Abstract
Background
This is an updated version of the Cochrane Review published in 2015.
Epilepsy is a chronic neurological disorder, characterised by recurring, unprovoked seizures. Vagus nerve stimulation (VNS) is a neuromodulatory treatment that is used as an adjunctive therapy for treating people with drug‐resistant epilepsy. VNS consists of chronic, intermittent electrical stimulation of the vagus nerve, delivered by a programmable pulse generator.
Objectives
To evaluate the efficacy and tolerability of VNS when used as add‐on treatment for people with drug‐resistant focal epilepsy.
Search methods
For this update, we searched the Cochrane Register of Studies (CRS), and MEDLINE Ovid on 3 March 2022. We imposed no language restrictions. CRS Web includes randomised or quasi‐randomised controlled trials from the Specialised Registers of Cochrane Review Groups, including Epilepsy, CENTRAL, PubMed, Embase, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform.
Selection criteria
We considered parallel or cross‐over, randomised, double‐blind, controlled trials of VNS as add‐on treatment, which compared high‐ and low‐level stimulation (including three different stimulation paradigms: rapid, mild, and slow duty‐cycle), and VNS stimulation versus no stimulation, or a different intervention. We considered adults or children with drug‐resistant focal seizures who were either not eligible for surgery, or who had failed surgery.
Data collection and analysis
We followed standard Cochrane methods, assessing the following outcomes:
1. 50% or greater reduction in seizure frequency 2. Treatment withdrawal (any reason) 3. Adverse effects 4. Quality of life (QoL) 5. Cognition 6. Mood
Main results
We did not identify any new studies for this update, therefore, the conclusions are unchanged.
We included the five randomised controlled trials (RCT) from the last update, with a total of 439 participants. The baseline phase ranged from 4 to 12 weeks, and double‐blind treatment phases from 12 to 20 weeks. We rated two studies at an overall low risk of bias, and three at an overall unclear risk of bias, due to lack of reported information about study design. Effective blinding of studies of VNS is difficult, due to the frequency of stimulation‐related side effects, such as voice alteration.
The risk ratio (RR) for 50% or greater reduction in seizure frequency was 1.73 (95% confidence interval (CI) 1.13 to 2.64; 4 RCTs, 373 participants; moderate‐certainty evidence), showing that high frequency VNS was over one and a half times more effective than low frequency VNS.
The RR for treatment withdrawal was 2.56 (95% CI 0.51 to 12.71; 4 RCTs, 375 participants; low‐certainty evidence). Results for the top five reported adverse events were: hoarseness RR 2.17 (99% CI 1.49 to 3.17; 3 RCTs, 330 participants; moderate‐certainty evidence); cough RR 1.09 (99% CI 0.74 to 1.62; 3 RCTs, 334 participants; moderate‐certainty evidence); dyspnoea RR 2.45 (99% CI 1.07 to 5.60; 3 RCTs, 312 participants; low‐certainty evidence); pain RR 1.01 (99% CI 0.60 to 1.68; 2 RCTs; 312 participants; moderate‐certainty evidence); paraesthesia 0.78 (99% CI 0.39 to 1.53; 2 RCTs, 312 participants; moderate‐certainty evidence).
Results from two studies (312 participants) showed that a small number of favourable QOL effects were associated with VNS stimulation, but results were inconclusive between high‐ and low‐level stimulation groups. One study (198 participants) found inconclusive results between high‐ and low‐level stimulation for cognition on all measures used. One study (114 participants) found the majority of participants showed an improvement in mood on the Montgomery–Åsberg Depression Rating Scale compared to baseline, but results between high‐ and low‐level stimulation were inconclusive.
We found no important heterogeneity between studies for any of the outcomes.
Authors' conclusions
VNS for focal seizures appears to be an effective and well‐tolerated treatment. Results of the overall efficacy analysis show that high‐level stimulation reduced the frequency of seizures better than low‐level stimulation. There were very few withdrawals, which suggests that VNS is well tolerated.
Adverse effects associated with implantation and stimulation were primarily hoarseness, cough, dyspnoea, pain, paraesthesia, nausea, and headache, with hoarseness and dyspnoea more likely to occur with high‐level stimulation than low‐level stimulation.
However, the evidence for these outcomes is limited, and of moderate to low certainty.
Further high‐quality research is needed to fully evaluate the efficacy and tolerability of VNS for drug‐resistant focal seizures.
Keywords: Adult; Child; Humans; Anticonvulsants; Anticonvulsants/therapeutic use; Cough; Drug Resistant Epilepsy; Drug Resistant Epilepsy/drug therapy; Drug Therapy, Combination; Dyspnea; Dyspnea/drug therapy; Hoarseness; Hoarseness/chemically induced; Hoarseness/drug therapy; Pain; Pain/drug therapy; Paresthesia; Paresthesia/chemically induced; Seizures; Seizures/drug therapy; Vagus Nerve Stimulation; Vagus Nerve Stimulation/adverse effects
Plain language summary
Vagus nerve stimulation for focal seizures
What did we want to find out? The aim of this review was to find the current evidence on how effective vagus nerve stimulation is in reducing the frequency of epileptic seizures, and any side effects associated with the treatment.
What is epilepsy and how is it treated? Epilepsy is a disorder in which unexpected electrical discharges from the brain cause seizures. Most seizures can be controlled by a single antiepileptic drug, but sometimes, seizures do not respond to drugs. Some people need more than one antiepileptic drug to control their seizures, especially if they originate from one area of the brain (focal epilepsy), instead of involving the whole brain.
The vagus nerve runs down the side of the neck, from the brain to the large intestines, and controls body systems, like the heart and digestion. The vagus nerve stimulator (VNS) is a device that is used as an add‐on treatment for epilepsy, if it does not respond well to drugs, and only affects one part of the brain. The device is connected to the vagus nerve, and sends mild electrical impulses to it. This is particularly important for treating people whose epilepsy did not respond well to drugs, who are not eligible for epilepsy surgery, or for whom surgery was not successful in reducing the frequency of their seizures.
What did we do? We did not identify any new studies for this update. We included five multicentre, randomised controlled trials (RCTs) from the last update, which recruited a total of 439 participants, and compared different types of VNS therapy. Three compared high‐level stimulation to low‐level stimulation in participants from 12 to 60 years old. One trial examined high frequency stimulation versus low frequency stimulation in children. One trial examined three different stimulation frequencies.
What did we find? Since we did not identify any new studies, the conclusions remain unchanged.
VNS seems to be an effective treatment for people with intractable focal epilepsy. High‐level stimulation seems to reduce the number of seizures people had compared to low‐level stimulation.
Common side effects were voice alteration and hoarseness, pain, shortness of breath, cough, feeling sickly, tingling sensation, headache, or infection at the site of the operation. Shortness of breath, voice alteration and hoarseness were more common in people receiving high‐level stimulation compared to people receiving low‐level stimulation.
What are the limitations of the evidence?
The evidence for the effectiveness and side effects of VNS therapy was limited and imprecise. There were a small number of studies and participants included in the review, and details about the design and conduct of the trials was sometimes lacking. We rated the evidence as moderate or low certainty. This means that further research is likely, or very likely, to have an important impact on our confidence in the estimate of the effect, and may change the estimate.
How up to date is this evidence?
The evidence is current to 3 March 2022.
Summary of findings
Summary of findings 1. High versus low stimulation for focal seizures.
| High versus low stimulation for focal seizures | ||||||
| Patient or population: people with focal seizures Settings: outpatients Intervention: high stimulation Comparison: low stimulation | ||||||
| Outcomes |
Illustrative comparative risks* (95% CI) For adverse effects (99% CI) |
Relative effect
(95% CI) For individual adverse effects (99% CI) |
No of Participants (studies) | Certainty of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Low stimulation | High stimulation | |||||
| 50% reduction in seizure frequency (responders) | 144 per 1000 | 249 per 1000 (163 to 380) | RR 1.73 (1.13 to 2.64) | 373 (4 studies) | ⊕⊕⊕⊝ moderatea | RR > 1 indicates outcome is more likely with high stimulation |
| Withdrawals | 10 per 1000 | 26 per 1000 (5 to 130) | RR 2.56 (0.51 to 12.71) | 375 (4 studies) | ⊕⊕⊝⊝ lowa,b | RR > 1 indicates outcome is more likely with high stimulation |
| Voice alteration or hoarseness | 251 per 1000 | 545 per 1000 (374 to 796) | RR 2.17 (1.49 to 3.17) | 330 (3 studies) | ⊕⊕⊕⊝ moderatea | RR > 1 indicates outcome is more likely with high stimulation |
| Cough | 291 per 1000 | 317 per 1000 (215 to 471) | RR 1.09 (0.74 to 1.62) | 334 (3 studies) | ⊕⊕⊕⊝ moderatea | RR > 1 indicates outcome is more likely on high stimulation |
| Dyspnoea | 74 per 1000 | 181 per 1000 (79 to 414) | RR 2.45 (1.07 to 5.60) | 312 (2 studies) | ⊕⊕⊝⊝ lowa,c | RR > 1 indicates outcome is more likely on high stimulation |
| Pain | 239 per 1000 | 241 per 1000 (143 to 402) | RR 1.01 (0.60 to 1.68) | 312 (2 studies) | ⊕⊕⊕⊝ moderatea | RR > 1 indicates outcome is more likely on high stimulation |
| Paraesthesias | 172 per 1000 | 134 per 1000 (67 to 263) | RR 0.78 (0.39 to 1.53) | 312 (2 studies) | ⊕⊕⊕⊝ moderatea | RR > 1 indicates outcome is more likely on high stimulation |
| *The basis for the assumed risk is provided in footnote d. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High certainty evidence: further research is very unlikely to change our confidence in the estimate of effect. Moderate certainty evidence: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low certainty evidence: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low certainty evidence: we are very uncertain about the estimate. | ||||||
aOne study that contributed to this outcome was judged to be at high risk of bias, as it had incomplete outcome data, which could not be analysed by an intention‐to‐treat approach. bWide, imprecise confidence interval of the pooled effect estimate due to low withdrawal rates in the included studies cWide, imprecise confidence interval of the pooled effect estimate due to low event rates in the included studies dAssumed Risk: the event rate in the low‐level stimulation group multiplied by 1000. The event rate is the proportion of the total in which the event occurred.
Background
This is an updated version of the Cochrane review published in 2015 (Panebianco 2015).
Description of the condition
Epilepsy is a condition characterised by a tendency for recurrent seizures, unprovoked by any known proximate insult. Epileptiform (relates to seizure patterns) discharges involve either a localised area of the brain resulting in a focal seizure, or the entire brain resulting in a generalised seizure. The prevalence of epilepsy is estimated to be five to eight per 1000 population in developed countries; in adults, the most common type is focal epilepsy (Forsgren 2005; Hauser 1975). The majority of people given a diagnosis of epilepsy have a good prognosis, and their seizures will be controlled by treatment with a single antiepileptic drug (AED). However, 20% (reported in population‐based studies) to 30% (reported in clinical (non‐population‐based) series) will develop drug‐resistant epilepsy, often requiring treatment with combinations of AEDs (Cockerell 1995; Kwan 2000). People in this population tend to have frequent, disabling seizures that limit their ability to work and participate in activities. Many of them also suffer from the chronic effects of long‐term, high‐dose AED polytherapy (treatment that uses more than one medication), while anxiety and depressive disorders are common in people with epilepsy. Therefore, the development of effective new therapies for the treatment of drug‐resistant seizures is of considerable importance.
Description of the intervention
Vagus nerve stimulation (VNS) is a neuromodulatory treatment (neuromodulation is the process by which nervous activity is regulated, by controlling the physiological levels of neurotransmitters), used as an adjunctive therapy for people with drug‐resistant epilepsy who are not eligible for epilepsy surgery, or for whom surgery has failed. In this procedure, a pacemaker‐like device (the Neuro‐Cybernetic Prosthesis (NCP)) is implanted under the skin of the chest. The stimulating electrodes of the NCP carry electrical signals from the generator to the left vagus nerve. By programming the device, the frequency, intensity, and duration of stimulation can be varied (the stimulation paradigm). In the initial trials, the vagus nerve was stimulated for 30 seconds, every five minutes (Sackeim 2001). During each 30‐second stimulation, the device delivered 500 microsecond pulses, at 30 Hz frequency. For each individual, the intensity of the current was set to the highest level they could tolerate, or to low‐level stimulation, depending on the allocated treatment group. Also, in an attempt to further abort seizures, participants could activate the device by placing a magnet over it when a seizure had occurred, or was about to occur. Participants enrolled in the initial randomised controlled trials of VNS had drug‐resistant focal epilepsy, and experienced a 24% to 28% median reduction in seizure frequency over a three‐month treatment period (Selway 1987).
