Abstract
Coronavirus disease 2019 (COVID-19) infection in pregnancy has been associated with preterm delivery and preeclampsia. A less frequent and underrecognized complication is extensive placental infection which is associated with high rates of perinatal morbidity and mortality. The frequency, early pathogenesis, and range of lesions associated with this infection are poorly understood. We conducted a population-based study of placental pathology from all mothers with COVID-19 (n=271) over an 18-month period delivering within our health system. The overall prevalence of diffuse severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis, as defined by typical histology and immunohistochemical (IHC) staining for SARS-CoV-2 spike protein, was 14.8/1000, but increased to 59/1000 in preterm births. We also identified 3 cases with isolated small foci of localized SARS-CoV-2 placentitis, characterized by focal perivillous fibrin and intervillositis, which illustrate the early pathogenesis and suggest that infection may be contained in some cases. Two other placental lesions were more common in mothers with COVID-19, high-grade maternal vascular malperfusion in preterm deliveries and high-grade chronic villitis at term (5/5 cases tested of the latter were negative by IHC for SARS-CoV-2). Additional investigation of diffuse and localized SARS-CoV-2 placentitis by IHC showed loss of BCL-2, C4d staining in surrounding villi, and an early neutrophil-predominant intervillous infiltrate that later became dominated by monocyte-macrophages. We propose a model of focal infection of syncytiotrophoblast by virally infected maternal leukocytes leading to loss of BCL-2 and apoptosis. Infection is then either contained by surrounding fibrinoid (localized) or initiates waves of aponecrosis and immune activation that spread throughout the villous parenchyma (diffuse).
Key Words: placenta, COVID-19, pathology, SARS-CoV-2, intervillositis, apoptosis, necrosis, perivillous fibrin, syncytiotrophoblast
Coronavirus disease 2019 (COVID-19), caused by the novel beta-coronavirus severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has led to a worldwide pandemic first appreciated in the United States in late January 2020.1,2 Early in the pandemic, concerns were raised regarding the possible adverse effects of viral infection on pregnant mothers and their offspring.3 These concerns centered around 3 potential issues: the known increased maternal susceptibility to severe respiratory infection associated with immune deviation during pregnancy, transplacental spread as seen with many other viruses, and severe neonatal morbidity due to the immaturity of the developing immune system at birth.4
Early results were somewhat reassuring demonstrating only a mildly increased maternal risk of severe infection, a relatively small increase in preterm deliveries, and only rare possible transplacental spread with the relatively mild and transient neonatal disease.5 However, shortly thereafter, reports of an uncommon severe diffuse placental infection associated with maternal SARS-CoV-2 infection, referred to as diffuse SARS-CoV-2 placentitis for the purpose of this study, began to appear in April 2020.6,7 Despite multiple case reports and small case series of this unique pattern of placental infection, many physicians and patients are unaware of this important placental lesion and its strong association with perinatal morbidity and mortality.
A partial explanation for the susceptibility of some placentas to SARS-CoV-2 infection is an expression of the viral receptor angiotensin-converting enzyme-2 (ACE2) and coreceptor TMSS on placental syncytiotrophoblast (ScT), the multinucleate trophoblastic cell lining the surface of chorionic villi at the fetomaternal interface.8 However, it is well-documented that ScT is exceptionally resistant to infection by most pathogens and the reason why a small subpopulation of mothers with COVID-19 develop severe infection while the remainder are apparently unaffected remains obscure.9,10
We now report the results of a prospective, population-based study of all placentas delivered to mothers with COVID-19 in our health system over an 18-month period beginning in May 2020. Our study had 3 primary goals: (1) to determine the population prevalence of diffuse SARS-CoV-2 placentitis, (2) to identify and characterize localized forms of placental infection, and (3) to better understand the pathogenesis of this unusual placental response. We approached this last goal through a review of all well-documented cases of diffuse SARS-CoV-2 placentitis and a comparative immunohistochemical (IHC) study of both localized and diffuse cases plus 2 non–COVID-related placental conditions with overlapping histologic features, chronic histiocytic intervillositis (CHI), and massive perivillous fibrin deposition (MPVF).
MATERIALS AND METHODS
Study Population
This was a prospective population-based study of all placentas delivered to mothers testing positive for SARS-CoV-2 by reverse transcription-polymerase chain reaction during pregnancy and delivering at 1 of the 5 hospitals providing obstetric services in the University Hospitals Health System between May 1, 2020, and October 31, 2021. Our health system covers a large geographic area in Northeast Ohio and provides health care to ∼33% of its residents. A total of 271 placentas from mothers testing positive for SARS-CoV-2 were examined, representing 2.7% of the 10,163 deliveries occurring over this time period. The institutional review board with the following stipulations approved the study. Available clinical data was limited to that normally accompanying the placenta (admission history and physical and selected additional information regarding the delivery). We also had no access to the records of liveborn infants delivered to affected mothers. Maternal COVID-19 symptoms on admission were described in 111 patients, 98 mild-moderate and 13 severe. Six mothers were hospitalized for COVID-19-related pneumonia. Placentas were evaluated by 1 or more of the authors, all members of our perinatal service which examines over 4000 placentas per year. Criteria for the histologic diagnosis of diffuse SARS-CoV-2 placentitis were as established in prior publications and described in detail below. The first 100 cases were rereviewed by 1 of the authors (R.W.R.) with prior experience through review of positive cases seen in consultation from other institutions. All cases of SARS-CoV-2 placentitis were confirmed by IHC for SARS-CoV-2 spike protein with appropriate positive (autopsy lung) and negative controls (see below), as specified in recent publications.11
Population-based Controls
A database of singleton placentas evaluated according to Amsterdam consensus criteria between January 2005 and December 2014 at our institution was utilized to compare the prevalence of placental lesions in the current cohort to placentas evaluated before the COVID-19 era.12 This database includes 8006 placentas with an overall submission rate of 40% to 50% of total deliveries occurring over this time period.
Definition and Histologic Grading of Other Placental Lesions
Other placental lesions observed in placentas from mothers with COVID-19 infection were separated into 4 categories based on the recent Amsterdam classification system, maternal vascular malperfusion (MVM), fetal vascular malperfusion (FVM), idiopathic chronic villitis (so-called villitis of unknown etiology [VUE]), and acute chorioamnionitis.13 These categories were further subdivided into low-grade and high-grade subgroups, as previously reported.12 In brief, MVM was defined by accelerated villous maturation with high-grade MVM having distal villous hypoplasia, multiple villous infarcts, or placental weight less than third percentile. FVM was defined by avascular villi with high-grade FVM having an average of either >15 avascular villi per slide or multiple fetal thrombi. VUE was defined by villous stromal chronic inflammation with high-grade VUE having >10 contiguous affected villi. Acute chorioamnionitis was defined by neutrophils in the chorionic portion of the placental membranes with high grade having neutrophils in the umbilical arterial wall and/or confluent inflammation of chorionic vessels.
