Abstract
Context
Novel fasting interventions have gained scientific and public attention. Periodic fasting has emerged as a dietary modification promoting beneficial effects on metabolic syndrome.
Objective
Assess whether periodic fasting reduces albuminuria and activates nephropathy-driven pathways.
Design/Participants
Proof-of-concept study where individuals with type 2 diabetes (n = 40) and increased albumin-to-creatinine ratio (ACR) were randomly assigned to receive a monthly fasting-mimicking diet (FMD) or a Mediterranean diet for 6 months with 3-month follow-up.
Main Outcomes Measures
Change in ACR was assessed by analysis of covariance adjusted for age, sex, weight loss, and baseline value. Prespecified subgroup analysis for patients with micro- vs macroalbuminuria at baseline was performed. Change in homeostatic model assessment for insulin resistance (HOMA-IR), circulating markers of dicarbonyl detoxification (methylglyoxal-derived hydroimidazolone 1, glyoxalase-1, and hydroxyacetone), DNA-damage/repair (phosphorylated histone H2AX), lipid oxidation (acylcarnitines), and senescence (soluble urokinase plasminogen activator receptor) were assessed as exploratory endpoints.
Results
FMD was well tolerated with 71% to 95% of the participants reporting no adverse effects. After 6 months, change in ACR was comparable between study groups [110.3 (99.2, 121.5) mg/g; P = 0.45]. FMD led to a reduction of ACR in patients with microalbuminuria levels at baseline [−30.3 (−35.7, −24.9) mg/g; P ≤ 0.05] but not in those with macroalbuminuria [434.0 (404.7, 463.4) mg/g; P = 0.23]. FMD reduced HOMA-IR [−3.8 (−5.6, −2.0); P ≤ 0.05] and soluble urokinase plasminogen activator receptor [−156.6 (−172.9, −140.4) pg/mL; P ≤ 0.05], while no change was observed in markers of dicarbonyl detoxification or DNA-damage/repair. Change in acylcarnitines was related to patient responsiveness to ACR improvement. At follow-up only HOMA-IR reduction [−1.9 (−3.7, −0.1), P ≤ 0.05]) was sustained.
Conclusions
Improvement of microalbuminuria and of markers of insulin resistance, lipid oxidation, and senescence suggest the potential beneficial effects of periodic fasting in type 2 diabetes.
Keywords: diabetic nephropathy, periodic fasting, insulin resistance, dicarbonyl detoxification, lipid oxidation, senescence
Diabetic nephropathy is the most common cause of end-stage renal disease, and therapeutic options for slowing its progression are limited to control of glycemia, lipidemia, and blood pressure (1). Current therapies, in particular, sodium-glucose cotransporter-2 (SGLT-2) inhibitors and angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers, exert nephroprotection to some degree (2, 3). However, the renal benefits attributed to SGLT-2 inhibitors cannot be explained by the modest improvement in glycemic, blood pressure, and lipid control (2). Once diabetic kidney disease (DKD) is established, it is only possible to slow its progression, whereas therapies for long-lasting remission are lacking (4).
Hyperglycemia may cause a decline in renal function leading to DKD via activation of the sorbitol pathway, activation of the hexosamine pathway, and an increase in oxidative stress, eventually resulting in fibrosis (5-7). Moreover, dicarbonyl stress and advanced glycation endproducts have long been implicated in the development of diabetic complications and DKD (8-12). Increased DNA damage and persistent DNA damage signaling as well as senescence have also been described in type 2 diabetes (13-16). Recently, it was shown in vivo that restoring DNA repair in experimental type 1 diabetes by overexpression of a nuclear phosphomimetic receptor for advanced glycation endproducts reduces DNA damage and inflammation and may improve renal function (17).
