Abstract
The aim was to examine whether clinical seizures and amplitude-integrated electroencephalogram (aEEG) patterns in infants with hypoxic ischemic encephalopathy (HIE) can predict the extent of brain injury on magnetic resonance images (MRI) and the long-term neurodevelopmental outcomes at 18∼24 months of age.
HIE infants who underwent therapeutic hypothermia (TH) between June 2014 and March 2017 were included in this study. Infants with clinical seizure were analyzed for aEEG patterns and the extent of brain injury on MRI findings. Clinical seizure, aEEG, and brain MRI were assessed and compared with neurodevelopmental outcomes at 18∼24 months of age.
Among the 97 HIE infants enrolled in this study with brain MRI scans, 78 (73.1%) TH-treated HIE infants exhibited clinical seizures. More abnormalities on a EEGs and more significant use of first and second antiepileptic drugs (AEDs) were significantly higher in the clinical-seizure group with longer hospitalized days. At a corrected 18 to 24 months of age, HIE infants in the clinical-seizure group with more extension of injury lesions on diffusion-weighted MRI scans exhibited significantly more delayed neurodevelopment. A risk factor analysis indicated that male infants who stayed in the hospital for more than 11 days were at a higher risk of having clinical seizures. The lesion size in MRI greater than 37 pixels was a risk factor with an 81.8% accuracy.
Seizures in HIE infants may predict abnormal brain MRI scans and abnormal neurodevelopment at 18 to 24 months of age.
Keywords: clinical risk factor, delayed development, diffusion-weighted MRI, hypoxia-ischemia, outcome, seizure, therapeutic hypothermia
1. Introduction
Hypoxic ischemic encephalopathy (HIE) after perinatal asphyxia affects 1 to 8 per 1000 live births annually and is an important cause of morbidity and mortality in newborns.[1,2] The majority of HIE newborns experience seizures, which occur at admission in approximately 1 to 3.5/1000 live term births.[3,4] Estimated prevalence of neonatal seizures range from 0.5% in full-term newborns to 22.2% in preterm newborns,[5] which are mainly due to early brain injuries such as hypoxic-ischemic injury involving perinatal risk factors, as well as ischemic and hemorrhagic strokes, which account for more than 70% of neonatal seizures.[6] Neonates with seizures are associated with death and disability, including cognitive impairment and post-neonatal epilepsy.[7] Therefore, seizures caused by HIE may be a marker of the severity of underlying injuries and may further harm the developing human brain. Early identification and prompt treatment of seizures are important to prevent ongoing injury to the brain. However, clinical detection of neonatal seizures is very challenging, because bedside observation of infants in a hospital setting is not continuous. Also, neonatal seizures are often very subtle, and sudden changes in the heart rate or blood pressure in neonatal intensive care unit (NICU) settings might not be uniquely caused by a seizure itself, but as a result of normal physiological responses as paroxysmal autonomic movements. To assess neonatal seizures more efficiently and accurately in our NICU, amplitude-integrated electroencephalography (aEEGs) is performed on high-risk infants to generate a simplified display on a bedside monitor. Typically, several hours of aEEG monitoring are conducted until a neonatologist or pediatric neurologist decides that no more seizure movements or abnormal amplitudes are found. Clinical seizures are confirmed when paroxysmal autonomic changes, such as a sudden change in the heart rate or blood pressure, occur in addition to the involvement of clinical features, such as apnea, eye deviation, focal tonic stiffening or movement, or pupillary dilatation. In our study, clinical seizures in addition to aEEG patterns were analyzed in HIE infants treated with therapeutic hypothermia (TH). The association with brain MRI findings and neurodevelopmental outcomes at 18 to 24 months of age were also studied after discharge. Our aim of this study was to observe whether seizures could serve as a predictive risk factor for the extent of injury on MR images. The relationship between seizures and neurodevelopmental outcomes at 18 to 24 months was also assessed.
2. Materials and methods
2.1. Data extraction
We included hypoxic encephalopathic term or late preterm infants (≥35 weeks of gestation with birth weights of ≥ 2000 g) who were treated with TH between June 2014 and March 2017 at Seoul St. Mary's Hospital, Catholic University of Korea (Seoul, Korea). Those data were retrospectively analyzed in this study.