IHC Studies
All immunostaining was performed by the IHC laboratory at University Hospitals Cleveland Medical Center. Briefly, unstained 4 μm sections on whole charged slides were prepared from paraffin blocks and baked for 30 minutes at 60°C. Primary antibodies were incubated at 37°C, and slides were counterstained onboard the automated instruments listed below. The Optiview Universal DAB Detection Kit (Roche Ventana) was used for all assays. Individual conditions for each antibody are as follows.
SARS-CoV-2 Spike Protein (1A9, 1/750 Dilution, 30 min; GeneTex)
IHC was performed following incubation with Bond Epitope Retrieval Solution II (Leica), an EDTA-based pH 9.0 solution at 100°C. Negative controls were placentas with CHI, and MPVF diagnosed before the COVID-19 era. Positive controls were lung specimens from COVID-19-infected patients obtained at autopsy and a commercial positive lung control (Bio SB, Santa Barbara, CA).
Other Antibodies
CD3 (clone LN10; Leica), CD68 (clone 514H12; Leica), and myeloperoxidase (MPO) (clone M9A5; Leica) were processed using a Bond III automated immunostainer following incubation with the Bond Epitope retrieval solution II. BCL-2 (clone 124; Roche Ventana), C4d (clone SP91; Roche Ventana), and CD56 (clone 123C3; Roche Ventana) were processed with a BenchMark Ultra Automated Immunostainer (Roche Ventana) following incubation with CC1 epitope retrieval solution, a Tris-based buffer with a slightly basic pH solution at 95°C.
Statistical Analysis
Comparison of categorical data regarding the prevalence of placental lesions in various subgroups was performed by χ2 analysis with 2 df with significance level set at P-value <0.05.
RESULTS
Population-based Prevalence and Clinical Features of Diffuse SARS-CoV-2 Placentitis
Diffuse SARS-CoV-2 placentitis is defined by a unique set of histopathologic features including large geographically distributed, contiguous areas of villous agglutination with ScT necrosis and variable components of histiocyte-predominant chronic inflammation and perivillous fibrin.14 Diffuse SARS-CoV-2 placentitis was observed in 4 of 271 placentas from SARS-CoV-2-infected mothers for an overall prevalence of 14.8/1000 births. Three of these cases occurred among the 51 cases delivered before 37 weeks gestation, yielding a much higher prevalence of 59/1000 among premature births. All diffuse cases showed the diagnostic pattern of extensive villous agglutination with ScT necrosis, perivillous fibrin, and chronic inflammation affecting between 50% and 90% of the placental parenchyma (Figs. 1A, C) and all had strong diffuse staining of affected areas by IHC for SARS-CoV-2 spike protein (Fig. 1B). Of note were clusters of ScT showing nuclear features consistent with apoptosis (Fig. 1D). These observations suggested a mixed pattern of ScT necrosis and apoptosis (aponecrosis). Clinical and pathologic details of these 4 cases of diffuse SARS-CoV-2 placentitis, plus 3 additional placentas with focally positive SARS-CoV-2 staining by IHC (localized SARS-CoV-2 placentitis, as described below), are provided in Table 1.
FIGURE 1.
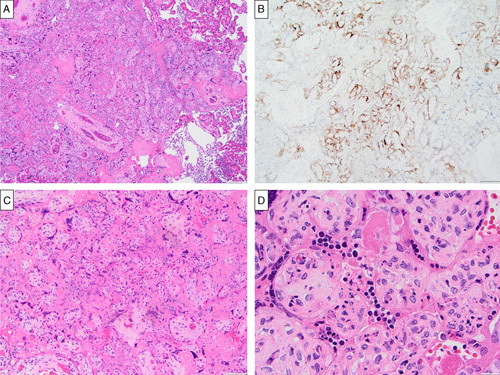
Histologic features of diffuse SARS-CoV-2 placentitis (case 1) with confirmation by IHC staining for SARS-CoV-2 spike protein. A, Extensive villous agglutination with ScT necrosis, perivillous fibrin (left), and chronic intervillositis (right) (hematoxylin and eosin). B, Strong positive staining for SARS-CoV-2 capsid protein involving nearly all ScT within areas of villous agglutination (SARS-CoV-2 immunostain). C, Extensive perivillous fibrin and ScT necrosis; manifesting features intermediate between non–COVID-related villous infarction and MPVF (hematoxylin and eosin). D, ScT nuclei with features suggestive of apoptosis: nuclear condensation, membrane blebbing, and cell shrinkage (hematoxylin and eosin).
TABLE 1.
Clinical and Pathologic Details of the 7 Study Cases Showing IHC Positivity for SARS-CoV-2 Spike Antigen
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | |
|---|---|---|---|---|---|---|---|
| Extent (% or # of foci, maximum size) | Diffuse (50%) | Diffuse (90%) | Diffuse (60%) | Diffuse (90%) | Localized, exudative (N=1, 1.9 mm) | Localized, organizing (N=2, 1.8 mm) | Localized, involuted (N=1, 1.6 mm) |
| Maternal age (y) | 25 | 32 | 27 | 28 | 19 | 39 | 36 |
| OB Index* | G1 P0 | G5 P1031 | G4 P0030 | G3 P1011 | G1 P0 | G3 P0020 | G2 P2022 |
| Gestational age (wk) | 27.7 | 37.0 | 32.7 | 29.1 | 38.7 | 40.6 | 35.1 |
| Positive CoV-2 test (predelivery) (d) | 5 | 11 | 7 | 17 | 16 | 1 | 51 |
| COVID-19 symptoms | Mild, recovered | Moderate, recovered | Mild, recovered | Moderate ×7 wk, recovered | Mild, recovered | Mild ×1 wk | Asymptomatic |
| Other maternal | Decreased platelets | Low PAPP-A | Low PAPP-A, preeclampsia | Class 3 obesity | None | Class 2 obesity | Di Di twins, twin B |
| Birth weight (size for GA) | 720 G (AGA) | 3020 G (AGA) | 1750 G (AGA) | 1070 G (AGA) | 2970 G (AGA) | 3400 G (AGA) | 2580 G (AGA) |
| Apgar scores 1/5/10 min | 1/4/9 | 3/7/8 | 2/6/9 | 0/0 (IUFD) | 8/9 | 7/9 | 9/9 |
| Placental weight (size for GA) | 145 G (AGA) | 372 G (AGA) | 269 G (AGA) | 188 G (AGA) | 379 G (AGA) | 505 G (AGA) | 354 G (AGA) |
| Placenta (other) | Fragmented | Accessory lobe | Accessory lobes | Intervillous thrombus | None | Long umbilical cord (82 cm) | Placenta B: Low-grade VUE |
Gravidity (total pregnancies); parity (previous full term, preterm, losses before 20 wk, living children).
AGA indicates appropriate for gestational age; Di Di, dichorionic, diamniotic; GA, gestational age; IUFD, intrauterine fetal demise; PAPP-A, pregnancy-associated plasma protein A.
