Abstract
Context
Decreased first-phase insulin response (FPIR) during intravenous glucose tolerance testing (IVGTT) is an early indicator of β-cell dysfunction and predictor of type 1 diabetes (T1D).
Objective
Assess whether oral glucose tolerance test (OGTT) measures could serve as FPIR alternatives in their ability to predict T1D in autoantibody positive (Aab+) subjects.
Design
OGTT and IVGTT were performed within 30 days of each other. Eleven OGTT variables were evaluated for (1) correlation with FPIR and (2) T1D prediction.
Setting
Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D” (TN-07) and Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) studies clinical sites.
Patients
TN-07 (n = 292; age 9.4 ± 6.1 years) and DPT-1 (n = 194; age 15.1 ± 10.0 years) Aab + relatives of T1D individuals.
Main outcome measures
(1) Correlation coefficients of OGTT measures with FPIR and (2) T1D prediction at 2 years using area under receiver operating characteristic (ROCAUC) curves.
Results
Index60 showed the strongest correlation in DPT-1 (r = -0.562) but was weaker in TN-07 (r = -0.378). C-peptide index consistently showed good correlation with FPIR across studies (TN-07, r = 0.583; DPT-1, r = 0.544; P < 0.0001). Index60 and C-peptide index had the highest ROCAUCs for T1D prediction (0.778 vs 0.717 in TN-07 and 0.763 vs 0.721 in DPT-1, respectively; P = NS), followed by FPIR (0.707 in TN-07; 0.628 in DPT-1).
Conclusions
C-peptide index was the strongest measure to correlate with FPIR in both studies. Index60 and C-peptide index had the highest predictive accuracy for T1D and were comparable. OGTTs could be considered instead of IVGTTs for subject stratification in T1D prevention trials.
Keywords: type 1 diabetes, first-phase insulin response, C-peptide index, Index60, oral glucose tolerance test, TrialNet
The first-phase insulin response (FPIR), obtained from the intravenous glucose tolerance test (IVGTT) and calculated within the first few minutes following administration of intravenous glucose, plays a critical role in the maintenance of glucose homeostasis (1). Loss of FPIR is one of the first observable defects during the progression to clinical diabetes (2-4), and reduced first-phase insulin release has been shown to be highly predictive for the development of type 1 diabetes (T1D) among high-risk autoantibody-positive individuals (4-7). In islet-cell autoantibody (ICA)-positive first-degree relatives of persons with T1D, a FPIR less than the first percentile was associated with a 30% to 50% risk of developing symptomatic T1D within 1 year (5, 8). Furthermore, an accelerated decline in FPIR is observed from 1.5 to 0.5 years before T1D diagnosis (4).
Based on this predictive ability, FPIR has been used to stratify participants in T1D prevention studies. In the Diabetes Prevention Trial-Type 1 Diabetes (DPT-1) study, ICA and insulin autoantibody (IAA)-positive subjects were randomized according to a predetermined FPIR threshold and oral glucose tolerance test (OGTT) results to either the parenteral insulin vs placebo trial (high-risk group) or oral insulin vs placebo trial (intermediate-risk group) (9). Although oral insulin did not delay or prevent T1D, a subgroup of subjects with high IAA levels (≥80 nU/mL) had an annualized diabetes rate of 6.2% compared with a rate of 10.4% in subjects who received placebo (hazard ratio, 0.566; 95% CI, 0.361-0.888; P < 0.015). The results from this post hoc analysis paved the way for the Type 1 Diabetes TrialNet study TN-07 “Oral Insulin for Prevention of Diabetes in Relatives as Risk for Type 1 Diabetes Mellitus,” which applied similar FPIR thresholds for subject stratification (10). Oral insulin treatment failed to delay or prevent T1D within the overall cohort, but in the stratum comprising autoantibody-positive (Aab+) subjects with a low FPIR (secondary stratum 1), the time to diabetes was significantly prolonged by oral insulin therapy (hazard ratio, 0.45; 95% CI, 0-0.82; P = 0.006). These data highlight the utility of FPIR in T1D trial design and risk prediction. FPIR is determined during an IVGTT. Briefly, blood samples are drawn at very frequent intervals over a 10-minute period after a pulse of intravenous glucose. The test can be technically demanding, making its application challenging, especially in large clinical trials. By contrast, the OGTT is a simpler test that is widely used to detect dysglycemia or establish a biochemical diagnosis of diabetes mellitus both clinically and in research. Studies that use the IVGTT usually also require an OGTT to determine glucose tolerance status, but the 2 tests (each of which requires fasting) cannot be performed on the same day.
The goal of this study was to identify OGTT measures capable of serving as alternatives to FPIR to reduce participant and clinical research protocol testing burden. To this end, we used data from a unique subset of individuals from the Type 1 Diabetes TrialNet TN-07 and DPT-1 studies who underwent paired IVGTT and OGTT tests within 30 days.
We then sought to compare the prognostic accuracy of OGTT-derived measures against FPIR for development of T1D over a 2-year period. Because both cohorts differed in the baseline risk for T1D at study entry (TN-07 comprised individuals with normal glucose tolerance, whereas DPT-1 allowed for either normal or abnormal glucose tolerance), data were not combined, and analyses were conducted separately for each cohort.
