Abstract
Older adults are the leading users of medications, where this can be associated with a high number of potentially inappropriate medications (PIMs) and of potentially inappropriate prescribing (PIP) and consequent harm to health. No Brazilian study evaluating potentially inappropriate prescribing in older patients with Alzheimer's disease (AD) was found. This study determined and analyzed the prevalence of PIP and PIM prescribed for older people with AD.
A cross-sectional study was carried out at the Specialty Drugs Pharmacy in the city of Sorocaba, São Paulo State, Brazil. The MEDEX system provided the register in older people with AD and data were collected during interviews with patients and/or caregivers between June and September 2017. The PIMs were identified according to the 2019 Beers Criteria. The association between PIMs and independent variables was analyzed by Poisson regression.
This study included 234 older patients with AD. The prevalence of PIP prescribed was 66.7% (n = 156). Of the 1073 medications prescribed, 30.5% (n = 327) were inappropriate with most affecting the central nervous system or cardiovascular, particularly quetiapine (12.8%) and acetylsalicylic acid (11.6%), respectively. Around 45.2% of the PIMs should be avoided in older people, especially sertraline (14.2%) and clonazepam (7.4%). After adjusted analysis, the PIMs were associated with the diagnosis of depression (P = 0.010) and the number of comorbidities (P = 0.005).
There was a high number of PIMs among older people, a substantial number of which should have been avoided in this population. Health care professionals can apply these findings to improve safety in the use of medications for treating patients with AD.
Keywords: aged, Alzheimer disease, drug prescriptions, potentially inappropriate medication list
1. Introduction
Alzheimer disease (AD) is a chronic neurodegenerative disorder characterized by the gradual impairment of cognitive functions, psychiatric and neuropsychiatric symptoms, and difficulty performing activities of daily living. It is classified as early-onset when it affects persons aged <65 years and as late-onset when onset occurs after 65 years of age.[1]
This disease is associated with the deposition of amyloid plaques in the brain, in addition to neurofibrillary tangles, which cause a reduction in the size of synapses. However, other causative agents seem to be associated with protective reactions of the organism against infectious, inflammatory, or toxic challenges.[2]
Neurocognitive disorders, heart disease, and cancer have been implicated as the main factors predisposing individuals to the morbidity and mortality of AD in America, with AD representing the most common type of dementia.[3] In the United States, an estimated 5.4 million Americans had the disease in 2016, a figure set to rise to 13.8 million by the middle of the 21st century.[4]
With population ageing, the number of people living with dementia is growing rapidly. Projections from the World Health Organization, the number of people living with dementia will triple in the coming years, reaching a projection of 152 million until the year 2050. Presently, around 10 million individuals develop dementia each year, of these, 6 million come from middle and low-income countries).[5] According to data from the Global Burden of Disease Study 2016,[6] Brazil had the second highest age-standardized prevalence with 1037 cases of dementia per 100,000 population. Pooled meta-analyses with Brazilian studies, based on DSM-IV, revealed a dementia prevalence rate of 6.2%.[7]
People with AD have higher levels of comorbidities and take more medications to treat these conditions than people without the disease.[8,9] Furthermore, achieving optimal medication use for this population is a challenge for clinicians, because older adults with dementia experience a greater sensitivity to the adverse effects of medications and are often excluded from drug trials, limiting the available evidence to guide prescribing practices.[10]
The potentially inappropriate medication (PIM) is a term used to describe the use of a medicine for which the associated risks outweigh the potential benefits, especially when alternatives more effective are available.[11] Studies show that PIM use can lead to avoidable adverse drug events,[12] including falls, fractures, and delirium and is associated with hospitalization[13,14] and mortality.[15,16] Therefore, the identification of PIM can help health professionals to elaborate strategies to minimize risks in this vulnerable population.
Beers et al[17] published an important tool for the analysis of PIMs for older people, known as the Beers Criteria. The American Geriatrics Society updated these criteria in 2012,[18] 2015,[19] and 2019[20] (version used in this article). A prospective cohort study carried out in France analyzed the prescriptions of 684 older people with AD using the Beers Criteria and identified that 25.3% of patients with mild-to-moderate dementia used PIMs.[21]
In Brazil, the medications used for the treatment of AD have been funded by the public health system since 2002, where the prescribing and dispensing of these medications is carried out according to the National Clinical Protocol and Therapeutic Guidelines for AD.[22]
Although there are Brazilian studies that evaluated PIM in the elderly, none of them described the prescription profile of older people with AD, and this fact prompted this study that evaluated aspects related to the safety of drug treatment in this population. Thus, the present study determined the prevalence of potentially inappropriate prescription (PIP), the prevalence of PIMs according to drugs group and the prevalence of these drugs that should be avoided in older patients, who obtained their medications for the treatment of AD under the Brazilian public health system.
2. Methods
2.1. Study design and data availability
This cross-sectional study was based on data from the Drugs of Exceptional Distribution system (MEDEX—Medicamento de Distribuição Excepcional) and from interviews carried out with older patients with AD and/or caregivers, at the Specialty Drugs Pharmacy in the city of Sorocaba, São Paulo State, Brazil. The data of this study can be made available, if requested.
2.2. Data setting
The Specialty Drugs Pharmacy is located in the Conjunto Hospitalar de Sorocaba (Sorocaba Hospital Complex), in Sorocaba city, State of São Paulo, Brazil. This unit is one of 40 in São Paulo State and caters to 48 municipalities under the XVI Regional Health Department (DRS-XVI).
The Health Secretary of the State, through the Coordination of Science, Technology, and Strategic Health Inputs, maintains a computerized MEDEX system for use by managers in that contain information from patient's medical file used to control dispensing from Specialty Drugs Pharmacies. This system provided access to patient registration details.
All patients with an active registration at the time of data collection (ie, with monthly dispensing) were eligible for participation in the study.
2.3. Study population and sample
Initially, we analyzed 12,869 registered individuals in the MEDEX system who received treatment for AD from 48 municipalities between December 2004 and February 2017. After the selection of Sorocaba municipality, we identified 2619 registered individuals.
We selected only the records that were active in this municipality. In this way, in the period between December 2016 and April 2017, 285 patients diagnosed with AD had active registration on the MEDEX system for collecting their medications. Of this group, 237 were older people and constituted the population of this study.
The participants were selected on the interview day, since the researchers did not have access by MEDEX, to the name of patient and the medication withdrawal date. The interviews also provided data on the other medications used by the patient.