2.2. Selection criteria
All infants experienced acute perinatal events were assessed for signs of HIE. When infants met greater than moderate HIE criteria, they were recruited for TH treatment within 6 hours of birth. Enrolled infants fulfilled one of the 2 parameters of (1) arterial or venous gas pH of ≤7.0 or a base deficit of >16 mmol/L within an hour of NICU admission or (2) an Apgar score of 5 at 10 minutes or continued respiratory support at 10 minutes as previously described in the CoolCap, NICHD, European Trial, and TOBY trials[8–11]: Cerebral function monitoring (CFM) (CFM, Natus Medical, Seattle, WA) or video EEG was initiated as early as possible to detect any possible electrographic seizures. The evidence of moderate or severe encephalopathy was distinguished using Sarnat clinical stages.[12] Seizures were clinically diagnosed by an experienced neonatologist or pediatric neurologist as a paroxysmal alteration in motor function.[9] Infants proceeded to whole-body cooling (Blanketrol III Hyper-Hypothermia System, Cincinnati Sub-Zero, Cincinnati, OH) with the core esophageal temperature maintained at 33.5°C for 72 hours). Brain MRI with MR diffusion was performed in all TH-treated infants (within at least 10 days of life) and radiologists were blinded to treatment and outcomes of the infants and reviewed the brain MR images. This was due to MR diffusion changes disappearing after the first week of life. The study was approved by the Ethics Committee of Seoul St. Mary's Hospital, The Catholic University of Seoul, Korea. No funding was received in this study.
2.3. Exclusion criteria
We excluded HIE infants who were with major congenital abnormalities, syndromes, or metabolic diseases were also excluded. Infants with a birth weight of ≤2000 g, a gestational age of ≤35 weeks of gestational age, overt bleeding, signs of infection, or those requiring ≥80% oxygen support were also excluded from this study.
2.4. Classification of aEEGs
EEG recordings continued for at least the first several hours or until a neonatologist or pediatric neurologist decided that no more seizure movements were being detected. The mild abnormal is when absence or decreased frequency of normal patterns with overall slightly decreased voltage is found. The moderately abnormal EEG is when asymmetries in voltage or frequencies were found. The severely abnormal is isoelectric or low-voltage activity with a burst-suppression pattern.
2.5. Classification of brain MRI images
MR images were reviewed by experienced radiologists who were blinded to the treatment and outcomes of the infants. The MRI was classified according to the NICHD pattern for brain injury: a score of 0 for normal MRI; 1A for minimal cerebral lesions only; 1B for more-extensive cerebral lesions without basal ganglia and thalamus (BGT), posterior limb of the internal capsule (PLIC), or anterior limb of the internal capsule (ALIC) involvement; 2A same as stage 1B with no cerebral lesions; 2B same as stage 2A with additional cerebral lesions; and 3 for cerebral hemispheric devastation.[9]
2.6. Neurodevelopmental assessments
The infants were followed up with the same neurologist and neonatologist at the outpatient clinics after discharge. At 18 to 24 months, TH infants returned for follow-up evaluations, completed the cognitive, language, and motor composites of the Bayley Scales of Infant and Toddler Development III, and were evaluated by certified examiners. Children were considered as having developmental delay or abnormal development if scores were < 85. If the score was ≥ 85, children were considered to have a normal developmental stage. Written informed parental consent was waived, as this study was a retrospective chart review, which was approved by the Ethics Committee of Seoul St. Mary's Hospital, The Catholic University of Seoul (Seoul, Korea). In addition, all methods were carried out in accordance with relevant guidelines and regulations of Ethics Committee of Seoul St. Mary's Hospital.
2.7. Imaging analysis
The MRIs were performed after a mild sedation with midazolam or fentanyl, which usually did not require intubation. Any marked lesions with injury on brain MR images and the size of the markings were analyzed and associated with long-term neurodevelopment. Radiologists were all blinded to clinical outcomes and reviewed the images for quality and acquired lesions. Additional statistical analyses were performed using more-granular and detailed information about abnormal features obtained directly from the MR images with programming tools. Lesion sizes in the brain were characterized by continuous values calculated programmatically by the number of pixels. Each lesion was separately counted from adjacent ones if there was spatial separation from one another without touching. The total number of lesions in each size group was counted. Normal scans had zero lesion counts for all groups. All calculations were performed with Python 3.5 using the scipy and numpy libraries to determine the presence of lesions in the image, and their counts and sizes. As a result, a table with total counts per size range and per patient was generated as input to determine correlations between abnormalities on brain MR images and neurodevelopmental abnormalities at 18 to 24 months of age.
2.8. Statistical analysis
All statistical analyses were 2-tailed, with statistical significance defined as a value of P < .05. All statistical analyses were performed with SPSS, vers. 15.0 (Statistical Package for the Social Sciences, SPSS-PC, Chicago, IL). Continuous variables are expressed as the mean ± standard deviation (SD) and were compared using Student t test or the Satterthwaite approximation when the variances were heterogeneous. Continuous variables are displayed as the median with the interquantile range (IQR) when the variables were not normally distributed and were compared with the Wilcoxon rank-sum test.
