Abstract
Background:
This study aimed to evaluate the efficacy of peri-induction forced air warming to prevent inadvertent perioperative hypothermia, defined as a reduction in body temperature to <36.0°C during the perioperative period, in intraoperatively warmed patients receiving major surgery lasting >120 minutes.
Methods:
In total, 130 patients scheduled for elective surgery under general anesthesia lasting >120 minutes were divided into 2 groups: peri-induction warming (n = 65) and control (n = 65). Patients in the peri-induction warming group were warmed during the anesthetic induction period using a forced-air warmer set at 47°C, whereas patients in the control group were covered passively with a cotton blanket. All patients were warmed with a forced-air warmer during surgery. Body temperature was measured using a tympanic membrane thermometer in the pre- and postoperative periods and using a nasopharyngeal temperature probe during surgery. Patients were evaluated for shivering scale score, thermal comfort scale score, and satisfaction score in the post-anesthesia care unit.
Results:
The incidence rates of intraoperative and postoperative hypothermia were lower in the peri-induction warming group than in the control group (19.0% vs 57.1%, P < .001; 3.3% vs 16.9%, P = .013, respectively). Body temperature was higher in the peri-induction warming group (P < .001). However, intraoperative blood loss, as well as postoperative thermal comfort scale score, shivering scale score, and patient satisfaction score, were similar between groups. Post-anesthesia care unit duration was also similar between groups.
Conclusions:
Peri-induction active forced air warming is an effective, simple, and convenient method to prevent inadvertent perioperative hypothermia in intraoperatively warmed patients undergoing major surgery lasting >120 minutes.
Keywords: forced-air warming, hypothermia, perioperative period
1. Introduction
Perioperative hypothermia is defined as a reduction in body temperature to <36.0°C during the perioperative period.[1] The rate of first-hour-onset perioperative hypothermia is approximately 65%, despite recent improvements in body temperature monitoring and management protocols (eg, continuous intraoperative warming).[2] Prevention of perioperative hypothermia is important because even mild hypothermia can cause complications including cardiac morbidity, poor drug metabolism, delayed recovery from anesthesia, greater blood loss related to platelet dysfunction and coagulopathy, delayed wound recovery, and greater frequency of surgical site infections.[1]
Central body temperature decreases by 1.6°C during the first hour of anesthesia; the effect of central-peripheral temperature redistribution due to anesthetic-induced vasodilation is 81%, and 46 kcal of heat is redistributed.[3] This redistribution hypothermia can be prevented by preoperative warming (pre-warming), as shown by several studies[4]; several guidelines for prevention of perioperative hypothermia recommend at least 30 minutes of pre-warming.[5–7] However, the efficacy and optimal timing of pre-warming have become controversial. Some studies have reported that short pre-warming has sufficient pre-warming efficacy to prevent perioperative hypothermia.[8,9] In contrast, conventional 60-minute pre-warming has been reported to not affect redistribution hypothermia in outpatients undergoing surgery with continuous intraoperative warming.[10]
Furthermore, pre-warming is inconvenient for use in actual clinical practice. Many pre-warming factors must be considered, such as the pre-anesthetic waiting area, warming devices, and time. In addition, the pre-warming protocol typically has an unwarmed period during transfer to the operating room, induction of anesthesia, and preparation for surgery. There may be a reduction in pre-warming efficacy due to loss of heat during these unwarmed periods.
Peri-induction warming, in which the patient is warmed during the anesthesia induction period, reduces heat loss by reducing the unwarmed period during induction of anesthesia. It is a simple method with a reasonable cost that does not delay the induction of anesthesia or surgery.
Few studies have investigated the efficacy of peri-induction warming to prevent perioperative hypothermia.[11–13] Therefore, this study evaluated the efficacy of peri-induction warming for prevention of perioperative hypothermia, defined as a reduction in body temperature to <36.0°C during the perioperative period, during a major surgery lasting >120 minutes.
2. Methods
This prospective randomized controlled trial was approved by our hospital's institutional Ethics Committee (SCHUH 2018–12–008-001) and was registered before patient enrollment in the clinical trials registry (Clinical Research Information Service in Korea, cris.nih.go.kr, KCT0003719). The trial was conducted from March 2019 to July 2019. All patients were given information regarding the trial, and all provided written informed consent to participate.
2.1. Study participants and inclusion/exclusion criteria
The trial included 130 patients (age ≥19 years) who had American Society of Anesthesiologists (ASA) physical status rating of I–III and who were scheduled for elective surgery under general anesthesia lasting more than 120 minutes in duration. The exclusion criteria were: body mass index (BMI) >35 kg/m2; preoperative body temperature >38°C or <36°C; regional anesthesia or combined regional-general anesthesia; and pregnancy.
2.2. Randomization and masking
The patients were divided randomly by a computer-generated number blocked table using Excel (Microsoft Excel 2016; block size 4 and 6) into a peri-induction warming group (n = 65) and a control group (n = 65) with an allocation rate of 1:1. The anesthesiologists were not blinded to the randomization information, but the study nurse who collected the preoperative and postoperative data was blinded to the randomization information.