2.4. Eligibility criteria
2.4.1. Inclusion criteria
Older people (aged ≥65 years) were considered eligible if diagnosed according to International Classification of Diseases code (ICD 10): F00 (dementia in AD), F00.0 (dementia in early-onset AD), F00.1 (dementia in late-onset AD), F00.2 (dementia in AD, atypical or mixed form), F00.9 (dementia, unspecified in AD) and G30 (AD), G30.0 (early-onset AD), G30.1 (late-onset AD), G30.8 (other forms of AD), G30.9 (AD, unspecified) and previously registered on the MEDEX system for medicine dispensing. Also, if taking at least 1 drug for the treatment of AD (donepezil, galantamine, rivastigmine and/or memantine) in addition to other concomitant drugs.
The interviewed was the patient and/or their caregiver (aged ≥18 years, considered as the person responsible for daily care). When the patient and caregiver were present, both were interviewed.
2.4.2. Exclusion criteria
Patients and/or caregivers who had difficulty reporting the data requested by the interviewer or those who refused to participate.
2.5. Data collection
The information collected from the MEDEX system were: date of patient inclusion in the program, patient health unit of origin, disease diagnosis (according to the ICD-10), medication used and dispensing date.
The interviews were carried out at the time of patient visits to the pharmacy to collect their medications. Patients that met the inclusion criteria were then invited to participate in the study. All interviews took place between July and September 2017. The following variables were collected: sex, age, comorbidities, medications used, level of reported autonomy, and type of medical care. When further data were needed, another interview was scheduled, or the additional information was collected by telephone.
The medications were classified according to the Anatomical Therapeutic Chemical Code, criteria adopted by the World Health Organization, into different groups and subgroups, according to the physiological system they act upon and to their chemical, pharmacological, and therapeutic properties. Polypharmacy was defined as the use of ≥5 drugs.[23] The level of autonomy was determined by the following question: “In your opinion, from 0 (totally dependent) to 10 (totally independent), how is the level of autonomy that do you have?.”
2.6. Potentially inappropriate prescribing and potentially inappropriate medications
The 2019 Beers Criteria were used to characterize the prevalence of PIP and identify the PIMs.[19] Based on these criteria, medications are divided into lists with the following descriptions: medications that should be avoided in older adults; medications that should be avoided in older adults with specific diseases or syndromes; medications that should be used with caution; drug–drug interactions that should be avoided in older adults; medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults; medications with strong anticholinergic properties.
2.7. Outcomes
The outcomes evaluated were the prevalence of older people with at least one PIP, the prevalence of PIM according to drugs group and the prevalence of PIMs that should be avoided in older people.
2.8. Data analysis
The outcomes were expressed as absolute and relative frequency. The frequency of PIP of medications was described by the therapeutic group. The frequency of PIP that should be avoided in older people was described according to pharmacological characteristics, the rationale for non-use, quality of evidence, and strength of recommendation, according to the 2019 Beers Criteria.
The profile of older patients with AD according to the presence of PIP was analyzed by the χ2 test for heterogeneity of proportions or Fisher exact test for the categorical variables and the chi-square test of the linear trend for the ordinal variables.
To assess the association between PIP and the exposure variables, crude and adjusted analysis for confounding factors, by means of Poisson regression, were performed. We used a conceptual model of analysis by levels, being that on the 1st level were included the demographic variables, on the 2nd level the health variables, at 3rd level the type of healthcare system and polypharmacy. We have kept in the adjusted analyses, only the variables with P < .20, ensuring the control for possible confounding factors. The statistical association was assessed by Wald tests for heterogeneity and linear trend. A significance level of 5% was adopted.
3. Results
Of the 237 older people with AD, 234 met the inclusion criteria and participated in the study (evaluation of 234 prescriptions, 1 prescription per patient). Of the 1073 medications prescribed (4.6 medications/patient), 30.5% (n = 327) were PIMs (Fig. 1). The prevalence of PIP was 66.7% (n = 156) (95% confidence interval: 60.6%–72.8%).
Figure 1.
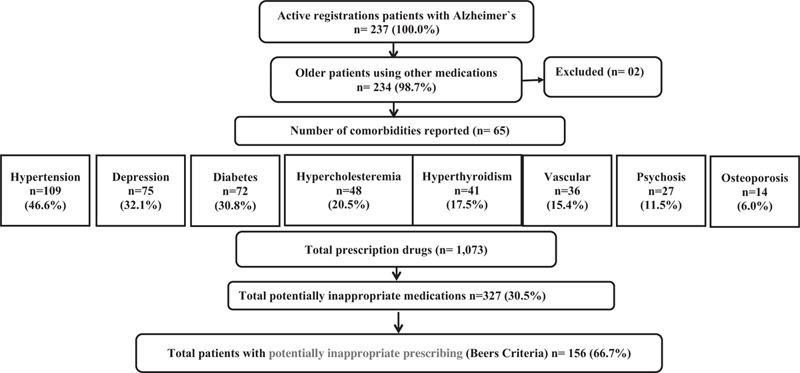
Study flow diagram.
Overall, the sample comprised predominantly subjects that were women (65.0%), aged between 75 and 84 (41.1%), in use of a single medication for the treatment of AD (81.6%), and users of both public and private healthcare systems (65.2%). The most reported diseases were hypertension (46.6%) and depression (32.1%). The most common treatment for AD was donepezil (41.6%) followed by galantamine (21.5%). Patients using PIMs have higher prevalence of diagnosis of depression (48.7% vs 1.3%; P < .001), psychosis (17.3% vs 0.0%; P < .001), and hypertension (53.8% vs 32.0%; P = 0.002), ≥4 comorbidities (32.7% vs 6.4%; P < .001), and polypharmacy (62.0% vs 24.0%; P < .001) (Table 1).
Table 1.