How the intervention might work
Left VNS is a promising, relatively new treatment for epilepsy. In 1997, VNS was approved in the United States as an adjunctive treatment for drug‐resistant focal‐onset seizures in adults and adolescents. For some people with focal‐onset seizures, the adverse effects of AEDs are intolerable; for others, no single AED or combination of anticonvulsant agents is effective. Cerebral resective surgery (a procedure during which the brain tissue is resected to remove the seizure focus) is an alternative to pharmacotherapy in some cases, but many people with focal‐onset seizures are not optimal candidates for intracranial (within the cranium) surgery (Schachter 1998).
The mechanism of action of VNS is not fully understood, but can be reasonably assumed to involve brainstem nuclei. The nucleus of the solitary tract, the main terminus for vagal afferents (fibres specialised to detect stimuli associated with physiological activity of visceral endings), has direct or indirect projections to the locus coeruleus, raphe nucleus, reticular formation, and other brainstem nuclei. These nuclei have been shown to influence cerebral seizure susceptibility, hence vagal modulation of one or more of these nuclei could plausibly represent the mechanism for seizure suppression (Krahl 2012). In this context, the immunomodulatory function (modulation of the immune system) of the vagus nerve is of particular interest. When inflamed, afferent signals can activate the so‐called cholinergic (receptors or synapses that use acetylcholine as neurotransmitter) anti‐inflammatory pathway. Through this pathway, efferent (motor) vagus nerve fibres inhibit the release of pro‐inflammatory cytokines (small secreted proteins with a specific effect on the interactions and communications between cells), and in this way, reduce inflammation. In recent years, inflammation has been strongly implicated in the development of seizures and epilepsy, and therefore, the activation of the anti‐inflammatory pathway by VNS could decrease the inflammatory response, and thereby, explain its clinical effects. In addition to anticonvulsive effects, VNS might have positive effects on behaviour, mood, and cognition (Panebianco 2016; Vonck 2014).
Why it is important to do this review
In this review, we summarised evidence from randomised controlled trials that investigated the efficacy and tolerability of VNS for people with drug‐resistant focal epilepsy, in order to aid clinical decision‐making.
Objectives
To evaluate the efficacy and tolerability of vagus nerve stimulation (VNS) when used as add‐on treatment for people with drug‐resistant focal epilepsy.
Methods
Criteria for considering studies for this review
Types of studies
Trials had to meet all the following criteria:
Randomised controlled trials;
Double‐blind trials;
Placebo‐controlled, with active control (low stimulation) or other intervention control groups; and
Parallel group or cross‐over studies.
Types of participants
Individuals of any age with focal epilepsy (i.e. experiencing simple focal, complex focal, or secondarily generalised tonic‐clonic seizures) who had failed to respond to at least one antiepileptic drug (AED), who were not eligible for surgery, or for whom surgery had previously failed.
Types of interventions
Vagus nerve stimulation (VNS) using high‐level stimulation (therapeutic) versus low‐level stimulation (presumed sub‐therapeutic)
VNS stimulation versus different stimulation of the VNS
VNS stimulation versus no stimulation
VNS stimulation versus a different intervention
Types of outcome measures
Primary outcomes
50% or greater reduction in seizure frequency
The proportion of participants with a 50% or greater reduction in seizure frequency during the treatment period, compared to the pre‐randomisation baseline period
Secondary outcomes
Treatment withdrawal
The proportion of participants having their allocated VNS paradigm stopped or altered during the course of the trial, for whatever reason was used as a measure of 'global effectiveness'. Treatment is likely to be withdrawn due to adverse effects, lack of efficacy, or a combination of both, and this was an outcome to which the individual made a direct contribution.
Adverse effects
We reported the incidence of adverse events in all VNS‐implanted participants, and according to randomised group. We chose to investigate the following adverse effects, which were the most common and important.
Infection at implantation site
Haemorrhage at implantation site
Voice alteration or hoarseness
Pain
Dyspnoea
Cough
Ataxia
Dizziness
Paraesthesias
Fatigue
Nausea
Somnolence
Headache
In addition, we reported the five most common adverse effects (if different from those stated above).
Quality of life
The difference between intervention and control group(s) means on quality of life measures used in the individual studies
Cognition
The difference between intervention and control group(s) means on cognitive assessments used in the individual studies
Mood
The difference between intervention and control group(s) means on mood assessments used in the individual studies
Search methods for identification of studies
Electronic searches
Searches were run for the original review in 2000. Subsequent searches were run in 2005, July 2007, January 2010, July 2012, September 2013, February 2015, December 2016, and May 2019. For the latest update, we searched the following databases on 3 March 2022.
The Cochrane Register of Studies (CRS Web), using the strategy outlined in Appendix 1. CRS Web includes randomised or quasi‐randomised, controlled trials from the Specialised Registers of Cochrane Review Groups, including Epilepsy, the Cochrane Central Register of Controlled Trials (CENTRAL), PubMed, Embase, ClinicalTrials.gov, and the World Health Organization International Clinical Trials Registry Platform (ICTRP).
MEDLINE Ovid (1946 to March 02, 2022), using the strategy outlined in Appendix 2. In MEDLINE Ovid, the coverage end date always lags a few days behind the search date.
We imposed no language restrictions.
Searching other resources
We checked reference lists of included studies to search for additional reports of relevant studies, and performed citation searches on key articles, as we did in Panebianco 2015. We contacted experts in the field for ongoing trials, and the manufacturer of VNS (Cyberonics) for additional information.
Data collection and analysis
Selection of studies
For this update, two review authors (MP and AR) independently assessed trials for inclusion. Any disagreements were resolved by discussion with a third author (AM). For the original version of this review, three review authors (MP, AR, and JW) extracted data and assessed the risk of bias; again, disagreements were resolved by discussion.
See Figure 1 for the flow‐chart of study identification and selection.
1.
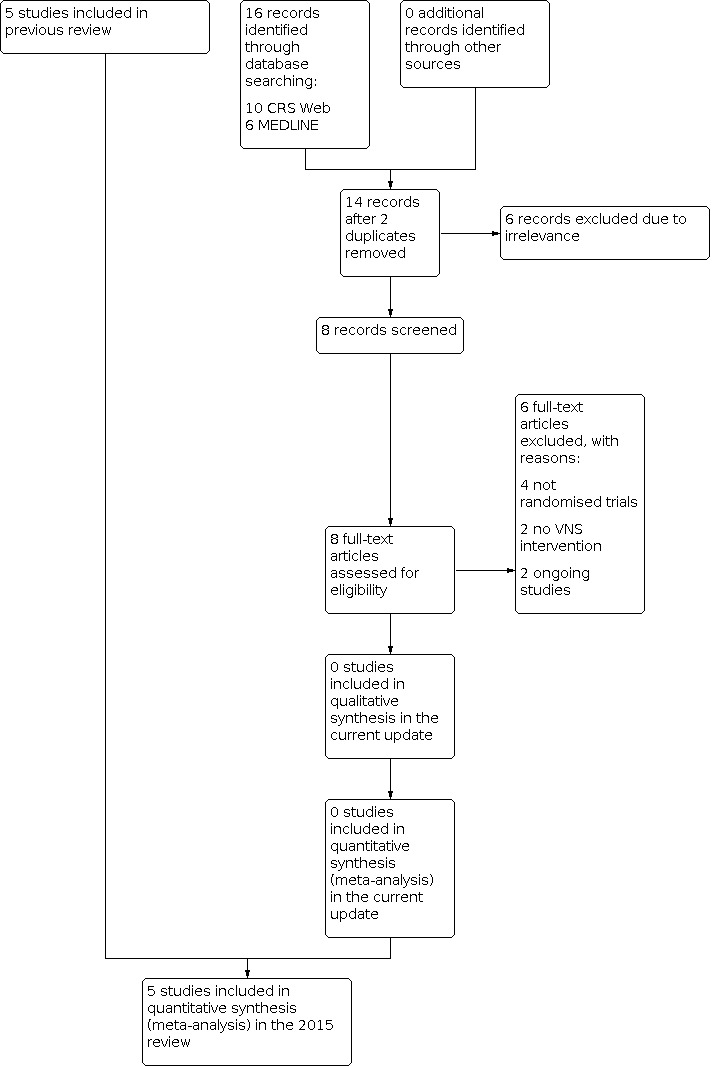
Study flow diagram (reflecting results of the search carried out on 3 March 2022)
Data extraction and management
The following data were extracted for each trial, using a data extraction form.
Methodological/trial design
Method of randomisation
Method of allocation concealment
Method of double‐blinding
Whether any participants were excluded from reported analyses
Duration of baseline period
Duration of treatment period
Frequency of VNS tested
Information on sponsorship/funding
Participant/demographic information
Total number of participants allocated to each treatment group
Age/sex
Number with focal/generalised epilepsy
Seizure types
Seizure frequency during the baseline period
Number of background drugs
Outcomes
We recorded the number of participants experiencing each outcome (see Types of outcome measures) per randomised group, and contacted authors of trials for any missing information.
Assessment of risk of bias in included studies
MP and AR independently assessed the risk of bias for each trial, using the Cochrane RoB 1 tool, described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreements were discussed and resolved. We rated all included studies as having a low, high, or unclear risk of bias on six domains applicable to randomised controlled trials: randomisation method, allocation concealment, blinding methods, incomplete outcome data, selective outcome reporting, and other sources of bias.
Measures of treatment effect
We analysed the primary outcome of seizure reduction as a binary outcome, and presented it as a risk ratio. We also analysed secondary outcomes, including adverse effects and treatment withdrawal, as binary outcomes, and presented risk ratios. We planned to analyse quality of life and cognition as continuous outcomes, using the standardised mean difference, but this was not possible, due to limited information from single studies for cognition outcomes, and heterogenous measurement scales for quality of life outcomes. Therefore, these outcomes were discussed narratively.
Unit of analysis issues
We did not encounter any unit of analysis issues, as we did not find any cross‐over studies; we analysed all outcomes using risk ratios, as planned, or discussed them narratively.
Dealing with missing data
We sought missing data by contacting the study authors. We carried out intention‐to‐treat (ITT), best‐case, and worst‐case analyses on the primary outcome, to account for any missing data (see Data synthesis). We presented all analyses in the main report.
Assessment of heterogeneity
We assessed clinical heterogeneity, by comparing the distribution of important individual participant factors among trials (for example age, seizure type, duration of epilepsy, number of AEDs taken at the time of randomisation), and trial factors (for example randomisation concealment, blinding, losses to follow‐up). We evaluated statistical heterogeneity among trials using the Chi² test with significance set at 0.1, along with the I² statistic. For the Chi² test, P > 0.1 indicated no significant statistical heterogeneity; P ≤ 0.1 indicated heterogeneity, according to percentage ranges of the I² statistic (Deeks 2011).
Assessment of reporting biases
We requested protocols from study authors for all included studies, to enable a comparison of outcomes of interest. If we suspected outcome reporting bias for any included study, we planned to further investigate, using the ORBIT matrix system (Kirkham 2010). We planned to exam asymmetry in funnel plots to assess the likelihood of publication bias, but we were unable to complete this assessment, due to the small number of studies included in the review.
Data synthesis
We used a fixed‐effect model in the meta‐analysis. We planned to stratify each comparison by the type of control group, that is level of stimulation (if any), and study characteristics, to ensure the appropriate combination of study data. Our preferred estimator for all binary outcomes was the Mantel‐Haenzsel risk ratio (RR). For the outcomes, 50% or greater reduction in seizure frequency, and treatment withdrawal, we used 95% confidence intervals (Cls). For individual adverse effects, we used 99% Cls, to make an allowance for multiple testing. Our analyses included all participants in the treatment groups to which they had been allocated following implantation of VNS. For the efficacy outcome (50% or greater reduction in seizure frequency), we undertook three analyses.
Primary (ITT) analysis. Participants not completing follow‐up, or with inadequate seizure data were assumed to be non‐responders. We analysed by ITT when this was reported by the included studies.To test the effect of this assumption, we undertook sensitivity analyses.
Worst‐case analysis. Participants not completing follow‐up, or with inadequate seizure data were assumed to be non‐responders in the high‐level stimulation group, and responders in the low‐level stimulation group.
Best‐case analysis. Participants not completing follow‐up, or with inadequate seizure data were assumed to be responders in the high‐level stimulation group, and non‐responders in the low‐level stimulation group.
Subgroup analysis and investigation of heterogeneity
We performed a subgroup analysis for adverse effects. We intended to investigate heterogeneity using sensitivity analysis, if deemed appropriate.
Sensitivity analysis
We planned to conduct the following sensitivity analyses to test the robustness of the meta‐analysis, where possible.
Repeating the analysis excluding unpublished studies.
Repeating the analysis excluding studies published only as abstracts.
These sensitivity analyses was not required, as all the included studies were published journal articles.
