Abstract
There are limited data to guide treatment recommendations for children with acute, symptomatic coronavirus disease 2019 (COVID-19). This review outlines a proposed management approach for children based on the published evidence to date and the approval of medications through drug regulatory agencies, as well as the known safety profile of the recommended drugs in this age group.
Keywords: 2019 novel coronavirus, infant, child, pediatrics, treatment, prevention, SARS-CoV-2, remdesivir, tocilizumab, dexamethasone, baricitinb, nirmatrelvir/ritonavir, budesonide, casirivimab-imdevimab
More than 2 years into the coronavirus disease 2019 (COVID-19) pandemic, ongoing waves of infection challenge hospital resources and public health responses worldwide. Most children are asymptomatic or have mild symptoms after exposure to severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) virus, including with the Delta and Omicron variants.1,2 Despite the low risk of severe COVID-19 in children, there has been an increase in the number of children requiring hospitalization and treatment as exposure to SARS-CoV-2 becomes near universal.3
To date, few clinical trials of chemotherapeutics for COVID-19 have included children, and therefore, there are limited data to guide treatment recommendations in this age group. This review outlines a proposed management approach for children with COVID-19 based on evidence from trials in adults, the registration of these medications through drug regulatory agencies, accessibility and known safety profiles of the treatments in children. Use of off-label or unlicensed medications for the treatment of COVID-19 outside of clinical trials requires informed consent from the parent or guardian, with clear discussion of the risks and benefits.
PROPOSED APPROACH
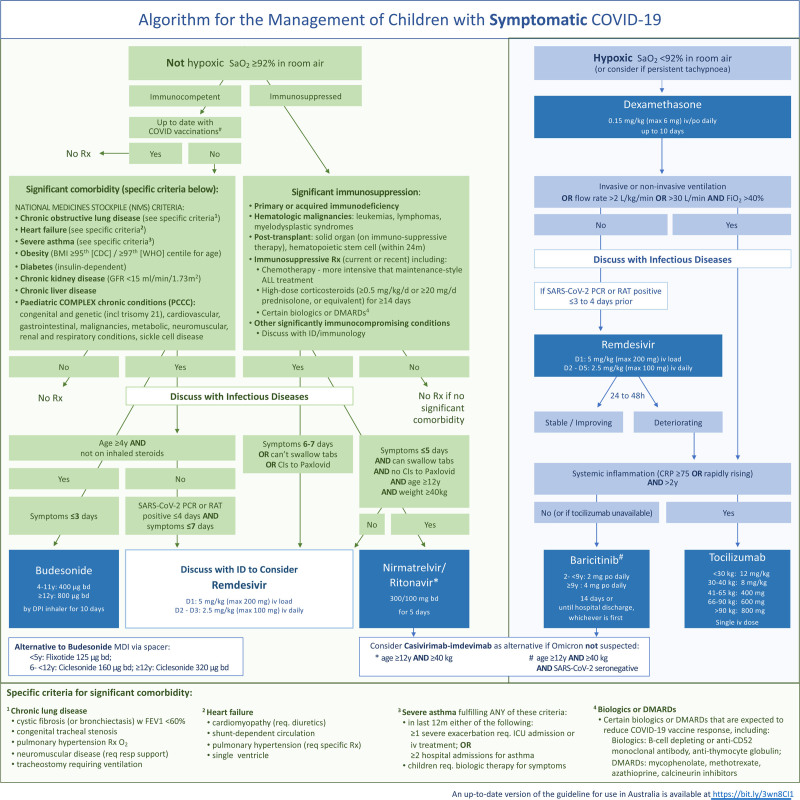
The proposed approach to the treatment of COVID-19 in children is outlined in Figure 1 and divided into 3 categories: (1) symptomatic children who are hypoxic with oxygen saturations (SaO2) <92% in room air; (2) symptomatic children who are not hypoxic (SaO2 ≥ 92% in room air); and (3) asymptomatic children exposed to SARS-CoV-2. Infection control and supportive care including thromboprophylaxis are also important in the management of children admitted to hospital because of COVID-19 but are beyond the scope of this treatment algorithm. Similarly, the treatment of pediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS) (also known as multisystem inflammatory system in children) is not addressed in this algorithm. For each recommended treatment, we outline the mechanism of action, adverse effects, evidence for use in COVID-19, indications for use and contraindications.
FIGURE 1.
Algorithm for the management of children with symptomatic COVID-19.
PART 1: SYMPTOMATIC CHILDREN WHO ARE HYPOXIC BECAUSE OF COVID-19
This section of the algorithm relates to children with COVID-19 who are hypoxic (Fig. 1).
Dexamethasone
Dexamethasone is a long-acting synthetic glucocorticoid with anti-inflammatory and immunosuppressive properties. Glucocorticoids bind to intracellular glucocorticoid receptors to regulate gene transcription, ultimately exerting an effect on a broad range of immune responses including decreased production of proinflammatory cytokines and suppression of neutrophil migration to inflammatory sites.4
The use of systemic corticosteroids compared with standard of care for adults with moderate-to-severe COVID-19 has been investigated in at least 11 randomized controlled trials (RCTs), the results of which have been further evaluated in 2 meta-analyses.5,6 The RECOVERY trial of 6425 hospitalized patients (2104 dexamethasone vs. 4321 standard care) showed that oral or intravenous (IV) dexamethasone at a dose of 6 mg once daily for up to 10 days reduced 28-day mortality across all subgroups receiving respiratory support [22.9% vs. 25.7%, risk ratio (RR), 0.83; 95% CI, 0.75–0.93].7 Notably, lower mortality rates with dexamethasone were also observed in those receiving oxygen without invasive mechanical ventilation (23.3% vs. 26.2%; RR, 0.82; 95% CI, 0.72–0.94).
In a 2020 meta-analysis of 7 RCTs including 1703 critically ill adults with COVID-19, steroids were found to reduce absolute mortality [32% (222/678) with steroids versus 41% (425/1025) with standard care or placebo, odds ratio (OR), 0.66; 95% CI, 0.53–0.82].6 In the subgroup analysis, findings were similar for dexamethasone and hydrocortisone, but a significant benefit was not demonstrated for methylprednisolone (OR, 0.91, 95% CI, 0.29–2.87).6 Similarly, a more recent meta-analysis found some evidence of systemic corticosteroids lowering all-cause mortality (RR, 0.89, 95% CI, 0.80–1.0).5 The COVID STEROID 2 RCT compared 6 and 12 mg of dexamethasone in 982 adults with severe hypoxemia (requiring >10 L/min oxygen or mechanical ventilation). The number of days alive without life support at 28 days, mortality at 90 days and serious adverse effects were all similar between the 2 groups.8 Children were not included in these trials.