Profile of elderly patients with AD according to presence of potentially inappropriate prescription (n = 234).
| Variables | Total patients, N (%) | Patients with PIM, N (%) | Patients without PIM, N (%) | P |
| Total | 201 (100.0) | 155 (100.0) | 45 (100.0) | |
| Sex | .208∗ | |||
| Female | 152 (65.0) | 97 (62.2) | 55 (70.5) | |
| Male | 82 (35.0) | 59 (37.8) | 23 (29.5) | |
| Age, y | .295∗∗ | |||
| 65–74 | 55 (23.8) | 36 (23.2) | 19 (25.0) | |
| 75–84 | 95 (41.1) | 69 (44.5) | 26 (34.2) | |
| ≥85 | 81 (35.1) | 50 (32,3) | 31 (40.8) | |
| Comorbidities (n) | <.001∗∗ | |||
| 0–1 | 70 (29.9) | 22 (14.1) | 48 (61.5) | |
| 2 | 62 (26.5) | 45 (28.9) | 17 (21.8) | |
| 3 | 46 (19.7) | 38 (24.4) | 8 (10.3) | |
| 4 + | 56 (23.9) | 51 (32.7) | 5 (6.4) | |
| Hypertension | 0.002∗ | |||
| No | 125 (53.4) | 72 (46.2) | 53 (68.0) | |
| Yes | 109 (46.6) | 84 (53.8) | 25 (32.0) | |
| Depression | <.001∗∗∗ | |||
| No | 159 (68.9) | 82 (51.3) | 77 (98.7) | |
| Yes | 75 (32.1) | 74 (48.7) | 1 (1.3) | |
| Diabetes | .229∗ | |||
| No | 162 (69.2) | 104 (66.7) | 58 (74.4) | |
| Yes | 72 (30.8) | 52 (33.3) | 20 (25.6) | |
| Psychosis | <.001∗∗∗ | |||
| No | 207 (88.5) | 129 (82.7) | 78 (100.0) | |
| Yes | 27 (11.5) | 27 (17.3) | 0 (0.0) | |
| Polypharmacy | <.001∗ | |||
| No (≤4 medications) | 128 (54.7) | 60 (38.5) | 68 (87.2) | |
| Yes (≥5 medications) | 106 (45.3) | 96 (61.5) | 10 (12.8) | |
| Medications for AD | .029∗ | |||
| Donepezil | 97 (41.6) | 54 (34.6) | 43 (55.8) | |
| Galantamine | 50 (21.5) | 32 (20.5) | 18 (23.4) | |
| Rivastigmine | 33 (14.2) | 27 (17.4) | 6 (7.8) | |
| Memantine | 10 (4.3) | 8 (5.2) | 2 (2.6) | |
| Donepezil + memantine | 20 (8.6) | 16 (10.3) | 4 (5.2) | |
| Galantamine + memantine | 15 (6.4) | 12 (7.7) | 3 (3.9) | |
| Rivastigmine + memantine | 8 (3.4) | 7 (4.5) | 1 (1.3) | |
| Time since diagnosis, y | .876∗∗ | |||
| <1 | 18 (8.0) | 9 (5.8) | 7 (15.2) | |
| 1–2 | 60 (26.8) | 39 (25.2) | 11 (23.9) | |
| >2–5 | 71 (31.7) | 50 (32.2) | 12 (26.1) | |
| >5–10 | 54 (24.1) | 43 (27.7) | 15 (32.6) | |
| >10 | 21 (9.4) | 6 (3.9) | 1 (2.2) | |
| Not stated | 10 (4.0) | 8 (5.2) | 0 (0) | |
| Level of autonomy (010) | .982∗∗ | |||
| None (0) | 20 (9.2) | 13 (8.4) | 6 (8.1) | |
| 1–5 | 63 (28.9) | 39 (25.2) | 22 (29.7) | |
| 6–9 | 114 (52.3) | 77 (49.7) | 39 (52.7) | |
| 10 | 21 (9.6) | 14 (9.0) | 7 (9.5) | |
| Not stated | 14 (6.9) | 12 (7.7) | 2 (4.3) | |
| Type of health care | .967∗ | |||
| Public | 65 (27.9) | 43 (27.6) | 22 (28.6) | |
| Private and public | 152 (65.2) | 101 (64.7) | 51 (66.2) | |
| Not stated | 16 (6.9) | 12 (7.7) | 4 (5.2) |
AD = Alzheimer disease, PIM = potentially inappropriate medication.
Test for heterogeneity of proportions.
Test of linear trend.
Fisher exact test.
Table 2 lists medications classified as PIMs according to Beers criteria. Nine therapeutic classes were identified, totaling 64 PIMs, prescribed 327 times, with medications acting mainly on the central nervous system and cardiovascular system. The most frequently prescribed PIMs were quetiapine (12.8%), acetylsalicylic acid (11.6%), escitalopram (6.1%), sertraline (6.1%), and hydrochlorothiazide (5.8%). Among benzodiazepines, the most prescribed was clonazepam (3.4%) and alprazolam (2.4%).
Table 2.
Frequency of prescriptions of PIM, 2019 Beer criteria, according to ATC classification (n = 327).
| ATC Classification (n = 9 classes, 60 medications) | Number of distinct PIM (n = 64) 65 | Frequency of PIM, n (%) | Description according to ATC classification n (%) |
| A—Alimentary tract metabolism (n = 6 medications) | Scopolamine 2,7 | 1 (0.3) | Gastrointestinal disturbances 1 (0.3) |
| Insulin 2 | 12 (3.7) | Insulin and analogs 12 (3.7) | |
| Glibenclamide 2 | 2 (0.6) | Antidiabetic 7 (2.1) | |
| Chlorpropamide 2 | 2 (0.6) | ||
| Pioglitazone 3 | 2 (0.6) | ||
| Ranitidine 6 | 1 (0.3) | Gastric acid 1 (0.3) | |
| B—Blood and blood forming organs (n = 4 medications) | AAs 4 | 38 (11.6) | Antithrombotic agent 53 (16.2) |
| Apixaban 6 | 1 (0.3) | ||
| Cilostazol 3 | 12 (3.7) | ||
| Dabigatran 4,6 | 2 (0.6) | ||
| C—Cardiovascular system (n = 11 medications) | Amiodarone 2,5 | 5 (1.5) | Antiarrhythmic 5 (1.5) |
| Digoxin 2 | 1 (0.3) | Arrhythmia and atrial fibrillation 1 (0.3) | |
| Diltiazem 3 | 2 (0.6) | Calcium channel blocker 2 (0.6) | |
| Propatylnitrate 4 | 6 (1.8) | Cardiac vasodilator 6 (1.8) | |
| Captopril 5 | 5 (1.5) | Antihypertensive 49 (15.0) | |
| Spironolactone 4 | 4 (1.2) | ||
| Furosemide 5 | 5 (1.5) | ||
| Hydrochlorothiazide 4 | 19 (5.8) | ||
| Hydrochlorothiazide+combinations 4 | 5 (1.5) | ||
| Nifedipine 2 | 4 (1.2) | ||
| Propranolol 4 | 7 (2.1) | ||
| G—Genitourinary system and sex hormones (n = 2 medications) | Doxazosin 2,3 | 5 (1.5) | Benign prostatic hypertrophy 6 (1.8) |
| Doxazosin + combinations 2,3 | 1 (0.3) | ||
| J—General anti-infectives for systemic use (n = 2 medications) | Nitrofurantoin 2,3,5 | 1 (0.3) | Anti-infective with systemic action 2 (0.6) |
| Ciprofloxacin 26 | 1 (0.3) | ||
| L—Antineoplastic and immunomodulating agents (n = 1 medication) | Infliximab 3,4,5 | 1 (0.3) | Antineoplastic and immunomodulation agent 1 (0.3) |
