Abstract
Fibromyalgia (FM) is a common and refractory chronic pain condition with multiple clinical phenotypes. The current diagnosis is based on a syndrome identification which can be subjective and lead to under or over-diagnosis. Therefore, there is a need for objective biomarkers for diagnosis, phenotyping, and prognosis (treatment response and follow-up) in fibromyalgia. Potential biomarkers are measures of cortical excitability indexed by transcranial magnetic stimulation (TMS). However, no systematic analysis of current evidence has been performed to assess the role of TMS metrics as a fibromyalgia biomarker. Therefore, this study aims to evaluate evidence on corticospinal and intracortical motor excitability in fibromyalgia subjects and to assess the prognostic role of TMS metrics as response biomarkers in FM. We conducted systematic searches on PubMed/Medline, Embase, and Cochrane Central databases for observational studies and randomized controlled trials on fibromyalgia subjects that used TMS as an assessment. Three reviewers independently selected and extracted the data. Then, a random-effects model meta-analysis was performed to compare fibromyalgia and healthy controls in observational studies. Also, to compare active versus sham treatments, in randomized controlled trials. Correlations between changes in TMS metrics and clinical improvement were explored. The quality and evidence certainty were assessed following standardized approaches. We included 15 studies (696 participants, 474 FM subjects). The main findings were: (1) fibromyalgia subjects present less intracortical inhibition (mean difference (MD) = −0.40, 95% confidence interval (CI) −0.69 to −0.11) and higher resting motor thresholds (MD = 6.90 μV, 95% CI 4.16 to 9.63 μV) when compared to controls; (2) interventions such as exercise, pregabalin, and non-invasive brain stimulation increased intracortical inhibition (MD = 0.19, 95% CI 0.10 to 0.29) and cortical silent period (MD = 14.92 ms, 95% CI 4.86 to 24.98 ms), when compared to placebo or sham stimulation; (3) changes on intracortical excitability are correlated with clinical improvements – higher inhibition moderately correlates with less pain, depression, and pain catastrophizing; lower facilitation moderately correlates with less fatigue. Measures of intracortical inhibition and facilitation indexed by TMS are potential diagnostic and treatment response biomarkers for fibromyalgia subjects. The disruption in the intracortical inhibitory system in fibromyalgia also provides additional evidence that fibromyalgia has some neurophysiological characteristics of neuropathic pain. Treatments inducing an engagement of sensorimotor systems (e.g., exercise, motor imagery, and non-invasive brain stimulation) could restore the cortical inhibitory tonus in FM and induce clinical improvement.
Keywords: biomarkers, cortical inhibition, fibromyalgia, transcranial magnetic stimulation
INTRODUCTION
Fibromyalgia (FM) is common and a hard-to-manage chronic pain condition with multiple clinical manifestations, including widespread pain, fatigue, mood disorders, cognitive impairments, and sleep problems 1,2. The lack of understating of the FM pathophysiology results in an imprecise identification of patients and the current scarcity of reliable therapies for the condition 2,3.
The current diagnosis consists of a syndrome identification (self-reported heterogeneous symptoms) which can be subjective and lead to under or over-diagnosis. Unlike other rheumatologic conditions, the FM diagnosis does not have specific visual clinical signs on physical examination or imaging exams 1. Usually, the patients could show hypersensitivity at specific tender points; however, this increased sensitivity can be present in some individuals regardless of the presence of FM 1,3,4. The pain characteristics also can be variable since they are affected by several distinct factors, such as physical or mental stress, comorbidities, and temperature 1,3,4. Besides, although widespread pain is the most common manifestation, patients with the disease also can experience symptoms involving almost all body systems, such as cognitive dysfunction, dyspepsia, and urinary tract disorders, besides other signs that can be difficult to distinguish between other chronic conditions 1,4,5.
The identification of biomarkers as potential objective measures of FM and its variety of clinical phenotypes is an ongoing endeavor in FM research 6. There is a need for objective biomarkers for diagnosis (case identification), phenotyping (subgroup identification), and prognosis (treatment response and follow-up) in FM. Comparisons between protein serums levels, genetic factors, and neuroimaging biomarkers have been described in the literature based on several potential mechanisms related to FM, such as central sensitization, immune system implications, and alterations in neurotransmitters 3,5,7,8. However, further research is necessary to validate these potential biomarkers 8.
Recent studies suggested that FM patients have alterations in cortico-subcortical processing. Flodin et al. 9 showed a decreased connectivity between the thalamus and premotor areas, and the right insula and primary sensorimotor areas, indicating reduced intrinsic coupling between pain-related brain regions and the primary sensorimotor areas in the absence of externally applied pain. Moreover, FM patients seem to have an unbalanced GABAergic network associated with central sensitization and altered motor cortical excitability 10,11. Thus, measurements of sensorimotor system excitability and central sensitization are useful options for the identification of FM biomarkers.
Transcranial magnetic stimulation (TMS) assessment can offer a potential solution as a non-invasive biomarker that can measure corticospinal and motor cortex intracortical excitability. The corticospinal activity can be evaluated by calculating motor evoked potentials (MEPs) and motor thresholds 12. The MEPs are the electrical signals recorded from the descending motor pathways and muscles following stimulation of the motor cortex (M1); and the motor threshold is the lowest transcranial stimulus intensity at which M1 produces an electromyographic response, i.e., an MEP in the rested or contracted target muscle 12.
Likewise, intracortical excitability (inhibition and facilitation) can be assessed by cortical silent period (CSP), short intracortical inhibition (SICI), and intracortical facilitation (ICF). The CSP is an electrical silence period after an MEP. It represents the short-term interruption of the muscle signal when the M1 is stimulated. It has been stipulated that gamma aminobutyric acid B (GABA-B)receptors mediate this “suppression” within the M1 13; therefore, longer CSP durations could reflect an increase in intracortical and interhemispheric inhibition 12. Finally, SICI and ICF are elicited when a first subthreshold conditioning stimulus follow a second suprathreshold stimulus at different interstimulus intensity (1 to 6 ms for SICI and 8 to 30 ms for ICF) producing an attenuation or an increase of the MEP, respectively. The SICI likely represents post-synaptic inhibition mediated by GABA-A; and the ICF likely represents excitatory glutamatergic circuits in M1 12. Therefore, TMS metrics could be used to assess motor cortex excitability in FM patients, as diagnostic and prognostic biomarkers. Although the measurements of excitability are restricted to the primary motor cortex, they actually provide interesting information for FM as it can assess plasticity changes in the sensorimotor system. Nevertheless, current results are heterogeneous and no systematic analysis of the evidence was performed to assess the role of TMS metrics as FM biomarkers.
The aim of this review is to systematically evaluate the evidence of corticospinal and intracortical motor excitability alterations in FM patients compared to healthy controls, assess the prognostic role of these metrics as treatment response biomarkers in this condition, and evaluate their associations with clinical outcomes after treatment. We hypothesize a decreased inhibitory tonus in FM patients and potential inhibition restoration after interventions that engaged sensorimotor networks such as exercise and brain stimulation. We expect that an increase in inhibitory tonus will be correlated to clinical improvement.
DATA AND METHODS
We followed the “Preferred Reporting Items for Systematic reviews and Meta-Analyzes” (PRISMA) 14 guidelines and the Cochrane Handbook for Systematic Reviews of Interventions 15. The PRISMA checklist is presented in Additional Table 1.
Literature search and inclusion criteria
We searched PubMed/Medline, Cochrane Central, and Embase databases from inception up to April 21, 2022 (search performed that day). The search strategy is presented in Additional Table 2. Additionally, we reviewed the references of the included studies. The eligibility criteria included: 1) observational studies or randomized controlled trials (RCTs) that included FM patients and assessed TMS metrics (resting motor threshold [rMT], MEP, CSP, SICI, or ICF) at baseline or longitudinally, 2) with or without a control group and including any type of intervention (if applicable), and 3) full-text accessible. We excluded the following articles: (1) animal studies; (2) review articles; (3) letters to the editor and editorials; and (4) conference abstracts. We did not exclude studies based on date or language.
Study selection and data extraction
After removing duplicates, three authors (DL, DP, and ES) independently reviewed records by titles and abstracts and then by full text using the Covidence platform (https://www.covidence.org/). Discrepancies were verified with a fourth reviewer (KPB), achieving consensus by discussion. Data extraction from each selected study was conducted independently. For each selected study, we extracted the five TMS metrics: rMT (in mV), MEP (in mV), CSP (in ms), SICI (as percentage), or ICF (as percentage). The TMS metrics were extracted from FM and control groups in the case of observational studies. For RCT, the TMS metrics from active and sham/placebo interventions were extracted. Also, we extracted the correlation coefficients between TMS metrics and clinical outcomes, if available. Additionally, we extracted characteristics of the study and study sample such as author, publication year, study design, sample size, type of control group, intervention type, FM diagnosis description, age, gender, pain intensity, depression, anxiety, and pain catastrophizing scores. Moreover, we extract the TMS experiment characteristics, including targeted cortical area, type of coil, outcome measures, and parameters. In addition to that, we used Web Plot Digitizer v.3.11 to extract raw values from published graphs 16. If authors did not answer our data request or raw data from graphs were not extractable, the study was excluded from the meta-analysis. The extracted data were tabulated, coded, and imported into a datasheet for analysis.
