Abstract
Background
Intraductal papillary mucinous neoplasms (IPMNs) represent a unique opportunity to treat and prevent a curable neoplasm before it has the chance to progress to incurable cancer. This prospect, however, has to be balanced with the real risk of over treating patients with lesions that would, in fact, never progress during the life of the patient.
Purpose
Informed clinical decisions in the treatment of IPMNs are first and foremost based on a deep understanding of the pathology of these lesions.
Conclusions
Here we review the pathology of IPMNs, with an emphasis on the clinical relevance of the important features that characterize these lesions.
Keywords: Intraductal papillary mucinous neoplasm, Intraductal oncocytic papillary neoplasm, Intraductal tubulopapillary neoplasm, Pathology, Pancreatic cancer, Pancreas cancer
Introduction
Our understanding of the pathology of intraductal papillary mucinous neoplasms (IPMNs) has evolved rapidly, and it is worth-while to review this history. It is hard to imagine, but in the 1970s, all cystic tumors of the pancreas were lumped together [1]. Entirely benign serous cystadenomas were grouped with precancerous mucin-producing neoplasms [1]. Then, in 1978, two groups, Compagno and Oertel at the Armed Forces Institute of Pathology and Hodgkinson and colleagues at the Mayo Clinic, separated mucin-producing neoplasms of the pancreas from serous cystadenomas [2–4]. Four years later, investigators from Japan, led by K. Ohhashi and colleagues, described what we recognize today as IPMNs, and in the years that followed, G. Zamboni and others clearly separated IPMNs from mucinous cystic neoplasms (MCNs) [5, 6]. It is now recognized that MCNs are defined by the presence of ovarian-type stroma, while IPMNs lack ovarian-type stroma and involve the pancreatic duct system [7–9]. The separation of IPMNs from the other cyst-forming neoplasms of the pancreas opened the doors to detailed studies of the gross, microscopic, and genetic features of these neoplasms. As a result, more recently, entities that were previously classified as variants of IPMN, such as intraductal oncocytic papillary neoplasms (IOPNs) and intraductal tubulopapillary neoplasms (ITPNs), are now recognized as distinctive neoplasms by the World Health Organization (WHO) classification scheme.
An integrated understanding of IPMNs, IOPNs, and ITPNs, one that incorporates gross, microscopic, and genetic features is now emerging. This new understanding has improved our ability to preoperatively classify cystic lesions of the pancreas and to prioritize lesions that are most likely to require surgical intervention.
Gross appearance
While the first IPMNs to be recognized were large lesions that diffusely involved the main pancreatic duct, over time, it became clear that some IPMNs predominantly involve the main pancreatic duct, while others predominantly involve branches of the main duct (Figs. 1, 2, and 3) [8–12]. Not surprisingly, IPMNs that involve the main pancreatic duct are designated “main-duct” IPMNs, those that are limited to branches off of the main duct are designated “branch-duct” IPMNs, and those that involve both the main and side-branches are designated “mixed” IPMNs.
Fig. 1.
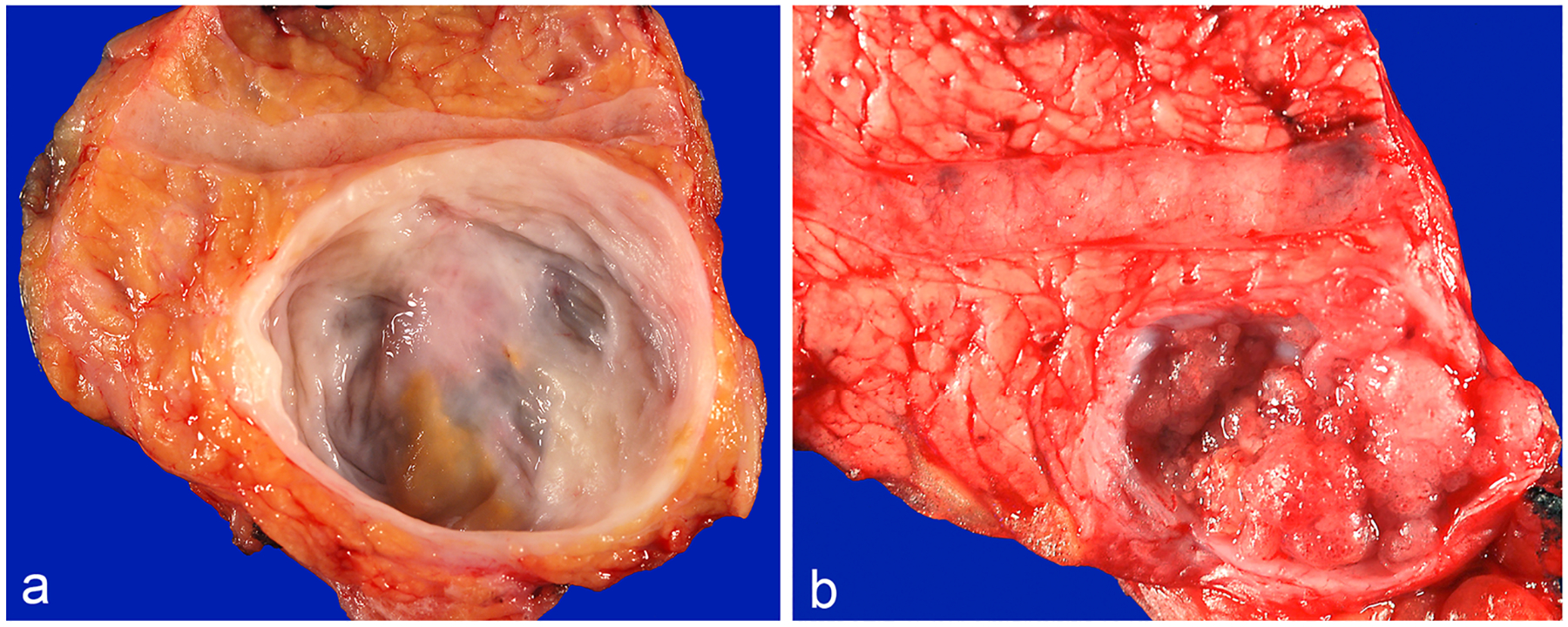
a An example of IPMN arising in a branch-duct without a mural nodule. b Branch-duct IPMN with a mural nodule
Fig. 2.
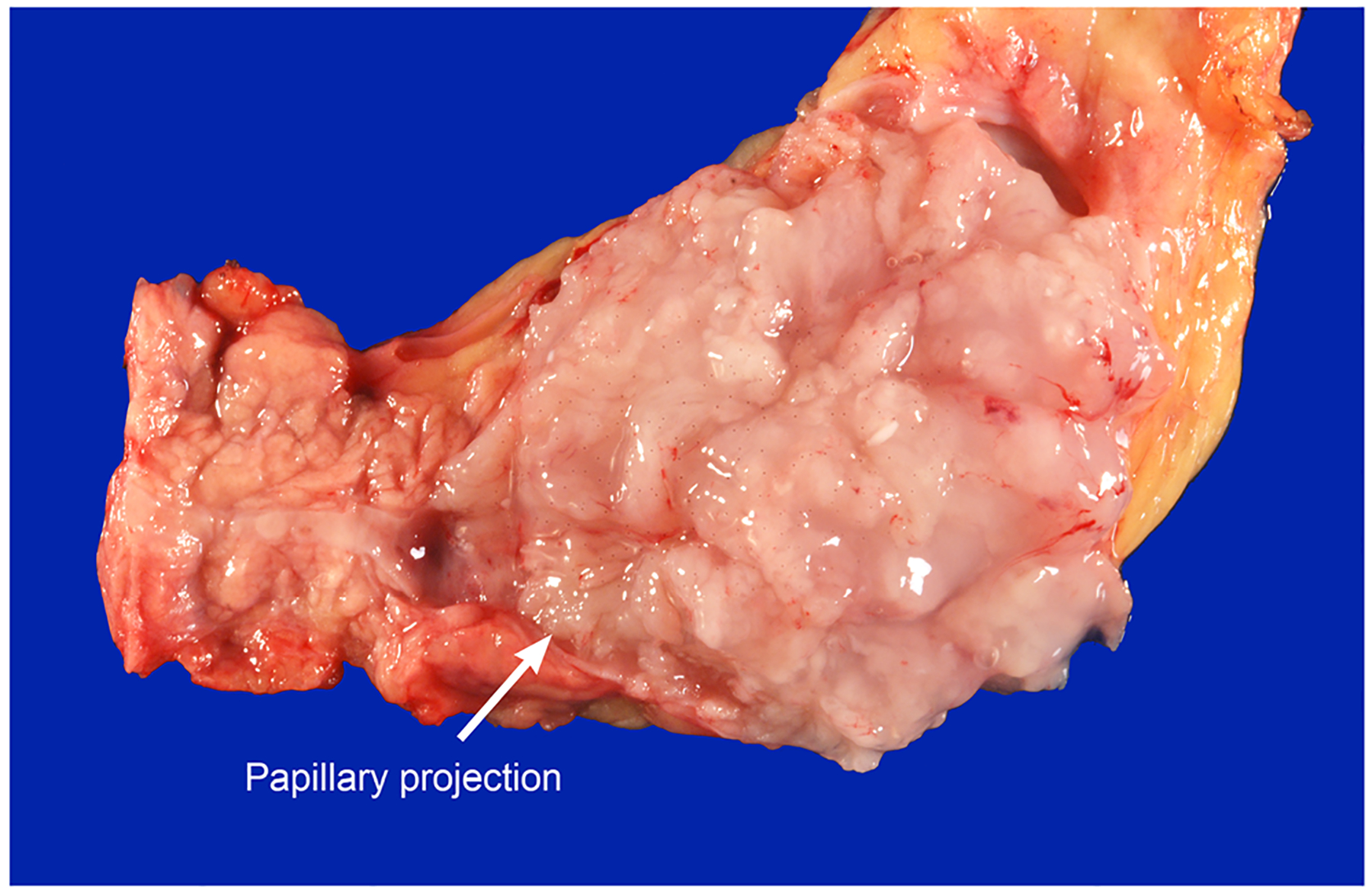
Main-duct IPMN with an associated mural nodule. A hint of papillary projection can be appreciated (arrow)
Fig. 3.