| M—Musculoskeletal system (n = 3 medications) | Etodolac 2 | 1 (0.3) | Anti-inflammatory and anti-rheumatic 4 (1.2) |
| Ibuprofen 2 | 1 (0.3) | ||
| Nimesulide 2,3 | 2 (0.6) | ||
| N—Central nervous system (n = 27 medications) | Alprazolam 2,3,5 | 8 (2.4) | Anxiolytic 16 (4.9) |
| Lorazepam2,3,5 | 2 (0.6) | ||
| Bromazepam 4 | 2 (0.6) | ||
| Diazepam 2,3,5,7 | 4 (1.2) | ||
| Amitriptyline2, 3,4,5 | 1 (0.3) | Antidepressant 75 (22.9) | |
| Citalopram 4,5,6 | 1 (0.3) | ||
| Duloxetine 4,5,6 | 5 (1.5) | ||
| Escitalopram 3,4,5 | 20 (6.1) | ||
| Fluoxetine 2,4,5 | 2 (0.6) | ||
| Sertraline 2,3,4,5 | 20 (6.1) | ||
| Sertraline + sodium valproate3 | 1 (0.3) | ||
| Trazodone 4,5 | 10 (3.0) | ||
| Venlafaxine, 2,5 | 2 (0.6) | ||
| Paroxetine2,3,4,5 | 5 (1.5) | ||
| Imipramine 2,5 | 2 (0.6) | ||
| Mirtazapine 3,4 | 6 (1.8) | ||
| Dipyrone 4 | 0 | Analgesic and antipyretic 7 (2.1) | |
| Dipyrone + combinations 4 | 5 (1.5) | ||
| Paracetamol 5 | 2 (0.6) | ||
| Clonazepam 2,3,5 | 11 (3.4) | Anti-epileptic 23 (7.0) | |
| Phenytoin 3,5 | 4 (1.2) | ||
| Phenobarbital + sertraline3 | 1 (0.3) | ||
| Phenobarbital 2 | 1 (0.3) | ||
| Lamotrigine 3 | 3 (0.9) | ||
| Oxcarbazepine 4 | 3 (0.9) | ||
| Levodopa + benserazide 3 | 2 (0.6) | Anti-parkinsonian 2 (0.6) | |
| Levomepromazine2 | 5 (1.5) | Antipsychotic 59 (18.0) | |
| Periciazine 2,4,5 | 1 (0.3) | ||
| Quetiapine4,5 | 42 (12.8) | ||
| Risperidone3,4,5 | 9 (2.7) | ||
| Haloperidol 2,4,5 | 2 (0.6) | ||
| R—Respiratory system (n = 5 medications) | Diphenhydramine 2,7 | 1 (0.3) | Systemic antihistamine 4 (1.2) |
| Dimenhydrinate 2,7 | 1 (0.3) | ||
| Meclizine 2,7 | 1 (0.3) | ||
| Promethazine 2,7 | 1 (0.3) | ||
| Tiotropium 3,5 | 2 (0.6) | Airway obstructions 2 (0.6) |
Superscript numbers indicate different Beers criteria lists as follows: 2) medications that should be avoided in older adults; 3) medications that should be avoided in older adults with specific diseases or syndromes; 4) medications that should be used with caution in older adults; 5) potentially clinically important drug-drug Interactions that should be avoided in older adults; 6) medications that should be avoided or have their dosage reduced with varying levels of kidney function in older adults; 7) updated list of medications with strong anticholinergic properties. ATC = anatomical therapeutic chemical, PIM = potentially inappropriate medications.
Table 3 lists PIMs that should be avoided in most of older people. Of the 60 medications inappropriately prescribed, 32 (56.7%) belonged to this category. In other words, of the 327 instances in which medications were inappropriately prescribed, 148 (45.2%) should be avoided in older people. Medications included those that act on the central nervous system, antidepressants (22.3%), contraindicated for inducing orthostatic hypotension; insulin (8.1%), for risk of hypoglycaemia without improvement in hyperglycemia management; and benzodiazepines (18.2%) that should be avoided for causing loss of balance.
Table 3.
Frequency of prescriptions containing medications that should be avoided in older adults, according to 2019 Beers criteria (n = 148).
| Classification of PIM by pharmacological group (n = 32 medications) | No. of prescriptions 148 (100.0%) | Rationale | Evidence/strength of recommendation |
| First-generation antihistamines | 4 (2.7) | Highly anticholinergic; clearance reduced with advanced age, and tolerance develops when used as hypnotic; risk of confusion, dry mouth, constipation, and other anticholinergic effects or toxicity. Use of diphenhydramine in situations such as acute treatment of severe allergic reaction may be appropriate. | Moderate/strong |
| Dimenhydrinate | |||
| Diphenhydramine | 1 (0.7) | ||
| Meclizine | 1 (0.7) | ||
| Promethazine | 1 (0.7) | ||
| 1 (0.7) | |||
| Antispasmodics | 1 (0.7) | Strong/moderate | |
| Scopolamine | 1 (0.7) | Highly anticholinergic; uncertain effectiveness | |
| Anti-infectives | 2 (1.3) | Long-term use of nitrofurantoin can cause hepatotoxicity, pulmonary toxicity and peripheral neuropathy. Avoid in individuals with creatinine clearance <30 mL/min or for long-term suppression. Ciprofloxacin worsens renal function and increases risk of CNS effects. | Low/strong |
| Nitrofurantoin | 1 (0.7) | ||
| Ciprofloxacin | 1 (0.7) | ||
| Cardiovascular | 16 (10.9) | ||
| Doxazosin | 6 (4.0) | Risk of orthostatic hypertension; not recommended as first choice for treating hypertension. Avoid use as anti-hypertensive | Moderate/strong |
| Digoxin | 1 (0.7) | Use in atrial fibrillation: should not be used as first-line agent. May be associated with increased mortality. Use in heart failure: conflicting effects on risk of hospitalization. May be associated with increased mortality in elderly patients with heart failure; high dosages may increase risk of toxicity and death Avoid as first-line therapy | Moderate/strong |
| Low/strong | |||
| Nifedipine | 4 (2.7) | Potential for hypotension; risk of precipitating myocardial ischemia | High/strong |
| Amiodarone | 5 (3.4) | Should be avoided as first-line therapy for atrial fibrillation unless patient has heart failure or substantial left-ventricular hypertrophy | High/strong |
