Abstract
Adipose tissue acts as an active endocrine organ secreting a number of adipokines and may be involved in biological mechanism of stroke. Vaspin, apelin, and visfatin play important roles in the regulation of vascular disorders.
Our aim was to evaluate whether the concentrations of vaspin, apelin, and visfatin were associated with stroke risk.
A total of 235 patients with stroke (156 patients with ischemic stroke and 79 patients with hemorrhagic stroke) and 235 age- and gender-matched healthy controls were included in this study. A sandwich ELISA was developed to measure the serum vaspin, apelin, and visfatin levels.
There was a statistically significant difference in the median levels of serum vaspin, apelin, and visfatin levels between stroke cases and controls (vaspin: 1.50 vs 1.07 ng/ml; apelin: 1.56 vs 1.32 pg/ml; visfatin: 23.40 vs 19.65 ng/ml; all P values <.001). Multiple logistic regression analysis showed that, serum vaspin and visfatin levels were significantly inversely associated with increased risk of stroke, and the odds ratios (ORs) in the highest tertile were 2.25 [95% confidence interval (CI) 1.38–3.67; P for trend <.001] for vaspin and 2.56 (95% CI 1.46–4.47; P for trend <.001) for visfatin, respectively, compared with the lowest tertile. Higher apelin levels were marginally associated with lower stroke risk (P for trend =.060).
Our study indicated that higher vaspin, apelin, and visfatin levels might be associated with increased stroke risk. Necessary prospective cohort studies should be conducted to confirm this association in the future.
Keywords: adipokines, apelin, stroke, vaspin, visfatin
1. Introduction
Stroke is the second cause of death worldwide and a leading cause of long-term disability in China[1,2]; however, its pathogenesis is still unknown until now.[3] Stroke patients occupy lots of medical resources and bring heavy economic pressure and mental burden on patients and families. Stroke shares many risk factors with cardiovascular diseases, such as age, smoking, hypertension, inactivity, overweight or obesity, and dyslipidemia.[4]
Adipose tissue is a metabolic and active secretory organ that produces a variety of messenger molecules. Adipocyte-derived hormones contribute to the regulation of many biological processes.[5] Adipokines, like adiponectin and leptin which are the most well-studied. These traditional adipokines play an important role in the pathogenesis of diabetes, dyslipidemia, inflammation, coronary artery disease, and carotid atherosclerosis.[6,7] The role of novel adipokines in stroke remains largely unknown.[8]
Emerging evidence suggests associations between novel adipokines such as vapin, apelin, visfatin between vascular risk factors and atherosclerotic diseases. Vaspin is an important and a new adpokines which mainly produced by adipocytes, and proposed as a biomarker along with other pro-inflammatory adipokines, in evaluating exposure-disease associations.[9] Vaspin has regulation characteristics in glucose and lipid mechanism. Recent findings illustrate vaspin improves glucose tolerance and insulin sensitivity.[10] Other study has showed vaspin expression levels were higher in ischemic stroke.[3,11] Paradoxically, another research has found that ischemic stoke had lower of vaspin serum concentrations.[12] Apelin encoded by APLN gene, is a novel endogenous ligand of the orphan G-protein-coupled receptor.[13] Some investigations documented the inverse relationship of serum apelin with atherosclerosis.[14,15] On the contrary, apelin serum levels have been suggested to be increased in patients with coronary atherosclerosis.[16] However, another report did not find any differences in apelin plasma levels between ischemic stroke patients and healthy controls.[12] Visfatin mainly secreted by visceral adipose tissue, is a novel adipokine with pleotropic functions.[17] Increased serum levels of visfatin have been found in diabetes, obesity, coronary artery disease, and carotid atherosclerosis.[7,18] Upregulated plasma visfatin levels have been found in ischemic stroke.[19]
Recent studies showed the novel adipokines may have an important role in the stroke. However, the effect of them on stroke is not yet well understood, and related studies are confusing. Meanwhile, there is less research on the relationship between stroke in the Chinese population. Therefore, in this study, we aimed to investigate the association between serum levels of 3 novel adipokines (vaspin, apelin, and visfatin) and stroke risk in a group of Chinese participants.
