Abstract
Context
The oocyte-secreted factors growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) play essential roles in follicle development and oocyte maturation, and aberrant regulation might contribute to the pathogenesis of polycystic ovary syndrome.
Objective
Are there measurable differences in concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer in small antral follicle fluids from women with and without polycystic ovaries (PCO)?
Design and Setting
Follicle fluids (n = 356) were collected from 4- to 11-mm follicles in unstimulated ovaries of 87 women undergoing ovarian tissue cryopreservation for fertility preservation.
Patients
Twenty-seven women with PCO were identified and 60 women without PCO-like characteristics (non-PCO women) were matched according to age and follicle size.
Main outcome measures
Intrafollicular concentrations of GDF9, BMP15, GDF9/BMP15 heterodimer, anti-Mullerian hormone (AMH), inhibin-A and -B, total inhibin, activin-B and -AB, and follistatin were measured using enzyme-linked immunosorbent assays.
Results
The detectability of GDF9, BMP15, and the GDF9/BMP15 heterodimer were 100%, 94.4%, and 91.5%, respectively, and concentrations were significantly negatively correlated with increasing follicle size (P < 0.0001). GDF9 was significantly higher in women with PCO (PCO: 4230 ± 189 pg/mL [mean ± SEM], n = 188; non-PCO: 3498 ± 199 pg/mL, n = 168; P < 0.03), whereas BMP15 was lower in women with PCO (PCO: 431 ± 40 pg/mL, n = 125; non-PCO: 573 ± 55 pg/mL, n = 109; P = 0.10), leading to a significantly higher GDF9:BMP15 ratio in women with PCO (P < 0.01). Significant positive associations between BMP15 and AMH, activins, and inhibins in non-PCO women switched to negative associations in women with PCO.
Conclusions
Intrafollicular concentrations of GDF9 and BMP15 varied inversely in women with PCO reflecting an aberrant endocrine environment. An increased GDF9:BMP15 ratio may be a new biomarker for PCO.
Keywords: PCOS, follicle fluid, antral follicle, BMP15, GDF9, cumulin
The oocyte plays a major role in the regulation of folliculogenesis and modifies its own follicular microenvironment by secretion of paracrine growth factors (1). Growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15) act as such paracrine factors secreted by the oocyte to regulate the function of the neighboring granulosa and cumulus cells within the follicular compartment (2). These factors play essential roles in follicle development, oocyte maturation, and ovulation (2-5), and studies have suggested that GDF9 and BMP15 contribute to the pathogenesis of polycystic ovary syndrome (PCOS) (6-10).
PCOS affects 5% to 10% of women of reproductive ages and is considered the most common endocrine disorder characterized by anovulation, ovarian cysts, hyperandrogenism, hirsutism, insulin resistance, obesity, and irregular menstrual bleeding leading to infertility (11-14). Mutations in GDF9 and BMP15 have been associated with PCOS in some studies (15-17); however, the prevalence of these nonsynonymous mutations were not significantly different from that in women without PCOS (18), and other studies have reported an absence of such associations (19, 20). Several studies have also reported dysregulated levels and aberrant expression of BMP15 and GDF9 in oocytes, cumulus cells, and granulosa cells from women with polycystic ovaries (PCO) or PCOS compared with controls (6-10, 21, 22); however, results have been contradicting and consistent conclusions are lacking.
GDF9 and BMP15 belong to the TGF-β superfamily, which is the largest family of secreted proteins in mammals, and like all other TGF-β superfamily proteins, they are produced as promature proteins that form dimers and require proteolytic cleavage by furin-like proteases to become active (23, 24). However, GDF9 and BMP15 differ structurally from other TGF-β superfamily ligands because they lack the conserved fourth cysteine residue and form noncovalent dimers to elicit their effects (25, 26). Based on their noncovalent dimer interaction, shared spatiotemporal expression pattern in the oocyte, close structural homology, and coimmunoprecipitation, it has been shown that GDF9 and BMP15 can interact physically ex vivo to form a GDF9:BMP15 heterodimer complex called cumulin (27-30). However, it is unknown exactly how GDF9 and BMP15 are processed and interact in vivo, and which primary bioactive forms are present in biological fluids in humans.
The GDF9 transcript is highly expressed in human oocytes of all follicle stages from primordial, primary, secondary, antral, to preovulatory follicles, and in mature MII oocytes, whereas BMP15 mRNA is expressed in human oocytes from the early secondary stage with increasing expression throughout follicle development to the preovulatory stage, and in mature MII oocytes (31-33). Because of assay limitations protein concentrations of GDF9 and BMP15 in oocytes, follicle fluids (FFs), and serum are less well characterized. Recent studies by Riepsamen and colleagues found low but relative uniform serum concentrations of GDF9 and BMP15 throughout the menstrual cycle in both healthy ovulatory women and infertile women undergoing in vitro fertilization, possibly reflecting the total population of oocytes within a woman’s ovaries, similar to anti-Mullerian hormone (AMH) (34, 35). However, no correlations between serum AMH and either GDF9 or BMP15 serum concentrations were found, nor with concentrations of FSH (34).
Intrafollicular concentrations of GDF9 and BMP15 are expected to be higher than in serum. The study by Riepsamen and colleagues showed higher detection rates of GDF9 and BMP15 in FF from preovulatory follicles compared with serum (34), and with a new highly sensitive commercially available ELISA assay our group recently reported high concentrations of GDF9 in human small antral follicles collected in the natural menstrual cycle (36). We also found that women with PCO had significantly reduced intrafollicular concentrations of inhibins and an aberrant regulation of the inhibin-activin-follistatin axis, as well as AMH, which may reflect underlying mechanisms characterizing aberrant follicle growth in PCOS (36).
Using newly developed specific ELISA (Ansh Labs, Webster, TX, USA) to detect native forms of human oocyte-secreted GDF9, BMP15, and the GDF9/BMP15 heterodimer cumulin, the aim of the current study was to measure concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer in fluid from 4- to 11-mm small antral follicles obtained from women with or without PCO. Furthermore, these intrafollicular concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer were related to the follicle diameter and associated to concentrations of other TGF-β members.
Materials and Methods
Study Population
The study included FF obtained from surplus ovarian tissue from unstimulated ovaries of 87 women (aged 16-38 years) undergoing ovarian tissue cryopreservation for fertility preservation at the Laboratory of Reproductive Biology, University Hospital of Copenhagen, Denmark, from 2011 to 2019. The women had 1 ovary laparoscopically removed at various times during their menstrual cycle. The ovarian cortical tissue was isolated and cryopreserved using the slow freezing technique (37). Surplus ovarian material, including antral follicles and FF, was donated for research by patients giving written consent after written and orally conveyed information (ethical approval: H-2-2011-044; Capital Region).
The diagnoses of the patients were breast cancer (n = 44), lymphoma (n = 12), sarcoma (n = 6), brain cancer (n = 10), colorectal cancer (n = 2), cervical cancer (n = 3), leukemia (n = 1), and other diseases including aplastic anemia, Blackfan diamond syndrome, and sclerosis (n = 9).
Collection of Small Antral Follicle Fluids
Follicle fluids were aspirated from individual small antral follicles visible from the outside of the ovary and located within the surplus medullary tissue. Fluids were gently aspirated with a 23G needle attached to a 1-mL syringe. Based on the aspirated FF volume, the diameter of the follicle was calculated assuming a spherical shape. Included FFs were obtained from follicles with a diameter of 4 to 11 mm. The FF and the granulosa cells were separated by centrifugation at 400g for 3 to 5 minutes, and the FF and granulosa cells were snap frozen in liquid nitrogen separately and stored at -80 °C until further analysis.
