Abstract
Context
Calorie restriction plus dietary advice is suggested as a preventive strategy for individuals with obesity and prediabetes; however, optimal diet is still debatable. We aimed to compare the effects of Mediterranean diet (MD) and Chinese diets high or low in plants on body weight and glucose homeostasis among high-risk Chinese.
Subjects and Methods
In this parallel-arm randomized controlled trial, 253 Chinese adults aged 25 to 60 years with a body mass index ≥ 24.0 kg/m2 and fasting blood glucose ≥ 5.6 mmol/L were randomly assigned to 3 isocaloric-restricted diets: MD (n = 84), a traditional Jiangnan diet high in plants (TJD, n = 85), or a control diet low in plants (CD, n = 84). During the 6-month trial, a 5-weekday full-feeding regimen was followed, along with mobile app–based monitoring. Abdominal fat measurement (magnetic resonance imaging), oral glucose tolerance test (OGTT), and continuous glucose monitoring (CGM) were conducted at baseline and 3 and 6 months.
Results
With a 25% calorie restriction for 6 months, weight deduction was 5.72 kg (95% confidence interval, 5.03-6.40) for MD, 5.05 kg (4.38-5.73) for TJD, and 5.38 kg (4.70-6.06) for CD (Ptime < 0.0001). No between-group differences were found for fasting glucose, insulin, and the Matsuda index from OGTT. Notably, CD had significantly longer time below range (glucose < 3.9 mmol/L) than MD (0.81% [0.21-1.40], P = 0.024) and marginally longer time than TJD (0.56% [-0.03 to 1.15], P = 0.065), as measured by CGM.
Conclusions
With the 6-month isocaloric-restricted feeding, TJD and MD achieved comparable weight deduction and improved glucose homeostasis, whereas CD showed a higher risk for hypoglycemia.
Keywords: overweight, prediabetes, Mediterranean diet, plant-based diet, oral glucose tolerance test, continuous glucose monitoring
The Mediterranean diet (MD) has been linked to lowering all causes of mortality and risks of obesity, cardiovascular disease, and type 2 diabetes (1). Likewise, traditional diets enriched with plant foods in many Asian countries have been associated with a lower risk of cardiometabolic morbidities in several cohort studies (2, 3). As one of the representative dietary patterns with a high plant content in China, the traditional Jiangnan diet (TJD), consumed by people in Southeast China, emphasizes ample vegetables and whole grains with limited red and processed meat as with MD, except containing plenty of soy products and moderate freshwater fish cooked using sunflower oil or various vegetable oils other than olive oil. Indeed, this type of diet is also consumed in many Asian countries (4). However, with the recent nutrition transition, Chinese people consumed fewer whole grains (91.0 g vs 14.6 g), but more red meat (18.2 g vs 64.4 g, mainly pork) and dietary fat (12.0% vs 32.3%) during 1982 through 2012 (5). During this time, an 11- to 15-fold higher prevalence of overweight/obesity and diabetes were also evidenced (6, 7). To date, few, if any, trials have compared MD with traditional diets from Asian countries (4). Thus, it would be of interest to simultaneously assess the health benefits of MD and TJD in Chinese adults.
Moderate calorie restriction (500-750 kcal/d) in combination with dietary advice is considered a common intervention for individuals with obesity and prediabetes (8). The 1-year result of PREDIMED–Plus revealed that MD plus calorie restriction and behavioral interventions aided more in weight loss and reduction in fasting glucose than solely MD intervention (9). However, only a few trials have compared the effects of different dietary patterns along with calorie restriction. Previously, the 2-year DIRECT study showed that isocaloric-restricted MD reduced body weight and fasting glucose more than that of low-fat diet in the subgroup of type 2 diabetes (10), whereas the isocaloric-restricted MD and vegetarian diet achieved similar improvement in body weight and fasting glucose in an education-based crossover trial (CARDIVEG Study) among 118 overweight participants (11). Given that it is still debatable which dietary pattern is more effective under calorie restriction, a feeding trial comparing different dietary patterns with moderate isocaloric-restriction fits well in this regard, which not only simulates the practical situations of individuals who follow the calorie-restricted recommendation, but also eliminates the impact of different caloric intake from various diets (12).
As an independent risk factor for diabetes complications and cardiovascular disease (13, 14), maintaining glycemic variability within a targeted range was newly recommended as a crucial metric in diabetes management (15, 16). Continuous glucose monitoring (CGM) can dynamically monitor glycemic variability and has frequently been used to facilitate adherence for lifestyle intervention trials in patients (17, 18). Meanwhile, other new health technologies such as portable devices and mobile apps are emerging as useful strategies to improve the quality of trials (19). Therefore, 3 isocaloric-restricted dietary patterns: MD, TJD, and a control diet low in plant content (CD) were provided in this 6-month controlled feeding trial, combining CGM and mobile app–based monitoring devices, to test our hypothesized that various dietary patterns under isocaloric restriction would achieve various improvements in body weight and glucose homeostasis among Chinese adults with prediabetes.
Materials and Methods
Study Design and Participants
This 6-month parallel-arm randomized controlled trial was performed from February to September 2019 at SAIC Volkswagen Automotive Company in Shanghai, China. The study protocol was approved by the institutional review boards of the Shanghai Institute of Nutritional and Health, Chinese Academy of Sciences, and Ruijin Hospital, Shanghai Jiao Tong University School of Medicine. All participants provided written informed consent before attendance.
Eligible participants aged 25 to 60 years were overweight/obese (body mass index [BMI] ≥ 24 kg/m2, defined by Chinese criteria (20)) with fasting glucose ≥ 5.6 mmol/L. The exclusion criteria included: (1) clinically diagnosed diabetes or use of hypoglycemic medications; (2) clinically diagnosed cardiovascular, kidney, liver, pituitary, alimentary tract, and thyroid diseases as well as cancer or mental health illnesses; (3) pregnancy or lactation; (4) undergoing gastrointestinal surgery within 1 year, except appendicitis or hernia; (5) current use of antidepressant(s); (6) alcohol consumption > 40 g/d or other substance abuse; (7) allergies to foods contained in the test meals; and (8) participation in other studies within the past 3 months. Before recruitment, screening visits were conducted for the participants who signed up online.
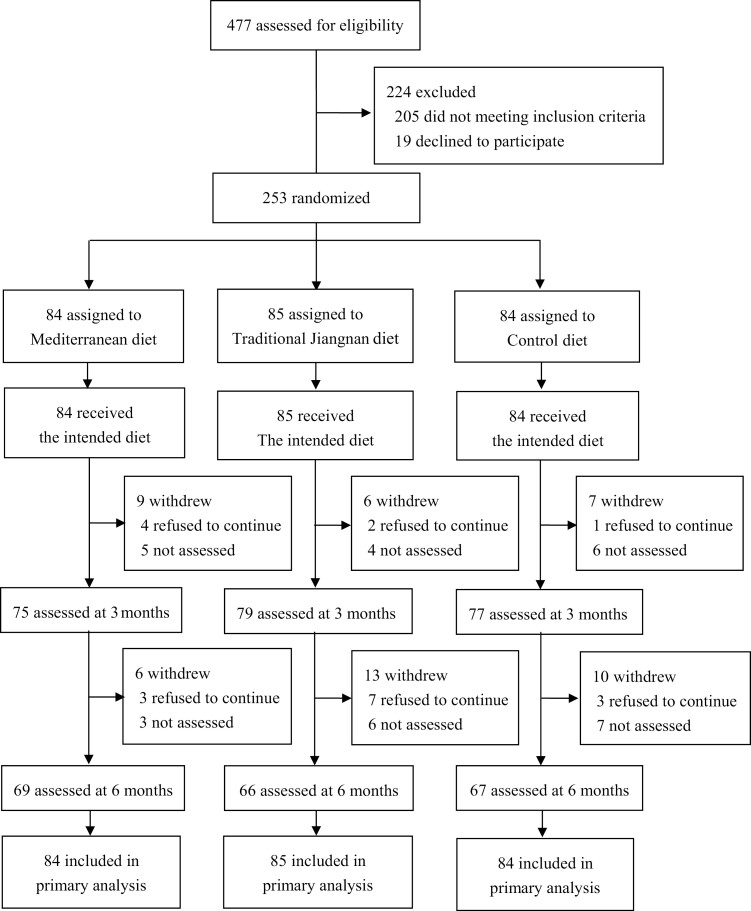
A total of 477 potential participants were recruited by advertisement and were prescreened from the electronic medical history of annual physical examinations by trained medical doctors in the company-affiliated hospital. After screening, 205 individuals did not meet the inclusion criteria and 19 persons refused to participate. A total of 253 eligible participants were randomly assigned to 3 diet groups: (1) MD (n = 84); (2) TJD (n = 85); and (3) CD (n = 84) (Fig. 1). An independent statistician performed randomization stratified by sex, median of screening BMI (26.5 kg/m²), and fasting glucose (6.1 mmol/L) via the SAS program and assigned color labels corresponding to the 3 groups. All clinical investigators who were responsible for enrollment, measurements, or data analyses were blinded to the group allocation. Only onsite staff was aware of the diet assignments for meal distribution. Each participant received a color label for taking meals instead of explicitly informing their group assignments.
