Abstract
Objectives
No studies have examined longitudinal patterns of naturally exhaled SARS-CoV-2 RNA viral load (VL) during acute infection. We report this using facemask sampling (FMS) and assessed the relationship between emitted RNA VL and household transmission.
Methods
Between December 2020 and February 2021, we recruited participants within 24 hours of a positive RT-qPCR on upper respiratory tract sampling (URTS) (day 0). Participants gave FMS (for 1 hour) and URTS (self-taken) on seven occasions up to day 21. Samples were analysed by RT-qPCR (from sampling matrix strips within the mask) and symptom diaries were recorded. Household transmission was assessed through reporting of positive URTS RT-qPCR in household contacts.
Results
Analysis of 203 FMS and 190 URTS from 34 participants showed that RNA VL peaked within the first 5 days following sampling. Concomitant URTS, FMS RNA VL, and symptom scores, however, were poorly correlated, but a higher severity of reported symptoms was associated with FMS positivity up to day 5. Of 28 participants who had household contacts, 12 (43%) reported transmission. Frequency of household transmission was associated with the highest (peak) FMS RNA VL obtained (negative genome copies/strip: 0% household transmission; 1 to 1000 copies/strip: 20%; 1001 to 10 000 copies/strip: 57%; >10 000 copies/strip: 75%; p = 0.048; age adjusted OR of household transmission per log increase in copies/strip: 4.97; 95% CI, 1.20–20.55; p = 0.02) but not observed with peak URTS RNA VL.
Discussion
Exhaled RNA VL measured by FMS is highest in early infection, can be positive in symptomatic patients with concomitantly negative URTS, and is strongly associated with household transmission.
Keywords: Airborne, Transmission, SARS-CoV-2, COVID-19, Viral load, Exhaled virus, Viral diagnostics, Respiratory viruses, Nasopharyngeal sampling, Facemask sampling
Abbreviations: SARS-CoV-2, Severe Acute Respiratory Syndrome - Coronavirus-2; COVID-19, Coronavirus Disease - 2019
Introduction
To enable transmission, most scientists agree that SARS-CoV-2 must be emitted from the respiratory tract [[1], [2], [3]]. The standard method of SARS-CoV-2 diagnosis is to obtain upper respiratory tract samples (URTS) from the nose and throat. While there are single point assessments of exhaled virus by different methods, no clear picture exists of the natural history of SARS-CoV-2 emission [[4], [5], [6], [7], [8]]. Facemask sampling (FMS) offers particular advantages for assessment of exhaled virus output over multiple sampling periods [9]. FMS can be performed within the comfort of patients' own homes, and the methodology is replicable in most routine laboratories. In this study, we provide a description of the longitudinal output of SARS-CoV-2 genomic RNA in exhaled breath from infected participants using FMS. We compare the FMS findings from these individuals with concomitant URTS results and assess relationships between FMS RNA viral load (VL), clinical symptoms, and subsequently detected infections in the same household.
Methods
Study settings
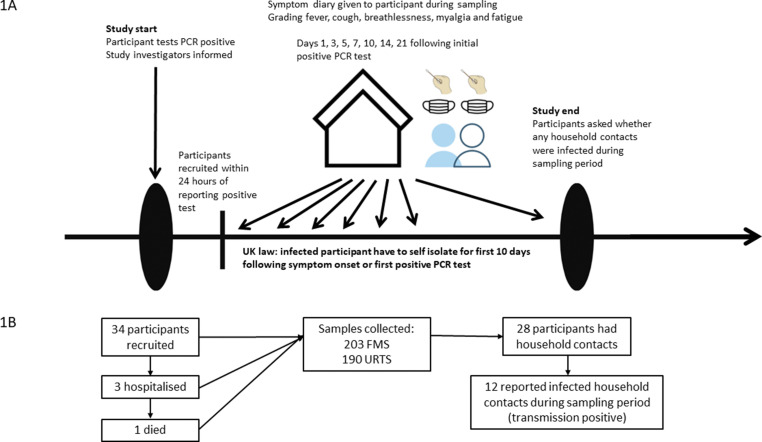
We enrolled healthcare workers (HCWs) who were URTS positive for SARS-CoV-2 at the University Hospitals of Leicester NHS Trust, Leicester, UK, between December 2020 and February 2021. This was in the middle of an alpha wave (December 2020 to March 2021) and when HCWs had just started to be vaccinated in January 2021, when very few had been vaccinated or previously infected [10,11]. HCWs took an URTS if (a) they were exhibiting symptoms of COVID-19; (b) had been in close contact at work or at home with someone with confirmed SARS-CoV-2; or (c) had worked on a hospital ward where there was an unexpected outbreak of COVID-19. We included HCWs who were within 24 hours of a routinely positive SARS-CoV-2 test by URTS and, at time of consent, did not require oxygen therapy (day 0). We then took up to seven serial FMS and URTS for analyses, on days 1, 3, 5, 7, 10, 14, and 21 days of their initial URTS. A timeline of the sampling plan is shown in Fig. 1 (a).
