Abstract
Context
Evidence from animal studies suggests that the gradual rise in follicle-stimulating hormone (FSH) during reproductive senescence may contribute to the change in adiposity distribution characteristic of menopause. The potential independent role the interrelationships of FSH and estradiol (E2) may play in postmenopausal adiposity changes are not well studied.
Objective
Our objective was to evaluate the associations of FSH and dual x-ray absorptiometry (DXA)-derived adiposity measures, with consideration of estradiol and postmenopausal hormone therapy use.
Methods
In a sample of 667 postmenopausal women from the Women’s Health Initiative Buffalo OsteoPerio Ancillary Study, we studied the associations of serum FSH and E2 levels with dual x-ray absorptiometry (DXA)-derived adiposity measures via cross-sectional and longitudinal analyses (5-year follow-up).
Results
In cross-sectional analyses, FSH levels were inversely associated with all measures of adiposity in models adjusted for age, years since menopause, smoking status, pack-years, and hormone therapy (HT) use; these associations were not influenced by adjustment for serum E2. In longitudinal analyses, the subset of women who discontinued HT over follow-up (n = 242) experienced the largest increase in FSH (+33.9 mIU/mL) and decrease in E2 (–44.3 pg/mL) and gains in all adiposity measures in unadjusted analyses. In adjusted analyses, an increase in FSH was associated with a gain in percentage of total body fat, total body fat mass, and subcutaneous adipose tissue (SAT).
Conclusion
While cross-sectional findings suggest that FSH is inversely associated with adiposity, our longitudinal findings suggest that greater increases in FSH were associated with greater increases in percentage of total body fat, total body fat mass, and SAT. Future studies are needed to provide additional insight into FSH-adiposity mechanisms in larger samples.
Keywords: follicle stimulating hormone (FSH), adiposity, body composition, endogenous hhormones, estradiol, women’s Health initiative (WHI)
Menopause is often accompanied by weight gain and/or changes in adiposity distribution, including increases in central fat mass and visceral adipose tissue (VAT), which are associated with adverse health conditions (1-3). The menopausal transition includes decreasing levels of endogenous estrogen and rising follicle-stimulating hormone (FSH); the FSH rise is attributable to loss of negative feedback by ovarian estrogen (4, 5).
Evidence from animal studies suggests that FSH may contribute to adiposity, where interrupting the interaction of FSH with its receptor (via a polyclonal antibody) reduced body fat, VAT and subcutaneous adipose tissue (SAT), and enhanced thermogenesis in mice (6, 7). The few clinical observational studies in humans to date on the association between FSH concentrations and adiposity in postmenopausal women are small and/or studied body mass index (BMI), a less robust measure of adiposity. In contrast to preclinical findings, cross-sectional studies in postmenopausal women (or stratified by menopausal status) suggest an inverse association of FSH with either BMI or visceral fat (8-12). In longitudinal studies, an inverse association of FSH and BMI has also been reported (13, 14); however, studies using more robust measures of adiposity (VAT and SAT or overall fat mass) found either no association (15), or a significant positive relationship of FSH and adiposity gain (16). These studies had fewer than 200 postmenopausal women, did not evaluate estradiol (E2), or did not account for changes in hormone therapy (HT) use.
Here, we performed both cross-sectional and longitudinal studies on the associations of FSH with dual x-ray absorptiometry (DXA)-assessed adiposity and adiposity change over a 5-year period in a sample of postmenopausal women, while also accounting for circulating E2 and HT use.
Materials and Methods
Study Sample
Our sample includes postmenopausal women enrolled in the Buffalo Osteoporosis and Oral Bone Loss (OsteoPerio) Study, an ancillary study to the Women’s Health Initiative (WHI) Observational Study. Study recruitment and data collection protocols have been described previously (17). In brief, postmenopausal women between ages 50 and 79 years at baseline were recruited for the OsteoPerio study from the group of women enrolled into the WHI Observational Study in Buffalo, New York, USA. Inclusion criteria included having 6 or more teeth, free of bone disease, no previous history of hip replacement, cancer in the last 10 years, or other serious illness. After all exclusions, a total of 1341 postmenopausal women were eligible, enrolled, and completed the first study visit.
Of the 1341 women who completed the OsteoPerio study baseline visit, 1026 women (75%) completed the 5-year follow-up visit. Our sample was restricted to 667 women who had a stored blood sample at both visits to assess reproductive hormone levels (17). DXA measures were available for all participants. Written informed consent was obtained, and the study was approved by the University at Buffalo Institutional Review Board.
Hormone Measurements
Fasting blood samples were collected by a trained phlebotomist and processed according to a standardized protocol (18). Samples were processed within 30 minutes and stored in 0.5-mL straws in liquid nitrogen at –196 ºC then moved to –80 ºC storage immediately before being sent for analysis (18). Twenty-seven pooled quality control serum samples were included and tested along with participants’ samples (one per each batch of 100 samples). Samples were sent to the Clinical & Epidemiologic Research Laboratory (Children’s Hospital, Boston, Massachusetts, USA) for measurement of serum E2 and FSH.
Serum E2 concentration was measured at baseline and follow-up by competitive electrochemiluminescence immunoassay (Roche E Modular system, Roche Diagnostics; Roche catalog No. 06656021190, RRID:AB_2905575). The limit of detection (LOD) of this assay is 5 pg/mL (18). The intra-assay coefficient of variation for the pooled quality control samples was 4.1%. There were 154 participants at baseline and 411 at follow-up with serum E2 below the LOD.