Summary of findings and assessment of the certainty of the evidence
We created Table 1, using the GRADE approach to assess the certainty of the evidence. We downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, or high probability of publication bias. We downgraded evidence by one level if we considered the limitation to be serious, and by two levels if very serious.
We included the primary outcome, 50% or greater reduction in seizure frequency, the secondary outcome, treatment withdrawal, and the five most commonly reported adverse events in the table.
Results
Description of studies
Results of the search
The latest search (carried out 3 March 2022) identified 16 records. We screened 14 records after duplicates were removed. We excluded six at this point, and requested eight full‐text articles to assess for eligibility. We contacted authors of these trials when their contact details were available, for more information. Following this, we excluded six studies. Two studies were ongoing. Thus, we did not include any new studies in this review.
Included studies
We did not find any new studies for this update.
The previous version of this review included five randomised controlled trials, which recruited a total of 439 participants (DeGiorgio 2005; Handforth 1998; Klinkenberg 2012; Michael 1993; VNS Study Group 1995). Trial characteristics are summarised below. For further information on each trial, please see Characteristics of included studies.
Three trials compared high‐level stimulation to low‐level stimulation, in participants aged 12 to 60 years (Handforth 1998; Michael 1993; VNS Study Group 1995), and another trial examined high‐level stimulation versus low‐level stimulation in children (Klinkenberg 2012). One trial examined three different stimulation paradigms (DeGiorgio 2005). We did not find any studies with different comparisons (different stimulation, no stimulation, different intervention).
One multicentre, parallel trial from the USA randomised 64 participants, older than 12 years, to one of three treatment arms, corresponding to rapid, medium, and slow duty‐cycles: group A, seven seconds on and 18 seconds off (N = 19); group B, 30 seconds on and 30 seconds off (N = 19); group C, 30 seconds on and three minutes off (N = 23). The baseline was four weeks long, with a treatment period of three months (DeGiorgio 2005). Another multicentre, parallel trial from the USA included 198 participants, aged 13 to 60 years, and had two treatment arms: intervention, high‐level stimulation (N = 95) and active control group (low stimulation; N = 103). This trial had a baseline period of 12 to 16 weeks, and a treatment period of 16 weeks (Handforth 1998). Dodrill 2000 and Amar 1998 are linked to this study.
A recent multicentre, parallel trial from the Netherlands investigated children only, and consisted of two treatment arms, including high‐level stimulation (N = 21) and low‐level stimulation (N = 20). The baseline period was 12 weeks long, followed by a treatment period of 20 weeks (Klinkenberg 2012). After the blinded phase, all participants underwent a non‐controlled follow‐up, in which they received high‐level stimulation (add‐on phase). Aalbers 2012 and Klinkenberg 2014 are linked to this study.
One multicentre, parallel trial from the USA and Europe had a pre‐randomisation period of 12 weeks, and a treatment period of 14 weeks, during which 22 adults were randomised to one of two treatment arms: high‐level stimulation, therapeutic (N = 10) or low‐level stimulation, sub‐therapeutic (N = 12). All participants completed the acute phase of the study and entered the extension phase (Michael 1993).
Another multicentre, parallel trial from the USA, Sweden, and Germany randomised 114 participants to one of two treatment arms: high‐level stimulation (N = 54) and low‐level stimulation (N = 60). This trial had a baseline period of 12 weeks, and a treatment period of 14 weeks. Participants exiting the study were offered indefinite extension treatment in an open trial (VNS Study Group 1995). Ben‐Menachem 1994, Ben‐Menachem 1995, Elger 2000, Ramsay 1994, Holder 1992, and Lötvall 1994 are linked to this study.
Excluded studies
In this update, we excluded six studies for the following reasons: four studies were not randomised trials; two studies assessed different interventions. For further information on each trial, please see Characteristics of excluded studies.
Ongoing studies
We identified two ongoing studies (please see Characteristics of ongoing studies).
Risk of bias in included studies
See Figure 2 and Figure 3 for a summary of the risk of bias in each included study. We allocated an overall rating for risk of bias of low, high, or unclear for each study. See below for specific domain ratings.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
We rated four studies at low risk of bias for sequence generation, because they reported using a computer‐generated randomisation schedule, random number tables, or random permuted blocks (DeGiorgio 2005; Handforth 1998; Klinkenberg 2012; VNS Study Group 1995). We rated one study as unclear due to a lack of details on the methods used (Michael 1993).
We rated the method by which allocation was concealed at low risk of bias In three trials (Handforth 1998; Klinkenberg 2012; VNS Study Group 1995). Two trials did not provide clear methods, and we rated them as unclear risk of bias (DeGiorgio 2005;Michael 1993).
Blinding
All of the studies achieved blinding by using identical implants in the different groups.
We judged blinding of participants as unclear in two papers, as they provided no details of the method of blinding (DeGiorgio 2005; Michael 1993). We rated the other three studies at low risk of bias for this particular domain, because to assure blinding, at each treatment‐phase visit, the device was temporarily turned off while the participant was assessed by the blinded interviewer (Handforth 1998;Klinkenberg 2012; VNS Study Group 1995).
An important issue in blinded trials on VNS is the difficulty of effectively blinding the participants, given the frequency of stimulation‐related side effects, such as voice alteration. This could limit the validity of the observed treatment effects.
Incomplete outcome data
We rated three studies at low risk of bias for incomplete outcome data, as intention‐to‐treat analysis was used, and there were no concerns of missing data having an effect on the overall outcome estimate (Handforth 1998; Klinkenberg 2012; Michael 1993). We rated the DeGiorgio 2005 study as unclear risk of bias, as three participants out of 64 exited early from the study, and an intention‐to‐treat analysis was not used; however, it was unclear if this approach influenced the results of the study. We rated the VNS Study Group 1995 at high risk of bias because 57 participants were randomised to low‐level stimulation; however, three participants allocated to high‐level stimulation had their stimulator programmed for low‐level stimulation, in error. These participants were analysed in the low‐level stimulation group rather than in the high‐level stimulation group, to which they were randomised, therefore, this was not an intention‐to‐treat analysis.
Selective reporting
None of the protocols for the included studies were available, therefore, we were unable to compare a priori methods and outcomes for the published reports. Because of this, we rated all but one study as unclear risk of bias (Klinkenberg 2012). However, It should be noted that based on the information contained in the publications, there was no suspicion of reporting bias. Klinkenberg 2012 described the outcome of IQ in the methods section, but did not report it in the results, thus, we assessed this study at high risk of reporting bias.
Other potential sources of bias
All studies were sponsored by Cyberonics, Inc, Webster (TX), the manufacturers of the device, and therefore, we rated them as unclear risk of bias for this domain.
Effects of interventions
See: Table 1
See: Table 1.
High‐level versus low‐level vagus nerve stimulation (VNS)
Primary outcomes
50% or greater reduction in seizure frequency
Data from four studies contributed to this outcome.
Intention‐to‐treat analysis
Results of a Chi² test showed no significant heterogeneity between trials for a response to VNS (Chi² = 3.67, df = 3, P = 0.30, I² = 18%). The overall risk ratio (RR) for a response to high‐level stimulation compared to low‐level stimulation, using the fixed‐effect model, was 1.73 (95% Cl 1.13 to 2.64; P = 0.01; 4 trials, 373 participants; moderate‐certainty evidence; Analysis 1.1), showing that participants receiving high‐level stimulation were more likely to show a 50% or greater reduction in seizure frequency.
1.1. Analysis.

Comparison 1: High versus low stimulation, Outcome 1: 50% responders
Best‐ and worst‐case scenarios
No significant heterogeneity was found for these outcomes. The overall worst‐case response to VNS was RR 1.61 (95% CI 1.07 to 2.43; P = 0.02; Chi² = 4.94; df = 3; P = 0.18; I² = 0%; Analysis 1.2); the best‐case response was RR 1.91 (95% CI 1.27 to 2.89; P = 0.002; Chi² = 2.13; df = 3; P = 0.55; I² = 39%; Analysis 1.3).
1.2. Analysis.

Comparison 1: High versus low stimulation, Outcome 2: 50% responders – worst‐case scenario
1.3. Analysis.

Comparison 1: High versus low stimulation, Outcome 3: 50% responders – best‐case scenario
All three analyses showed that high‐level stimulation was more likely to reduce the frequency of seizures by 50% or more than low‐level stimulation.
Secondary outcomes
Treatment withdrawal
Data from four studies contributed to this outcome. Five participants withdrew from high‐level stimulation and three participants withdrew from low‐level stimulation in Handforth 1998 and Klinkenberg 2012 combined. No participants withdrew from either stimulation paradigm in Michael 1993 and VNS Study Group 1995. A Chi² test revealed no significant statistical heterogeneity (Chi² = 0.11, df = 1, P = 0.74). The overall risk ratio (RR) for withdrawal for any reason was 2.56 (95% CI 0.51 to 12.71; P = 0.2; 4 studies, 375 participants; low‐certainty evidence; Analysis 1.4). None of the four analyses showed a difference in withdrawal between high‐ and low‐level stimulation.
1.4. Analysis.

Comparison 1: High versus low stimulation, Outcome 4: Withdrawals
Adverse effects
Four studies reported adverse effects. The risk ratios (RR) were as follows.
Voice alteration and hoarseness: more likely to be reported in the high‐level stimulation group (RR 2.17, 99% CI 1.49 to 3.17; 3 studies, 334 participants; moderate‐certainty evidence; Analysis 1.5; (Handforth 1998; Michael 1993; VNS Study Group 1995)).
Cough: the results between the two groups were inconclusive (RR 1.09; 99% CI 0.74 to 1.62; 3 studies, 334 participants; moderate‐certainty evidence; Analysis 1.6; (Handforth 1998; Michael 1993; VNS Study Group 1995)).
Dyspnoea: more likely to be reported in the high‐level stimulation group (RR 2.45, 99% CI 1.07 to 5.60; 2 studies, 312 participants; Analysis 1.7; (Handforth 1998; VNS Study Group 1995)).
Pain: the results between the two groups were inconclusive (RR 1.01, 99% CI 0.60 to 1.68; 2 studies, 312 participants; Analysis 1.8; (Handforth 1998; VNS Study Group 1995)).
Paraesthesia: more likely to be reported in the low‐level stimulation group (RR 0.78; 99% CI 0.39 to 1.53; 2 studies, 312 participants; Analysis 1.9; (Handforth 1998; VNS Study Group 1995)).
Nausea: the results between the two groups were inconclusive (RR 0.89, 99% CI 0.42 to 1.90; 2 studies, 312 participants; Analysis 1.10; (Handforth 1998; Michael 1993)).
Headache: more likely to be reported in the low‐level stimulation group (RR 0.90, 99% CI 0.48 to 1.69; 2 studies, 220 participants; Analysis 1.11; (Handforth 1998; VNS Study Group 1995)).
1.5. Analysis.

Comparison 1: High versus low stimulation, Outcome 5: Voice alteration or hoarseness
1.6. Analysis.

Comparison 1: High versus low stimulation, Outcome 6: Cough
1.7. Analysis.

Comparison 1: High versus low stimulation, Outcome 7: Dyspnoea
1.8. Analysis.

Comparison 1: High versus low stimulation, Outcome 8: Pain
1.9. Analysis.

Comparison 1: High versus low stimulation, Outcome 9: Paraesthesias
1.10. Analysis.

Comparison 1: High versus low stimulation, Outcome 10: Nausea
1.11. Analysis.

Comparison 1: High versus low stimulation, Outcome 11: Headache
Overall, this showed that high‐level stimulation led to voice alteration and hoarseness, and dyspnoea. None of the studies reported on haemorrhage at implantation site, ataxia, dizziness, fatigue, or somnolence.
Quality of life (QoL)
Two studies reported data on QoL. We were unable to pool the data because of heterogeneous measurement scales, so reported the results narratively (Handforth 1998; VNS Study Group 1995).
Using the Short‐Form Health Survey (SF‐36), high‐level stimulation led to better physical function and social function, but the results were inconclusive for the other domains. Using the Washington Psychosocial Seizure Inventory, high‐level stimulation led to improved financial status. Participants, investigators, and companions reported improved QoL compared to baseline for both groups using Global Rating Scales. However, the results were inconclusive when they compared the results between raters. Participants who had at least 50% seizure reduction exhibited signs of slight improvement in QoL compared to those who did not demonstrate this degree of seizure reduction. The results were inconclusive between groups when measured with the Quality of Life in Epilepsy‐31 scale, the Medical Outcomes Study, and Health‐Related Hardiness Scale. Overall, a small number of favourable QoL effects were associated with low‐levels stimulation that are now typically used clinically. On the Washington Psychosocial Seizure Inventory, only financial status was reported to be significantly different between groups.