We reviewed the literature and identified 56 previously published and well-documented cases of diffuse SARS-CoV-2 placentitis.6,7,14–26 A summary of the pregnancy-related features of these, together with our 4 new cases, is provided in Table 2. Notable findings include a high overall frequency of preterm delivery and low 5-minute Apgar scores among liveborn cases and frequently associated large intervillous thrombi, early preterm delivery (<32 wk), male predominance, and longer interval between positive COVID-19 test and delivery among stillborns. Pertinent negative findings include the relative rarity of fetal or placental growth restriction or severe maternal COVID-19 symptoms. Evidence supporting maternal to the fetal transmission of SARS-CoV-2 has been reported for 12 of these cases.26 However, some uncertainty remains since testing was not uniform and criteria for fetal positivity included focal IHC positivity limited to the placental villous stroma.
TABLE 2.
Summary of 56 Previously Published Cases of Diffuse SARS-CoV-2 Placentitis Plus the Additional 4 Cases in This Report
| n (%) | |||
|---|---|---|---|
| All | Liveborn Infants | Stillbirths | |
| N | 60* | 31 | 22 |
| Maternal age, median (range) (y) | 32 (19-46) | 35 (19-46) | 30 (22-38) |
| Timing of positive CoV-2 test (days predelivery), median (range) | 4.5 (0-51) | 0 (0-51) | 10 (0-17) |
| Primiparity | 10/25 (40) | 6/13 (46) | 2/7 (29) |
| Gestational age <37 wk | 42 (70) | 19 (61) | 20 (91) |
| Gestational age <32 wk | 22 (37) | 7 (23) | 14 (64) |
| Preeclampsia | 4 (6.7) | 3 (9.7) | 1 (4.5) |
| COVID-19: asymptomatic | 5 (8.3) | 2 (6.5) | 1 (4.5) |
| Mild | 27 (45) | 16 (52) | 9 (41) |
| Severe | 1 (1.7) | 1 (3.2) | 0 |
| Male fetus/infant | 15/25 (60) | 6/13 (46) | 9/12 (75) |
| Birth weight <10th percentile | 3 (5.0) | 3 (9.7) | 0 |
| Apgar score 5 min <7 | — | 6/17 (35) | — |
| Placenta | |||
| Weight <10th percentile | 6 (10) | 4 (13) | 2 (9.1) |
| MVM | 4 (6.7) | 1 (3.2) | 2 (9.1) |
| FVM | 5 (8.3) | 1 (3.2) | 1 (4.5) |
| VUE | 5 (8.3) | 3 (9.7) | 0 |
| Intervillous thrombi | 6 (10) | 2 (6.5) | 4 (18) |
Seven published cases were not classified as either stillborn or liveborn.
Identification and Characterization of Localized SARS-CoV-2 Placentitis
Early in the course of the study, an unusual lesion characterized by small clusters of terminal villi with focal ScT necrosis surrounded by a recent blood clot and intervillous inflammation was appreciated in a subset of the placentas of SARS-CoV-2-infected mothers. Eight placentas with this finding were further investigated by IHC for SARS-CoV-2 spike protein, and 3 showed IHC positivity. Since not all placentas were evaluated by IHC for spike protein, we cannot exclude that other cases of localized disease escaped detection. Clinical and pathologic details of the 3 placentas (cases 5 to 7) with localized SARS-CoV-2 placentitis are provided in Table 1. Timing of positive SARS-CoV-2 testing and clinical symptoms relative to the date of delivery correlated poorly with the histology of the localized lesions, a finding also observed in other reported cases of diffuse SARS-CoV-2 placentitis.5,14 Case 5 demonstrated what appears to be an early, exudative pattern characterized by focal acute intervillositis with perivillous fibrin (Fig. 2A). Positive staining for SARS-CoV-2 spike protein within this lesion was limited to a short strip of contiguous ScT nuclei and a few maternal leukocytes in the adjacent intervillous space (Fig. 2B). Higher magnification images show recently coagulated maternal blood and focal acute inflammation in areas contiguous to degenerating ScT (Figs. 2C, D). Case 6 demonstrated what appears to be an organizing pattern with 2 small well-circumscribed foci, each showing serpiginous streaks of positive staining for SARS-CoV-2 spike antigen emanating from a central nidus of necrotic ScT and surrounded by fibrinoid matrix and focal extravillous trophoblast (Figs. 2E, F). Case 7 demonstrated what appears to be a remote, involuted pattern, seen in 1 of 2 twin placentas delivered to an asymptomatic mother with a positive SARS-CoV-2 test 51 days before delivery. Histologically, a basal intervillous thrombus surrounded a small cluster of completely involuted villi that showed residual IHC staining for SARS-CoV-2 spike protein (Figs. 2G, H). Interestingly, this case also had foci of low-grade VUE distant from the intervillous thrombus that were negative for SARS-CoV-2 by IHC.
FIGURE 2.
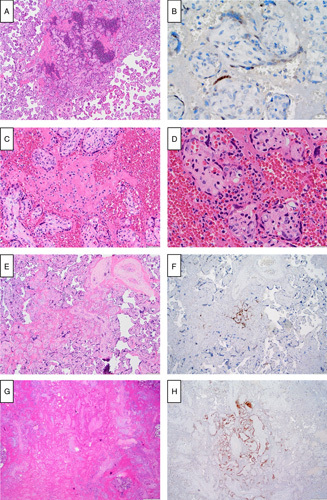
Histologic features of early/localized SARS-CoV-2 placentitis with confirmation by IHC staining for SARS-CoV-2 spike protein. A, Localized, exudative pattern (case 5): sharply circumscribed focus of recent perivillous villous fibrin with scattered inflammatory cells and focal villous agglutination (hematoxylin and eosin). B, Localized, exudative pattern (case 5): cytoplasmic staining for SARS-CoV-2 capsid protein of a short, well-circumscribed strip of viable ScT (a few IHC-positive maternal leukocytes are seen in the intervillous space, other similar positive cells not shown) (SARS-CoV-2 immunostain). C, Localized, exudative pattern (case 5): maternal leukocytes (neutrophils, activated macrophages, and an eosinophil) embedded in a recent intervillous blood clot with focal ScT necrosis in adjacent villi (hematoxylin and eosin). D, Localized, exudative pattern (case 5): focally intense perivillous neutrophilic exudate with early ScT degeneration (hematoxylin and eosin). E, Localized, organizing pattern (case 6): small perivillous fibrin plaque with focally necrotic ScT (hematoxylin and eosin). F, Localized, organizing pattern (case 6): serpiginous strands of central positivity for SARS-CoV-2 capsid protein (SARS-CoV-2 immunostain). G, Localized, involuted pattern (case 7): ghost-like outlines of involuted villi surrounded by fibrinoid matrix and peripheral extravillous trophoblast (right). Inflammatory cells and viable ScT are not identified. (hematoxylin and eosin). H, Localized, involuted pattern (case 7): persisting positive staining for SARS-CoV-2 capsid protein outlines the contours of involuted central villi; surrounding villi, fibrinoid, and extravillous trophoblast lack staining (SARS-CoV-2 immunostain).
