Abstract
Context
Adiponectin is an adipokine mainly secreted by adipocytes that regulates the metabolism of lipids and glucose. Liver receptor homolog-1 (LRH-1), also named NR5A2, is a nuclear receptor that regulates lipid metabolism and homeostasis.
Objective
The purpose of this study was to compare adiponectin and LRH-1 messenger RNA (mRNA) expression in adipose tissue and LRH-1 expression in skeletal muscle between men and women at baseline and to study the effects of aerobic exercise (AEX) training or weight loss (WL) on their expression.
Methods
This hospital and university setting study included 62 overweight and obese men (n = 23) and women (n = 39) older than 45 years, of whom 41 completed 6 months of WL (n = 21) or AEX (n = 20). Outcomes included abdominal and gluteal adipose tissue and skeletal muscle gene expression.
Results
Adiponectin and LRH-1 mRNA expression in adipose tissue and LRH-1 mRNA expression in skeletal muscle is higher in women than in men (P < .05). Adiponectin mRNA expression in gluteal and abdominal adipose tissue did not change significantly after AEX or WL. LRH-1 mRNA expression increased both in adipose tissue and skeletal muscle after AEX (P < .05) and the change in muscle LRH-1 was different between the groups (P < .05). Adiponectin was positively correlated to LRH-1 in adipose tissue (P < .001). The change in maximal oxygen consumption related to the change in LRH-1 mRNA (r = 0.43; P = .01).
Conclusion
LRH-1, as a nuclear reporter, may activate adiponectin mRNA expression in adipose tissue and increases after AEX.
Keywords: adiponectin, liver receptor homolog-1 (LRH-1), adipose tissue, skeletal muscle, obesity
Adiponectin is an anti-inflammatory, antiatherogenic, and insulin-sensitizing adipokine [1]. Lower plasma concentrations and expression levels are reported in obese individuals and adults with type 2 diabetes [2]. Adiponectin activates insulin receptor substrate-1 (IRS-1)–mediated phophatidylinositol-3 kinase (PI-3K) and glucose uptake in skeletal muscle cells, enhances muscle beta-oxidation via the activation of adenosine 5′-monophosphate (AMP)-kinase, and suppresses hepatic glucose production [3]. It also has antiatherogenic effects, suppressing monocyte adhesion to endothelial cells by reducing nuclear factor-κB signaling and the messenger RNA (mRNA) expression of adhesion molecules in endothelial cells [4]. Adiponectin mRNA expression is higher in lean individuals compared to obese individuals [5]. There are also sex differences in circulating levels of adiponectin in humans. Adiponectin levels are significantly higher in women than men [6] and negatively associated with obesity, fasting glucose, and insulin levels and insulin resistance [6-9]. Adiponectin mRNA expression in epicardial adipose tissue is higher in women than men undergoing some type of heart surgery [10]. A reduced level of high-molecular-weight adiponectin levels is more strongly associated with metabolic syndrome in women than men [11]. In animal models, treatment with testosterone reduces plasma adiponectin, and in adipocyte cell culture, testosterone reduces adiponectin secretion [12], suggesting androgens may play a role in sex differences of adiponectin.
Liver receptor homolog-1 (LRH-1) is a monomeric orphan nuclear receptor expressed in the liver, pancreas (islets, β-cells), intestine, ovary, adrenal glands, preadipocytes, adipose tissue, and skeletal muscle [13, 14]. It is mostly recognized for its role in early development, cholesterol homeostasis, and cancer. LRH-1 has emerged as an upstream regulator of the glucokinase-carbohydrate response element binding protein axis. LRH-1 controls the first step of hepatic glucose uptake through direct transcriptional regulation of the glucokinase gene. Thus, LRH-1 may play a role in insulin sensitivity.
The LRH response element is located downstream of the peroxisome-proliferator response element in the human adiponectin promoter and in mature adipocytes enhances the transcription of adiponectin [3]. It is interesting to speculate that increasing LRH-1 would lead to the increased transcription of adiponectin, which would improve insulin sensitivity. However, the role of LRH-1 and insulin sensitivity has not been examined. Further, there are no studies that have examined the effect of exercise and diet on LRH-1 response. In contrast, there are several studies that use aerobic exercise training (AEX), weight loss (WL), or both that have shown adiponectin mRNA expression increases [2, 15-17] or does not change [18, 19]. The reasons for the discrepancies among studies is unclear; however, participant characteristics such as age may play a role.
The purpose of this study was to compare adipose tissue adiponectin mRNA expression and adipose and skeletal muscle LRH-1 mRNA expression between older overweight and obese men and women before the interventions and study the effects of 6-month WL and AEX interventions on their expression. We hypothesized that adiponectin and LRH-1 mRNA expression would be related to each other and to insulin sensitivity and that 6-month WL and AEX interventions would increase adiponectin and LRH-1 expression in adipose tissue and skeletal muscle.
Materials and Methods
This study was conducted in men and women with a body mass index (BMI) greater than 25 but less than 50. Additional inclusion criteria were age older than 45 years, women had to be postmenopausal for more than 1 year, and nonsmokers (> 5 years). Any medications including insulin, hypoglycemic agents, anti-inflammatory medications, or hormone replacement therapy were exclusionary. Participants needed to be weight stable (< 2 kg weight change in past year) and sedentary (< 20 minutes of structured aerobic activity) to eliminate the influence of individual differences in dietary composition, physical activity, and weight fluctuations on baseline metabolic variables. This study was conducted according to the guidelines of the Declaration of Helsinki and each study participant provided written consent with methods and procedures approved by the institutional review board at University of Maryland School of Medicine and the VA Research and Development Committee (HP-00040472; January 19, 2010). Interested participants were screened and underwent a physical examination including a comprehensive past medical history, fasting blood profile, and a graded exercise treadmill test.
Testing
Participants underwent the following tests before and after the interventions: whole-body dual-energy x-ray absorptiometry, VO2max, 3-hour (80 mU.m2.min–1) hyperinsulinemic-euglycemic clamp, vastus lateralis skeletal muscle biopsy (basal and 120 minutes into clamp), and abdominal and gluteal adipose tissue biopsies. Height (cm) and weight (kg) were measured to calculate BMI. Percentage body fat mass was determined by dual-energy x-ray absorptiometry (iDXA, LUNAR Radiation Corp). VO2max was measured using a continuous treadmill test protocol [20].
