Abstract
Caloric restriction (CR) and metabolic glucoprivation affect spontaneous physical activity (SPA), but it's unknown whether these treatments similarly affect SPA in selectively bred obesity-prone (OP) and -resistant (OR) rats. OR rats have greater basal SPA and are more responsive to treatments that modulate SPA, such as orexin A administration. We hypothesized that OR rats would be more sensitive to other treatments modulating SPA. To test this, continuous 24-h SPA was measured before and during acute (24 h) and chronic (8 wk) CR in OR, OP, and Sprague-Dawley rats. Pharmacological glucoprivation was produced by injection of 2-deoxyglucose (2-DG), and SPA was measured 5 h postinjection. Acute CR increased SPA in all groups; however, the effect was dependent on the index of SPA and time interval during the 24-h time period. In contrast to OR rats, chronic CR increased distance traveled, ambulatory episodes, and time spent in ambulation and stereotypy during the time interval preceding anticipation of food in OP and Sprague-Dawley rats. Although the effects of 2-DG treatment on SPA were minimal, OR rats had significantly greater SPA than OP and Sprague-Dawley rats independent of treatment. That chronic CR failed to result in significant changes in SPA in OR rats suggests that these rats may be especially unresponsive to treatments modulating feeding. This insensitivity coupled with elevated basal SPA levels may in part mediate phenotypic traits of lean rats.
Keywords: food deprivation, locomotor activity, diet-induced obesity
the rising prevalence of obesity indicates that a sustained positive imbalance between energy intake and expenditure exists at the population level. However, it's unclear which factor, excessive intake or reduced energy expenditure, has contributed more to the obesity epidemic (19). Given the strong influence of genetics and environment on body weight gain, it's intriguing that spontaneous physical activity (SPA) has a strong familial and genetic component (65) and that lean adults and children display greater SPA than their obese counterparts (25, 38). Similar to lean humans and nonhuman primates (63), rats selected for low weight gain [obesity-resistant rats, (OR)] have greater SPA (57), body-weight adjusted SPA-associated energy expenditure (22), and nonresting energy expenditure (40). The response to treatments affecting food intake also differ between obese and lean rodents: weight gain following consumption of highly palatable diets (30) and weight regain after 50% caloric restriction (CR) is lower in OR rats (35). Further, fat oxidation was elevated in chow-fed OR rats (20). Moreover, total fat pad weight adjusted for body weight was significantly less in OR rats in response to early postweaning exercise (47). Hence, it's plausible that a divergent response to treatments influencing feeding and physical activity function to perpetuate body weight gain differences in obese and lean rats.
CR reduces total energy expenditure (17, 27), but the effects of CR on individual components of energy expenditure and whether the effect is comparable between obese and lean rodents and humans are less understood (26, 27, 40). In contrast to acute CR, which stimulates physical activity, prolonged CR dampens physical activity levels in rodents (54). There are divergent effects of SPA across species (2, 10–12), and the effect of CR on physical activity is dependent on the duration, severity (14, 54), and age of onset of the CR period (11) in rodents.
We have demonstrated that despite a similar feeding response following intracranial orexin A infusion, OR rats have greater basal and orexin A-induced SPA and elevated brain orexin receptor mRNA compared with obesity prone (OP) rats (57). It is plausible that reduced body weight gain among OR rats may be due, in part, to enhanced responsiveness to agents or manipulations that stimulate physical activity and, in turn, energy expenditure. Therefore, we sought to determine whether OR rats were also more responsive to another stimuli that promotes SPA, CR. We reasoned that like their heightened SPA response to orexin A, OR rats would have an enhanced physical activity response to CR and enhanced protection against obesity. We tested three methods of CR on SPA: 1) acute (24 h) food restriction; 2) chronic food restriction (8 wk); and 3) pharmacological glucoprivation (5 h) induced by 2-deoxyglucose (2-DG), a glucose analog that limits cellular glucose availability.
Another goal of the study was to determine whether feeding-associated activity (FAA), which was first described by Curt Richter [reviewed by (45)] and defined as the increase in locomotor activity prior to a meal (44), was phenotype dependent. FAA, a component of SPA, is reduced in mice lacking orexin neurons, indicating the importance of normal orexin signaling for maintaining FAA (43). Given the potential differences in orexin signaling between OP and OR rats, it is possible that differences in FAA exist between OP and OR rats that may contribute to body weight differences; hence, we determined whether there was a differential effect of CR on FAA between OP and OR rats.
MATERIALS AND METHODS
Animals
Four-month-old male Sprague-Dawley and selectively bred male OP and OR rats (Charles River, Kingston, NY) were housed individually in cages with a 12:12-h light-dark cycle (lights on at 0700) in a temperature-controlled room (21–22°C). The selectively bred OP and OR rats were obtained commercially from a colony of high-fat-fed outbred Sprague-Dawley rats (Charles River, Kingston, NY) that were selected for their weight gain status. Although a high-fat diet was used during the selective breeding process, consumption of a high-fat diet is not required to observe phenotypic differences in body weight, as OP and OR rats remain obese and lean, respectively, when maintained on a low-fat diet (32, 49). Standard rodent chow (Harlan Teklad 8604) and water were allowed ad libitum except where noted. Studies were approved by the local Institutional Animal Care and Use Committee at the Veterans Affairs Medical Center and the University of Minnesota.
SPA Measurement
SPA was measured using customized, high-precision racks of infrared activity sensors (Med Associates, St. Albans, VT) placed around a square acrylic cage (17.0“ × 17.0”), as previously described (57, 59). Briefly, three 16-beam infrared arrays with two arrays in the “x” direction and an elevated “x” array measured ambulation and vertical movement. An activity unit was recorded and time stamped each time a beam was interrupted, and therefore, movement was simultaneously detected in all three axes. Rodent chow was placed on the chamber floor (except where noted), and water was provided in test tubes with stoppers, which were secured in the corners of the chambers two inches from the chamber floor. Thus, vertical movement was not confounded by activity due to eating or drinking. We report several measures of SPA: distance traveled, time spent in ambulatory, (locomotor activity), vertical (rearing or standing on two limbs), and stereotypic movement (small movements, including grooming/feeding) and ambulatory episodes (the number of times movement was initiated).
Specific experimental designs
Study 1. Effect of chronic CR on SPA.