Quality assessment
Two reviewers (DL and DP) conducted a quality assessment of TMS experiments using the checklist developed by Chipchase et al. 17. A third reviewer (KPB) solved discrepancies. This checklist evaluates 26 factors that should be reported and/or controlled in TMS studies that target the motor cortex. We assigned a value of zero if they do not report and justify the criteria in the manuscript and one if they do so. Then, we calculate the total score and relative score (percentage) per each study, a higher number corresponds to high quality.
Evidence certainty assessment
The certainty of the evidence was assessed using the “Grading of Recommendations Assessment, Development, and Evaluation” (GRADE). The GRADE evaluation includes the domains of risk of bias, inconsistency, indirect evidence, imprecision, and publication bias 18. We did not conduct a publication bias assessment due to the small number of included studies, thus we waived that item from the evaluation. For the meta-analysis including only RCTs, the certainty evaluation started as “high quality”, with the possibility to lower it by either one or two levels in each item according to the extent of the limitations. For the meta-analysis that included observational studies, the evaluation certainty was set at ‘low’ with the same possibility to lower it, but also to increase it if the domains were conducted appropriately.
Statistical analysis
We conducted meta-analyses of continuous outcomes with random-effects models since we expected a priori a high between-study heterogeneity, according to the DerSimonian and Laird method 19. We performed two sets of meta-analyses: 1) Comparison of TMS metrics between FM patients and the control group; 2) Comparison of TMS metrics changes in FM patients who received active interventions and who received sham/placebo interventions. Each TMS metric (repetitive magnetic stimulation [rTMS], MEP, CSP, SICI, and ICF) was analyzed separately. We calculated effect sizes as weighted mean differences (MDs) with 95% confidence intervals (CIs) since we combined studies with similar outcomes (standardized TMS metrics) 15. Moreover, we used the Hartung-Knapp adjustment for random-effects models, which calculated more adequate error rates, especially when the number of included studies is small 20. We tested for statistical heterogeneity of pooled estimates using the Chi-square test and the degree of heterogeneity using the I2 statistic, considering that heterogeneity might not be substantial when I2 < 40% 18. We performed subgroup analyses by control type (for observational studies) and intervention type (for RCTs), but we did not perform a publication bias assessment due to the low number of studies we found 21. The data was processed with R Studio 4.1.1 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Systematic search and included studies
Details of the selection process can be found in Figure 1. The literature search resulted in 255 articles after duplicates were eliminated. Based on titles and abstracts screening, 230 articles were excluded. Then, the 25 remaining articles were screened by reading the full text for TMS metrics in FM. In this phase, 10 were excluded as they did not report measures of cortical excitability or they had incorrect study design, and 15 articles were included (n = 696 participants). Of those studies, eight were observational (n = 388, 166 FM patients and 222 controls). Seven had control groups (healthy controls, major depression, osteoarthritis, rheumatoid arthritis, and myofascial pain). On the other hand, we included seven RCTs (n = 308 FM patients, 149 received active treatment, and 159 received sham/placebo). The identified interventions were M1 repetitive magnetic stimulation (rTMS), M1 anodal transcranial direct current stimulation (tDCS), exercise, pregabalin, and low-pressure hyperbaric oxygen treatment. The detailed descriptions of the included studies and their TMS assessments are reported in Tables 1 and 2.
Figure 1. Study selection flow-chart.
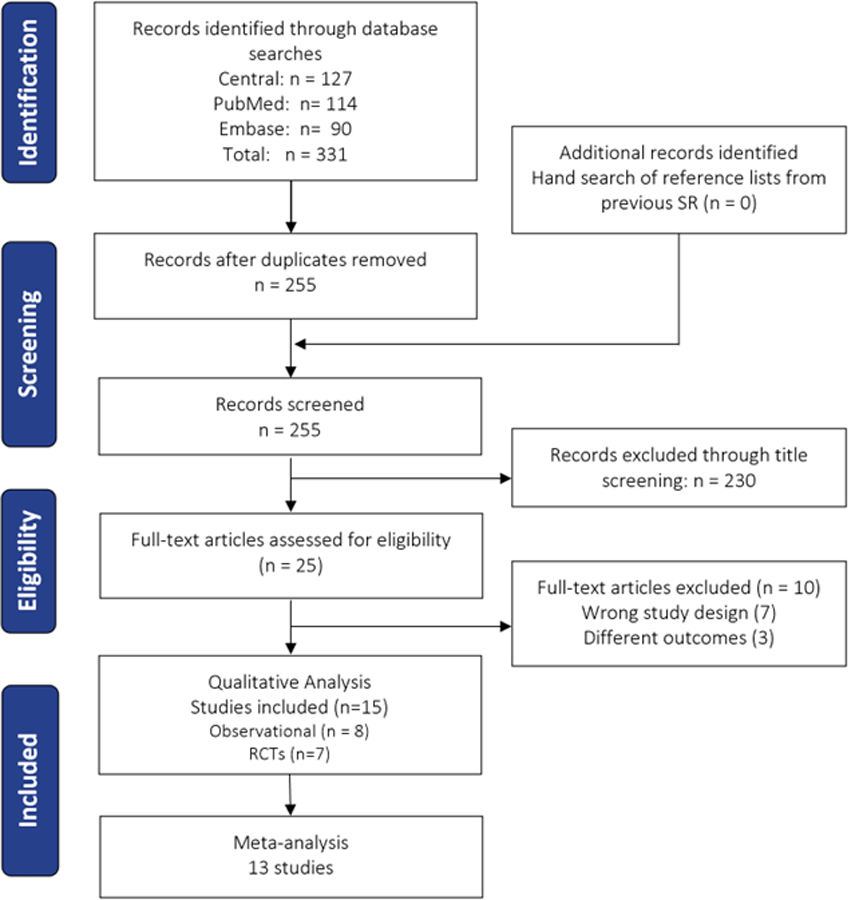
RCT: Randomized controlled trial, SR: systematic review.
Table 1.
Characteristics of included studies
| Study | Type of study | Sample size | Fibromyalgia description | Age (mean ± SD or range) | Gender | Pain intensity (mean ± SD) | Pain tool/scale | Depression score | Anxiety score | Pain catastrophizing score | Type of control/ intervention comparison |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Beement (2014) | RCT/cross-over | 15 | Diagnosed by a Rheumatologist | 53.7 ± 9.9 | 15 F | 47.8 ± 18.8 | Fibromyalgia Impact Questionnaire | N/A | N/A | N/A | Quiet rest |
| Deitos (2018) | Cross-over | 27 | According to the 2010 American College of Rheumatology criteria | FM: 50.5 ± 8.7 HC: 43.7 ± 9.4 |
27 F | 7.1 ± 1.8 – last 24 h 7.9 ± 1.9 – last 7 d |
VAS | Beck Depression Inventory II | State and trait anxiety | Brazilian Portuguese Catastrophi-zing Scale | Healthy subjects |
| Mahla (2011) | RCT | 40 | According to the 2010 American College of Rheumatology criteria | Active rTMS: 51.8 ± 11.6 Sham rTMS: 49.6 ± 10.0 |
40 F | Active rTMS: 6.2 ± 1.4 Sham rTMS: 6.5 ± 1.8 |
Brief pain inventory | 21-item Hospital Anxiety and Depression
Scaleand 13-item short form of the Beck Depression Inventory |
21-item Hospital Anxiety and Depression Scale | Pain Catastrophi-zing Scale | Sham rTMS |
| Mahla (2010) | RCT | 67 | According to the 2010 American College of Rheumatology criteria | 50.8 ± 10.3 | 67 F | Patients being treated with psychotropic drug:
6.2 ± 1.5 Patients without treatment: 5.5 ± 1.3 |
Brief pain inventory | 21-item Hospital Anxiety and Depression Scale
and 13-item short form of the Beck Depression Inventory |
21-item Hospital Anxiety and Depression Scale | Pain Catastrophi-zing Scale | Healthy subjects and treatment with a psychotropic drug |
| Mendonca (2016) | RCT | 45 | Modified criteria from the American College of Rheumatology criteria | 47.4 ± 12.1 | 44 F/1 M | tDCS/AE: 7.3 ± 1.75 AE: 6.8 ± 2.0 tDCS: 7.2 ± 1.27 |
VNS | Beck Depression Inventory | VNS for anxiety | N/A | Sham aerobic exercisend sham tDCS |
| Schwenkreis (2010) | RCT | 62 | According to the 2010 American College of Rheumatology criteria | MD: 41.0 ± 10.4 FM: 48.7 ± 8.4 HC: 37.7 ± 11.5 |
32 F/ 30 M | N/A | N/A | N/A | N/A | N/A | Muscular dystrophy and Healthy control |
| Alventosa (2020) | RCT | 49 | 2016 American College of Rheumatology: generalized pain for at least 3 months and a widespread pain index ⩾ 7 and symptom severity scale ⩾ 5 or a widespread pain index of 4–6 and a symptom severity scale score ⩾ 9] | 53.30 ± 7.86 | 49 F | Physical exercise group (n =
16): 6.13 ± 2.22. Hyperbaric oxigeny therapy (n = 17): 7.35 ± 1.66. Control: 5.63 ± 1.75 |
VAS | N/A | N/A | N/A | Subjects not submitted to the interventions. |
| Carinal (2019) | Cross-sectional | 63 | 2016 American College of Rheumatology: generalized pain for at least 3 mon and a widespread pain index ⩾ 7 and symptom severity scale ⩾ 5 or a widespread pain index of 4–6 and a symptom severity scale score ⩾ 9] | FM: 50.5 ± 8.7 MDD: 45.2 ± 15.9 HC: 43.8 ± 13.0 |
63 F | 6.7 (5.8–8.2) | VAS | Beck Depression Inventory II | State and trait anxiety | Pain Catastrophi-zing Scale | Major depressive
disorder and Healthy controls |
| Caumo (2016) | Cross-sectional | 114 | According to the 2010 American College of Rheumatology criteria | FM: 50.42 ± 8.84 MPS: 46.13 ± 12.10 OA: 64.42 ± 7.81 HS: 32.43 ± 10.81 |
114 F | Last 7 days: FM: 7.94 ± 1.89 MPS: 7.23 ± 2.19 OA: 6.26 ± 2.15 HS: Not availahle Last 24 h: FM: 7.10 ± 1.88 MPS: 6.11 ± 2.59 OA: 5.37 ± 2.47 HS: NA |
VAS | Beck Depression Inventory II | N/A | Pain Catastrophi-zing Scale | Myofascial pain syndrome; Osteoarthritis
and Healthy subjects |
| Clampide (2017) | Cross-sectional | 47 | According to the 2010 American College of Rheumatology criteria. | 54.2 ± 9.9 | 47 F | 22.8 ± 7.2 | VAS | N/A | N/A | N/A | Healthy controls |
| Kaziyama (2020) | Cross-sectional | 43 | According to the 2010 American College of Rheumatology criteria | Symmetrical FM: 49.4 ±
6.2 Asymmetrical FM: 55.0 ± 13.7 |
43 F | Symmetrical FM: 22.7 ±
7.5 Asymmetrical FM: 28.3 ± 3.9 |
BPI VAS | 21-item Hospital Anxiety and Depression Scale | 21-item Hospital Anxiety and Depression Scale | N/A | Symmetrical Fibromyalgia; Asymmetrical fibromyalgia and Health controls |