Glucose Clamp and Skeletal Muscle Biopsies
Body weight was measured to ensure that all participants were weight stabilized (± 2%) for at least 2 weeks before metabolic testing before and after the interventions and were provided all meals as a eucaloric diet for 2 days before the clamp by a registered dietitian to control nutrient intake [21]. All testing was performed in the morning after a 12-hour overnight fast and 36 to 48 hours after the last exercise bout for AEX group. Whole-body insulin sensitivity was measured using the hyperinsulinemic-euglycemic clamp technique [22]. Arterialized blood was obtained from a dorsal heated hand vein [23]. Blood samples were obtained every 5 and 10 minutes for the determination of plasma glucose and insulin levels. A 10-minute priming and continuous infusion of insulin (80 mU.m–2.min–1 Humulin, Eli Lilly Co) was performed for 180 minutes with a continuous infusion of 20% glucose solution starting at 10 minutes. Blood samples were collected in heparinized syringes, placed in prechilled test tubes containing 1.5 mg EDTA/mL of blood, and centrifuged at 4 °C for plasma glucose and stored at –70 °C until analysis for plasma insulin. Plasma glucose concentrations were measured using the glucose oxidase method (2300 STAT Plus, YSI). Plasma insulin was measured in duplicate by radioimmunoassay (Millipore). The homeostatic model assessment of insulin resistance (HOMA) was calculated by ([fasting insulin [µU/mL] × fasting glucose [mmol/L]])/22.5 [24]. M (glucose utilization) was calculated from the amount of glucose infused after correction for glucose equivalent space (glucose space correction). Insulin levels during the clamp were not statistically different before and after each intervention (WL: 1080 ± 87 vs 1175 ± 47 pmol/L and AEX: 1215 ± 42 vs 1171 ± 60 pmol/L). Before the start of the clamp and 120 minutes during the glucose clamp, a vastus lateralis percutaneous needle muscle biopsy was taken from each participant under local anesthesia using a 5-mm Bergström needle (Stille) for the measurement of LRH-1 mRNA expression. Muscle samples were frozen immediately in clamps cooled in liquid nitrogen and stored at –80 °C until assay.
Adipose Tissue Biopsy
On a separate occasion, participants underwent abdominal and gluteal adipose tissue aspirations. Participants were weight stable (± 2%) at least 2 weeks before baseline testing and maintained the Therapeutic Lifestyle Changes diet throughout the study. Participants were provided with an eucaloric diet for 2 days before the fat aspirations, which were performed after a 12-hour overnight fast and 36 to 48 hours after the last exercise bout for the AEX group. Subcutaneous adipose tissue was aspirated under local anesthesia (0.5% xylocaine) from both the abdominal and gluteal regions using a 10-mm mini-cannula. Adipose tissue was rinsed off for blood with saline and was immediately freeze-clamped and stored at −80 °C until assay.
Interventions
Participants were recruited by group and randomly assigned to WL or AEX. Participants in the WL group maintained the American Heart Association diet and reduced calories by 250 to 350 kcal/day. They attended weekly group WL classes led by a registered dietician for instruction in the principles of hypocaloric diet according to the Therapeutic Lifestyle Changes guidelines [25]. Diets were monitored by 7-day food records (or 24-hour recalls) using the American Diabetes Association exchange list system. Participants in the AEX group walked on motorized treadmills on which the intensity of exercise began at 50% to 60% of heart rate reserve (HRR) for 30 to 40 minutes during week 1, 55% to 65% HRR for 45 to 50 minutes at week 2, 60% to 65% HRR for 50 minutes at week 3, and 65% to 75% HRR at week 4. By week 5 to 8, they exercised for 50 minutes at approximately 70% to 80% HRR. Each exercise session included a 5- to 10-minute stretching and warmup phase and a 5- to 10-minute cool down phase. HR was monitored during exercise training sessions using HR monitors (Polar Electro Inc).
Laboratory Methods
Approximately 1 g of adipose tissue was used for RNA isolation and adiponectin and LRH-1 gene expression. Approximately 50 to 80 mg of muscle was used for RNA isolation and LRH-1 gene expression. Total RNA was extracted by Trizol (ThermoFisher; catalog No. 15596018) method and RNA concentrations were measured in a spectrophotometer. A total of 1 μg of total RNA for each sample was reverse-transcribed into first-strand complementary DNA (cDNA) using Transcriptor First Strand cDNA Synthesis Kit (04 896 866 001; Roche Applied Science) according to the detailed manufacturer’s protocol. The random primer was used as the primer and the RT reaction was performed at 10 minutes at 25 °C and then 55 °C for 30 minutes in a volume of 20 μL. The reaction was inactivated by incubating at 85 °C for 10 minutes and stopped by placing the tube on ice. A reverse-transcription control (master mix without reverse-transcription enzyme) was performed.
Quantitative real-time polymerase chain reaction (PCR) and data analysis for adiponectin and LRH-1 were performed in a LightCycler 480 real-time PCR system with LightCycler 480 software (Roche Applied Science). LightCycler 480 Multiwell plate 384 (04 729 748 001; Roche Applied Science), LightCycler 480 Probes Master kit (04 887 301 001; Roche Applied Science), and TaqMan gene expression primer/probe set (ThermoFisher, adiponectin: assay ID HS00605917-m1, LRH-1: assay ID HS00187067-m1) were used. Each quantitative PCR was performed in a final volume of 10 μL, consisting of 2 μL 1:4 diluted template cDNA, 5 μL LightCycler 480 Probes Master, 0.5 μL TaqMan gene expression primer and probe mix, and 2.5 μL nuclease-free water. Water instead of cDNA served as the no template control. According to the manufacturer’s instructions, the quantitative PCR protocol was adopted for all samples after incubation at 95 °C for 10 minutes to activate the DNA polymerase, 45 cycles of 95 °C for 10 seconds, and 60 °C for 30 seconds each were performed to facilitate the PCR. For normalization, 36B4 served as an internal control. Data acquisition occurred in real-time during the annealing/elongation incubation at 60 °C. All samples were amplified in triplicate from the same RNA preparation. Gene expression data were analyzed by Roche LightCycler 480 system software version 1.5 advanced relative quantification program. The average of 3 determinations for each sample and the normalized ratio of target-to-reference were used in statistical analyses. The cDNA synthesis and quantitative Real-Time PCR were performed using our standard laboratory methods [26].
Statistical Analyses
The WL group was directly compared to the AEX group using the t test (unpaired) on baseline characteristics, within-group change scores (paired-measures t tests), and between-groups change scores on measures between baseline and 6 months. An analysis of covariance of changes in outcomes was also performed covarying for sex. Normality was examined using the Shapiro-Wilk test. Nonparametric tests specifically, Spearman correlation coefficients were used to assess relationships between key variables. Statistical significance was set at a 2-tailed P value less than .05. Data were analyzed using SPSS (SPSS Inc); results are expressed as mean ± SEM.
