Abstract
Introduction
Ochratoxin A (OTA) is a mycotoxin notably produced by Aspergillus and Penicillium spp. Bacillus subtilis fermentation extract (BSFE) contains specific enzymes which hydrolyse OTA. This study evaluated the efficiency of BSFE in ameliorating the immunotoxic and nephrotoxic effects of OTA in broiler chickens.
Material and Methods
Day-old broiler chicks were divided equally into four groups of ten: control, OTA (0.5 mg/kg feed), BSFE product (1 mL/L water) and OTA + BSFE at the same concentrations. The chicks were vaccinated against avian influenza, Newcastle disease, and infectious bronchitis, and lymphoproliferation was induced in all birds by phytohaemagglutinin-P (PHA-P). Serum samples were taken before sacrifice and organ tissue samples were taken after, in which renal function biomarkers were assayed and the presence of OTA residue was evaluated by high-performance thin-layer chromatography. Protein markers of apoptosis were determined by qPCR, and tissue lesions were examined histopathologically.
Results
Exposure to OTA significantly decreased the antibody response to the vaccines and the lymphoproliferative response to PHA-P, and significantly elevated the renal function indicators: serum urea, uric acid and creatinine. It also induced oxidative stress (reduced catalase activity and glutathione concentration), lipid peroxidation (increased malondialdehyde content), apoptosis (increased Bax and Caspase-3 and decreased Bcl-2 gene levels) and pathological lesions in kidney, bursa of Fabricius, spleen and thymus tissue. Residues of OTA were detected in the serum and tissue. BSFE mitigated most of these toxic effects.
Conclusion
BSFE counters OTA-induced immunotoxicity and nephrotoxicity because of its content of carboxypeptidase and protease enzymes.
Keywords: apoptosis, Bacillus subtilis, carboxypeptidase, mycotoxins, oxidative stress
Introduction
Ochratoxin (OT), one of the mycotoxins contaminating food, is produced as a secondary metabolite during the growth of some toxigenic fungal species, e.g. Aspergillus and Penicillium. The favourable conditions for fungal growth are warmth, high humidity and lack of ventilation, together with deficient adherence to quality control procedures, which are almost unavailable to implement in the developing countries. These circumstances can lead to the accumulation of mycotoxins to levels which are harmful to humans and animals (28). Barley, wheat and oats, cereal products, potatoes, nuts and wine are frequently spoiled with OT. Ochratoxin A (OTA) is an extremely toxic and frequent type of ochratoxin, of which A, B and C variants are known. The chemical structure of OTA comprises two parts; a dihydroisocoumarin (OTα) and L-phenylalanine, which are linked via an amide group (15). Previous studies suggested that lipid peroxidation, suppression of protein synthesis, inhibition of ATP production by mitochondria and apoptosis are the main mechanisms of OTA intoxication (13).
Ingestion of OTA-contaminated food produces a well-known disease in humans, mammals and birds called ochratoxicosis. Ochratoxin A, a specific nephrotoxin, is a cause of the condition in humans called Balkan endemic nephropathy, as well as one of the main causes of chicken nephritis (17). Moreover, OTA induces apoptosis in the kidney nephrons (27). However, the diversity of the modes of action of OTA results in a variety of toxic impacts, such as immunotoxicity, hepatotoxicity, mutagenicity and teratogenicity (17). Several studies demonstrated that OTA reduced the primary immune tissue size (the bursa of Fabricius, spleen and thymus) (6). Previous studies proved that the maximum recommended level of OTA (0.1 mg OTA/kg) spiked in poultry feed diminished thymic tissue size and decreased total protein, albumin and globulin concentrations in serum; however, other variables including performance, liver enzyme levels, haematology parameters and kidney function were not changed (19). Ochratoxin A-induced suppression of the immune functions of birds was mentioned in a previous report (12).
As is well-known, food contamination with OTA cannot be completely avoided. Consequently, control measures including methods to inhibit fungal growth, remove toxin from contaminated foodstuffs or reduce its bioavailability in organs and systems are in practice. Using more than one control measure is the correct way to decrease OTA contamination and mitigate its expected adverse effects. A Bacillus subtilis fermentation extract (BSFE) product (IFT Animal Health/Avial, Cairo, Egypt) provides two counteractions to this toxin. The first is the breakdown of OTA to nontoxic ochratoxin α (OTα) by the enzymes in Bacillus subtilis fermentation extract (16). Bacillus subtilis is commonly used as a probiotic in the food industry because of its low price, safety and ability to produce enzymes such as proteases, amylase, lipase and carboxypeptidase (20). In particular, carboxypeptidase A, an enzyme first extracted from the bovine pancreas, is responsible for the hydrolysis of the amide link of OTA to phenylalanine and the non-toxic ochratoxin α (18). The second toxin neutralising property derives from its content of the vitamins pantothenic acid, riboflavin, pyridoxine and thiamine, the organic citric, phosphoric and malic acids, and the salts of these acids. These valuable nutrients ameliorate the toxic effects of OTA by boosting the activity of the liver and kidneys as well as improving overall immune parameters (14).
Despite previous studies that discussed the OTA-induced toxic impacts in broiler chickens and different attempts to protect poultry against these harmful effects, the mechanism of toxicity of OTA in different organs is still under investigation. To our knowledge, no study has explored the ability of a combination of Bacillus subtilis fermentation extract, vitamins and organic acids against the toxicity of OTA. Therefore, the present study aims to investigate the mechanistic pathways by which OTA can induce its immunotoxic and nephrotoxic effects and also to test the ability of BSFE to ameliorate them.
Material and Methods
Ochratoxin A. The toxin was produced by culturing a pure strain of Aspergillus ochraceus in Erlenmeyer flasks of 1L capacity containing broken wheat grains and leaving the contents to ferment for 14 days at 28℃ (24). After the incubation period, water : acetonitrile (1 : 6) was prepared for the extraction of OTA from the fermented wheat, and then the OTA quantity was evaluated using ELISA.
Birds and experimental design. Forty Cobb broiler chicks at the age of one day were obtained from a local poultry flock and were housed in a healthy and disinfected environment. They were allowed three days for acclimatisation to the new laboratory conditions. They were next divided randomly into four experimental groups; each group contained 10 birds and was divided into two duplicates (5 birds each). The chicks were fed on a formulated diet based on corn and soybean according to the US National Research Council poultry nutrient requirements. All chicks were provided with water and feed ad libitum for 42 days. Care and management were approved by the Institutional Animal Care and Use Committee of Cairo University and complied with the guidelines of the US National Institutes of Health. The experimental groups were allocated as follows:
Group I (Control): did not receive any treatment;
Group II (OTA): received OTA at 0.5 mg/kg feed on the precedent set by Elaroussi et al. (6);
Group III (BSFE): received BSFE product at 1 mL/L drinking water, according to the recommendations of the manufacturer, IFT Animal Health/Avial (Cairo, Egypt);
Group IV (OTA + BSFE): received OTA and BSFE at the concentrations adopted for the other groups.