FSH levels were measured at both study visits by a sandwich electrochemiluminescence immunoassay on the Roche Cobas 6000 system (Roche Diagnostics; Roche catalog No. 11775863122, RRID:AB_2800499). The lower LOD is 0.10 mIU/mL and the upper limit of detection is 200 mIU/mL. The intra-assay coefficient of variation was 3.3%. One participant had baseline and follow-up serum FSH above the upper LOD.
Circulating free estradiol (fE2) was calculated using measured serum E2, serum sex hormone–binding globulin (SHBG), and albumin via the calculations described in Mazer (19) and based on the method of Rinaldi et al (20). We calculated fE2 at the baseline visit only because SHBG and albumin were measured only at baseline. For participants with baseline serum E2 below the lower LOD, the midpoint (2.5 pg/mL) was imputed and used for calculation of fE2. SHBG was measured by a competitive electrochemiluminescence immunoassay on the Roche E Modular system (Roche Diagnostics; Roche catalog No. 03052001160, RRID: AB_2891165).
Anthropometry
Height was measured to the nearest 0.1 cm using a wall-mounted stadiometer. Weight was measured to the nearest 0.1 kg on a balance bean scale. BMI was calculated as weight in kilograms divided by height in meters squared.
Dual X-ray Absorptiometry Body Composition Measurements
All participants completed a whole-body DXA scan at the baseline visit (QDR-4500A; Hologic Inc) and at the 5-year follow-up visit (Discovery A; Hologic Inc) according to a standardized protocol by a trained and certified DXA technician. DXA instruments were calibrated by Hologic because of scanner upgrades. Standardized procedure for participant positioning and scan analysis was used. Phantom scans and a random sampling of scans were reviewed to monitor machine and technician performance.
Whole-body DXA scans were used to estimate total body fat mass (TBF, kg), VAT (cm2), and SAT (cm2). Percentage of total body fat was computed (TBF, kg/total body mass, kg). To estimate abdominal VAT and SAT, DXA images at both time points were reanalyzed using new software (Hologic APEX 4.0 software toolbox). The proprietary procedures outlined in the Hologic Operator Manual (MAN-03644 Revision 005) were used to estimate VAT and SAT in an abdominal region of interest 5 cm wide at approximately the fourth lumbar vertebrae, avoiding the iliac crest and limiting bony interference with the soft-tissue measures.
Covariate Measurements
Demographic information and potential confounding variables were collected via self-administered questionnaires including age, self-identified race and ethnicity, years since menopause, education, diabetes, smoking status at baseline, pack years of smoking at baseline, and HT use at baseline and the 5-year follow-up visit.
For HT use, we categorized women at baseline and at the 5-year follow-up visit into the following categories: never/never, former/former, current/former, or current/current, where never/never users were women who had no history of HT use at baseline and were not using HT at year 5. Those in the “never/never” and “former/former” category were combined because of sample size. There were 15 women categorized as “former/current” or “never/current” or “never/former,” and 2 were missing information on HT use at baseline or follow-up; these women were excluded from longitudinal analyses.
Statistical Analysis
Descriptive statistics were computed to characterize the sample and examine distributions for normality. Bivariate associations were analyzed using parametric and nonparametric one-way analysis of variance for continuous variables and chi-square tests for categorical variables, as appropriate.
Cross-sectional Analyses
Age-adjusted partial Spearman correlation coefficients between serum hormones and adiposity measures at baseline were calculated.
We used multivariate linear regression models to test the associations of baseline FSH and free E2 with baseline DXA-derived adiposity measures (VAT, SAT, TBF, percentage of total body fat, and BMI). FSH and fE2 were entered simultaneously and separately into models, with adjustment for the covariates. Covariates were selected based on an association with FSH and adiposity in our sample and were parameterized as follows: age (years), years since menopause, HT use (dummy variables; current, former, never), smoking status (current, former, never), smoking (pack-years).
Longitudinal Analyses
We used separate multivariate linear regression models to estimate associations of baseline FSH (on a continuous scale) and 5-year change in adiposity measures (VAT, SAT, TBF, percentage of total body fat, and BMI; on a continuous scale), as well as 5-year change in FSH and 5-year change in adiposity measures. Both models adjusted for age (years), years since menopause, fE2 at baseline (pg/mL), pack-years of smoking (years), smoking status (current, former, never), change in E2 (pg/mL), change in weight (kg), and the respective baseline adiposity measure. In models of percentage of total body fat and BMI, change in weight was not included in the final model as the measures of change in percentage of total body fat and BMI fundamentally includes changes in mass.
Exploratory Analyses
We explored the longitudinal associations of FSH and adiposity according to years since menopause (≤ 10 or > 10 years since menopause) using stratified linear regression models.
Hypotheses were tested using Wald tests of the regression coefficients at the α level of .05. All tests for multiplicative interaction were considered significant at an α level of .10. All analyses were performed using SAS (release 9.4; SAS Institute Inc).
Results
Cross-sectional Analyses
Demographic and adiposity statistics according to tertiles of FSH change over the 5-year follow-up are shown in Table 1. Women who experienced a gain in FSH during follow-up (tertile 3) tended to be slightly younger, have higher fE2 at baseline, and were more likely to have stopped using HT (current/former HT user). Women who had the largest FSH gain also experienced the largest gain in SAT, TBF, and percentage of total body fat compared to women with decreasing or more stable FSH. Women with a relatively stable FSH (tertile 2) were slightly older and had approximately 2 more years since menopause compared to women in tertiles 1 or 3.