Materials and Methods
Subjects
Included participants from the TN-07 and DPT-1 studies came from both the intervention and placebo arms because both studies were negative. However, a subgroup of subjects, assigned to the treatment arm of their respective study, demonstrated a prolonged time to T1D development or decreased annualized diabetes rate. These subjects were excluded from our analyses as described in the following section. The described analyses were exploratory for both studies. Baseline characteristics for study participants are shown in Table 1.
Table 1.
Baseline characteristics for DPT-1 and TN-07 participants who had a paired IVGTT and OGTT within 30 days before study randomization
| Baseline characteristics | DPT-1 (N = 194) | TN-07 (N = 292) | P valuea | |
|---|---|---|---|---|
| Age, y | ||||
| Mean (SD) | 15.1 (10.0) | 9.4 (6.1) | <0.0001 | |
| Median [Q1, Q3] | 11.8 [8.7, 17.2] | 8.1 [5.5, 12.2] | ||
| BMI percentileb | ||||
| Mean (SD) | 52.8 (31.0) | 59.5 (28.6) | 0.0259 | |
| Median [Q1, Q3] | 57.8 [24.8, 79.8] | 63.1 [36.8, 84.8] | ||
| Missing | 28 | 0 | ||
| Sex, n (%) | ||||
| Female | 100 (51.6) | 115 (39.4) | 0.0082 | |
| Male | 94 (48.5) | 177 (60.6) | ||
| Race, n (%) | ||||
| White | 176 (91.7) | 264 (96.4) | 0.0301 | |
| Non-White | 16 (8.3) | 10 (3.7) | ||
| Unknown | 2 | 18 | ||
| Relationship to proband, n (%) | ||||
| First degree | 177 (91.2) | 263 (90.1) | 0.6665 | |
| Second degree | 17 (8.8) | 29 (9.9) | ||
| HLA DR3 or DR4, n (%) | ||||
| Present | 155 (80.7) | 245 (84.2) | 0.3235 | |
| Absent | 37 (19.3) | 46 (15.8) | ||
| Unknown | 2 | 1 | ||
| Duration between IVGTT and OGTT, d | ||||
| Mean (SD) | 15.7 (10.8) | 21.3 (7.6) | <0.0001 | |
| Median [Q1, Q3] | 20.0 [3.0, 26.0] | 23.0 [17.5, 28.0] | ||
| T1D diagnosis reported within 5 y, n (%) | ||||
| Yes | 80 (41.2) | 84 (28.8) | ||
| No | 114 (58.8) | 208 (71.2) | ||
| Follow-up for T1D, y | ||||
| Median (range) | 4.7 (0.0-7.2) | 5.1 (0.0-11.5) |
Abbreviations: BMI, body mass index; DPT-1, Diabetes Prevention Trial-Type 1 Diabetes; IVGTT, intravenous glucose tolerance test; OGTT, oral glucose tolerance test; T1D, type 1 diabetes; TN-07, Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D.”
a Kruskal-Wallis test for continuous variables and χ2 test or Fisher exact test for categorical variables.
b For participants older than 20 years of age, BMI percentile values for age 20 years were applied.
TN-07 Study Cohort
The TN-07 study evaluated whether oral insulin could delay T1D onset in autoantibody-positive (Aab+) relatives of individuals with T1D. Subjects were initially screened for antibodies to microinsulin, glutamic acid decarboxylase, and insulinoma-associated antigen-2. ICA was measured if at least 1 of these antibodies was positive. Aab+ subjects (ie, at a minimum having tested positive for the microinsulin autoantibody on 2 sample collections) were required to have normal glucose tolerance at study entry. Subjects were stratified according to the FPIR threshold used in DPT-1 and randomized to receive either oral insulin or placebo. Study follow-up continued until T1D onset or study end on December 31, 2016. The median follow-up for our study cohort was 5.1 years (range, 0.0-11.5 years). Details about study eligibility, follow-up scheme, and results have been previously published (10).
Data were available from 306 Aab+ subjects who underwent paired OGTT and IVGTT within 30 days before randomization. Subjects who were randomized to oral insulin in secondary stratum 1, the stratum that showed significantly prolonged time to diabetes by oral insulin therapy, were excluded (n = 14) resulting in a total 292 subjects included for analyses.
DPT-1 Study Cohort
The DPT-1 study evaluated whether parenteral or oral insulin could delay the development of T1D. ICA and IAA-positive subjects were defined as having high-risk for T1D (5-year risk > 50%) if the OGTT was abnormal and/or the FPIR was below threshold. For subjects aged 3 to 7 years or parents of probands with T1D, the FPIR threshold was 60 μU/mL. For siblings or offspring aged 8 to 45 years or second-degree relatives aged 8 to 20 years, the threshold was 100 μU/mL. Aab+ subjects with FPIR above the threshold (ie, normal insulin secretion) and normal glucose tolerance were defined as having intermediate risk (5-year risk of 26%-50%). High-risk subjects were randomized to the parenteral insulin vs placebo trial and those with intermediate risk to the oral insulin vs placebo trial (9). Participants were followed until diabetes onset or study end (5-year follow-up). The median follow-up for our study cohort was 4.7 years (range, 0.0-7.2 years).
Data from 222 Aab+ subjects with paired IVGTT and OGTT performed within 30 days before randomization were available. In this cohort, subjects with confirmed IAA ≥ 80 nU/mL at baseline and who were then randomized to active drug treatment, that is, the group that showed a lower annualized diabetes rate compared with placebo in a post hoc analysis, were excluded (n = 28), resulting in a total of 194 subjects included for analyses.