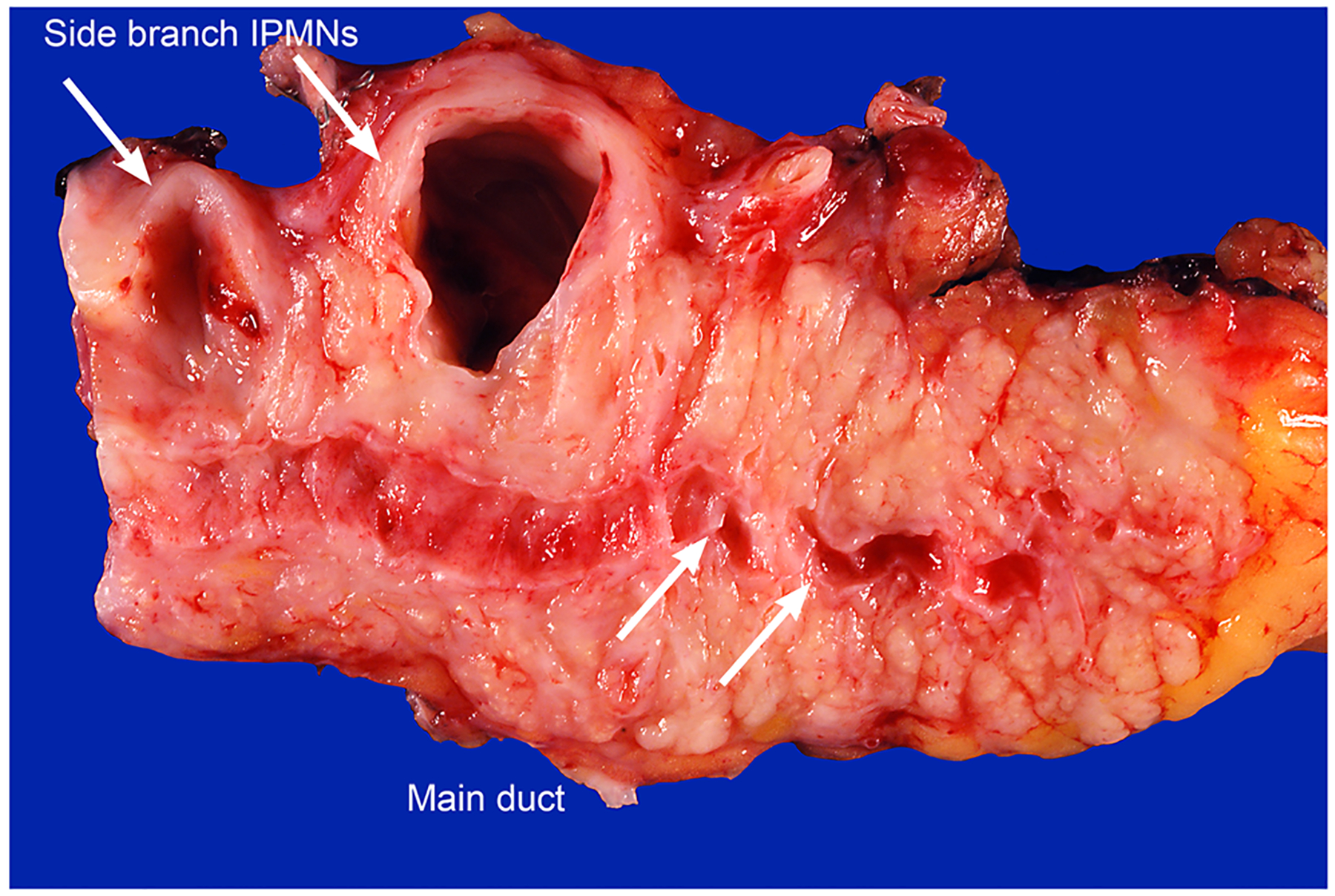
Two distinct, clearly separated, branch-duct IPMNs
Studies correlating the gross type of IPMN with the microscopic degree of dysplasia have shown that main-duct IPMNs are significantly more likely to have high-grade dysplasia or an associated invasive carcinoma than are branch-duct IPMNs [13–15]. For example, a collaborative analysis of 1028 surgically resected IPMNs found that 71% of the main-duct IPMNs had “high-risk disease” (high-grade dysplasia or an associated invasive carcinoma) compared to only 29% of the branch-duct IPMNs [14]. Similarly, Fernandez-Del Castillo and colleagues reported on 145 surgically resected branch-duct IPMNs, and only 22% had high-grade dysplasia or an associated invasive carcinoma [13]. This has immediate clinical implications as main-duct and branch-duct IPMNs can be distinguished on imaging, providing a preoperative measure of risk that has proven critical in the clinical management of patients with an IPMN [10, 11, 16–18]. Most main-duct IPMNs are surgically resected because the risk of high-grade dysplasia or an associated invasive carcinoma is so high, while small branch-duct IPMNs without high-risk stigmata, such as associated symptoms, can be safely followed.
Mural nodules are grossly found in some IPMNs (Figs. 1b and 2) [12]. Some of these nodules are formed by aggregated bunches of papillae or by more complex solid masses of neoplastic cells. Mural nodules are more likely to harbor epithelium with high-grade dysplasia than are the flatter areas of an IPMN. These mural nodules can be detected on imaging, providing another preoperative measure of risk that has proven valuable in the clinical management of patients with an IPMN [19]. Several studies, including the collaborative study of 1028 patients mentioned earlier, have shown that the finding of a mural nodule on imaging is associated with high-risk disease [14, 20]. The finding of a mural nodule on imaging is therefore another indication in favor of surgery in the current management guidelines [11, 18, 21]. However, gross examination of surgically resected IPMNs has revealed that some mural nodules are composed of acellular globs of mucin or polyps of reactive/inflammatory cells secondary to erosion of the cyst wall. These harmless mural nodules can mimic a neoplastic mural nodule on imaging and can lead a surgeon to resect an IPMN that, in retrospect, did not need to be resected.
Gross examination of surgically resected IPMNs has also demonstrated that 20–40% are grossly multifocal (Fig. 3) [22]. For example, H. Matthaei and colleagues described 34 grossly multifocal IPMNs [22]. S. Fritz and colleagues reported that 18% of 287 patients who underwent surgical resection of an IPMN had multifocal disease [23]. The multifocality of IPMNs contrasts with mucinous cystic neoplasms (the neoplasms with ovarian stroma) which are almost always unifocal. As will be discussed later in this review, the multifocality of IPMNs has significant clinical implications [8, 9, 24].
Colloid carcinoma (CC), which is a rare variant of ductal adenocarcinoma of the pancreas, typically arises in an association with IPMN or MCN. Grossly, CC usually forms well-demarcated gelatinous stromal nodules (Fig. 4) [25].
Fig. 4.
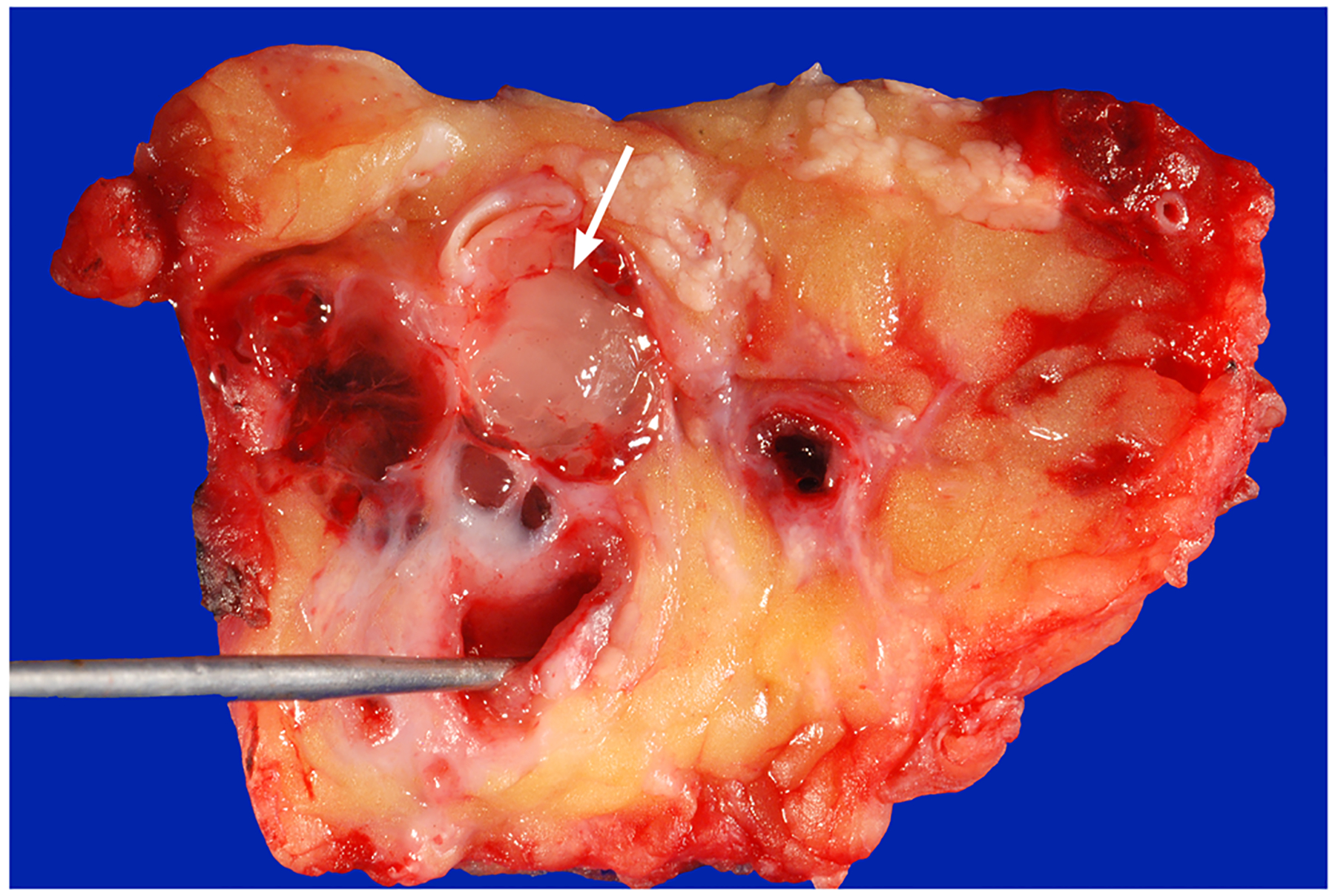
Colloid carcinoma (arrow) arising in association with a main-duct IPMN, the probe is in the main duct
Microscopy
Microscopic examination of surgically resected IPMNs has revealed that the neoplastic epithelium can have a variety of directions of differentiation. These include intestinal, gastric-foveolar, and pancreatobiliary. However, individual IPMNs can show a mixture of directions of differentiations, and further research is needed to determine if pancreatobiliary and intestinal IPMNs are truly separate entities or if they represent neoplastic progression of a gastric-type IPMN [26, 27].
The neoplastic epithelium in IPMNs with intestinal differentiation typically forms long finger-like (villous) papillae, and the neoplastic cells have basophilic cytoplasm and enlarged oval and hyperchromatic nuclei (Fig. 5a) [12, 28]. Pseudostratification can be seen, and there is usually moderate to high-grade dysplasia. Basically, these IPMNs histologically resemble villous adenomas of the colon. Virtually all colloid carcinomas of the pancreas arise from an intestinal-type IPMN [25, 29].
Fig. 5.
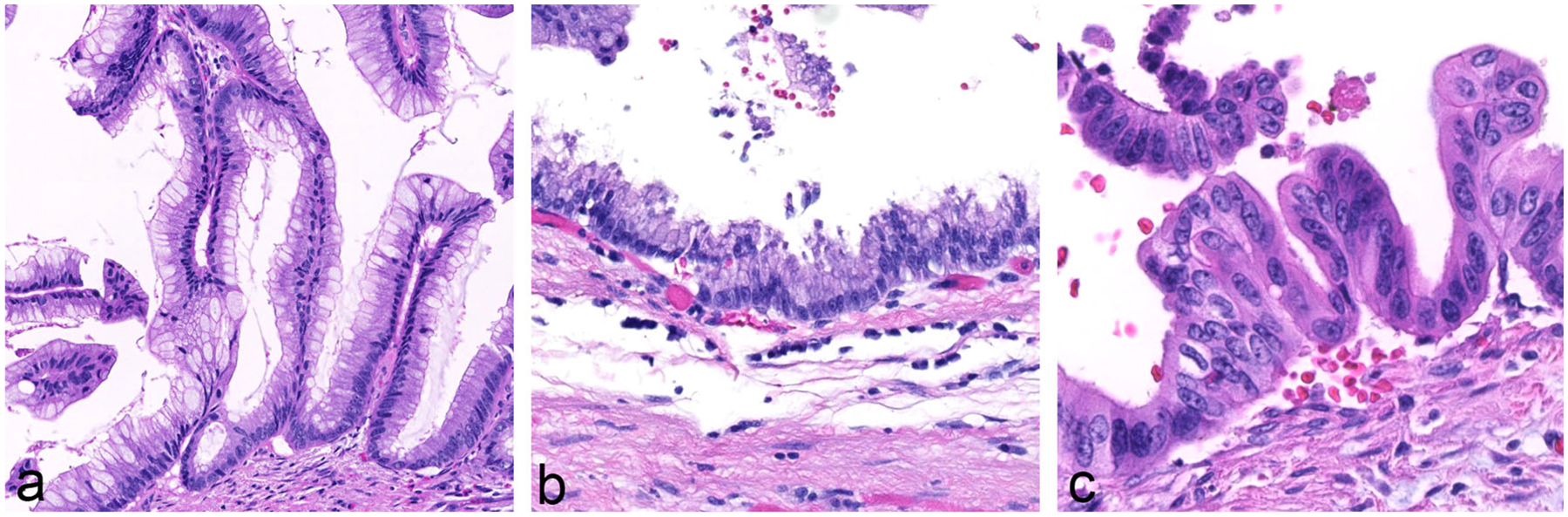
a An IPMN with intestinal differentiation forming villous papillae. Note the basophilic cytoplasm and enlarged oval and hyperchromatic nuclei. b IPMN with gastric-foveolar differentiation. Note the flat epithelium with basally placed nuclei and abundant mucin cap resembling gastric epithelium. c IPMN with pancreatobiliary differentiation. The neoplastic cells are more cuboidal in contrast to IPMNs with other directions of differentiation. The cytoplasm is amphophilic and the nuclei often are enlarged
The neoplastic epithelium in IPMNs with gastric-foveolar differentiation often forms broader (thicker) papillae, and the cytoplasm of the neoplastic cells is eosinophilic, with basally placed nuclei (Fig. 5b) [12, 28]. The epithelium can also be flat, and typically has only low-grade dysplasia. Branch-duct IPMNs often have gastric-foveolar differentiation.