Table 1.
Demographic characteristics and key variables by tertiles of 5-year follicle-stimulating hormone change (n = 663)
| Mean (SD) or N (%) | FSH change Tertile 1 (–77.2 to 0.30 mIU/mL) N = 221 |
FSH change Tertile 2 (0.33 to 19.7 mIU/mL) N = 221 |
FSH change Tertile 3 (19.7 to 121.3 mIU/mL) N = 221 |
P a |
|---|---|---|---|---|
| Baseline variables | ||||
| Age, y | 65.5 (6.8) | 66.3 (6.7) | 64.7 (6.5) | .039 |
| Y since menopause | 16.7 (8.4) | 18.5 (8.6) | 16.9 (8.1) | .047 |
| Education | .907 | |||
| High school | 43 (19.8%) | 41 (18.8%) | 44 (20.2%) | |
| College | 91 (41.9%) | 101 (46.3%) | 93 (42.7%) | |
| Post college | 83 (38.3%) | 76 (34.9%) | 81 (37.2%) | |
| Missing | 4 | 3 | 3 | |
| Ethnicity | ≥ .999 | |||
| Hispanic | 2 (0.9%) | 2 (0.9%) | 2 (0.9%) | |
| Non-Hispanic | 219 (99.1%) | 219 (99.1%) | 219 (99.1%) | |
| Race | .198 | |||
| White | 215 (97.3%) | 221 (100.0%) | 219 (99.1%) | |
| American Indian/ Alaskan Native |
3 (1.4%) | 0 (0.0%) | 1 (0.5%) | |
| Asian American/ Pacific Islander |
1 (0.5%) | 0 (0.0%) | 1 (0.5%) | |
| African American | 2 (0.9%) | 0 (0.0%) | 0 (0.0%) | |
| Oophorectomy | .777 | |||
| No | 188 (85.8%) | 185 (84.9%) | 181 (83.4%) | |
| Yes | 31 (14.2%) | 33 (15.1%) | 36 (16.6%) | |
| Missing | 2 | 3 | 4 | |
| Diabetes | .185 | |||
| No | 210 (95.0%) | 212 (95.9%) | 217 (98.2%) | |
| Yes | 11 (5.0%) | 9 (4.1%) | 4 (1.8%) | |
| Pack-y of smoking | 9.2 (16.0) | 7.9 (14.8) | 8.9 (17.1) | .424 |
| Free E 2 , pg/mL | 0.23 (0.25) | 0.41 (0.59) | 0.78 (0.67) | < .001 |
| VAT, cm 2 | 100.6 (52.3) | 109.5 (59.2) | 98.3 (49.8) | .236 |
| SAT, cm 2 | 310.0 (115.5) | 323.2 (117.4) | 312.2 (118.9) | .343 |
| Total body fat, kg | 28.2 (8.5) | 29.7 (9.5) | 28.0 (8.7) | .147 |
| TBF, % | 39.7 (5.4) | 40.2 (5.5) | 39.2 (5.4) | .134 |
| BMI | 26.2 (4.7) | 27.2 (5.3) | 26.3 (4.7) | .073 |
| Change during 5-y follow-up | ||||
| Change in hormone therapy use | < .001 | |||
| Never/Never | 88 (40.0%) | 85 (38.6%) | 15 (6.8%) | |
| Former/Former | 83 (37.7%) | 39 (17.7%) | 5 (2.3%) | |
| Current/Current | 20 (9.1%) | 38 (17.3%) | 32 (14.5%) | |
| Current/Former | 18 (8.2%) | 55 (25.0%) | 168 (76.0%) | |
| Change in E 2, pg/mL | –3.1 (18.9) | –11.5 (21.0) | –48.6 (33.7) | < .001 |
| Change in VAT, cm 2 | 5.0 (21.6) | 4.1 (24.8) | 8.3 (24.2) | .170 |
| Change in SAT, cm 2 | –1.7 (52.3) | –2.9 (52.6) | 8.9 (56.4) | .033 |
| Change in total body fat, kg | –0.29 (3.2) | –0.51 (3.6) | 0.58 (3.5) | .0003 |
| Change in TBF, % | –0.02 (2.4) | 0.03 (2.4) | 0.89 (2.5) | < .001 |
| Change in BMI | 0.11 (1.8) | –0.07 (1.9) | 0.20 (1.9) | .290 |
Values in bold indicate statistical significance (p<0.05)
Abbreviations: BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone; SAT, subcutaneous adipose tissue; TBF, total body fat; VAT, visceral adipose tissue.
a Chi-square tests used for categorical variables; nonparametric one-way analysis of variance used for continuous variables.
Hormone concentrations and adiposity measures at baseline according to HT use are shown in Table 2. At the baseline visit, current HT users had lower FSH, higher E2, and higher fE2 compared to never and former HT users (all P < .001). Current HT users also had significantly lower VAT (P < .017) and lower percentage of total body fat (P < .003) than never and former HT users.
Table 2.
