Abstract
We present a case in which lung ultrasound (LUS) was relevant to reach an early diagnosis of lung tuberculosis and to manage the patient in the right setting. Moreover, ultrasound allowed to detect and treat massive pleural effusion through an ultrasound‐guided thoracentesis.
Keywords: bedside, lung ultrasound, pleural effusion, tuberculosis
A 36‐year‐old Caucasian policeman was referred to our Emergency Department for dyspnea. He reported persistent cough and fever with night sweats for about 2 weeks, for which antibiotic therapy for 5 days without clinical benefit. Laboratory investigations revealed a C‐reactive protein of 15.10 mg/dl (n.v. 0.00–0.50). We performed a bedside lung ultrasound (LUS) that showed a massive finely corpuscular anechoic pleural effusion with thin branches of fibrin on the left, with a consolidated lung parenchyma collapsing to the hilum as for complete atelectasis; in the context of the consolidated lung parenchyma, multiple round hypo‐anechoic formations were seen, with finely irregular margins, not assuming colordoppler signal suspicious for cavitations.
1. INTRODUCTION
Tuberculosis (TB) is one of the leading causes of death due to infectious diseases, and it is a global health threat. TB incidence has undergone a rapid increase in the last decade, attributable to the increase in risk factors such as HIV co‐infections, drug resistance, and emigrations from areas where the disease is endemic. Today, TB is the major infectious causes of death in the world, with 1.6 million deaths in 2017. 1 Here, we present the case of a young policeman referred to our hospital for dyspnea and respiratory failure. Utilization of an ultrasound allowed for establishment of a fast and specific diagnostic program, and to perform a diagnostic thoracentesis. Subsequently, we performed a narrative review on the role of ultrasound in diagnosing lung tuberculosis.
2. CASE PRESENTATION
A 36‐year‐old Caucasian policeman was referred to our Emergency Department for dyspnea. He reported persistent cough and fever with night sweats for about 2 weeks, for which antibiotic therapy with Levofloxacin 750 mg daily for 5 days was prescribed without clinical benefit. His past medical history was not suggestive for any disease.
Blood pressure (BP) and cardiac rhythm were normal (BP: 130/75 mmHg; heart rate: 91 bpm, sinus rhythm), but the arterial blood gas analysis revealed a respiratory failure (peripheral oxygen saturation: 87% on room air; pO2: 53 mmHg; pCO2: 41 mmHg). The physical examination showed a hypo‐expansion of the left hemithorax, reduced tactile fremitus, and medium‐basal hypophonesis with abolished vesicular murmur.
Blood laboratory tests showed a blood glucose level of 60 mg/dl (normal value 74–106), a C‐reactive protein of 15.10 mg/dl (n.v. 0.00–0.50), and the following blood count: white blood cells 10,760/µl (n.v. 4000–11,000), red blood cells 4,850,000/µl (n.v. 4,500,000–5,300,000), hemoglobin 14.2 g/dl (n.v. 13–16.0), neutrophils 7810/µl (n.v. 2100–7100), lymphocytes 1800/µl (n.v. 1100–3000), monocytes 1080/µl (n.v. 200–960), eosinophils 50/µl (n.v. 0–500), and basophils 20/µl (n.v. 0−200).
Urea, creatinine, sodium, potassium, calcium, transaminases, gamma‐glutamyl transpeptidase, amylase, lipase, lactate dehydrogenase, total and fractionated bilirubin, and procalcitonin were normal.
Chest X‐ray was required, and we performed a bedside lung ultrasound (LUS) to complete the physical examination.
LUS showed a massive finely corpuscular anechoic pleural effusion with thin branches of fibrin on the left, with a consolidated lung parenchyma collapsing to the hilum as for complete atelectasis; in the context of the consolidated lung parenchyma, multiple round hypo‐anechoic formations were seen, with finely irregular margins, not assuming colordoppler signal: cavitations? Abscesses? (Figures 1 and 2).
FIGURE 1.