Dexamethasone is licensed for use in children, is widely accessible and has a well-established safety profile. Based on the data from trials in adults, we therefore recommend dexamethasone should be given to children with hypoxemia, defined as SaO2 oxygen saturation <92% in room air (note this commonly used pediatric definition differs from most adult trials in which hypoxemia was defined as <94% in room air5). The recommended dose is 0.15 mg/kg/day (maximum 6 mg) given orally or intravenously for up to 10 days or until discharge from hospital, based on the RECOVERY trial.7
Dexamethasone was not associated with an increased risk of serious adverse events in the trials for COVID-19. However, important potential adverse effects include hyperglycemia, gastritis and gastrointestinal bleeding, behavioral changes, hypertension, adrenal suppression and risk related to infection (bacterial, fungal and strongyloidiasis).
Remdesivir
Remdesivir is a RNA-dependent RNA polymerase inhibitor with in vitro inhibitory effects on coronaviruses, including SARS-CoV-2. Trials evaluating remdesivir for the treatment of COVID-19 have reported conflicting findings. The World Health Organization (WHO) SOLIDARITY trial found no significant effect of remdesivir on mortality in patients hospitalized with COVID-19 (no severity classification).9 However, a double-blind, randomized trial [Adaptive COVID-19 Treatment Trial 1 (ACTT-1)] found that, compared with placebo, remdesivir was associated with a shorter time to recovery (clinical improvement at day 15; OR, 1.5; 95% CI, 1.2–1.9), but there was no significant reduction in mortality (hazard ratio, 0.73; 95% CI, 0.52–1.03).10 A meta-analysis of 5 RCTs of remdesivir for COVID-19 found no significant reduction in mortality (RR 0.93; 95% CI, 0.82–1.06).11 However, when stratified by disease severity, a mortality benefit was observed in those with moderate-to-severe disease who did not require mechanical ventilation (invasive or noninvasive).12
Remdesivir was the first antiviral approved for the treatment of COVID-19 in October 2020. The Food and Drug Administration (FDA) and European Medicines Agency (EMA) have both approved the use of remdesivir in children ≥12 years and ≥40 kg with COVID-19 pneumonia requiring supplemental oxygen. The FDA has emergency use authorization (EUA) for children weighing between 3.5 and 40 kg who are hospitalized with COVID-19 and who are at an increased risk for progression to severe disease.
In our proposed treatment algorithm, remdesivir is recommended in those with a positive SARS-CoV-2 polymerase chain reaction (PCR) test within the previous 3–4 days. Although RCTs have evaluated both 5- and 10-day treatment courses, a 5-day treatment duration is recommended as it is associated with fewer serious adverse events.11,13 Of note, there is a paucity of data on the efficacy of remdesivir in immunocompromised patients. The effect of remdesivir may be impacted by the timing of administration in relation to the illness course. In the ACTT-1 trial, clinical improvement was greater when remdesivir was given early.10 The DisCoVeRy trial found no benefit of remdesivir in hospitalized adults with hypoxia; however, the median time to starting treatment was 9 days after symptom onset.14
Remdesivir is administered intravenously for 3–5 days with dosing regimens varying by weight and age (see Table 1). It is recommended that children <12 years only receive the lyophilized powder formulation. Trials to date have not included adults with severe liver or renal disease. Remdesivir should not be used in patients with elevated creatinine or an alanine aminotransferase (ALT) greater than five times the upper limit of normal (ULN) by local laboratory measure or an estimated glomerular filtration rate (eGFR) of <30 mL/min. Patients should have baseline liver functions tests, electrolytes, creatinine and full blood examination with monitoring whilst on therapy. It is recommended that remdesivir be discontinued if any of the above parameters are met.
TABLE 1.
Drug Dosing Regimens
| Drug | Age | Dose | Duration | Contraindications | Precautions and Comments |
|---|---|---|---|---|---|
| Baricitinib | 2 to <9 y ≥9 y |
2 mg PO daily 4 mg PO daily |
14 d OR until discharged, whichever is first | • Renal impairment (creatinine clearance <15 mL/min/1.73 m2) • Lymphopenia (<2 × 109 cells/L) • Neutropenia (<0.5 × 109 cells/L) • Transaminitis (ALT or AST >5× ULN) • Active TB and other opportunistic infections |
• Due to its immunosuppressive effects should be used with caution in immunocompromised patients • Cytopenia may occur during treatment; cease if lymphocyte count <0.5 × 109 cells/L, hemaglobin <80 g/L or ANC <1 × 109 cells/L • Dose adjustment required in patients with renal impairment |
| Budesonide | ≥4 to 11 y ≥12 y |
400 µg by DPI BD 800 µg by DPI BD |
10 d | • Patients unable to appropriately inhale from a DPI • Patients already on inhaled corticosteroids |
• Rinse mouth following use to minimize risk of oral candidiasis |
| Casirivimab-imdevimab | ≥12 y and ≥40 kg | For treatment single dose based on disease severity: - Mild: 1200 mg IV or SC - Moderate-critical: 8000 mg IV For postexposure prophylaxis: Single dose 1200 mg IV or SC Ongoing 600 mg IV or SC 4 weekly or until no longer required (max 6 doses) |
Not applicable | • No known contraindications | • IV route preferred for 1200 mg due to medication volume• Not recommended for infection with Omicron variant (BA.1) |
| Ciclesonide | ≥6 to 11 y ≥12 y |
160 µg by MDI BD ± spacer 320 µg by MDI BD ± spacer |
10 d | • Patients already on inhaled corticosteroids | • Alternative for patients ≥6 y unable to inhale from a DPI • Rinse mouth following use to minimize the risk of oral candidiasis |
| Dexamethasone | All ages | 0.15 mg/kg (maximum 6 mg) IV or PO daily | Up to 10 d or until discharge (whatever is first) | • Not recommended for infection with Omicron variant (BA.1) | |
| Fluticasone | <5 y | 125 µg BD by MDI + spacer | 10 d | • Patients already on inhaled corticosteroids | • Alternative for patients <5 y unable to inhale from a DPI • Rinse mouth following use to minimize risk of oral candidiasis |
| Nirmatrelvir/ritonavir | ≥12 y and ≥40 kg | 300 mg nirmatrelvir + 100 mg ritonavir BD | 5 d | • Contraindicated in patients receiving drugs highly dependent on CYP3A for clearance* OR potent CYPA3A inducers | • Dose adjustment in renal impairment • No safety or efficacy data for children and adolescents <18 y |
| Remdesivir | ≥12 y and ≥40 kg <12 y and/or <40 kg |
200 mg IV loading dose 100 mg IV daily 5 mg/kg IV loading dose 2.5 mg/kg IV daily |
Day 1 Days 2–5 Day 1 Days 2–5 |
• Known hypersensitivity to the drug, the metabolites or formulation excipient | • Severe bradycardia and mild-moderate increase in AST and ALT reported in patients receiving remdesivir for COVID-19; discontinue if hepatic impairment develops |
| Sotrovimab | ≥12 y and ≥40 kg | 500 mg IV as single dose | Not applicable | • No known contraindications | • No dosage adjustment for hepatic or renal impairment • Monitor for possible anaphylactic or infusion reaction during the infusion and until 1 h post infusion • Not recommended for infection with Omicron variant (BA.2) |
| Tocilizumab | >2 y | Single IV dose: <30 kg: 12 mg/kg 30–40 kg: 8 mg/kg 41–65 kg: 400 mg 66–90 kg: 600 mg >90 kg: 800 mg |
Not applicable | • Known hypersensitivity to tocilizumab • Severe hepatic impairment (AST or ALT >10× ULN) • Severe, active infection (including TB and other opportunistic infections) |
• Due to its immunosuppressive effects should be used with caution in immunocompromised patients • No dose adjustment required in renal impairment |
*See product information statement for full list of contraindicated medications.