Hormone concentrations and adiposity characteristics at baseline and follow-up according to hormone therapy use
| Baseline characteristics (N = 666) | ||||
|---|---|---|---|---|
| Mean (SD) | Never HT use(N = 202) | Former HT use(N = 132) | Current HT use(N = 332) | P a |
| FSH, mIU/mL | 70.3 (25.6) | 72.2 (28.3) | 43.2 (24.2) | < .001 |
| E2, pg/mL | 10.6 (13.1) | 11.5 (14.6) | 51.1 (33.3) | < .001 |
| Free E2, pg/mL | 0.22 (0.3) | 0.23 (0.3) | 0.72 (0.6) | < .001 |
| VAT, cm2 | 111.2 (57.9) | 107.5 (58.1) | 95.9 (48.9) | .017 |
| SAT, cm2 | 322.6 (117.6) | 322.1 (132.8) | 307.0 (109.8) | .370 |
| Total body fat mass, kg | 29.8 (8.9) | 29.3 (10.4) | 27.7 (8.2) | .059 |
| TBF, % | 40.7 (5.1) | 39.9 (6.2) | 38.9 (5.3) | .003 |
| BMI | 27.1 (5.1) | 26.9 (5.9) | 26.1 (4.4) | .085 |
| Changes in hormones and adiposity during 5-y follow-up (N = 650). | ||||
| Mean (SD) |
Non-HT use/non-HT use
b
(N = 318) |
Current/former HT use
b
(N = 242) |
Current/current HT use
b
(N = 90) |
P a |
| 5-y change in FSH, mIU/mL | 0.16 (12.3) | 33.9 (26.6) | 15.8 (26.2) | < .001 |
| 5-y change in E2, pg/mL | –3.8 (16.0) | –44.3 (30.3) | –23.5 (40.6) | < .001 |
| 5-y change in VAT, cm2 | 3.2 (23.8) | 9.7 (24.4) | 6.1 (19.0) | .008 |
| 5-y change in SAT, cm2 | –4.1 (53.1) | 10.4 (55.7) | –1.16 (49.5) | .001 |
| 5-y change in total body fat mass, kg | –0.61 (3.4) | 0.68 (3.6) | –0.08 (2.8) | < .001 |
| 5-y change in TBF, % | –0.11 (2.4) | 0.91 (2.5) | 0.20 (2.4) | < .001 |
| 5-y change in BMI | –0.07 (1.8) | 0.32 (2.0) | 0.01 (1.7) | .021 |
Abbreviations: BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone; HT, hormone therapy; SAT, subcutaneous adipose tissue; TBF, total body fat; VAT, visceral adipose tissue.
a Chi-square tests or Kruskal-Wallis one-way analysis of variance, as appropriate.
b Non-HT use/Non-HT users were not using HT at baseline or at 5-year follow-up. Current/Former HT users stopped using HT during follow-up. Current/Current HT users were using HT at baseline and follow-up.
Women who stopped using HT (current/former) during follow-up had the largest decrease in circulating E2 (44.3 compared to 3.8 pg/mL in noncurrent users) and the largest increase in FSH (33.9 vs 0.16 mIU/mL in noncurrent users); see Table 2. We observed significant differences in all adiposity measures according to HT use category. Women who stopped using HT (current/former) experienced gains in all measures of adiposity; these adiposity gains were larger in magnitude compared to women who stayed on HT.
In cross-sectional analyses at the baseline visit, we observed significant inverse age-adjusted Spearman correlations between FSH and adiposity measures (correlation coefficients ranging from –0.12 to -0.22 across DXA measures). We observed weak and nonsignificant positive age-adjusted Spearman correlations between fE2 and adiposity (0.02-0.07 across DXA measures; data not shown).
Cross-sectional linear regression models are shown in Table 3. FSH is inversely associated with all measures of adiposity when entered in the model without fE2 (all P < .001). When fE2 was entered into each adiposity model without FSH, fE2 was positively associated with all adiposity measures (all P < .05). When both FSH and fE2 were entered into the models simultaneously, the significant inverse associations between FSH and adiposity measures persisted and were minimally influenced by the addition of fE2 into the model.
Table 3.
Cross-sectional study, linear regression models for follicle-stimulating hormone (FSH) and free estradiol (fE2) with adiposity measures. FSH and fE2 modeled separately and together (n = 654)
| FSH modela (no fE2 in model) |
fE2 modela (no FSH in model) |
FSH and fE2 entered together in modela | ||||||
|---|---|---|---|---|---|---|---|---|
| FSH β (SE), mIU/mL | P | fE2 β (SE), pg/mL | P | FSH β (SE), mIU/mL | P | fE2 β (SE), pg/mL | P | |
| VAT, cm2 | –0.60 (0.1) | < .001 | 13.4 (4.2) | .001 | –0.57 (0.1) | < .001 | 8.1 (4.1) | .048 |
| SAT, cm2 | –1.30 (0.2) | < .001 | 28.5 (9.1) | .002 | –1.24 (0.2) | < .001 | 16.9 (8.9) | .059 |
| Total fat, kg | –0.10 (0.01) | < .001 | 1.9 (0.7) | .007 | –0.10 (0.01) | < .001 | 0.97 (0.7) | .153 |
| TBF, % | –0.04 (0.01) | < .001 | 0.95 (0.4) | .026 | –0.04 (0.01) | < .001 | 0.56 (0.4) | 0.190 |
| BMI | –0.06 (0.01) | < .001 | 1.3 (0.4) | .001 | –0.06 (0.01) | < .001 | 0.77 (0.4) | .039 |
Abbreviations: BMI, body mass index; FSH, follicle-stimulating hormone; fE2, free estradiol; HT, hormone therapy; SAT, subcutaneous adipose tissue; TBF, total body fat; total fat, total body fat mass; VAT, visceral adipose tissue.
a Adjusted for age, years since menopause, smoking status, pack-years, and HT use.
However, the positive associations of fE2 and adiposity were attenuated and became borderline or nonsignificant.