2. Methods
2.1. Study population
The study was a case-control study conducted from January 2016 to September 2019. 235 patients with a first stoke were consecutively recruited from the neurology department at 2 hospitals: Chengdu Tianfu New District People's Hospital and Sichuan Provincial People's Hospital. There were 156 patients with ischemic stroke and 79 patients with hemorrhagic stroke. According to Trial of Org 10172 in Acute Stroke Treatment (TOAST) criteria,[20] ischemic stroke patients were divided into large-artery atherosclerosis (LAA), small-artery occlusion (SAO), cardioembolism (CE), other determined etiology (SOE), and undetermined etiology, and there were 89 LAA cases, 50 SAO cases, 10 CE cases, 3 SOE cases, and 4 undetermined cases included.
The inclusion criteria were as follows:
-
(1)
age over 18 years;
-
(2)
stroke diagnosed according to the World Health Organization criteria, confirmed by CT or MRI;
-
(3)
no medications were taken before the blood test, and
-
(4)
stayed in the hospitals no more than 7 days.
Exclusion criteria included patients:
-
(1)
with a history of cerebral hemorrhage, intracranial space occupying lesions, acute cerebral embolism, lacunar cerebral infarction, coronary heart disease, atrial fibrillation, and malignant tumors; and
-
(2)
taking any lipid-lowering drugs, anticoagulants, steroids, etc.
The controls were healthy check participants from 2 hospitals mentioned above and were age and gender-matched without a history of cardiovascular diseases.
All participants or their legal representatives gave their informed consent, and study protocol was approved by the ethical committee of Sichuan Provincial People's Hospital.
2.2. Clinical data
Demographic and clinic characteristics were obtained including age, body mass index, education, income, smoking status, hypertension and diabetes mellitus. Smoking was defined as having smoked ≥1 cigarette per day for ≥1 year. Hypertension was defined as a systolic blood pressure of ≥140 mm Hg and/or a diastolic blood pressure ≥90 mm Hg. Diabetes mellitus was defined as a fasting glucose level ≥7.0 mmol/L or postprandial blood glucose ≥11.1 mmol/L.
2.3. Vaspin, apelin, visfatin level measurements
Blood samples were collected in siliconized tubes after the admission. The specimens were centrifuged at 1500 g for 10 minutes and the separated serum stored at −80°C until laboratory examination.
Vaspin, apelin, and visfatin were analyzed by commercial Human Vaspin ELISA Kit (Cat No: E-EL-H1762c, Elabscience, Wuhan, China), Human Apelin ELISA Kit (Cat No: E-EL-H0456c, Elabscience, Wuhan, China), and Human Visfatin ELISA Kit (Cat No: E-EL-H1763c, Elabscience, Wuhan, China) according to manufacturer's instructions. The absorbance value of the samples was read at 450 nm using a Bio-Rad Model 680 ELISA reader. The concentrations were calculated from the calibrated standard curve, which was constructed using the standards provided in the kit. Each sample was assayed in duplicate, and the level was calculated as the average of the 2 measurements. The laboratory technicians who performed the measurements were blinded to the characteristics of the study participants. The lower detection limits, intra- and inter-assay coefficients of variations (CV%) were 0.1 ng/ml, 6.2% and 8.8% for vaspin, 0.10 ng/ml, 7.5% and 9.4% for apelin, and 3 pg/ml, 8.5% and 10.2% for visfatin, respectively.
2.4. Statistical analysis
Data of normally distributed continuous variables were expressed as the mean value ± SD, student t test was used for comparing parametric data between the groups. Categorical variables were expressed as percentages, and Chi-Squared test was used for comparing classified variables between groups. In further analysis, vaspin, apelin, and vifatin levels were divided into 3 groups according to tertiles.
Conditional logistic regression analyses were performed to estimate the odds ratios (ORs) and 95% confidence intervals (CIs) for the risk of stroke. Potential confounders were selected into multivariable adjusted models. In model 1, we only adjusted age. In model 2, we further adjusted BMI, education, income, smoking status, and hypertension and diabetes mellitus. Tests for trend were calculated by including the categorical variables as continuous variables.
