on average during the V12 stage, which exceeded the P requirement threshold of crops (15 mg kg–1),22 resulting in the soil available-P content being saturated. Therefore, nutrient supply during this growing period was not the determining factor in yield increase. The activity of photosynthesis-related enzymes such as AGPase and ATP synthesis significantly influenced photosynthesis, and the net photosynthetic rate was positively correlated with yield and PUE. The amount of available-P in the soil was positively correlated with root phosphatase activity, while root phosphatase was significantly positively correlated with yield and PUE. Moreover, root phosphatase could accelerate the conversion of organophosphorus to inorganic P in plants, as well as stimulate the transport of inorganic P from senescent to tender tissue.23 The endogenous hormones such as CTK and ABA also showed significant positive correlations with maize yield and PUE.
Figure 5.
Correlation analysis of yield and PUE with their related indicators (A) and principal component analysis (B). AP (soil available-P), NO3– (soil NO3–-N), NH4+ (soil NH4+-N), GA (gibberellin acid), IAA (indole-3-acetic acid), ABA (abscisic acid), CTK (cytokinin), ATP8 (ATP synthase), PEPC (phosphoenolpyruvate carboxylase), AGPase (ADP-glucose pyrophosphorylase), PPDK (pyruvate phosphate dikinase), Photo (photosynthetic rate), soil AP (soil acid phosphatase), soil ALP (soil alkaline phosphatase), root AP (soil acid phosphatase), and root ALP (soil alkaline phosphatase).
Spearman correlation is an approach to accessing correlations between indicators. And 22 related indicators, including soil physical and chemical properties and growth indicators, contributed 40.5 and 23.3% to PC1 and PC2, respectively, in the principal component analysis (Figure 5B). The repeatability of the treatments was good, with the same treatments being accurately clustered together in the graph. Soil acid and alkaline phosphatase contents were significantly and positively correlated with maize yield when the phosphatase contents were increased significantly in the CDAP treatments, resulting in maize yield being improved. The results are like those shown in Figure 5A. Changes in endogenous hormones, such as CTK and ABA, as well as photosynthesis-related enzymes, were found to have a significant impact on maize yield in the ZNC-added treatments. Hence, ZNC improved photosynthesis-related enzyme activity, soil acid and alkaline phosphatase, and endogenous hormone content, all of which increased maize production.18 When CDAP was combined with ZNC, the positive and negative correlation indexes worked together to increase maize yield.
Discussion
Effect of DAP Combined with ZNC on Maize Yield and PUE
Combining several types of fertilizers and efficiency enhancement measures could significantly increase crop yield in theory. In this study, DAP combined with ZNC significantly increased the soil available-P content by 25.6 and 51.9% at the maize V12 and silking stages, respectively, while the NO3–-N content was increased by 80.8% at the jointing stage. Thus, these improved the intensity of nutrient supply during the critical fertility period of maize, resulting in an increase of 11.7% and 12.1 percentage points in crop yield and PUE, respectively. The possible reason is that ZNC could increase the number of soil rhizosphere microbial populations and beneficial microbial populations, and further improve the soil structure and soil available nutrient activity through rhizosphere microbial metabolites.24 This is consistent with the experimental results of Chen,25 who discovered that applying ZNC to DAP in fields could improve maize yield by 3.56% compared with DAP. It is possible that the IAA content of DAP combined with ZNC was significantly increased by 33.6% in this study because ZNC could induce the expression of genes related to IAA synthesis in roots such as YUC3 and YUC9,18 thus promoting the growth and yield increase of maize. Moreover, ZNC could not only directly act on crops to promote their physiological functions and metabolic processes but also act on soil and soil microorganisms to improve physicochemical structures such as soil pores and promote the absorption of nutrients by the root system.17,26
However, the yield difference between DAP combined with ZNC and CDAP was not significant. The possible reasons were that the majority of the P absorbed by the crop was delivered to plant organs rather than seeds and the nutrient supply of CDAP was sustainable enough to meet the nutrient demand of crops. In addition, the practical application of trace ZNC combined with fertilizers in the field was prone to producing problems such as leaching loss and degradation, and ZNC applied on the fertilizer surface was at risk of inactivation or degradation.20 Therefore, the effect of adding ZNC to DAP was not superior to that of CDAP.