Hormone Measurements
ELISAs developed by Ansh Labs were used to measure intrafollicular concentrations of GDF9 (38), BMP15 (39), GDF9/BMP15 heterodimer (40), picoAMH (41), inhibin-A (42), inhibin-B (43), total inhibin (44), activin-B (45), activin AB (46), and follistatin (47). The assays were performed according to the manufacturer’s protocol with an appropriate dilution of the FF samples using the supplied assay buffer. Analytical characteristics of the assays for picoAMH, inhibins, activins, and follistatin were described in Kristensen et al (36). Analytical assay characteristics for GDF9, BMP15, and the GDF9/BMP15 heterodimer are described in supplemental Table 1 (48). Samples were diluted 1:5 in the GDF9 calibrator A before measuring in GDF9 ELISA. The standard for the GDF9/BMP15 heterodimer assay had not yet been calibrated into protein concentrations at the time of measurements and is therefore defined in terms of arbitrary units (AU).
Blood samples from the patients were collected before cryopreservation of ovarian tissue and serum levels of AMH, FSH, LH, estradiol, progesterone, and testosterone were measured as routine clinical samples by the clinical biochemical departments at the university hospitals of Copenhagen, Odense, and Aarhus, Denmark.
Immunofluorescence Analysis
Immunofluorescence analysis was performed to detect GDF9 and BMP15 expression in cumulus-oocyte complexes (COCs) obtained from small antral follicles in PCO. The analysis was performed as described previously (49). In brief, 5-µm tissue sections including the oocyte were deparaffinated in xylene, rehydrated in ethanol followed by antigen retrieval in Tris-egtazic acid (EGTA) buffer (10 mM Tris, 0.5 mM EGTA, pH 9) and blocking in Tris-buffered saline with 1% BSA. Sections were incubated overnight at 4 °C in mouse monoclonal antibodies against GDF9 or BMP15 (generously provided by Ansh Labs) together with rabbit monoclonal antibody against 17β-Hydroxy Steroid Dehydrogenase B1 (HSD17B1, Abcam, Cambridge, UK, catalog no. ab51045) (50) diluted 1:100 in blocking reagent. After incubation with primary antibodies sections were rinsed in Tris-buffered saline with Tween20, incubated 1 hour in appropriate secondary antibody, rinsed, and nuclear DAPI stained (Invitrogen by Thermo Fisher, Roskilde, Denmark, catalog no. D21490) and mounted in Prolong Gold (Invitrogen, catalog no. P36930). Universal negative control serum (BioCare Medical) and antibody dilution buffer was used in place of primary antibody and showed no staining, data published in supplemental Figure 1 (51) and as supplemental data in Mamsen et al (52).
Statistics
The statistical analysis was performed in R version 3.4.3 using a linear mixed-effect model with random intercept for each woman, and a linear effect of follicle diameter and case (PCO/non-PCO) as well as the interaction between follicle diameter and case as fixed effects. Tests of statistical significance were carried out using Wald tests with a significance level of 0.05. Spearman correlations between the intrafollicular levels of all measured TGF-β members in the non-PCO and PCO groups were performed as well.
Results
Patient Demographics and Definitions of Non-PCO and PCO Groups
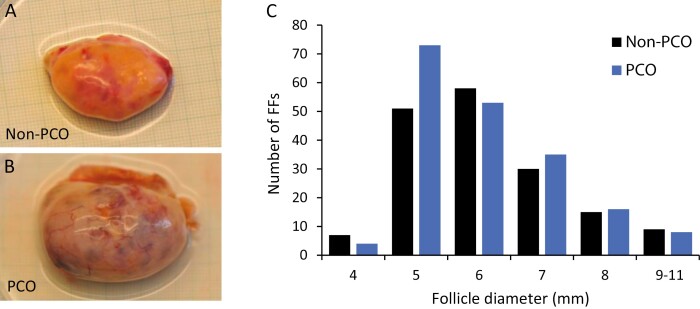
Women with and without PCO were identified based on ovarian volume, number of aspirated antral follicles, and serum hormone profiles (Table 1). The trimmed ovary was weighed upon arrival to the laboratory and the ovarian volume was determined using the conversion of 1 g of tissue corresponding to 1 mL (53). Women were characterized as having PCO when the ovarian volume exceeded 10 mL and an ovarian PCO appearance was observed with a high number of small antral follicles visible in the periphery of the ovary (Fig. 1A and 1B). Furthermore, the characterization of PCO included aspiration of 7 or more FF. The total number of aspirated FF per ovary was, however, not comparable with a clinical antral follicle count (AFC) by ultrasound. The number of aspirated FF comprised only a fraction of the actual AFC and was used as an indicative parameter of AFC. The morphological findings of PCO were supported by hormone profiles (increased AMH, LH, and LH/FSH ratio), and in total 27 women were included in the PCO group and 60 women without PCO-like characteristics were matched according to age and follicle size (Table 1). The mean age of women without PCO (28.2 ± 5.1 years [± SD]; range, 16-38 years) and with (28.4 ± 5.6 years; range, 16-37 years) was similar. The ovarian volume was significantly higher in the PCO group (13.8 ± 2.9 mL; range, 10-20.9 mL) compared with the non-PCO (6.9 ± 1.9 mL; range, 2.4-9.5 mL) (Table 1). The median number of collected FF per woman was also significantly higher in the PCO group (median of 10 FF) compared with the non-PCO group (median, 4 FF). Clinical hormone data showed that the PCO group had significantly higher serum AMH concentrations (43.2 ± 4.3 pmol/L) compared with non-PCO (14.7 ± 1.2 pmol/L) (Table 1). Serum FSH concentrations were similar in the 2 groups, whereas LH concentrations were significantly higher in the PCO group (9.6 ± 1.2 IU/L) compared with non-PCO women (6.5 ± 0.6 IU/L), and consequently the LH/FSH ratio was significantly higher in PCO group (2.1 ± 0.2) compared with the non-PCO group (1.1 ± 0.1) (Table 1). Serum concentrations of estradiol, progesterone, and testosterone were not statistically significantly different in the 2 groups (data not shown). Clinical information regarding menstrual cycle or body mass index was not available, and a clinical diagnosis of PCOS according to the Rotterdam criteria was rarely possible in the current setting as the women were referred for fertility preservation with the primary aim of preserving follicles within a very limited time frame.
Table 1.
Patient demographics and clinical information
| Non-PCO | PCO | P | |
|---|---|---|---|
| Woman, no. | 60 | 27 | |
| Age in years (mean ± SD), [range] | 28.2 ± 5.1 [16–38] | 28.4 ± 5.6 [16–37] | NS |
| Ovarian volume in mL (mean ± SD), [range] | 6.9 ± 1.9 [2.4–9.5] | 13.8 ± 2.9 [10.0–20.9] | <0.001 |
| Median number of FF collected per woman, [range] | 4 [1–7] | 10 [7–14] | <0.001 |
| Serum AMH in pmol/L (mean ± SEM) | 14.7 ± 1.2 | 43.2 ± 4.3 | <0.001 |
| Serum FSH in IU/L (mean ± SEM) | 6.0 ± 0.4 | 5.1 ± 0.4 | NS |
| Serum LH in IU/L (mean ± SEM) | 6.5 ± 0.6 | 9.6 ± 1.2 | < 0.02 |
| LH/FSH ratio (mean ± SEM) | 1.1 ± 0.1 | 2.1 ± 0.2 | P < 0.001 |
| Follicle diameter, mm | 3.9-10.7 | 4.2-10.7 | |
| Total no. of FF analyzed | 170 | 189 | |
| Median no. of FF analyzed per woman, [range] | 2 [1-7] | 8 [2-12] | |
| Diagnosis, no. | Breast cancer (32) Lymphoma (8) Sarcoma (2) Brain cancer (8) Cervical cancer (1) Colorectal cancer (1) Leukemia (1) Others (7) |
Breast cancer (12) Lymphoma (4) Sarcoma (4) Brain cancer (2) Cervical cancer (2) Colorectal cancer (1) Others (2) |
Abbreviations: AMH, anti-Mullerian hormone; FF, follicle fluid; NS, not significant; PCO, polycystic ovary.