| Kukseymen (2020) | Cross-sectional | 26 | According to the 2010 American College of Rheumatology criteria | 53 (47 to 58) | 23F/3 M | 5.98 ± 2.01 | VAS | Beck Depression Inventory | VAS for anxiety | N/A | Combined aerobic exercise and tDCS |
| Salerno (2000) | Cross-sectional | 31 | According to the 1990 American College of Rheumatology criteria | FM: 50.07 ± 5.6 RA: 50.04 ± 5.1 |
31 F | N/A | N/A | N/A | N/A | N/A | Healthy controls (age-matched) and Rheumatoid Arthritis |
| Tiwari (2021) | Cross-sectional | 64 | 2016 American College of Rheumatology: generalized pain for at least 3 mons and a widespread pain index) ⩾ 7 and symptom severity scale ⩾ 5 or a widespread pain index of 4–6 and a symptom severity scale score ⩾ 9] | FM: 40.6 ± 8.7; Pain-free controls: 36.5 ± 9.2 |
64 F | N/A | N/A | N/A | N/A | N/A | Pain-free controls |
| Antal (2010) | RCT/cross-over | 23 | Diagnosed by a neurologist at least two years before the start of the study | 28–70 | 6 M/17 F | 7.11±1.2 – Anodal
tDCS; 7.0 ±1.5 – Sham tDCS; 5.8 ±2.2/2.95 ±2.2 – Active and sham tDCS |
VAS | N/A | N/A | N/A | Sham tDCS |
Note: AE: aerobic excercise, BPI: brief pain inventory, F: females, HC: healthy controls, FM: fibromyalgia, M: males, MD: muscular dystrophy, MDD: major depression disorder, MPS: myofascial pain syndrome, N/A: not available or not applied, OA: osteoarthritis, RA: rheumatoid arthritis, RCT: randomized controlled trial, rTMS: repetitive transcranial magnetic stimulation, tDCS: transcranial direct current stimulation, VAS: visual analogue scale, VNS: visual numeric scale, SD: standard deviation.
Table 2.
TMS experiments description
| Study | Area of interest | Type of coil | Muscle Evaluated | Outcome measure | Parameters | Risk of Bias score |
|---|---|---|---|---|---|---|
| Bement (2014) | Motor cortex (M1) | Round coil | Brachioradialis muscle | Motor evoked potential (MEP) amplitude | They determined the MEP threshold by applying a single pulse of maximum stimulator output in M1 to generate an MEP in the muscle evaluated - at an intensity of 40%–70%- and used the intensity of 120% of this threshold in each session. | 17 |
| Carinal (2019) | M1 | Figure-eight coils | First dorsal interosseous (FDI) | Short intracortical inhibition (SICI), intracortical facilitation (ICF), MEP and cortical silent period (CSP) | The motor threshold (MT) was assessed as the lowest stimuli to induce 50% of the evoked potential in the resting FDI. They used a single-pulse TMS protocol with 130% of the intensity of the MT applied to record ten MEP. The CSP was measured during muscle activity - twenty percent of maximal force). The authors also used an interstimulus interval of 2 and 12 ms and paired-pulse to measure ICF and SICI. | 15 |
| Caumo (2016) | M1 | Figure-eight coils | FDI | Short ICF, SICI, MEP and CSP. | The authors determined the resting MT
(RMT) using 50% of ten consecutive trials MEPs and then recorded another ten MEPs using 130% intensity of the individual RMT. The CSPs were measured during muscle activity assessed on a dynamometer set to approximately 20% of the maximal force. Also, the authors used paired pulse to measure ICI and SICI, with an interstimulus interval of 2 and 12 ms, respectively. |
14 |
| Ciampide Andrade (2017) | M1 | Circular shaped coil | Thenar eminences | Rest MT (RMT), MEP, Short intracortical Inhibition (SICI) and ICF |
SICI and ICF were measured at an interstimulus interval of 2 and 4 ms and at 10 and 15 ms, respectively. The authors didn’t describe how they assess the RMT and MEP. | 12 |
| Kaziyama (2020) | M1 | Circular coil | FDI | RMT, MEPs, SICI, and ICF | The RMT was assessed as the lower intensity to elicit an MEP of at least 50 μV in 50% of trials. They investigate intracortical modulation using paired pulse; interstimulus intervals (ISIs) of 2 and 4 ms were used to measure SICI, while ISIs of 10 and 15 ms were used to assess ICF. | 15 |
| Kukseymen (2020) | M1 | figure-of-eight coil | FDI | RMT, MEPs, SICI, and ICF | The authors assessed the RMT as the lower intensity to elicit an MEP of at least 100 μV in 3/5 of trials in the relaxed muscle. The MEP was assessed as 120% of the MT intensity and the ISI for SICI and ICF was 2 ms and 10 ms, using the paired pulse protocol. | 15 |
| Deitos (2018) | M1 | Figure-eigh coil | FDI | CSP, SICI, RMT and MEP | The RMT was defined as the lower intensity to elicit an MEP of at least 50 μV in 10 consecutive trials. The MEP was recorded as 130% of the MT intensity, and the CSPs were assessed with a dynamometer as 20% of the maximal force during the muscle contraction and recorded using an intensity of 130% of the RMT. SICI and ICF were measured using a paired-pulse TMS protocol, using an ISI of 2 and 4 ms for the SICI and 9 and 12 ms for the ICF. | 12 |
| Mhala (2011) | M1 | figure-8-shaped coil | First interosseous muscle (FDI) | MEP, RMT, SICI and ICF | The authors tested the RMT as the minimal intensity to elicit an MEP of at least 50 μV in 50% of trials. Using a paired-pulse protocol, they recorded the intensity of the MEP test stimuli as 120% of the RMT. They also used ISIs of 2 and 4 ms for SICI and 10 and 15 ms for ICF. | 12 |
| Mendonca (2016) | M1 | Figure-of-eight coil | Adductor muscle of the thumb | MT, MEP, intracortical inhibition, and ICF | RMT was determined as the lower intensity to generate an MEP of at least 50 mV in 10 trials; to determine the MEP, they used 120% of the resting MT. The inter stimulus interval was 10 ms for ICF and 2 ms for intracortical inhibition, using the paired-pulse technic. | 14 |
| Mhala (2010) | M1 | Figure-of-eight-shaped coil | First interosseous muscle (FDI) | RMT, MEP, short ICF and ICF | They investigate SICI and ICF with an ISI of 2 and 4 ms and 10 and 15 ms, respectively. Single-pulse stimulation was used to measure the RMT, using paired pulses with the Intensity of the conditioning stimulus as 80% of the RMT. For MEPs, the test stimuli were measured as 120% of the RMT. | 13 |
| Schwenkreis (2010) | M1 | Circular coil | Flexor muscle of the forearm | MT, CSP, SICI and ICF | The MT was defined as the minimum intensity to produce five motors evoked potentials > 50 mV out of 10 trials. The authors measured the ICI and ICF at inhibitory ISIs of 2 and 4 ms and facilitatory intervals of 10 and 15 ms. They also assessed the CSP by applying a stimulation 50% above the MT while the subject was contracting muscle with 20–30% of maximum voluntary strength. | 13 |
| Alventosa (2020) | M1 | Figure-of-eight-shaped | First dorsal interosseous | RMT | The authors only describe the RMT, and defined it as the minimum stimuli to elicit an evoked motor potential of at least 50 microvolts in 50% of the tests. | 9 |
| Salerno (2000) | M1 | Double cone coil | First dorsal interosseous muscle (FDI) and tibialis anterior | RMT, MEP, SICI and ICF | The TMS was performed with a “double cone” coil, targeting the FDI muscle. The stimulation was set at 150% of the relaxed motor threshold. The pair-pulse protocol was performed with 4, 25, 55, 85, 100, 155, 200, 255, and 355 ms intervals. | 13 |
| Tiwari (2021) | M1 | Figure-of-8 coil | Thumb muscles | RMT and MEP | The authors defined the RMT as the minimum stimulus to generate an evoked potential with a peak-to-peak amplitude of at least 50 mV at 50% of ten trials, and the MEP measure as the peak-to-peak amplitude elicited by averaging ten consecutive stimuli at 100% of the RMT. | 14 |
| Antal (2010) | M1 | Standard double 70 mm coil | FDI | RMT, active MT, CSP, SICI and ICF | RMT was defined as the minimum output to induce an MEP of 50 mV in at least three of six consecutive trials. The authors also described the active MT as the lowest stimulus intensity to generate three of six consecutive MEP in the tonically contracting FDI muscle. SICI was measured with ISIs of 2 and 4 ms, and ICF with ISIs of 9 and 12 ms. | 17 |
Note: CSP: cortical silent period, FDI: First dorsal interosseous, ICF: intracortical facilitation, ICI: intracortical inhibition, ISIs: interstimulus intervals, M1:Motor cortex, MEP: Motor evoked potential
MEP: motor evocate potential, MT: Motor threshold, RMT: resting motor threshold, TMS: transcranial magnetic stimulation, SICI: Short intracortical inhibition.