Figure 1.
Trial flow diagram.
Dietary Interventions
A weekly 5-day feeding regimen was followed for 6 months and 3 isocaloric-restricted diets attributed to 1600 kcal/d for men and 1300 kcal/d for women (~25% caloric restriction), according to the Dietary Reference Intakes (DRIs) of energy requirement for Chinese adult men (2250 kcal/d) and women (1800 kcal/d) with low level of physical activity (21). The MD adopted typical ingredients of this diet, including extra-virgin olive oil, marine fish, nuts, and wholegrain products like buckwheat bread and pasta (22). Nevertheless, sauces and spices specific to the Mediterranean region were not fully included in the modified MD menus, and the meals were prepared habitually with relatively lower cooking temperatures to promote acceptability in Chinese participants. The TJD was designed according to the traditional cuisines in Southeast China, which are enriched with vegetables, fruits, legumes, soy products, freshwater fish, and shrimp, combined with moderate amounts of whole grains, such as brown rice (5, 23). The CD was designed to represent the current dietary pattern, characterized by relatively large portions of saturated fat, pork, and refined rice in megacities like Shanghai, according to the China Health and Nutrition Survey (24). Calories intake from protein accounted for 20% in all 3 diets, whereas from carbohydrate and fat were 43% and 37% (<10% saturated fat) in the MD, 50% and 30% (<10% saturated fat) in the TJD, and 43% and 37% (>10% saturated fat) in the CD, respectively.
Over the 6-month period, the participants received 3 meals/day for 5 weekdays in 1 of 8 nearby dining rooms inside the company and had their family meals ad libitum during the weekend. Trained dietitians weekly updated the 5-day cycle menus for 3 diets with seasonal vegetables and fruits using Nutrition Star Software (Zhending Co., Ltd, Shanghai, China) that incorporates the Chinese Food Composition Table (25). Another full-time dietitian closely supervised the overall process of meal preparation in a center kitchen to ensure that the designed menus were followed. Participants were requested to consume their lunch in the assigned dining room, and trained staff members recorded information on meal-taking and weight-measured leftovers. Meanwhile, the participants were allowed to take breakfast and dinner home and were asked to record the consumption information by sending photos to the staff members. The compliance was evaluated by dividing the number of consumed meals by the number of provided meals.
Outcomes and Measurements
The primary outcomes were 6-month changes in body weight, and glucose homeostasis assessed by oral glucose tolerance test (OGTT) and CGM (FreeStyle Libre; Abbott Diabetes Care, Alameda, CA). A group of metrics was obtained to indicate glucose homeostasis, including (1) fasting glucose, insulin, Matsuda index, area under the curve (AUC) for glucose and insulin in OGTT; and (2) mean, time below range (TBR; < 3.9 mmol/L), time above range (> 10.0 mmol/L), and SD of interstitial glucose from CGM (26). The secondary outcomes were 6-month changes in abdominal fat, blood pressure, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol (LDL-cholesterol), triglycerides, and glycated hemoglobin.
At the beginning of the intervention, each participant received an electronic weight scale (Nutriease, Zhejiang, China) and a smart band (QingNiu, Shenzhen, China) connected to a mobile app to monitor daily changes of body weight and walking steps, and to collect information on satiety and leftover photos. The satiety information included the feeling of fullness, hunger, prospective consumption, and eating capacity from visual analog scales immediately before lunch or dinner, on a weekly basis. All participants were encouraged to wear the smart band continuously except during sleeping or showering, in addition to maintaining their habitual physical activity levels for the entire intervention period.
At baseline and 3 and 6 months, the participants were invited to attend a physical examination. At the baseline visit, information on demographic properties, lifestyle factors, health status, and medication use were obtained in a face-to-face interview by trained dietitians with a standard questionnaire. A 3-day dietary record was used to obtain dietary intake (2 weekdays plus 1 weekend day). During each visit, physical activity levels were assessed using a Short-Form International Physical Activity Questionnaire (27), in addition to wearing a smart band for the entire trial. Weight and height were measured in light clothing without shoes by a calibrated weighing scale (Seca-882; ScalesGalore) and a wall-mounted stadiometer (Seca-214; Scales-Galore), respectively. BMI was calculated as weight (kg) divided by the square of height (m). Waist circumference was measured halfway between the last rib and the iliac crest using an anthropometric tape. Blood pressure was measured 3 times using a validated semiautomatic oscillometer (Omron HEM-7000) after 5 minutes of rest between measurements. Abdominal fat including visceral adipose tissue (VAT) and subcutaneous adipose tissue were assessed through magnetic resonance imaging scans (MRI; uMR 560, United Imaging Healthcare, Shanghai, China).
During each visit, overnight fasting blood samples and samples at 30, 60, and 120 minutes after consuming 75 g of glucose were collected and stored at −80°C until laboratory assays. The AUC of the plasma glucose and insulin response was calculated using the trapezoidal method. Insulin sensitivity was estimated by using the Matsuda Index = 10,000/√ [(fasting insulin (mU/L) × fasting glucose (mg/dL)) × (mean OGTT insulin (mU/L) × (mean OGTT glucose (mg/dL))] (28). Additionally, a CGM sensor was placed subcutaneously on the arm. Interstitial glucose concentrations were measured every 15 minutes over the subsequent 14 days.
Laboratory Measurements
Plasma concentrations of glucose, insulin, triglycerides, total cholesterol, high-density lipoprotein cholesterol, and LDL-cholesterol were measured on an automatic analyzer (Hitachi 7080) using commercial kits from Wako Pure Chemical Industries. All assessments were blinded to group allocation.
Statistical Analysis
The power calculation was only performed for body weight because there was not enough a priori data for the metrics of glucose homeostasis from OGTT and CGM. Based on a previous study comparable to our design (10), the SD of weight change was assumed as 6 kg. To detect a difference of 1.5 kg (0.25 SD) in weight change, a sample size of 53 per group was estimated with a power of 80% and 2-sided alpha as 0.05. An actual sample size of 253 was allowed to have a 37% dropout rate.
The main analyses were conducted in line with the intention-to-treat principle. Descriptive statistics are presented as means ± SDs, medians (interquartile range), and median numbers (percentages) for variables with normal distribution or skewed distribution, and categorical variables respectively, unless otherwise stated. R package “cgmanalysis” was used to calculate the metrics from CGM. Changes in all continuous variables were calculated by subtracting the baseline values from the values at 3 or 6 months. All outcomes were evaluated through skewness (skewed distribution if the absolute value of skewness > 1) and quantile-quantile plots for normality before analysis. The square-root method was used for normalization of metrics with skewed distribution. For each outcome, outliers were defined as 3 times the interquartile range above the upper quartile (75th percentile) or below the lower quartile (25th percentile) within each group (29). No significant differences were observed with and without outliers, which were involved in the final analysis.