Interestingly, recent research has shown that periodic fasting can have protective effects by reducing oxidative damage, inflammation, and supporting cellular protection (18-22). Moreover, previous work on cardiorenal benefits of SGLT-2 inhibitors postulated that enhanced ketogenesis may account for their favorable effects due to a shift in fuel metabolism from glucose oxidation to fat and subsequent utilization of ketone bodies shifting energy metabolism to ketolysis (23-26). Since kidney metabolism relies highly on lipolysis and ketone bodies, periodic fasting may be beneficial by activating lipolysis. It is yet unclear whether periodic fasting reduces systemic dicarbonyl stress, DNA damage, and markers of senescence.
The aim of this proof-of-concept study was to test whether a periodic fasting-mimicking diet (FMD) may lead to change in albumin-to-creatinine ratio (ACR) and to changes in markers of insulin resistance, dicarbonyl detoxification, lipid oxidation, DNA damage/repair, and senescence.
Methods
Study Design and Study Population
This randomized controlled open study was performed at the Clinic of Endocrinology, Diabetology, Metabolism and Clinical Chemistry, at the University Hospital of Heidelberg. The ethics committee of the University Hospital of Heidelberg approved all study procedures (Ethic-Nr. S-682/2016) in compliance with national guidelines and the declaration of Helsinki. This study was registered at the German Clinical Trials Register (Deutsches Register Klinischer Studien DRKS; DRKS-ID: DRKS00014287).
Patients with type 2 diabetes with good glycemic and blood pressure control, optimized treatment according to current guidelines (27), and increased ACR assessed from 2 consecutive early morning spot urine samples according to Kidney Disease Improving Global Outcomes guidelines were recruited (28). All participants gave written informed consent. Inclusion criteria were age 50 to 75 years, estimated glomerular filtration rate (eGFR) > 30 mL/min/173 cm² from Chronic Kidney Disease Epidemiology Collaboration formula, and body mass index 23 to 40 kg/m². Exclusion criteria included legally incapacitated persons; other form of diabetes (ie, diabetes mellitus type 1, pancreatogenic diabetes, or steroid-induced diabetes); acute infection/fever; severe heart; kidney; hematological or liver diseases; heart failure of New York Heart Association class IV; nondiabetic liver disease (ie, primary biliary cirrhosis, primary sclerosing cholangitis, Morbus Wilson, hemochromatosis, autoimmune hepatitis); severe peripheral artery disease (stage IV); nondiabetic glomerulopathy; immunosuppressive therapy; alcohol or drug abuse; history of cancer disease in the last 5 years prior to study; infectious hepatitis B, C, or E; HIV infection; autoimmune diseases or immunosuppressive therapy; participation in other interventional studies; anemia or hematological disease; other causes of polyneuropathy (autoimmune, alcohol-induced, or vitamin B12 deficiency, collagenosis); pacemaker; and food allergy (nuts, tomato, soja, or other ingredients enlisted in the diet program). Withdrawal criteria included subject’s request and lack of increase in blood and/or urine ketone bodies.
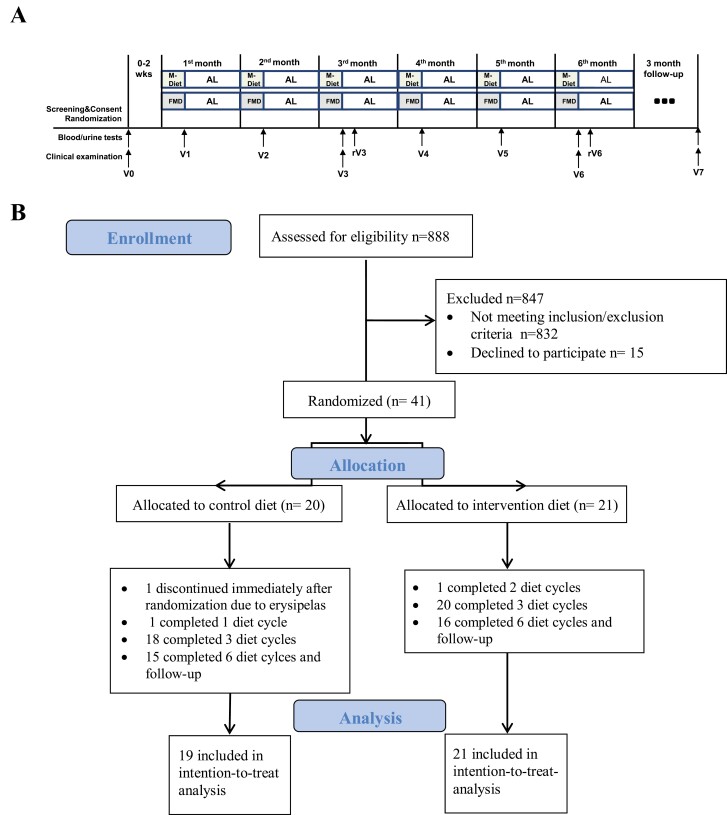