Quality of included studies
We evaluated the quality of the 15 included studies, the range of points was from 9 to 17 (out of 26 possible applicable domains), which represents a range from low to moderate quality (35 to 65% of maximum score). They mostly did not report adequately the following domains: “Coil location and stability (with or without a neuronavigation system)” (95%), “Amount of relaxation/contraction of target muscles” (95%), “Subjects prescribed medication” (77%), and “Prior motor activity of the muscle to be tested” (77%). All studies did not report the “Level of relaxation of muscles other than those being tested,” “Any medical conditions,” “History of specific repetitive motor activity,” and “Subject attention (level of arousal) during testing” (Additional Table 3).
Baseline comparison
Of the eight observational studies identified, seven were included in the meta-analysis (one study was excluded due to the lack of a control group), but not all studies reported the five TMS metrics. We compared FM patients against any type of control. The pooled estimate was statistically significant different for rMT (MD = 6.90 μV, 95% CI 4.16 to 9.63 μV) and SICI (MD = −0.40, 95% CI −0.69 to −0.11) in FM patients (n = 226) compared to controls (n = 255). Thus, FM patients have higher rMT and less intracortical inhibition compared to controls. The rMT pooled estimate had smaller between-study heterogeneity (I2 = 0%), compared to the SICI meta-analysis (I2 = 93%). The SICI was also lower when compared to only healthy subjects (MD = −0.57, 95% CI −1.04 to −0.10, I2=93%) and patients with major depression disorder (MD = −0.48, 95% CI −0.78 to −0.18), but no different than other musculoskeletal conditions (such as osteoarthritis and myofascial pain) (Figure 2). We found no difference between MEP, CSP, and ICF (Additional Figures 1–3).
Figure 2 – Forest plots of cross-sectional comparison.
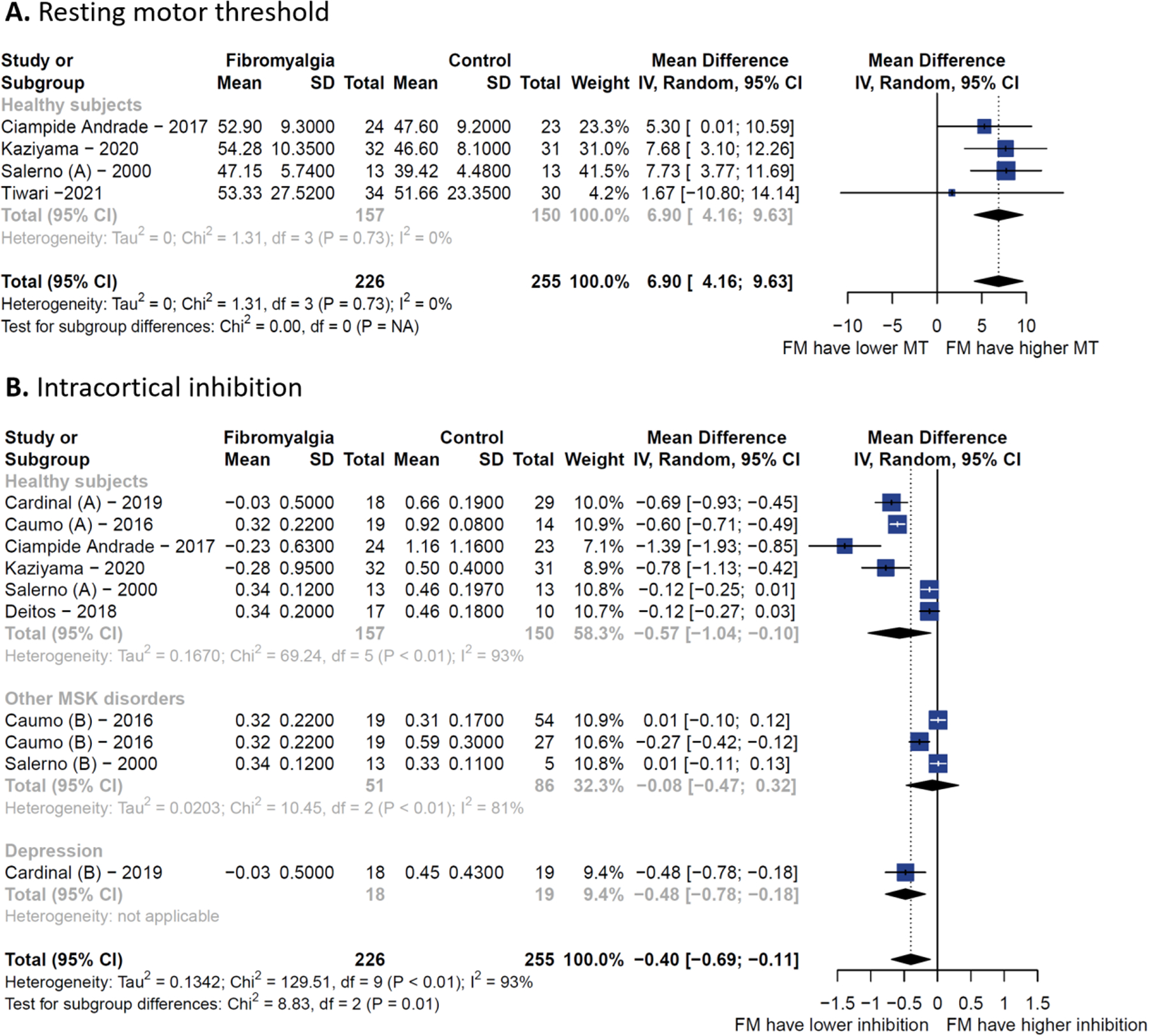
(A) Resting motor threshold. (B) Short intracortical inhibition.
Longitudinal changes after treatment
When analyzing longitudinal changes of TMS metrics from RCTs (six out of seven included, one study was excluded due to lack of extractable data), we found that SICI (MD=0.19, 95% CI 0.10 to 0.29) and CSP (MD = 14.92 ms, 95% CI 4.86 to 24.98 ms) were increased after active interventions (n = 178) compared to sham/placebo (n = 151). Therefore, the disrupted intracortical inhibition of FM patients was temporarily restored after active treatments. The SICI pooled estimate had larger between-study heterogeneity (I2 = 66%), compared to the CSP meta-analysis (I2 = 0%). The interventions included in the meta-analysis were M1 rTMS, M1 tDCS, exercise, and pregabalin. For SICI, non-invasive brain stimulation (MD = 0.21, 95% CI 0.05 to 0.37, I2 = 74%) and pregabalin (MD = 0.20, 95% CI 0.05 to 0.35) were the subgroups that reported the largest intracortical inhibitions improvements (Figure 3). We did not find any difference in rMT, MEP, and ICF (Additional Figures 4–6).