Baseline characteristics between groups were compared using 1-way ANOVA or χ2 tests when appropriate. Mixed-effects linear models with unstructured variance structure were used to analyze the effect of group; time and group by time interactions with adjusting for age, sex, and baseline BMI (except for weight); baseline fasting glucose (except for fasting glucose); physical activity; and baseline values. Group, time, and their interaction were treated as fixed effects, whereas participant was treated as random effects. Estimated marginal means (95% CI) were the adjusted values from the these models. Post hoc pairwise comparisons were applied with Bonferroni correction. All analyses were performed using SAS (version 9.4) and R software (version 3.2.3). A 2-sided P value < 0.05 was considered statistically significant.
Results
Of the 253 eligible participants, 202 (80%) participants completed the 6-month intervention (Fig. 1), and they had similar baseline body weight and glucose homeostatic metrics as those who withdrew from the trial (STable 1 (30)). Among the dropouts, 30 persons refused to attend the visits and the rest either disliked the assigned diets or had personal reasons (Fig. 1). The baseline characteristics of the participants are shown in Table 1. Eighty-five percent of them were men aged 37.7 ± 8.7 years with 26.7 ± 3.0 kg/m² BMI and 6.04 ± 0.72 mmol/L fasting glucose.
Table 1.
Baseline characteristics of participantsa
| Dietary group | |||
|---|---|---|---|
| MD (n = 84) | TJD (n = 85) | CD (n = 84) | |
| Male, no. (%) | 71 (84) | 72 (85) | 73 (87) |
| Age, y | 38 ± 8 | 37 ± 9 | 38 ± 9 |
| Education, no. (%) | |||
| 0-12 y | 4 (5) | 8 (9) | 4 (5) |
| ≥13 y | 80 (95) | 77 (91) | 80 (95) |
| Current smoker, no. (%) | 16 (19) | 11 (13) | 20 (24) |
| Alcohol drinker, no. (%) | 67 (80) | 58 (68) | 61 (73) |
| Medications, no. (%) | |||
| Lipid-lowering drugs | 1 (1.2) | 0 (0) | 1 (1.2) |
| Antihypertensive drugs | 9 (11) | 15 (18) | 11 (13) |
| Physical activity, no. (%)b | |||
| High | 9 (11) | 16 (19) | 14 (21) |
| Moderate | 60 (72) | 47 (56) | 55 (21) |
| Low | 14 (17) | 21 (25) | 15 (21) |
| Dietary intakec | |||
| Energy, male, kcal/d | 1973 ± 410 | 1996 ± 513 | 2003 ± 484 |
| Energy, female, kcal/d | 1893 ± 412 | 1785 ± 276 | 1538 ± 378 |
| Total fat (%) | 33.9 ± 6.4 | 33.0 ± 6.5 | 33.8 ± 7.6 |
| Carbohydrate (%) | 49.7 ± 7.2 | 49.7 ± 7.2 | 48.7 ± 8.6 |
| Protein (%) | 16.8 ± 2.2 | 17.3 ± 2.7 | 17.5 ± 2.7 |
| Cholesterol, mg/d | 456 ± 180 | 498 ± 216 | 524 ± 258 |
| Fiber, g/d | 9.0 ± 3.8 | 9.2 ± 3.7 | 8.5 ± 3.0 |
| Anthropometrics and MRId | |||
| Weight, kg | 78.9 ± 12.5 | 79.7 ± 10.9 | 79.5 ± 12.3 |
| BMI, kg/m2 | 26.6 ± 3.2 | 26.8 ± 3.0 | 26.7 ± 3.0 |
| SBP, mmHg | 129 ± 13.3 | 130 ± 16.6 | 126 ± 12.6 |
| DBP, mmHg | 85.8 ± 10.0 | 85.9 ± 11.6 | 84.1 ± 10.4 |
| Abdominal fat, cm2e | 261 (126) | 273 (102) | 270 (109) |
| VAT, cm2e | 86.7 (53.4) | 95.4 (57.5) | 82.6 (77.6) |
| SAT, cm2e | 172 (90.0) | 187 (96.1) | 176 (74.1) |
| OGTT | |||
| 0-h glucose, mmol/Le | 6.13 (0.81) | 6.03 (0.60) | 5.97 (0.63) |
| AUC glucose, mmol*h/Le | 16.4 (5.52) | 17.0 (4.16) | 17.2 (4.74) |
| Matsuda Index | 13.1 ± 7.0 | 11.7 ± 6.4 | 12.0 ± 6.5 |
| CGMf | |||
| Mean glucose, mmol/Le | 5.90 (0.81) | 5.85 (0.60) | 5.99 (0.80) |
| TBR, %e | 1.49 (3.98) | 1.07 (3.67) | 1.04 (3.79) |
| TAR, %e | 0.21 (1.48) | 0.42 (1.26) | 0.52 (1.67) |
| Nighttime SD, mmol/L | 0.65 ± 0.27 | 0.59 ± 0.25 | 0.61 ± 0.23 |
No significant between-group difference was observed among all characteristics above.
Abbreviations: AUC, area under the curve; CD, control diet; CGM, continuous glucose monitoring; DBP, diastolic blood pressure; MD, Mediterranean diet; OGTT, oral glucose tolerance test; SAT, subcutaneous adipose tissue; SBP, systolic blood pressure; SD, standard deviation; TAR, time above range (> 10.0 mmol/L); TBR, time below range (< 3.9 mmol/L); TJD, traditional Jiangnan diet; VAT, visceral adipose tissue.
a Data are mean ± standard deviation or n (%) unless otherwise stated.
b Physical activity was calculated based on the International Physical Activity Questionnaire.
c Dietary intake data were calculated from the 3-day dietary record.
d A total of 183 participants had valid magnetic resonance imaging records at baseline, 54 in Mediterranean group, 65 in traditional Jiangnan group, and 64 in control group.
e Data are medians (interquartile range).
f 200 participants had valid CGM records at baseline, 63 in Mediterranean group, 68 in traditional Jiangnan group, and 69 in control group.
The nutrient and food composition of the provided menus during the entire feeding trial are presented in Table 2. Both the MD and the TJD had large amounts of high-quality carbohydrates (MD: buckwheat products 62 g/d; TJD: brown rice 36 g/d). In contrast, the CD had larger amounts of refined cereals, meat, and a significantly higher percentage of calories from saturated fatty acids (all Pbetween-group < 0.001). Moreover, the TJD had a higher fraction of soy products and plant-based protein than the CD (Pbetween-group < 0.01), whereas the MD included more nuts and marine fish than the TJD and the CD (all Pbetween-group < 0.01).
Table 2.