A decision tree methodology was adopted as a machine learning algorithm for establishing risk classification systems based upon on number of covariates for developing prediction algorithms for the target variable of clinical seizures. A tree represents a segmentation of the data that is created by applying a series of simple rules. These rule-based models generate predictions through an iterative process of splitting and pruning. CART (Classification and Regression Tree) is the most commonly used approach for decision tree algorithms. The generalized Gini index was incorporated as a loss function in CART.
All brain lesions that ware present on abnormal scans ware characterized by their volume size in pixels and total counts for each MR image. All lesion feature calculations were performed using Python 3.5 programming language. The absence of abnormalities was characterized by a total volume size of 0.
3. Results
Between 2014 and 2017, the study recruited 97 HIE infants with brain MR images. Among the 97 patients, 78 (73.1%) TH-treated infants in this study manifested clinical seizures. For the MR image assessment with programming tools, patients with diffusion-weighted MRI scans were analyzed for the number of lesions. Descriptive clinical characteristics of TH-treated infants with outcomes of clinical seizures are presented in Table 1. More abnormalities on an aEEG and a higher classification of an abnormal aEEG were significantly more often found in the clinical-seizure group. More use of first and second AED were significantly associated with the clinical-seizure group. The mean length of AED treatment for phenobarbital was 17 days, Keppra for 14 days, and phenytoin for 19 days.
Table 1.
Clinical characteristics of the seizure group in therapeutic hypothermia (TH)-treated HIE newborns (n = 97).
| No clinical seizure (n = 19) | Clinical seizure (n = 78) | P | |
| Gestational age, wks | 40 (39.07∼40.14) | 39.64 (38.29∼40.14) | .512 |
| Birth weight, g | 3260 (3155∼3560) | 3175 (2942.5∼3437.5) | .100 |
| Maternal age, yr | 32.42 (2.67) | 32.5 (4.75) | .945 |
| Apgar score at 1 min | 1 (15 (78.9)) | 53 (67.9) | .429 |
| Apgar score at 5 min | 2 (3 (15.8)) | 20 (25.6) | .430 |
| Outborn, n (%) | 3 (15.8) | 23 (29.5) | .358 |
| Male, n (%) | 4 (21.1) | 36 (46.2) | .083 |
| MAS, n (%) | 2 (10.5) | 6 (7.7) | 1.000 |
| SGA, n (%) | 1 (5.3) | 7 (9) | .950 |
| Fetal heart deceleration | 14 (73.7) | 61 (78.2) | .907 |
| Emergent csearean section | 14 (73.7) | 69 (88.5) | .201 |
| Initial serum pH < 7.0 | 19 (100) | 78 (100) | 1.000 |
| Initial BE | 8.37 (4.81) | 6.95 (4.58) | .231 |
| LDH (initial) | 1234.53 (392) | 1232.94 (525.7) | .990 |
| CPK (initial) | 1122.16 (819.82) | 903.38 (838.3) | .309 |
| Abnormal aEEG | .008 | ||
| Mild abnormal | 1 (5.3) | 6 (7.7) | |
| Moderate abnormal | 6 (31.6) | 50 (64.1) | |
| Severe abnormal | 0 (0) | 4 (5.1) | |
| AED, Phenobarbital | 12 (63.2) | 77 (98.7) | <.001 |
| AED, keppra | 0 (0) | 37 (47.4) | <.001 |
| AED, phenytoin | 0 (0) | 10 (12.8) | .220 |
| Ventilator care, d | 2.63 (2.14) | 3.53 (2.93) | .212 |
| Full feeding days | 6.79 (1.62) | 8.04 (3.4) | .123 |
| Hospital days | 10.89 (2) | 13.26 (4.44) | .026 |
| PPHN (%) | 2 (10.5) | 4 (5.1) | .730 |
| AEP refer | 3 (15.8) | 9 (11.7) | .628 |
| Expired = 0 (%) | 19 (100) | 78 (100) | 1.000 |
AED = antiepileptic drug, aEEG = amplitude-integrated electroencephalogram, AEP = auditory evoked potential, BE = base excess, CPK = creatine phosphokinase, HIE = hypoxic ischemic encephalopathy, LDH = lactate dehydrogenase, MAS = meconium aspiration syndrome, MRI = magnetic resonance imaging, NSD = normal spontaneous delivery, PPHN = persistent pulmonary hypertension, SGA = small for gestational age.
Furthermore, the number of hospitalized days was significantly longer in the clinical-seizure group (Table 1).