Male age-matched rats (n = 10–12/group) were ranked by body weight and then alternatively assigned by rank to a treatment group (ad libitum or chronic CR) to ensure that body weight of the subgroups within a given treatment condition were not significantly different (P = 0.9076). Mean 24-h food intake in the home cage was determined by averaging 24-h food intake for 2 days. Then, 24-h SPA was measured following a 24-h acclimation period in the SPA chambers. Following the 24-h measurement period, rats were returned to their home cages and CR began. Rats in the chronic CR group were given 70% of their mean 24-h food intake plus additional food to account for spillage. Rats in the ad libitum group had ad libitum access to food. Food was given to the chronic CR group daily 2–3 h (1600–1700) before lights off at 1900. The restricted-feeding paradigm was continued until differences in body weight were no longer statistically significant between the calorically restricted OP and ad libitum fed OR rats, a period of 8 wk (body weight: calorically restricted OP: 505 ± 12.7 and ad libitum-fed OR: 500.7 ± 27.5, P = 0.8813). At that time, 24-h SPA was measured again following a 24-h reacclimation period to the SPA chambers. Body weight was measured weekly, and food intake was measured 2 or 3 times per week. To increase the number of animals in each group and for feasibility, study 1 was repeated in a second group of OP, OR, and Sprague-Dawley rats. Therefore, study 1 was completed in two phases, and the data sets from phases 1 and 2 were combined after it was determined that the 24 h of SPA preceding the restricted-feeding paradigm was not significantly different between rats in phases 1 and 2 for each phenotype (OP: F1,17 = 0.6, P = 0.4307, OR: F1,17 = 2.1, P = 0.1643 and Sprague-Dawley: F1,18 = 1.8, P = 0.1998).
Study 2. Effect of acute CR on SPA.
A repeated-measures Latin-square design was used, in which half of the animals in each group (OP, OR, and Sprague-Dawley) received each treatment (ad libitum or acute CR), and both treatments were represented on each treatment day. Therefore, male age-matched rats (n = 10–12/group) were randomly assigned to a treatment group (ad libitum or acute CR) in which body weight of the subgroups within a given treatment condition was not significantly different (P = 0.5986). Rats were acclimated for 24 h to the SPA chambers prior to a continuous 24-h measurement period. Then, rats were returned to their home cages for 72 h with ad libitum access to food and water. Finally, treatments (ad libitum or acute CR) were reversed, and the 24-h acclimation and measurement periods were repeated. Rodents had ad libitum access to rodent chow and water throughout the acclimation and measurement periods, except during the acute 24-h CR measurement period during which food was withheld. Body weight was measured on the first and last treatment day and was used to calculate mean body weight for the study.
Study 3. Effect of 2-DG-induced glucoprivation on SPA.
A repeated-measures Latin-square design was used, in which half of the animals in each group (OP, OR, and Sprague-Dawley) received each treatment (vehicle or 2-DG) consistent with study 2. Male age-matched rats (n = 10–12/group) were randomly assigned to a treatment group (saline or 2-DG, 400 mg/kg, dissolved in saline, Sigma, St. Louis, MO), such that body weights of the subgroups within a given treatment condition were not significantly different (P = 0.1026). Rats from study 2 were acclimated to the SPA chambers for 6 h on three separate occasions prior to the start of this study. Rodents had ad libitum access to rodent chow and water throughout the acclimation period. Saline or 2-DG was injected subcutaneously at 1800, 1 h before the start of the dark cycle. Continuous SPA was measured for 5 h postinjection, during which time food was unavailable. Then rats were returned to their home cages for at least 48 h with food and water ad libitum, treatment groups (vehicle or 2-DG) were reversed, and continuous SPA was measured for 5 h postinjection. Body weight was measured on the first and last treatment day and was used to calculate mean body weight for this study.
Statistical Analyses
Data were analyzed using Statview 5.0 (Cary, NC) and are expressed as means ± SE. An alpha level of 0.05 was used for all statistical tests. Data were analyzed by three-factor ANOVA with repeated measures on one (study 1) or two (studies 2 and 3) factors, in which phenotype (OP, OR, Sprague-Dawley) was the between-subjects factor and treatment (study 1: ad libitum or chronic CR; study 2: ad libitum or acute CR; study 3: saline or 2-DG) was the within-subjects factor. For studies 1 and 2, data were divided into 4-h time bins across the 24-h measurement period and were reported as follows: 0900–1300, 1300–1700, 1800–2100, 2100–0100, 0100–0500, and 0500–0900. In addition, we analyzed data in the cumulative 12-h light cycle, 12-h dark cycle, and the 24-h time interval. Data from study 3 were analyzed for the 5-h time interval postinjection. A separate analysis was completed for each time interval for the following dependent variables: distance traveled, time ambulatory, time vertical, time stereotypic, and ambulatory episodes). When there was a significant group-by-treatment interaction or when both main effects were significant, a paired-test was completed to determine differences between ad libitum and CR treatments within each group of OP, OR, and Sprague-Dawley rats. Mean body weight (studies 2 and 3), body weight (study 1: initial and body weight change), initial food intake (study 1) were analyzed by one-factor ANOVA followed by Fisher's paired least significant difference test to determine significant differences between group means.
RESULTS
Study 1. Effect of Chronic CR on SPA
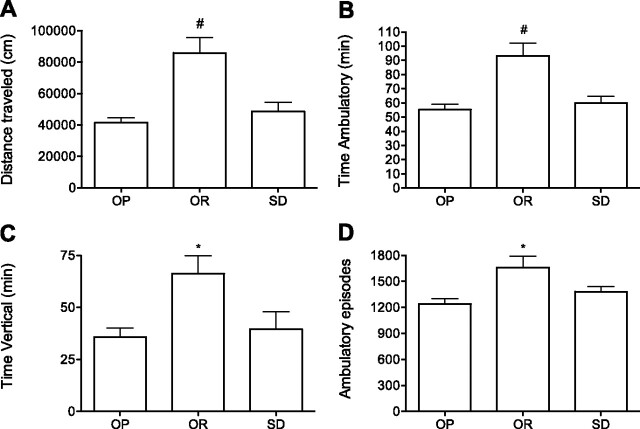
Before the start of the restricted-feeding paradigm, OR rats weighed significantly less than OP and Sprague-Dawley rats, and body weights between OP and Sprague-Dawley rats were comparable (Table 1). Mean 24-h food intake and change in body weight after the restricted-feeding paradigm, respectively, were not significantly different between groups (Table 1). Hence, OP rats were not hyperphagic, and the rate of weight loss was comparable between groups during the restricted-feeding regime. Last, before the restricted-feeding paradigm, OR rats traveled significantly farther, initiated more ambulatory episodes, and spent more time in ambulatory and vertical movement than both the OP and SD rats during the 24-h period (Fig. 1). Time spent in stereotypic movement was similar between groups (data not shown).
Table 1.