ANC, absolute neutrophil count; AST, aspartate aminotransferase; BD, twice daily; DPI, dry powder inhaler; ECMO, extracorporeal membrane oxygenation; MDI, metered dose inhaler; PO, orally; SC, subcutaneous; TB tuberculosis.
Tocilizumab
Tocilizumab is a humanized monoclonal antibody that interferes with interleukin (IL)-6 receptor binding. IL-6 is a potent proinflammatory cytokine which activates signal transduction mediated by Janus (JAK) and other kinases, upregulating the production of additional proinflammatory mediators, B cell and T cell differentiation.15 Elevated levels of IL-6 are implicated in the development of cytokine release syndrome and multiorgan failure in severe COVID-19.15,16 Tocilizumab inhibits signal transduction and prevents the subsequent proinflammatory cascade.
Early RCTs of tocilizumab in adults with COVID-19 were underpowered, had variable inclusion criteria and produced conflicting results.17–20 The largest trial of tocilizumab to date (RECOVERY) found a reduction in 28-day all-cause mortality (31% tocilizumab vs. 35% standard care, 95% CI, 0.76–0.95, P = 0.0028).21 Two key inclusion criteria were used: hypoxia (SaO2 <92% on room air) and systemic inflammation, defined as a C-reactive protein (CRP) ≥75 mg/L. Of note, most (82%) participants were already receiving steroids, enrollment occurred early in the hospital admission (median 2, range 1–5 days), and only a small proportion (14%) were mechanically ventilated. No participants received remdesivir. The REMAP-CAP trial included adults recently admitted to the intensive care unit (ICU) (within 24 hours) requiring respiratory or cardiovascular support and did not include a CRP cutoff for enrollment, although the median CRP value was 150 mg/L.22 The trial showed improved survival in the IL-6 receptor antagonist group (353 tocilizumab; 48 sarilumab) compared with control (hazard ratio 1.61 at 90 days; 95% credible interval, 1.25–2.08). In contrast, despite a similar median CRP, the COVACTA trial, which included 294 adults who received tocilizumab compared with 144 who received placebo, showed no improvement in clinical status or reduction in mortality at 28 days.23 The WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group meta-analysis, which included 27 randomized trials, found an IL-6 receptor antagonist was associated with a lower 28-day all-cause mortality in hospitalized adults with COVID-19 (summary OR, 0.86; 95% CI, 0.79–0.95, P = 0.003).24
There is no evidence for the use of tocilizumab in children with symptomatic COVID-19 outside of case series and cohort studies, which included only a small proportion of children receiving experimental therapies.25,26 However, a trial of tocilizumab for children with COVID-19 is currently underway (RECOVERY NCT04381936). Safety data for tocilizumab in children are primarily derived from trials of rheumatological diseases and it is overall, well-tolerated.27 The most commonly reported side effects are infections, cytopenia and infusion-related reactions. In trials in adults with COVID-19, drug-related adverse effects included secondary bacterial infections, hepatotoxicity, thrombocytopenia and neutropenia.24
Tocilizumab is currently licensed by the FDA and EMA for use in children >2 years with juvenile idiopathic arthritis and cytokine release syndrome induced by chimeric antigen receptor T cell therapy. There is currently a worldwide shortage of tocilizumab due to COVID-19. This algorithm recommends a single dose of tocilizumab for children ≥2 years of age, with hypoxemia and evidence of systemic inflammation (CRP ≥75 mg/L or rapidly rising) who are either: (1) receiving invasive or noninvasive ventilation or (2) not yet requiring ventilation but clinically deteriorating 24–48 hours after starting steroids. Of note, only the FDA has approved the use of tocilizumab for COVID-19 in the pediatric age group. In the RECOVERY, COVACTA and REMAP-CAP trials, a second dose was given (8-24 hours later) to participants deemed to not be improving at the treating clinician’s discretion. This occurred in only 22%–29% of participants.22,23
The dose of IV tocilizumab is stratified by weight (Table 1). As tocilizumab inhibits the proinflammatory cascade, following its administration CRP should not be used as a marker of monitoring progress. Tocilizumab is not recommended in children with impaired liver function (defined as aspartate aminotransferase/ALT > 10× the ULN) or thrombocytopenia (<50 × 103/µL).
Baricitinib
Baricitinib is an orally administered selective JAK 1 and 2 inhibitor. It exerts its anti-inflammatory effects by reversibly inhibiting the intracellular signaling of multiple cytokines implicated in the immunopathology of COVID-19. It is also proposed to have antiviral properties against SARS-CoV-2, interfering with viral entry and reducing ACE2 expression on bronchial and upper airway epithelial cells.28 Baricitinib is approved for use in adults with moderate-to-severe rheumatoid arthritis and in some countries for severe atopic dermatitis, and is being studied in clinical trials for the treatment of atopic dermatitis in children.
The role of bariticinib in adults with COVID-19 has been assessed in 2 phase III, randomized, double-blind, placebo-controlled clinical trials.29,30 In 1033 hospitalized adults with COVID-19 (evidence of systemic inflammation was not required for enrollment), the ACTT-2 trial found that participants who received baricitinib plus remdesivir had a faster median time to recovery by 1 day compared with those who received placebo plus remdesivir (median 7 vs. 8 days; rate ratio for recovery 1.16; 95% CI, 1.01–1.32; P = 0.03), but no difference in 28-day mortality was observed.29 Of note, the difference in time to recovery was greatest in those patients requiring high-flow oxygen or noninvasive ventilation (median 10 vs. 18 days; rate ratio for recovery 1.51; 95% CI, 1.10–2.08). The COV-BARRIER trial was a placebo-controlled RCT evaluating baricitinib in combination with standard of care in 1525 hospitalized adults with COVID-19.30 This trial found a lower 28-day all-cause mortality in the baricitinib group compared with placebo (8% vs. 13%; hazard ratio, 0.57; 96% CI, 0.41–0.78). Participants required at least 1 elevated marker of systemic elevation for enrollment (CRP, D-dimer, lactate dehydrogenase or ferritin above the ULN). Standard of care treatment included systemic corticosteroids (79.3%) and remdesivir (18.9%). Neither trial showed a significant increase in serious adverse events, serious infections or thromboembolic events with baricitinib. However, no trials have evaluated the role of baricitinib in children with COVID-19. The pharmacokinetics of baricitinib in children has been evaluated in a cohort of 18 patients [median age 12.5 years (range 1.2–24.1); weight 8.5–84 kg] with inherited interferon disorders enrolled in a compassionate use program. Weight and renal function significantly impacted on volume of distribution and clearance, respectively. The half-life of baricitinib was significantly shorter in children <40 kg.28
Based on the above data, the FDA has provided EUA for baricitinib for the treatment of COVID-19 in hospitalized adults and children ≥2 years of age requiring supplemental oxygen, noninvasive or invasive mechanical ventilation or extracorporeal membrane oxygenation. The EMA has not yet approved baricitinib for use in children. The recommended oral dosage is stratified based on age (see Table 1) and duration of therapy is 14 days or until hospital discharge, whichever occurs earlier. Baricitinib is not recommended for patients with renal impairment (creatinine clearance <15 mL/min/1.73 m2), lymphopenia (lymphocytes < 2 × 109 cells/L), neutropenia (neutrophils 0.5 × 109 cells/L), transaminitis (ALT or aspartate aminotransferase > 5 × ULN) or active tuberculosis.
