Abstract
Context
Younger age at treatment onset with conventional therapy (phosphate salts and active vitamin D; Pi/D) is associated with improved growth and skeletal outcomes in children with X-linked hypophosphatemia (XLH). The effect of age on burosumab efficacy and safety in XLH is unknown.
Objective
This work aimed to explore the efficacy and safety of burosumab vs Pi/D in younger (< 5 years) and older (5-12 years) children with XLH.
Methods
This post hoc analysis of a 64-week, open-label, randomized controlled study took place at 16 academic centers. Sixty-one children aged 1 to 12 years with XLH (younger, n = 26; older, n = 35) participated. Children received burosumab starting at 0.8 mg/kg every 2 weeks (younger, n = 14; older, n = 15) or continued Pi/D individually titrated per recommended guidelines (younger, n = 12; older, n = 20). The main outcome measure included the least squares means difference (LSMD) in Radiographic Global Impression of Change (RGI-C) rickets total score from baseline to week 64.
Results
The LSMD in outcomes through 64 weeks on burosumab vs conventional therapy by age group were as follows: RGI-C rickets total score (younger, +0.90; older, +1.07), total Rickets Severity Score (younger, −0.86; older, −1.44), RGI-C lower limb deformity score (younger, +1.02; older, +0.91), recumbent length or standing height Z-score (younger, +0.20; older, +0.09), and serum alkaline phosphatase (ALP) (younger, −31.15% of upper normal limit [ULN]; older, −52.11% of ULN). On burosumab, dental abscesses were not reported in younger children but were in 53% of older children.
Conclusion
Burosumab appears to improve outcomes both in younger and older children with XLH, including rickets, lower limb deformities, growth, and ALP, compared with Pi/D.
Keywords: burosumab, fibroblast growth factor 23, X-linked hypophosphatemia, rickets, children
X-linked hypophosphatemia (XLH) is a rare, heritable skeletal disorder characterized by excess production and circulating concentrations of fibroblast growth factor 23 (FGF23), resulting in renal phosphate wasting, hypophosphatemia, and defective bone mineralization that causes rickets, osteomalacia, skeletal deformities, short stature, pain, decreased linear growth, and reduced physical function (1-3). Previous conventional therapy for XLH included high doses of phosphate salts and active vitamin D (Pi/D); however, complications of XLH (eg, nephrocalcinosis and hyperparathyroidism) may persist or eventually be exacerbated with Pi/D, and gastrointestinal symptomatology may limit compliance with the requirement for multiple daily doses (4-6). Furthermore, Pi/D often may not correct biochemistry, resolve the radiographic features of rickets, or prevent dental abscesses and enthesopathy (7, 8).
A randomized, open-label, phase 3 study comparing continuation of Pi/D vs switching to burosumab, a fully human immunoglobulin G1 monoclonal antibody to FGF23, enrolled children aged 1 to 12 years with XLH. Treatment with burosumab led to significantly better renal phosphate reabsorption and serum phosphate levels, as well as improved rickets, lower limb deformity, linear growth, and mobility compared with Pi/D therapy (9). Given that initiation of previous conventional therapy at a younger age (< 1 year) in XLH has been reported to improve growth and skeletal outcomes compared with initiation at later ages (10, 11), we assessed the efficacy of burosumab vs Pi/D in younger vs older children with XLH. Because randomization for this study was stratified by age younger than 5 and 5 years and older to ensure even age distribution between treatment arms, a post hoc analysis was possible to explore the efficacy and safety of burosumab vs Pi/D in these 2 subgroups. We also assessed the difference in the response specifically to burosumab in younger compared with older children.
Materials and Methods
Participants
The randomized, active-controlled, open-label, phase 3 study (CL301) has been published (9). The study was conducted at 16 academic health centers in the United States (n = 5), Japan (n = 3), Canada (n = 3), the United Kingdom (n = 2), Sweden (n = 1), South Korea (n = 1), and Australia (n = 1). Principal inclusion criteria were children aged 1 to 12 years with XLH, fasting serum phosphorus less than 3.0 mg/dL (< 0.97 mmol/L), PHEX mutation or variant of unknown significance confirmed in the child or a family member, total Rickets Severity Score (RSS) 2.0 or greater, and treatment with conventional therapy for 6 consecutive months or more for children younger than 3 years or 12 months or more for children aged 3 years and older.
Principal exclusion criteria were Tanner stage 4 or 5; height above the 50th percentile for age and sex based on country-specific norms; use of growth hormone therapy in the 12 months before screening; plasma intact parathyroid hormone (iPTH) greater than 180 pg/mL (19 pmol/L); hypocalcemia or hypercalcemia; renal ultrasound indicating grade 4 nephrocalcinosis (on a scale of 0-4) (12); or planned orthopedic surgery.
The study was conducted in accordance with the Declaration of Helsinki and the Good Clinical Practice guidelines developed at the International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use. The institutional review board at each participating site approved the protocol. Parents or guardians provided written informed consent for study participation. Children gave written consent or assent per local guidelines, and an independent committee monitored safety.
Study Design, Treatment, and End Points
As previously described (9), following a 7-day washout period of Pi/D, children were randomly assigned 1:1 using an Interactive Web Response System to receive open-label subcutaneous burosumab starting at 0.8 mg/kg every 2 weeks or to restart Pi/D, with doses titrated individually and consistent with published recommendations (oral phosphate 20-60 mg/kg per day, and alfacalcidol 40-60 ng/kg per day or calcitriol 20-30 ng/kg per day) (5, 6). The dose of burosumab could be increased to 1.2 mg/kg every 2 weeks if 2 consecutive predose fasting serum phosphorus concentrations were less than 3.2 mg/dL (< 1.03 mmol/L) and serum phosphorus had increased by less than 0.5 mg/dL (< 0.16 mmol/L) from baseline on any single measurement. Pi/D doses and dose adjustments were made at the discretion of the treating physician in line with the recommended standard-of-care guidelines (5, 6). Randomization was performed in blocks and stratified by RSS (≤ 2.5 vs > 2.5), age (< 5 vs ≥ 5 years), and whether the child was enrolled from Japan or elsewhere. All participants received their assigned treatment for 64 weeks. Treatment compliance was assessed by site personnel during scheduled biweekly telephone visits; empty vials were to be returned to the study site for accountability records. Supplementation (eg, cholecalciferol) was allowed if serum 25-hydroxyvitamin D (25[OH]D) levels fell below 20 ng/mL.
Post hoc Subgroup Analysis
Randomization was stratified by age to ensure equal distribution of ages between the two treatment arms. In this descriptive post hoc analysis, the efficacy and safety of burosumab vs Pi/D during the 64-week randomized treatment period were initially compared in children younger than 5 years (ie, younger children) and in those aged 5 to 12 years (ie, older children). Next, the differences in treatment effect (ie, burosumab vs Pi/D) for each efficacy outcome were compared between the younger and older children; the differences in efficacy outcomes were also specifically compared between younger and older children who received burosumab.
As reported previously (9), the primary end point of the study was change in rickets severity per the Radiographic Global Impression of Change (RGI-C) rickets total score at week 40. In this post hoc analysis, efficacy assessments to study week 64 included fasting serum phosphorus, tubular maximum for phosphate reabsorption per glomerular filtration rate (TmP/GFR; limited to children aged > 5 years), 1,25-dihydroxyvitamin D (1,25[OH]2D), 25(OH)D, alkaline phosphatase (ALP), RGI-C rickets total score (at the wrists and knees), total RSS, the RGI-C lower limb deformity score, and the recumbent length or standing height Z-score. The RGI-C is a 7-point ordinal scale (−3, severe worsening; −2, moderate worsening; −1, minimal worsening; 0, no change; +1, minimal healing; +2, substantial healing; +3, complete healing) whereby radiologists provided bilateral wrist and knee scores, and an RGI-C total score that reflects the changes both in the wrists and knees (13). Reviews were performed independently by 3 radiologists were not among the authors. Lower limb deformities were also assessed using RGI-C. The RSS assigns a total score ranging from 0 (no rickets) to 10 (severe rickets) based on the sum of scores from the more severely affected wrist (0-4) and knee (0-6) (14). Safety assessments included adverse events (AEs), renal ultrasound nephrocalcinosis scores, and plasma iPTH levels. Laboratory samples were assessed by a central laboratory.