Effect of CDAP Combined with ZNC on Yield and PUE
The coated fertilizer penetrated the outer water through the membrane layer, increasing the internal osmotic pressure so that the membrane shell was cracked or broken, and the nutrients were released slowly, thus allowing for one-time fertilization to meet the nutritional demand of crops for the whole growth period.27,28 CDAP regulated nutrient release according to the P demand of crops at different growth stages, which could improve the soil available nutrient content and promote the absorption and utilization of nutrients.29 When DAP was coated, it was isolated from the soil, avoiding the adsorption and precipitation between phosphate root and stratified silicate minerals such as magnesium and aluminum in the soil. Thus, the soil available-P content of this study was increased by 15.0%, which was consistent with the experimental results of Chen et al. that applied synergistic CDAP on maize.20 The application of DAP after coating also significantly increased the activities of photosynthesis-related enzymes such as ATP8 and PPDK by 28.6 and 46.9%, and the yield and PUE significantly increased by 13.6% and 24.5 percentage points, respectively. That may be because the available-P absorbed by plants can be used in the photosynthetic phosphorylation process to generate ATP, and sufficient P supply promoted the photosynthetic phosphorylation process in maize to provide energy, increased CO2 consumption in the leaf flesh cells, and decreased the photosynthetic CO2 compensation point, thus increasing the photosynthetic activity and stomatal conductance of the leaf flesh,30,31 resulting in a significant improvement in maize photosynthesis. Furthermore, according to the principal component analysis, the net photosynthetic rate of maize leaves was positively correlated with yield because the enhanced photosynthesis promoted the production and accumulation of organic matter required by maize (Figure 5).
According to recent research findings, ZNC can stimulate PTI through the FLG22 and chitin signaling pathways to enhance plant disease resistance, enhance the expression of IAA and N absorption-related genes, and improve plant metabolism to promote plant growth.32 When DAP compounded coating ZNC, it was able to keep ZNC alive, limit the release of ZNC and nutrients together, promote the growth and development of crops, and further improve maize yield and PUE. However, the results of this study showed that ZNC combined with CDAP had a better yield-increasing effect in 2017 than the treatments CDAP or DAP combined with ZNC, increasing maize yield by 9.6 and 14.3%, respectively, and the yield difference was not significant in 2018. The ZNC contained a variety of carbohydrates, amino acids, and alkyl structures, and the recommended concentration ranged from 37.5 to 75.0 g ha–1.14,19 It was possible that the amount of ZNC used in this experiment, 3.08 mg kg–1 DAP, was not ideal and the effect was unstable due to the extremely low dosage, resulting in the combination of the two failing to show a synergistic yield increase in 2018. Additionally, there may be differences in objective environmental elements such as outdoor temperature and humidity, as well as weather conditions in the seasons of 2017 and 2018, resulting in differences in experimental results between the two years. Furthermore, maize is a P-efficient crop, and soil moisture content and temperature of the maize season were higher, which could improve phytase and glycerin phosphatase enzyme activities and increase the content of soil available-P, conducive to the maize root system to absorb through enhanced phosphorus transport,33 making maize less sensitive to the discretion of the soil P content and resulting in unstable results. Therefore, in the later relevant experiments, different gradients of ZNC could be used with controlled-release fertilizers to investigate whether there is an optimal amount of ZNC that could achieve synergistic crops when combined with slow and controlled-release fertilizers, as well as the application effect of slow and controlled-release fertilizers combined with ZNC on wheat and other crops.
