Abstract
Dibenzothiophene (DBT), and in particular substituted DBTs, are resistant to hydrodesulfurization (HDS) and can persist in fuels even after aggressive HDS treatment. Treatment by Rhodococcus sp. strain ECRD-1 of a middle distillate oil whose sulfur content was virtually all substituted DBTs produced extensive desulfurization and a sulfur level of 56 ppm.
To meet regulated sulfur levels, petroleum fuels must be treated to remove organic sulfur. This is accomplished mainly by hydrodesulfurization (HDS), which converts organic sulfur in the feed to hydrogen sulfide in the presence of a transition metal catalyst and hydrogen. The extent of desulfurization achieved by HDS is determined by the reaction conditions, with higher hydrogen pressures and temperatures giving greater sulfur removal (25). In middle distillate (diesel range) fractions, the sulfur that remains after aggressive HDS treatment is typically in the form of substituted dibenzothiophene (DBT) compounds. The most refractory DBTs have substitutions at the 4 and 6 positions, which are adjacent to the sulfur moiety and are believed to sterically hinder access of the sulfur atom to the catalyst surface (12). As regulations on sulfur levels in fuels become stricter, more of the HDS-refractory compounds must be removed. This requires increasingly more severe and inherently more costly HDS. As a result, HDS-refractory sulfur compounds represent a significant barrier to reaching very low sulfur levels in middle and heavy distillate range fuels.
Early work on biodesulfurization focused on organisms that degraded DBT. The pathways involved relied on oxidation and mineralization of the DBT carbon skeleton instead of sulfur removal and thus reduced the fuel value of the desulfurized product (9, 10, 15–17, 19, 20, 31; K. A. Malik and D. Claus, Abstr. Fifth Int. Ferment. Symp., abstr. 23.03, p. 421, 1976). In contrast, Rhodococcus sp. strain ECRD-1 uses a sulfur-selective oxidative pathway to remove sulfur from organic sulfur compounds and is capable of desulfurizing both DBT and sterically hindered DBT compounds (18). A number of other bacteria that use a similar sulfur-selective oxidative desulfurization pathway have been isolated (1, 2, 6, 11, 14, 21, 29, 30). This pathway, initially referred to as the 4S pathway (1), involves sequential oxidation of the sulfur moiety and cleavage of the carbon-sulfur bonds. This system consists of two monooxygenases, DszC and DszA, which sequentially oxidize DBT to DBT sulfone and 2-hydroxybiphenyl-2′-sulfinic acid, an NADH-flavin mononucleotide oxidoreductase (DszD) which supplies the two monooxygenases with reduced flavin, and a desulfinase (DszB) which converts 2-hydroxybiphenyl-2′-sulfinic acid to the desulfurized end product 2-hydroxybiphenyl (3, 5, 22).
Work on the sulfur oxidative pathway has focused on model compounds, and little has been reported on the biodesulfurization of real refinery feeds, limiting the ability to assess the commercial potential of biodesulfurization. We previously described the desulfurization by Rhodococcus sp. strain ECRD-1 (ATCC 55309) of a straight-run middle distillate fraction that had not been HDS treated (7). In these experiments the sulfur content of the oil was reduced by 30%, from approximately 2.0% to 1.4%, and 60% of the remaining sulfur was found to be in an oxidized form. Here we evaluate desulfurization by Rhodococcus sp. strain ECRD-1 (18) of an extensively HDS-treated oil containing primarily substituted DBTs. The extent of sulfur removal and chemical nature of the residual sulfur are described.
The oil used for this study was an extensively HDS desulfurized, 175 to 350°C boiling range, catalytic cracker middle distillate light cycle oil (LCO) from Exxon Company U.S.A., Baytown, Tex. The sulfur content of the oil was 669 ppm. DBT was the first sulfur compound to elute upon gas chromatography (GC) analysis (49.8 min), representing <5% of the total sulfur. All other sulfur compounds eluted after DBT, indicating that the majority of the sulfur compounds in the LCO oil were alkylated DBT compounds.