The abnormal aEEG group was significantly more associated with clinical seizures and more first AED use. The emergent call was significantly more found in the abnormal MRI group experiencing seizure (P = .004). Patients with more injury lesions on diffusion-weighted MRI scans were significantly related to the seizure group. Similarly, the abnormal aEEG group was also significantly related to the delayed-neurodevelopmental group assessed at a corrected 18 to 24 months of age. At 18 to 24 months, infants were evaluated by certified examiners for neurodevelopmental (ND) delays using the Bayley Scales of Infant and Toddler Development III; 22 (50%) infants with both abnormal aEEG and MRI findings exhibited significant abnormal ND stages. Three infants (14.3%) with abnormal aEEG, but normal brain MRI group showed 14.3% of abnormal ND stages at 18 to 24 months. Despite normal aEEG, 5 (25%) infants with abnormal MRI manifested abnormal ND stages. According to NICHD MRI scoring, more lesions involved in the basal ganglia and thalamus, or posterior limb of internal capsule as grouped in stages 2A and 2B were significantly associated with abnormal ND outcomes at 18 to 24 months of age (P < .001). The most severe stage for cerebral hemispheric devastation as stage 3 was not found in both groups in this study. The lesion size of brain injury was also significantly larger in pixel numbers in the abnormal aEEG group (310.77 vs 141.5, P = .007), which meant more injurious lesions on MR images were present in the aEEG group (Table 2). The receiver operating characteristic (ROC) curve for seizures using the lesion size had a specificity of 63.1%, and it was 62.7% for lesion size (Fig. 1).
Table 2.
Clinical characteristics of the abnormal amplitude-integrated electroencephalogram (aEEG) group (n = 97).
| Normal EEG (n = 32) | Abnormal EEG (n = 65) | |||||
| Normal MRI (n = 12) | Abnormal MRI (n = 20) | P value | Normal MRI (n = 21) | Abnormal MRI (n = 44) | P | |
| Gestational age, wks | 39.29 (38.89∼40.21) | 39.86 (38.43∼40.14) | 0.769 | 40.14 (39.14–40.57 | 39.36 (38.43∼40.00) | .200 |
| Birth weight, kg | 3.32 (0.28) | 3.2 (0.34) | 0.35 | 3.29 (0.37) | 3.16 (0.44) | .271 |
| Maternal age, yr | 31.58 (2.64) | 32.1 (4.97) | 0.743 | 32.29 (4.03) | 32.55 (4.72) | .829 |
| Emergent call | 10 (83.3) | 14 (73.7) | 0.165 | 12 (57.1) | 30 (68.2) | .040 |
| Apgar score at 1 min | 4.42 (2.27) | 4.85 (2.06) | 0.583 | 5.05 (2.94) | 4.73 (2.18) | .632 |
| Apgar score at 5 min | 6.75 (1.73) | 6.6 (1.82) | 0.879 | 7.24 (2.07) | 6.52 (2.13) | .328 |
| Outborn | 3 (25) | 5 (25) | 1.000 | 8 (38.1) | 12 (27.3) | .918 |
| Male, n (%) | 3 (25) | 7 (35) | 0.844 | 12 (57.1) | 19 (43.2) | .783 |
| MAS, n (%) | 1 (8.3) | 3 (15) | 1 | 2 (9.5) | 1 (2.3) | .530 |
| SGA, n (%) | 0 (0) | 2 (10) | 0.706 | 2 (9.5) | 4 (9.1) | 1.000 |
| Fetal heart deceleration | 8 (66.7) | 17 (85) | 0.44 | 14 (66.7) | 38 (86.4) | .087 |
| Initial BE | 8.11 (2.84) | 6.34 (4.01) | 0.198 | 8.21 (5.64) | 6.52 (4.42) | .207 |
| LDH (initial) | 1345.92 (532.03) | 1382.9 (542.53) | 0.948 | 1213.1 (434.6) | 1133.68 (480.83) | .721 |
| CPK | 1103.08 (646.17) | 1086.3 (1108.76) | 0.962 | 855.75 (796.94) | 874.93 (644.4) | .390 |
| Clinical seizures | 3 (25) | 17 (85) | 0.003 | 17 (81) | 41 (93.2) | .648 |
| AED, Phenobarbital | 6 (50) | 19 (95) | 0.011 | 21 (100) | 44 (100) | 1.000 |
| AED, Keppra | 3 (25) | 86 (40) | 0.631 | 7 (33) | 21 (47.7) | .643 |
| AED, phenytoin | 1 (8.3) | 2 (10) | 1.000 | 4 (19) | 3 (6.8) | .32 |
| Ventilator care, d | 3.67 (5.21)) | 1.79 (2.02) | 0.166 | 4.19 (3.22) | 3.7 (1.73) | .431 |
| Hospital days | 11.58 (5.45) | 11.78 (2.6) | 0.896 | 13.75 (4.36) | 13.05 (4.34) | .559 |
| Death | 12 (100) | 18 (100) | 1.000 | 20 (100) | 40 (100) | 1.000 |
| AEP refer | 2 (16.7) | 1 (5.3) | 0.165 | 2 (10) | 1 (2.5) | .878 |
| Delayed development | 1 (8.3) | 5 (25) | 0.483 | 3 (14.3) | 22 (50) | .013 |
| NICHD MRI∗ | 0.011 | <.001 | ||||
| 0 | 9 (75) | 2 (10.5) | 13 (65) | 5 (11.4) | ||
| 1A | 3 (25) | 9 (47.4) | 6 (30) | 16 (36.4) | ||
| 1B | 0 (0) | 2 (10.5) | 1 (5) | 4 (9.1) | ||
| 2A | 0 (0) | 5 (26.3) | 0 (0) | 10 (22.7) | ||
| 2B | 0 (0) | 1 (5.3) | 0 (0) | 9 (20.5) | ||
AED = antiepileptic drug, aEEG = amplitude-integrated electroencephalogram, aEEG = amplitude-integrated electroencephalogram, AEP = auditory evoked potential, BE = base excess, CPK = creatine phosphokinase, CS = cesarean section, LDH = lactate dehydrogenase, MAS = meconium aspiration syndrome, PPHN = persistent pulmonary hypertension, SGA = small for gestational age.