Results
Baseline
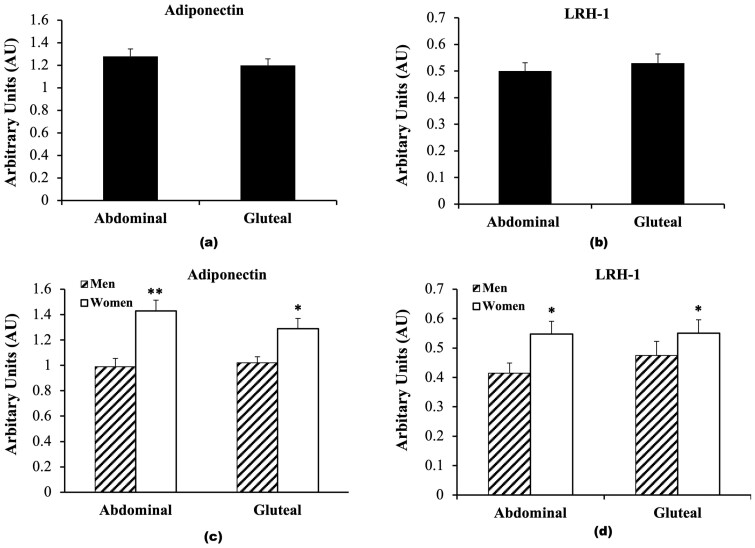
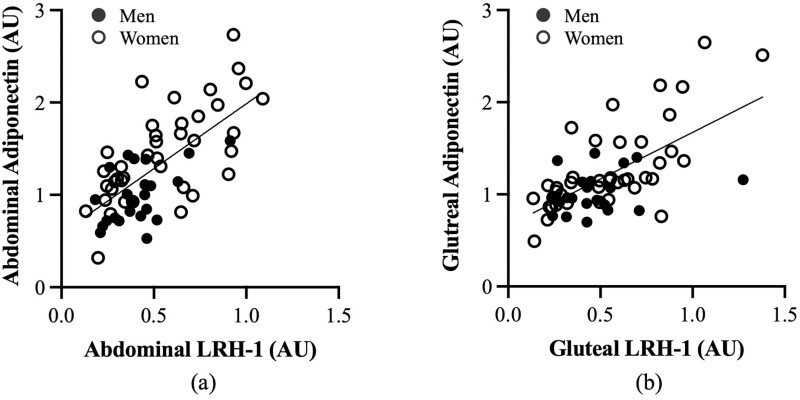
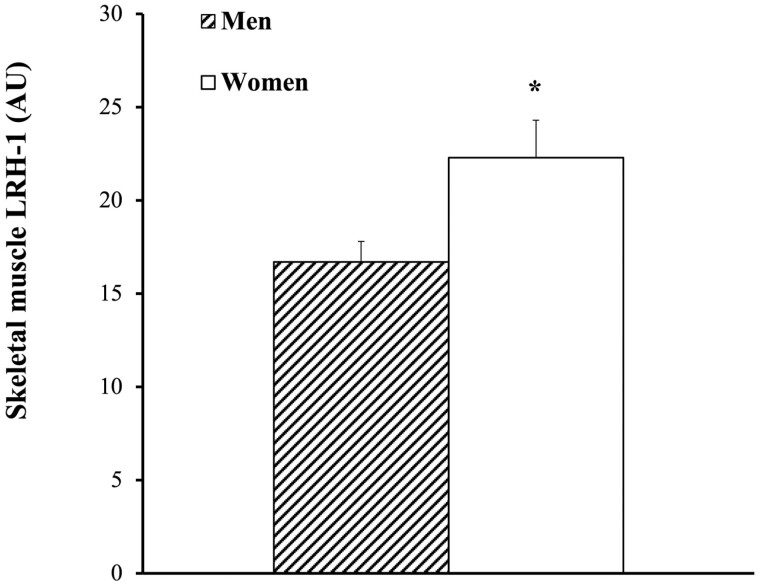
The study enrolled 92 overweight and obese men (40%) and women (60%). The physical and metabolic characteristics of the 62 individuals (n = 23 men and n = 39 women) who completed the biopsies at baseline are presented in Table 1. At baseline, there were no significant differences between men and women in age, BMI, fat mass, 120-minute glucose, fasting and 120-minute insulin, and HOMA-IR. Women had higher percentage body fat (P < .001) and M (expressed in μmol.kg–1.min–1; P < .05 and expressed in μmol.kgFFM–1.min–1; P < .01) than men. Women had lower fat free mass (FFM) (P < .001), VO2max (P < .001), and fasting glucose (P < .05) than men. At baseline, there was no difference between abdominal and gluteal adipose tissue adiponectin expression (Fig. 1A). Abdominal and gluteal adipose tissue LRH-1 mRNA expression were also not significantly different (Fig. 1B). There were, however, sex differences in that women (n = 39) had higher adiponectin mRNA expression both in abdominal (36%; P < .001) and gluteal (23%; P < .05) adipose tissue regions compared to men (n = 23) (Fig. 1C). Men had 28% lower abdominal adipose tissue LRH-1 expression than women (P < .05) and 15% lower gluteal LRH-1 expression than women (P = .05; Fig. 1D). Abdominal adipose tissue adiponectin and abdominal LRH-1 expression were highly correlated (r = 0.68; P < .001; Fig. 2A). Likewise, there was a correlation between gluteal adipose tissue expression of adiponectin and LRH-1 (r = 0.64; P < .001; Fig. 2B). Basal skeletal muscle LRH-1 was lower in men than women (Fig. 3; P < .05). Relationships between gene expression and metabolic variables at baseline (n = 62) indicate that gluteal adiponectin expression was associated with fasting and 120-minute glucose (r = –0.27; P < .05 and r = –0.28; P < .05, respectively). Abdominal adipose adiponectin expression was not associated with fasting or 120-minute glucose. There were no relationships between abdominal and gluteal adiponectin with fasting and 120-minute insulin, VO2max (mL/kg/min), or HOMA-IR. Abdominal adiponectin mRNA was associated with M (expressed in μmol.kg–1.min–1; r = 0.27; P < .05 and expressed in μmol.kgFFM–1.min–1; r = 0.30; P < .05). Likewise, gluteal adiponectin mRNA was associated with M (expressed in μmol.kg–1.min–1; r = 0.25; P = .07 and expressed in μmol.kgFFM-–1.min–1; r = 0.30; P < .05). However, LRH in abdominal and gluteal adipose tissue and LRH-1 expression in skeletal muscle were not related to fasting and 120-minute glucose or insulin, VO2max, HOMA-IR, or M.
Table 1.