In September 2021, the FDA issued a black box warning for baricitinib in relation to the risk of malignancy (lymphoma and nonmelanoma skin cancer), thrombosis and serious infection, including tuberculosis. Baricitinib has been associated with thrombocytosis and increases in serum creatinine, lipids, liver enzymes, bilirubin and creatinine kinase. These laboratory markers should be monitored along with hematologic indices during treatment.28
Casirivimab-imdevimab (Ronapreve)
Casirivimab and imdevimab are recombinant IgG1 monoclonal antibodies that bind to the spike protein of SARS-CoV-2 preventing its interaction with the ACE2 receptor thereby inhibiting cell infection.31
The role of casirivimab-imdevimab in hospitalized patients ≥12 years with COVID-19 was first investigated in an open-label RCT by the RECOVERY group comparing usual care with experimental therapies.32 In seronegative patients, casirivimab-imdevimab reduced 28-day mortality caused by COVID-19 [396/1633 (24%) intervention vs. 451/1520 (30%) usual care; rate ratio, 0.80; 95% CI, 0.70–0.91]. Although a reduction in mortality was also seen in seropositive patients, there was a significant difference in mortality benefit between seropositive and seronegative patients (P value for heterogeneity = 0.001). Furthermore, when data were combined for those with an unknown, seropositive and seronegative baseline antibody status, there was no significant improvement in clinical outcome (rate ratio, 0.94; 95% CI, 0.86–1.03), suggesting a maximum benefit in seronegative patients.
Casirivimab-imdevimab was initially authorized for use by the FDA and EMA for the treatment of COVID-19 in hospitalized patients ≥12 years and ≥40 kg. However, because of in vitro evidence showing reduced activity against certain mutations including Omicron BA.1, casirivimab-imdevimab is not recommended for infection with the Omicron variant.33 The FDA has specified it should only be used in patients infected with or exposed to a susceptible variant, and the National Institute of Health COVID-19 Treatment Guidelines Panel has recommended against its use. However, a recent study suggests casirivimab-imdevimab may have restored activity against the Omicron BA.2 variant.34
PART 2: SYMPTOMATIC CHILDREN WHO ARE NOT HYPOXIC
This section of the algorithm relates to children with symptoms of COVID-19 who are not hypoxic (Fig. 1). There are currently insufficient data to quantify the risk of severe COVID-19 in children associated with specific risk factors. The suggested criteria for treatment in this algorithm are therefore informed by both the published evidence and expert subspecialist opinion. As the risk of severe COVID-19 even in children with comorbidities is likely to be lower than the risk in healthy adults, decisions in relation to the treatment of symptomatic children who are not hypoxic should always be discussed with an infectious diseases specialist and made on a case-by-case basis. Given the significant protection against severe disease conferred by COVID-19 vaccination, these therapeutics are not recommended for immunocompetent children who have been fully vaccinated. Children receiving disease-modifying antirheumatic drugs or specific biologics expected to reduce the response to the COVID-19 vaccination should be considered as immunosuppressed in this approach.35
Risk Factors for Severe COVID-19 in Children
Children and adolescents with underlying health problems are at higher risk of hospitalization, admission to an ICU and mortality.36–39 In a systematic review of children with severe COVID-19, comorbid conditions were associated with an increased risk of severe disease and mortality with a relative RR of 1.79 (95% CI, 1.27–2.51) and 2.81 (95% CI, 1.31–6.02), respectively.36 The most frequently reported comorbidities in children with severe COVID-19 include obesity, complex neurodevelopmental disorders, respiratory diseases including asthma, immunodeficiency and malignancy. In a multicenter study in 25 European countries including 582 children with PCR-confirmed COVID-19, 25% had comorbidities which were associated with an increased risk of ICU admission in a multivariate analysis (OR, 3.27; P = 0.0015).25 In this study, of the 145 children with comorbidities, the most common were chronic pulmonary diseases (predominantly asthma and bronchopulmonary dysplasia) (5%), followed by hematologic or solid tumor malignancy (5%), neurologic disorders (4%) and congenital heart disease (4%). Of note, in a prospective study of 1527 immunocompromised children from 46 hospitals in the United Kingdom, only 4 children required hospital admission with confirmed SARS-CoV-2 infection, and there were no cases of severe COVID-19 or death.40
Obesity is consistently reported to confer a significant risk for severe disease in both adults and children.36,41 This is important in many high-income countries where a large proportion of children and adolescents are classified as obese (eg, Australia 8.2%; the United States 19.3% including 6.1% with severe obesity).42–44 A large systematic review reported a relative risk of 2.87 (95% CI, 1.16–7.07) for severe disease in obese children.36 Pooled data from 42 studies of children with severe COVID-19 found obesity was the most common comorbidity associated with severe disease or ICU admission (64/358).36
A recently published national cohort study in Scotland found that children from 5 to 17 years of age with poorly controlled asthma (defined as the requirement for oral corticosteroid use or previous hospital admission for asthma) had a higher rate of hospitalization for COVID-19 compared with those with well-controlled or no asthma [HR, 6.40 (95% CI 3.27–12.53) for those with a previous admission within the previous 2-year period; HR, 3.38 (95% CI, 1.84–6.21) for those who had received 3 or more courses of corticosteroids within the previous 2-year period].45 Sickle cell disease (SCD) was found in a retrospective population-level study based in the United Kingdom to be associated with a 4-fold increased risk of hospitalization and 2.6-fold increased risk of death.46 This study only identified 5 children with SCD requiring hospitalization, none of whom died. The mortality rate of children with SCD with COVID-19 in an international registry is reported as 0.2%.47
Sotrovimab
Sotrovimab is a monoclonal antibody that binds the highly preserved epitope on the virus’s spike (S) protein receptor binding domain, neutralizing sarbecoviruses, including SARS-CoV-2. Its 2-amino acid Fc modification enables a long half-life and is thought to improve bioavailability in the respiratory mucosa.48,49
The COMET-ICE RCT evaluated sotrovimab in nonhospitalized, unvaccinated, symptomatic adults with SARS-CoV-2 infection in the first 5 days from symptom onset who had oxygen saturations ≥94% in room air and at least one risk factor for disease progression.50 Those who received sotrovimab were significantly less likely to progress to severe disease, requiring hospitalization for ≥24 hours (relative risk reduction 85%) or death. Risk factors for disease progression included diabetes requiring medication, obesity (body mass index >30 kg/m2), chronic kidney disease (eGFR <60 mL/min/1.73 m2), chronic obstructive pulmonary disease, moderate-to-severe asthma (requiring oral steroids within the past year or an inhaled steroid to control symptoms), congestive heart failure (New York Heart Association class II or higher) or age ≥55 years. Sotrovimab was well tolerated, with the only reported side effect being mild diarrhea and infusion-related reactions in a small number of patients.50
Sotrovimab previously had EUA by the FDA and was approved by the EMA for use in children (≥12 years and ≥40 kg) and adults with SARS-CoV-2 infection who are at increased risk of hospitalization and/or death, and who do not require supplemental oxygen.