Assessment of antibody response. Chicks were immunised against H5N1 avian influenza (AI) and Newcastle disease (ND) by subcutaneous injection of inactivated vaccines on day 8. They were also vaccinated against infectious bronchitis (IB) on day 8 through intra-ocular instillation. After 21 days, the wing veins were punctured to collect the blood, and then serum was obtained by centrifugation (3,000 rpm for 10 min). Antibody titres against AI and ND were measured using a haemagglutination inhibition test (HI) according to Allan and Gough (2). However, ELISA was used to determine the titre of anti-IB antibodies, following the instructions of the ProFLOK kit (Zoetis, Edison, NJ, USA).
Lymphoproliferative response to phytohaemagglutinin-P. Phytohaemagglutinin-P (PHA-P), a mitogen that induces the proliferation of lymphocytes, was obtained from Sigma-Aldrich (St. Louis, MO, USA). The birds were injected with 50 μg of PHA-P/100 μL phosphate-buffered saline (PBS) intradermally into the toe web skin of the right foot. The left foot was injected with 100 μL of PBS as a control. Twenty-four hours post injection, the toe web thickness was measured with a micrometer (Global Sources, Shanghai, China). Subsequently, the difference between the thickness of the skin of the right toe web and that of the left toe web was calculated (5).
Sample collection and preparation. On the last day of the experiment (day 42), the chickens were deprived of food for 12 h and then blood was collected from the wing vein and centrifuged to obtain serum samples, which were preserved at −70℃ for biochemical analysis. The chickens were then euthanised by exsanguination and samples of kidney, bursa of Fabricius, spleen, thymus, liver and muscle tissue were obtained and processed in three different ways to suit the proceeding laboratory work. The first sample portion was specified for histological examination and fixed in 10% neutral buffer formalin. The second portion was flash-frozen and stored at −70℃ for gene expression analysis and detection of OTA residues. The third portion was homogenised with a glass Teflon homogeniser in 10 mM potassium phosphate cold buffer (pH 7.4) to obtain 10% tissue homogenate then centrifuged at 4,000 rpm for 15 min, after which the supernatant was transferred to another tube and preserved at −70℃ for subsequent oxidative stress analysis.
Serum biochemical analysis. The levels of serum metabolic wastes creatinine (CRE), uric acid (UA) and blood urea nitrogen (BUN) were analysed using purchased kits (Biodiagnostic, Cairo, Egypt). All preparations and procedures were performed following the recommended instructions.
Measurement of oxidative stress and lipid peroxidation parameters. Catalase (CAT) and glutathione (GSH) levels as respective enzymatic and non-enzymatic antioxidants, and the malondialdehyde (MDA) level were determined in the kidney, bursa, spleen and thymus tissue samples using commercially available kits (Biodiagnostic).
Determination of ochratoxin A residues in serum and edible tissue samples. Approximately 2 g of kidney, liver and muscle tissue and 2 mL of serum were used to determine the amount of OTA contained in these samples.
Extraction and clean-up. A 2 g mass of each tissue sample was homogenised using 10 mL of chloroform, and the homogenate was then centrifuged at 3,500 rpm for 15 min and filtered. A rotary evaporator was then used to evaporate the elute, and the remaining part was dissolved in methanol. The whole extract was cleaned up using the solid-phase extraction technique and OchraTest WB immunoaffinity columns (Vicam, Milford, MA, USA). The columns were washed and dried, and then the OTA was eluted with 5 mL methanol : acetic acid (49 : 1). The solution was evaporated under a stream of nitrogen, and the residues were dissolved again in 200 μL toluene : acetic acid (99 : 1) mobile phase, giving a solution ready for analysis. The method was validated by the protocol approved by the Association of Official Analytical Chemists (25).
For serum samples, 2 mL of serum were added to an acidified solution consisting of 0.1 M MgCl2 and 5 mL of chloroform in a centrifuge tube, and then the tube was rotated for 10 min (20 turns/min). The solution was subsequently centrifuged at 1,600 rpm for 10 min. A nitrogen stream was used to evaporate 2 mL of the chloroform extract, and the residues were then redissolved in 1 mL of methanol (10).
High-performance thin-layer chromatography (HPTLC). A standard OTA solution (40 μL/mL) for TLC (Sigma-Aldrich, St. Louis, MO, USA) was dissolved in toluene/acetic acid (99 : 1). A 30 μL volume of the standard solution and an identical volume of the residues were spotted onto TLC silica gel plates 20 cm × 10 cm (Merck, Darmstadt, Germany). The base of the plate was soaked in toluene : ethyl acetate : formic acid (6 : 3 : 1) and then dried. As the next step, the plate was observed under ultraviolet light (365 nm) and the standard fluorescence intensity was compared to those of the samples. The OTA concentration was calculated by comparing the area of the chromatographic peak of the sample to that of the standard curve by densitometry analysis (25).
Histopathological examination. Tissue specimens from kidney, the bursa of Fabricius, spleen, and thymus were preserved in 10% neutral buffer formalin for 48 h, then treated by the classical method utilising an ascending alcohol gradient and xylene. Subsequently, the treated samples were embedded in paraffin wax to form a block, which was sliced with an ordinary microtome into 4 μm-thick sections and stained with haematoxylin and eosin. The sections were examined under an Olympus BX43 light microscope and captured by an Olympus DP27 camera sending data to cellSens Dimension software (23).
The semiquantitative multi-parametric ordinal scoring system was used to grade the microscopic lesions observed in the examined organs according to criteria applied previously (9, 22). Briefly, all the lesions of various types observed in different organs, such as cellular degeneration and necrosis, interstitial oedema, haemorrhage, and inflammatory cell infiltration, were assessed and scored on a scale of 0–5 according to the percentage of damaged tissue per total microscopic field. Score 0 indicated no tissue damage and 1, 2, 3, 4 and 5 corresponded to <10%, 10–25%, 25–50%, 50–75%, and >75% tissue damage, respectively. Furthermore, the observed focal lesions; namely, focal inflammation, necrosis, and haemorrhage, were scored in the method reported by Hassanen et al. (8) as follows: 0 - no foci, 1 - <3 foci, 2 - 3–6 foci, 3 - 7–12 foci and 4 - >12 foci/field at low magnification power (100 ×). All analyses were performed blinded.