Figure 1.
Distribution of analyzed follicle fluids from women with non-PCO and PCO in relation to follicle diameter. (A) Ovary from a woman representing the non-PCO group. (B) Ovary from a woman representing the PCO group. (C) Number of follicle fluids (FFs) analyzed according to follicle diameter (4–11 mm) in the non-PCO and PCO groups.
The 2 groups do not reflect the prevalence of PCO-like phenotypes within the entire cohort of patients undergoing ovarian tissue cryopreservation because they were explicitly selected according to specific phenotypes for this study.
Sample Details
In total, 356 FF were collected and analyzed from 87 women. Of these samples, 187 were included in our previous study (36), which did not include measurements of BMP15 and the GDF9/BMP15 heterodimer. In the current study a total of 170 and 189 FF were included in the non-PCO and PCO groups, respectively. The diameters of the follicles ranged from 3.9 to 10.7 mm and were similar in both groups (Table 1; Fig. 1C). The median number of analyzed FF per woman was higher in the PCO group (n = 8 FF/woman; range, 2-12) compared with non-PCO (n = 2 FF/woman; range, 1-7) (Table 1).
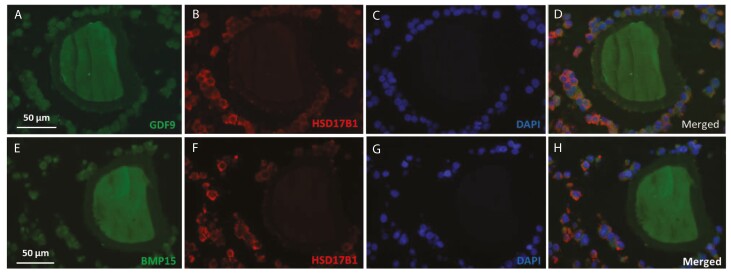
GDF9 and BMP15 Expressed in the Oocytes of COCs
Immunofluorescent analysis showed localization of GDF9 and BMP15 in both the oocyte and cumulus cells of COCs from women with PCO (Fig. 2). Staining for HSD17B1 showed cumulus-specific localization in the COCs (Fig. 2).
Figure 2.
GDF9 and BMP15 expressed in human oocytes. Immunofluorescent analysis showing localization of (A-D) GDF9 and (E-H) BMP15 in the oocyte and cumulus cells of COCs from women with PCO. (A + E) GDF9/BMP15; (B + F) HSD17B1 showing cumulus-specific localization in the COCs; (C + G) DAPI nuclear staining; (D + H): merged images.
Detectability of GDF9, BMP15, and the GDF9/BMP15 Heterodimer
A total of 356 samples were analyzed for GDF9, which was detectable in all samples. A total of 248 samples were analyzed for BMP15; BMP15 was detectable in 234 of the samples, resulting in an overall detectability of 94.4%. A total of 342 samples were analyzed for the GDF9/BMP15 heterodimer, and the heterodimer was detectable in 313 of the samples resulting in an overall detectability of 91.5%. BMP15 and the GDF9/BMP15 heterodimer were measured in fewer samples compared to GDF9 because of insufficient sample material.
Intrafollicular Concentrations of GDF9, BMP15, and the GDF9/BMP15 Heterodimer
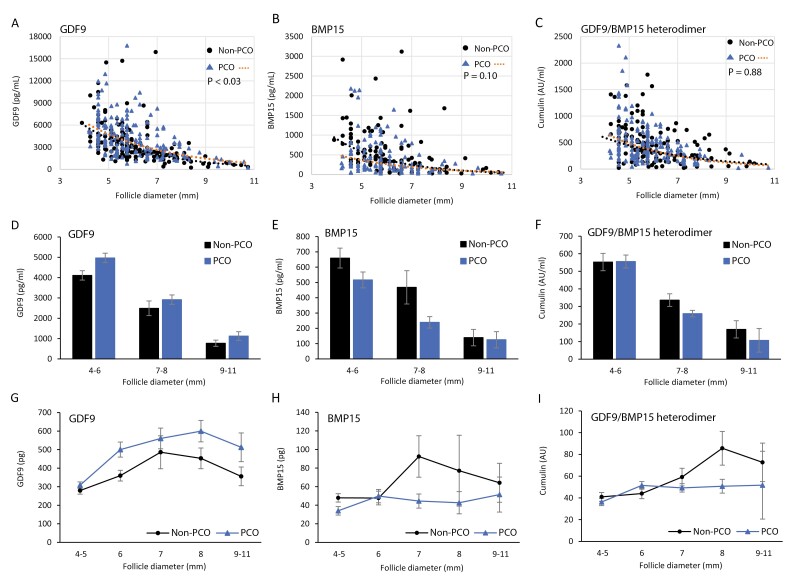
Overall, the concentration of GDF9 (3884 ± 138 pg/mL; mean ± SEM) was approximately 8-fold higher than BMP15 (497 ± 33 pg/mL) based on the standards developed for each of the assays (Table 2). The concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer were significantly negatively correlated with increasing follicle size (P < 0.0001 for all 3 proteins) (Fig. 3A, 3B, and 3C).
Table 2.
Intrafollicular concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer in small antral follicle fluids from non-PCO and PCO
| All samples (n = 356) |
Non-PCO n = 168 |
PCO n = 188 |
P | |
|---|---|---|---|---|
| GDF9, pg/mL | 3884 ± 138 | 3498 ± 199 | 4230 ± 189 | P = 0.03 |
| BMP15, pg/mLa | 497 ± 33 | 573 ± 55 | 431 ± 40 | P = 0.10 |
| GDF9/BMP15 heterodimer, AU/mLb | 477 ± 23 | 483 ± 37 | 473 ± 29 | P = 0.88 |
Values are mean ± SEM. P values show significant differences between non-PCO and PCO.
Abbreviations: BMP15, bone morphogenetic protein 15; GDF9, growth differentiation factor 9; PCO, polycystic ovary.
a 234 follicle fluids with detectable measurements (109 non-PCO and 125 PCO).
b 313 follicle fluids with detectable measurements (147 non-PCO and 167 PCO).
Figure 3.