Statistical Analysis
In this post hoc analysis, efficacy data were summarized with means and (SDs), medians with interquartile ranges, least squares (LS) means with SE, and LS means difference (LSMD) with 95% CIs, as appropriate. Serum phosphorus, TmP/GFR, 1,25(OH)2D, 25(OH)D, ALP, and iPTH were summarized with means and SD; serum ALP was expressed as the percentage of the age- and sex-appropriate upper limit of normal (ULN). AEs were summarized descriptively as the number and proportion of children in each age and treatment group. There was no imputation for missing data.
In this post hoc analysis, within the younger and older subgroups, the generalized estimating equations (GEE) model with exchangeable covariance structure was used to assess all clinical outcomes with repeated measures (ie, the RGI-C rickets total score, total RSS, RGI-C lower limb deformity score, and recumbent length or standing height Z-score), as reported (9). Variables in the model included treatment group, study visit, interaction between treatment group and study visit, and between baseline stratification factors and covariates. For recumbent length or standing height Z-scores, baseline age and height or length Z-scores were included as continuous covariates. LS means of the treatment effects (burosumab vs Pi/D) assessed within an age subgroup were considered statistically significant if P values were below .05.
In the present post hoc analysis, differences in the response to treatment by age group, and the difference in the response specifically to burosumab by age group, were explored using the GEE model for RGI-C rickets total score, total RSS, RGI-C lower limb deformity score, and recumbent length or standing height. Specifically, the GEE model compared the response to burosumab alone on efficacy outcomes at week 64 between younger and older children, as well as the treatment effect (burosumab vs Pi/D) between younger and older children. LSMD (burosumab vs Pi/D) between age subgroups were considered statistically significant if P values were below .05.
Differences between age subgroups in exposure to burosumab, phosphate, calcitriol, and alfacalcidol were assessed using a 2-sample t test. P values below .05 were considered statistically significant.
Results
Patients
Patients were recruited from August 3, 2016, to May 8, 2017. Of 122 children initially screened, 61 were ineligible (9). In total, 61 children with XLH were randomly assigned to treatment (Pi/D, n = 32; burosumab, n = 29); 26 were younger (ie, aged 1-< 5 years; Pi/D, n = 12; burosumab, n = 14), and 35 were older (ie, aged ≥ 5-12 years; Pi/D, n = 20; burosumab, n = 15). No children discontinued the study. Demographics and baseline characteristics by age groups are summarized in Table 1.
Table 1.
Demographics and baseline disease characteristics
| Age < 5 y | Age ≥ 5 y | |||
|---|---|---|---|---|
| Conventional therapy (n = 12) |
Burosumab (n = 14) |
Conventional therapy (n = 20) |
Burosumab (n = 15) |
|
| Median (IQR) age, y | 3.0 (2.0 to 3.7) | 3.1 (2.2 to 3.7) | 8.7 (6.2 to 9.8) | 7.8 (6.4 to 10.2) |
| Sex, n (%) | ||||
| Male | 5 (42) | 3 (21) | 9 (45) | 10 (67) |
| Female | 7 (58) | 11 (79) | 11 (55) | 5 (33) |
| Race, n (%) | ||||
| White | 11 (92) | 13 (93) | 14 (70) | 12 (80) |
| Asian | 1 (8) | 1 (7) | 5 (25) | 1 (7) |
| Other | 0 | 0 | 1 (5) | 2 (13) |
| Height Z-score | ||||
| Mean (SD) | –2.27 (1.01) | –2.27 (1.15) | –1.91 (0.76) | –2.36 (1.22) |
| Median (IQR) | –2.18 (–2.71 to –1.53) | –2.31 (–2.77 to –1.68) | –2.06 (–2.38 to –1.40) | –2.02 (–3.20 to –1.40) |
| Mean (SD) serum phosphorus, mg/dL | 2.39 (0.22) | 2.54 (0.22) | 2.25 (0.27) | 2.31 (0.22) |
| Mean (SD) serum phosphorus, mmol/L | 0.77 (0.07) | 0.82 (0.07) | 0.73 (0.09) | 0.75 (0.07) |
| Mean (SD) TmP/GFR, mg/dLa | 1.95 (0.33) | 2.35 (0.32) | 2.05 (0.33) | 2.08 (0.38) |
| Mean (SD) TmP/GFR, mmol/La | 0.63 (0.11) | 0.76 (0.10) | 0.66 (0.11) | 0.67 (0.12) |
| Mean (SD) serum 1,25(OH)2D, pg/mL | 45.9 (14.2) | 52.2 (22.4) | 36.9 (14.6) | 39.8 (15.8) |
| Mean (SD) serum 1,25(OH)2D, pmol/L | 110.2 (34.1) | 125.3 (53.7) | 88.5 (35.1) | 95.4 (37.9) |
| Mean (SD) serum 25(OH)D, ng/mL | 32.1 (8.1) | 31.6 (8.9) | 31.6 (11.3) | 32.8 (12.0) |
| Mean (SD) serum 25(OH)D, nmol/L | 80.1 (20.2) | 79.0 (22.3) | 78.8 (28.1) | 81.9 (30.0) |
| Mean (SD) serum alkaline phosphatase, U/L | 534.5 (134.9) | 529.4 (99.0) | 516.8 (168.1) | 493.3 (146.4) |
| Mean (SD) serum alkaline phosphatase, % of ULN | 166.1 (45.6) | 166.7 (33.8) | 157.5 (43.1) | 153.2 (53.5) |
| Mean (SD) duration of conventional therapy, y | 1.7 (0.6) | 1.5 (0.8) | 5.9 (2.8) | 5.1 (3.5) |
| Mean (SD) total RSS | 3.50 (1.48) | 3.29 (1.14) | 3.00 (0.87) | 3.07 (0.82) |
| Tanner stage, n (%) | ||||
| 1 | 12 (100) | 14 (100) | 19 (95) | 13 (87) |
| 2 | 0 | 0 | 1 (5) | 2 (13) |
Abbreviations: 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; IQR, interquartile range; RSS, Rickets Severity Score; TmP/GFR, tubular maximum for phosphate reabsorption per glomerular filtration rate; ULN, upper limit of normal.
a TmP/GFR data were available for 22 children younger than 5 years (conventional therapy, n = 12; burosumab, n = 10) and 32 children 5 years and older (conventional therapy, n = 18; burosumab, n = 14).
Exposure
As reported previously for the total population, median daily doses of oral Pi were within the recommended range, and median daily doses of active vitamin D were typically within the recommended range (9). Twenty-six of 29 children received all doses of burosumab, except for 3 children, who each missed 1 dose (9). Eight of 29 (28%) children increased to a burosumab dose of 1.2 mg/kg because of low serum phosphorus (9). In this analysis, there were no significant differences in mean (SD) weight-based burosumab doses between younger and older children (Table 2). Although prestudy and on-study doses of oral Pi (per kg) appeared to be higher in older children than younger children, no statistically significant differences were found. Similarly, although prestudy and on-study doses of calcitriol (per kg) appeared to be higher in younger children than in older children, no statistically significant differences were found.
Table 2.
Exposure to conventional therapy and burosumab
| Conventional therapy (n = 32) | Burosumab (n = 29) | |||||
|---|---|---|---|---|---|---|
| Age < 5 y (n = 12) | Age ≥ 5 y (n = 20) | P a | Age < 5 y (n = 14) | Age ≥ 5 y (n = 15) | P a | |
| Phosphate, mg/kg | ||||||
| No. | 12 | 20 | 14 | 15 | ||
| Baseline meanb (SD) | 32.9 (15.6) | 38.1 (16.3) | .3856 | 33.7 (45.8) | 27.4 (14.8) | .63 |
| Wk 40 mean (SD) | 36.6 (15.0) | 43.6 (23.5) | .3627 | – | – | |
| Wk 64 mean (SD) | 41.7 (16.5) | 48.3 (32.8) | .3269 | – | – | |
| Calcitriol, µg/kg | ||||||
| n | 10 | 12 | 11 | 14 | ||
| Baseline meanb (SD) | 23.3 (8.7) | 20.1 (6.1) | .3193 | 24.3 (12.6) | 19.2 (6.6) | .24 |
| Wk 40 mean (SD) | 30.0 (15.8) | 23.5 (10.7) | .2688 | – | – | |
| Wk 64 mean (SD) | 30.5 (16.0) | 24.4 (10.7) | .3004 | – | – | |
| Alfacalcidol, µg/kg | ||||||
| n | 2 | 7c | 3 | 1 | ||
| Baseline meanb (SD) | 49.5 (21.2) | 86.2 (73.8) | .5270 | 55.6 (6.8) | 205.5 | – |
| Wk 40 mean (SD) | 57.1 (32.0) | 95.6 (66.9) | .6029 | – | – | |
| Wk 64 mean (SD) | 58.1 (33.3) | 94.7 (64.8) | .6171 | – | – | |
| Total burosumab dose, mg/kg | ||||||
| n | 14 | 15 | ||||
| Baseline mean (SD) | – | – | 0.80 (0.07) | 0.81 (0.03) | .88 | |
| Wk 40 mean (SD) | – | – | 0.84 (0.11) | 0.91 (0.18) | .21 | |
| Wk 64 mean (SD) | – | – | 0.89 (0.17) | 0.90 (0.20) | .84 |
a Differences in exposure between age groups were compared with a 2-sample t test. Values less than .05 indicate statistical significance.
b Values from before study entry.
c One child was omitted because they had received eldecalcitol (19.5 ng/kg per day) at baseline and switched to alfacalcidol at week 32 with no dose adjustments thereafter.
