Abstract
Neurotoxicology is the study of adverse effects on the structure or function of the developing or mature adult nervous system following exposure to chemical, biological, or physical agents. The development of more informative alternative methods to assess developmental (DNT) and adult (NT) neurotoxicity induced by xenobiotics is critically needed. The use of such alternative methods including in silico approaches that predict DNT or NT from chemical structure (e.g., statistical-based and expert rule-based systems) is ideally based on a comprehensive understanding of the relevant biological mechanisms. This paper discusses known mechanisms alongside the current state of the art in DNT/NT testing. In silico approaches available today that support the assessment of neurotoxicity based on knowledge of chemical structure are reviewed, and a conceptual framework for the integration of in silico methods with experimental information is presented. Establishing this framework is essential for the development of protocols, namely standardized approaches, to ensure that assessments of NT and DNT based on chemical structures are generated in a transparent, consistent, and defendable manner.
Keywords: In silico, Computational toxicology, Neurotoxicity, Developmental neurotoxicity, In silico toxicology protocols, Hazard identification, Risk assessment, QSAR, Expert system, Profilers, Read-across
Introduction
The scope and complexity of the nervous system (NS) is vast and its study spans examination of simple changes in lipid bilayers to psychological evaluation. In humans and other animals, the NS is generally considered to consist of the central nervous system (CNS; comprising chiefly the brain and spinal cord) and the peripheral nervous system (PNS; further subdivided into the somatic nervous system and the autonomic nervous system). The NS has unique features linked to its sensory, motor, and cognitive capabilities [1] including reception, integration, transmission, and storage of information. It can be adversely impacted by extrinsic stressors or even by an overabundance of endogenous substances. Such adverse effects (reversible or irreversible) may be due to acute or repeated exposures to xenobiotics. The developing NS is generally more vulnerable than the mature adult NS, and even transient effects on nervous system development can result in life-long impacts [2]. Finally, in contrast to other organs that can regenerate in response to damage, the nervous system has limited capacity to regenerate [3].
Neurotoxicology encompasses the multi-disciplinary study of adverse effects on the structure or function of the developing or adult (mature or aging) nervous system following exposure to chemical, biological, or physical agents [4,5]. Toxicant-induced alterations of the integrity of the NS may result in neurochemical, anatomical, physiological, or behavioral changes that can be immediate or delayed, as well as transient or persistent [5]. Neuropathological effects are structural changes in the NS; neurochemical, neurophysiological, or behavioral changes are referred to as functional effects and include adverse changes in somatic/autonomic, sensory, motor, and cognitive function [4,6].
While many of the effects of neurotoxicity are readily observable, the underlying mechanisms are poorly understood due to the extraordinary complexity of the nervous system’s structures and functions. Indeed, a major uncertainty in neurotoxicity is the inability, in many cases, to identify the underlying pathobiology responsible for the observed adverse effects [7,8]. This is especially true for developmental neurotoxicity (DNT), which is further complicated by the complexity of the developing brain as well as the developmental window at the time of exposure [9,10]. The complexity of neurotoxicity can be further increased by interindividual variability in response to chemical stressors [11].
DNT has been defined as “potential functional and morphological hazards to the nervous system which may arise in the offspring from exposure of the mother during pregnancy and lactation”[12]. This definition should also include effects occurring during early childhood and adolescence. Adverse effects on the developing nervous system can be caused by both kinetic factors (processes of absorption, distribution, metabolism, and excretion) and dynamic factors (chemical interactions with biochemical, molecular, cellular, and physiological processes) [13–15]. These factors also include the complexity of the developing CNS which involves critical events occurring at different stages, resulting in windows of vulnerability when neurodevelopment can be disturbed by xenobiotics exposure [10,16].
Regulatory-driven neurotoxicity testing mostly employs in vivo animal tests that can also be used to supplement information obtained from human clinical or epidemiological studies. In a regulatory framework, neurotoxicity testing is generally performed in an iterative manner [17,18]. For example, in the European Union (EU), when systematic testing from standard single dose toxicity studies such as Test Guideline (TG) 402 [19], TG 403 [20], TG 420 [21], TG 423 [22], and TG 425 [23] or repeated dose toxicity studies such as TG 407 [24] and TG 408 [25] provide indications of adverse effects on the nervous system, neurotoxicity testing is then conducted using specific guidelines including TG 424 [26], TG 418 [27], TG 419 [28], or TG 426 [29]. Higher tier tests are generally required for certain classes of chemicals (pesticides), but may also be triggered by structure activity relationships or information on a specific toxicological mechanism [17]. Supplementary material for this paper provides additional details about the test guidelines available to examine markers of neurotoxicity. Undesirable effects on the NS are assessed by evaluating neurological functions with tests that look for changes in clinical signs, motor activity, sensory/motor functions, autonomic responses, learning and memory, and neuropathology. These tests are sometimes followed by more comprehensive studies, if needed, including electrophysiological and neurochemical methods, and/or additional behavioral tests or specialized neurohistological procedures [30]. Common endpoints observed in animal testing to detect functional and structural effects on the nervous system following exposure to neurotoxicants [4] are listed in Table 1.
Table 1.
Examples of potential endpoints for developmental and adult neurotoxicity in animal studies (adapted and modified from EPA [4]).
| Endpoints | Examples of indicators of neurotoxic effect |
|---|---|
|
| |
| Behavioral | Delays in the ontogeny of behaviors |
| Changes in clinical signs (e.g., touch, sight, sound, motor coordination, weakness, paralysis, abnormal movement or posture, tremor, seizures, body temperature) | |
| Altered grip strength or limb splay | |
| Increases or decreases in motor activity | |
| Increases or decreases in amplitude or latency of sensorimotor reflex | |
| Changes in learning, memory, and attention | |
| Neurophysiological | Changes in velocity, amplitude, or refractory period of nerve conduction |
| Changes in latency or amplitude of sensory-evoked potential | |
| Changes in electroencephalographic patterns | |
| Neurochemical | Alterations in synthesis, release, uptake, degradation of neurotransmitters |
| Alterations in second-messenger-associated signal transduction | |
| Alterations in membrane-bound enzymes regulating neuronal activity | |
| Inhibition and aging of neuropathy target esterase Increases in glial fibrillary acidic protein in adults | |
| Structural or neuropathological | Gross changes in morphology, including brain
weight Quantitative and qualitative histologic changes in neurons or glia (neuronopathy, axonopathy, myelinopathy) |
Rodents, such as mice and rats, are the most common species used to study structural, biochemical, electrophysiological, developmental, epigenetic, and behavioral effects following exposure to potential neurotoxicants. The features characterizing the rodent systems as compared to humans (including similar progression of neurodevelopment) allow for the assessment of many neurological outcomes [31]. However, as with most in vivo testing, neurotoxicity testing raises a number of issues that have been used to argue for advancing the development of testing strategies based on in vitro and in silico models [8,32–35]. First, testing multiple endpoints is needed as one single in vivo method does not allow for the identification of all possible neurotoxic effects. Moreover, in vivo studies are expensive, time consuming, and require large numbers of animals, and thus are not suitable for screening large numbers of chemicals. Finally, experts have raised concerns that the current battery of in vivo animal tests may not predict all relevant human neurotoxicity [32].
For these reasons, there has been a focus on the development and potential use of new alternative methods (New Approach Methodologies, NAMs), including in vitro and in silico approaches [36,37]. Application of alternative approaches has been strongly linked to the concepts of Adverse Outcome Pathway (AOP) and Integrated Approaches to Testing and Assessment (IATA) [35,38–40]. The eventual aim of such approaches is that information derived from appropriate combinations of alternative approaches (including in silico and in vitro) that target key events (KEs) within well-defined AOPs should aid hazard and risk assessments by guiding minimal but well-informed higher tier testing.
In silico toxicology (IST) methods (also referred to as computational approaches) can play a crucial role in the context of an IATA by informing the relationship between chemical structures and effects [41]. Development of IST protocols (i.e., standardized procedures) is a fundamental step in the integration of computational methods within mechanistically-informed alternative strategies [42,43].
Table 2 describes several situations related to neurotoxicity safety assessments that would benefit from standardized procedures that guide the use of IST methods. Given the large numbers of chemicals used in all aspects of global commerce, standardized procedures would be beneficial for compliance across related regulatory programs (e.g., FDA and EFSA, TSCA and REACH). NAMs, including those based on computational methods, are currently employed to inform weight of evidence approaches for hazard assessments, and may one day replace animal testing which is in line with the US EPA’s aim to reduce animal testing by 2025 and eliminate all mammal study requests and funding by 2035 [44].
Table 2.
Examples of potential applications of in silico toxicology for developmental (DNT) and (mature) adult (NT) neurotoxicity.
| Context | Discussion |
|---|---|
|
| |
| U.S. Food & Drug Administration (FDA) | All drugs under regulatory review should generally undergo testing in a broad receptor selectivity battery [45]. Among this battery are the major classes of neurotransmitter receptors. Drugs or major metabolites that exhibit binding at receptors of concern should be subsequently tested in functional assays and may prompt focused animal studies for possible neurotoxic effects on structure (expanded neurohistopathology) and function (neurobehavioral testing for effects on sensory, motor, and cognitive functions). Currently, there are no in silico neurotoxicity models that are used to support regulatory review of pharmaceuticals. |
| United States Environmental Protection Agency – Federal Insecticide, Fungicide, and Rodenticide Act (EPA-FIFRA) | For regulation of pesticides and other compounds under FIFRA, EPA has the legal authority to request neurotoxicity [46] and developmental neurotoxicity guideline studies [47]. For new pesticide compound registration, an acute and subchronic neurotoxicity guideline study is generally required. However, a guideline DNT study is not required, and it is only triggered when neurotoxicity is observed in other guideline studies or is a concern due to structure–activity relationships linked to a NT or DNT effect, or other information. |
| Under FIFRA, alternative approaches, including in silico methods, can be utilized, and there is growing interest to use these approaches as part of “weight-of-evidence” methods in decision making [48]. | |
| Eventually, application of these approaches can guide the development of more focused in vivo studies that can be conducted more quickly and economically, especially when predictive relationships between in vitro and in vivo outcomes can be postulated or are established. | |
| United States Environmental Protection Agency – Toxic Substances Control Act (EPA-TSCA) |
Under TSCA, new chemicals are submitted to the Agency often with little information other than structure, basic chemical properties, intended use, and estimated production volumes. Data on health and environmental effects, byproducts, and impurities, if available, also need to be submitted. In contrast to FIFRA however, no specific testing is required. EPA has 90 days in which to make a safety determination (for Premanufacturing Notices, or PMNs) regarding intended and reasonably foreseeable conditions of use of new compounds. In addition, there are thousands of existing chemicals currently in US commerce for which little toxicity information is available, especially for NT and DNT neurotoxicity hazards. The Lautenberg Chemical Safety Act of 2016 updated the TSCA legislation and among other things: 1) required that EPA begin performing risk evaluations on existing chemicals under TSCA, and 2) specifically mandated that EPA reduce testing in vertebrate species when requesting information for new or existing chemicals [49]. Due to the numbers of new and existing chemicals regulated under TSCA and the new requirement to reduce testing in vertebrates, in silico and alternative approaches, in general, have a large role to play in the screening and prioritization of compounds for different potential hazardous outcomes, including NT and DNT. |
| European Chemicals Agency (ECHA) | According to ECHA, both under REACH (Registration, Evaluation, Authorisation and Restriction of Chemical Substances) [50] and the Biocidal Products Regulation (BPR) [51,52], NT and DNT testing are triggered if the test chemical or a structurally similar substance has been shown to cause NT or DNT adverse effects, or has a mode of action which is linked to NT or DNT effects. ECHA considers read-across as an adequate approach for investigating NT and DNT when sufficiently reliable and relevant information exists from structurally similar substances to justify its application. In the context of read-across, QSAR (Quantitative Structure Activity Relationship) models and in vitro methods are usually applied to support the predictions. There is a recognized need to standardize and validate non-animal methods in order to use them to screen for neurotoxicity potential, trigger further studies, and integrate data from these methods as elements in weight of evidence (WoE) approaches, in support of read-across methodology, and discovery of mechanisms underpinning toxicity [51,53]. |
| European Food Safety Authority (EFSA) | Pesticides represent a class of chemicals of special interest for the assessment of neurotoxicity as some pesticides are specifically designed to be neurotoxic (e.g., organophosphates, carbamates, pyrethroids, organochlorines). In the EU, guideline neurotoxicity studies consist of animal-based testing in rodents are not mandatory and are only performed on those active substances with indicators of potential neurotoxicity [54]. EFSA, which is involved in the pesticides peer-review system in Europe, has devoted great effort in exploring alternative methods (e.g., nonmammalian animal models, in vitro tests, and in silico and read-across methods) to support neurotoxicity assessment [18,55,56]. More specifically, in silico methods can assist a mechanistically-based IATA in the selection of the most relevant tests to use. EFSA has specifically recognized DNT as a key area where alternative methods including in silico, in vitro, and alternative species can play an important role in hazard decisions [56]. |
| Pharmaceuticals (product development) | Some in silico models predicting, for example, blood brain barrier (BBB) penetration, neuronal bioactivity, or off-target panel interaction are currently integrated in the research and development process of pharmaceutical companies; they will not necessarily reveal high-resolution tructure-activity relationships (SAR) for compound sets, but such models can provide means of compound prioritization by filtering out the “worst offenders” prior to subjecting these compounds to in vitro screens.There is a clear need in the preclinical phase for the development of evaluation frameworks that combine information from different sources (in silico, in vitro, and in vivo models) for the evaluation of adverse effects of pharmaceuticals on the nervous systems; it was noted that current alternative methods performed during the research and development process poorly predict such potential adverse effects [57]. |
The present work proposes a general schema that supports the use of in silico information in combination with data originating from other sources (e.g., in vivo and in vitro alternative methods) for the evaluation of DNT and NT. The development of this schema requires the organization and classification of available and emerging experimental approaches and the underlying endpoints that are going to be integrated in this evaluation [42]. Importantly, such a process is essential for the development and use of in silico models that may adequately support the assessment of adverse neurotoxicological effects.
The output of the current endeavor is a draft decision assessment framework that builds on the emerging vision of mechanistically-informed approaches. This framework pragmatically identifies the building blocks serving as a basis for the subsequent development of an IST protocol that standardizes the use of in silico approaches for the prediction of neurotoxicity from chemical structure. The IST protocol initiative is a collaborative project involving several international organizations and summarizes the status of in silico toxicology for hazard identification and characterization. Its goal is the definition of principles for generating, recording, communicating, archiving, and then evaluating assessments which make use of in silico methods in a uniform, consistent, and reproducible manner [42]. Not only do the standardization principles defined by the IST protocol provide a means to support a more transparent analysis of the results, but they will also facilitate use and acceptability of both the methods and the corresponding predictions by end users, colleagues, collaborators, and regulators.
Overview
Conceptual framework
IST protocols combine both relevant in silico results and available experimental data for the assessment of major toxicological endpoints [42,43]. This integration of data should be complemented by the evaluation of reliability and completeness of the experiment and/or in silico results along with an assessment of confidence that takes into consideration the quality of the information used to derive the assessment and the overall uncertainty in the assessment [58].
To develop a neurotoxicology in silico protocol, an assessment framework first needs to be established wherein in silico predictions can be integrated with other existing data. The overall process should result in an in silico prediction workflow [42] that follows a consistent and well-documented construct comprising:
a description of adverse effects or mechanisms to predict and relevant experimental data to consider;
a list of in silico methodologies and approaches to use;
recommendation on generation of the predictions and on assessment of relevant experimental data;
indications on the performance of the in silico analysis that generates the result, including expert review;
transparency, via recommendations on reporting formats to share the results and the corresponding uncertainties.
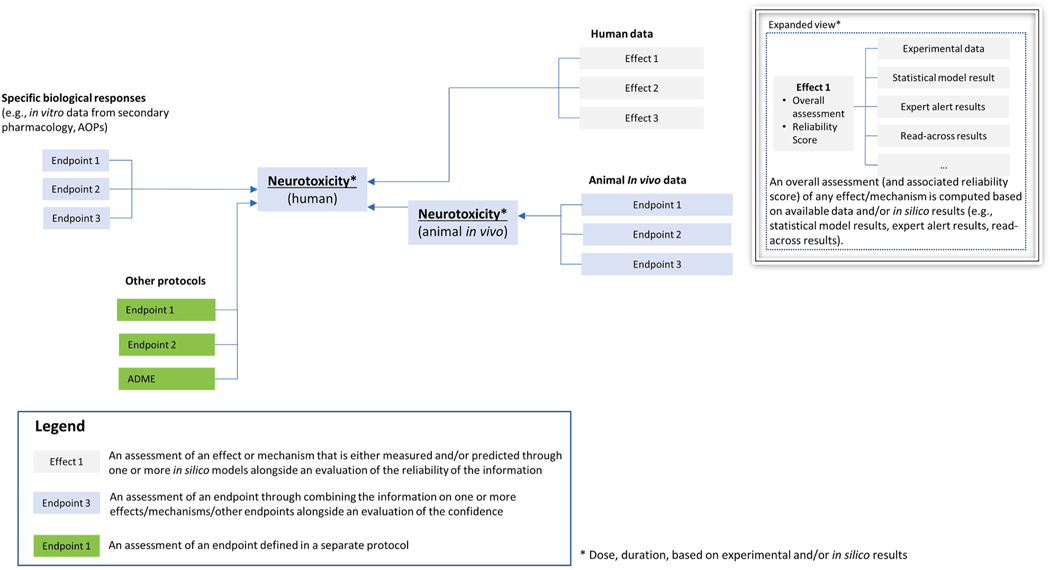
Following the basic schema proposed for the organ toxicity protocol [59], a draft, general outline of the hazard assessment decision framework for neurotoxicity was created (Fig. 1). This decision framework combines mechanistic information from in vitro models, results from in vivo experiments and human data, and it allows for the integration of in silico predictions according to standardized principles (i.e., the neurotoxicology in silico protocol). Other processes related to chemical absorption, distribution, metabolism, and excretion (ADME), that are regulated by different protocols, should also be considered. Effects or mechanisms (light grey boxes in Fig. 1) refer to a result as specifically coming from experimental measurements (in vitro, in vivo animal, or human data) and/or predictions by one (or more) in silico model(s) alongside an evaluation of the reliability of the information; the endpoint (blue boxes in Fig. 1) refers to an assessment resulting from a combination of information on one or more effects/mechanisms/other endpoints alongside an evaluation of the confidence.
Fig. 1.
General outline of potential hazard assessment decision framework for neurotoxicity [59]. Data from different sources are integrated in the decision framework: in vitro data (e.g., biological responses from receptor-based assays), in vivo results, and human data. Other protocols (e.g., ADME or other organs) can contribute useful information (e.g., information on ADME processes is important to the interpretation of data from in vitro testing and complementary to, and supportive of, the prediction of neurotoxicity for human health hazard assessments; considerations on kinetic processes are necessary to relate in vitro findings to in vivo findings). Available information (e.g., environmental, drug, consumer, accidental) should also be used to supplement the protocol. Effects (predicted by in silico methods or measured experimentally) are combined for the assessment of a given endpoint. Combination of data is supplemented by an evaluation of the confidence that takes into consideration the quality of the information used to derive the assessment and the overall uncertainty in the assessment.
Identification of relevant effects/mechanisms and endpoints together with their mutual relationships forms the scaffold of the overall mechanistically-informed assessment framework. Although such a framework will need to continuously adapt to the fast-paced advances in toxicology and the ever-increasing understanding of the biological pathways underlying NT and DNT, the present work attempts to pragmatically capture current knowledge to provide a construct that integrates the use of in silico methods for DNT and NT assessments and that informs future development of in silico approaches.
ADME
Regardless of the toxicological endpoint, toxicokinetic studies on ADME provide data on the tissue-specific internal dose of the test chemical by describing its entry into the body, persistence within the body, distribution in the organs, metabolic reactions involved in its elimination, and routes of excretion from the body [60]. The internal dose eliciting toxic effects depends on these ADME processes. Biotransformation of xenobiotics that leads to formation of either inactive or reactive metabolites, may play an important role in determining the relevance of observed neurotoxic effects [61,62].
In the particular case of chemical-induced toxicity to the mature adult CNS, neurotoxicants cannot exert their adverse effects unless they cross the brain barrier systems (e.g., blood brain barrier and the blood-cerebrospinal fluid barrier) [63,64]. These anatomical barriers can isolate the CNS from the systemic blood circulation protecting it from some potentially damaging substances. Many substances can cross the blood brain barrier (BBB) by simple transmembrane diffusion, a mechanism generally favored for substances with low molecular weight and high lipophilicity. For example, the major route for some drugs to access the brain from the bloodstream is by passive diffusion [65]. Molecules can also be actively transported in and out of the brain by transporters, a complex mechanism regulated by the different carriers that mediate the influx/efflux of endogenous substances (e.g., nutrients) or of some xenobiotics including certain toxins (e.g., methylmercury) [31,66,67].
ADME (Q)SAR models are available for the prediction of ADME properties [68], and specific models for the prediction of BBB penetration will be discussed further below. Quantitative descriptions of the temporal change in the concentration of chemicals and/or their metabolites in any tissue of the exposed organism may be computed with physiologically based kinetic (PBK) modeling, also commonly referred to PBPK (physiologically based pharmacokinetic) and PBTK (physiologically based toxicokinetic) modeling [69–73]. PBK modeling has been used to improve the interpretation of the neurotoxicity of several chemicals [74–76].
Within testing strategies based on NAMs, consideration of kinetic processes are necessary to relate in vitro findings to in vivo findings [77,78]. These kinetic parameters expound the relationship between media and cellular target concentrations that are controlled by a number of assay variables (e.g., binding to serum proteins, plastics, cell density, and any metabolic capacity of the test system) [72,79]. ADME information, integrated into the non-animal strategies with quantitative modeling, informs a quantitative in vitro-in vivo extrapolation [80–82]. There are now examples were in vitro neurotoxicity data combined with ADME data computed from PBK modeling has predicted in vivo concentrations that help inform whether in vivo bioactivity might be expected (e.g., [83]). In vitro experiments have provided chemical-specific pharmacokinetic and pharmacodynamic parameters for large numbers of chemicals [84,85] that have improved in vitro-in vivo extrapolation for many chemicals including neurotoxicants [85,86].
Information on ADME processes is important to the interpretation of in vitro testing that investigates the potential neurotoxic effects induced by chemicals, while also complementary to and supportive of the prediction of neurotoxicity for human health hazard assessments [61,87]. Understanding the metabolic capacity of NAMs is critical to their use in predictive models and hazard assessments. Many in vitro models have limited or unknown metabolic capacity (e.g., [78,88]), but when applied to screen large numbers of chemicals for prioritization, lack of information on metabolic capability may be acceptable based on the regulatory need. However, for some classes or specific chemicals, this lack of knowledge of metabolic capability in NAMs will add uncertainty to hazard decisions. Toxicokinetic data are also key elements to support the acceptability and applicability of predictions generated by in silico methods, like read-across or grouping approaches [89].