Fig. 1.
(a) Timeline of participant recruitment into the study. (b) Flowchart of participants through the study.
Sampling procedure
Our sampling methods have been described in detail previously [12]. Briefly, each participant wore a duckbilled surgical mask (Integrity 600-3004) containing two 1 × 9 cm three-dimensional printed polyvinyl-alcohol sampling matrix strips placed horizontally across the inside of the mask [13]. Participants were asked to wear the mask for 1 hour on the allocated day at the same time. The study had ethical approval from the West Midlands Research Ethics Committee (REC Reference 20/WM/0153). All participants gave written, informed consent prior to any study procedures.
Sample processing and controls
Detailed description is provided in our previous publication [12]. In brief, for FMS processing, two polyvinyl-alcohol strips were dissolved in a mixture of molecular grade water and QIAamp ACL buffer and underwent RNA extraction using the QIAampl DSP Circulating Nucleic Acid Kit (Qiagen, Hilden, Germany; Cat. no. 61 504). For URTS, the sampled material was first eluted from the swab head into water by vortexing then RNA extracted using RNeasy mini kits (Qiagen, Cat. no. 74 104). For both sample types, target RNA was detected and quantified using the QuantiNova Probe RT-qPCR Kit (Qiagen, Cat. no. 208 356) and a Rotor-Gene Q thermocycler (Qiagen, Cat. no. 9 001 590). Quantification results were normalised to per sampling strip for FMS, and to per 100μL of swab eluate for URTS. Sample positivity was determined with assays directed to the E gene. All positive samples were quantified for genome copy number in a single E gene-directed RT-qPCR run (see previous work for standard curve) [12,14].
Clinical data, outcomes, definitions, and symptom diaries
We collected clinical data on the following: age, gender, ethnicity, and comorbidities, as well as whether participants lived in the same household. Outcome data included household transmission, admission to hospital, or death. During the period of the study, the isolation guidance was for both the infected persons and their household contacts to isolate for a minimum of 10 days following symptom onset or a day 0 positive URTS (whichever came first). Household contacts had free access to one URTS RT-qPCR, which they would request for if they developed COVID-19 symptoms [15].
We defined household transmission within one household if positive SARS-CoV-2 tests in household contacts were reported 2 to 14 days after the day 0 URTS from our study participant for those who did not live alone, and where there were two participants, defining the index as the individual with the earliest onset and excluding the latter participant. Each study participant was also given a symptom diary, whereby they were asked to grade the severity of fever, cough, breathlessness, myalgia, and fatigue on the day that they provided a concomitant FMS and URTS on a 5-point Likert scale.
Statistical analysis
Continuous variables are expressed as median and interquartile range. Categorical variables are displayed as numbers and percentages (%). Pearson's χ2 test and Fisher's exact row test were used to compare categorical variables between groups. Student's t-test and Kruskal-Wallis were used to compare continuous variables between groups depending on the normality of distribution.