Efficacy
Biochemistry
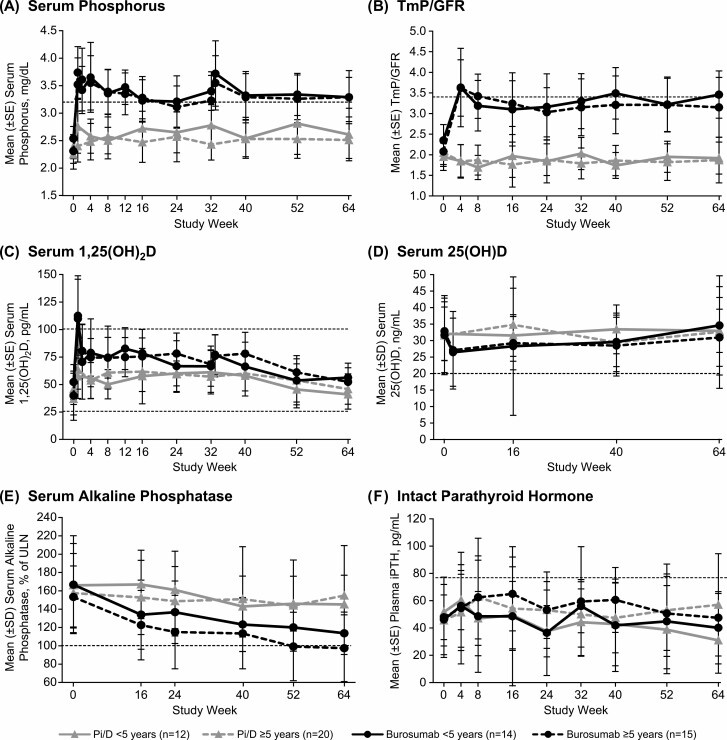
In both age groups, children who received burosumab had sustained increases in fasting serum phosphorus and TmP/GFR, whereas those who received Pi/D had minimal changes in these 2 parameters (Fig. 1A and 1B). Younger and older children both had peak increases in serum 1,25(OH)2D following the initial dose of burosumab; subsequent values were within the normal range (Fig. 1C). In both age groups, serum 25(OH)D decreased slightly after the first dose of burosumab and subsequently increased throughout the treatment period, whereas serum 25(OH)D levels were relatively stable among children who received Pi/D (Fig. 1D). Among younger children, mean (SD) serum 25(OH)D for the treatment period was 30.3 (11.0) ng/mL in those who received burosumab and 32.4 (6.9) ng/mL in those who received Pi/D. Among older children, mean (SD) serum 25(OH)D for the treatment period was 29.7 (13.8) ng/mL in those who received burosumab and 32.0 (10.4) ng/mL in those who received Pi/D. No children had serum 25(OH)D in the range associated with rickets and osteomalacia (ie, < 10 ng/mL) at any time point. Among younger children, serum 25(OH)D between 10 and less than 20 ng/mL occurred in 2 children at baseline (burosumab, n = 1; Pi/D, n = 1), in 3 children at week 2 (burosumab, n = 3), in 2 children at week 16 (burosumab, n = 2), and in 2 children at week 40 (burosumab, n = 2). Among older children, serum 25(OH)D between 10 and less than 20 ng/mL occurred in 3 at baseline (burosumab, n = 1; Pi/D, n = 2), in 3 at week 2 (burosumab, n = 3), in 7 at week 16 (burosumab, n = 6; Pi/D, n = 1), in 4 at week 40 (burosumab, n = 1; Pi/D, n = 3), and in 4 at week 64 (burosumab, n = 2; Pi/D, n = 2). In both age groups, burosumab resulted in greater decreases in serum ALP than Pi/D (Fig. 1E).
Figure 1.
Mean (SD) A, serum phosphorus; B, TmP/GFR; C, 1,25(OH)2D; D, 25(OH)D; E, alkaline phosphatase; and F, plasma iPTH from study weeks 0 to 64 in children younger than 5 and aged 5 years and older who received Pi/D or burosumab every 2 weeks. Dashed lines indicate the LLN for serum phosphorus (3.2-6.1 mg/dL), TmP/GFR (3.4-5.8 mg/dL), the LLN and ULN for 1,25(OH)2D (25.8-101.5 pg/mL), the 25(OH)D status benchmarked to 20 ng/mL, the ULN for alkaline phosphatase, and the ULN for plasma iPTH (14-72 pg/mL). Alkaline phosphatase is shown as the percentage of the ULN for age and sex, and the ULN is labeled as 100%, calculated from the following normal ranges: girls aged 1 to 4 years, 317 U/L; girls aged 4 to 7 years, 297 U/L; girls aged 7 to 10 years, 325 U/L; girls aged 10 to 15 years, 300 U/L; boys aged 1 to 4 years, 383 U/L; boys aged 4 to 7 years, 345 U/L; boys aged 7 to 10 years, 309 U/L; and boys aged 10 to 15 years, 385 U/L. For PTH, the dashed line indicates the upper limit of the normal for range (14-72 pg/mL). 1,25(OH)2D, 1,25-dihydroxyvitamin D; 25(OH)D, 25-hydroxyvitamin D; iPTH, intact parathyroid hormone; LLN, lower limit of normal; Pi/D, phosphate salts and active vitamin D; TmP/GFR, tubular maximum for phosphate reabsorption per glomerular filtration rate; ULN, upper limit of normal.