Conclusions
P. variotii extract (ZNC) could promote the absorption and utilization of nutrients by regulating endogenous hormone levels and encouraging the growth and development of crops. When ZNC was combined with DAP, the activity of crucial enzymes such as AMPSS, PRPPAT, and IAA increased by 38.8, 17.3, and 33.6%, respectively, improving the maize yield by 11.5% and PUE by 11.1 percentage points. In addition, the combination of CDAP and ZNC increased the net photosynthetic rate by 32.0% at the crucial growth stage, improving maize yield and PUE by 15.2% and 17.6 percentage points, respectively, compared with DAP combined with ZNC. In short, the combined application of CDAP and ZNC could increase crop yield and PUE by the combined effect on available soil nutrients, photosynthesis enzymes, and endogenous hormones, reducing agricultural nonpoint source pollution caused by excessive phosphate fertilizer application and maintaining the ecological environment. This study was supposed to provide a scientific approach for the combination of CDAP and ZNC, as well as research and development of summer maize fertilizer products.
Experimental Section
Materials
The test soil was collected from the 0–20 cm cultivated layer of the experimental base of the National Engineering Laboratory for Efficient Utilization of Soil Fertility Resources (NELEUSFR), Shandong Agricultural University (SDAU), China. The soil was classified as Typic-Hapli-Udic Argosols (Chinese Soil Taxonomy, CRGCST, 2001), Typic Hapludalf (Soil Survey Staff, USDA, 1999), and Hapic Luvisols (IUSS Working Group WRB, 2015). The properties of the soil were as follows: 12.10 g kg–1 organic matter, 0.32 g kg–1 total P, 13.50 mg kg–1 available-P, 71.45 mg kg–1 NO3–-N, 9.45 mg kg–1 NH4+-N, 92.32 mg kg–1 available-K, and pH 7.83 (1:2.5 soil to water ratio).
The controlled-release P fertilizer, CDAP (17.2% N, 44.0% P2O5), was prepared by NELEUSFR, SDAU, China. The coating was made up of 10% paraffin and 90% polyurethane. Resin-coated controlled-release urea (43.0% N; 3-month release period) was purchased from Kingenta Ecological Engineering Group Co., Ltd., Shandong, China. The ZNC (commercial name: Zhi Neng Cong) was extracted from Paecilomeyces variotii and isolated from the root system of wild seabuckthorn, which was obtained from Shandong Peng Bo Bio-Technology Co., Ltd.18 The fertilizer of CDAP combined with ZNC was prepared by spraying ZNC twice, once on the surface of DAP prior to coating and once on the film after coating.20 The remaining fertilizers, urea (46% N), DAP (18.0% N, 46.0% P2O5), and potassium chloride (60.0% K2O), were acquired from the local market. The test crop was the summer maize variety “Zhengdan 958”, with 96 days of fertility and a thousand grains weighing 302 g.
Experimental Design and Treatments
The pot experiment was carried out at the research farm of NELEUSFR, SDAU, China. The site is located at the central region of Shandong Province, with an average annual temperature of about 13 °C and a warm-temperate continental monsoon climate. The following seven treatments were set up, each with four replications: (1) Control groups: CK (no P control), P100% (DAP at 75 kg of P2O5 ha–1), and CP100% (CDAP at 75 kg of P2O5 ha–1); (2) Test groups: P100%Z (DAP at 75 kg of P2O5 ha–1 combined with ZNC), P80%Z (DAP at 60 kg of P2O5 ha–1 combined with ZNC), CP100%Z (CDAP at 75 kg of P2O5 ha–1 combined with ZNC), and CP80%Z (CDAof P at 60 kg of P2O5 ha–1 combined with ZNC). The data for CK, P100%, and CP100% treatments as the Control group in this study was published in Frontiers in Plant Science by Chen.34
One kilogram of sand was first placed at the bottom of each ceramic pot (36.0 cm in height, 30.0 cm in diameter) to improve aeration and promote more oxygen supply to the root system,35 and then 20 kg of soil was placed on the top of the sand layer. The soil used was air-dried, blended equally, and sieved before use. The test sand (0.35–0.5 mm) was purchased from the local market. Soil moisture was kept at 70 ± 5% of the field water-holding capacity with an automatic drip irrigation system.36 For the control treatment, nitrogen and potassium fertilizers were applied once as a basal fertilizer at 225 kg of N ha–1 and 150 kg of K2O ha–1, respectively, whereas for the other treatments, the N–P2O5–K2O rate was applied at 225–150–75 kg ha–1 and the 20% phosphorus reduction treatment was applied at 225–120–75 kg ha–1, with ZNC added at 100 mg acre–1.37 The phosphate fertilizer rate applied to one maize plant was 1.80 or 1.44 g pot–1 (P2O5) based on standard growth practices by local growers (83 325 maize plants ha–1). The amount of fertilizer applied was doubled for the pot experiment. For all treatments, both coated controlled-release nitrogen and conventional nitrogen were used to provide 60 and 40% of the total applied nitrogen, respectively.37,38
On June 20, 2017, three seeds of maize were sown in each container. At the three-leaf stage, the seedlings were reduced to one. Agricultural management, such as pest and weed control, was carried out as needed in accordance with local customs. In 2018, the experiment was repeated using the same pots.