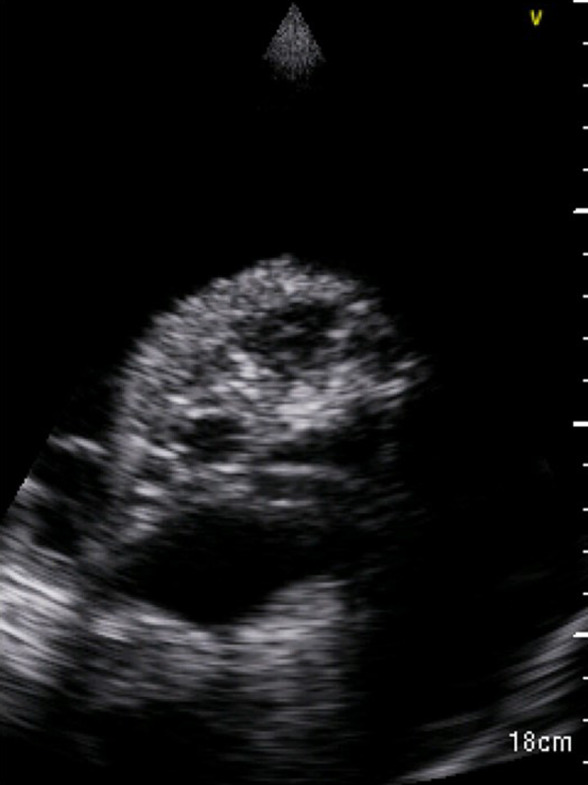
Bedside thoracic US of the left hemithorax showing massive pleural effusion, complete lung atelectasis, and cavitations in the context of the consolidated parenchyma
FIGURE 2.
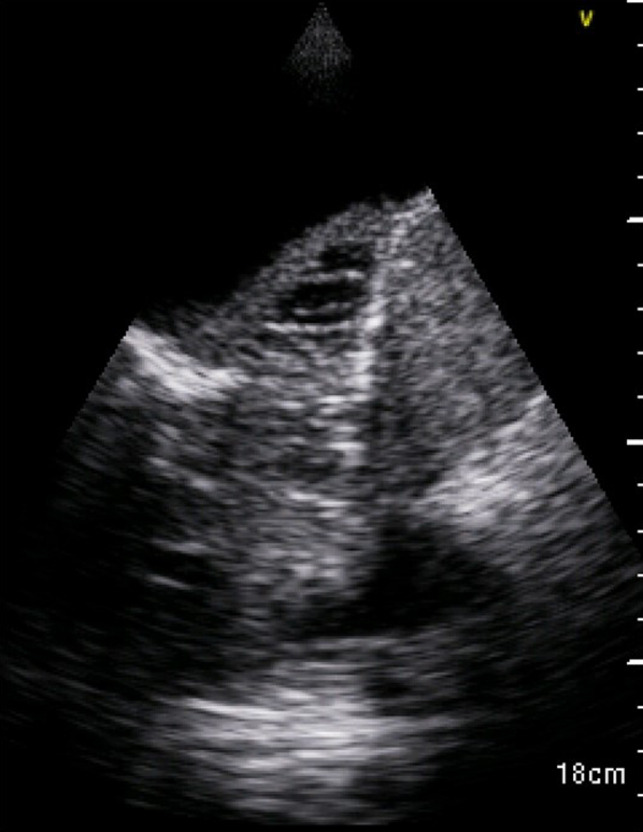
Bedside thoracic US of the left hemithorax showing cavitations in collapsed left lung
Therefore, chest X‐ray confirmed the pleural effusion associated with consensual parenchymal hypoventilation and showed a widespread non‐specific thickening of the bronchial walls. No pleural effusion on the right or no signs of pneumothorax were observed. The cardiac shape was within limits. Surprisingly, the radiographic images did not show the anechoic roundish formations identified by US.
Therapy with oxygen supplementation was set, and the patient was admitted to the infectious diseases ward and isolated according to the US findings.
