Abstract
Context
Hyperprolactinemia suppresses gonadotropin-releasing hormone (GnRH)-induced luteinizing hormone (LH) pulses. The hypothalamic neuropeptide kisspeptin potently stimulates the secretion of GnRH. The effects of exogenous kisspeptin administration on GnRH pulse generation in the setting of hyperprolactinemia have not previously been explored.
Objective
This work aimed to examine the effects of kisspeptin on GnRH secretion, as reflected by LH secretion, in women with hyperprolactinemia.
Methods
Women with hyperprolactinemia (n = 11) participated in two 12-hour visits. Before study visits, participants underwent washout of dopamine agonist and/or combined oral contraceptive. Frequent blood sampling was performed (1 sample was collected every 10 minutes). Visit 1 involved no intervention, to examine baseline LH pulsatility. During visit 2, kisspeptin 112–121 (0.24 nmol/kg) was administered every 1 hour, for 10 hours. At hour 11, one intravenous bolus of GnRH (75 ng/kg) was administered.
Results
Repetitive intravenous bolus kisspeptin administration increased the total number of LH pulses in the setting of hyperprolactinemia. The interpulse interval declined during the same time frames. LH pulse amplitude did not change, but the mean LH rose. In 6 participants with progesterone levels suggestive of an anovulatory state, mean LH and estradiol levels increased significantly at visit 2. In the entire cohort, follicle-stimulating hormone and prolactin levels did not change significantly across the 2 visits. A total of 73% of subjects exhibited an LH pulse within 30 minutes of first kisspeptin dose.
Conclusion
Kisspeptin is capable of stimulating hypothalamic GnRH-induced LH pulses in the setting of hyperprolactinemia.
Keywords: kisspeptin, hyperprolactinemia
Pregnancy and lactation are the most common physiologic causes of hyperprolactinemia. Pituitary stalk compression, drug side effects, and pituitary adenomas are common pathophysiologic causes, with adenomas being particularly important to detect. Prolactinomas can lead to mechanical compression of local structures such as the optic nerves, leading to visual field disturbances and, in more severe cases, loss of vision. Therefore, the consequences of untreated prolactinomas can be severe.
Although excellent medical therapy (dopamine agonists; DAs) is available for patients with hyperprolactinemia, resistance to these drugs is becoming increasingly recognized. Resistance can occur in the setting of a macroprolactinoma (1), cavernous sinus invasion (2), certain gene mutations (3), male sex (2), with bromocriptine DA treatment (4) and less commonly with cabergoline treatment (2). Although DAs are usually well tolerated, an important safety consideration related to their use is pathological impulse control disorder (5), which may include gambling, compulsive shopping, hypersexuality, binge eating, or repetitive activities. Given the potentially serious consequences and the paucity of other treatment options, there is a medical need to find alternative therapies for patients with hyperprolactinemia.
Hyperprolactinemia inhibits the pulsatile release of gonadotropin-releasing hormone (GnRH), which in turn inhibits the release of both luteinizing hormone (LH) and follicle-stimulating hormone (FSH) from the pituitary (5-10). The hyperprolactinemia-induced reductions of both the frequency and amplitude of LH pulses result in suppression of ovarian and testicular function, leading to hypogonadism, erectile dysfunction, amenorrhea, decreased libido, and infertility. Pulsatile GnRH replacement can reverse the infertility induced by hyperprolactinemia (11, 12), suggesting that the principal mechanism of hyperprolactinemia-associated infertility is prolactin (PRL)-induced suppression of GnRH release. For many years, the pathomechanism underlying PRL-induced suppression of GnRH release was unknown, as only a few GnRH neurons express prolactin receptors (13-15), suggesting the existence of PRL-sensitive afferents.
Kisspeptin neurons are critical afferent regulators of GnRH neurons, providing direct synaptic input and powerful depolarizing action via their G-protein–coupled receptors. As gatekeepers of sexual maturation across mammalian species, kisspeptin’s role in reproduction was revealed by the discovery that loss-of-function mutations in the kisspeptin receptor cause GnRH deficiency in humans and mice (16-18). Kisspeptin’s importance is supported by its powerful stimulation of GnRH secretion in mice (19), rats (20), sheep (21), nonhuman primates (22), and humans (23), its ability to advance sexual maturation (20, 24), and the ability of a receptor antagonist to block ovulation and suppress LH (24, 25). Recent evidence suggests that a subset of kisspeptin neurons serve as the elusive “pulse generator” that regulates the pulsatile activities of GnRH neurons (26).
In female mice with hyperprolactinemia, kisspeptin administration was shown to restore GnRH and gonadotropin secretion and ovarian cyclicity (27). We sought to determine whether kisspeptin administration can restore GnRH-induced gonadotropin secretion in patients with hyperprolactinemia.
Materials and Methods
Participants
Individuals were recruited from postings (eg, ClinicalTrials.gov), advertisements (eg, Rally at Partners), and the Mass General Brigham patient population using the Partners Research Patient Data Registry. The study team contacted patients’ endocrinologists or primary care physicians to determine if study participation was appropriate. Potential participants were then contacted through their clinical provider by phone, email, letter, or in person. Individuals then contacted the study team for further information and completed a screening process to assess their eligibility. Screening laboratory tests were performed by LabCorp, MGH Laboratory Services, and Quest Diagnostics. Individuals with hyperprolactinemia due to a macroadenoma (≥ 10 mm) were excluded. This protocol was approved by the institutional review board of Mass General Brigham (formerly known as Partners Health Care) and the Food and Drug Administration. All participants gave written informed consent. The kisspeptin study was registered with ClinicalTrials.gov (NCT02956447).
Dopamine Agonist and Oral Contraceptive Pill Washout
Before admission to the Translational Clinical Research Center, if necessary, eligible individuals underwent DA (≥ 2 weeks) and combined oral contraceptive (COC; ≥ 8 weeks) washout.
Materials
Kisspeptin 112–121 and GnRH were synthesized using good manufacturing practices by PolyPeptide Laboratories in San Diego, California. Resuspended aliquots underwent additional tests for sterility, pyrogenicity, purity, and concentration.
Frequent Blood Sampling
Participants were admitted to the Translational Clinical Research Center of Massachusetts General Hospital on 2 separate occasions. Whenever possible, visits were scheduled on consecutive days to maintain comparable sex steroid levels between the 2 visits. During each admission, a blood sample was drawn every 10 minutes for 12 hours.
Neuropeptide Administration
During the second admission, kisspeptin was administered at a dose of 0.24 nmol/kg (0.313 μg/kg) intravenous bolus (IVB) hourly beginning at the 1-hour time point and concluding at the 10-hour time point, for a total of 10 IVB administrations. GnRH was administered at a dose of 75 ng/kg IVB at the 11-hour time point.