Gene expression analysis. To obtain total RNA from the kidney, bursa of Fabricius, spleen and thymus tissue, the commercial Biospin RNA Extraction Kit (Bioer, Hangzhou, China) was used and the manufacturer’s instructions were followed. After this step, DNase I enzyme was added to obtain DNA-free RNA, and the purity and yield of the total extracted RNA was determined using a NanoDrop ND 1000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). Reverse transcriptase (Bio Basic, Burlington, ON, Canada) and oligo-dT were then added to the extracted RNA to obtain complementary DNA (cDNA), following the instructions. Then BioEasy SYBR Green Master Mix (Bioer, Hangzhou, China) and primers were added to the cDNA, and the expression levels of B-cell lymphoma 2 (Bcl-2), B-cell lymphoma 2–associated X protein (Bax), Caspase-3 and β-actin were measured by real-time PCR. Agarose gel electrophoresis was used to confirm the amplicon size. The gene : β-actin ratio was calculated and gene expression in different groups was also calculated, this as relative ratio to the control group. A 1.0 value was considered the control group value. The calculated values were equalised to β-actin. The primers’ base sequences are contained in Table 1.
Table 1.
The primer sequences used in gene expression analysis
| Primer | Sequence | Accession number | Amplicon size (bp) |
|---|---|---|---|
| Bcl-2 | F: TTCAAGCGAAAACAGGGTGG R: CTCTGAGCACATGGAAAGCC |
NM_205339.2 | 167 |
| Bax | F: CACCTTTGTCTCACCTGTGC R: GATGGCAGTGATGAGCATGG |
XM_015290060.2 | 241 |
| CASP-3 | F: TTGAAGCAGACAGTGGACCA R: GTTCAAGTTTCCTGGCGTGT |
NM_204725.1 | 177 |
| β-actin | F: CCCACACCCCTGTGATGAAAR: TAGAACTTTGGGGGCGTTCG |
NM_205518.1 | 177 |
Bcl-2 – B-cell protein 2; Bax – Bcl-2-associated x protein; CASP-3 – caspase 3
Statistical analysis. Results are shown as mean ± standard deviation (SD) and the statistical analysis was carried out using a one-way ANOVA test in SPSS software (Chicago, IL, USA). Tukey’s post-hoc test was used to compare the mean values of different groups. The difference was considered statistically significant at P < 0.05.
Results
Antibody response to vaccination. The levels of specific antibodies against AI, ND and IB were significantly reduced in the OTA group as opposed to the control group. However, the administration of BSFE alone resulted in a non-significant change in the antibody titre from the control birds’ titre. The concurrent administration of OTA and BSFE engendered a non-significant difference in antibody levels in comparison to the control birds, while the antibody levels were significantly increased over those of the OTA group (Fig. 1).
Fig. 1.
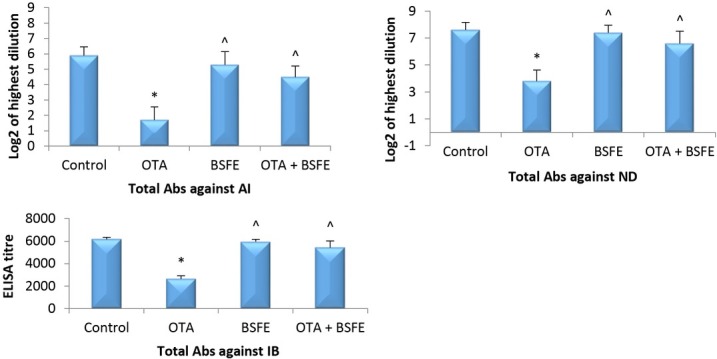
Impacts of ochratoxin A (OTA) and/or Bacillus subtilis fermentation extract (BSFE) on the specific antibody titres 21 days after vaccination (n = 5 birds/group)
* – P < 0.05 versus control group; ^ – P < 0.05 versus OTA group
Lymphoproliferative response to phytohaemagglutinin-P. The foot skin thickness of the OTA-intoxicated birds was significantly decreased from that of the control birds. On the other hand, administration of BSFE alone or concurrently with OTA did not produce a significant difference in the toe web thickness compared with that of the control birds. Moreover, the skin thickness was significantly increased in the OTA + BSFE group over that of the OTA birds (Fig. 2).
Fig. 2.
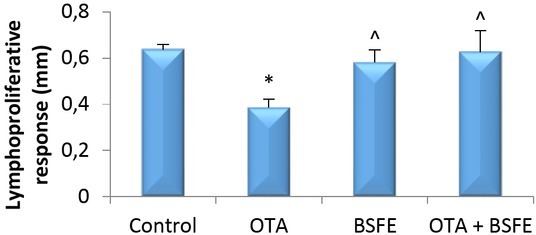
Impacts of ochratoxin A (OTA) and/or Bacillus subtilis fermentation extract (BSFE) on swelling of the toe web skin 21 days after phytohaemagglutinin-P injection (n = 5 birds/group)
* – P < 0.05 versus control group; ^ – P < 0.05 versus OTA group
Changes in serum biochemical parameters. The levels of CRE, UA and BUN were increased significantly in the OTA birds relative to the control birds. Additionally, there was no significant change in CRE, UA or BUN between the control, BSFE and OTA + BSFE groups. However, these levels were significantly reduced in the OTA + BSFE birds from those of the OTA birds (Fig. 3).
Fig. 3.
