Background:
Clinical presentation for extrapulmonary tuberculosis (EPTB) in children can be variable and nonspecific, leading to delayed diagnosis, disease and death. We describe the age-specific clinical presentation and identify risk factors for EPTB among children in Pakistan.
Methods:
In 2015–2016 in 4 facilities in Sindh, Pakistan, children were diagnosed with TB either through bacteriologic confirmation or clinical-radiologic criteria. EPTB comprised any form of TB disease that did not involve the lungs. Among children with TB disease, we report demographics, clinical characteristics and symptoms, family medical history and diagnostic test results for children with and without EPTB. We conduct age-specific regression analyses to identify factors associated with an EPTB diagnosis among children age 0–4, 5–9 and 10–14 years.
Results:
A total of 1163 children were diagnosed with TB disease, of which 157 (13.5%) had EPTB. Of those, 46 (29.3%) were 0–4, 53 (33.8%) were 5–9 and 58 (36.9%) were 10–14 years old. Of children with EPTB, the most frequently reported sites were lymph node (113, 72.4%) and abdominal (31, 19.9%). Weight loss was associated with an increased risk of EPTB in the 0–4-year-old (adjusted odds ratio: 2.80, 95% confidence interval: 1.05–7.47) and 10–14-year-old (adjusted odds ratio: 2.79, 95% confidence interval: 1.28–6.07) groups, and the presence of cough was associated with a decreased risk of EPTB.
Conclusions:
This study provides new knowledge about age-specific clinical presentation and risk factors of EPTB in children in Pakistan. Our results can help to optimize clinical algorithms designed to achieve a timely diagnosis in children with EPTB along with improved treatment outcomes.
Keywords: pediatric, epidemiology, diagnosis, extrapulmonary
In 2019, extrapulmonary tuberculosis (EPTB) accounted for about 16% of 7.1 million reported TB cases globally.1 Children are more likely to present with EPTB compared with adults, with younger age and developing immunity contributing to elevated disease risk.2,3 In 2020, 1.1 million children (<15 years old) were estimated to develop TB.4 The global burden of EPTB among children specifically remains unknown.
Challenges to estimating the burden of EPTB include difficulty with bacteriologic confirmation of pediatric disease as well as under-reporting of pediatric cases.5 The pathogenesis of EPTB generally consists of primary lung disease with hematogenous dissemination of the Mycobacterium tuberculosis to other parts of the body.3 Specific barriers for diagnosing EPTB include variable and nonspecific clinical presentation, which present unique diagnostic challenges.6 These lead to delays in diagnosis of EPTB, which in turn contributes to poor treatment outcomes. There is an urgent need to understand which children are at higher risk of EPTB to improve TB diagnosis, prompt treatment initiation and subsequent treatment outcomes.
As a top 30 high TB burden country, Pakistan accounts for 5.8% of all estimated incident TB cases worldwide.4 Additionally, Pakistan has a high number of EPTB cases, which accounts for 20% of all notified TB cases.2 Children in Pakistan are burdened with high levels of acute and chronic malnutrition, which contribute to these high TB burdens.7 A descriptive analysis performed in Pakistan among 3607 children (age 0–14 years) with TB registered nationwide in 2016 found the following frequency of EPTB disease by site: abdominal TB (38.4%), lymphatic TB (22.4%), pleural TB (14.0%), central nervous system (CNS; 6.0%), osteoarticular (3.9%) and site not specified (12.1%).2 Beyond that report, there are limited data in the literature about the clinical presentation of EPTB and risk factors for EPTB in children, by age group.
To fill this gap, we describe the age-specific clinical presentation and risk factors for EPTB among children diagnosed with TB disease in Pakistan.
MATERIALS AND METHODS
Setting, Intervention and Definitions
Between October 2014 and March 2016, 4 public sector health facilities in Jamshoro District, Pakistan, implemented a screening program designed to improve diagnosis of children with TB disease. Children were screened for symptoms and contact history by health workers and were referred to a TB medical officer for a clinical evaluation for TB. Elsewhere, we describe details of the screening procedures used.8–10 Children received a clinical evaluation, including chest radiograph, complete blood count and erythrocyte sedimentation rate, and—if they could produce sputum—additional testing such as Xpert MTB/RIF assay and acid fast bacilli smear. If indicated, abdominal ultrasound, computed tomography and/or fine-needle aspiration/biopsy were performed. Gastric aspirates and induced sputum procedures were not available. During the first quarter of the program (October 2014 to December 2014), audit of the clinical data raised a possibility of overdiagnosis of abdominal TB. We retrained all the project medical officers in the next quarter (January 2015 to March 2015) and put in place a mechanism that required discussion of EPTB diagnosis with the project pediatrician. A random sample of all TB patients diagnosed was also independently validated by the district TB coordinator throughout the remainder of the program. Thus, in this analysis, we include children diagnosed with EPTB after the implementation of quality checks to improve EPTB diagnoses, between April 2015 and March 2016.