Baseline physical and metabolic characteristics of all participants (n = 62) and in men (n = 23) and women (n = 39)
| All (n = 62) | Men (n = 23) | Women (n = 39) | |
|---|---|---|---|
| Age, y | 61 ± 1 | 61 ± 2 | 60 ± 1 |
| Body weight, kg | 94.4 ± 2.4 | 103.0 ± 4.1 | 89.4 ± 2.7b |
| BMI | 33 ± 1 | 33 ± 1 | 33 ± 1 |
| VO2max, L/min | 2.2 ± 0.1 | 2.7 ± 0.1 | 1.9 ± 0.1c |
| VO2max, mL/kg/min | 23.2 ± 0.6 | 26.6 ± 0.8 | 21.1 ± 0.6c |
| VO2max, mL/kgFFM/min | 40.4 ± 0.8 | 41.4 ± 1.1 | 39.8 ± 1.0 |
| % Body fat | 42.4 ± 1.1 | 35.6 ± 1.3 | 46.6 ± 1.1c |
| Fat mass, kg | 41.4 ± 1.8 | 37.4 ± 2.6 | 43.9 ± 2.3 |
| FFM, kg | 54.5 ± 1.5 | 65.3 ± 1.7 | 47.7 ± 1.1c |
| Fasting glucose, mg/dL | 99 ± 2 | 104 ± 2 | 96 ± 2a |
| 120-min glucose, mg/dL | 148 ± 6 | 162 ± 11 | 139 ± 8 |
| Fasting insulin, pmol/L | 116 ± 9 | 130 ± 14 | 107 ± 11 |
| 120-min insulin, pmol/L | 712 ± 64 | 792 ± 116 | 654 ± 71 |
| M, μmol.kg–1.min–1 | 27.5 ± 1.6 | 23.2 ± 2.7 | 30.4 ± 1.7a |
| M, μmol.kgFFM–1.min–1 | 47.1 ± 2.8 | 34.5 ± 3.6 | 56.4 ± 3.1c |
| HOMA-IR | 4.2 ± 0.3 | 4.9 ± 0.6 | 3.7 ± 0.4 |
Values are mean ± SEM.
Abbreviations: BMI, body mass index; FFM, fat free mass; HOMA-IR, homeostatic model assessment of insulin resistance; VO2max, maximal oxygen consumption.
Statistically significant different pre and post intervention:
a P ≤ .05;
b P < .01; and
c P < .001.
Figure 1.
A, Adiponectin, and B, liver receptor homolog-1 (LRH-1) messenger RNA (mRNA) expression in abdominal and gluteal adipose tissue in older adults. C, Abdominal and gluteal adipose tissue adiponectin mRNA expression in men (n = 23, hashed) and women (n = 39, solid white). *P < .05; **P < .001. D, Abdominal and gluteal adipose tissue LRH-1 mRNA expression in men (n = 23, hashed) and women (n = 39, solid white). *P < .05.
Figure 2.
Relationship between adiponectin and liver receptor homolog-1 (LRH-1) expression in A, abdominal adipose tissue (r = 0.68; P < .001), and B, gluteal adipose tissue (r = 0.64; P < .001)).
Figure 3.
Skeletal muscle liver receptor homolog-1 (LRH-1) messenger RNA expression in men (n = 23, hashed) and women (n = 39, solid white). *P < .05.
Effects of the Interventions
There were 21 participants who completed WL and 20 who completed AEX. Body composition and fitness results are presented in Table 2. There were between-group differences in the change in body weight, BMI, VO2max, fat mass, and FFM (all P < .001). In the WL group, there was a 9% decrease in body weight (P < .0001) and an average 3-unit decrease in BMI (P < .05). Percentage body fat decreased (P < .0001), fat mass decreased (P < .0001), and FFM also decreased (P < .0001). Percentage body fat significantly decreased in the AEX group (P < .01), FFM increased (P = .05), fat mass decreased slightly (P = .09), and body weight did not change. There was a 12% increase in VO2 max after AEX (P < .0001) with no change in the WL group. Covarying for sex did not change the significance of the body composition and fitness results (Table 3).
Table 2.
Physical and metabolic characteristics before and after weight loss and aerobic exercise
| WL (n = 21) | AEX (n = 20) | |||
|---|---|---|---|---|
| Pre | Post | Pre | Post | |
| Age, y | 60 ± 1 | – | 62 ± 2 | – |
| Body weight, kg | 102.9 ± 3.9 | 93.7 ± 3.5g,a | 92.7 ± 3.6 | 91.5 ± 4.1 |
| BMI | 36 ± 1 | 33 ± 1c,a | 32 ± 1 | 32 ± 1 |
| VO2max, L/min | 2.3 ± 0.1 | 2.3 ± 0.1a | 2.4 ± 0.1 | 2.7 ± 0.1g |
| VO2max, mL/kg/min | 23.0 ± 1.2 | 24.6 ± 1.3e,a | 25.1 ± 1.1 | 29.2 ± 1.3e |
| VO2max, mL/kgFFM/min | 40.0 ± 1.0 | 40.0 ± 1.1 | 41.7 ± 1.47 | 44.4 ± 3.0 |
| % Body fat | 44.2 ± 1.7 | 41.2 ± 2.0g | 38.3 ± 1.8 | 36.9 ± 2.1d |
| Fat mass, kg | 45.7 ± 2.5 | 38.9 ± 2.4g,a | 36.3 ± 2.5 | 35.0 ± 2.9 |
| FFM, kg | 57.4 ± 2.8 | 55.9 ± 2.7g,a | 57.6 ± 2.4 | 58.4 ± 2.6c |
| Fasting glucose, mg/dL | 101 ± 3 | 92 ± 2g,b | 96 ± 2 | 95 ± 2 |
| 120-min glucose, mg/dL | 150 ± 10 | 128 ± 9e | 131 ± 9 | 127 ± 9 |
| Fasting insulin, pmol/L | 109 ± 11 | 85 ± 8f | 100 ± 9 | 90 ± 9a |
| 120-min insulin, pmol/L | 685 ± 93 | 472 ± 57f | 595 ± 92 | 505 ± 56 |
| M, μmol.kg–1.min–1 | 22.1 ± 2.9 | 31.2 ± 2.8e | 31.7 ± 2.6 | 37.3 ± 2.8c |
| M, μmol.kgFFM–1.min–1 | 40.8 ± 4.6 | 55.2 ± 4.7d | 52.2 ± 4.7 | 59.6 ± 5.1c |
| HOMA-IR | 4.0 ± 0.5 | 2.8 ± 0.3e | 3.4 ± 0.3 | 3.1 ± 0.3 |
All participants (n = 41) before and after the AEX (n = 20) and WL (n = 21) intervention. Values are mean ± SEM.
Abbreviations: AEX, aerobic exercise; BMI, body mass index; FFM, fat free mass; HOMA-IR, homeostatic model assessment of insulin resistance; VO2max, maximal oxygen consumption; WL, weight loss.
Statistically significant different pre and post intervention WL vs AEX:
a P < .001;
b P < .05;
c P ≤ .05;
d P < .01;
e P < .001;
f P˂.005; and
g P ≤ .0001.