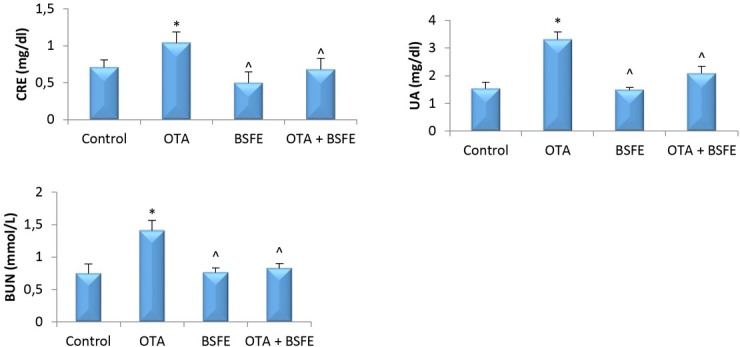
Analysis of serum metabolic wastes in chickens after exposure to ochratoxin A (OTA) and/or Bacillus subtilis fermentation extract (BSFE) (n = 5 birds/group)
CRE – creatinine; UA – uric acid; BUN – blood urea nitrogen; * – P < 0.05 versus control group; ^ – P <0.05 versus OTA group
Changes in oxidative and anti-oxidative parameters. The results showed that the GSH and CAT enzyme contents were significantly decreased, while the level of MDA was significantly increased in the kidney, bursa, spleen and thymus tissue of the OTA-intoxicated birds relative to the control birds. Additionally, non-significant changes in the contents of GSH, CAT and MDA were noticed between the control and BSFE and control and OTA + BSFE birds. The GSH and CAT levels were also significantly elevated, and the MDA level significantly reduced in the OTA + BSFE group as opposed to the OTA birds (Fig. 4).
Fig. 4.
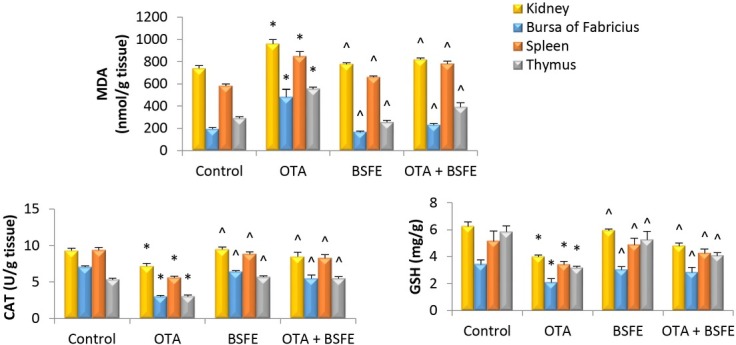
Impacts of ochratoxin A (OTA) and/or Bacillus subtilis fermentation extract (BSFE) on the levels of oxidative and antioxidative parameters in the kidney and immune tissues (n = 5 birds/group)
MDA – malondialdehyde; CAT – catalase; GSH – glutathione; * – P < 0.05 versus control group; ^ – P < 0.05 versus OTA group
Ochratoxin A residues in serum and different tissues. The results showed OTA content in the serum and tissues of the control and BSFE groups as undetectable. The highest OTA residues were detected in the renal tissue, and progressively lower residues were noted in the hepatic tissue, serum and muscles of broilers after exposure to OTA. The concentration of OTA was significantly decreased in the kidney, liver, muscle and serum when BSFE was administered concurrently with OTA (Table 2).
Table 2.
Ochratoxin A levels in serum and tissues
| Sample | Control | OTA | BSFE | OTA + BSFE |
|---|---|---|---|---|
| Serum (μg/L) | < LOD1 | 11.3 ± 1.7 | < LOD | 6.6 ± 1.37 ^ |
| Kidney (μg/Kg) | < LOD | 26.67 ± 6.03 | < LOD | 12.3 ± 4.36 ^ |
| Liver (μg/Kg) | < LOD | 14.83 ± 2.84 | < LOD | 5.25 ± 1.52 ^ |
| Muscle (μg/Kg) | < LOD | 8.77 ± 1.97 | < LOD | 2.9 ± 0.85 ^ |
OTA – ochratoxin A; BSFE - Bacillus subtilis fermentation extract; LOD – limit of detection; ^ – P < 0.05 versus ochratoxin A (OTA) group; 1– LOD was 1 μg/kg and 1 μg/L. Values are presented as mean ± SD (n = 3 birds/group)
Histopathological evaluation. Kidney sections obtained from birds in the control group and those receiving the BSFE product had a normal histological structure (Fig. 5a). Kidney sections of the group receiving OTA showed severe histopathological alterations in both tubules and glomeruli. The epithelial lining renal tubules showed either hyperplasia or vacuolation and necrosis followed by desquamation (Fig. 5b). There were focal to diffuse lymphocytic infiltrations in the interstitium (Fig. 5c). There were oedema and haemorrhages along with severe vascular congestion in the interstitial tissue (Fig. 5d). Most of the glomeruli showed proliferative glomerulopathy manifested by hypercellularity with thicker glomerular area and basement membranes. On the other hand, the group receiving BSFE + OTA exhibited less extensive damage in histopathology of kidney sections. Neither necrosis nor proliferation was observed in the renal tubules or glomeruli. Some sections showed mild to moderate degenerative changes in the tubular epithelium (Fig. 5e). Small focal areas of lymphocytic exocytosis were also evident in the interstitium of some sections (Fig. 5f).
Fig. 5.
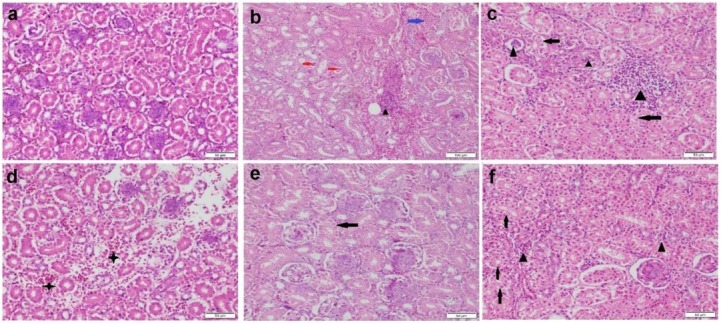
Photomicrograph of kidney tissue sections stained by haematoxylin and eosin. a – control group with normal histological structure; b–d – ochratoxin A (OTA) group showing vacuolar degeneration (black arrows), necrosis (blue arrow) and desquamation (red arrows) of tubular epithelium, tubular and interstitial inflammatory cell infiltration (black triangles) and interstitial haemorrhage (black stars); e–f – Bacillus subtilis fermentation extract + OTA group showing moderate vacuolar degeneration (black arrows) in some tubular epithelial cells and mild interstitial inflammatory cell infiltration (black triangles)
Spleen sections of the birds from the control group and those receiving BSFE showed normal histological organisation (Fig. 6a), while those obtained from the OTA group showed mild to moderate histopathological alterations. Some lymphoid follicles had moderate lymphocytolysis (Fig. 6b), while others had been replaced by coagulative necrotic area (Fig. 6c). The red pulp displayed congestion and a mild degree of haemorrhaging with fibrin deposition in the splenic sinuses. In other respects, the spleen sections obtained from birds receiving BSFE + OTA presented a normal histological structure (Fig. 6d).