MRI were classified according to the NICHD pattern for brain injury: score of 0 for normal MRI; 1A for minimal cerebral lesions only; 1B for more extensive cerebral lesions without basal ganglia and thalamus (BGT), or posterior limb of internal capsule (PLIC) or anterior limb of internal capsule (ALIC) involvement and no area of watershed infarction; 2A for any BGT, PLIC, or ALIC involvement or watershed infarction without any cerebral lesions; 2B for any BGT, PLIC, or ALIC involvement or watershed infarction with additional cerebral lesions; and 3 for cerebral hemispheric devastation.
Figure 1.
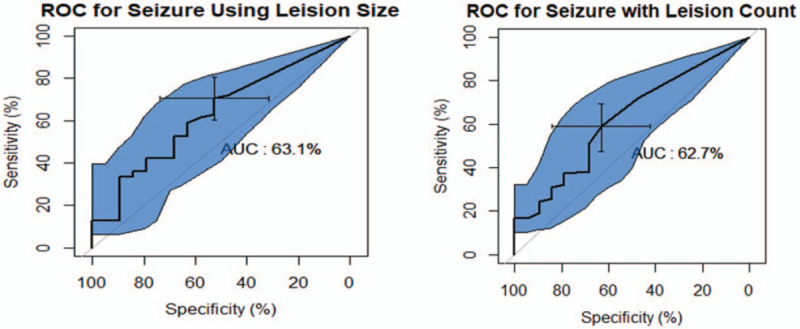
The receiver operating characteristic (ROC) for seizure lesion size versus lesion count.
3.1. Clinical risk factor prediction
The decision tree for risk factor prediction was built in several steps: first, an EEG was found which best split the data into 2 groups based on minimizing the Gini index. The data began with 78 cases with clinical seizures, which were then were separated depending on whether the EEG was normal or abnormal. This process was separately applied to each subgroup, and so on recursively until no improvement was evident. When a patient had a normal EEG, the next splitting criteria was whether the MR image was normal or abnormal. If the infant had a normal MR image, it was predicted that the infant would have no clinical seizures with a 75% (9/12) accuracy. In contrast, when the infant had an abnormal EEG and had stayed in the hospital for more than 14 days, a higher risk of being predicted to have clinical seizures with 100% (20/20) accuracy was shown. This process continued to every node, and the last node indicated that male patients who stayed in the hospital for more than 11 days were at a higher risk of having clinical seizures if the lesion size was greater than 37 pixels with 81.8% (9/11) accuracy (Fig. 2).
Figure 2.
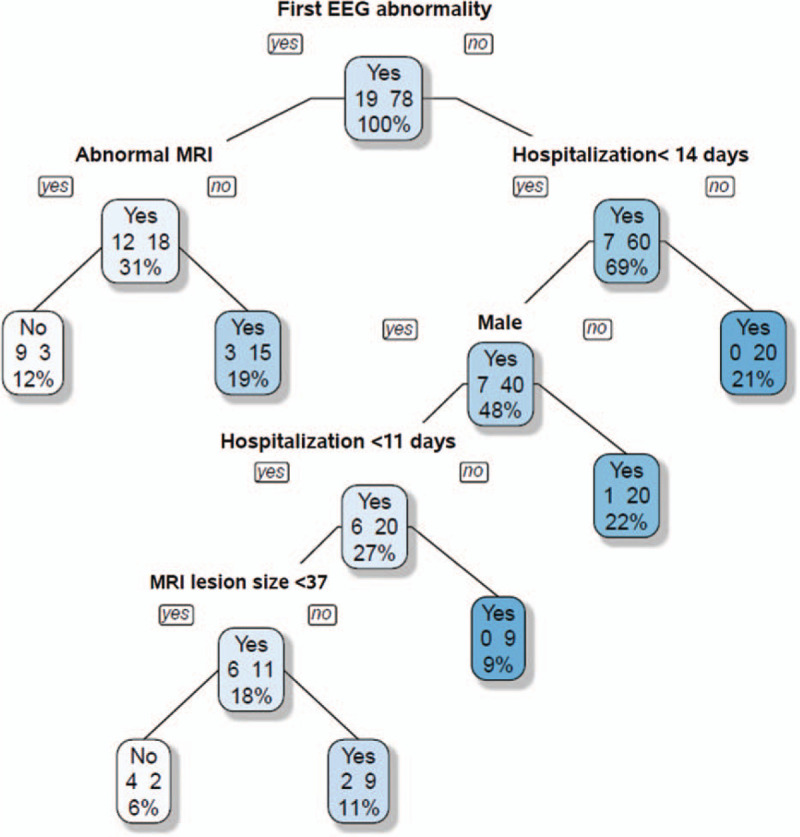
Clinical risk factor prediction. EEG = electroencephalogram, MRI = magnetic resonance imaging.