Mechanisms and AOPs
Important to predictive models is knowledge of the biological mechanisms by which chemicals cause toxicity in the nervous system tissues. Herein, the terms “mechanistic” and “mechanism” are used to describe the biochemical interactions through which a chemical substance produces its effect on the initial biochemical target (MIE, molecular initiating events within the AOP framework) or through downstream processes (KE, key events within the AOP framework). The term mode-of-action, as commonly used, describes the measurable neurochemical, physiological, or anatomical changes that occur. Within the AOP framework, the mechanism of action is tied to the MIE, and the mode-of-action arises from the downstream KEs. While there are known mechanisms for some forms of NT (e.g., peripheral neuropathies), there is limited information about the mechanisms responsible for relatively common human neurodevelopmental disorders (e.g., ADHD, autism).
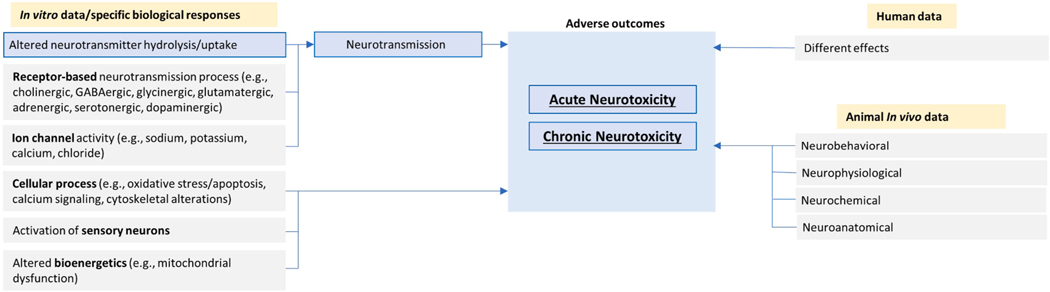
Neurotoxicological outcomes have been classified by their impacts on functional (behavioral), neurochemical, neurophysiological, and neuropathological outcomes [90–93]. Neurotoxicants can act on different neuronal tissues and result in a diverse array of adverse outcomes. For example, neurotoxicants may damage peripheral axons, which results in an axonopathy and paresis [93]; or, they may interfere with the process of neurotransmission, as in the case of organophosphates that inhibit acetylcholinesterase leading to a buildup of synaptic acetylcholine and subsequent neurotoxicity [94]. A detailed analysis of mechanistic information on acute systemic toxicity for about 100 chemicals highlights that the nervous system is one of the most frequent sites of toxicity induced by high doses of chemicals with changes in neurotransmission being a critical mechanism [95].
At the molecular level, categories of endpoints related to neurotoxicity can be formulated based on similarities of the underlying biological mechanisms of the adverse effects so that each group of endpoints is characterized by common pathways and key events [18]. There are a number of mechanistic categories known to be linked to neurotoxic outcomes, and these include alterations in: a) receptor-based neurotransmission processes such as cholinergic and GABAergic (gamma-aminobutyric acid) processes (e.g., blocking of GABAA receptors by organochlorines leading to seizures [96]); b) activity of ion channels such as sodium, potassium, calcium, and chloride channels (e.g., pyrethroid effects on voltage-gated sodium channels [97]); c) cellular processes such as oxidative stress/apoptosis; d) mitochondrial dysfunction (e.g., apoptosis and oxidative stress by androgen steroids [98]); e) hormonal systems (e.g., alteration of volume of the sexually dimorphic nuclei in the preoptic area of the brain [99]); f) immunomodulation (e.g., neurotoxicity predominantly in the form of peripheral neuropathy by immunomodulatory drugs such as thalidomide [100]); g) neurotoxic adverse events by cancer immunotherapies [101–103]); h) epigenetics (e.g., astrocyte-mediated neuroinflammation and neurotoxicity [104]). Table 3 shows selected examples of mechanistically-based categories of endpoints as adapted from a recent EFSA external scientific report on neurotoxicity testing methods [18,105].
Table 3.
Selected examples of mechanistically-based endpoint categories adapted from an EFSA report on neurotoxicity testing methods [18]. Endpoints related to neurotoxicity can be categorized according to similarities of the underlying biological pathways leading to adverse effects.
| Category type | Endpoint category |
|---|---|
|
| |
| Receptor-based neurotransmission | cholinergic GABAergic glycinergic
glutamatergic adrenergic serotonergic dopaminergic other neuronal receptors |
| Ion channel activity | sodium channels potassium channels calcium channels chloride channels |
| Cellular processes | oxidative stress/apoptosis oxidative stress
mitochondrial dysfunction calcium signaling and other 2nd messenger
signaling (e.g., PKC, cAMP) cytoskeletal alterations |
Abbreviations: PKC (Protein kinase C), cAMP (cyclic adenosine monophosphate).
For DNT, the mechanisms underpinning DNT compounds are mostly unknown (e.g., [106]). Therefore, in building in vitro NAMs, there is international consensus that measurement of disturbances in more downstream fundamental neurodevelopmental processes will reflect the integration of chemical disruptions in multiple upstream molecular events [107,108]. These in vitro assessments are further discussed below, and include evaluation of cellular processes such as: neural stem/progenitor cell proliferation, differentiation, migration, apoptosis, neurite outgrowth, myelination, synapse formation, and neural network formation and function [32,109,110].
Linking molecular and cellular mechanisms to downstream adverse outcomes can be facilitated by use of the AOP framework [38]. A key strength of AOPs is that they identify molecular targets associated with MIE as well as “common KEs” (CKEs); knowledge of these MIEs and KEs allows for the development and use of high-throughput in vitro screening methods that generate empirical data sets for large chemical libraries [111]. Such data is critical in the development and validation of predictive in silico models (e.g., [112]).
Several research activities are on-going to investigate AOPs related to NT and DNT, but the number of such AOPs is still currently limited [7,113–116]. A list of AOPs extracted from the AOP-Wiki are reported in the supplementary material (Table S2). This includes MIEs involving glutamate receptors, glucocorticoid receptors, GABA receptors, N-methyl-D-aspartate (NMDA) receptors, sodium/iodide symporters, serotonin transporters, and thyroperoxidase. It is important to note only a limited number of these AOPs have undergone the OECD (Organisation for Economic Co-operation and Development) review process. To date, the only MIE for developmental neurotoxicity that has led to an in silico predictive model involves thyroperoxidase [117,118].
Data and endpoints
Human data
Human neurotoxicology research addresses the development and validation of methodologies to detect and measure functional deficits and neurotoxic effects in adults as well as susceptible populations [119]. The developing nervous system is particularly susceptible to neurotoxic effects; the aging brain may be particularly vulnerable to neurotoxic insult as a consequence of its reduced capacity to compensate for impairment; genetic factors may also alter an individual’s predisposition to neurotoxic disease [2,9,11].
Accidental and occupational exposures, case-studies, clinical evaluations, epidemiological studies, field studies, and laboratory studies represent the sources for human data related to neurotoxicity induced by xenobiotics including environmental chemical agents and drugs [119]. Experimental human studies (i.e., where humans experience a controlled exposure to a test substance) generally cover a small number of volunteers, involve low doses, and measure acute effects. This limits insights on health risks following chronic exposure to potential neurotoxicants; moreover, the statistical power may be reduced due to small sample sizes and the use of low doses [120].
Epidemiological studies investigate the relationships between exposure and occurrence of neurological diseases and can employ large sample sizes. Generally, these types of studies do not perform in-depth assessments of individual subjects. Furthermore, since exposure is not controlled, they cannot unambiguously ascribe causation to any specific substance [120]. Some examples of chemicals with epidemiological studies that have informed risk assessments include perchlorate [121], paraquat and rotenone [122], and methyl mercury [123,124].
In the context of pharmaceuticals, neurotoxicity significantly contributes, along with cardiotoxicity and hepatotoxicity, to safety liabilities and adverse drug reactions (ADRs) that can occur during the pharmaceutical life cycle, including the post-approval stage. This can lead to withdrawal of marketed drugs [125]. ADRs are unwanted phenotypic responses to drug treatments that can be reported by consumers directly to regulatory agencies and collected in pharmacovigilance databases. Data from clinical case studies permit the identification of new hazards and characterization of toxic manifestations in exposed people, but they have some limitations, including the small number of individuals analyzed and the fact that they generally do not describe quantitative dose–response relationships [120]. There are several resources on side-effects, clinical trials information, and pharmacovigilance [126] that may contain relevant information about chemically-induced adverse effects on the human nervous system including the Side Effect Resource (SIDER) [127], FDA Adverse Event Reporting System (FAERS) [128], Vigibase [129], and EudraVigilance [130].
Neurotoxicity is an important element of occupational safety and health programs [131]. Occupations involving exposure to pesticides and solvents have been correlated with higher rates of neurodegenerative diseases [132,133]. A common concern in occupational and environmental settings is the exposure to lower levels of potentially neurotoxic agents for long periods of time. In addition, adverse neurotoxic effects may be delayed or not become evident until sometime after initial exposure [134]. For example, adverse neurologic effects from exposure may not manifest until people age or undergo other stressors [131]. Indeed, a progressive accumulation of damage that becomes evident only over a prolonged period may be caused by low-level exposure to neurotoxic agents [131]. Typically, neurotoxicity testing in humans is based on batteries of tests designed to cover many different neural targets and functions [135–137]. Major classes of occupational and environmental neurotoxicants are metals (e.g., lead, mercury, manganese), organic solvents, pesticides (e.g., organophosphorus compounds, carbamates, pyrethroids), and persistent organic chemicals (e. g., polychlorinated biphenyls (PCBs), polybrominated diphenyl ethers (PBDEs), polychlorinated dibenzofurans (PCDFs)) [131].
In vivo data
Animal data, especially from rodents, cover the multiple levels of nervous system organization, including the behavioral, neurophysiological, neurochemical, and neuroanatomical levels [119]. Functional examinations, including clinical observations and the Functional Observational Battery (FOB), cover the autonomic nervous system, sensorimotor, neuromuscular, and other behavioral parameters [138–141]. Alterations in clinical observations or FOB endpoints are indicative of acute, chronic, and developmental neurotoxicity. More selective tests and evaluations are also performed using less subjective, automated neurological methods, including automated tests of motor and sensory function, learning and memory, neurophysiological assessments (e.g., nerve conduction velocity, evoked potentials), neurochemical assessments (e.g., biomarkers, neuroimaging), and neuropathological methods [142]. Interpretation of in vivo neurotoxicity studies can be confounded by regulatory requirements to induce either clear neurotoxicity, or some systemic toxicity, and without additional studies, it can be difficult to determine if the neurotoxic effects are separate from, or a secondary effect of the systemic toxicity.
In vivo guideline tests (see also Table S1 in the supplementary material) for investigating neurotoxic effects range from non-specific studies (e.g., rodent and non-rodent acute or repeat-dose studies) to more specific methods that test for changes in behavior and neuroanatomy. The findings coming from these in vivo animal experiments can be broadly categorized as shown in Table 4.
Table 4.
Broad categorization of the observations from in vivo animal experiments associated with alterations of the integrity of the nervous system.
| Categories | Endpoints |
|---|---|
|
| |
| Neurobehavioral | Clinical signs |
| Motor function | |
| Sensory function | |
| Affective behavior | |
| Attentional behavior | |
| Social behavior | |
| Learning and Memory | |
| Neurophysiological | Electroencephalography |
| Evoked Potentials (EPs) | |
| Peripheral nerve conduction velocity | |
| Electromyography (EMG) | |
| Synaptic function | |
| Neurochemical | General neurochemistry |
| Cell-specific markers (e.g., glial proteins) | |
| Neurotransmitters | |
| Neurospecific enzymes such as acetylcholinesterase (AChE) | |
| Neuroanatomical | Gross evaluations |
| Brain Weight | |
| Microscopic qualitative evaluations | |
| Microscopic quantitative evaluations (morphometry) | |
| Computed tomography (CT) | |
| Magnetic resonance imaging (MRI) | |
| Positive emission tomography (PET) | |
In vitro data
In vitro models can provide data to characterize potential toxicity and also gain mechanistic insights; they can then supplement the hazard assessment from animal studies by enhancing the interpretation of in vivo data [30,87,119]. Understanding the correlation between in vitro assays and in vivo studies is a crucial step in translating the in vitro marker into a quantifiable risk in the corresponding in vivo model. In vitro results that identify chemicals that alter structural or functional metrics are a cause for concern, and they can add to the weight of evidence for NT or DNT. Adding toxicokinetic considerations to estimate internal dosimetry will provide context and improve the understanding of risk. In the absence of any toxicological information, the in vitro data could influence regulatory decision-making and, for example, result in requests for additional data.
In hazard assessments, method selection for NT or DNT in vitro testing should be regulatory-needs based [8]. For example, chemicals lacking hazard data could first rely on in silico and in vitro test batteries, and, depending on the regulatory need, follow-up testing could include orthogonal in vitro assays, alternative species (e.g., C. elegans, zebrafish, planaria), or in vivo mammalian methods [143]. Both regulatory and scientific communities are currently working to speed the development of testing batteries of in vitro assays capable of screening chemicals for the potential to disrupt function and structure in both the developing and mature adult nervous systems [35,110,144,145].
In the in vitro assays related to NT, endpoint measurements are commonly assessed after relatively short exposure durations (e.g., 1–24hr) and using cell culture models with characteristics that are relatively stable [146]. In the in vitro DNT assays, exposures should encompass relatively longer time periods that coincide with the dynamic changes in cell status associated with neurodevelopmental processes (e.g., from relatively immature into more stable adult-like cultures [147]), or examine processes that are generally recognized to have a much greater role in neurodevelopment than in the adult brain (e.g., proliferation, neurite outgrowth, differentiation, synaptogenesis).
In vitro testing methods for neurotoxicity include numerous models of increasing biological complexity, ranging from monocultures of individual neural cell types to systems that reproduce multiple aspects of the respective tissue found in vivo (e.g., pure neuronal cultures, primary mixed neuronal and glial cultures, three-dimensional cultures, organotypic brain slices) [18,61,148]. In vitro DNT models are based on stem cell, progenitor cell, or primary cell cultures, as well as some immortalized neuronal cell lines that are either rodent or human in origin [30,31,56]. However, it has been recommended that developments of new in vitro methods focus on human cell-based models with the aim to reduce the uncertainty of the extrapolation of the in vitro animal data to humans [110].
High-throughput and high-content assays measuring the effects of chemical substances on neuronal function are available for a variety of NT and DNT endpoints and processes [143,149,150]. Examples are cell-based assays measuring key neurodevelopmental processes (e.g., proliferation, receptor-ligand assays, synaptogenesis, migration) in vitro [18,151–156]. Moreover, the effects of compounds on neuronal network function can be measured, both for acute and developmental changes, using microelectrode array (MEA) technology in a high-throughput manner [147,157]. While cell-based assays that assess key neurodevelopmental processes provide insights about chemical effects on structural changes, the MEA approach provides information on functional alterations.
As with all in vitro assays, it is important to clarify the role of cell stress and/or cytotoxicity in any observed changes. Examples include activation of cell stress pathways, disruption of proteins or membranes, or broad low-affinity non-covalent interactions. This can be achieved using concurrent measures of cell stress or viability within NT or DNT assays, or using data from many different cell types [158]. The former provides evidence of specificity of results from DNT assays, and the latter informs the specificity of the DNT effect relative to non-specific effects across a wide biological spectrum.
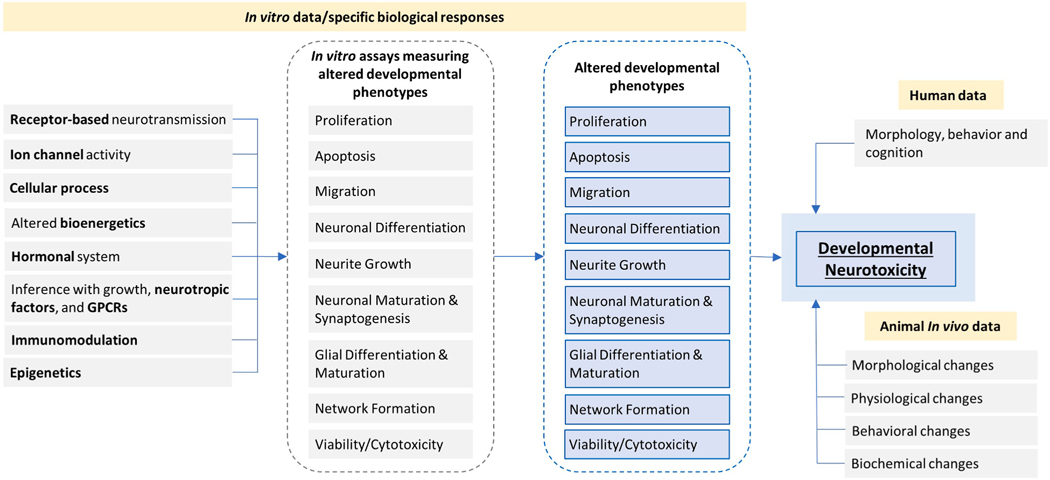
Although the mechanisms underlying the action of DNT compounds are mostly unknown, international consensus supports the hypothesis that if a chemical alters key neurodevelopmental processes (see Table 5) in vitro, it has potential to do the same in vivo [108,159]. It is important to clearly point out that the specificity of these endpoints for correct prediction of in vivo adverse outcomes is currently not known. Such concepts have been examined by the EFSA/OECD DNT in vitro battery testing project [143]. This is an initial attempt with a limited number of chemicals tested in vitro, and an even smaller number tested in vivo, to identify different modes of action targeting compound-specific sensitivities for a number of endpoints associated with key neurodevelopmental processes [143].
Table 5.
Key neurodevelopmental processes modeled in the DNT in vitro battery [160].
| Process | Description |
|---|---|
|
| |
| Proliferation | Division of neural stem cells and neural progenitor cells resulting in an increase in cell number. Changes in proliferation can result in an incorrect cell number (increase or decrease) and altered brain growth. It is measured directly by assessing the number of cells undergoing DNA replication or inferred by measuring an increase in cell number over time. |
| Apoptosis | Programmed death of cells resulting in a decrease in cell number. Changes in apoptosis can result in increases or decreases in neuron cell numbers and altered brain growth. It is measured by assessment of cell nucleus morphology or detection of biochemical markers specific to the apoptosis pathway. |
| Migration | Movement of neural progenitor, glial, or neuronal cells from their point of origin to a final location. Changes in migration can result in cells in the wrong location, resulting in abnormal brain structure and function. It is measured by assessing the number of cells moving into a defined area or the distance moved by individual cells. |
| Neuronal Differentiation | Process in which a neural progenitor cell changes to a specific type of neuron. Changes in differentiation can result in altered cell numbers for specific populations of neurons, resulting in altered brain structure and function. It is measured by assessment of the number of cells expressing markers specific for neurons and neuronal subtypes. |
| Neurite Growth | Outgrowth of morphological processes relatively early in neuronal differentiation. Neurites eventually develop into dendrites or axons. Changes in neurite growth can alter the number and length of axons and dendrites, changing brain structure and connectivity between neurons. It is measured by counting the number of cells elaborating processes or morphological assessment of neurite length. |
| Neuronal Maturation & Synaptogenesis | Maturation of neurites into the specialized processes of dendrites and axons which then form synapses responsible for communication between neurons. Changes in neuronal maturation and synaptogenesis alter neuronal connectivity, changing network formation and brain function. They are measured by morphological assessment of axon and dendrite length and counting of the number of synapses. |
| Glial Differentiation & Maturation | Process in which a neural progenitor cell changes to a specific type of glia (radial glia that support formation of cortical architecture, astrocytes that support neuronal function, and oligodendrocytes that myelinate axons). Changes in differentiation can result in altered numbers of glia and reduced myelination, changing brain structure and function. Differentiation and maturation are measured by assessment of the number of cells expressing markers specific for glial subtypes. |
| Network Formation | Process in which neurons and glia grow and make functional contacts with each other, exemplified by spontaneous generation and propagation of electrical action potentials within a network. Changes in network formation and function can result in reduced neural connectivity and altered brain function. It is measured by electrophysiological assessment of coordinated electrical activity of neurons and glia grown on microelectrode arrays. |
| Viability/Cytotoxicity | Test methods for each neurodevelopmental process should also include at least one concurrent measure of cell viability (or its converse, cytotoxicity) as a baseline for comparison of potential non-specific effects of chemical exposure. Cell viability can be assessed by counting cells with normal morphology (based on cell body and nucleus size), delineation of live/dead cells based on uptake or exclusion of vital dyes, or biochemical assessment of active cell metabolism. Cytotoxicity is typically assessed by measuring parameters associated with loss of cell membrane integrity, including leakage of intracellular proteins and enzymes, or exposure of DNA. |
As mentioned above, development of new in vitro test methods is shifting away from the use of immortalized cells in favor of systems that examine cellular and biochemical mechanisms of human neurotoxicity based on primary human cells (e.g., neural progenitor cells (hNPC) and human induced pluripotent stem cell (hiPSC)-derived neural cells) that can be differentiated into different types of postmitotic neurons, astrocytes, and oligodendrocytes. Current models derived from stem cells are deemed useful to investigate DNT rather than adult neurotoxicity [87]. Cell cultures based on stem cell lines need standardization for their reporting [161] to ensure appropriate use in regulatory decisions.
Several in vitro assays based on different cell types enable the study of alterations of the receptor-based neurotransmission processes, ion channel activity, and other cellular processes (Table 3) that may lead to neurotoxicity [18]. In addition, predictions of the potential for acute-, chronic-, or developmental-neurotoxicity may be assessed using other NAMs (e.g., axonal transport, vesicular release, ion channel function, neuronal-glial interaction, neural stem cell commitment and proliferation, cell migration, axonal and dendritic elongation).
In the context of drug discovery and development, safety pharmacology investigations make use of in vitro models for the evaluation of potential neurotoxicants [162]. These, for example, include hippocampal brain slice electrophysiology [163,164] and multi-electrode arrays for seizure liability [165–168].
In vitro receptor-ligand assays
In the context of in vitro screening, receptor-ligand assays test the compound activity against targets expressed in the CNS (e.g., neurotransmitters, ion channels, G-protein coupled receptors, enzymes, transporters) that have been associated with clinical adverse effects or that could potentially have some impact on physiological functions [169]. Supplementary information for this paper (Tables S3, S4 and S5) provides examples of molecular targets potentially associated with neurotoxicity.
The pharmaceutical industry explores off-target interactions (referred to as secondary pharmacology profiling) by means of in vitro high throughput screens (HTS) against a large number of unintended targets, including some neurotransmitters and ion channels, with the aim of limiting off-target interactions and thus reducing liabilities leading to toxicity [169,170]. From the comparison of the in vitro pharmacological profiling campaigns of different pharmaceutical companies, a seminal safety minimal panel of 44 targets was collated and published, linking the biological targets to various ADRs [169]. Another compilation of molecular targets has been collated by AbbVie using a different literature curation process resulting in a battery linked to potential functional and pathological side effects to the different organs including the NS [171]. Molecular targets were also specifically associated with seizure liability [172] and abuse liability [173]. Information from human genetics and pharmacology was used to compile an extended list of off-targets linked to different target organ toxicities including CNS potential adverse effects [174].