Laboratory Assays
Each sample was assayed for LH. For visit 1, FSH, PRL, and estradiol values were determined in 6 pooled samples representing a 120-minute interval. For visit 2, FSH, PRL, and estradiol values were also determined on pooled specimens before and during kisspeptin administration, as well as post GnRH administration, for a total of 7 pooled samples. One progesterone value was obtained for both visit 1 and visit 2 using the first time point. LH (Immulite 2000, limit of detection: 0.1 mIU/mL, Siemens, RRID: AB_2756388), FSH (Immulite 2000, limit of detection: 0.1 mIU/mL, Siemens, RRID: AB_2756389), PRL (Immulite 2000, limit of detection: 0.5 ng/mL; RRID: AB_2827375), and progesterone (Immulite 2000, limit of detection: 0.1 ng/mL; RRID: AB_2756392) assays were performed by the UVA Ligand Core Laboratory. The mean value of intra-assay and interassay coefficients generated by the Core annually across the years 2018 to 2019 is reported for assays run by UVA Ligand Core Laboratory. The mean intra-assay coefficient of variation for LH was less than 3.7% and its interassay coefficient of variation was less than 6.0%. The mean intra-assay coefficient of variation for FSH was less than 3.0% and its interassay coefficient of variation was less than 5.2%. The mean intra-assay coefficient of variation for PRL was less than 2.5% and its interassay coefficient of variation was less than 5.0%. The mean intra-assay coefficient of variation for progesterone was less than 4.2% and its interassay coefficient of variation was less than 6.7%. Estradiol assays were determined using liquid chromatography–mass spectrometry (LC/MS, limit of detection: 1 ng/mL, in-house methods) by the Brigham Research Assay Core (BRAC). The intra-assay coefficient of variation for estradiol was less than 5% and its interassay coefficient of variation was less than 12% as reported by the laboratory.
Pulse Identification and Calculated Pulse Characteristics
Pulse characteristics were limited to data obtained between hours 1 and 11, thereby not including pulses that occurred pre-kisspeptin or post-GnRH administration. LH pulses were identified using a modification of the Santen and Bardin criteria (28, 29). Interpulse intervals (IPIs) were calculated by determining the number of hours from the nadir of each LH pulse to the nadir of the next consecutive pulse (30). For visit 1, the mean of LH pulse amplitude was calculated by subtracting the nadir from the highest peak of the pulse as defined by using a modification of the Santen and Bardin criteria (29). For visit 2, the kisspeptin-induced LH pulse amplitude was determined by the LH values following only the first kisspeptin bolus, as the subsequent frequency of kisspeptin administration precluded LH levels from returning to baseline.
Statistical Analysis
Summary data was assessed for normal distribution by Shapiro-Wilk test. Two-tailed Pearson correlation analyses were used to assess significance of data with normal distribution. Two-tailed Spearman correlation analyses were used to assess significance of data that was not normally distributed. Paired, two-tailed t-tests were used to assess significance of data with normal distribution. Wilcoxon matched-pairs signed rank tests were used to assess data that was not normally distributed. Subset analyses (Fig. 6 and Fig. 7) of “anovulatory” and “ovulatory” groups were performed without correction, due to our interpretation that these analyses are not multiple comparisons but rather address distinct scientific questions from the analysis of the entire, combined cohort.
Figure 6.
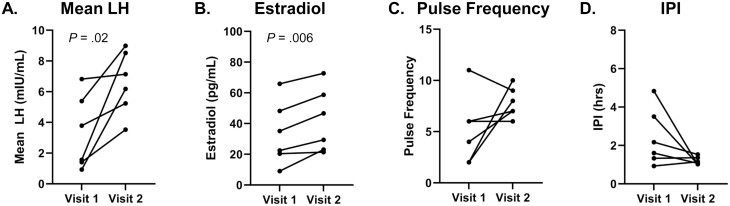
Hormone profiles of individuals with anovulatory progesterone levels (n = 6). For visit 1 and visit 2, analyses were restricted to time frame of kisspeptin administration (hour 1-hour 11), except for estradiol values from visit 1, for which all values were included (hour 0-hour 12). A, Mean LH; B, estradiol; C, pulse frequency; and D, inter-pulse interval (IPI). Statistically significant P-values are included in figure.
Figure 7.
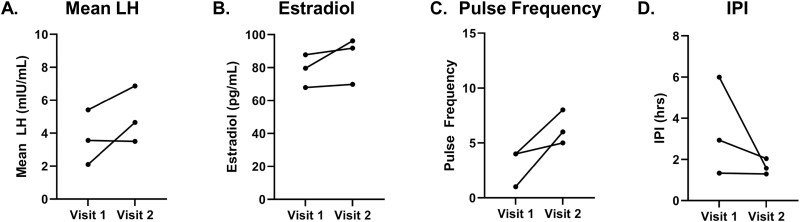
Hormone profiles of individuals with ovulatory progesterone levels (n = 3). These analyses were restricted to time frame of kisspeptin administration (hour 1-hour 11), except for estradiol values from visit 1, for which all values were included (hour 0-hour 12). A, Mean LH; B, estradiol; C, pulse frequency; and D, inter-pulse interval (IPI). Statistically significant P-values are included in figure.
Results
Characteristics of Participant Cohort at Initial Clinical Presentation With Hyperprolactinemia
Eleven women completed the study. The age at diagnosis of hyperprolactinemia ranged from 15 to 44 years, and the age at the first study visit ranged from 23 to 44 years. The mean PRL level at the time of clinical presentation was 77.4 ± 10.9 ng/mL, with a range of 32.0 to 147.2 ng/mL. Six of 11 individuals presented with amenorrhea, ranging in duration from 7 weeks to 8 years. Five of 11 participants presented with galactorrhea. Although these 2 clinical features are commonly associated with hyperprolactinemia, several study participants had 1 feature but not the other. Four patients had amenorrhea without galactorrhea and 2 patients had galactorrhea without menstrual abnormalities. However, on brain magnetic resonance imaging at clinical presentation, all 11 participants were found to have a pituitary lesion smaller than 10 mm (mean of largest dimension: 4.4 ± 0.8 mm; ranging from no discrete mass to 8 mm), consistent with a microadenoma (Tables 1 and 2).
Table 1.
Participant characteristics at clinical presentation with hyperprolactinemia
| ID | Age at diagnosis, y | Menstrual cycle | Galactorrhea | Microadenoma, mm | Prolactin, ng/mL |
|---|---|---|---|---|---|
| 1 | 22 | Amenorrhea × uns. mo | Nob,d | 5 | 32d |
| 2 | 15 | Irregular × every 60 d | Yes | 3 | 105.5d |
| 3 | 39 | Regular × every 3 wka | Yes | 5 | 78.1 |
| 4 | 44 | Amenorrhea × 1 y | Yes | 3 | 112.4 |
| 5 | 37 | Amenorrhea × 8 ya | No | 4 | 44.4 |
| 6 | 30 | Amenorrhea × 1 ya | No | 8 | 147.2 |
| 7 | 22 | Oligomenorrhea × 3 y | No | No discrete massc | 45.7 |
| 8 | 23 | DUB × 3 ya | No | 7 | 96.4 |
| 9 | 31 | Regular × every 4 wk | Yes | 4 | 56 |
| 10 | 22 | Amenorrhea × 7 wk | No | 1 | 46 |
| 11 | 26 | Amenorrhea × uns. moa | Yes | 8 | 88 |
Unless otherwise indicated, data were gathered from visit notes and medical records from initial presentation to Mass General Brigham Endocrinology Practices and at a time when individual was not yet taking any medication to treat hyperprolactinemia.