Body weight and food intake for studies 1 to 3
| Obesity Prone | Obesity Resistant | Sprague-Dawley | ||||
|---|---|---|---|---|---|---|
| Study one: Effect of Chronic Caloric Restriction on SPA | ||||||
| Initial FI, g | 26.2±1.0 | 24.4±1.3 | 25.4±1.1 | |||
| Initial BW, g | 579±7 | 456±15* | 547±18 | |||
| BW change, g | −68±8 | −53±8 | −51±24 | |||
| Study Two: Effect of Acute Caloric Restriction on SPA | ||||||
| Mean BW, g | 573±5.9 | 475±14.6* | 568±15.3 | |||
| Study Three: Effect of 2-Deoxyglucose on SPA | ||||||
| Mean BW, g | 566±5.9 | 478±15.5* | 566±15.7 | |||
Values are expressed as means ± SE of food intake (FI) and body weight (BW).
P < 0.0001 as compared to obesity prone and Sprague-Dawley rats. Initial food intake represents the mean of daily food intake over 2 days prior to starting the restricted-feeding paradigm (n = 9 or 10/group).
Fig. 1.
Basal 24-h SPA prior to starting the chronic caloric restriction-feeding paradigm. Distance traveled (A), time ambulatory (B), time vertical (C) and ambulatory episodes (D) in obesity prone (OP), obesity resistant (OR), and Sprague-Dawley rats (SD). *P < 0.05 and #P < 0.005 compared with both obesity-prone and Sprague-Dawley rats; n = 9–10/group. Values are presented as means ± SE.
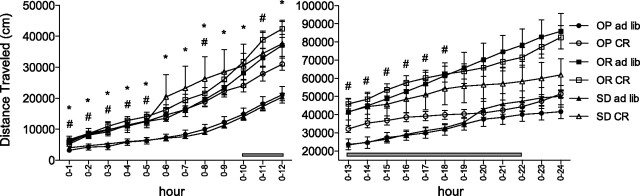
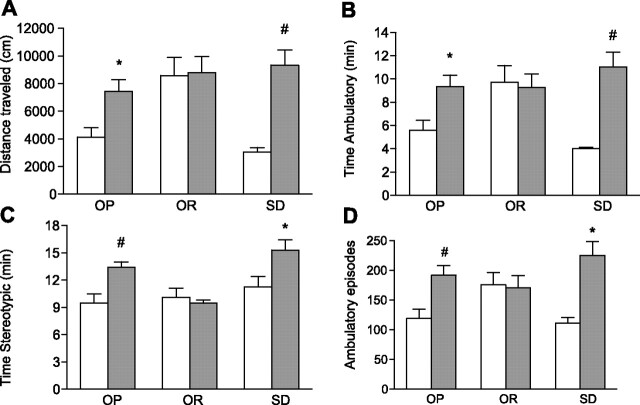
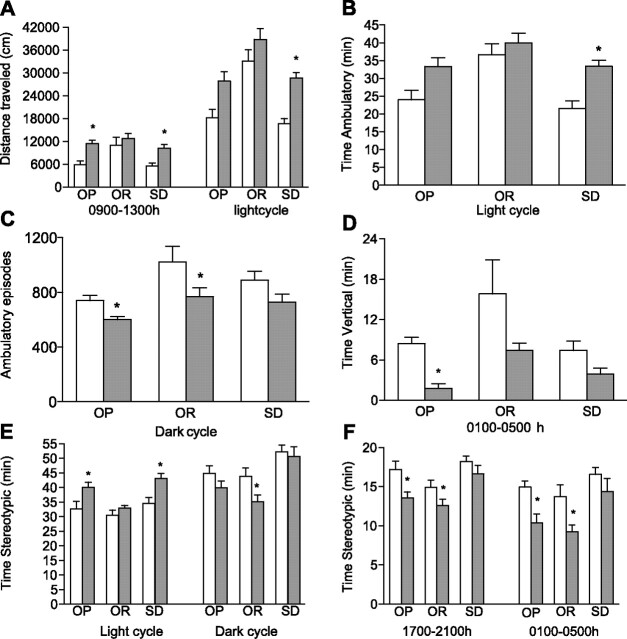
There was a differential effect of chronic CR on SPA between OP, OR, and Sprague-Dawley rats that was dependent on the time interval during the 24-h period and the index of SPA measured (Figs. 2 –4). First, chronic CR increased SPA in OP and Sprague-Dawley rats but failed to increase SPA in OR rats during the 1300–1700 time interval, which corresponds to the time interval preceding meal anticipation (Fig. 3). During this time interval, the interaction between phenotype and treatment was significant for distance traveled, time ambulatory, time stereotypic, and ambulatory episodes. Chronic CR significantly increased distance traveled 55% (P = 0.0161), time ambulatory 59% (P = 0.0232), time stereotypic 29% (P = 0.0027) and ambulatory episodes 62% (P = 0.0056) in OP rats (Fig. 3). In a similar manner, Sprague-Dawley rats traveled significantly farther (P = 0.0011) and had significantly increased time ambulatory (P = 0.0015), time stereotypic (P = 0.0513), and ambulatory episodes (P = 0.0062) in response to chronic CR (Fig. 3). Second, there was a differential effect of chronic CR between groups during the cumulative 12-h light-dark cycle (Fig. 4). During the light cycle, chronic CR had no effect on OR rats, but significantly increased time stereotypic (P = 0.0206) in OP rats and distance traveled (P = 0.0004), time ambulatory (P = 0.0032) and time stereotypic (P = 0.0181) in Sprague-Dawley rats. Chronic CR also increased distance traveled and time ambulatory in OP rats; however, these results failed to reach statistical significance (P = 0.0555 and P = 0.0931, respectively). During the dark cycle, chronic CR reduced time stereotypic in OR rats (P = 0.0023) and reduced ambulatory episodes in both OR and OP rats (P = 0.0070 and P = 0.0137, respectively, Fig. 4). Third, group-dependent effects of chronic CR were observed for several additional time intervals (Fig. 4). Chronic CR increased distance traveled in OP and Sprague-Dawley rats (P = 0.0115 and P = 0.0030, respectively) during the 0900–1300 time interval and reduced time vertical in OP rats (P < 0.0001) during the 0100–0500 time interval but had no effect on OR rats. Finally, chronic CR decreased time stereotypic in OP and OR rats during the 1700–2100 (P = 0.0085 and P = 0.0136, respectively) and 0100–0500 (P = 0.0127 and P = 0.0025, respectively) time intervals.
Fig. 2.
Cumulative distance traveled during the 24-h time interval in response to chronic caloric restriction [70% caloric restriction for 8 wk (open symbols) or ad libitum feeding (solid symbols)] in OP, OR, and SD rats. *P < 0.05 between ad libitum fed and calorically restricted obesity-prone rats and #P < 0.05 between ad libitum fed and calorically restricted SD rats. Shaded bar = dark cycle; n = 9–10/group. Values are presented as means ± SE.
Fig. 3.