Nutrient and food composition of the provided menus for the 3 dietary patterns (based on 1600 kcal/d)a
| Dietary groups | P b | |||
|---|---|---|---|---|
| MD (n = 84) | TJD (n = 85) | CD (n = 84) | ||
| Nutrients | ||||
| Total energy, kcal | 1607 ± 6 | 1608 ± 4 | 1608 ± 6 | 0.66 |
| Carbohydrate, % | 43.4 ± 0.5 | 50.4 ± 1.3 | 43.8 ± 4 | < 0.0001 |
| Fat, % | 36.7 ± 0.6 | 29.6 ± 0.8 | 36 ± 5.9 | < 0.0001 |
| SFAc | 7.6 ± 1.4 | 7.0 ± 0.9 | 11.5 ± 0.9 | < 0.0001 |
| MUFAc | 18.9 ± 2.9 | 8.0 ± 1.0 | 13.1 ± 1.0 | < 0.0001 |
| PUFAc | 5.1 ± 2.1 | 11.6 ± 1.2 | 10.8 ± 2.3 | < 0.0001 |
| Protein, % | 20.0 ± 0.5 | 19.9 ± 1.8 | 20.2 ± 2.0 | 0.75 |
| Animal sourcec | 10.7 ± 1.0 | 9.5 ± 1.3 | 11.4 ± 2.7 | 0.082 |
| Plant sourcec | 9.3 ± 1.2 | 10.2 ± 1.1 | 8.5 ± 2.4 | 0.005 |
| Cholesterol, mg | 360 ± 55 | 350 ± 51 | 539 ± 325 | 0.065 |
| Fiber, g/1000 kcal | 9.0 ± 1.3 | 7.2 ± 1.6 | 5.5 ± 1.6 | < 0.0001 |
| Food groups, g/d | ||||
| Refined cereals | 104 ± 46 | 178 ± 28 | 177 ± 34 | < 0.0001 |
| Brown rice | 14 ± 5 | 36 ± 8 | 0 ± 0 | < 0.0001 |
| Buckwheat product | 62 (0-120) | 14 (0-85) | 13 (0-70) | < 0.0001 |
| Fruits | 101 ± 55 | 107 ± 62 | 70 ± 45 | 0.021 |
| Vegetables | 575 ± 95 | 520 ± 138 | 444 ± 86 | 0.023 |
| Meat | 23 (0-110) | 25 (0-75) | 120 (90-170) | < 0.0001 |
| Poultry | 36 (0-110) | 18 (0-100) | 56 (0-140) | 0.0045 |
| Freshwater fish | 61 (0-130) | 93 (50-150) | 25 (0-145) | < 0.0001 |
| Marine fish | 98 (0-210) | 49 (0-150) | 45 (0-200) | 0.0075 |
| Nut | 29 (20-40) | 6 (0-20) | 0 (0-3) | < 0.0001 |
| Soy product | 80 (20-190) | 183 (65-340) | 52 (0-180) | < 0.0001 |
| Olive oil | 24 ± 3 | 0 ± 0 | 0 ± 0 | < 0.0001 |
| Sunflower oil | 0 ± 0 | 20 ± 4 | 17 ± 4 | < 0.0001 |
Abbreviations: CD, control diet; MD, Mediterranean diet; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; SFA, saturated fatty acid; TJD, traditional Jiangnan diet.
a Data were calculated from designed menus by the Nutrition Star Software (Zhending Co., Ltd, Shanghai, China), which incorporates the Chinese food composition table. Means ± SD and means (range) were used to represent variables with normal distribution and variables with skewed distribution.
b P value was calculated using 1-way ANOVA or χ2 tests for variables with normal distribution or skewed distribution.
c Data are percentage of total energy intake.
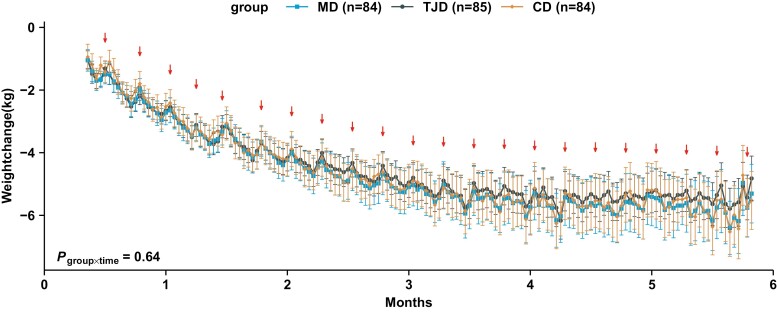
Overall, the average weight loss was about 7% at 6 months from baseline, accounting for 5.72 kg (95% CI, 5.03-6.40) for MD, 5.05 kg (95% CI, 4.38-5.73) for TJD, and 5.38 kg (95% CI, 4.70-6.06) for CD (Ptime < 0.0001), but no effect of group and no interaction of group × time was detected (Table 3). About 0.5-kg rebound was observed during the weekends or holidays when participants had meals with family members (Fig. 2). Similarly, significant time effects in abdominal fat, including VAT and subcutaneous adipose tissue, were also documented in 187 participants with valid MRI data, and no group differences were found (all Ptime < 0.01, Table 3).
Table 3.
Baseline values, and changes at 3 and 6 Months of bodyweight, abdominal fat, glucose, and insulina
| Dietary groups | P groupb | P timeb | P group × timeb | |||
|---|---|---|---|---|---|---|
| MD (n = 84) | TJD (n = 85) | CD (n = 84) | ||||
| Weight, kg | 0.52 | < 0.0001 | 0.64 | |||
| Baseline | 78.9 (12.5) | 79.7 (10.9) | 79.5 (12.3) | |||
| 3-mo change | −5.11 (−5.79 to −4.44) | −4.72 (−5.38 to −4.06) | −4.97 (−5.64 to −4.31) | |||
| 6-mo change | −5.72 (−6.40 to −5.03) | −5.05 (−5.73 to −4.38) | −5.38 (−6.06 to −4.70) | |||
| Abdominal fat, cm2c | 0.34 | < 0.0001 | 0.17 | |||
| Baseline | 261 (126) | 273 (102) | 270 (109) | |||
| 3-mo change | −56.6 (−68.2 to −44.9) | −45.8 (−56.4 to −35.2) | −47.1 (−57.4 to −36.7) | |||
| 6-mo change | −65.6 (−77.5 to −53.6) | −53.9 (−65.3 to −42.5) | −65.2 (−76.0 to −54.5) | |||
| VAT, cm2c | 0.12 | 0.0090 | 0.44 | |||
| Baseline | 86.7 (53.4) | 95.4 (57.5) | 82.6 (77.6) | |||
| 3-mo change | −34.1 (−41.6 to −26.50) | −30.8 (−37.7 to −23.9) | −29.0 (−35.7 to −22.3) | |||
| 6-mo change | −39.88 (−47.7 to −32.1) | −36.2 (−43.7 to −28.6) | −40.5 (−47.5 to −33.5) | |||
| SAT, cm2c | 0.77 | < 0.0001 | 0.26 | |||
| Baseline | 172 (90.0) | 187 (96.1) | 176 (74.1) | |||
| 3-mo change | −22.4 (−28.2 to −16.6) | −15.2 (−20.6 to −9.90) | −17.7 (−22.9 to −12.5) | |||
| 6-mo change | −25.8 (−31.8 to −19.7) | −17.7 (−23.6 to −11.9) | −24.4 (−29.8 to −19.0) | |||
| 0-h glucose, mmol/L | 0.72 | < 0.0001 | 0.30 | |||
| Baseline | 6.13 (0.81) | 6.03 (0.60) | 5.97 (0.63) | |||
| 3-mo change | −0.12 (−0.22 to −0.01) | −0.14 (−0.24 to −0.04) | −0.12 (−0.22 to −0.02) | |||
| 6-mo change | −0.38 (−0.48 to −0.27) | −0.34 (−0.44 to −0.23) | −0.28 (−0.38 to −0.17) | |||
| AUC glucose, mmol*h/L | 0.10 | < 0.0001 | 0.96 | |||
| Baseline | 16.4 (5.52) | 17.0 (4.16) | 17.2 (4.74) | |||
| 3-mo change | 0.23 (−0.29 to 0.75) | 0.86 (0.37-1.35) | 0.66 (0.17-1.15) | |||
| 6-mo change | −0.93 (−1.46 to −0.40) | −0.19 (−0.73 to 0.34) | −0.47 (−0.99 to 0.05) | |||
| 0-h insulin, uIU/mL | 0.65 | 0.12 | 0.32 | |||
| Baseline | 12.2 (8.40) | 14.2 (9.10) | 14.4 (9.40) | |||
| 3-mo change | −3.98 (−5.57 to −2.40) | −2.55 (−4.10 to −1.00) | −4.16 (−5.72 to −2.60) | |||
| 6-mo change | −4.62 (−6.25 to −2.98) | −4.31 (−5.97 to −2.65) | −4.09 (−5.74 to −2.44) | |||
| AUC insulin, uIU*h/mL | 0.77 | 0.78 | 0.33 | |||
| Baseline | 161 (128) | 197 (113) | 178 (140) | |||
| 3-mo change | −15.0 (−34.0 to 3.95) | −31.7 (−49.9 to −13.4) | −30.5 (−48.5 to −12.6) | |||
| 6-mo change | −28.0 (−47.4 to −8.64) | −27.7 (−47.5 to −8.00) | −26.0 (−45.0 to −7.09) | |||
| Matsuda Index | 0.49 | 0.016 | 0.74 | |||
| Baseline | 11.19 (8.30) | 9.94 (9.26) | 10.29 (6.66) | |||
| 3-mo change | 3.62 (2.02-5.22) | 2.76 (1.23-4.30) | 2.29 (0.78-3.81) | |||
| 6-mo change | 4.84 (3.19-6.50) | 3.62 (1.94-5.31) | 4.06 (2.46-5.66) |
Abbreviations: AUC, area under the curve; CD, Control diet; MD, Mediterranean diet; SAT, subcutaneous adipose tissue; TJD, Traditional Jiangnan diet; VAT, visceral adipose tissue.
a Baseline values are the observed mean (SD) or median (interquartile range). The within-group changes from baseline are expressed as estimated marginal means (95% CI).
b The effects of group, time, and their interaction on variables were examined by mixed-effects linear models with unstructured variance structure, which adjusted for age, sex, baseline body mass index (except for weight), baseline fasting glucose (except for fasting glucose), physical activity, and baseline values.
c A total of 187 participants had valid MRI records, 58 in the MD group, 66 in TJD, and 65 in CD.