A case of TB disease was determined by a physician on review of the clinical, laboratory, radiologic and/or histopathologic test results. A case of EPTB disease was defined as having any form of TB disease that did not involve the lungs. Pulmonary TB (PTB) was defined as having TB disease with lung involvement. A patient with both PTB and EPTB was classified as a case of PTB.11 Children diagnosed with TB were started on treatment per the National TB Program guidelines of Pakistan.12
Study Population and Data Collection
Children were included in this analysis if they were between 0 and 14 years of age. Demographic and clinical characteristics were collected and entered into a custom-built electronic data collection tool.8 Demographic information included sex and age. Clinical characteristics included weight-for-age percentile, recorded using the World Health Organization’s (WHO) growth charts for children 0–2 years old and the U.S. Centers for Disease Control and Prevention’s growth charts for children older than 2 years old.13 We classified each child according to whether their weight-for-age was at or below the fifth percentile, or higher than the fifth percentile. Other clinical characteristics recorded were symptoms—such as cough for 2 weeks or longer, cough duration (characterized as no cough, less than 2 weeks, between 2 and 3 weeks, and greater than 3 weeks), fever lasting 2 or more weeks, and weight loss—and the presence of Bacillé Calmette-Guerin (BCG) vaccination scars. Family medical history included history of TB, family member TB type (EPTB or PTB), family member with TB (mother, father, or other) and whether the family member was sputum positive. Diagnostic test results included chest examination, abdominal examination, lymph node examination, chest radiograph, abdominal ultrasound and histopathology suggestive of TB. Type of TB was characterized as EPTB or PTB. Sites of EPTB disease included abdominal, lymph node, bone, CNS, pleural or other.
Statistical Analysis
We report demographics, symptoms, medical and family history and clinical examination results for children diagnosed with any form of TB disease and compare these characteristics across children diagnosed with PTB and EPTB using χ2 or Fisher exact tests. We report the odds ratios (OR), 95% confidence intervals (CIs) and P values produced from univariable and multivariable analyses for 6 different regression analyses. Model 1 identifies risk factors associated with being diagnosed with EPTB for all children (0–14 years old) who were diagnosed with any form of TB disease. Models 2–4 also identify risk factors for EPTB for children in each of the following age groups: 0–4-, 5–9-, and 10–14-year-olds. Models 5 and 6 aim to identify risk factors associated with being diagnosed with abdominal and lymph node TB, respectively, for all children (0–14 years old) who were diagnosed with EPTB. These EPTB subtypes were chosen as they are known to be the most prevalent in children in Pakistan.2
All univariable analyses assessed the associations of gender, age, weight percentile, cough, cough duration, fever, weight loss, presence of a BCG scar and family history of TB with the specific outcome. Multivariable analyses were restricted to only the following variables being eligible for potential inclusion: cough, fever and weight loss were eligible for models 1–4; age group, cough, fever and weight loss were eligible for models 5 and 6. Backward selection was used to determine inclusion of these potential variables in the final multivariable models; those with a P value <0.05 were retained in the final model. Among children with EPTB, we also assessed the percentage that had EPTB at 6 different sites (abdomen, lymph node, bone, CNS, pleural and other) by age group. Analyses were completed in SAS version 9.4 (SAS Institute Inc., Cary, NC).
Ethics
Ethics review was conducted by the Institutional Review Board of Interactive Research and Development, Karachi, Pakistan, for the original screening study. Verbal informed consent was obtained from all children’s guardians as well as from children over the age of 7. The Harvard Medical School Institutional Review Board determined analysis of the deidentified dataset to be exempt from review.