Longitudinal Analyses
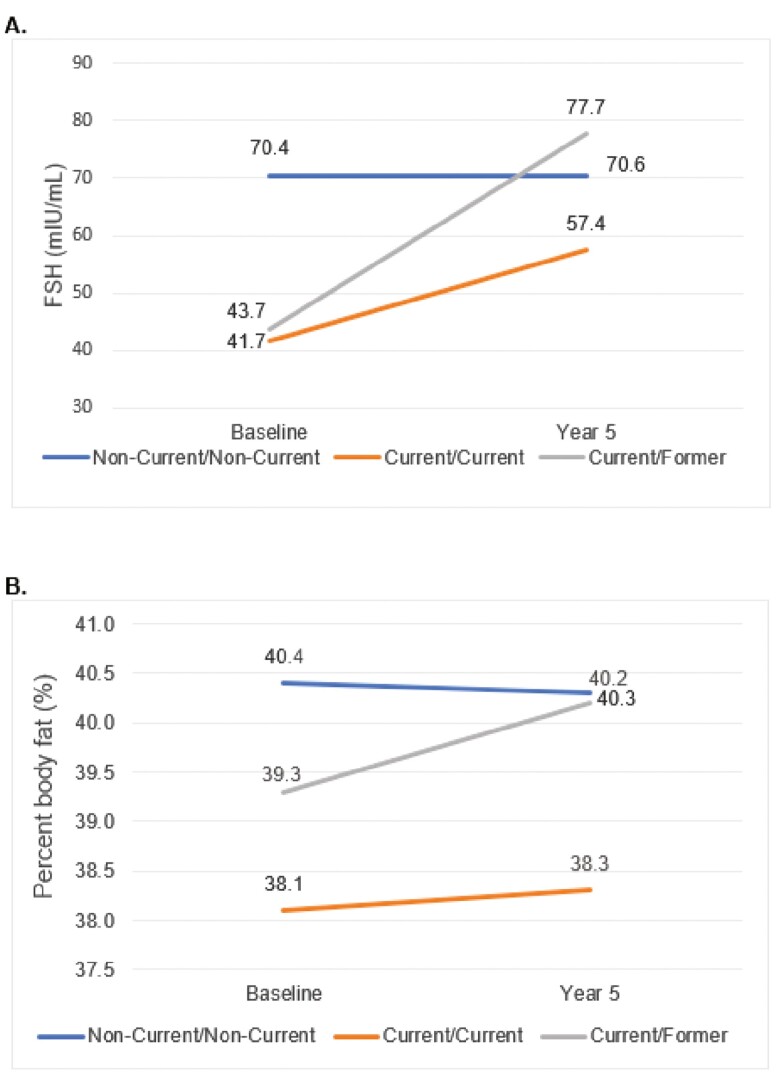
Fig. 1 shows changes in FSH and percentage of fat between baseline and 5-year follow-up by change in HT use category, where never/never and former/former categories are collapsed into a “noncurrent/noncurrent” category. The current/former HT user group experienced the largest mean increase in FSH from baseline to 5-year follow-up (34.0 mIU/mL). The current/current HT group experienced a relatively smaller FSH increase (15.7 mIU/mL). The noncurrent/noncurrent HT use group experienced no meaningful FSH change (0.16 mIU/mL). On average, the current/former HT group had a percentage fat gain (mean percent fat gain = 1.0%). The current/current HT group and the noncurrent/noncurrent HT group experienced percentage fat fluctuations of 0.2% and –0.2%, respectively (see Fig. 1B).
Figure 1.
Five-year mean change in A, follicle-stimulating hormone (FSH), and B, percent body fat (%) stratified by change in hormone therapy use category.
We observed significant inverse associations between baseline FSH and 5-year change in percentage of body fat and TBF that switched to positive associations when we modeled 5-year FSH change, where positive β coefficients indicate that increasing FSH is associated with increased TBF, percentage of total body fat, and SAT (Table 4).
Table 4.
Longitudinal analysis, linear regression models of baseline, and 5-year follicle-stimulating hormone change predicting 5-year change in adiposity measures (N = 633)
| Baseline FSH, mIU/mL, predicting change in adiposity | 5-y change in FSH, mIU/mL, predicting change in adiposity | |||||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted model | Adjusted modela | Unadjusted model | Adjusted modela | |||||
| FSH β (SE) | P | FSH β (SE) | P | FSH β (SE) | P | FSH β (SE) | P a | |
| ΔVAT, cm2 | –0.05 (0.03) | .131 | –0.04 (0.03) | .137 | 0.04 (0.04) | .260 | 0.03 (0.03) | .383 |
| ΔSAT, cm2 | –0.08 (0.07) | .286 | –0.10 (0.06) | .076 | 0.14 (0.08) | .083 | 0.17 (0.07) | .016 |
| ΔTotal fat, kg | –0.01 (0.01) | .151 | –0.005 (0.003) | .042 | 0.01 (0.01) | .006 | 0.01 (0.003) | < .001 |
| ΔTBF, % | –0.01 (0.03) | .004 | –0.01 (0.004) | .005 b | 0.02 (0.004) | < .001 | 0.01 (0.005) | .011 b |
| ΔBMI | –0.001 (0.003) | .622 | –0.004 (0.003) | .169b | –0.001 (0.003) | .804 | –0.004 (0.003) | .198b |
Abbreviations: BMI, body mass index; E2, estradiol; FSH, follicle-stimulating hormone; SAT, subcutaneous adipose tissue; TBF, total body fat; total fat, total body fat mass; VAT, visceral adipose tissue.
a Adjusted for age, years since menopause, baseline free E2, smoking status, pack-years, change in E2, change in weight, and respective adiposity measure at baseline.
b Adjusted for age, years since menopause, baseline free E2, smoking status, pack-years, change in E2, and respective adiposity measure at baseline.