Rickets
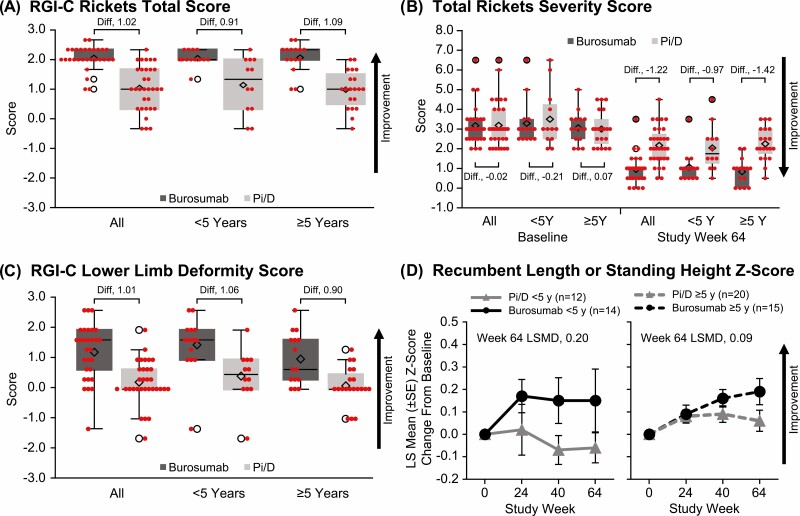
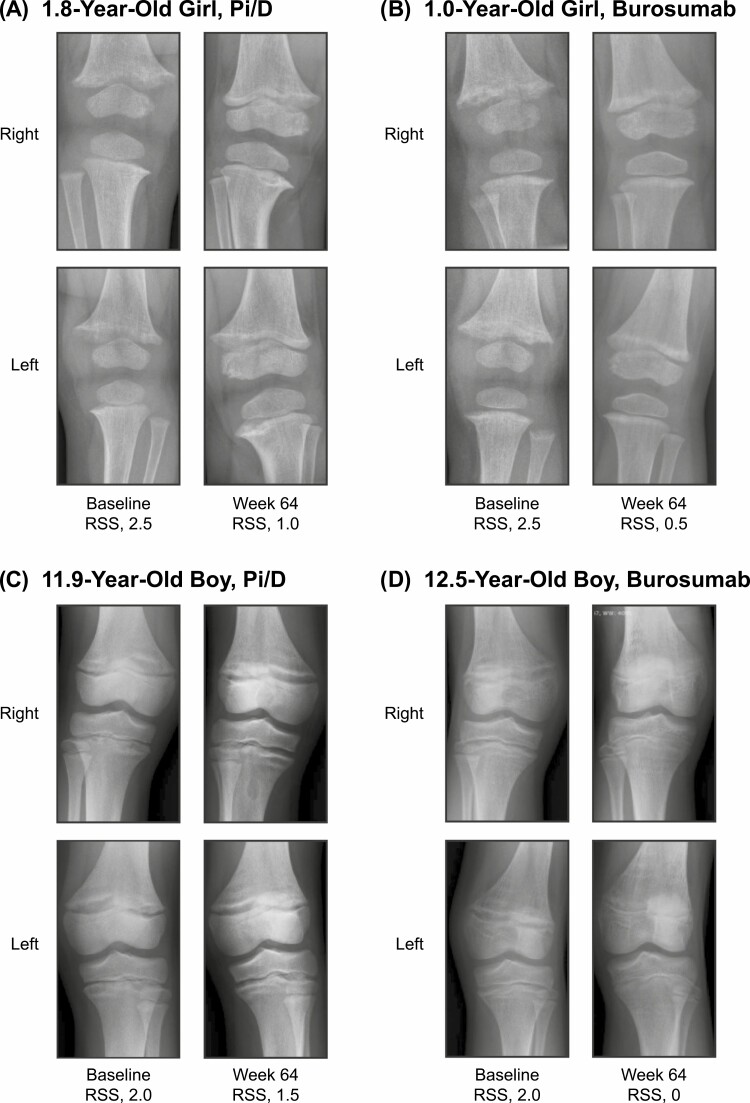
Treatment with burosumab, compared with Pi/D, significantly improved LS mean (SE) RGI-C rickets total score (indicated by positive changes) at week 64 both in younger children (2.04 [0.06] vs 1.14 [0.27]; LSMD, +0.90 [95% CI, 0.34-1.46]; P = .002) and in older children (2.06 [0.12] vs 0.99 [0.14]; LSMD, +1.07 [95% CI, 0.71-1.43; P < .001]; Fig. 2A). LSMD (95% CI) RGI-C rickets total score was not significantly different between younger and older children (−0.17 [−0.82 to 0.49]; P = .62) or between younger and older children who received burosumab (−0.03 [−030 to 0.25]; P = .85). Treatment with burosumab compared with Pi/D resulted in significantly improved LS mean (SE) total RSS at week 64, both in younger children (−2.26 [0.12] vs − 1.40 [0.28]; LSMD, −0.86 [95% CI, −1.45 to −0.27]; P = .005) and in older children (−2.20 [0.184] vs − 0.76 [0.158]; LSMD, −1.44 [95% CI, −1.91 to −0.96]; P < .001; Fig. 2B). LSMD (95% CI) total RSS was not significantly different between younger and older children (+0.57 [−0.19 to 1.34]; P = .14) or between younger and older children who received burosumab (+0.14 [−0.31 to 0.58]; P = .54). Examples of imaging showing rickets healing during treatment with Pi/D and burosumab are provided in Fig. 3.
Figure 2.
Rickets, lower limb deformity, and growth evaluations in children younger than 5 and 5 years and older who received conventional therapy or burosumab. A, RGI-C rickets total score at study week 64. RGI-C scale: +3.0, complete healing; +2.0, substantial healing; +1.0, minimal healing; 0, unchanged. B, Total RSS at baseline and study week 64. C, RGI-C lower limb deformity score at study week 64. D, Least squares mean (SE) change from baseline to study weeks 24, 40, and 64 in standing height Z-score. LS, least squares; RGI-C, Radiographic Global Impression of Change; RSS, Rickets Severity Score. In panels A to C, diamonds indicate means, horizontal lines indicate medians with ranges, and empty circles indicate outliers.
Figure 3.
Knee radiographs showing rickets improvement in children younger than 5 and 5 years and older on conventional therapy or burosumab. Baseline and week 64 knee radiographs and total RSS scores in A, a 1.8-year-old girl who received Pi/D; B, a 1.0-year-old girl who received burosumab; C, an 11.9-year-old boy who received Pi/D;, and D, in a 12.5-year-old boy who received burosumab. Pi/D, phosphate salts and active vitamin D; RSS, Rickets Severity Score.
Lower limb deformity
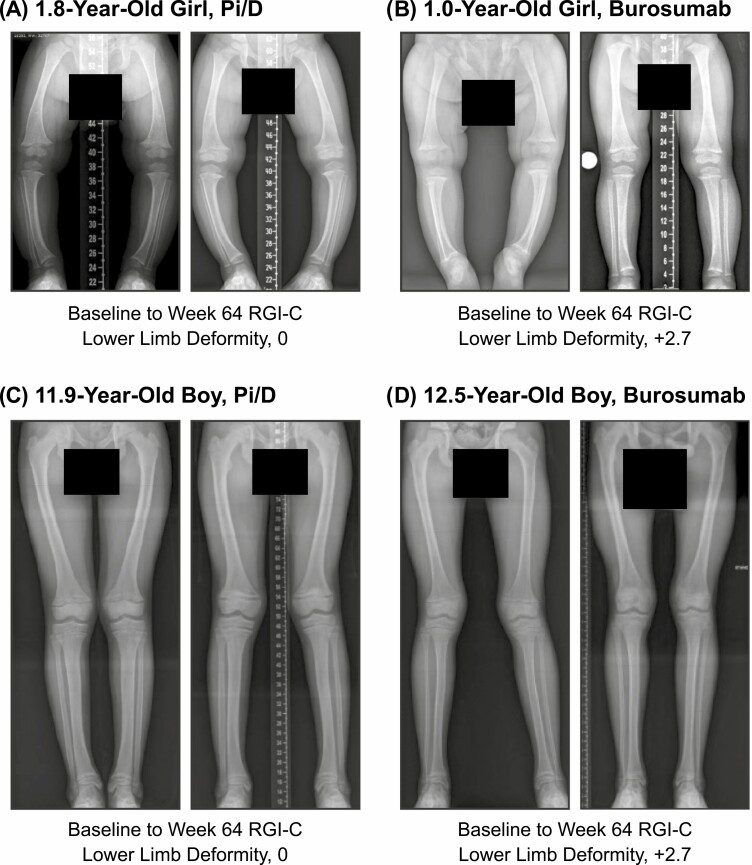
LS mean (SE) RGI-C lower limb deformity score at week 64 was significantly improved by treatment with burosumab, compared with Pi/D, both in younger children (1.48 [0.26] vs 0.47 [0.19]; LSMD, +1.02 [95% CI, 0.39-1.64]; P = .002) and in older children (1.02 [0.22] vs 0.12 [0.14]; LSMD, +0.91 [95% CI, 0.40-1.41]; P = .001; Fig. 2C). LSMD (95% CI) RGI-C lower limb deformity score was not significantly different between younger and older children (+0.12 [−0.69 to 0.94]; P = .77) or between younger and older children who received burosumab (+0.46 [−0.21 to 1.12]; P = .18). Examples of imaging showing improvement of lower limb deformities during treatment with Pi/D and burosumab are provided in Fig. 4.
Figure 4.
Radiographs showing improvement in lower limb deformity in children younger than 5 and 5 years and older on conventional therapy or burosumab. Baseline and week 64 knee radiographs and baseline to week 64 RGI-C lower limb deformity scores of A, a 1.8-year-old girl who received Pai/D; B, a 1.0-year-old girl who received burosumab; C, a 11.9-year-old boy who received Pi/D; and D, a 12.5-year-old boy who received burosumab. Pi/D, phosphate salts and active vitamin D; RGI-C, Radiographic Global Impression of Change.