Biodesulfurization of LCO was performed by growing Rhodococcus sp. strain ECRD-1 in mineral salts sulfur-free medium supplemented with vitamins and minerals (7), with LCO as sulfur source. Oil was autoclaved in sealed jars for 15 min at 121°C and 15 lb/in2 prior to addition to cultures. One part hydrotreated LCO was diluted in four parts decane, and 20 ml of the diluted oil was added per liter of culture.
Inocula for the biodesulfurization experiments were prepared from cells grown in Luria-Bertani broth (24) as previously described (7). Biodesulfurization cultures were incubated for 7 days at 25°C with shaking at 200 rpm on a rotary shaker. The pH of the cultures was monitored at 1- to 2-day intervals with pH paper (J. T. Baker Inc.) and adjusted to pH 7.0 with 1 M phosphoric acid if the pH rose more than 1 pH unit. Sterile, uninoculated controls were prepared and treated in an identical manner. After incubation, cultures were brought to a pH of 2 with 1 N HCl and extracted with methylene chloride as previously described (7). Culture extracts were concentrated by evaporation under a stream of nitrogen gas at room temperature to approximately 3 ml prior to analysis by GC and sulfur K-edge X-ray absorption-edge spectroscopy. For total sulfur analysis, a portion of the concentrated sample was evaporated under a stream of nitrogen gas to constant weight to remove residual methylene chloride and decane.
GC analysis was performed using FID (flame ionization detection) and SCD (sulfur chemiluminescence detection) in tandem as previously described (7). Sulfur removal was determined by the difference in sulfur content between sterile control oil and oil treated with Rhodococcus sp. strain ECRD-1. Total weight percent sulfur was determined in duplicate by Galbraith Laboratories, Inc., Knoxville, Tenn. Samples were first combusted in an oxygen atmosphere, and the resulting sulfate ion was analyzed by ion chromatography.
Sulfur K-edge X-ray absorption-edge spectroscopy was used to determine the chemical state of sulfur compounds in the treated and untreated oils as previously described (7). This technique is capable of determining (i) the relative amounts of sulfidic versus thiophenic sulfur and (ii) the oxidation state, e.g., sulfoxide versus sulfone, of the sulfur compounds present in an oil. Sulfur K-edge X-ray absorption-edge spectra were obtained on beamline 6-2 at the Stanford Synchrotron Radiation Laboratory. Sulfur K-edge X-ray absorption-edge spectra trend toward higher absorbance energies in the order sulfidic to thiophenic to oxidized species. The relative amounts of different sulfur types in biodesulfurized and control oils were determined from the combination and proportion of model compound spectra that gave the best fit to the spectra of the oil, as previously described (7).
The compounds present in the oil were modeled using DBT as a model thiophenic compound and benzylsulfide (BS) as a model aliphatic sulfide. DBT sulfoxide and sulfone have been identified as intermediates of DBT desulfurization by Rhodococcus sp. strain ECRD-1 (18). The compounds 2-hydroxybiphenyl-2′-sulfinic acid and 2-hydroxybiphenyl-2′-sulfonic acid, cyclized during acid extraction to dibenz[c,e][1,2]-oxanthiin 6-oxide (HBP-sultine) and dibenz[c,e][1,2]-oxanthiin 6,6-dioxide (HBP-sultone), respectively, have been identified as intermediates of DBT desulfurization by Rhodococcus sp. strain IGTS8, which also uses a sulfur-selective oxidative desulfurization pathway (5). Potential intermediates of biodesulfurization were modeled using dimethyl sulfoxide, DBT sulfone, HBP-sultine, and HBP-sultone.
DBT, BS, and dimethyl-sulfoxide were purchased from Aldrich. DBT sulfone was synthesized as previously described (18), and dibenz[c,e][1,2]-oxanthiin 6-oxide and dibenz[c,e][1,2]-oxanthiin 6,6-dioxide were synthesized from 2-hydroxybiphenyl by the method of Hanson and Kemp (8). All compounds were greater than 98% pure as determined by GC analysis.