Spearman correlation analysis was used assessed the correlation between the vaspin, apelin, and vifatin levels and National Institutes of Health Stroke Scale (NIHSS) scores among stroke cases. Correlation coefficient is represented by rs.
For statistical analysis, the statistics program SPSS18.0 was used. The values P < .05 were considered statistically significant.
3. Results
3.1. Baseline characteristics of patients and matched controls
A total of 235 patients with stroke and 235 age and gender-matching controls were included in our study. Comparing the characteristics of the stroke cases with the controls are described in Table 1. There was no statistically significant difference in the age, BMI, education level, and diabetes mellitus between stroke patients and stroke-free controls (P > .05). The income level of the stroke patients group was lower than the control group. 106 (45.11%) patients were smokers in stroke group and in control group were 78 (33.19%), which is significantly higher in stroke patients (P = .008). There were 172 (73.19%) hypertension in stroke patients group and 138 (58.72%) in the control group, the hypertension of stroke patients group were significantly higher (P = .023).
Table 1.
Baseline characteristics of patients and matched controls.
| Characteristics | Controls (n = 235) | Stroke cases (n = 235) | t/χ2 | P |
| Age, years | 57.16 ± 13.38 | 58.43 ± 10.75 | 1.134 | .260 |
| Body mass index, kg/m2 | 23.97 ± 3.14 | 24.25 ± 3.63 | 0.894 | .372 |
| Education, years, n (%) | 1.591 | .451 | ||
| < 6 | 71 (30.21) | 82 (34.89) | ||
| 6–12 | 118 (50.21) | 115 (48.94) | ||
| >12 | 46 (19.57) | 38 (16.17) | ||
| Income, yuan/month/person, n (%) | 6.476 | .039 | ||
| ≤3000 | 40 (17.02) | 62 (26.38) | ||
| 3001–6000 | 87 (37.02) | 83 (35.32) | ||
| >6000 | 108 (45.96) | 90 (38.30) | ||
| Smoking status, n (%) | 7.002 | .008 | ||
| Yes | 78 (33.19) | 106 (45.11) | ||
| No | 157 (66.81) | 129 (54.89) | ||
| Hypertension, n (%) | 5.198 | .023 | ||
| Yes | 138 (58.72) | 172 (73.19) | ||
| No | 97 (41.28) | 63 (26.81) | ||
| Diabetes mellitus, n (%) | 1.308 | .253 | ||
| Yes | 82 (34.89) | 94 (40.00) | ||
| No | 153 (65.11) | 141 (60.00) | ||
| NIHSS score | - | 5.24 ± 2.48 | – | – |
| Vaspin, ng/ml | 1.07 ± 0.55 | 1.50 ± 0.79 | 6.85 | <.001 |
| Apelin, ng/ml | 1.32 ± 0.58 | 1.56 ± 0.76 | 3.85 | <.001 |
| Visfatin, pg/ml | 19.65 ± 8.34 | 23.40 ± 8.89 | 4.75 | <.001 |
Continuous variables were described by means ± SD and categorical variables were described by n (%). NIHSS = National Institutes of Health Stroke Scale.
Mean serum vaspin levels were found to be 1.50 ± 0.79 ng/ml in stroke group compared with 1.07 ± 0.55 ng/ml in control group. Mean serum apelin levels were found to be 1.56 ± 0.76 ng/ml in stroke group compared with 1.32 ± 0.58 ng/ml in control group. Mean serum visfatin levels were found to be 23.40 ± 8.89 pg/ml in stroke group compared with 19.65 ± 8.34 pg/ml in control group. The stroke patients had significantly higher serum vaspin, apelin and visfatin than control (all P values <.001) (Fig. 1).
Figure 1.