4. Discussion
In our study, we evaluated whether neonatal seizures in HIE infants treated with TH could be a prognostic clinical factor for long-term neurodevelopmental outcomes at 18 to 24months of age. In our study, the presence of clinical seizures or aEEG abnormalities together with the severity of neonatal encephalopathy on brain MR images was indicated to be powerful predictors of neurodevelopmental outcomes at 18 to 24 months of age in HIE infants. A lesser response to initial AEDs, the presence of greater injury on brain MR images, and infants with clinical seizures were significantly related to worse neurodevelopmental outcomes. Until now, reliable early predictors of morbidity and mortality due to HIE in infants were shown to be the Apgar score, an aEEG, and the Sarnat score.[13] Clinical risk factors for adverse outcomes reported low serum pH on the first day of life, an abnormal neurologic examination for >72 hours, uncontrolled seizure and deep-gray or brainstem injury on MR images.[14] Despite many studies, the prediction of neurodevelopmental outcomes still remains challenging. Experimental studies in animals showed that the immature brain such as newborns in our clinical study is more susceptible to developing seizures, although the immature brain is less susceptible to damage induced by seizures.[15,16] Because neonatal seizures can contribute to the delayed neurodevelopment in a long term, aggressive control of seizure is important. Greater use of first and second AEDs was found to be significant in the clinical-seizure group (Table1Tables 1). Infants who failed to respond to initial anticonvulsant treatment had up to a 4-fold increased risk of developing epilepsy.[17,18] This is in accordance to the animal studies that highlighted how recurrent neonatal seizures may impair neurogenesis in the dentate gyrus[19] and could lead to persistent enhancement of neocortical excitability with increased susceptibility to seizure generation.[20] Our study revealed that the severity of seizures were strongly associated with the basal ganglia/thalamus predominant pattern. This is consistent with previous observations relating deep gray nuclei injury with profound asphyxia and severe encephalopathy.[21] Additional study supports that seizures were most severe in newborns with total brain injury.[22] A correlation between the location of injury and development of infantile spasms[23] was also studied, which emphasized the importance of the location of the brain injury. Lesions in the basal ganglia and thalamus are often associated with abnormalities in neurological outcome.[24] In our previous study, an abnormal signal intensity in the basal ganglia and thalamus was also a powerful predictor of abnormal neurodevelopmental outcomes[25] at 18 to 24 months of age (Table 2). Lesions in the basal ganglia and thalamus are usually consistent with a severe acute hypoxic-ischemic insult often associated with abnormalities in the cortex and adjacent subcortical white matter and may be graded as a severe HIE.[26] Those infants ≥ moderate lesions in the basal ganglia and thalamus and severe white matter lesions are associated with cerebral palsy.[27] In our study, neurodevelopmental outcomes were favorable in the group that did not manifest clinical seizures or who had mild muscle tone and reflex abnormalities on a neurological examination (Table 1).
Until now, the best way to try to stop brain damage or additional on-going cascades of brain damage is to stop clinical seizures, in addition to initiating TH as early as possible. Many studies showed that seizures frequently occur with HIE at presentation, during or following hypothermia, which were noted in 30% to 90% of infants.[28–30]
The positive predictive value of an abnormal aEEG pattern at the age of 3 to 6 hours was useful and can be best predictor of poor outcomes.[31] Continuous aEEG recordings were also shown to be useful beyond the first 6 hours. In our study, more clinical seizures and more AED use were significantly associated with abnormal brain MR images and were significantly related to abnormal neurodevelopmental outcomes at 18 to 24 months of age. Glass et al[30] showed that a cohort of 56 newborns with moderate-to-severe HIE, who underwent TH, and had a status epilepticus, multifocal seizures, and seizures that were resistant to a single loading dose of 20 mg/kg of phenobarbital, was associated with moderate-to-severe injury on neonatal MR images. MRI assessment of the location and extent of injury patterns can be a valuable predictor of neurodevelopmental outcomes in neonatal HIE infants. For prognosis, HIE children who were treated with TH had good survival rates and prognoses. This study had some limitations. Several factors may have contributed to a potential selection bias in our review: first, this was a retrospective study design, which might be unable to fully confirm the examined relationships; second, we only had a relatively small sample size in study group; third, hidden disabilities may subsequently have become apparent, and many infants might have important developmental lags that were not classified as impairments. However, with greater involvement of the basal ganglia and thalamus and a trend toward more-abnormal scans, the prognosis was worse.[32] In the future, studies on longer-follow up data as in school-age years of HIE infants are warranted.