Abbreviations: DUB, dysfunctional uterine bleeding; ID, identification; uns., unspecified.
a Self-reported data from screening medical history.
b Gathered from visit note subsequent to initial clinical presentation.
c The amount 0 mm was used for calculation of mean.
d Individual was taking dopamine agonist medication at time of data point. Data point without dopamine agonist medication was not present in the medical records.
Table 2.
Individual characteristics at participation
| ID | Age at participation, y | Combined oral contraceptive washout, wk | Dopamine agonist, wk | Prolactin at screening, ng/mL | Prolactin at visit 1, ng/mL | Prolactin at visit 2, ng/mL | Progesterone at visit 1, ng/mL | Progesterone at visit 2, ng/mL |
|---|---|---|---|---|---|---|---|---|
| 1a | 40 | 9.7 | 2.0 | 19.4 | 28.4 | 29.8 | 2.09 | 3.15 |
| 2 | 38 | 7.7b | n/a | 61.2 | 24.6 | 34.3 | 0.20 | 11.3 |
| 3a | 41 | n/a | 2.1 | 41.5 | 51.6 | 44.5 | 4.94 | 0.40 |
| 4a | 44 | n/a | 2.6 | 40.5 | 57.8 | 54.9 | 2.92 | 1.15 |
| 5a | 39 | n/a | 3.6 | 21.5 | 34.9 | 35.3 | 6.09 | 6.58 |
| 6a | 36 | n/a | 14.7b,c | 331.5 | 150d | 150d | 0.25 | 0.29 |
| 7 | 23 | n/a | 17b,c | 37.5 | 34.1 | 35.0 | 0.39 | 0.45 |
| 8a | 24 | 10.3 | 6.6 | < 1.0 | 24.8 | 24.9 | 9.19 | 9.25 |
| 9 | 35 | n/a | n/a | 50.6 | 63.3 | 61.2 | 0.44 | 0.20 |
| 10a | 24 | n/a | n/a | 34.6 | 77.2 | 75.9 | 8.64 | 6.78 |
| 11a | 30 | 8.9 | n/a | 36.9 | 27.0 | 28.2 | 1.00 | 0.31 |
Data gathered from documentation in each participant’s study file. Age at participation was calculated using the date of visit 1. Length of washout was calculated by subtracting start date of washout from date of visit 1. Prolactin at visit 1 includes the mean value from all study pools from visit 1. Prolactin at visit 2 includes the mean value from study pools that were collected during kisspeptin administration (hour 1-hour 11).
Abbreviations: ID, identification; n/a, individuals who were not taking these types of medication and therefore did not undergo washout.
a Pulse occurred within 30 minutes of first kisspeptin administration.
b The last day of the month was used as the start date of washout as only the month was recorded for start date of washout.
c Participants began their washout before screening visit as part of their clinical care.
d This sample returned value above the limit of detection (upper range 150 ng/mL was recorded for this individual).
Characteristics of Participant Cohort After Medication Washout
Nine of 11 participants were treated with either a DA or a COC at the time of their participation (see Table 1). After medication washout (DA: > 2.0 weeks’ duration, COCs: > 7.7 weeks’ duration), the mean PRL level of the cohort was 52.2 ± 11.2 ng/mL during the baseline visit (“visit 1”), ranging from 24.6 to greater than 150 ng/mL (reportable range, 0.5-150 ng/mL) (see Table 2). The mean PRL level of the cohort was also 52.2 ± 10.9 ng/mL during the kisspeptin administration visit (“visit 2”), ranging from 24.9 to greater than 150 ng/mL (see Table 2). These mean values indicate that the 2 frequent sampling visits (in the presence or absence of kisspeptin) were performed in the setting of modest hyperprolactinemia.
Neuroendocrine Profile of Study Cohort at Baseline (Visit 1)
All 11 participants underwent frequent blood sampling (every 10 minutes) for 12 hours, to detect LH pulses, which serve as a well-validated surrogate measure of GnRH secretory pulses. During the baseline frequent sampling period (“visit 1”), the mean estradiol levels of individual patients ranged from 9 to 87.8 pg/mL. Using a threshold of 20 pg/mL, only one participant was frankly hypogonadal (with estradiol of 9.0 pg/mL).
Owing to the challenge inherent to recruiting patients during comparable phases of the menstrual cycle, particularly given the considerable cycle irregularities seen in hyperprolactinemia, the hormone milieu of the participant cohort at the time of visit 1 was heterogeneous. Five of the 11 participants had progesterone levels that were comparable between the 2 visits and that were below 3.0 ng/mL, suggesting that no ovulation had occurred within the prior 2 weeks (see Table 2). One additional participant had progesterone levels that were similar between the 2 visits, but that fell on either side of the 3.0 ng/mL boundary value (see Table 2). In 3 of the 11 participants, progesterone levels were also similar between visits but above 3.0 ng/mL, suggesting recent ovulation. Two patients had progesterone values that differed between the 2 visits, 1 of which indicated that ovulation likely had occurred between visits. Therefore, the hormone milieu of the research cohort at the time of the first frequent blood sampling session (visit 1) differed between 2 participant cohorts—1 that was comparable to what is observed in the early and mid-follicular phases of the menstrual cycle and another that was comparable to levels observed during the luteal phase (31). Analyses were initially performed on the entire 11-patient cohort. Analysis of participant subsets, defined by the aforementioned progesterone categories, were later performed and are presented at the end of this section.
During visit 1, mean serum LH ranged from 0.93 to 6.82 mIU/mL, with a cohort-level mean of 3.32 ± 0.60 mIU/mL (see Table 3). Between hour 1 and hour 11, the pulse frequency ranged from 1 to 11 pulses during the 10-hour period, with a cohort-level mean of 4.5 ± 0.9 pulses (see Table 3). The LH peak amplitude ranged from 1.13 to 24.25 mIU/mL, with a cohort-level mean of 4.60 ± 2.03 mIU/mL (see Table 3). The cohort-level mean FSH level of all participants was 4.6 ± 1.0 mIU/mL (see Table 3).
Table 3.
Summary of responses to kisspeptin 112 to 121 (0.24 nmol/kg)
| Pulse frequency, pulses | LH pulse amplitude, mIU/mL | Mean LH, mIU/mL | IPI, h | FSH across visitsb, mIU/mL | FSH first kisspeptin administrationc, mIU/mL | Estradiol, pg/mLd | Estradiol after DA washout, pg/mL | |
|---|---|---|---|---|---|---|---|---|
| Visit 1 mean | 4.5 ± 0.9 | 4.60 ± 2.03 | 3.32 ± 0.60 | 2.7 ± 0.5 | 4.6 ± 1.0 | 4.6 ± 1.0 | 48.8 ± 8.2 | 54.1 ± 9.5 |
| Visit 2 mean | 7.5 ± 0.5 | 3.11 ± 0.84a | 5.91 ± 0.65 | 1.3 ± 0.1 | 5.2 ± 0.9 | 4.6 ± 0.9 | 54.2 ± 9.1 | 53.7 ± 11.4 |
Summary of mean values for characteristics used to assess response to kisspeptin administration (hour 1-hour 11).