Controls and Other Cases With Negative IHC Staining for SARS-CoV-2 Spike Protein
Twenty-nine of 271 study placentas (10.7%) were evaluated by IHC for expression of the SARS-CoV-2 spike protein, and 22 lacked positive staining. These IHC negative cases included twelve with placental thromboinflammatory lesions: 5 with lesions suspicious for localized infection as described above, 5 with high-grade VUE, and 1 case each from SARS-CoV-2-infected mothers with features of CHI and MPVF but lacking ScT necrosis. Ten placentas from COVID-19-infected mothers without thromboinflammatory lesions were also negative by IHC, 5 from mothers with COVID-19-related pneumonia, and 5 from mothers with mild-moderate symptoms. Control placentas with CHI and MPVF from the pre-COVID-19 era also, as expected, lacked positive staining.
Relative Frequency of Other Patterns of Placental Injury by Grade and Gestational Age in Mothers With COVID-19 During Pregnancy
The relative frequencies of other important placental lesions stratified by histologic grade (see the Materials and methods section) were compared with gestational age-matched controls from our institutional database of over 8000 placentas evaluated in a 10-year study before the COVID-19 pandemic (Table 3).12 While prevalence figures for the comparison group may be artificially elevated due to submission bias, lesions more common in the cohort from mothers with COVID-19 are worthy of further consideration. The frequency of most of these other placental lesions was equivalent in mothers with COVID-19 and controls for both term or preterm births. However, 2 lesions, high-grade VUE in term placentas and high-grade MVM among preterm deliveries, were significantly increased among SARS-CoV-2-infected mothers.
TABLE 3.
Frequency of Other Major Patterns of Placental Injury by Gestational Age and Maternal SARS-CoV-2 Status
| ≥37 Weeks Gestational Age | <37 Weeks Gestational Age | |||
|---|---|---|---|---|
| Placental Lesions | SARS-CoV-2 Positive | Controls | SARS-CoV-2 Positive | Controls |
| N | 220 | 5311 | 51 | 2389 |
| MVM | ||||
| Grade 1 | 5.5* | 9.3 | 5.9 | 13.6 |
| Grade 2 | 0.9 | 4.9 | 11.8** | 4.6 |
| FVM | ||||
| Grade 1 | 6.4 | 10.4 | 7.8 | 6.4 |
| Grade 2 | 1.4 | 1.0 | 0 | 1.4 |
| VUE | ||||
| Grade 1 | 2.7 | 4.1 | 0 | 2.7 |
| Grade 2 | 5.9** | 3.2 | 3.9 | 2.1 |
| Acute chorioamnionitis | ||||
| Grade 1 | 9.6 | 14.8 | 7.8 | 9.6 |
| Grade 2 | 3.6 | 7.8 | 2.0 | 12.2 |
Column percentages.
P<0.050, χ2.
IHC Analysis of the Necroinflammatory Response in Localized and Diffuse SARS-CoV-2 Placentitis and Non–COVID-19-related Conditions With Overlapping Features
We took advantage of the distinct histologic features in 2 of our localized cases and one of the diffuse cases to investigate the different types of host responses to SARS-CoV-2 infection and compare them to 2 non–COVID-19-related conditions with overlapping features, CHI and MPVF. Case 7, the remote, involuted lesion, lacked inflammation and viable ScT and was therefore not further evaluated. Diffuse SARS-CoV-2 placentitis (case 1) showed diffusely decreased BCL-2 staining in virally infected ScT at the center of areas of villous agglutination (Fig. 3A). A much smaller focus of decreased ScT BCL-2 expression was seen in case 5, the localized early, exudative lesion (Fig. 3B). Case 6, containing 2 small organizing, localized lesions, showed a complete absence of BCL-2 staining in affected villi (Fig. 3C). Extensive ScT C4d deposition accompanied diffuse SARS-CoV-2 placentitis, but in contrast to BCL-2, staining was most prominent in viable villi surrounding the more necrotic areas (Fig. 3D). There was no C4d staining within the localized early, exudative lesion, but a few small foci of C4d positive ScT were seen in histologically normal villi a short distance away (Fig. 3E). The 2 small organizing lesions lacked any C4d staining (Fig. 3F). The inflammatory infiltrate in diffuse SARS-CoV-2 placentitis was composed primarily of CD68-positive macrophages (Fig. 3G) with a lesser component of MPO-positive neutrophils (Fig. 3H), while the localized early, exudative lesion (case 5) showed the inverse pattern, a predominance of MPO-positive neutrophils with lesser numbers of CD68 macrophages (Figs. 3I, J). The 2 small organizing, localized, lesions showed only scant degenerating MPO-positive neutrophils in the center and relatively rare CD68-positive macrophages at the periphery (Figs. 3K, L). Other pertinent results not illustrated were as follows. Scattered lesional CD3-positive T cells were observed in both localized and diffuse SARS-CoV-2 placentitis, while no lesional CD56-positive natural killer (NK) cells were seen in any of the cases tested. Abundant CD56-positive cells in the adjacent maternal decidua served as an internal positive control. Cases of CHI and MPVF not associated with SARS-CoV-2 infection were distinct. While positive for CD68-positive macrophages, CHI had no areas of decreased BCL-2 or positive C4d staining and few MPO-positive neutrophils. MPVF, on the other hand, had diffusely decreased BCL-2 staining, but only rare foci of C4d positivity and lacked CD68-positive monocyte-macrophages.
FIGURE 3.
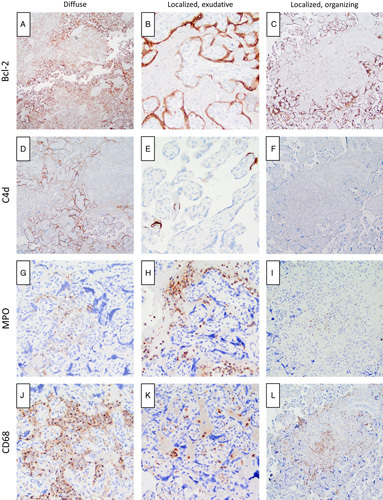
IHC characterization of the necroinflammatory response in diffuse and localized SARS-CoV-2 placentitis. A, Diffuse pattern (case 1): BCL-2 reactivity is diffusely decreased in the ScT at the center of clusters of degenerating villi (BCL-2 immunostain). B, Localized, exudative pattern (case 5): BCL-2 reactivity is focally reduced in a circumscribed strip of viable ScT (BCL-2 immunostain). C, Localized, organizing pattern (case 6): BCL-2 reactivity is largely absent within the area of perivillous fibrin (BCL-2 immunostain). D, Diffuse pattern (case 1): C4d deposition is seen in the ScT of villi at the periphery of areas of villous agglutination (C4d immunostain). E, Localized, exudative pattern (case 5): focal C4d deposition is limited to rare ScT located 0.5 to 1.0 mm from the focal lesion (C4d immunostain). F, Localized, organizing pattern (case 6): C4d deposition is absent (C4d immunostain). G, Diffuse pattern (case 1): only a few scattered MPO-positive neutrophils are present (MPO immunostain). H, Localized, exudative pattern (case 5): perivillous MPO-positive neutrophils predominate (MPO immunostain). I, Localized, organizing pattern (case 6): only rare degenerating MPO-positive neutrophils are present (MPO immunostain). J, Diffuse pattern (case 1): a dense intervillous infiltrate of CD68-positive monocyte-macrophages is present (CD68 immunostain). K, Localized, exudative pattern (case 5): CD68-positive monocyte-macrophages are relatively rare compared with neutrophils (H) at this stage (CD68 immunostain). L, Localized, organizing pattern (case 6): a patchy infiltrate of CD68-positive monocyte-macrophages surrounds the focus of perivillous fibrin; a few degenerating positively staining cells are present at the center of the plaque (CD68 immunostain).