We previously found age to be associated with both FMS and URTS RNA VL [12]. Thus, we calculated a priori age adjusted OR for household transmission using two logistic regression models: one for the highest (peak) FMS RNA VL taken from single individual, and another for peak URTS RNA VL. We also assessed the associations between FMS test results and household transmission on days 1 and 3, contingency analyses, together with sensitivity and specificity with positive and negative predictive values for household transmission. Data was analysed using GraphPad Prism (version 9; San Diego, CA, USA), Excel (Microsoft 2010; Redmond, WA, USA), and STATA (version 16.1; College Station, TX, USA). All tests were two-tailed and p less than 0.05 were regarded as significant.
Results
Description of cohort and RNA VL detected
Fig. 1(b) shows the flow of participants through the study. Table 1 shows the demographics of the 34 study participants who were enrolled in this study. The median age of the cohort was 37 (interquartile range 30–45) and most were female (n = 26, 76%). Most study participants were of white ethnicity and did not have any comorbidities; only three had received one dose of the BNT162b2 vaccine, in each case more than a week prior to testing positive for SARS-CoV-2 (11, 14, and 17 days).
Table 1.
Demographics of the cohort
| Variable (n = 34) | Median (IQR) or n (%) |
|---|---|
| Age (y) | 37 (30– 45) |
| Sex (female) | 26 (76) |
| Ethnicity | |
| White | 16 (47) |
| Asian | 15 (44) |
| Black | 3 (9) |
| Comorbidities | |
| Asthma | 1 (3%) |
| T-cell lymphocytic leukaemia | 1 (3%) |
| HIV (well controlled) | 1 (3%) |
| Hypertension | 1 (3%) |
| Vaccination | |
| One dose of Pfizer vaccine (compared to none) | 3 (8) |
| Number of days since vaccination | 14 (11– 17) |
| Clinical symptoms | |
| Symptomatic | 28 (82) |
| Days symptomatic prior to sampling | 2 (0–3) |
| Outcomes | |
| Hospitalised for COVID-19 | 2(6) |
| Died | 1 (3) |
| Household data | |
| More than one person in household | 31 (91) |
| Participants living in the same household | 6 (18); 2 per household |
| Household transmissiona | 12 (46) |
Continuous variables are displayed as number (n) and percentages (%). Categorical variables are denoted as median and IQR.
IQR, interquartile range.
Household transmission is defined as self-reported positive SARS-CoV-2 tests in household contacts 2 to 14 days after the initial positive test for the study participant, after excluding participants who lived alone, and where there were two participants, defining the index as the individual with the earliest onset and excluding the latter participant.
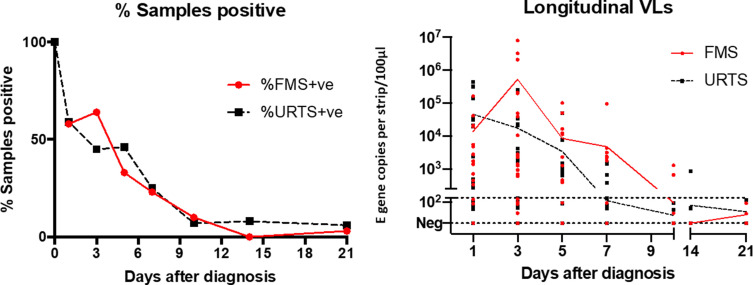
Of the samples, 203 FMS and 190 URT were collected from 34 HCWs; 76% produced one or more positive FMS samples. This was in the middle of an alpha wave (December 2020 to March 2021) and when HCWs had just started to be vaccinated in January 2021, when very few had been vaccinated or previously infected [10,11]. The overall pattern of FMS and URTS positivity and RNA VL are shown in Fig. 2 . Viral RNA detected by FMS ranged over five orders of magnitude (<10 to 7.8 × 106 genome copies/strip). Between day 1 and day 3, FMS RNA VL increased in 12 individuals, while URTS RNA VL declined in 20 (respectively 50% and 80% of available samples), thereafter the overall rate of decline was similar for the two sample types.
Fig. 2.
Proportion of facemask sampling and upper respiratory tract sampling positive samples over 21 days and complete dataset with lines showing daily mean values (biased toward high RNA viral load [VL]). Results from individuals giving negative results throughout were excluded. RNA VL are classified as viral genome copies per strip for FMS or per 100 μL for URTS. The dotted line at 250 genomes indicates the lower limit of quantification. VL, viral load.