2.3. General procedures
An 18G cannula was inserted into the forearm vein approximately 30 minutes before the induction of anesthesia in the general ward, and fluid stored at room temperature was administered. Immediately after arrival in the operating room, forced-air warming (Warm Touch 6000, Covidien, Mansfield, MA) was started using a full-body blanket under the patient's cotton blanket at 47°C in the peri-induction warming group; passive warming was performed with the patient's cotton blanket in the control group. Each patient's entire body was covered, except for an arm for the arterial line and the neck for a central venous catheter. Patients in the peri-induction warming group were warmed for the time required to induce anesthesia and attach monitoring equipment, including an arterial line and a central venous catheter as needed.
A standardized anesthesia induction protocol was followed using 2 mg/kg 1% propofol and 0.6 mg/kg rocuronium. The nasopharyngeal thermometer (ETP1040, Ewha Biomedics, Goyang, Korea) was inserted at a depth of 9 to 10 cm in the nasopharynx, immediately after induction of anesthesia.[14] Catheters were inserted into the urethra, radial artery, and internal jugular vein as needed with minimal exposure of the skin to ambient air. Anesthesia was maintained using an inhaled anesthetic (sevoflurane or desflurane) and remifentanil. After induction of anesthesia, the forced-air warmer was switched off and patients were covered with an upper or lower body blanket, depending on the surgical site; the surgical area was then prepared. Immediately after completion of surgical draping, intraoperative warming was started using the forced-air warmer. The heating temperature was adjusted to 45°C when the core body temperature was <36.5°C; it was adjusted to 40°C when the core body temperature was 36.5°C to 37.5°C, and was switched off when the core body temperature was >37.5°C.
At the end of the surgery, the patient's neuromuscular blockade was reversed using pyridostigmine and glycopyrrolate. After patient consciousness and spontaneous respiration had been restored, the nasopharyngeal thermometer and tracheal tube were removed. The patients were then transferred to the post-anesthesia care unit (PACU). If postoperative hypothermia occurred, warming was actively performed using the forced-air warmer adjusted to the patient's body temperature in the same manner as that used during intraoperative warming.
2.4. Measurements
Preoperative demographic characteristics were recorded, including age, sex, weight, height, BMI, ASA physical status classification, and surgery type. The primary endpoint was the incidence of perioperative hypothermia, defined as a reduction in body temperature to < 36.0°C, including intraoperative hypothermia and postoperative hypothermia. Each patient's tympanic temperature (Thermoscan, infrared tympanic thermometer IRT 4020; Braun, Bethlehem, PA) was measured by a masked nurse in the pre-anesthetic holding area and PACU at 10-minute intervals until 30 min after arrival in the PACU.[15,16] The right and left tympanic temperatures were measured, and the average of the measured temperatures was recorded. To measure intraoperative core temperature, the nasopharyngeal temperature was measured using a thermometer (ETP1040, Ewha Biomedics, Goyang, Korea) immediately after induction of anesthesia.[14] Insertion of the nasopharyngeal probe was regarded as time 0 minutes for intraoperative core temperature readings; subsequent readings were recorded at 15-minute intervals.
The secondary endpoints were perioperative temperature changes, patient postoperative thermal comfort scale score, postoperative shivering scale score, patient satisfaction about temperature management, and PACU duration. All patients participating in this study were trained on the thermal comfort scale (100-mm visual analog scale: 0 mm = coldest imaginable, 50 mm = pleasant, 100 mm = warmest imaginable) in the ward on the day before surgery. After arrival in the PACU and at 10-minute intervals until 30 minute after arrival in the PACU, the masked study nurse asked patients to indicate their comfort using a thermal comfort scale and shivering using a four-point shivering scale (0 = no shivering, 1 = intermittent, low intensity, 2 = moderate shivering, 3 = continuous intense shivering). Before leaving the PACU, patients were asked to rate perioperative temperature management using a five-point Likert satisfaction scale (0 = very dissatisfied, 1 = dissatisfied, 2 = neutral, 3 = satisfied, 4 = very satisfied). The length of stay in the PACU and any adverse effects from forced-air warming was also recorded.
In addition, ambient temperatures in the operating room and PACU were recorded when the patients arrived and departed; the average of the measured temperatures was used for analyses. The durations of the peri-warming period, unwarmed period (from arrival in the operating room or end of peri-warming to resumption of continuous warming), and anesthetic period were recorded. The intraoperative fluid administration volume, blood loss volume, and transfusion volume were measured and recorded by the anesthesiologist.
2.5. Statistical analyses
Our previous study revealed that the incidence of intraoperative hypothermia was 10.7% in patients who received a 10-minute pre-warming period, whereas it was 28.6% in the control group (patients warmed intraoperatively only).[17] Assuming that the incidence of intraoperative hypothermia in the present study would be reduced by a similar degree and assuming that the warming period during induction of anesthesia was approximately 10 minutes, we calculated that 58 patients per group were required with an α = 0.05 and power of 80%. Considering an estimated drop-out rate of 10%, we planned for 65 patients per group.
Statistical analyses were performed using IBM SPSS Statistics, version 26.0 (IBM Corp., Armonk, NY). The 2 groups were compared using Student t test or the Mann–Whitney U test for continuous data, after normality had been verified using the Kolmogorov–Smirnov test. Categorical data were compared with the χ2 analysis or Fisher exact test.
A standardized difference >0.1 is conventionally considered to indicate a statistical imbalance between groups.[18] Variables representing a standardized difference >0.1 are considered confounding factors for adjustment. We used univariable logistic regression to analyze the odds ratio (OR) of perioperative hypothermia according to the group assignment and adjusted for possible confounding factors in multivariable logistic regression.