We evaluated associations of FSH change and adiposity change according to years since menopause (≤ 10 years, > 10 years) and observed a similar association where FSH gain was associated with percentage of body fat gain in the more than 10 years since menopause strata (N = 497). The association was of similar magnitude in the 10 years or less since menopause group (N = 136) but did not reach statistical significance. The P for interaction for years since menopause and FSH change among all models was nonsignificant (Pinteraction > 0.10; data not shown).
Discussion
To our knowledge, this is the largest longitudinal study of FSH and DXA-derived adiposity in postmenopausal women and the first to evaluate the influence of circulating E2 and HT use on this association. This consideration of estrogen is compelled by the complexity of the FSH-E2 feedback loop and dependence on either adipose-derived or HT-derived E2 postovarian failure. A unique aspect of this population is the decrease in HT use that occurred between our baseline (1997-2000) and 5-year follow-up (2002-2005) visits, likely owing to the release of the WHI HT trial results in 2002 and 2004 (21, 22). Approximately 73% of current HT users at baseline stopped using HT during our study. As a result, an increase in FSH that is uncharacteristic of postmenopausal women was observed among these participants, providing a natural experiment for the study of the effects of FSH change. Our cross-sectional results suggest an inverse association between FSH and measures of adiposity, where women with higher FSH have lower values of all adiposity measures examined. However, evidence from our longitudinal study suggests that FSH gain is associated with percentage of TBF, TBF, and SAT gain over a 5-year postmenopausal period, although the effect is small.
The previous 2 studies that used gold-standard estimates of body fat are in agreement. The first study was conducted in a sample of 77 women with adiposity measured via magnetic resonance imaging from the Penn Ovarian Aging Study (12). Women in the lowest VAT tertile had significantly higher mean FSH levels (90.5 mIU/mL) than women in the highest VAT tertile (40.0 mIU/mL). Similar results were reported for SAT; however, differences were no longer significant after adjustment for age, race, and menopausal status (12).
In another cross-sectional study of 238 older postmenopausal women from the Reykjavik-AGES cohort who were not HT users, women in the highest quartile of FSH had significantly lower mean VAT (157.6 cm2) measured via quantitative computed tomography compared to the lowest FSH quartile (185.4 cm2) following adjustment for age, subgroup, E2, and testosterone. This group reported similar nonsignificant inverse trends for SAT (11).
In our cross-sectional study, we corroborate these inverse association of FSH with all DXA-derived measures of adiposity and demonstrate that the associations are not influenced by E2. Our findings further show a statistically significant positive association of fE2 and all DXA-derived adiposity measures. In both prior studies, FSH was more strongly associated with VAT than SAT, whereas we observed a stronger association for SAT. Differences in SAT findings may reflect age differences between study cohorts, as distribution of different compartments of abdominal fat (as well as VAT to SAT ratios) is dynamic early and late in the menopause years (23). While the Penn Ovarian Aging cohort sample was closer to menopause (mean age, 52.2 ± 3.18 years), the Reykjavik-AGES cohort was exclusively late postmenopausal women (mean age, 80.8 ± 4.2 years), whereas our sample has a broader age representation.
The only longitudinal study that used quantitative computed tomography–derived measures of adiposity was in a sample of 162 postmenopausal women (mean age, 82 ± 4 years) from the Reykjavik-AGES cohort who were not HT users (11). The authors reported no significant association between baseline FSH and annualized absolute change in any measure of adiposity over 3 years of follow-up with adjustment for age, subgroup, E2, testosterone, diabetes status, estimated glomerular filtration rate, and current smoking status (15). Our results for baseline FSH predicting adiposity change, albeit with absolute change over 5-year follow-up vs annualized change over 3 years, are largely congruent to the Reykjavik-AGES study, where most adiposity measures are not significant. The exception is the inverse finding of baseline FSH and percentage of body fat change that we identified. However, the older age of the sample, relatively small sample size, and shorter duration of follow-up contrast with our study and may explain the differences.
Results from 2 longitudinal studies that used BMI to represent adiposity are more in line with cross-sectional studies. In 196 women from the Penn Ovarian Aging Study who became postmenopausal by the end of 12-years of follow-up, obese women had significantly lower mean FSH levels (42.0 mIU/mL) on average compared to women with normal BMI (59.3 mIU/mL) at each respective time point, indicating an inverse relationship (13). By contrast, in our study that included women who were postmenopausal at the start of the study, we found a gain in adiposity in women who had a rise in FSH levels. While we were focused on the influence of FSH on adiposity (as reflected in our modeling and hypotheses driven by preclinical data), other studies have analyzed the influence of adiposity on FSH, essentially modeling the linear associations in reverse. In the Study of Women Across the Nation (SWAN), which included women across the menopausal transition, BMI gain was associated with decreasing FSH concentrations over a 3-year period (14). In menopause-stratified results, the largest effect size was observed among 114 women who were postmenopausal at the end of follow-up (14). However, neither study considered E2 or HT use, and used BMI rather than directly measured adipose tissue.