Intrafollicular concentrations and content of GDF9, BMP15, and the GDF9/BMP15 heterodimer in non-PCO and PCO. (A-C) Scatter plots and linear regression analysis showing intrafollicular concentrations of (A) GDF9, (B) BMP15, and the (C) GDF9/BMP15 heterodimer in relation to follicle diameters in non-PCO and PCO groups. (D-F) Intrafollicular concentrations of GDF9, BMP15, and the GDF9/BMP15 heterodimer in small antral follicles grouped by follicle diameter and non-PCO/PCO. Number of follicle fluids analyzed according to follicle diameters (non-PCO/PCO); GDF9 (D): 4-6 mm (n = 114/127), 7-8 mm (n = 45/51), 9-11 mm (n = 8/8); BMP15 (E): 4-6 mm (n = 70/88), 7-8 mm (n = 33/33), 9-11 mm (n = 6/4); GDF9/BMP15 heterodimer (F): 4-6 mm (n = 104/121), 7-8 mm (n = 37/42), 9-11 mm (n = 6/3). (G-I) Follicle content of GDF9 (G), BMP15 (H), and the GDF9/BMP15 heterodimer (I) calculated by follicle volume and concentration according to follicle diameters. Values are mean ± SEM.
Concentrations of GDF9 were significantly higher (P < 0.03) in the PCO group (4230 ± 189 pg/mL; n = 188) compared with the non-PCO group (3498 ± 199 pg/mL; n = 168), whereas no significant differences in BMP15 concentrations were found between the PCO group (431 ± 40 pg/mL; n = 125) and the non-PCO group (573 ± 55 pg/mL; n = 109), but BMP15 concentrations were overall lower in the PCO group (Table 2; Fig. 3A and 3B). No significant differences in the concentrations of the GDF9/BMP15 heterodimer were found between the PCO group (473 ± 29 AU/mL; n = 167) and the non-PCO group (483 ± 37 AU/mL; n = 147) (Table 2).
Intrafollicular concentrations of GDF9 and BMP15 in relation to follicle size grouped according to diameter clearly showed a consistently higher concentration of GDF9 in the PCO group compared to non-PCO (Fig. 3D). In contrast, intrafollicular concentrations of BMP15 were consistently lower in the PCO group compared with the non-PCO group in the smallest follicles until follicle selection (ie, diameters of around 7-8 mm), whereas levels were similar in 9- to 10-mm follicles (Fig. 3E). No clear tendencies were observed between the non-PCO and PCO groups for concentrations of the GDF9/BMP15 heterodimer according to follicle diameters (Fig. 3F).
Follicle Content of GDF9, BMP15, and GDF9/BMP15 Heterodimer
When concentrations of GDF9 were analyzed as follicle content (volume × concentration), the content of GDF9 was similar in follicles with diameters of 4 to 5 mm in the PCO and non-PCO groups but markedly higher in the PCO group compared with non-PCO in follicles with diameters exceeding 6 mm (Fig. 3G). The follicle content of GDF9 increased with increasing follicle diameter and peaked in 8-mm follicles (Fig. 3G). The follicle content of BMP15 was similar and stabile between the PCO and non-PCO groups in follicles with diameters of 4 to 6 mm, but the BMP15 content peaked around follicle selection in 7- to 8-mm follicles in the non-PCO group, whereas it remained low with increasing follicle diameter in the PCO group (Fig. 3H). The follicle content of the GDF9/BMP15 heterodimer according to increasing follicle diameter was similar to BMP15 but the peak in the non-PCO group appeared a little bit later (Fig. 3I).
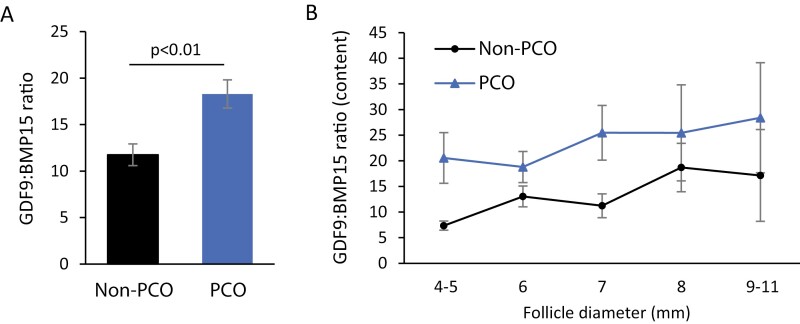
An Increased GDF9:BMP15 Ratio in PCO Follicle Fluids
The inverse relationship in intrafollicular concentrations of GDF9 and BMP15 resulted in a significantly higher GDF9:BMP15 ratio in the PCO group compared with the non-PCO group (non-PCO: 12 ± 1 [mean ± SEM]; PCO: 18 ± 2; P < 0.01) (Fig. 4A). Further analysis showed that the GDF9:BMP15 ratio increased with increasing follicle diameter when calculated according to follicle content and was consistently higher in the PCO group at all follicle diameters compared with the non-PCO group (Fig. 4B).
Figure 4.
An increased GDF9:BMP15 ratio in PCO follicle fluids. (A) The GDF9:BMP15 ratio in the non-PCO group (n = 109) and the PCO group (n = 122). A significantly increased GDF9:BMP15 ratio was found in the PCO group compared with the non-PCO group (P < 0.01). (B) The GDF9:BMP15 ratio based on intrafollicular content of GDF9 and BMP15 and depicted according to follicle diameter.
Intrafollicular Correlations Between TGF-β Growth Factors in non-PCO/PCO
A significant positive correlation between GDF9 and BMP15 was found in both PCO and non-PCO, with the most pronounced association and significance in the non-PCO group (r = 0.52, P < 0.001) compared with the PCO group (r = 0.36, P < 0.05) (supplemental Table 2 (54)). The GDF9/BMP15 heterodimer was significantly positively correlated with GDF9 and BMP15 in both PCO and non-PCO (supplemental Table 2 (54)).
To elucidate on the potential associations between the oocyte-secreted proteins and other TGF-β growth factors, Spearman correlations were also performed for GDF9, BMP15, and the GDF9/BMP15 heterodimer, and AMH, inhibins, activins and follistatin (supplemental Table 2 (54)). Significant negative correlations were found between GDF9 and the inhibins (inhibin-A, inhibin-B, and total inhibin), whereas significant positive correlations were found for GDF9 and the activins (activin-A and -B) and follistatin, which was consistent in both non-PCO and PCO (supplemental Table 2 (54)). The correlations between the GDF9/BMP15 heterodimer and the inhibins, activins, and follistatin were similar to that of GDF9 showing a tendency to more significant negative correlations with the inhibins in the PCO group compared with the non-PCO group (supplemental Table 2 (54)). Interestingly, Spearman correlations showed that significant positive correlations between BMP15 and AMH (r = 0.13, P < 0.03), inhibins (inhibin-B: r = 0.20, P < 0.01; inhibin-A: r = 0.03, P > 0.05; total inhibin: r = 0.12, P > 0.05), activins (activin-B: r = 0.28, P < 0.001; activin-AB: r = 0.17, P < 0.05), and follistatin (r = 0.33, P < 0.001) in the non-PCO women switched to negative correlations in the PCO women (AMH: r = -0.27, P > 0.05; inhibin-B: r = -0.45, P < 0.02; inhibin-A: r = -0.37, P < 0.05; total inhibin: r = -0.44, P < 0.02; activin-B: r = -0.11, P > 0.05; activin-AB: r = -0.31, P > 0.05; follistatin: r = -0.20, P > 0.05) (supplemental Table 2 (54)). Furthermore, the significant positive correlation between GDF9 and AMH in the non-PCO group (r = 0.39, P < 0.001) also switched to a nonsignificant correlation in the PCO group (r = 0.01, P > 0.05). No changes in the correlations between the GDF9/BMP15 heterodimer and AMH were observed between the non-PCO group (r = 0.27, P < 0.001) and the PCO group (r = 0.31, P < 0.001) (supplemental Table 2 (54)).