Perioperative body temperatures were plotted and evaluated using a mixed-effects model with a first-order autoregressive covariance structure. The fixed effects in the mixed-effects model included the group, time, and interaction between the group and time; patients were included as a random effect. Pairwise group comparisons were tested post hoc using Bonferroni method if the results of the mixed-effects model were statistically significant.
All continuous data are presented as means ± standard deviations or medians (1Q, 3Q); categorical data are presented as frequencies (percentages). P values <.05 were considered statistically significant. A clinically significant temperature difference in hypothermic patients between intervention and control groups was defined as 0.2°C, in accordance with the guidelines of the National Institute for Health and Clinical Excellence in the UK.[5]
3. Results
In total, 134 patients were screened. Four patients were excluded because they did not meet the inclusion criteria; thus, 130 patients were ultimately enrolled in the study and divided randomly into the peri-induction warming group (n = 65) and control group (n = 65). In the peri-induction warming group, 2 patients whose intraoperative warming was discontinued were withdrawn from the study (one was due to an anesthesiologist error and the other was due to loss of power to a machine). In the control group, one patient who was not continuously warmed intraoperatively because of an anesthesiologist error and another who showed a high body temperature in the pre-anesthetic holding area (38.1°C) were withdrawn from the study. Data from 126 patients were available for analysis: 63 patients in the peri-induction warming group and 63 patients in the control group. Of these, 2 patients assigned to the peri-induction warming group and four patients assigned to the control group transferred to the intensive care unit; thus, postoperative data from these 6 patients could not be obtained. Furthermore, 2 patients (one in the control group and the other in peri-induction warming group) showed postoperative delirium in the PACU; therefore, data subjectively obtained from these 2 patients (thermal comfort scale score and satisfaction score) could not be assessed. The remaining data were analyzed in this study (Fig. 1).
Figure 1.
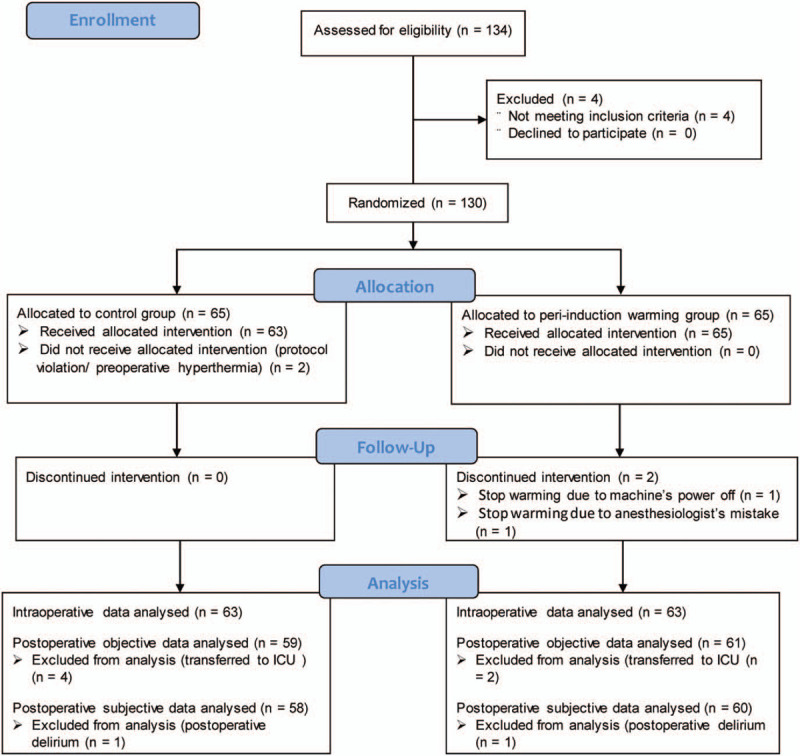
CONSORT diagram.
The baseline characteristics of the patients (age, sex, weight, height, BMI, and ASA physical status classification) are shown in Table 1. The following surgical characteristics are also shown in Table 1: surgery type, anesthesia duration, initial body temperature upon arrival in the pre-anesthetic holding area, operating room ambient temperature, PACU ambient temperature, and fluid administration volume. Among these characteristics, the standard differences for some factors (age, weight, BMI, initial body temperature, and operating room ambient temperature) were larger than the chosen threshold of 0.10, which was determined to require adjustment. Other variables did not differ between groups (Table 1).
Table 1.
Baseline characteristics and the peri-induction warming variables.