Table 3.
Changes in physical and metabolic characteristics before and after weight loss and aerobic exercise covarying by sex
| WL | AEX | P corrected model | P sex | P arm | |||
|---|---|---|---|---|---|---|---|
| Change | X (SEM) | CI | X (SEM) | CI | |||
| Body weight, kg | –9.51 (0.72) | –11.0 to –8.1 | –1.22 (0.75) | –2.74 to –0.0.30 | < .001 | .60 | < .001 |
| VO2max, L/min | –0.11 (0.06) | –0.23 to 0.01 | 0.31 (0.06) | 0.18 to 0.43 | < .001 | .17 | < .001 |
| VO2max, mL/kg/min | 0.07 (0.94) | –1.84 to 1.97 | 3.99 (1.01) | 1.94 to 6.04 | .008 | .19 | .008 |
| Fat mass, kg | –6.98 (0.77) | –8.54 to –5.42 | –1.29 (0.79) | –2.89 to 0.32 | < .001 | .86 | < .001 |
| FFM, kg | –1.43 (0.37) | –2.18 to –0.69 | 0.82 (0.39) | 0.03 to 1.60 | < .001 | .42 | < .001 |
| Fasting glucose, mg/dL | –8.0 (1.7) | –11.4 to –4.7 | –0.9 (1.7) | –4.4 to 2.7 | .02 | .62 | .005 |
| 120-min glucose mg/dL | –30.8 (9.6) | –50.3 to –11.4 | –2.6 (10.3) | –23.6 to 18.3 | .14 | .45 | .06 |
| Fasting insulin, pmol/L | –25.8 (6.6) | –39.2 to –12.4 | –12.4 (6.6) | –25.8 to 1.0 | .09 | .06 | .16 |
| 120-min insulin, pmol/L | –191.3 (65.5) | –324.4 to –58.0 | –84.2 (65.5) | –217.4 to 49.0 | .47 | .51 | .25 |
| M, μmol.kg–1.min–1 | 7.2 (2.5) | 2.2 to 12.2 | 5.3(2.4) | 0.4 to 10.2 | .62 | .47 | .58 |
| M, μmol.kgFFM–1.min–1 | 13.4 (3.2) | 6.8 to 19.9 | 6.9 (3.1) | 0.6 to 13.3 | .31 | .72 | .17 |
| HOMA-IR | –1.4 (0.31) | –2.0 to –0.76 | –0.54 (0.31) | –1.16 to 0.09 | .04 | .04 | .06 |
Abbreviations: AEX, aerobic exercise; BMI, body mass index; FFM, fat free mass; HOMA-IR, homeostatic model assessment of insulin resistance; VO2max, maximal oxygen consumption; WL, weight loss; X, mean.
The results for glucose metabolism are also presented in Table 2. There were between-group differences in the changes in fasting glucose (P < .05) but no other variables of glucose metabolism. With WL, fasting glucose decreased 9% (P < .0001), 120-minute glucose decreased (P < .001), fasting insulin decreased 22% (P < .005), and HOMA-IR decreased. With AEX, fasting insulin decreased 10% (P = .05) without significant changes in fasting glucose and in HOMA-IR. M increased when expressed as μmol.kg–1.min–1 after WL (P < .0001) and AEX (P < .05) as well as expressed per μmol.kgFFM–1.min–1 with WL and AEX (both P < .05). Covarying for sex did not change the significance of the results (Table 3) except the change in HOMA-IR was significantly different between groups (P = .04).
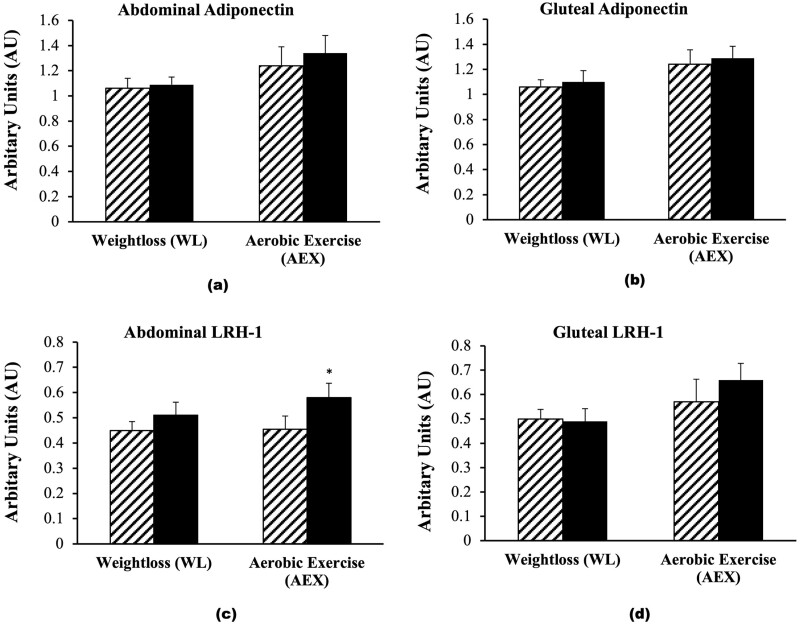
There were no between-group difference in the changes in adipose tissue adiponectin or LRH-1 expression. Adiponectin mRNA expression in the abdominal region did not change significantly (WL: 4.3%; AEX: 7.5%). Likewise, adiponectin mRNA expression in the gluteal region did not change significantly after WL (6.5%) or AEX (4.4%). LRH-1 mRNA expression in abdominal adipose tissue increased after AEX (P = .05) but the 12% increase after WL was not statistically significant (Fig. 4C). LRH-1 expression in gluteal adipose tissue tended to increase after AEX (P = .07) and did not change after WL (Fig. 4D). There were no significant correlations between changes in abdominal or gluteal adiponectin expression and changes in fasting glucose, insulin, HOMA-IR, or M. Likewise, changes in abdominal and gluteal LRH-1 did not correlate with changes in metabolic variables.
Figure 4.
Adipose tissue adiponectin and liver receptor homolog-1 (LRH-1) messenger RNA (A, abdominal adiponectin; and B, gluteal adiponectin; C, abdominal LRH-1; and D, gluteal LRH-1 expression before [hashed] and after weight loss [WL] or aerobic exercise [AEX] [solid black]). *P = .05.