RESULTS
A total of 1166 children were diagnosed with TB disease during the intervention period. Of those, the type of TB (PTB or EPTB) was reported in 1163 (99.7%) children; 1006 (86.5%) had PTB while 157 (13.5%) had EPTB. Of those with EPTB, 46 (29.3%) were 0–4, 53 (33.8%) were 5–9 and 58 (36.9%) were 10–14 years old (Table 1). This age breakdown was significantly different from that observed for children with PTB, in which 519 (51.6%) were 0–4, 293 (29.1%) were 5–9 and 194 (19.3%) were 10–14 years old (P < 0.001). Compared with children with PTB, less children with EPTB were under the 5th weight percentile (86.3% vs. 57.2%; P < 0.001), reported cough (98.1% vs. 78.8%; P < 0.001), had a family history of TB (94.7% vs. 88.5%; P = 0.003) and had a chest radiograph suggestive of TB disease (98.1% vs. 26.6%; P < 0.001). On the contrary, compared with children with PTB, more children with EPTB reported a fever (85.1% vs. 92.4%; P = 0.014) or weight loss (75.2% vs. 87.1%; P = 0.001).
TABLE 1.
Demographics, Clinical Characteristics and Symptoms, Family Medical History, and Diagnostic Results of Children With Pulmonary and Extrapulmonary Tuberculosis (N = 1163)
| Characteristics | Screening Group (N = 1163) | |||
|---|---|---|---|---|
| Total Children With TB N = 1163 (100%) (n, %) | Children With EPTB N = 157 (13.5%) (n, %) | Children With PTB N = 1006 (86.5%) (n, %) | P Value | |
| Demographic information | ||||
| Female | 499 (42.9) | 73 (46.5) | 426 (42.4) | 0.328 |
| Age | ||||
| 0–4 | 565 (48.6) | 46 (29.3) | 519 (51.6) | <0.001 |
| 5–9 | 346 (29.8) | 53 (33.8) | 293 (29.1) | |
| 10–14 | 252 (21.7) | 58 (36.9) | 194 (19.3) | |
| Clinical characteristics and symptoms | ||||
| Weight percentile <5th (N = 1150) | 948 (81.5) | 87 (57.2) | 861 (86.3) | <0.001 |
| Cough (N = 1122) | 1072 (92.2) | 115 (78.8) | 957 (98.1) | <0.001 |
| Cough duration (N = 1066) | ||||
| No cough | 86 (7.4) | 56 (50.0) | 30 (3.1) | <0.001 |
| <2 wk | 90 (7.7) | 12 (10.7) | 78 (8.2) | |
| 2–3 wk | 289 (24.8) | 19 (17.0) | 270 (28.3) | |
| >3 wk | 601 (51.7) | 25 (22.3) | 576 (60.4) | |
| Fever (N = 1162) | 1000 (86.0) | 145 (92.4) | 855 (85.1) | 0.014 |
| Weight loss (N = 1158) | 889 (76.4) | 135 (87.1) | 754 (75.2) | 0.001 |
| Presence of a BCG scar (N = 1056) | 741 (63.7) | 91 (65.5) | 650 (70.9) | 0.194 |
| Family medical history | ||||
| Family history of TB | 1092 (93.9) | 139 (88.5) | 953 (94.7) | 0.003 |
| Family TB type EPTB (N = 1082) | 1078 (92.7) | 133 (97.1) | 945 (100.0) | N/A |
| Family member with TB (N = 1090) | ||||
| Mother | 1043 (89.7) | 125 (89.9) | 918 (96.5) | <0.001 |
| Father | 3 (0.3) | 0 (0) | 3 (0.3) | |
| Other | 44 (3.8) | 14 (10.1) | 30 (3.2) | |
| Family member with sputum positive TB (N = 1091) | 1041 (89.5) | 129 (92.8) | 912 (95.8) | 0.115 |
| Diagnostic test results | ||||
| Chest examination suggestive of TB (N = 1105) | 992 (85.3) | 39 (27.9) | 953 (98.8) | <0.001 |
| Abdominal examination suggestive of TB (N = 1105) | 60 (5.2) | 38 (27.1) | 22 (2.3) | <0.001 |
| Lymph node examination suggestive of TB (N = 1105) | 128 (11.0) | 95 (67.9) | 33 (3.4) | <0.001 |
| Chest radiograph suggestive of TB (N = 1145) | 1021 (87.8) | 38 (26.6) | 983 (98.1) | <0.001 |
| Ultrasound abdomen suggestive of TB (N = 98) | 94 (8.1) | 39 (97.5) | 55 (94.8) | 0.643* |
| Histopathology suggestive of TB (N = 19) | 19 (1.6) | 18 (100.0) | 1 (100.0) | N/A |
Fisher’s exact text used; otherwise, χ2 test used.