The FDA now recommends against sotrovimab in areas where Omicron BA.2 is dominant due to reduced neutralizing activity against this subvariant. As this subvariant has become predominant worldwide, sotrovimab is no longer included in our proposed treatment algorithm.
Casirivimab-imdevimab
In a phase 1 to 3, double-blind, placebo-controlled RCT of nonhospitalized adult patients with symptomatic COVID-19, there was both a lower rate of medical visits and hospitalization, in association with a significant decrease in viral load in those receiving casirivimab-imdevimab.51 Of note, patients were enrolled within 72 hours of symptom onset and positive PCR, the median age of enrollment was 43 years and inclusion was not restricted on the basis of comorbidities or baseline serology. Emergency outpatient use of casirivimab-imdevimab was granted in November 2021 by the FDA and EMA for adolescents ≥12 years of age and adults with mild-to-moderate COVID-19 with risk factors for progression to severe disease. Subsequently, a large retrospective cohort study of 1392 adults with mild-to-moderate COVID-19, with one or more risk factors for high-risk disease (including age ≥65 years, cardiometabolic disease or immunosuppression) found that adults treated within 10 days of symptom onset with casirivimab-imdevimab had lower hospitalization rates within 28 days of infusion (1.6% vs. 4.8%; absolute difference: 3.2%; 95% CI, 1.4%–5.1%).52 In both studies, casirivimab-imdevimab was well tolerated, with no serious adverse events.
Inhaled corticosteroids
Inhaled glucocorticoids commonly used for the treatment of inflammatory respiratory disease, including asthma, have been shown to reduce SARS-CoV-2 replication in airway epithelial cells and decrease the expression of angiotensin-converting enzyme-2 (ACE2) and transmembrane serine protease 2 (TMPRSS2), required for viral particle cell entry.53,54 Ciclesonide has been shown to also have direct antiviral activity and prevent SARS-CoV-2 replication, in addition to its anti-inflammatory effects.55
Two open-label RCTs, the STOIC and PRINCIPLE trials, reported favorable outcomes with the use of inhaled budesonide compared with standard care in adults with mild COVID not requiring hospital admission.56,57 The STOIC trial randomized 146 adults with COVID-19 within 7 days of symptom onset to usual care or budesonide (800 µg twice daily). In the intention-to-treat analysis, only 2 patients in the budesonide treated group met the primary outcome of a COVID-19-related emergency presentation or hospitalization, compared with 11 patients in the standard of care group (3% vs. 15%; 95% CI, 0.03–0.21; P = 0.009). The number needed to treat to prevent deterioration with COVID-19 was 8.56 Only a small proportion of participants had underlying comorbidities, such as cardiovascular disease, diabetes or asthma. Of note, the median duration of symptoms was 3 days in both groups. The PRINCIPLE trial included 1856 adults age ≥65 or ≥50 years of age with comorbidities who were randomized to receive budesonide (800 µg twice daily) or standard of care within 14 days of symptom onset.57 No immunocompromised adults were included. Participants taking budesonide had a reduced time to self-reported recovery by 2.9 days compared with those on usual care (11.8 vs. 14.7 days; 95% Bayesian CI, 1.19–5.12). In addition, fewer patients taking budesonide were hospitalized or died due to COVID-19 at 28 days compared with those receiving usual care (6.8% vs. 8.8%; OR, 0.75; 95% Bayesian CI, 0.55–1.02). However, this result did not meet the study’s predefined threshold for significance. Budesonide was well tolerated in both trials with only mild and reversible adverse effects.
Due to its promising in vitro activity against SARS-CoV-2, 2 RCTs have studied the efficacy of ciclesonide in reducing symptoms of COVID-19. The CONTAIN RCT compared resolution of symptoms in 203 adults with symptomatic PCR-proven infection, who received either placebo or ciclesonide (600 µg inhaled twice daily or 200 µg intranasal once daily). There was no difference in resolution of symptoms between the 2 groups [40% intervention vs. 35% control, absolute adjusted risk difference 5.5% (−7.8 to 18.8)].58 Of note, most started ciclesonide early in the illness course, with the median time to enrollment 3 days after symptom onset. Similarly, a placebo-controlled RCT of 400 adolescents (≥12 years) and adults enrolled within three days of a positive PCR result found the median duration to symptom resolution was 19 days in both groups. However, those in the ciclesonide group had less emergency department visits and hospital admissions for COVID-19 (OR, 0.2; 95% CI, 0.0–0.9).59
As the clinical efficacy of inhaled steroids was only modest and children were not included in these trials, inhaled corticosteroids should only be considered for unvaccinated children with symptomatic COVID-19 with significant risk factors for severe disease. Budesonide is only available as a dried powder inhaler which is challenging to administer to younger children or those with limited inspiratory capacity (such as those children with complex neurodevelopmental disorders). Therefore, a proposed alternative is ciclesonide or fluticasone which are available as metered dose inhalers that can be administered via a spacer and face mask (Table 1). Ciclesonide is licensed in the United States for use in children ≥12 years for asthma and allergic rhinitis; however, in other countries, it is approved for use in children ≥6 years, including Australia. Although there are currently no clinical data on the efficacy of fluticasone for COVID-19, in vitro data suggest it also reduces ACE2 expression.60
Remdesivir
The PINETREE RCT was the first trial to focus on the effect of early administration of remdesivir on disease progression in an unvaccinated outpatient population with significant comorbidities [hypertension, cardiovascular or cerebrovascular disease, obesity (body mass index ≥ 30), immune compromise, chronic mild or moderate kidney disease, chronic liver disease, chronic lung disease, current cancer or SCD] without hypoxia.61 This trial included adults and adolescents ≥12 years of age within seven days of symptom onset, and reported a significant decrease in COVID-19-associated hospitalizations in the remdesivir group (0.7% vs. 5.3%; hazard ratio, 0.1; 95% CI, 0.0–0.6). Of note, the median time of symptom duration before remdesivir infusion was 5 days, with a median of 2 days from SARS-CoV2 PCR positivity. Interestingly, clinical improvement was not associated with upper airway viral load. No deaths occurred in either groups by day 28.61 Remdesivir was well tolerated with few serious adverse effects, the most common being nausea, headache and cough.