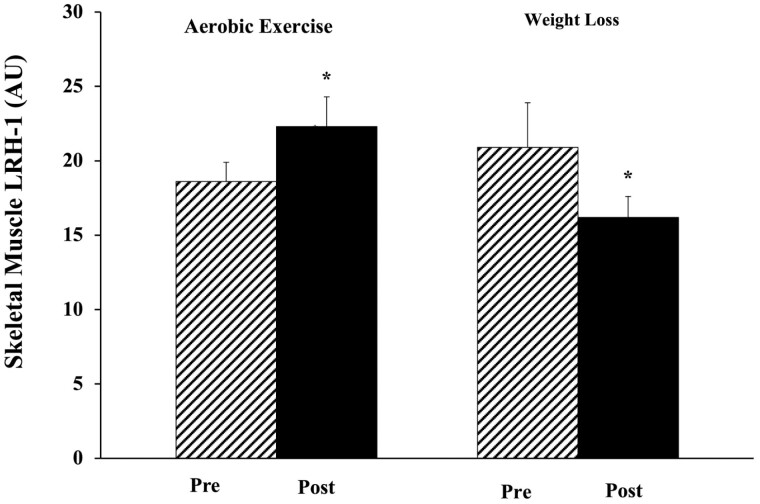
There was a significant group × time interaction in the change in basal LRH-1 (P < .05) with an increase after AEX and decrease after WL (Fig. 5). Basal LRH-1 skeletal muscle expression increased after AEX (P < .05). There was no change in LRH-1 mRNA expression with insulin stimulation during the clamp in the AEX group (basal vs insulin pre: 18.6 ± 1.3 vs 19.3 ± 1.3 AU; post 22.3 ± 2.0 vs 25.4 ± 2.4 AU) or WL group (basal vs insulin: pre: 20.9 ± 3.0 vs 18.0 ± 1.8 AU; post 16.2 ± 1.4 vs 19.2 ± 1.7 AU). The change in VO2max related to the change in LRH-1 mRNA (r = 0.43; P = .01). There were no significant relationships between changes in LRH-1 and changes in fasting glucose, insulin, HOMA-IR, or M.
Figure 5.
Skeletal muscle liver receptor homolog-1 (LRH-1) messenger RNA expression before (hashed) and after (solid) weight loss (WL) or aerobic exercise (AEX). *P < .05 before vs after AEX and *change between WL vs AEX.
Discussion
The present study indicates that expression of LRH-1 predicts the expression of adiponectin both in abdominal and gluteal human adipose tissue with sex differences in adiponectin and LRH-1 expression. A 6-month AEX program significantly increases abdominal LRH-1 expression and skeletal muscle LRH-1 whereas WL results in a decrease in muscle LRH-1 without changes in abdominal and gluteal adipose tissue LRH-1 expression suggesting differential effects of the interventions on muscle LRH-1. In support of this, improvements in fitness predicted increased skeletal muscle LRH-1 expression. Our results also indicate that WL or AEX did not change adiponectin expression in abdominal and gluteal adipose tissue.
Adiponectin, a 29-kDa adipocyte-derived circulating protein, plays a critical role in the maintenance of whole-body homeostasis and metabolism [26, 27]. In visceral fat obesity, the plasma level of adiponectin is lower [28]. WL or AEX increase adiponectin expression, the release in abdominal and gluteal subcutaneous adipose tissue, and circulating adiponectin [16, 29, 30]. Our data show that adiponectin expression was not changed in abdominal and gluteal adipose tissue after AEX intervention, but support findings of sex differences in adiponectin demonstrating that women have higher plasma levels and abdominal adiponectin RNA expression than men [5, 31]. Our data would add that adiponectin RNA expression both in abdominal and gluteal tissue were significantly higher in women than in men and is also consistent with a prior study [17] that there is not a significant difference between abdominal and gluteal adiponectin RNA expression.
Although WL decreased body weight, FFM, and percentage body fat, WL did not change the adiponectin RNA expression in adipose tissue from both abdominal and gluteal regions. We have shown plasma adiponectin levels are negatively related to body fat and positively with insulin sensitivity by the glucose clamp in adult women across a wide range of age and obesity [32]. Furthermore, adiponectin is negatively correlated with truncal fat, both in men and women [33]. It is possible that the amount of WL in this study was not sufficient to induce a significant change in adipose tissue adiponectin gene expression as we have shown that moderate WL did not change circulating adiponectin levels in postmenopausal women [34]. It also may be attributable to whether measures are made in the circulation or tissue, as in our case. Moreover, it is well-known that fat provides the most important form of fuel for aerobic exercise and aerobic sports activities at low to medium intensity exercise (25%-65% of VO2max) [35]. As AEX increased both VO2max and LRH-1 mRNA expression in abdominal adipose tissue, it is possible that adipose tissue-derived LRH-1 is involved in the fat utilization during exercise.
LRH-1 is a nuclear receptor that plays an important role in lipid homeostasis and embryogenesis. It is expressed in multiple tissues or organs such as the digestive system, urinary system, and respiratory system. It exists mainly in cells involved in fatty acid/glucose metabolism such as hepatocytes, brown adipocytes, and cardiomyocytes, and neurons involved in the regulation of food intake such as the arcuate nucleus in the hypothalamus and paraventricular nucleus of thalamus [36]. Our data indicate that LRH-1 is expressed both in adipose tissue and muscle. Moreover, we found no significant difference between LRH-1 expressions in abdominal and gluteal adipose tissue. However, we did observe a significant difference by sex whereby women have significantly higher LRH-1 expression both in adipose tissue and skeletal muscle than men. Iwaki et al [3] reported that LRH-1 is expressed in mature adipocytes and plays a significant role in the transcriptional activation of the adiponectin gene. Our results further show that LRH-1 is positively correlated with adiponectin both in abdominal and gluteal adipose tissues, indicating a relationship between these 2 genes. We are unaware of other studies examining sex differences or the effects of either AEX or WL alone on LRH-1 expression in adipose tissue and skeletal muscle. Since changes in LRH-1 expression were also associated with changes in fitness and LRH-1 increased in skeletal muscle after AEX, it is reasonable to suggest that it acts as a myokine.
Although LRH-1 is involved in hepatic glucose metabolism, we investigated its role in whole-body insulin sensitivity measured by the gold-standard glucose clamp technique. Our results indicate that neither adipose tissue nor skeletal muscle LRH-1 expression were related to fasting glucose or glucose utilization. In IR mouse models, the LRH-1 agonist ligand (dilauroyl phosphatidylcholine) decreases serum glucose and improves glucose homeostasis [37]. In a rodent model with the deletion of liver LRH-1, whole-body glucose infusion rate is not different from control mice but hepatic LRH-1 deficiency does impair the liver’s role in glucose metabolism [38]. Our data show AEX significantly improves cardiorespiratory fitness and insulin sensitivity, and increases skeletal muscle LRH-1 RNA expression. This finding indicates that LRH-1 is inducible on AEX training.