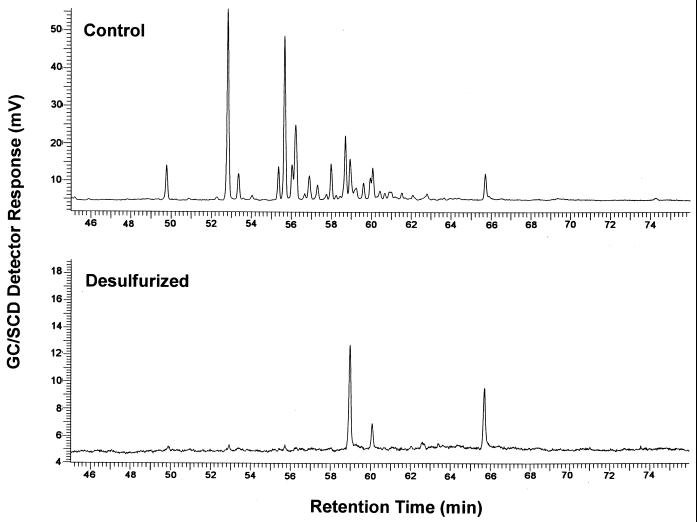
The profile of sulfur-containing compounds present in the untreated LCO is displayed by the GC/SCD chromatogram shown in Fig. 1. There are relatively few sulfur compounds present, and most are fairly well resolved. This is in contrast to the unresolved complex mixture of sulfur compounds typically observed in crude oil middle distillate fractions that have not received HDS treatment (7). Sulfur K-edge X-ray absorption-edge spectroscopy indicates that the sulfur in the LCO is entirely thiophenic in nature (Table 1), consistent with the expected reactivities of sulfur compounds toward HDS.
FIG. 1.
GC/SCD chromatograms of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized LCO oil.
TABLE 1.
Chemical state of sulfur species in LCO
| Sulfur type | Relative % total S in indicated chemical state
|
|
|---|---|---|
| Sterile control | ECRD-1 desulfurized | |
| Aliphatic sulfide | 0 | 0 |
| Thiophenic sulfur | 100 | 62 |
| Sulfoxide | 0 | 1 |
| Sulfone | 0 | 20 |
| Sultine | 0 | 17 |
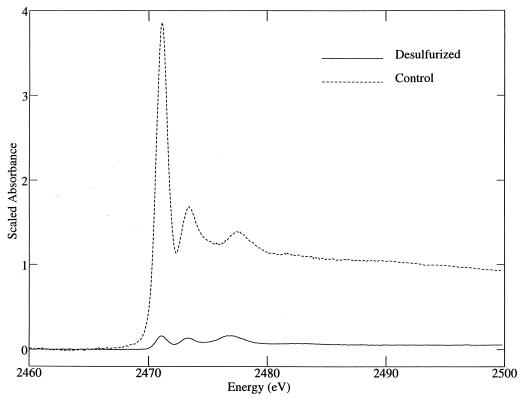
Biodesulfurization of LCO by Rhodococcus sp. strain ECRD-1 resulted in extensive removal of sulfur and dramatically reduced the number of sulfur compounds present in the oil, with only three significant peaks remaining (Fig. 1). Analysis of the sulfur content of the treated oil revealed that 92% of the sulfur had been removed, reducing the sulfur content from 669 (±40) ppm to 56 (±4) ppm. The sulfur K-edge X-ray absorption-edge spectra of the oils are shown in Fig. 2. Comparison of these spectra shows a marked decrease in the biodesulfurized oil of the 2,471-eV absorbance peak characteristic of sulfur in DBT-like compounds. The best fit to the spectrum of the biodesulfurized oil included 38% oxidized species, with the majority represented as sulfone (20%) and sultine (17%) (Table 1). In contrast, the GC/FID chromatograms of the biodesulfurized and control oils are almost identical, indicating no significant change in the hydrocarbon profile resulted from treatment (data not shown).
FIG. 2.