Highly significant correlations between all three inhibins (inhibin-B, inhibin-A, total inhibin) and activin-B were observed in the non-PCO group, which switched to nonsignificant correlations in the PCO group (supplemental Table 3 (55)). In contrast, no significant correlations were found between inhibins and activin-AB in the non-PCO group, whereas strong significant correlations were found in the PCO (supplemental Table 3 (55)). Follistatin showed significant, strong correlations with activin-B and activin-AB in both groups, but the correlations between follistatin and the inhibins switched from significant negative correlations in non-PCO women to positive or nonsignificant correlations in the PCO group (supplemental Table 3 (55)). These correlations between the inhibins, activins, and follistatin in non-PCO and PCO corroborates our previous findings in Kristensen et al (36).
Discussion
This is the first study to perform quantitative measurements of GDF9, BMP15, and the GDF9/BMP15 heterodimer proteins in a large sample set including 356 FF from human small antral follicles collected during the natural cycle. The concentrations of GDF9 and BMP15 were present in surprisingly high concentrations given that only the oocyte itself secretes these growth factors, which enforces the view that the oocyte actively influences the local environment within the follicle. In addition, it is likely that accumulation of these proteins takes place within small antral follicles. The intrafollicular concentrations of GDF9 were approximately 8-fold higher than BMP15, and GDF9 and BMP15 concentrations varied inversely in FF from women with PCO, which resulted in a significantly higher GDF9:BMP15 ratio in the PCO group compared with the non-PCO group. Furthermore, the GDF9/BMP15 heterodimer was measured for the first time in biological fluids and intrafollicular concentrations were similar to BMP15 and between the PCO and non-PCO groups.
Oocyte-specific proteins such as GDF9 and BMP15 are ideal candidates as potential diagnostic biomarkers of oocyte quality and function. However, quantification of these proteins in biological fluids has proven difficult, primarily because of the lack of specific monoclonal antibodies available and the atypical structural features of the BMP15 and GDF9 proteins. Only a few studies to date have quantified GDF9 and BMP15 in human serum and FF using either in-house developed ELISAs or a commercially available ELISA for GDF9 (34-36, 56). However, serum concentrations of GDF9 and BMP15 have not yet proven useful as diagnostic biomarkers of female fecundity or oocyte quality as the current assays show low detectability and high variability between patients (34, 35). Recently, Riepsamen and colleagues measured GDF9 and BMP15 in preovulatory FF from 138 women undergoing in vitro fertilization and found that BMP15 and GDF9 were detectable in 76% and 60% of samples, respectively, compared with a detectability of 67% and 29% in serum (34). In our current study, we found higher detectability in fluids from small antral follicles with the GDF9, BMP15, and GDF9/BMP15 heterodimer being measured in 100%, 94.4%, and 91.5% of all samples, respectively. The higher detectability of GDF9 and BMP15 in small antral follicles compared with preovulatory follicles is in line with the significant negative correlations that we found between the concentrations of GDF9 and BMP15 in relation to increasing follicle size in the current study. Thus, the highest intrafollicular concentrations of GDF9 and BMP15 appeared to be found in small follicles prior to follicle selection. Interestingly, our data also show that small antral follicles contained GDF9 in abundance compared with BMP15, indicating that GDF9 exerts functions on its own during follicle development because the vast majority of GDF9 will not be trapped in the heterodimer complex. It is also noticeable that BMP15 in the non-PCO group appeared to peak just around follicle selection at 7 to 8 mm, enforcing that this particular developmental stage is an important landmark for the hormonal regulation of human follicles.
Several studies in mammalian species have shown improved oocyte competence when the culture medium was supplemented with recombinant GDF9 and/or recombinant BMP15 (30, 57-59). In 2001, GDF9/BMP15 double knockout mice studies by Yan and colleagues showed that GDF9 and BMP15 cooperate, indicating redundant or synergistic biological actions of GDF9 and BMP15 (60). However, to date, it is still unknown whether this cooperation is generated by GDF9 and BMP15 homodimers separately or by the GDF9/BMP15 heterodimer. Interestingly, in vitro studies have shown that the recombinant human GDF9/BMP15 heterodimer potently activates Smad2/3 signalling in mammalian granulosa cells and improve embryo development during oocyte in vitro maturation compared to human GDF9 and BMP15 homodimers (29, 30, 59). However, our current findings indicate similar intrafollicular concentrations of the GDF9/BMP15 heterodimer and BMP15 in small antral follicles, suggesting that only a minor proportion of the total GDF9 would potentially form a heterodimer with BMP15 in vivo, and that GDF9 performs actions on its own as a homodimer. Moreover, our findings showed no differences in the intrafollicular concentrations of the GDF9/BMP15 heterodimer between the women with and without PCO. The follicle content of the GDF9/BMP15 heterodimer in relation to follicle diameter also followed the same pattern of expression as BMP15 in both non-PCO and PCO samples. Thus, a role for the GDF9/BMP15 heterodimer in the abnormalities in follicle growth in PCOS appears to be questionable based on our results.
Our results showed that intrafollicular concentrations of GDF9 were significantly higher in women with PCO, whereas BMP15 concentrations were lower in women with PCO. The increased protein concentrations of GDF9 in FF from women with PCO are in line with 2 previous studies showing higher expression of GDF9/GDF9 at both mRNA and protein level in oocytes from PCOS patients compared with a control group undergoing controlled ovarian stimulation (7, 10). In striking contrast, another study found that the expression of GDF9 mRNA was lower in stimulated mature oocytes from women with PCOS compared with women without PCO (8). The discrepancies in the published data are unknown but may be attributed to stage-dependent differences or oocyte quality, follicle diameter, or sensitivity and specificity of the quantitative PCR analysis (10). Interestingly, these previous studies also found a concomitant higher or lower expression of BMP15/BMP15 in the oocytes from PCOS patients compared with controls, which contrasts with our results. Our findings are the only data showing an inverse relationship between GDF9 and BMP15 in women with PCO. Thus, the expression dynamics of GD9 and BMP15 in human follicles remains controversial, but current findings support the concept that any changes in especially GDF9 protein levels can disrupt ovarian function and female fertility (61). Further studies assessing the expression of GDF9 and BMP15 in FF and corresponding oocytes and cumulus or granulosa cells in women with PCO/PCOS during the natural cycle are needed to reveal the complex interplay between the oocyte and the follicle compartment in ovarian pathologies like PCOS.
That intrafollicular concentrations of GDF9 and BMP15 varied inversely in women with PCO compared to women without PCO resulted in a significantly increased intrafollicular GDF9:BMP15 ratio in women with PCO in our study. The GDF9:BMP15 ratio is known to play an important role in mammalian ovulation rate and fecundity by mechanisms involving GDF9 and BMP15 regulation of LH receptor function on granulosa cells (62, 63). Interestingly, the studies by Riepsamen and colleagues suggested an increased GDF9:BMP15 ratio in serum with increasing oocyte numbers in women without PCOS, but not in women with PCOS (34). Thus, it can be speculated whether the GDF9:BMP15 ratio could be developed as a potential biomarker and diagnostic tool in women with and without PCOS. However, with the low detectability of GDF9 and BMP15 in serum further research is needed using even more sensitive assays to reveal whether these essential oocyte-secreted growth factors could prove useful in a clinical context.