Association of demographic and clinical outcomes by RNA VL on FMS and URTS
Of participants, 82% were symptomatic. Of these participants, 29% were recruited the same day they developed symptoms and 75% were recruited within two days of symptom onset (see Supplementary material, Fig. S1). Six individuals reported asymptomatic throughout the 21 days of sampling. Three participants were hospitalised during the study; one study participant died following the provision of one concomitant FMS and URTS sample.
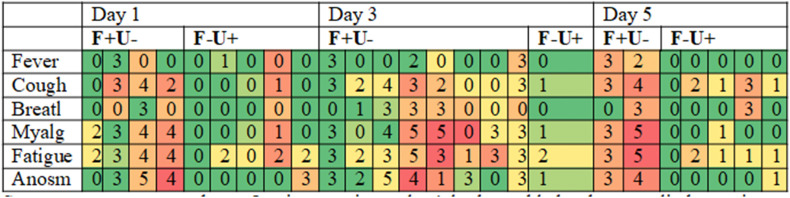
Table 2 shows heat maps of days 1, 3, and 5 symptom diaries associated with the subgroups of participants who were concomitantly FMS RNA VL of >200 and URTS negative (FMS+/URTS-); FMS negative and URTS RNA VL > 200 (FMS-/URTS+). We found that in early infection, a higher severity of symptoms was associated with FMS positivity rather than URTS positivity. On day 1, FMS+/URTS- reported different median total symptom scores compared to those who were FMS-/URTS+ (15 vs. 3, p = 0.04). Combining results for days 3 and 5, participants reported higher median symptom scores in the FMS+/URTS- group compared to the FMS-/URTS + group (15 vs. 3, p = 0.0017). Those who were FMS+/URTS + had a moderate degree of symptom severity. For both FMS and URTS, we found no overall relationship between RNA VL and the presence of clinical symptoms.
Table 2.
Symptom scores related to FMS + ve/URTS –ve and the converse results on days 1, 3, and 5
Symptoms were reported on a 5-point severity scale. A lookup table has been applied to assist comparisons. Each table section refers to individuals with a specific combination of FMS and URTS abbreviating F for FMS and U for URTS.
Anosm, anosmia; Breathl, breathlessness; FMS, facemask sampling; Myalg, myalgia; URTS, upper respiratory tract sampling.
Associations with transmission
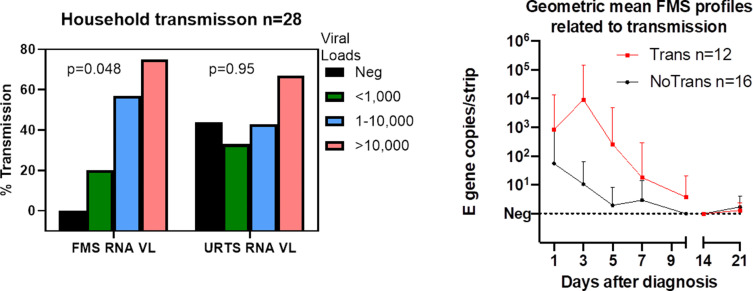
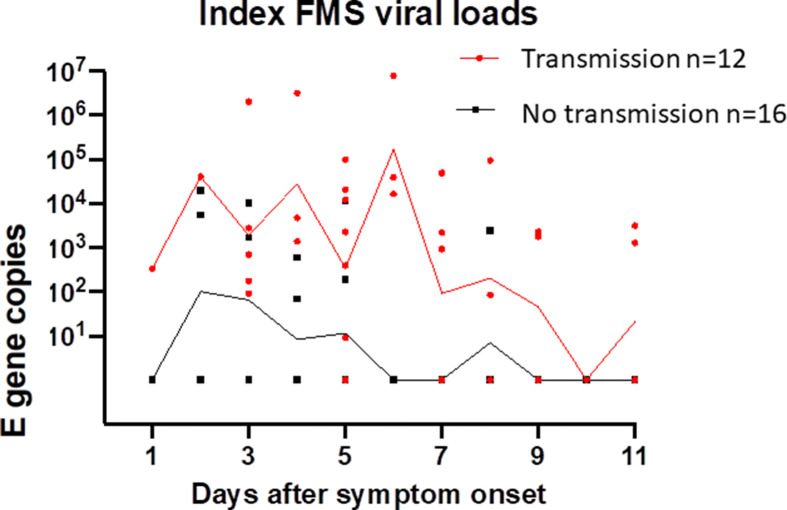
Of participants, 28 reported results of RT-qPCR tests taken by household contacts after their enrolment; 12 reported positive RT-qPCR tests in contacts. None of the participants who were hospitalised reported household transmission. Associations between household transmission and clinical data are shown in the Supplementary material, Table S1. As shown in Fig. 3 (a), we noted an association between peak FMS RNA VL and percentage of participants who reported household transmission, which was not apparent in URTS. In an age-adjusted logistic regression model for household transmission, for every logarithmic increase in peak exhaled viral RNA in a study participant the probability of transmission to household contacts increased by 5-fold and up to 20-fold (age adjusted OR 4.97, 95% CI, 1.20–20.55; p = 0.048). The proportion and strength of longitudinal FMS positive samples for each participant who reported positive household transmission was also higher compared to those who were transmission negative (Fig. 3(b) and Supplementary material, Table S2).
Fig. 3.