Sulfur K-edge X-ray absorption spectra of sterile control and Rhodococcus sp. strain ECRD-1-desulfurized LCO oil. Spectra are scaled relative to the levels of sulfur in the oils.
The extent of sulfur removal obtained by biodesulfurization reflects (i) the bioavailability of the substrate, (ii) the substrate range of the enzymes involved, and (iii) the rate of reaction. Due to the inherently low water solubility of the organosulfur compounds within the boiling range of the LCO, it is unlikely that the bioavailability of one type of sulfur compound over another is the limiting factor determining the extent of sulfur removal. However, the substrate range of oxidative desulfurizing bacteria has been shown to be restricted. Rhodococcus sp. strain ECRD-1 was shown to utilize compounds containing the DBT or benzothiophene nucleus and benzylphenyl sulfide as sulfur sources, while thiophene derivatives and phenyl sulfide could not be used (18). Similar utilization patterns have been seen with other desulfurization strains (11, 13, 21). Alkyl substitutions to the DBT nucleus have also been shown to affect the rate of desulfurization. Using a mixture of DBT and 4,6-diethyl DBT, it was shown that Rhodococcus sp. strain ECRD-1 preferentially desulfurized DBT before significant desulfurization of the alkylated derivative (7). Similarly, it was shown with Rhodococcus sp. strain I-19 that DBTs reacted at different rates depending on the extent of alkyl group substitution (designated Cx-DBTs, where x is the number of alkyl groups attached; e.g., C2-DBTs include all dimethyl- and monoethyl-substituted DBTs) (4). The half-saturation rate constants obtained for DBT, C1-DBTs, and C2-DBTs were approximately 100 ppm, 182 ppm for C3-DBTs, 425 ppm for C4-DBTs, 1,538 for C5-DBTs, and 2,000 ppm for higher Cx-DBTs.
The presence of oxidized sulfur compounds remaining in the oil after biodesulfurization suggests that the monooxygenases DszC and DszA that oxidize the sulfur atom to produce sulfones and sulfinic acids (5) have broader substrate ranges than the desulfinase, DszB, that catalyzes the removal of sulfur from sulfinic acids. We have previously observed the accumulation of oxidized sulfur species with a straight-run middle distillate fraction treated with ECRD-1 (7). The presence of oxidized sulfur compounds in oil biodesulfurized by Rhodococcus sp. strain I-19 was also observed (4). Metabolic processes other than desulfurization may have produced oxidized sulfur compounds. To evaluate this possibility, ECRD-1 was grown in the presence of a middle distillate oil containing 2.1% sulfur (7) under the same conditions as used for biodesulfurization of LCO, with the addition of 1 mM SO42−, which has previously been shown to repress desulfurization activity (18). Sulfur K-edge X-ray absorption-edge spectroscopy of the oil after treatment revealed essentially no oxidized sulfur compounds (data not shown). The fact that oxidized species remain after biodesulfurization suggests that the substrate range and/or kinetic rates of subsequent enzymes in the desulfurization pathway limits the extent of desulfurization. This would argue that improving the activity of the limiting enzymes, using techniques such as directed evolution, could increase the extent of desulfurization.
In response to the Clean Air Act Amendments of 1990, the sulfur content of transportation fuels has been reduced, with current U.S. regulations setting the sulfur content of on-road diesel fuel at a maximum of 500 ppm (26). In 1999, the U.S. Environmental Protection Agency published an advanced notice of proposed rulemaking on diesel fuel quality, which, along with the proposed emission standards for vehicles, may require sulfur levels as low as 30 ppm to meet engine manufacturers' needs (27, 28). Similar standards and future reductions are also under way in Europe, Japan, and elsewhere. As sulfur regulations continue to tighten, the need for ultra-low-sulfur fuels will continue to increase. To meet this need, low-cost technology capable of producing very low sulfur content fuels will be required. The results shown here indicate that biodesulfurization in conjunction with conventional HDS technology can reach very low sulfur levels in diesel range fuel. However, to be commercially viable, conversion rates, biocatalyst cost and stability, and overall process economics must outperform competing technology.