Maize ears were harvested after maturity on September 29, 2017, and September 26, 2018. To deactivate the enzymes, kernels and plant samples were oven-dried at 105 °C for 15 min and then dried at 65 °C to a constant weight.39 The biomass and yield of the maize were determined.
Sampling Analysis
Soil samples were collected at depths ranging from 0 to 20 cm, fully mixed at each point, and brought back to the laboratory where they were naturally dried in the light, ground, passed through a 2 mm sieve, and stored for testing. In 2017, at the growth stages of seedling (V3), six-leaf (V6), 12-leaf (V12), silking stage (R1), and maturity stage (R6), soil samples were taken from 0 to 20 cm layer of each pot, air-dried, ground, and sieved to <2 mm; plant height was measured from the soil surface to the top of the plant stem; the diameter of the maize stem was measured at the middle of the third node from the soil surface. The readings from the Soil Plant Analysis Development (SPAD) chlorophyll meter were taken between 09:00 and 11:00 a.m. (SPAD-502, Minolta, Japan). Soil available-P was extracted with 0.5 M NaHCO3 (pH = 8.5) and quantified with an automatic chemical analyzer (Smartchem200, AMS, Italy). Soil NO3–-N and NH4+-N were extracted with 0.01 M CaCl2 (1:10 soil to water ratio) and measured with a continuous-flow injection analyzer (AA3-A001-02E, Bran-Luebbe, Germany).40,41 A flame photometer was used to determine soil available-K after extracting it with 1.0 M CH3COONH4.42
The photosynthetic rates during the V12 stage were determined in 2018 between 09:00 and 11:00 a.m. using an LI-6400XT portable photosynthesis system (LI-Cor, Lincoln, NE). Then, the fresh plant leaves, roots, and soil were sampled and frozen in liquid nitrogen for biochemical analysis. Contents of phosphoenolpyruvate carboxylase (PEPC), adenosine triphosphatase (ATP synthase, ATP8), pyruvate phosphate dikinase (PPDK), adenosine diphosphate pyrophosphorylase (AGPase), adenylosuccinate synthase (AMPSS), phosphoribosyl pyrophosphatase (PRPP), phosphoribosyl pyrophosphate acyltransferase (PRPPAT), succinyl coenzyme A synthetase (SCS), glyceraldehyde-3-phosphate dehydrogenase (GAPDH), auxin-indole-3-acetic acid (IAA), cytokinin (CTK), abscisic acid (ABA), and gibberellin (GA) of maize leaves were measured using the ELISA kit from Shanghai HengYuan Biological Technology Co., Ltd. (Shanghai, China) according to the manufacturer’s instructions. The acid phosphatase (AP) and alkaline phosphatase (ALP) activity of maize root and soil were measured using the ELISA kit. At harvest time, the stalks and kernels were killed in an oven at 105 °C for 15 min, then dried in an oven at 65 °C to a constant weight, and finally weighed and ground for measurement. These indicators were used to investigate soil nutrient supply intensity, photosynthetic properties, and maize growth status.