Figure 2.
Changes in body weight monitored by scales at home during the intervention. The within-group changes from baseline are presented as estimated marginal means (Mediterranean diet [MD], n = 84; traditional Jiangnan diet [TJD], n = 85; control diet [CD], n = 84). Error bars show 95% CI. ↓ represent rebounds at weekends or on holidays. P group × time was examined by mixed-effects linear models with unstructured variance structure, which was adjusted for age, sex, baseline fasting glucose, physical activity, and baseline body weight.
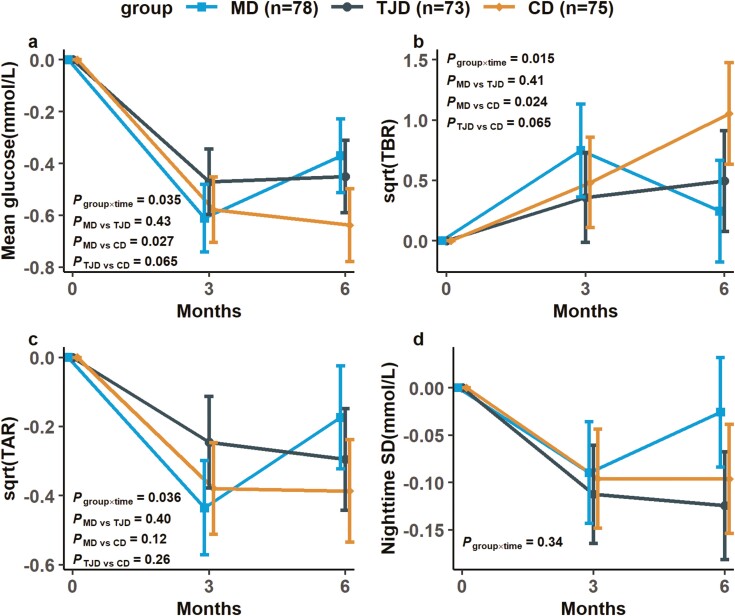
OGTT results at baseline and 3 and 6 months are indicated in Table 3. Significant effects of time were identified in 0-h glucose, AUC glucose, and the Matsuda index after a 6-month intervention with no group differences (all Ptime < 0.05). Valid CGM data were obtained from 226 participants, and there was a significant interaction of group × time on the mean glucose, TBR, and time above range over the 6 months (Pgroup × time < 0.05, Fig. 3). After post hoc pairwise comparisons, the participants in the CD group had significantly higher TBR than those in the MD group (0.81% [95% CI, 0.21-1.40], Pbetween-group = 0.024, Fig. 3B). Moreover, the stratified analysis by sex showed that both MD and TJD significantly suppressed the increase in TBR compared with CD in the male but not in female participants (STable 2, Pheterogeneity < 0.05 (30)), whereas the stratified analysis by obesity status (BMI, 28 kg/m2) showed a similar trend of results in CGM metrics between overweight and obese participants (STable 3, Pheterogeneity > 0.05 (30)).
Figure 3.
Changes in whole-day glucose homeostasis and nocturnal glucose fluctuation from CGM. The within-group changes from baseline are expressed as estimated marginal means (Mediterranean diet [MD], n = 78; traditional Jiangnan diet [TJD], n = 73; control diet [CD], n = 75). Error bars show 95% CI. (A) Mean glucose. (B) TBR was calculated and displayed in square-root form. (C) TAR was calculated and displayed in square-root form. (D) Nighttime SD. P group × time on variables were examined by mixed-effects linear models with unstructured variance structure, which adjusted for age, sex, baseline BMI, baseline fasting glucose, physical activity, and baseline values. P MD vs TJD, P MD vs CD, P TJD vs CD were P values for the comparison between Mediterranean diet and traditional Jiangnan diet, Mediterranean diet and control diet, traditional Jiangnan diet and control diet at 6 months with Bonferroni adjustment. Abbreviations: AUC, area under the curve hourly; CGM, continuous glucose monitoring; TAR, time above range (>10 mmol/L); TBR, time below range (<3.9 mmol/L).
After the 6-month intervention, there were significant time effects on systolic and diastolic blood pressure or circulating total and LDL-cholesterol (all Ptime < 0.05, Table 4). However, no significant group differences and interactions of group × time were observed on any variable mentioned.
Table 4.
Baseline values, and changes at 3 and 6 months of blood pressure and lipidsa
| Dietary groups | P groupb | P timeb | P group × timeb | |||
|---|---|---|---|---|---|---|
| MD (n = 84) | TJD (n = 85) | CD (n = 84) | ||||
| SBP, mmHg | 0.62 | < 0.0001 | 0.41 | |||
| Baseline | 129 (13.3) | 130 (16.6) | 126 (12.6) | |||
| 3-mo change | −6.31 (−8.22 to −4.39) | −7.49 (−9.36 to −5.62) | −7.56 (−9.46 to −5.65) | |||
| 6-mo change | −9.50 (−11.5 to −7.51) | −10.6 (−12.6 to −8.57) | −8.99 (−11.0 to −6.97) | |||
| DBP, mmHg | 0.84 | < 0.0001 | 0.13 | |||
| Baseline | 85.8 (10.0) | 85.9 (11.6) | 84.1 (10.4) | |||
| 3-mo change | −7.92 (−9.40 to −6.45) | −6.71 (−8.15 to −5.27) | −8.24 (−9.70 to −6.78) | |||
| 6-mo change | −9.37 (−10.9 to −7.84) | −9.55 (−11.1 to −8.00) | −8.83 (−10.4 to −7.29) | |||
| Cholesterol, mmol/L | 0.20 | 0.045 | 0.50 | |||
| Baseline | 4.89 (1.16) | 4.98 (1.30) | 4.78 (0.93) | |||
| 3-mo change | −0.23 (−0.36 to −0.11) | −0.34 (−0.46 to −0.21) | −0.33 (−0.45 to −0.2) | |||
| 6-mo change | −0.12 (−0.25 to 0.01) | −0.22 (−0.35 to −0.08) | −0.30 (−0.44 to −0.17) | |||
| HDL-cholesterol, mmol/L | 0.82 | 0.49 | 0.24 | |||
| Baseline | 1.23 (0.34) | 1.27 (0.28) | 1.21 (0.29) | |||
| 3-mo change | 0.04 (−0.01 to 0.09) | 0.07 (0.02-0.11) | 0.08 (0.04-0.13) | |||
| 6-mo change | 0.07 (0.02-0.12) † | 0.03 (−0.02 to 0.09) | 0.05 (0.00 to 0.10) | |||
| LDL-cholesterol, mmol/L | 0.56 | < 0.0001 | 0.56 | |||
| Baseline | 3.19 (0.78) | 3.19 (0.77) | 3.18 (0.75) | |||
| 3-mo change | −0.10 (−0.22 to 0.02) | −0.08 (−0.20 to 0.04) | −0.12 (−0.24 to 0.00) | |||
| 6-mo change | −0.22 (−0.35 to −0.09) | −0.24 (−0.37 to −0.12) | −0.34 (−0.47 to −0.21) | |||
| Triglyceride, mmol/L | 0.31 | 0.84 | 0.81 | |||
| Baseline | 1.54 (0.98) | 1.33 (0.83) | 1.53 (1.23) | |||
| 3-mo change | −0.15 (−0.32 to 0.02) | −0.31 (−0.48 to −0.14) | −0.31 (−0.48 to −0.14) | |||
| 6-mo change | −0.2 (−0.38 to −0.03) | −0.27 (−0.45 to −0.09) | −0.33 (−0.51 to −0.15) |
Abbreviations: CD, control diet; DBP, diastolic blood pressure; HDL-cholesterol, high-density lipoprotein cholesterol; LDL-cholesterol, low-density lipoprotein cholesterol; MD, Mediterranean diet; SBP, systolic blood pressure; TJD, Traditional Jiangnan diet.