Using the Gene Ontology database, protein targets from the ToxCast Novascreen assays were identified and used to develop classification predictions for potential adverse effects on the nervous system [175]. These are further discussed in Table S5 of the supplementary material. The Tox21/ToxCast data can be used to alert for potential neurotoxic effects, and such data include both receptor-based assays and other cellular assays. Additional assays that have been associated with neuronal targets selected from Tox21/ToxCast are reported in Table S6 of the supplementary material. The data on the interaction of a wide variety of environmental compounds with ToxCast assays can be found on the ToxCast Dashboard maintained by the US EPA [176,177]. In the future, Tox21/ToxCast assay data can be used to develop additional predictive models, identify and measure KEs or endpoints proximal to KEs, as there are assays for enzymes (e.g., AChE), ion channels, and second messengers, among other endpoints. One example of the different off-targets that can be associated with potential adverse effects to the CNS is the vesicular monoamine transporter 2 (VMAT2). It is a neuronal membrane protein that transports biogenic monoamines (e.g., dopamine, serotonin) into synaptic vesicles for storage within the neuron. It has been found that some drugs that interact with VMAT2 can alter the uptake and release of biogenic amines, which can lead to neuropsychiatric consequences [178–180]. However, the function and expression of biogenic monoamines at VMAT2 are not commonly tested as part of the secondary pharmacology assays for new drugs, which can result in missed safety signals.
Although safety screen panels based on molecular targets associated with neurotoxicity are available (e.g., Table S3 of the supplementary material) and widely employed [125,169,181], an industry-regulatory consensus on the list of off-targets is currently lacking [182]. The complexity of a neuron and its environment in vivo can limit the predictive accuracy on the functional consequences in the intact in vivo organism. Still, these types of results, which may also be predicted by suitable in silico models, can trigger higher-tier testing for further investigations of potential physiological consequences [30,183].
In vitro electrophysiological methods
NS studies based on in vitro electrophysiological techniques measure the electrical activity from cell cultures or brain slices exploiting the ionic conductance of ion channels and transient modulation of the membrane potential of a neuron [183,184]. This is a growing area of interest in developing new automated and semi-automated high-throughput assays as well as understanding the in vitro-in vivo extrapolation of the electrophysiological assays [184]. Such in vitro assays can be employed to address various adverse effects induced by chemical agents or drugs in the nervous system (e.g., seizure liability, cognitive deficits, sedative effects) [183] and paired with other testing, may provide useful insights into NS liabilities [184]. Electrophysiological approaches can also provide mechanism of action information, often at the level of an individual ion channel or receptor. Methods such as patch clamp electrophysiology have been used for both target- and safety- based screening [185]. MEA recordings provide information on how drugs and chemicals can impact the function of intact networks of neurons grown in two (for review, see [147,157]) or three dimensional cultures [186]. Such recordings of rodent or human neural networks in vitro have been used to characterize a wide variety of compound effects on neural network function (reviewed in [147]). More recently, several publications indicate that rodent and human networks provide similar but not identical responses following compound treatment [187–189]. While most evaluations of chemical effects on neural network function using MEAs have focused on acute effects, about 200 chemicals have been evaluated for effects on the formation of neural networks [190–194]. While these approaches focus on the CNS, there are also screening systems available that allow assessment of compound effects on peripheral nerve function [195,196].
In vitro methods for blood brain barrier permeability
In vitro assays are widely used to understand the BBB function in physiological and pathological situations as well as a screening strategy to triage compounds for in vivo studies in early drug development. There are several in vitro approaches that can be utilized to measure BBB permeability, including static and dynamic platforms which make use of different cell lines (e.g., primary cells, stem cells, iPSC) [197,198]. Commonly employed static BBB assays are cell-based monolayer transport assays and parallel-artificial membrane permeability assay (PAMPA-BBB). Cell-based monolayer transport assays are used to measure permeability of the molecules across the lipid membrane [199]. These assays are reported to have good correlation with the in vivo assays that determine the rate of brain penetration such as brain uptake index or microdialysis [200]. The most common cell-based BBB assay employed in research uses multidrug resistance protein 1-Madin Darby canine kidney (MDR1-MDCK) cells for screening purposes. PAMPA-BBB is a method that predicts the ability of the compounds to enter the brain providing a clear distinction between CNS + and CNS- compounds that can enter the brain through passive diffusion. One of the limitations of this method is that it does not distinguish between compounds that are actively influxed, effluxed, or metabolized. PAMPA-BBB has been reported to have a good correlation with MDCK cell-based transport assays in determining the rate of brain penetration [200]. Currently, additional in vitro microphysiological models of the neurovascular unit are being developed and in the near future, they may improve evaluation of compound penetration across the BBB [201–204]. However, in silico BBB models are most commonly constructed using in vivo data [205]. Among the commonly reported in vivo assays are brain/plasma ratio (B/P) at equilibrium, microdialysis, and in situ brain perfusion [199,200].
Non-mammalian species
Nonmammalian vertebrates, such as zebrafish (Danio rerio), and invertebrates, such as Drosophila melanogaster, C. elegans and planarians are proving to be valid alternative test organisms to investigate DNT and NT [206–211]. Zebrafish can be used to study morphological, behavioral, and molecular endpoints related to neurotoxicity [212]; in safety pharmacology, this species has been employed to evaluate CNS adverse effects, including seizure potential [162,213]. Zebrafish embryos represent an increasingly important high-throughput screening model to investigate chemical-induced DNT by identifying behavioral and anatomical changes [208,212]. Indeed, the zebrafish is considered a practical model for an efficient and rapid evaluation of potential neurodevelopmental neurotoxicants [207]. C. elegans is also becoming useful to predict possible effects of developmental neurotoxicants; such studies can be employed to advance knowledge on the molecular pathways underlying neurodevelopmental disorders [210]. Recent studies using planaria have demonstrated the ability to evaluate 24 endpoints, including lethality, morphology, growth, and various behavioral endpoints in both developing and adult planaria in response to chemical exposures, another potentially useful approach [211]. Hence, available information from alternative species should be integrated alongside other in silico, in vitro, and in vivo data.
Data libraries
Data libraries for NT and DNT assays can be important sources of information when conducting integrated assessments. Several available data libraries are shown in Table 6.
Table 6.
Summary of databases including data associated with neurotoxicity and developmental neurotoxicity (non-exhaustive list).
| Database | Description | Link and reference |
|---|---|---|
|
| ||
| Alternatives Assessment Dashboard Hazard Database | Collection of hazard data compiled for the Alternatives Assessment Dashboard | https://catalog.data.gov/dataset/alternatives-assessment-dashboard-hazard-database-version-1-0-generated-12-07-2018 |
| BindingDB | Binding Database | https://www.bindingdb.org/ |
| ChemBL/PubChem | Database of bioactive drug-like small molecules | https://www.ebi.ac.uk/chembl/ |
| DNT-DIVER | Developmental NeuroToxicity Data Integration and Visualization Enabling Resource | https://sandbox.ntp.niehs.nih.gov/neurotox/ |
| eChemPortal | The Global Portal to Information on Chemical
Substances |
https://www.echemportal.org/echemportal/ |
| ExCAPE-DB | Exascale Compound Activity Prediction Engine Database | https://solr.ideaconsult.net/search/excape/ |
| HSDB | Hazardous Substances Data Bank | https://www.nlm.nih.gov/databases/download/hsdb.html |
| OpenFoodTox | European Food Safety Authority chemical hazards database | https://data.europa.eu/euodp/en/data/dataset/openfoodtox-efsa-s-chemical-hazards-database |
| PDSP Ki database | The NIMH Psychoactive Drug Screening Program
(PDSP) Ki database |
https://pdsp.unc.edu/databases/kidb.php |
| RTECS | Registry of Toxic Effects of Chemical Substances (National Institute for Occupational Safety and Health (NIOSH)) | https://www.cdc.gov/niosh/rtecs/default.html |
| SIDER | Side Effect Resource database | http://sideeffects.embl.de/ |
| ToxCast/Tox21 | Chemical screening data from the ToxCast project and Tox21 collaboration | https://www.epa.gov/chemical-research/exploring-toxcast-data-downloadable-data |
| ToxRefDB | The Toxicity Reference Database developed by the U.S. Environmental Protection Agency | https://github.com/USEPA/CompTox-ToxRefDB |
Libraries of pharmaceuticals may include information resulting from drug use originating from clinical trials and pharmacovigilance [126]. As an example, the SIDER database [127] reports adverse effects from clinical trials and ADRs, and includes data related to nervous system disorders, neuralgia, neuropathy, neurosis, peripheral motor neuropathy, and polyneuropathy [214]. This database collects information from public documents and package inserts on marketed medicines and their recorded adverse drug reactions.
The Toxicity Reference Database (ToxRefDB) developed by the US EPA contains results from over 5900 in vivo studies conducted in accordance with guidelines and/or from open literature [215]. The ToxRefDB version 2 database includes results for effects that are indicative of DNT obtained from 185 studies for 124 chemicals and NT for 18 chemicals from 18 in vivo experiments [216].
The National Toxicology Program (NTP) has developed the Developmental NeuroToxicity Data Integration and Visualization Enabling Resource (DNT-DIVER), a database aimed at analyzing, comparing, and visualizing multiple DNT assays in an interactive web-application [217]. The DNT-DIVER provides a chemical library of 80 chemicals, that cover chemical categories including drugs, flame retardants, industrial chemicals, polycyclic aromatic hydrocarbons (PAHs), and pesticides. Some of these are chemicals with known DNT or NT data (that are linked to corresponding references).
The OpenFoodTox EFSA’s chemical hazards database contains about 100 substances, mostly pesticides and food contaminants, with neurotoxicity data whose corresponding studies are usually discussed in detail [218,219]. In addition, results of the testing of 119 compounds across the current DNT in vitro testing battery are available on the EFSA website [143].
Data related to neurotoxicity are included in the dissemination portal by ECHA that provides electronic public access to information on chemical substances manufactured or imported in Europe [220]. Such information is not peer-reviewed and originates from the registration dossiers, submitted by companies to ECHA within the framework of the REACH Regulation [221]. This data can also be accessed from the eChemPortal database [222].
Several publications summarizing neurotoxicity data are available in the literature. DNT studies for pesticides submitted to the US EPA Office of Pesticide Programs (OPP) were reviewed for use in regulatory decisions by Raffaele et al. [223] and found that positive DNT findings for 15 of 69 chemicals were used as a point of departure for one or more risk assessment scenarios. Mundy and co-workers reviewed the literature for approximately 500 chemicals with evidence of developmental neurotoxicity in animals and humans, and compiled a list of about 100 reference chemicals for use in in vitro assay development and validation [106]. A similar effort resulted in a shorter list of both positive and negative DNT compounds, also for use in assay development [224]. Some examples of reviews focused on specific classes of neurotoxicants include organophosphates [225], neonicotinoids [226], and pyrethroids [227,228].
In silico approaches
Overview
IST comprises different methodologies including category formation (grouping) and read-across, Structure-Activity Relationship (SAR), Quantitative Structure-Activity Relationship (QSAR), and expert systems. Computational approaches may bring two main contributions within an IATA: 1) organization of existing information and, 2) prediction of MIEs or other KEs as defined in an AOP of interest [229]. Computational models developed for MIEs can be used to link chemicals to mechanisms related to neurotoxicity; they can foster development of in vitro screening methods for MIEs (e.g., cholinesterase inhibition), and inform the relevance of such in vitro methods for molecular targets (e.g., whether the cellular targets of interest occur in the cellular model) [230].
In silico studies that focus on the adverse effects of chemical agents on the NS are limited (e.g., [231–233]). They can be generally classified into the following types: a) screening models based on specific and selected biological mechanisms; b) models built against classes of chemicals and these also include structural alerts identified in known neurotoxicants; c) global prediction models for behavior-based endpoints; and d) read-across. A selection of references in silico methods (for the prediction of developmental or adult neurotoxicity) based on structure–activity relationship is included in Table S7 of the supplementary material.
Mechanistic profiling
Models based on specific and selected biological pathways can be applied to profile chemicals and to develop chemical categories based upon similar mechanisms as part of an AOP paradigm; such in silico profilers can be used to screen data sets in order to identify chemicals with the potential to induce a specific type of toxicity (e.g., [234]). The development of an in silico profiler usually involves grouping of chemicals into categories based upon structural similarity. A mechanistic analysis is then performed for each category, allowing for the development of mechanism-based structural alerts which are then combined together to form an in silico profiler [235]. Profilers are different from expert rule-based approaches because structural alerts of expert-based systems are used in a predictive manner (i.e., providing a positive or negative prediction for a given toxicological endpoint), whereas structural alerts of in silico profilers give no indication of toxicity but rather relate to a defined molecular mechanism [236,237]. Toxicity route-agnostic profilers are then used to gain mechanistic insight rather than to provide a toxicity prediction and they can be employed to screen any in vivo or in vitro database. Once a mechanism has been recognized by an in silico profiler, an expert review is needed to derive a conclusion on the potential toxicity for any specific chemical under investigation. Importantly, these in silico predictions can be confirmed using targeted in vitro assays.
In silico profilers have been developed for neurotoxicity-relevant endpoints, some of which are listed in Table 7. For example, a profiler for mitochondrial inhibition embeds machine learning models together with a series of scaffolds that reflect features of known mitochondrial inhibitors implemented as substructure patterns. The mitochondrial system is involved in providing the energy for neurotransmission and the profiler allows for classification of test substances as potential mitochondrial inhibitors [238]. Enoch and co-workers also developed a profiler for mitochondrial dysfunction based on categories that specifically group chemicals according to uncoupling of the oxidative phosphorylation pathway (OxPhos) [239,240]. In silico profilers to identify the cholinergic potential of compounds have also been developed using the knowledge extracted from a database of most known cholinergic compounds, namely about ~ 20,000 compounds that either do or do not interact with the nicotinic (nAChR) and muscarinic receptors (mAChR) and acetylcholinesterase (AChE) [232]. In addition, other profilers have been developed for neuroreceptors (see Table 7), such as the major inhibitory neurotransmitters (GABAA, GABAB, and glycine), as well as serotonin (5HT2A and 5HT3). An in silico profiler for retinoic acid receptor (RAR) binding is integrated in the OECD QSAR Toolbox; over activation of the RAR signaling pathway is associated with CNS developmental defects [240,241].
Table 7.
In silico profilers flagging possible mechanisms relevant for neurotoxicity.
| In silico profiler linked to specific mechanism | Mechanism-type | Description | Reference |
|---|---|---|---|
|
| |||
| Binding to GABAergic system | Receptor-based | Binding to Gamma-Aminobutyric Acid (GABA; GABAA; GABAB) | [243,244] |
| Binding to the glycinergic system | Receptor-based | Binding to ligand-gated ion channel: glycine receptors | [243,244] |
| Binding to the serotonergic system | Receptor-based | Binding to rhodopsin-like receptor: serotonin G-protein coupled receptor (GPCR) (5HT2A) Binding to ligand-gated ion channel: serotonin cys-loop (5HT3) | [243,244] |
| Binding to the cholinergic system | Receptor-based | Binding to: -acetylcholinesterase (AChE) -muscarinic receptor (mAChR) -nicotinic receptor (nAChR) | [232] |
| Alerting structures: pyrethroid, carbamate, organophosphate | Receptorbased | Interactions with the voltage-gated sodium channel is the mode of action of the pyrethroid insecticides Organophosphates and carbamates are potent cholinesterase inhibitors | [232,245,246] |
| Binding to retinoic acid receptor (RAR) | Receptor-based | Binding to the RAR nuclear receptor | [240] |
| Activation of sensory neurons | Receptor-based | Activation of sensory neurons via TRPV1 (Transient receptor potential vanilloid 1) and TRPA1 (Transient receptor potential channel ankyrin 1) | [247] |
| Neural-toxicity mediated by mitochondria | Cellular Processes | Categories that group chemicals according to
known subtarget features associated with mitochondrial inhibition
(i.e., inhibition of Complexes I–V or uncoupling through protonophore action) |
[238] |
| Neural-toxicity mediated by mitochondria | Cellular Processes | Protonophoric activity in mitochondria | [239] |
Protein-ligand docking might also be useful for profiling chemicals in a weight-of-evidence approach and to gain further insights on MIEs and potential mechanisms of action. Such methodology, not yet well-established, requires knowledge of the three-dimensional structure of the molecular target of interest.
Structural features associated with potential neurotoxicity can be identified by mining appropriate datasets. For example, this approach was applied to MEA-based data to find chemicals with acute effects on neural network function [242]. More specifically, data mining was aimed at identifying chemical substructures significantly enriched with MEA actives relative to the total test set (e.g., biphenyls and alkyl phenols); the selected substructures were also used to single out other mechanistically-relevant ToxCast assays (e.g., ion-channel assays) significantly enriched with the MEA substructures. Such an approach is useful for generating hypotheses and building weight-of-evidence arguments that help guide SAR investigations and inform future research.
Predictive models for DNT/NT
Some software tools are available for the prediction of DNT and NT. For example, the Leadscope Model Applier [248] contains neurotoxicity statistical models for rodent pup behavior. Derek Nexus is another tool that estimates adult neurotoxicity using 8 structural alerts: gamma-diketone or precursor; acrylamide or glycidamide; nitroimidazole; carbon disulphide or precursor; pyrethroid; 1-methyl-1,2,3,6-tetrahydropyridine; lead or lead compound; organophosphorus ester [233]. PALLAS HazardExpert v3.6.2.1 and PASS also include models relevant to neurotoxicity [233]. Table S7 of the supplemental material provides additional details on in silico models and approaches as extracted from publications.
Analysis of the performance of selected QSAR tools for neurotoxicity concluded that the absence of neurotoxic potential could not be predicted with certainty by the use of an individual model or a combination of two approaches [233]. Thus, even with combinations of models, a negative in silico prediction is insufficient to ensure lack of neurotoxic potential at this time. On the other hand, useful models exist to identify neurotoxicants. It was thus proposed that the in silico approaches could be used to identify positives by means of QSAR followed by the use of read-across to identify negatives [233].
Read-across
Read-across approaches are usually supported by in silico methods, and they are increasingly being applied for filling data gaps, based on the identification of similar compounds (analogues) that share the same type of mechanism. As neurotoxicity mechanisms are poorly understood, an agreed procedure that uses endpoint-specific profilers for analogue identification is currently lacking; consequently, analogues are mainly identified based only on structural similarity. Application of read-across for characterization of Parkinsonian Hazard Liability was demonstrated in a case study based on an AOP approach for the safety assessment of structurally related mitochondrial complex I inhibitors (i.e., deguelin and rotenone); docking was also integrated in this case study [249].
In silico ADME
In silico models that predict the ability of a chemical agent to cross the BBB provide important information in relation to the evaluation of the adverse effects to the nervous system. They provide both relevance (i.e., does the chemical get into the brain) and inform tissue dose estimates. BBB modeling is also a focus in drug discovery and development to study the brain uptake of potential drug candidates that target the CNS [250,251].
Over the past several decades in silico models have been developed using a variety of datasets and QSAR modeling methodologies to predict BBB permeability [251–253]. Both free and commercial software tools are implementing BBB models to identify chemicals that can cross the BBB [68,87,233]. Most of the QSAR models are based on in vivo Log BB, which is the logarithm of total drug concentration in the brain over plasma concentration measured at equilibrium; such data are the most readily available in the public domain. Additionally, models have been developed based on permeability-surface area (log PS) and unbound brain-to-plasma concentration (Kp,uu,brain); however, their applicability may be limited due to a lack of publicly available data [254,255]. Furthermore, molecular descriptors such as lipophilicity, polar surface area, and hydrogen bonding have been utilized to construct BBB models. The most commonly reported methodologies consist of multiple linear regression (MLR), partial least square (PLS) analysis, variable selection, genetic algorithms, random forest (RF), support vector machine (SVM), and artificial neural networks (ANN) [251]. BBB models have been criticized because most do not account for differences in passive diffusion and active influx/efflux that are due to the presence of various transporters [251,256,257]. However, some QSAR models do include the role of efflux transporters in predictions of BBB permeability [258].
In the case of DNT, the ontogeny of the blood brain barrier has been thought to be functional in the early embryonic period in mammals. However, full maturation (e.g., including metabolism and efflux) does not occur until later in gestation [14,259]. In addition, chemical agents also need to pass the placental barrier to reach the fetus. Thus, the ability of a chemical agent to cross both the placental barrier and BBB will involve not only physicochemical properties, but also dynamic developing biological processes that may affect the developmental neurotoxic potential [87]. Existing QSARs relevant for DNT are based on the physicochemical and structural properties of the substance enabling placental transfer, and do not include the ontogeny of the BBB, nor the DNT effect itself [56]. Five QSAR models for placental transfer have been developed by Hewitt et al. [260] based on different data sets of drugs. In general, it was observed that placental transfer is positively correlated with compound lipophilicity [261].
DNT is known to be impacted by ADME processes. For early neonatal exposures through breast feeding, ADME is dependent on maternal exposure parameters (e.g., diet, inhalation), physiological parameters, personal habits, as well as physicochemical properties of the chemical [262]. Species differences can be an additional factor to consider due to differences in the timing of development and maturation of NS cell type and structure during gestation and lactation [263]. Critical developmental windows for some brain regions exist prenatally in humans and postnatally in rodents, and ADME models should be adjusted to reflect such species differences in compound transfer via the placenta or breast milk. Indeed, the development and application of in silico methods have been recommended for DNT [34,264,265].
In general, in silico predictions of ADME and toxicokinetics rely on rather mature approaches, especially in the context of pharmaceuticals (where the wording pharmacokinetics is instead used) [266–268]. Several models and software tools are available and have been discussed and examined [68,269–271]. Compartmental TK (toxicokinetic) models and PBTK models are increasingly being applied in human hazard assessment [272,273]. Analogous PBPK modeling approaches are being implemented for predictions of distribution of drugs to the human CNS [274,275]. In neurotoxicity risk assessment, threshold dose levels might be estimated by integrating neurotoxicity data measured in vitro with PBPK modeling [83,85,276–278].
Discussion
The goal of a hazard assessment framework underpinning an IST protocol is to integrate computational and/or experimental results (i.e., in vitro, in vivo, human data) in the assessment of the effects of a chemical on a given endpoint (Fig. 1). Development of such a decision framework for the evaluation of neurotoxicity (DNT or NT), where adverse effects are predicted by in silico methods and/or based on empirical measurements, requires that endpoints and effects relevant for the evaluation of neurotoxicity are organized, classified, and combined.
A standardized hazard assessment framework provides the basis for the future development of a corresponding in silico toxicology protocol that guides the use of in silico approaches; this has been shown for genetic toxicology and skin sensitization [279,280] or discussed in relation to the development of IST protocols for carcinogenicity and organ toxicity [59,281,282].