After digestion with H2SO4–H2O2, the total P content of the plant was determined using the molybdenum–antimony method.42
Statistical Analysis
All data were collected and processed with Microsoft Excel 2019 and IBM SPSS Statistics 22 software (SPSS Inc., IL), and figures were created with Origin 2021 (Version 2021b, Origin Lab Corporation, MA). One-way analysis of variance (ANOVA) was used between treatments. SPSS was used to analyze the significance of different treatments using analysis of variance (ANOVA) with Duncan testing (P < 0.05).
Acknowledgments
This study was supported by the National Natural Science Foundation of Shandong Province (No. ZR2020MD106) and the Key Research and Development Program of Shandong Province (No. 2019GNC106011).
Glossary
Abbreviations
- ZNC
Paecilomyces variotii extracts
- P
phosphorus
- N
nitrogen
- DAP
diammonium phosphate
- CDAP
coated diammonium phosphate
- CRP
controlled-release phosphate fertilizer
The authors declare no competing financial interest.
References
- Geng J. B.; Chen J. Q.; Sun Y. B.; Zheng W. K.; Tian X. F.; Yang Y. C.; Li C. L.; Zhang M. Controlled Release Urea Improved Nitrogen Use Efficiency and Yield of Wheat and Corn. Agron. J. 2016, 108, 1666–1673. 10.2134/agronj2015.0468. [DOI] [Google Scholar]
- Guo J. J.; Fan J. L.; Xiang Y. Z.; Zhang F. C.; Yan S. C.; Zhang X. Y.; Zheng J.; Li Y. P.; Tang Z. J.; Li Z. J. Coupling effects of irrigation amount and nitrogen fertilizer type on grain yield, water productivity and nitrogen use efficiency of drip-irrigated maize. Agric. Water Manage. 2022, 261, 107389 10.1016/j.agwat.2021.107389. [DOI] [Google Scholar]
- Li G. H.; Cheng G. G.; Li L.; Lu D. L.; Lu W. P. Effects of slow-released fertilizer on maize yield, biomass production, and source-sink ratio at different densities. J. Plant Nutr. 2020, 43, 725–738. 10.1080/01904167.2019.1701027. [DOI] [Google Scholar]
- Naomi M. R.; Supriyono; Nurmalasari I. A.; Pardono Role of phosphate fertilizer on growth and yield of hybrid maize (Zea mays L.). IOP Conf. Ser.: Earth Environ. Sci. 2021, 637, 012070 10.1088/1755-1315/637/1/012070. [DOI] [Google Scholar]
- Gerke J.; Hermann R. Adsorption of Orthophosphate to Humic-Fe-Complexes and to Amorphous Fe-Oxide. J. Plant Nutr. Soil Sci. 1992, 155, 233–236. 10.1002/jpln.19921550313. [DOI] [Google Scholar]
- Bender R. R.; Jason W. H.; Matias L. R.; Fred E. Nutrient uptake, partitioning, and remobilization in modern, transgenic insect-protected maize hybrids. Agron. J. 2013, 105, 161–170. 10.2134/agronj2012.0352. [DOI] [Google Scholar]
- Hinsinger P. Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. plant soil. Plant. Soil 2001, 237, 173–195. 10.1023/A:1013351617532. [DOI] [Google Scholar]
- Fu J. J.; Wang C. Y.; Chen X. X.; Huang Z. W.; Chen D. M. Classification research and types of slow controlled release fertilizers (SRFs) used - a review. Commun. Soil Sci. Plant Anal. 2018, 49, 2219–2230. 10.1080/00103624.2018.1499757. [DOI] [Google Scholar]
- Gu N.; Zhao L. P.; Zhao X. M. A Review and Perspective on Slow and Controlled Release Fertilizer in China. Appl. Mech. Mater. 2014, 535, 222. 10.4028/www.scientific.net/AMM.535.222. [DOI] [Google Scholar]