a Baseline values are the observed mean (SD) or median (interquartile range). The within-group changes from baseline are expressed as estimated marginal means (95% CI).
b The effects of group, time, and their interaction on variables were examined by mixed-effects linear models with unstructured variance structure, which adjusted for age, sex, baseline body mass index, baseline fasting glucose, physical activity, and baseline values.
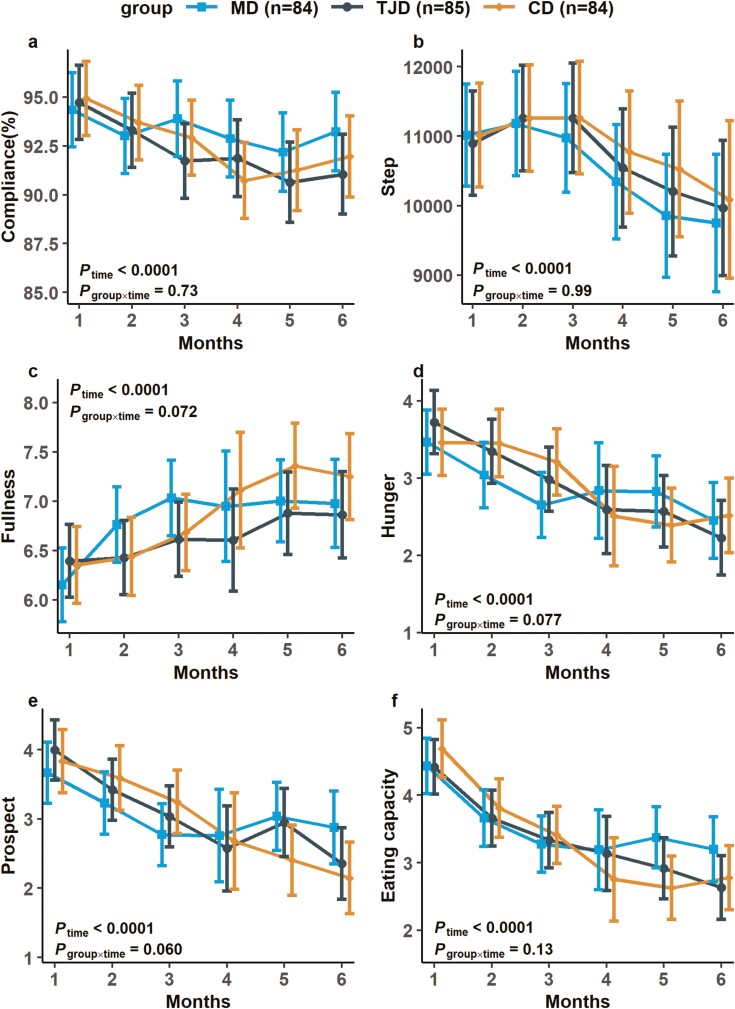
All 3 groups had > 90% compliance rates, and no between-group differences were observed (Fig. 4). There were considerable effects of time on fullness, hunger, prospective consumption, and eating capacity (all Ptime < 0.0001, Fig. 4), with no significant group differences and interactions of group × time during the 6-month period. Participants in all 3 groups had comparable physical activity levels evaluated using questionnaires and step records from the app over the 6-month trial (Fig. 4).
Figure 4.
Compliance, step record and appetitive measurements during intervention. The values are presented as observed means (Mediterranean diet [MD], n = 84; traditional Jiangnan diet [TJD], n = 85; control diet [CD], n = 84). Error bars indicate 95% CI. P group × time on variables were examined by mixed-effects linear models with unstructured variance structure, which adjusted for age, sex, baseline BMI, baseline fasting glucose, physical activity, and baseline values. (A) The rate of compliance. (B) Step record. (C) Fullness. (D) Hunger. (E) Prospective consumption. (F) Eating capacity.
All reported adverse events during the 6-month trial were mild and frequently reported in previous weight-loss interventions (31), such as constipation, nausea, diarrhea, and headache. One participant dropped out because of seafood allergies. Meanwhile, the rates of adverse events were similar across the 3 groups (STable 4 (30)).
Discussion
To our best knowledge, it was the first study comparing the effects of MD with Chinese diets high or low in plants on weight loss and glucose homeostasis in Chinese adults with prediabetes. By adapting app-based monitoring devices, a weekly 5-day feeding with 3 isocaloric-restricted diets for 6 months achieved comparable weight loss and enhanced glucose tolerance in conventional measurements. CGM data revealed the MD induced less increase in TBR than the CD.
Though the 3 dietary patterns had quite different macronutrient compositions and food sources, our trial supported that dietary pattern provides little additional benefit on weight loss beyond the 25% caloric restriction. Previously, the POUNDS LOST trial exhibited similar weight loss when 811 overweight adults were assigned to 1 of the 4 isocaloric-restricted diets with varied macronutrient compositions for 2 years (32). Besides body weight, our MRI results also showed that 3 isocaloric-restricted diets induced comparable reduction in VAT, in line with the finding from an 18-month intervention with isocaloric-restricted MD and low-fat diet in 278 adults with metabolic disorders (33). However, this study further indicated that MD was more potent in lowering hepatic and pancreatic fat than the low-fat diet. Thus, it remains to be elucidated whether fat accumulation on major metabolic organs could be more sensitive to the specific dietary pattern(s).
As in the case of weight loss, all 3 diet groups equally improved most glycemic metrics measured using OGTT at 6 months, somewhat resembling a few earlier calorie-restricted feeding trials (34, 35). For instance, replacing beef with isocaloric plant protein in the Dietary Approaches to Stop Hypertension diet did not change fasting glucose and insulin levels following a 6-week weight-loss phase in 62 adults with metabolic syndrome (34). In another 12-week controlled feeding trial, replacing refined grains with whole grains did not alter fasting glycemia beyond caloric restriction in 29 obese adults (35). By adopting CGM covering a mean 12-day period in our study, participants in the MD group had significantly less increase in TBR than those in the CD group at the end of the intervention. Similarly, TJD showed marginally less increase in TBR than CD among all study participants (P = 0.065). As expected, the observed longer duration of TBR in the CD group could be ascribed to the higher percentage of refined rice and lower dietary fiber content, which may accelerate postprandial digestion and absorption as well as alter glycemic response (36) when compared with MD and TJD groups. The marginal difference between TJD and CD is possibly because both diets had similar amounts of refined cereals. Meanwhile, slightly higher fiber in TJD and a higher percentage of fat in CD may both retard and prolong the postprandial glycemic response (13), thus diminishing the between-group difference. We observed that men but not women had a significantly lower increase in TBR induced by TJD rather than CD. Although the underlying mechanism remains largely unknown, we recently revealed that sex hormones and gut microbiota exerted an influence on glucose homeostasis (37). Certainly, more future studies are needed in this regard. Considering that hypoglycemia is a nonnegligible side effect and could cause adverse cardiovascular outcomes (38), the MD was a more favorable choice to avoid hypoglycemia under caloric-restricted conditions, particularly for those with impaired glucose homeostasis.