By contrast, a longitudinal study of overall body fat (measured via bioelectrical impedance), indicated a positive association of FSH and adiposity that is similar to our own (16). In a SWAN study of 543 women across the menopausal transition (including 81 women classified as postmenopausal by the end of 6-year follow-up), increasing FSH levels were associated with increasing TBF with adjustment for age and baseline fat mass (16). Our study also indicated a small but significantly positive association between increasing FSH and increasing TBF.
Tepper et al (24) reported on E2 and FSH trajectories in a sample of 1316 postmenopausal women in SWAN, where measurements were included before and after the menopause transition. When considering E2 and FSH trajectories during the menopause transition, having a low FSH trajectory (out of 3 possible) and flat E2 (out of 4 possible) trajectory was more often observed in obese women. This highlights the importance of characterizing obesity at different menopause stages in studying FSH-adiposity associations, because if women are already obese (at our baseline visit), they may have lower FSH levels due to obesity at the start of menopause that could influence the inverse associations we observed here in our postmenopausal sample.
In vivo studies support our findings of a positive association between FSH and adipose tissue. FSH receptor expression in visceral and subcutaneous fat provides evidence that adipose tissue is responsive to FSH signaling (25). Recent studies of blocking binding of the ligand FSHβ to the FSH receptor reduced body fat, triggered adipocyte beiging, and increased thermogenesis in ovariectomized and intact mice (6, 7). When considering these findings in the human context, these findings support our own results and previous longitudinal work by others, where increasing FSH is associated with body fat gain in postmenopausal women (16).
Our study has several strengths, including the use of robust tools for measurement of adiposity (DXA). To date, this is also the largest study to evaluate the association between FSH and adiposity in postmenopausal women. Other strengths include our ability to evaluate the influences of circulating E2 (including fE2 at baseline) and change in HT use over time. Change from current user to former HT user over 5 years of follow-up led to a spike in FSH over follow-up among aging postmenopausal women, which provided a natural experiment in this subset of participants.
An important limitation is generalizability. The WHI OsteoPerio study is a cohort of predominantly White women; therefore, our findings cannot necessarily be generalized to other race/ethnic groups. Randolph et al (14) provided interesting data indicating differences in FSH and E2 levels by race/ethnic group that will warrant further exploration in larger, more representative samples. Because our WHI sample includes only postmenopausal women, we are unable to study FSH rise during the earlier part of menopause, nor do we have power to study associations stratified by HT where we observed the largest FSH increase (in former HT users). As this was not an intentional weight loss study, no wasting diseases were included, and there may have been insufficient follow-up to observe dramatic changes in body composition. Studying changes in abdominal adiposity earlier in menopause would more closely coincide with the FSH increase and may provide additional information on the FSH associations. Study size precluded robust stratified analyses; larger studies are needed.
The inverse cross-sectional association between FSH and adiposity may simply reflect weight status, while longitudinal results suggest a positive association between FSH and adiposity over time among postmenopausal women, in alignment with preclinical models. Replication of these findings in a larger, diverse sample is critical in this population at high risk for obesity-associated chronic diseases.
Glossary
Abbreviations
- BMI
body mass index
- DXA
dual x-ray absorptiometry
- E2
estradiol
- fE2
free estradiol
- FSH
follicle-stimulating hormone
- HT
hormone therapy
- LOD
limit of detection
- SAT
subcutaneous adipose tissue
- SHBG
sex hormone–binding globulin
- SWAN
Study of Women Across the Nation
- TBF
total body fat mass
- WHI
Women’s Health Initiative
- VAT
visceral adipose tissue
Contributor Information
Lindsey J Mattick, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York 14214, USA.
Jennifer W Bea, Department of Health Promotion Sciences, Mel and Enid Zuckerman College of Public Health, University of Arizona, Tucson, Arizona 85724, USA; Department of Medicine, University of Arizona, Tucson, Arizona 85724, USA.
Lawanya Singh, Jacobs School of Medicine and Biomedical Sciences, University at Buffalo, The State University of New York, Buffalo, New York 14203, USA.
Kathleen M Hovey, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York 14214, USA.
Hailey R Banack, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York 14214, USA.
Jean Wactawski-Wende, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York 14214, USA.
JoAnn E Manson, Department of Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston, Massachusetts 02115, USA; Department of Epidemiology, Harvard T. H. Chan School of Public Health, Boston, Massachusetts 02115, USA.
Janet L Funk, Department of Medicine, University of Arizona, Tucson, Arizona 85724, USA.
Heather M Ochs-Balcom, Department of Epidemiology and Environmental Health, School of Public Health and Health Professions, University at Buffalo, The State University of New York, Buffalo, New York 14214, USA.
Financial Support
This work was supported by the Breast Cancer Research and Education Fund through New York State Department of Health (contract No. C34926GG). The opinions expressed here are solely those of the authors and do not necessarily reflect those of the Health Research Science Board, the New York State Department of Health, or the State of New York. Additional grants include NHLBI-CSB-WH-2016-01-CM, DOD No. OS950077, and NIH R01 DE13505.
The WHI program is funded by the National Heart, Lung, and Blood Institute through contract Nos. HHSN268201600018C, HHSN268201600001C, HHSN268201600002C, HHSN268201600003C, and HHSN268201600004C. The WHI program is also funded by the National Heart, Lung, and Blood Institute through contract Nos. 75N92021D00001, 75N92021D00002, 75N92021D00003, 75N92021D00004, and 75N92021D00005.
This manuscript was prepared in collaboration with WHI investigators and has been reviewed and approved by the WHI. WHI investigators are listed at https://www-whi-org.s3.us-west-2.amazonaws.com/wp-content/uploads/WHI-Investigator-Short-List.pdf.