The current review was performed in preparation for a neurotoxicology (inclusive of DNT) in silico protocol and to support the identification of relevant effects and endpoints, with correlative and/or causative relationships, that inform the decision framework for neurotoxicity hazard assessment. This effort has resulted in a draft assessment framework for DNT and NT (see Fig. 2 and Fig. 3). Importantly, this process served to identify data gaps (see below) in the development of a comprehensive in silico neurotoxicity protocol.
Fig. 2.
Draft assessment framework potentially underlying an IST protocol for the assessment of NT. The grey boxes correspond to an assessment of an effect or mechanism that is either measured and/or predicted through one or more in silico models along with an evaluation of the reliability of the information. The blue boxes correspond to an assessment of an endpoint as coming from the combination of information on one or more effects/mechanisms/other endpoints along with an evaluation of confidence. The NT hazard assessment framework needs to be combined with other relevant decision frameworks (e.g., ADME, IVIVE). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Draft assessment framework potentially underlying an IST protocol for the assessment of DNT. The grey boxes correspond to an assessment of an effect or mechanism based on experimental measurements/observations (e.g., using in vitro assays for critical neurodevelopment processes) and/or predicted through one or more in silico models along with an evaluation of the reliability of the information. The blue boxes correspond to an assessment of an endpoint as coming from the combination of information on one or more effects/mechanisms/other endpoints along with an evaluation of the confidence. The DNT hazard assessment framework would be combined with other relevant decision frameworks (e.g., ADME, IVIVE). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The complexity of DNT and NT, coupled with limited knowledge of the underlying biological pathways makes the development of a comprehensive and mechanistically-informed assessment framework challenging. Nevertheless, the proposed draft hazard assessment frameworks for DNT and NT aim to summarize current knowledge (see for example Table 3 and Table 5) necessary for the development and application of predictive in silico models. This development process will necessarily evolve to incorporate the increasing understanding of biological pathways that is ongoing, as well as to account for emerging NAMs that are being developed for assessing NT and DNT. For example, high-throughput transcriptomics [283] as well as high-throughput phenotypic profiling [284,285] are currently being utilized as screening approaches with non-neuronal cells, and are an integral part of future approaches [286]. It is expected that in the near future, these approaches will also be applied to neuronal models.
Draft assessment framework and role of in silico approaches
Effects identified in the hazard assessment decision frameworks (Fig. 2 and Fig. 3) can be evaluated by existing in silico approaches including QSAR, profilers, and read-across. For example, profilers providing insights into potential mechanisms of action triggering neurotoxicity are available to screen compounds for their interaction with cholinergic, GABAergic, serotonergic, and glycinergic systems, as well as for the activation of sensory neurons as shown in Table 7.
Future directions
Development of IST protocols is a fundamental step in the integration of computational methods within mechanistically-informed alternative strategies [42]. In the context of neurotoxicity, these protocols will provide standardized approaches for the use of in silico methods for different purposes including:
screening large numbers of chemicals for prioritization;
chemical-class-specific screening;
potential hazard identification and characterization of a single chemical;
neuro-mechanism specific identification;
grouping of chemicals according to their properties to support read-across and/or provide information on the mechanism underpinning toxicity;
identification of triggers for higher tier tests;
mixture risk assessment to evaluate combined effects;
identification of potential reactive metabolites;
prediction of ADME such as blood–brain barrier permeability and general bioavailability.
The information provided by the application of in silico approaches may inform experimental investigations by supporting, for example, the identification of follow-up in vitro or targeted in vivo assays. In silico predictions may also help to identify whether a given neuro-specific in vitro assay is relevant for a given mechanistic effect (e.g., Do compounds that interfere with synaptogenesis inhibit the assay or is the assay not sensitive? Are given in vitro platforms responsive or refractory to specific MIEs?). In silico methods can help identify chemical classes, such as surfactants or chemically reactive compounds, that can non-specifically interact with protein receptors at high concentrations [287,288] and therefore confound in vitro results.
A mechanistically-driven analysis of in silico approaches for the prediction of toxicity to different organs (i.e., liver, kidney, heart, lung) discussed critical areas supporting the development and application of (Q)SARs for target organ toxicity; the conclusions reached for those target organs may also be considered valid topics for improving in silico approaches in the context of neurotoxicity [59,282]. A key data gap in development of in silico models for NT or DNT is the availability of relevant reproducible and reliable in vivo, ex vivo, or in vitro experimental data. Emerging elucidations of MIEs and KEs need to be complemented with the development of corresponding tests that would then allow for the development of appropriate in silico models. Critical to this effort is the testing of chemical libraries with large and diverse structures in NT and DNT assays [287,289]. In addition, a better understanding of the relationship between in vitro results and in vivo outcomes is needed. As an example, VMAT2 is emerging as a potential off-target site associated with adverse effects on the CNS [179], but due to a lack of testing, limited data are currently available to develop an in silico model for this endpoint. More testing covering a broad chemical space will need to be conducted for all NT and DNT endpoints to allow for future model development.
Another challenging area is the lack of integrated in vivo neurotoxicity databases that contain annotated information, including positive and negative results, on endpoints at tested timepoints and concentrations all linked to chemical structures. This has been accomplished, to a limited degree, with NT studies submitted for pesticide registrations in the United States [215,290].
Similarly, there have been repeated calls for the development of publicly accessible databases of in vitro NT and DNT data [8,33,35,110]. Some progress has been made in this area, including: 1) data from MEA for the ToxCast library chemicals are now available in the Toxcast database [176,191,194,291,292]; 2) data from the OECD/EFSA project has been released by EFSA [143]; and 3) NTP has released a web-based searchable database for about 80 chemicals and 9 different datasets related to DNT [144]. There is also an ongoing international project to analyze all available data from the DNT in vitro battery at the EPA using the in vitro data analysis pipeline [293]. This effort will facilitate the use of standardized analyses and foster similar terminology for integration of information from different sources.
(Q)SARs that predict dose/timepoints need to be further developed (an overview of models is summarized in Table S7 of the supplemental material of this work), and use of ordinal models and read-across approaches may help in this regard. Additionally, in silico methods focusing on identifying molecular initiating events for specific neuronal targets would provide a valuable contribution in the context of NT and DNT. These would include profilers that investigate the interaction of chemicals with, for instance, neuronal receptors and sensory neuron signaling targets, as well as non-specific targets enriched in the nervous system.
Within the AOP paradigm, using data from large numbers of chemicals that are linked to MIEs or KEs (e.g., high-throughput and high-content assays) will support the identification of a greater number of DNT/NT active compounds. However, data associated with KEs cannot differentiate multiple possible upstream MIEs. Thus, development of corresponding target-specific in silico models using KE-based data, as compared to models directly linked to MIEs, will likely reduce the overall accuracy of such models.
ADME considerations are also key determinants that need to be integrated in any hazard assessment framework based on NAMs. Information on in vitro and in vivo kinetics are critical for any in vitro-in vivo extrapolation that supports any estimate of target effect concentrations. Several in silico models are available for some ADME processes linked to NT and DNT, such as BBB penetration and placental transport. The performance of such models is limited by the fact that they are based on limited chemical libraries and do not generally account for differences in passive diffusion and active influx/efflux processes, or developmental age.
Finally, the key characteristics (KCs) concept, first introduced in the context of identifying carcinogenic compounds [294–296] might also be useful for organizing relevant mechanistic and other information for the human health assessments of possible adult and developmental neurotoxicants. The carcinogenic KCs derive from the empirically observed and common properties of human carcinogens, and while they provide a broad, holistic consideration of mechanistic evidence, they avoid a narrow focus on specific pathways and hypotheses [295–297]. Because KCs may have several mechanistic endpoints, they are not themselves mechanisms or AOPs, but instead provide a pragmatic rationale for structuring information.
Key characteristics for (developmental) neurotoxicants are under development by an international expert working group [298]. The current list of KCs includes alterations in or abnormal initiation of: 1) patterns of normal cell death, proliferation, or differentiation; 2) cytoskeletal architecture or function (e.g., axon transport); 3) neurotransmission; 4) cellular Ca2+ homeostasis; 5) protein homeostasis; 6) energy metabolism; 7) redox balance; 8) sterol or lipid metabolism; 9) neuroanatomical structure (including BBB structure or function); and 10) neuroinflammation.
The use of KCs to clearly identify toxicants is challenging at this time. Becker and co-workers [299] concluded that identification of carcinogens was not possible when a KC-based analysis was conducted using ToxCast/Tox21 high throughput assay data only, but more importantly, that the approach requires “explicit, transparent, and scientifically robust procedures to evaluate the relevance and reliability of mechanistic datasets and the process of integrating mechanistic results with extant animal toxicity findings and human epidemiology.” This is true also for the identification of (developmental) neurotoxicants based on a KC framework which might facilitate the combination of different pieces of evidence to create a systematic approach for the evaluation and integration of mechanistic evidence with other types of evidence, while allowing for hazard classifications that are scientifically defensible and appropriate for regulatory decision-making [300].
Conclusions
The ever-expanding chemical universe requires higher throughput analytical toxicological methods alongside mechanistically-based approaches that can lead to greater confidence and efficiency in characterizing the hazards that may be associated with chemical exposures. Incorporation and integration of in silico approaches to assess neurotoxicity hazards, are critical components of advancing human health risk assessments in the 21st century. In silico methods continue to evolve as increasingly valuable and, in some cases, essential for drug development and, more recently, regulatory review.
Herein, the current knowledge of neurotoxicity and the status of in silico approaches for the prediction of neurotoxicity was summarized and future areas for the development of novel computational models were identified. The present work also proposes a pragmatic, rational, and transparent methodology to integrate complex and diverse endpoints related to neurotoxicity and developmental neurotoxicity into a framework that would be acceptable for use in hazard identification and ultimately as a tool for risk assessment. Similar to the IATA concept, this proposed hazard assessment framework integrates data from different sources including data arising from mechanistically-informed alternative strategies. It is meant to foster discussion and support the incorporation of in silico methods that will be eventually applied according to standardized principles to provide clearer expectations of what characteristics are required when making predictions for NT and DNT.
Supplementary Material
Acknowledgements
Research reported in this publication was supported by the National Institute of Environmental Health Sciences of the National Institutes of Health under Award Number R44ES026909. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors would like to thank Dr Louis (Gino) Scarano (US EPA, NW Washington, DC) for his constructive comments on the manuscript.
Abbreviations:
- AChE
Acetylcholinesterase
- ADHD
Attention Deficit Hyperactivity Disorder
- ADME
Absorption, distribution, metabolism, and excretion
- ADR
Adverse drug reaction
- ANN
artificial neural networks
- AOP
Adverse outcome pathway
- BBB
Blood brain barrier
- BPR
Biocidal Products Regulation
- cAMP
Cyclic adenosine monophosphate
- CNS
Central nervous system
- CT
Computed tomography
- DNT
Developmental neurotoxicity
- DNT-DIVER
Developmental NeuroToxicity Data Integration and Visualization Enabling Resource
- ECHA
European Chemicals Agency
- EPA
United States Environmental Protection Agency
- EFSA
European Food Safety Authority
- FAERS
FDA Adverse Event Reporting System
- FDA
U.S. Food & Drug Administration
- FIFRA
Federal Insecticide, Fungicide, and Rodenticide Act
- FOB
Functional observational battery
- GABA
Gamma aminobutyric acid
- GPCR
G-protein coupled receptor
- hiPSC
Human induced pluripotent stem cell
- hNPC
Human neural progenitor cell
- IATA
Integrated approaches to testing and assessment
- IST
In silico toxicology
- IVIVE
in vitro-in vivo extrapolation
- KC
Key characteristics
- KE
Key events
- mAChR
Muscarinic acetylcholine receptor
- MDR1-MDCK
protein 1-Madin Darby canine kidney with MDR1 gene
- MEA
Microelectrode array
- MIE
Molecular initiating event
- MLR
Multiple linear regression
- MRI
Magnetic resonance imaging
- nAChR
Nicotinic acetylcholine receptor
- NAMs
New approach methodologies
- NIOSH
National Institute for Occupational Safety and Health
- NMDA
N-methyl-D-aspartate
- NS
Nervous system
- NT
Neurotoxicity (adult)
- NTP
National toxicology program
- OECD
Organisation for Economic Co-operation and Development
- OPP
US EPA Office of Pesticide Programs
- OxPhos
Oxidative phosphorylation pathway
- PAHs
Polycyclic aromatic hydrocarbons
- PAMPA
Parallel-artificial membrane permeability assay
- PBDEs
Polybrominated diphenyl ethers
- PBK
Physiologically based kinetic
- PBPK
Physiologically based pharmacokinetic
- PBTK
Physiologically based toxicokinetic
- PCBs
Polychlorinated biphenyls
- PCDFs
Polychlorinated dibenzofurans
- PET
Positive emission tomography
- PKC
Protein kinase C
- PLS
Partial least square
- PNS
Peripheral nervous system
- QSAR
Quantitative structure–activity relationship
- RAR
Retinoic acid receptor
- RF
Random forest
- REACH
Registration, Evaluation, Authorisation and Restriction of Chemical Substances
- RTECS
Registry of Toxic Effects of Chemical Substances
- SAR
Structure-activity relationship
- SIDER
Side Effect Resource
- SVM
Support vector machine
- TG
Test guideline
- ToxRefDB
Toxicity Reference Database
- TRPV1
Transient receptor potential vanilloid 1
- TRPA1
Transient receptor potential ankyrin subtype 1
- TSCA
Toxic Substances Control Act
- VMAT2
Vesicular monoamine transporter 2
- WoE
Weight of evidence
Footnotes
Disclaimer
DOD disclaimer
The views and opinions presented herein are those of the author(s) and do not necessarily represent the views of DoD or its Components.
CDC disclaimer
The findings and conclusions in this article are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention/the Agency for Toxic Substances and Disease Registry.
EPA disclaimer
Preparation of this document has been funded by the U.S. Environmental Protection Agency. This document has been subjected to review by the Center for Computational Toxicology and Exposure and approved for publication. Approval does not signify that the contents reflect the views of the Agency, nor does mention of trade names or commercial products constitute endorsement or recommendation for use.
FDA disclaimer
This article reflects the views of the authors and should not be construed to represent FDA’s views or policies. The mention of commercial products, their sources, or their use in connection with material reported herein is not to be construed as either an actual or implied endorsement of such products by the Department of Health and Human Services.
OECD disclaimer
The opinions expressed and arguments employed herein are those of the authors and do not necessarily reflect the official views of the OECD or of the governments of its member countries.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Myatt reports grants from NIH during the conduct of the study; and FDA’s Center for Drug Evaluation and Research (CDER) and Leadscope Inc. (an Instem company) are parties to a formal Research Collaboration Agreement (RCA).
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.comtox.2022.100223.
References
- [1].NRC, NRC, Environmental Neurotoxicology, National Academies Press; (US), Washington, 1992. https://www.ncbi.nlm.nih.gov/books/NBK234245. [PubMed] [Google Scholar]
- [2].Rodier PM, Developing brain as a target of toxicity, Environ. Health Perspect 103 (Suppl 6) (1995) 73–76, 10.1289/ehp.95103s673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Hayashi Y, Jinnou H, Sawamoto K, Hitoshi S, Adult neurogenesis and its role in brain injury and psychiatric diseases, J. Neurochem 147 (5) (2018) 584–594, 10.1111/jnc.14557. [DOI] [PubMed] [Google Scholar]
- [4].Epa Guidelines for Neurotoxicity Risk Assessment, U.S. Environmental Protection Agency; 1998. Washington, DC. [Google Scholar]
- [5].FDA, Chapter IV.C.10. Neurotoxicity Studies, in: Center for Food Safety and Applied Nutrition (Ed.), Redbook 2000. Toxicological Principles for the Safety Assessment of Food Ingredients, FDA, 2000. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/redbook-2000-ivc10-neurotoxicity-studies. [Google Scholar]
- [6].WHO, IPCS Risk Assessment Terminology, World Health Organization, Geneva, Switzerland, 2004. https://www.who.int/publications/i/item/9241562676. [Google Scholar]
- [7].Bal-Price A, Crofton KM, Sachana M, Shafer TJ, Behl M, Forsby A, Hargreaves A, Landesmann B, Lein PJ, Louisse J, Monnet-Tschudi F, Paini A, Rolaki A, Schrattenholz A, Suñol C, van Thriel C, Whelan M, Fritsche E, Putative adverse outcome pathways relevant to neurotoxicity, Crit. Rev. Toxicol 45 (1) (2015) 83–91, 10.3109/10408444.2014.981331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Crofton KM, Mundy WR, Shafer TJ, Developmental neurotoxicity testing: a path forward, Congenital Anomalies 52 (2012) 140–146, 10.1111/j.1741-4520.2012.00377.x. [DOI] [PubMed] [Google Scholar]
- [9].Rice D, Barone S, Critical periods of vulnerability for the developing nervous system: evidence from humans and animal models, Environ. Health Perspect 108 (suppl 3) (2000) 511–533, 10.1289/ehp.00108s3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Rodier PM, Vulnerable periods and processes during central nervous system development, Environ. Health Perspect 102 (Suppl 2) (1994) 121–124, 10.1289/ehp.94102121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zeise L, Bois FY, Chiu WA, Hattis D, Rusyn I, Guyton KZ, Addressing human variability in next-generation human health risk assessments of environmental chemicals, Environ. Health Perspect 121 (1) (2013) 23–31, 10.1289/ehp.1205687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].US EPA, Health Effects Test Guidelines OPPTS 870.6300 Developmental Neurotoxicity Study, United States Environmental Protection Agency, 1996. https://nepis.epa.gov/Exe/ZyPDF.cgi/P100G6UI.PDF?Dockey=P100G6UI.PDF. [Google Scholar]
- [13].Reiter LW, Age-related effects of chemicals on the nervous system, in: Hunt VR, Smith MK, Worth D (Eds.), Environmental Factors in Human Growth and Development, Banbury Report II, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, 1982: pp. 245–267. [Google Scholar]
- [14].Adinolfi M, The development of the human blood-CSF-brain barrier, Dev. Med. Child Neurol 27 (1985) 532–537, 10.1111/j.1469-8749.1985.tb04581.x. [DOI] [PubMed] [Google Scholar]
- [15].Ek CJ, Dziegielewska KM, Habgood MD, Saunders NR, Barriers in the developing brain and Neurotoxicology, NeuroToxicology. 33 (3) (2012) 586–604, 10.1016/j.neuro.2011.12.009. [DOI] [PubMed] [Google Scholar]
- [16].Giordano G, Costa LG, Developmental neurotoxicity: some old and new issues, ISRN Toxicol. 2012 (2012) 1–12, 10.5402/2012/814795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Legradi JB, Di Paolo C, Kraak MHS, van der Geest HG, Schymanski EL, Williams AJ, Dingemans MML, Massei R, Brack W, Cousin X, Begout M-L, van der Oost R, Carion A, Suarez-Ulloa V, Silvestre F, Escher BI, Engwall M, Nilén G, Keiter SH, Pollet D, Waldmann P, Kienle C, Werner I, Haigis A-C, Knapen D, Vergauwen L, Spehr M, Schulz W, Busch W, Leuthold D, Scholz S, Vom Berg CM, Basu N, Murphy CA, Lampert A, Kuckelkorn J, Grummt T, Hollert H, An ecotoxicological view on neurotoxicity assessment, Environ. Sci. Eur 30 (2018) 46, 10.1186/s12302-018-0173-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Masjosthusmann S, Barenys M, El-Gamal M, Geerts L, Gerosa L, Gorreja A, Kühne B, Marchetti N, Tigges J, Viviani B, Witters H, Fritsche E, Literature review and appraisal on alternative neurotoxicity testing methods, EFSA Supporting Publications. 15 (2018) 1410E. doi: 10.2903/sp.efsa.2018.EN-1410. [DOI] [Google Scholar]
- [19].OECD, Test No. 402: Acute Dermal Toxicity, OECD Publishing, Paris, 2017. doi: 10.1787/9789264070585-en. [DOI] [Google Scholar]
- [20].OECD, Test No. 403: Acute Inhalation Toxicity, OECD Publishing, Paris, 2009. doi: 10.1787/9789264070608-en. [DOI] [Google Scholar]
- [21].OECD, Test No. 420: Acute Oral Toxicity - Fixed Dose Procedure, OECD Publishing, Paris, 2002. doi: 10.1787/9789264070943-en. [DOI] [Google Scholar]
- [22].OECD, Test No. 423: Acute Oral toxicity - Acute Toxic Class Method, OECD Publishing, Paris, 2002. doi: 10.1787/9789264071001-en. [DOI] [Google Scholar]
- [23].OECD, Test No. 425: Acute Oral Toxicity: Up-and-Down Procedure, OECD Publishing, Paris, 2008. doi: 10.1787/9789264071049-en. [DOI] [Google Scholar]
- [24].OECD, Test No. 407: Repeated Dose 28-day Oral Toxicity Study in Rodents, OECD Publishing, Paris, 2008. doi: 10.1787/9789264070684-en. [DOI] [Google Scholar]
- [25].OECD, Test No. 408: Repeated Dose 90-Day Oral Toxicity Study in Rodents, OECD Publishing, Paris, 2018. doi: 10.1787/9789264070707-en. [DOI] [Google Scholar]
- [26].OECD, Test No. 424: Neurotoxicity Study in Rodents, OECD Publishing, Paris, 1997. doi: 10.1787/9789264071025-en. [DOI] [Google Scholar]
- [27].OECD, Test No. 418: Delayed Neurotoxicity of Organophosphorus Substances Following Acute Exposure, OECD Publishing, Paris, 1995. doi: 10.1787/9789264070905-en. [DOI] [Google Scholar]
- [28].OECD, Test No. 419: Delayed Neurotoxicity of Organophosphorus Substances: 28- day Repeated Dose Study, OECD Publishing, Paris, 1995. doi: 10.1787/9789264070929-en. [DOI] [Google Scholar]
- [29].OECD, Test No. 426: Developmental Neurotoxicity Study, OECD Publishing, Paris, 2007. doi: 10.1787/9789264067394-en. [DOI] [Google Scholar]
- [30].Staflin K, Misner D, Dambach D, Utilization of in vitro neurotoxicity models in pre-clinical toxicity assessment, in: Sahu SC (Ed.), Stem Cells in Toxicology and Medicine, John Wiley & Sons Ltd, Chichester, UK, 2016, pp. 155–178, 10.1002/9781119135449.ch9. [DOI] [Google Scholar]
- [31].Fretham S, Caito S, Martinez-Finley E, Giordano G, Costa L, Aschner M, Neurotoxicology, in: Hayes A, Kruger C (Eds.), Hayes’ Principles and Methods of Toxicology, Sixth Edition, CRC Press, 2014: pp. 1579–1600. doi: 10.1201/b17359-37. [DOI] [Google Scholar]
- [32].Bal-Price A, Hogberg HT, Crofton KM, Daneshian M, FitzGerald RE, Fritsche E, Heinonen T, Bennekou SH, Klima S, Piersma AH, Sachana M, Shafer TJ, Terron A, Monnet-Tschudi F, Viviani B, Waldmann T, Westerink RHS, Wilks MF, Witters H, Zurich M-G, Leist M, Recommendation on test readiness criteria for new approach methods (NAM) in toxicology: exemplified for developmental neurotoxicity (DNT), ALTEX. 35 (2018) 306–352. 10.14573/altex.1712081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Crofton KM, Mundy WR, Lein PJ, Bal-Price A, Coecke S, Seiler AEM, Knaut H, Buzanska L, Goldberg A, Developmental neurotoxicity testing: recommendations for developing alternative methods for the screening and prioritization of chemicals, ALTEX 28 (2011) 9–15. [PubMed] [Google Scholar]
- [34].Fritsche E, Grandjean P, Crofton KM, Aschner M, Goldberg A, Heinonen T, Hessel EVS, Hogberg HT, Bennekou SH, Lein PJ, Leist M, Mundy WR, Paparella M, Piersma AH, Sachana M, Schmuck G, Solecki R, Terron A, Monnet-Tschudi F, Wilks MF, Witters H, Zurich M-G, Bal-Price A, Consensus statement on the need for innovation, transition and implementation of developmental neurotoxicity (DNT) testing for regulatory purposes, Toxicol. Appl. Pharmacol 354 (2018) 3–6, 10.1016/j.taap.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Sachana M, Bal-Price A, Crofton KM, Bennekou SH, Shafer TJ, Behl M, Terron A, International regulatory and scientific effort for improved developmental neurotoxicity testing, Toxicol. Sci 167 (2019) 45–57, 10.1093/toxsci/kfy211. [DOI] [PubMed] [Google Scholar]
- [36].ECHA, New approach methodologies in regulatory science - Proceedings of a scientific workshop Helsinki, 19–20 April 2016, Publications Office of the EU, 2016. doi: 10.2823/543644. [DOI] [Google Scholar]
- [37].Kavlock RJ, Bahadori T, Barton-Maclaren TS, Gwinn MR, Rasenberg M, Thomas RS, Accelerating the pace of chemical risk assessment, Chem. Res. Toxicol 31 (5) (2018) 287–290, 10.1021/acs.chemrestox.7b00339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ankley GT, Bennett RS, Erickson RJ, Hoff DJ, Hornung MW, Johnson RD, Mount DR, Nichols JW, Russom CL, Schmieder PK, Serrrano JA, Tietge JE, Villeneuve DL, Adverse outcome pathways: a conceptual framework to support ecotoxicology research and risk assessment, Environ. Toxicol. Chem 29 (3) (2010) 730–741, 10.1002/etc.34. [DOI] [PubMed] [Google Scholar]
- [39].Sakuratani Y, Horie M, Leinala E, Integrated approaches to testing and assessment: OECD activities on the development and use of adverse outcome pathways and case studies, Basic Clin. Pharmacol. Toxicol 123 (Suppl 5) (2018) 20–28, 10.1111/bcpt.12955. [DOI] [PubMed] [Google Scholar]
- [40].OECD, Guidance document on an Integrated Approach on Testing and Assessment (IATA) for skin corrosion and irritation, 2017. doi: 10.1787/9789264274693-en. [DOI] [Google Scholar]
- [41].Cronin MTD, Richarz A-N, Relationship between adverse outcome pathways and chemistry-based in silico models to predict toxicity, Appl. In Vitro Toxicol 3 (4) (2017) 286–297, 10.1089/aivt.2017.0021. [DOI] [Google Scholar]
- [42].Myatt GJ, Ahlberg E, Akahori Y, Allen D, Amberg A, Anger LT, Aptula A, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Burden N, Cammerer Z, Cronin MTD, Cross KP, Custer L, Dettwiler M, Dobo K, Ford KA, Fortin MC, Gad-McDonald SE, Gellatly N, Gervais V, Glover KP, Glowienke S, Van Gompel J, Gutsell S, Hardy B, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Hughes K, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kim MT, Kruhlak NL, Kulkarni SA, Kümmerer K, Leavitt P, Majer B, Masten S, Miller S, Moser J, Mumtaz M, Muster W, Neilson L, Oprea TI, Patlewicz G, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Ruiz P, Schilter B, Serafimova R, Simpson W, Stavitskaya L, Stidl R, Suarez-Rodriguez D, Szabo DT, Teasdale A, Trejo-Martin A, Valentin J-P, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Hasselgren C, In silico toxicology protocols, Regul. Toxicol. Pharmacol. 96 (2018) 1–17, 10.1016/j.yrtph.2018.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Myatt GJ, Bassan A, Bower D, Crofton KM, Cross KP, Graham JC, Hasselgren C, Jolly RA, Miller S, Pavan M, Tice RR, Zwickl C, Johnson C, Increasing the acceptance of in silico toxicology through development of protocols and position papers, Comput. Toxicol 21 (2022) 100209, 10.1016/j.comtox.2021.100209. [DOI] [Google Scholar]
- [44].US EPA, Administrator Memo Prioritizing Efforts to Reduce Animal Testing, September 10, 2019, (2019). https://www.epa.gov/research/administrator-memo-prioritizing-efforts-reduce-animal-testing-september-10-2019.