In our study, all 3 calorie-restricted diet groups also achieved significant improvements in multiple cardiometabolic risks besides glycemic control at 6 months. Control of blood pressure and lipid metabolism is required for diabetes prevention in high-risk populations (1). Following our findings, a recent meta-analysis including 121 trials suggested that modest weight loss in 6 months could result in substantial improvements in blood pressure (39). Nevertheless, a crossover feeding study conducted in 164 adults with metabolic disorder showed that partial substitution of carbohydrates with monounsaturated fat for 6 weeks could further lower blood pressure and improve lipids, which was not observed in our study (40). The discrepancies could be due to the moderate calorie restriction in the present study, whereas body weight was maintained in that crossover trial. Thus, calorie restriction induced weight loss seemed to be critical to improve blood pressure and lipids.
Notably, all 3 diets had comparable compliance (>90%) and dropout rate during 6-month feeding period. The relatively high compliance in our study could partially attribute to the “5 + 2” regimen, in which the participants had 5-day calorie-restricted full feeding plus 2 weekend days enjoying family meals for 6 months. Meanwhile, the participants were told to wear an app-connected weight scale and smart band to monitor weight fluctuation and maintain pretrial physical activity levels. In addition, the MD menus were tailored to enhance their acceptability for Chinese people, while reserving its traditional concepts and core components. Collectively, all the efforts could facilitate our participants to achieve the mean weight loss of 7% (5.5 kg), which met the goal in previous diabetes prevention studies (1). Nonetheless, considering higher associability and comparable metabolic benefits, TJD could serve as a regional alternative of MD in Chinese adults and in populations with similar dietary patterns.
The major strengths of this study included the following: (1) simultaneously evaluated effects of MD with 2 Chinese dietary patterns reflecting before and after nutrition transition on weight loss and glucose homeostasis in Chinese adults with prediabetes, which could be necessary for the regions or countries experiencing similar shifting from diet high in plants to diet low in plants; (2) the “5 + 2” regimen combined with mobile app-based devices enhanced adherence; and (3) applications of CGM and MRI provided more insights and aspects of diet-induced changes in adiposity status and glycemic homeostasis. Admittedly, our study also has certain limitations. First, the sample size may have been insufficient to detect between-group differences in glycemic metrics from OGTT and CGM because of the unavailable priori power calculations. Second, not all meals were consumed under supervision, though all participants were asked to weigh or upload photos of leftovers through the app. Third, the dropout rate was 20% even though a sensitivity analysis with multiple imputation was applied to compensate for this bias. Finally, readable CGM measurements may also have an influence on the overall improvement of glucose homeostasis in addition to the dietary intervention in our study.
In conclusion, the isocaloric-restricted TJD and MD exerted comparable impacts on weight loss and glucose homeostasis, whereas the CD raised hypoglycemic risk beyond weight loss in Chinese adults with prediabetes. Future studies are merited to confirm our findings in various populations with longer durations and uncover underlying mechanisms.
Acknowledgments
We thank Quan Xiong, Shaofeng Huo, Zhenhua Niu, Di Wang, Huan Yun, Shuanshuan Chen, and Qianlu Jin from Shanghai Institute of Nutrition and Health and Lingxia Ye, Ling Zhang, Shaoqian Zhao, Yansong Liu, Yusong Chen, Dongqin Gu, and Wenting Su from Ruijin Hospital for their kind assistances at various stages of this trial; Yueqin Cao, Sipei Kang, and other staff members of SAICVOLKSWAGEN helped in feeding intervention. We are also grateful to the Wilmar Group for donating partial food ingredients used in intervention meals and Zhejiang Nutriease Health Technology Co. Ltd for providing support of APP monitoring system.
Glossary
Abbreviations
- AUC
area under the curve
- BMI
body mass index
- CD
control diet
- CGM
continuous glucose monitoring
- LDL
low-density lipoprotein
- MD
Mediterranean diet
- MRI
magnetic resonance imaging
- OGTT
oral glucose tolerance test
- TBR
time below range
- TJD
traditional Jiangnan diet
- VAT
visceral adipose tissue
Contributor Information
Yaogan Luo, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Jiqiu Wang, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Liang Sun, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Weiqiong Gu, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Geng Zong, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Boyu Song, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Chongrong Shen, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Puchen Zhou, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Yufei Chen, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Yanpu Wu, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Huibin Lin, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
He Zheng, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Mengshan Ni, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Xiaowei Yang, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Yanru Chen, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Xinming Xu, Shanghai Medical College, Fudan University, Shanghai, 200032, China.
Juan Zhang, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Juan Shi, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Ru Zhang, SAIC Volkswagen Automotive Company Limited, Shanghai, 201805, China.
Jinfen Hu, SAIC Volkswagen Automotive Company Limited, Shanghai, 201805, China.
Hong Hou, SAIC Volkswagen Automotive Company Limited, Shanghai, 201805, China.
Ling Lu, Key Laboratory of Systems Health Science of Zhejiang Province, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, 310024, China; Key Laboratory of Systems Biology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Hangzhou, 310024, China.
Xiaoqiang Xu, Aimigene Institute, Shenzhen, 518063, China.
Liming Liang, Department of Epidemiology and Department of Biostatistics, Harvard T H Chan School of Public Health, Boston, MA, 02115, USA.
Ruixin Liu, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Xiaoran Liu, Department of Internal Medicine, Rush University Medical Center, Chicago, IL, 60612, USA.
Huaixing Li, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China.
Jie Hong, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Weiqing Wang, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Xu Lin, Shanghai Institute of Nutrition and Health, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Shanghai, 200031, China; Key Laboratory of Systems Health Science of Zhejiang Province, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Hangzhou, 310024, China; Key Laboratory of Systems Biology, Hangzhou Institute for Advanced Study, University of Chinese Academy of Sciences, Chinese Academy of Sciences, Hangzhou, 310024, China.
Guang Ning, Department of Endocrine and Metabolic Diseases, Shanghai Institute of Endocrine and Metabolic Diseases, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China; Shanghai National Clinical Research Center for metabolic Diseases, Key Laboratory for Endocrine and Metabolic Diseases of the National Health Commission of the PR China, Shanghai National Center for Translational Medicine, Ruijin Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, 200025, China.
Financial Support
The study was funded by the Strategic Priority CAS Project (XDB38000000), Chinese Academy of Sciences (ZDBS-SSW-DQC-02, KJZD-EW-G20-02), Ministry of Science and Technology of China (2018YFC1313800 and 2018YFC1314800), the National Natural Science Foundation of China (81970684, 91957124, and 81822009), Shanghai Municipal Science and Technology Major Project (2017SHZDZX01), the Outstanding Academic Leader Project of Shanghai Municipal Health Commission (2018BR01), and Shanghai Medicine and Health Development Foundation (DMRFP_I_08). The funders had no role in the design and conduct of the study; collection, analysis, and interpretation of the data; and decision to submit the manuscript for publication.
Author Contributions
G.N., X.L., and J.W. conceived the study and designed the intervention. L.S., W.G., G.Z., H.L.., R.L., J.H., and W.W. contributed to the study design. Y.L., J.W., L.S., W.G., G.Z., B.S., C.S., P.Z., Y.C., Y.W., H.L., H.Z., M.N., X.Y., Y.R.C., X.X., J.Z., S.J., R.Z., J.H., H.H., and L.L. conducted the research. Y.L. analyzed the data with support from X.Q.X. and L.M.L. and wrote the initial paper. L.S., J.W., X.R.L., X.L., and G.N. edited the paper. All authors reviewed the manuscript and approved the final version.
Disclosures
None of the authors have relevant disclosures or conflicts of interest to disclose.
Trial Registration
Clinicaltrials.gov registration no. NCT03856762 (registered February 27, 2019).