Abbreviations: DA, dopamine agonist; FSH, follicle-stimulating hormone; IPI, interpulse interval; LH, luteinizing hormone.
a Pulse amplitude value for Visit 2 only includes pulse amplitude value following first kisspeptin bolus.
b Visit 1 mean includes values across all baseline sampling time frame (hour 0—hour 12).
c Visit 1 mean includes values across all baseline sampling timepoints. Visit 2 includes values collected during the first 2 hours after kisspeptin administration.
d This column includes the entire cohort (n = 11). Visit 1 mean includes values across all baseline sampling time frame (hour 0-hour 12).
e This column includes individuals who underwent DA washout (n = 7). Visit 1 mean includes values across all baseline sampling time frame.
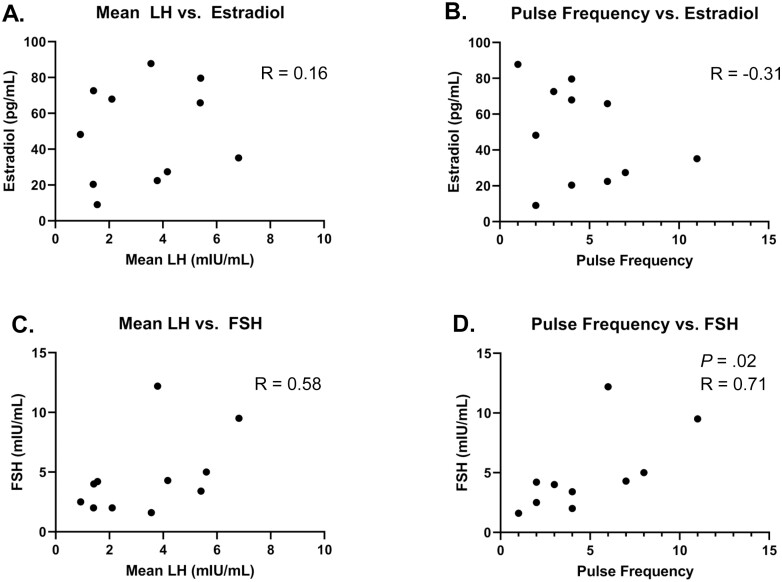
At the participant level, no correlation was observed between mean serum levels of LH and estradiol (see Fig. 1A), or between LH pulse frequency and estradiol (see Fig. 1B). Mean serum LH and FSH levels were also not correlated (see Fig. 1C). However, LH pulse frequency and FSH levels were positively correlated (P = .02; R = 0.68) (see Fig. 1D).
Figure 1.
Relationships among gonadotropins (luteinizing hormone [LH] and follicle-stimulating hormone [FSH]) and estradiol of individuals at visit 1 (n = 11). Mean LH and pulse frequency are calculated across hour 1 to hour 11. Values for FSH and estradiol span the entire sampling period of visit 1 (hour 0-hour 12). A, Mean LH vs estradiol; B, pulse frequency vs estradiol; C, mean LH vs FSH; D, pulse frequency vs FSH. Statistically significant P and R values are included in the figure.
Neuroendocrine Profile of Study Cohort During Kisspeptin Administration (Visit 2)
After completing this first frequent sampling session to establish baseline GnRH secretory patterns, participants were asked to return to complete a second 12-hour session of frequent blood sampling (“visit 2”). Nine of 11 individuals completed visit 2 the next consecutive day, and 2 patients completed visit 2 on a nonconsecutive day within the subsequent 14 days. At the 1-hour time point during visit 2, participants received an IVB of 0.24 nmol/kg (0.313 μg/kg) of the human kisspeptin decapeptide, followed by a bolus once every hour. At the 11-hour time point, participants received an IVB of 75 ng/kg of GnRH.
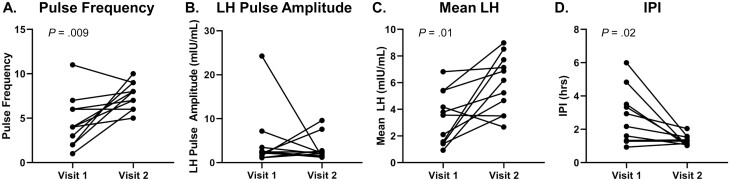
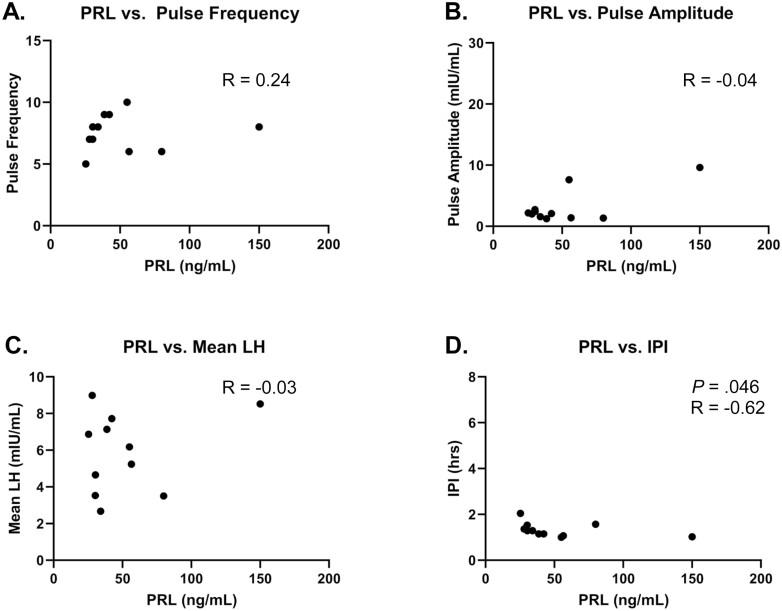
In 8 out of 11 participants, kisspeptin induced an immediate LH pulse (within 30 minutes of kisspeptin administration), regardless of the timing of the previous endogenous pulse. The following analyses (see Table 3 and Fig. 2) were conducted across the same time frame both at visit 1 and visit 2, which corresponds to the time frame when kisspeptin was administered (hour 1-hour 11). Kisspeptin stimulation during visit 2 resulted in a significant increase in the number of pulses compared to visit 1 (visit 1: 4.5 ± 0.9 pulses vs visit 2: 7.5 ± 0.5 pulses; P = .009). Kisspeptin stimulation also decreased the IPI (visit 1: 2.7 ± 0.5 hours vs visit 2: 1.3 ± 0.1 hours; P = .02). Using the LH pulse amplitude, as determined by the LH values following only the first kisspeptin bolus, the LH amplitude was similar between the 2 visits (visit 1: 4.60 ± 2.03 mIU/mL vs visit 2, first kisspeptin bolus: 3.11 ± 0.84 mIU/mL; P = .8). The kisspeptin-induced changes both to LH pulse amplitude and LH pulse frequency at visit 2 combine to increase the mean LH levels during kisspeptin administration, compared to the mean LH levels during visit 1 (visit 1: 3.32 ± 0.60 mIU/mL vs visit 2: 5.91 ± 0.65 mIU/mL; P = .01). At the individual level, no correlation was found between PRL levels at visit 2 and kisspeptin-influenced LH pulse frequency, LH pulse amplitude, or mean LH (see Fig. 3A-3C). Interestingly, an inverse correlation was observed between PRL levels at visit 2 and kisspeptin-influenced IPI (Fig. 3D), perhaps because IPI conveys information about the distribution of LH pulses across a period of time, which pulse frequency does not.