DISCUSSION
Despite its unique histopathologic features and many previous case reports and short case series, diffuse SARS-CoV-2 placentitis (also known as CHI with trophoblast necrosis and placental SARS-CoV-2 infection with extensive perivillous fibrin and chronic intervillositis) remains a largely unappreciated and serious complication of maternal COVID-19 infection during pregnancy. Our study provides a snapshot of the placental consequences of COVID-19 in women delivering in Northeast Ohio over the first 18 months of the pandemic. We examined placentas from all mothers with positive SARS-CoV-2 infection; ∼3% of the over 10,000 deliveries occurring over this time period. Four of these placentas showed diffuse SARS-CoV-2 placentitis for an overall prevalence of 14.8/1000 infected mothers. Notably, the prevalence of diffuse placentitis was higher in preterm deliveries, affecting ∼59/1000 of all SARS-CoV-2-infected mothers delivering before 37 weeks. Worthy of mention, all 4 cases occurred during periods of high COVID-19 spread in our area (cases 1 to 3, April-May 2021, and case 4, late October 2021). Interestingly, 3 additional cases were identified in the 5 weeks following the closure of the study, a period of high SARS-CoV-2 transmission in Northeast Ohio with a predominance of the delta variant.
A number of characteristic clinical features of diffuse SARS-CoV-2 placentitis have emerged, as illustrated by the review of our 4 new cases and 56 other well-characterized cases in the literature. Placental disease shows little or no correlation with the timing and severity of maternal COVID symptoms. This could reflect the presence of persistent reservoirs of SARS-CoV-2 at other sites. Cases also show little correlation with the severity of COVID-19 symptoms. Many cases have arisen in asymptomatic mothers, and immunostaining of the placentas of 5 mothers with severe COVID-19-related pneumonia in our study revealed no cases of positivity for SARS-CoV-2. There is no strong association of placentitis with severe underlying maternal diseases, although obesity and hypertension are relatively frequent. Despite the extensive disease, fetoplacental growth restriction is rare, suggesting that placentitis probably develops relatively close to the time of delivery. The extent of disease, which can approach 90% in some cases, likely explains the strong association with adverse outcomes such as stillbirth, premature delivery, and neonatal distress. To what extent diffuse SARS-CoV-2 placentitis accounts for the recently documented 4-fold overall increase in the rate of stillbirth in COVID mothers remains to be determined, but all 3 stillbirths occurring in mothers with COVID-19 in our hospital (1 in this report and 2 subsequent cases) had diffuse SARS-CoV-2 placentitis.27 While there is no overall fetal sex predilection for the development of placentitis, affected stillborns are more likely to be male. However, the significance of this observation is uncertain since male predominance for severe adverse outcomes is a common feature of many pregnancy complications.28 Importantly, despite extensive placental disease, transmission to the fetus appears to be uncommon, and liveborn infants rarely show clinical signs and symptoms of COVID-19 infection.
Early in the course of the study, we detected unusual focal placental lesions characterized by intervillous fibrin and increased neutrophils. These lesions bear a superficial resemblance to those seen in some cases of maternal bacterial sepsis at term and in severe chorioamnionitis in the second trimester.29,30 IHC staining for SARS-CoV-2 spike protein was positive in 3 of 8 such cases that were evaluated, defining a heretofore unrecognized, localized variant of SARS-CoV-2 placentitis. We suggest that these 3 cases may represent different histologic stages of localized infection. Case 5, an early, exudative lesion with a single small focus of intervillous hemorrhage and small clusters of neutrophils, showed early ScT necrosis with one short strip of ScT testing positive for SARS-CoV-2 spike protein by IHC. Interestingly, this early strip-like staining pattern resembles that previously reported in an in vitro study of normal term villi infected with SARS-CoV-2 virus in organ culture and evaluated 72 hours post infection.25 A second, organizing pattern with 2 small lesions in close apposition to one another (case 6) showed agglutinated villi with focal central SARS-CoV-2 staining surrounded by fibrinoid matrix and extravillous trophoblast. The third, late involuted pattern was observed in our case 7, an asymptomatic woman who had tested positive for SARS-CoV-2 51 days before birth. Staining for SARS-CoV-2, in this case, was limited to a small focus of ghost-like villi surrounded by a large bland intervillous thrombus. These cases suggest that early lesions may sometimes be “walled-off” by a combination of fibrin-type (maternal coagulation-derived) and matrix-type (intervillous extravillous trophoblast-derived) fibrinoid deposition and later involute completely.31 Importantly, we cannot exclude that the 5 other, histologically similar localized lesions identified in our study may have had levels of viral antigen below the threshold of detection of our IHC assay or that there was the loss of antigenicity due to prolonged intervals before fixation.
A controversial topic in the previous literature is whether gestational COVID-19 infection during pregnancy is associated with any of the classic patterns of placental injury, as defined by the Amsterdam classification. Early reports found associations with maternal and FVM that were not observed in subsequent studies.32–35 We took advantage of our large institutional database to examine the relative frequency of these other lesions, stratified by grade and gestational age, in patients with and without COVID-19. While selection bias in unavoidable, it should be noted that our overall placental examination rate is high (40% to 50% of all deliveries). We found a significantly increased rate of high-grade MVM in the placentas of preterm infants and an increased rate of high-grade VUE in the placentas of term infants delivered from mothers with COVID-19. We favor prepregnancy risk factors or COVID-19-related increased susceptibility, rather than direct viral causation, as potential explanations for these findings. Of note, the 5 cases of high-grade VUE tested were all negative for IHC for SARS-CoV-2 spike protein.