All participants received 1 individual dietary counseling before the baseline visit according to recommendations of the American Diabetes Association (27). Baseline measures were performed before the first diet cycle. Participants were seen at baseline and each month after the diet cycle (the sixth day after the 5-day diet intervention), for a total of 6 diet cycles. Participants were seen at refeeding timepoints: 1 week after the third and after the sixth diet cycle. Adherence to the study regimen was assessed from dietary protocols and measurement of ketone bodies in blood and urine. Follow-up timepoint was 3 months after study completion. Study design is shown in Figure 1A.
Figure 1.
Study design (A) and CONSORT flow diagram (B). Abbreviations: AL, ad libitum; FMD, fasting-mimicking diet; M-Diet, Mediterranean diet; rV3, visit at refeeding 1 week after the third diet cycle; rV6, visit at refeeding one week after the sixth diet cycle; V0, visit at baseline; V1, visit after the first diet cycle; V2, visit after the second diet cycle; V3, visit after the third diet cycle; V4, visit after the fourth diet cycle; V5, visit after the fifth diet cycle; V6, visit after the sixth diet cycle; V7, follow-up visit 3 months after study completion.
Randomization
Participants were randomized (1:1) by a stratified computed procedure (Randomizer Version 2.0.3© Institute for Medical Informatics, Statistics and Documentation, Medical University of Graz) for body mass index (cutoff value 30.0 kg/m²) and hemoglobin A1c (cutoff value 7.0%) to intervention or control group. The randomization list was only accessible to assigned randomization list managers.
Diet Intervention
Participants were instructed to comply for 5 consecutive days each month, either with FMD (intervention group) or with a Mediterranean diet (M-Diet; control group) and to return to their normal diet until the next diet cycle that was initiated about 25 days later. FMD was a plant-based diet that mimics fasting-like effects on glucose and ketone bodies: day 1 supplied 4600 kJ (11% protein, 46% fat, and 43% carbohydrates), whereas days 2 to 5 provided 3000 kJ (9% protein, 44% fat, and 47% carbohydrate) per day (21). M-Diet had no change in caloric intake compared to participant’s diet before the study and was adapted according to the Mediterranean diet score (MDS) (29). Weight loss of <10% was accepted for both study groups. Patients who were on insulin therapy were instructed to discontinue short-acting insulin and to reduce the long-acting insulin by 50% when taking FMD. Oral antidiabetic therapy was also discontinued during FMD. Glycemic levels were self-monitored (fasting and 2-hour postprandial levels, at least 4 measurements daily) with a capillary blood glucose monitoring system (Accu-Check Guide®, Roche), and a 24-hour telephone platform was available for participants to report hypo- or hyperglycemic episodes. Antihyperglycemic medication was reduced in case of a reduction of fasting plasma glucose by more than 20% compared to the previous measurement (21). Antihypertensive medication was reduced if a participant reported hypotension (lower than 100 mmHg for systolic and lower than 60 mmHg for diastolic values). The aim was to maintain comparable glycemic and blood pressure control between the study groups throughout the study. All participants were instructed to avoid excessive physical activity during FMD or M-Diet and to return to their normal physical activity afterward. Participants were asked to write their individual diet and physical activity protocol during the study. Health-related quality of life and physical health was assessed using the 12-Item Short-form Health Survey (30). Somatic and depression symptoms were evaluated using the Patient Health Questionnaire (31). Thyroid function was controlled during the study by measuring thyroid-stimulating hormones levels in plasma.