- Lu H.; Tian H. Y.; Liu Z. G.; Zhang M.; Zhao C. H.; Guo Y. L.; Guan R.; Chen Q.; Yu X. J.; Wang H. L.; Zheng L. Polyolefin Wax Modification Improved Characteristics of Nutrient Release from Biopolymer-Coated Phosphorus Fertilizers. ACS Omega 2019, 4, 20402–20409. 10.1021/acsomega.9b03348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaseen M.; Aziz M. Z.; Manzoor A.; Naveed M.; Hamid Y.; Noor S.; Khalid M. A. Promoting growth, yield, and phosphorus-use efficiency of crops in maize–wheat cropping system by using polymer-coated diammonium phosphate. Commun. Soil Sci. Plant Anal. 2017, 48, 646–655. 10.1080/00103624.2017.1282510. [DOI] [Google Scholar]
- Jing S. C.; He D. S. Present development situation and prospects of China’s phosphate fertilizer industry. Mod. Chem. 2018, 38, 5–9. [Google Scholar]
- Salmiaton A.; Firoozeh D. Controlled-Release Fertilizers: Advances and Challenges. Life Sci. J. 2015, 12, 33–45. [Google Scholar]
- Wang Q. B.; Peng C. E.; Shi L. R.; Liu Z. G.; Zhou D. F.; Meng H.; Zhao H. L.; Li F. C.; Zhang M. A technical system for the large-scale application of metabolites from Paecilomyces variotii SJ1 in agriculture. Front. Bioeng. Biotechnol. 2021, 9, 671879 10.3389/fbioe.2021.671879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng C. E.; Zhang A. L.; Wang Q. B.; Song Y. Z.; Zhang M.; Ding X. H.; Li Y.; Geng Q. Z.; Zhu C. X. Ultrahigh-activity immune inducer from Endophytic Fungi induces tobacco resistance to virus by SA pathway and RNA silencing. BMC Plant Biol. 2020, 20, 169. 10.1186/s12870-020-02386-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaitieng S.; Kanokkorn S.; Pornpairin R.; Suphachai A. Arbuscular mycorrhiza fungi applications and rock phosphate fertilizers enhance available phosphorus in soil and promote plant immunity in robusta coffee. Soil Sci. Plant Nutr. 2021, 67, 97–101. 10.1080/00380768.2020.1848343. [DOI] [Google Scholar]
- Wang X. Q.; Yao Y. Y.; Chen B. C.; Zhang M.; Liu Z. G.; Wang Q. B.; Ma J. Z. Paecilomyces variotii extracts and controlled-release urea synergistically increased nitrogen use efficiency and rice yield. ACS Omega 2020, 5, 13303–13311. 10.1021/acsomega.0c01348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C. C.; Liu H. F.; Jiang D. P.; Wang L. L.; Jiang Y. K.; Tang S. Y.; Hou X. W.; Han X. Y.; Liu Z. G.; Zhang M.; Chu Z. H.; Ding X. H. Paecilomyces variotii extracts (ZNC) enhance plant immunity and promote plant growth. Plant Soil 2019, 441, 383–397. 10.1007/s11104-019-04130-w. [DOI] [Google Scholar]
- Wang X. Q.; Yao Y. Y.; Liu Z. G.; Chen B. C.; Zhang M.; Wang Q. B.; Ma J. Z. Highly active biostimulant Paecilomyces variotii extracts reduced controlled-release urea application while maintaining rice yield. J. Sci. Food Agric. 2022, 102, 1883–1893. 10.1002/jsfa.11525. [DOI] [PubMed] [Google Scholar]
- Chen Q.; Li Z. L.; Qu Z. M.; Zhou H. Y.; Qi Y. J.; Liu Z. G.; Zhang M. Maize yield and root morphological characteristics affected by controlled-release diammonium phosphate and Paecilomyces variotii extracts. Field Crops Res. 2020, 255, 107862 10.1016/j.fcr.2020.107862. [DOI] [Google Scholar]