Fig. 6.
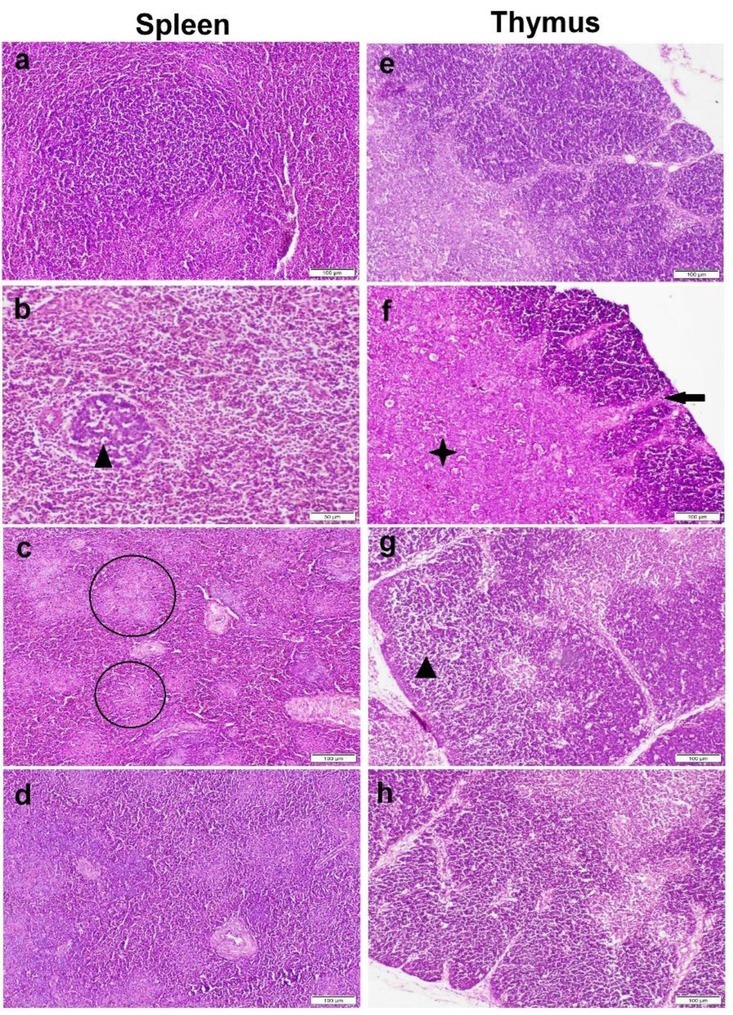
Photomicrograph of spleen (a–d) and thymus (e–h) tissue sections stained by haematoxylin and eosin. a and e – control group with normal histological structure; b and f, c and g – ochratoxin A (OTA) group showing lymphocytic cell depletion and lymphocytolysis (black triangles), multifocal areas of necrosis (circles), thinning in the cortical layer (black arrow) and expansion with extensive necrosis of medulla (black star); d and h – Bacillus subtilis fermentation extract + OTA group showing normal histological structure
The thymus sections from both the control group and the BSFE group illustrated similar and normal histological organisation (Fig. 6e). The thymus of the OTA-administered birds showed severe histopathological lesions. There was marked thinning of the cortical layer with the presence of multifocal areas of necrosis (Figs. 6f and 6g). The medulla had congestion and haemorrhages in most sections. In contrast, thymus sections obtained from birds receiving BSFE in parallel with OTA showed a marked improvement in the microscopic picture and appeared similar to those of the control group (Fig. 6h).
The bursa of Fabricius tissue sections obtained from birds in the control group and those administered BSFE presented normal histological architecture (Fig. 7a). Sections from the OTA group, however, had undergone a severe degree of pathological changes in most cases. The most prominent lesions observed in the bursal lymphoid follicles were lymphoid cell depletion, thinning of the lymphoid cortex and prominent corticomedullary basement membranes (Fig. 7b). Some follicles were in a state of complete destruction with the presence of large numbers of phagocytic macrophages. The interfollicular septa showed a marked widening with congestion, oedema, inflammatory cell infiltrations, and sometimes a small area of haemorrhaging (Fig. 7c and 7d). The group administered BSFE in combination with OTA presented a different picture of noticeably less degradation to some bursal lymphoid follicles, while other follicles still exhibited lymphocytolysis with the presence of tingible macrophages (Fig. 7e). Some sections evinced widening of the interfollicular septa by oedema and infiltration of a few inflammatory cells (Fig. 7f).
Fig. 7.
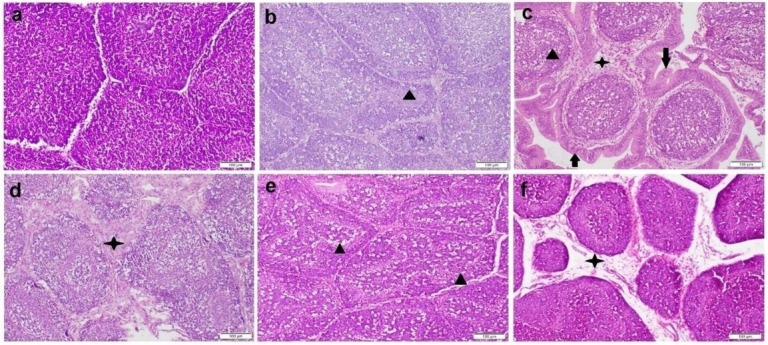
Photomicrograph of bursal tissue sections stained by haematoxylin and eosin. a – control group with normal histological architecture; b–d – ochratoxin A (OTA) group showing lymphocytic cell necrosis with large number of phagocytic cells (black triangles), interstitial widening by fibrinous exudate and inflammatory cell infiltration (black stars) and atrophy of the lobules with extensive corrugation of the basement membrane (black arrows); e and f – Bacillus subtilis fermentation extract + OTA group showing mild medullary lymphocytolysis (black triangles) and widening of the interfollicular septa by oedema and infiltration by a few inflammatory cells (black star)
The results of the microscopic lesion scoring of all examined organs are summarised in Table 3. The OTA-administered group was scored the highest for lesions in all organs among all the groups, being at greatest variance from the control group. There were also significantly lower lesion scores in the group receiving BSFE + OTA compared with the OTA-administered group. In addition, there were no significant differences between the lesion scores of the examined organs of the BSFE group and those of the control group.
Table 3.