We acknowledge a larger sample size in each intervention group might have provided the ability to examine the participants by glucose tolerance status or by sex. Our results did not change after covarying for sex except for changes in HOMA-IR with the interventions. Other limitations are the lack of measurement of circulating total or high-molecular-weight adiponectin and muscle adiponectin expression. To our knowledge, there are no current assays to measure human LRH-1 in plasma. Measurement of protein expression of adiponectin and LRH-1 is another limitation. Future studies could also include the measurement of isotopes to depict changes in liver sensitivity and a no intervention control group. Yet, our well-controlled interventions of long duration, advanced methods for determining insulin action, and measurement of adiponectin and LRH-1 in the specific tissue (adipose and skeletal muscle) are strengths of the study design.
Conclusion
In summary, our study provides evidence that aerobic exercise training of 6 months’ duration elicits increases in LRH-1 gene expression both in adipose tissue and skeletal muscle in overweight and obese adults. We suggest that an increase in LRH-1 expression is observed before an increase in adiponectin expression when there are improvements in fitness and that LRH-1 may act as a myokine. Additional studies could aim to examine the adiponectin receptors with lifestyle interventions. Future investigations are also necessary to examine what factors drive changes in tissue LRH-1 with exercise training.
Glossary
Abbreviations
- AEX
aerobic exercise
- BMI
body mass index
- cDNA
complementary DNA
- FFM
fat free mass
- HOMA
homeostatic model assessment of insulin resistance
- HRR
heart rate reserve
- LRH-1
liver receptor homolog-1
- mRNA
messenger RNA
- PCR
polymerase chain reaction
- VO2max
maximal oxygen consumption
- WL
weight loss
Contributor Information
Alice S Ryan, Email: aryan@som.umaryland.edu, VA Research Service, VA Maryland Health Care System, Baltimore, Maryland 21201, USA; Department of Medicine, Division of Geriatric and Palliative Medicine, University of Maryland School of Medicine, Baltimore, Maryland 21201, USA; Baltimore VA Medical Center Geriatric Research, Education and Clinical Center, Baltimore, Maryland 21201, USA.
Guoyan Li, Department of Medicine, Division of Geriatric and Palliative Medicine, University of Maryland School of Medicine, Baltimore, Maryland 21201, USA.
Financial Support
This work was supported by a Senior Research Career Scientist Award (to A.S.R.) from the US Department of Veterans Affairs (VA) Rehabilitation R&D (Rehab RD) Service, a VA Merit Award (No. 1153486 to A.S.R.) Clinical Service R&D, VA Medical Center Baltimore Geriatric Research, Education and Clinical Center (GRECC), and the National Institutes of Health (grant Nos. P30-AG028747 and P30 DK072488).
Author Contributions
A.S.R. designed the research, performed the experiments, collected and analyzed the data, and wrote the manuscript; and G.L. performed the experiments and contributed to the writing of the manuscript.
Disclosures
The authors have nothing to disclose.
Data Availability
Data will be provided on reasonable request.
Clinical Trial Information
Clinical trial registration No. NCT00753363 (registered April, 2007).
Conflict of Interest
The authors have no conflict of interest.
References
- 1. Ouchi N, Walsh K. Adiponectin as an anti-inflammatory factor. Clin Chim Acta. 2007;380(1-2):24-30. doi: 10.1016/j.cca.2007.01.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Nigro E, Scudiero O, Monaco ML, et al. . New insight into adiponectin role in obesity and obesity-related diseases. Biomed Res Int. 2014;2014:658913. doi: 10.1155/2014/658913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Iwaki M, Matsuda M, Maeda N, et al. . Induction of adiponectin, a fat-derived antidiabetic and antiatherogenic factor, by nuclear receptors. Diabetes. 2003;52(7):1655-1663. doi: 10.2337/diabetes.52.7.1655 [DOI] [PubMed] [Google Scholar]
- 4. Ouchi N, Kihara S, Arita Y, et al. . Adiponectin, an adipocyte-derived plasma protein, inhibits endothelial NF-kappaB signaling through a cAMP-dependent pathway. Circulation. 2000;102(11):1296-1301. doi: 10.1161/01.cir.102.11.1296 [DOI] [PubMed] [Google Scholar]
- 5. Kern PA, Di Gregorio GB, Lu T, Rassouli N, Ranganathan G. Adiponectin expression from human adipose tissue: relation to obesity, insulin resistance, and tumor necrosis factor-alpha expression. Diabetes. 2003;52(7):1779-1785. doi: 10.2337/diabetes.52.7.1779 [DOI] [PubMed] [Google Scholar]
- 6. Ohman-Hanson RA, Cree-Green M, Kelsey MM, et al. . Ethnic and sex differences in adiponectin: from childhood to adulthood. J Clin Endocrinol Metab. 2016;101(12):4808-4815. doi: 10.1210/jc.2016-1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yamamoto Y, Hirose H, Saito I, et al. . Correlation of the adipocyte-derived protein adiponectin with insulin resistance index and serum high-density lipoprotein-cholesterol, independent of body mass index, in the Japanese population. Clin Sci (Lond). 2002;103(2):137-142. doi: 10.1042/cs1030137 [DOI] [PubMed] [Google Scholar]
- 8. Cnop M, Havel PJ, Utzschneider KM, et al. . Relationship of adiponectin to body fat distribution, insulin sensitivity and plasma lipoproteins: evidence for independent roles of age and sex. Diabetologia. 2003;46(4):459-469. doi: 10.1007/s00125-003-1074-z [DOI] [PubMed] [Google Scholar]
- 9. Salas-Salvadó J, Granada M, Bulló M, Corominas A, Casas P, Foz M. Plasma adiponectin distribution in a Mediterranean population and its association with cardiovascular risk factors and metabolic syndrome. Metabolism. 2007;56(11):1486-1492. doi: 10.1016/j.metabol.2007.06.014 [DOI] [PubMed] [Google Scholar]
- 10. Iglesias MJ, Eiras S, Piñeiro R, et al. . Gender differences in adiponectin and leptin expression in epicardial and subcutaneous adipose tissue. Findings in patients undergoing cardiac surgery [article in Spanish]. Rev Esp Cardiol. 2006;59(12):1252-1260. [PubMed] [Google Scholar]
- 11. Eglit T, Lember M, Ringmets I, Rajasalu T. Gender differences in serum high-molecular-weight adiponectin levels in metabolic syndrome. Eur J Endocrinol. 2013;168(3):385-391. doi: 10.1530/EJE-12-0688 [DOI] [PubMed] [Google Scholar]