a (left): Relationships between peak viral loads and probable household transmission for FMS and URTS. FMS, facemask sampling; TR+, transmission positive; URTS, upper respiratory tract sampling. b (right): Higher and more prolonged FMS positivity associated with household transmission due to infectious participants (red), compared to no household transmission from non-infectious participants (black). E gene copies/strip expressed as geometric means + 95% CIs. Viral load units are classified as viral genome copies per strip for FMS.
We also found that all five participants who gave consistently negative FMS throughout the 21 days of the study were in households assessed to be transmission negative; three out of eight participants who consistently gave negative URTS results from day 1 onwards reported household transmission. For participants who did not produce FMS RNA VL in excess of 1000 copies per strip (excluding individuals who only provided one sample), the negative predictive value (NPV) for household transmission was 89% (95% CI, 57%–99% p = 0.02). Contingency analyses, together with sensitivity, specificity, positive and NPV for association between FMS test results and transmission on day 1 and day 3 are shown in the Supplementary material, Table S3(a).There was strong association between FMS positivity and transmission on day 3. The same analyses applied to the URTS showed no association with transmission (see Supplementary material, Table S3(b)). Since URTS from seven individuals were all negative after day 0, we considered the possibility that their initial tests may have been false positives for infectious virus (perhaps because of transient colonisation of the upper respiratory tract) and repeated the analyses excluding these individuals, with similar findings (see Supplementary material, Table S3; results labelled with ∗). FMS NPV was high following exclusion of the URTS negative individuals. Finally, symptom onset adjusted, rather than day 0 patterns of RNA VL on FMS, are shown in the Supplemental material, Fig. S2, with FMS VL being consistently higher in those who had reported positive household transmission compared to those who did not report transmission.
Discussion
We describe the first real-world study to longitudinally measure exhaled SARS-CoV-2 RNA over the entire course of acute illness. Although our cohort included only 34 participants, we achieved rapid recruitment within 24 hours of diagnosis and were able to determine exhaled RNA VL throughout the course of infection, allowing us to make several novel observations.
We found that exhaled RNA VL is highest in early disease. Previous studies using sampling from modified facemasks have not assessed longitudinal RNA VL kinetics [[5], [6], [7], [8], [9],12]. Our findings are consistent with findings from the Gesundheit II-exhaled breath collector (GII) [16]. Here, higher RNA VL were observed in exhaled breath within those who were sampled once, on day 3 after symptom onset [17,18]. The convenience of FMS allows us to sample participants within their own homes in a simple and efficient manner, thereby allowing us to perform multiple measurements that would have been more challenging with the GII. In contrast to GII, FMS would not be able to discriminate between large respiratory droplets that could drop to surfaces or be deposited in the upper airway and smaller particles that may remain airborne. However, both can transmit infection.