The neoplastic epithelium in IPMNs with pancreatobiliary differentiation forms thin, branching, complex papillae (Fig. 5c) [12, 28]. The neoplastic cells are not as columnar as in IPMNs with other directions of differentiation. The cytoplasm is amphophilic and the nuclei often are enlarged. These IPMNs typically have high-grade dysplasia, and if an associated invasive carcinoma is present, it is almost always a tubular (ductal) type of invasive adenocarcinoma.
The neoplastic epithelium in IOPNs, previously known as oncocytic subtype of IPMNs, forms thick, complex papillae with intracellular and intraepithelial lumina (Fig. 6) [12, 28]. The cytoplasm of the neoplastic cells is abundant and distinctly oncocytic (eosinophilic). The nuclei are round and typically contain prominent nucleoli. These IPMNs almost always have high-grade dysplasia. As will be discussed in the section on genetics, distinct translocations have been identified in these neoplasms with oncocytic differentiation [30]. They should therefore be clearly designated as “intraductal oncocytic papillary neoplasms,” and classified separately from the other IPMNs.
Fig. 6.
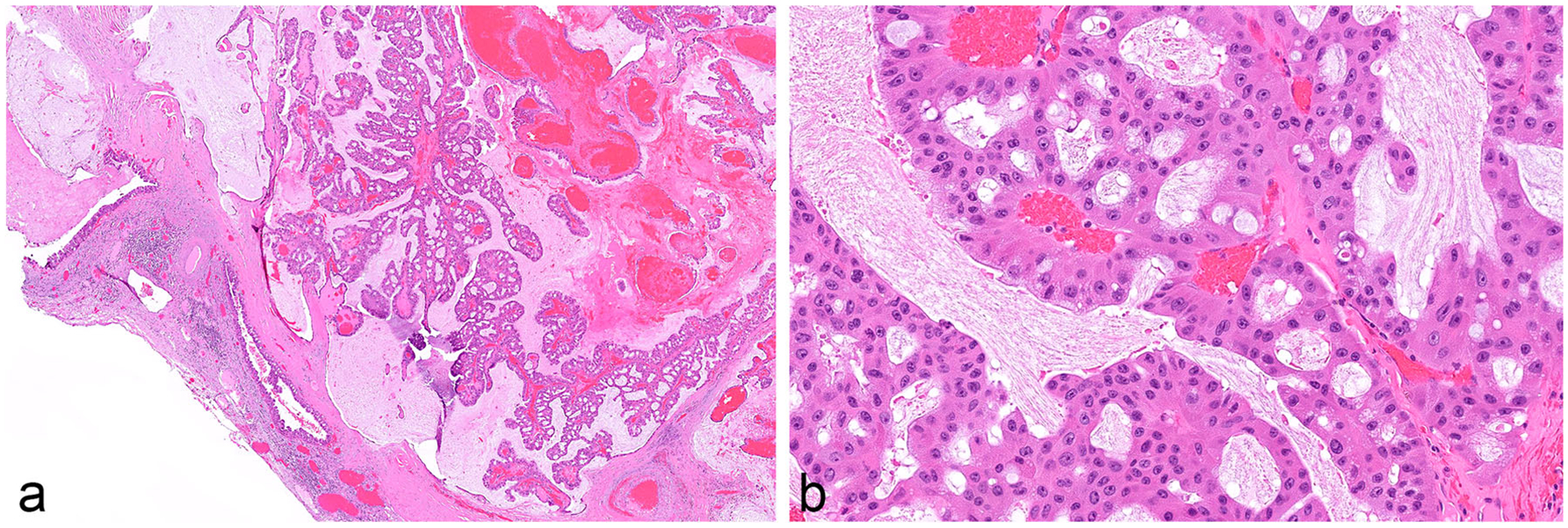
a Intraductal oncocytic papillary neoplasm (IOPN). Note the thick, complex papillae. b The neoplastic cells have abundant and distinctly oncocytic (eosinophilic) cytoplasm. The nuclei are round and typically contain prominent nucleoli
ITPNs are composed of small, tightly packed glands that form intraductal nodules (Fig. 7) [12, 28, 31–33]. The neoplastic cells are cuboidal and, on hematoxylin and eosin staining, typically do not contain significant mucin. These neoplasms can pose a significant diagnostic challenge for pathologists, as it can be difficult to distinguish noninvasive neoplastic cells growing in the branching duct system, from a nodule of neoplastic cells invading into the stroma. ITPNs are now classified separately from the other IPMNs.
Fig. 7.
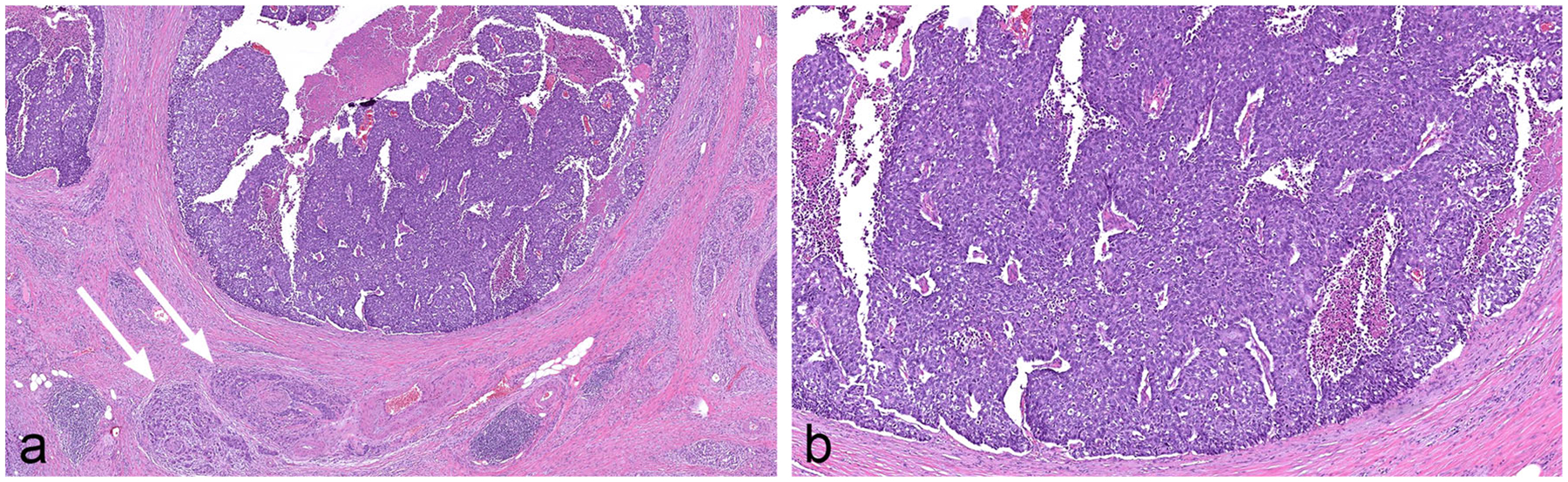
Intraductal tubulopapillary neoplasm (ITPN) composed of small, tightly packed glands forming intraductal nodules. The neoplastic cells are cuboidal with no significant mucin. This particular case shows foci of comedo-type necrosis resembling intraductal carcinoma of the breast and it also has an associated invasive carcinoma with perineural invasion (arrows)
Histologic examination of IPMNs, IOPNs, and ITPNs has also revealed a spectrum of dysplasia. Although a three-tier grading system was employed originally, things have been simplified, and the degree of dysplasia in these neoplasms is currently assigned one of two grades [8, 9, 34, 35]. Low-grade neoplasms have only mild to moderate atypia, simple papillae, rare mitoses, and minimal to moderate pleomorphism [8, 9, 34, 35]. In general, the cells are well-oriented towards the lumina, and there is minimal stratification of the neoplastic cells. By contrast, high-grade neoplasms have marked atypia, complex papillae, marked pleomorphism, and nuclear stratification with loss of polarity [8, 9, 34, 35]. Mitoses can also be seen. If both low-grade and high-grade dysplasia are present, the neoplasms should be assigned the higher grade.
Although grade and direction of differentiation are not completely independent, essentially all studies have found that the grade of dysplasia is clinically more important than the direction of differentiation [36, 37]. High-grade neoplasms are more likely to have an associated invasive carcinoma, and, as discussed in greater detail below, high-grade dysplasia in a surgically resected intraductal neoplasm turns out to be a significant risk factor for recurrent disease in the remnant pancreas after surgery [36, 37]. Therefore clinical efforts are focused on identifying and treating high-grade neoplasms while avoiding the overtreatment of patients with a low-grade neoplasm [11, 14, 18, 21].
Histologic examination has also confirmed the radiologic and gross findings that IPMNs are often multifocal [22, 23]. This multifocality can be dramatic at the light microscopic level and includes not only multifocal IPMNs, but importantly, histologic examination has shown that the different locules that comprise a single IPMN can have different grades of dysplasia [34]. Furthermore, when multiple anatomically separated IPMNs are present, they too can have different grades of dysplasia. As discussed later, this heterogeneity has significant clinical implications.
Cytopathology
Fine-needle aspiration (FNA) plays an important role in the initial diagnosis of a cystic lesion suspected to be an IPMN on radiological imaging. The ability to bring the ultrasound transducer closer to the pancreas via endoscopic ultrasound (EUS) has revolutionized diagnostic sampling of even sub-centimeter cysts with good diagnostic yield. EUS-FNA has the ability to sample IPMNs throughout the entire length of the pancreas. A successful EUS-FNA diagnostic approach requires the presence of a well-trained cytotechnologist or cytopathologist for rapid on-site evaluation (ROSE) at the time of the procedure. ROSE ensures a higher diagnostic accuracy, reducing the number of unnecessary FNA passes and helps triage the aspirated fluid for ancillary testing such as chemistry and molecular analysis, as needed.
The cytopathologic evaluation of an IPMN includes a multimodal approach and is carefully done after correlation with radiological imaging findings as well as the chemical analysis of the aspirated cyst fluid for carcinoembryonic antigen (CEA) and amylase levels [38, 39]. Although it is generally recommended that each lab should establish its own cut-off values for CEA, a value of approximately 200 ng/ml or higher is considered strongly supportive of a neoplastic mucin-producing cyst. It is important to realize that cyst fluid CEA levels do not help differentiate between an IPMN and mucinous cystic neoplasm (MCN), as well as in distinguishing low-grade from high-grade IPMNs. A significantly high amylase level supports the diagnosis of a pseudocyst or IPMN, a common differential for a cystic lesion. Serous cystadenoma typically will show a low CEA and amylase levels. Molecular analysis of the aspirated cyst fluid is being increasingly performed in several practices and significantly enhances the diagnostic accuracy of an FNA interpretation [40, 41].
Cytopathologic evaluation not only establishes the diagnosis of an IPMN, but also further characterizes the grade of dysplasia as well as evaluates for the presence or absence of invasive component. In order to use a standardized terminology for reporting FNA of IPMN and other cystic lesions of the pancreas, the Papanicolaou Society of Cytopathology (PSC) has put forward its recommended guidelines [42]. IPMN is presently reported on FNA under the general category of “Neoplastic” with the added statement of “Neoplastic Mucinous Cyst.” Also included in the diagnosis is a discrete statement further elaborating on the “presence or absence of high-grade dysplasia or carcinoma.”
Cytomorphologic characteristics of the aspirated cyst form the cornerstone for accurate interpretation of IPMNs and the degree of the associated dysplasia [43]. The evaluation starts at the time of ROSE with the gross features of the aspirated fluid having a thick, viscous, glistening, and mucoid appearance. On microscopy, at low magnification, the majority of cases will display significant extracellular mucin. IPMNs often show thick inspissated mucin having a “colloid-like” appearance, also visible to the naked eye on the glass slide (Fig. 8). Most cases will contain mucinous glandular epithelium in the form of well-preserved intact, sheet-like tissue fragments (Fig. 9). A papillary architecture may or may not be evident in all cases but when present is considered quite specific (Fig. 10). The neoplastic cells appear disorganized with enlarged and crowded nuclei. Intracytoplasmic mucin is readily identifiable in most cells. Gastrointestinal tract material (particularly when EUS-FNA is performed through the gastric wall) may confound interpretation in heavily contaminated aspirates. Occasional cases of IPMN may not contain appreciable mucin, especially cysts with thin degenerated mucin.
Fig. 8.