A number of recent studies have shown that GDF9 and BMP15 modulate FSH-induced secretion of a number of granulosa and cumulus cell-secreted proteins and hormones including other TGF-β members (26, 64-67), which suggests that the oocyte itself is actively engaged in the control of follicle development. It is therefore noticeable that the associations between BMP15 and the concentrations of other TGF-β members show positive associations in the non-PCO group that turn into negative associations in the PCO group. This reflects aberrant regulation of follicle growth in the PCO group, but this study is unable to pinpoint the underlying mechanisms. We also found that the significant positive associations between both GDF9 and BMP15 and AMH in the non-PCO group were nonexisting in the PCO group. Studies in mice and human granulosa and cumulus cells have shown that GDF9 and BMP15 induce AMH expression (66, 68-71). Interestingly, studies also showed that FSH inhibits the GDF9/BMP15-induced AMH expression (66, 71). It may be hypothesized that the fine-tuned interactions of growth factors with FSH in the control of granulosa and cumulus cell functions is disturbed in the PCO group. Collectively, alterations in the associations between GDF9, BMP15, and other TGF-β members in our study probably reflect aberrant regulation of these TGF-β members in women with PCO, and our findings complement those of previous studies implicating members of the TGF-β superfamily in the pathophysiology of PCOS (21, 36, 72-75).
A limitation to the current study is that women with PCO were not diagnosed clinically with PCOS according to the Rotterdam criteria. However, these data were not recorded nor required in management of these women for whom the priority was fertility preservation. Selected patients in the PCO group had an average ovarian volume of 14 mL, a high number of small antral follicles, high serum concentrations of AMH and LH, and a high LH/FSH ratio, which collectively indicate a potential PCO-like phenotype. Furthermore, the data set was not large enough to analyze the within-patient variation of the growth factors at specific follicle diameters nor the potential impact that a cancer diagnosis may have on baseline ovarian hormone levels. Finally, only a limited number of samples from small antral follicles larger than 8 mm in diameter were available for analysis and results obtained in these larger antral follicles should be considered with caution.
Conclusion
Concentrations of oocyte-secreted factors GDF9 and BMP15 varied inversely in small antral FF from PCO and an increased intrafollicular GDF9:BMP15 ratio could be a biomarker for PCO. However, measurements in circulation are required to determine whether an increased GDF9/BMP15 ratio could have clinical potential as a biomarker for PCO or PCOS. Further, our results indicate that the bioactive forms of GDF9 and BMP15 in vivo probably act as homodimers and the role of a GDF9/BMP15 heterodimer needs further clarification. Collectively, our findings provide new perspectives for understanding the pathogenesis of PCOS.
Acknowledgments
The authors thank all the women who consented and donated samples for the study. We acknowledge all personnel involved with the fertility preservation program in Denmark for their passionate work.
Glossary
Abbreviations
- AFC
antral follicle count
- AMH
anti-Mullerian hormone
- AU
arbitrary unit
- BMP15
bone morphogenetic protein 15
- COC
cumulus-oocyte complex
- FF
follicle fluid
- GDF9
growth differentiation factor 9
- PCO
polycystic ovary
- PCOS
polycystic ovary syndrome
Contributor Information
Stine Gry Kristensen, Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, 2100 Copenhagen, Denmark.
Ajay Kumar, Ansh Labs LLC, Webster, TX 77598, USA.
Linn Salto Mamsen, Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, 2100 Copenhagen, Denmark.
Bhanu Kalra, Ansh Labs LLC, Webster, TX 77598, USA.
Susanne Elisabeth Pors, Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, 2100 Copenhagen, Denmark.
Jane Alrø Bøtkjær, Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, 2100 Copenhagen, Denmark.
Kirsten Tryde Macklon, The Fertility Clinic, Copenhagen University Hospital, Rigshospitalet, 2100 Copenhagen, Denmark.
Jens Fedder, Centre of Andrology & Fertility Clinic, Odense University Hospital, 5000 Odense, Denmark.
Erik Ernst, Department of Gynecology and Obstetrics, Horsens Regional Hospital, 8700 Horsens, Denmark.
Kate Hardy, Institute of Reproductive and Developmental Biology, Imperial College London, Hammersmith Hospital, London W12 0NN, United Kingdom.
Stephen Franks, Institute of Reproductive and Developmental Biology, Imperial College London, Hammersmith Hospital, London W12 0NN, United Kingdom.
Claus Yding Andersen, Laboratory of Reproductive Biology, The Juliane Marie Centre for Women, Children and Reproduction, University Hospital of Copenhagen, 2100 Copenhagen, Denmark; Faculty of Health and Medical Sciences, University of Copenhagen, 2100 Copenhagen, Denmark.
Funding
The project received support from the Research Council of Rigshospitalet (no. E22055-01 to S.G.K.), the Novo Nordisk Foundation (no. NNF17OC0029848 to C.Y.A.), and an MRC Project Grant MR/M012638/1 (£490,635) to S.F., K.H., and C.Y.A.: “Role of growth factors of the TGFβ superfamily in aberrant follicle development in PCOS”, 2015-2018.
Conflict of Interest
The authors assert they have no conflicts of interest.
Disclosures
The authors have no conflicts of interest and nothing to disclose. A.K. and B.K. are employed by Ansh Labs. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Data Availability
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.
References
- 1. Gilchrist RB. Recent insights into oocyte-follicle cell interactions provide opportunities for the development of new approaches to in vitro maturation. Reprod Fertil Dev. 2011;23(1):23-31. [DOI] [PubMed] [Google Scholar]
- 2. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14(2):159-177. [DOI] [PubMed] [Google Scholar]
- 3. Knight PG, Glister C. TGF-beta superfamily members and ovarian follicle development. Reproduction 2006;132(2):191-206. [DOI] [PubMed] [Google Scholar]
- 4. Chang HM, Qiao J, Leung PC. Oocyte-somatic cell interactions in the human ovary-novel role of bone morphogenetic proteins and growth differentiation factors. Hum Reprod Update. 2017;23(1):1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sanfins A, Rodrigues P, Albertini DF. GDF-9 and BMP-15 direct the follicle symphony. J Assist Reprod Genet. 2018;35(10):1741-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Teixeira Filho FL, Baracat EC, Lee TH, et al. . Aberrant expression of growth differentiation factor-9 in oocytes of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2002;87(3):1337-1344. [DOI] [PubMed] [Google Scholar]
- 7. Zhao SY, Qiao J, Chen YJ, Liu P, Li J, Yan J. Expression of growth differentiation factor-9 and bone morphogenetic protein-15 in oocytes and cumulus granulosa cells of patients with polycystic ovary syndrome. Fertil Steril. 2010;94(1):261-267. [DOI] [PubMed] [Google Scholar]