Figure 3. Forest plots of longitudinal changes.
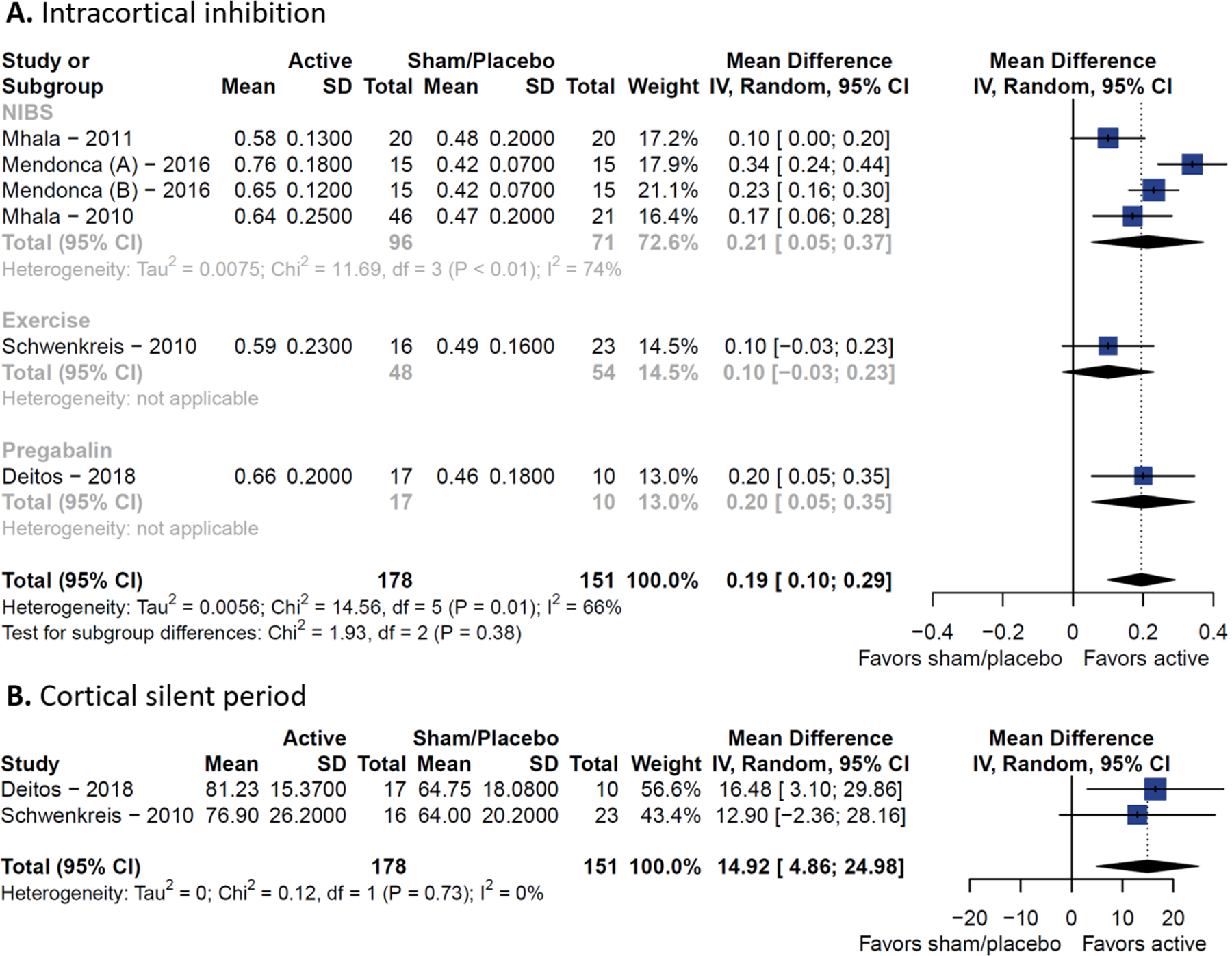
(A) Resting motor threshold. (B) Short intracortical inhibition.
A summary of baseline and longitudinal comparison of TMS metrics by subgroups is presented in Table 3.
Table 3.
Summary of pooled effects
| Metric | n | Mean differences | 95% confidence interval | I 2 |
|---|---|---|---|---|
| Biomarker | ||||
| MT* | 4 | 6.9 | 4.16 to 9.63 | 0% |
| ICI | 10 | −0.4 | −0.69 to −0.11 | 93% |
| Healthy | 6 | −0.57 | −1.04 to −0.10 | 93% |
| Other MSK | 3 | −0.08 | −0.47 to 0.32 | 81% |
| Depression | 1 | −0.48 | −0.78 to −0.18 | - |
| CSP | 7 | −7.82 | −40.77 to 25.14 | 94% |
| Healthy | 4 | −20.34 | −92.66 to 51.98 | 96% |
| Other MSK | 2 | 2.61 | −37.00 to 42.21 | 0% |
| Depression | 1 | 18.63 | 8.08 to 29.18 | - |
| ICF | 7 | −0.25 | −0.69 to 0.20 | 93% |
| Healthy | 4 | −0.15 | −0.92 to 0.62 | 95% |
| Other MSK | 2 | −0.11 | −1.10 to 0.89 | 0% |
| Depression | 1 | −1.06 | −1.53 to −0.59 | - |
| MEP | 10 | 0.31 | −0.59 to 1.21 | 88% |
| Healthy | 6 | 0.35 | −0.86 to 1.57 | 83% |
| Other MSK | 3 | 0.65 | −4.43 to 5.74 | 90% |
| Depression | 1 | −0.28 | −0.54 to −0.02 | - |
| Predictor | ||||
| CSP** | 2 | 14.92 | 4.86 to 24.98 | 0% |
| ICI | 6 | 0.19 | 0.10 to 0.29 | 66% |
| NIBS | 4 | 0.21 | 0.05 to 0.37 | 74% |
| Exercise | 1 | 0.1 | −0.03 to 0.23 | - |
| Pregabalin | 1 | 0.2 | 0.05 to 0.35 | - |
| ICF | 5 | 0.78 | −0.27 to 1.82 | 94% |
| NIBS | 4 | 1.03 | −0.16 to 2.21 | 93% |
| Exercise | 1 | −0.19 | −0.78 to 0.40 | - |
| MT** | 5 | −1.79 | −5.62 to 2.03 | 0% |
| MEP** | 2 | 0.21 | −0.42 to 0.83 | 95% |
Note: CSP: cortical silent period, ICI: intracortical inhibition, MEP: motor evocate potential, MSK: musculoskeletal disorders, MT: Motor threshold, NIBS: non-invasive brain stimulation.
all vs healthy subjects
all vs sham/placebo.
Correlation of TMS changes and clinical improvement
Of the eight RCTs, only four studies reported a correlational analysis between TMS metrics and clinical outcomes (changes in pain intensity, pain thresholds, fatigue, depression, and pain catastrophizing). Two studies found that changes in SICI (increased of inhibition) after treatments (M1 rTMS and M1 anodal tDCS) were moderately correlated with pain intensity reduction (r = −0.64, P = 0.007), and one study found a weak correlation between depression and pain catastrophizing improvements (r = 0.32, P = 0.04, r = 0.42, P = 0.01, respectively). Similarly, two studies found that reductions in ICF after M1 rTMS were moderately correlated with fatigue reduction (r = 0.47, P = 0.02). Lastly, one study reported that a small MEP after exercise was correlated with less pain intensity and higher pain thresholds (r = −0.51, P = 0.004, r = 0.51, P = 0.01, respectively). A visual summary of the correlational findings is reported in Figure 4.
Figure 4. Pearson’s correlation betweenTMS metrics changes and clinical improvement.
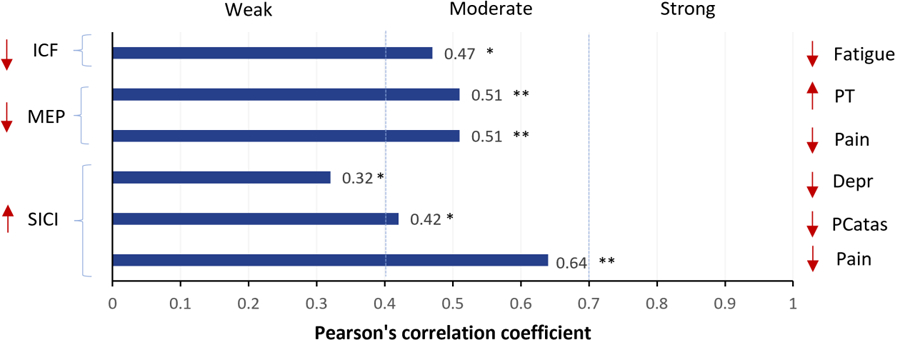
Note: ICF and SICI data were extracted from 2 studies (Mhala et al. 2010 and 2011), the active intervention was M1 rTMS. MEP data was extracted from 1 study (Bement et al. 2014), the active intervention was exercise. *P < 0.05, **P < 0.01. Depr: depression; ICF: Intracortical facilitation; MEP: motor-evoked potential; Pcatas: pain catastrophizing; PT: pain threshold; SICI: short intracortical inhibition.