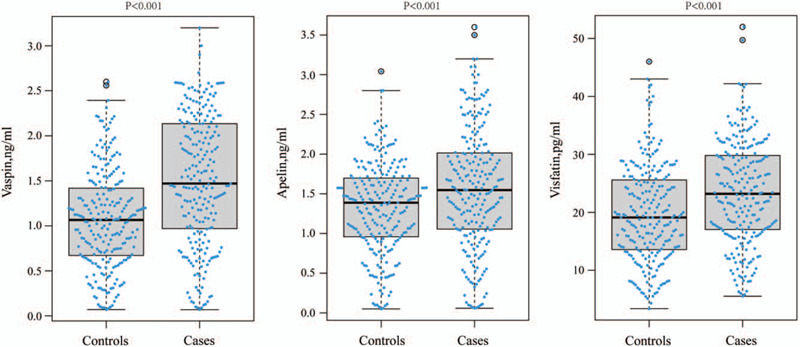
Distribution of serum vaspin, apelin, and visfatin levels among stroke cases and controls.
3.2. Associations between the serum vaspin, apelin, and visfatin levels and stroke risk
As shown in Table 2, the levels of vaspin, apelin, and visfatin were then grouped according to tertiles. Conditional logistic regression analysis revealed that there was a positive association between serum vaspin and stroke (P for trend <.001). Compared with the lowest tertile of serum vaspin levels, the risk of stroke was significantly increased in the higher 2 tertiles (tertile 2: OR = 1.67, 95% CI: 1.02–2.72; tertile 3: OR = 2.25, 95% CI: 1.38–3.67). As serum apelin levels decreased, stroke risk increased, but this difference was marginally significant (tertile2: OR = 1.553, 95% CI: 0.949–2.539; tertile 3: OR = 1.618, 95% CI: 0.997–2.625; P for trend =.060). There was a significant positive correlation between serum visfatin levels and stroke (P for trend <.001). With the lowest tertile of serum visfatin level distribution, in the higher 2 tertiles, the risk of stroke was significantly increased (tertile2: OR = 1.978, 95% CI: 1.107–3.533; tertile 3: OR = 2.557, 95% CI: 1.463–4.471).
Table 2.
Odds ratio (95% CIs) of stroke risk for tertiles of serum vaspin, apelin, and visfatin.
| Tertiles of serum vaspin, apelin, and visfatin levels | |||
| T1 | T2 | T3 | |
| Vaspin | |||
| Quartiles, ng/ml | <1.28 | 1.28–2.01 | ≥2.01 |
| N (case/control) | 42/78 | 74/79 | 119/78 |
| OR1 (95% CI)a | 1.00 | 1.67 (1.02–2.72) | 2.25 (1.38–3.67) |
| OR2 (95% CI)b | 1.00 | 1.62 (1.03–2.54) | 2.16 (1.43–3.26) |
| P | <0.001 | ||
| Apelin | |||
| Quartiles, pg/ml | <1.23 | 1.23–1.97 | ≥1.97 |
| N (case/control) | 55/78 | 88/79 | 92/78 |
| OR1 (95% CI)a | 1.00 | 1.597 (0.961,2.653) | 1.637 (0.995,2.693) |
| OR2 (95% CI)b | 1.00 | 1.553 (0.949, 2.539) | 1.618 (0.997, 2.625) |
| P | 0.060 | ||
| Visfatin | |||
| Quartiles, ng/ml | <15.81 | 15.81–23.96 | ≥23.96 |
| N (case/control) | 39/78 | 84/79 | 112/78 |
| OR1 (95% CI)a | 1.00 | 1.978 (1.107,3.533) | 2.557 (1.463,4.471) |
| OR2 (95% CI)b | 1.00 | 1.835 (1.009, 3.336) | 2.469 (1.396, 4.368) |
| P | <0.001 | ||
BMI = education, income, OR1 = adjusted for age, OR2 = adjusted for age, smoking status, and hypertension and diabetes mellitus.
Adjustment for age.
Adjustment for age, BMI, education, income, smoking status, hypertension, and diabetes mellitus.
Spearman correlation analysis indicated that the NIHSS scores for cases were significantly correlated with levels of vaspin (rs = 0.219, P = .001), apelin (rs = 0.198, P = .002), and visfatin (rs = 0.152, P = .021), respectively.