Ritonavir-boosted nirmatrelvir (Paxlovid)
Ritonavir-boosted nirmatrelvir, a combination of 2 oral antivirals with activity against SARS-CoV-2, was recently given EUA by the FDA for adults ≥12 years and ≥40 kg with mild-to-moderate COVID-19 who are at high-risk for disease progression. The EMA has recently approved its use in adults ≥18 years. This follows release of interim results of the phase 2/3 RCT EPIC-HR showing administration within five days reduced the risk of hospitalization or death from COVID-19 in unvaccinated adults [8/1039 (0.8%) in treatment group vs. 66/1046 (6.3%) in placebo group].62 Outcomes in the vaccinated cohort of this trial with a breakthrough infection have not been reported. Phase 2 and 3 trials in children 6 to 17 years of age are underway. Ritonavir-boosted nirmatrelvir is a strong inhibitor of cytochrome (CYP) P450 3A. Therefore, co-administration with other drugs that induce or are metabolized by CYP3A results in either reduced drug concentrations and inferior virologic response; or increased drug concentrations with associated adverse effects, respectively. Contraindicated concomitant drugs include antiarrhythmics (eg, amiodarone and flecainide), anticancer drugs (eg, vincristine and venetoclax), anticoagulants (eg, warfarin and rivaroxaban), anticonvulsants (eg, carbamazepine), anti-HIV medications (eg, raltegravir), antimicrobials (eg, voriconazole, rifampicin, clarithromycin) and systemic corticosteroids.
PART 3: CHEMOPROPHYLAXIS OF CHILDREN EXPOSED TO COVID-19
As the risk of severe disease in healthy children exposed to SARS-CoV-2 is low and reduced further with vaccination,63,64 there are no recommendations for routine postexposure prophylaxis in asymptomatic children who are not fully vaccinated. Decisions regarding chemoprophylaxis should be on a case-by-case basis.
Casirivimab-imdevimab
For those with risk factors for progression to severe disease, casirivimab-imdevimab has also received EUA from the FDA for postexposure prophylaxis for children ≥12 years and ≥40 kg. The evidence for its effectiveness in preventing symptomatic SARS-CoV-2 is shown in a 2-part placebo-controlled RCT of unvaccinated, seronegative adolescents ≥12 years and adults. In Part A of the trial, uninfected household contacts of a positive case were less likely to develop symptomatic SARS-CoV-2 if they received casirivimab-imdevimab within 96 hours of exposure [11/753 (1.5%) vs. 59/752 (7.8%) with placebo; relative risk reduction 81.4%]. Notably, the duration of symptoms in those who proceeded to develop SARS-CoV-2 was also shorter in the casirivimab-imdevimab group (3.2 vs. 1.2 weeks). This was associated with a reduced duration of a high viral load (>104 copies/ml).65 In Part B, infected close contacts randomized to receive casirivimab-imdevimab also had reduced progression to symptomatic COVID-19 [(29/100 (29.0%) vs. 44/104 (42.3%) with placebo; OR, 0.54; 95% CI, 0.30–0.97)].66 Casirivimab-imdevimab was well tolerated. As previously discussed, given its lack of efficacy against the Omicron variant, casirivimab-imdevimab is not currently recommended. Its efficacy against new variants will need to be assessed.
Tixagevimab and Cilgavimab (Evusheld)
Tixagevimab and cilgavimab is a combination of 2 monoclonal antibodies that bind to the spike protein of SARS-CoV-2 to prevent infection. It is the first long-acting antibody combination for pre-exposure prophylaxis approved by the EMA for adults and has been given EUA by the FDA for children ≥12 years and ≥40 kg. It is only approved for those who are immunocompromised or who have a vaccine allergy preventing COVID-19 vaccination.
The PROVENT RCT phase III clinical trial randomized 5197 unvaccinated adults in a 2:1 ratio to combination tixagevimab and cilgavimab or placebo. Tixagevimab and cilgavimab is given as a single dose by 2 sequential intramuscular injections. The majority of trial participants had at least one comorbidity and all had negative SARS-CoV-2 serology. In the tixagevimab and cilgavimab group, there were no severe cases or deaths attributed to COVID-19, and there was an overall 77% reduction in the risk of developing COVID-19. In contrast, of 1737 participants in the placebo group, there are 3 (0.17%) cases of severe COVID-19 and 2 (0.12%) deaths. Notably, tixagevimab and cilgavimab was well tolerated.67 However, recent in vitro data suggest that neutralizing activity against Omicron is reduced (12- to 30-fold) and the clinical significance of this requires further study.68,69
PRECAUTIONARY NOTE
The major limitation of the described approach to treating COVID-19 is the extrapolation of evidence from trials in adult to children in the absence of dedicated pediatric studies. Some of the drugs described, including tocilizumab, budesonide and dexamethasone, have established pharmacokinetic and safety data in children. However, newer medications, including remdesivir, baricitinib, casirivimab-imdevimab, ritonavir-boosted nirmatrelvir, tixagevimab and cilgavimab and sotrovimab, do not. Although case series and cohort studies report the widespread use of these drugs in younger age groups, there have been no RCTs to demonstrate safety and efficacy. This is particularly problematic in younger children in whom drug pharmacokinetics often differ (<2 years). In addition, a major limitation of all guidelines is the rapidity in which they can become outdated in light of new evidence, particularly as new strains emerge. Finally, the applicability of this algorithm will vary according to accessibility to therapeutics across different national jurisdictions within a timely manner.
CONCLUSION
This article outlines a proposed approach to the management of COVID-19 in children based on the published evidence to date. Dedicated pediatric trials are needed to ensure the safety and efficacy of treatments for COVID-19 in children. Due to the lack of pediatric safety data for many of the therapeutic options for the treatment of COVID-19, pharmacovigilance and adverse event reporting, data collection to contribute to the existing literature, and parent/guardian informed consent is required.
Acknowledgments
Royal Children’s Hospital COVID-19 Treatment Working Group: Jonathan Akikusa, Jim Buttery, Penelope Bryant, Simon Carter, Michael Cheung, Vanessa Clifford, Theresa Cole, Trevor Duke, Katherine Frayman, Gabrielle Haeusler, Joshua Osowicki, Shivanthan Shanthikumar, Mike Starr, Andrew Steer and Shidan Tosif.
Footnotes
The authors have no funding or conflicts of interest to disclose.
Contributor Information
Alison Boast, Email: alison.boast@rch.org.au.
Johanna Holschier, Email: hanna.holschier@rch.org.au.
Rachael Purcell, Email: rachael.purcell@gmail.com.
Samantha Bannister, Email: samantha.bannister@rch.org.au.
Christine Plover, Email: christine.plover@rch.org.au.
David Burgner, Email: david.burgner@rch.org.au.
Suzanne L. Boyce, Email: suzanne.boyce@rch.org.au.
Sarah McNab, Email: sarah.mcnab@rch.org.au.
Amanda Gwee, Email: amanda.gwee@rch.org.au.