Acknowledgments
Stanford Synchrotron Radiation Laboratory (SSRL) is operated by the Department of Energy, Office of Basic Energy Sciences. The SSRL Biotechnology Program is supported by the National Center for Research Resources Biomedical Research Technology Program, National Institutes of Health, and by the Office of Biological and Environmental Research, Department of Energy.
REFERENCES
- 1.Campbell I M. An orphaned child makes good—the story of US DoE/PETC's foray into fossil fuel biodesulphurization. Am Chem Soci Divi Petroleum Chem Preprints. 1993;38(2):275–278. [Google Scholar]
- 2.Chang J H, Rhee S-K, Chang Y K, Chang H N. Desulfurization of diesel oils by a newly isolated dibenzothiophene-degrading Nocardia sp. strain CYKS2. Biotechnol Prog. 1998;14:851–855. doi: 10.1021/bp9800788. [DOI] [PubMed] [Google Scholar]
- 3.Denome S A, Oldfield C, Nash L J, Young K D. Characterization of the desulfurization genes from Rhodococcus sp. strain IGTS8. J Bacteriol. 1994;176:6707–6716. doi: 10.1128/jb.176.21.6707-6716.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Folsom B R, Schieche D R, DiGrazia P M, Werner J, Palmer S. Microbial desulfurization of alkylated dibenzothiophenes from a hydrodesulfurized middle distillate by Rhodococcus erythropolis I-19. Appl Environ Microbiol. 1999;65:4967–4972. doi: 10.1128/aem.65.11.4967-4972.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gray K A, Pogrebinsky O S, Mrachko G T, Xi L, Monticello D J, Squires C H. Molecular mechanisms of biocatalytic desulfurization of fossil fuels. Nat Biotechnol. 1996;14:1705–1709. doi: 10.1038/nbt1296-1705. [DOI] [PubMed] [Google Scholar]
- 6.Grossman M J. Microbial removal of organic sulfur from fuels: a review of past and present approaches. In: Occelli M L, Chianelli R, editors. Hydrotreating technology for pollution control: catalysts, catalysis, and processes. New York, N.Y: Marcel Dekker; 1996. pp. 345–359. [Google Scholar]
- 7.Grossman M J, Lee M K, Prince R C, Garrett K K, George G N, Pickering I J. Microbial desulfurization of a crude oil middle-distillate fraction: analysis of the extent of sulfur removal and the effect of removal on remaining sulfur. Appl Environ Microbiol. 1999;65:181–188. doi: 10.1128/aem.65.1.181-188.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanson G, Kemp D S. Convenient routes to 4,4"-functionalized O-terphenyls and 2,2′-functionalized biphenyls. J Org Chem. 1981;46:5441–5443. [Google Scholar]
- 9.Hartdegen F J, Coburn J M, Roberts R L. The microbial desulfurization of petroleum. Chem Eng Prog. 1984;80:63–67. [Google Scholar]
- 10.Hou C T, Laskin A I. Microbial conversion of dibenzothiophene. Dev Ind Microbiol. 1976;17:351–362. [Google Scholar]
- 11.Izumi Y, Ohshiro T, Ogino H, Hine Y, Shimao M. Selective desulfurization of dibenzothiophene by Rhodococcus erythropolis D-1. Appl Environ Microbiol. 1994;60:223–226. doi: 10.1128/aem.60.1.223-226.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kabe T, Ishihara A, Tajima H. Hydrodesulfurization of sulfur-containing polyaromatic compounds in light oil. Ind Eng Chem Res. 1992;31:1577–1580. [Google Scholar]
- 13.Kayser K J, Bielaga-Jones B A, Jackowski K, Odusan O, Kilbane J J., II Utilization of organosulfur compounds by axenic and mixed cultures of Rhodococcus rhodochrous IGTS8. J Gen Microbiol. 1993;139:3123–3129. [Google Scholar]
- 14.Kilbane J J, Bielaga B A. Toward sulfur-free fuels. CHEMTECH. 1990;20:747–751. [Google Scholar]