Figure 2.
Measures of luteinizing hormone (LH) pulsatility profile at visits 1 and 2 (n = 11). Pulse frequency, mean LH, and interpulse interval (IPI) were calculated across the time frame of kisspeptin administration (hour 1-hour 11). LH pulse amplitude for visit 2 includes value from response to first kisspeptin bolus only. One participant experienced only one pulse during visit 1. For this individual, hour 11 was used as the equivalent to the nadir of subsequent pulse for IPI calculations. A, pulse frequency; B, LH pulse amplitude; C, mean LH levels; and D, IPI. Statistically significant P values are included in the figure.
Figure 3.
Relationship between prolactin (PRL) levels of individuals at visit 2 and features of luteinizing hormone (LH) response to kisspeptin at visit 2 (n = 11). PRL level from visit 2 before kisspeptin administration (hour 0-hour 1) is included in this analysis. Pulse frequency, mean LH, and interpulse interval (IPI) were calculated across the time frame of kisspeptin administration (hour 1-hour 11). LH pulse amplitude from response to first kisspeptin bolus is included in this analysis. A, PRL vs pulse frequency; B, PRL vs LH pulse amplitude; C, PRL vs mean LH levels; and D, PRL vs IPI. Statistically significant P and R values are included in the figure.
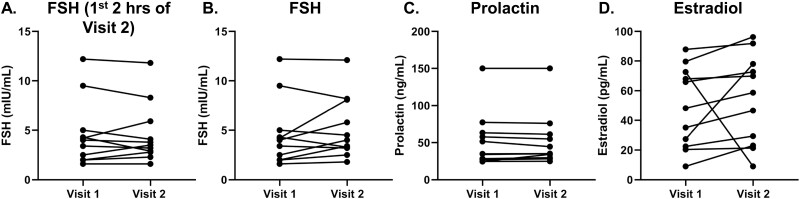
Although LH is a surrogate marker of GnRH secretion, FSH secretion is regulated by multiple other factors, including activin. During the first 2 hours of kisspeptin administration, FSH level did not change in comparison to visit 1 levels (visit 1: 4.6 ± 1.0 mIU/mL vs visit 2: 4.6 ± 0.9 mIU/mL; P = .8; see Table 3 and Fig. 4A). In addition, overall mean FSH levels did not differ between the 2 frequent sampling studies (see Table 3 and Fig. 4B, P = .3). Of note, each participant’s circulating prolactin levels were nearly identical at Visits 1 and 2 (Fig. 4C).
Figure 4.
Comparison of hormone levels between visits 1 and 2 (n = 11). Estradiol and prolactin for visit 1 were calculated as mean of all visit 1 time points (hour 0-hour 12). Follicle-stimulating hormone (FSH) values include all FSH levels during visit 1 baseline sampling (hour 0-hour 12) and FSH levels across kisspeptin administration during visit 2 (hour 1-hour 11). FSH First 2H of Kisspeptin administration includes all FSH levels during visit 1 baseline sampling and FSH across first 2 hours of kisspeptin administration during visit 2 (hour 1-hour 3). A, FSH visit 1 vs. visit 2 restricted to first 2Hrs of kisspeptin administration; B, FSH; C, prolactin; and D, estradiol.
Estradiol Levels
The estradiol levels of the entire cohort (all 11 participants) were similar between visits 1 and 2 (Visit 1: 48.8 ± 8.2 pg/mL vs visit 2: 54.2 ± 9.1 pg/mL; P = .5; see Table 3 and Fig. 4D)
Response to Exogenous Gonadotropin-releasing Hormone
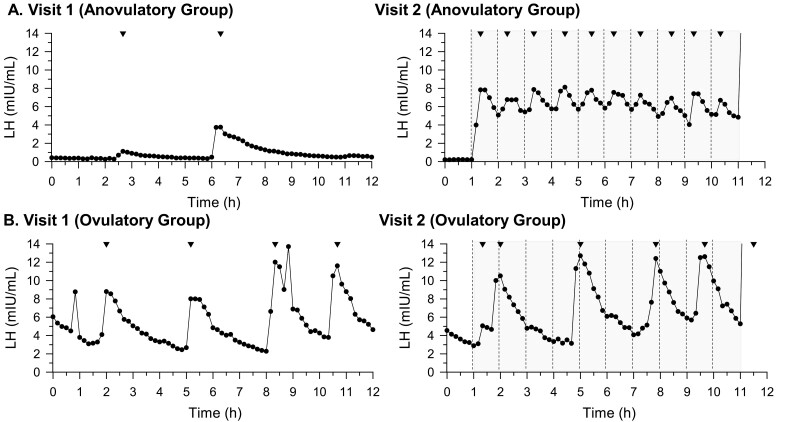
All participants exhibited robust LH responses to a single bolus of exogenous GnRH, demonstrating normal pituitary gonadotroph function (Fig. 5).
Figure 5.
Representative luteinizing hormone (LH) pulse profiles from participant subsets. A, This section shows results from a representative individual (participant 4) from subset with progesterone levels suggestive of anovulatory state (n = 6), with a suppressed hypothalamic-pituitary-gonadal (HPG) axis at visit 1, showing dampened LH pulsatility and hypogonadal state expected in the context of high prolactin levels; B, This section shows results from a representative individual (participant 8) from a subset with progesterone levels suggestive of ovulatory state (n = 3), with an HPG axis that was not fully suppressed. The shaded region represents the time frame of kisspeptin administration. Dashed lines represent kisspeptin bolus administration. Inverted triangles represent pulses as defined by modified Santen and Bardin method.
Subset Analysis
Nine of the 11 participants had comparable progesterone levels between their 2 frequent sampling visits, providing a unique opportunity to examine neuroendocrine responses in the setting of a constant sex steroid milieu. Five individuals had progesterone levels less than 3 ng/mL, suggesting that both visits occurred in an anovulatory context. A sixth participant was included in this first group because her progesterone levels were similar between her 2 visits, although her progesterone level at visit 2 was 3.15 ng/mL, slightly above the 3.0 ng/mL threshold. Three patients had progesterone levels greater than 3 ng/mL at each visit, suggesting they had recently ovulated. Within the anovulatory group, mean LH (visit 1: 3.32 ± 0.99 mIU/mL vs visit 2: 6.60 ± 0.84 mIU/mL; P = .02), and estradiol levels (visit 1: 33.5 ± 8.5 pg/mL vs visit 2: 42.0 ± 8.5 pg/mL; P = .006) showed a significant increase from visit 1 to visit 2 (Fig. 6A and 6B). LH pulse frequency and IPI for this anovulatory group did not change significantly across the subset, although 4 of 6 participants had a clear increase in pulse frequency and 4 of 6 individuals had a clear decrease in IPI (Fig. 6C and 6D). Within the ovulatory group (n = 3), mean LH, LH pulse frequency, and estradiol all trended upward from visit 1 to visit 2, and IPI trended downward, but these trends did not reach statistical significance (Fig. 7).