In the ward, an ultrasound‐guided diagnostic‐evacuative thoracentesis was performed with drainage of yellow citrine liquid. Chest computed tomography (CT) was performed to better characterize the images observed with US and not confirmed by chest X‐ray showing an excavated formation with fluid‐air levels in the apical segment of the left lower lobe of about 6 × 3.5 cm (Figure 3). This formation was surrounded by an area of slightly increased density of the pulmonary parenchyma, with ground‐glass appearance.
FIGURE 3.
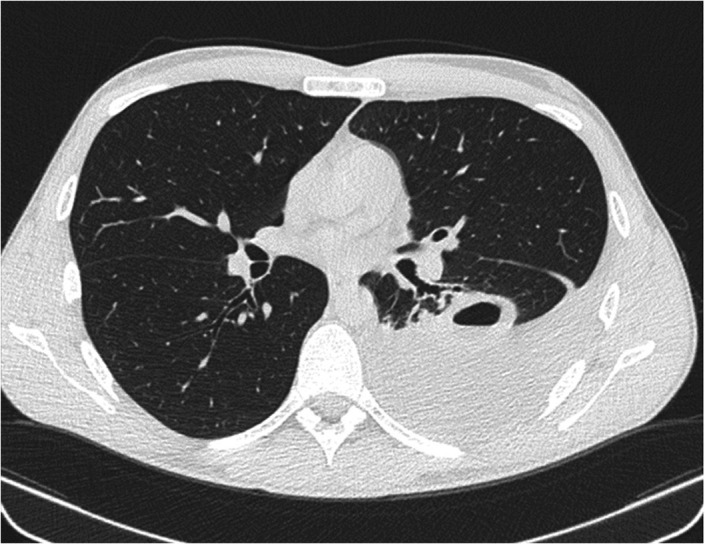
Chest CT after thoracentesis confirming the presence of excavated formation with fluid‐air levels in the left lung
Another subpleural area of parenchymal consolidation with pseudonodular morphology was present in the apicodorsal segment of the left upper lobe and appeared as excavated and communicating with the adjacent bronchus. A parenchymal consolidation area with appreciable subpleural morphology was present in the apical segment of the right upper lobe, showing a maximum axial diameter of about 2.2 cm and appeared excavated. Atelectasis striae in the lower segment of the lingula and in the basal pyramid of the left lower lobe were present. The findings described were compatible with TB disease with cavitations. A fibrobronchoscopy was performed showing marked inflammation of the mucous membrane of the left bronchial hemisystem, where whitish secretions were detected.
The analysis of pleural fluid and bronchoalveolar lavage fluid, and microbiological examination on sputum were positive for alcohol‐acid resistant elements, confirming the diagnosis of pleuro‐pulmonary TB. First‐line specific antibiotic therapy was established, and the patient was discharged on the seventh day in good clinical condition with indication for outpatient follow‐up. The final diagnosis was pleuro‐pulmonary cavitary tuberculosis.
3. DISCUSSION AND LITERATURE REVIEW
In past years, LUS has shown increased utilization in the diagnostic field alongside traditional diagnostic tests. Although the presence of an air interface below the pleura prevents the visualization of the lung in depth, the evaluation of sonographic artifacts allows for evaluation of several pathological conditions and it is a useful tool to address a more focused and rapid diagnosis.