REFERENCES
- 1.Zimmermann P, Curtis N. Coronavirus infections in children including COVID-19: An overview of the epidemiology, clinical features, diagnosis, treatment and prevention options in children. Pediatr Infect Dis J. 2020;39:355–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimmermann P, Curtis N. Why is COVID-19 less severe in children? A review of the proposed mechanisms underlying the age-related difference in severity of SARS-CoV-2 infections. Arch Dis Child. 2021;106:429–439. [DOI] [PubMed] [Google Scholar]
- 3.Ong R, Hart J, Russell F. COVID-19 and Children’s surveillance report – Compiled 08 February 2022. Available at: https://www.mcri.edu.au/sites/default/files/media/documents/covid-19_and_childrens_surveillance_report_9_080222.pdf. Accessed February 13, 2022.
- 4.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17:233–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wagner C, Griesel M, Mikolajewska A, et al. Systemic corticosteroids for the treatment of COVID-19. Cochrane Database Syst Rev. 2021;8:CD014963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: a meta-analysis. JAMA. 2020;324:1330–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The COVID STEROID 2 Trial Group. Effect of 12 mg vs 6 mg of dexamethasone on the number of days alive without life support in adults with COVID-19 and severe hypoxemia: the COVID STEROID 2 randomized trial. JAMA. 2021;326:1807–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Solidarity Trial Consortium. Repurposed antiviral drugs for Covid-19 - interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beigel JH, Tomashek KM, Dodd LE, et al. ; ACTT-1 Study Group Members. Remdesivir for the treatment of Covid-19 - Final Report. N Engl J Med. 2020;383:1813–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaka AS, MacDonald R, Greer N, et al. Major update: remdesivir for adults with COVID-19: a living systematic review and meta-analysis for the American College of Physicians Practice Points. Ann Intern Med. 2021;174:663–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.National COVID-19 clinical evidence taskforce. 2022. Available at: https://covid19evidence.net.au/ Accessed February 13, 2022.
- 13.Rezagholizadeh A, Khiali S, Sarbakhsh P, et al. Remdesivir for treatment of COVID-19; an updated systematic review and meta-analysis. Eur J Pharmacol. 2021;897:173926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ader F, Bouscambert-Duchamp M, Hites M, et al. Remdesivir plus standard of care versus standard of care alone for the treatment of patients admitted to hospital with COVID-19 (DisCoVeRy): a phase 3, randomised, controlled, open-label trial. Lancet Infect Dis. 2022;22:209–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang C, Wu Z, Li JW, et al. Cytokine release syndrome in severe COVID-19: interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int J Antimicrob Agents. 2020;55:105954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone JH, Frigault MJ, Serling-Boyd NJ, et al. ; BACC Bay Tocilizumab Trial Investigators. Efficacy of tocilizumab in patients hospitalized with Covid-19. N Engl J Med. 2020;383:2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermine O, Mariette X, Tharaux PL, et al. ; CORIMUNO-19 Collaborative Group. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Salvarani C, Dolci G, Massari M, et al. ; RCT-TCZ-COVID-19 Study Group. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med. 2021;181:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta S, Wang W, Hayek SS, et al. ; STOP-COVID Investigators. Association between early treatment with tocilizumab and mortality among critically ill patients with COVID-19. JAMA Intern Med. 2021;181:41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2021;397:1637–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.REMAP-CAP Investigators. Interleukin-6 receptor antagonists in critically ill patients with Covid-19. N Engl J Med. 2021;384:1491–1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosas IO, Bräu N, Waters M, et al. Tocilizumab in hospitalized patients with severe Covid-19 pneumonia. N Engl J Med. 2021;384:1503–1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Götzinger F, Santiago-García B, Noguera-Julián A, et al.; ptbnet COVID-19 Study Group. COVID-19 in children and adolescents in Europe: a multinational, multicentre cohort study. Lancet Child Adolesc Health. 2020;4:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feldstein LR, Tenforde MW, Friedman KG, et al. ; Overcoming COVID-19 Investigators. Characteristics and outcomes of US children and adolescents with Multisystem Inflammatory Syndrome in Children (MIS-C) compared with severe acute COVID-19. JAMA. 2021;325:1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schiff MH, Kremer JM, Jahreis A, et al. Integrated safety in tocilizumab clinical trials. Arthritis Res Ther. 2011;13:R141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jorgensen SCJ, Tse CLY, Burry L, et al. Baricitinib: a review of pharmacology, safety, and emerging clinical experience in COVID-19. Pharmacotherapy. 2020;40:843–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kalil AC, Patterson TF, Mehta AK, et al. ; ACTT-2 Study Group Members. Baricitinib plus remdesivir for hospitalized adults with Covid-19. N Engl J Med. 2021;384:795–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marconi VC, Ramanan AV, de Bono S, et al. ; COV-BARRIER Study Group. Efficacy and safety of baricitinib for the treatment of hospitalised adults with COVID-19 (COV-BARRIER): a randomised, double-blind, parallel-group, placebo-controlled phase 3 trial. Lancet Respir Med. 2021;9:1407–1418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang Y, Yang C, Xu XF, et al. Structural and functional properties of SARS-CoV-2 spike protein: potential antivirus drug development for COVID-19. Acta Pharmacol Sin. 2020;41:1141–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.RECOVERY Group Collaborative. Casirivimab and imdevimab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet. 2022;399:665–676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.VanBlargan LA, Errico JM, Halfmann PJ, et al. An infectious SARS-CoV-2 B.1.1.529 Omicron virus escapes neutralization by therapeutic monoclonal antibodies. Nat Med. 2022;28:490–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takashita E, Kinoshita N, Yamayoshi S, et al. Efficacy of antiviral agents against the SARS-CoV-2 omicron subvariant BA.2. N Engl J Med. 2022;386:1475–1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Australian Technical Advisory Group on Immunisation. Reccomendations on the use of a 3rd primary dose of COVID-19 vaccine in individuals who are severely immunocompromised. Available at: https://www.health.gov.au/sites/default/files/documents/2021/10/atagi-recommendations-on-the-use-of-a-third-primary-dose-of-covid-19-vaccine-in-individuals-who-are-severely-immunocompromised_1.pdf. Accessed February 13, 2022.
- 36.Tsankov BK, Allaire JM, Irvine MA, et al. Severe COVID-19 infection and pediatric comorbidities: a systematic review and meta-analysis. Int J Infect Dis. 2021;103:246–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swann OV, Holden KA, Turtle L, et al. ; ISARIC4C Investigators. Clinical characteristics of children and young people admitted to hospital with covid-19 in United Kingdom: prospective multicentre observational cohort study. BMJ. 2020;370:m3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shekerdemian LS, Mahmood NR, Wolfe KK, et al. ; International COVID-19 PICU Collaborative. Characteristics and outcomes of children with Coronavirus Disease 2019 (COVID-19) infection admitted to US and Canadian pediatric intensive care units. JAMA Pediatr. 2020;174:868–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCormick DW, Richardson LC, Young PR, et al. ; Pediatric Mortality Investigation Team. Deaths in children and adolescents associated with COVID-19 and MIS-C in the United States. Pediatrics. 2021;148:e2021052273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chappell H, Patel R, Driessens C, et al. Immunocompromised children and young people are at no increased risk of severe COVID-19. J Infect. 2022;84:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sjögren L, Stenberg E, Thuccani M, et al. Impact of obesity on intensive care outcomes in patients with COVID-19 in Sweden-A cohort study. PLoS One. 2021;16:e0257891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cole TJ, Bellizzi MC, Flegal KM, et al. Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ. 2000;320:1240–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Australian Institute of Health and Welfare. Overweight and obesity among Australia children and adolescents. 2020. Available at: https://www.aihw.gov.au/getmedia/ac61b7d7-7991-4e15-8fa6-a7973479fa8b/aihw-phe-274.pdf.aspx?inline=true. Accessed February 13, 2022.