- 15.Kodama K, Nakatani S, Umehara K, Shimizu K, Minoda Y, Yamada K. Microbial conversion of petrosulfur compounds. Part III. Isolation and identification of products from dibenzothiophene. Agric Biol Chem. 1970;34:1320–1324. [Google Scholar]
- 16.Kodama K, Umehara K, Shimizu K, Nakatani S, Minoda Y, Yamada K. Identification of microbial products from dibenzothiophene and its proposed oxidation pathway. Agric Biol Chem. 1973;37:45–50. [Google Scholar]
- 17.Laborde A, Gibson D T. Metabolism of dibenzothiophene by Beijerinckia species. Appl Environ Microbiol. 1977;34:783–790. doi: 10.1128/aem.34.6.783-790.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee M K, Senius J D, Grossman M J. Sulfur-specific microbial desulfurization of sterically hindered analogs of dibenzothiophene. Appl Environ Microbiol. 1995;61:4362–4366. doi: 10.1128/aem.61.12.4362-4366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Monticello D J, Finnerty W R. Microbial desulfurization of fossil fuels. Annu Rev Microbiol. 1985;39:371–389. doi: 10.1146/annurev.mi.39.100185.002103. [DOI] [PubMed] [Google Scholar]
- 20.Monticello D J, Bakker D, Finnerty W R. Plasmid-mediated degradation of dibenzothiophene by Pseudomonas species. Appl Environ Microbiol. 1985;49:756–760. doi: 10.1128/aem.49.4.756-760.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Omori T, Monna L, Saiki Y, Kodama T. Desulfurization of dibenzothiophene by Corynebacterium sp. Stain SY1. Appl Environ Microbiol. 1992;58:911–915. doi: 10.1128/aem.58.3.911-915.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piddington C S, Kovacevich B R, Rambosek J. Sequence and molecular characterization of a DNA region encoding the dibenzothiophene desulfurization operon of Rhodococcus sp. strain IGTS8. Appl Environ Microbiol. 1995;61:468–475. doi: 10.1128/aem.61.2.468-475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhee S-K, Chang J H, Chang Y K, Chang H N. Desulfurization of dibenzothiophene and diesel oils by a newly isolated Gordona strain, CYKS1. Appl Environ Microbiol. 1998;64:2327–2331. doi: 10.1128/aem.64.6.2327-2331.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Speight J G. The desulfurization of heavy oils and residua. New York, N.Y: Marcel Dekker; 1981. [Google Scholar]
- 26.U.S. Environmental Protection Agency. Clean Air Act Amendments of 1990. U.S. Washington, D.C.: Environmental Protection Agency; 1990. [Google Scholar]
- 27.U.S. Environmental Protection Agency, Office of Mobile Sources. Diesel fuel quality, advance notice of proposed rulemaking. EPA 420-F-99-011. U.S. Washington, D.C.: Environmental Protection Agency; 1999. [Google Scholar]
- 28.U.S. Environmental Protection Agency, Office of Mobile Sources. Proposed “Tier 2” emission standards for vehicles and gasoline sulfur standards for refineries. EPA 420-F-99-010. U.S. Washington, D.C.: Environmental Protection Agency; 1999. [Google Scholar]
- 29.Van Afferden M, Schacht S, Klein J, Truper H G. Degradation of dibenzothiophene by Brevibacterium sp. DO Arch Microbiol. 1990;153:324–328. [Google Scholar]
- 30.Wang P, Krawiec S. Desulfurization of dibenzothiophene to 2-hydroxybiphenyl by some newly isolated bacterial strains. Arch Microbiol. 1994;161:266–271. [Google Scholar]
- 31.Yen K M, Gunsalus I C. Plasmid gene organization: naphthalene/salicylate oxidation. Proc Natl Acad Sci USA. 1982;79:874–878. doi: 10.1073/pnas.79.3.874. [DOI] [PMC free article] [PubMed] [Google Scholar]