Distance traveled (A), time ambulatory (B), time stereotypic (C), and ambulatory episodes (D) during the time interval preceding meal anticipation (1300–1700) in response to chronic caloric restriction, 70% caloric restriction for 8 wk, (shaded bars), or ad libitum feeding (open bars) in OP, OR, and SD rats. *P < 0.05 and #P < 0.005 compared with ad libitum feeding within the same group; n = 9–10/group. Values are presented as means ± SE.
Fig. 4.
Distance traveled (A), time ambulatory (B), ambulatory episodes (C), time vertical (D), and time stereotypic in response to chronic caloric restriction (E and F), 70% caloric restriction for 8 wk (shaded bars), or ad libitum feeding (open bars) in OP, OR, and SD rats in specific time intervals across the 24-h measurement period. *P < 0.05 compared with ad libitum feeding within the same group; n = 9–10/group. Values are presented as means ± SE.
Study 2. Effect of Acute CR on SPA
Mean body weight of OR rats was significantly less than OP (P < 0.0001) and Sprague-Dawley (P < 0.0001) rats, and mean body weight was similar between OP and Sprague-Dawley rats (Table 1). There was a differential effect of acute CR on SPA between groups, which was dependent on the type of SPA and the time interval (Fig. 5). Acute CR increased distance traveled in OR rats (67%) during the 1300–1700 time interval (P = 0.0328) and in OP rats (138%) during 0500–0900 (P = 0.0343). Acute CR increased time vertical in OR (20%) and Sprague-Dawley (29%) rats during the light cycle (P = 0.0238 and P = 0.0086, respectively) and ambulatory episodes in OP rats (18%) during the cumulative 24-h time interval (P = 0.0113), Fig. 5. Finally, there was a main effect of treatment on distance traveled, time ambulatory, time vertical, and ambulatory episodes for the cumulative light cycle and the 1300–1700 and 0500–0900 time intervals, which indicates that acute CR increased SPA (P < 0.05, all comparisons, data not shown).
Fig. 5.
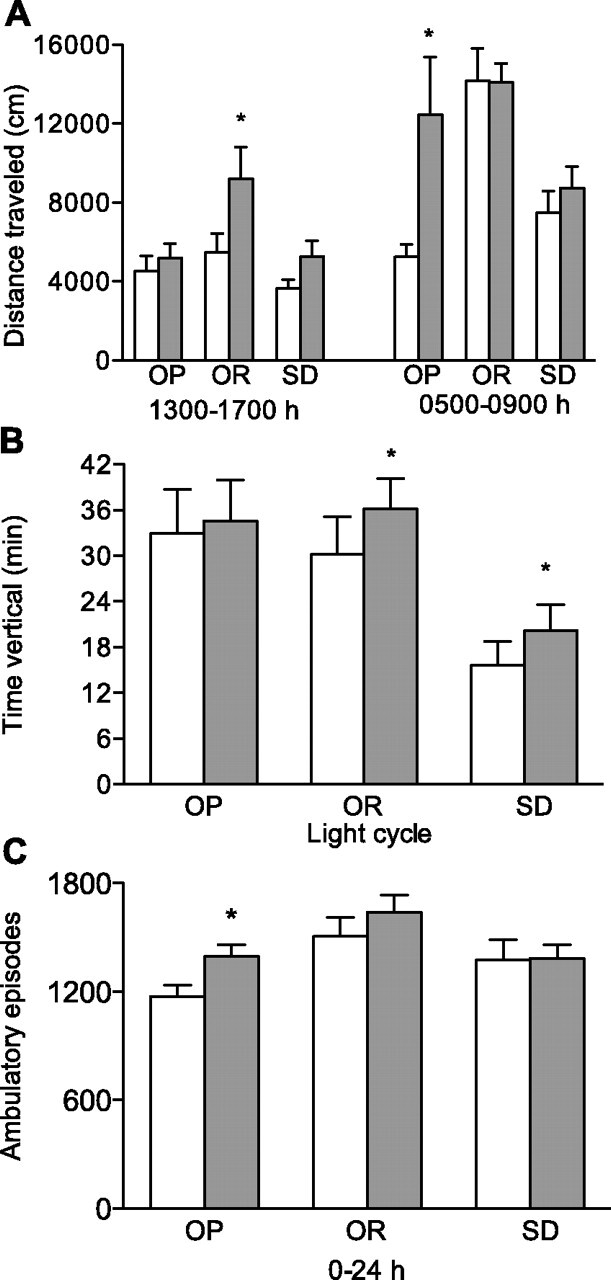
Distance traveled (A), time vertical (B), and ambulatory (C) episodes in response to acute caloric restriction (24-h caloric restriction, shaded bars) or ad libitum feeding (open bars) in OP, OR, and SD rats. *P < 0.05 compared with ad libitum feeding within the same group; n = 9–10/group. Values are presented as means ± SE.
Study 3. Effect of 2-DG-Induced Glucoprivation on SPA
Mean body weight of OR rats was significantly less than OP (P < 0.0001) and Sprague-Dawley (P < 0.0001) rats and mean body weight was similar between OP and Sprague-Dawley rats (Table 1). In general, 2-DG was without effect on SPA with two exceptions (Table 2). In contrast to OP and Sprague-Dawley rats, OR rats had fewer ambulatory episodes in response to 2-DG (vehicle: 177.9 ± 11.3 and 2-DG: 137.9 ± 15.9, P = 0.0446) and 2-DG treatment decreased time stereotypic in all rats (P = 0.0161). Finally, OR rats traveled significantly farther and had greater time ambulatory and time vertical compared with OP and Sprague-Dawley rats independent of treatment (P < 0.05 all comparisons, Table 2).
Table 2.
Effect of 2-deoxyglucose on spontaneous physical activity in obesity-prone, obesity-resistant, and Sprague-Dawley rats
| Spontaneous Physical Activity Parameter |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Distance Traveled, cm† | Time Ambulatory, min* | Time Vertical, min† | Time Stereotypic, min‡ | Ambulatory Episodes | |||||
| Obesity-prone vehicle | 17780.0±1935.4 | 24.2±2.4 | 18.2±1.4 | 23.6±1.1 | 527.8±40.7 | ||||
| 2-Deoxyglucose | 18484.0±1755.2 | 23.7±2.4 | 21.1±2.4 | 22.1±1.3 | 529.5±51.7 | ||||
| Obesity-resistant vehicle | 33479.9±4408.7 | 38.7±4.1 | 25.8±1.8 | 19.2±1.4 | 614.3±50.5 | ||||
| 2-Deoxyglucose | 30460.4±3389.2 | 32.4±3.9 | 25.5±2.1 | 19.3±0.9 | 537.0±53.3 | ||||
| Sprague-Dawley vehicle | 19495.3±1675.5 | 23.5±1.7 | 15.2±1.6 | 24.1±1.3 | 481.1±26.3 | ||||
| 2-Deoxyglucose | 16325.6±1903.8 | 19.5±2.2 | 14.0±2.1 | 19.3±1.1 | 417.7±42.3 | ||||
Values are expressed as means ± SE
P < 0.005 and
P < 0.0005 main effect of group;
P = 0.0161 main effect of treatment (n = 9 or 10/group).