Procedures
Intravenous and oral glucose tolerance tests
For IVGTTs, a solution of 25% dextrose (0.5 g/kg body weight up to a maximum of 35 g) was infused in fasting subjects over 3 minutes. Blood samples for determination of glucose and insulin levels were drawn at -10 and -4 minutes and at 1, 3, 5, 7, and 10 minutes after the glucose infusion. For both studies, FPIR was calculated as the sum of the plasma insulin values at the first and third minutes (11).
OGTTs were performed on a separate day and participants were advised to follow a diet containing at least 150 g of carbohydrates for at least 3 days before the test. Fasting participants ingested an oral glucose load of 1.75 g/kg (maximum, 75 g). In both TN-07 and DPT-1, blood samples were obtained for C-peptide and glucose, and in TN-07 samples for insulin were also collected. Samples were collected at the fasting timepoint and then at 30, 60, 90, and 120 minutes after ingestion of the oral glucose load.
We performed a broad approach evaluating several OGTT-derived variables commonly used to describe metabolic status, and selected 11 variables to compare against FPIR (Table 2). The peak C-peptide was defined as the maximum point of all measurements. Area under the curve (AUC) of C-peptide was calculated using the trapezoidal rule (12). The insulinogenic index was defined as the change in insulin from 0 to 30 minutes divided by the change in glucose from 0 to 30 minutes (ΔI0-30/ΔG0-30); similarly, the C-peptide index was defined as the change in C-peptide from 0 to 30 minutes divided by the change in glucose from 0 to 30 minutes (ΔC-pep0-30/ΔG0-30) (13). The oral disposition index was calculated as the product of the insulinogenic index and 1/fasting insulin ([ΔI0-30/ΔG0-30] × 1/fasting insulin) (14). Index60 was calculated as 0.3695 × (log fasting C-peptide [ng/mL]) + 0.0165 × (60-minute glucose [mg/dL]) – 0.364 × (60-minute C-peptide [ng/mL]) (15). The sum of glucose and sum of C-peptide were defined as the addition of all the glucose or C-peptide time points (fasting and post glucose load) during the OGTT.
Table 2.
Spearman correlation between FPIR and 11 OGTT variables in relatives at risk for T1D in the TN-07 study cohort
| Metabolic variables | N | Spearman correlation coefficient | 95% confidence limits | P valuea | |
|---|---|---|---|---|---|
| Insulinogenic Index | 292 | 0.605 | 0.527 | 0.673 | <0.0001 |
| C-peptide Index | 292 | 0.583 | 0.501 | 0.654 | <0.0001 |
| C-Peptide AUC | 292 | 0.537 | 0.450 | 0.614 | <0.0001 |
| Sum of C-peptide | 292 | 0.515 | 0.425 | 0.595 | <0.0001 |
| Fasting C-peptide | 292 | 0.505 | 0.414 | 0.586 | <0.0001 |
| C-peptide 30-0 min | 292 | 0.500 | 0.408 | 0.581 | <0.0001 |
| Peak of C-Peptide | 292 | 0.481 | 0.388 | 0.565 | <0.0001 |
| Index60 | 292 | -0.378 | -0.472 | -0.275 | <0.0001 |
| Oral Disposition Index | 292 | 0.209 | 0.096 | 0.316 | 0.0004 |
| Fasting glucose | 292 | 0.196 | 0.083 | 0.304 | 0.0008 |
| Sum of glucose | 292 | -0.142 | -0.253 | -0.028 | 0.0149 |
Abbreviations: AUC, area under the curve; FPIR, first-phase insulin response; OGTT, oral glucose tolerance test; T1D, type 1 diabetes; TN-07, Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D.”
a Adjusted P values using the Benjamini-Hochberg method to control for false-discovery rates.
Laboratory tests
Glucose was measured using the glucose oxidase method. In DPT-1, C-peptide was measured exclusively by a radioimmunoassay (RIA), whereas in TN-07, it was measured exclusively using a 2-site immunoenzymometric assay (Tosoh Bioscience, South San Francisco, CA) performed on a Tosoh II 600 autoanalyzer. The interassay coefficient of variation for the C-peptide RIA was 6.9% in a reference pool with relatively high values, and 7.8% in a reference pool with relatively low values. For the C-peptide Tosoh assay, the intra-assay coefficient of variation was 2.0% in a control sample with a mean C-peptide of 5.23 ± 0.11 ng/mL, and 2.8% in a control sample with a mean C-peptide of 1.71 ± 0.05 ng/mL. The interassay precision coefficient of variation was 3.1% in a control sample with a mean C-peptide of 5.06 ± 0.16 ng/mL, and 2.6% in a control sample with a mean C-peptide of 1.65 ± 0.04 ng/mL. In a previously reported analysis, C-peptide measurements by both RIA and Tosoh assays showed very good correlation (r = 0.961; TOSOH = 0.96 RIA + 0.1) (16).