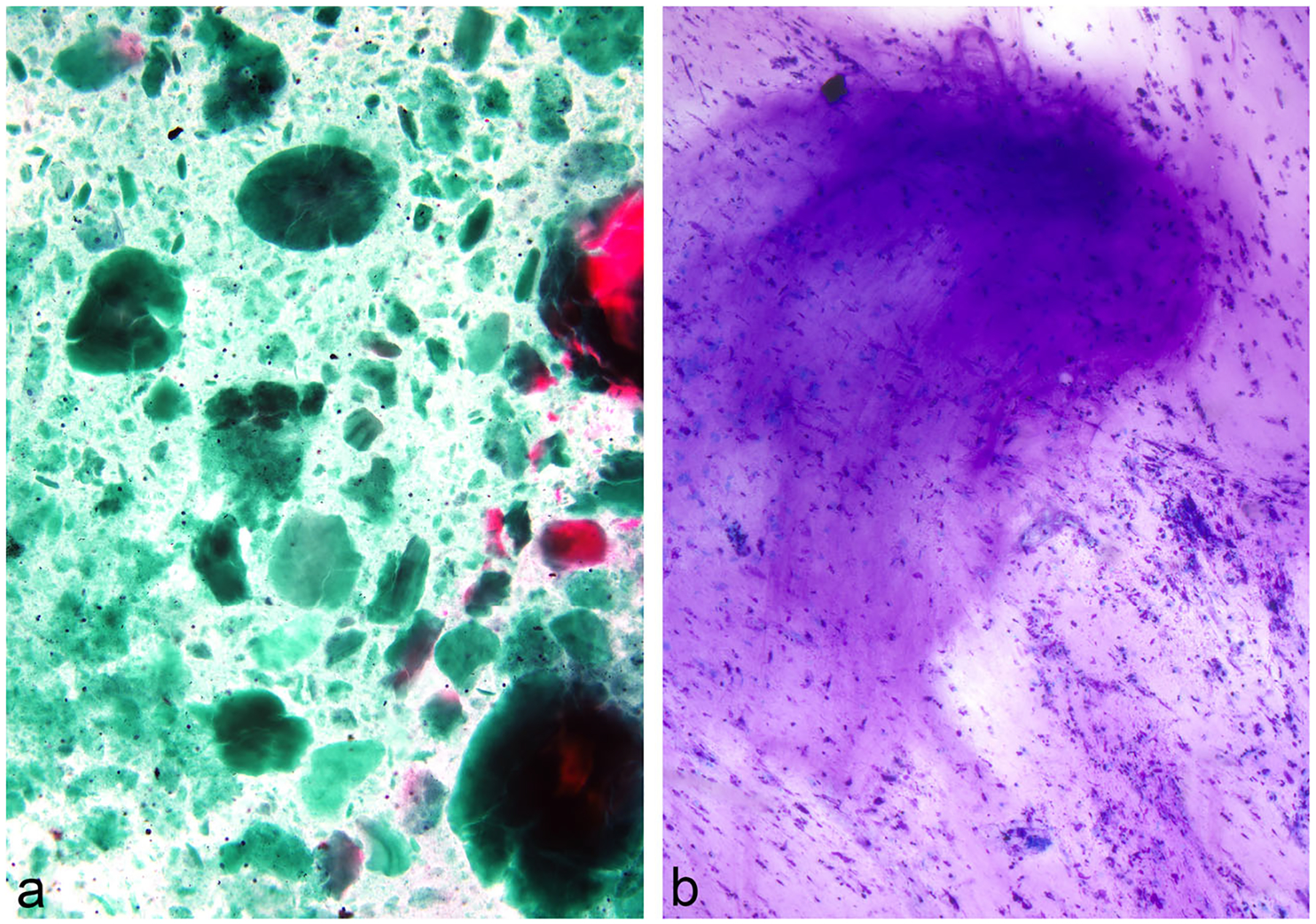
IPMN, EUS-FNA; a-b. Occasional cases of IPMN display abundant, thick and inspissated mucin with a “colloid-like” appearance, which is considered diagnostic in the right clinicoradiologic setting (Papanicolaou and Diff-Quik stains)
Fig. 9.
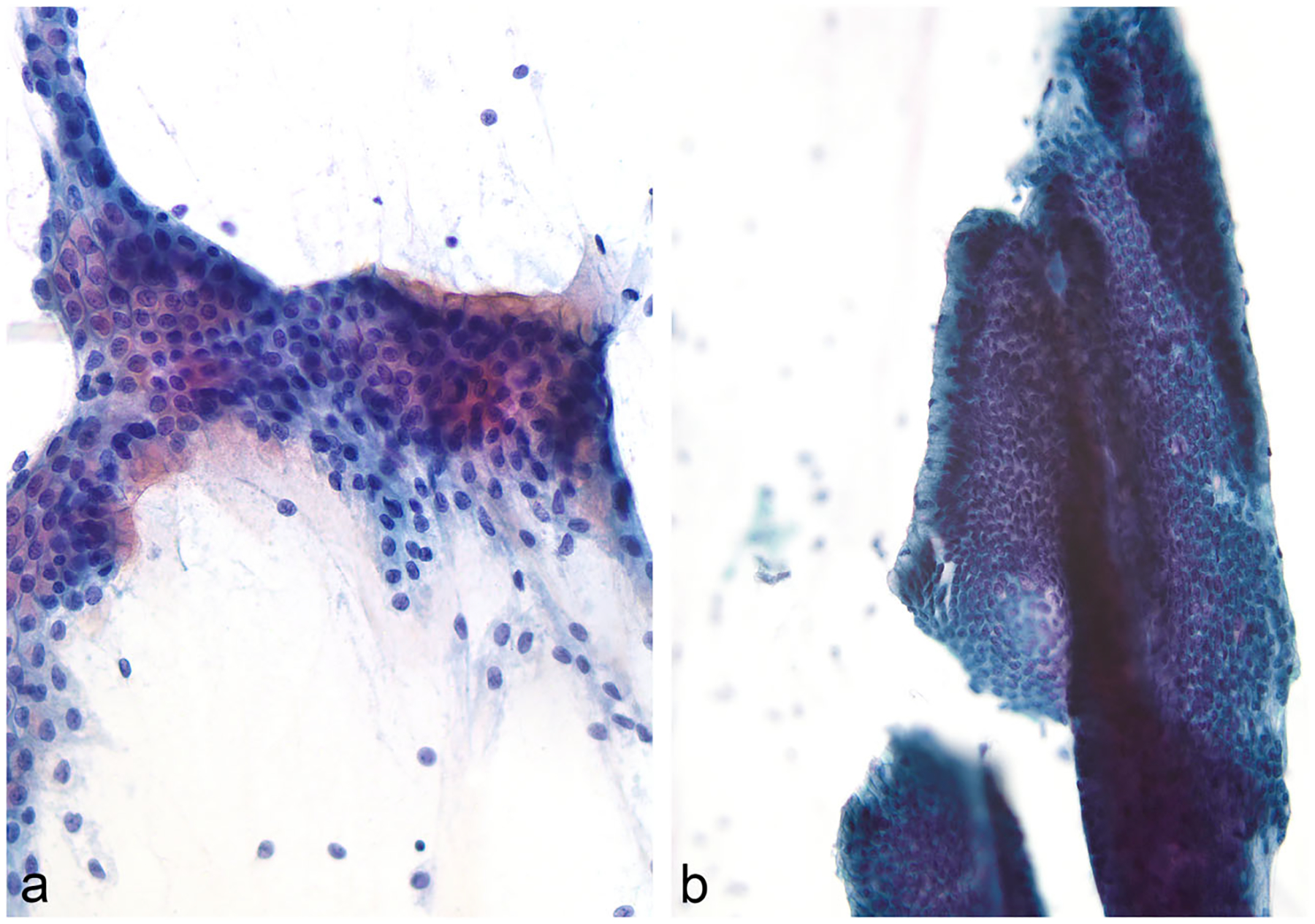
IPMN, EUS-FNA; a-b Images showing mucinous glandular epithelium with a well-preserved and intact, sheet-like architecture. Intracytoplasmic mucin is readily identifiable (Papanicolaou stain)
Fig. 10.
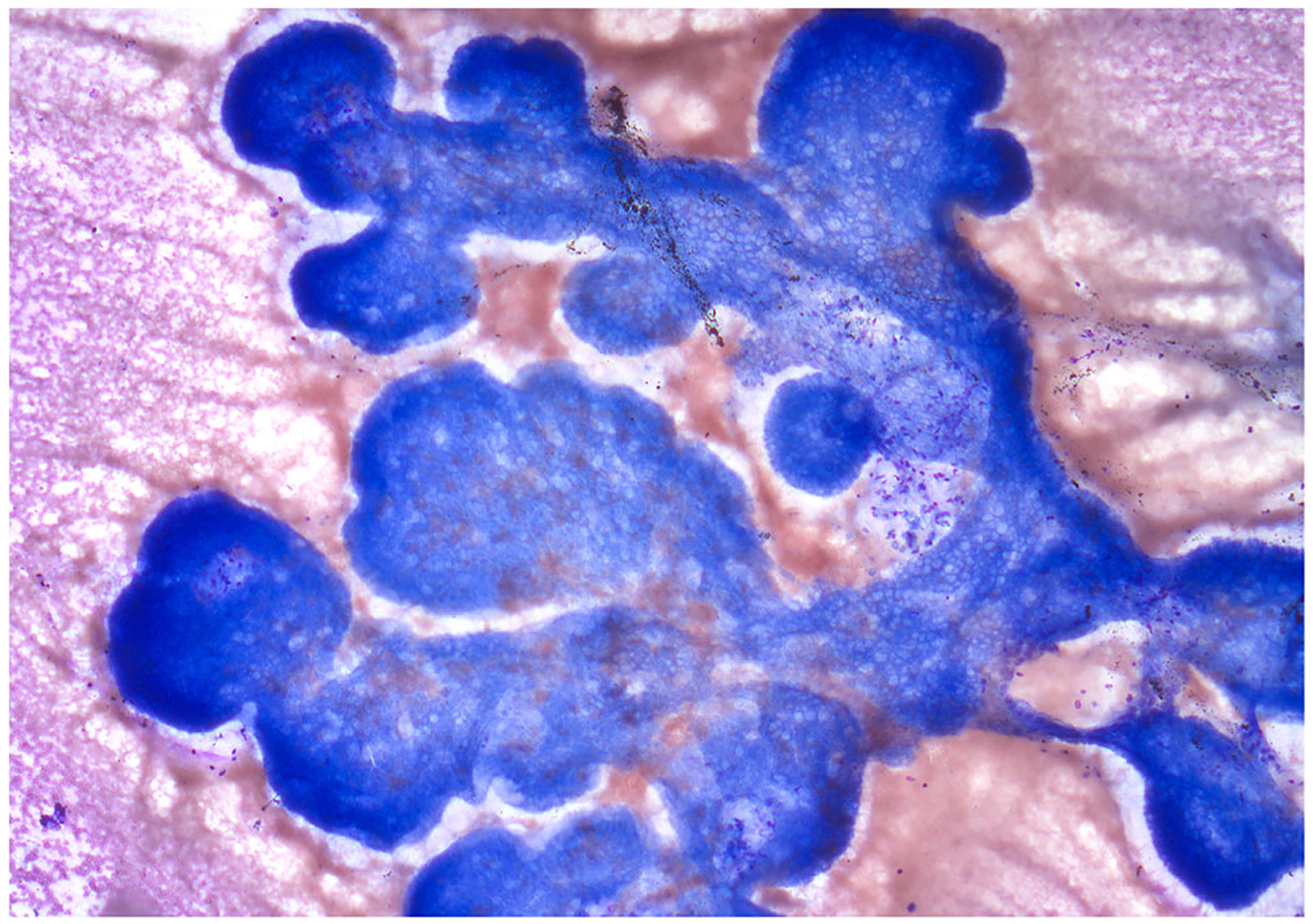
IPMN, EUS-FNA; An IPMN depicting neoplastic mucinous epithelium with a classic papillary architecture (Papanicolaou stain)
Another critical aspect of cytomorphologic evaluation of IPMN aspirates is to assess for the presence or absence of high-grade dysplasia or carcinoma [44]. A cellular sample with well-preserved epithelial component is definitely needed to exclude high-grade dysplasia or carcinoma. For high-grade dysplasia, the most important cellular characteristics are cells with significantly increased nuclear to cytoplasmic (N/C) ratios, abnormal chromatic patterns, and background necrosis [45] (Fig. 11). Nucleolar prominence is unusual and can be seen even in the absence of a high-grade dysplasia.
Fig. 11.