Evidence certainty
Of the ten outcomes (five TMS metrics from baseline analyses and five from longitudinal analyses), eight had a very low evidence certainty, mostly due to the moderate to high risk of bias, high heterogeneity (inconsistency), and imprecise CI. The pooled estimates of SICI and CSP after treatments had the most reliable evidence certainty. The outcome of longitudinal SICI changes presented a low certainty (due to moderate risk of bias, and substantial heterogeneity), and the outcome of longitudinal CSP changes presented moderate certainty (only penalized due to moderate risk of bias) (Table 4).
Table 4.
GRADE assessment - summary of findings
| Outcomes | Anticipated absolute
effects |
№ of participants (N° estimates; studies’ design) | Certainty of the evidence (GRADE) | Comments | |
|---|---|---|---|---|---|
| Range of Means in Control | Mean in Group, estimate and 95% CI | ||||
| MT | 39.42 to 51.66 | MD 6.90 higher (4.16 to 9.63) | 200 (4; non-RCTs) | ⨁◯◯◯ Very low a, b, c, d, e |
Patients with fibromyalgia have higher MT. |
| ICI | 0.31 to 1.16 | MD 0.40 lower (−0.69 to −0.11) | 481 (10; non-RCTs) | ⨁◯◯◯ VERY LOW a, b, c, e, f |
Patients with fibromyalgia have lower inhibition (ICI). |
| CSP | 48.58 to 185.85 | MD 7.82 lower (−40.77 to 25.14) | 289 (7; non-RCTs) | ⨁◯◯◯ VERY LOW a, b, c, f, g |
Patients with fibromyalgia have no differences in CSP. |
| ICF | 0.71 to 1.89 | MD 0.25 lower (−0.69 to 0.20) | 346 (7; non-RCTs) | ⨁◯◯◯ VERY LOW a, b, c, f, g |
Patients with fibromyalgia have no differences in facilitation. |
| MEP | 0.34 to 1.64 | MD 0.31 higher (−0.59 to 1.21) | 454 (10; non-RCTs) | ⨁◯◯◯ VERY LOW a, b, c, f, g |
Patients with fibromyalgia have no differences in MEP. |
| MT | 45.25 to 71.70 | MD 1.79 lower (−5.62 to 2.03) | 212 (5; RCTs) | ⨁◯◯◯ VERY LOW h, i, c, d, g |
Patients with fibromyalgia have no changes in MT after treatment. |
| ICI | 0.42 to 0.49 | MD 0.19 higher (0.10 to 0.29) | 233 (6 RCTs) | ⨁⨁◯◯ LOW h, b, c, f, e |
Patients with fibromyalgia have increases in ICI after treatment. |
| CSP | 64.00 to 64.75 | MD 14.92 higher (4.86 to 24.98) | 66 (2 RCTs) | ⨁⨁⨁◯ MODERATE h, b, c, d, e |
Patients with fibromyalgia have increases in CSP after treatment. |
| ICF | 1.91 to 3.13 | MD 0.78 higher (−0.27 to 1.82) | 206 (5 RCTs) | ⨁◯◯◯ VERY LOW h, b, c, f, g |
Patients with fibromyalgia have no changes in ICF after treatment. |
| MEP | 0.60 to 1.44 | MD 0.21 higher (−0.42 to 0.83) | 60 (2 RCTs) | ⨁◯◯◯ VERY LOW h, b, c, f, g |
Patients with fibromyalgia have no changes in MEP after treatment. |
Note:
The certainty of the evidence started at low due to the inclusion of non-RCTs.
In most of the included studies moderate risk of bias was detected (40–60% of the items were correct). We downgraded one level due to risk of bias.
The population included is extrapolatable to the population of the research question. We did not downgrade due to indirectness.
There is overlap of confidence intervals suggesting only small variation. No statistical heterogeneity. We did not downgrade for inconsistency.
The confidence intervals were relatively narrow and did not include the point of no effect. We did not downgrade due to imprecision.
There is moderate overlap of confidence intervals suggesting considerable variations. Substantial statistical heterogeneity (I2 higher 60%). We downgraded one level due to inconsistency.
The confidence intervals were broad and included the point of no effect. We downgraded one level due to imprecision.
The certainty of the evidence started at high due to the inclusion of RCTs only.
The included studies presented high to moderate risk of bias. We downgraded two levels due to risk of bias.
CSP: cortical silent period,ICI: intracortical inhibition, ICF: intracortical facilitation, MD: mean differences, MEP: motor evocate potential, MT: Motor threshold, RCT: randomized controlled trial.
DISCUSSION
Main findings
Our meta-analysis suggested that: (1) FM subjects have an unbalanced inhibitory-excitatory motor cortex regulation with less intracortical inhibition (MD=-0.40, 95% CI −0.69 to −0.11) and higher resting motor thresholds (MD = 6.90 μV, 95% CI 4.16 to 9.63 μV) when compared to controls. (2) This inhibitory dysfunction can be modulated by some interventions such as exercise, pregabalin, and non-invasive brain stimulation, which can increase intracortical inhibition (MD = 0.19, 95% CI 0.10 to 0.29) and CSP (MD = 14.92 ms, 95% CI 4.86 to 24.98 ms), when compared to placebo or sham interventions. This neurophysiological profile is also similar to neuropathic pain syndromes such as phantom limb pain 22,23. Finally, (3) changes in intracortical inhibition and facilitation are correlated with clinical improvements: higher inhibition moderately correlates with less pain, depression, and pain catastrophizing; on the other hand, lower facilitation moderately correlates with less fatigue. These associations were observed after exercise and non-invasive brain stimulation. However, due to high between-study heterogeneity, and the high risk of bias, the evidence certainty ranged from very low to moderate.
Motor cortex inhibition is altered in FM subjects
We found a robust difference in SICI when comparing FM subjects to controls, confirming a decreased inhibitory tonus in FM. These findings are aligned with our previous studies 24, also with the previous meta-analysis in mixed chronic pain populations 25. The exact pathophysiology of FM is unknown, although neuroimaging studies have associated the syndrome with alterations in the balance of inhibitory/excitatory networks when compared to healthy individuals 26,27. The findings of these studies suggest a dysregulated excitatory/inhibitory system that is mediated by GABA and glutamate 28. Pomares et al. 29performed a randomized, double-blinded, crossover trial using a positron emission tomography with Flumazenil tracer to identify an upregulation in GABA-A receptors compared to healthy controls. The total mean of the GABAa receptor in the FM group was 11–31% higher than the control. Also, these authors demonstrate the concentration of this receptor is positively associated with increased functional impairment and pain scores. These findings suggest that some of the symptomatology observed in FM patients is linked to inhibitory maladaptation, which is congruent with the findings of this review, since it has been reported that SICI is primarily depending on GABA-A neurotransmission30. Therefore, we hypothesize that there is an upregulation of the GABA-A receptors secondary to low availability of the neurotransmitter and therefore lower inhibition capacity in FM patients compared to healthy individuals. However, the initial trigger of this inhibitory dysregulation is unclear, although recent evidence suggests sensory deafferentation due to small fiber neuropathy 31 and a neuroimmune response 32. Interestingly, we found no differences in SICI when comparing FM to other musculoskeletal disorders. Although this opens up the question of the specificity of our findings, there were only three studies with small sample sizes and the results were mixed. Also, there is a possibility that there is an index of neuropathic pain characteristics across other musculoskeletal conditions that deserves future investigation. Future studies with multimodal assessment and transdiagnostic approaches are needed to elucidate this question.
Motor control dysfunction in FM
One of our findings suggested higher corticospinal excitability in FM, which is related to motor function and motor control. The framework that defines motor dysfunction as a feature of FM syndrome has been initially supported by data showing that FM patients perform worse in clinical parameters of motor functionality than controls – as evidenced by worse motor and walking speed and other kinematics of gait and balance 33–36. A recent experimental trial found FM to be even more severely impaired in these metrics than chronic low back pain subjects 37. Moreover, this composition has been supported by neurophysiological data pointing to deficits specifically in psychomotor processing during motor control/inhibition tasks. Canny et al. showed that FM patients had impairment of fine motor function and manual dexterity than healthy controls, as assessed with the Box and Block Test of Manual Dexterity 38. These findings were confirmed by Pérez-de-Heredia-Torres et al. (39 that further uncovered such impaired dexterity to be also present in activities of daily life (e.g., writing, turning over cards, stacking checkers), in a sample of FM women without upper-limp sensory symptoms. Interestingly, that was not associated with clinical pain, disease duration, or severity. More recently, Rasouli et al. showed that FM patients performed statistically worse than healthy controls in gross motor function, as measured by reaction time to gait initiation in response to auditory stimuli (adjusted mean difference 0.034; 95% CI 0.013 to 0.054, P = 0.001), while also presented with worse fine motor skills, although failing to reach statistical significance 40. We believed the motor functional alterations and their neurophysiological surrogates could be included in the evaluation of FM patients, even though they are not part of the diagnosis criteria, they can severely impact the quality of life.