Microscopic lesion scoring of the examined organs in different groups
| Lesion | Control | OTA | BSFE | OTA + BSFE |
|---|---|---|---|---|
| Microscopic renal lesion scoring | ||||
| RTD | 0 a | 5 b | 0 a | 3 c |
| RTN | 0 a | 5 b | 0 a | 3 c |
| RTP | 0 a | 3 b | 0 a | 2 c |
| Inflammation | 0 a | 5 b | 0 a | 1 c |
| Haemorrhaging | 0 a | 2 b | 0 a | 1 c |
| Congestion | 0 a | 3 c | 1 b | 1 b |
| Glomerular hypercellularity | 0 a | 3 b | 0 a | 0 a |
| Glomerular degeneration | 0 a | 4 b | 0 a | 2 c |
| Microscopic splenic lesion scoring | ||||
| Lymphocytolysis | 0 a | 3 b | 0 a | 0 a |
| Congestion | 0 a | 3 b | 0 a | 1 c |
| Microscopic thymic lesion scoring | ||||
| Lymphocytolysis | 0 a | 4 b | 0 a | 0 a |
| Congestion | 0 a | 2 b | 0 a | 0 a |
| Microscopic bursal lesion scoring | ||||
| Lymphocytolysis | 0 a | 5 b | 0 a | 1 c |
| Congestion | 0 a | 2 b | 0 a | 0 a |
| 0 a | 4 b | 0 a | 0 a | |
OTA – ochratoxin A; BSFE – Bacillus subtilis fermentation extract; RTD – renal tubular degeneration; RTN – renal tubular necrosis; RTP – renal tubular proliferation. Data are presented as median (n = 7 microscopic field/5 sections representing 5 birds/group). Different letters in the same column indicate a significant difference at P < 0.05
Gene expression analysis. The analysis of the apoptosis-related gene expression in different tissues is shown in Fig. 8. The OTA-intoxicated birds showed a significant elevation in the mRNA levels of Bax and CASP-3, while the Bcl-2 level was reduced significantly measured against the control birds. A non-significant difference was noted between the control and BSFE groups. In contrast, there was a significant increase in Bax and CASP-3 expression levels and a significant decrease in Bcl-2 expression level in the OTA + BSFE birds relative to the control birds. Conversely, the levels of Bax and CASP-3 were reduced significantly, whereas that of Bcl-2 was significantly raised in the OTA + BSFE birds in comparison to the OTA birds.
Fig. 8.
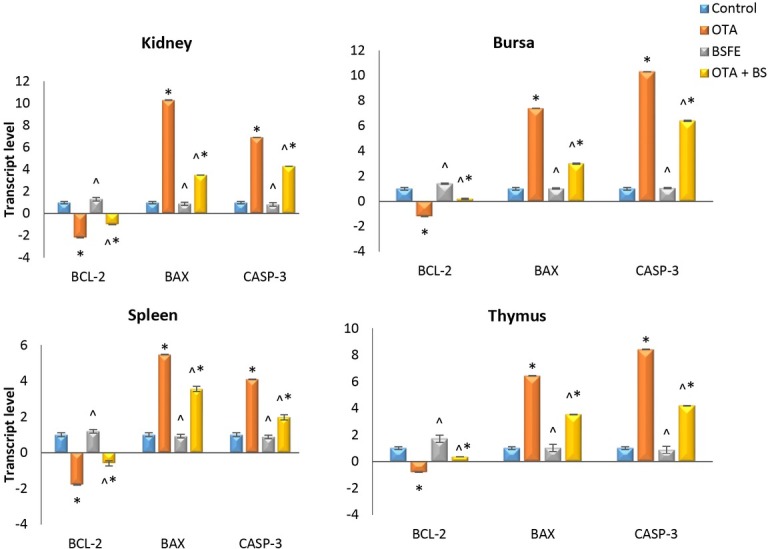
Impacts of ochratoxin A (OTA) and/ or Bacillus subtilis fermentation extract (BSFE) on gene expression levels of apoptosis-related genes in different tissues of broiler chickens (n = 3 birds/ group)
BCL-2 – B-cell lymphoma 2 protein; BAX – BCL-2-associated X protein; CASP-3 – caspase 3; * – P < 0.05 versus control group; ^ – P < 0.05 versus OTA group
Discussion
Recently, much effort has been made to find effective methods for the detoxification of mycotoxins contaminating food. The widespread harmful effects of OTA make attempts to find effective OTA mitigation materials of great interest. For this reason, we selected a source of Bacillus subtilis fermentation extract to examine its efficacy against OTA-induced immunotoxicity and nephrotoxicity in broiler chickens.
Adaptive immunity consists of two main responses, humoral and cellular. The humoral immune response depends mainly on specific antibodies, which are produced by activated B-lymphocytes against foreign antigens. As for cellular immunity, it depends on the proliferation of immune cells (T-lymphocytes and phagocytes) responding to specific antigens. Many previous studies discussed the immunotoxic impacts of OTA on various animals and cell lines. A previous experiment showed an evident reduction in antibody production in a dose-dependent manner in OTA-exposed broiler chickens after injection of sheep RBCs. The researchers also observed a weak lymphocyte response to PHA-P injected into the toe web skin (6). Furthermore, Liu et al. (12) reported that exposure of peripheral blood mononuclear cells to ochratoxin A results in cell cycle arrest in the G1 phase and induces apoptosis. Our results are in line with those of previous studies and showed a significant decrease in specific antibody production after AI, ND and IB vaccination in the OTA group. There was also a reduction in the measured toe web thickness after cutaneous PHA-P injection in OTA-exposed chickens.
Additionally, our results concur with those of another previous study (3) in that OTA induced kidney damage and produced a significant elevation in serum biochemical wastes (CRE, UA and BUN) when compared to the control group.
This OTA-induced immunosuppression and kidney damage may be attributable to oxidative damage resulting from increased ROS production, leading to the elevation of the MDA level and the reduction of GSH and CAT, which are normally found in cells as endogenous antioxidants (1). OTA-induced oxidative stress and lipid peroxidation also affect the histological structure of tissues and gene expression. The observed degenerative changes and necrosis of the proximal tubular cells after exposure to OTA were also demonstrated in a previous study (11). Degeneration, necrosis and enlargement of the periportal areas were also observed in liver tissue exposed to OTA (4), and lymphocytic depletion was observed in the splenic tissue of OTA-exposed pigs (7). Our results also showed degenerative changes and apoptosis in renal cells and depletion in lymphoid follicles with thinning of the cortical area in the birds consuming the OTA-contaminated diet.