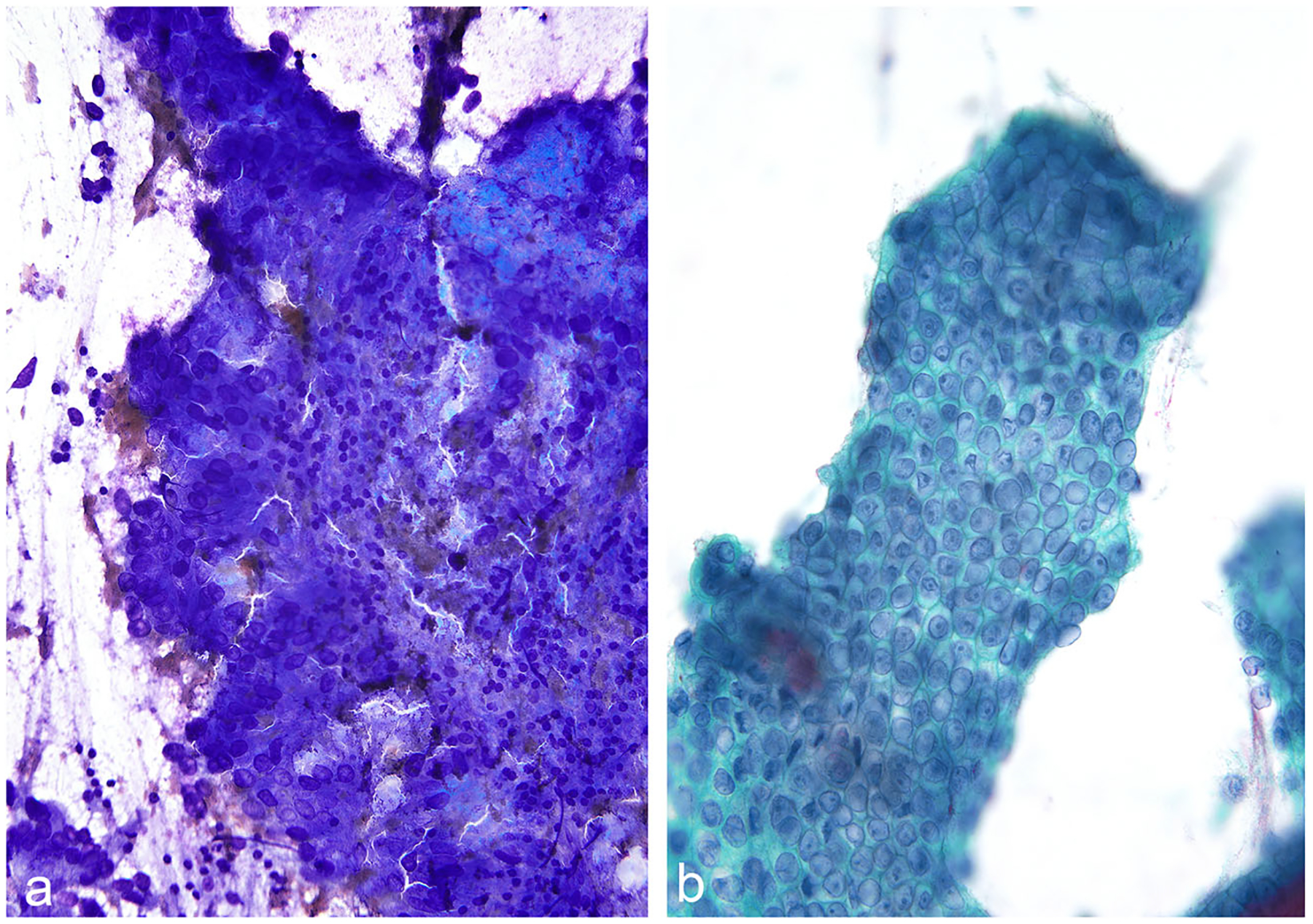
IPMN, EUS-FNA; a-b Two cases of IPMN with cytomorphologic features consistent with at least high-grade dysplasia. The neoplastic cells have significantly high N/C ratios and show abnormal chromatin patterns. (Diff Quik and Papanicolaou stains)
Immunolabeling
Several studies have examined the immunolabeling profiles of IPMNs. Most IPMNs label with antibodies to pancytokeratin (AE1/AE3) and with antibodies to cytokeratins 7, 8, and 19 [8, 9]. The expression of the oncoproteins CEA and CA 19.9 is also common [8, 9]. IPMNs with intestinal differentiation label with antibodies to cytokeratin 20 [8, 9]. As shown in Table 1, the pattern of labeling for mucins depends on the direction of differentiation of the IPMN [46–48]. IOPNs label with antibodies to mitochondrial proteins, 111.3, CD117, and with hepatocyte-1 (HepPAR-1) antibodies [49, 50].
Table 1.
Patterns of immunolabeling in IPMNs, IOPNs and ITPNs
| Tumor type | MUC1 | MUC2 | MUC5AC | MUC6 | CDX2 |
|---|---|---|---|---|---|
| Gastric-foveolar IPMN | − | − | + | ± | − |
| Intestinal IPMN | − | + | + | ± | + |
| Pancreatobiliary IPMN | + | − | + | + | − |
| IOPN | + | * | * | + | − |
| ITPN | + | − | − | + | − |
Restricted to goblet cells. IOPN, intraductal oncocytic papillary neoplasm; IPMN, intraductal papillary mucinous neoplasm; ITPN, intraductal tubulopapillary neoplasm
While immunolabeling can be used to determine the direction of differentiation of an IPMN, this classification, as noted above, is not as important as the degree of dysplasia and is of minimal clinical importance. The recognition of which oncoproteins are expressed in IPMNs is, however, indirectly clinically important as high serum levels of CA19.9 in a patient with an IPMN correlate with increased risk of malignancy [51].
Somatic and germline mutations
The molecular revolution has changed our understanding of the genetic drivers of human neoplasms, and IPMNs are no exception. The exomes of IPMNs have been sequenced, and a number of driver genes have been identified (Table 2). Somatic activating mutations in the KRAS and GNAS genes are the most prevalent alterations, and a number of other genes, KLF4, PIK3CA, p16/CDKN2A, RNF43, SMAD4, TGFBR2, and TP53, can also be targeted [52–57]. Somatic BRAF mutations also occur, but are rare (6% in one series) [56]. Gene fusions, including ATP1B1-PRKACB and DNAJB1-PRKACA, are almost universally found in, and restricted to, IOPNs [30, 58]. Mutations in SMAD4, TP53, and TGFBR2 appear to be late events, occurring primarily in high-grade dysplasia and in the invasive components of invasive carcinomas arising in association with an IPMN. By contrast, KLF4 mutations are found more commonly in low-grade IPMNs than they are in high-grade IPMNs [59].
Table 2.
Genes commonly targeted in IPMNs
| Gene | Chromosome | Function ofgene product |
|---|---|---|
| BRAF | 7q | Serine/threonine kinase that functions in MAP kinase/ERK signaling pathway |
| GNAS | 20q | Guanine nucleotide-binding protein functions in adenylyl cyclase activation |
| KLF4 | 9q | A Kruppel family transcription factor with a number of functions, including p53 mediated DNA damage repair |
| KRAS | 12p | Member of the small GTPase family |
| PIK3CA | 3q | This phosphoinositide-3-kinase participates in cellular signaling by phosphorylating downstream targets |
| P16/CDKN2A | 9p | Cell cycle control |
| RNF43 | 17q | A RING-type E3 ubiquitin ligase that functions in Wnt signaling |
| SMAD4 | 18q | Transforming growth factor—beta signaling |
| TGFBR2 | 3p | Transforming growth factor—beta signaling |
| TP53 | 17p | Induces cell cycle arrest, apoptosis and other changes in response to cellular stresses |
McCune Albright syndrome is caused by somatic mutations in GNAS that occur very early in development resulting in individuals who are mosaic for GNAS mutations [60]. In addition to polyostotic fibrous dysplasia and café-au-lait spots, many individuals with this syndrome develop IPMNs with GNAS mutations [61].
Germline genetic alterations have been reported in patients with IPMNs. N. Roberts and colleagues sequenced the germline of 315 patients with a surgically resected and pathologically confirmed IPMN, and 23 (7.3%) of the 315 patients had a deleterious germline variant associated with cancer risk [62]. These included deleterious variants in the ATM, PTCH1, and SUFU genes [62]. IPMNs have been reported in patients with the Peutz-Jeghers syndrome, and M. Goggins and colleagues have demonstrated biallelic inactivation of the STK11 gene in IPMNs resected from patients with this syndrome [63, 64].
The somatic genetic changes in IPMNs can be used as highly specific tools to study the pathology of these lesions. The analysis of the noninvasive and invasive components of IPMNs with an associated invasive cancer has demonstrated the same exact somatic mutation in each component, establishing, beyond a shadow of a doubt, that IPMNs are bona fide precursors to invasive cancer [59, 65]. The findings, however, are more complex than a simple progression. Genetic analyses have shown that some invasive cancers located adjacent to an IPMN, did not, in fact, arise from the IPMN [66]. This phenomenon, “neighbors but not always relatives,” is clinically important because it suggests that even if we were to predict the grade of an IPMN with perfect accuracy, we will not be able to rule out an independent cancer arising in the parenchyma adjacent to the IPMN [65, 66].
Matthaei and colleagues used somatic GNAS mutations as an IPMN marker, and examined small precursors in the pancreas that were in between PanINs and IPMNs in size [67]. They found that some of these lesions, histologically too small to be technically classified as an IPMN, harbored a GNAS mutation, suggesting that they were incipient IPMNs [67].
Genetic tools have also been used to examine the multifocality of IPMNs. L. Wood and colleagues have used the pattern of somatic mutations to show that anatomically distinct IPMNs in the same pancreas harbor different somatic alterations, confirming that IPMNs can be multifocal [68, 69]. When Wood and colleagues took the sequencing to the single cell level, they found even greater heterogeneity [70]. Some grossly unifocal IPMNs contained multiple distinct genetic clones, establishing significant genetic heterogeneity in IPMNs [70].
The neoplastic cells lining cysts in the pancreas shed their DNA into the cyst fluid. As a result, sequencing cyst fluid aspirated at the time of endoscopy can provide preoperative insight into cyst type [41, 71–74]. For example, A. Singhi and colleagues applied next generation sequencing to over 600 cyst fluid samples obtained by EUS-FNA and reported that KRAS/GNAS mutations were highly sensitive for mucin-producing cysts, including IPMNs [41, 75]. They further suggested that the combination of TP53/PIK3CA/PTEN mutations was indicative of high-grade dysplasia. Lennon and colleagues, in a study of 436 patients with a pancreatic cyst, reported that sequencing of cyst fluid can be highly sensitive and specific for cyst type, especially when combined with protein markers (VEGFA and CEA) and clinical findings [72]. These molecular approaches, particularly when combined with clinical features, are extremely exciting, but are imperfect when it comes to determining the degree of dysplasia. As discussed below in clinical implications, we believe that this, in large part, comes from the underlying heterogeneity of the cysts in IPMNs.
Advances in our understanding of the somatic genetic alterations in intraductal neoplasms have also helped improve the classification of these lesions. For example, for years, pathologists debated whether or not IOPNs should be considered a variant of an IPMN, or a separate entity. The discovery that virtually all IOPNs harbor fusion specific fusion genes (ATP1B1-PRKACB or DNAJB1-PRKACA) that are not found in other intraductal neoplasms, helped establish that IOPNs should be considered a completely separate entity from the other IPMNs [30, 58].
Methylation
The patterns of DNA methylation in IPMNs have also been described [76–79]. Targeted and whole genome analyses have revealed distinct patterns of methylation in IPMNs compared to normal ducts, and some genes, including BNIP3, CDO1, EBF3, NXPH1, PTCHD2, and SOX17, have been reported to be more commonly methylated in high-grade IPMNs than in low-grade IPMNs [79, 80]. Future studies may exploit whole genome methylation profiles to refine IPMN subtyping and classification, as is currently the practice for brain tumor classification and as is emerging for pancreatic neuroendocrine tumors [81]. Methylation profiles can be detected in cyst fluid, suggesting that the analysis of the patterns of methylation in EUS-FNA obtained cyst fluid could be used aid in the clinical classification of cyst type [76].
Microbiome
When we think of the microbiome, we tend to think of the tubular gastrointestinal tract. Recently, however, several reports have documented the presence of bacteria in some IPMNs [82–85]. It is unclear if these bacteria are harmless passengers or potential drivers of disease progression [86].
Clinical implications
The dramatic improvements in our understanding of the pathology and genetics of IPMNs, IOPNs, and ITPNs that has occurred over the past two decades have a number of significant clinical implications.