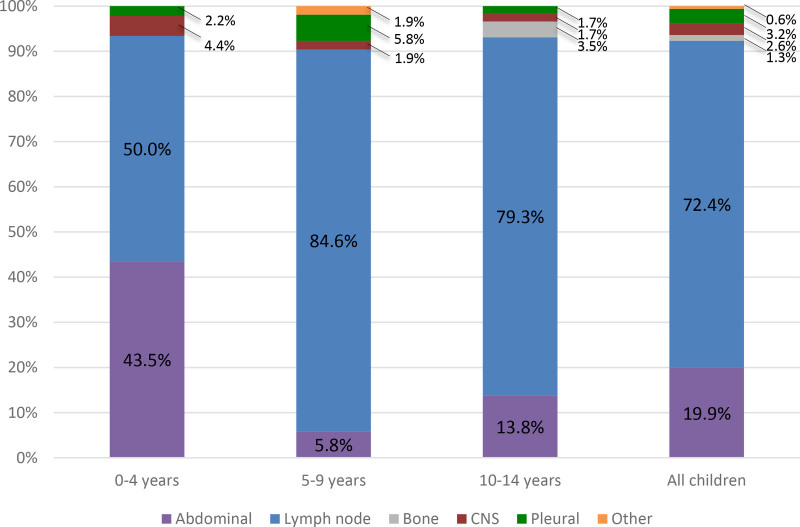
Among children with EPTB, the most frequently reported sites were the abdomen (19.9% overall; 43.5%, 5.8%, and 13.8% in children 0–4, 5–9 and 10–14 years old, respectively) and lymph nodes (72.4% overall; 50.0%, 84.6% and 79.3% in children 0–4, 5–9 and 10–14 years old, respectively; Fig. 1). The site of disease was not reported for 1 child with EPTB.
FIGURE 1.
Age-specific percentages of site of EPTB disease in children (n = 156).
In univariable regression analysis, we observed that increasing age, presence of fever and weight loss were all associated with increased odds of EPTB disease in children 0–14 years old who were diagnosed with TB (Table 2). Weight below the 5th percentile, presence of cough, and a family history of TB were all associated with lower odds of EPTB disease. In multivariable analysis, cough was associated with lower odds of having EPTB disease (OR: 0.18, 95% CI: 0.14–0.24; P < 0.001) while weight loss was associated with higher odds of EPTB (OR: 2.02, 95% CI: 1.29–3.14; P = 0.002).
TABLE 2.
Risk Factors for EPTB in Children 0–14 Years Old With TB Disease (n = 1163)
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Female | 1.16 (0.86–1.55) | 0.328 | ||
| Age | ||||
| 0–4 | Ref | Ref | ||
| 5–9 | 1.88 (1.30–2.73) | <0.001 | ||
| 10–14 | 2.83 (1.98–4.04) | <0.001 | ||
| Weight percentile <5th | 0.29 (0.22–0.38) | <0.001 | ||
| Cough | 0.17 (0.13–0.23) | <0.001 | 0.18 (0.14–0.24) | <0.001 |
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 0.20 (0.12–0.35) | <0.001 | ||
| 2–3 wk | 0.10 (0.06–0.16) | <0.001 | ||
| >3 wk | 0.06 (0.04–0.10) | <0.001 | ||
| Fever | 1.96 (1.11–3.44) | 0.020 | ||
| Weight loss | 2.04 (1.30–3.20) | 0.002 | 2.02 (1.29–3.14) | 0.002 |
| Presence of a BCG scar | 0.81 (0.58–1.11) | 0.192 | ||
| Family history of TB | 0.50 (0.33–0.77) | 0.002 | ||
Across univariable models for all 3 separate age groups, weight below the 5th percentile, presence of cough and increasing cough duration was significantly associated with lower odds of EPTB (Table 3). Additionally, in the 0–4- and 10–14-year-old models, weight loss was associated with higher odds of EPTB; and in the 0–4-year-old model, the presence of BCG scars and family history of TB also associated with higher odds of EPTB. In multivariable analyses, cough was associated with lower odds of EPTB in children 0–4 (OR: 0.12, 95% CI: 0.07–0.19; P < 0.001), 5–9 [OR: 0.22 (95% CI: 0.13–0.39); P < 0.001] and 10–14 (OR: 0.25, 95% CI: 0.18–0.35; P < 0.001). In children 0–4 and 10–14, weight loss was associated with higher odds of EPTB (OR: 2.80, 95% CI: 1.05–7.47; P = 0.039 and OR: 2.79, 95% CI: 1.28–6.07; P = 0.010, respectively).