Measurements of insulin levels for FPIR calculation were performed with an RIA in DPT-1, and with both RIA and the Tosoh assay in the TN-07 study. The interassay coefficient of variation for the insulin RIA used in DPT-1 and TN-07 was 4.5% in the high reference pool and 6.9% in the low reference pool. For the insulin Tosoh assay, the intra-assay coefficient of variation was 1.5% for a control sample with a mean insulin of 164.4 ± 2.44 µU/mL, 1.4% for a control sample with mean insulin of 92.2 ± 1.32 µU/mL, and 2.3% for a control sample with a mean insulin of 12.3 ± 0.28 µU/mL. The interassay precision coefficient of variation was 2.5% for a control sample with a mean insulin of 165.5 ± 4.15 µU/mL, 2.1% for a control sample with mean insulin of 93.0 ± 1.94 µU/mL, and 4.4% for a control sample with a mean insulin of 12.4 ± 0.54 µU/mL. Correlation of FPIR by RIA and Tosoh methods was high (r = 0.96, P < 0.0001; n = 292). For consistency between studies, we have used the FPIR obtained by the RIA method for our analyses because in DPT-1, only the RIA method was used.
Statistical Analysis
Nonparametric correlation analysis was performed to assess whether and how strongly pairs of variables were related. Spearman rank correlation coefficient and 95% CIs summarized the direction and strength of association between paired variables. Fisher Z-score tests were used to determine if correlations between paired variables were significantly different from 0. A Spearman rank correlation coefficient threshold ≥ 0.520 was set to select the OGTT variables more strongly correlated with FPIR. The overall prognostic performance of FPIR and OGTT variables, measured at baseline as continuous variables, in predicting progression to type 1 diabetes within 2 years was summarized by the area under the receiver operating characteristic (ROC) curve and its 95% CI. ROC curves were compared using the empirical (nonparametric) methods described by DeLong et al (17). Pairwise comparisons were adjusted using the Benjamini-Hochberg method, which controls the false-discovery rate. All P values were 2-sided, with values < 0.05 considered to be statistically significant. Analyses were performed with SAS version 9.4 (SAS Institute, Cary, NC) software.
Results
Baseline Participant Characteristics
In the TN-07 cohort, subjects were younger than in DPT-1, 9.4 ± 6.1 years vs 15.1 ± 10 years (P < 0.0001), had higher body mass index percentile 59.5 ± 28.6 vs 52.8 ± 31.0 (P = 0.0259), and the majority were male (60.6%) and White (96.4%). For those who progressed to stage 3 (clinical) T1D, median follow-up time was 5.1 years (interquartile range, 0.0-11.5) and 84 subjects (28.8%) developed T1D (Table 1). In the DPT-1 cohort, there were slightly more females (51.6%) and 91.7% were White. The median follow-up time to stage 3 T1D was 4.7 years (interquartile range, 0.0-7.2) and a total of 80 subjects (41.2%) developed T1D (Table 1).
Correlations between OGTT-derived variables and FPIR
Eleven OGTT variables commonly used to describe metabolic status were compared with FPIR.
In the TN-07 cohort, the highest correlation coefficients with FPIR were observed with the insulinogenic index (r = 0.605, P < 0.0001) and C-peptide index (r = 0.583, P < 0.0001), followed by the C-peptide AUC (r = 0.537, P < 0.0001) (Table 2). By contrast, the oral disposition index, the glucose sum, and fasting glucose showed poor correlation. We also found that the insulinogenic index highly correlated with the C-peptide index (r = 0.91; P < 0.0001).
We next evaluated these relationships in the DPT-1 cohort. Of note, insulin was not measured during OGTTs in DPT-1, which prevented the evaluation of the insulinogenic index. Notably, and in contrast with the TN-07 data, Index60 had the highest correlation to FPIR (r = -0.562, P < 0.0001), closely followed by the C-peptide index (r = 0.544, P < 0.0001) and the C-peptide 30-0 minutes (r = 0.534, P < 0.0001) (Table 3). Poorly correlated measures included fasting C-peptide, sum of glucose, and fasting glucose.
Table 3.
Spearman correlation between FPIR and 9 OGTT variables in relatives at risk for T1D in the DPT-1 study cohort
| Metabolic variablesa | N | Spearman correlation coefficient | 95% confidence limits | P valueb | |
|---|---|---|---|---|---|
| Index60 | 194 | -0.562 | -0.651 | -0.457 | < 0.0001 |
| C-peptide Index | 194 | 0.544 | 0.437 | 0.636 | < 0.0001 |
| C-peptide 30-0 min | 194 | 0.534 | 0.425 | 0.627 | < 0.0001 |
| C-Peptide AUC | 194 | 0.484 | 0.368 | 0.585 | < 0.0001 |
| Sum of C-peptide | 194 | 0.466 | 0.348 | 0.569 | < 0.0001 |
| Peak of C-peptide | 194 | 0.436 | 0.315 | 0.544 | < 0.0001 |
| Fasting C-peptide | 194 | 0.369 | 0.241 | 0.485 | < 0.0001 |
| Sum of glucose | 194 | -0.341 | -0.460 | -0.210 | < 0.0001 |
| Fasting glucose | 194 | 0.030 | -0.111 | 0.170 | 0.6758 |
Abbreviations: AUC, area under the curve; DPT-1, Diabetes Prevention Trial-Type 1 Diabetes; FPIR, first-phase insulin response; OGTT, oral glucose tolerance test; T1D, type 1 diabetes.
a Insulinogenic index and oral disposition index could not be calculated in DPT-1 because samples for insulin testing were not collected during OGTTs.
b Adjusted P values using the Benjamini-Hochberg method to control for false-discovery rates.