First and foremost, as discussed earlier, cyst fluid can be aspirated at the time of endoscopic ultrasound and the patterns of gene and protein alterations in the cyst fluid, when coupled with imaging and other patient characteristics, can be used to characterize cyst type with high accuracy [41, 71–74]. These analyses can also give a good, but imperfect, indication of the degree of dysplasia in a lesion. The challenge is, and we understand this because we understand the pathology, that the multiple locules that comprise IPMNs can be heterogeneous. Fluid aspirated from one locule provides incomplete information on the other locules in that IPMN. For example, an IPMN could be composed of five locules, four of which have high-grade dysplasia and one of which has low-grade dysplasia. If the endoscopist’s needle happens to sample the locule with low-grade dysplasia, even a perfect test of that fluid will miss the high-grade dysplasia in the adjacent locules. This is compounded by the fact that IPMNs are often multifocal, and not all foci can be practically sampled. Thus, the heterogeneity of IPMNs poses a significant hurdle to the preoperative clinical management of these patients.
The multifocality of IPMNs also has significant clinical implications for patients who undergo surgery for an IPMN [87–91]. For example, the Japanese Pancreas Society reported that 155 (15%) of 1074 patients who underwent surgical resection of an IPMN developed a postoperative recurrence [90]. The risk in the remnant pancreas appears greatest after the resection of main-duct IPMNs and IPMNs with high-grade dysplasia [90, 92]. This risk of metachronous disease is almost certainly a manifestation of the multifocality of IPMNs as it is not seen with mucinous cystic neoplasms, a neoplasm which is almost always a solitary lesion [24, 69]. These results suggest that patients should be carefully followed clinically after the surgical resection of an IPMN, especially if the IPMN is a main-duct IPMN or has high-grade dysplasia [87, 90]
An important clinical issue is the clinical relevance of IPMN lesions at surgical transection margins. The data are controversial, in part due to great variability of the definition “positive transection margin” [93]. It is well accepted that high-grade dysplasia or invasive carcinoma at the transection margin requires further surgical intervention, to prevent progression or recurrence [87]. Pflüger and colleagues reported that the size (>0.5 cm) of the dysplastic focus at the margin and the presence of high-grade dysplasia are associated with relapse of disease in patients with an IPMN [87]. However, Dhar and colleagues found that the majority of positive transection margins have only low-grade dysplasia and that these low-grade lesions are not associated with the development of recurrent disease in the remnant pancreas [94]. Furthermore, genetic analyses have suggested that a significant proportion of dysplastic foci at positive surgical margins are actually separate PanIN lesions unrelated to the primary IPMN [87].
Resection margins during intraoperative frozen section should be always regarded with caution. Tumor heterogeneity and de-epithelialization can mask the real histological picture of the lesion [93]. Good communication between pathologist and surgeon is therefore critical, and the clinical value of resecting additional margin should be carefully balanced with the potential harm caused by resecting pancreatic parenchyma [87, 93, 94].
On a positive note, the discovery of the genetic drivers of IPMNs has identified several potential therapeutic targets. Most IPMNs harbor KRAS mutations, and novel inhibitors of KRAS G12C mutations have been developed, and Zhou and colleagues reported on the use of bispecific antibodies to target neoplastic cells with KRAS mutations [95–97]. Similarly, RNF43 mutations are prevalent in IPMNs, and tumors with RNF43 loss of function mutations may be particularly susceptible to therapies that inhibit the Wnt pathway [98]. Targeted therapies such as these have the potential to be useful in not only treating IPMNs, but, perhaps more importantly, in treating the invasive cancers that arise from IPMNs.
Summary
Our understanding of IPMNs, IOPNs, and ITPNs has come far, but we still have far to go. Too many patients with lesions that mimic IPMNs and too many patients with low-grade IPMNs undergo surgery and are over treated. We need new approaches to more accurately predict the degree of dysplasia in an IPMN and the risk of progression. We need new approaches to account for heterogeneity and to define the risk of the entire pancreas, and we need to do a better job of predicting who will recur after surgery. We believe that improvements in our understanding of pancreatic pathology will form the basis for these advances.
Footnotes
Ethical approval This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest Under a license agreement between, Thrive Earlier Detection Corp., a subsidiary of Exact Sciences Corp., and the Johns Hopkins University, Dr. Hruban and the University are entitled to royalty distributions related to the GNAS invention described in this review. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest’s policies.
References
- 1.Hruban RH, Klimstra DS, Zamboni G, Klöppel G (2020) A semi-centennial of pancreatic pathology: the genetic revolution is here, but don’t throw the baby out with the bath water! Hum Pathol 95: 99–112 [DOI] [PubMed] [Google Scholar]
- 2.Compagno J, Oertel JE (1978) Microcystic adenomas of the pancreas (glycogen-rich cystadenomas): a clinicopathologic study of 34 cases. Am J Clin Pathol 69(3):289–298 [DOI] [PubMed] [Google Scholar]
- 3.Compagno J, Oertel JE (1978) Mucinous cystic neoplasms of the pancreas with overt and latent malignancy (cystadenocarcinoma and cystadenoma). A clinicopathologic study of 41 cases. Am J Clin Pathol 69(6):573–580 [DOI] [PubMed] [Google Scholar]
- 4.Hodgkinson DJ, ReMine WH, Weiland LH (1978) A clinicopathologic study of 21 cases of pancreatic cystadenocarcinoma. Ann Surg 188(5):679–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zamboni G, Scarpa A, Bogina G, Iacono C, Bassi C, Talamini G, Sessa F, Capella C, Solcia E, Rickaert F, Mariuzzi GM, Klöppel G (1999) Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol 23(4):410–422 [DOI] [PubMed] [Google Scholar]
- 6.Ohhashi K, M Y, Takekoshi T (1982) Four cases of “mucin producing” cancer of the pancreas on specific findings of the papilla of Vater (Abstr). Prog Diagn Endosc 20:348–351 [Google Scholar]
- 7.Wilentz RE, Albores-Saavedra J, Hruban RH (2000) Mucinous cystic neoplasms of the pancreas. Semin Diagn Pathol 17(1):31–42 [PubMed] [Google Scholar]
- 8.Bosman FT, Carneiro F, Hruban RH, Theise ND (2010) WHO classification of tumours of the digestive system, 4th edn. International Agency for Research on Cancer, Lyon, pp 300–313 [Google Scholar]
- 9.Hruban RH, Pitman MB, Klimstra DS (2007) Tumors of the pancreas. Washington, D.C, American Registry of Pathology [Google Scholar]
- 10.Oyama H, Tada M, Takagi K, Tateishi K, Hamada T, Nakai Y, Hakuta R, Ijichi H, Ishigaki K, Kanai S, Kogure H, Mizuno S, Saito K, Saito T, Sato T, Suzuki T, Takahara N, Morishita Y, Arita J, Hasegawa K, Tanaka M, Fukayama M, Koike K (2020) Long-term risk of malignancy in branch-duct intraductal papillary mucinous neoplasms. Gastroenterology. 158(1):226–237 e225 [DOI] [PubMed] [Google Scholar]
- 11.Tanaka M, Fernandez-Del Castillo C, Kamisawa T et al. (2017) Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 17(5):738–753 [DOI] [PubMed] [Google Scholar]
- 12.Adsay V, Mino-Kenudson M, Furukawa T, Basturk O, Zamboni G, Marchegiani G, Bassi C, Salvia R, Malleo G, Paiella S, Wolfgang CL, Matthaei H, Offerhaus GJ, Adham M, Bruno MJ, Reid MD, Krasinskas A, Klöppel G, Ohike N, Tajiri T, Jang KT, Roa JC, Allen P, Fernández-del Castillo C, Jang JY, Klimstra DS, Hruban RH, Members of Verona Consensus Meeting, 2013 (2016) Pathologic evaluation and reporting of intraductal papillary mucinous neoplasms of the pancreas and other tumoral intraepithelial neoplasms of pancreatobiliary tract: recommendations of Verona consensus meeting. Ann Surg 263(1):162–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodriguez JR, Salvia R, Crippa S, Warshaw AL, Bassi C, Falconi M, Thayer SP, Lauwers GY, Capelli P, Mino–Kenudson M, Razo O, McGrath D, Pederzoli P, Fernández–del Castillo C (2007) Branch-duct intraductal papillary mucinous neoplasms: observations in 145 patients who underwent resection. Gastroenterology. 133(1):72–79 quiz 309–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Attiyeh MA, Fernandez-Del Castillo C, Al Efishat M et al. (2018) Development and validation of a multi-institutional preoperative nomogram for predicting grade of dysplasia in intraductal papillary mucinous neoplasms (IPMNs) of the pancreas: a report from the pancreatic surgery consortium. Ann Surg 267(1):157–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirono S, Kawai M, Okada KI et al. (2017) Factors associated with invasive intraductal papillary mucinous carcinoma of the pancreas. JAMA Surg 152(3):e165054. [DOI] [PubMed] [Google Scholar]
- 16.Boraschi P, Tarantini G, Donati F, Scalise P, Cervelli R, Caramella D (2020) Side-branch intraductal papillary mucinous neoplasms of the pancreas: outcome of MR imaging surveillance over a 10 years follow-up. Eur J Radiol Open 7:100250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.D’Onofrio M, Tedesco G, Cardobi N, de Robertis R, Sarno A, Capelli P, Martini PT, Giannotti G, Beleù A, Marchegiani G, Gobbo S, Butturini G, Bogdan M, Salvia R, Bassi C (2021) Magnetic resonance (MR) for mural nodule detection studying Intraductal papillary mucinous neoplasms (IPMN) of pancreas: Imaging-pathologic correlation. Pancreatology. 21(1):180–187 [DOI] [PubMed] [Google Scholar]
- 18.European Study Group on Cystic Tumours of the Pancreas (2018) European evidence-based guidelines on pancreatic cystic neoplasms. Gut. 67(5):789–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee JE, Choi SY, Min JH, Yi BH, Lee MH, Kim SS, Hwang JA, Kim JH (2019) Determining malignant potential of intraductal papillary mucinous neoplasm of the pancreas: CT versus MRI by Using Revised 2017 International Consensus Guidelines. Radiology. 293(1):134–143 [DOI] [PubMed] [Google Scholar]
- 20.Akita H, Takeda Y, Hoshino H, Wada H, Kobayashi S, Marubashi S, Eguchi H, Tanemura M, Mori M, Doki Y, Nagano H (2011) Mural nodule in branch duct-type intraductal papillary mucinous neoplasms of the pancreas is a marker of malignant transformation and indication for surgery. Am J Surg 202(2):214–219 [DOI] [PubMed] [Google Scholar]
- 21.Canto MI, Harinck F, Hruban RH, Offerhaus GJ, Poley JW, Kamel I, Nio Y, Schulick RS, Bassi C, Kluijt I, Levy MJ, Chak A, Fockens P, Goggins M, Bruno M, International Cancer of Pancreas Screening (CAPS) Consortium (2013) International Cancer of the Pancreas Screening (CAPS) Consortium summit on the management of patients with increased risk for familial pancreatic cancer. Gut. 62(3):339–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matthaei H, Norris AL, Tsiatis AC, Olino K, Hong SM, dal Molin M, Goggins MG, Canto M, Horton KM, Jackson KD, Capelli P, Zamboni G, Bortesi L, Furukawa T, Egawa S, Ishida M, Ottomo S, Unno M, Motoi F, Wolfgang CL, Edil BH, Cameron JL, Eshleman JR, Schulick RD, Maitra A, Hruban RH (2012) Clinicopathological characteristics and molecular analyses of multifocal intraductal papillary mucinous neoplasms of the pancreas. Ann Surg 255(2):326–333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fritz S, Schirren M, Klauss M, Bergmann F, Hackert T, Hartwig W, Strobel O, Grenacher L, Büchler MW, Werner J (2012) Clinicopathologic characteristics of patients with resected multifocal intraductal papillary mucinous neoplasm of the pancreas. Surgery. 152(3 Suppl 1):S74–S80 [DOI] [PubMed] [Google Scholar]