5. Conclusion
Higher incidences of clinical seizure and greater use of AEDs to control seizures were significantly higher in groups with abnormal brain MR images and delayed neurodevelopment. More lesions in the basal ganglia or thalamus and posterior limb of the internal capsule on MRI scans were predictive of later abnormal neurodevelopment at 18 to 24 months of age.
Acknowledgment
The authors would like to thank Taipei Medical University and Seoul St. Mary's Hospital for offering this chance to collaborate in this paper.
Author contributions
Conceptualization: YoungAh Youn.
Data curation: Seok Hwang-Bo, Yu-Mi Seo, YoungAh Youn.
Formal analysis: Yen-Kuang Lin.
Investigation: Seok Hwang-Bo, Yu-Mi Seo, YoungAh Youn.
Methodology: Yen-Kuang Lin, YoungAh Youn.
Project administration: YoungAh Youn.
Resources: Seok Hwang-Bo, Yu-Mi Seo.
Software: Yen-Kuang Lin.
Supervision: YoungAh Youn.
Validation: Yen-Kuang Lin.
Writing – original draft: YoungAh Youn.
Writing – review & editing: YoungAh Youn.
Footnotes
Abbreviations: AED = antiepileptic drugs, aEEG = amplitude electroencephalogram, BE = base excess, BG = basal ganglia, CFM = cerebral function monitoring, CP = cerebral palsy, HIE = hypoxic–ischemic encephalopathy, MRI = magnetic resonance imaging, TH = therapeutic hypothermia.
How to cite this article: Lin YK, Hwang-Bo S, Seo YM, Youn YA. Clinical seizures and unfavorable brain MRI patterns in neonates with hypoxic ischemic encephalopathy. Medicine. 2021;100:12(e25118).
We have no financial relationship with any organization. No honorarium, grant, or other form of payment was received to produce this manuscript. We do not have any sources of financial assistance or potential conflicts of interest. The study was approved by the Ethics Committee of Seoul St. Mary's Hospital.
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
- [1].Nelson KB. Neonatal encephalopathy: etiology and outcome. Dev Med Child Neurol 2005;47:292. [DOI] [PubMed] [Google Scholar]
- [2].Badawi N, Felix JF, Kurinczuk JJ, et al. Cerebral palsy following term newborn encephalopathy: a population-based study. Dev Med Child Neurol 2005;47:293–8. [DOI] [PubMed] [Google Scholar]
- [3].Glass HC, Pham TN, Danielsen B, et al. Antenatal and intrapartum risk factors for seizures in term newborns: a population-based study, California 1998-2002. J Pediatr 2009;154:24–8.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Glass HC, Wu YW. Epidemiology of neonatal seizures. J Pediatr Neurol 2009;7:13–7. [Google Scholar]
- [5].Scher MS. Neonatal seizure classification: a fetal perspective concerning childhood epilepsy. Epilepsy Res 2006;70: (Suppl 1): S41–57. [DOI] [PubMed] [Google Scholar]
- [6].Glass HC, Shellhaas RA, Wusthoff CJ, et al. Contemporary profile of seizures in neonates: a prospective cohort study. J Pediatr 2016;174:98–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Ronen GM, Buckley D, Penney S, et al. Long-term prognosis in children with neonatal seizures: a population-based study. Neurology 2007;69:1816–22. [DOI] [PubMed] [Google Scholar]
- [8].Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet 2005;365:663–70. [DOI] [PubMed] [Google Scholar]
- [9].Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med 2005;353:1574–84. [DOI] [PubMed] [Google Scholar]
- [10].Simbruner G, Mittal RA, Rohlmann F. Systemic hypothermia after neonatal encephalopathy: outcomes of neo. nEURO. network RCT. Pediatrics 2010;126:e771–778. [DOI] [PubMed] [Google Scholar]
- [11].Azzopardi DV, Strohm B, Edwards AD, et al. Moderate hypothermia to treat perinatal asphyxial encephalopathy. N Engl J Med 2009;361:1349–58. [DOI] [PubMed] [Google Scholar]
- [12].Sarnat HB, Sarnat MS. Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Arch Neurol 1976;33:696–705. [DOI] [PubMed] [Google Scholar]