| Peri-induction warming group | Control group | |||
| Baseline variables | (n = 63) | (n = 63) | Standardized difference | P |
| Age, y | 58.2 ± 14.5 | 60.0 ± 14.5 | 0.124 | .488 |
| Sex (M/F) | 23/40 | 25/38 | 0.065 | .855 |
| Weight (kg) | 63.9 (54.4, 74.0) | 60.0 (52.3, 69.3) | 0.104 | .283 |
| Height, cm | 162 (156, 166) | 160 (155, 169) | 0.033 | .758 |
| BMI, kg/m2 | 24.7 ± 3.4 | 24.1 ± 4.1 | 0.181 | .310 |
| ASA classification (1/2/3) | 22/31/10 | 23/30/10 | 0.035 | 1.000 |
| Surgery type | 0.000 | 1.000 | ||
| Laparoscopic abdominopelvic surgery | 39 | 39 | ||
| Open abdominopelvic surgery | 8 | 8 | ||
| Head and neck surgery | 12 | 12 | ||
| Orthopedic surgery | 4 | 4 | ||
| Duration of anesthesia, min | 270 (200, 360) | 275 (182, 368) | 0.087 | .738 |
| Initial body temperature (°C) | 37.1 ± 0.3 | 37.0 ± 0.3 | 0.332 | .065 |
| OR ambient temperature (°C) | 22.4 ± 1.1 | 22.3 ± 1.1 | 0.151 | .398 |
| PACU ambient temperature (°C) | 26.3 (25.5, 26.6) | 26.2 (25.6, 26.6) | 0.095 | .850 |
| Fluid administration | 1000 (500, 1550) | 1000 (500, 1600) | 0.011 | .986 |
| Peri-induction warming variables | ||||
| Whole preperation duration, min | 39.5 ± 12.6 | 42.3 ± 14.6 | 0.209 | .243 |
| Periwarming duration, min | 20.2 ± 8.7 | 0 | 3.264 | <.001 |
| Duration of unwarmed period, min | 17 (11, 25) | 40 (34, 52) | 1.863 | <.001 |
Values are presented as numbers (%) for categorical data. Values are presented as means ± standard deviations or medians (1Q, 3Q), as appropriate, for continuous data.
ASA = American Society of Anesthesiologists, BMI = body mass index, OR = operating room, PACU = post-anesthesia care unit.
The whole preparation duration (time from arrival in the operating room to the start of active intraoperative warming) did not differ between groups (39.5 ± 12.59 vs 42.3 ± 14.55 minutes, P = .243). The peri-warming duration in the peri-induction warming group was 20.2 ± 8.7 minutes; the duration of unwarming (time from arrival in the operating room or end of peri-warming to resumption of continuous warming) was significantly reduced in the peri-induction warming group (17 vs 40 minutes, P < .001) (Table 1).
The incidence of intraoperative hypothermia was significantly lower in the peri-induction warming group than in the control group (19.0% vs 57.1%; mean difference [95% confidence interval, CI]: 0.381 [0.2159–0.5261], P < .001), and the effect of peri-induction warming was moderate (phi = −0.392). The incidence of first-hour-onset hypothermia was also significantly lower in the peri-induction warming group than in the control group (14.3% vs 42.9%; mean difference [95% CI]: 0.286 [0.1310–0.4306], P < .001), and the effect was moderate (Cramer V = 0.392). The severity (very mild: 35.5°C–35.9°C, mild: 35.0°C–35.4°C, moderate: <35.0°C) of intraoperative hypothermia was also significantly different between groups (P < .001), and the effect size was relatively strong (Cramer V = 0.401) (Table 2).
Table 2.
Intraoperative and postoperative outcome variables.
| Outcome variables | Peri-induction warming group | Control group | Differences (control-periwarming) (95% CI) | Effect size | P |
| Intraoperative variables | (n = 63) | (n = 63) | |||
| Intraoperative hypothermia | 12 (19.0%) | 36 (57.1%) | 0.381 (0.2159–0.5261) | −0.392∗ | <.001 |
| Onset of hypothermia | 0.392∗∗ | <.001 | |||
| Normothermia | 51 (81.0%) | 27 (42.9%) | −0.381 (−0.5261 to −0.2159) | ||
| Within 1 h | 9 (14.3%) | 27 (42.9%) | 0.286 (0.1310 to 0.4306) | ||
| After 1 h | 3 (4.8%) | 9 (14.3%) | 0.095 (−0.0087 to 0.2097) | ||
| Hypothermia severity | 0.407∗∗ | <.001 | |||
| Normothermia | 51 (81.0%) | 27 (42.9%) | −0.381 (−0.5261 to −0.2159) | ||
| Very mild (35.5°C –35.9°C) | 10 (15.9%) | 23 (36.5%) | 0.206 (0.0535 to 0.3533) | ||
| Mild (35.0°C –35.4°C) | 2 (3.2%) | 10 (15.9%) | 0.127 (0.0277 to 0.2417) | ||
| Moderate (<35.0°C) | 0 (0.0%) | 3 (4.8%) | 0.048 (−0.1314 to 0.0117) | ||
| Last intraoperative core temperature | 36.94 ± 0.66 | 36.58 ± 0.70 | 0.36 (0.12 to 0.60) | 0.5227∗∗∗ | .004 |
| Blood loss, mL | 150 (50, 400) | 200 (100, 300) | .907 | ||
| Transfusion | 1 (1.6%) | 3 (4.8%) | .619 | ||
| PACU objective variables | (n = 61) | (n = 59) | |||
| PACU hypothermia | 2 (3.3%) | 10 (16.9%) | 0.136 (0.0329 to 0.2573) | −0.228∗ | .013 |
| Shivering grade (0/1/2) | 56/2/3 | 54/4/1 | 0.118∗∗ | .538 | |
| LOS at PACU, min | 40 (35, 50) | 39 (35, 49) | .744 | ||
| PACU subjective variables | (n = 60) | (n = 58) | |||
| Highest TCS | 50 (50, 50) | 50 (50, 50) | .797 | ||
| Lowest TCS | 50 (50, 50) | 50 (50, 50) | .294 | ||
| Patient satisfaction (4/3/2) | 34/22/4 | 33/20/5 | 0.040∗∗ | .960 |
Values are presented as numbers (%) for categorical data. Values are presented as means ± standard deviations or medians (1Q, 3Q), as appropriate, for continuous data. Effect sizes of χ2 test are ∗phi or ∗∗Cramer V, as appropriate; effect size of continuous data is ∗∗∗Cohen d.