- 8. Wei LN, Liang XY, Fang C, Zhang MF. Abnormal expression of growth differentiation factor 9 and bone morphogenetic protein 15 in stimulated oocytes during maturation from women with polycystic ovary syndrome. Fertil Steril. 2011;96(2):464-468. [DOI] [PubMed] [Google Scholar]
- 9. Wei LN, Huang R, Li LL, Fang C, Li Y, Liang XY. Reduced and delayed expression of GDF9 and BMP15 in ovarian tissues from women with polycystic ovary syndrome. J Assist Reprod Genet. 2014;31(11):1483-1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. de Resende LO, Vireque AA, Santana LF, et al. . Single-cell expression analysis of BMP15 and GDF9 in mature oocytes and BMPR2 in cumulus cells of women with polycystic ovary syndrome undergoing controlled ovarian hyperstimulation. J Assist Reprod Genet. 2012;29(10):1057-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Franks S. Polycystic ovary syndrome. New Engl J Med. 1995;333(13):853-861. [DOI] [PubMed] [Google Scholar]
- 12. Fauser BC, Tarlatzis BC, Rebar RW, et al. . Consensus on women’s health aspects of polycystic ovary syndrome (PCOS): the Amsterdam ESHRE/ASRM-sponsored 3rd PCOS Consensus Workshop Group. Fertil Steril. 2012;97(1):28-38.e25. [DOI] [PubMed] [Google Scholar]
- 13. Legro RS, Arslanian SA, Ehrmann DA, et al. . Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bozdag G, Mumusoglu S, Zengin D, Karabulut E, Yildiz BO. The prevalence and phenotypic features of polycystic ovary syndrome: a systematic review and meta-analysis. Hum Reprod. 2016;31(12):2841-2855. [DOI] [PubMed] [Google Scholar]
- 15. Wang TT, Ke ZH, Song Y, et al. . Identification of a mutation in GDF9 as a novel cause of diminished ovarian reserve in young women. Hum Reprod. 2013;28(9):2473-2481. [DOI] [PubMed] [Google Scholar]
- 16. Liu J, Wang B, Wei Z, et al. . Mutational analysis of human bone morphogenetic protein 15 in Chinese women with polycystic ovary syndrome. Metabolism 2011;60(11):1511-1514. [DOI] [PubMed] [Google Scholar]
- 17. Mehdizadeh A, Sheikhha MH, Kalantar SM, Aali BS, Ghanei A. Mutation analysis of exon1 of bone morphogenetic protein-15 gene in Iranian patients with polycystic ovarian syndrome. Int J Reprod Biomed. 2016;14(8):527-532. [PMC free article] [PubMed] [Google Scholar]
- 18. Belli M, Shimasaki S. Molecular aspects and clinical relevance of GDF9 and BMP15 in ovarian function. Vitam Horm. 2018;107:317-348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takebayashi K, Takakura K, Wang H, Kimura F, Kasahara K, Noda Y. Mutation analysis of the growth differentiation factor-9 and -9B genes in patients with premature ovarian failure and polycystic ovary syndrome. Fertil Steril. 2000;74(5):976-979. [DOI] [PubMed] [Google Scholar]
- 20. Sproul K, Jones MR, Mathur R, Azziz R, Goodarzi MO. Association study of four key folliculogenesis genes in polycystic ovary syndrome. BJOG 2010;117(6):756-760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Haouzi D, Assou S, Monzo C, Vincens C, Dechaud H, Hamamah S. Altered gene expression profile in cumulus cells of mature MII oocytes from patients with polycystic ovary syndrome. Hum Reprod. 2012;27(12):3523-3530. [DOI] [PubMed] [Google Scholar]
- 22. Karagül MI, Aktaş S, Coşkun Yılmaz B, Yılmaz M, Orekici Temel G. GDF9 and BMP15 expressions and fine structure changes during folliculogenesis in polycystic ovary syndrome. Balkan Med J. 2018;35(1):43-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simpson CM, Stanton PG, Walton KL, et al. . Activation of latent human GDF9 by a single residue change (Gly 391 Arg) in the mature domain. Endocrinology 2012;153(3):1301-1310. [DOI] [PubMed] [Google Scholar]
- 24. Al-Musawi SL, Walton KL, Heath D, Simpson CM, Harrison CA. Species differences in the expression and activity of bone morphogenetic protein 15. Endocrinology 2013;154(2):888-899. [DOI] [PubMed] [Google Scholar]
- 25. McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-beta superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268(5):3444-3449. [PubMed] [Google Scholar]
- 26. Richani D, Constance K, Lien S, et al. . Cumulin and FSH cooperate to regulate inhibin b and activin b production by human granulosa-lutein cells in vitro. Endocrinology 2019;160(4):853-862. [DOI] [PubMed] [Google Scholar]
- 27. McNatty KP, Moore LG, Hudson NL, et al. . The oocyte and its role in regulating ovulation rate: a new paradigm in reproductive biology. Reproduction 2004;128(4):379-386. [DOI] [PubMed] [Google Scholar]
- 28. McIntosh CJ, Lun S, Lawrence S, Western AH, McNatty KP, Juengel JL. The proregion of mouse BMP15 regulates the cooperative interactions of BMP15 and GDF9. Biol Reprod. 2008;79(5):889-896. [DOI] [PubMed] [Google Scholar]
- 29. Peng J, Li Q, Wigglesworth K, et al. . Growth differentiation factor 9:bone morphogenetic protein 15 heterodimers are potent regulators of ovarian functions. Proc Natl Acad Sci USA. 2013;110(8):E776-E785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mottershead DG, Sugimura S, Al-Musawi SL, et al. . Cumulin, an oocyte-secreted heterodimer of the transforming growth factor-β family, is a potent activator of granulosa cells and improves oocyte quality. J Biol Chem. 2015;290(39):24007-24020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Grøndahl ML, Borup R, Vikea J, Ernst E, Andersen CY, Lykke-Hartmann K. The dormant and the fully competent oocyte: comparing the transcriptome of human oocytes from primordial follicles and in metaphase II. Mol Hum Reprod. 2013;19(9):600-17. [DOI] [PubMed] [Google Scholar]
- 32. Kristensen SG, Andersen K, Clement CA, Franks S, Hardy K, Andersen CY. Expression of TGF-beta superfamily growth factors, their receptors, the associated SMADs and antagonists in five isolated size-matched populations of pre-antral follicles from normal human ovaries. Mol Hum Reprod. 2014;20(4):293-308. [DOI] [PubMed] [Google Scholar]
- 33. Zhang Y, Yan Z, Qin Q, et al. . Transcriptome landscape of human folliculogenesis reveals oocyte and granulosa cell interactions. Mol Cell. 2018;72(6):1021-1034.e4. [DOI] [PubMed] [Google Scholar]
- 34. Riepsamen AH, Chan K, Lien S, et al. . Serum concentrations of oocyte-secreted factors BMP15 and GDF9 during IVF and in women with reproductive pathologies. Endocrinology 2019;160(10):2298-2313. [DOI] [PubMed] [Google Scholar]
- 35. Riepsamen AH, Donoghoe MW, Baerwald A, et al. . Exploratory analysis of serum concentrations of oocyte biomarkers growth differentiation factor 9 and bone morphogenetic protein 15 in ovulatory women across the menstrual cycle. Fertil Steril. 2021;116(2):546-557. [DOI] [PubMed] [Google Scholar]
- 36. Kristensen SG, Kumar A, Kalra B, et al. . Quantitative differences in TGF-beta family members measured in small antral follicle fluids from women with or without PCO. J Clin Endocrinol Metab. 2019;104(12):6371-6384. [DOI] [PubMed] [Google Scholar]
- 37. Kristensen SG, Liu Q, Mamsen LS, et al. . A simple method to quantify follicle survival in cryopreserved human ovarian tissue. Hum Reprod. 2018;33(12):2276-2284. [DOI] [PubMed] [Google Scholar]
- 38.RRID: AB_2783706, https://scicrunch.org/resolver/AB_2783706
- 39.RRID: AB_2909503, https://scicrunch.org/resolver/AB_2909503
- 40.RRID: AB_2909504, https://scicrunch.org/resolver/AB_2909504