Hematogenous infections of the placenta fall into several distinct groups. Best known are so-called TORCH infections, such as cytomegalovirus, which cause chronic villitis in the placenta, have a high rate of spread to the fetus, and are associated with congenital anomalies.36 A second group are infections, such as Zika virus, that cause only focal placental inflammation but also have a high rate of spread to the fetus with frequent malformations.37 The third group are antenatal infections, such as Parvovirus B19, where organisms cross the placenta and infect the fetus but do not cause placental inflammation.38 SARS-CoV-2 placentitis falls into a fourth group of very uncommon placental infections (eg, Ebolavirus, Chlamydia psittici, and Coxiella burnetti), where inflammation and necrosis are most severe in the ScT lining the villi and in the adjacent maternal intervillous space.39–41 The unique feature of organisms in this fourth group is the ability to replicate within ScT, a cell type known to be unusually resistant to infection compared with both other placental cells and cells in the remainder of the body. Unusual aspects of SARS-CoV-2, not seen with any of the other hematogenous infections, are its low prevalence among infected mothers, its exclusive (not just predominant) localization to ScT, and its low rate of spread to the fetus.
Our IHC analysis revealed some interesting additional details regarding pathogenesis. First, we found that BCL-2 staining is decreased in ScT, even at apparently early stages of infection. This decrease may predispose to apoptosis, which has previously been documented in diffuse SARS-CoV-2 placentitis by immunostaining for caspase-3.15 Interestingly, in vitro experiments of trophoblast in tissue culture have demonstrated a unique pattern where focal apoptosis can spread rapidly in waves to involve the entire cellular monolayer.42 This may reflect the fact that ScT is a syncytium without the cell-cell junctions that normally impede such spread in other cell types. Second, as previously reported, we found strong C4d staining in diffuse SARS-CoV-2 placentitis, but in our cases, this staining was largely restricted to the surrounding villi, rather than those directly infected by SARS-CoV-2 as indicated by positive IHC.14 Complement is activated in SARS-CoV-2 infections by both the classical (antigen-antibody complexes) and lectin (mannosylated SARS-CoV-2 S protein and MASP activation) pathways.43 Decreases in complement regulatory proteins in damaged ScT may further enhance local activation, and complement fragments such as C5a likely contribute to the brisk inflammatory response seen in diffuse cases. With regard to the inflammatory response, we found that the localized early, the exudative lesion showed both MPO-positive neutrophils and CD68-positive monocyte-macrophages with the former predominating while the later, organizing localized lesion contained only rare degenerating neutrophils and a few scattered surrounding monocyte-macrophages. Diffuse placentitis, on the other hand, was associated with abundant neutrophils and monocyte-macrophages, with the latter predominating. While a few scattered CD3-positive T cells were seen adjacent to all lesions, there were no CD56-positive NK cells. This is somewhat surprising for 3 reasons. First, NK cells are a prominent responding cell in pulmonary COVID-19 infections. Second, abundant CD56-positive cells are frequent in the immediately adjacent decidua. Third, one study suggests that NK cells may play an important role in controlling hematogenous placental infection caused by Listeria monocytogenes.44,45 Finally, CHI and MPVF unrelated to COVID-19 infection have some overlapping histologic features but have not been directly compared with SARS-CoV-2 placentitis in previous studies. We found their immunoprofile to be distinct with fewer neutrophils, normal BCL-2 and absent C4d staining in CHI and absent inflammation, and only focal C4d staining in MPVF. Of note, recent studies suggest that MPVF and CHI are phenotypes showing some overlap and that distinct clinical subsets may exist with different IHC profiles.46,47
Based on the findings of this and previous studies, we propose the following working model for placentitis associated with maternal COVID-19 infection. First, placentitis is rare because of the known intrinsic ability of ScT to resist infection. We suggest that decreased resistance to infection involves loss of function in known protective pathways, such as high levels of interferon-delta, inflammasome activation, or C19 miRNA expression.48–50 Loss of function could be due to intrinsic genetic abnormalities, such as the interferon pathway mutations described in some adults, or to extrinsic factors such as high levels of circulating virus, upregulation of ACE2 viral receptor expression, or focal ScT hypoxia.51 An example of an intrinsic factor predisposing to the related placental lesion, MPVF, is fetal long-chain 3-hydroxyacyl-CoA dehydrogenase deficiency.52 An example of an extrinsic factor is the recently demonstrated colocalization of increased ACE2 receptor in areas of villous hypoxia as demonstrated by IHC for carbonic anhydrase IX.53 Once productively infected, ScT, despite its known high levels of endogenous autophagy, may be limited in its ability to kill the virus due to the known propensity of SARS-CoV-2 to persist and replicate in autophagocytic vesicles avoiding fusion with lysosomes.54–56 Infected ScT might then progress along 1 of 2 pathways: early complete apoptosis with surrounding fibrinoid deposition (localized SARS-CoV-2 placentitis) or uncontrolled waves of incomplete apoptosis (aponecrosis) resulting in a diffuse MPVF-like response.57 Interestingly, similar MPVF-type responses have recently been reported with other placental infections including as cytomegalovirus, coxsackievirus, and syphilis.47 While this diffuse MPVF-type pattern may partially protect the mother, and possibly the fetus, from high-level infection, it does so at the expense of extensive placental damage that can lead to adverse pregnancy outcomes. Persisting high levels of virus in ScT may lead to continuing generation of complement fragments, inflammasome-derived cytokines, and other chemotactic factors that act in a feed-forward fashion to elicit the monocyte-macrophage predominant inflammatory response that further aggravates placental dysfunction. These factors together could explain the unique destructive lesion known as diffuse SARS-CoV-2 placentitis.
Strengths of our study include its population-based design spanning the entire pandemic period up to November 2021, the uniform pathologic analysis by a single group of perinatal pathologists with extensive experience in evaluating a large number of placentas using criteria as defined by the Amsterdam consensus classification, and a large pre-COVID-19 placental database from the same patient population for comparisons. Weaknesses include selection bias in the comparison group since only 40% to 50% of all placentas were evaluated, the fact that only 11% of COVID-19 placentas were stained for SARS-CoV-2 spike protein by IHC, the intrinsic limitations of inferring causality and timing by analyzing pathologic specimens at any one instant in time, and the lack of neonatal follow-up.
From a practical perspective, we find that all placentas showing the 3 classical features, diffuse villous agglutination, ScT necrosis, and a histiocyte-predominant intervillous inflammatory infiltrate with variable amounts of perivillous fibrin, are positive for SARS-CoV-2 by IHC, and hence a presumptive diagnosis can be provided to the clinician before, or even without, confirmation. In our experience, other patchy-diffuse lesions lacking 1 or more of the defining characteristics were never IHC positive. However, localized lesions containing SARS-CoV-2 spike protein by IHC exist and can show several distinct patterns. With experience, such lesions are easily identifiable by hematoxylin and eosin stain and can be further evaluated using either IHC or RNA in situ hybridization. However, given the limited availability of these tests and the absence of clinical data regarding clinical significance, the role of ancillary testing remains to be established.