Growth
In younger children, the LS mean (SE) recumbent length or standing height Z-score increased from baseline to week 64 with burosumab (0.15 [0.12]) and decreased from baseline to week 64 with Pi/D (−0.05 [0.07]; LSMD, +0.20 [95% CI, −0.07 to 0.47]; P = .14; Fig. 2D). In older children, the LS mean (SE) recumbent or standing height length Z-score increased from baseline to week 64 with burosumab (0.17 [0.05]) and with Pi/D (0.08 [0.04]; LSMD, +0.09 [95% CI, −0.03 to 0.22]; P = .15). In younger children who received burosumab, the change from baseline in recumbent length or standing height Z-score was greatest during the first 24 weeks of treatment and then remained stable (ie, appropriate for age and sex compared with the healthy reference population) (3) for the remainder of the treatment period, indicating sustained, improved growth. LSMD (95% CI) recumbent length or standing height Z-score was not significantly different between younger and older children (+0.10 [−0.21 to 0.42]; P = .52) or between younger and older children who received burosumab (−0.03 [−0.31 to 0.24]; P = .80).
Most children were prepubertal at baseline (younger, n = 26 [100%]; older, n = 32 [91%]). In total, 11 of 61 (18%) children, all of whom were in the older subgroup, had postbaseline Tanner stage greater than 1, of whom 8 were Tanner stage 1 at baseline. There was no apparent relationship between Tanner stage and growth during this 64-week treatment period.
Safety and Tolerability
Safety for the full study population was reported previously (9). On-study AEs are summarized in Table 3. The incidence of treatment-emergent AEs (TEAEs) was similar between younger and older children who received Pi/D (10/12 [83%] vs 17/20 [85%]) and who received burosumab (14/14 [100%] vs 15/15 [100%]; see Table 3). Treatment-related AEs occurred less frequently in younger than in older children, both with Pi/D (0/12 [0%] vs 7/20 [35%]) and burosumab (6/14 [43%] vs 11/15 [73%]).
Table 3.
Summary of adverse events
| Age < 5 y | Age ≥ 5 y | |||
|---|---|---|---|---|
| Conventional therapy (n = 12) | Burosumab(n = 14) | Conventional therapy (n = 20) | Burosumab(n = 15) | |
| Patients with any treatment-emergent AE, n (%) | 10 (83) | 14 (100) | 17 (85) | 15 (100) |
| Patients with any treatment-related AE, n (%) | 0 | 6 (43) | 7 (35) | 11 (73) |
| Patients with any serious treatment-emergent AE, n (%) | 0 | 2 (14) | 3 (15) | 1 (7) |
| Patients with any serious treatment-related AE, n (%) | 0 | 0 | 0 | 0 |
| Patients with grade 3 or 4 treatment-emergent AEs, n (%) | 0 | 1 (7) | 3 (15) | 3 (20) |
| Predefined treatment-emergent AEs of interest, n (%) | ||||
| Injection site reactions | 0 | 7 (50) | 0 | 8 (53) |
| Hypersensitivity | 3 (25) | 8 (57) | 3 (15) | 3 (20) |
| Treatment-emergent AEs occurring in ≥ 20% of patients in any group, n (%) | ||||
| Pyrexia | 3 (25) | 10 (71) | 3 (15) | 6 (40) |
| Headache | 2 (17) | 1 (7) | 4 (20) | 9 (60) |
| Cough | 2 (17) | 8 (57) | 4 (20) | 7 (47) |
| Arthralgia | 2 (17) | 5 (36) | 8 (40) | 8 (53) |
| Pain in extremity | 3 (25) | 3 (21) | 7 (35) | 8 (53) |
| Tooth abscess | 3 (25) | 0 | 0 | 8 (53) |
| Nasopharyngitis | 4 (33) | 6 (43) | 10 (50) | 5 (33) |
| Vomiting | 4 (33) | 6 (43) | 4 (20) | 6 (40) |
| Dental caries | 0 | 5 (36) | 2 (10) | 4 (27) |
| Injection site erythema | 0 | 4 (29) | 0 | 5 (33) |
| Vitamin D decreaseda | 0 | 1 (7) | 1 (5) | 5 (33) |
| Constipation | 0 | 4 (29) | 0 | 1 (7) |
| Diarrhea | 1 (8) | 4 (29) | 1 (5) | 3 (20) |
| Injection site reaction | 0 | 4 (29) | 0 | 3 (20) |
| Nasal congestion | 1 (8) | 1 (7) | 0 | 4 (27) |
| Rhinorrhea | 1 (8) | 3 (21) | 1 (5) | 4 (27) |
| Gastroenteritis | 1 (8) | 1 (7) | 2 (10) | 1 (7) |
| Influenza | 3 (25) | 2 (14) | 3 (15) | 2 (13) |
| Viral gastroenteritis | 3 (25) | 2 (14) | 0 | 0 |
| Oropharyngeal pain | 0 | 3 (21) | 1 (5) | 2 (13) |
| Rash | 2 (17) | 3 (21) | 0 | 0 |
| Injection site pruritus | 0 | 0 | 0 | 3 (20) |
| Injection site swelling | 0 | 0 | 0 | 3 (20) |
| Upper abdominal pain | 2 (17) | 0 | 1 (5) | 3 (20) |
Abbreviation: AE, adverse event.
a Includes the preferred terms, vitamin D decreased and vitamin D deficiency, per Common Terminology Criteria for Adverse Events, volume 4.0.
As reported previously (9), no children had serious treatment-related AEs, whereas serious TEAEs (all grade 1 or 2) were reported for 3 children who received Pi/D (craniosynostosis, bilateral genu varum, and hematuria) and 3 children who received burosumab (craniosynostosis, viral infection, and migraine; see Table 3). Serious TEAEs were less frequent in younger than older children who received Pi/D (0/12 [0%] vs 3/20 [15%]) but more frequent in younger than older children who received burosumab (2/14 [14%] vs 1/15 [7%]).
As reported previously (9), grade 3 TEAEs were reported for 3 children who received Pi/D (food allergy anaphylaxis, arthralgia, and craniosynostosis) and 4 children who received burosumab (dysuria, high urine ketone, viral gastroenteritis, and arthralgia); all resolved. Only the arthralgia in the burosumab arm was treatment related. No grade 4 or 5 AEs were reported, and no AEs resulted in study or treatment discontinuation (9). In this analysis, grade 3 or 4 TEAEs occurred less often in younger than older children, both with Pi/D (0/12 [0%] vs 3/20 [15%]) and burosumab (1/14 [7%] vs 3/15 [20%]).
Injection site reaction, a predefined AE of interest, occurred only among children who received burosumab, and with similar frequency between younger and older children (7/14 [50%] vs 8/15 [53%]; see Table 3). Hypersensitivity, reported more often with burosumab than Pi/D, occurred more frequently in younger than older children who received Pi/D (3/12 [25%] vs 3/20 [15%]) and who received burosumab (8/14 [57%] versus 3/15 [20%]). Notably, no child discontinued burosumab for hypersensitivity (or for any other reason) during the 64-week study.
The most commonly reported TEAEs were pyrexia, headache, and cough, which are common in the pediatric setting. Pyrexia, which occurred more frequently with burosumab than Pi/D (16/29 [55%] vs 6/32 [19%]), was more frequent in younger than older children, both with Pi/D (3/12 [25%] vs 3/20 [15%]) and burosumab (10/14 [71%] vs 6/15 [40%]). Headache occurred in similar proportions of younger and older children who received Pi/D (2/12 [17%] vs 4/20 [20%]) but was less frequent in younger than older children who received burosumab (1/14 [7%] vs 9/15 [60%]). Cough, which was reported more frequently with burosumab than Pi/D (15/29 [52%] vs 6/32 [19%]), occurred in similar proportions of younger and older children who received Pi/D (2/12 [17%] vs 4/20 [20%]) and burosumab (8/14 [57%] vs 7/15 [47%]). Arthralgia, which was reported more frequently with burosumab than Pi/D (13/29 [45%] vs 10/32 [31%]), occurred less often in younger than older children, both with Pi/D (2/12 [17%] vs 8/20 [40%]) and burosumab (5/14 [36%] vs 8/15 [53%]). Extremity pain occurred less often in younger than older children, both with Pi/D (3/12 [25%] vs 7/20 [35%]) and burosumab (3/14 [21%] vs 8/15 [53%]). Timing of reported extremity pain varied, being distributed throughout the study period.