To our knowledge, there are few data about US findings in lung TB 2 focusing on the following fields of interest: detection of pleural effusion, assessment of residual pleural thickening, the execution of trans‐thoracic needle biopsy, assessment of mediastinal lymphadenopathies, and detection of pulmonary involvement in miliary TB. 3
Sonographic pattern of pulmonary TB can be visualized as consolidations, subpleural nodules, pleural thickenings, fibrosis, pleural effusion, and pneumothorax together with miliary pattern. 2 , 4 , 5 The miliary pattern has been described by Hunter et al. 6 as characterized by bilateral vertical artifacts (B‐lines and/or “comet‐tail artifacts”) in multiple lung areas. This pattern may be also characterized by sub‐pleural granular changes, which may be more characteristic of miliary TB 6 versus other presentations. However, these findings are not so specific, since similar bilateral vertical artifacts have been described in patients with Pneumocystis jirovecii or cytomegaly virus pneumonia and sub‐pleural US alterations can be found in other pulmonary conditions such as metastatic thyroid cancer. 6
Agostinis et al. 2 classified the sonographic findings in patients with lung TB into two categories: lung (subpleural nodule, pleural effusion, miliary pattern, and cavitations) and extra‐lung (pericardial effusion, splenomegaly, abdomen lymph nodes, hepatomegaly, and ascites). 2 The hypoechoic subpleural nodules (SUNs) were the most frequently identified in sixty adults with diagnosis of lung TB in a rural African setting. 2 Consolidations were detected in about half of the patients and are indistinguishable from bacterial pneumonia; cavitations were observed in three patients and described as anechoic or hypoechoic areas within solid lung consolidation. 2 SUNs were not identified in subjects without TB, suggesting an important role in diagnosis of lung TB. 2
The accuracy of LUS in the diagnosis of pulmonary TB in adults has been investigated in a recent study. 4 Montuori et al. 4 demonstrated that the combination of apical consolidations and SUNs found in the same patients has a specificity of 96% and a sensibility of 31%, when at least one of these signs is identified, the sensitivity and the specificity are of 86% and 63%, 4 respectively. According to Agostinis et al., 2 cavitations were found to be the least common sign and not sensitive enough also in this study, probably due to the presence of air in cavitations or due to the fact that a high number of lesions do not reach the pleura. 4
In our case, the identification of the non‐vascularized hypo‐anechoic roundish lesions compatible with cavitations was fundamental in the diagnostic suspicion of cavitary TB and for the early isolation of our patient in order to avoid the spreading of the infection in the crowded environment of the Emergency Room. It must be considered that our patient had not apparent risk factors for TB in relation to the clinical context (patient medical history and lifestyle, HIV‐negative test), and that the chest X‐ray did not show the cavitations identified by US, so that the patient would have been hospitalized in a medical setting without the necessary isolation measures. Even if chest CT and microbiological and laboratory tests were fundamental in the different diagnosis between cavitations and lung abscesses, the findings provided by bedside LUS were relevant to confirm our diagnostic suspicion. This diagnosis subsequently allowed establishing an effective therapeutic approach, as has been already described in critical care. 7 In our case, we did not identify SUNs and apical consolidations because the lung parenchyma was completely collapsed due to the massive pleural effusion. However, the chest CT performed after evacuation of pleural fluid documented parenchymal consolidations at the level of the apical segments of the left upper and lower lobes, and of the right upper lobe, which are typical lung fields where consolidations can be found in post‐primary tuberculosis. 8
LUS has been increasingly used to safely perform bedside thoracentesis. 7 , 9 , 10 , 11 In our case, the possibility of identifying the corpuscular nature of the pleural fluid has contributed to the diagnostic suspicion, 5 , 12 and the US quantification of the fluid was fundamental to guide the therapeutic intervention. 13
We did not perform a complete US evaluation of the abdomen since we focused on the immediate evaluation of the lung parenchyma to diagnose the possible life‐threatening condition given the Emergency setting and the need to evaluate the respiratory status of our patient who presented with a respiratory failure. However, the abdominal CT performed during hospitalization did not show lesions compatible with the dissemination of the disease.
Other possible applications of thoracic US in the diagnosis of TB are described in literature, especially in patients with suspected mediastinal TB (Table 1). Diagnosis of mediastinal TB is difficult due to non‐specific clinical features and lack of characteristic radiographic features. 14 In order to obtain histopathological confirmation, a CT‐guided fine‐needle aspiration biopsy (FNAB) or invasive procedures such as mediastinoscopy or open surgical biopsy are often required. 14 US‐guided FNAB has proven to be safe, and effective in the diagnosis of mediastinal TB, 14 despite its limitations for centrally located lesions due to the lack of a good acoustic window. 15 More recently, some authors have investigated the possibility of performing endobronchial ultrasound (EBUS). 16 , 17 This may be useful in investigating peripheral lung lesions and in guiding transbronchial FNAB and endoscopic aspiration from esophagus in suspected mediastinal TB. 18 , 19
TABLE 1.