3.3. Serum vaspin, apelin, and visfatin levels between stroke cases and controls by subtype
Among 235 stroke patients, there were 156 ischemic stroke patients and 79 hemorrhagic stroke patients. We further compared the serum vaspin, apelin, and visfatin levels in ischemic stroke and hemorrhagic stroke. Compared to the corresponding control group, regardless of ischemic stroke patients or hemorrhagic stroke patients, the serum levels of vaspin, apelin, and visfatin were all markedly elevated. And the serum levels of vaspin, apelin, and visfatin in ischemic stroke patients were higher than in hemorrhagic stroke patients (Table 3).
Table 3.
Serum vaspin, apelin and visfatin levels between stroke cases and controls by subtype.
| Ischemic Strokes | Hemorrhagic Strokes | |||||||
| Stroke cases (n = 156) | Controls (n = 156) | t | P | Stroke cases (n = 79) | Controls (n = 79) | t | P | |
| Vaspin, ng/ml | 1.59 ± 0.78 | 1.06 ± 0.54 | 8.564 | <.001 | 1.32 ± 0.79 | 1.09 ± 0.57 | 3.62 | <.001 |
| Apelin, pg/ml | 1.61 ± 0.74 | 1.32 ± 0.51 | 4.947 | <.001 | 1.45 ± 0.79 | 1.31 ± 0.69 | 2.046 | .042 |
| Visfatin, ng/ml | 24.03 ± 8.69 | 19.62 ± 8.15 | 5.674 | <.001 | 22.12 ± 9.21 | 19.72 ± 8.75 | 2.896 | .004 |
4. Discussion
In this case–control study, vaspin, apelin, and visfatin were significantly higher in the stroke patients compared to control. Higher levels of vaspin, apelin, and visfatin were associated with increased stroke risk. Stratified analysis by stroke subtype shows similar findings.
Stroke has been ranked as the first leading cause of mortality and long-term disability in world and China. As the aging of society and the drastic changes in lifestyle, cerebrovascular disease has become the main disease that endangers the middle-aged and elderly population in China.[1,2,21] According to epidemiological statistics, about 2 million new strokes occur each year in the country, about 1.5 million people die of cerebrovascular disease each year, and about 6 to 7 million disabled patients, bringing long-term and multifaceted aspects burden to patients, families, and society.[21,22] At present, there is no ideal curable treatment for cerebrovascular disease. Therefore, it is of great significance to find its risk factors and actively intervene to reduce the incidence. The present study aimed to explore the serum level vaspin, apelin, and visfatin as predictive biomarkers of stroke for early detection and prevention.
Vaspin is a novel adipokine that was first found by Japanese scholar.[23] Vaspin is considered to be a member of serine protease family.[24] There are studies suggesting that there is a relationship between the serum levels of vaspin and stroke. Aust et al[25] found that the serum vaspin concentration of patients with ischemic cerebrovascular events was lower than that of the normal control group, and further believed that the occurrence of ischemic events was related to the decrease of vaspin serum concentration; and Kadoglou et al[12] also found that the serum vaspin level were lower in stroke patients with carotid atherosclerosis than control group. However, there were inconsistent results. Cura et al[11] found that serum vaspin levels in patients with acute stroke were higher than those in the normal control group. In our study, serum vaspin levels were lower in stroke patients than control group. The reason for this contradiction may be the difference in the selected participants in these studies. In Aust and Kadoglou studies, the serum levels of vaspin were lower in the stroke patients with carotid atherosclerosis. However, in our study, we did not specifically classify the diseases and symptoms associated with stroke.