LOS = Length of stay, PACU = post-anesthesia care unit, TCS = Thermal comfort scale.
The last intraoperative nasopharyngeal temperature was higher in the peri-induction warming group than in the control group (36.94°C ± 0.66°C vs 36.58°C ± 0.70°C; mean difference [95% CI]: 0.36 [0.12–0.60]; P = .004), and the effect size was medium (Cohen d = 0.523). The incidence of immediate postoperative hypothermia in the PACU was lower in the peri-induction warming group than in the control group (3.3% vs. 16.9%; mean difference [95% CI]: 0.136 [0.0329–0.2573], P = .013) and the effect was moderate (phi = −.228) (Table 2).
Confounding factors were adjusted for primary outcomes. Based on univariable and multivariable logistic regression analyses, the odds of intraoperative hypothermia in the peri-induction warming group did not change appreciably after adjustments for age, BMI, operating room ambient temperature, and initial body temperature (crude odds ratio [OR] [95% CI]: 0.176 [0.079–0.394]; P < .001; adjusted OR [95% CI]: .170 [0.068–0.426]; P < .001). Univariable and multivariable logistic regression analyses showed that the odds of PACU hypothermia (immediate postoperative hypothermia) in the peri-induction warming group also did not change appreciably after adjustments for age, BMI, operating room ambient temperature, and initial body temperature (crude OR [95% CI]: 0.163 [0.034–0.781]; P = .023; adjusted OR [95% CI]: 0.185 [0.037–0.931]; P = .041) (Table 3).
Table 3.
Odds ratios of perioperative hypothermia.
| Outcome variables | Crude OR with 95% CI | P | Adjusted OR∗ with 95% CI | P |
| Intraoperative hypothermia | 0.176 (0.079–0.394) | <.001 | 0.170 (0.068–0.426) | <.001 |
| PACU hypothermia | 0.163 (0.034–0.781) | .023 | 0.185 (0.037–0.931) | .041 |
Adjustment for confounding factors, including age, BMI, operating room ambient temperature, and initial body temperature.
BMI = body mass index, CI = confidence interval, OR = odds ratio, PACU = post-anesthesia care unit.
Blood loss and transfusion requirement during the intraoperative period did not differ between groups (P = .907 and P = .619, respectively). Postoperative shivering scale score (P = .538) and the highest and lowest thermal comfort scale scores (P = .797 and P = .294, respectively) in the PACU did not significantly differ between groups. Furthermore, the patients’ satisfaction score for the warming protocol did not differ between groups (P = .960); the length of PACU stay also did not differ significantly between groups (P = .744) (Table 2). In all patients, there were no adverse effects from forced-air warming, such as skin irritation or burns.
The body temperature change between groups over time differed significantly (P < .001) (Table 4, Fig. 2). The differences between groups from induction of anesthesia to the end of PACU recovery were statistically significant (post hoc Bonferroni comparison, all P < .05) and were >0.2°C, which was regarded as a significant clinical difference in patients with hypothermia (Table 4, Fig. 2).[5] In addition, the significant body temperature reduction, compared with the preoperative initial body temperature, lasted up to 210 minutes after anesthesia had been induced in the peri-induction warming group, and 360 minutes after anesthesia had been induced in the control group (Table 4, Fig. 2).
Table 4.
Mean (SE) changes in perioperative core temperature (°C) at 30-minute intervals post-induction.
| Peri-induction warming group | Control group | Mean difference between groups∗∗ | ||||
| Time, min | Change in core temperature∗ | n | Change in core temperature∗ | N | (95% CI) | P ∗∗ |
| 0 | −0.45 (0.02)∗ | 63 | −0.59 (0.02)∗ | 63 | 0.246 (0.065–0.427) | .008 |
| 30 | −0.61 (0.03)∗ | 63 | −0.89 (0.03)∗ | 63 | 0.390 (0.210–0.571) | <.001 |
| 60 | −0.59 (0.04)∗ | 63 | −0.96 (0.04)∗ | 63 | 0.473 (0.292–0.654) | <.001 |
| 90 | −0.51 (0.04)∗ | 63 | −0.88 (0.04)∗ | 63 | 0.478 (0.297–0.658) | <.001 |
| 120 | −0.44 (0.05)∗ | 60 | −0.75 (0.05)∗ | 60 | 0.415 (0.234–0.586) | <.001 |
| 150 | −0.38 (0.05)∗ | 53 | −0.64 (0.05)∗ | 56 | 0.374 (0.192–0.556) | <.001 |
| 180 | −0.32 (0.06)∗ | 50 | −0.58 (0.06)∗ | 45 | 0.361 (0.177–0.546) | <.001 |
| 210 | −0.25 (0.06)∗ | 44 | −0.50 (0.06)∗ | 39 | 0.357 (0.169–0.545) | <.001 |
| 240 | −0.2 (0.06) | 41 | −0.46 (0.06)∗ | 36 | 0.368 (0.176–0.559) | <.001 |
| 270 | −0.16 (0.07) | 26 | −0.43 (0.07)∗ | 29 | 0.373 (0.176–0.570) | <.001 |
| 300 | −0.19 (0.07) | 20 | −0.40 (0.07)∗ | 20 | 0.318 (0.114–0.523) | .002 |
| 330 | −0.17 (0.08) | 16 | −0.38 (0.08)∗ | 19 | 0.317 (0.110–0.523) | .003 |
| 360 | −0.17 (0.08) | 13 | −0.36 (0.08)∗ | 14 | 0.303 (0.106–0.499) | .003 |
| PACU arrival | 0.02 (0.07) | 63 | −0.24 (0.07) | 62 | 0.364 (0.183–0.544) | <.001 |
| PACU 10 min | −0.02 (0.07) | 61 | −0.24 (0.07) | 59 | 0.329 (0.148–0.510) | <.001 |
| PACU 20 min | −0.04 (0.07) | 61 | −0.24 (0.07) | 59 | 0.308 (0.126–0.489) | .001 |
| PACU 30 min | −0.01 (0.07) | 60 | −0.21 (0.07) | 59 | 0.304 (0.123–0.486) | .001 |
P < .05 in group × time comparison compared with preoperative time using mixed-effects model with post hoc Bonferroni test.