TABLE 3.
Age Group-specific Risk Factors for EPTB in Children 0–4, 5–9 and 10–14 Years Old Who Were Diagnosed With TB
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| 0–4-year-old model (n = 565) | ||||
| Female | 0.94 (0.53–1.65) | 0.820 | ||
| Weight-for-age <5th percentile | 0.23 (0.13–0.41) | <0.001 | ||
| Cough | 0.11 (0.07–0.19) | <0.001 | 0.12 (0.07–0.19) | <0.001 |
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 0.20 (0.07–0.57) | 0.002 | ||
| 2–3 wk | 0.12 (0.05–0.28) | <0.001 | ||
| >3 wk | 0.06 (0.02–0.13) | <0.001 | ||
| Fever | 6.89 (0.96–49.2) | 0.055 | ||
| Weight loss | 3.04 (1.11–8.33) | 0.031 | 2.80 (1.05–7.47) | 0.039 |
| BCG scars | 0.53 (0.29–0.97) | 0.040 | ||
| Family history of TB | 0.31 (0.16–0.59) | <0.001 | ||
| 5–9-year-old model (n = 346) | ||||
| Female | 0.88 (0.53–1.47) | 0.628 | ||
| Weight percentile <5th | 0.24 (0.15–0.39) | <0.001 | ||
| Cough | 0.22 (0.13–0.39) | <0.001 | 0.22 (0.13–0.39) | <0.001 |
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 0.17 (0.06–0.49) | 0.001 | ||
| 2–3 wk | 0.10 (0.05–0.20) | <0.001 | ||
| >3 wk | 0.04 (0.02–0.10) | <0.001 | ||
| Fever | 1.93 (0.73–5.11) | 0.184 | ||
| Weight loss | 1.37 (0.70–2.67) | 0.359 | ||
| BCG scars | 1.08 (0.61–1.90) | 0.805 | ||
| Family history of TB | 0.48 (0.20–1.13) | 0.091 | ||
| 10–14-year-old model (n = 252) | ||||
| Female | 1.47 (0.93–2.35) | 0.102 | ||
| Weight percentile <5th | 0.35 (0.23–0.55) | <0.001 | ||
| Cough | 0.24 (0.16–0.34) | <0.001 | 0.25 (0.18–0.35) | <0.001 |
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 0.35 (0.16–0.76) | 0.008 | ||
| 2–3 wk | 0.12 (0.05–0.29) | <0.001 | ||
| >3 wk | 0.12 (0.07–0.22) | <0.001 | ||
| Fever | 1.38 (0.67–2.81) | 0.383 | ||
| Weight loss | 2.50 (1.19–5.23) | 0.015 | 2.79 (1.28–6.07) | 0.010 |
| BCG scars | 0.71 (0.43–1.18) | 0.183 | ||
| Family history of TB | 0.86 (0.39–1.90) | 0.717 | ||
In multivariable analysis in the cohort of children with EPTB, compared with children age 0–4 years, older age groups [age 5–9 (OR: 0.12, 95% CI: 0.04–0.37; P < 0.001) and 10–14 (OR: 0.30, 95% CI: 0.15–0.60; P < 0.001)] were associated with lower odds of abdominal TB disease, while the presence of cough (OR: 3.04, 95% CI: 1.04–8.91; P = 0.043) was associated with higher odds of abdominal TB disease (Table 4). Compared with children age 0–4 years, older age groups [age 5–9 (OR: 1.69, 95% CI: 1.24–2.31; P < 0.001) and 10–14 (OR: 1.59; 95% CI: 1.6–2.18; P < 0.004)] were associated with higher odds of lymph node TB.
TABLE 4.