Apelin is a bioactive peptide that was originally identified as the endogenous ligand of the orphan G-protein-coupled receptor. Apelin is widely expressed in the heart, brain, limbs, kidneys, retina, lungs, skin, and adipose tissue.[26,27] Apelin participate in many physiological activities, such as the regulation of blood pressure, the regulation of myocardial contractility, immunity, glucose metabolism, water balance, cell proliferation, and angiogenesis.[26,28,29] Apelin signaling pathway is closely related to stroke, especially to regulate a variety of physiological, and pathological processes of ischemic stroke. It promotes the generation of neonatal collateral vessels after ischemic stroke,[30] inhibits neurons and nerve cell damage,[31] increase the stability of atherosclerotic plaques[32] and regulate post-stroke inflammatory response,[17] and play a neuroprotective role. In this study, we found the serum apelin levels were higher in stroke patients than control group, but the serum apelin levels did not add any diagnostic value of stroke. Recent studies had shown the relationship of the serum levels of apelin and stroke. Differently from our findings, a recently reported study did not find any differences in apelin plasma levels between stroke patients and health controls,[12] while the author's another report had found the inverse association of apelin serum levels with recent stroke (within 15 days).[7] They conducted further analysis and found that apelin levels were lower in the stroke patients with carotid atherosclerosis compared to stroke-patients without carotid atherosclerosis.[12] The differences in apelin serum levels may come from the adopted study subjects and the period of the occurrence of stroke for measuring serum levels. The study found that as different from the time of the occurrence of ischemic stroke, apelin expression also changed, apelin expression in the ischemic phase of ischemic stroke was increased, while the expression of Apelin during reperfusion is decreased.[33]
Visfatin is a recently described adipokine highly produced in and secreted by adipose tissue and also produced in animal and human brain,[34] is also known as nicotinamide phosphoribosylttansferase. Visfatin is expressed in multiple organs, tissues, and cells of the human, such as bone marrow, liver, skeletal muscle cells, kidneys, heart, lungs and immune-active lymphocytes, monocytes, neutrophils.[35] Visfatin is involved in vital cellular functions such as transcription, translation, cell signaling, and fundamental energy metabolism.[35,36] Role of visfatin in stroke has been studied and reported by several researches. The increased plasma visfatin levels in acute ischemic stroke had been proved.[12,37] Visfatin may be an independent risk factor for ischemic stroke.[19] In our study, we found the similar results that the serum visfatin levels were higher in stroke patients compared to control group. And the increased visfatin levels were especially higher in ischemic group than in the hemorrhagic group. This observation is consistent with a recently report.[38]
There are some limitations in our research. First, selection bias is difficult to rule out in hospital-based case-control studies. In this study, selection bias was minimized by recruitment of both cases and control groups from a similar target population. Second, the study was a 2-center Cohort and the sample size was relatively small. These characteristics limit the external validity of the findings. Third, serum samples were collected after the cases were diagnosed. The low concentrations may have been a consequence of the stroke. Forth, though we have tried our best to adjust a wide range of potential confounders in our analysis, some residual confounding factors may still remain. For example, factors such as physical activity, parity, family history of obesity were suggested to be associated with obesity,[39] which may also affect serum adipokine levels, but these factors were not adjusted in our analysis. Finally, the assessment of serum adipokines was based on a single measurement, without taking into account the reliability of measures, which may have caused some misclassification. In addition, we did not have serum levels of adipokines tracking and detection, it is unclear the vaspin, apelin and visfatin persistently changed in response to the stroke.
In conclusion, our data suggested that vaspin, apelin, and visfatin levels might be associated with increased stroke risk. However, the use of serum vaspin, apelin, and visfatin as indicators for stroke needs to be confirmed by further large-scale prospective studies.
Author contributions
Data curation: Dalin Yu.
Investigation: Bin Wu.
Methodology: Dalin Yu, Bin Wu.
Project administration: Bin Huang.
Software: Dalin Yu.
Validation: Bin Wu.
Writing – original draft: Dalin Yu.
Writing – review & editing: Dalin Yu, Bin Huang, Jun Xiao.
Footnotes
Abbreviations: CI = confidence interval, NIHSS = National Institutes of Health Stroke Scale, ORs = odds ratios.
How to cite this article: Yu D, Huang B, Wu B, Xiao J. Association of serum vaspin, apelin, and visfatin levels and stroke risk in a Chinese case-control study. Medicine. 2021;100:12(e25184).