DISCUSSION
We determined the effect of acute and chronic CR and metabolic glucoprivation on SPA and demonstrate that the effect of CR on SPA not only differed between selectively bred OP, OR, and Sprague-Dawley rats but was also dependent on the method of CR tested, index of SPA measured, and time interval during the 24-h time period. In general, acute and chronic CR stimulated SPA and the effect of 2-DG treatment on SPA was minimal. On the basis of differences in glucosensing between OP and OR rats (28, 34, 36), effects of CR on orexin neurotransmission (21, 39, 53), our previous work showing heightened sensitivity to orexin A following glucoprivation in Sprague-Dawley rats (58, 59) and greater orexin A-induced SPA and orexin receptor mRNA in OR rats (57), we hypothesized that OR rats would be more sensitive to treatments modulating SPA. Instead. chronic CR stimulated SPA in OP and Sprague-Dawley rats and, in general, was without effect in OR rats. Effects of acute CR were not confined to a specific group such that the stimulatory effect of acute CR was typically observed in all groups with few group-specific differences noted. Pharmacological glucoprivation reduced stereotypic movement in all groups, but lean OR rats still had significantly greater (baseline) SPA than OP and Sprague-Dawley rats. That OR rats remain lean despite their complete and partial failure to increase SPA in response to chronic and acute CR, respectively, suggests that either this particular group of selectively bred OR rats was at their maximal baseline SPA levels already, they were not necessarily sensitive to all conditions that gave rise to elevated SPA, or as suggested by our previous work, they appear to be specifically sensitive to SPA behavior related to orexin receptor activity. Alternatively, the lack of increase in CR-induced SPA in OR rats may be related to a preferential loss in fat-free mass in response to CR as suggested previously (35), since reduced fat-free mass would be expected to negatively augment SPA. Nonetheless, that lean rats were less responsive to chronic CR than obese rats, despite a comparable rate of weight loss between groups, suggests that insensitivity to this specific feeding-related signal coupled with elevated basal SPA levels in the ad libitum-fed condition together perpetuates the lean phenotype of OR rats. This idea is plausible in the context of reduced food availability, as elevated SPA or foraging in obese rats would increase the probability of finding and consuming food.
Basal SPA, prior to starting the chronic CR-feeding paradigm, was significantly greater in OR rats relative to OP and SD rats, which agrees with and extends our previous findings (57; Fig. 1). Further, the stimulatory effect of CR on SPA shown here is consistent with previous studies demonstrating CR-induced increases in overall physical activity in rodents (2, 11, 13, 18, 64), zebrafish (46), and nonhuman primates (48, 61). Although others have shown no effect of CR on physical activity in rodents (13), nonhuman primates (24), and humans (27), the discrepancies are likely due to methodological inconsistencies between studies since effects of CR on physical activity are species specific and dependent on the duration, severity, modality, and the age of onset of the CR period (2, 11, 24, 41). Unlike mild or intermittent CR, which had no effect on physical activity, severe and continuous CR increased physical activity (54). Additional discrepancies are likely related to the modality used to measure physical activity (18) or the components of physical activity reported (11, 24, 48). Further, the final conclusion of studies testing whether CR effects SPA may differ based on the modality and testing environment [e.g., open field chambers vs. running wheels (55) or respiratory chambers vs. free-living condition], time of measurement interval, and the mode of reporting [e.g., total vs. individual components of physical activity or energy expenditure (27, 42, 51)]. For example, CR increased running wheel revolutions during the light cycle but reduced total 24-h wheel revolutions (29). Consistent with the aforementioned study, we found CR increased SPA during the light cycle in our open-field environment, but we did not observe robust effects of CR on 24-h SPA. Moreover, previous studies found no effect of CR on SPA measured in respiratory chambers (27, 42), despite a reduction in free-living physical activity level (total energy expenditure adjusted for resting metabolic rate), as determined by doubly labeled water in humans (42). Together, the divergent results noted above and the finding that SPA measured in respiratory chambers and the free-living environment are highly associated (56, 62) highlight the complexity of interpreting studies testing the effect of CR on SPA.
We demonstrate that the SPA-promoting effect of CR was dependent on the time interval measured during the 24-h time period. Of particular interest, we show that chronic CR increased SPA during the time interval preceding meal anticipation [food anticipatory activity (FAA)] in OP and Sprague-Dawley rats but failed to stimulate SPA in OR rats. In contrast, acute CR caused OR rats to travel farther during this time period, while OP and Sprague-Dawley rats were unaffected (Figs. 3A and 5A). Although others demonstrated that CR increased FAA in rodents (11), monkeys (48, 61), and hamsters (1), this is the first report to show a differential effect of acute and chronic CR on FAA between obese and lean rats. The findings that OR, but not OP, rats failed to increase FAA in response to chronic CR and that the reverse was true during acute CR suggest a differential sensitivity to this specific feeding-related signal, which could plausibly perpetuate phenotypic differences in body weight between OP and OR rats. This idea is consistent with studies showing OP rats were more sensitive to the anorectic effects of central insulin (8) and leptin (31, 33) administration, despite a similar feeding response to orexin A (57). Alternatively, perhaps the current group of OR rats were at a ceiling level of SPA or that the strength of the stimulus (chronic CR) was not sufficient to increase SPA in these OR rats, suggesting that this group of OR rats could not be further stimulated for additional SPA by chronic CR. However, we have observed a wide variation in SPA between groups of rats (22). Although OR rats are always at a higher SPA level than OP rats, the absolute levels vary considerably, and we have previously observed greater mean FAA in ad libitum-fed OR rats, relative to the OR rats in the current chronic food restriction CR study. This would support the idea that the OR rats in our current study were not at their maximum SPA levels, and instead it suggests that the OR rats simply were less sensitive to this feeding signal.