- [45].ICH, ICH S7A Safety pharmacology studies for human pharmaceuticals, European Medicines Agency, 2000. https://database.ich.org/sites/default/files/S7A_Guideline.pdf. [Google Scholar]
- [46].US EPA, OPPTS 870.6200 Neurotoxicity Screening Battery [EPA 712–C–98–238], (1998). https://www.regulations.gov/document/EPA-HQ-OPPT-2009-0156-0041.
- [47].US EPA, OPPTS 870.6300 Developmental Neurotoxicity Study [EPA 712–C–98–239], (1998). https://www.regulations.gov/document/EPA-HQ-OPPT-2009-0156-0042.
- [48].US EPA, Transmittal of Meeting Minutes and Final Report of the Federal Insecticide, Fungicide and Rodenticide Act, Scientific Advisory Panel (FIFRA SAP) Virtual Meeting held on September 15–18, 2020, (2020). https://www.regulations.gov/document/EPA-HQ-OPP-2020-0263-0054.
- [49].US EPA, Alternative Test Methods and Strategies to Reduce Vertebrate Animal Testing, US EPA. (2017). https://www.epa.gov/assessing-and-managing-chemicals-under-tsca/alternative-test-methods-and-strategies-reduce (accessed May 28, 2021). [Google Scholar]
- [50].ECHA, Guidance on information requirements and chemical safety assessment Chapter R.7a: endpoint specific guidance. Version 6.0, Publications Office of the EU, 2017. doi: 10.2823/337352. [DOI] [Google Scholar]
- [51].ECHA, Non-animal approaches Current status of regulatory applicability under the REACH, CLP and Biocidal Products regulations, Publications Office of the EU, 2017. doi: 10.2823/000784. [DOI] [Google Scholar]
- [52].ECHA, Guidance on the Biocidal Products Regulation Volume III: human health, assessment & evaluation (Parts B+C) Version 4.0, Publications Office of the EU, 2017. doi: 10.2823/143042. [DOI] [Google Scholar]
- [53].ECHA, Read-Across Assessment Framework (RAAF), Publications Office of the EU, 2017. 10.2823/619212. [DOI] [Google Scholar]
- [54].EU, Commission Regulation (EU) No 283/2013 setting out the data requirements for active substances in accordance with Regulation (EC) No 1107/2009 and of the Council concerning the placing of plant protection products on the market, OJ. L 93 (2013) 1–84. http://data.europa.eu/eli/reg/2013/283/oj. [Google Scholar]
- [55].EFSA, OECD/EFSA Workshop on Developmental Neurotoxicity (DNT): the use of non-animal test methods for regulatory purposes, (2016). http://www.efsa.europa.eu/it/events/event/161018b. [DOI] [PubMed]
- [56].Fritsche E, Alm H, Baumann J, Geerts L, Håkansson H, Masjosthusmann S, Witters H, Literature review on in vitro and alternative Developmental Neurotoxicity (DNT) testing methods, EFSA Supporting Publication. 12(4):EN- 778 (2015) 186. 10.2903/sp.efsa.2015.EN-778. [DOI] [Google Scholar]
- [57].EU, Improving the preclinical prediction of adverse effects of pharmaceuticals on the nervous system (H2020 Programme, European Commission), (2017). https://cordis.europa.eu/programme/id/H2020_IMI2-2017-13-10 (accessed May 28, 2021).
- [58].Johnson C, Anger LT, Benigni R, Bower D, Bringezu F, Crofton KM, Cronin MTD, Cross KP, Dettwiler M, Frericks M, Melnikov F, Miller S, Roberts DW, Suarez-Rodrigez D, Roncaglioni A, Lo Piparo E, Tice RR, Zwickl C, Myatt GJ, Evaluating confidence in toxicity assessments based on experimental data and in silico predictions, Computational, Toxicology 21 (2022) 100204, 10.1016/j.comtox.2021.100204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Bassan A, Alves VM, Amberg A, Anger LT, Auerbach S, Beilke L, Bender A, Cronin MTD, Cross KP, Hsieh J-H, Greene N, Kemper R, Kim MT, Mumtaz M, Noeske T, Pavan M, Pletz J, Russo DP, Sabnis Y, Schaefer M, Szabo DT, Valentin J-P, Wichard J, Williams D, Woolley D, Zwickl C, Myatt GJ, In silico approaches in organ toxicity hazard assessment: current status and future needs in predicting liver toxicity, Comput. Toxicol 20 (2021) 100187, 10.1016/j.comtox.2021.100187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].White RE, Role of ADME/PK in Drug Discovery, Safety Assessment, and Clinical Development, in: Comprehensive Medicinal Chemistry III, Elsevier, 2017: pp. 1–33. doi: 10.1016/B978-0-12-409547-2.12364-9. [DOI] [Google Scholar]
- [61].Coecke S, Eskes C, Gartlon J, Kinsner A, Price A, van Vliet E, Prieto P, Boveri M, Bremer S, Adler S, Pellizzer C, Wendel A, Hartung T, The value of alternative testing for neurotoxicity in the context of regulatory needs, Environ. Toxicol. Pharmacol 21 (2) (2006) 153–167, 10.1016/j.etap.2005.07.006. [DOI] [PubMed] [Google Scholar]
- [62].Deza-Ponzio R, Herrera ML, Bellini MJ, Virgolini MB, Hereñú CB, Aldehyde dehydrogenase 2 in the spotlight: The link between mitochondria and neurodegeneration, Neurotoxicology. 68 (2018) 19–24, 10.1016/j.neuro.2018.06.005. [DOI] [PubMed] [Google Scholar]
- [63].Umezu T, Sano T, Hayashi J, Shibata Y, Simultaneous blood and brain microdialysis in a free-moving mouse to test blood-brain barrier permeability of chemicals, Toxicol. Rep 7 (2020) 1542–1550, 10.1016/j.toxrep.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Zheng W, Aschner M, Ghersi-Egea J-F, Brain barrier systems: a new frontier in metal neurotoxicological research, Toxicol. Appl. Pharmacol 192 (1) (2003) 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Di L.i., Artursson P, Avdeef A, Ecker GF, Faller B, Fischer H, Houston JB, Kansy M, Kerns EH, Kramer SD, Lennern¨ as H, Sugano K, Evidence-based ¨ approach to assess passive diffusion and carrier-mediated drug transport, Drug Discovery Today. 17 (15–16) (2012) 905–912, 10.1016/j.drudis.2012.03.015. [DOI] [PubMed] [Google Scholar]
- [66].Banks WA, Characteristics of compounds that cross the blood-brain barrier, BMC Neurol. 9 (Suppl 1) (2009) S3, 10.1186/1471-2377-9-S1-S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Gearhart JM, Clewell HJ, Crump KS, Shipp AM, Silvers A, Pharmacokinetic Dose Estimates of Mercury in Children and Dose-Response Curves of Performance Tests in a Large Epidemiological Study, in: Porcella DB, Huckabee JW, Wheatley B (Eds.), Mercury as a Global Pollutant, Springer; Netherlands, Dordrecht, 1995: pp. 49–58. doi: 10.1007/978-94-011-0153-0_6. [DOI] [Google Scholar]
- [68].Shin HK, Kang Y-M, No KT, Predicting ADME Properties of Chemicals, in: Leszczynski J (Ed.), Handbook of Computational Chemistry, Springer, Dordrecht, Netherlands, 2016: pp. 1–37. doi: 10.1007/978-94-007-6169-8_59-1. [DOI] [Google Scholar]
- [69].Clewell H III, Clewell R, Andersen M, Physiologically Based Pharmacokinetic and Toxicokinetic Models, in: Hayes A, Kruger C (Eds.), Hayes’ Principles and Methods of Toxicology, Sixth Edition, CRC Press, 2014: pp. 247–294. doi: 10.1201/b17359-8. [DOI] [Google Scholar]
- [70].Krishnan K, Andersen M, Physiologically Based Pharmacokinetic and Toxicokinetic Models, in: Hayes A (Ed.), Principles and Methods of Toxicology, Fifth Edition, CRC Press, London, 2008: pp. 231–292. doi: 10.1201/b14258. [DOI] [Google Scholar]
- [71].Mumtaz M, Fisher J, Blount B, Ruiz P, Application of physiologically based pharmacokinetic models in chemical risk assessment, J. Toxicol 2012 (2012) 1–11, 10.1155/2012/904603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Paini A, Leonard JA, Joossens E, Bessems JGM, Desalegn A, Dorne JL, Gosling JP, Heringa MB, Klaric M, Kliment T, Kramer NI, Loizou G, Louisse J, Lumen A, Madden JC, Patterson EA, Proença S, Punt A, Setzer RW, Suciu N, Troutman J, Yoon M, Worth A, Tan YM, Next generation physiologically based kinetic (NG-PBK) models in support of regulatory decision making, Comput. Toxicol 9 (2019) 61–72, 10.1016/j.comtox.2018.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].van der Merwe D, Gehring R, Buur JL, Chapter 8 – Toxicokinetics in Veterinary Toxicology, in: Gupta RC(Ed.), Veterinary Toxicology (Third Edition), Academic Press, 2018: pp. 133–143. doi: 10.1016/B978-0-12-811410-0.00008-8. [DOI] [Google Scholar]
- [74].Knaak JB, Dary CC, Zhang X, Gerlach RW, Tornero-Velez R, Chang DT, Goldsmith R, Blancato JN, Parameters for pyrethroid insecticide QSAR and PBPK/PD models for human risk assessment, Rev. Environ. Contam. Toxicol 219 (2012) 1–114, 10.1007/978-1-4614-3281-4_1. [DOI] [PubMed] [Google Scholar]
- [75].Nong A, Tan Y-M, Krolski ME, Wang J, Lunchick C, Conolly RB, Clewell HJ, Bayesian calibration of a physiologically based pharmacokinetic/ pharmacodynamic model of carbaryl cholinesterase inhibition, J. Toxicol. Environ. Health, Part A 71 (20) (2008) 1363–1381, 10.1080/15287390802271608. [DOI] [PubMed] [Google Scholar]
- [76].Timchalk C, Poet TS, Development of a physiologically based pharmacokinetic and pharmacodynamic model to determine dosimetry and cholinesterase inhibition for a binary mixture of chlorpyrifos and diazinon in the rat, Neurotoxicology 29 (3) (2008) 428–443, 10.1016/j.neuro.2008.02.004. [DOI] [PubMed] [Google Scholar]
- [77].Proença S, Escher BI, Fischer FC, Fisher C, Grégoire S, Hewitt NJ, Nicol B, Paini A, Kramer NI, Effective exposure of chemicals in in vitro cell systems: A review of chemical distribution models, Toxicol. In Vitro 73 (2021) 105133, 10.1016/j.tiv.2021.105133. [DOI] [PubMed] [Google Scholar]
- [78].Tice RR, Austin CP, Kavlock RJ, Bucher JR, Improving the human hazard characterization of chemicals: A Tox21 update, Environ. Health Perspect 121 (7) (2013) 756–765, 10.1289/ehp.1205784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kramer NI, Di Consiglio E, Blaauboer BJ, Testai E, Biokinetics in repeated-dosing in vitro drug toxicity studies, Toxicol. In Vitro 30 (1) (2015) 217–224, 10.1016/j.tiv.2015.09.005. [DOI] [PubMed] [Google Scholar]
- [80].Thomas RS, Philbert MA, Auerbach SS, Wetmore BA, Devito MJ, Cote I, Rowlands JC, Whelan MP, Hays SM, Andersen ME, Meek MEB, Reiter LW, Lambert JC, Clewell HJ, Stephens ML, Zhao QJ, Wesselkamper SC, Flowers L, Carney EW, Pastoor TP, Petersen DD, Yauk CL, Nong A, Incorporating new technologies into toxicity testing and risk assessment: moving from 21st century vision to a data-driven framework, Toxicol. Sci 136 (1) (2013) 4–18, 10.1093/toxsci/kft178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Tsaioun K, Evidence-based absorption, distribution, metabolism, excretion (ADME) and its interplay with alternative toxicity methods, ALTEX. (2016) 343–358. doi: 10.14573/altex.1610101. [DOI] [PubMed] [Google Scholar]
- [82].Wetmore BA, Wambaugh JF, Ferguson SS, Sochaski MA, Rotroff DM, Freeman K, Clewell HJ, Dix DJ, Andersen ME, Houck KA, Allen B, Judson RS, Singh R, Kavlock RJ, Richard AM, Thomas RS, Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment, Toxicol. Sci 125 (1) (2012) 157–174, 10.1093/toxsci/kfr254. [DOI] [PubMed] [Google Scholar]
- [83].Forsby A, Blaauboer B, Integration of in vitro neurotoxicity data with biokinetic modelling for the estimation of in vivo neurotoxicity, Hum. Exp. Toxicol 26 (4) (2007) 333–338, 10.1177/0960327106072994. [DOI] [PubMed] [Google Scholar]
- [84].Nicolas CI, Mansouri K, Phillips KA, Grulke CM, Richard AM, Williams AJ, Rabinowitz J, Isaacs KK, Yau A, Wambaugh JF, Rapid experimental measurements of physicochemical properties to inform models and testing, Sci. Total Environ 636 (2018) 901–909, 10.1016/j.scitotenv.2018.04.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Yoon M, Kedderis GL, Yan GZ, Clewell HJ, Use of in vitro data in developing a physiologically based pharmacokinetic model: carbaryl as a case study, Toxicology. 332 (2015) 52–66, 10.1016/j.tox.2014.05.006. [DOI] [PubMed] [Google Scholar]
- [86].Mallick P, Moreau M, Song G, Efremenko AY, Pendse SN, Creek MR, Osimitz TG, Hines RN, Hinderliter P, Clewell HJ, Lake BG, Yoon M, Development and application of a life-stage Physiologically Based Pharmacokinetic (PBPK) model to the assessment of internal dose of pyrethroids in humans, Toxicol. Sci 173 (2020) 86–99, 10.1093/toxsci/kfz211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Bal-Price A, Pistollato F, Application of Non-Animal Methods to More Effective Neurotoxicity Testing for Regulatory Purposes, in: Aschner M, Costa L (Eds.), Cell Culture Techniques, Springer, New York, NY, 2019: pp. 283–299. doi: 10.1007/978-1-4939-9228-7_15. [DOI] [Google Scholar]
- [88].Thomas RS, Paules RS, Simeonov A, Fitzpatrick SC, Crofton KM, Casey WM, Mendrick DL, The US Federal Tox21 Program: A strategic and operational plan for continued leadership, ALTEX. 35 (2018) 163–168. doi: 10.14573/altex.1803011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].OECD, Test No. 417: Toxicokinetics, OECD Publishing, Paris, 2010. doi: 10.1787/9789264070882-en. [DOI] [Google Scholar]
- [90].Blake BL, Toxicology of the nervous system, in: Hodgson E(Ed.), A Textbook of Modern Toxicology, 4th ed., John Wiley & Sons, Hoboken, N.J., 2010, pp. 303–322, https://www.wiley.com/en-us/A+Textbook+of+Modern+Toxicology%2C+4th+Edition-p-9780470462065. [Google Scholar]
- [91].Costa LG, Giordano G, Guizzetti M, Vitalone A, Neurotoxicity of pesticides: a brief review, Front. Biosci 13 (2008) 1240–1249, 10.2741/2758. [DOI] [PubMed] [Google Scholar]
- [92].Moser VC, Aschner M, Richardson RJ, Philbert M, Toxic response of the nervous system, in: Casarett LJ, Doull J, Klaassen CD (Eds.), Casarett and Doull’s Toxicology: The Basic Science of Poisons, 7th ed, McGraw-Hill, New York, 2008, pp. 631–664. [Google Scholar]
- [93].Spencer PS, Schaumburg HH, Ludolph AC (Eds.), Experimental and clinical neurotoxicology, 2nd ed., Oxford University Press, New York, 2000. [Google Scholar]
- [94].Padilla S, Regulatory and research issues related to cholinesterase inhibition, Toxicology. 102 (1–2) (1995) 215–220, 10.1016/0300-483X(95)03050-P. [DOI] [PubMed] [Google Scholar]
- [95].Prieto P, Graepel R, Gerloff K, Lamon L, Sachana M, Pistollato F, Gribaldo L, Bal-Price A, Worth A, Investigating cell type specific mechanisms contributing to acute oral toxicity, ALTEX. 36 (2019) 39–64. doi: 10.14573/altex.1805181. [DOI] [PubMed] [Google Scholar]
- [96].Suñol C, Tusell JM, Gelpí E, Rodríguez-Farré E, GABAergic modulation of lindane (gamma-hexachlorocyclohexane)-induced seizures, Toxicol. Appl. Pharmacol 100 (1989) 1–8, 10.1016/0041-008x(89)90086-0. [DOI] [PubMed] [Google Scholar]
- [97].Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML, Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment, Toxicology. 171 (1) (2002) 3–59. [DOI] [PubMed] [Google Scholar]
- [98].Pomara C, Neri M, Bello S, Fiore C, Riezzo I, Turillazzi E, Neurotoxicity by synthetic androgen steroids: oxidative stress, apoptosis, and neuropathology: A review, Curr. Neuropharmacol 13 (2015) 132–145, 10.2174/1570159X13666141210221434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Houtsmuller EJ, Brand T, de Jonge FH, Joosten RNJMA, van de Poll NE, Slob AK, SDN-POA volume, sexual behavior, and partner preference of male rats affected by perinatal treatment with ATD, Physiol. Behav 56 (3) (1994) 535–541, 10.1016/0031-9384(94)90298-4. [DOI] [PubMed] [Google Scholar]
- [100].Patel UH, Mir MA, Sivik JK, Raheja D, Pandey MK, Talamo G, Central neurotoxicity of immunomodulatory drugs in multiple myeloma, Hematol. Rep 7 (1) (2015) 5704, 10.4081/hr.2015.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Acharya UH, Dhawale T, Yun S, Jacobson CA, Chavez JC, Ramos JD, Appelbaum J, Maloney DG, Management of cytokine release syndrome and neurotoxicity in chimeric antigen receptor (CAR) T cell therapy, Expert Rev. Hematol 12 (3) (2019) 195–205, 10.1080/17474086.2019.1585238. [DOI] [PubMed] [Google Scholar]
- [102].Hunter BD, Jacobson CA, CAR T-cell associated neurotoxicity: mechanisms, clinicopathologic correlates, and future directions, J. Natl. Cancer Inst 111 (2019) 646–654, 10.1093/jnci/djz017. [DOI] [PubMed] [Google Scholar]
- [103].Willis MD, Robertson NP, Neurotoxicity of novel cancer immunotherapies, J. Neurol 266 (8) (2019) 2087–2089, 10.1007/s00415-019-09444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Neal M, Richardson JR, Epigenetic regulation of astrocyte function in neuroinflammation and neurodegeneration, Biochim. Biophys. Acta, Mol. Basis Dis 1864 (2) (2018) 432–443, 10.1016/j.bbadis.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fritsche E, Tigges J, Hartmann J, Kapr J, Serafini MM, Viviani B, Neural In Vitro Models for Studying Substances Acting on the Central Nervous System, in: Schäfer-Korting M, Stuchi Maria-Engler S, Landsiedel R (Eds.), Organotypic Models in Drug Development, Springer International Publishing, Cham, 2021: pp. 111–141. doi: 10.1007/164_2020_367. [DOI] [PubMed] [Google Scholar]
- [106].Mundy WR, Padilla S, Breier JM, Crofton KM, Gilbert ME, Herr DW, Jensen KF, Radio NM, Raffaele KC, Schumacher K, Shafer TJ, Cowden J, Expanding the test set: Chemicals with potential to disrupt mammalian brain development, Neurotoxicol. Teratol 52 (2015) 25–35, 10.1016/j.ntt.2015.10.001. [DOI] [PubMed] [Google Scholar]
- [107].Bal-Price A, Crofton KM, Leist M, Allen S, Arand M, Buetler T, Delrue N, FitzGerald RE, Hartung T, Heinonen T, Hogberg H, Bennekou SH, Lichtensteiger W, Oggier D, Paparella M, Axelstad M, Piersma A, Rached E, Schilter B, Schmuck G, Stoppini L, Tongiorgi E, Tiramani M, Monnet-Tschudi F, Wilks MF, Ylikomi T, Fritsche E, International STakeholder NETwork (ISTNET): creating a developmental neurotoxicity (DNT) testing road map for regulatory purposes, Arch. Toxicol 89 (2) (2015) 269–287, 10.1007/s00204-015-1464-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Radio NM, Mundy WR, Developmental neurotoxicity testing in vitro: Models for assessing chemical effects on neurite outgrowth, NeuroToxicol. 29 (3) (2008) 361–376, 10.1016/j.neuro.2008.02.011. [DOI] [PubMed] [Google Scholar]
- [109].Carlson LM, Champagne FA, Cory-Slechta DA, Dishaw L, Faustman E, Mundy W, Segal D, Sobin C, Starkey C, Taylor M, Makris SL, Kraft A, Potential frameworks to support evaluation of mechanistic data for developmental neurotoxicity outcomes: a symposium report, Neurotoxicol. Teratol 78 (2020) 106865, 10.1016/j.ntt.2020.106865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Fritsche E, OECD/EFSA workshop on developmental neurotoxicity (DNT): The use of non-animal test methods for regulatory purposes, ALTEX. (2017) 311–315. doi: 10.14573/altex.1701171. [DOI] [PubMed] [Google Scholar]