P value in group × time comparison compared with peri-induction warming group – control group using mixed-effects model with post hoc Bonferroni test.
CI = confidence interval, PACU = post-anesthesia care unit, SE = Standard error.
Figure 2.
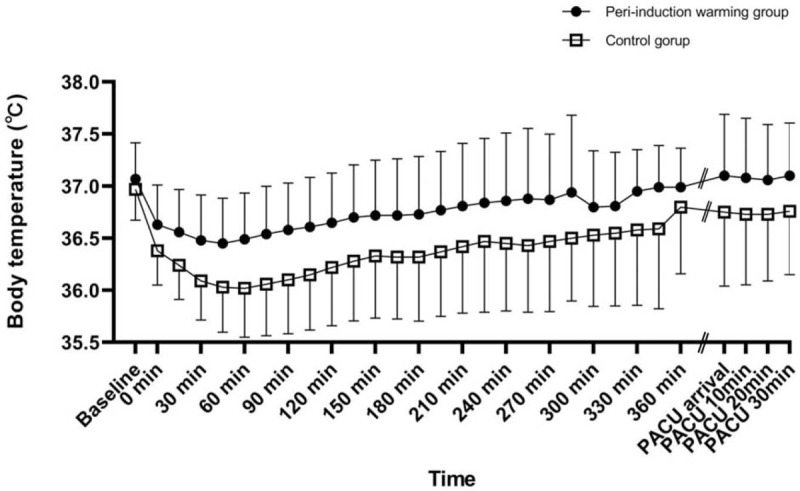
Perioperative body temperature. Error bars indicate ± 1 standard deviation of temperature at each time. Preoperative and postoperative core temperatures of patients were measured using tympanic membrane thermometer. Intraoperative core temperature was recorded at 15-minute intervals via nasopharyngeal probe after induction of anesthesia. Temperature was higher in peri-warming group from induction of anesthesia to end of recovery in PACU. Baseline: immediately after arrival in pre-anesthetic holding area; intraoperative 0 min: immediately after insertion of nasopharyngeal probe; PACU arrival: immediately after arrival at PACU; PACU 10, 20, and 30 minutes: 10, 20, and 30 minutes after arrival at PACU. PACU = post-anesthesia care unit.
4. Discussion
This study demonstrated that peri-induction warming at 47°C reduced the incidence and severity of inadvertent perioperative hypothermia in intraoperatively warmed patients undergoing major surgery lasting more than 120 minutes. The core-to-peripheral redistribution of body heat in patients receiving peri-induction warming reduced core temperature by approximately 0.6°C during the first hour after induction; the temperature difference between groups during the first hour after induction was approximately 0.47°C, which is consistent with the findings of previous studies regarding conventional pre-warming.[9,10,19,20] These significant temperature differences between groups continued until the recovery period and led to a reduction in the rate of postoperative hypothermia.
Akhtar et al[10] reported no difference between pre-warmed and control groups despite 60 minutes of pre-warming due to effective passive insulation of a warming suit used in their control group, as well as an improved clinical environment.[10] The study by Akhtar et al[10] involved short and simple outpatient surgery, whereas our study yielded different results for patients undergoing major surgery lasting >120 minutes with a longer unwarmed period. The unwarmed duration in our control group was 40 minutes, compared with previous studies in which it was 20 minutes.[9,20] Therefore, although a cotton blanket was used for passive insulation and covered the whole body in our control group, greater redistribution of body heat occurred, compared with previous studies.[9,10,20]
Our study showed similar results to pre-warming for >30 minutes, despite the average warming time of 20 minutes.[10] One factor that may have contributed to the effect of peri-induction warming was reduction of the unwarmed period. This approach may also have enhanced heat transfer efficiency by rapidly transferring heat because of the anesthesia-induced reductions in thermoregulation and peripheral vasodilation; conventional pre-warming did not transfer much heat above the threshold because the thermoregulatory system (eg, thermal discomfort and sweating) was strongly maintained.