Logistic Regression Results to Identify Risk Factors for Abdominal and Lymph Node TB in Children Diagnosed With EPTB
| Characteristics | Univariable Analysis | Multivariable Analysis | ||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Abdominal TB model (n = 156) | ||||
| Female | 0.74 (0.39–1.41) | 0.358 | ||
| Age | ||||
| 0–4 | Ref | Ref | Ref | Ref |
| 5–9 | 0.13 (0.04–0.42) | <0.001 | 0.12 (0.04–0.37) | <0.001 |
| 10–14 | 0.32 (0.15–0.65) | 0.002 | 0.30 (0.15–0.60) | <0.001 |
| Weight percentile <5th | 6.87 (2.18–21.60) | 0.001 | ||
| Cough | 2.54 (0.83–7.80) | 0.104 | 3.04 (1.04–8.91) | 0.043 |
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 18.33 (4.44–75.70) | <0.001 | ||
| 2–3 wk | 13.03 (3.08–55.02) | <0.001 | ||
| >3 wk | 9.90 (2.31–42.52) | 0.002 | ||
| Fever | 2.50 (0.37–16.77) | 0.345 | ||
| Weight loss | 2.16 (0.56–8.38) | 0.264 | ||
| BCG scars | 0.06 (0.02–0.19) | <0.001 | ||
| Family history of TB | 1.89 (0.49–7.27) | 0.354 | ||
| Lymph node TB model (n = 156) | ||||
| Female | 1.11 (0.91–1.34) | 0.303 | ||
| Age | ||||
| 0–4 | Ref | Ref | Ref | Ref |
| 5–9 | 1.69 (1.24–2.31) | <0.001 | 1.69 (1.24–2.31) | <0.001 |
| 10–14 | 1.59 (1.16–2.18) | 0.004 | 1.59 (1.16–2.18) | 0.004 |
| Weight percentile <5th | 0.67 (0.55–0.81) | <0.001 | ||
| Cough | 1.05 (0.80–1.37) | 0.729 | ||
| Cough duration | ||||
| No cough | Ref | Ref | ||
| <2 wk | 0.36 (0.16–0.80) | 0.013 | ||
| 2–3 wk | 0.40 (0.22–0.72) | 0.002 | ||
| >3 wk | 0.69 (0.51–0.94) | 0.017 | ||
| Fever | 0.86 (0.65–1.13) | 0.273 | ||
| Weight loss | 1.05 (0.77–1.42) | 0.778 | ||
| BCG scars | 2.51 (1.70–3.70) | <0.001 | ||
| Family history of TB | 0.85 (0.68–1.08) | 0.178 | ||
DISCUSSION
Our study highlights the variable clinical presentation and risk factors for children with EPTB by age group. For clinical presentation, we found that lymph node TB was the most commonly reported site of EPTB for children in all groups, followed by abdominal TB. In the age 5–9 group, abdominal TB and pleural TB had similar percentages (5.8%). Our findings are consistent with prior literature on EPTB in children, as lymph node TB is the most common form of extrathoracic TB in children.3,14 Our findings are also consistent with a prior study of EPTB in Pakistan, which found that abdominal TB and lymphatic TB were the most common clinical manifestations of EPTB in children.2
We found that weight loss was associated with increased risk of EPTB for all age groups (except age 5–9). Of note, the symptom of weight loss was associated with EPTB in our study, while weight percentile was not associated with EPTB. Since 81.5% of children in our cohort had a weight percentile <5th percentile, one explanation for this finding is that if the children experienced weight loss, it was less pronounced given that it made up a small absolute amount, though a significant proportion of their initial weight. In all age groups, cough was associated with decreased risk of EPTB. This is consistent with our definition of EPTB as any form of TB disease that did not involve the lungs. Prior studies have evaluated risk factors for EPTB in children. In the low TB burden setting of Spain, EPTB in children was found to be associated with immigrant status, immune disorders and drug resistance.6 In the high TB burden setting of China, age less than 5 years and female sex were associated with extrathoracic TB.15
In terms of the risk factors for subtypes of EPTB, our study found that cough was associated with an increased risk of abdominal TB, while ages 5–9 and 10–14 was associated with a decreased risk of abdominal TB. Our finding that, compared with younger children, those children ages 5–9 and 10–14 have a lower risk of abdominal TB is a unique finding, given that prior studies in Pakistan have not examined the presentation of EPTB by age group in children.2 For lymph node TB, our study found that ages 5–9 and 10–14 were associated with an increased risk of lymph node TB. This is consistent with findings from a study performed in Ethiopia, another high TB burden country, which reported a higher prevalence of lymph node TB in older children (5–14 years), compared with children under 5.16 Hypotheses for increased risk of lymph node TB in older children include age-specific pathophysiology and missed diagnoses of EPTB in younger children.3
Overall, the proportion of EPTB in our cohort of pediatric TB cases was 13.5%. This is on the lower end of the range reported in prior studies, which found that the proportion of EPTB cases ranged between 13% and 50% from both high and low TB burden settings.2,6,17,18 Compared with children with PTB, children with EPTB reported significantly more fever or weight loss. Prior literature supports the association of fever and weight loss with EPTB in children.19 We did not find significant variations in gender between children with EPTB and PTB. The role of malnutrition on the clinical presentation of EPTB remains unclear, particularly as malnutrition is a key driver of TB, but TB also leads to malnutrition.2 There also may also be geographic differences in clinical presentation of TB, as there are different percentages of extrapulmonary TB reported across the WHO regions.1
Strengths of our study include analysis of a prospectively collected data set with comprehensive information on demographics, symptoms, medical and family history and clinical examination results for children diagnosed with EPTB in a high burden TB country. An additional strength is that clinicians refined their diagnostic algorithm and underwent quality assurance measures when diagnosing children with TB.8
Limitations to our study include the inability to assess all variables in the multivariable analysis when broken down by age group. However, it is important to note that our sample size is relatively large in relation to overall EPTB literature. We may have underestimated the number of EPTB diagnoses due to the limitations of classification of EPTB. We were unable to assess the impact of HIV status, immunocompromised status or disseminated disease for our cohort as this information was not collected in the data set.