Cognition
Data from one study contributed to this outcome (Handforth 1998). The study authors reported no statistically significant differences in interaction effects between groups for all four measures used: Wonderlic Personnel Test, Digit Cancellation, Stroop Test, and Symbol Digit Modalities.
Mood
Data from one study contributed to this outcome (VNS Study Group 1995). Mood was measured according to the Montgomery–Åsberg Depression Rating Scale (MADRS) at baseline, three months, and six months. A MADRS total score of between 10 and 20 (maximum score 20) indicates mild depressive mood disorder.
In the low‐level stimulation group (N = 5), three participants at baseline, two at three months, and one participant at six months had MADRS scores higher than 10. In the high‐level stimulation group (N = 6), four participants at baseline, two at three months, and one participant at six months had MADRS scores higher than 10. Overall, four of five participants in the low‐level stimulation group, and five of six participants in the high‐level stimulation group showed decreases in MADRS scores over the study, but the difference between groups was inconclusive (Mann‐Whitney's test P < 0.10).
Rapid versus medium versus slow duty‐cycle VNS
Primary outcomes
50% or greater reduction in seizure frequency
Data from one study contributed to this comparison (DeGiorgio 2005).
The reduction in seizure frequency was 22% for rapid‐cycle (P = 0.0078), 26% for medium‐cycle (P = 0.0270), and 29% for slow‐cycle VNS (P = 0.0004). When all three groups were combined, the reduction in seizure frequency was 40%. Study authors reported that between‐group comparisons found no statistically significant differences in seizure frequency (Kruskal Wallis test, P value was not reported).
A responder rate > 50% was the same for all three groups (six participants in each group achieved 50% or greater reduction in seizure frequency).
Secondary outcomes
Treatment withdrawal
Three participants withdrew during the study: one developed a device infection, one was lost to follow‐up, and one could not tolerate stimulation (rapid cycle). The randomisation assignments of the first two participants were unknown; presumably, the study authors deemed this to be irrelevant to the conclusion.
Adverse effects
The combined adverse effects from all three groups were: postoperative pain at the generator 21.3%, throat pain and pharyngitis 9.8%, cough 9.8%, voice alteration 4.9%, vocal cord paralysis 1.6%, abdominal pain and diarrhoea 1.6%. Cough and voice alteration were more common during rapid‐cycle stimulation (26%), versus 5% during medium‐cycle, and 9% during slow‐cycle. The study authors did not list the other adverse effects by treatment groups.
Discussion
Summary of main results
We found no new studies that met the selection criteria during this update.
We included five randomised controlled trials, which recruited 439 participants, from the previous version of the review (Panebianco 2015). All trials were sponsored by Cyberonics, Inc., Webster, TX, USA.
Results of the overall efficacy analysis showed that participants who received high‐level vagus nerve stimulation (VNS) were 1.73 times more likely to have at least a 50% reduction in seizures compared to those who received low‐level stimulation (moderate‐certainty evidence). This effect did not vary substantially for either the best‐ or worst‐case scenarios, which accounted for missing outcome data in one study.
One study compared three different duty‐cycle paradigms (rapid versus mild versus slow), and did not find that one duty cycle reduced seizure frequency more or less than the others.
The total withdrawal rate was 4.7%, which suggests the treatment was well tolerated. The most common adverse events were voice alteration and hoarseness, cough, dyspnoea, pain, paraesthesias, nausea, and headache. Voice alteration, hoarseness, and dyspnoea were more than twice as likely to be reported in participants who received high‐level VNS. However, there was some uncertainty and imprecision in reported differences for the other adverse events between groups; there were often wide confidence intervals, making it difficult to draw conclusions.
A small number of favourable quality of life effects resulted from VNS stimulation, but the results between high‐ and low‐level stimulation were inconclusive. The results were also inconclusive between groups for cognition and mood, although the majority of participants reported improvement in their mood.
Overall completeness and applicability of evidence
Currently, there are only five studies that examined the effects of VNS for focal seizures, with fewer than 500 participants in total. The addition of further evidence from future studies may change the results and conclusions of this review. This review focused on the use of VNS in drug‐resistant focal seizures. The results cannot be extrapolated to other populations, such as those with generalised epilepsy. The results of this review suggest that VNS is an effective add‐on treatment for drug‐resistant seizures, but we cannot state how VNS compares to other antiepileptic treatments, because it was tested in an active control situation, whereas antiepileptic drugs are tested against placebo. Head‐to‐head trials are needed to assess the relative efficacy and tolerability of antiepileptic treatments.
Quality of the evidence
Out of the five included studies, we rated two studies at an overall low risk of bias; the other three studies as unclear risk of bias, due to lack of methodological detail concerning study design. We used the GRADE approach to rate the level of certainty of the evidence for each outcome (see Table 1).
We rated the certainty of the evidence as moderate for the main outcome of 50% reduction in seizure frequency, due to incomplete outcome data from one study contributing to the analysis. We rated tolerability outcomes (withdrawal and adverse effects) as moderate to low‐certainty evidence, due to the imprecision of pooled results and incomplete outcome data from one study contributing to the analysis.
Potential biases in the review process
Although we requested all trial protocols from the study authors, the time frame in which the majority of the studies were conducted made retrieval of all of these difficult. This could lead to potential bias through omitted information to which we did not have access.
Agreements and disagreements with other studies or reviews
The studies included in this review were essentially active control trials, thus results may be difficult to compare against other meta‐analyses of antiepileptic drugs that were compared against placebo. The magnitude of the risk ratio for high‐level VNS compared to control would tend to be reduced by any anti‐seizure effect of the low‐level stimulation. A higher risk ratio may have been found if high‐level VNS was compared against no stimulation, while with low‐level stimulation participants were less likely to guess which treatment they were receiving. Another review on VNS supported our conclusions regarding a positive outcome with VNS (Morris 2013). This review also described an association between VNS and mood in adult participants, and included children‐specific analyses.
Authors' conclusions
Implications for practice.
Vagus nerve stimulation (VNS) appears to be an effective treatment for people with drug‐resistant focal epilepsy, as an add‐on treatment. High‐level VNS reduced seizure frequency better than low‐level VNS. Both high‐ and low‐level VNS seemed to be well tolerated and withdrawals were rare; however, limited withdrawal information was available for this review, so important differences between high‐ and low‐level stimulation cannot be excluded. Adverse effects associated with implantation and stimulation were primarily hoarseness, cough, dyspnoea, pain, paraesthesia, nausea, and headache; voice alteration and dyspnoea were more likely to occur with high‐level stimulation than low‐level stimulation. The adverse effect profile was substantially different from the adverse effect profile associated with antiepileptic drugs, making VNS a potential alternative for people who have difficulty tolerating antiepileptic drug adverse effects.
Implications for research.
Identifying the adverse effect profile of VNS was rather complex, because treatment involves both the implantation of the device and intermittent stimulation, each with slightly different adverse effects. In addition, these studies were essentially active control trials.
Further research is needed to determine:
the mode of action of VNS;
the long‐term effects of VNS;
the details of effective stimulation paradigms and protocols;
the effectiveness of VNS compared to antiepileptic drugs currently available.
Feedback
Query regarding analysis 01.01, 21 May 2006
Summary
The following comment was made on 21 May 2006 regarding Analysis 01.01. Comparison 01: High stimulation versus low stimulation, Outcome 01: 50% responders.
The two groups in the VNS Study Group consists of 54 people and 60 people and NOT 2 x 57 people. This mistake is shown in many of the analyses.
Reply
Whilst there may be a discrepancy between the data presented in this review and the original published report by the VNS Study Group, the authors have not made a mistake in the preparation of this review.
In the VNS study group trial, 57 patients were randomised to high stimulation and 57 were randomised to low stimulation. However, three patients allocated to high stimulation had their stimulator programmed for low stimulation in error. For the paper published in Neurology, these three participants were analysed in the low stimulation group rather than in the group to which they had been allocated. The authors of this Cochrane Review preferred to use the more conservative intention‐to‐treat analysis, in which participants are analysed in the treatment group to which they were allocated.
Reply made by Dr Tony Marson (Co‐ordinating Editor, Cochrane Epilepsy Group) on behalf of the review authors (06 August 2008).
Update 2014. Following re‐review of the data extracted from the VNS study Group manuscripts, most of the data used in the analyses in this review are presented for the high stimulation group of 54 people, and the low stimulation group of 60 people; in other words, groups according to the treatment received rather than treatment allocated. In the analysis of the previous version of the review, the event rates reported for the groups of 54 and 60 patients respectively were used in the analysis, but with group totals assigned as 57 participants per group, as an intention‐to‐treat analysis.
However, following consideration for this update, given that separate data for the three participants randomised to high stimulation, but who received low stimulation are not presented; and assuming that event rates in the treatment‐received groups would be the same as in the treatment‐allocated groups may not necessarily be correct; for this reason, the authors of the updated review presented results in terms of the treatment‐received groups. They acknowledge that this is not an intention‐to‐treat approach, and reflected this in the risk of bias table for the VNS Study Group 1995 study.
Contributors
Comment made by Dr Jesper Erdal
What's new
| Date | Event | Description |
|---|---|---|
| 21 March 2022 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 3 March 2022 | New search has been performed | Searches updated 3 March 2022; no new relevant studies were identified. |
History
Protocol first published: Issue 1, 2001 Review first published: Issue 1, 2002
| Date | Event | Description |
|---|---|---|
| 2 May 2019 | New search has been performed | Searches updated 02 May 2019; no new studies added. The term 'partial' has been replaced by 'focal', in accordance with the most recent classification of epilepsies of the International League Against Epilepsy (Scheffer 2017). |
| 2 May 2019 | New citation required but conclusions have not changed | Conclusions are unchanged. |
| 20 December 2016 | New search has been performed | Searches updated 20 December 2016; no new studies added. |
| 20 December 2016 | New citation required but conclusions have not changed | Conclusions remain unchanged. |
| 23 February 2015 | New search has been performed | The searches were updated on 23 February 2015. |
| 23 February 2015 | New citation required but conclusions have not changed | Three new studies have been included (identified from a search carried out in September 2013); however the conclusions remain unchanged. A pre‐publication search carried out on 23rd February 2015 identified two potentially relevant studies (Klinkenberg 2014a; NCT02089243). These will be addressed at the next update. |
| 7 August 2009 | Amended | Copyedits made at editorial base. |
| 24 October 2008 | Amended | Search strategy amended to comply with RevMan 5. |
| 11 August 2008 | Feedback has been incorporated | A response has now been made to the feedback originally left on 21 May 2006. |
| 31 July 2008 | Amended | Converted to new review format. |
| 19 July 2007 | New search has been performed | Searches updated 6 July 2007; no new studies identified. |
Acknowledgements
This review update was supported by the National Institute for Health and Care Research, via Cochrane Infrastructure funding to the Epilepsy Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS, or the Department of Health and Social Care.
We acknowledge Jennifer Weston for contributions to previous updates.
Cochrane Epilepsy supported the authors in the development of this review update. The following people conducted the editorial process for this update.
Managing Editor (provided editorial guidance to authors, edited the update, conducted editorial policy checks): Rachael Kelly
Copy Editor (copy‐editing and production): Victoria Pennick
Appendices
Appendix 1. CRS Web search strategy
1. MeSH DESCRIPTOR Vagus Nerve Explode All AND CENTRAL:TARGET
2. (vagus or vagal) NEAR3 stimul* AND CENTRAL:TARGET
3. #1 OR #2
4. MESH DESCRIPTOR Epilepsies, Partial EXPLODE ALL AND CENTRAL:TARGET
5. ((partial or focal) and (seizure* or epilep*)):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
6. (secondar* and (generalized or generalised) and seizure*):AB,KW,KY,MC,MH,TI AND CENTRAL:TARGET
7. #4 OR #5 OR #6
8. #3 AND #7
Appendix 2. MEDLINE search strategy
This strategy includes a modification of the Cochrane Highly sensitive search strategy for identifying randomised trials (Lefebvre 2019).
1. exp Vagus Nerve/
2. ((vagus or vagal) adj3 stimul$).mp.
3. 1 or 2
4. exp Epilepsies, Partial/
5. ((partial or focal) and (seizure$ or epilep$)).mp.
6. (secondar$ and generali?ed and seizure$).mp.
7. 4 or 5 or 6
8. exp controlled clinical trial/ or (randomi?ed or placebo or randomly).ab.
9. clinical trials as topic.sh.
10. trial.ti.
11. 8 or 9 or 10
12. exp animals/ not humans.sh.