We show that detection of exhaled SARS-CoV-2 RNA was more strongly associated with transmission compared to URTS. In a cohort of participants sampled within six days of symptom onset or less, using a mobile laboratory that drove to peoples' homes, Alsved et al. found that exhaled SARS-CoV-2 RNA had similar findings, but again, because of logistical constraints, only sampled at one point in time [19]. In contrast, Marks et al. found that URTS VL was a strong driver of transmission in 314 patients and 753 of their contacts [20]. Since both FMS and URTS VL are from the respiratory tract, both may be related to transmission but in differing strengths of association.
A controlled human challenge study has demonstrated that lateral flow tests were strongly associated with viable virus from the upper respiratory tract [3]. Despite this, in an analysis linking six sources of empirical evidence from the United Kingdom, Deeks et al. found that rapid antigen tests miss a substantial number of infectious individuals [21]. It may be that despite having low URTS RNA VL (below the threshold for detection by rapid antigen tests), infectious individuals may continue to be exhaling large amounts of virus. Our study supports the hypothesis that if SARS-COV-2 is exhaled in the air it can pose a potential risk of infection to others who may inhale it. Around one fifth (18%) of study participants accounted for the majority of total FMS RNA VL captured in our study, which if linked to individual infectivity, aligns with studies on overdispersion and the predominance of superspreading events in SARS-CoV-2 transmission dynamics [22].
Finally, we note that the presence (or absence) of clinical symptoms in early disease did not relate to RNA VL from FMS/URTS, in line with other studies [3]. FMS could therefore be used to screen asymptomatic or pre-symptomatic individuals [23]. Given the high negative predictive value identified for FMS, our method could also identify those who are SARS-CoV-2 URTS positive but no longer infectious, allowing them to be de-escalated from isolation rooms in hospital, or allow HCWs to return to work without infecting their patients.
Our study had several limitations. Ours was a pilot study, designed to explore the direct measurement of emitted SARS-CoV-2 and to inform sample size calculations for future transmission studies. Household contacts were not directly recruited into the study; sampling, genome sequencing, and serology of index participants and their contacts may have enhanced precision of the assignment of transmission but would have required considerably larger resources. However, all participants in this study were HCWs and experienced in both URTS sampling and the wearing of facemasks; their household contacts at the time of study were bound by UK law to stay at home, and none reported previous SARS-CoV-2 infection. Therefore, the context in which this study was performed offers a relatively well-defined setting enabling assessment of forward SARS-CoV-2 transmission. Indeed, such was the strength of the FMS NPV that mis-assignment of six determinations (three positives and three negatives) would still retain a FMS NPV of 73% on day 3 following an inital positive URTS. We may have also underestimated household transmission if household contacts had been infected but asymptomatic (and thus did not request for URTS) or if symptomatic household contacts became infected following a negative URTS. However, given that most transmission events occur in early infection, the latter appears to be unlikely. Around half of households in our study were transmission positive, which is comparable to existing studies on household transmission [24]. Finally, we did not perform viral culture. Other studies have shown cultivable virus from exhaled breath at high RNA VL, consistent with our conclusions that high FMS RNA VL may be associated with transmission [17].
In conclusion, we found that the majority of exhaled SARS-CoV-2 as measured by FMS is emitted early on in infection; that patients with severe respiratory symptoms may be FMS positive but URTS negative during their acute illness, and FMS may be a better marker of transmission to close contacts than RNA VL captured from the upper respiratory tract. Our results emphasise the importance of reducing exposure to and transmission of airborne SARS-CoV-2 through universal masking, physical distancing, and increased room ventilation.
Transparency declaration
MP reports grants from Sanofi, grants and personal fees from Gilead Sciences, and personal fees from QIAGEN outside the submitted work. All other authors have no conflicts of interest.