Disclosures
The authors have nothing to disclose.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Sun Y, Liu B, Snetselaar LG, et al. Association of normal-weight central obesity with all-cause and cause-specific mortality among postmenopausal women. JAMA Netw Open. 2019;2(7): e197337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sahakyan KR, Somers VK, Rodriguez-Escudero JP, et al. Normal-weight central obesity: implications for total and cardiovascular mortality. Ann Intern Med. 2015;163(11):827-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Leeners B, Geary N, Tobler PN, Asarian L. Ovarian hormones and obesity. Hum Reprod Update. 2017;23(3):300-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Butler L, Santoro N. The reproductive endocrinology of the menopausal transition. Steroids. 2011;76(7):627-635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prior JC. Perimenopause: the complex endocrinology of the menopausal transition. Endocr Rev. 1998;19(4):397-428. [DOI] [PubMed] [Google Scholar]
- 6. Liu P, Ji Y, Yuen T, et al. Blocking FSH induces thermogenic adipose tissue and reduces body fat. Nature. 2017;546(7656):107-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Han X, Guan Z, Xu M, et al. A novel follicle-stimulating hormone vaccine for controlling fat accumulation. Theriogenology. 2020;148:103-111. [DOI] [PubMed] [Google Scholar]
- 8. Gavaler JS, Rosenblum E. Predictors of postmenopausal body mass index and waist hip ratio in the Oklahoma Postmenopausal Health Disparities Study. J Am Coll Nutr. 2003;22(4):269-276. [DOI] [PubMed] [Google Scholar]
- 9. Jung ES, Choi EK, Park BH, Chae SW. Serum follicle-stimulating hormone levels are associated with cardiometabolic risk factors in post-menopausal Korean women. J Clin Med. 2020;9(4):1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wang N, Shao H, Chen Y, et al. Follicle-stimulating hormone, its association with cardiometabolic risk factors, and 10-year risk of cardiovascular disease in postmenopausal women. J Am Heart Assoc. 2017;6(9):e005918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Veldhuis-Vlug AG, Woods GN, Sigurdsson S, et al. Serum FSH is associated with BMD, bone marrow adiposity, and body composition in the AGES-Reykjavik study of older adults. J Clin Endocrinol Metab. 2021;106(3):e1156-e1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Senapati S, Gracia CR, Freeman EW, et al. Hormone variations associated with quantitative fat measures in the menopausal transition. Climacteric. 2014;17(2):183-190. [DOI] [PubMed] [Google Scholar]
- 13. Freeman EW, Sammel MD, Lin H, Gracia CR. Obesity and reproductive hormone levels in the transition to menopause. Menopause. 2010;17(4):718-726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Randolph JF Jr, Sowers M, Bondarenko IV, Harlow SD, Luborsky JL, Little RJ. Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab. 2004;89(4):1555-1561. [DOI] [PubMed] [Google Scholar]
- 15. Wu KC, Ewing SK, . Li X, et al. FSH level and changes in bone mass and body composition in older women and men. J Clin Endocrinol Metab. 2021;106(10):2876-2889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sowers M, Zheng H, Tomey K, et al. Changes in body composition in women over six years at midlife: ovarian and chronological aging. J Clin Endocrinol Metab. 2007;92(3):895-901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wactawski-Wende J, Hausmann E, Hovey K, Trevisan M, Grossi S, Genco RJ. The association between osteoporosis and alveolar crestal height in postmenopausal women. J Periodontol. 2005;76(11 Suppl):2116-2124. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, LaMonte MJ, Hovey KM, et al. Association of serum 17β-estradiol concentration, hormone therapy, and alveolar crest height in postmenopausal women. J Periodontol. 2015;86(4):595-605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazer NA. A novel spreadsheet method for calculating the free serum concentrations of testosterone, dihydrotestosterone, estradiol, estrone and cortisol: with illustrative examples from male and female populations. Steroids. 2009;74(6):512-519. [DOI] [PubMed] [Google Scholar]
- 20. Rinaldi S, Geay A, Déchaud H, et al. Validity of free testosterone and free estradiol determinations in serum samples from postmenopausal women by theoretical calculations. Cancer Epidemiol Biomarkers Prev. 2002;11(10 Pt 1):1065-1071. [PubMed] [Google Scholar]
- 21. Rossouw JE, Anderson GL, Prentice RL, et al. Writing Group for the Women’s Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA. 2002;288(3):321-333. [DOI] [PubMed] [Google Scholar]
- 22. Anderson GL, Limacher M, Assaf AR, et al. Women’s Health Initiative Steering Committee. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women’s Health Initiative randomized controlled trial. JAMA. 2004;291(14):1701-1712. [DOI] [PubMed] [Google Scholar]
- 23. Lovejoy JC, Champagne CM, de Jonge L, Xie H, Smith SR. Increased visceral fat and decreased energy expenditure during the menopausal transition. Int J Obes (Lond). 2008;32(6):949-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tepper PG, Randolph JF Jr, McConnell DS, et al. Trajectory clustering of estradiol and follicle-stimulating hormone during the menopausal transition among women in the Study of Women’s Health across the Nation (SWAN). J Clin Endocrinol Metab. 2012;97(8):2872-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liu XM, Chan HC, Ding GL, et al. FSH regulates fat accumulation and redistribution in aging through the Gαi/Ca(2+)/CREB pathway. Aging Cell. 2015;14(3):409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.