Cortical inhibition markers can be used for monitoring treatment response
One important finding is that the alteration of cortical inhibition in FM seems to be reversible after using treatments that engaged sensorimotor systems (e.g., exercise and non-invasive motor cortex stimulation) and inhibitory networks (e.g., pregabalin). This is aligned with basic findings on GABAergic neuroplasticity. It is well known that external stimuli have the potential to interact with the GABAergic systems, for instance sensory experience can induce specific plastic changes at inhibitory synapses 41, as well as, motor stimuli, as Ziemann et al. 42 showed in humans that a decrease in GABA-related inhibition facilitates practice-dependent plasticity in the cortex. Particularly, tDCS can modulate GABA and glutamate neurotransmission, especially after combination with a behavioral intervention such as exercise or motor imagery 23,43–45. Also, there is evidence in the literature that high‐intensity exercise physical exercise can increase sensorimotor cortex GABA concentration increased by 20% 46. Taken together, these interventions can restore the inhibitory tonus in FM and could be monitored longitudinally using TMS metrics.
Interestingly, we also found that changes in SICI correlate with clinical improvement (pain and mood), which provides initial validation of cortical inhibition measures as a biomarker of treatment response in FM. Previous studies from our research group found that TMS metrics (including SICI) do not correlate to clinical manifestations at baseline (e.g., pain and mood) in FM 24 and phantom limb patients 22, likely due to compensatory mechanisms. Conversely, based on our findings, SICI appears to be reactive to the acute engagement of sensorimotor systems, thus having longitudinal association instead of cross-sectional. Although promising, the validation of intracortical inhibition as a treatment response biomarker requires well-powered longitudinal study designs, which at the moment are inexistent in the FM field.
Another important point is to understand motor intracortical inhibition as a neurophysiological concept that can be measured by TMS, but also by another type of assessments. TMS, despite being a valuable way of measuring cortical inhibition, is an assessment with some challenges for large-scale, real-world applications, such as the operator-dependent factor and high cost. Simpler assessments, such as the Stop-Signal Reaction Time task - a type of go/no-go task - could prove to be a feasible option to measure cortical inhibition – and, therefore, the GABAergic system in FM. For instance, Tran et al. 47 showed that the Stop-Signal Reaction Time task is correlated with the strength of GABA-A-mediated short-intracortical inhibition (r = 0.442, P < 0.009). More comparative studies are needed to explore more feasible metrics of motor intracortical inhibition.
Research recommendations
In order to accelerate the translation to clinic of cortical inhibition measures for FM, several methodological and analytical issues need to be refined in future studies. The proper reporting of TMS parameters following a standardized checklist could facilitate reproducibility, data interpretation, and meta-research 17. Furthermore, another issue observed in the reviewed studies was the lack of standardization between MEP intensities and interstimulus intervals. Some of the most important findings of our study were related to SICI measurements; however, the interpretability of the data was difficult to assess since some authors failed to report the direct amount of inhibition when measuring this parameter. Further studies should clarify the TMS metrics calculation and, if possible, report greater values of ICI as a larger amount of inhibition to improve the interpretability 17. Finally, to further assess the role of ICI and CSP as prognostic and surrogate biomarkers, adjusted correlation analysis (with multivariate regression models) is required to link these surrogate outcomes to clinical outcomes. Few studies in this meta-analysis performed this correlation. Further research also requires assessing causal pathways of treatment response using mediation analysis and machine learning algorithms.
Strengths and limitations
The present study comprises a systematic search throughout the most updated databases and integrates different study designs. Therefore, the findings are a compilation of what is known about the role of TMS metrics in FM. Also, the included studies were critically evaluated (quality and evidence certainty assessments), thus providing suggestions for future research. However, the evidence on TMS metrics in FM is still scarce and heterogeneous, so our results are mostly exploratory and require further validation, although we performed subgroup and sensitivity analyses were made to understand this heterogenicity, many papers are poorly reported, which limits the results’ interpretation.
CONCLUSIONS
Measures of intracortical inhibition and facilitation indexed by TMS are potential diagnostic and treatment response biomarkers for FM patients. Treatments targeting the sensorimotor systems (e.g., exercise, motor imagery, and non-invasive brain stimulation) could restore the cortical inhibitory tonus in FM and induce clinical improvement. Measures of cortical inhibition could be used to objectively monitor treatment response. Finally, treatments developed for neuropathic pain may have positive outcomes in FM given the findings of this study. Future studies need to validate these metrics with larger sample sizes and more standardized assessments.
Supplementary Material
Additional Table 1: PRISMA Checklist
Additional Table 2: Search terms.
Additional Table 3: Risk of bias evaluation of included TMS studies.
Additional Figure 1: Forest plot of baseline cortical silent period differences.
Additional Figure 2: Forest plot of baseline intracortical facilitation differences.
Additional Figure 3: Forest plot of baseline motor evocate potential differences.
Additional Figure 4: Forest plot of longitudinal intracortical facilitation.
Additional Figure 5: Forest plot of longitudinal motor evoked potential.
Additional Figure 6: Forest plot of longitudinal rest motor threshold.
Funding:
This work is supported by NIH grant R01 AT009491-01A1.
Footnotes
Conflicts of interest
The authors declare no competing financial interests.
Availability of data and materials
All data generated or analyzed during this study are included in this published article and its supplementary information files.
REFERENCES
- 1.Sarzi-Puttini P, Giorgi V, Marotto D, Atzeni F. Fibromyalgia: an update on clinical characteristics, aetiopathogenesis and treatment. Nature Reviews Rheumatology. 2020;16:645–660. [DOI] [PubMed] [Google Scholar]
- 2.Jones GT, Atzeni F, Beasley M, Flüß E, Sarzi‐Puttini P, Macfarlane GJ. The prevalence of fibromyalgia in the general population: a comparison of the American College of Rheumatology 1990, 2010, and modified 2010 classification criteria. Arthritis & rheumatology. 2015;67:568–575. [DOI] [PubMed] [Google Scholar]
- 3.Siracusa R, Paola RD, Cuzzocrea S, Impellizzeri D. Fibromyalgia: pathogenesis, mechanisms, diagnosis and treatment options update. International Journal of Molecular Sciences. 2021;22:3891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singh L, Kaur A, Bhatti MS, Bhatti R. Possible molecular mediators involved and mechanistic insight into fibromyalgia and associated co-morbidities. Neurochemical research. 2019;44:1517–1532. [DOI] [PubMed] [Google Scholar]
- 5.Kocak I, Hizmetli S, Tas A, Karadag A, Zontul C, Silig Y. High levels of cathepsin S and cystatin C in patients with fibromyalgia syndrome. International journal of rheumatic diseases. 2020;23:966–969. [DOI] [PubMed] [Google Scholar]
- 6.Castelo-Branco L, Cardenas-Rojas A, Pacheco-Barrios K, Teixeira PEP, Gonzalez-Mego P, Vasquez-Avila K, Cortez PC, Marduy A, Rebello-Sanchez I, Parente J. Can neural markers be used for fibromyalgia clinical management? Principles and Practice of Clinical Research. 2022;8:28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kumbhare D, Hassan S, Diep D, Duarte FCK, Hung J, Damodara S, West DWD, Selvaganapathy PR. Potential role of blood biomarkers in patients with fibromyalgia: a systematic review with meta-analysis. Pain. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Donadel DG, Zortea M, Torres ILS, Fregni F, Caumo W. The mapping of cortical activation by near-infrared spectroscopy might be a biomarker related to the severity of fibromyalgia symptoms. Scientific reports. 2021;11:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Flodin P, Martinsen S, Löfgren M, Bileviciute-Ljungar I, Kosek E, Fransson P. Fibromyalgia is associated with decreased connectivity between pain-and sensorimotor brain areas. Brain connectivity. 2014;4:587–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Oliveira Franco Á, da Silveira Alves CF, Vicuña P, Bandeira J, de Aratanha MA, Torres ILS, Fregni F, Caumo W. Hyper-connectivity between the left motor cortex and prefrontal cortex is associated with the severity of dysfunction of the descending pain modulatory system in fibromyalgia. Plos one. 2022;17:e0247629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caumo W, Deitos A, Carvalho S, Leite J, Carvalho F, Dussán-Sarria JA, Lopes Tarrago MdG, Souza A, Torres ILdS, Fregni F. Motor cortex excitability and BDNF levels in chronic musculoskeletal pain according to structural pathology. Frontiers in Human Neuroscience. 2016;10:357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rossini PM, Burke D, Chen R, Cohen LG, Daskalakis Z, Di Iorio R, Di Lazzaro V, Ferreri F, Fitzgerald PB, George MS. Non-invasive electrical and magnetic stimulation of the brain, spinal cord, roots and peripheral nerves: Basic principles and procedures for routine clinical and research application. An updated report from an IFCN Committee. Clinical neurophysiology. 2015;126:1071–1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hupfeld KE, Swanson CW, Fling BW, Seidler RD. TMS-induced silent periods: A review of methods and call for consistency. Journal of Neuroscience Methods. 2020;346:108950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chandler J, Cumpston M, Li T, Page MJ, Welch VA. Cochrane handbook for systematic reviews of interventions. John Wiley & Sons; 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PlotDigitizer. https://plotdigitizer.com.