Prognostic accuracy of FPIR- and OGTT-derived measures for T1D development
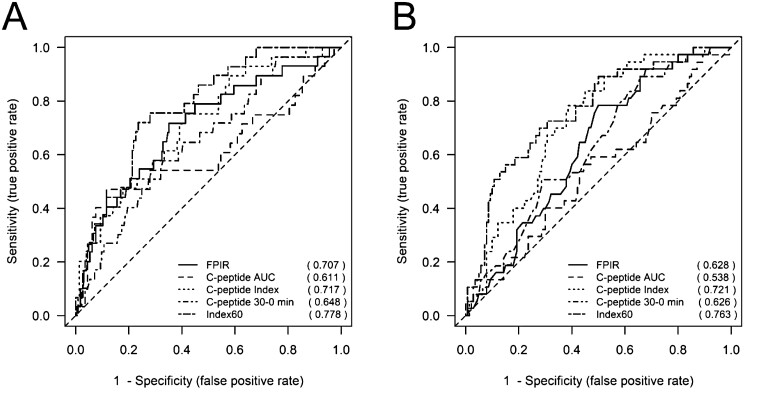
We next compared the prognostic accuracy of FPIR- and the OGTT-derived measures that were more strongly correlated to FPIR in each respective study cohort (ie, C-peptide index, C-peptide AUC, Index60, and C-peptide 30-0min) for the development of T1D within 2 years in both studies. This 2-year time frame was specifically selected for its importance in designing prevention protocols for subjects at high risk for T1D development. In the TN07 cohort at baseline, Index60 had the highest ROCAUC at 0.778 (95% CI, 0.698-0.858), followed by C-peptide index at 0.717 (95% CI, 0.621-0.813), and FPIR at 0.707 (95% CI, 0.602-0.812) (Table 4, Fig. 1A). Similarly, in the DPT-1 cohort, Index60 had the highest area under the ROC curve for a baseline measure at 0.763 (95% CI, 0.677-0.848), followed by C-peptide index at 0.721 (95% CI, 0.638-0.803), and FPIR at 0.628 (95% CI, 0.537-0.719) (Table 4, Fig. 1B).
Table 4.
AUC and 95% CIs for 2-y ROC curves for FPIR and OGTT-derived measures across studies
| TN-07 | DPT-1 | |||
|---|---|---|---|---|
| AUC | (95% CI) | AUC | (95% CI) | |
| FPIR | 0.707 | (0.602-0.812) | 0.628 | (0.537-0.719) |
| C-Peptide AUC | 0.611 | (0.479-0.742) | 0.538 | (0.432-0.643) |
| C-Peptide Index | 0.717 | (0.621-0.813) | 0.721 | (0.638-0.803) |
| C-Peptide 30-0 min | 0.648 | (0.546-0.751) | 0.626 | (0.532-0.721) |
| Index60 | 0.778 | (0.698-0.858) | 0.763 | (0.677-0.848) |
Abbreviations: AUC, area under the curve; DPT-1, Diabetes Prevention Trial-Type 1 Diabetes; FPIR, first-phase insulin response; OGTT, oral glucose tolerance test; TN-07, Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D.”
Figure 1.
Time-dependent ROC curves for T1D prediction over 2 years for FPIR and OGTT derived measures. (A) The TN-07 study (n = 292) and (B) the DPT-1 study (n = 194). In TN-07, no significant differences were noted in ROCAUC between FPIR and OGTT measures. In DPT-1, Index60 ROCAUC was significantly higher than FPIR ROCAUC (P = 0.0120).
We then compared the FPIR 2-year ROCAUC vs the OGTT-derived 2-year ROCAUC across both studies to determine if OGTT measures performed similarly or outperformed FPIR in its predictive accuracy. In TN-07, the OGTT-derived measures performed similarly to FPIR in (P = NS) (Tables 5). However, in DPT-1, the accuracy of Index60 to predict T1D within 2 years was significantly higher than FPIR (P = 0.012), whereas the other OGTT measures were not significantly different (Table 5).
Table 5.
Adjusted P values comparing FPIR 2-y AUC vs OGTT-derived measures 2-y AUC across studies
| TN-07a | DPT-1a | |
|---|---|---|
| FPIR vs | ||
| Index60 | 0.3493 | 0.0120 |
| C-Peptide Index | 0.8403 | 0.1402 |
| C-peptide AUC | 0.3380 | 0.1483 |
| C-Peptide 30-0 min | 0.3493 | 0.9731 |
Abbreviations: AUC, area under the curve; DPT-1, Diabetes Prevention Trial-Type 1 Diabetes; FPIR, first-phase insulin response; OGTT, oral glucose tolerance test; TN-07, Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D.”
a Adjusted P values using the Benjamini-Hochberg method to control for false-discovery rates.
Last, we compared the OGTT-derived measures 2-year ROCAUC against each other across both studies. Notably, Index60 and C-peptide index were not significantly different (P = NS), and both had higher prediction accuracy to detect T1D within 2 years compared with C-peptide 30-0 min and C-peptide AUC (Table 6).
Table 6.