- Wang L.; Zheng J. D.; You J. J.; Li J.; Qian C.; Leng S. H.; Yang G.; Zuo Q. S. Effects of Phosphorus Supply on the Leaf Photosynthesis, and Biomass and Phosphorus Accumulation and Partitioning of Canola (Brassica napus L.) in Saline Environment. Agronomy 2021, 11, 1918. 10.3390/agronomy11101918. [DOI] [Google Scholar]
- Bai Z. H.; Li H. G.; Yang X. Y.; Zhou B. K.; Shi X. J.; Wang B. R.; Li D. C.; Shen J. B.; Chen Q.; Qin W.; Oenema O.; Zhang F. S. The critical soil P levels for crop yield, soil fertility and environmental safety in different soil types. Plant Soil 2013, 372, 27–39. 10.1007/s11104-013-1696-y. [DOI] [Google Scholar]
- Duff S. M. G.; Gautam S.; William C. P. The role of acid phosphatases in plant phosphorus metabolism. Physiol. Plant 1994, 90, 791–800. 10.1111/j.1399-3054.1994.tb02539.x. [DOI] [Google Scholar]
- Wang H. F.; Geng Q. Z.; Kong B.; Zhang X. Y.; Ding X. H.; Wang Q. B.; Chen D. Y. Research Progress of Brassinolide and ZNC Immune Inducer. Mod. Agric. Tech. 2020, 19, 127–130. [Google Scholar]
- Chen Q.; Liu Z. G.; Zhang M.; Li Z. L.; Qu Z. M.; Yang M. F.; Sun L. L. Diammonium phosphate wrapped with xanthate to improve wheat yield and soil nutrient supply intensity. Soil Sci. 2018, 55, 1472–1484. [Google Scholar]
- Moreno-Gavíra A.; Fernando D.; Brenda S. M.; Mila S. Paecilomyces variotii as A Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 597. 10.3390/agronomy10040597. [DOI] [Google Scholar]
- Tian H. Y.; Li Z. L.; Lu P. F.; Wang Y.; Jia C.; Wang H. L.; Liu Z. G.; Zhang M. Starch and castor oil mutually modified, cross-linked polyurethane for improving the controlled release of urea. Carbohydr. Polym. 2021, 251, 117060 10.1016/j.carbpol.2020.117060. [DOI] [PubMed] [Google Scholar]
- Li R. C.; Gao Y. X.; Chen Q.; Li Z. L.; Gao F.; Meng Q. M.; Li T. G.; Liu A. R.; Wang Q.; Wu L.; Wang Y.; Liu Z. G.; Zhang M. Blended controlled-release urea with straw returning improved soil nitrogen availability, soil microbial community, and root morphology of wheat grown. Soil. Till Res. 2021, 212, 105045 10.1016/j.still.2021.105045. [DOI] [Google Scholar]
- Le Pioufle O.; Matike G.; Maryline C. S.; Fatma B. D.; Stéphane D. Rhizophagus irregularis MUCL 41833 improves phosphorus uptake and water use efficiency in maize plants during recovery from drought stress. Front. Plant Sci. 2019, 10, 897. 10.3389/fpls.2019.00897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos E. F.; Paula P.; Rodrigues R. A.; Silveira R. F. H.; Antunes A. R.; White P. J.; Lavres J. Unravelling homeostasis effects of phosphorus and zinc nutrition by leaf photochemistry and metabolic adjustment in cotton plants. Sci. Rep. 2021, 11, 13746 10.1038/s41598-021-93396-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao H.; Chen H. K.; Conde L. D.; Chen Z. J. Effects of phosphorus availability on plant growth and soil nutrient status in the rice/soybean rotation system on newly cultivated acidic soils. Am. J. Agric. For. 2015, 2, 309. 10.11648/j.ajaf.20140206.23. [DOI] [Google Scholar]