List and review of previously published works on the application of thoracic ultrasound in patients affected by TB
| Article | Patients | US method | Findings |
|---|---|---|---|
| Agostinis P et al. Trop Doct. 2017 2 | 60 adult patients diagnosed with lung TB | TUS | The most frequent finding was a SUN, which was mostly multiple and also found in radiologically normal areas. Other findings were lung consolidations, cavitations, miliary patterns made of miniature SUNs, and pleural and pericardial effusions. Chest US is a complementary tool in evaluating patients with suspected lung TB in resource‐limited settings where the disease has high prevalence. |
| Di Gennaro F et al. Int J Environ Res Public Health 2018 3 | Review article | TUS | Five main fields of chest US application in TB were identified: (1) Detection, characterization, and quantification of TB; (2) detection of residual pleural thickening after evacuation; (3) chest ultrasound‐guided needle biopsy; (4) identification of pathologic mediastinal lymph nodes in children; and (5) identification of parenchymal ultrasound patterns. Effusion was also detected, in early stages, with signs of organization in 24% of patients. CUS was able to identify mediastinal lymph nodes in as many as 67% of patients with negative chest radiography. |
| Montuori M et al. Eur J Intern Med 2019 4 | Patients admitted with clinical suspicion of PTB. PTB was confirmed in 51 out of 102 patients. | TUS | Multiple consolidations, apical consolidations, superior quadrant consolidations, and subpleural nodules were significantly associated with PTB diagnosis. Apical consolidation and subpleural nodules retained a significant association in a multivariate model, with an overall accuracy of 0.799. |
| Hunter L et al. Infection 2016 8 | 10 patients with miliary TB | TUS | B‐lines and comet‐tail artifacts disseminated throughout multiple lung areas and a pattern of sub‐pleural granularity are seen in lung ultrasound of patients with pulmonary miliary TB diagnosed by chest radiography. |
| Jones PW, et al. CHEST 2003 9 | 605 patients referred to interventional radiology for a diagnostic and/or therapeutic ultrasound‐guided thoracentesis between August 1997 and September 2000 | TUS | The complication rate with ultrasound‐guided thoracentesis is lower than that reported for non–image‐guided thoracentesis. |
| Feller‐Kopman D. CHEST 2006 10 | Review article | TUS | Ultrasonography is a portable and easily learned procedure that enhances the physical examination, and can provide real‐time evaluation of the pleural space. It is becoming the standard of care for procedural guidance since its use has been associated with a reduction of complications due to thoracentesis. |
| Mercaldi CJ and Lanes SF. CHEST 2013 11 | 61,261 patients who had a thoracentesis, including 26,838 (44%) who had US guidance. | TUS | US‐guided thoracentesis is associated with decreased risk of pneumothorax. |
| Chen HJ et al. J Ultrasound Med. 2006 12 | 73 patients with lung cancer‐related pleural effusions and 93 with tuberculous pleural effusions | TUS | A complex septated pattern in the sonographic appearance is a useful predictor of tuberculosis in lymphocyte‐rich exudative pleural effusions. |
| Hew M. and Tay T. Eur Respir Rev 2016 13 | Review article | TUS | For bedside US of the pleura, there is evidence supporting diagnostic accuracy efficacy, and efficacy in guiding pleural interventions. Chest US for the lung parenchyma has an impact on diagnostic accuracy and decision‐making for patients presenting with acute respiratory failure or breathlessness. |
| Gulati M et al. Int J Tuberc Lung Dis. 2000 14 | 26 patients with a proven diagnosis of mediastinal TB | TUS | US‐guided FNAB is a safe, effective technique in the diagnosis of mediastinal TB. |