Outcomes
Primary endpoint was the change in ACR between study groups. A prespecified subgroup analysis for participants with micro- vs macroalbuminuria levels at baseline was performed for the primary endpoint. Prespecified exploratory endpoints comprised change in homeostatic model assessment for insulin resistance (HOMA-IR) as marker for insulin resistance, change in plasma methylglyoxal-derived hydroimidazolone 1 (MG-H1), plasma methylglyoxal (MG), glyoxalase-1 (Glo-1) activity, and expression of phosphorylated Glo-1 (pGlo-1) in white blood cells (WBC), hydroxyacetone in red blood cells as markers of dicarbonyl detoxification, change in plasma acylcarnitine (AC) profile as a marker of fatty acid oxidation, change in phosphorylated histone H2AX (yH2Ax) expression in WBC as marker of DNA damage/repair, and change in soluble urokinase plasminogen activator receptor (suPAR), as a marker of kidney injury and senescence.
Safety
Safety was monitored by assessment of vital signs, physical examination, electrocardiogram, adverse events following the Common Terminology Criteria for Adverse Events (v4.0), and laboratory results at each visit.
Power Calculation
According to previous studies, a reduction of albuminuria by at least 30% is considered clinically relevant (32, 33). Thus, the actual study required a total sample size of 34 patients to detect a mean difference between groups of 30% decrease of ACR from baseline, assuming an SD of 30% for both groups with a 2-sample t-test and a 2-sided significance level of α = 5% and a power of at least 80%. An estimated dropout rate of 15% resulted in 40 participants. Sample size calculation was performed in PASS 16.0.12.
Blood Chemistry
Blood samples were drawn in fasting state and immediately processed in the Central Laboratory of the University Hospital of Heidelberg under standardized conditions. Beta-hydroxybutyrate was measured at each visit in venous blood (StatStrip® Glucose/Ketone Meter System, Nova® Biomedical).
Quantification of Acylcarnitines
ACs were determined in serum by electrospray ionization tandem mass spectrometry according to a modified method as previously described (34), using a Quattro Ultima triple quadrupole mass spectrometer (Micromass, Manchester, UK) equipped with an electrospray ion source and a Micromass MassLynx data system. In particular, 5 µL of plasma was placed on a 4.7-mm filter paper punch, dried at room temperature overnight, and extracted with 100 µL of deuterium-labeled standard solution in methanol (34).
Measurement of MG, MG-H1, Hydroxyacetone, and Glo-1 Activity
Plasma concentration of MG and plasma and urine concentration of MG-H1 was determined by stable isotopic dilution, liquid chromatography with tandem mass spectrometry as described previously (35). Hydroxyacetone was determined using O-(2,3,4,5,6-pentafluorophenyl) methyl hydroxylamine as a derivatizing agent (36). Activity of Glo-1 was determined spectrophotometrically as described previously (36).
Phosphorylated Glyoxalase-1
Total protein was isolated from WBC using immunoprecipitation buffer, and protein concentration was determined using the Bradford reagent (37). Proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (15% Mini-PROTEAN TGX, Biorad) and transferred onto a 0.2-µM nitrocellulose membrane and blocked using 5% milk powder in phosphate-buffered saline (PBS) and 0.05% Tween 20 (PBS-T) at room temperature for 1 hour. Membranes were then incubated overnight at 4°C with antibodies against phosphorylated Y136 Glo-1 rabbit polyclonal self-made (1:250 dilution; Teleman-Dkfz, Glo1_Y136, RRID: AB_2909423), Glo-1 antibody guinea pig polyclonal self-made (1:1000 dilution; Teleman-Dkfz, Glo1_Drosophila, RRID: AB_2909427), and Calnexin rabbit polyclonal antibody (1:1000 dilution Enzo Life Sciences ADI-SPA-860, RRID: AB_10616095) in 5% bovine serum albumin in PBS-T. Blots were washed and then incubated with an appropriate anti-rabbit horseradish peroxidase-conjugated antibody (Jackson ImmunoResearch Labs, 111-035-003, RRID: AB_2313567) and anti-guinea horseradish peroxidase-conjugated antibody (Jackson ImmunoResearch Labs, 106-035-003, RRID: AB_2337402; 1:5000 in 5% milk powder in PBS-T) for 1 hour at room temperature. Proteins were visualized on a ChemiDoc system using Western Lightning Plus-ECL (PerkinElmer) with varying exposure times (0.1-3 minutes). Protein expression was determined using the software Image-master (Bio-Rad) and normalized to calnexin.