We predicted that 2-DG administration would function like acute CR and, therefore, stimulate physical activity. We further hypothesized that 2-DG SPA augmentation would differ between groups based on reported differences in glucosensing between obese and lean rats (28, 34, 36), elevated orexin receptor mRNA and orexin A stimulation of SPA in OR rats (57), 2-DG activation of orexin neurons (5) and an elevated feeding response to orexin A following 2-DG administration (59). In this study, 2-DG, at a dose that prevented cellular glucose utilization (6) and increased food intake (9, 16, 50), was administered 1 h before lights off. Food was unavailable during the 5-h testing period postinjection. Despite our attempt to induce nutritional and pharmacological glucoprivation and contrary to our expectations, there was a minimal effect of 2-DG on SPA as 2-DG reduced ambulatory episodes in OR rats and reduced time in stereotypy in all rats. In agreement, others have shown no effect of 2-DG-supplemented diets or chronic 2-DG injections on locomotor activity (41, 60). It is plausible that we failed to detect an effect of 2-DG on SPA because of the timing of the SPA measurements. In contrast to the acute and chronic CR studies, in which SPA was measured for 24 h, SPA was measured during 5 h of CR primarily during the dark cycle in this study. Finally, the failure to observe 2-DG-stimulated SPA may be due to a ceiling effect where the dose of 2-DG was insufficient to stimulate SPA above baseline levels during the measurement interval of the dark cycle. This idea is consistent with the stimulatory effect of acute and chronic CR to on SPA during the light cycle, despite no effect of CR during the dark cycle.
The evolutionary advantage conferred by increased SPA following CR is unknown, but it is plausible that CR-induced SPA represents a purposeful increase in foraging behavior or a heightened motivation to seek food. Therefore, elevated FAA in response to chronic CR in OP and Sprague-Dawley rats may indicate that these rats are more motivated to search for food, which is consistent with operant studies, demonstrating greater food-motivated behavior in obese rats (23). Elevated SPA increases energy expenditure. Therefore, an alternative suggestion is that increased FAA in OP and SD rats occurs in response to interoceptive cues of the obese phenotype to achieve homeostatic energy balance. Irrespectively, the differential sensitivity to chronic CR between obese and lean rats has implications for future studies of CR on factors that influence energy balance.
A group of Sprague-Dawley rats was included in each study to determine which group (OP or OR) was more similar to Sprague-Dawley rats. Obesity-prone and -resistant rats were selectively bred from outbred Sprague-Dawley rats. It might be expected that the mean response to CR in Sprague-Dawley rats would lie between that observed in OP and OR rats; however, this was not the case. Body weight and effects of CR on SPA, in general, were most similar between OP and Sprague-Dawley rats, which is consistent with our previous work, showing that body weight and baseline SPA levels in OP and Sprague-Dawley rats were more similar (57). Because Sprague-Dawley rats would be representative of a general population of laboratory rats, these data indicate that the lean phenotype and heightened basal activity levels displayed by OR rats is atypical. As the ability to remain lean is of current difficulty in humans, the neuromolecular underpinnings that govern the lean phenotype of OR rats deserve further investigation.
The mechanism(s) underlying the effects of acute and chronic CR on SPA in OP, OR, and Sprague-Dawley rats is unclear. Caloric restriction increases mRNA and protein levels for orexin (53) and its receptors (21, 39), dopamine receptor signaling (7), and neuropeptide Y, and reduces proopiomelanocortin (4), which augment physical activity. It is possible that the effect of CR on neuropeptide levels differs between these groups of rats, which then contributes to the observed divergent SPA response to CR in OP, OR, and Sprague-Dawley rats. This idea is supported by studies demonstrating differences in brain neuropeptide activity between OP and OR rats. Selectively bred OR rats have elevated orexin one and two receptor mRNA (57), and outbred OP rats have decreased dopamine turnover (37) and dopamine beta hydroxylase, the rate-limiting enzyme required for dopamine synthesis, which modulates physical activity. In addition, hypothalamic projections from the arcuate nucleus of the hypothalamus (ARC), leptin-induced signaling in the ARC, and ARC neurite growth differ between OP and OR rats (3). Hence, these studies suggest CR-induced changes in brain neuropeptide levels likely contribute to the differential effect of CR on SPA between OP and OR rats observed here. Together, our studies indicate there is an unequal effect of acute and chronic CR on SPA between obese and lean rodents and suggest that these effects on SPA may reinforce obesity status.
Perspectives and Significance
The widespread prevalence of obesity will continue to challenge society since the environment promotes using labor-saving devices and consumption of energy-dense foods. Theoretically, behavioral therapy targeted toward reducing caloric intake and increasing physical activity-energy expenditure should slow rates of obesity. However, behavioral therapy yields uneven results, which may be due, in part, to the high interindividual variability in weight loss and regain following CR alone or in combination with increased physical activity. Studies of CR in humans indicate that resting energy expenditure is reduced (27). CR in humans also induces a disproportionately large reduction in nonresting energy expenditure, due to increased skeletal muscle work efficiency during low levels of physical activity (52). However, others show that SPA and energy expenditure remained unchanged after 6 mo of CR in humans (42), and nonresting energy expenditure in OR rats was greater than preobese, obesity-prone and weight-reduced rats (40). Although methodological differences between studies likely contribute to divergent results, these studies highlight the need to clarify how CR affects components of nonresting energy expenditure and suggests that differences in metabolic adaptation related to nonresting energy expenditure, specifically low levels of physical activity, may contribute to the high recidivism rate among those formerly obese. Consistent with ideas suggested previously (35) and studies showing effects of CR on changes in fat and fat-free mass (15), the data presented here suggest that compensatory mechanisms in response to CR differ on the basis of body weight phenotype. We found that the magnitude of weight loss in response to CR was similar between lean and obese rats, despite a greater SPA after CR in obese rats, which would be expected to augment nonresting energy expenditure. These data have implications for behavioral therapy and suggest that despite a similar level of CR, the magnitude of SPA required to induce weight loss when combined with CR may be dependent on body weight phenotype.
GRANTS
Funding for this research and publication was provided by an American College of Sports Medicine Foundation Research Grant, the University of Minnesota Doctoral Dissertation Fellowship, Department of Veterans Affairs, and the Minnesota Department of Employment and Economic Development from the state's legislative appropriation for the Minnesota Partnership for Biotechnology and Medical Genomics.