It was reported that OTA-induced ROS overproduction led to the initiation of apoptosis through the suppression of survival pathways which restrain mitosis and initiate the death cascade (29, 30). In the current study, the expression levels of Bax (a proapoptotic protein) and Caspase-3 (an important enzyme in the execution phase of apoptosis) were significantly elevated and that of Bcl-2 (an antiapoptotic protein) was significantly reduced, which is evidence of the induction of apoptosis as a result of the overproduction of ROS induced by OTA.
Shi et al. (21) found that viable bacterial cells of Bacillus subtilis can inhibit the growth of Aspergillus ochraceus and Aspergillus carbonarius in contaminated maize. However, this effect may have been expected to be due to the competition between organisms for nutrients, and to exclude this explanation, cell-free supernatant of Bacillus subtilis was examined instead of viable bacterial cells, and the inhibitory effect of the cell-free supernatant was the same as that of the viable bacterial cells. Moreover, the cell-free supernatant also degraded the ochratoxin produced by fungi. The same authors concluded that Bacillus subtilis secretes metabolites in the culture medium, which are responsible for these effects. This effect was attributed to the carboxypeptidase and protease enzymes in the fermentation extract of Bacillus subtilis (26). In this study, we observed that the addition of BSFE product to drinking water concurrently with OTA-contaminated feed decreased the absorption and distribution of OTA to the tissue of different organs. The concentration of OTA in serum was significantly lower in the OTA + BSFE birds than it was in the OTA birds, which means that BSFE hydrolysed most of the OTA in the gastrointestinal tract. The distribution of OTA to the tissues in the intoxicated and BSFE-administered group was also significantly less than in the OTA group, and that explains the ameliorative effect of BSFE on kidney and immune tissues. The OTA + BSFE group exhibited cellular and humoral immune responses similar to those of the control group, and this group’s serum biochemical wastes were significantly less concentrated compared to those of the OTA group. This was attributable to the preservation of the normal histological structure of the kidney and immune organs due to the maintenance of the cellular oxidative and antioxidative status as well as the augmentation of the immune organ, liver and kidney functions due to the organic acid and vitamin content of the BSFE product. Moreover, the expression of apoptosis-related genes was reduced significantly in the OTA + BSFE group which is evidence of cell survival and the restraint of apoptosis.
In the current study, we tested a source of Bacillus subtilis fermentation extract to study its beneficial impacts on OTA-induced nephrotoxicity and immunotoxicity in broiler chickens. Exposure to OTA induced oxidative stress, apoptosis and different pathological injuries in the kidney and organs of immunity. We found that BSFE maintained intact renal and immunological functions. This protective effect is attributable to the carboxypeptidase and other protease enzymes in the Bacillus subtilis fermentation extract, which hydrolyse the OTA to non-toxic OTα. Further in vivo studies are needed to explore the ability of Bacillus subtilis fermentation extract to protect against other types of mycotoxins and to investigate other potential mechanisms of protection against these mycotoxins.
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: The source of funding of research and the article was Cairo University.
Animal Rights Statement: The authors declare that the experiments on animals were conducted in accordance with the Institutional Animal Care and Use Committee of Cairo University laws and regulations as regards the care and use of laboratory animals.
References
- 1.Abdelrahman R.E., Khalaf A.A.A., Elhady M.A., Ibrahim M.A., Hassanen E.I., Noshy P.A.. Quercetin ameliorates ochratoxin A-Induced immunotoxicity in broiler chickens by modulation of PI3K/AKT pathway. Chem Biol Interact. 2022;351:109720. doi: 10.1016/J.CBI.2021.109720. [DOI] [PubMed] [Google Scholar]
- 2.Allan W.H., Gough R.E.. A standard haemagglutination inhibition test for Newcastle disease. (2) Vaccination and challenge. Vet Rec. 1974;95:147–149. doi: 10.1136/vr.95.7.147. [DOI] [PubMed] [Google Scholar]
- 3.Andretta E., Longobardi C., Laselva M., Lauritano C., Avantaggiato G., Schiavone A., Jarriyawattanachaikul W., Florio S., Damiano S., Ciarcia R.. Protective effects of new antioxidants in OTA-treated chicken kidney. Med Sci Forum. 2021;2:18. doi: 10.3390/cahd2020-08617. [DOI] [Google Scholar]
- 4.Aydin G., Ozçelik N., Çiçek E., Soyöz M.. Histopathologic changes in liver and renal tissues induced by Ochratoxin A and melatonin in rats. Hum Exp Toxicol. 2003;22:383–391. doi: 10.1191/0960327103ht354oa. [DOI] [PubMed] [Google Scholar]
- 5.Corrier D.E.. Comparison of phytohemagglutinin-induced cutaneous hypersensitivity reactions in the interdigital skin of broiler and layer chicks. Avian Dis. 1990;34:369–373. doi: 10.2307/1591421. [DOI] [PubMed] [Google Scholar]
- 6.Elaroussi M.A., Mohamed F.R., El Barkouky E.M., Atta A.M., Abdou A.M., Hatab M.H.. Experimental ochratoxicosis in broiler chickens. Avian Pathol. 2006;35:263–269. doi: 10.1080/03079450600817115. [DOI] [PubMed] [Google Scholar]
- 7.Gan F., Hou L., Zhou Y., Liu Y., Huang D., Chen X., Huang K.. Effects of ochratoxin A on ER stress, MAPK signaling pathway and autophagy of kidney and spleen in pigs. Environ Toxicol. 2017;32:2277–2286. doi: 10.1002/tox.22443. [DOI] [PubMed] [Google Scholar]