| Central nervous system | 69 (46.6) | High/strong | |
| Antidepressants | |||
| Amitriptyline | 33 (22.3) | Highly anticholinergic, sedating, and cause orthostatic hypotension | |
| Fluoxetine | 1 (0.7) | ||
| Imipramine | 2 (1.3) | ||
| Sertraline | 2 (1.3) | ||
| Paroxetine | 21 (14.2) | ||
| Venlafaxine | 5 (3.4) | ||
| 2 (1.3) | |||
| Antipsychotics (conventional and atypical) | 8 (5.4) | Increased risk of cerebrovascular accident (stroke) and greater rate of cognitive decline and mortality in persons with dementia. Avoid antipsychotics for behavioral problems of dementia or delirium, unless nonpharmacological options (eg, behavioral interventions) have failed or are not possible and older adult is threatening substantial harm to self or others. Avoid, except in schizophrenia or bipolar disorder, or for short-term use as antiemetic during chemotherapy | Moderate/strong |
| Haloperidol | |||
| Levomepromazine | 2 (1.3) | ||
| Periciazine | 5 (3.4) | ||
| 1 (0.7) | |||
| Barbiturates | 1 (0.7) | High rate of physical dependence, tolerance to sleep benefits, greater risk of overdose at low dosages | High/strong |
| Phenobarbital | 1 (0.7) | ||
| Benzodiazepines | 27 (18.2) | Older adults have increased sensitivity to benzodiazepines and decreased metabolism of long-acting agents; in general, this class increases risk of cognitive impairment, delirium, falls, fractures and motor-vehicle crashes in older adults | Moderate/strong |
| Alprazolam | 8 (5.4) | ||
| Bromazepam | 2 (1.3) | ||
| Clonazepam | 11 (7.4) | ||
| Diazepam | 4 (2.7) | ||
| Lorazepam | 2 (1.3) | ||
| Endocrine system | 16 (10.8) | Higher risk of hypoglycemia without improvement in hyperglycemia management with regimens of only short or rapid-acting insulin to control or prevent hyperglycemia, without concurrent use of basal or long-acting insulin. Oral medications can potentially cause risk of prolonged hypoglycemia in older adults | Moderate/strong |
| Insulin | 12 (8.1) | ||
| Glibenclamide | 2 (1.3) | ||
| Chlorpropamide | 2 (1.3) | ||
| Pain medications (NSAIDs) | 4 (2.7) | Increased risk of gastrointestinal bleeding or peptic ulcer disease in high-risk groups, including those >75 years or taking oral or parenteral corticosteroids, anticoagulants or antiplatelet agent. Avoid chronic use, unless other alternatives are not effective | Moderate/strong |
| Etodolac | 1 (0.7) | ||
| Ibuprofen | 1 (0.7) | ||
| Nimesulide | 2 (1.3) |
CNS = Central Nervous System, NSAIDs = nonsteroidal anti-inflammatory drugs, PIM = potentially inappropriate medications.
Table 4 shows the prevalence of PIM and crude and adjusted analyses of this outcome according to demographic and health variables, type of healthcare system, and polypharmacy. After the adjusted analysis diagnosis of depression (P = 0.010) and the number of comorbidities (P = 0.005) were associated with PIM.
Table 4.
Prevalence of PIM and crude and adjusted analyses of this outcome according to demographic and health variables, type of healthcare system and polypharmacy in elderly patients with Alzheimer's disease. (n = 234).
| Variables | % PIM | Crude analyses prevalence ratio (95% CI) | P valor | Adjusted analyses P (95% CI) | P | |
| Sex | .467∗ | .559∗ | ||||
| 1st level | Male | 72.0 | 1.00 | 1.00 | ||
| Female | 63.8 | 0.89 (0.64–1.23) | 0.90 (0.65–1.25) | |||
| Age, y | .715∗∗ | .754∗∗ | ||||
| 65–74 | 65.5 | 1.00 | 1.00 | |||
| 75–84 | 72.6 | 1.11 (0.74–1.66) | 1.12 (0.75–1.67) | |||
| >85 | 61.7 | 0.94 (0.61–1.45) | 0.95 (0.62–1.47) | |||
| Time since diagnosis, y | .805∗∗ | .761∗∗ | ||||
| <1 | 55.6 | 1.00 | 1.00 | |||
| 1–2 | 66.7 | 1.20 (0.60–2.40) | 1.16 (0.56–2.41) | |||
| >2–5 | 69.0 | 1.24 (0.63–2.45) | 1.20 (0.59–2.47) | |||
| >5 | 65.3 | 1.18 (0.60–2.32) | 1.17 (0.57–2.48) | |||
| Hypertension | .070∗ | .338∗ | ||||
| No | 57.6 | 1.00 | 1.00 | |||
| Yes | 77.1 | 1.34 (0.98–1.83) | 1.18 (0.84–1.64) | |||
| 2nd level | Depression | <.001∗ | .010∗ | |||
| No | 51.6 | 1.00 | 1.00 | |||
| Yes | 98.7 | 1.91 (1.40–2.62) | 1.57 (1.11–2.20) | |||
| Psychosis | .025∗∗ | .288∗ | ||||
| No | 62.3 | 1.00 | 1.00 | |||
| Yes | 100.0 | 1.05 (1.01–1.10) | 1.03 (0.98–1.08) | |||
| Comorbidities (n) | <.001∗∗ | .005∗∗ | ||||
| 0–1 | 31.4 | 1.00 | 1.00 | |||
| 2 | 72.6 | 2.31 (1.39–3.85) | 2.03 (1.20–3.43) | |||
| 3 | 82.6 | 2.62 (1.55–4.44) | 2.20 (1.27–3.82) | |||
| 4 or + | 91.1 | 2.90 (1.76–4.78) | 2.35 (1.39–4.02) | |||
| Level of autonomy (0–10) | .929∗∗ | .545∗∗ | ||||
| None (0) | 70.0 | 1.00 | 1.00 | |||
| 1–5 | 65.1 | 0.93 (0.51–1.71) | 0.74 (0.40–1.40) | |||
| 6–9 | 65.8 | 0.94 (0.53–1.66) | 0.76 (0.42–1.38) | |||
| 10 | 66.7 | 0.95 (0.45–1.99) | 0.72 (0.33–1.57) | |||
| Type of health care | .981∗ | .531∗ | ||||
| Public | 66.2 | 1.00 | 1.00 | |||
| 3rd level | Private and public | 66.5 | 1.00 (0.70–1.44) | 0.89 (0.62–1.28) | ||
| Polypharmacy | <.001∗ | .158∗ | ||||
| No (≤4 medications) | 46.9 | 1.00 | 1.00 | |||
| Yes (≥5 medications) | 90.6 | 1.93 (1.40–2.67) | 1.38 (0.88–2.17) |
CI = confidence interval, PIM = potentially inappropriate medications.