Discussion
Although kisspeptin’s ability to stimulate GnRH-induced LH secretion is well known, quantification of the neuroendocrine responses of individuals to exogenous kisspeptin has not been explored systematically, particularly in patients with reproductive disorders. This study demonstrates that exogenous kisspeptin administration can stimulate GnRH-induced LH pulses in women with hyperprolactinemia. The ability of kisspeptin to reactivate the gonadotropic axis in women with hyperprolactinemia supports the contention that the suppressive effects of PRL on the reproductive cascade are mediated through kisspeptin.
Repetitive bolus administration of kisspeptin at a physiologic dose (30, 32) and at a frequency of 1 bolus/hour increased mean LH levels and LH pulse frequency in the entire cohort (n = 11) of hyperprolactinemic women. Thus, this study extends the findings of a previous report of 2 women with hyperprolactinemia who received exogenous kisspeptin via infusion for 12 hours (33). As kisspeptin is released in a pulsatile manner (34), intermittent, repetitive kisspeptin administration was chosen as the method of administration in this study to mimic human physiology more closely.
In our study, LH frequency and mean LH rose, but LH pulse amplitude did not. Neither estradiol nor FSH levels changed in the cohort (n = 11) as a whole. However, in a subset of participants with progesterone levels that suggested no recent ovulation (n = 6), estradiol increased significantly in the presence of kisspeptin (visit 2). This phenomenon indicates kisspeptin influences sex steroid levels in female patients with hyperprolactinemia in an anovulatory state.
As noted earlier, hyperprolactinemia often results in hypogonadotropic hypogonadism both in males and females. Initially, prolactin was thought to suppress LH secretion through suppression of GnRH release, although few GnRH neurons contain prolactin receptors (13). However, the localization of PRL receptors on kisspeptin neurons suggested that kisspeptin might mediate the inhibitory effects of hyperprolactinemia on GnRH secretion (13, 14). In support of this hypothesis, PRL infusions have been shown to reduce the kisspeptin and GnRH expression, resulting in anovulation (27). In addition, during lactation, elevated PRL inhibits kisspeptin expression in the arcuate nucleus (35). Finally, conditional knockout of PRL receptors specifically in arcuate kisspeptin cells has been shown to be sufficient to prevent PRL’s suppression of pulsatile LH secretion (36).
Despite these insights, very little is known about the effect of hyperprolactinemia-based reductions in arcuate kisspeptin expression on the expression of the kisspeptin receptor on GnRH neurons themselves. In this study, the very first kisspeptin bolus stimulated a GnRH-induced LH response in women with hyperprolactinemia. This stands in contrast to patients with idiopathic hypogonadotropic hypogonadism (IHH), in which the same weight-based dose of IV kisspeptin failed to stimulate an LH response (37). Moreover, the mean LH amplitude in response to the first kisspeptin IVB in hyperprolactinemic women was comparable to that observed in normoprolactinemic female volunteers in the midluteal phase (32). While the reason between the differences in the responsiveness between an acquired form of hypogonadotropism (hyperprolactinemia) and a congenital form of hypogonadotropism (IHH) is not clear, this study suggests that the GnRH neurons in hyperprolactinemic women exist in a greater state of “functional readiness,” compared to IHH patients.
After all 7 relevant participants completed medication washout, in the setting of their ambient hyperprolactinemia, the full 11-patient cohort had a mean estradiol level of (48.8 ± 8.2 pg/mL; see Table 3). This value is comparable to the estradiol levels observed in healthy volunteers in the early follicular phase of the menstrual cycle undergoing a similar protocol (35 ± 3 pg/mL) (32). However, the research participants in this study appeared to respond to kisspeptin more robustly than healthy volunteers in the early follicular phase who received the same weight-based dose. A total of 73% of these individuals exhibited an LH pulse within 30 minutes of the first kisspeptin dose, compared to 50% of healthy volunteers in the follicular phase with a higher mean LH amplitude (see Table 2). Further studies will be necessary to refine the relationship between prevailing estradiol levels and kisspeptin responsiveness.
Although PRL suppresses kisspeptin expression in hyperprolactinemic states, the relationship between the 2 proteins has not been fully elucidated in the normoprolactinemic state. It is theoretically possible that kisspeptin administration itself could increase serum PRL levels. Depending on the species and the model, kisspeptin’s role in PRL’s regulation varies. Kisspeptin has been reported not to affect circulating levels of PRL in adult rats (38), ewes (39), rhesus monkeys (40), or healthy women (41). However, kisspeptin does increase PRL in bovine (42) and fish (43) pituitary cell cultures. Moreover, intracerebral kisspeptin administration has been shown to increase PRL release in estradiol-treated ovariectomized rats (44). In this study, no statistically significant difference in circulating levels of PRL was identified between the 2 frequent sampling admissions (see Fig. 4C).
One of the strengths of this study is the homogeneity of the study population—participants shared numerous biochemical and clinical features. At presentation and at the time of study enrollment, all participants had PRL levels less than 200 ng/mL (with exception of 1 individual whose PRL at visit 1 was above the detectable limit of 150 ng/mL and thus not definable). Brain magnetic resonance imaging scans revealed either pituitary microadenomas (< 10 mm) or normal pituitary glands without any visible lesion.
One of the challenges of this study was limiting the disruption of study participants’ clinical care, and by extension, the length of time that study individuals were off their medications (either DAs or COCs). After patients completed their medication washouts, they performed their frequent sampling, both without and with kisspeptin administration, as quickly as possible, in effort to balance scientific objectives with expediency (specifically, limiting study participants’ time off medication). Thus, it was not possible to perform repeated neuroendocrine assessments, particularly once patients “achieved” hyperprolactinemia in the absence of their DA therapy. In addition, neuroendocrine studies of the hypothalamic-pituitary-gonadal axis in women are frequently anchored to the early follicular phase of the menstrual cycle as a reference point. Such anchoring was not possible in this study. Although most patients were anovulatory after medication washout (as evidenced by progesterone levels < 3 ng/mL), 2 of 11 patients demonstrated discordant progesterone levels between the first and second admission. Therefore, 18% of study individuals did not have the same sex hormone milieu in both of their frequent sampling studies.
In summary, intermittent administration of exogenous kisspeptin is able to restore GnRH-induced LH pulses in this proof-of-concept study of women with hyperprolactinemia. Thus, the functional capacity of the GnRH neuronal network appears to be intact in women with this condition. The robust response to exogenous kisspeptin suggests that endogenous kisspeptin tone is relatively low in patients with excess PRL. These observations differ from those observed in individuals with congenital hypogonadotropic hypogonadism, who, by and large, are unable to respond to the same weight-based dose of kisspeptin. The reversal of hyperprolactinemia-associated GnRH suppression by kisspeptin is of clinical relevance in the potential management of hypogonadism in patients with hyperprolactinemia, particularly in those who have contraindications to estrogen or DAs, those in whom DAs are either not tolerated or not effective, those who cannot discontinue medications that raise PRL, and those who seek fertility. Overcoming GnRH inhibition through kisspeptin administration could potentially address an important unmet need in such patients.