- 44.Fryar C, Carroll M, Afful J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018 [internet]. Division of Health and Nutrition Examination Surveys, Centre for Disease Control and Prevention. Available at: https://www.cdc.gov/nchs/data/hestat/obesity-child-17-18/obesity-child.htm. Accessed February 13, 2022.
- 45.Shi T, Pan J, Katikireddi SV, et al. Risk of COVID-19 hospital admission among children aged 5–17 years with asthma in Scotland: a national incident cohort study. The Lancet Resp Med. 2022;10:191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clift AK, Saatci D, Coupland CAC, et al. ; International Investigator Group for Ethnicity and COVID-19. Sickle Cell Disorders and Severe COVID-19 Outcomes: A Cohort Study. Ann Intern Med. 2021;174:1483–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Surveillance epidemiology of coronavirus (COVID-19) under research exclusion. Available at: https://covidsicklecell.org/updates-data/ Accessed February 13, 2022.
- 48.Ko SY, Pegu A, Rudicell RS, et al. Enhanced neonatal Fc receptor function improves protection against primate SHIV infection. Nature. 2014;514:642–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early treatment for Covid-19 with SARS-CoV-2 neutralizing antibody sotrovimab. N Engl J Med. 2021;385:1941–1950. [DOI] [PubMed] [Google Scholar]
- 50.Gupta A, Gonzalez-Rojas Y, Juarez E, et al. Early COVID-19 treatment with SARS-CoV-2 neutralizing antibody sotrivumab. 2021. [DOI] [PubMed]
- 51.Weinreich DM, Sivapalasingam S, Norton T, et al. ; Trial Investigators. REGN-COV2, a neutralizing antibody cocktail, in outpatients with Covid-19. N Engl J Med. 2021;384:238–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Razonable RR, Pawlowski C, O’Horo JC, et al. Casirivimab-Imdevimab treatment is associated with reduced rates of hospitalization among high-risk patients with mild to moderate coronavirus disease-19. EClinicalMedicine. 2021;40:101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Peters MC, Sajuthi S, Deford P, et al. COVID-19-related genes in sputum cells in Asthma. Relationship to demographic features and corticosteroids. Am J Respir Crit Care Med. 2020;202:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Finney LJ, Glanville N, Farne H, et al. Inhaled corticosteroids downregulate the SARS-CoV-2 receptor ACE2 in COPD through suppression of type I interferon. J Allergy Clin Immunol. 2021;147:510–519.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jeon S, Ko M, Lee J, et al. Identification of antiviral drug candidates against SARS-CoV-2 from FDA-approved drugs. Antimicrob Agents Chemother. 2020;64:e00819–e00820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ramakrishnan S, Nicolau DV, Jr, Langford B, et al. Inhaled budesonide in the treatment of early COVID-19 (STOIC): a phase 2, open-label, randomised controlled trial. Lancet Respir Med. 2021;9:763–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu LM, Bafadhel M, Dorward J, et al. ; PRINCIPLE Trial Collaborative Group. Inhaled budesonide for COVID-19 in people at high risk of complications in the community in the UK (PRINCIPLE): a randomised, controlled, open-label, adaptive platform trial. Lancet. 2021;398:843–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ezer N, Belga S, Daneman N, et al. Inhaled and intranasal ciclesonide for the treatment of covid-19 in adult outpatients: CONTAIN phase II randomised controlled trial. BMJ. 2021;375:e068060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clemency BM, Varughese R, Gonzalez-Rojas Y, et al. Efficacy of inhaled ciclesonide for outpatient treatment of adolescents and adults with symptomatic COVID-19: a randomized clinical trial. JAMA Intern Med. 2022;182:42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nakazono A, Nakamaru Y, Ramezanpour M, et al. Fluticasone propionate suppresses poly(I:C)-induced ACE2 in primary human nasal epithelial cells. Front Cell Infect Microbiol. 2021;11:655666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gottlieb RL, Vaca CE, Paredes R, et al. ; GS-US-540-9012 (PINETREE) Investigators. Early remdesivir to prevent progression to severe Covid-19 in outpatients. N Engl J Med. 2022;386:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pfizer’s novel COVID-19 oral antiviral treatment candidate reduced risk of hospitalization or death by 89% in interim analysis of phase 2/3 EPIC-HR study. Press Release November 5th 2021. Available at: https://www.pfizer.com/news/press-release/press-release-detail/pfizers-novel-covid-19-oral-antiviral-treatment-candidate. Accessed February 13, 2022.
- 63.Olson SM, Newhams MM, Halasa NB, et al. ; Overcoming Covid-19 Investigators. Effectiveness of BNT162b2 vaccine against critical Covid-19 in adolescents. N Engl J Med. 2022;386:713–723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Walter EB, Talaat KR, Sabharwal C, et al. ; C4591007 Clinical Trial Group. Evaluation of the BNT162b2 Covid-19 vaccine in children 5 to 11 years of age. N Engl J Med. 2022;386:35–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O’Brien MP, Forleo-Neto E, Musser BJ, et al. ; Covid-19 Phase 3 Prevention Trial Team. Subcutaneous REGEN-COV antibody combination to prevent Covid-19. N Engl J Med. 2021;385:1184–1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.O’Brien MP, Forleo-Neto E, Sarkar N, et al. ; COVID-19 Phase 3 Prevention Trial Team. Effect of subcutaneous casirivimab and imdevimab antibody combination vs placebo on development of symptomatic COVID-19 in early asymptomatic SARS-CoV-2 infection: a randomized clinical trial. JAMA. 2022;327:432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.AZD7442 PROVENT Phase III prophylaxis trial met primary endpoint in preventing COVID-19. Press Release 20th August 2021. Available at: https://www.astrazeneca.com/media-centre/press-releases/2021/azd7442-prophylaxis-trial-met-primary-endpoint.html#:~:text=First%20long%2Dacting%20antibody%20combination,19%2C%20the%20trial’s%20primary%20endpoint.D= Accessed March 20 2022.
- 68.Planas D, Saunders N, Maes P, et al. Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature. 2022;602:671–675. [DOI] [PubMed] [Google Scholar]
- 69.Cameroni E, Bowen JE, Rosen LE, et al. Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature. 2022;602:664–670. [DOI] [PMC free article] [PubMed] [Google Scholar]