Several challenges exist in relation to diagnosis and characterization of childhood EPTB. There are diagnostic challenges, given various and nonspecific clinical manifestations and difficulties of culture diagnosis in children. In addition, there is a broad differential for lymphadenopathy and other EPTB findings, including malignancy and other alternative diagnoses.20 Given that EPTB contributes less to transmission than PTB, EPTB may receive less attention when designing TB case-finding interventions. In addition, EPTB is characterized in various ways in different studies. In children, extrapulmonary TB can also be referred to as “extrathoracic” tuberculosis, which refers to disease outside of the chest cavity that excludes miliary TB, pleural effusion and mediastinal lymphadenopathy.3 In addition, there are studies that define children with both PTB and EPTB as cases of EPTB,6 and others that characterize both PTB and EPTB as disseminated TB.21 Our study characterized EPTB according to the WHO definition, which may explain the lower proportion of EPTB cases in this cohort.11
Our study adds important knowledge about age-specific clinical presentation and risk factors for EPTB disease in children in Pakistan. Our results can be used to optimize clinical algorithms to ensure that children with EPTB receive a timely diagnosis and have successful outcomes. It is important to recognize fever, weight loss, cervical lymphadenopathy and abdominal pain as presenting signs of EPTB. Further studies are needed to evaluate the risk of EPTB disease by individual age and determine clinical rationale for high rates of lymph node and abdominal TB in children.
ACKNOWLEDGMENTS
The authors acknowledge the TB Program of Sindh Province, participating facilities and medical and project personnel involved in the implementation of this intervention.
Footnotes
This project was supported through Stop TB Partnership’s TB REACH initiative, the William F. Milton Fund at Harvard University (M.B.B.), the Center for Global Health Delivery at Harvard Medical School (M.B.B. and M.C.B.), the Dubai Harvard Medical Foundation for Medical Research (M.B.B. and M.C.B.), and the National Institutes of Allergy and Infectious Diseases at the National Institutes of Health (K01-AI151083 to M.B.B. and T32-AI007433 to M.M.D.). TB REACH is generously supported by Global Affairs Canada. This manuscript’s contents are solely the responsibility of the authors and do not necessarily represent the official views of these organizations.
The authors have no conflicts of interest to disclose.
M. M. Dubois, M. B. Brooks, M. C. Becerra and H. Hussain contributed equally to this study.
Contributor Information
Melanie M. Dubois, Email: Melanie.Dubois@childrens.harvard.edu.
Meredith B. Brooks, Email: Meredith_Brooks@hms.harvard.edu.
Amyn A. Malik, Email: amyn.malik@ird.global.
Sara Siddiqui, Email: sarsid@gmail.com.
Junaid F. Ahmed, Email: ahmedjunaid921@gmail.com.
Maria Jaswal, Email: mariaraufjaswal@gmail.com.
Farhana Amanullah, Email: farhana.maqbool@ird.global.
Hamidah Hussain, Email: hamidah.hussain@ird.global.
REFERENCES
- 1.World Health Organization. Global Tuberculosis Report 2020. Published online 2020. Available at: https://www.who.int/publications/i/item/9789240013131. Accessed October 30, 2020.
- 2.Tahseen S, Khanzada FM, Baloch AQ, et al. Extrapulmonary tuberculosis in Pakistan-a nation-wide multicenter retrospective study. PLoS One. 2020;15:e0232134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Starke JR, Donald PR, eds. Handbook of Child and Adolescent TB. Oxford University Press; 2016. [Google Scholar]
- 4.World Health Organization. Global Tuberculosis Report 2021. Published online 2021. Available at: https://www.who.int/publications/i/item/9789240037021. Accessed October 23, 2021.