| Yuan A et al. Thorax. 1993 15 | 13 patients | TUS | US can direct the needle to the most suitable part of a lesion to obtain the relevant specimens. The diagnostic yield is high, and the procedure is relatively safe. It is especially helpful in patients with negative results of sputum and bronchoscopic examinations. |
| Chan A et al. BMC Pulm Med 2015 16 | 123 patients with computed tomographic evidence of PLLs who underwent radial EBUS‐guided bronchoscopy | EBUS | EBUS‐FNA is useful in investigating PLLs in a high TB incidence setting. Radial EBUS is a more rapid diagnosis technique for tuberculous lesions. |
| Thangakunam B et al. Indian J Tuberc 2017 17 | 138 patients who EBUS | EBUS | In high TB prevalence countries, EBUS‐FNA diagnoses a higher number of granulomatous than malignant diseases. |
| Chalela R et al. Med Clin (Barc) 2016 18 | 46 patients with mediastinal lymphadenopathy without pulmonary involvement | EBUS | EBUS‐TBNA is a safe and effective technique in the diagnosis of patients with suspected mediastinal TB. |
| Sharma M et al. Lung India 2016 19 | 266 patients underwent endoscopic ultrasound‐guided fine‐needle aspiration, and 134 cases were diagnosed as mediastinal tuberculosis | EUS | EUS‐FNA should be the investigation of choice for diagnosis of mediastinal tuberculosis. |
Abbreviations: EBUS‐FNA, endobronchial ultrasound‑guided fine‐needle aspiration from bronchus; EBUS‐TBNA, transbronchial needle aspiration guided by endoscopic ultrasonography; EUS‐FNA, endoscopic ultrasound‑guided aspiration from esophagus; PLLs, peripheral lung lesions; SUN, subpleural nodule; TB, tuberculosis; TUS, thoracic ultrasound; US, ultrasound.
4. CONCLUSIONS
Bedside US is a safe, non‐invasive, portable, versatile, easily repeatable, and cost‐effective imaging modality. 4 , 7 Its use can lead to the identification of specific pleuro‐pulmonary pathological conditions by strengthening the clinical‐diagnostic suspicion. While there are few data about LUS evaluation in tuberculosis, in our case it was fundamental in the diagnosis of the characteristic lung lesion through direct visualization of and early isolation of the patient; early suspicion allowed prompt diagnostic investigations and initiation of targeted therapy. Our case has also confirmed that LUS is important for the identification, quantization, and characterization of pleural effusion and for the execution of diagnostic and therapeutic ultrasound‐guided thoracentesis.
CONFLICT OF INTEREST
None.
ETHICAL APPROVAL
There are no ethical concerns relating to this case report.
CONSENT
Written informed consent was obtained from the patient to publish this report in accordance with the journal's patient consent policy.
ACKNOWLEDGEMENT
None.
Cocco G, Boccatonda A, Rossi I, et al. Early detection of pleuro‐pulmonary tuberculosis by bedside lung ultrasound: A case report and review of literature. Clin Case Rep. 2022;10:e05739. doi: 10.1002/ccr3.5739
Giulio Cocco and Andrea Boccatonda have equally contributed to this work.
DATA AVAILABILITY STATEMENT
In accordance with the DFG Guidelines on the Handling of Research Data, we will make all data available upon request.
REFERENCES
- 1. Reid MJA, Arinaminpathy N, Bloom A, et al. Building a tuberculosis‐free world: the Lancet Commission on tuberculosis. Lancet. 2019;393(10178):1331‐1384. doi: 10.1016/S0140-6736(19)30024-8 [DOI] [PubMed] [Google Scholar]
- 2. Agostinis P, Copetti R, Lapini L, Badona Monteiro G, N’Deque A, Baritussio A. Chest ultrasound findings in pulmonary tuberculosis. Trop Doct. 2017;47(4):320‐328. doi: 10.1177/0049475517709633 [DOI] [PubMed] [Google Scholar]