- 8.Hassanen E.I., Korany R.M.S., Bakeer A.M.. Cisplatin-conjugated gold nanoparticles-based drug delivery system for targeting hepatic tumors. J Biochem Mol Toxicol. 2021;35:e22722. doi: 10.1002/jbt.22722. [DOI] [PubMed] [Google Scholar]
- 9.Hassanen E.I., Morsy E.A., Hussien A.M., Farroh K.Y., Ali M.E.. Comparative assessment of the bactericidal effect of nanoparticles of copper oxide, silver, and chitosan–silver against Escherichia coli infection in broilers. Biosci Rep. 2021;41 doi: 10.1042/BSR20204091. BSR 20204091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hult K., Hokby E., Gatenbeck S., Rutqvist L.. Ochratoxin A in blood from slaughter pigs in Sweden: Use in evaluation of toxin content of consumed feed. Appl Environ Microbiol. 1980;39:828–830. doi: 10.1128/aem.39.4.828-830.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li K., Cao Z., Guo Y., Tong C., Yang S., Long M., Li P., He J.. Selenium Yeast Alleviates Ochratoxin A-Induced Apoptosis and Oxidative Stress via Modulation of the PI3K/AKT and Nrf2/Keap1 Signaling Pathways in the Kidneys of Chickens. Oxid Med Cell Longev. 2020;2020:4048706. doi: 10.1155/2020/4048706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Wang Y., Cui J., Xing L., Shen H., Wu S., Lian H., Wang J., Yan X., Zhang X.. Ochratoxin A induces oxidative DNA damage and G1 phase arrest in human peripheral blood mononuclear cells in vitro. Toxicol Lett. 2012;211:164–171. doi: 10.1016/j.toxlet.2012.03.800. [DOI] [PubMed] [Google Scholar]
- 13.Mally A., Hard G.C., Dekant W.. Ochratoxin A as a potential etiologic factor in endemic nephropathy: Lessons from toxicity studies in rats. Food Chem Toxicol. 2007;45:2254–2260. doi: 10.1016/j.fct.2007.05.021. [DOI] [PubMed] [Google Scholar]
- 14.McDowell L.R. Vitamins in Animal and Human Nutrition, Second Edition. Iowa State University Press; Ames, IA: 2000. [DOI] [Google Scholar]
- 15.Otero P., Rodríguez P., Botana A.M., Alfonso A., Botana L.M. Fanali S., Haddad P.R., Poole C.F., Schoenmakers P., Lloyd D. Liquid Chromatography: Applications. Elsevier, Amsterdam; 2013. Chapter 15 Analysis of Natural Toxins; pp. 411–430. edited by. [DOI] [Google Scholar]
- 16.Petchkongkaew A., Taillandier P., Gasaluck P., Lebrihi A.. Isolation of Bacillus spp. from Thai fermented soybean (Thuanao): Screening for aflatoxin B1 and ochratoxin A detoxification. J Appl Microbiol. 2008;104:1495–1502. doi: 10.1111/j.1365-2672.2007.03700.x. [DOI] [PubMed] [Google Scholar]
- 17.Pfohl-Leszkowicz A., Manderville R.A.. Ochratoxin A: An overview on toxicity and carcinogenicity in animals and humans. Mol Nutr Food Res. 2007;51:1192. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 18.Pitout M.J.. The hydrolysis of ochratoxin A by some proteolytic enzymes. Biochem Pharmacol. 1969;18:485–491. doi: 10.1016/0006-2952(69)90224-X. [DOI] [PubMed] [Google Scholar]
- 19.Pozzo L., Salamano G., Mellia E., Gennero M.S., Doglione L., Cavallarin L., Tarantola M., Forneris G., Schiavone A.. Feeding a diet contaminated with ochratoxin A for chickens at the maximum level recommended by the EU for poultry feeds (0.1 mg/kg) 1. Effects on growth and slaughter performance, haematological and serum traits. J Anim Physiol Anim Nutr. 2013;97:13–22. doi: 10.1111/jpn.12050. [DOI] [PubMed] [Google Scholar]
- 20.Sathe S.K.. Dry bean protein functionality. Crit Rev Biotech. 2002;22:175–223. doi: 10.1080/07388550290789487. [DOI] [PubMed] [Google Scholar]
- 21.Shi L., Liang Z., Li J., Hao J., Xu Y., Huang K., Tian J., He X., Xu W.. Ochratoxin A biocontrol and biodegradation by Bacillus subtilis CW 14. J Sci Food Agric. 2014;94:1879–1885. doi: 10.1002/jsfa.6507. [DOI] [PubMed] [Google Scholar]
- 22.Shih W., Hines W.H., Neilson E.G.. Effect of cyclosporin A on the development of immune-mediated interstitial nephritis. Kidney Int. 1988;33:1113–1118. doi: 10.1038/ki.1988.119. [DOI] [PubMed] [Google Scholar]
- 23.Suvarna K.S., Layton C., Bancroft J.D. Bancroft’s theory and practice of histological techniques, Eighth Edition. Elsevier; Amsterdam: 2018. [Google Scholar]
- 24.Trenk H.L., Butz M.E., Chu F.S.. Production of ochratoxins in different cereal products by Aspergillus ochraceus. Appl Microbiol. 1971;21:1032–1035. doi: 10.1128/aem.21.6.1032-1035.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Der Merwe K.J., Steyn P.S., Fourie L., Scott D.B., Theron J.J.. Ochratoxin A, a toxic metabolite produced by Aspergillus ochraceus Wilh. Nature. 1965;205:1112–1113. doi: 10.1038/2051112a0. [DOI] [PubMed] [Google Scholar]
- 26.Yin H., Jia F., Huang J.. The variation of two extracellular enzymes and soybean meal bitterness during solid-state fermentation of Bacillus subtilis. Grain Oil Sci Technol. 2019;2:39–43. doi: 10.1016/j.gaost.2019.05.001. [DOI] [Google Scholar]
- 27.Yu Z., Wu F., Tian J., Guo X., An R.. Protective effects of compound ammonium glycyrrhizin, L-arginine, silymarin and glucurolactone against liver damage induced by ochratoxin A in primary chicken hepatocytes. Mol Med Rep. 2018;18:2551–2560. doi: 10.3892/mmr.2018.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zain M.E.. Impact of mycotoxins on humans and animals. J Saudi Chem Soc. 2011;15:129–144. doi: 10.1016/j.jscs.2010.06.006. [DOI] [Google Scholar]
- 29.Zhang T.Y., Wu R.Y., Zhao Y., Xu C.S., Zhang W.D., Ge W., Liu J., Sun Z.Y., Zou S.H., Shen W.. Ochratoxin A exposure decreased sperm motility via the AMPK and PTEN signaling pathways. Toxicol Appl Pharmacol. 2018;340:49–57. doi: 10.1016/j.taap.2017.12.011. [DOI] [PubMed] [Google Scholar]
- 30.Zhang T.Y., Sun X.F., Li L., Ma J.M., Zhang R.Q., Li N., Liu X.L., Dyce P.W., Shen W.. Ochratoxin A Exposure Impairs Porcine Granulosa Cell Growth via the PI3K/AKT Signaling Pathway. J Agric Food Chem. 2019;67:2679–2690. doi: 10.1021/acs.jafc.8b06361. [DOI] [PubMed] [Google Scholar]