Acknowledgments
We thank the research participants, members of the Massachusetts General Hospital Reproductive Endocrine Unit for discussions and reading of the manuscript, staff of the Harvard Catalyst Clinical Research Center for assistance with the frequent sampling studies, the Massachusetts General Hospital Investigational Drug Service (Clinical Trials Pharmacy), and the Massachusetts General Hospital Clinical Laboratory Research Core. We thank Alexander Faje, MD, for assistance in recruitment of research participants. We thank Isabella McDonald for contributions to the collection and analysis of data. We thank Andrew Dwyer, PhD, FNP-BC, FNAP, FAAN, for assistance with applying relevant criteria and technique to pulse analysis.
Glossary
Abbreviations
- COC
combined oral contraceptive
- DA
dopamine agonist
- FSH
follicle-stimulating hormone
- GnRH
gonadotropin-releasing hormone
- IHH
idiopathic hypogonadotropic hypogonadism
- IPI
interpulse interval
- IV
intravenous
- IVB
intravenous bolus
- LH
luteinizing hormone
- PRL
prolactin
Contributor Information
Katerina Hoskova, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Nora Kayton Bryant, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Margaret E Chen, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Lisa B Nachtigall, Neuroendocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Margaret F Lippincott, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Ravikumar Balasubramanian, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Stephanie B Seminara, Reproductive Endocrine Unit, Department of Medicine, Massachusetts General Hospital, Boston, Massachusetts 02114, USA.
Financial Support
This work was supported by the Eunice K. Shriver National Institute for Child Health and Human Development (grant No. R37 HD043341-19), the US Food and Drug Administration (grant No. R01 FD005712-04), and the Harvard Catalyst/Harvard Clinical and Translational Science Center (National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health Awards Nos. 1UL1TR002541-01 and 1UL1TR001102). S.B.S. is supported by the Eunice K. Shriver National Institute for Child Health and Human Development (grant No. P50HD104224-01). M.F.L. is supported by the Eunice K. Shriver National Institute for Child Health and Human Development (grant No. K23HD097296-03). The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University, the National Center for Research Resources, the National Center for Advancing Translational Science, or the National Institutes of Health.
Author Contributions
Designing research studies: M.F.L., L.N., and S.B.S.; conducting experiments: K.H., M.C., and M.F.L.; acquiring data: K.H., M.C., and M.F.L.; analyzing data: K.H., N.K.B., M.C., M.F.L., and S.B.S.; writing the manuscript: K.H., N.K.B., R.B., and S.B.S.
Disclosures
The authors have nothing to disclose.
Clinical Trial Information
ClinicalTrials.gov number NCT02956447 (registered November 6, 2016).
Data Availability
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”
References
- 1. Vroonen L, Jaffrain-Rea ML, Petrossians P, et al. Prolactinomas resistant to standard doses of cabergoline: a multicenter study of 92 patients. Eur J Endocrinol. 2012;167(5):651-662. [DOI] [PubMed] [Google Scholar]
- 2. Delgrange E, Daems T, Verhelst J, Abs R, Maiter D. Characterization of resistance to the prolactin-lowering effects of cabergoline in macroprolactinomas: a study in 122 patients. Eur J Endocrinol. 2009;160(5):747-752. [DOI] [PubMed] [Google Scholar]
- 3. Vergès B, Boureille F, Goudet P, et al. Pituitary disease in MEN type 1 (MEN1): data from the France-Belgium MEN1 multicenter study. J Clin Endocrinol Metab. 2002;87(2):457-465. [DOI] [PubMed] [Google Scholar]
- 4. Molitch ME. Pharmacologic resistance in prolactinoma patients. Pituitary. 2005;8(1):43-52. [DOI] [PubMed] [Google Scholar]
- 5. Weintraub D, David AS, Evans AH, Grant JE, Stacy M. Clinical spectrum of impulse control disorders in Parkinson’s disease. Mov Disord. 2015;30(2):121-127. [DOI] [PubMed] [Google Scholar]
- 6. Cohen-Becker IR, Selmanoff M, Wise PM. Inhibitory effects of exogenously induced hyperprolactinemia on the endogenous cyclic release of luteinizing hormone and prolactin in the estrogen-primed ovariectomized rat. Endocrinology. 1986;119(4):1718-1725. [DOI] [PubMed] [Google Scholar]
- 7. Fox SR, Hoefer MT, Bartke A, Smith MS. Suppression of pulsatile LH secretion, pituitary GnRH receptor content and pituitary responsiveness to GnRH by hyperprolactinemia in the male rat. Neuroendocrinology. 1987;46(4):350-359. [DOI] [PubMed] [Google Scholar]
- 8. Matsuzaki T, Azuma K, Irahara M, Yasui T, Aono T. Mechanism of anovulation in hyperprolactinemic amenorrhea determined by pulsatile gonadotropin-releasing hormone injection combined with human chorionic gonadotropin. Fertil Steril. 1994;62(6):1143-1149. [DOI] [PubMed] [Google Scholar]
- 9. Ordög T, Chen MD, O’Byrne KT, et al. On the mechanism of lactational anovulation in the rhesus monkey. Am J Physiol. 1998;274(4):E665-E676. [DOI] [PubMed] [Google Scholar]
- 10. Klibanski A, Beitins IZ, Zervas NT, McArthur JW, Ridgway EC. alpha-Subunit and gonadotropin responses to luteinizing hormone-releasing hormone in hyperprolactinemic women before and after bromocriptine. J Clin Endocrinol Metab. 1983;56(4):774-780. [DOI] [PubMed] [Google Scholar]
- 11. Polson DW, Sagle M, Mason HD, Adams J, Jacobs HS, Franks S. Ovulation and normal luteal function during LHRH treatment of women with hyperprolactinaemic amenorrhoea. Clin Endocrinol (Oxf). 1986;24(5):531-537. [DOI] [PubMed] [Google Scholar]
- 12. Lecomte P, Lecomte C, Lansac J, Gallier J, Sonier CB, Simonetta C. Pregnancy after intravenous pulsatile gonadotropin-releasing hormone in a hyperprolactinaemic woman resistant to treatment with dopamine agonists. Eur J Obstet Gynecol Reprod Biol. 1997;74(2):219-221. [DOI] [PubMed] [Google Scholar]
- 13. Grattan DR, Jasoni CL, Liu X, Anderson GM, Herbison AE. Prolactin regulation of gonadotropin-releasing hormone neurons to suppress luteinizing hormone secretion in mice. Endocrinology. 2007;148(9):4344-4351. [DOI] [PubMed] [Google Scholar]
- 14. Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152(2):526-535. [DOI] [PubMed] [Google Scholar]
- 15. Brown RSE, Piet R, Herbison AE, Grattan DR. Differential actions of prolactin on electrical activity and intracellular signal transduction in hypothalamic neurons. Endocrinology. 2012;153(5): 2375-2384. [DOI] [PubMed] [Google Scholar]