- Cao J.; Liu B. Y.; Xu X. N.; Zhang X. Y.; Zhu C. X.; Li Yang.; Ding X. H. Plant endophytic fungus extract ZNC improved potato immunity, yield, and quality. Front. Plant Sci. 2021, 12, 707256 10.3389/fpls.2021.707256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X.-j.; Chen Q.; Shi W. C.; Gao Z.; Sun X.; Dong J. J.; Li J.; Wang H. T.; Gao J. G.; Liu Z. G. Interactions between phosphorus availability and microbes in a wheat-maize double cropping system: a reduced fertilization scheme. J. Integr. Agric. 2022, 21, 840–854. 10.1016/S2095-3119(20)63599-7. [DOI] [Google Scholar]
- Chen Q.; Qu Z. M.; Li Z. L.; Zhang Z. X.; Ma G. H.; Liu Z. G.; Wang Y. F.; Wu L.; Fang F.; Wei Z. B.; Zhang M. Coated diammonium phosphate combined with humic acid improves soil phosphorus availability and photosynthesis and the yield of maize. Front. Plant Sci. 2021, 12, 759929 10.3389/fpls.2021.759929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. L.; Liu Z. G.; Zhang M.; Chen Q.; Zheng L.; Li Y. C.; Sun L. L. The combined application of controlled-release urea and fulvic acid improved the soil nutrient supply and maize yield. Arch. Agron. Soil Sci. 2021, 67, 633–646. 10.1080/03650340.2020.1742326. [DOI] [Google Scholar]
- Liu Z. G.; Zhang M.; Geng J. B.; Chen B. C.; Yang Y. C.; Li C. L.. An Automatic Irrigation and Moisture Monitoring Device for Crop Pot Experiment. Chinese Patent CN104663373B, 2017.
- Zheng W. K.; Zhang M.; Liu Z. G.; Zhou H. Y.; Lu H.; Zhang W. T.; Yang Y. C.; Li C. L.; Chen B. C. Combining controlled-release urea and normal urea to improve the nitrogen use efficiency and yield under wheat-maize double cropping system. Field Crops Res. 2016, 197, 52–62. 10.1016/j.fcr.2016.08.004. [DOI] [Google Scholar]
- Qu Z. M.; Qi X. C.; Shi R. G.; Zhao Y. J.; Hu Z. P.; Chen Q.; Li C. L. Reduced N fertilizer application with optimal blend of controlled-release urea and urea improves tomato yield and quality in greenhouse production system. J. Soil Sci. Plant Nutr. 2020, 20, 1741–1750. 10.1007/s42729-020-00244-8. [DOI] [Google Scholar]
- Gao Y. X.; Song X.; Liu K. X.; Li T. G.; Zheng W. K.; Wang Y.; Liu Z. G.; Zhang M.; Chen Q.; Li Z. L.; Li R. C.; Zheng L.; Liu W. L.; Miao T. Y. Mixture of controlled-release and conventional urea fertilizer application changed soil aggregate stability, humic acid molecular composition, and maize nitrogen uptake. Sci. Total Environ. 2021, 789, 147778 10.1016/j.scitotenv.2021.147778. [DOI] [PubMed] [Google Scholar]
- Houba V. J. G.; Novozansky I.; Huybregts A. W. M.; Van der Lee J. J. Comparison of soil extractions by 0.01 M CaCl2, by EUF and by some conventional extraction procedures. Plant Soil 1986, 96, 433–437. 10.1007/BF02375149. [DOI] [Google Scholar]
- Dou H.; Alva A. K.; Appel T. An evaluation of plant-available soil nitrogen in selected sandy soils by electro-ultrafiltration, KCl, and CaCl2 extraction methods. Biol. Fertil. Soils 2000, 30, 328–332. 10.1007/s003740050011. [DOI] [Google Scholar]
- Lu R. K.The Analysis Method of Soil Agro-Chemistry; Chinese Agricultural Academic Press: Beijing, 2000. [Google Scholar]