Wald test for heterogeneity.
Wald test for linear trend.
4. Discussion
4.1. Principal findings and comparison with previous studies
This study revealed a high prevalence of PIP among older patients with AD who obtain their treatment from the Public Health System. Two systematic reviews also have demonstrated that the prevalence of PIP among older people with dementia is high.[24,25]
We found an independent association between PIM and depression. Our findings are consistent with previous studies.[26,27] Most PIMs can cause adverse effects including confusion, agitation, and depression. However, neuropsychiatric symptoms such as depression often accompany progressive neurodegeneration among dementia patients. Thus, PIMs may be prescribed to treat these conditions, leading to a cycle of increasing use of them.
In this study, the number of comorbidities was a predictor of PIM prescription. Although this association is not a novel finding,[26,27] older people suffering dementia have a range of needs and pharmacological treatments to manage the array of comorbidities associated with aging. Thus, this result could be an indicator of inappropriate medication management for conditions in such a vulnerable population.
There were 327 instances of inappropriately prescribed medications, totaling 60 PIMs, mainly prescribed for diseases associated with the central nervous system and cardiovascular. The most frequently prescribed PIM was the antipsychotic quetiapine. Although non-pharmacologic options are consistently recommended as the first line for the management of behavioral and psychological symptoms of dementia, several limitations exist with nonpharmacologic therapies, which hinder their utility in everyday clinical practice. Furthermore, antipsychotics are recommended within this approach as appropriate for the management of severe agitation and other behavioral and psychological symptoms of dementia with low initial dose and regular review every 1 to 3 months for deprescribing.[28] Therefore, the decision to use an antipsychotic drug in older people with AD should be considered with caution.
Other important prescribed PIMs identified were acetylsalicylic acid, which may increase the risk of bleeding in older adults higher and equal to 70 years’ old; antidepressants (escitalopram and sertraline), which may cause orthostatic hypotension, risk of ataxia, impairment in psychomotor function, syncope, and falls; and hydrochlorothiazide, that can exacerbate or cause the syndrome of inappropriate secretion of antidiuretic hormone or hyponatremia.
Two patients aged >80 years were found to be at risk of severe drug interaction, due to concomitant use of acetylsalicylic acid with ramipril or enalapril. The mechanism of interaction is pharmacodynamic antagonism, which may attenuate antihypertensive effects and cause a significant decline in renal function.[20]
Around 45% of instances of improperly prescribed medications involved medications that should be avoided in older people, most notably antidepressants for causing orthostatic hypotension; insulin because it leads to hypoglycemia without improvement of glycemic control; and benzodiazepines (especially clonazepam) due to loss of balance.
The population studied was similar to that investigated by Montastruc et al[21] in a prospective multicenter cohort study conducted in France involving 684 patients with mild-to-moderate AD. Participants had an average age of 78 years, lived with the family, were also predominantly women, and used mainly donepezil. The authors noted that 46.7% of patients had at least 1 PIP (according to the list of Laroche et al[29]), a lower rate compared to the present study.
In this study, although benzodiazepines were not the main PIMs, they were widely used, reinforcing the findings in Montastruc et al,[21] showing that the majority of older people with AD were in intermediate to long-term use of benzodiazepines.
A cross-sectional study carried out in Argentina,[30] interviewed 215 noninstitutionalized older people. The study identified PIP according to 2002 Beers criteria,[31] the Priscus list (2008),[32] and the STOPP criteria. (2008)[33] It was observed that 25.5%, 31.9%, and 30.0% of patients, respectively; had prescriptions containing PIM. The main PIMs were also benzodiazepines, especially clonazepam.
The identification and management of neuropsychiatric symptoms in patients with AD pose a major challenge for clinicians due to the ambiguity between symptoms of psychiatric disorders and those of dementia, and the lack of effective treatments that can be safely used in older people.[3] This might explain the high number of prescriptions for psychiatric symptoms such as quetiapine, escitalopram, sertraline, alprazolam, diazepam, and clonazepam.
The Beers criteria[20] considers all benzodiazepines inappropriate for older people aged ≥65 years and recommends their use be avoided due to increased risk of cognitive impairment, delirium, falls, and fractures. A systematic review showed that the risk of older patients developing dementia doubled when using benzodiazepines for >30 days. This link is also associated with the presence of comorbidities such as diabetes, dyslipidemia, arterial fibrillation, hypertension, stroke, heart disease, hyperlipidemia, hypercholesterolemia, epilepsy, insomnia, and anxiety; use of medications (statins, platelet antiaggregants, anticoagulants and antihypertensives); habits such as smoking, alcoholism and drugs; and with age and sex.[34]
With regard to the results obtained in this study, it is important to point out some of the most relevant differences in the current version of the Beers criteria[20] compared with its previous version.[19] One such change involves the prescription of H2 receptor antagonists (omeprazole and pantoprazole was used chronically by 11 and 8 patients, respectively, in the present study) which were removed from the “avoid criteria" in patients with cognitive impairment. The rationale given was that the quality of evidence is weak to adverse cognitive effects.
There was a reduction in age, from ≥80 to ≥70 years, for the use of acetylsalicylic acid among older adults in the primary prevention of cardiovascular disease and colorectal cancer, with moderate quality of evidence. In this study, 26 older patients used acetylsalicylic acid for the prevention of primary thromboembolic events.
Another relevant change was the use of anticonvulsants in association with serotonin and norepinephrine reuptake inhibitors, which should be avoided in older people with a history of falls and/or fragility fractures. Seven antidepressants of this class were prescribed to 55 patients, but the association (citalopram with phenobarbital and sertraline with sodium valproate) was used by only two patients. However, it was not possible to determine whether these individuals had a history of falls and/or fragility fractures.
Dabigatran and rivaroxaban were included for use with caution in older people due to the increased risk of gastrointestinal bleeding when compared to warfarin and other novel anticoagulants. The guidelines recommend use with caution in patients aged ≥75 years for long-term treatment, such as prevention of venous thromboembolism and atrial fibrillation. Too glimepiride was included as a PIM for older people due to its potential to pose a long-term risk of hypoglycemia, joining glibenclamide and chlorpropamide in this listing.