Adjusted P value comparisons of 2-y AUC of OGTT-derived measures across studies
| TN-07a | DPT-1a | |
|---|---|---|
| C-Peptide Index vs Index60 | 0.3202 | 0.2025 |
| C-Peptide Index vs C-peptide 30-0 min | 0.1091 | 0.0026 |
| C-peptide Index vs C-peptide AUC | 0.0724 | 0.0002 |
| Index60 vs C-peptide 30-0 min | 0.0724 | 0.0017 |
| Index60 vs C-peptide AUC | 0.0724 | <0.0001 |
| C-peptide 30-0 min vs C-peptide AUC | 0.3202 | 0.0464 |
Abbreviations: AUC, area under the curve; DPT-1, Diabetes Prevention Trial-Type 1 Diabetes; OGTT, oral glucose tolerance test; TN-07, Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D.”
a Adjusted P values using the Benjamini-Hochberg method to control for false-discovery rates.
Discussion
To our knowledge, this is the first assessment of the relationship between FPIR- and OGTT-derived measures in Aab+ individuals at high genetic risk for T1D. In our current analysis, several measures showed a good correlation with FPIR within either the TN-07 or DPT-1 cohort but were not maintained in the other study cohort. On the other hand, the C-peptide index showed good correlation in both cohorts.
We have previously reported on the prognostic accuracy of individual OGTT-derived indices for T1D development in the DPT-1 study cohort and showed that the peak C-peptide, FPIR, and FPIR/homeostasis model assessment of insulin resistance provided modest but significant prognostic values for 5-year risk with a similar level of ROCAUC ranging between 0.60 and 0.70 (18). In our current study, we further expanded these observations with a particular focus on the ability of OGTT-derived indices to predict progression to stage 3 T1D within 2 years because this would help in identifying a more vulnerable population potentially justifying the use of immunomodulatory therapies in the context of prevention clinical trials. In both TN-07 and DPT-1, Index60 and C-peptide index demonstrated the highest baseline ROCAUCs for the development of T1D over a 2-year period and were comparable in their predictive accuracy (ROCAUC 0.778 vs 0.717 in TN-07 [P = 0.320]; and ROCAUC 0.763 vs 0.721 in DPT-1 [P = 0.203], respectively). Index60 was the only measure for which the ROCAUC was significantly higher than the FPIR in DPT-1. Although the Index60 ROCAUC was appreciably higher than the FPIR ROCAUC in TN07, the difference was not significant. The ROCAUC for the C-peptide index was comparable to that of the FPIR in TN-07 and DPT-1. It is important to note that subjects in DPT-1 were older (15.1 ± 10.0 vs 9.4 ± 6.1 years), comprised more females (51.6 vs 39.4%), and underwent paired metabolic tests within a shorter timeframe (15.7 ± 10.8 vs 21.3 ± 7.6 days) compared with TN-07. Another important distinction is that subjects in TN07 were required to have a normal OGTT, whereas subjects in DPT-1 could have abnormal OGTT results (below the threshold for diabetes diagnosis) at study entry. These factors, in addition to potential differences in the number of subjects going through puberty within each study cohort, may have accounted for differences in FPIR correlation with OGTT measures and T1D predictive accuracy between studies (19-21). Also, when interpreting our data, we need to consider that although both TN07 and DPT-1 included children and adults, most of the patients included in our study cohorts were children and therefore our results may not be generalizable to adults.
Because the OGTT-derived C-peptide index and Index60 were comparable or superior to FPIR as predictors of T1D, and OGTTs serve to metabolically stage T1D, the continued use of the FPIR in clinical research studies might be questioned. The elimination of IVGTTs would reduce the time and expense of large-scale screening, as well as inconvenience for participants. However, there are some caveats. Although the correlations of the FPIR with some OGTT measures were substantial, such correlations do not necessarily mean that those measures address the specific information about the early insulin response provided by the FPIR. Our analyses suggest that for purposes of predicting progression to T1D, the 30- or 60-minute time points used in calculating the C-peptide index or the Index60, respectively, contribute information related to diabetes risk. FPIR is measured in the first few minutes after stimulation and, thus, in studies where assessment of β-cell function is important, may provide information that cannot be obtained from the OGTT studies. Further, a frequently sampled intravenous glucose tolerance test allows for the measurement of insulin sensitivity and glucose effectiveness by use of the minimal model, an advantage over the OGTT (22). Thus, the FPIR may still be useful for some studies.
Other studies have evaluated the relationship between the FPIR- and OGTT-derived measures, although not in subjects at risk for T1D. In a study of children with normal glucose tolerance that examined the correlation between OGTT-derived and fasting-based indices of insulin secretion and the FPIR (calculated with the MINMOD computer program Millennium version 6.02, Richard N. Bergman, 2004) during a frequently sampled intravenous glucose tolerance test, the insulinogenic index was shown to be the variable with the highest correlation coefficient against FPIR (r = 0.80, P < 0.05) (23). Also, Mari et al evaluated adult subjects with glucose tolerance ranging from normal to overt type 2 diabetes and showed a strong correlation between the FPIR (calculated as the mean insulin concentration increment above the fasting value from 0 to 8 minutes after intravenous glucose administration with samples collected every minute) and the insulinogenic index in the entire dataset (r = 0.68, P < 0.001), whereas the relationship was not significant in the type 2 diabetes subgroup (r = 0.26, P = NS) (24). In our study, the insulinogenic index showed the strongest correlation to FPIR in TN-07 but could not be calculated in DPT-1. Nonetheless, considering its robust correlation with the C-peptide index, it is conceivable it may have also shown good correlation to FPIR in DPT-1 had insulin levels been measured. Of note, because we could not use the insulinogenic index in both studies, it was not included in our T1D prediction analyses.