The authors have no funding and conflicts of interests to disclose.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Alonso DLM, Gutierrez-Fernandez M, Romano M, et al. Strategies to improve recovery in acute ischemic stroke patients: Iberoamerican Stroke Group Consensus. Int J Stroke 2014;9:503–13. [DOI] [PubMed] [Google Scholar]
- [2].Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res 2017;120:439–48. [DOI] [PubMed] [Google Scholar]
- [3].Rashad NM, Ahmed HS, Ashour W, et al. Association of vaspin gene expression and its serum level on the risk of ischemic stroke in type 2 diabetic Egyptian patients: prospective case-control study. Biotechnol Appl Biochem 2019;67:912–9. [DOI] [PubMed] [Google Scholar]
- [4].Slomka A, Switonska M, Sinkiewicz W, et al. Haemostatic factors do not account for worse outcomes from ischaemic stroke in patients with higher C-reactive protein concentrations. Ann Clin Biochem 2017;54:378–85. [DOI] [PubMed] [Google Scholar]
- [5].Opatrilova R, Caprnda M, Kubatka P, et al. Adipokines in neurovascular diseases. Biomed Pharmacother 2018;98:424–32. [DOI] [PubMed] [Google Scholar]
- [6].Katsiki N, Mantzoros C, Mikhailidis DP. Adiponectin, lipids and atherosclerosis. Curr Opin Lipidol 2017;28:347–54. [DOI] [PubMed] [Google Scholar]
- [7].Kadoglou NP, Sailer N, Moumtzouoglou A, et al. Adipokines: a novel link between adiposity and carotid plaque vulnerability. Eur J Clin Invest 2012;42:1278–86. [DOI] [PubMed] [Google Scholar]
- [8].Boden-Albala B, Sacco RL, Lee HS, et al. Metabolic syndrome and ischemic stroke risk: Northern Manhattan Study. Stroke 2008;39:30–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Eichelmann F, Rudovich N, Pfeiffer AF, et al. Novel adipokines: methodological utility in human obesity research. Int J Obes (Lond) 2017;41:976–81. [DOI] [PubMed] [Google Scholar]
- [10].Kloting N, Kovacs P, Kern M, et al. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011;54:1819–23. [DOI] [PubMed] [Google Scholar]
- [11].Cura HS, Ozdemir HH, Demir CF, et al. Investigation of vaspin level in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis 2014;23:453–6. [DOI] [PubMed] [Google Scholar]
- [12].Kadoglou NP, Fotiadis G, Lambadiari V, et al. Serum levels of novel adipokines in patients with acute ischemic stroke: potential contribution to diagnosis and prognosis. Peptides 2014;57:12–6. [DOI] [PubMed] [Google Scholar]
- [13].Chandrasekaran B, Dar O, McDonagh T. The role of apelin in cardiovascular function and heart failure. Eur J Heart Fail 2008;10:725–32. [DOI] [PubMed] [Google Scholar]
- [14].Lv D, Li H, Chen L. Apelin and APJ, a novel critical factor and therapeutic target for atherosclerosis. Acta Biochim Biophys Sin (Shanghai) 2013;45:527–33. [DOI] [PubMed] [Google Scholar]
- [15].Kadoglou NP, Lampropoulos S, Kapelouzou A, et al. Serum levels of apelin and ghrelin in patients with acute coronary syndromes and established coronary artery disease--KOZANI STUDY. Transl Res 2010;155:238–46. [DOI] [PubMed] [Google Scholar]
- [16].Karbek B, Bozkurt NC, Topaloglu O, et al. Relationship of vaspin and apelin levels with insulin resistance and atherosclerosis in metabolic syndrome. Minerva Endocrinol 2014;39:99–105. [PubMed] [Google Scholar]
- [17].Lago F, Dieguez C, Gomez-Reino J, et al. Adipokines as emerging mediators of immune response and inflammation. Nat Clin Pract Rheumatol 2007;3:716–24. [DOI] [PubMed] [Google Scholar]
- [18].Kadoglou NP, Sailer N, Moumtzouoglou A, et al. Visfatin (NAMPT) and ghrelin as novel markers of carotid atherosclerosis in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 2010;118:75–80. [DOI] [PubMed] [Google Scholar]
- [19].Lu LF, Yang SS, Wang CP, et al. Elevated visfatin/pre-B-cell colony-enhancing factor plasma concentration in ischemic stroke. J Stroke Cerebrovasc Dis 2009;18:354–9. [DOI] [PubMed] [Google Scholar]