13. 11 not 12
14. 3 and 7 and 13
15. remove duplicates from 14
Data and analyses
Comparison 1. High versus low stimulation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 50% responders | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.13, 2.64] |
| 1.2 50% responders – worst‐case scenario | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.61 [1.07, 2.43] |
| 1.3 50% responders – best‐case scenario | 4 | 373 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.27, 2.89] |
| 1.4 Withdrawals | 4 | 375 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.56 [0.51, 12.71] |
| 1.5 Voice alteration or hoarseness | 3 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.17 [1.49, 3.17] |
| 1.6 Cough | 3 | 334 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.09 [0.74, 1.62] |
| 1.7 Dyspnoea | 2 | 312 | Risk Ratio (M‐H, Fixed, 99% CI) | 2.45 [1.07, 5.60] |
| 1.8 Pain | 2 | 312 | Risk Ratio (M‐H, Fixed, 99% CI) | 1.01 [0.60, 1.68] |
| 1.9 Paraesthesias | 2 | 312 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.78 [0.39, 1.53] |
| 1.10 Nausea | 2 | 220 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.89 [0.42, 1.90] |
| 1.11 Headache | 2 | 312 | Risk Ratio (M‐H, Fixed, 99% CI) | 0.90 [0.48, 1.69] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
DeGiorgio 2005.
| Study characteristics | ||
| Methods | Randomised, prospective, active‐control study Pre‐randomisation baseline period: 4 weeks Duration of treatment: 3 months. This period included month 1, month 2, and month 3 time points. |
|
| Participants | A multicentre trial (USA) 64 people randomised Group A (rapid cycle): 19 participants; mean output current at completion of study 0.87 mA Group B (mild cycle): 19 participants; mean output current at completion of study 0.80 mA Group C (low cycle): 23 participants; mean output current at completion of study 0.93 mA |
|
| Interventions | Randomised comparison of three distinct duty‐cycles in treatment of refractory focal seizures: Group A: on/off time 7 s/18 s, duty‐cycle 28%, frequency 20 Hz, pulse width 500 sec rapid cycle; Group B: on/off time 30 s/30 s, duty‐cycle 50%, frequency 20 Hz, pulse width 250 seconds mild cycle; Group C: on/off time 30 s/3 min, duty‐cycle 14%, frequency 30 Hz, pulse width 500 seconds slow cycle. All participants had an identical implantation of the vagus nerve stimulation device (NeuroCybernetic Prosthesis, Cyberonics). |
|
| Outcomes | Primary outcome: Seizure frequency (50% and 75% reduction of seizures) Secondary outcomes:
|
|
| Notes | Supported by a grant from Cyberonics, Inc., Webster, TX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation occurred in blocks of six (2 for each group), with a unique predetermined randomisation schedule for each site. |
| Allocation concealment (selection bias) | Unclear risk | No details of concealment of allocation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Identical implants used, but no details of method of blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 out 64 participants exited early: one developed a device infection, so the device had to be removed; one could not tolerate rapid cycle stimulation, so was converted to a standard duty‐cycle and removed from the study; and one participant was lost to follow‐up. No intention‐to‐treat analysis was performed. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable, but it appeared all expected and prespecified outcomes were reported. |
| Other bias | Unclear risk | All studies were sponsored by Cyberonics, the manufacturer of the device. |
Handforth 1998.
| Study characteristics | ||
| Methods | Randomised, prospective, double‐blind, active‐control study Pre‐randomisation baseline period: 12 to 16 weeks Duration of treatment: 16 weeks. This period included 2, 12, and 16‐week time points. |
|
| Participants | A multicentre trial (USA) 198 people randomised High stimulation group: 95 participants; mean age 32.1 years; 51.6% male and 48.4% female; mean seizures/day 1.59; mean duration of epilepsy 22.1 years Low stimulation group: 103 participants; mean age 34.2 years; 42.7% male and 57.3% female; mean seizures/day 0.97; mean duration of epilepsy 23.7 years |
|
| Interventions | Comparison of high (on/off cycles of 30 seconds every 5 minutes, each 'on' period consisting of 500 µs duration pulses at 30 Hz frequency) and low stimulation (on/off cycles of 30 seconds every 3 hours, each 'on' cycle of 130 µs duration pulse at 1 Hz frequency) in treatment of refractory focal seizures All participants had an identical implantation of the vagus nerve stimulation device (NeuroCybernetic Prosthesis, Cyberonics). |
|
| Outcomes | Primary outcome: Seizure frequency (50% reduction of seizures) Secondary outcomes:
|
|
| Notes | Supported by a grant from Cyberonics, Inc., Webster, TX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation schedule was generated by a statistical consultant before study initiation. |
| Allocation concealment (selection bias) | Low risk | Telephone communication to obtain randomised treatment assignment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study personnel and participants were blinded. Identical implants used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The study data were analysed by a blinded interviewer. Identical implants used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 participants withdrew, but the reasons for exclusion were reported. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section of the paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | All studies were sponsored by Cyberonics, the manufacturer of the device. |
Klinkenberg 2012.
| Study characteristics | ||
| Methods | Randomised, double‐blinded add‐on, active‐control study in children with refractory focal seizures Pre‐randomisation baseline period: 12 weeks Duration of treatment phase: 20 weeks |
|
| Participants | A multicentre trial (the Netherlands) 41 people randomised High‐output stimulation group: 21 participants; mean age at onset 2:10 (y:mo); median age at onset 1:2 (y:mo); mean age at implantation 10:11 (y:mo); 11/10 male/female; median seizures/day 2.1; localisation‐related 90% (symptomatic 71%, cryptogenic 19%); generalised 10% (Idiopathic 0, symptomatic 10%) Low‐output stimulation group: 20 participants; mean age at onset 1:8 (y:mo); median age at onset 1:2 (y:mo); mean age at implantation 11:6 (y:mo);12/8 male/female; median seizures/day 0.9; localisation‐related 80% (symptomatic 50%, cryptogenic 30%); generalised 20% (Idiopathic 10%, symptomatic 10%) A small sample (6 participants) had generalised epilepsy (2 idiopathic and 2 symptomatic). |
|
| Interventions | Comparison of high (output current 0.25 mA, duty‐cycle on/off 30 s/5 min, frequency 30 Hz, pulse width 0.5 ms) and low (output current 0.25 mA, duty‐cycle on/off 14 s/60 min, frequency 1 Hz, pulse width 0.1 ms) stimulation frequency in treatment of refractory focal seizures. In the treatment group, the current was increased stepwise at 2‐week intervals to the maximally tolerated output current (maximum 1.75 mA). All participants had an identical implantation of the vagus nerve stimulation device (NeuroCybernetic Prosthesis, Cyberonics). |
|
| Outcomes | Primary outcomes: Seizure frequency (50% reduction of seizures) Secondary outcomes:
|
|
| Notes | Supported by Cyberonics, Inc., Webster, TX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation method used |
| Allocation concealment (selection bias) | Low risk | Participants were allocated to a treatment condition by one trial nurse, using a computer program. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Neurologist, participants, and parents were blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Investigators were blinded. Identical implants used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 participants withdrew, but the reasons for exclusion were reported. |
| Selective reporting (reporting bias) | High risk | The secondary outcome of IQ, described in the methods, was not reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | All studies were sponsored by Cyberonics, the manufacturer of the device. |
Michael 1993.
| Study characteristics | ||
| Methods | Randomised, controlled, double‐blind, active‐control study of participant with refractory focal seizures Pre‐randomisation baseline period: 12 weeks Duration of treatment: 14 weeks. Efficacy was determined at the end of the acute phase. At the end of the 14‐week double‐blind phase, participants entered an extension phase, in which low stimulation participants were switched to high stimulation. |
|
| Participants | A multicentre trial (USA) 22 people randomised High stimulation group: 10 participants Low stimulation group: 12 participants Mean age 32 years (range 15 to 56); seizure frequency 2 per day; mean duration of epilepsy 19 years (range 5 to 32) |
|
| Interventions | Comparison of high (output current 1.0 to 3.0 mA, on/off time 30 s/5 min, frequency 30 Hz, pulse width 500 µs) and low (output current 0.25 to 0.5 mA, on/off time 30 s/60 to 90 min, frequency 1 Hz, pulse width 130 µs) stimulation in treatment of refractory focal seizures All participants had an identical implantation of the vagus nerve stimulation device (NeuroCybernetic Prosthesis, Cyberonics). |
|
| Outcomes | Primary outcome: seizure frequency (mean seizure frequency percent reduction) Secondary outcomes:
|
|
| Notes | Supported by Cyberonics, Inc., Webster, TX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. Identical implants used. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in the analysis. |
| Selective reporting (reporting bias) | Unclear risk | Protocol unavailable, but it appeared all expected and prespecified outcomes were reported. |
| Other bias | Unclear risk | All studies were sponsored by Cyberonics, the manufacturer of the device. |
VNS Study Group 1995.
| Study characteristics | ||
| Methods | Randomised, prospective, double‐blind, active‐control study Pre‐randomisation baseline period: 12 weeks Duration of treatment: 14 weeks. Efficacy was determined at the 12‐week time point. At the end of the 14 week double‐blind phase, participants entered an extension phase in an open trial. |
|
| Participants | A multicentre trial (USA, Canada, Sweden, and Germany) 114 people randomised High stimulation group: 54 participants; mean age 33.1 years; 61% male and 39% female; mean seizures/day 1.49; median seizures/day 0.73; mean duration of epilepsy 23.1 years; simple focal seizures 24 participants; complex focal seizures 50 participants; secondarily generalised seizures 38 participants Low stimulation group: 60 participants; mean age 33.5 years; 63% male and 37% female; mean seizures/day 1.71; median seizures/day 0.82; mean duration of epilepsy 20.0 years; simple focal seizures 25 participants; complex focal seizures 58 participants; secondarily generalised seizures 33 participants |
|
| Interventions | Comparison of high (output current 0.25 to 3.0 mA, on/off time 30 to 90 s/5 to 10 min, frequency 20 to 50 Hz, pulse width 500 µs) and low (output current 0.25 to 1.75 mA, on/off time 30 s/60 to 180 min, frequency 1 to 2 Hz, pulse width 130 µs) stimulation in treatment of refractory focal seizures All participants had an identical implantation of the vagus nerve stimulation device (NeuroCybernetic Prosthesis, Cyberonics). |
|
| Outcomes | Primary outcome: Seizure frequency (50% and 75% reduction of seizures) Secondary outcomes:
|
|
| Notes | Supported by a grant from Cyberonics, Inc., Webster, TX | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables were developed by an independent statistician with assignments, based on the participant's identification code. |
| Allocation concealment (selection bias) | Low risk | Assignments were made automatically by a look‐up table in the computer software used to program the generator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants were blinded and one investigator for each site was unblinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The interviewer remained blinded to treatment group. Identical implants used. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 57 participants were randomised to low stimulation. 3 participants allocated to high stimulation had their stimulator programmed for low stimulation in error. These participants were analysed in the low stimulation group according to the treatment they received rather than the treatment they were randomised to. This was not an intention‐to‐treat analysis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes stated in the methods section of the paper were reported in the results. There was no protocol available to check a priori outcomes. |
| Other bias | Unclear risk | All studies were sponsored by Cyberonics, the manufacturer of the device. |
µs: microseconds Hz: hertz IQ: intelligent quotient mA: milliampere min: minutes mo: month ms: milliseconds QOL: quality of life s: seconds VNS = vagus nerve stimulation y: year
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bauer 2016 | Different intervention (transcutaneous vagus nerve stimulation: a noninvasive technique to stimulate the left auricular branch of the vagus nerve at the ear conch) |
| Boon 2015 | Different outcomes (this study evaluated the performance of a CBSDA incorporated into a closed‐loop VNS device) |
| Brodtkorb 2019 | Not a randomised controlled trial |
| Clarke 1997 | Not a randomised controlled trial |
| Colicchio 2010 | No comparison group |
| Cramer 2001 | Not a randomised controlled trial |
| Cukiert 2013 | Ineligible population (participants with Lennox‐Gastaut Syndrome) |
| Cukiert 2015 | Not a randomised controlled trial |
| DeGiorgio 2000 | Not a randomised controlled trial |
| Dibue‐Adjei 2019 | Not a randomised controlled trial |
| Dumoulin 2018 | Different intervention (transcutaneous auricular vagus nerve stimulation) |
| Ernst 2019 | Different intervention (RNS System) |
| Fisher 2016 | Not a randomised controlled trial |
| Galbarriatu 2015 | Not a randomised controlled trial |
| Garcia‐Navarrete 2013 | Not a randomised controlled trial |
| Geller 2017 | Different intervention (brain‐responsive neurostimulation) |
| George 1994 | Not a randomised controlled trial |
| Ghani 2015 | Not a randomised controlled trial |
| He 2015 | Different intervention (transcutaneous auricular vagus nerve stimulation (tVNS)) |
| Ho 2016 | No comparison group |
| Hornig 1997 | Not a randomised controlled trial |
| Jobst 2017 | Different intervention (brain‐responsive neurostimulation (RNS)) |
| Lee 2013 | Not a randomised controlled trial |
| Liu 2017 | Not a randomised controlled trial |
| Madaan 2021 | Not a randomised controlled trial |
| Marras 2013 | Not a randomised controlled trial |
| Marrosu 2003 | Not a randomised controlled trial |
| Marrosu 2005 | Not a randomised controlled trial |
| Morris 1999 | Not a randomised controlled trial |
| Muller 2010 | Ineligible population (participants with Lennox‐Gastaut Syndrome) |
| NCT00215215 | This study was terminated, and no data were available. |
| NCT01118455 | This study was terminated, and no data were available (insufficient enrolment). |
| NCT01178437 | This study was terminated, and no data were available. |
| NCT01910129 | This study was terminated, and no data were available. |
| NCT02385526 | Not a randomised controlled trial |
| NCT02465970 | Different intervention (transcranial direct current stimulation (TDCS)) |
| NCT02603991 | Not a randomised controlled trial |
| Pelot 2018 | Ineligible population (no epilepsy) |
| Perez‐Carbonell 2020 | Not a randomised controlled trial |
| Robinson 2013 | Not a randomised controlled trial |
| Ronkainem 2006 | Not a randomised controlled trial |
| Rossignol 2009 | Not a randomised controlled trial |
| Salanova 2021 | Different intervention (stimulation of the anterior nucleus of the thalamus) |
| Salinsky 1996 | Not a randomised controlled trial |
| Scherrrmann 2001 | Not a randomised controlled trial |
| Shimizu 1995 | Not a randomised controlled trial |
| Sirven 2000 | Not a randomised controlled trial |
| Tormasiova 2014 | Not a randomised controlled trial |
| Uthman 1993 | Not a randomised controlled trial |
| Wang 2004 | Not a randomised controlled trial |
| Wang 2009 | Not a randomised controlled trial |
| Zambrelli 2016 | Not a randomised controlled trial |
CBSDA: cardiac‐based seizure detection algorithm TDCS: transcranial direct current stimulation VNS: vagus nerve stimulation
Characteristics of ongoing studies [ordered by study ID]
EUCTR2018‐003464‐32‐HU.