- 24.Griffin JF, Page AJ, Samaha GJ et al. (2017) Patients with a resected pancreatic mucinous cystic neoplasm have a better prognosis than patients with an intraductal papillary mucinous neoplasm: a large single institution series. Pancreatology 17(3):490–496 [DOI] [PubMed] [Google Scholar]
- 25.Adsay NV, Merati K, Basturk O, Iacobuzio-Donahue C, Levi E, Cheng JD, Sarkar FH, Hruban RH, Klimstra DS (2004) Pathologically and biologically distinct types of epithelium in intraductal papillary mucinous neoplasms: delineation of an “intestinal” pathway of carcinogenesis in the pancreas. Am J Surg Pathol 28(7):839–848 [DOI] [PubMed] [Google Scholar]
- 26.Noë M, Brosens LAA (2020) Gastric- and intestinal-type IPMN: two of a kind? Virchows Arch 477(1):17–19 [DOI] [PubMed] [Google Scholar]
- 27.Kobayashi T, Omori Y, Ono Y et al. (2021) Pathways for the development of multiple epithelial types of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol 56(6):581–592 [DOI] [PubMed] [Google Scholar]
- 28.Furukawa T, Klöppel G, Volkan Adsay N, Albores-Saavedra J, Fukushima N, Horii A, Hruban RH, Kato Y, Klimstra DS, Longnecker DS, Lüttges J, Offerhaus GJA, Shimizu M, Sunamura M, Suriawinata A, Takaori K, Yonezawa S (2005) Classification of types of intraductal papillary-mucinous neoplasm of the pancreas: a consensus study. Virchows Arch 447(5):794–799 [DOI] [PubMed] [Google Scholar]
- 29.Seidel G, Zahurak M, Iacobuzio-Donahue C, Sohn TA, Adsay NV, Yeo CJ, Lillemoe KD, Cameron JL, Hruban RH, Wilentz RE (2002) Almost all infiltrating colloid carcinomas of the pancreas and periampullary region arise from in situ papillary neoplasms: a study of 39 cases. Am J Surg Pathol 26(1):56–63 [DOI] [PubMed] [Google Scholar]
- 30.Singhi AD, Wood LD, Parks E, Torbenson MS, Felsenstein M, Hruban RH, Nikiforova MN, Wald AI, Kaya C, Nikiforov YE, Favazza L, He J, McGrath K, Fasanella KE, Brand RE, Lennon AM, Furlan A, Dasyam AK, Zureikat AH, Zeh HJ, Lee K, Bartlett DL, Slivka A (2020) Recurrent rearrangements in PRKACA and PRKACB in intraductal oncocytic papillary neoplasms of the pancreas and bile duct. Gastroenterology. 158(3):573–582 e572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim H, Ro JY (Mar 2018) Intraductal tubulopapillary neoplasm of the pancreas: an overview. Arch Pathol Lab Med 142(3):420–423 [DOI] [PubMed] [Google Scholar]
- 32.Basturk O, Adsay V, Askan G, Dhall D, Zamboni G, Shimizu M, Cymes K, Carneiro F, Balci S, Sigel C, Reid MD, Esposito I, Baldaia H, Allen P, Klöppel G, Klimstra DS (2017) Intraductal Tubulopapillary neoplasm of the pancreas: a clinicopathologic and immunohistochemical analysis of 33 cases. Am J Surg Pathol 41(3):313–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yamaguchi H, Shimizu M, Ban S, Koyama I, Hatori T, Fujita I, Yamamoto M, Kawamura S, Kobayashi M, Ishida K, Morikawa T, Motoi F, Unno M, Kanno A, Satoh K, Shimosegawa T, Orikasa H, Watanabe T, Nishimura K, Ebihara Y, Koike N, Furukawa T (2009) Intraductal tubulopapillary neoplasms of the pancreas distinct from pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 33(8):1164–1172 [DOI] [PubMed] [Google Scholar]
- 34.Basturk O, Hong SM, Wood LD, Adsay NV, Albores-Saavedra J, Biankin AV, Brosens LA, Fukushima N, Goggins M, Hruban RH, Kato Y, Klimstra DS, Klöppel G, Krasinskas A, Longnecker DS, Matthaei H, Offerhaus GJ, Shimizu M, Takaori K, Terris B, Yachida S, Esposito I, Furukawa T, Baltimore Consensus Meeting (2015) A revised classification system and recommendations from the Baltimore consensus meeting for neoplastic precursor lesions in the pancreas. Am J Surg Pathol 39(12):1730–1741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hruban RH, Takaori K, Klimstra DS, Adsay NV, Albores-Saavedra J, Biankin AV, Biankin SA, Compton C, Fukushima N, Furukawa T, Goggins M, Kato Y, Klőppel G, Longnecker DS, Lűttges J, Maitra A, Offerhaus GJA, Shimizu M, Yonezawa S (2004) An illustrated consensus on the classification of pancreatic intraepithelial neoplasia and intraductal papillary mucinous neoplasms. Am J Surg Pathol 28(8):977–987 [DOI] [PubMed] [Google Scholar]
- 36.Amini N, Habib JR, Blair A et al. (2020) Invasive and non-invasive progression after resection of non-invasive intraductal papillary mucinous neoplasms. Ann Surg. 10.1097/SLA.0000000000004488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rezaee N, Barbon C, Zaki A, He J, Salman B, Hruban RH, Cameron JL, Herman JM, Ahuja N, Lennon AM, Weiss MJ, Wood LD, Wolfgang CL (2016) Intraductal papillary mucinous neoplasm (IPMN) with high-grade dysplasia is a risk factor for the subsequent development of pancreatic ductal adenocarcinoma. HPB (Oxford) 18(3):236–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cizginer S, Turner BG, Bilge AR, Karaca C, Pitman MB, Brugge WR (Oct 2011) Cyst fluid carcinoembryonic antigen is an accurate diagnostic marker of pancreatic mucinous cysts. Pancreas. 40(7): 1024–1028 [DOI] [PubMed] [Google Scholar]
- 39.Jhala N, Srimunta P, Jhala D (2020) Role of ancillary testing on endoscopic US-guided fine needle aspiration samples from cystic pancreatic neoplasms. Acta Cytol 64(1–2):124–135 [DOI] [PubMed] [Google Scholar]
- 40.Rosenbaum MW, Jones M, Dudley JC, Le LP, Iafrate AJ, Pitman MB (2017) Next-generation sequencing adds value to the preoperative diagnosis of pancreatic cysts. Cancer Cytopathol 125(1):41–47 [DOI] [PubMed] [Google Scholar]
- 41.Singhi AD, McGrath K, Brand RE, Khalid A, Zeh HJ, Chennat JS, Fasanella KE, Papachristou GI, Slivka A, Bartlett DL, Dasyam AK, Hogg M, Lee KK, Marsh JW, Monaco SE, Ohori NP, Pingpank JF, Tsung A, Zureikat AH, Wald AI, Nikiforova MN (2018) Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut. 67(12):2131–2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pitman MB, Centeno BA, Ali SZ, Genevay M, Stelow E, Mino-Kenudson M, Castillo CF, Schmidt CM, Brugge WR, Layfield LJ (2014) Standardized terminology and nomenclature for pancreatobiliary cytology: The Papanicolaou Society of Cytopathology Guidelines. Cytojournal. 11(Suppl 1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michaels PJ, Brachtel EF, Bounds BC, Brugge WR, Pitman MB (2006) Intraductal papillary mucinous neoplasm of the pancreas: cytologic features predict histologic grade. Cancer 108(3):163–173 [DOI] [PubMed] [Google Scholar]
- 44.Smith AL, Abdul-Karim FW, Goyal A (2016) Cytologic categorization of pancreatic neoplastic mucinous cysts with an assessment of the risk of malignancy: a retrospective study based on the Papanicolaou Society of Cytopathology guidelines. Cancer Cytopathol 124(4):285–293 [DOI] [PubMed] [Google Scholar]
- 45.Pitman MB, Centeno BA, Daglilar ES, Brugge WR, Mino-Kenudson M (2014) Cytological criteria of high-grade epithelial atypia in the cyst fluid of pancreatic intraductal papillary mucinous neoplasms. Cancer Cytopathol 122(1):40–47 [DOI] [PubMed] [Google Scholar]
- 46.Adsay NV, Merati K, Andea A, Sarkar F, Hruban RH, Wilentz RE, Goggins M, Iocobuzio-Donahue C, Longnecker DS, Klimstra DS (2002) The dichotomy in the preinvasive neoplasia to invasive carcinoma sequence in the pancreas: differential expression of MUC1 and MUC2 supports the existence of two separate pathways of carcinogenesis. Mod Pathol 15(10):1087–1095 [DOI] [PubMed] [Google Scholar]
- 47.Lüttges J, Zamboni G, Longnecker D, Klöppel G (2001) The immunohistochemical mucin expression pattern distinguishes different types of intraductal papillary mucinous neoplasms of the pancreas and determines their relationship to mucinous noncystic carcinoma and ductal adenocarcinoma. Am J Surg Pathol 25(7):942–948 [DOI] [PubMed] [Google Scholar]
- 48.Terris B, Dubois S, Buisine MP, Sauvanet A, Ruszniewski P, Aubert JP, Porchet N, Couvelard A, Degott C, Fléjou JF (2002) Mucin gene expression in intraductal papillary-mucinous pancreatic tumours and related lesions. J Pathol 197(5):632–637 [DOI] [PubMed] [Google Scholar]
- 49.Basturk O, Chung SM, Hruban RH, Adsay NV, Askan G, Iacobuzio-Donahue C, Balci S, Zee SY, Memis B, Shia J, Klimstra DS (2016) Distinct pathways of pathogenesis of intraductal oncocytic papillary neoplasms and intraductal papillary mucinous neoplasms of the pancreas. Virchows Arch 469(5):523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mattiolo P, Hong SM, Paolino G et al. (2020) CD117 is a specific marker of intraductal papillary mucinous neoplasms (IPMN) of the pancreas, oncocytic subtype. Int J Mol Sci 21(16):5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ciprani D, Morales-Oyarvide V, Qadan M, Hank T, Weniger M, Harrison JM, Rodrigues C, Horick NK, Mino-Kenudson M, Ferrone CR, Warshaw AL, Lillemoe KD, Fernández-del Castillo C (Jun 2020) An elevated CA 19-9 is associated with invasive cancer and worse survival in IPMN. Pancreatology. 20(4):729–735 [DOI] [PubMed] [Google Scholar]
- 52.Wu J, Jiao Y, Dal Molin M et al. (2011) Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc Natl Acad Sci U S A 108(52):21188–21193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wu J, Matthaei H, Maitra A et al. (2011) Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci Transl Med 3(92):92ra66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fujikura K, Hosoda W, Felsenstein M et al. (2020) Multiregion whole-exome sequencing of intraductal papillary mucinous neoplasms reveals frequent somatic KLF4 mutations predominantly in low-grade regions. Gut 70(5):928–939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schonleben F, Qiu W, Ciau NT, et al. PIK3CA mutations in intraductal papillary mucinous neoplasm/carcinoma of the pancreas. Clin Cancer Res. Jun 15 2006;12(12):3851–3855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Amato E, Molin MD, Mafficini A et al. (2014) Targeted next-generation sequencing of cancer genes dissects the molecular profiles of intraductal papillary neoplasms of the pancreas. J Pathol 233(3):217–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Huang B, Trujillo MA, Fujikura K, Qiu M, Chen F, Felsenstein M, Zhou C, Skaro M, Gauthier C, Macgregor-Das A, Hutchings D, Hong SM, Hruban RH, Eshleman JR, Thompson ED, Klein AP, Goggins M, Wood LD, Roberts NJ (2020) Molecular characterization of organoids derived from pancreatic intraductal papillary mucinous neoplasms. J Pathol 252(3):252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vyas M, Hechtman JF, Zhang Y, Benayed R, Yavas A, Askan G, Shia J, Klimstra DS, Basturk O (Apr 2020) DNAJB1-PRKACA fusions occur in oncocytic pancreatic and biliary neoplasms and are not specific for fibrolamellar hepatocellular carcinoma. Mod Pathol 33(4):648–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Noë M, Niknafs N, Fischer CG et al. (2020) Genomic characterization of malignant progression in neoplastic pancreatic cysts. Nat Commun 11(1):4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson C, Estrada A, Zaheer A et al. (2018) Clinical and radio-graphic gastrointestinal abnormalities in McCune-Albright syndrome. J Clin Endocrinol Metab 103(11):4293–4303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wood LD, Noë M, Hackeng W, Brosens LAA, Bhaijee F, Debeljak M, Yu J, Suenaga M, Singhi AD, Zaheer A, Boyce A, Robinson C, Eshleman JR, Goggins MG, Hruban RH, Collins MT, Lennon AM, Montgomery EA (2017) Patients with McCune-Albright syndrome have a broad spectrum of abnormalities in the gastrointestinal tract and pancreas. Virchows Arch 470(4):391–400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Skaro M, Nanda N, Gauthier C, Felsenstein M, Jiang Z, Qiu M, Shindo K, Yu J, Hutchings D, Javed AA, Beckman R, He J, Wolfgang CL, Thompson E, Hruban RH, Klein AP, Goggins M, Wood LD, Roberts NJ (2019) Prevalence of germline mutations associated with cancer risk in patients with intraductal papillary mucinous neoplasms. Gastroenterology. 156(6):1905–1913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sato N, Rosty C, Jansen M, Fukushima N, Ueki T, Yeo CJ, Cameron JL, Iacobuzio-Donahue CA, Hruban RH, Goggins M (2001) STK11/LKB1 Peutz-Jeghers gene inactivation in intraductal papillary-mucinous neoplasms of the pancreas. Am J Pathol 159(6):2017–2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sahin F, Maitra A, Argani P, Sato N, Maehara N, Montgomery E, Goggins M, Hruban RH, Su GH (2003) Loss of Stk11/Lkb1 expression in pancreatic and biliary neoplasms. Mod Pathol 16(7): 686–691 [DOI] [PubMed] [Google Scholar]