Our high warming temperature (47°C) to increase heat transfer could have been another factor associated with efficacy, although the warming time was short. Previous studies have shown that the blanket-to-surface temperature gradient and resulting heat flow exhibits a clear linear relationship.[21,22] An earlier study by our group reported that a 47°C pre-warming period could be effectively used without eliciting significant thermal discomfort.[17,23] In the present study, it was impossible to ask about thermal discomfort because warming was performed during the patient lost consciousness; however, there were no problems caused by warming, such as skin changes or sweating, during the peri-induction warming period.
Two studies have reported the efficacy of peri-induction warming to prevent hypothermia in patients undergoing cardiac surgery.[12,13] They showed that peri-induction warming reduced intraoperative hypothermia without delaying the induction of anesthesia or the timing of surgery. However, the warming times were longer in these previous studies than in our study (49.7 ± 9.9 and 35 ± 6 vs 20.2 ± 8.7 minutes) because of additional monitoring, such as the pulmonary artery catheterization required for cardiac surgery. Our study findings are applicable to patients undergoing various major surgeries with a shorter induction time than cardiac surgery. Shenoy et al[11] reported that pre-warming for 60 minutes before induction of anesthesia does not confer any added advantage for patients undergoing intraoperative continuous warming and peri-induction warming, which was regarded as “co-warming” by those authors. Based on their results and our present results, we suggest that peri-induction warming alone sufficiently reduces the heat redistribution that can be obtained during pre-warming.
The peri-induction warming protocol used in this study has some advantages that make it more promising and useful than conventional pre-warming in terms of convenience. Peri-induction warming has no limitations in terms of place, time, or device. No additional time or place is required because it is performed during anesthesia induction in the operating room; additional devices are not required because the device prepared for intraoperative warming can be used. In addition, peri-induction warming does not delay the induction of anesthesia or the timing of surgery.[12,13] However, in this study, reduced perioperative complications and enhanced patient satisfaction due to the low incidence of hypothermia were not confirmed because of small power of this study to prove it. The lack of a difference in intraoperative blood loss and the transfusion requirement may have been partially influenced by changes in clinical treatment (eg, minimally invasive surgery) over time. Continuous full-body warming after surgery in the PACU may have contributed to the absence of differences in postoperative shivering scale, thermal discomfort scale, and patient satisfaction scores.
This study had several limitations. First, the results may not apply to surgeries lasting less than 120 minutes, which have shorter induction durations than our population; the present study was conducted on patients undergoing major surgery for more than 120 minutes, with an average induction time of approximately 40 minutes. Second, the pre- and postoperative temperatures differed from the intraoperative nasopharyngeal core temperature because the tympanic membrane temperature during the pre- and postoperative periods was used to reduce patient discomfort. However, the tympanic temperature is the most accurate and precise among other peripheral temperature measurements, so bias was presumably minimized.[15,16] Third, the peri-induction warming method used in this study focused on the process performed during the induction of anesthesia; thus, the duration of warming was not fixed. However, the warming duration in this study was more similar to a clinical situation and lasted approximately 20 minutes; this was effective and more likely to reflect real-world practices. Fourth, heat content and peripheral temperature were estimated because the values were not measured directly. However, given that core temperature is the primary variable that determines perioperative hypothermia and affects hypothermic outcome, it may have been sufficient. Fifth, the association with perioperative complications is unknown, because the power was small to prove it and no long-term follow-up was performed after patients exited the PACU. Further randomized controlled trials with larger sample sizes, including an evaluation of long-term complications from perioperative mild hypothermia, will be required in the future.
In conclusion, peri-induction active forced air warming was an effective, simple, and convenient method to prevent inadvertent perioperative hypothermia during intraoperative warming of patients receiving major surgery lasting >120 minutes.
Author contributions
Conceptualization: Jae Hwa Yoo, Si Young Ok.
Data analysis and interpretation: Jae Hwa Yoo, Hong Chul Oh, Sang Ho Kim, Ji Won Chung, Sun Young Park
Data curation: Ji Won Chung, Sun Young Park, Mun Gyu Kim, Hong Chul Oh.
Formal analysis: Jae Hwa Yoo, Sang Ho Kim, Ji Won Chung, Sun Young Park, Hong Chul Oh.
Investigation: Mun Gyu Kim, Ho Bum Cho, Sang Hoon Song, Chae Yeon Cho.
Methodology: Jae Hwa Yoo, Hong Chul Oh.
Patient recruitment and data collection: Mun Gyu Kim, Ho Bum Cho, Sang Hoon Song, Chae Yeon Cho
Writing – original draft: Jae Hwa Yoo.
Writing – review & editing: Jae Hwa Yoo, Si Young Ok, Hong Chul Oh.
Footnotes
Abbreviations: ASA = American Society of Anesthesiologists, BMI = Body mass index, CI = confidence interval, OR = Odds ratio, PACU = post-anesthesia care unit.
How to cite this article: Yoo JH, Ok SY, Kim SH, Chung JW, Park SY, Kim MG, Cho HB, Song SH, Cho CY, Oh HC. Efficacy of active forced air warming during induction of anesthesia to prevent inadvertent perioperative hypothermia in intraoperative warming patients: Comparison with passive warming, a randomized controlled trial. Medicine. 2021;100:12(e25235).
The authors report no conflicts of interests.
The data used in this study are available by request to the corresponding author. Due to protection of sensitive patient data, the data used are not publicly available.