Dental abscesses occurred in 3 of 12 (25%) younger children who received Pi/D compared with 0 of 20 (0%) of the younger children who received burosumab, whereas 8 of 15 (53%) older children who received burosumab had dental abscesses compared with 0 of 20 (0%) who received Pi/D. Dental caries, which were reported more frequently with burosumab than Pi/D (9/29 [31%] vs 2/32 [6%]), occurred slightly more often in older than younger children who received Pi/D (2/20 [10%] vs 0/12 [0%]) and slightly more often in younger than older children who received burosumab (5/14 [36%] vs 4/15 [27%]). Other reported AEs, such as constipation, diarrhea, and nasal congestion, were infrequent.
At baseline, nephrocalcinosis was less frequent in younger than older children in both treatment groups (Pi/D, 2/12 [17%] vs 7/20 [35%]; burosumab, 1/14 [7%] vs 4/15 [27%]; Table 4). Most children had stable (unchanged) nephrocalcinosis scores, regardless of age or treatment. Among older children, an increase in nephrocalcinosis score from 0 to 1 occurred in 3 of 20 (15%) who received Pi/D and 1 of 15 (7%) who received burosumab; no increases occurred in younger children. Decreases in nephrocalcinosis score were uncommon, occurring in 1 older child out of 20 (5%) who received Pi/D, 1 older child out of 15 (7%) who received burosumab, and 1 younger child out of 12 (8%) who received Pi/D. In both age groups and treatment arms, changes in plasma iPTH concentrations during the study period were minimal, and mean concentrations remained within the normal range (see Fig. 1E).
Table 4.
Summary of renal ultrasound assessments of nephrocalcinosis through study week 64
| Age < 5 y | Age ≥ 5 y | |||
|---|---|---|---|---|
| Conventional therapy (n = 12) | Burosumab(n = 14) | Conventional therapy (n = 20) | Burosumab(n = 15) | |
| Baseline score, n (%) | ||||
| 0 | 10 (83) | 13 (93) | 13 (65) | 11 (73) |
| 1 | 0 | 1 (7) | 3 (15) | 1 (7) |
| 2 | 1 (8) | 0 | 2 (10) | 2 (13) |
| 3 | 1 (8) | 0 | 2 (10) | 1 (7) |
| Maximum postbaseline score increase, n (%) | ||||
| 0-1 | 0 | 0 | 3 (15) | 1 (7) |
| Maximum postbaseline score decline, n (%) | ||||
| 2-1 | 0 | 0 | 0 | 1 (7) |
| 3-2 | 1 (8) | 0 | 1 (5) | 0 |
| Stable nephrocalcinosis score, n (%) | ||||
| 0-0 | 10 (83) | 13 (93) | 10 (50) | 10 (67) |
| 1-1 | 0 | 1 (7) | 3 (15) | 1 (7) |
| 2-2 | 1 (8) | 0 | 2 (10) | 1 (7) |
| 3-3 | 0 | 0 | 1 (5) | 1 (7) |
Discussion
In this post hoc subgroup analysis using data from the phase 3 study of burosumab vs Pi/D in children with XLH aged 1 to 12 years (9), we explored the effect of burosumab vs Pi/D on phosphate homeostasis, growth, lower limb deformity, and safety outcomes for those younger (aged 1-< 5 years) vs older (aged 5-12 years) at study onset. In both age categories, we found that improvements in phosphate metabolism (ie, normalization of serum phosphorous and TmP/GFR) and in rickets/osteomalacia healing (ie, declines in ALP) were consistently greater with burosumab than Pi/D. Normalization of phosphate homeostasis with burosumab treatment also was reflected in clinical and radiographic characteristics including improved growth, rickets healing, and lower limb deformity.
Regarding the response to burosumab and conventional therapy in the 2 age categories, at the biochemical level, improvements with burosumab were similar, irrespective of age group (and greater for all measured parameters compared with the corresponding age groups who received Pi/D). Serum ALP was lower at week 64 both in older and younger children who received burosumab compared with those who received conventional therapy, reflecting a decrease in bone turnover. Serum ALP declined to a greater extent in older compared with younger children who received burosumab. This observation may partly reflect the normal decrease in bone turnover that occurs in older children as they approach skeletal maturity.
Interestingly, serum 1,25(OH)2D levels were similar at 64 weeks in both treatment arms, which we hypothesize is likely due to 2 entirely different mechanisms. In both age groups on burosumab, 1,25(OH)2D rose quickly (within 1-2 weeks), which we postulate is due, at least in part, to blockade of excess FGF23 activity and consequently, release of inhibition on renal 1-alpha-hydroxylase. Thereafter, 1,25(OH)2D declined progressively to week 64 on burosumab, which we suggest is the appropriate physiologic response to restoration of serum phosphate levels when FGF23 overproduction is effectively treated. In both age groups who received Pi/D, serum 1,25(OH)2D levels remained within the normal range but lower than those of children who received burosumab, reflective of excess FGF23 activity causing a reduction in renal 1-alpha-hydroxylase activity, and thereby, 1,25(OH)2D synthesis (15, 16). In addition, FGF23 also increases expression of 24-hydroxylase, which catabolizes 25(OH)D and 1,25(OH)2D (15, 16). Together, these observations affirm that neutralization of excess FGF23 with burosumab restores not only a normal FGF23-phosphate axis, but also calcitriol regulation.
Greater improvements in rickets healing and lower limb deformity—2 key complications of XLH—occurred in children who received burosumab vs those who received Pi/D, irrespective of age. In addition, there were similar magnitudes of improvement in rickets healing and lower limb deformity on burosumab specifically, irrespective of age. In the phase 3 study of burosumab vs Pi/D, linear growth was modestly improved after 64 weeks with burosumab compared with Pi/D (mean recumbent length or standing height Z-score difference, 0.14; 95% CI, 0-0.29; P = .05) (9). Given the greater improvement with burosumab vs Pi/D in not only radiographic rickets healing but also lower limb deformity, both of these abnormalities may have benefited from the improved growth with burosumab. In a prior study, children with XLH aged 2 to 12 years who received conventional therapy had declining growth velocity compared with healthy children of the same age, resulting in declining height Z-scores in the XLH cohort (3). Consistent with this observation, in the Pi/D arm of the present analysis, the linear growth rate declined in younger children, whereas in older children, the growth rate initially improved in the first 24 weeks, followed by progressive decline. Among younger children who received burosumab, linear growth was greatest during the first 24 weeks of treatment and thereafter remained stable (ie, tracked appropriately for age and sex); this means that, during the remainder of the treatment period, children who received burosumab had sustained, improved growth. Among older children who received burosumab, the change from baseline in height Z-scores continued to increase throughout the 64-week treatment period. Overall, our subanalysis did not show that starting burosumab between age 1 and younger than 5 years was associated with improved growth relative to initiation at an older age; however, a study with a larger sample size that is specifically powered to evaluate the effect of age at burosumab initiation would be required to address this issue more definitively. Ideally, such a study would also include infants who manifested renal Pi wasting and signs of rickets, since linear growth is rapid during the first year of life, and therefore, the first year may be a particularly sensitive time for optimization of growth plate mineralization.
In a small retrospective study, Mäkitie et al (10) showed that treatment with conventional therapy initiated before age 1 year modestly improved final or adult height Z score; however, it did not prevent the need for surgical intervention in some patients. Quinlan et al (11) similarly demonstrated some improvements in growth among children with XLH who started treatment mostly before vs after age 1 year. Importantly, we note that those 2 studies differ from ours in 2 ways. First, the studies by Mäkitie et al and Quinlan et al evaluated the effect of age on clinical outcomes from the initiation of conventional therapy (10, 11). Therefore, the children were treatment naive before initiating conventional therapy, which means that older children had accrued a greater height deficit before treatment. In contrast, all of the children in our study had received conventional therapy before enrollment, which presumably resulted in prestudy benefit to growth. Second, the children in our study would have been categorized as “older” in the previous studies because they were older than 1 year at enrollment—the average age of our younger group was 3.1 years. Although the results of our study are encouraging in that burosumab demonstrated benefit to growth, lower limb deformity, and rickets healing in both age groups, it remains unknown whether treatment with burosumab before the first birthday can reduce, or obviate completely, the need for surgical correction of leg deformity. Further studies are therefore also warranted to examine the effect of burosumab on lower limb deformity when initiated before age 1 year and continued throughout the growth period.