- [13].Shankaran S, Barnes PD, Hintz SR, et al. Brain injury following trial of hypothermia for neonatal hypoxic-ischaemic encephalopathy. Arch Dis Child Fetal Neonatal 2012;97:F398–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Glass HC, Grinspan ZM, Shellhaas RA. Outcomes after acute symptomatic seizures in neonates. Semin Fetal Neonatal Med 2018;23:218–22. [DOI] [PubMed] [Google Scholar]
- [15].Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr 2006;18:628–33. [DOI] [PubMed] [Google Scholar]
- [16].Williams PA, Dou P, Dudek FE. Epilepsy and synaptic reorganization in a perinatal rat model of hypoxia-ischemia. Epilepsia 2004;45:1210–8. [DOI] [PubMed] [Google Scholar]
- [17].Toet MC, Groenendaal F, Osredkar D, et al. Postneonatal epilepsy following amplitude-integrated EEG-detected neonatal seizures. Pediatr Neurol 2005;32:241–7. [DOI] [PubMed] [Google Scholar]
- [18].Garfinkle J, Shevell MI. Cerebral palsy, developmental delay, and epilepsy after neonatal seizures. Pediatr Neurol 2011;44:88–96. [DOI] [PubMed] [Google Scholar]
- [19].McCabe BK, Silveira DC, Cilio MR, et al. Reduced neurogenesis after neonatal seizures. J Neurosci 2001;21:2094–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Isaeva E, Isaev D, Savrasova A, et al. Recurrent neonatal seizures result in long-term increases in neuronal network excitability in the rat neocortex. Eur J Neurosci 2010;31:1446–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Sie LT, van der Knaap MS, Oosting J, et al. MR patterns of hypoxic-ischemic brain damage after prenatal, perinatal or postnatal asphyxia. Neuropediatrics 2000;31:128–36. [DOI] [PubMed] [Google Scholar]
- [22].Steven P Miller, Ramaswamy V, Michelson D, et al. Patterns of brain injury in term neonatal encephalopathy. J Pediatr 2005;146:453–60. [DOI] [PubMed] [Google Scholar]
- [23].Gano D, Sargent MA, Miller SP, et al. MRI findings in infants with infantile spasms after neonatal hypoxic-ischemic encephalopathy. Pediatr Neurol 2013;49:401–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cowan F, Rutherford M, Groenendaal F, et al. Origin and timing of brain lesions in term infants with neonatal encephalopathy. Lancet 2003;361:736–42. [DOI] [PubMed] [Google Scholar]
- [25].Rutherford MA, Pennock JM, Counsell SJ, et al. Abnormal magnetic resonance signal in the internal capsule predicts poor neurodevelopmental outcome in infants with hypoxic-ischemic encephalopathy. Pediatrics 1998;102:323–8. [DOI] [PubMed] [Google Scholar]
- [26].Okereafor A, Allsop J, Counsell SJ, et al. Patterns of brain injury in neonates exposed to perinatal sentinel events. Pediatrics 2008;121:906–14. [DOI] [PubMed] [Google Scholar]
- [27].Barkovich AJ, Miller SP, Bartha A, et al. MR imaging, MR spectroscopy, and diffusion tensor imaging of sequential studies in neonates with encephalopathy. AJNR Am J Neuroradiol 2006;27:533–47. [PMC free article] [PubMed] [Google Scholar]
- [28].Wusthoff CJ, Dlugos DJ, Gutierrez-Colina A, et al. Electrographic seizures during therapeutic hypothermia for neonatal hypoxic-ischemic encephalopathy. J Child Neurol 2011;26:724–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Glass HC, Wusthoff CJ, Shellhaas RA, et al. Seizures and magnetic resonance imaging-detected brain injury in newborns cooled for hypoxic-ischemic encephalopathy. J Pediatr 2011;159:731–5. e731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Nash KB, Bonifacio SL, Glass HC, et al. Video-EEG monitoring in newborns with hypoxic-ischemic encephalopathy treated with hypothermia. Neurology 2011;76:556–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Thoresen M, Hellstrom-Westas L, Liu X, et al. Effect of hypothermia on amplitude-integrated electroencephalogram in infants with asphyxia. Pediatrics 2010;126:e131–139. [DOI] [PubMed] [Google Scholar]
- [32].Rutherford M, Ramenghi LA, Edwards AD, et al. Assessment of brain tissue injury after moderate hypothermia in neonates with hypoxic-ischaemic encephalopathy: a nested substudy of a randomised controlled trial. Lancet Neurol 2010;9:39–45. [DOI] [PMC free article] [PubMed] [Google Scholar]