4.2. Study limitations and strengths
To our knowledge, this is the first study to examine the prevalence of PIP in older people with AD in Brazil. The study sample included 80% of older people with an active registration for medication collection at the Specialty Medications Pharmacy in the city of Sorocaba. Strengths include the devising of a tailored data collection instrument which may, after adaptation, be employed in other studies. In addition, a pilot study was performed to refine the instrument. It is too important to emphasize that the findings of this study are limited to individuals with AD whose medications were provided by the public health system in a large Brazilian city. However, the lack of information regarding this population reinforces the need for this study.
This study drew on information obtained directly from patients and/or caregivers, where some had difficulties remembering the name of the diseases, medications used, and other information. Although MEDEX system data were used, much of the information could not be collected because access was not authorized. To minimize this bias, the information was complemented with data from medical prescriptions, telephone calls, or by scheduling another interview with the patient and/or their caregiver. Another limitation of the study was not to access the information about the classification concerning the stages of dementia (early or late stages and atypical or mixed form, among others).
We used the Beers Criteria to evaluate the prescription of PIMs. This tool cannot replace clinical judgment, and a medication identified as PIM using such a tool may subsequently be found to be appropriate following a full clinical assessment. Furthermore, the Beers Criteria is not specific criteria for dementia, which could be affecting the results of this study. Although there has been a considerable research effort to develop criteria to assess PIMs in this population, most focus is on the advanced stages of dementia.[9] Thus, the use of Beers Criteria should be a starting point for a comprehensive process of identifying and improving medication appropriateness and safety in older people with dementia.
One limitation of the cross-sectional study is that the exposure and outcome are simultaneously assessed. Then, there is no evidence of a temporal relationship between exposure and outcome. In this way, the association between PIM and diagnosis of depression may have been affected by reverse causality, since exposure and outcome were measured at the same time. Longitudinal studies are important to elucidate the associations described herein.
4.3. Implications for clinical practice and research
The projected rise in dementia cases, predominantly AD, together with the high prevalence of PIMs found in older people assessed, highlights the need for strategies to ensure the safe use of medications in this population.
Possible strategies include a multidisciplinary care team, pharmaceutical care interventions, access to reliable information so that the ”risk/benefit paradox of treatment" can be better evaluated by health professionals, establishing of clinical protocols that include the monitoring of medication use among older people,[20] and that these recommendations can be applied in clinical practice.
The pharmacotherapy used by this population treats signs and symptoms associated with comorbidities related to the aging process but may cause or increase cognitive decline. The effectiveness and safety of this pharmacotherapy should be regularly monitored, and strict prescription and withdrawal protocols followed.
Researchers, managers, prescribers, caregivers, and patients can use the findings of this study to minimize risks associated with the use of medications in older people with AD. There is a lack of data in the national and international literature on the subject, suggesting the need for studies. Further primary studies on the safety of drug treatment in older people with AD may complement these findings.
5. Conclusion
This study evaluated the prescriptions for older with AD in Brazil and identified a high prevalence of PIP in this population. There are few prescribing guidelines addressing the specific needs of patients living with this disease and other dementias. Although the PIM lists are not designed to replace clinical judgment, health care professionals may benefit from these findings by improving safety in the use of medications for treating AD and comorbidities. Further studies should investigate whether the PIP is associated with adverse outcomes and whether the application of the specific PIMs list can help improving prescription appropriateness for older people with AD.
Author contributions
Cristiane de Cássia Bergamaschi is the project manager, coinvestigator and contributed to the writing and revision of the manuscript. Luciane Cruz Lopes and Fabiane Raquel Motter are coinvestigators and contributed to the writing and revision of the manuscript.
Conceptualization: Tânia Regina Ferrreira, Luciane Cruz Lopes, Cristiane de Cássia Bergamaschi.
Data curation: Tânia Regina Ferrreira.
Formal analysis: Tânia Regina Ferrreira, Fabiane Raquel Motter.
Investigation: Tânia Regina Ferrreira.
Methodology: Tânia Regina Ferreira, Fabiane Raquel Motter, Luciane Cruz Lopes, Cristiane de Cássia Bergamaschi.
Project administration: Cristiane de Cássia Bergamaschi.
Supervision: Cristiane de Cássia Bergamaschi.
Validation: Cristiane de Cássia Bergamaschi.
Visualization: Luciane Cruz Lopes, Fabiane Raquel Motter, Cristiane de Cássia Bergamaschi.
Writing – original draft: Tânia Regina Ferrreira, Luciane Cruz Lopes, Fabiane Raquel Motter.
Writing – review & editing: Tânia Regina Ferreira, Fabiane Raquel Motter, Cristiane de Cássia Bergamaschi, Luciane Cruz Lopes.
Footnotes
Abbreviations: AD = Alzheimer disease, ATC Code = Anatomical Therapeutic Chemical Code, DRS-XVI = Regional Health Department-XVI, ICD-10 = International Classification of Diseases code 10, MEDEX = Drugs of Exceptional Distribution, PIM = potentially inappropriate medication, PIP = potentially inappropriate prescribing.
How to cite this article: Ferreira TR, Lopes LC, Motter FR, de Cássia Bergamaschi C. Potentially inappropriate prescriptions to Brazilian older people with Alzheimer disease: A cross-sectional study. Medicine. 2021;100:12(e25015).
The authors report no conflicts of interest.
Ethical approval: This study was approved by the Ethics Committee on Research of the University of Sorocaba (protocol number: 1860724). All subjects enrolled in the study received explanations regarding the aims of the studies and signed a form attesting that their participation was voluntary.
Informed consent: Each participant signed and provided written informed consent.
Consent for publication: Not applicable.
The datasets generated during and/or analyzed during the present study are not publicly available, but are available from the corresponding author on reasonable request.