- [111].Judson RS, Magpantay FM, Chickarmane V, Haskell C, Tania N, Taylor J, Xia M, Huang R, Rotroff DM, Filer DL, Houck KA, Martin MT, Sipes N, Richard AM, Mansouri K, Setzer RW, Knudsen TB, Crofton KM, Thomas RS, Integrated model of chemical perturbations of a biological pathway using 18 in vitro high-throughput screening assays for the estrogen receptor, Toxicol. Sci 148 (1) (2015) 137–154, 10.1093/toxsci/kfv168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Mansouri K, Abdelaziz A, Rybacka A, Roncaglioni A, Tropsha A, Varnek A, Zakharov A, Worth A, Richard AM, Grulke CM, Trisciuzzi D, Fourches D, Horvath D, Benfenati E, Muratov E, Wedebye EB, Grisoni F, Mangiatordi GF, Incisivo GM, Hong H, Ng HW, Tetko IV, Balabin I, Kancherla J, Shen J, Burton J, Nicklaus M, Cassotti M, Nikolov NG, Nicolotti O, Andersson PL, Zang Q, Politi R, Beger RD, Todeschini R, Huang R, Farag S, Rosenberg SA, Slavov S, Hu X, Judson RS, CERAPP: collaborative estrogen receptor activity prediction project, Environ. Health Perspect 124 (7) (2016) 1023–1033, 10.1289/ehp.1510267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Bal-Price A, Pistollato F, Sachana M, Bopp SK, Munn S, Worth A, Strategies to improve the regulatory assessment of developmental neurotoxicity (DNT) using in vitro methods, Toxicol. Appl. Pharmacol 354 (2018) 7–18, 10.1016/j.taap.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bal-Price A, Meek MEB, Adverse outcome pathways: Application to enhance mechanistic understanding of neurotoxicity, Pharmacol. Ther 179 (2017) 84–95, 10.1016/j.pharmthera.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Li J, Settivari R, LeBaron MJ, Marty MS, An industry perspective: a streamlined screening strategy using alternative models for chemical assessment of developmental neurotoxicity, Neurotoxicology. 73 (2019) 17–30, 10.1016/j.neuro.2019.02.010. [DOI] [PubMed] [Google Scholar]
- [116].Sachana M, Rolaki A, Bal-Price A, Development of the Adverse Outcome Pathway (AOP): Chronic binding of antagonist to N-methyl-d-aspartate receptors (NMDARs) during brain development induces impairment of learning and memory abilities of children, Toxicol. Appl. Pharmacol 354 (2018) 153–175, 10.1016/j.taap.2018.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Paul Friedman K, Watt ED, Hornung MW, Hedge JM, Judson RS, Crofton KM, Houck KA, Simmons SO, Tiered high-throughput screening approach to identify thyroperoxidase inhibitors within the ToxCast phase I and II chemical libraries, Toxicol. Sci 151 (1) (2016) 160–180, 10.1093/toxsci/kfw034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Rosenberg SA, Watt ED, Judson RS, Simmons SO, Paul Friedman K, Dybdahl M, Nikolov NG, Wedebye EB, QSAR models for thyroperoxidase inhibition and screening of U.S. and EU chemical inventories, Comput. Toxicol 4 (2017) 11–21, 10.1016/j.comtox.2017.07.006. [DOI] [Google Scholar]
- [119].WHO, Neurotoxicity Risk Assessment for Human Health: Principles and Approaches, World Health Organization, Geneva, Switzerland, 2001. http://www.inchem.org/documents/ehc/ehc/ehc223.htm#_223318000. [Google Scholar]
- [120].Boyes WK, Moser VC, Geller AM, Benignus VA, Bushnell PJ, Kamel F, Integrating epidemiology and toxicology in neurotoxicity risk assessment, Hum. Exp. Toxicol 26 (4) (2007) 283–293, 10.1177/0960327106070481. [DOI] [PubMed] [Google Scholar]
- [121].Tarone RE, Lipworth L, McLaughlin JK, The epidemiology of environmental perchlorate exposure and thyroid function: a comprehensive review, J. Occup. Environ. Med 52 (2010) 653–660, 10.1097/JOM.0b013e3181e31955. [DOI] [PubMed] [Google Scholar]
- [122].Ockleford C, Adriaanse P, Berny P, Brock T, Duquesne S, Grilli S, Hernandez-Jerez AF, Bennekou SH, Klein M, Kuhl T, Laskowski R, Machera K, Pelkonen O, Pieper S, Smith R, Stemmer M, Sundh I, Teodorovic I, Tiktak A, Topping CJ, Wolterink G, Angeli K, Fritsche E, Hernandez-Jerez AF, Leist M, Mantovani A, Menendez P, Pelkonen O, Price A, Viviani B, Chiusolo A, Ruffo F, Terron A, Bennekou SH, Investigation into experimental toxicological properties of plant protection products having a potential link to Parkinson’s disease and childhood leukaemia, EFS2. 15 (3) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Davidson PW, Cory-Slechta DA, Thurston SW, Huang L-S, Shamlaye CF, Gunzler D, Watson G, van Wijngaarden E, Zareba G, Klein JD, Clarkson TW, Strain JJ, Myers GJ, Fish consumption and prenatal methylmercury exposure: Cognitive and behavioral outcomes in the main cohort at 17 years from the Seychelles child development study, NeuroToxicol. 32 (6) (2011) 711–717, 10.1016/j.neuro.2011.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Grandjean P, Weihe P, White RF, Debes F, Araki S, Yokoyama K, Murata K, Sørensen N, Dahl R, Jørgensen PJ, Cognitive deficit in 7-year-old children with prenatal exposure to methylmercury, Neurotoxicol. Teratol 19 (6) (1997) 417–428. [DOI] [PubMed] [Google Scholar]
- [125].Weaver RJ, Valentin J-P, Today’s challenges to de-risk and predict drug safety in human “Mind-the-Gap”, Toxicol. Sci 167 (2019) 307–321, 10.1093/toxsci/kfy270. [DOI] [PubMed] [Google Scholar]
- [126].Pawar G, Madden JC, Ebbrell D, Firman JW, Cronin MTD, In silico toxicology data resources to support read-across and (Q)SAR, Front. Pharmacol 10 (2019) 561, 10.3389/fphar.2019.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Kuhn M, Letunic I, Jensen LJ, Bork P, The SIDER database of drugs and side effects, Nucl. Acids Res 44 (D1) (2016) D1075–D1079, 10.1093/nar/gkv1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Fang H, Su Z, Wang Y, Miller A, Liu Z, Howard PC, Tong W, Lin SM, Exploring the FDA adverse event reporting system to generate hypotheses for monitoring of disease characteristics, Clin. Pharmacol. Ther 95 (5) (2014) 496–498, 10.1038/clpt.2014.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Lindquist M, VigiBase, the WHO Global ICSR Database System: Basic Facts, Drug Inf. J 42 (5) (2008) 409–419, 10.1177/009286150804200501. [DOI] [Google Scholar]
- [130].Postigo R, Brosch S, Slattery J, van Haren A, Dogné J-M, Kurz X, Candore G, Domergue F, Arlett P, EudraVigilance medicines safety database: publicly accessible data for research and public health protection, Drug Saf. 41 (7) (2018) 665–675, 10.1007/s40264-018-0647-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Boyes WK, Neurotoxicology and Behavior, in: Bingham E, Cohrssen B, Powell CH (Eds.), Patty’s Toxicology, 5th ed., John Wiley & Sons Inc., New York, NY, 2012: pp. 35–74. doi: 10.1002/0471435139.tox025.pub2. [DOI] [Google Scholar]
- [132].Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG, Touchstone JA, Dosemeci M, Potential occupational risks for neurodegenerative diseases, Am. J. Ind. Med 48 (1) (2005) 63–77, 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- [133].Schulte PA, Burnett CA, Boeniger MF, Johnson J, Neurodegenerative diseases: occupational occurrence and potential risk factors, 1982 through 1991, Am. J. Public Health 86 (1996) 1281–1288. doi: 10.2105/ajph.86.9.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Reuhl KR, Delayed expression of neurotoxicity: the problem of silent damage, Neurotoxicology. 12 (1991) 341–346. [PubMed] [Google Scholar]
- [135].Kent Anger W, Neurobehavioural tests and systems to assess neurotoxic exposures in the workplace and community, Occup. Environ. Med 60 (7) (2003) 531–538, 10.1136/oem.60.7.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Anger WK, Worksite behavioral research. Results, sensitive methods, test batteries and the transition from laboratory data to human health, Neurotoxicology 11 (1990) 627–717. [PubMed] [Google Scholar]
- [137].Rohlman DS, Lucchini R, Anger WK, Bellinger DC, van Thriel C, Neurobehavioral testing in human risk assessment, Neurotoxicology 29 (3) (2008) 556–567, 10.1016/j.neuro.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Gad SC, A neuromuscular screen for use in industrial toxicology, J. Toxicol. Environ. Health 9 (5–6) (1982) 691–704, 10.1080/15287398209530197. [DOI] [PubMed] [Google Scholar]
- [139].Irwin S, Comprehensive observational assessment: Ia. A systematic, quantitative procedure for assessing the behavioral and physiologic state of the mouse, Psychopharmacologia. 13 (3) (1968) 222–257. [DOI] [PubMed] [Google Scholar]
- [140].Moser VC, The functional observational battery in adult and developing rats, Neurotoxicology 21 (2000) 989–996. [PubMed] [Google Scholar]
- [141].Moser VC, Applications of a neurobehavioral screening battery, J. Am. Coll. Toxicol 10 (6) (1991) 661–669, 10.3109/10915819109078658. [DOI] [Google Scholar]
- [142].Chang LW, Slikker W, eds., Neurotoxicology: approaches and methods, 1st Edition, Academic Press, San Diego, 1995. https://www.elsevier.com/books/neurotoxicology/chang/978-0-12-168055-8. [Google Scholar]
- [143].Masjosthusmann S, Blum J, Bartmann K, Dolde X, Holzer A, Stürzl L, Keßel EH, Förster N, Dönmez A, Klose J, Pahl M, Waldmann T, Bendt F, Kisitu J, Suciu I, Hübenthal U, Mosig A, Leist M, Fritsche E, Establishment of an a priori protocol for the implementation and interpretation of an in-vitro testing battery for the assessment of developmental neurotoxicity, EFS3 17 (2020) 1938E, 10.2903/sp.efsa.2020.EN-1938. [DOI] [Google Scholar]
- [144].Behl M, Ryan K, Hsieh J-H, Parham F, Shapiro AJ, Collins BJ, Sipes NS, Birnbaum LS, Bucher JR, Foster PMD, Walker NJ, Paules RS, Tice RR, Screening for developmental neurotoxicity at the National Toxicology Program: the future is here, Toxicol. Sci 167 (2019) 6–14, 10.1093/toxsci/kfy278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].US EPA, Chemical Safety for Sustainability Strategic Research Action Plan 2019–2022, (2020). https://www.epa.gov/research/chemical-safety-sustainability-strategic-research-action-plan-2019-2022.
- [146].Vassallo A, Chiappalone M, De Camargos Lopes R, Scelfo B, Novellino A, Defranchi E, Palosaari T, Weisschu T, Ramirez T, Martinoia S, Johnstone AFM, Mack CM, Landsiedel R, Whelan M, Bal-Price A, Shafer TJ, A multi-laboratory evaluation of microelectrode array-based measurements of neural network activity for acute neurotoxicity testing, NeuroToxicology 60 (2017) 280–292, 10.1016/j.neuro.2016.03.019. [DOI] [PubMed] [Google Scholar]
- [147].Shafer TJ, Application of Microelectrode Array Approaches to Neurotoxicity Testing and Screening, in: Chiappalone M, Pasquale V, Frega M(Eds.), Vitro Neuronal Networks, Springer International Publishing, Cham, 2019, pp. 275–297, 10.1007/978-3-030-11135-9_12. [DOI] [PubMed] [Google Scholar]
- [148].Barbosa DJ, Capela JP, de Lourdes Bastos M, Carvalho F, In vitro models for neurotoxicology research, Toxicol. Res 4 (4) (2015) 801–842, 10.1039/C4TX00043A. [DOI] [Google Scholar]
- [149].Fritsche E, Barenys M, Klose J, Masjosthusmann S, Nimtz L, Schmuck M, Wuttke S, Tigges J, Current availability of stem cell-based in vitro methods for Developmental Neurotoxicity (DNT) testing, Toxicol. Sci 165 (2018) 21–30, 10.1093/toxsci/kfy178. [DOI] [PubMed] [Google Scholar]
- [150].Wilson MS, Graham JR, Ball AJ, Multiparametric High Content Analysis for assessment of neurotoxicity in differentiated neuronal cell lines and human embryonic stem cell-derived neurons, NeuroToxicology. 42 (2014) 33–48, 10.1016/j.neuro.2014.03.013. [DOI] [PubMed] [Google Scholar]
- [151].Hoelting L, Klima S, Karreman C, Grinberg M, Meisig J, Henry M, Rotshteyn T, Rahnenführer J, Blüthgen N, Sachinidis A, Waldmann T, Leist M, Stem cell-derived immature human dorsal root ganglia neurons to identify peripheral neurotoxicants, Stem Cells Transl. Med 5 (2016) 476–487, 10.5966/sctm.2015-0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Moors M, Rockel TD, Abel J, Cline JE, Gassmann K, Schreiber T, Schuwald J, Weinmann N, Fritsche E, Human neurospheres as three-dimensional cellular systems for developmental neurotoxicity testing, Environ. Health Perspect 117 (7) (2009) 1131–1138, 10.1289/ehp.0800207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Nimtz L, Hartmann J, Tigges J, Masjosthusmann S, Schmuck M, Keßel E, Theiss S, Köhrer K, Petzsch P, Adjaye J, Wigmann C, Wieczorek D, Hildebrandt B, Bendt F, Hübenthal U, Brockerhoff G, Fritsche E, Characterization and application of electrically active neuronal networks established from human induced pluripotent stem cell-derived neural progenitor cells for neurotoxicity evaluation, Stem Cell Res. 45 (2020) 101761, 10.1016/j.scr.2020.101761. [DOI] [PubMed] [Google Scholar]
- [154].Nyffeler J, Karreman C, Leisner H, Kim YJ, Lee G, Waldmann T, Leist M, Design of a high-throughput human neural crest cell migration assay to indicate potential developmental toxicants, ALTEX. 34 (2017) 75–94. doi: 10.14573/altex.1605031. [DOI] [PubMed] [Google Scholar]
- [155].Pistollato F, de Gyves EM, Carpi D, Bopp SK, Nunes C, Worth A, Bal-Price A, Assessment of developmental neurotoxicity induced by chemical mixtures using an adverse outcome pathway concept, Environ. Health 19 (2020) 23, 10.1186/s12940-020-00578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [156].Pistollato F, Louisse J, Scelfo B, Mennecozzi M, Accordi B, Basso G, Gaspar JA, Zagoura D, Barilari M, Palosaari T, Sachinidis A, Bremer-Hoffmann S, Development of a pluripotent stem cell derived neuronal model to identify chemically induced pathway perturbations in relation to neurotoxicity: effects of CREB pathway inhibition, Toxicol. Appl. Pharmacol 280 (2) (2014) 378–388, 10.1016/j.taap.2014.08.007. [DOI] [PubMed] [Google Scholar]
- [157].Johnstone AFM, Gross GW, Weiss DG, Schroeder O-U, Gramowski A, Shafer TJ, Microelectrode arrays: A physiologically based neurotoxicity testing platform for the 21st century, NeuroToxicology 31 (4) (2010) 331–350, 10.1016/j.neuro.2010.04.001. [DOI] [PubMed] [Google Scholar]
- [158].Judson R, Houck K, Martin M, Richard AM, Knudsen TB, Shah I, Little S, Wambaugh J, Woodrow Setzer R, Kothya P, Phuong J, Filer D, Smith D, Reif D, Rotroff D, Kleinstreuer N, Sipes N, Xia M, Huang R, Crofton K, Thomas RS, Editor’s highlight: Analysis of the effects of cell stress and cytotoxicity on in vitro assay activity across a diverse chemical and assay space, Toxicol. Sci 152 (2) (2016) 323–339, 10.1093/toxsci/kfw092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Lein P, Locke P, Goldberg A, Meeting report: alternatives for developmental neurotoxicity testing, Environ. Health Perspect 115 (5) (2007) 764–768, 10.1289/ehp.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Crofton KM, Mundy WR, External scientific report on the interpretation of data from the developmental neurotoxicity in vitro testing assays for use in integrated approaches for testing and assessment, EFSA Supporting Publication. (2021) EN-6924. 42pp. doi: 10.2903/sp.efsa.2021.EN-6924. [DOI] [Google Scholar]
- [161].Krebs A, Waldmann T, Wilks MF, Van Vugt-Lussenburg BMA, Van der Burg B, Terron A, Steger-Hartmann T, Ruegg J, Rovida C, Pedersen E, Pallocca G, Luijten M, Leite SB, Kustermann S, Kamp H, Hoeng J, Hewitt P, Herzler M, Hengstler JG, Heinonen T, Hartung T, Hardy B, Gantner F, Fritsche E, Fant K, Ezendam J, Exner T, Dunkern T, Dietrich DR, Coecke S, Busquet F, Braeuning A, Bondarenko O, Bennekou SH, Beilmann M, Leist M, Template for the description of cell-based toxicological test methods to allow evaluation and regulatory use of the data, ALTEX. 36 (2019) 682–699. doi: 10.14573/altex.1909271. [DOI] [PubMed] [Google Scholar]
- [162].Authier S, Arezzo J, Delatte MS, Kallman M-J, Markgraf C, Paquette D, Pugsley MK, Ratcliffe S, Redfern WS, Stevens J, Valentin J-P, Vargas HM, Curtis MJ, Safety pharmacology investigations on the nervous system: An industry survey, J. Pharmacol. Toxicol. Methods 81 (2016) 37–46, 10.1016/j.vascn.2016.06.001. [DOI] [PubMed] [Google Scholar]
- [163].Easter A, Sharp TH, Valentin J-P, Pollard CE, Pharmacological validation of a semi-automated in vitro hippocampal brain slice assay for assessment of seizure liability, J. Pharmacol. Toxicol. Methods 56 (2) (2007) 223–233, 10.1016/j.vascn.2007.04.008. [DOI] [PubMed] [Google Scholar]
- [164].Oliver AP, Hoffer BJ, Wyatt RJ, The hippocampal slice: a system for studying the pharmacology of seizures and for screening anticonvulsant drugs, Epilepsia 18 (4) (1977) 543–548, 10.1111/j.1528-1157.1977.tb05002.x. [DOI] [PubMed] [Google Scholar]
- [165].Bradley JA, Luithardt HH, Metea MR, Strock CJ, In vitro screening for seizure liability using microelectrode array technology, Toxicol. Sci 163 (2018) 240–253, 10.1093/toxsci/kfy029. [DOI] [PubMed] [Google Scholar]
- [166].Bradley JA, Strock CJ, Screening for neurotoxicity with microelectrode array, Curr. Protoc. Toxicol 79 (2019), e67, 10.1002/cptx.67. [DOI] [PubMed] [Google Scholar]
- [167].Fan J, Thalody G, Kwagh J, Burnett E, Shi H, Lewen G, Chen S-J, Levesque P, Assessing seizure liability using multi-electrode arrays (MEA), Toxicol. In Vitro 55 (2019) 93–100, 10.1016/j.tiv.2018.12.001. [DOI] [PubMed] [Google Scholar]
- [168].Kreir M, Van Deuren B, Versweyveld S, De Bondt A, Van den Wyngaert I, Van der Linde H, Lu HR, Teuns G, Gallacher DJ, Do in vitro assays in rat primary neurons predict drug-induced seizure liability in humans? Toxicol. Appl. Pharmacol 346 (2018) 45–57, 10.1016/j.taap.2018.03.028. [DOI] [PubMed] [Google Scholar]
- [169].Bowes J, Brown AJ, Hamon J, Jarolimek W, Sridhar A, Waldron G, Whitebread S, Reducing safety-related drug attrition: the use of in vitro pharmacological profiling, Nat. Rev. Drug Discovery 11 (12) (2012) 909–922, 10.1038/nrd3845. [DOI] [PubMed] [Google Scholar]
- [170].Bendels S, Bissantz C, Fasching B, Gerebtzoff G, Guba W, Kansy M, Migeon J, Mohr S, Peters J-U, Tillier F, Wyler R, Lerner C, Kramer C, Richter H, Roberts S, Safety screening in early drug discovery: An optimized assay panel, J. Pharmacol. Toxicol. Methods 99 (2019) 106609, 10.1016/j.vascn.2019.106609. [DOI] [PubMed] [Google Scholar]
- [171].Lynch JJ, Van Vleet TR, Mittelstadt SW, Blomme EAG, Potential functional and pathological side effects related to off-target pharmacological activity, J. Pharmacol. Toxicol. Methods 87 (2017) 108–126, 10.1016/j.vascn.2017.02.020. [DOI] [PubMed] [Google Scholar]
- [172].Easter A, Bell ME, Damewood JR, Redfern WS, Valentin J-P, Winter MJ, Fonck C, Bialecki RA, Approaches to seizure risk assessment in preclinical drug discovery, Drug Discovery Today 14 (17–18) (2009) 876–884, 10.1016/j.drudis.2009.06.003. [DOI] [PubMed] [Google Scholar]
- [173].FDA, Assessment of Abuse Potential of Drugs, U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER), 2017. https://www.fda.gov/media/116739/download. [Google Scholar]
- [174].Deaton AM, Fan F, Zhang W, Nguyen PA, Ward LD, Nioi P, Rationalizing secondary pharmacology screening using human genetic and pharmacological evidence, Toxicol. Sci 167 (2019) 593–603, 10.1093/toxsci/kfy265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [175].Chushak YG, Shows HW, Gearhart JM, Pangburn HA, In silico identification of protein targets for chemical neurotoxins using ToxCast in vitro data and read-across within the QSAR toolbox, Toxicol. Res 7 (3) (2018) 423–431, 10.1039/C7TX00268H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [176].US EPA, Comptox Chemicals Dashboard, (2021). https://comptox.epa.gov/dashboard/assay_endpoints/ (accessed September 10, 2021).