- 65.Omori Y, Ono Y, Tanino M, Karasaki H, Yamaguchi H, Furukawa T, Enomoto K, Ueda J, Sumi A, Katayama J, Muraki M, Taniue K, Takahashi K, Ambo Y, Shinohara T, Nishihara H, Sasajima J, Maguchi H, Mizukami Y, Okumura T, Tanaka S (2019) Pathways of progression from intraductal papillary mucinous neoplasm to pancreatic ductal adenocarcinoma based on molecular features. Gastroenterology. 156(3):647–661 e642 [DOI] [PubMed] [Google Scholar]
- 66.Felsenstein M, Noë M, Masica DL, Hosoda W, Chianchiano P, Fischer CG, Lionheart G, Brosens LAA, Pea A, Yu J, Gemenetzis G, Groot VP, Makary MA, He J, Weiss MJ, Cameron JL, Wolfgang CL, Hruban RH, Roberts NJ, Karchin R, Goggins MG, Wood LD (2018) IPMNs with co-occurring invasive cancers: neighbours but not always relatives. Gut. 67(9):1652–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matthaei H, Wu J, Dal Molin M, Shi C, Perner S, Kristiansen G, Lingohr P, Kalff JC, Wolfgang CL, Kinzler KW, Vogelstein B, Maitra A, Hruban RH (2014) GNAS sequencing identifies IPMN-specific mutations in a subgroup of diminutive pancreatic cysts referred to as “incipient IPMNs”. Am J Surg Pathol 38(3):360–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fischer CG, Beleva Guthrie V, Braxton AM, Zheng L, Wang P, Song Q, Griffin JF, Chianchiano PE, Hosoda W, Niknafs N, Springer S, Dal Molin M, Masica D, Scharpf RB, Thompson ED, He J, Wolfgang CL, Hruban RH, Roberts NJ, Lennon AM, Jiao Y, Karchin R, Wood LD (2019) Intraductal papillary mucinous neoplasms arise from multiple independent clones, each with distinct mutations. Gastroenterology. 157(4):1123–1137 e1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pea A, Yu J, Rezaee N, Luchini C, He J, Dal Molin M, Griffin JF, Fedor H, Fesharakizadeh S, Salvia R, Weiss MJ, Bassi C, Cameron JL, Zheng L, Scarpa A, Hruban RH, Lennon AM, Goggins M, Wolfgang CL, Wood LD (2017) Targeted DNA sequencing reveals patterns of local progression in the pancreatic remnant following resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Surg 266(1):133–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kuboki Y, Fischer CG, Beleva Guthrie V, Huang W, Yu J, Chianchiano P, Hosoda W, Zhang H, Zheng L, Shao X, Thompson ED, Waters K, Poling J, He J, Weiss MJ, Wolfgang CL, Goggins MG, Hruban RH, Roberts NJ, Karchin R, Wood LD (2019) Single-cell sequencing defines genetic heterogeneity in pancreatic cancer precursor lesions. J Pathol 247(3):347–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hata T, Dal Molin M, Hong SM et al. (2017) Predicting the grade of dysplasia of pancreatic cystic neoplasms using cyst fluid dna methylation markers. Clin Cancer Res 23(14):3935–3944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Springer S, Masica DL, Dal Molin M et al. (2019) A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med 11(501):eaav4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Springer S, Wang Y, Dal Molin M, Masica DL, Jiao Y, Kinde I, Blackford A, Raman SP, Wolfgang CL, Tomita T, Niknafs N, Douville C, Ptak J, Dobbyn L, Allen PJ, Klimstra DS, Schattner MA, Schmidt CM, Yip-Schneider M, Cummings OW, Brand RE, Zeh HJ, Singhi AD, Scarpa A, Salvia R, Malleo G, Zamboni G, Falconi M, Jang JY, Kim SW, Kwon W, Hong SM, Song KB, Kim SC, Swan N, Murphy J, Geoghegan J, Brugge W, Fernandez-del Castillo C, Mino-Kenudson M, Schulick R, Edil BH, Adsay V, Paulino J, van Hooft J, Yachida S, Nara S, Hiraoka N, Yamao K, Hijioka S, van der Merwe S, Goggins M, Canto MI, Ahuja N, Hirose K, Makary M, Weiss MJ, Cameron J, Pittman M, Eshleman JR, Diaz LA Jr, Papadopoulos N, Kinzler KW, Karchin R, Hruban RH, Vogelstein B, Lennon AM (2015) A combination of molecular markers and clinical features improve the classification of pancreatic cysts. Gastroenterology. 149(6):1501–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kadayifci A, Atar M, Wang JL, Forcione DG, Casey BW, Pitman MB, Brugge WR (2017) Value of adding GNAS testing to pancreatic cyst fluid KRAS and carcinoembryonic antigen analysis for the diagnosis of intraductal papillary mucinous neoplasms. Dig Endosc 29(1):111–117 [DOI] [PubMed] [Google Scholar]
- 75.Schmitz D, Kazdal D, Allgauer M et al. (2021) KRAS/GNAS-testing by highly sensitive deep targeted next generation sequencing improves the endoscopic ultrasound-guided workup of suspected mucinous neoplasms of the pancreas. Genes Chromosom Cancer 60(7):489–497 [DOI] [PubMed] [Google Scholar]
- 76.Faias S, Duarte M, Pereira L et al. (2020) Methylation changes at the GNAS imprinted locus in pancreatic cystic neoplasms are important for the diagnosis of malignant cysts. World J Gastrointest Oncol 12(9):1056–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hong SM, Kelly D, Griffith M, Omura N, Li A, Li CP, Hruban RH, Goggins M (2008) Multiple genes are hypermethylated in intraductal papillary mucinous neoplasms of the pancreas. Mod Pathol 21(12):1499–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Sato N, Ueki T, Fukushima N, Iacobuzio–Donahue CA, Hruban RH, Goggins M, Yeo CJ, Cameron JL (2002) Aberrant methylation of CpG islands in intraductal papillary mucinous neoplasms of the pancreas. Gastroenterology. 123(1):365–372 [DOI] [PubMed] [Google Scholar]
- 79.Hong SM, Omura N, Vincent A et al. (2012) Genome-wide CpG island profiling of intraductal papillary mucinous neoplasms of the pancreas. Clin Cancer Res 18(3):700–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fujiyama Y, Kumamoto Y, Nishizawa N, Nakamoto S, Harada H, Yokota K, Tanaka Y, Igarashi K, Oiki H, Okuwaki K, Iwai T, Kajita S, Takahashi H, Tajima H, Kaizu T, Sasaki J, Watanabe M, Yamashita K (2020) Promoter DNA hypermethylation of the cysteine dioxygenase 1 (CDO1) gene in intraductal papillary mucinous neoplasm (IPMN). Ann Surg Oncol 27(10):4007–4016 [DOI] [PubMed] [Google Scholar]
- 81.Chan CS, Laddha SV, Lewis PW et al. (2018) ATRX, DAXX or MEN1 mutant pancreatic neuroendocrine tumors are a distinct alpha-cell signature subgroup. Nat Commun 9(1):4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morgell A, Reisz JA, Ateeb Z et al. (2021) Metabolic characterization of plasma and cyst fluid from cystic precursors to pancreatic cancer patients reveal metabolic signatures of bacterial infection. J Proteom Res 20(5):2725–2738 [DOI] [PubMed] [Google Scholar]
- 83.Alkharaan H, Lu L, Gabarrini G, Halimi A, Ateeb Z, Sobkowiak MJ, Davanian H, Fernández Moro C, Jansson L, del Chiaro M, Özenci V, Sällberg Chen M (2020) Circulating and salivary antibodies to fusobacterium nucleatum are associated with cystic pancreatic neoplasm malignancy. Front Immunol 11:2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaiser RA, Halimi A, Alkharaan H, Lu L, Davanian H, Healy K, Hugerth LW, Ateeb Z, Valente R, Fernández Moro C, del Chiaro M, Sällberg Chen M (2019) Enrichment of oral microbiota in early cystic precursors to invasive pancreatic cancer. Gut. 68(12):2186–2194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li S, Fuhler GM, Bn N et al. (2017) Pancreatic cyst fluid harbors a unique microbiome. Microbiome. 5(1):147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Riquelme E, Zhang Y, Zhang L et al. (2019) Tumor microbiome diversity and composition influence pancreatic cancer outcomes. Cell. 178(4):795–806 e712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Pflüger MJ, Griffin JF, Hackeng WM et al. (2020) The impact of clinical and pathological features on intraductal papillary mucinous neoplasm recurrence after surgical resection: long-term follow-up analysis. Ann Surg. 10.1097/SLA.0000000000004427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chari ST, Yadav D, Smyrk TC, DiMagno EP, Miller LJ, Raimondo M, Clain JE, Norton IA, Pearson RK, Petersen BT, Wiersema MJ, Farnell MB, Sarr MG (2002) Study of recurrence after surgical resection of intraductal papillary mucinous neoplasm of the pancreas. Gastroenterology. 123(5):1500–1507 [DOI] [PubMed] [Google Scholar]
- 89.Kim HS, Han Y, Kang JS et al. (2020) Fate of patients with intraductal papillary mucinous neoplasms of pancreas after resection according to the pathology and margin status: continuously increasing risk of recurrence even after curative resection suggesting necessity of lifetime surveillance. Ann Surg. 10.1097/SLA.0000000000004478 [DOI] [PubMed] [Google Scholar]
- 90.Hirono S, Shimizu Y, Ohtsuka T, Kin T, Hara K, Kanno A, Koshita S, Hanada K, Kitano M, Inoue H, Itoi T, Ueki T, Shimokawa T, Hijioka S, Yanagisawa A, Nakamura M, Okazaki K, Yamaue H (2020) Recurrence patterns after surgical resection of intraductal papillary mucinous neoplasm (IPMN) of the pancreas; a multicenter, retrospective study of 1074 IPMN patients by the Japan Pancreas Society. J Gastroenterol 55(1):86–99 [DOI] [PubMed] [Google Scholar]
- 91.Al Efishat M, Attiyeh MA, Eaton AA et al. (2018) Progression patterns in the remnant pancreas after resection of non-invasive or micro-invasive intraductal papillary mucinous neoplasms (IPMN). Ann Surg Oncol 25(6):1752–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Marchegiani G, Mino-Kenudson M, Sahora K, Morales-Oyarvide V, Thayer S, Ferrone C, Warshaw AL, Lillemoe KD, Fernández-del Castillo C (May 2015) IPMN involving the main pancreatic duct: biology, epidemiology, and long-term outcomes following resection. Ann Surg 261(5):976–983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paini M, Crippa S, Scopelliti F et al. (2014) Extent of surgery and implications of transection margin status after resection of IPMNs. Gastroenterol Res Pract 2014:269803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Dhar VK, Merchant NB, Patel SH, Edwards MJ, Wima K, Imbus J, Abbott DE, Weber SM, Louie R, Kim HJ, Martin RCG, Scoggins CR, Bentrem DJ, LeCompte MT, Idrees K, Lopez-Aguiar AG, Maithel SK, Kooby DA, Franco DA, Yakoub D, Ahmad SA (2018) Does surgical margin impact recurrence in noninvasive intraductal papillary mucinous neoplasms?: a multi-institutional study. Ann Surg 268(3):469–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Arbour KC, Rizvi H, Plodkowski AJ et al. (2021) Treatment outcomes and clinical characteristics of patients with KRAS-G12C-mutant non-small cell lung cancer. Clin Cancer Res 27(8):2209–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Douglass J, Hsiue EH, Mog BJ et al. (2021) Bispecific antibodies targeting mutant RAS neoantigens. Sci Immunol 6(57):eabd5515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Thein KZ, Biter AB, Hong DS (2021) Therapeutics targeting mutant KRAS. Annu Rev Med 72:349–364 [DOI] [PubMed] [Google Scholar]
- 98.Yu J, Yusoff PAM, Woutersen DTJ et al. (2020) The functional landscape of patient-derived RNF43 mutations predicts sensitivity to Wnt inhibition. Cancer Res 80(24)):5619–5632 [DOI] [PubMed] [Google Scholar]