Data Availability
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Evert AB, Dennison M, Gardner CD, et al. Nutrition therapy for adults with diabetes or prediabetes: a consensus report. Diabetes Care. 2019;42(5):731-754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Li Y, Wang DD, Ley SH, et al. Time trends of dietary and lifestyle factors and their potential impact on diabetes burden in China. Diabetes Care. 2017;40(12):1685-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gan ZH, Cheong HC, Tu YK, Kuo PH. Association between plant-based dietary patterns and risk of cardiovascular disease: a systematic review and meta-analysis of prospective cohort studies. Nutrients. 2021;13(11):3952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuehn BM. Heritage diets and culturally appropriate dietary advice may help combat chronic diseases. JAMA. 2019;322(23):2271-2273. [DOI] [PubMed] [Google Scholar]
- 5. He Y, Li Y, Yang X, et al. The dietary transition and its association with cardiometabolic mortality among Chinese adults, 1982-2012: a cross-sectional population-based study. Lancet Diabetes Endocrinol. 2019;7(7):540-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang YF, Sun MX, Xue H, et al. [Understanding the China Blue Paper on Obesity Prevention and Control and policy implications and recommendations for obesity prevention and control in China.] Zhonghua yu fang yi xue za zhi [Zhonghua Yu Fang Yi Xue Za Zhi]. 2019;53(9):875-884. [DOI] [PubMed] [Google Scholar]
- 7. Li Y, Teng D, Shi X, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ (Clin Res Ed). 2020;369:m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American Diabetes Association. 5. Lifestyle management: standards of medical care in Diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S46-S60. [DOI] [PubMed] [Google Scholar]
- 9. Salas-Salvadó J, Díaz-López A, Ruiz-Canela M, et al. Effect of a lifestyle intervention program with energy-restricted Mediterranean diet and exercise on weight loss and cardiovascular risk factors: one-year results of the PREDIMED-plus trial. Diabetes Care. 2019;42(5):777-788. [DOI] [PubMed] [Google Scholar]
- 10. Shai I, Schwarzfuchs D, Henkin Y, et al. Weight loss with a low-carbohydrate, Mediterranean, or low-fat diet. N Engl J Med. 2008;359(3):229-241. [DOI] [PubMed] [Google Scholar]
- 11. Sofi F, Dinu M, Pagliai G, et al. Low-calorie vegetarian versus Mediterranean diets for reducing body weight and improving cardiovascular risk profile: CARDIVEG study (cardiovascular prevention with vegetarian diet). Circulation. 2018;137(11):1103-1113. [DOI] [PubMed] [Google Scholar]
- 12. Ludwig DS, Ebbeling CB, Heymsfield SB. Improving the quality of dietary research. JAMA. 2019;322(16):1549-1550. [DOI] [PubMed] [Google Scholar]
- 13. Tay J, Thompson CH, Brinkworth GD. Glycemic variability: assessing glycemia differently and the implications for dietary management of diabetes. Annu Rev Nutr. 2015;35:389-424. [DOI] [PubMed] [Google Scholar]
- 14. Lu J, Wang C, Shen Y, et al. Time in range in relation to all-cause and cardiovascular mortality in patients with type 2 diabetes: a prospective cohort study. Diabetes Care. 2021;44(2):549-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. American Diabetes Association. 6. Glycemic targets: standards of medical care in Diabetes-2021. Diabetes Care. 2021;44(Suppl 1):S73-S84. [DOI] [PubMed] [Google Scholar]
- 16. Ceriello A, Prattichizzo F, Phillip M, Hirsch IB, Mathieu C, Battelino T. Glycaemic management in diabetes: old and new approaches. Lancet Diabetes Endocrinol. 2022;10(1):75-84. [DOI] [PubMed] [Google Scholar]
- 17. Zeevi D, Korem T, Zmora N, et al. Personalized nutrition by prediction of glycemic responses. Cell. 2015;163(5):1079-1094. [DOI] [PubMed] [Google Scholar]
- 18. Ye L, Gu W, Chen Y, et al. The impact of shift work on glycemic characteristics assessed by CGM and its association with metabolic indices in non-diabetic subjects. Acta Diabetol. 2020;57(1):53-61. [DOI] [PubMed] [Google Scholar]
- 19. American Diabetes Association. 7. Diabetes technology standards of medical care in diabetes. Diabetes Care. 2021;44(suppl 1):S85-S99. [DOI] [PubMed] [Google Scholar]
- 20. Zhou B; Coorperative Meta-Analysis Group of China Obesity Task Force. [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population.] Zhonghua Liu Xing Bing Xue Za Zhi Zhonghua Liuxingbingxue Zazhi. 2002;23(1):5-10. [PubMed] [Google Scholar]
- 21. Chinese Nutrition Society. Dietary Guidelines for Chinese Residents. Beijing: People’s Medical Publishing House; 2016;35. [Google Scholar]
- 22. Willett WC, Sacks F, Trichopoulou A, et al. Mediterranean diet pyramid: a cultural model for healthy eating. Am J Clin Nutr. 1995;61(6 suppl):1402S-1406S. [DOI] [PubMed] [Google Scholar]
- 23. Wang J, Lin X, Bloomgarden ZT, Ning G. The Jiangnan diet, a healthy diet pattern for Chinese. J Diabetes. 2020;12(5):365-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhai FY, Du SF, Wang ZH, Zhang JG, Du WW, Popkin BM. Dynamics of the Chinese diet and the role of urbanicity, 1991-2011. Obes Rev. 2014;15(suppl 1):16-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang Y, Food C.. Composition 2004. Beijing, China: Peking University Medical Press; 2005. [Google Scholar]
- 26. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care. 2017;40(12):1631-1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Craig CL, Marshall AL, Sjöström M, et al. International physical activity questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35(8):1381-1395. [DOI] [PubMed] [Google Scholar]
- 28. Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22(9):1462-1470. [DOI] [PubMed] [Google Scholar]
- 29. Kwak SK, Kim JH. Statistical data preparation: management of missing values and outliers. Korean J Anesthesiol. 2017;70(4):407-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo YG, Wang JQ, Sun Let al. Data. In: Isocaloric-restricted Mediterranean diet and Chinese diets high or low in plants in adults with prediabetes. Github Deposited. March 20, 2022. https://github.com/yaoganluo/JCEM-online-material [DOI] [PMC free article] [PubMed]
- 31. Pi-Sunyer FX. Short-term medical benefits and adverse effects of weight loss. Ann Intern Med. 1993;119(7 Pt 2):722-726. [DOI] [PubMed] [Google Scholar]
- 32. Sacks FM, Bray GA, Carey VJ, et al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859-873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gepner Y, Shelef I, Schwarzfuchs D, et al. Effect of distinct lifestyle interventions on mobilization of fat storage pools: central magnetic resonance imaging randomized controlled trial. Circulation. 2018;137(11):1143-1157. [DOI] [PubMed] [Google Scholar]
- 34. Hill AM, Harris Jackson KA, Roussell MA, West SG, Kris-Etherton PM. Type and amount of dietary protein in the treatment of metabolic syndrome: a randomized controlled trial. Am J Clin Nutr. 2015;102(4):757-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Raatz SK, Torkelson CJ, Redmon JB, et al. Reduced glycemic index and glycemic load diets do not increase the effects of energy restriction on weight loss and insulin sensitivity in obese men and women. J Nutr. 2005;135(10):2387-2391. [DOI] [PubMed] [Google Scholar]
- 36. Slavin J. Why whole grains are protective: biological mechanisms. Proc Nutr Soc. 2003;62(1):129-134. [DOI] [PubMed] [Google Scholar]
- 37. Gao AB, Su JL, Liu RX, et al. Sexual dimorphism in glucose metabolism is shaped by androgen-driven gut microbiome. Nat Commun. 2021;12(1):7080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Greenway FL; Look AHEAD Research Group (Appendix A). Severe hypoglycemia in the Look AHEAD Trial. J Diabetes Its Complicat. 2016;30(5):935-943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ge L, Sadeghirad B, Ball GDC, et al. Comparison of dietary macronutrient patterns of 14 popular named dietary programmes for weight and cardiovascular risk factor reduction in adults: systematic review and network meta-analysis of randomised trials. BMJ (Clin Res Ed). 2020;369:m696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Appel LJ, Sacks FM, Carey VJ, et al. Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294(19):2455-2464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all datasets generated during and/or analyzed during the current study are not publicly available but are available from the corresponding author on reasonable request.