| Study name | Effect of mirtazapine on seizure frequency in epileptic patients with vagal nerve stimulation device |
| Methods | Clinical randomised trial |
| Participants | The study population consisted of adults participants with refractory epilepsy |
| Interventions | VNS therapy |
| Outcomes | 1) Seizure frequency 2) Quality of life (QOLIE‐89) 3) Depression (HAM‐D; BDI) |
| Starting date | May 2019 |
| Contact information | No details |
| Notes |
Ji 2019.
| Study name | Vagus nerve stimulation for paediatric patients with intractable epilepsy between 3 and 6 years of age: study protocol for a double‐blind, randomised control trial |
| Methods | Prospective randomised trial |
| Participants | The study population consisted of paediatric participants with intractable epilepsy |
| Interventions | VNS therapy |
| Outcomes | 1) Seizure frequency 2) Side effects |
| Starting date | February 2017 |
| Contact information | No details |
| Notes |
NCT00782249.
| Study name | Trial comparing different stimulation paradigms in patients treated with vagus nerve stimulation for refractory epilepsy |
| Methods | Prospective randomised trial |
| Participants | People with refractory epilepsy |
| Interventions | Comparison of three different paradigms in participants treated with VNS therapy |
| Outcomes | 1) Seizure frequency 2) Side effects 3) Quality of life |
| Starting date | October 2005 |
| Contact information | Veerle De Herdt, veerle.deherdt@ugent.be |
| Notes |
NCT01281293.
| Study name | Vagus nerve stimulation clinical outcomes measured prospectively in patients stimulated (V‐COMPAS) |
| Methods | Long‐term, prospective, observational, multi‐site outcome study to follow the clinical course and seizure reduction of people with refractory seizures who are being treated with adjunctive VNS therapy |
| Participants | The study population consisted of participants with a diagnosis of refractory seizures who were treated with adjunctive VNS therapy. |
| Interventions | VNS therapy |
| Outcomes | 1) Efficacy 2) Safety and tolerability |
| Starting date | January 2011 |
| Contact information | Mark Bunker, PharmD mark.bunker@cyberonics.com |
| Notes |
NCT01378611.
| Study name | Does VNS interact with the serotonergic and immune system in children with intractable epilepsy? |
| Methods | Clinical randomised controlled observer‐blinded add‐on design |
| Participants | The study population consisted of children (age 4 to 18 years) with intractable epilepsy who were not eligible for resective surgery. |
| Interventions | VNS therapy |
| Outcomes | Seizure frequency |
| Starting date | March 2006 |
| Contact information | No details |
| Notes |
NCT02089243.
| Study name | Controlled randomised vagus nerve stimulation (VNS) therapy versus resection (CoRaVNStiR) |
| Methods | A randomised, single‐blind, parallel study |
| Participants | The study population consist of subjects (child and adults) 12 to 60 years with pharmacoresistant focal seizures. |
| Interventions | VNS therapy |
| Outcomes | 1) Seizure frequency 2) Neuropsychological examination 3) Responder rates 4) Mean seizure‐free interval 5) Seizure severity 6) Quality of life 7) Complications |
| Starting date | June 2014 |
| Contact information | Yanchun YC Deng, China, +86 29 84773994 yanchund@fmmu.edu.cn |
| Notes |
NCT02378792.
| Study name | Clinical trial of vagus nerve stimulation for treatment of refractory epilepsy (VNSRE) |
| Methods | A randomised, double‐blind, parallel study |
| Participants | The study population consisted of subjects (child and adults) 6 to 60 years with refractory epilepsy. |
| Interventions | VNS therapy |
| Outcomes | 1) Changes in seizure frequency from baseline to the seizure count evaluation period at 6 months 2) Changes in seizure frequency from baseline to 4, 8, 12, 16, 20, and 24 months 3) Overall Quality of Life in Epilepsy‐31 (QOLIE‐31) score in participants with baseline and at least one post‐baseline QOLIE assessment 4) Changes in the number of antiepileptic drugs in participants with less then a 50% reduction in seizures 5) Changes from baseline in the Engel and McHugh description 6) Changes from baseline in 24‐hour ECG description |
| Starting date | August 2014 |
| Contact information | Jia Fumin, China, +86 010‐59361265 pins_medical@163.com |
| Notes |
NCT05180916.
| Study name | Priming the epileptic brain: tVNS to improve efficacy of add‐on AED in patients with focal epilepsy |
| Methods | Randomised controlled trial |
| Participants | The study population consisted of adults with refractory focal epilepsy |
| Interventions | VNS therapy |
| Outcomes | 1) Seizure reduction 2) Improvement of cognition 3) Improvement of quality of life |
| Starting date | January 2022 |
| Contact information | Angelique A Stuurman, Netherlands, +31 0402279777 prep@tue.nl |
| Notes |
AED: antiepileptic drug ECG: electrocardiogram QOLIE‐31: Overall Quality of Life in Epilepsy‐31 tVNS: transcutaneous vagus nerve stimulation VNS: vagal nerve stimulation
Differences between protocol and review
The previous version of this review changed the inclusion criteria slightly to include active controlled trials (Panebianco 2015).
We replaced the term 'partial' with 'focal', in accordance with the most recent classification of epilepsies of the International League Against Epilepsy (Scheffer 2017).
Contributions of authors
Mariangela Panebianco was primarily responsible for the writing of this update, and completed data extraction and risk of bias assessments. Alexandra Rigby assessed studies for eligibility, extracted data, and assessed risk of bias. Anthony G Marson provided guidance and manuscript feedback during the update process.
Sources of support
Internal sources
-
no internal support, Other
no internal support
External sources
National Institute for Health and Care Research, UK
Declarations of interest
MP: none known AR: none known AGM: a consortium of pharmaceutical companies (GSK, EISAI, UCB Pharma) funded the National Audit of Seizure Management in Hospitals (NASH) through grants paid to the University of Liverpool. Professor Marson is funded in part by the NIHR Applied Research Collaboration, North West Coast (NIHR ARC NWC). Professor Marson is a National Institute for Health and Care Research (NIHR) Senior Investigator. The views expressed in this article are those of the author(s) and not necessarily those of the NIHR, or the Department of Health and Social Care. Professor Marson is the Co‐ordinating Editor of the Cochrane Epilepsy Group; however, he was not involved in the editorial process of this review update.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
DeGiorgio 2005 {published data only}
- DeGiorgio C, Heck C, Bunch S, Britton J, Green P, Lancman M, et al. Vagus nerve stimulation for epilepsy: randomized comparison of three stimulation paradigms. Neurology 2005;65(2):317-9. [DOI: 10.1212/01.wnl.0000168899.11598.00] [PMID: ] [DOI] [PubMed] [Google Scholar]
Handforth 1998 {published data only}
- Amar AP, Heck C, Levy M, Smith T, DeGiorgio CM, Oviedo S, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique and outcome. Neurosurgery 1998;43(6):1265-76. [DOI: 10.1097/00006123-199812000-00001] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Morris GL. Effects of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy & Behavior 2001;2(1):46-53. [DOI: 10.1006/ebeh.2000.0148] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Handforth A, DeGiorgio CM, Schachter SC, Uthman BM, Naritoku DK, Tecoma ES, et al. Vagus nerve stimulation therapy for partial-onset seizures: a randomized active-control trial. Neurology 1998;51(1):48-55. [DOI: 10.1212/wnl.51.1.48] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klinkenberg 2012 {published data only}
- Aalbers MW, Klinkenberg S, Rijkers K, Verschuure P, Kessels A, Aldenkamp A, et al. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in children with refractory epilepsy: an exploratory study. Neuroimmunomodulation 2012;19(6):352-8. [DOI: 10.1159/000341402] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Klinkenberg S, Aalbers MW, Vles JSH, Cornips EMJ, Rijkers K, Leenen L, et al. Vagus nerve stimulation in children with intractable epilepsy: a randomized controlled trial. Developmental Medicine & Child Neurology 2012;54(9):855-61. [DOI: 10.1111/j.1469-8749.2012.04305.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Klinkenberg S, Van den Borne CJ, Aalbers MW, Verschuure P, Kessels AG, Leenen L, et al. The effects of vagus nerve stimulation on tryptophan metabolities in children with intractable epilepsy. Epilepsy & Behavior 2014;37:133-8. [DOI: 10.1016/j.yebeh.2014.06.001] [PMID: ] [DOI] [PubMed] [Google Scholar]
Michael 1993 {published data only}
- Michael JE, Wegener K, Barners DW. Vagus nerve stimulation for intractable seizures: one year follow-up. Journal of Neuroscience Nursing 1993;25(6):362-6. [DOI: 10.1097/01376517-199312000-00007] [PMID: ] [DOI] [PubMed] [Google Scholar]
VNS Study Group 1995 {published data only}
- Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Research 1995;20(3):221-7. [DOI: 10.1016/0920-1211(94)00083-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, Mañon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures. Epilepsia 1994;35(3):616-26. [DOI: 10.1111/j.1528-1157.1994.tb02482.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Research 2000;42(2-3):203-10. [DOI: 10.1016/s0920-1211(00)00181-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Holder LK, Wernicke JF, Tarver WB. Treatment of refractory partial seizures: preliminary results of a controlled study. Pacing & Clinical Electrophysiology 1992;15(10 Pt 2):1557-71. [DOI: 10.1111/j.1540-8159.1992.tb02934.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Lötvall J, Lunde H, Augustinson LE, Hedner T, Svedmyr N, Ben-Menachem E. Airway effects of direct left-sided cervical vagal stimulation in patients with complex partial seizures. Epilepsy Research 1994;18(2):149-54. [DOI: 10.1016/0920-1211(94)90007-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Ramsay RE, Uthman BM, Augustinsson LE, Upton ARM, Naritoku D, Willis J, et al, First International Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. Epilepsia 1994;35(3):627-36. [DOI: 10.1111/j.1528-1157.1994.tb02483.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
- Vagus Nerve Stimulation Study Group. A randomized controlled trial of chronic vagus nerve stimulation for treatment of medically intractable seizures. Neurology 1995;45(2):224-30. [DOI: 10.1212/wnl.45.2.224] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bauer 2016 {published data only}