Overall, the safety profile of burosumab in children with XLH was consistent with prior studies that have shown burosumab was overall well tolerated (9, 17, 18). We observed that extremity pain occurred more frequently with burosumab than Pi/D in older children but not in younger children. Although leg pain is a frequent complaint in children with XLH, it is unclear from these results whether extremity pain was synonymous with leg pain and, if so, why extremity pain would be more frequent in older children receiving burosumab compared with Pi/D. We note that children who received burosumab exhibited greater healing of rickets and osteomalacia, as well as greater improvement in growth and lower limb deformity, compared with Pi/D. Interestingly, adults also have experienced leg pain following burosumab initiation (9, 17, 19, 20), which we hypothesize may have been due to increased physical activity and/or the healing of osteomalacia following restoration of euphosphatemia. In the present study, we postulate that the extremity pain that occurred just after the prerandomization washout period might have been due to transient exacerbation of the osteomalacia, whereas extremity pain that occurred later in the study might have resulted from osteomalacia healing or increased physical activity. Overall, the etiology, duration, and intensity of leg pain during treatment with burosumab require further study.
Dental abscesses are an important manifestation of XLH. In this study, none of the younger children who received burosumab experienced dental abscesses, whereas dental abscesses occurred in 53% of the older children who received burosumab. Based on current understanding, inadequate mineralization of the dentin with subsequent enlargement of the pulp chamber (a portal of entry for microbes) is the main mechanism by which XLH predisposes to dental abscess formation (21-23). It remains unknown if, and to what extent, mineralization of the dentin might improve with restoration of normal phosphate homeostasis on burosumab, and whether such restoration at a younger age will reduce the predisposition to dental abscesses. Understanding whether there is a window of opportunity in children to optimally mineralize the dentin and positively affect the risk of abscess formation merits further exploration.
Cough and headache were frequently reported among children who received burosumab in the total population in this trial (52% and 34%, respectively) and in the phase 2 trial (44% and 50%, respectively) (9, 17). In this analysis, although the incidence of cough was similar (57% vs 47%) among younger and older children who received burosumab, the incidence of headache was less (7% vs 60%) among younger children than older children who received burosumab. Further study is needed to determine whether there is any association between age and the occurrence of cough and headache with burosumab treatment.
Nephrocalcinosis, a complication of conventional Pi/D therapy for XLH (5, 24), was more frequent at baseline in older than in younger children. This could reflect the longer durations of conventional therapy in the older children. In this study nephrocalcinosis scores did not increase over 64 weeks in any of the younger children in either treatment group, whereas nephrocalcinosis scores did increase in a small number of the older children. Preventing or mitigating nephrocalcinosis in children with XLH remains an important area of investigation. Consistent with prior observations (9), plasma iPTH concentrations changed little during the study period, and concentrations remained within the normal range, regardless of age group or treatment arm.
A strength of the present study was the participation of international sites, which generalized the results to a broad population of children, and the randomized, controlled study design. On the other hand, our post hoc subgroup analysis was limited by the small sample sizes among age subgroups, the open-label design of the trial, the variation in Pi/D dosing, and that adherence to Pi/D was not measured by drug accountability; however, the number of missed dosing days were captured (9). Six-minute walk time could not be assessed in this analysis, as the study was designed to assess it only in children who were at least aged 5 years and able to complete the test (9). Nevertheless, clinically meaningful differences with burosumab vs Pi/D were observed in younger and older children for key clinical outcomes, including growth and lower limb deformity. A further limitation was our inability to evaluate the effect of pubertal growth among the older children, as pubertal stage increased from Tanner stage 1 at baseline to Tanner stage 2 or higher at week 64 in only 8 children. Thus, further studies are needed to evaluate the influence of burosumab on pubertal growth among children.
In conclusion, improvements in phosphate homeostasis, rickets, and lower limb deformities were greater switching to burosumab than continuing Pi/D both in younger and older children with XLH. Further clinical studies that are sufficiently powered are needed to specifically address whether initiating burosumab at an earlier (rather than later) age ameliorates growth, lower limb deformity, and the risk of dental abscesses. The relationship between the PHEX genotype and various XLH outcomes on different treatment regimens also merits further study. The XLH Disease Monitoring Program (ClinicalTrials.gov No. NCT03651505) will provide long-term (10 years) longitudinal evaluation of patients with XLH with or without treatment with burosumab.
Acknowledgments
Valerie Wollberg, RN, coordinated participant care and clinical evaluations, and Vinieth Bijanki, MS, CCRP, performed local data entry and monitoring and processed and laboratory samples for the CL301 study at the Shriners Hospitals for Children–St. Louis.
Glossary
Abbreviations
- 1,25(OH)2D
1,25-dihydroxyvitamin D
- 25(OH)D
25-hydroxyvitamin D
- AE
adverse event
- ALP
alkaline phosphatase
- FGF23
fibroblast growth factor 23
- GEE
generalized estimating equation
- iPTH
intact parathyroid hormone
- LSMD
least squares means difference
- Pi/D
phosphate salts and active vitamin D
- RGI-C
Radiographic Global Impression of Change
- RSS
Rickets Severity Score
- TEAE
treatment-emergent adverse event
- TmP/GFR
tubular maximum for phosphate reabsorption per glomerular filtration rate
- ULN
upper limit of normal
- XLH
X-linked hypophosphatemia
Contributor Information
Leanne M Ward, Department of Pediatrics, Faculty of Medicine, University of Ottawa, Ottawa, Ontario K1H 8L1, Canada.
Francis H Glorieux, Shriners Hospitals for Children, Canada, McGill University, Montreal, Quebec H4A OA9, Canada.
Michael P Whyte, Shriners Hospitals for Children St Louis, St Louis, Missouri 63110, USA.
Craig F Munns, Child Health Research Centre, The University of Queensland, Brisbane, Queensland 4072, Australia; Department of Endocrinology and Diabetes, Queensland Children’s Hospital, Brisbane, Queensland 4101, Australia.
Anthony A Portale, Department of Pediatrics, University of California, San Francisco, San Francisco, California 94143, USA.
Wolfgang Högler, Department of Pediatrics and Adolescent Medicine, Johannes Kepler University Linz, Linz 4040, Austria; Institute of Metabolism and Systems Research, University of Birmingham, Birmingham, B15 2TT, UK.
Jill H Simmons, Department of Pediatrics, Division of Endocrinology and Diabetes, Vanderbilt University School of Medicine, Vanderbilt University, 680-8570 Nashville, Tennessee, 63110, USA.
Gary S Gottesman, Shriners Hospitals for Children St Louis, St Louis, Missouri 63110, USA.
Raja Padidela, Department of Paediatric Endocrinology, Royal Manchester Children’s Hospital, Manchester M13 9WL, UK.
Noriyuki Namba, Department of Pediatrics, Osaka Hospital, Japan; Community Healthcare Organization; Osaka University Graduate School of Medicine, Osaka 553-0003, Japan; Division of Pediatrics and Perinatology, Tottori University Faculty of Medicine, Yonago, Japan.
Hae Il Cheong, Department of Pediatrics, Seoul National University Children’s Hospital, Seoul 14068, South Korea.
Ola Nilsson, Division of Pediatric Endocrinology and Center for Molecular Medicine, Karolinska Institutet, Stockholm 17177, Sweden; School of Medical Sciences, Department of Pediatrics, Örebro University and University Hospital, Örebro S-703 62, Sweden.
Meng Mao, Ultragenyx Pharmaceutical Inc, Novato, California 94949, USA.
Angel Chen, Ultragenyx Pharmaceutical Inc, Novato, California 94949, USA.
Alison Skrinar, Ultragenyx Pharmaceutical Inc, Novato, California 94949, USA.
Mary Scott Roberts, Ultragenyx Pharmaceutical Inc, Novato, California 94949, USA.
Erik A Imel, Department of Medicine and Department of Pediatrics, Indiana University School of Medicine, Indianapolis, Indianapolis 46202, USA.
Financial Support
This work was sponsored and funded by Ultragenyx Pharmaceutical Inc. in partnership with Kyowa Kirin International plc. Medical writing support was provided by Ben Scott, PhD (Scott Medical Communications, LLC), and was funded by Ultragenyx Pharmaceutical Inc. Chao-Yin Chen, PhD. performed biostatistical analyses for the clinical study as an employee of Ultragenyx Pharmaceutical Inc. Dr Ward is supported by the University of Ottawa Clinical Research Chair Program.