- 5.Seddon JA, Jenkins HE, Liu L, et al. Counting children with tuberculosis: why numbers matter. Int J Tuberc Lung Dis. 2015;19(Suppl 1):9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santiago-García B, Blázquez-Gamero D, Baquero-Artigao F, et al. ; EREMITA Study Group. Pediatric extrapulmonary tuberculosis: clinical spectrum, risk factors and diagnostic challenges in a low prevalence region. Pediatr Infect Dis J. 2016;35:1175–1181. [DOI] [PubMed] [Google Scholar]
- 7.UNICEF Pakistan. National Nutrition Survey 2018: Key Findings Report. Published online 2018. Available at: https://www.unicef.org/pakistan/media/1951/file/Final%20Key%20Findings%20Report%202019.pdf. Accessed August 10, 2020.
- 8.Malik AA, Amanullah F, Codlin AJ, et al. Improving childhood tuberculosis detection and treatment through facility-based screening in rural Pakistan. Int J Tuberc Lung Dis. 2018;22:851–857. [DOI] [PubMed] [Google Scholar]
- 9.Malik AA, Hussain H, Creswell J, et al. The impact of funding on childhood TB case detection in Pakistan. Trop Med Infect Dis. 2019;4:E146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brooks MB, Dubois MM, Malik AA, et al. Age-specific effectiveness of a tuberculosis screening intervention in children. PLoS One. 2022;17:e0264216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.World Health Organization. Treatment of Tuberculosis Guidelines: Fourth Edition. Published online 2010. Available at: https://apps.who.int/iris/handle/10665/44165. Accessed October 23, 2021. [PubMed] [Google Scholar]
- 12.National TB Control Program, Pakistan. Doctor’s Desk Guide-Management of Childhood Tuberculosis Pakistan. Published online 2017. Available at: http://ntp.gov.pk/ntp-old/uploads/Doctors_Desk_Guide_Childhood_TB.pdf. Accessed October 23, 2021.
- 13.Centers for Disease Control and Prevention. National Center for Health Statistics. Growth charts. Published online 2010. Available at: https://www.cdc.gov/growthcharts/who_charts.htm#The%20WHO%20Growth%20Charts. Accessed November 12, 2020.
- 14.Marais BJ, Wright CA, Schaaf HS, et al. Tuberculous lymphadenitis as a cause of persistent cervical lymphadenopathy in children from a tuberculosis-endemic area. Pediatr Infect Dis J. 2006;25:142–146. [DOI] [PubMed] [Google Scholar]
- 15.Pan Y, Yang Z, Liu R, et al. Host and microbial predictors of childhood extrathoracic tuberculosis and tuberculosis meningitis. Pediatr Infect Dis J. 2015;34:1289–1295. [DOI] [PubMed] [Google Scholar]
- 16.Ramos JM, Pérez-Butragueño M, Tesfamariam A, et al. Comparing tuberculosis in children aged under 5 versus 5 to 14 years old in a rural hospital in southern Ethiopia: an 18-year retrospective cross-sectional study. BMC Public Health. 2019;19:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oshi DC, Chukwu JN, Nwafor CC, et al. Does intensified case finding increase tuberculosis case notification among children in resource-poor settings? A report from Nigeria. Int J Mycobacteriol. 2016;5:44–50. [DOI] [PubMed] [Google Scholar]
- 18.Laghari M, Sulaiman SAS, Khan AH, et al. Epidemiology of tuberculosis and treatment outcomes among children in Pakistan: a 5 year retrospective study. PeerJ. 2018;6:e5253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lal SB, Bolia R, Menon JV, et al. Abdominal tuberculosis in children: a real-world experience of 218 cases from an endemic region. JGH Open. 2020;4:215–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakoor S, Mir F, Hasan R. Common alternative diagnoses among a pediatric hospital-based cohort evaluated for tuberculosis in Karachi, Pakistan: the need for facilitated referral in tuberculosis clinics. Int J Mycobacteriol. 2019;8:42–47. [DOI] [PubMed] [Google Scholar]
- 21.Aygun D, Akcakaya N, Cokugras H, et al. Evaluation of clinical and laboratory characteristics of children with pulmonary and extrapulmonary tuberculosis. Medicina (Kaunas). 2019;55:E428. [DOI] [PMC free article] [PubMed] [Google Scholar]