In conclusion, we report an overall prevalence of ∼1% to 2% for diffuse SARS-CoV-2 placentitis in mothers with gestational COVID-19 infection and a >10-fold increase in preterm births. We define and characterize a novel localized form of SARS-CoV-2 placentitis and provide some additional insights into the pathogenesis of both the localized and diffuse variants. We also report anecdotal evidence for increased prevalence during periods of COVID-19 surges in our area, especially with respect to the recent delta variant-related surge. Outstanding questions include further details regarding the molecular pathways leading to localized versus diffuse placental involvement and the frequency, pathogenesis, and long-term consequences of any fetal infections occurring in this setting.
ACKNOWLEDGMENTS
The authors thank Dr Jason Hornick (Brigham and Women’s Hospital) and Amad Awadallah for advice on and performance of the immunohistochemical studies.
Footnotes
Conflicts of Interest and Source of Funding: Funding was through an internal grant from the University Hospitals Cleveland Medical Center Pathology Technical Development Program. The authors have disclosed that they have no significant relationships with, or financial interest in, any commercial companies pertaining to this article.
Contributor Information
Raymond W. Redline, Email: raymondw.redline@uhhospitals.org.
Sanjita Ravishankar, Email: sanjita.ravishankar@uhhospitals.org.
Christina Bagby, Email: christina.bagby@uhhospitals.org.
Shahrazad Saab, Email: shahrazad.saab@uhhospitals.org.
Shabnam Zarei, Email: shabnam.zarei@uhhospitals.org.
REFERENCES
- 1.Kontis V, Bennett JE, Rashid T, et al. Magnitude, demographics and dynamics of the effect of the first wave of the COVID-19 pandemic on all-cause mortality in 21 industrialized countries. Nat Med. 2020;26:1919–1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J, Lai S, Gao GF, et al. The emergence, genomic diversity and global spread of SARS-CoV-2. Nature. 2021;600:408–418. [DOI] [PubMed] [Google Scholar]
- 3.Dashraath P, Wong JLJ, Lim MXK, et al. Coronavirus disease 2019 (COVID-19) pandemic and pregnancy. Am J Obstet Gynecol. 2020;222:521–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wadman M. Why infection poses a special risk to pregnant women. Science. 2020;369:606–607. [DOI] [PubMed] [Google Scholar]
- 5.Schwartz DA. An analysis of 38 pregnant women with COVID-19, their newborn infants, and maternal-fetal transmission of SARS-CoV-2: maternal coronavirus infections and pregnancy outcomes. Arch Pathol Lab Med. 2020;144:799–805. [DOI] [PubMed] [Google Scholar]
- 6.Hosier H, Farhadian SF, Morotti RA, et al. SARS-CoV-2 infection of the placenta. J Clin Invest. 2020;130:4947–4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vivanti AJ, Vauloup-Fellous C, Prevot S, et al. Transplacental transmission of SARS-CoV-2 infection. Nat Commun. 2020;11:3572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taglauer E, Benarroch Y, Rop K, et al. Consistent localization of SARS-CoV-2 spike glycoprotein and ACE2 over TMPRSS2 predominance in placental villi of 15 COVID-19 positive maternal-fetal dyads. Placenta. 2020;100:69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delorme-Axford E, Sadovsky Y, Coyne CB. The placenta as a barrier to viral infections. Annu Rev Virol. 2014;1:133–146. [DOI] [PubMed] [Google Scholar]
- 10.Zeldovich VB, Clausen CH, Bradford E, et al. Placental syncytium forms a biophysical barrier against pathogen invasion. PLoS Pathog. 2013;9:e1003821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roberts DJ, Edlow AG, Romero RJ, et al. A standardized definition of placental infection by SARS-CoV-2, a consensus statement from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development SARS-CoV-2 Placental Infection Workshop. Am J Obstet Gynecol. 2021;225:593.e1–593.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou YY, Ravishankar S, Luo G, et al. Predictors of high grade and other clinically significant placental findings by indication for submission in singleton placentas from term births. Pediatr Dev Pathol. 2020;23:274–284. [DOI] [PubMed] [Google Scholar]
- 13.Khong TY, Mooney EE, Nikkels PGJ, Morgan TK, Gordjin SJ, eds. Pathology of the Placenta A Practical Guide. Cham, Switzerland: Springer Nature; 2018. [Google Scholar]
- 14.Watkins JC, Torous VF, Roberts DJ. Defining severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) placentitis. Arch Pathol Lab Med. 2021;145:1341–1349. [DOI] [PubMed] [Google Scholar]
- 15.Cribiu FM, Erra R, Pugni L, et al. Severe SARS-CoV-2 placenta infection can impact neonatal outcome in the absence of vertical transmission. J Clin Invest. 2021;131:e145427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schwartz DA, Morotti D. Placental pathology of COVID-19 with and without fetal and neonatal infection: trophoblast necrosis and chronic histiocytic intervillositis as risk factors for transplacental transmission of SARS-CoV-2. Viruses. 2020;12:1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patane L, Morotti D, Giunta MR, et al. Vertical transmission of coronavirus disease 2019: severe acute respiratory syndrome coronavirus 2 RNA on the fetal side of the placenta in pregnancies with coronavirus disease 2019-positive mothers and neonates at birth. Am J Obstet Gynecol MFM. 2020;2:100145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sisman J, Jaleel MA, Moreno W, et al. Intrauterine transmission of SARS-COV-2 infection in a preterm infant. Pediatr Infect Dis J. 2020;39:e265–e267. [DOI] [PubMed] [Google Scholar]
- 19.Facchetti F, Bugatti M, Drera E, et al. SARS-CoV2 vertical transmission with adverse effects on the newborn revealed through integrated immunohistochemical, electron microscopy and molecular analyses of Placenta. EBioMedicine. 2020;59:102951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Linehan L, O’Donoghue K, Dineen S, et al. SARS-CoV-2 placentitis: an uncommon complication of maternal COVID-19. Placenta. 2021;104:261–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marton T, Hargitai B, Hunter K, et al. Massive perivillous fibrin deposition and chronic histiocytic intervillositis a complication of SARS-CoV-2 infection. Pediatr Dev Pathol. 2021;24:450–454. [DOI] [PubMed] [Google Scholar]
- 22.Sharps MC, Hayes DJL, Lee S, et al. A structured review of placental morphology and histopathological lesions associated with SARS-CoV-2 infection. Placenta. 2020;101:13–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirtsman M, Diambomba Y, Poutanen SM, et al. Probable congenital SARS-CoV-2 infection in a neonate born to a woman with active SARS-CoV-2 infection. CMAJ. 2020;192:E647–E650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts J, Cheng JD, Moore E, et al. Extensive perivillous fibrin and intervillous histiocytosis in a SARS-CoV-2 infected placenta from an uninfected newborn: a case report including immunohistochemical profiling. Pediatr Dev Pathol. 2021;24:581–584. [DOI] [PubMed] [Google Scholar]