REFERENCES
- 1.Bae HH, Larkin JE, Zucker I. Juvenile Siberian hamsters display torpor and modified locomotor activity and body temperature rhythms in response to reduced food availability. Physiol Biochem Zool 76: 858–867, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Blank JL, Desjardins C. Differential effects of food restriction on pituitary-testicular function in mice. Am J Physiol Regul Integr Comp Physiol 248: R181–R189, 1985. [DOI] [PubMed] [Google Scholar]
- 3.Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB. Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7: 179–185, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brady LS, Smith MA, Gold PW, Herkenham M. Altered expression of hypothalamic neuropeptide mRNAs in food-restricted and food-deprived rats. Neuroendocrinology 52: 441–447, 1990. [DOI] [PubMed] [Google Scholar]
- 5.Briski KP, Sylvester PW. Hypothalamic orexin-A-immunpositive neurons express Fos in response to central glucopenia. Neuroreport 12: 531–534, 2001. [DOI] [PubMed] [Google Scholar]
- 6.Brown J. Effects of 2-deoxyglucose on carbohydrate metablism: review of the literature and studies in the rat. Metabolism 11: 1098–1112, 1962. [PubMed] [Google Scholar]
- 7.Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience 119: 1157–1167, 2003. [DOI] [PubMed] [Google Scholar]
- 8.Clegg DJ, Benoit SC, Reed JA, Woods SC, Dunn-Meynell A, Levin BE. Reduced anorexic effects of insulin in obesity-prone rats fed a moderate-fat diet. Am J Physiol Regul Integr Comp Physiol 288: R981–R986, 2005. [DOI] [PubMed] [Google Scholar]
- 9.Clegg DJ, Edwards GL, Martin RJ. Central insulin potentiates eating elicited by 2-deoxy-d-glucose. Physiol Behav 78: 331–336, 2003. [DOI] [PubMed] [Google Scholar]
- 10.Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int 7: 113–124, 1990. [DOI] [PubMed] [Google Scholar]
- 11.Duffy PH, Feuers RJ, Hart RW. Effect of chronic caloric restriction on the circadian regulation of physiological and behavioral variables in old male B6C3F1 mice. Chronobiol Int 7: 291–303, 1990. [DOI] [PubMed] [Google Scholar]
- 12.Duffy PH, Leakey JE, Pipkin JL, Turturro A, Hart RW. The physiologic, neurologic, and behavioral effects of caloric restriction related to aging, disease, and environmental factors. Environ Res 73: 242–248, 1997. [DOI] [PubMed] [Google Scholar]
- 13.Evans SA, Parsons AD, Overton JM. Homeostatic responses to caloric restriction: influence of background metabolic rate. J Appl Physiol 99: 1336–1342, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Even PC, Nicolaidis S. Adaptive changes in energy expenditure during mild and severe feed restriction in the rat. Br J Nutr 70: 421–431, 1993. [DOI] [PubMed] [Google Scholar]
- 15.Forbes GB. Body fat content influences the body composition response to nutrition and exercise. Ann NY Acad Sci 904: 359–365, 2000. [DOI] [PubMed] [Google Scholar]
- 16.Giraudo SQ, Kim EM, Grace MK, Billington CJ, Levine AS. Effect of peripheral 2-DG on opioid and neuropeptide Y gene expression. Brain Res 792: 136–140, 1998. [DOI] [PubMed] [Google Scholar]
- 17.Heilbronn LK, de Jonge L, Frisard MI, DeLany JP, Larson-Meyer DE, Rood J, Nguyen T, Martin CK, Volaufova J, Most MM, Greenway FL, Smith SR, Deutsch WA, Williamson DA, Ravussin E. Effect of 6-month calorie restriction on biomarkers of longevity, metabolic adaptation, and oxidative stress in overweight individuals: a randomized controlled trial. JAMA 295: 1539–1548, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol 42: 78–81, 1987. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery RW, Harnack LJ. Evidence implicating eating as a primary driver for the obesity epidemic. Diabetes 56: 2673–2676, 2007. [DOI] [PubMed] [Google Scholar]
- 20.Ji H, Friedman MI. Reduced capacity for fatty acid oxidation in rats with inherited susceptibility to diet-induced obesity. Metabolism 56: 1124–1130, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Karteris E, Machado RJ, Chen J, Zervou S, Hillhouse EW, Randeva HS. Food deprivation differentially modulates orexin receptor expression and signaling in rat hypothalamus and adrenal cortex. Am J Physiol Endocrinol Metab 288: E1089–E1100, 2005. [DOI] [PubMed] [Google Scholar]
- 22.Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol 294: R699–R710, 2008. [DOI] [PubMed] [Google Scholar]
- 23.la Fleur SE, Vanderschuren LJ, Luijendijk MC, Kloeze BM, Tiesjema B, Adan RA. A reciprocal interaction between food-motivated behavior and diet-induced obesity. Int J Obes (Lond) 31: 1286–1294, 2007. [DOI] [PubMed] [Google Scholar]
- 24.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci USA 93: 4159–4164, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lanningham-Foster L, Jensen TB, Foster RC, Redmond AB, Walker BA, Heinz D, Levine JA. Energy expenditure of sedentary screen time compared with active screen time for children. Pediatrics 118: e1831–e1835, 2006. [DOI] [PubMed] [Google Scholar]
- 26.Leibel RL. Molecular physiology of weight regulation in mice and humans. Int J Obes (Lond) 32 Suppl 7: S98–S108, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibel RL, Rosenbaum M, Hirsch J. Changes in energy expenditure resulting from altered body weight. N Engl J Med 332: 621–628, 1995. [DOI] [PubMed] [Google Scholar]
- 28.Levin BE. Intracarotid glucose induced norepinephrine response and the development of diet-induced obesity. Int J Obes Relat Metab Disord 16: 451–457, 1992. [PubMed] [Google Scholar]
- 29.Levin BE, Dunn-Meynell AA. Chronic exercise lowers the defended body weight gain and adiposity in diet-induced obese rats. Am J Physiol Regul Integr Comp Physiol 286: R771–R778, 2004. [DOI] [PubMed] [Google Scholar]
- 30.Levin BE, Dunn-Meynell A. A. Defense of body weight depends on dietary composition and palatability in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 282: R46–R54, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Levin BE, Dunn-Meynell AA. Reduced central leptin sensitivity in rats with diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 283: R941–R948, 2002. [DOI] [PubMed] [Google Scholar]
- 32.Levin BE, Dunn-Meynell AA, Balkan B, Keesey RE. Selective breeding for diet-induced obesity and resistance in Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 273: R725–R730, 1997. [DOI] [PubMed] [Google Scholar]
- 33.Levin BE, Dunn-Meynell AA, Banks WA. Obesity-prone rats have normal blood-brain barrier transport but defective central leptin signaling before obesity onset. Am J Physiol Regul Integr Comp Physiol 286: R143–R150, 2004. [DOI] [PubMed] [Google Scholar]