- Bauer S, Baier H, Baumgartner C, Bohlmann K, Fauser S, Graf W, et al. Transcutaneous vagus nerve stimulation (tVNS) for treatment of drug-resistant epilepsy: a randomized, double-blind clinical trial (cMPsE02). Brain Stimulation 2014;9(3):356-63. [DOI: 10.1016/j.brs.2015.11.003] [DOI] [PubMed] [Google Scholar]
Boon 2015 {published data only}
- Boon P, Vonck K, Van Rijckevorsel K, El Tahry R, Elger CE, Mullatti N. A prospective, multicenter study of cardiac-based seizure detection to activate vagus nerve stimulation. Seizure 2015;32:52-61. [DOI: 10.1016/j.seizure.2015.08.011] [PMID: ] [DOI] [PubMed] [Google Scholar]
Brodtkorb 2019 {published data only}
- Brodtkorb E, Samsonsen C, Jorgensen JV, Helde G. Epilepsy patients with and without perceived benefit from vagus nerve stimulation. Seizure 2019;72:28-32. [DOI] [PubMed] [Google Scholar]
Clarke 1997 {published data only}
- Clarke BM, Upton ARM, Griffin H, Fitzpatrick D, DeNardis M. Seizure control after stimulation of the vagus nerve: clinical outcome measures. Canadian Journal of Neurological Sciences 1997;24(3):222-5. [PMID: ] [DOI] [PubMed] [Google Scholar]
Colicchio 2010 {published data only}
- Colicchio G, Policicchio D, Barbati G, Cesaroni E, Fuggetta F, Meglio M, et al. Vagal nerve stimulation for drug-resistant epilepsies in different age, aetiology and duration. Child's Nervous System: ChNS: Official Journal of the International Society for Pediatric Neurosurgery 2010;26(6):811-9. [DOI: 10.1007/s00381-009-1069-2] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cramer 2001 {published data only}
- Cramer JA. Exploration of changes in health-related quality of life after 3 months of vagus nerve stimulation. Epilepsy & Behavior 2001;2(5):460-5. [DOI: 10.1006/ebeh.2001.0248] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cukiert 2013 {published data only}
- Cukiert A, Cukiert CM, Burattini JA, LIma AM, Forster CR, Baise C, et al. A prospective long-term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation 2013;16(6):551-6. [DOI: 10.1111/j.1525-1403.2012.00522.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Cukiert 2015 {published data only}
- Cukiert A. Vagus nerve stimulation for epilepsy: an evidence-based approach. Progress in Neurological Surgery 2015;29:39-52. [DOI: 10.1159/000434654] [PMID: ] [DOI] [PubMed] [Google Scholar]
DeGiorgio 2000 {published data only}
- DeGiorgio CM, Schachter SC, Handforth A, Salinsky M, Thompson J, Uthman B, et al. Prospective long term study of vagus nerve stimulation for the treatment of refractory seizures. Epilepsia 2000;41(9):1195-2000. [PMID: ] [DOI] [PubMed] [Google Scholar]
Dibue‐Adjei 2019 {published data only}
- Dibue-Adjei M, Brigo F, Yamammoto T, Vonck K, Trinka E. Vagus nerve stimulation in refractory and super-refractory status epilepticus – A systematic review. Brain Stimulation 2019;12(5):1101-10. [DOI] [PubMed] [Google Scholar]
Dumoulin 2018 {published data only}
- Dumoulin M, Liberati G, Mouraux A, El Tahry R. Optimization of vagus nerve therapy: a study of the behavioral and electrophysiological effects of transcutaneous VNS. Brain Stimulation 2019;12(2):454, Abstract no: 360. [DOI: 10.1016/j.brs.2018.12.473] [DOI] [Google Scholar]
Ernst 2019 {published data only}
- Ernst LD, Krause KL, Kellog MA, Raslan AM, Spencer DC. Novel use of responsive neurostimulation (RNS System) in the treatment of super refractory status epilepticus. Journal of Clinical Neurophysiology 2019;36(3):242-5. [DOI] [PubMed] [Google Scholar]
Fisher 2016 {published data only}
- Fisher RS, Afra P, Macken M, Minecan DN, Bagic A, Benbadis SR, et al. Automatic vagus nerve stimulation triggered by ictal tachycardia: clinical outcomes and device performance – the U.S. E-37 trial. Neuromodulation 2016;19(2):188-95. [DOI: 10.1111/ner.12376] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Galbarriatu 2015 {published data only}
- Galbarriatu L, Pomposo I, Aurrecoechea J, Marinas A, Agundez M, Gomez JC, et al. Vagus nerve stimulation therapy for treatment-resistant epilepsy: a 15-year experience at a single institution. Clinical Neurology & Neurosurgery 2015;137:89-93. [DOI: 10.1016/j.clineuro.2015.06.023] [PMID: ] [DOI] [PubMed] [Google Scholar]
Garcia‐Navarrete 2013 {published data only}
- Garcia-Navarrete E, Torres CV, Gallego I, Navas M, Pastor J, Sola RG. Long-term results of vagal nerve stimulation for adults with medication-resistant epilepsy who have been on unchanged antiepileptic medication. Seizure 2013;22(1):9-13. [DOI: 10.1016/j.seizure.2012.09.008] [PMID: ] [DOI] [PubMed] [Google Scholar]
Geller 2017 {published data only}
- Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, et al. Brain-responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia 2017;58(6):994-1004. [DOI: 10.1111/epi.13740] [PMID: ] [DOI] [PubMed] [Google Scholar]
George 1994 {published data only}
- George R, Salinsky M, Kuzniecky R, Rosenfeld W, Bergen D, Tarver WB, et al. Vagus nerve stimulation for treatment of partial seizures: 3. Long-term follow-up on first 67 patients exiting a controlled study. Epilepsia 1994;35(3):637-43. [DOI: 10.1111/j.1528-1157.1994.tb02484.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ghani 2015 {published data only}
- Ghani S, Vilensky J, Turner B, Tubbs RS, Loukas M. Meta-analysis of vagus nerve stimulation treatment for epilepsy: correlation between device setting parameters and acute response. Child's Nervous System 2015;31(12):2291-304. [DOI: 10.1007/s00381-015-2921-1] [PMID: ] [DOI] [PubMed] [Google Scholar]
He 2015 {published data only}
- He W, Wang X-Y, Zhou L, Li Z-M, Jing X-H, Lv Z-L, et al. Transcutaneous auricular vagus nerve stimulation for pediatric epilepsy: study protocol for a randomized controlled trial. Trials 2015;16:371. [DOI: 10.1186/s13063-015-0906-8] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ho 2016 {published data only}
- Ho B, Baker M, Lai M. Vagus nerve stimulation: effects on cholinergic neural networks? International Journal of Surgery 2016;36(Suppl 1):S40, Abstract no: 1307. [DOI: 10.1016/j.ijsu.2016.08.050] [DOI] [Google Scholar]
Hornig 1997 {published data only}
- Hornig GW, Murphy JV, Schallert G, Tilton C. Left vagus nerve stimulation in children with refractory epilepsy: an update. Southern Medical Journal 1997;90(5):484-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Jobst 2017 {published data only}
- Jobst BC, Kapur R, Barkley GL, Bazil CW, Berg MJ, Bergey GK, et al. Brain-responsive neurostimulation in patients with medically intractable seizures arising from eloquent and other neocortical areas. Epilepsia 2017;58(6):1005-14. [DOI: 10.1111/epi.13739] [PMID: ] [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee D, Isojarvi J, Benbadis SR. Response to clobazam in vagus nerve stimulation (VNS) vs non-VNS patients. Annals of Neurology 2013;74(Suppl 17):S74, Abstract no: M2221. [DOI: 10.1002/ana.24068] [DOI] [Google Scholar]
Liu 2017 {published data only}
- Liu H, Yang Z, Huang L, Qu W, Hao H, Li L. Heart-rate variability indices as predictors of the response to vagus nerve stimulation in patients with drug-resistant epilepsy. Epilepsia 2017;58(6):1015-22. [DOI: 10.1111/epi.13738] [PMID: ] [DOI] [PubMed] [Google Scholar]
Madaan 2021 {published data only}
- Madaan P, Gupta A, Gulati S. Pediatric epilepsy surgery: indications and evaluation. Indian Journal of Pediatrics 2021;88(10):1000-6. [DOI] [PubMed] [Google Scholar]
Marras 2013 {published data only}
- Marras CE, Chiesa V, De Benedictis A, Franzini A, Rizzi M, Villani F, et al. Vagus nerve stimulation in refractory epilepsy: new indications and outcome assessment. Epilepsy & Behavior 2013;28(3):374-8. [DOI: 10.1016/j.yebeh.2013.05.021] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marrosu 2003 {published data only}
- Marrosu F, Serra A, Maleci A, Puligheddu M, Biggio G, Piga M. Correlation between GABA-A receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Research 2003;55(1-2):59-70. [DOI: 10.1016/s0920-1211(03)00107-4] [PMID: ] [DOI] [PubMed] [Google Scholar]
Marrosu 2005 {published data only}
- Marrosu F, Santoni F, Puligheddu M, Barberini L, Maleci A, Ennas F, et al. Increase in 20-50 Hz (gamma frequencies) power spectrum and synchronization after chronic vagal nerve stimulation. Clinical Neurophysiology 2005;116(9):2026-36. [DOI: 10.1016/j.clinph.2005.06.015] [PMID: ] [DOI] [PubMed] [Google Scholar]
Morris 1999 {published data only}
- Morris GL 3rd, Mueller WM, Vagus Nerve Stimulation Group. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. Neurology 1999;53(8):1731-5. [DOI: 10.1212/wnl.53.8.1731] [PMID: ] [DOI] [PubMed] [Google Scholar]
Muller 2010 {published data only}
- Muller K, Fabo D, Entz L, Kelemen A, Halasz P, Rasonyi G, et al. Outcome of vagus nerve stimulation for epilepsy in Budapest. Epilepsia 2010;51(Suppl.3):98-101. [DOI: 10.1111/j.1528-1167.2010.02620.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
NCT00215215 {published data only}
- NCT00215215. Effectiveness study comparing treatment with drug(s) or adjunctive VNS therapy for pharmacoresistant partial seizures. clinicaltrials.gov/show/NCT00215215 (first received 22 September 2005).
NCT01118455 {published data only}
- NCT01118455. Trial to assess vagus nerve stimulation therapy vs. anti-epileptic drug treatment in children with refractory seizures. clinicaltrials.gov/show/NCT01118455 (first received 6 May 2010).
NCT01178437 {published data only}
- NCT01178437. Transcutaneous non-invasive stimulation of the vagus nerve for the treatment of difficult-to-treat epilepsy. clinicaltrials.gov/show/NCT01178437 (first received 10 August 2010).
NCT01910129 {published data only}
- NCT01910129. Neurostimulation to the vagus nerve for the reduction in frequency of seizures associated with epilepsy. clinicaltrials.gov/show/NCT01910129 (first received 29 July 2013).
NCT02385526 {published data only}
- NCT02385526. Vagus nerve stimulation titration protocol to improve tolerance and accelerate adaptation. clinicaltrials.gov/show/NCT02385526 (first received 11 March 2015).
NCT02465970 {published data only}
- NCT02465970. Effects of transcranial direct current stimulation (TDCS) in drug-resistant partial epilepsy. clinicaltrials.gov/show/NCT02465970 (first received 9 June 2015).
NCT02603991 {published data only}
- NCT02603991. Prospective cohort study on the efficacy of vagus nerve stimulation for children and adolescents with epilepsy. clinicaltrials.gov/show/NCT02603991 (first received 13 November 2015).