- 41.RRID: AB_2783675, https://scicrunch.org/resolver/AB_2783675
- 42.RRID: AB_2783674, http://antibodyregistry.org/AB_2783674
- 43.RRID: AB_2783661, https://scicrunch.org/resolver/AB_2783674
- 44.RRID: AB_2783681, https://scicrunch.org/resolver/AB_2783681
- 45.RRID: AB_2783689, https://scicrunch.org/resolver/AB_2783689
- 46.RRID: AB_2783692, https://scicrunch.org/resolver/AB_2783692
- 47.RRID: AB_2783669, https://scicrunch.org/resolver/AB_2783669
- 48. Kristensen SG. Supplemental table 1 for “Intrafollicular concentrations of the oocyte-secreted factors GDF9 and BMP15 vary inversely in polycystic ovaries”. Figshare, 2022. Deposited March 10, 2022. doi: 10.6084/m9.figshare.19336631.v1 [DOI] [PMC free article] [PubMed]
- 49. Mamsen LS, Zafeiri A, Bøtkjær JA, et al. . Expression of the insulin-like growth factor system in first- and second-trimester human embryonic and fetal gonads. J Clin Endocrinol Metab. 2020;105(9):dgaa470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.RRID: AB_870570, https://scicrunch.org/resolver/AB_870570
- 51. Kristensen SG. Supplemental figure 1 for “Intrafollicular concentrations of the oocyte-secreted factors GDF9 and BMP15 vary inversely in polycystic ovaries”. Figshare, 2022. Deposited March 20, 2022. doi: 10.6084/m9.figshare.19388036.v2 [DOI] [PMC free article] [PubMed]
- 52. Mamsen LS, Kristensen SG, Pors SE, et al. . Consequences of β-thalassemia or sickle cell disease for ovarian follicle number and morphology in girls who had ovarian tissue cryopreserved. Front Endocrinol (Lausanne) 2021;11:593718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Rosendahl M, Ernst E, Rasmussen PE, Andersen CY. True ovarian volume is underestimated by two-dimensional transvaginal ultrasound measurement. Fertil Steril. 2010;93(3):995-998. [DOI] [PubMed] [Google Scholar]
- 54. Kristensen SG. Supplemental table 2 for “Intrafollicular concentrations of the oocyte-secreted factors GDF9 and BMP15 vary inversely in polycystic ovaries”. Figshare, 2022. Deposited March 10, 2022. doi: 10.6084/m9.figshare.19336802.v1 [DOI] [PMC free article] [PubMed]
- 55. Kristensen SG. Supplemental table 3 for “Intrafollicular concentrations of the oocyte-secreted factors GDF9 and BMP15 vary inversely in polycystic ovaries”. Figshare, 2022. Deposited March 21, 2022. doi: 10.6084/m9.figshare.19336808.v3 [DOI] [PMC free article] [PubMed]
- 56. Berberoglu Z, Aktas A, Fidan Y, Yazici AC, Aral Y. Association of plasma GDF-9 or GDF-15 levels with bone parameters in polycystic ovary syndrome. J Bone Miner Metab. 2015;33(1):101-108. [DOI] [PubMed] [Google Scholar]
- 57. Sudiman J, Sutton-McDowall ML, Ritter LJ, et al. . Bone morphogenetic protein 15 in the pro-mature complex form enhances bovine oocyte developmental competence. PLoS One. 2014;9(7):e103563e103563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Li JJ, Sugimura S, Mueller TD, et al. . Modifications of human growth differentiation factor 9 to improve the generation of embryos from low competence oocytes. Mol Endocrinol. 2015;29(1):40-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Stocker WA, Walton KL, Richani D, et al. . A variant of human growth differentiation factor-9 that improves oocyte developmental competence. J Biol Chem. 2020;295(23):7981-7991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Yan C, Wang P, DeMayo J, et al. . Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15(6):854-866. [DOI] [PubMed] [Google Scholar]
- 61. Simpson CM, Robertson DM, Al-Musawi SL, et al. . Aberrant GDF9 expression and activation are associated with common human ovarian disorders. J Clin Endocrinol Metab. 2014;99(4):E615-E624. [DOI] [PubMed] [Google Scholar]
- 62. Crawford JL, McNatty KP. The ratio of growth differentiation factor 9: bone morphogenetic protein 15 mRNA expression is tightly co-regulated and differs between species over a wide range of ovulation rates. Mol Cell Endocrinol. 2012;348(1):339-343. [DOI] [PubMed] [Google Scholar]
- 63. Christoforou ER, Pitman JL. Intrafollicular growth differentiation factor 9: bone morphogenetic 15 ratio determines litter size in mammals. Biol Reprod. 2019;100(5):1333-1343. [DOI] [PubMed] [Google Scholar]
- 64. Hobeika E, Armouti M, Kala H, et al. . Oocyte-secreted factors synergize with FSH to promote aromatase expression in primary human cumulus cells. J Clin Endocrinol Metab. 2019;104(5):1667-1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hobeika E, Armouti M, Fierro MA, et al. . Regulation of insulin-like growth factor 2 by oocyte-secreted factors in primary human granulosa cells. J Clin Endocrinol Metab. 2020;105(1):327-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Convissar S, Armouti M, Fierro MA, et al. . Regulation of AMH by oocyte-specific growth factors in human primary cumulus cells. Reproduction 2017;154(6):745-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Esfandyari S, Winston NJ, Fierro MA, Scoccia H, Stocco C. Oocyte-secreted factors strongly stimulate sFRP4 expression in human cumulus cells. Mol Hum Reprod. 2021;27(6):gaab031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Salmon NA, Handyside AH, Joyce IM. Oocyte regulation of anti-Müllerian hormone expression in granulosa cells during ovarian follicle development in mice. Dev Biol. 2004;266(1):201-208. [DOI] [PubMed] [Google Scholar]
- 69. McNatty KP, Juengel JL, Reader KL, et al. . Bone morphogenetic protein 15 and growth differentiation factor 9 co-operate to regulate granulosa cell function in ruminants. Reproduction 2005;129(4):481-487. [DOI] [PubMed] [Google Scholar]
- 70. Pierre A, Estienne A, Racine C, et al. . The bone morphogenetic protein 15 up-regulates the anti-Müllerian hormone receptor expression in granulosa cells. J Clin Endocrinol Metab. 2016;101(6):2602-2611. [DOI] [PubMed] [Google Scholar]
- 71. Roy S, Gandra D, Seger C, et al. . Oocyte-derived factors (GDF9 and BMP15) and FSH regulate AMH expression via modulation of H3K27AC in granulosa cells. Endocrinology 2018;159(9):3433-3445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Owens LA, Kristensen SG, Lerner A, et al. . Gene expression in granulosa cells from small antral follicles from women with or without polycystic ovaries. J Clin Endocrinol Metab. 2019;104(12):6182-6192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Magoffin DA, Jakimiuk AJ. Inhibin A, inhibin B and activin A concentrations in follicular fluid from women with polycystic ovary syndrome. Hum Reprod. 1998;13(10):2693-2698. [DOI] [PubMed] [Google Scholar]
- 74. Fujiwara T, Sidis Y, Welt CK, et al. . Dynamics of inhibin subunit and follistatin mRNA during development of normal and PCOS follicles. J Clin Endocrinol Metab. 2001;86(9):4206-415. [DOI] [PubMed] [Google Scholar]
- 75. Welt CK, Taylor AE, Fox J, Messerlian GM, Adams JM, Schneyer AL. Follicular arrest in polycystic ovary syndrome is associated with deficient inhibin A and B biosynthesis. J Clin Endocrinol Metab. 2005;90(10):5582-5587. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during the current study are not publicly available but are available from the corresponding author on reasonable request.