This work was supported by funding from the PROTECT COVID-19 National Core Study on transmission and environment, managed by the Health and Safety Executive on behalf of Her Majesty's Government. DP is supported by an NIHR Doctoral Research Fellowship (Award number: NIHR302338). MP is funded by a NIHR Development and Skills Enhancement Award and is supported by NIHR Leicester Biomedical Research Centre (BRC). SS and CW are supported by NIHR Academic Clinical Lectureships. Funding also provided by National Institute for Health and Social Care Research, University of Leicester LD3/MRC Confidence in Concept grant and the UK National Core Study: PROTECT (Transmission and the Environment).
Author contributions
DP, CMW, MP, and MRB conceived the study. DP, SS, SA, and SA recruited the participants. JN, JD, RH, and EF processed the samples within the laboratory. DP and MRB analysed the data. DP wrote the initial draft of the manuscript. All authors were involved in the review and editing that resulted in the final version of the manuscript for publication. MRB acquired financial support for the project leading to the publication.
Acknowledgements
We gratefully acknowledge support from staff and patients of University Hospitals of Leicester in completing this work. We dedicate this study to the participant who died during the conduct of the study from COVID-19, as well as their family. Tylon Smith is acknowledged for his contribution to polyvinyl-alcohol strip production and mask assembly. Finally, we gratefull acknowledge the National Instutite for Health and Social Care Research (NIHR), UK National Core Study (PROTECT) and the University of Leicester for their funding of this work.
Editor: R. Chemaly
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cmi.2022.07.005.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
cmi_figure s1.tif.
cmi_figure s2.tif.
References
- 1.Leung N.H.L., Chu D.K.W., Shiu E.Y.C., Chan K.H., McDevitt J.J., Hau B.J.P., et al. Respiratory virus shedding in exhaled breath and efficacy of face masks. Nat Med. 2020;26:676–680. doi: 10.1038/s41591-020-0843-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rutter H., Parker S., Stahl-Timmins W., Noakes C., Smyth A., Macbeth R., et al. Visualising SARS-CoV-2 transmission routes and mitigations. BMJ. 2021;375 doi: 10.1136/bmj-2021-065312. [DOI] [PubMed] [Google Scholar]
- 3.Killingley B., Mann A.J., Kalinova M., Boyers A., Goonawardane N., Zhou J., et al. Safety, tolerability and viral kinetics during SARS-CoV-2 human challenge in young adults. Nat Med. 2022;28:1031–1041. doi: 10.1038/s41591-022-01780-9. [DOI] [PubMed] [Google Scholar]
- 4.Ma J., Qi X., Chen H., Li X., Zhang Z., Wang H., et al. Coronavirus disease 2019 patients in earlier stages exhaled millions of severe acute respiratory syndrome coronavirus 2 per hour. Clin Infect Dis. 2021;72 doi: 10.1093/cid/ciaa1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sriraman K., Shaikh A., Parikh S., Udupa S., Chatterjee N., Shastri J., et al. Non-invasive adapted N-95 mask sampling captures variation in viral particles expelled by COVID-19 patients: implications in understanding SARS-CoV2 transmission. PLoS One. 2021;16 doi: 10.1371/journal.pone.0249525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smolinska A., Jessop D.S., Pappan K.L., De Saedeleer A., Kang A., Martin A.L., et al. The SARS-CoV-2 viral load in COVID-19 patients is lower on face mask filters than on nasopharyngeal swabs. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92665-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng D.H.L., Sim M.Y., Huang H.H., Sim J.X.Y., Low J.G.H., Lim J.K.S. Feasibility and utility of facemask sampling in the detection of SARS-CoV-2 during an ongoing pandemic. Eur J Clin Microbiol Infect Dis. 2021;40:2489–2496. doi: 10.1007/s10096-021-04302-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen P.Q., Soenksen L.R., Donghia N.M., Angenent-Mari N.M., de Puig H., Huang A., et al. Wearable materials with embedded synthetic biology sensors for biomolecule detection. Nat Biotechnol. 2021;39:1366–1374. doi: 10.1038/s41587-021-00950-3. [DOI] [PubMed] [Google Scholar]
- 9.Kanaujia R., Biswal M., Angrup A., Ray P. Inhale, then exhale: start afresh to diagnose severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) by non-invasive face-mask sampling technique. Clin Microbiol Infect. 2020;26:1701–1702. doi: 10.1016/j.cmi.2020.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Office for National Statistics United Kingdom Government Coronavirus (COVID-19) infection survey technical article: waves and lags of COVID-19 in England, June 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/articles/coronaviruscovid19infectionsurveytechnicalarticle/wavesandlagsofcovid19inenglandjune2021 Available at: Accessed.