Pelot 2018 {published data only}
- Pelot NA, Grill WM. Effects of vagal neuromodulation on feeding behaviour. Brain Research 2018;1693(Pt B):180-7. [DOI: 10.1016/j.brainres.2018.02.003] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Perez‐Carbonell 2020 {published data only}
- Perez-Carbonell L, Faulkner H, Higgins S, Koutroumanidis M, Leschziner G. Vagus nerve stimulation for drug-resistant epilepsy. Practical Neurology 2020;20(3):189-98. [DOI] [PubMed] [Google Scholar]
Robinson 2013 {published data only}
- Robinson R, European Paediatric VNS Study Group. Vagus nerve stimulation in children with intractable epilepsy: a randomized controlled trial. Developmental Medicine and Child Neurology 2013;55(2):195. [DOI: 10.1111/dmcn.12026] [PMID: ] [DOI] [PubMed] [Google Scholar]
Ronkainem 2006 {published data only}
- Ronkainen E, Korpelainen JT, Heikkinen E, Myllyla VV, Huikuri HV, Isojarvi JIT. Cardiac autonomic control in patients with refractory epilepsy before and during vagus nerve stimulation treatment: a one-year follow-up study. Epilepsia 2006;47(3):556-62. [DOI: 10.1111/j.1528-1167.2006.00467.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Rossignol 2009 {published data only}
- Rossignol E, Lortie A, Thomas T, Bouthiller A, Scavarda D, Mercier C, et al. Vagus nerve stimulation in pediatric epileptic syndromes. Seizure 2009;18(1):34-7. [DOI: 10.1016/j.seizure.2008.06.010] [PMID: ] [DOI] [PubMed] [Google Scholar]
Salanova 2021 {published data only}
- Salanova V, Sperling MR, Gross RE, Irwin CP, Vollhaber JA, Giftakis JE. The SANTE study at 10 years of follow-up: effectiveness, safety, and sudden unexpected death in epilepsy. Epilepsia 2021;62(6):1306-17. [DOI] [PubMed] [Google Scholar]
Salinsky 1996 {published data only}
- Salinsky MC, Uthman BM, Ristanovic RK, Wernicke JF, Tarver WB, Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for the treatment of medically intractable seizures: results of a 1-year open-extension trial. Archives of Neurology 1996;53(11):1176-80. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scherrrmann 2001 {published data only}
- Scherrmann J, Hoppe C, Kral T, Schramm J, Elger CE. Vagus nerve stimulation: clinical experience in a large patient series. Journal of Clinical Neurophysiology 2001;18(5):408-14. [PMID: ] [DOI] [PubMed] [Google Scholar]
Shimizu 1995 {published data only}
- Shimizu H, Ishijima B, Nakamura K, Asakura T, Ohtsuki T, Yoshimoto T, et al. Effect of vagal stimulation on intractable epilepsy. Psychiatry and Clinical Neurosciences 1995;49(3):S254-5. [DOI: 10.1111/j.1440-1819.1995.tb02194.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Sirven 2000 {published data only}
- Sirven JI, Sperling M, Naritoku D, Schachter S, Labar D, Holmes M, et al. Vagus nerve stimulation therapy for epilepsy in older adults. Neurology 2000;54(3):1179-82. [PMID: ] [DOI] [PubMed] [Google Scholar]
Tormasiova 2014 {published data only}
- Tormasiova M, Kollova A, Bratsky L. Immediate post-implantation effect of baseline stimulation parameters of vagus nerve stimulation in patients with refractory epilepsy. Stereotactic and Functional Neurosurgery 2014;92(Suppl 2):216, Abstract no: 224. [Google Scholar]
Uthman 1993 {published data only}
- Uthman BM, Wilder BJ, Penry JK, Dean C, Ramsay RE, Reid SA, et al. Treatment of epilepsy by stimulation of the vagus nerve. Neurology 1993;43(7):1338-45. [PMID: ] [DOI] [PubMed] [Google Scholar]
Wang 2004 {published data only}
- Wang LN, Zhao LF, Wang YZ. Basic and clinical study of vagus nerve stimulation in the treatment of epilepsy. Chinese Journal of Clinical Rehabilitation 2004;8(13):2516-7. [Google Scholar]
Wang 2009 {published data only}
- Wang H, Chen X, Lin Z, Shao Z, Sun B, Shen H, et al. Long-term effect of vagus nerve stimulation on interictal epileptiform discharges in refractory epilepsy. Journal of the Neurological Sciences 2009;284(1-2):96-102. [DOI: 10.1016/j.jns.2009.04.012] [DOI] [PubMed] [Google Scholar]
Zambrelli 2016 {published data only}
- Zambrelli E, Saibene AM, Furia F, Chiesa V, Vignoli A, Pipolo C. Laryngeal motility alteration: a missing link between sleep apnea and vagus nerve stimulation for epilepsy. Epilepsia 2016;57(1):e24-7. [DOI: 10.1111/epi.13252] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
EUCTR2018‐003464‐32‐HU {published data only}
- EUCTR2018-003464-32-HU. Effect of mirtazapine on seizure frequency in epileptic patients with vagal nerve stimulation device. www.clinicaltrialsregister.eu/ctr-search/trial/2018-003464-32/HU (first entered 11 August 2019).
Ji 2019 {published data only}
- Ji T, Yang Z, Liu Q, Liao J, Yin F, Chen Y, et al. Vagus nerve stimulation for pediatric patients with intractable epilepsy between 3 and 6 years of age: a study protocol for a double-blind, randomized control trial. Trials 2019;20(1):44. [DOI: 10.1186/s13063-018-3087-4] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00782249 {published data only}
- NCT00782249. Trial comparing different stimulation paradigms in patients treated with vagus nerve stimulation for refractory epilepsy. clinicaltrials.gov/show/NCT00782249 (first received 31 October 2008).
NCT01281293 {published data only}
- NCT01281293. Vagus nerve stimulation Clinical Outcomes Measured prospectively in PAtients Stimulated (V-COMPAS). clinicaltrials.gov/show/NCT01281293 (first received 21 January 2011).
NCT01378611 {published data only}
- NCT01378611. Does VNS interact with the serotonergic and immune system in children with intractable epilepsy? clinicaltrials.gov/show/NCT01378611 (first received 14 June 2011).
NCT02089243 {published data only}
- NCT02089243. Controlled Randomized Vagus Nerve Stimulation (VNS) therapy versus Resection (CoRaVNStiR). clinicaltrials.gov/show/NCT02089243 (first received 13 March 2014).
NCT02378792 {published data only}
- NCT02378792. Clinical trial of vagus nerve stimulation for treatment of refractory epilepsy. clinicaltrials.gov/show/NCT02378792 (first received 27 February 2015).
NCT05180916 {published data only}
- NCT05180916. Priming the epileptic brain: tVNS to improve efficacy of add-on AED in patients with focal epilepsy. clinicaltrials.gov/show/NCT05180916 (first received 2 July 2021).
Additional references
Aalbers 2012
- Aalbers MW, Klinkenberg S, Rijkers K, Verschuure P, Kessels A, Aldenkamp A, et al. The effects of vagus nerve stimulation on pro- and anti-inflammatory cytokines in children with refractory epilepsy: an exploratory study. Neuroimmunomodulation 2012;19(6):352-8. [DOI] [PubMed] [Google Scholar]
Amar 1998
- Amar AP, Heck CN, Levy ML, Smith T, DeGiorgio CM, Oviedo S, et al. An institutional experience with cervical vagus nerve trunk stimulation for medically refractory epilepsy: rationale, technique, and outcome. Neurosurgery 1998;43(6):1265-76. [DOI] [PubMed] [Google Scholar]
Ben‐Menachem 1994
- Ben-Menachem E, Manon-Espaillat R, Ristanovic R, Wilder BJ, Stefan H, Mirza W, et al, First International Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for treatment of partial seizures: 1. A controlled study of effect on seizures.. Epilepsia 1994;35(3):616-26. [DOI] [PubMed] [Google Scholar]
Ben‐Menachem 1995
- Ben-Menachem E, Hamberger A, Hedner T, Hammond EJ, Uthman BM, Slater J, et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Research 1995;20(3):221-7. [DOI] [PubMed] [Google Scholar]
Cockerell 1995
- Cockerell OC, Johnson AL, Sander JW, Hart YM, Shorvon SD. Remission of epilepsy: results from the National General Practice Study of Epilepsy. Lancet 1995;346(8968):140-4. [DOI: 10.1016/s0140-6736(95)91208-8] [PMID: ] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9. Analysing data and undertaking meta-analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Dodrill 2000
- Dodrill CB, Morris GL. Effect of vagal nerve stimulation on cognition and quality of life in epilepsy. Epilepsy & Behavior 2001;2(1):46-53. [DOI: 10.1006/ebeh.2000.0148] [PMID: ] [DOI] [PubMed] [Google Scholar]
Elger 2000
- Elger G, Hoppe C, Falkai P, Rush AJ, Elger CE. Vagus nerve stimulation is associated with mood improvements in epilepsy patients. Epilepsy Research 2000;42(2-3):203-10. [DOI: 10.1016/S0920-1211(00)00181-9] [PMID: ] [DOI] [PubMed] [Google Scholar]
Forsgren 2005
- Forsgren L, Beghi E, Oun A, Sillanpaa M. The epidemiology of epilepsy in Europe – a systematic review. European Journal of Neurology 2005;12(4):245-53. [DOI: 10.1111/j.1468-1331.2004.00992.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Hauser 1975
- Hauser WA, Kurland LT. The epidemiology of epilepsy in Rochester, Minnesota, 1935 through 1967. Epilepsia 1975;16(1):1-66. [DOI: 10.1111/j.1528-1157.1975.tb04721.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from training.cochrane.org/handbook/archive/v5.1/.
Holder 1992
- Holder LK, Wernicke JF, Tarver WB. Treatment of refractory partial seizures: preliminary results of a controlled study. Pacing & Clinical Electrophysiology 1992;15(10 Pt2):1557-71. [DOI: 10.1111/j.1540-8159.1992.tb02934.x] [PMID: ] [DOI] [PubMed] [Google Scholar]
Kirkham 2010
- Kirkham JJ, Dwan KM, Altman DG, Gamble C, Dodd S, Smyth R, et al. The impact of outcome reporting bias in randomised controlled trials on a cohort of systematic reviews. BMJ 2010;340:c365. [DOI: ] [PMID: ] [DOI] [PubMed] [Google Scholar]
Klinkenberg 2014
- Klinkenberg S, den Borne CJ, Aalbers MW, Verschuure P, Kessels AG, Leenen L, et al. The effects of vagus nerve stimulation on tryptophan metabolites in children with intractable epilepsy. Epilepsy & Behavior 2014;37:133-8. [DOI] [PubMed] [Google Scholar]
Krahl 2012
- Krahl SE, Clark KB. Vagus nerve stimulation for epilepsy: a review of central mechanisms. Surgical Neurology International 2012;3(Suppl 4):S255-9. [DOI: 10.4103/2152-7806.103015] [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kwan 2000
- Kwan P, Brodie MJ. Early identification of refractory epilepsy. New England Journal of Medicine 2000;342(5):314-9. [PMID: ] [DOI] [PubMed] [Google Scholar]
Lefebvre 2019
- Lefebvre C, Glanville J, Briscoe S, Littlewood A, Marshall C, Metzendorf M-I, et al. Technical Supplement to Chapter 4: Searching for and selecting studies. In: Higgins JPT, Thomas J, Chandler J, Cumpston MS, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 6. Cochrane, 2019. Available from training.cochrane.org/handbook/archive/v6.
Lötvall 1994
- Lötvall J, Lunde H, Augustinson LE, Hedner T, Svedmyr N, Ben-Menachem E. Airway effects of direct left-sided cervical vagal stimulation in patients with complex partial seizures. Epilepsy Research 1994;18(2):149-54. [DOI] [PubMed] [Google Scholar]
Morris 2013
- Morris GL 3rd, Gloss D, Buchhalter J, Mack KJ, Nickels K, Harden C. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology 2013;81(16):1453-9. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Panebianco 2016
- Panebianco M, Zavanone C, Dupont S, Restivo DA, Pavone A. Vagus nerve stimulation therapy in partial epilepsy: a review. Acta Neurologica Belgica 2016;116(3):241-8. [PMID: ] [DOI] [PubMed] [Google Scholar]
Ramsay 1994
- Ramsay RE, Uthman BM, Augustinsson LE, Upton AR, Naritoku D, Willis J, et al, First International Vagus Nerve Stimulation Study Group. Vagus nerve stimulation for treatment of partial seizures: 2.Safety, side effects, and tolerability. Epilepsia 1994;35(3):627-36. [DOI] [PubMed] [Google Scholar]
Sackeim 2001
- Sackeim HA, Rush AJ, George MS, Marangell LB, Husain MM, Nahas Z, et al. Vagus nerve stimulation (VNS) for treatment-resistant depression: efficacy, side effects and predictors of outcome. Neuropsychopharmacology 2001;25(5):713–28. [PMID: ] [DOI] [PubMed] [Google Scholar]
Schachter 1998
- Schachter SC, Saper CB. Vagus nerve stimulation. Epilepsia 1998;39(7):677-86. [PMID: ] [DOI] [PubMed] [Google Scholar]
Scheffer 2017
- Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia 2017;58(4):512-21. [PMID: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Selway 1987
- Selway R, Nashef L. Vagus nerve stimulation. In: Membranes to Mankind – A Practical Guide to Epilepsy, chapter 48. Chalfont St Peter, Bucks: Epilepsy Society, 1987. [Google Scholar]
Vonck 2014
- Vonck K, Raedt R, Naulaerts J, De Vogelaere F, Thiery E, Van Roost D, et al. Vagus nerve stimulation… 25 years later! What do we know about the effects on cognition? Neuroscience & Biobehavioral Reviews 2014;45:63-71. [DOI: 10.1016/j.neubiorev.2014.05.005] [PMID: ] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Panebianco 2015
- Panebianco M, Rigby A, Weston J, Marson AG. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews 2015, Issue 4. Art. No: CD002896. [DOI: 10.1002/14651858.CD002896.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Privitera 2002
- Privitera MD, Welty TTE, Ficker DDM, Welge J. Vagus nerve stimulation for partial seizures. Cochrane Database of Systematic Reviews 2002, Issue 1. Art. No: CD002896. [DOI: 10.1002/14651858.CD002896] [DOI] [PubMed] [Google Scholar]