- [20].Adams HP, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- [21].Liu M, Wu B, Wang WZ, et al. Stroke in China: epidemiology, prevention, and management strategies. Lancet Neurol 2007;6:456–64. [DOI] [PubMed] [Google Scholar]
- [22].Sun H, Zou X, Liu L. Epidemiological factors of stroke: a survey of the current status in china. J Stroke 2013;15:109–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hida K, Wada J, Zhang H, et al. Identification of genes specifically expressed in the accumulated visceral adipose tissue of OLETF rats. J Lipid Res 2000;41:1615–22. [PubMed] [Google Scholar]
- [24].Hida K, Wada J, Eguchi J, et al. Visceral adipose tissue-derived serine protease inhibitor: a unique insulin-sensitizing adipocytokine in obesity. Proc Natl Acad Sci USA 2005;102:10610–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Aust G, Richter O, Rohm S, et al. Vaspin serum concentrations in patients with carotid stenosis. Atherosclerosis 2009;204:262–6. [DOI] [PubMed] [Google Scholar]
- [26].Liu MQ, Chen Z, Chen LX. Endoplasmic reticulum stress: a novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol Sin 2016;37:425–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xie F, Liu W, Feng F, et al. Apelin-13 promotes cardiomyocyte hypertrophy via PI3K-Akt-ERK1/2-p70S6K and PI3K-induced autophagy. Acta Biochim Biophys Sin (Shanghai) 2015;47:969–80. [DOI] [PubMed] [Google Scholar]
- [28].He L, Xu J, Chen L, et al. Apelin/APJ signaling in hypoxia-related diseases. Clin Chim Acta 2015;451:191–8. [DOI] [PubMed] [Google Scholar]
- [29].Galanth C, Hus-Citharel A, Li B, et al. Apelin in the control of body fluid homeostasis and cardiovascular functions. Curr Pharm Des 2012;18:789–98. [DOI] [PubMed] [Google Scholar]
- [30].Zhang J, Liu Q, Fang Z, et al. Hypoxia induces the proliferation of endothelial progenitor cells via upregulation of Apelin/APLNR/MAPK signaling. Mol Med Rep 2016;13:1801–6. [DOI] [PubMed] [Google Scholar]
- [31].Zeng XJ, Yu SP, Zhang L, et al. Neuroprotective effect of the endogenous neural peptide apelin in cultured mouse cortical neurons. Exp Cell Res 2010;316:1773–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Zhou Y, Wang Y, Qiao S. Apelin: a potential marker of coronary artery stenosis and atherosclerotic plaque stability in ACS patients. Int Heart J 2014;55:204–12. [DOI] [PubMed] [Google Scholar]
- [33].Wu Y, Wang X, Zhou X, et al. Temporal expression of apelin/apelin receptor in ischemic stroke and its therapeutic potential. Front Mol Neurosci 2017;10:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Huang Q, Dai WM, Jie YQ, et al. High concentrations of visfatin in the peripheral blood of patients with acute basal ganglia hemorrhage are associated with poor outcome. Peptides 2013;39:55–8. [DOI] [PubMed] [Google Scholar]
- [35].Zhang LQ, Van Haandel L, Xiong M, et al. Metabolic and molecular insights into an essential role of nicotinamide phosphoribosyltransferase. Cell Death Dis 2017;8:e2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang P, Miao CY. NAMPT as a therapeutic target against stroke. Trends Pharmacol Sci 2015;36:891–905. [DOI] [PubMed] [Google Scholar]
- [37].Yin CG, Jiang L, Tang B, et al. Prognostic significance of plasma visfatin levels in patients with ischemic stroke. Peptides 2013;42:101–4. [DOI] [PubMed] [Google Scholar]
- [38].Ilhan N, Susam S, Canpolat O, et al. The emerging role of leptin, adiponectin and visfatin in ischemic/hemorrhagic stroke. Br J Neurosurg 2019;33:504–7. [DOI] [PubMed] [Google Scholar]
- [39].Hajian-Tilaki KO, Heidari B. Prevalence of obesity, central obesity and the associated factors in urban population aged 20-70 years, in the north of Iran: a population-based study and regression approach. Obes Rev 2007;8:3–10. [DOI] [PubMed] [Google Scholar]