- 3. Di Gennaro F, Pisani L, Veronese N, et al. Potential diagnostic properties of chest ultrasound in thoracic tuberculosis — a systematic review. Int J Environ Res Public Health. 2018;15(10):2235. doi: 10.3390/ijerph15102235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Montuori M, Casella F, Casazza G, et al. Lung ultrasonography in pulmonary tuberculosis: a pilot study on diagnostic accuracy in a high‐risk population. Eur J Intern Med. 2019;66:29‐34. doi: 10.1016/j.ejim.2019.06.002 [DOI] [PubMed] [Google Scholar]
- 5. Heuvelings CC, Bélard S, Janssen S, et al. Chest ultrasonography in patients with HIV: a case series and review of the literature. Infection. 2016;44:1‐10. doi: 10.1007/s15010-015-0780-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hunter L, Bélard S, Janssen S, van Hoving DJ, Heller T. Miliary tuberculosis: sonographic pattern in chest ultrasound. Infection. 2015;44(2):243‐246. doi: 10.1007/s15010-015-0865-8 [DOI] [PubMed] [Google Scholar]
- 7. Bouhemad B, Zhang M, Lu Q, Rouby J‐J. Clinical review: bedside lung ultrasound in critical care practice. Crit Care. 2007;11(1):205. doi: 10.1186/cc5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Nachiappan AC, Rahbar K, Shi X, et al. Pulmonary tuberculosis: role of radiology in diagnosis and management. Radiographics. 2017;3(1):52‐72. doi: 10.1148/rg.2017160032 [DOI] [PubMed] [Google Scholar]
- 9. Jones PW, Moyers JP, Rogers JT, Rodriguez RM, Lee YCG, Light RW. Ultrasound‐guided thoracentesis: is it a safer method? Chest. 2003;123(2):418‐423. doi: 10.1378/chest.123.2.418 [DOI] [PubMed] [Google Scholar]
- 10. Feller‐Kopman D. Ultrasound‐guided thoracentesis. Chest. 2006;129(6):1709‐1714. doi: 10.1378/chest.129.6.1709 [DOI] [PubMed] [Google Scholar]
- 11. Mercaldi CJ, Lanes SF. Complications and improves the cost of care among patients undergoing thoracentesis and paracentesis. Chest. 2013;143(2):532‐538. doi: 10.1378/chest.12-0447 [DOI] [PubMed] [Google Scholar]
- 12. Chen H‐J, Hsu W‐H, Tu C‐Y, et al. Sonographic septation in lymphocyte‐rich exudative pleural effusions. J Ultrasound Med. 2006;25:857‐863. [DOI] [PubMed] [Google Scholar]
- 13. Hew M, Tay TR. The efficacy of bedside chest ultrasound: from accuracy to outcomes. Eur Respir Rev. 2016;25(141):230‐246. doi: 10.1183/16000617.0047-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gulati M, Venkataramu NK, Gupta S, et al. Ultrasound guided fine needle aspiration biopsy in mediastinal tuberculosis. Int J Tuberc Lung Dis. 2000;4(12):1164‐1168. [PubMed] [Google Scholar]
- 15. Yuan A, Yang PC, Chang DB, et al. Ultrasound guided aspiration biopsy for pulmonary tuberculosis with unusual radiographic appearances. Thorax. 1993;48(2):167‐170. doi: 10.1136/thx.48.2.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chan A, Devanand A, Low SY, Koh MS. Radial endobronchial ultrasound in diagnosing peripheral lung lesions in a high tuberculosis setting. BMC Pulm Med. 2015;15:90. doi: 10.1186/s12890-015-0089-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thangakunam B, Isaac BTJ, Christopher DJ. Endobronchial ultrasound experience in a high tuberculosis prevalence setting. Indian J Tuberc. 2017;64(3):196‐200. doi: 10.1016/j.ijtb.2016.11.035 [DOI] [PubMed] [Google Scholar]
- 18. Chalela R, Sánchez‐Font A, Domínguez‐Álvarez M, Badenes‐Bonet D, Pijuan L, Curull V. Utilidad de la punción aspirativa transbronquial guiada con ultrasonografía endobronquial en el diagnóstico de la tuberculosis mediastínica. Med Clin (Barc). 2016;146(12):532‐535. doi: 10.1016/j.medcle.2016.07.022 [DOI] [PubMed] [Google Scholar]
- 19. Sharma M, Ecka RS, Somasundaram A, Shoukat A, Kirnake V. Endoscopic ultrasound in mediastinal tuberculosis. Lung India. 2016;33(2):129‐134. doi: 10.4103/0970-2113.177451 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
In accordance with the DFG Guidelines on the Handling of Research Data, we will make all data available upon request.