- 25.Argueta LB, Lacko LA, Bram Y, et al. SARS-CoV-2 infects syncytiotrophoblast and activates inflammatory responses in the placenta. bioRxiv. 2021. doi: 10.1101/2021.06.01.446676. [DOI] [Google Scholar]
- 26.Schwartz DA, Baldewijns M, Benachi A, et al. Hofbauer cells and COVID-19 in pregnancy. Arch Pathol Lab Med. 2021;145:1328–1340. [DOI] [PubMed] [Google Scholar]
- 27.DeSisto CL, Wallace B, Simeone RM, et al. Risk for stillbirth among women with and without COVID-19 at delivery hospitalization—United States, March 2020-September 2021. MMWR Morb Mortal Wkly Rep. 2021;70:1640–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clifton VL. Review: sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31(suppl):S33–S39. [DOI] [PubMed] [Google Scholar]
- 29.Bendon R, Bornstein S, Faye-Petersen O. Two fetal deaths associated with maternal sepsis and with thrombosis of the intervillous space of the placenta. Placenta. 1998;19:385–389. [DOI] [PubMed] [Google Scholar]
- 30.Redline RW. Placental inflammation. Semin Neonatol. 2004;9:265–274. [DOI] [PubMed] [Google Scholar]
- 31.Frank HG, Malekzadeh F, Kertschanska S, et al. Immunohistochemistry of two different types of placental fibrinoid. Acta Anat (Basel). 1994;150:55. [DOI] [PubMed] [Google Scholar]
- 32.Baergen RN, Heller DS. Placental pathology in COVID-19 positive mothers: preliminary findings. Pediatr Dev Pathol. 2020;23:177–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shanes ED, Mithal LB, Otero S, et al. Placental pathology in COVID-19. Am J Clin Pathol. 2020;154:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hecht JL, Quade B, Deshpande V, et al. SARS-CoV-2 can infect the placenta and is not associated with specific placental histopathology: a series of 19 placentas from COVID-19-positive mothers. Mod Pathol. 2020;33:2092–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suhren JT, Meinardus A, Hussein K, et al. Meta-analysis on COVID-19-pregnancy-related placental pathologies shows no specific pattern. Placenta. 2021;117:72–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bittencourt AL, Garcia AG. The placenta in hematogenous infections. Pediatr Pathol Mol Med. 2002;21:401–432. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg AZ, Yu W, Hill DA, et al. Placental pathology of zika virus: viral infection of the placenta induces villous stromal macrophage (Hofbauer cell) proliferation and hyperplasia. Arch Pathol Lab Med. 2017;141:43–48. [DOI] [PubMed] [Google Scholar]
- 38.Caul EO, Usher MJ, Burton PA. Intrauterine infection with human parvovirus B19: a light and electron microscopy study. J Med Virol. 1988;24:55–66. [DOI] [PubMed] [Google Scholar]
- 39.Ben Amara A, Ghigo E, Le Priol Y, et al. Coxiella burnetii, the agent of Q fever, replicates within trophoblasts and induces a unique transcriptional response. PLoS One. 2010;5:e15315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hyde SR, Benirschke K. Gestational psittacosis: case report and literature review. Mod Pathol. 1997;10:602–607. [PubMed] [Google Scholar]
- 41.Muehlenbachs A, de la Rosa Vazquez O, Bausch DG, et al. Ebola virus disease in pregnancy: clinical, histopathologic, and immunohistochemical findings. J Infect Dis. 2017;215:64–69. [DOI] [PubMed] [Google Scholar]
- 42.Longtine MS, Barton A, Chen B, et al. Live-cell imaging shows apoptosis initiates locally and propagates as a wave throughout syncytiotrophoblasts in primary cultures of human placental villous trophoblasts. Placenta. 2012;33:971–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Song WC, FitzGerald GA. COVID-19, microangiopathy, hemostatic activation, and complement. J Clin Invest. 2020;130:3950–3953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crespo AC, Mulik S, Dotiwala F, et al. Decidual NK cells transfer granulysin to selectively kill bacteria in trophoblasts. Cell. 2020;182:1125–1139.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Narni-Mancinelli E, Vivier E. Clues that natural killer cells help to control COVID. Nature. 2021;600:226–227. [DOI] [PubMed] [Google Scholar]
- 46.Benachi A, Rabant M, Martinovic J, et al. Chronic histiocytic intervillositis: manifestation of placental alloantibody-mediated rejection. Am J Obstet Gynecol. 2021;225:662–662.e1l. [DOI] [PubMed] [Google Scholar]
- 47.Redline RW. Extending the spectrum of massive perivillous fibrin deposition (maternal floor infarction). Pediatr Dev Pathol. 2021;24:10–11. [DOI] [PubMed] [Google Scholar]
- 48.Bayer A, Lennemann NJ, Ouyang Y, et al. Type III interferons produced by human placental trophoblasts confer protection against zika virus infection. Cell Host Microbe. 2016;19:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Megli C, Morosky S, Rajasundaram D, et al. Inflammasome signaling in human placental trophoblasts regulates immune defense against Listeria monocytogenes infection. J Exp Med. 2021;218:e20200649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sadovsky Y, Mouillet JF, Ouyang Y, et al. The function of trophomirs and other microRNAs in the human placenta. Cold Spring Harb Perspect Med. 2015;5:a023036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wadman M. Flawed interferon response spurs severe illness. Science. 2020;369:1550–1551. [DOI] [PubMed] [Google Scholar]
- 52.Griffin AC, Strauss AW, Bennett MJ, et al. Mutations in long-chain 3-hydroxyacyl coenzyme a dehydrogenase are associated with placental maternal floor infarction/massive perivillous fibrin deposition. Pediatr Dev Pathol. 2004;15:368–374. [DOI] [PubMed] [Google Scholar]
- 53.Mao Q, Chu S, Shapiro S, et al. Increased placental expression of angiotensin-converting enzyme 2, the receptor of SARS-CoV-2, associated with hypoxia in twin anemia-polycythemia sequence (TAPS). Placenta. 2021;105:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Curtis S, Jones CJ, Garrod A, et al. Identification of autophagic vacuoles and regulators of autophagy in villous trophoblast from normal term pregnancies and in fetal growth restriction. J Matern Fetal Neonatal Med. 2013;26:339–346. [DOI] [PubMed] [Google Scholar]
- 55.Ghosh S, Dellibovi-Ragheb TA, Kerviel A, et al. Beta-coronaviruses use lysosomes for egress instead of the biosynthetic secretory pathway. Cell. 2020;183:1520–1535.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wong HH, Sanyal S. Manipulation of autophagy by (+) RNA viruses. Semin Cell Dev Biol. 2020;101:3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Formigli L, Papucci L, Tani A, et al. Aponecrosis: morphological and biochemical exploration of a syncretic process of cell death sharing apoptosis and necrosis. J Cell Physiol. 2000;182:41–49. [DOI] [PubMed] [Google Scholar]