References
- [1].Oh ES, Rabins PV. Dementia. Ann Intern Med 2019;171:ITC33–48. [DOI] [PubMed] [Google Scholar]
- [2].Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med 2011;1:a006189-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Wolinsky D, Drake K, Bostwick J. Diagnosis and management of neuropsychiatric symptoms in Alzheimer's disease. Curr Psychiatry Rep 2018;20:117. [DOI] [PubMed] [Google Scholar]
- [4].[2019] Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2019; 15(3):321-87. doi: available at: 10.1016/j.jalz.2019.01.010. [DOI] [Google Scholar]
- [5].World Health Organization. Dementia: number of people affected to triple in next 30 years. News release. Geneva. 7 December 2017. Available https://www.who.int/news/item/07-12-2017-dementia-number-of-people-affected-to-triple-in-next-30-years. Accessed March 12, 2021. [Google Scholar]
- [6].GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer's disease and other dementias, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2019;18:88–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Farina N, Ibnidris A, Alladi S, et al. A systematic review and meta-analysis of dementia prevalence in seven developing countries: A STRiDE project [published online ahead of print, 2020 Jul 13]. Glob Public Health 2020;1–6. [DOI] [PubMed] [Google Scholar]
- [8].Delgado J, Bowman K, Clare L. Potentially inappropriate prescribing in dementia: a state- of-the-art review since 2007. BMJ Open 2020;10:e029172-e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Parsons C. Polypharmacy and inappropriate medication use in patients with dementia: an underresearched problem. Ther Adv Drug Saf 2017;8:31–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Marengoni A, Onder G. Guidelines, polypharmacy, and drug-drug interactions in patients with multimorbidity. BMJ (Clinical research ed) 2015;350:h1059. [DOI] [PubMed] [Google Scholar]
- [11].Renom-Guiteras A, Meyer G, Thurmann PA. The EU(7)-PIM list: a list of potentially inappropriate medications for older people consented by experts from seven European countries. Eur J Clin Pharmacol 2015;71:861–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hedna K, Hakkarainen KM, Gyllensten H, et al. Potentially inappropriate prescribing and adverse drug reactions in the elderly: a population-based study. Eur J Clin Pharmacol 2015;71:1525–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Cabre M, Elias L, Garcia M, et al. Avoidable hospitalizations due to adverse drug reactions in an acute geriatric unit. Analysis of 3,292 patients. Med Clin 2018;150:209–14. [DOI] [PubMed] [Google Scholar]
- [14].Price SD, Holman CD, Sanfilippo FM, et al. Association between potentially inappropriate medications from the Beers criteria and the risk of unplanned hospitalization in elderly patients. Ann Pharmacother 2014;48:6–16. [DOI] [PubMed] [Google Scholar]
- [15].Lau DT, Kasper JD, Potter DE, et al. Hospitalization and death associated with potentially inappropriate medication prescriptions among in elderly nursing home residents. Arch Intern Med 2005;165:68–74. [DOI] [PubMed] [Google Scholar]
- [16].Muhlack DC, Hoppe LK, Weberpals J, et al. The association of potentially inappropriate medication at older age with cardiovascular events and overall mortality: a systematic review and meta-analysis of cohort studies. J Am Med Dir Assoc 2017;18:211–20. [DOI] [PubMed] [Google Scholar]
- [17].Beers MH, Ouslander JG, Rollingher I, et al. Explicit criteria for determining inappropriate medication use in nursing home residents. UCLA Division of Geriatric Medicine. Arch Intern Med 1991;151:1825–32. [PubMed] [Google Scholar]
- [18].American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2012;60:616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].American Geriatrics Society 2015 Beers Criteria Update Expert Panel. American Geriatrics Society 2015 Updated Beers Criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2015;63:2227–46. [DOI] [PubMed] [Google Scholar]
- [20].2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 Updated AGS Beers Criteria(R) for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674–94. [DOI] [PubMed] [Google Scholar]
- [21].Montastruc F, Gardette V, Cantet C, et al. Potentially inappropriate medication use among patients with Alzheimer disease in the REAL FR cohort: be aware of atropinic and benzodiazepine drugs!. Eur J Clin Pharmacol 2013;69:1589–97. [DOI] [PubMed] [Google Scholar]
- [22].Brasil. Ministério da Saúde. Portaria n°1554 de 30 de julho de 2013. Dispõe sobre as regras de financiamento e execução do Componente Especializado da Assistência Farmacêutica no âmbito do Sistema Único de Saúde (CONITEC). 30 Julie, 2013. Available http://bvsms.saude.gov.br/bvs/saudelegis/gm/2013/prt1554_30_07_2013.html. Accessed March 12, 2021. [Google Scholar]
- [23].Masnoon N, Shakib S, Kalisch-Ellett L, et al. What is polypharmacy? A systematic review of definitions. BMC Geriatr 2017;17:230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Hukins D, Macleod U, Boland JW. Identifying potentially inappropriate prescribing in older people with dementia: a systematic review. Eur J Clin Pharmacol 2019;75:467–81. [DOI] [PubMed] [Google Scholar]
- [25].Redston MR, Hilmer SN, McLachlan AJ, et al. Prevalence of potentially inappropriate medication use in older inpatients with and without cognitive impairment: a systematic review. J Alzheimers Dis 2018;61:1639–52. [DOI] [PubMed] [Google Scholar]
- [26].Koyama A, Steinman M, Ensrud K, et al. Ten-year trajectory of potentially inappropriate medications in very old women: importance of cognitive status. J Am Geriatr Soc 2013;61:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lau DT, Mercaldo ND, Harris AT, et al. Polypharmacy and potentially inappropriate medication use among community-dwelling elders with dementia. Alzheimer Dis Assoc Disord 2010;24:56–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reus VI, Fochtmann LJ, Eyler AE, et al. The American Psychiatric Association Practice Guideline on the use of antipsychotics to treat agitation or psychosis in patients with dementia. Focus (Am Psychiatr Publ) 2017;15:81–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Laroche ML, Charmes JP, Nouaille Y, et al. Is inappropriate medication use a major cause of adverse drug reactions in the elderly? Br J Clin Pharmacol 2007;63:177–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Regueiro M, Mendy N, Cañás M, et al. Uso de medicamentos en adultos mayores no institucionalizados. Rev Peru Med Exp Salud Publica 2011;28:643–7. [PubMed] [Google Scholar]
- [31].Fick DM, Cooper JW, Wade WE, et al. Updating the Beers criteria for potentially inappropriate medication use in older adults: results of a US consensus panel of experts. Arch Intern Med 2003;163:2716–24. [DOI] [PubMed] [Google Scholar]
- [32].Holt S, Schmiedl S, Thurmann PA. Potentially inappropriate medications in the elderly: the PRISCUS list. Dtsch Arztebl Int 2010;107:543–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Gallagher P, Ryan C, Byrne S, et al. STOPP (Screening Tool of Older Person's Prescriptions) and START (Screening Tool to Alert doctors to Right Treatment). Consensus validation. Int J Clin Pharmacol Ther 2008;46:72–83. [DOI] [PubMed] [Google Scholar]
- [34].Islam MM, Iqbal U, Walther B, et al. Benzodiazepine use and risk of dementia in the elderly population: a systematic review and meta-analysis. Neuroepidemiology 2016;47:181–91. [DOI] [PubMed] [Google Scholar]