It is not surprising that the earliest measures derived from an OGTT are the ones more closely correlated to FPIR because the latter is measured within the first 3 to 10 minutes of the IVGTT. Therefore, another important limitation of this study is that the earliest sample collected during the OGTT was at 30 minutes, a time-point that far exceeds the time at which FPIR occurs. Indeed, a study by Bacha et al comprising 26 healthy children (mean age, 9.9 ± 1.1 years), of which 13 were normal weight and 13 were overweight, showed that the C-peptide index calculated from OGTT samples obtained at 15 minutes was a better correlate of FPIR measured during a hyperglycemic clamp than the 30-minute C-peptide index (25). Thus, collection of earlier samples during the OGTT may allow for the calculation of measures with more robust correlations with the FPIR, but it is unclear whether such a strategy would provide superior ability to predict T1D. In addition, it is conceivable that an abbreviated OGTT (1-hour duration) may suffice for risk stratification and T1D prediction compared with a full OGTT in subjects at risk, although this approach would not allow diabetes staging.
In conclusion, the C-peptide index and Index60 derived from the OGTT performed at least comparably to the FPIR to predict progression to stage 3 T1D within 2 years in at-risk individuals. Thus, the IVGTT may not be necessary for subject stratification in T1D prevention trials.
Acknowledgments
The authors thank all TN-07 and DPT-1 study participants and their families.
Glossary
Abbreviations
- Aab+
autoantibody positive
- AUC
area under the curve
- DPT-1
Diabetes Prevention Trial-Type 1 Diabetes
- FPIR
first-phase insulin response
- IAA
insulin autoantibody
- ICA
islet-cell autoantibody
- IVGTT
intravenous glucose tolerance test
- OGTT
oral glucose tolerance test
- RIA
radioimmunoassay
- ROC
receiver operating curve
- T1D
type 1 diabetes
- TN-07
Type 1 Diabetes TrialNet “Oral Insulin for Prevention of Diabetes in Relatives at Risk for T1D
Contributor Information
David A Baidal, Department of Medicine and the Diabetes Research Institute, Leonard Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Megan Warnock, Data Analysis & Research, Office of Data Management & Information Systems, West Virginia Department of Education, Charleston, WV 25305, USA.
Ping Xu, Late Development Statistics, Biostatistics and Research Decision Sciences, Merck Research Laboratories, Rahway, NJ 07065-4607, USA.
Susan Geyer, Health Sciences Research, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester, MN 55905, USA.
Jennifer B Marks, Department of Medicine and the Diabetes Research Institute, Leonard Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Antoinette Moran, Department of Pediatrics, University of Minnesota, Minneapolis, MN 55454, USA.
Jay Sosenko, Department of Medicine and the Diabetes Research Institute, Leonard Miller School of Medicine, University of Miami, Miami, FL 33136, USA.
Carmella Evans-Molina, Departments of Medicine and Pediatrics and the Herman B Wells Center for Pediatric Research, Indiana University School of Medicine, Indianapolis, IN 46202, USA.
Financial Support
This work was funded by the National Institutes of Health (NIH) through the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institute of Allergy and Infectious Diseases, the Eunice Kennedy Shriver National Institute of Child Health and Human Development through cooperative agreements U01 DK061010, U01 DK061034, U01 DK061042, U01 DK061058, U01 DK085465, U01 DK085453, U01 DK085461, U01 DK085466, U01 DK085499, U01 DK085504, U01 DK085509, U01 DK103180, U01 DK103153, U01 DK085476, U01 DK103266, U01 DK103282, U01 DK106984, U01 DK106994, U01 DK107013, U01 DK107014, UC4 DK106993, UC4 DK117009-01, and the Juvenile Diabetes Research Foundation. The contents of this article are solely the responsibility of the authors and do not necessarily represent the official views of the NIH or the JDRF.
Disclosures
The authors have nothing to disclose.