- 11.Public Health England . Sage; 2021. SARS-CoV-2 variants of concern and variants under investigation in England; pp. 1–50. [Google Scholar]
- 12.Williams C.M., Pan D., Decker J., Wisniewska A., Fletcher E., Sze S., et al. Exhaled SARS-CoV-2 quantified by face-mask sampling in hospitalised patients with COVID-19. J Infect. 2021;82:253–259. doi: 10.1016/j.jinf.2021.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Taie A., Han X., Williams C.M., Abdulwhhab M., Abbott A.P., Goddard A., et al. 3-D printed polyvinyl alcohol matrix for detection of airborne pathogens in respiratory bacterial infections. Microbiol Res. 2020;241 doi: 10.1016/j.micres.2020.126587. [DOI] [PubMed] [Google Scholar]
- 14.Han M.S., Byun J.H., Cho Y., Rim J.H. RT-PCR for SARS-CoV-2: quantitative versus qualitative. Lancet Infect Dis. 2020;21:165. doi: 10.1016/S1473-3099(20)30424-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.United Kingdom Government Stay at home: guidance for households with possible or confirmed coronavirus (COVID-19) infection. https://www.gov.uk/government/publications/covid-19-stay-at-home-guidance/stay-at-home-guidance-for-households-with-possible-coronavirus-covid-19-infection Available at: Accessed.
- 16.McDevitt J.J., Koutrakis P., Ferguson S.T., Wolfson J.M., Fabian M.P., Martins M., et al. Development and performance evaluation of an exhaled-breath bioaerosol collector for influenza virus. Aerosol Sci Technol. 2013;47:444–451. doi: 10.1080/02786826.2012.762973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Adenaiye O.O., Lai J., Mesquita J., Hong F., Youssefi S., German J., et al. Infected SARS-CoV-2 in exhaled aerosols and efficacy of masks during early mild infection. Clin Infect Dis. 2021:ciab797. doi: 10.1093/cid/ciab797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coleman K.K., Tay D.J.W., Tan K Sen, Ong S.W.X., Than T.S., Koh M.H., et al. Viral load of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in respiratory aerosols emitted by patients with coronavirus disease 2019 (COVID-19) while breathing, talking, and singing. Clin Infect Dis. 2022;74:1722–1728. doi: 10.1093/cid/ciab691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alsved M., Nygren D., Thuresson S., Medstrand P., Fraenkel C., Löndahl J. SARS-CoV-2 in exhaled aerosol participants from covid-19 cases and its association to household transmission. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac202. ciac202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marks M., Millat-Martinez P., Ouchi D., Roberts C.H., Alemany A., Corbacho-Monné M., et al. Transmission of COVID-19 in 282 clusters in Catalonia, Spain: a cohort study. Lancet Infect Dis. 2021;21:629–636. doi: 10.1016/S1473-3099(20)30985-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deeks J.J., Singanayagam A., Houston H., Sitch A.J., Hakki S., Dunning J., et al. SARS-CoV-2 antigen lateral flow tests for detecting infectious people: linked data analysis. BMJ. 2022;376 doi: 10.1136/bmj-2021-066871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen P.Z., Bobrovitz N., Premji Z., Koopmans M., Fisman D.N., Gu F.X. Heterogeneity in transmissibility and shedding SARS-CoV-2 via droplets and aerosols. Elife. 2021;10 doi: 10.7554/eLife.65774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mina B.M.J., Andersen K.G. COVID-19 testing: one size does not fit all. Science. 2021;371:126–127. doi: 10.1126/science.abe9187. [DOI] [PubMed] [Google Scholar]
- 24.Madewell Z.J., Yang Y., Longini I.M., Halloran M.E., Dean N.E. Factors associated with household transmission of SARS-CoV-2: an updated systematic review and meta-analysis. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.22240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.