- 17.Chipchase L, Schabrun S, Cohen L, Hodges P, Ridding M, Rothwell J, Taylor J, Ziemann U. A checklist for assessing the methodological quality of studies using transcranial magnetic stimulation to study the motor system: an international consensus study. Clinical Neurophysiology. 2012;123:1698–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schünemann H, Brożek J, Guyatt G. GRADE Handbook, GRADE working group. Available at Accessed April. 2019;3. [Google Scholar]
- 19.DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled clinical trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 20.IntHout J, Ioannidis J, Borm GF. The Hartung-Knapp-Sidik-Jonkman method for random effects meta-analysis is straightforward and considerably outperforms the standard DerSimonian-Laird method. BMC medical research methodology. 2014;14:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guyatt GH, Oxman AD, Montori V, Vist G, Kunz R, Brozek J, Alonso-Coello P, Djulbegovic B, Atkins D, Falck-Ytter Y. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of clinical epidemiology. 2011;64:1277–1282. [DOI] [PubMed] [Google Scholar]
- 22.Pacheco-Barrios K, Pinto CB, Velez FGS, Duarte D, Gunduz ME, Simis M, Gianlorenco ACL, Barouh JL, Crandell D, Guidetti M. Structural and functional motor cortex asymmetry in unilateral lower limb amputation with phantom limb pain. Clinical Neurophysiology. 2020;131:2375–2382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunduz ME, Pacheco-Barrios K, Bonin Pinto C, Duarte D, Vélez FGS, Gianlorenco ACL, Teixeira PEP, Giannoni-Luza S, Crandell D, Battistella LR. Effects of combined and alone transcranial motor cortex stimulation and mirror therapy in phantom limb pain: A randomized factorial trial. Neurorehabilitation and Neural Repair. 2021;35:704–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Uygur-Kucukseymen E, Castelo-Branco L, Pacheco-Barrios K, Luna-Cuadros MA, Cardenas-Rojas A, Giannoni-Luza S, Zeng H, Gianlorenco AC, Gnoatto-Medeiros M, Shaikh ES. Decreased neural inhibitory state in fibromyalgia pain: A cross-sectional study. Neurophysiologie Clinique. 2020;50:279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chang W-J, O’Connell NE, Beckenkamp PR, Alhassani G, Liston MB, Schabrun SM. Altered primary motor cortex structure, organization, and function in chronic pain: a systematic review and meta-analysis. The Journal of Pain. 2018;19:341–359. [DOI] [PubMed] [Google Scholar]
- 26.Pomares FB, Roy S, Funck T, Feier NA, Thiel A, Fitzcharles M-A, Schweinhardt P. Upregulation of cortical GABAA receptor concentration in fibromyalgia. Pain. 2020;161:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Foerster BR, Petrou M, Edden RAE, Sundgren PC, Schmidt‐Wilcke T, Lowe SE, Harte SE, Clauw DJ, Harris RE. Reduced insular γ‐aminobutyric acid in fibromyalgia. Arthritis & Rheumatism. 2012;64:579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petroff OAC. Book review: GABA and glutamate in the human brain. The Neuroscientist. 2002;8:562–573. [DOI] [PubMed] [Google Scholar]
- 29.Pomares FB, Funck T, Feier NA, Roy S, Daigle-Martel A, Ceko M, Narayanan S, Araujo D, Thiel A, Stikov N. Histological underpinnings of grey matter changes in fibromyalgia investigated using multimodal brain imaging. Journal of Neuroscience. 2017;37:1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Di Lazzaro V, Oliviero A, Saturno E, Dileone M, Pilato F, Nardone R, Ranieri F, Musumeci G, Fiorilla T, Tonali P. Effects of lorazepam on short latency afferent inhibition and short latency intracortical inhibition in humans. The Journal of physiology. 2005;564:661–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grayston R, Czanner G, Elhadd K, Goebel A, Frank B, Üçeyler N, Malik RA, Alam U. A systematic review and meta-analysis of the prevalence of small fiber pathology in fibromyalgia: Implications for a new paradigm in fibromyalgia etiopathogenesis. Seminars in arthritis and rheumatism. 2019;48:933–940. [DOI] [PubMed] [Google Scholar]
- 32.Fanton S, Sandström A, Tour J, Kadetoff D, Schalling M, Jensen KB, Sitnikov R, Ellerbrock I, Kosek E. The translocator protein gene is associated with endogenous pain modulation and the balance between glutamate and γ-aminobutyric acid in fibromyalgia and healthy subjects: a multimodal neuroimaging study. Pain. 2022;163:274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Auvinet B, Bileckot R, Alix A-S, Chaleil D, Barrey E. Gait disorders in patients with fibromyalgia. Joint Bone Spine. 2006;73:543–546. [DOI] [PubMed] [Google Scholar]
- 34.Heredia Jiménez JM, Aparicio García-Molina VA, Porres Foulquie JM, Delgado Fernández M, Soto Hermoso VM. Spatial-temporal parameters of gait in women with fibromyalgia. Clinical rheumatology. 2009;28:595–598. [DOI] [PubMed] [Google Scholar]
- 35.Costa IdS, Gamundí A, Miranda JGV, França LGS, De Santana CN, Montoya P. Altered functional performance in patients with fibromyalgia. Frontiers in human neuroscience. 2017;11:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gentile E, Ricci K, Delussi M, de Tommaso M. Motor cortex function in fibromyalgia: a pilot study involving near-infrared spectroscopy and co-recording of laser-evoked potentials. Functional Neurology. 2019;34:107–118. [PubMed] [Google Scholar]
- 37.Mingorance JA, Montoya P, Miranda JGV, Riquelme I. An Observational Study Comparing Fibromyalgia and Chronic Low Back Pain in Somatosensory Sensitivity, Motor Function and Balance. Healthcare. 2021;9:1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Canny ML, Thompson JM, Wheeler MJ. Reliability of the box and block test of manual dexterity for use with patients with fibromyalgia. The American Journal of Occupational Therapy. 2009;63:506–510. [DOI] [PubMed] [Google Scholar]
- 39.Pérez-de-Heredia-Torres M, Martínez-Piédrola RM, Cigarán-Méndez M, Ortega-Santiago R, Fernández-de-Las-Peñas C. Bilateral deficits in fine motor control ability and manual dexterity in women with fibromyalgia syndrome. Experimental brain research. 2013;226:137–143. [DOI] [PubMed] [Google Scholar]
- 40.Rasouli O, Fors EA, Borchgrevink PC, Öhberg F, Stensdotter A-K. Gross and fine motor function in fibromyalgia and chronic fatigue syndrome. Journal of pain research. 2017;10:303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Griffen T, Maffei A. GABAergic synapses: their plasticity and role in sensory cortex. 2014;8. doi: 10.3389/fncel.2014.00091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ziemann U, Muellbacher W, Hallett M, Cohen LG. Modulation of practice-dependent plasticity in human motor cortex. Brain. 2001;124:1171–1181. [DOI] [PubMed] [Google Scholar]
- 43.Fregni F, El-Hagrassy MM, Pacheco-Barrios K, Carvalho S, Leite J, Simis M, Brunelin J, Nakamura-Palacios EM, Marangolo P, Venkatasubramanian G. Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. International Journal of Neuropsychopharmacology. 2021;24:256–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pacheco-Barrios K, Cardenas-Rojas A, Thibaut A, Costa B, Ferreira I, Caumo W, Fregni F. Methods and strategies of tDCS for the treatment of pain: current status and future directions. Expert review of medical devices. 2020;17:879–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Castelo-Branco L, Kucukseymen EU, Duarte D, El-Hagrassy MM, Pinto CB, Gunduz ME, Cardenas-Rojas A, Pacheco-Barrios K, Yang Y, Gonzalez-Mego P. Optimised transcranial direct current stimulation (tDCS) for fibromyalgia—targeting the endogenous pain control system: a randomised, double-blind, factorial clinical trial protocol. BMJ open. 2019;9:e032710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Coxon JP, Cash RFH, Hendrikse JJ, Rogasch NC, Stavrinos E, Suo C, Yücel M. GABA concentration in sensorimotor cortex following high-intensity exercise and relationship to lactate levels. J Physiol. 2018;596:691–702. doi: 10.1113/JP274660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tran DMD, Chowdhury NS, McNair NA, Harris JA, Livesey EJ. Linking cortical and behavioural inhibition: Testing the parameter specificity of a transcranial magnetic stimulation protocol. Brain Stimulation. 2020;13:1381–1383. doi: 10.1016/j.brs.2020.07.010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Table 1: PRISMA Checklist
Additional Table 2: Search terms.
Additional Table 3: Risk of bias evaluation of included TMS studies.
Additional Figure 1: Forest plot of baseline cortical silent period differences.
Additional Figure 2: Forest plot of baseline intracortical facilitation differences.
Additional Figure 3: Forest plot of baseline motor evocate potential differences.
Additional Figure 4: Forest plot of longitudinal intracortical facilitation.
Additional Figure 5: Forest plot of longitudinal motor evoked potential.
Additional Figure 6: Forest plot of longitudinal rest motor threshold.