Data Availability
TrialNet and DPT-1 data can be requested from the NIDDK public repository. The data sets generated and analyzed during the present study will be made available by request from the NIDDK Central Repository at https://repository.niddk.nih.gov/search/study/
References
- 1. Del Prato S, Tiengo A. The importance of first-phase insulin secretion: implications for the therapy of type 2 diabetes mellitus. Diabetes Metab Res Rev. 2001;17(3):164-174. [DOI] [PubMed] [Google Scholar]
- 2. Evans-Molina C, Sims EK, DiMeglio LA, et al. β Cell dysfunction exists more than 5 years before type 1 diabetes diagnosis. JCI Insight. 2018;3(15):e120877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Williams AJ, Long AE. Following the fate of the failing β-cell: new insights from first-phase insulin responses. Diabetes. 2013;62(12):3990-3992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sosenko JM, Skyler JS, Beam CA, et al. ; Type 1 Diabetes T, Diabetes Prevention Trial-Type 1 Study G. Acceleration of the loss of the first-phase insulin response during the progression to type 1 diabetes in diabetes prevention trial-type 1 participants. Diabetes. 2013;62(12):4179-4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vardi P, Crisa L, Jackson RA. Predictive value of intravenous glucose tolerance test insulin secretion less than or greater than the first percentile in islet cell antibody positive relatives of type 1 (insulin-dependent) diabetic patients. Diabetologia. 1991;34(2):93-102. [DOI] [PubMed] [Google Scholar]
- 6. Srikanta S, Ganda OP, Rabizadeh A, Soeldner JS, Eisenbarth GS. First-degree relatives of patients with type I diabetes mellitus. Islet-cell antibodies and abnormal insulin secretion. N Engl J Med. 1985;313(8):461-464. [DOI] [PubMed] [Google Scholar]
- 7. Chase HP, Voss MA, Butler-Simon N, Hoops S, O’Brien D, Dobersen MJ. Diagnosis of pre-type I diabetes. J Pediatr. 1987;111(6 Pt 1):807-812. [DOI] [PubMed] [Google Scholar]
- 8. Chase HP, Garg SK, Butler-Simon N, et al. Prediction of the course of pre-type I diabetes. J Pediatr. 1991;118(6):838-841. [DOI] [PubMed] [Google Scholar]
- 9. Diabetes Prevention Trial--Type 1 Diabetes Study G. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N Engl J Med. 2002;346(22):1685-1691. [DOI] [PubMed] [Google Scholar]
- 10. Krischer JP, Schatz DA, Bundy B, Skyler JS, Greenbaum CJ; Writing Committee for the Type 1 Diabetes TrialNet Oral Insulin Study Group. Effect of oral insulin on prevention of diabetes in relatives of patients with type 1 diabetes: a randomized clinical trial. JAMA. 2017;318(19):1891-1902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bingley PJ, Colman P, Eisenbarth GS, et al. Standardization of IVGTT to predict IDDM. Diabetes Care. 1992;15(10):1313-1316. [DOI] [PubMed] [Google Scholar]
- 12. Gabrielsson J, Weiner D. Non-compartmental analysis. In: Reisfeld B, Mayeno AN, Totowa, NJ, eds. Computational Toxicology: Volume I. Humana Press; 2012:377-389. [Google Scholar]
- 13. Tfayli H, Bacha F, Gungor N, Arslanian S. Islet cell antibody-positive versus -negative phenotypic type 2 diabetes in youth: does the oral glucose tolerance test distinguish between the two? Diabetes Care. 2010;33(3):632-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Utzschneider KM, Prigeon RL, Faulenbach MV, et al. Oral disposition index predicts the development of future diabetes above and beyond fasting and 2-h glucose levels. Diabetes Care. 2009;32(2):335-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sosenko JM, Skyler JS, DiMeglio LA, et al. ; Type 1 Diabetes TrialNet Study Group. A new approach for diagnosing type 1 diabetes in autoantibody-positive individuals based on prediction and natural history. Diabetes Care. 2015;38(2):271-276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sosenko JM, Mahon J, Rafkin L, et al. ; Diabetes Prevention Trial-Type 1 and TrialNet Study Groups. A comparison of the baseline metabolic profiles between Diabetes Prevention Trial-Type 1 and TrialNet Natural History Study participants. Pediatr Diabetes. 2011;12(2):85-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837-845. [PubMed] [Google Scholar]
- 18. Xu P, Beam CA, Cuthbertson D, Sosenko JM, Skyler JS, Krischer JP; DPT-1 Study Group. Prognostic accuracy of immunologic and metabolic markers for type 1 diabetes in a high-risk population: receiver operating characteristic analysis. Diabetes Care. 2012;35(10):1975-1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goran MI, Gower BA. Longitudinal study on pubertal insulin resistance. Diabetes. 2001;50(11):2444-2450. [DOI] [PubMed] [Google Scholar]
- 20. Ball GD, Huang TT, Gower BA, et al. Longitudinal changes in insulin sensitivity, insulin secretion, and beta-cell function during puberty. J Pediatr. 2006;148(1):16-22. [DOI] [PubMed] [Google Scholar]
- 21. Ma X, Becker D, Arena VC, Vicini P, Greenbaum C. The effect of age on insulin sensitivity and insulin secretion in first-degree relatives of type 1 diabetic patients: a population analysis. J Clin Endocrinol Metab. 2009;94(7):2446-2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bergman RN. Lilly lecture 1989. Toward physiological understanding of glucose tolerance. Minimal-model approach. Diabetes. 1989;38(12):1512-1527. [DOI] [PubMed] [Google Scholar]
- 23. Henderson M, Baillargeon JP, Rabasa-Lhoret R, Chiasson JL, Hanley J, Lambert M. Estimating insulin secretion in youth using simple indices derived from the oral glucose tolerance test. Diabetes Metab. 2012;38(4):309-315. [DOI] [PubMed] [Google Scholar]
- 24. Mari A, Tura A, Pacini G, Kautzky-Willer A, Ferrannini E. Relationships between insulin secretion after intravenous and oral glucose administration in subjects with glucose tolerance ranging from normal to overt diabetes. Diabet Med. 2008;25(6):671-677. [DOI] [PubMed] [Google Scholar]
- 25. Bacha F, Gungor N, Arslanian SA. Measures of beta-cell function during the oral glucose tolerance test, liquid mixed-meal test, and hyperglycemic clamp test. J Pediatr. 2008;152(5):618-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
TrialNet and DPT-1 data can be requested from the NIDDK public repository. The data sets generated and analyzed during the present study will be made available by request from the NIDDK Central Repository at https://repository.niddk.nih.gov/search/study/