- 16. de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proc Natl Acad Sci U S A. 2003;100(19):10972-10976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seminara SB, Messager S, Chatzidaki EE, et al. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349(17):1614-1627. [DOI] [PubMed] [Google Scholar]
- 18. Topaloglu AK, Tello JA, Kotan LD, et al. Inactivating KISS1 mutation and hypogonadotropic hypogonadism. N Engl J Med. 2012;366(7):629-635. [DOI] [PubMed] [Google Scholar]
- 19. Gottsch ML, Cunningham MJ, Smith JT, et al. A role for kisspeptins in the regulation of gonadotropin secretion in the mouse. Endocrinology. 2004;145(9):4073-4077. [DOI] [PubMed] [Google Scholar]
- 20. Matsui H, Takatsu Y, Kumano S, Matsumoto H, Ohtaki T. Peripheral administration of metastin induces marked gonadotropin release and ovulation in the rat. Biochem Biophys Res Commun. 2004;320(2):383-388. [DOI] [PubMed] [Google Scholar]
- 21. Caraty A, Smith JT, Lomet D, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148(11):5258-5267. [DOI] [PubMed] [Google Scholar]
- 22. Shahab M, Mastronardi C, Seminara SB, Crowley WF, Ojeda SR, Plant TM. Increased hypothalamic GPR54 signaling: a potential mechanism for initiation of puberty in primates. Proc Natl Acad Sci U S A. 2005;102(6):2129-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dhillo WS, Chaudhri OB, Patterson M, et al. Kisspeptin-54 stimulates the hypothalamic-pituitary gonadal axis in human males. J Clin Endocrinol Metab. 2005;90(12):6609-6615. [DOI] [PubMed] [Google Scholar]
- 24. Kinoshita M, Tsukamura H, Adachi S, et al. Involvement of central metastin in the regulation of preovulatory luteinizing hormone surge and estrous cyclicity in female rats. Endocrinology. 2005;146(10):4431-4436. [DOI] [PubMed] [Google Scholar]
- 25. Roseweir AK, Kauffman AS, Smith JT, et al. Discovery of potent kisspeptin antagonists delineate physiological mechanisms of gonadotropin regulation. J Neurosci. 2009;29(12):3920-3929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clarkson J, Han SY, Piet R, et al. Definition of the hypothalamic GnRH pulse generator in mice. Proc Natl Acad Sci U S A. 2017;114(47):E10216-E10e23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sonigo C, Bouilly J, Carré N, et al. Hyperprolactinemia-induced ovarian acyclicity is reversed by kisspeptin administration. J Clin Invest. 2012;122(10):3791-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spratt DI, Carr DB, Merriam GR, Scully RE, Rao PN, Crowley WF Jr. The spectrum of abnormal patterns of gonadotropin-releasing hormone secretion in men with idiopathic hypogonadotropic hypogonadism: clinical and laboratory correlations. J Clin Endocrinol Metab. 1987;64(2):283-291. [DOI] [PubMed] [Google Scholar]
- 29. Santen RJ, Bardin CW. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest. 1973;52(10):2617-2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chan YM, Butler JP, Pinnell NE, et al. Kisspeptin resets the hypothalamic GnRH clock in men. J Clin Endocrinol Metab. 2011;96(6):E908-E915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Filicori M, Santoro N, Merriam GR, Crowley WF Jr. Characterization of the physiological pattern of episodic gonadotropin secretion throughout the human menstrual cycle. J Clin Endocrinol Metab. 1986;62(6):1136-1144. [DOI] [PubMed] [Google Scholar]
- 32. Chan YM, Butler JP, Sidhoum VF, Pinnell NE, Seminara SB. Kisspeptin administration to women: a window into endogenous kisspeptin secretion and GnRH responsiveness across the menstrual cycle. J Clin Endocrinol Metab. 2012;97(8):E1458-E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Millar RP, Sonigo C, Anderson RA, et al. Hypothalamic-pituitary-ovarian axis reactivation by kisspeptin-10 in hyperprolactinemic women with chronic amenorrhea. J Endocr Soc. 2017;1(11):1362-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology. 2008;149(8):4151-4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Araujo-Lopes R, Crampton JR, Aquino NS, et al. Prolactin regulates kisspeptin neurons in the arcuate nucleus to suppress LH secretion in female rats. Endocrinology. 2014;155(3):1010-1020. [DOI] [PubMed] [Google Scholar]
- 36. Brown RSE, Khant Aung Z, Phillipps HR, et al. Acute suppression of LH secretion by prolactin in female mice is mediated by kisspeptin neurons in the arcuate nucleus. Endocrinology. 2019;160(5):1323-1332. [DOI] [PubMed] [Google Scholar]
- 37. Chan YM, Lippincott MF, Butler JP, et al. Exogenous kisspeptin administration as a probe of GnRH neuronal function in patients with idiopathic hypogonadotropic hypogonadism. J Clin Endocrinol Metab. 2014;99(12):E2762-E2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Navarro VM, Castellano JM, Fernández-Fernández R, et al. Characterization of the potent luteinizing hormone-releasing activity of KiSS-1 peptide, the natural ligand of GPR54. Endocrinology. 2005;146(1):156-163. [DOI] [PubMed] [Google Scholar]
- 39. Smith JT, Saleh SNH, Clarke IJ. Seasonal and cyclical change in the luteinizing hormone response to kisspeptin in the ewe. Neuroendocrinology. 2009;90(3):283-291. [DOI] [PubMed] [Google Scholar]
- 40. Plant TM, Ramaswamy S, Bhat GK, Stah CD, Pohl CR, Mann DR. Effect of transient hypothyroidism during infancy on the postnatal ontogeny of luteinising hormone release in the agonadal male rhesus monkey (Macaca mulatta): implications for the timing of puberty in higher primates. J Neuroendocrinol. 2008;20(10):1203-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jayasena CN, Comninos AN, Narayanaswamy S, et al. Acute and chronic effects of kisspeptin-54 administration on GH, prolactin and TSH secretion in healthy women. Clin Endocrinol (Oxf). 2014;81(6):891-898. [DOI] [PubMed] [Google Scholar]
- 42. Kadokawa H, Suzuki S, Hashizume T. Kisspeptin-10 stimulates the secretion of growth hormone and prolactin directly from cultured bovine anterior pituitary cells. Anim Reprod Sci. 2008;105(3-4):404-408. [DOI] [PubMed] [Google Scholar]
- 43. Yang B, Jiang Q, Chan T, Ko WK, Wong AO. Goldfish kisspeptin: molecular cloning, tissue distribution of transcript expression, and stimulatory effects on prolactin, growth hormone and luteinizing hormone secretion and gene expression via direct actions at the pituitary level. Gen Comp Endocrinol. 2010;165(1):60-71. [DOI] [PubMed] [Google Scholar]
- 44. Szawka RE, Ribeiro AB, Leite CM, et al. Kisspeptin regulates prolactin release through hypothalamic dopaminergic neurons. Endocrinology. 2010;151(7):3247-3257. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Some or all data generated or analyzed during this study are included in this published article or in the data repositories listed in “References.”