- 34.Levin BE, Govek EK, Dunn-Meynell AA. Reduced glucose-induced neuronal activation in the hypothalamus of diet-induced obese rats. Brain Res 808: 317–319, 1998. [DOI] [PubMed] [Google Scholar]
- 35.Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol Regul Integr Comp Physiol 274: R412–R419, 1998. [DOI] [PubMed] [Google Scholar]
- 36.Levin BE, Planas B. Defective glucoregulation of brain alpha 2-adrenoceptors in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 264: R305–R311, 1993. [DOI] [PubMed] [Google Scholar]
- 37.Levin BE, Triscari J, Sullivan AC. Metabolic features of diet-induced obesity without hyperphagia in young rats. Am J Physiol Regul Integr Comp Physiol 251: R433–R440, 1986. [DOI] [PubMed] [Google Scholar]
- 38.Levine J, Lanningham-Foster L, McCrady S, Krizan A, Olson L, Kane P, Jensen M, Clark M. Interindividual variation in posture allocation: possible role in human obesity. Science 307: 584–586, 2005. [DOI] [PubMed] [Google Scholar]
- 39.Lu XY, Bagnol D, Burke S, Akil H, Watson SJ. Differential distribution and regulation of OX1 and OX2 orexin/hypocretin receptor messenger RNA in the brain upon fasting. Horm Behav 37: 335–344, 2000. [DOI] [PubMed] [Google Scholar]
- 40.MacLean PS, Higgins JA, Johnson GC, Fleming-Elder BK, Donahoo WT, Melanson EL, Hill JO. Enhanced metabolic efficiency contributes to weight regain after weight loss in obesity-prone rats. Am J Physiol Regul Integr Comp Physiol 287: R1306–R1315, 2004. [DOI] [PubMed] [Google Scholar]
- 41.Mamczarz J, Bowker JL, Duffy K, Zhu M, Hagepanos A, Ingram DK. Enhancement of amphetamine-induced locomotor response in rats on different regimens of diet restriction and 2-deoxy-d-glucose treatment. Neuroscience 131: 451–464, 2005. [DOI] [PubMed] [Google Scholar]
- 42.Martin CK, Heilbronn LK, de Jonge L, DeLany JP, Volaufova J, Anton SD, Redman LM, Smith SR, Ravussin E. Effect of calorie restriction on resting metabolic rate and spontaneous physical activity. Obesity 15: 2964–2973, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. J Neurosci 24: 10493–10501, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mistlberger RE. Circadian food-anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev 18: 171–195, 1994. [DOI] [PubMed] [Google Scholar]
- 45.Moran TH, Tamashiro KL. Curt Richter: spontaneous activity and food intake. Appetite 49: 368–375, 2007. [DOI] [PubMed] [Google Scholar]
- 46.Novak CM, Jiang X, Wang C, Teske JA, Kotz CM, Levine JA. Caloric restriction and physical activity in zebrafish (Danio rerio). Neurosci Lett 383: 99–104, 2005. [DOI] [PubMed] [Google Scholar]
- 47.Patterson CM, Dunn-Meynell AA, Levin BE. Three weeks of early-onset exercise prolongs obesity resistance in DIO rats after exercise cessation. Am J Physiol Regul Integr Comp Physiol 294: R290–R301, 2008. [DOI] [PubMed] [Google Scholar]
- 48.Ramsey JJ, Colman RJ, Binkley NC, Christensen JD, Gresl TA, Kemnitz JW, Weindruch R. Dietary restriction and aging in rhesus monkeys: the University of Wisconsin study. Exp Gerontol 35: 1131–1149, 2000. [DOI] [PubMed] [Google Scholar]
- 49.Ricci MR, Levin BE. Ontogeny of diet-induced obesity in selectively bred Sprague-Dawley rats. Am J Physiol Regul Integr Comp Physiol 285: R610–R618, 2003. [DOI] [PubMed] [Google Scholar]
- 50.Ritter S, Taylor JS. Vagal sensory neurons are required for lipoprivic but not glucoprivic feeding in rats. Am J Physiol Regul Integr Comp Physiol 258: R1395–R1401, 1990. [DOI] [PubMed] [Google Scholar]
- 51.Rosenbaum M, Ravussin E, Matthews DE, Gilker C, Ferraro R, Heymsfield SB, Hirsch J, Leibel RL. A comparative study of different means of assessing long-term energy expenditure in humans. Am J Physiol Regul Integr Comp Physiol 270: R496–R504, 1996. [DOI] [PubMed] [Google Scholar]
- 52.Rosenbaum M, Vandenborne K, Goldsmith R, Simoneau JA, Heymsfield S, Joanisse DR, Hirsch J, Murphy E, Matthews D, Segal KR, Leibel RL. Effects of experimental weight perturbation on skeletal muscle work efficiency in human subjects. Am J Physiol Regul Integr Comp Physiol 285: R183–R192, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92: 573–585, 1998. [DOI] [PubMed] [Google Scholar]
- 54.Severinsen T, Munch IC. Body core temperature during food restriction in rats. Acta Physiol Scand 165: 299–305, 1999. [DOI] [PubMed] [Google Scholar]
- 55.Sherwin CM. Voluntary wheel running: a review and novel interpretation. Anim Behav 56: 11–27, 1998. [DOI] [PubMed] [Google Scholar]
- 56.Snitker S, Tataranni PA, Ravussin E. Spontaneous physical activity in a respiratory chamber is correlated to habitual physical activity. Int J Obes Relat Metab Disord 25: 1481–1486, 2001. [DOI] [PubMed] [Google Scholar]
- 57.Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol 291: R889–R899, 2006. [DOI] [PubMed] [Google Scholar]
- 58.Thorpe AJ, Mullett MA, Wang C, Kotz CM. Peptides that regulate food intake: regional, metabolic, and circadian specificity of lateral hypothalamic orexin A feeding stimulation. Am J Physiol Regul Integr Comp Physiol 284: R1409–R1417, 2003. [DOI] [PubMed] [Google Scholar]
- 59.Thorpe AJ, Teske JA, Kotz CM. Orexin A induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol 289: R367–R372, 2005. [DOI] [PubMed] [Google Scholar]
- 60.Wan R, Camandola S, Mattson MP. Intermittent fasting and dietary supplementation with 2-deoxy-d-glucose improve functional and metabolic cardiovascular risk factors in rats. FASEB J 17: 1133–1134, 2003. [DOI] [PubMed] [Google Scholar]
- 61.Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav 62: 97–103, 1997. [DOI] [PubMed] [Google Scholar]
- 62.Westerterp KR, Kester AD. Physical activity in confined conditions as an indicator of free-living physical activity. Obes Res 11: 865–868, 2003. [DOI] [PubMed] [Google Scholar]
- 63.Wolden-Hanson T, Davis GA, Baum ST, Kemnitz JW. Insulin levels, physical activity, and urinary catecholamine excretion of obese and non-obese rhesus monkeys. Obes Res 1: 5–17, 1993. [DOI] [PubMed] [Google Scholar]
- 64.Yu BP, Masoro EJ, McMahan CA. Nutritional influences on aging of Fischer 344 rats: I. Physical, metabolic, and longevity characteristics. J Gerontol 40: 657–670, 1985. [DOI] [PubMed] [Google Scholar]
- 65.Zurlo F, Ferraro RT, Fontvielle AM, Rising R, Bogardus C, Ravussin E. Spontaneous physical activity and obesity: cross-sectional and longitudinal studies in Pima Indians. Am J Physiol Endocrinol Metab 263: E296–E300, 1992. [DOI] [PubMed] [Google Scholar]