Funding sources: This work was supported by the Soonchunhyang University Research Fund.
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Sessler DI. Perioperative thermoregulation and heat balance. Lancet 2016;387:2655–64. [DOI] [PubMed] [Google Scholar]
- [2].Sun Z, Honar H, Sessler DI, et al. Intraoperative core temperature patterns, transfusion requirement, and hospital duration in patients warmed with forced air. Anesthesiology 2015;122:276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Matsukawa T, Sessler DI, Sessler AM, et al. Heat flow and distribution during induction of general anesthesia. Anesthesiology 1995;82:662–73. [DOI] [PubMed] [Google Scholar]
- [4].Connelly L, Cramer E, DeMott Q, et al. The optimal time and method for surgical prewarming: a comprehensive review of the literature. J Perianesth Nurs 2017;32:199–209. [DOI] [PubMed] [Google Scholar]
- [5].Health NIf, Excellence C. Hypothermia: prevention and management in adults having surgery. NICE clinical guideline [CG65])(Published 2016 Accessed July 28, 2019) https://www nice org uk/guidance/cg65/chapter/Recommendations View in Article. 2008. [Google Scholar]
- [6].Forbes SS, Eskicioglu C, Nathens AB, et al. Evidence-based guidelines for prevention of perioperative hypothermia. J Am Coll Surg 2009;209:492–503.e491. [DOI] [PubMed] [Google Scholar]
- [7].Horn EP, Klar E, Hocker J, et al. [Prevention of perioperative hypothermia: Implementation of the S3 guideline]. Chirurg 2017;88:422–8. [DOI] [PubMed] [Google Scholar]
- [8].Brauer A, Waeschle RM, Heise D, et al. [Preoperative prewarming as a routine measure. First experiences]. Anaesthesist 2010;59:842–50. [DOI] [PubMed] [Google Scholar]
- [9].Horn EP, Bein B, Bohm R, et al. The effect of short time periods of pre-operative warming in the prevention of peri-operative hypothermia. Anaesthesia 2012;67:612–7. [DOI] [PubMed] [Google Scholar]
- [10].Akhtar Z, Hesler BD, Fiffick AN, et al. A randomized trial of prewarming on patient satisfaction and thermal comfort in outpatient surgery. J Clin Anesth 2016;33:376–85. [DOI] [PubMed] [Google Scholar]
- [11].Shenoy L, Krishna HM, Kalyan N, et al. A prospective comparative study between prewarming and cowarming to prevent intraoperative hypothermia. J Anaesthesiol Clin Pharmacol 2019;35:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Cho YJ, Lee SY, Kim TK, et al. Effect of prewarming during induction of anesthesia on microvascular reactivity in patients undergoing off-pump coronary artery bypass surgery: a randomized clinical trial. PLoS One 2016;11:e0159772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Kim JY, Shinn H, Oh YJ, et al. The effect of skin surface warming during anesthesia preparation on preventing redistribution hypothermia in the early operative period of off-pump coronary artery bypass surgery. Eur J Cardiothorac Surg 2006;29:343–7. [DOI] [PubMed] [Google Scholar]
- [14].Lee J, Lim H, Son KG, et al. Optimal nasopharyngeal temperature probe placement. Anesth Analg 2014;119:875–9. [DOI] [PubMed] [Google Scholar]
- [15].Gasim GI, Musa IR, Abdien MT, et al. Accuracy of tympanic temperature measurement using an infrared tympanic membrane thermometer. BMC Res Notes 2013;6:194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Asadian S, Khatony A, Moradi G, et al. Accuracy and precision of four common peripheral temperature measurement methods in intensive care patients. Med Devices (Auckl) 2016;9:301–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yoo JH, Ok SY, Kim SH, et al. Effects of 10-minutes of pre-warming on inadvertent perioperative hypothermia in intraoperative warming patients: a randomized controlled trial. Anesth Pain Med 2020;15:356–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Schulte PJ, Mascha EJ. Propensity score methods: theory and practice for anesthesia research. Anesth Analg 2018;127:1074–84. [DOI] [PubMed] [Google Scholar]
- [19].Just B, Trevien V, Delva E, et al. Prevention of intraoperative hypothermia by preoperative skin-surface warming. Anesthesiology 1993;79:214–8. [DOI] [PubMed] [Google Scholar]
- [20].Perl T, Peichl LH, Reyntjens K, et al. Efficacy of a novel prewarming system in the prevention of perioperative hypothermia. A prospective, randomized, multicenter study. Minerva Anestesiol 2014;80:436–43. [PubMed] [Google Scholar]
- [21].Brauer A, English MJ, Lorenz N, et al. Comparison of forced-air warming systems with lower body blankets using a copper manikin of the human body. Acta Anaesthesiol Scand 2003;47:58–64. [DOI] [PubMed] [Google Scholar]
- [22].Brauer A, English MJ, Steinmetz N, et al. Comparison of forced-air warming systems with upper body blankets using a copper manikin of the human body. Acta Anaesthesiol Scand 2002;46:965–72. [DOI] [PubMed] [Google Scholar]
- [23].Yoo JH, Ok SY, Kim SH, et al. The effect of 10 minutes of prewarming for prevention of inadvertent perioperative hypothermia: comparison with 30 minutes of prewarming. Anesth Pain Med 2018;13:447–53. [Google Scholar]