- [177].Williams AJ, Grulke CM, Edwards J, McEachran AD, Mansouri K, Baker NC, Patlewicz G, Shah I, Wambaugh JF, Judson RS, Richard AM, The CompTox Chemistry Dashboard: a community data resource for environmental chemistry, J. Cheminf 9 (2017) 61, 10.1186/s13321-017-0247-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Nickell JR, Siripurapu KB, Vartak A, Crooks PA, Dwoskin LP, The vesicular monoamine transporter-2: an important pharmacological target for the discovery of novel therapeutics to treat methamphetamine abuse, Adv. Pharmacol. (San Diego, CA, U. S.). 69 (2014) 71–106. 10.1016/B978-0-12-420118-7.00002-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Wimalasena K, Vesicular monoamine transporters: structure-function, pharmacology, and medicinal chemistry, Med. Res. Rev 31 (4) (2011) 483–519, 10.1002/med.20187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Yasumoto S, Tamura K, Karasawa J, Hasegawa R, Ikeda K, Yamamoto T, Yamamoto H, Inhibitory effect of selective serotonin reuptake inhibitors on the vesicular monoamine transporter 2, Neurosci. Lett 454 (3) (2009) 229–232, 10.1016/j.neulet.2009.03.049. [DOI] [PubMed] [Google Scholar]
- [181].Valentin J-P, Guillon J-M, Jenkinson S, Kadambi V, Ravikumar P, Roberts S, Rosenbrier-Ribeiro L, Schmidt F, Armstrong D, In vitro secondary pharmacological profiling: an IQ-DruSafe industry survey on current practices, J. Pharmacol. Toxicol. Methods 93 (2018) 7–14, 10.1016/j.vascn.2018.07.001. [DOI] [PubMed] [Google Scholar]
- [182].Whitebread S, Dumotier B, Armstrong D, Fekete A, Chen S, Hartmann A, Muller PY, Urban L, Secondary pharmacology: screening and interpretation of off-target activities – focus on translation, Drug Discov. Today 21 (8) (2016) 1232–1242, 10.1016/j.drudis.2016.04.021. [DOI] [PubMed] [Google Scholar]
- [183].Teuns G, Easter A, Predicting organ toxicity in vivo – central nervous system, in: Will Y, McDuffie JE, Olaharski AJ, Jeffy BD (Eds.), Drug Discovery Toxicology, John Wiley & Sons Ltd, 2016, pp. 214–226, 10.1002/9781119053248.ch15. [DOI] [Google Scholar]
- [184].Accardi MV, Pugsley MK, Forster R, Troncy E, Huang H, Authier S, The emerging role of in vitro electrophysiological methods in CNS safety pharmacology, J. Pharmacol. Toxicol. Methods 81 (2016) 47–59, 10.1016/j.vascn.2016.03.008. [DOI] [PubMed] [Google Scholar]
- [185].Dunlop J, Bowlby M, Peri R, Vasilyev D, Arias R, High-throughput electrophysiology: an emerging paradigm for ion-channel screening and physiology, Nat. Rev. Drug Discovery 7 (4) (2008) 358–368, 10.1038/nrd2552. [DOI] [PubMed] [Google Scholar]
- [186].Zafeiriou M-P, Bao G, Hudson J, Halder R, Blenkle A, Schreiber M-K, Fischer A, Schild D, Zimmermann W-H, Developmental GABA polarity switch and neuronal plasticity in Bioengineered Neuronal Organoids, Nat. Commun 11 (2020) 3791, 10.1038/s41467-020-17521-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Saavedra L, Wallace K, Freudenrich TF, Mall M, Mundy WR, Davila J, Shafer TJ, Wernig M, Haag D, Comparison of acute effects of neurotoxic compounds on network activity in human and rodent neural cultures, Toxicol. Sci 180 (2) (2021) 295–312, 10.1093/toxsci/kfab008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Tukker AM, Wijnolts FMJ, de Groot A, Westerink RHS, Applicability of hiPSC-derived neuronal cocultures and rodent primary cortical cultures for in vitro seizure liability assessment, Toxicol. Sci 178 (2020) 71–87, 10.1093/toxsci/kfaa136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [189].Tukker AM, Bouwman LMS, van Kleef RGDM, Hendriks HS, Legler J, Westerink RHS, Perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) acutely affect human α1β2γ2L GABAA receptor and spontaneous neuronal network function in vitro, Sci. Rep 10 (2020) 5311, 10.1038/s41598-020-62152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Brown JP, Hall D, Frank CL, Wallace K, Mundy WR, Shafer TJ, Evaluation of a microelectrode array-based assay for neural network ontogeny using training set chemicals, Toxicol. Sci 154 (2016) 126–139, 10.1093/toxsci/kfw147. [DOI] [PubMed] [Google Scholar]
- [191].Frank CL, Brown JP, Wallace K, Mundy WR, Shafer TJ, Developmental neurotoxicants disrupt activity in cortical networks on microelectrode arrays: Results of screening 86 compounds during neural network formation, Toxicol. Sci 160 (2017) 121–135, 10.1093/toxsci/kfx169. [DOI] [PubMed] [Google Scholar]
- [192].Hogberg HT, Sobanski T, Novellino A, Whelan M, Weiss DG, Bal-Price AK, Application of micro-electrode arrays (MEAs) as an emerging technology for developmental neurotoxicity: evaluation of domoic acid-induced effects in primary cultures of rat cortical neurons, NeuroToxicology 32 (1) (2011) 158–168, 10.1016/j.neuro.2010.10.007. [DOI] [PubMed] [Google Scholar]
- [193].Robinette BL, Harrill JA, Mundy WR, Shafer TJ, In vitro assessment of developmental neurotoxicity: use of microelectrode arrays to measure functional changes in neuronal network ontogeny, Front. Neuroeng 4 (2011), 10.3389/fneng.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Shafer TJ, Brown JP, Lynch B, Davila-Montero S, Wallace K, Friedman KP, Evaluation of chemical effects on network formation in cortical neurons grown on microelectrode arrays, Toxicol. Sci 169 (2019) 436–455, 10.1093/toxsci/kfz052. [DOI] [PubMed] [Google Scholar]
- [195].Nguyen H, McCoy L, Willey H, Sharma AD, Curley L, Moore M, Nerve-on-a-chip platform for assessing chemotherapy-induced peripheral neuropathy, J. Pharmacological and Toxicol. Methods 99 (2019) 106595, 10.1016/j.vascn.2019.05.088. [DOI] [Google Scholar]
- [196].Sharma AD, McCoy L, Willey H, Nguyen H, Curley L, Moore M, Novel high-throughput human cells-based co-culture screen for chemotherapeutics induced neurotoxicity, J. Pharmacol. Toxicol. Methods 99 (2019) 106595, 10.1016/j.vascn.2019.05.182. [DOI] [Google Scholar]
- [197].Bagchi S, Chhibber T, Lahooti B, Verma A, Borse V, Jayant RD, In-vitro blood-brain barrier models for drug screening and permeation studies: an overview, Drug Des., Dev. Ther 13 (2019) 3591–3605, 10.2147/DDDT.S218708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Sivandzade F, Cucullo L, In-vitro blood-brain barrier modeling: a review of modern and fast-advancing technologies, J. Cereb. Blood Flow Metab. 38 (10) (2018) 1667–1681, 10.1177/0271678X18788769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [199].Di L.i., Kerns EH, Carter GT, Strategies to assess blood-brain barrier penetration, Expert Opin Drug Discov. 3 (6) (2008) 677–687, 10.1517/17460441.3.6.677. [DOI] [PubMed] [Google Scholar]
- [200].Di L.i., Kerns EH, Bezar IF, Petusky SL, Huang Y, Comparison of blood-brain barrier permeability assays: in situ brain perfusion, MDR1-MDCKII and PAMPA-BBB, J. Pharm. Sci 98 (6) (2009) 1980–1991, 10.1002/jps.21580. [DOI] [PubMed] [Google Scholar]
- [201].Alcendor DJ, Block III FE, Cliffel DE, Daniels J, Ellacott KL, Goodwin CR, Hofmeister LH, Li D, Markov DA, May JC, McCawley LJ, McLaughlin B, McLean JA, Niswender KD, Pensabene V, Seale KT, Sherrod SD, Sung H-J, Tabb DL, Webb DJ, Wikswo JP, Neurovascular unit on a chip: implications for translational applications, Stem Cell Res. Ther 4 (2013) S18, 10.1186/scrt379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [202].Brown JA, Pensabene V, Markov DA, Allwardt V, Neely MD, Shi M, Britt CM, Hoilett OS, Yang Q, Brewer BM, Samson PC, McCawley LJ, May JM, Webb DJ, Li D, Bowman AB, Reiserer RS, Wikswo JP, Recreating blood-brain barrier physiology and structure on chip: a novel neurovascular microfluidic bioreactor, Biomicrofluidics. 9 (5) (2015) 054124, 10.1063/1.4934713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [203].Lee CS, Leong KW, Advances in microphysiological Blood-Brain Barrier (BBB) models towards drug delivery, Curr. Opin. Biotechnol 66 (2020) 78–87, 10.1016/j.copbio.2020.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [204].Seo S, Kim H, Sung JH, Choi N, Lee K, Kim HN, Microphysiological systems for recapitulating physiology and function of blood-brain barrier, Biomaterials. 232 (2020) 119732, 10.1016/j.biomaterials.2019.119732. [DOI] [PubMed] [Google Scholar]
- [205].OECD, Guidance Document on the Characterisation, Validation and Reporting of PBK Models for Regulatory Purposes, OECD Publishing, Paris, 2021. http://www.oecd.org/chemicalsafety/testing/series-testing-assessment-publications-number.htm. [Google Scholar]
- [206].Avila D, Helmcke K, Aschner M, The Caenorhabiditis elegans model as a reliable tool in neurotoxicology, Hum. Exp. Toxicol 31 (3) (2012) 236–243, 10.1177/0960327110392084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [207].d’Amora M, Giordani S, The utility of zebrafish as a model for screening developmental neurotoxicity, Front. Neurosci 12 (2018) 976, 10.3389/fnins.2018.00976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [208].Garcia GR, Noyes PD, Tanguay RL, Advancements in zebrafish applications for 21st century toxicology, Pharmacol. Ther 161 (2016) 11–21, 10.1016/j.pharmthera.2016.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [209].Quevedo C, Behl M, Ryan K, Paules RS, Alday A, Muriana A, Alzualde A, Detection and prioritization of developmentally neurotoxic and/or neurotoxic compounds using zebrafish, Toxicol. Sci 168 (2019) 225–240, 10.1093/toxsci/kfy291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [210].Ruszkiewicz JA, Pinkas A, Miah MR, Weitz RL, Lawes MJA, Akinyemi AJ, Ijomone OM, Aschner M, elegans C as a model in developmental neurotoxicology, Toxicol. Appl. Pharmacol 354 (2018) 126–135, 10.1016/j.taap.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Zhang S, Hagstrom D, Hayes P, Graham A, Collins E-M-S, Multi-behavioral endpoint testing of an 87-chemical compound library in freshwater planarians, Toxicol. Sci 167 (2019) 26–44, 10.1093/toxsci/kfy145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [212].Kiper KG, Freeman JL, Zebrafish as a Tool to Assess Developmental Neurotoxicity, in: Aschner M, Costa L (Eds.), Cell Culture Techniques, Springer, New York, NY, 2019: pp. 169–193. 10.1007/978-1-4939-9228-7_9. [DOI] [Google Scholar]
- [213].Koseki N, Deguchi J, Yamashita A, Miyawaki I, Funabashi H, Establishment of a novel experimental protocol for drug-induced seizure liability screening based on a locomotor activity assay in zebrafish, J. Toxicol. Sci 39 (4) (2014) 579–600, 10.2131/jts.39.579. [DOI] [PubMed] [Google Scholar]
- [214].Jamal S, Goyal S, Shanker A, Grover A, Predicting neurological Adverse Drug Reactions based on biological, chemical and phenotypic properties of drugs using machine learning models, Sci. Rep 7 (2017) 1–12, 10.1038/s41598-017-00908-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [215].Watford S, Pham LL, Wignall J, Shin R, Martin MT, Friedman KP, ToxRefDB version 2.0: Improved utility for predictive and retrospective toxicology analyses, Reproductive Toxicol. 89 (2019) 145–158, 10.1016/j.reprotox.2019.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Watford S, Pham LL, Friedman KP, Toxicity Reference Database User Guide - Version 1 - US Environmental Protection Agency Office of Research and Development National Center for Computational Toxicology Research Triangle Park, North Carolina, (2019). https://github.com/USEPA/CompTox-ToxRefDB/blob/master/ToxRefDB_2_0_UserGuide_Final.pdf. [Google Scholar]
- [217].NTP, Integrative Screening Strategies for Neurotoxicity at the National Toxicology Program (NTP), (2018) 002–00062-0001–0000–0001. 10.22427/NTP-DATA-002-00062-0001-0000-1. [DOI]
- [218].Baldin R, Fioravanzo E, Pavan M, Bassan A, Report on “Data collection and data entry for EFSA’s chemical hazards database NP/EFSA/EMRISK/2011/01,” EFSA Supporting Publications. 10 (2013) 458E. doi: 10.2903/sp.efsa.2013.EN-458. [DOI] [Google Scholar]
- [219].Dorne JLCM, Richardson J, Livaniou A, Carnesecchi E, Ceriani L, Baldin R, Kovarich S, Pavan M, Saouter E, Biganzoli F, Pasinato L, Zare Jeddi M, Robinson TP, Kass GEN, Liem AKD, Toropov AA, Toropova AP, Yang C, Tarkhov A, Georgiadis N, Di Nicola MR, Mostrag A, Verhagen H, Roncaglioni A, Benfenati E, Bassan A, EFSA’s OpenFoodTox: An open source toxicological database on chemicals in food and feed and its future developments, Environ. Int 146 (2021) 106293, 10.1016/j.envint.2020.106293. [DOI] [PubMed] [Google Scholar]
- [220].ECHA, European Chemical Agency (ECHA) website, (2021). https://echa.europa.eu/en(accessed August 27, 2021).
- [221].EU, Regulation (EC) No 1907/2006 of the European Parliament and of the Council of 18 December 2006 Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), establishing a European Chemicals Agency, amending Directive 1999/45/EC and repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC, OJ. L 396 (2006) 1–849. http://data.europa.eu/eli/reg/2006/1907/oj. [Google Scholar]
- [222].OECD, eChemPortal version 3.0, (2020). https://www.echemportal.org/echemportal/(accessed April 23, 2020).
- [223].Raffaele KC, Rowland J, May B, Makris SL, Schumacher K, Scarano LJ, The use of developmental neurotoxicity data in pesticide risk assessments, Neurotoxicol. Teratol 32 (5) (2010) 563–572, 10.1016/j.ntt.2010.04.053. [DOI] [PubMed] [Google Scholar]
- [224].Aschner M, Ceccatelli S, Daneshian M, Fritsche E, Hasiwa N, Hartung T, Hogberg HT, Leist M, Li A, Mundy WR, Padilla S, Piersma AH, Bal-Price A, Seiler A, Westerink RH, Zimmer B, Lein PJ, Reference compounds for alternative test methods to indicate developmental neurotoxicity (DNT) potential of chemicals: example lists and criteria for their selection and use, ALTEX. 34 (2017) 49–74. doi: 10.14573/altex.1604201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [225].Voorhees JR, Rohlman DS, Lein PJ, Pieper AA, Neurotoxicity in preclinical models of occupational exposure to organophosphorus compounds, Front. Neurosci 10 (2017), 10.3389/fnins.2016.00590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [226].Sheets LP, Li AA, Minnema DJ, Collier RH, Creek MR, Peffer RC, A critical review of neonicotinoid insecticides for developmental neurotoxicity, Crit. Rev. Toxicol 46 (2) (2016) 153–190, 10.3109/10408444.2015.1090948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Weiner ML, Nemec M, Sheets L, Sargent D, Breckenridge C, Comparative functional observational battery study of twelve commercial pyrethroid insecticides in male rats following acute oral exposure, Neurotoxicology. 30 (Suppl 1) (2009) S1–S16, 10.1016/j.neuro.2009.08.014. [DOI] [PubMed] [Google Scholar]
- [228].Wolansky MJ, Gennings C, Crofton KM, Relative potencies for acute effects of pyrethroids on motor function in rats, Toxicol. Sci 89 (2006) 271–277, 10.1093/toxsci/kfj020. [DOI] [PubMed] [Google Scholar]
- [229].Tollefsen KE, Scholz S, Cronin MT, Edwards SW, de Knecht J, Crofton K, Garcia-Reyero N, Hartung T, Worth A, Patlewicz G, Applying Adverse Outcome Pathways (AOPs) to support Integrated Approaches to Testing and Assessment (IATA), Regul. Toxicol. Pharmacol 70 (3) (2014) 629–640, 10.1016/j.yrtph.2014.09.009. [DOI] [PubMed] [Google Scholar]
- [230].Gearhart JM, Chushak YG, Hinckley S, Bang AG, Ott DK, Pangburn HA, QSAR analysis of chemical neurotoxins using human neuronal stem cell microelectrode array measurements (MEA) and Tox21 high-throughput data, The Toxicologist, Supplement to Toxicological Sciences. 150 (1) (2017) Abstract #1391. https://www.toxicology.org/pubs/docs/Tox/2017Tox.pdf. [Google Scholar]
- [231].Burton J, Worth AP, Tsakovska I, Diukendjieva A, In silico models for acute systemic toxicity, in: Benfenati E (Ed.), In Silico Methods for Predicting Drug Toxicity, Humana Press, New York, NY, 2016: pp. 177–200. doi: 10.1007/978-1-4939-3609-0_10. [DOI] [PubMed] [Google Scholar]
- [232].Wijeyesakere SJ, Wilson DM, Sue Marty M, Prediction of cholinergic compounds by machine-learning, Comput. Toxicol 13 (2020) 100119, 10.1016/j.comtox.2020.100119. [DOI] [Google Scholar]
- [233].Worth A, Fuart-Gatnik M, Lapenna S, Serafimova R, Applicability of QSAR analysis in the evaluation of developmental and neurotoxicity effects for the assessment of the toxicological relevance of metabolites and degradates of pesticide active substances for dietary risk assessment, EFSA Supporting Publications 8 (2011) 169E, 10.2903/sp.efsa.2011.EN-169. [DOI] [Google Scholar]
- [234].Nelms MD, Mellor CL, Cronin MTD, Madden JC, Enoch SJ, Development of an in silico profiler for mitochondrial toxicity, Chem. Res. Toxicol 28 (10) (2015) 1891–1902, 10.1021/acs.chemrestox.5b00275. [DOI] [PubMed] [Google Scholar]
- [235].Enoch SJ, Cronin MTD, Ellison CM, The use of a chemistry-based profiler for covalent DNA binding in the development of chemical categories for read-across for genotoxicity, ATLA, Altern. Lab. Anim 39 (2) (2011) 131–145, 10.1177/026119291103900206. [DOI] [PubMed] [Google Scholar]
- [236].Enoch SJ, Cronin MTD, A review of the electrophilic reaction chemistry involved in covalent DNA binding, Crit. Rev. Toxicol 40 (8) (2010) 728–748, 10.3109/10408444.2010.494175. [DOI] [PubMed] [Google Scholar]
- [237].Schultz TW, Chapter 14: Adverse Outcome Pathways: A way of linking chemical structure to in vivo toxicological hazards, in: Cronin MTD, Madden JC(Eds.), Silico Toxicology: Principles and Applications, Royal Society of Chemistry, 2010, pp. 346–371, 10.1039/9781849732093-00346. [DOI] [Google Scholar]
- [238].Wijeyesakere SJ, Wilson D, Auernhammer T, Parks A, Kovacs D, Marty MS, Hybrid machine-learning/SMARTS profiling model for mitochondrial inhibition, Appl. In Vitro Toxicol 5 (4) (2019) 196–204, 10.1089/aivt.2019.0010. [DOI] [Google Scholar]
- [239].Enoch SJ, Schultz TW, Popova IG, Vasilev KG, Mekenyan OG, Development of a Decision Tree for Mitochondrial Dysfunction: Uncoupling of Oxidative Phosphorylation, Chem. Res. Toxicol 31 (8) (2018) 814–820, 10.1021/acs.chemrestox.8b00132. [DOI] [PubMed] [Google Scholar]
- [240].OECD, The OECD QSAR Toolbox, Toolbox 4.4, https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm, 2020. https://www.oecd.org/chemicalsafety/risk-assessment/oecd-qsar-toolbox.htm (accessed September 23, 2021).