- 12. Nishizawa H, Shimomura I, Kishida K, et al. . Androgens decrease plasma adiponectin, an insulin-sensitizing adipocyte-derived protein. Diabetes. 2002;51(9):2734-2741. doi: 10.2337/diabetes.51.9.2734 [DOI] [PubMed] [Google Scholar]
- 13. Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004;14(5):250-260. doi: 10.1016/j.tcb.2004.03.008 [DOI] [PubMed] [Google Scholar]
- 14. Bolado-Carrancio A, Riancho JA, Sainz J, Rodríguez-Rey JC. Activation of nuclear receptor NR5A2 increases Glut4 expression and glucose metabolism in muscle cells. Biochem Biophys Res Commun. 2014;446(2):614-619. doi: 10.1016/j.bbrc.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 15. Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290(5):E961-E967. doi: 10.1152/ajpendo.00506.2005 [DOI] [PubMed] [Google Scholar]
- 16. Christiansen T, Paulsen SK, Bruun JM, Ploug T, Pedersen SB, Richelsen B. Diet-induced weight loss and exercise alone and in combination enhance the expression of adiponectin receptors in adipose tissue and skeletal muscle, but only diet-induced weight loss enhanced circulating adiponectin. J Clin Endocrinol Metab. 2010;95(2):911-919. doi: 10.1210/jc.2008-2505 [DOI] [PubMed] [Google Scholar]
- 17. Moghadasi M, Mohebbi H, Rahmani-Nia F, Hassan-Nia S, Noroozi H, Pirooznia N. High-intensity endurance training improves adiponectin mRNA and plasma concentrations. Eur J Appl Physiol. 2012;112(4):1207-1214. doi: 10.1007/s00421-011-2073-2 [DOI] [PubMed] [Google Scholar]
- 18. Polak J, Klimcakova E, Moro C, et al. . Effect of aerobic training on plasma levels and subcutaneous abdominal adipose tissue gene expression of adiponectin, leptin, interleukin 6, and tumor necrosis factor alpha in obese women. Metabolism. 2006;55(10):1375-1381. doi: 10.1016/j.metabol.2006.06.008 [DOI] [PubMed] [Google Scholar]
- 19. Sjögren P, Sierra-Johnson J, Kallings LV, et al. . Functional changes in adipose tissue in a randomised controlled trial of physical activity. Lipids Health Dis. 2012;11:80. doi: 10.1186/1476-511X-11-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ryan AS, Ortmeyer HK, Sorkin JD. Exercise with calorie restriction improves insulin sensitivity and glycogen synthase activity in obese postmenopausal women with impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2012;302(1):E145-E152. doi: 10.1152/ajpendo.00618.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ryan AS, Nicklas BJ, Berman DM. Aerobic exercise is necessary to improve glucose utilization with moderate weight loss in women. Obesity (Silver Spring). 2006;14(6):1064-1072. doi: 10.1038/oby.2006.122 [DOI] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237(3):E214-EE23. doi:10.1152/ajpendo.1979.237.3.E214 [DOI] [PubMed] [Google Scholar]
- 23. McGuire EA, Helderman JH, Tobin JD, Andres R, Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976;41(4):565-573. doi: 10.1152/jappl.1976.41.4.565 [DOI] [PubMed] [Google Scholar]
- 24. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 25. Lichtenstein AH, Appel LJ, Brands M, et al. . Summary of American Heart Association Diet and Lifestyle Recommendations revision 2006. Arterioscler Thromb Vasc Biol. 2006;26(10):2186-2191. doi: 10.1161/01.ATV.0000238352.25222.5e [DOI] [PubMed] [Google Scholar]
- 26. Ryan AS, Li G, Blumenthal JB, Ortmeyer HK. Aerobic exercise + weight loss decreases skeletal muscle myostatin expression and improves insulin sensitivity in older adults. Obesity (Silver Spring). 2013;21(7):1350-1356. doi: 10.1002/oby.20216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maeda N, Funahashi T, Matsuzawa Y, Shimomura I. Adiponectin, a unique adipocyte-derived factor beyond hormones. Atherosclerosis. 2020;292:1-9. doi: 10.1016/j.atherosclerosis.2019.10.021 [DOI] [PubMed] [Google Scholar]
- 28. Arita Y, Kihara S, Ouchi N, et al. . Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79-83. doi: 10.1006/bbrc.1999.0255 [DOI] [PubMed] [Google Scholar]
- 29. Højbjerre L, Rosenzweig M, Dela F, Bruun JM, Stallknecht B. Acute exercise increases adipose tissue interstitial adiponectin concentration in healthy overweight and lean subjects. Eur J Endocrinol. 2007;157(5):613-623. doi: 10.1530/EJE-07-0213 [DOI] [PubMed] [Google Scholar]
- 30. Wang X, You T, Murphy K, Lyles MF, Nicklas BJ. Addition of exercise increases plasma adiponectin and release from adipose tissue. Med Sci Sports Exerc. 2015;47(11):2450-2455. doi: 10.1249/MSS.0000000000000670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cui J, Wu X, Andrel J, Falkner B. Relationships of total adiponectin and molecular weight fractions of adiponectin with free testosterone in African men and premenopausal women. J Clin Hypertens (Greenwich). 2010;12(12):957-963. doi: 10.1111/j.1751-7176.2010.00383.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ryan AS, Berman DM, Nicklas BJ, et al. . Plasma adiponectin and leptin levels, body composition, and glucose utilization in adult women with wide ranges of age and obesity. Diabetes Care. 2003;26(8):2383-2388. doi: 10.2337/diacare.26.8.2383 [DOI] [PubMed] [Google Scholar]
- 33. Turer AT, Khera A, Ayers CR, et al. . Adipose tissue mass and location affect circulating adiponectin levels. Diabetologia. 2011;54(10):2515-2524. doi: 10.1007/s00125-011-2252-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ryan AS, Nicklas BJ, Berman DM, Elahi D. Adiponectin levels do not change with moderate dietary induced weight loss and exercise in obese postmenopausal women. Int J Obes Relat Metab Disord. 2003;27(9):1066-1071. doi: 10.1038/sj.ijo.0802387 [DOI] [PubMed] [Google Scholar]
- 35. Horowitz JF, Klein S. Lipid metabolism during endurance exercise. Am J Clin Nutr. 2000;72(2 Suppl):558S-563S. doi: 10.1093/ajcn/72.2.558S [DOI] [PubMed] [Google Scholar]
- 36. Higashiyama H, Kinoshita M, Asano S. Expression profiling of liver receptor homologue 1 (LRH-1) in mouse tissues using tissue microarray. J Mol Histol. 2007;38(1):45-52. doi: 10.1007/s10735-007-9077-6 [DOI] [PubMed] [Google Scholar]
- 37. Lee JM, Lee YK, Mamrosh JL, et al. . A nuclear-receptor-dependent phosphatidylcholine pathway with antidiabetic effects. Nature. 2011;474(7352):506-510. doi: 10.1038/nature10111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Oosterveer MH, Mataki C, Yamamoto H, et al. . LRH-1-dependent glucose sensing determines intermediary metabolism in liver. J Clin Invest. 2012;122(8):2817-2826. doi: 10.1172/jci62368 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data will be provided on reasonable request.