Author Contributions
The following individuals were clinical trial site investigators and therefore contributed to data collection; these individuals also critically reviewed and edited the manuscript (L.M.W., F.H.G., M.P.W., C.F.M., A.A.P., W.H., J.H.S., G.S.G., R.P., N.N., H.I.C., O.N., and E.A.I.). M.M. collected the study data, A.C. contributed to statistical analyses and data interpretation, A.S. collected and interpreted the study data, and M.S.R. helped design the supervise the study, collect the data, and interpret the findings. L.M.W. and E.A.I. critically edited the first draft of the methods, results, tables, and figures, cowrote the first draft of the introduction and discussion, and finalized revisions from all of the coauthors.
Clinical Trial Information
ClinicalTrials.gov registration No. NCT02915705 (registered September 27, 2016.
Disclosures
L.M.W. has been a consultant to, and participated in clinical trials with, Ultragenyx Pharmaceutical Inc. (with funds to Dr Ward’s institution). F.H.G. has been a consultant to, and participated in clinical trials with, Ultragenyx Pharmaceutical Inc. M.P.W. has no conflicts to disclose within the last 2 years. C.F.M. is a consultant for Kyowa Kirin and has received research funding from Kyowa Kirin. A.A.P. has been a consultant to, and served as an investigator in clinical trials with, Ultragenyx Pharmaceutical Inc. W.H. served as an investigator in clinical trials with, and as a consultant for, Ultragenyx Pharmaceutical Inc. and serves as a clinical investigator in clinical trials with, and has received research funding from, Kyowa Kirin. J.H.S. has received institutional research funding from and personal honoraria for participation in an advisory board from Ultragenyx Pharmaceutical Inc. G.S.G. has been a consultant for Ultragenyx Pharmaceutical Inc. R.P. has no conflicts to disclose within the last 2 years. N.N. has been a consultant to, and participated in clinical trials with, Kyowa Kirin. H.I.C. has been a consultant to, and participated in clinical trials with, Ultragenyx Pharmaceutical Inc. O.N. has received speakers’ honoraria from Kyowa Kirin, Abbott, and Biomarin, consulting fees from Kyowa Kirin and Biomarin, and research support from Kyowa Kirin. M.M., A.C., A.S., and M.S.R. report employment by, and stock ownership in, Ultragenyx Pharmaceutical Inc. E.A.I. has been a consultant to, and participated in clinical trials with, Ultragenyx Pharmaceutical Inc.
Data Availability
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Carpenter TO, Shaw NJ, Portale AA, Ward LM, Abrams SA, Pettifor JM. Rickets. Nat Rev Dis Primers. 2017;3:17101. [DOI] [PubMed] [Google Scholar]
- 2. Gohil A, Imel EA. FGF23 and associated disorders of phosphate wasting. Pediatr Endocrinol Rev. 2019;17(1):17-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mao M, Carpenter TO, Whyte MP, et al. Growth curves for children with X-linked hypophosphatemia. J Clin Endocrinol Metab. 2020;105(10):3243-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Carpenter TO. New perspectives on the biology and treatment of X-linked hypophosphatemic rickets. Pediatr Clin North Am. 1997;44(2):443-466. [DOI] [PubMed] [Google Scholar]
- 5. Carpenter TO, Imel EA, Holm IA, Jan de Beur SM, Insogna KL. A clinician’s guide to X-linked hypophosphatemia. J Bone Miner Res. 2011;26(7):1381-1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Linglart A, Biosse-Duplan M, Briot K, et al. Therapeutic management of hypophosphatemic rickets from infancy to adulthood. Endocr Connect. 2014;3(1):R13-R30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Connor J, Olear EA, Insogna KL, et al. Conventional therapy in adults with X-linked hypophosphatemia: effects on enthesopathy and dental disease. J Clin Endocrinol Metab. 2015;100(10): 3625-3632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Beck-Nielsen SS, Mughal Z, Haffner D, et al. FGF23 and its role in X-linked hypophosphatemia-related morbidity. Orphanet J Rare Dis. 2019;14(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Imel EA, Glorieux FH, Whyte MP, et al. Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet. 2019;393(10189):2416-2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mäkitie O, Doria A, Kooh SW, Cole WG, Daneman A, Sochett E. Early treatment improves growth and biochemical and radiographic outcome in X-linked hypophosphatemic rickets. J Clin Endocrinol Metab. 2003;88(8):3591-3597. [DOI] [PubMed] [Google Scholar]
- 11. Quinlan C, Guegan K, Offiah A, et al. Growth in PHEX-associated X-linked hypophosphatemic rickets: the importance of early treatment. Pediatr Nephrol. 2012;27(4):581-588. [DOI] [PubMed] [Google Scholar]
- 12. Verge CF, Lam A, Simpson JM, Cowell CT, Howard NJ, Silink M. Effects of therapy in X-linked hypophosphatemic rickets. N Engl J Med. 1991;325(26):1843-1848. [DOI] [PubMed] [Google Scholar]
- 13. Whyte MP, Fujita KP, Moseley S, Thompson DD, McAlister WH. Validation of a novel scoring system for changes in skeletal manifestations of hypophosphatasia in newborns, infants, and children: the radiographic global impression of change scale. J Bone Miner Res. 2018;33(5): 868-874. [DOI] [PubMed] [Google Scholar]
- 14. Thacher TD, Pettifor JM, Tebben PJ, et al. Rickets severity predicts clinical outcomes in children with X-linked hypophosphatemia: utility of the radiographic Rickets Severity Score. Bone. 2019;122:76-81. [DOI] [PubMed] [Google Scholar]
- 15. Chanakul A, Zhang MYH, Louw A, et al. FGF-23 regulates CYP27B1 transcription in the kidney and in extra-renal tissues. PLoS One. 2013;8(9):e72816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shimada T, Hasegawa H, Yamazaki Y, et al. FGF-23 is a potent regulator of vitamin D metabolism and phosphate homeostasis. J Bone Miner Res. 2004;19(3):429-435. [DOI] [PubMed] [Google Scholar]
- 17. Carpenter TO, Whyte MP, Imel EA, et al. Burosumab therapy in children with X-linked hypophosphatemia. N Engl J Med. 2018;378(21):1987-1998. [DOI] [PubMed] [Google Scholar]
- 18. Whyte MP, Carpenter TO, Gottesman GS, et al. Efficacy and safety of burosumab in children aged 1-4 years with X-linked hypophosphataemia: a multicentre, open-label, phase 2 trial. Lancet Diabetes Endocrinol. 2019;7(3):189-199. [DOI] [PubMed] [Google Scholar]
- 19. Insogna KL, Briot K, Imel EA, et al. ; AXLES 1 Investigators. A randomized, double-blind, placebo-controlled, phase 3 trial evaluating the efficacy of burosumab, an anti-FGF23 antibody, in adults with X-linked hypophosphatemia: week 24 primary analysis. J Bone Miner Res. 2018;33(8):1383-1393. [DOI] [PubMed] [Google Scholar]
- 20. Insogna KL, Rauch F, Kamenický P, et al. Burosumab improved histomorphometric measures of osteomalacia in adults with X-linked hypophosphatemia: a phase 3, single-arm, international trial. J Bone Miner Res. 2019;34(12):2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McKee MD, Hoac B, Addison WN, Barros NMT, Millán JL, Chaussain C. Extracellular matrix mineralization in periodontal tissues: noncollagenous matrix proteins, enzymes, and relationship to hypophosphatasia and X-linked hypophosphatemia. Periodontol 2000. 2013;63(1):102-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coyac BR, Falgayrac G, Penel G, et al. Impaired mineral quality in dentin in X-linked hypophosphatemia. Connect Tissue Res. 2018;59(Suppl 1):91-96. [DOI] [PubMed] [Google Scholar]
- 23. Salmon B, Bardet C, Coyac BR, et al. Abnormal osteopontin and matrix extracellular phosphoglycoprotein localization, and odontoblast differentiation, in X-linked hypophosphatemic teeth. Connect Tissue Res. 2014;55(Suppl 1):79-82. [DOI] [PubMed] [Google Scholar]
- 24. Dahir K, Roberts MS, Krolczyk S, Simmons JH. X-linked hypophosphatemia: a new era in management. J Endocr Soc. 2020;4(12):bvaa151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data sets generated during and/or analyzed during the present study are not publicly available but are available from the corresponding author on reasonable request.