- [241].Yasuhara R, Yuasa T, Williams JA, Byers SW, Shah S, Pacifici M, Iwamoto M, Enomoto-Iwamoto M, Wnt/beta-catenin and retinoic acid receptor signaling pathways interact to regulate chondrocyte function and matrix turnover, J. Biol. Chem 285 (2010) 317–327, 10.1074/jbc.M109.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Strickland JD, Martin MT, Richard AM, Houck KA, Shafer TJ, Screening the ToxCast phase II libraries for alterations in network function using cortical neurons grown on multi-well microelectrode array (mwMEA) plates, Arch. Toxicol 92 (1) (2018) 487–500, 10.1007/s00204-017-2035-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [243].Marty MS, Wijeyesakere SJ, Auernhammer T, Parks A, Wilson D, Use of ToxCast high-throughput in vitro data to develop a computational model to identify compounds that interact with neurological receptors, The Toxicologist, Supplement to Toxicological Sciences. 150 (1), Abstract #2535 (2018). https://www.toxicology.org/pubs/docs/Tox/2018Tox.pdf. [Google Scholar]
- [244].Wijeyesakere SJ, Wilson D, Auernhammer TR, Parks A, Marty MS, Developing Highly Accurate Computational Models for Neuronal Targets, The Toxicologist, Supplement to Toxicological Sciences. 168 (1), Abstract #1329 (2019). https://www.toxicology.org/pubs/docs/Tox/2019Tox.pdf. [Google Scholar]
- [245].Field LM, Emyr Davies TG, O’Reilly AO, Williamson MS, Wallace BA, Voltage-gated sodium channels as targets for pyrethroid insecticides, Eur. Biophys. J 46 (7) (2017) 675–679, 10.1007/s00249-016-1195-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [246].Ju H, Kandimalla VB, CHAPTER 2 – Biosensors for pesticides, in: Zhang X, Ju H, Wang J (Eds.), Electrochemical Sensors, Biosensors and Their Biomedical Applications, Academic Press, San Diego, 2008: pp. 31–56. doi: 10.1016/B978-012373738-0.50004-0. [DOI] [Google Scholar]
- [247].Krieger SM, Wijeyesakere SJ, Auernhammer TR, Parks A, Hotchkiss JA, Wilson D, Marty MS, Development of a computational model for the Transient Receptor Potential Vanilloid Subfamily Type 1 Protein (TRPV1), The Toxicologist, Supplement to Toxicological Sciences. 168 (1), Abstract #2028 (2019). https://www.toxicology.org/pubs/docs/Tox/2019Tox.pdf. [Google Scholar]
- [248].Instem, Instem – Computational Toxicology, (2021). https://www.instem.com/solutions/insilico/computational-toxicology.php (accessed December 10, 2021).
- [249].OECD, Case Study on the Use of Integrated Approaches to Testing and Assessment for Identification and Characterisation of Parkinsonian Hazard Liability of Deguelin by an AOP-Based Testing and Read Across approach, OECD Publishing, Paris, 2020. http://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=ENV/JM/MONO(2020)22&docLanguage=en. [Google Scholar]
- [250].Cecchelli R, Berezowski V, Lundquist S, Culot M, Renftel M, Dehouck M-P, Fenart L, Modelling of the blood–brain barrier in drug discovery and development, Nat. Rev. Drug Discovery 6 (8) (2007) 650–661, 10.1038/nrd2368. [DOI] [PubMed] [Google Scholar]
- [251].Chen H, Winiwarter S, Engkvist O, In Silico Tools for Predicting Brain Exposure of Drugs, in: Di L, Kerns EH(Eds.), Blood-Brain Barrier in Drug Discovery, John Wiley & Sons, Inc, Hoboken, NJ, 2015: pp. 167–187. 10.1002/9781118788523.ch9. [DOI] [Google Scholar]
- [252].Brito-Sánchez Y, Marrero-Ponce Y, Barigye SJ, Yaber-Goenaga I, MoréllPerez C, Le-Thi-Thu H, Cherkasov A, Towards better BBB passage prediction using an extensive and curated data set, Mol. Inf 34 (5) (2015) 308–330, 10.1002/minf.201400118. [DOI] [PubMed] [Google Scholar]
- [253].Morales JF, Montoto SS, Fagiolino P, Ruiz ME, Current state and future perspectives in QSAR models to predict blood- brain barrier penetration in central nervous system drug R&D, Mini-Rev. Med. Chem 17 (2017) 247–257, 10.2174/1389557516666161013110813. [DOI] [PubMed] [Google Scholar]
- [254].Fridén M, Winiwarter S, Jerndal G, Bengtsson O, Wan H, Bredberg U, Hammarlund-Udenaes M, Antonsson M, Structure-brain exposure relationships in rat and human using a novel data set of unbound drug concentrations in brain interstitial and cerebrospinal fluids, J. Med. Chem 52 (20) (2009) 6233–6243, 10.1021/jm901036q. [DOI] [PubMed] [Google Scholar]
- [255].Liu X, Tu M, Kelly RS, Chen C, Smith BJ, Development of a computational approach to predict blood-brain barrier permeability, Drug Metab. Dispos 32 (1) (2004) 132–139, 10.1124/dmd.32.1.132. [DOI] [PubMed] [Google Scholar]
- [256].Begley DJ, ABC transporters and the blood-brain barrier, Curr. Pharm. Des 10 (2004) 1295–1312, 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- [257].International Transporter Consortium, Giacomini KM, Huang, Tweedie, Benet LZ, Brouwer, Chu X, Dahlin A, Evers, Fischer V, Hillgren KM, Hoffmaster, Ishikawa T, Keppler, Kim RB, Lee CA, Niemi M, Polli JW, Sugiyama Y, Swaan, Ware JA, Wright SH, Yee SW, Zamek-Gliszczynski MJ, Zhang L, Membrane transporters in drug development, Nat Rev, Drug Discovery 9 (2010) 215–236, 10.1038/nrd3028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [258].Garg P, Dhakne R, Belekar V, Role of breast cancer resistance protein (BCRP) as active efflux transporter on blood-brain barrier (BBB) permeability, Mol. Diversity 19 (1) (2015) 163–172, 10.1007/s11030-014-9562-2. [DOI] [PubMed] [Google Scholar]
- [259].Saili KS, Zurlinden TJ, Schwab AJ, Silvin A, Baker NC, Hunter ES, Ginhoux F, Knudsen TB, Blood-brain barrier development: Systems modeling and predictive toxicology, Birth Defects Res. 109 (2017) 1680–1710, 10.1002/bdr2.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [260].Hewitt M, Madden JC, Rowe PH, Cronin MTD, Structure-based modelling in reproductive toxicology: (Q)SARs for the placental barrier, SAR QSAR Environ. Res 18 (1–2) (2007) 57–76, 10.1080/10629360601053893. [DOI] [PubMed] [Google Scholar]
- [261].Giaginis C, Zira A, Theocharis S, Tsantili-Kakoulidou A, Application of quantitative structure-activity relationships for modeling drug and chemical transport across the human placenta barrier: a multivariate data analysis approach, J. Appl. Toxicol 29 (2009) 724–733, 10.1002/jat.1466. [DOI] [PubMed] [Google Scholar]
- [262].Clewell RA, Gearhart JM, Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure, Environ. Health Perspect 110 (2002) A333–A337, 10.1289/ehp.021100333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [263].Howdeshell KL, A model of the development of the brain as a construct of the thyroid system, Environ. Health Perspect 110 (Suppl 3) (2002) 337–348, 10.1289/ehp.02110s3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [264].Coecke S, Goldberg AM, Allen S, Buzanska L, Calamandrei G, Crofton K, Hareng L, Hartung T, Knaut H, Honegger P, Jacobs M, Lein P, Li A, Mundy W, Owen D, Schneider S, Silbergeld E, Reum T, Trnovec T, Monnet-Tschudi F, Bal-Price A, Workgroup report: incorporating in vitro alternative methods for developmental neurotoxicity into international hazard and risk assessment strategies, Environ. Health Perspect 115 (6) (2007) 924–931, 10.1289/ehp.9427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [265].Grandjean P, Landrigan PJ, Neurobehavioural effects of developmental toxicity, Lancet Neurol. 13 (3) (2014) 330–338, 10.1016/S1474-4422(13)70278-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [266].Effinger A, O’Driscoll CM, McAllister M, Fotaki N, In Vitro and In Silico ADME Prediction, in: Talevi A, Quiroga PAM (Eds.), ADME Processes in Pharmaceutical Sciences, Springer, Cham, 2018: pp. 301–330. doi: 10.1007/978-3-319-99593-9_13. [DOI] [Google Scholar]
- [267].Lombardo F, Desai PV, Arimoto R, Desino KE, Fischer H, Keefer CE, Petersson C, Winiwarter S, Broccatelli F, In silico Absorption, Distribution, Metabolism, Excretion, and Pharmacokinetics (ADME-PK): utility and best practices. An industry perspective from the International Consortium for Innovation through Quality in Pharmaceutical Development, J. Med. Chem 60 (22) (2017) 9097–9113, 10.1021/acs.jmedchem.7b00487. [DOI] [PubMed] [Google Scholar]
- [268].van de Waterbeemd H, Gifford E, ADMET in silico modelling: towards prediction paradise? Nat. Rev. Drug Discovery 2 (3) (2003) 192–204, 10.1038/nrd1032. [DOI] [PubMed] [Google Scholar]
- [269].Ghosh J, Lawless MS, Waldman M, Gombar V, Fraczkiewicz R, Modeling ADMET, in: Benfenati E (Ed.), In Silico Methods for Predicting Drug Toxicity, Humana Press, New York, NY, 2016: pp. 63–83. doi: 10.1007/978-1-4939-3609-0_4. [DOI] [PubMed] [Google Scholar]
- [270].Madden JC, In Silico Approaches for Predicting ADME Properties, in: Puzyn T, Leszczynski J, Cronin MT (Eds.), Recent Advances in QSAR Studies, Springer, Dordrecht, 2010: pp. 283–304. doi: 10.1007/978-1-4020-9783-6_10. [DOI] [Google Scholar]
- [271].Mostrag-Szlichtyng A, Worth A, Review of QSAR Models and Software Tools for predicting Biokinetic Properties, Luxembourg, 2010. doi: 10.2788/94537. [DOI] [Google Scholar]
- [272].EFSA, Modern methodologies and tools for human hazard assessment of chemicals, EFSA J. 12 (2014) 3638. 10.2903/j.efsa.2014.3638. [DOI] [Google Scholar]
- [273].WHO, Characterization and application of physiologically based pharmacokinetic models in risk assessment, World Health Organization, Geneva, 2010. http://www.who.int/ipcs/methods/harmonization/areas/pbpk_models.pdf?ua=1. [Google Scholar]
- [274].de Lange ECM, PBPK Modeling Approach for Predictions of Human CNS Drug Brain Distribution, in: Di L, Kerns EH (Eds.), Blood-Brain Barrier in Drug Discovery, John Wiley & Sons, Ltd, 2015: pp. 296–323. doi: 10.1002/9781118788523.ch14. [DOI] [Google Scholar]
- [275].Yamamoto Y, Välitalo PA, Wong YC, Huntjens DR, Proost JH, Vermeulen A, Krauwinkel W, Beukers MW, Kokki H, Kokki M, Danhof M, van Hasselt JGC, de Lange ECM, Prediction of human CNS pharmacokinetics using a physiologically-based pharmacokinetic modeling approach, Eur. J. Pharm. Sci 112 (2018) 168–179, 10.1016/j.ejps.2017.11.011. [DOI] [PubMed] [Google Scholar]
- [276].Boyes WK, Simmons JE, Eklund C, Benignus VA, Janssen P, Bushnell PJ, Applications of dosimetry modeling to assessment of neurotoxic risk, Environ. Toxicol. Pharmacol 19 (3) (2005) 599–605, 10.1016/j.etap.2004.12.025. [DOI] [PubMed] [Google Scholar]
- [277].Croom EL, Shafer TJ, Evans MV, Mundy WR, Eklund CR, Johnstone AFM, Mack CM, Pegram RA, Improving in vitro to in vivo extrapolation by incorporating toxicokinetic measurements: A case study of lindane-induced neurotoxicity, Toxicol. Appl. Pharmacol 283 (1) (2015) 9–19, 10.1016/j.taap.2014.11.006. [DOI] [PubMed] [Google Scholar]
- [278].Ramoju SP, Mattison DR, Milton B, McGough D, Shilnikova N, Clewell HJ, Yoon M, Taylor MD, Krewski D, Andersen ME, The application of PBPK models in estimating human brain tissue manganese concentrations, NeuroToxicology. 58 (2017) 226–237, 10.1016/j.neuro.2016.12.001. [DOI] [PubMed] [Google Scholar]
- [279].Hasselgren C, Ahlberg E, Akahori Y, Amberg A, Anger LT, Atienzar F, Auerbach S, Beilke L, Bellion P, Benigni R, Bercu J, Booth ED, Bower D, Brigo A, Cammerer Z, Cronin MTD, Crooks I, Cross KP, Custer L, Dobo K, Doktorova T, Faulkner D, Ford KA, Fortin MC, Frericks M, Gad-McDonald SE, Gellatly N, Gerets H, Gervais V, Glowienke S, Van Gompel J, Harvey JS, Hillegass J, Honma M, Hsieh J-H, Hsu C-W, Barton-Maclaren TS, Johnson C, Jolly R, Jones D, Kemper R, Kenyon MO, Kruhlak NL, Kulkarni S, Kümmerer K, Leavitt P, Masten S, Miller S, Moudgal C, Muster W, Paulino A, Lo Piparo E, Powley M, Quigley DP, Reddy MV, Richarz A-N, Schilter B, Snyder RD, Stavitskaya L, Stidl R, Szabo DT, Teasdale A, Tice RR, Trejo-Martin A, Vuorinen A, Wall BA, Watts P, White AT, Wichard J, Witt KL, Woolley A, Woolley D, Zwickl C, Myatt GJ, Genetic toxicology in silico protocol, Regul. Toxicol. Pharmacol 107 (2019) 104403, 10.1016/j.yrtph.2019.104403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [280].Johnson C, Ahlberg E, Anger LT, Beilke L, Benigni R, Bercu J, Bobst S, Bower D, Brigo A, Campbell S, Cronin MTD, Crooks I, Cross KP, Doktorova T, Exner T, Faulkner D, Fearon IM, Fehr M, Gad SC, Gervais V, Giddings A, Glowienke S, Hardy B, Hasselgren C, Hillegass J, Jolly R, Krupp E, Lomnitski L, Magby J, Mestres J, Milchak L, Miller S, Muster W, Neilson L, Parakhia R, Parenty A, Parris P, Paulino A, Paulino AT, Roberts DW, Schlecker H, Stidl R, Suarez-Rodrigez D, Szabo DT, Tice RR, Urbisch D, Vuorinen A, Wall B, Weiler T, White AT, Whritenour J, Wichard J, Woolley D, Zwickl C, Myatt GJ, Skin sensitization in silico protocol, Regul. Toxicol. Pharmacol 116 (2020) 104688, 10.1016/j.yrtph.2020.104688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [281].Tice RR, Bassan A, Amberg A, Anger LT, Beal MA, Bellion P, Benigni R, Birmingham J, Brigo A, Bringezu F, Ceriani L, Crooks I, Cross K, Elespuru R, Faulkner DM, Fortin MC, Fowler P, Frericks M, Gerets HHJ, Jahnke GD, Jones DR, Kruhlak NL, Piparo EL, Lopez-Belmonte J, Luniwal A, Luu A, Madia F, Manganelli S, Manickam B, Mestres J, Mihalchik-Burhans AL, Neilson L, Pandiri A, Pavan M, Rider CV, Rooney JP, Trejo-Martin A, Watanabe-Sailor KH, White AT, Woolley D, Myatt GJ, In silico approaches in carcinogenicity hazard assessment: current status and future needs, Comput. Toxicol 20 (2021) 100191, 10.1016/j.comtox.2021.100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [282].Bassan A, Alves VM, Amberg A, Anger LT, Beilke L, Bender A, Bernal A, Cronin MTD, Hsieh J-H, Johnson C, Kemper R, Mumtaz M, Neilson L, Pavan M, Pointon A, Pletz J, Ruiz P, Russo DP, Sabnis Y, Sandhu R, Schaefer M, Stavitskaya L, Szabo DT, Valentin J-P, Woolley D, Zwickl C, Myatt GJ, In silico approaches in organ toxicity hazard assessment: current status and future needs for predicting heart, kidney and lung toxicities, Comput. Toxicol 20 (2021) 100188, 10.1016/j.comtox.2021.100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [283].Harrill JA, Everett LJ, Haggard DE, Sheffield T, Bundy JL, Willis CM, Thomas RS, Shah I, Judson RS, High-throughput transcriptomics platform for screening environmental chemicals, Toxicol. Sci 181 (2021) 68–89, 10.1093/toxsci/kfab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [284].Nyffeler J, Willis C, Lougee R, Richard A, Paul-Friedman K, Harrill JA, Bioactivity screening of environmental chemicals using imaging-based high-throughput phenotypic profiling, Toxicol. Appl. Pharmacol 389 (2020) 114876, 10.1016/j.taap.2019.114876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [285].Wawer MJ, Li K, Gustafsdottir SM, Ljosa V, Bodycombe NE, Marton MA, Sokolnicki KL, Bray M-A, Kemp MM, Winchester E, Taylor B, Grant GB, Hon C-Y, Duvall JR, Wilson JA, Bittker JA, Dančík V, Narayan R, Subramanian A, Winckler W, Golub TR, Carpenter AE, Shamji AF, Schreiber SL, Clemons PA, Toward performance-diverse small-molecule libraries for cell-based phenotypic screening using multiplexed high-dimensional profiling, Proc. Natl. Acad. Sci. U. S. A 111 (30) (2014) 10911–10916, 10.1073/pnas.1410933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [286].Thomas RS, Bahadori T, Buckley TJ, Cowden J, Deisenroth C, Dionisio KL, Frithsen JB, Grulke CM, Gwinn MR, Harrill JA, Higuchi M, Houck KA, Hughes MF, Hunter ES, Isaacs KK, Judson RS, Knudsen TB, Lambert JC, Linnenbrink M, Martin TM, Newton SR, Padilla S, Patlewicz G, Paul-Friedman K, Phillips KA, Richard AM, Sams R, Shafer TJ, Setzer RW, Shah I, Simmons JE, Simmons SO, Singh A, Sobus JR, Strynar M, Swank A, Tornero-Valez R, Ulrich EM, Villeneuve DL, Wambaugh JF, Wetmore BA, Williams AJ, The next generation blueprint of computational toxicology at the U.S. Environmental Protection Agency, Toxicol. Sci 169 (2) (2019) 317–332, 10.1093/toxsci/kfz058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [287].Richard AM, Huang R, Waidyanatha S, Shinn P, Collins BJ, Thillainadarajah I, Grulke CM, Williams AJ, Lougee RR, Judson RS, Houck KA, Shobair M, Yang C, Rathman JF, Yasgar A, Fitzpatrick SC, Simeonov A, Thomas RS, Crofton KM, Paules RS, Bucher JR, Austin CP, Kavlock RJ, Tice RR, The Tox21 10K compound library: collaborative chemistry advancing toxicology, Chem. Res. Toxicol 34 (2) (2021) 189–216, 10.1021/acs.chemrestox.0c0026410.1021/acs.chemrestox.0c00264.s003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [288].Thorne N, Auld DS, Inglese J, Apparent activity in high-throughput screening: origins of compound-dependent assay interference, Curr. Opin. Chem. Biol 14 (3) (2010) 315–324, 10.1016/j.cbpa.2010.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [289].Richard AM, Judson RS, Houck KA, Grulke CM, Volarath P, Thillainadarajah I, Yang C, Rathman J, Martin MT, Wambaugh JF, Knudsen TB, Kancherla J, Mansouri K, Patlewicz G, Williams AJ, Little SB, Crofton KM, Thomas RS, ToxCast chemical landscape: paving the road to 21st century toxicology, Chem. Res. Toxicol 29 (8) (2016) 1225–1251, 10.1021/acs.chemrestox.6b00135. [DOI] [PubMed] [Google Scholar]
- [290].Martin MT, Judson RS, Reif DM, Kavlock RJ, Dix DJ, Profiling chemicals based on chronic toxicity results from the U.S. EPA ToxRef Database, Environ. Health Perspect 117 (3) (2009) 392–399, 10.1289/ehp.0800074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [291].Kosnik MB, Strickland JD, Marvel SW, Wallis DJ, Wallace K, Richard AM, Reif DM, Shafer TJ, Concentration-response evaluation of ToxCast compounds for multivariate activity patterns of neural network function, Arch. Toxicol 94 (2) (2020) 469–484, 10.1007/s00204-019-02636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [292].US EPA, ToxCast Owner’s Manual – Guidance for Exploring Data, US EPA. (2017). https://www.epa.gov/chemical-research/toxcast-owners-manual-guidance-exploring-data (accessed February 12, 2021). [Google Scholar]
- [293].Shafer TJ, Personal communication, (2022). [Google Scholar]
- [294].IARC, Tumour Site Concordance and Mechanisms of Carcinogenesis, WHO Press, World Health Organization, Switzerland, 2019. https://publications.iarc.fr/Book-And-Report-Series/Iarc-Scientific-Publications/Tumour-Site-Concordance-And-Mechanisms-Of-Carcinogenesis-2019 (accessed February 17, 2020). [Google Scholar]
- [295].Smith MT, Guyton KZ, Kleinstreuer NC, Borrel A, Cardenas A, Chiu WA, Felsher DW, Gibbons CF, Goodson III WH, Houck KA, Kane A, La Merrill M, Lebrec H, Lowe L, McHale CM, Minocherhomji S, Rieswjik L, Sandy MM, Sone H, Wang A, Zhang L, Zeise L, Fielden M, The Key Characteristics of Carcinogens: Relationship to the Hallmarks of Cancer, Relevant Biomarkers, and Assays to Measure Them, in press (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [296].Smith MT, Guyton KZ, Gibbons CF, Fritz JM, Portier CJ, Rusyn I, DeMarini DM, Caldwell JC, Kavlock RJ, Lambert PF, Hecht SS, Bucher JR, Stewart BW, Baan RA, Cogliano VJ, Straif K, Key characteristics of carcinogens as a basis for organizing data on mechanisms of carcinogenesis, Environmental Health Perspectives. 124 (6) (2016) 713–721, 10.1289/ehp.1509912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [297].E. National Academies of Sciences, Using 21st Century Science to Improve Risk-Related Evaluations, 2017. doi: 10.17226/24635. [DOI] [PubMed] [Google Scholar]
- [298].Lein PJ, Fritsche E, Shafer TJ, et al. , Personal communication (key characteristics of neurotoxicity), (2022). [Google Scholar]
- [299].Becker RA, Dreier DA, Manibusan MK, Cox LAT, Simon TW, Bus JS, How well can carcinogenicity be predicted by high throughput “characteristics of carcinogens” mechanistic data? Regul. Toxicol. Pharmacol 90 (2017) 185–196, 10.1016/j.yrtph.2017.08.021. [DOI] [PubMed] [Google Scholar]
- [300].Goodman J, Lynch H, Improving the International Agency for Research on Cancer’s consideration of mechanistic evidence, Toxicol. Appl. Pharmacol 319 (2017) 39–46, 10.1016/j.taap.2017.01.020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.