Isolation of White Blood Cells
Blood samples were processed immediately after taking the blood in fasting conditions as described in the previous discussion. All following steps were done on ice at 4°C to avoid sample degradation. One syringe of 9-mL EDTA blood (Sarstedt AG & Co. KG, Nümbrecht, Germany) was spun down at 3000 rpm for 5 minutes and 2 mL of plasma supernatant were transferred in a separate tube and spun again at 1400 rpm for 1 minute. These plasma samples were frozen at −80°C until they were used for further analyses. One syringe of 9 mL EDTA blood (Sarstedt AG & Co. KG, Nümbrecht, Germany) was lysed in 40 mL erythrocyte lysis buffer (ECL; 1.5 M ammonium chloride, 100 mM sodium bicarbonate, 13 mM EDTA pH7.3, and, before use, diluted 1:10) for 15 minutes. Cells were spun down at 1700 rpm for 5 minutes. Afterward, the cells were washed once with ECL and once with NaCl 0.9% and spun down at 1300 rpm for 5 minutes. The cells were resuspended in fetal calve serum (FCS, Sigma-Aldrich, St. Louis, MO, USA) and frozen in FCS with 10% dimethyl sulfoxide (~3 Mio cells per tube) at −80°C until the samples were used for further analyses.
Determination of Comet Tail Length and yH2Ax-Positivity in WBC
For each patient, 1 vial with ~3 million cells were thawed in a 37°C waterbath and immediately transferred into 10 mL prewarmed PBS (Sigma-Aldrich) and spun down at 1400 rpm for 10 minutes. Following a second wash step with 10 mL PBS, cells were incubated with 1 mL of 200 ng DNase (Sigma-Aldrich) in PBS and 5 mM MgCl for 30 minutes at room temperature and spun down again. After resuspension in 1 mL PBS with 10% FCS and 1 mM EDTA (FACS buffer), 10 µL per patient were transferred to a V-shaped 96-well plate for the Comet Assay (Trevigen, Gaithersburg, MD, USA). The remaining material was spun down, resuspended in 200 µL FACS buffer, and 180 µL transferred to another V-shaped 96-well plate (Sigma-Aldrich) for the FACS analysis of yH2Ax. Remaining 20 µL per sample were pooled and used for controls.
Comet Assay
Comet assay was performed with the Reagent Kit for Higher Throughput Single Cell Gel Electrophoresis Assay (4252-040-K, Trevigen; Gaithersburg, MD, USA). The assay was performed according to the protocol, but with the following changes. For each sample 10 µL of cells were resuspended with 100 µL LMAgarose, and 15 µL were spread on the comet plate. To adjust for the used electrophoresis apparatus, the alternative alkaline unwinding and electrophoresis solutions were used. Electrophoresis was run with 380 mL at 15 V for 1 hour in an ice bath. Comets were stained with SYBRGreen 1:10 000 (S7563, ThermoFisherScientific, Waltham, MA, USA). Plates were washed twice after staining. Pictures were taken with Olympus IX81 Widefield microscope using Olympus ScanR acquisition, and comets were analyzed with OpenComet (ImageJ). Mean TailDNA% was used as readout.
yH2Ax Positivity Measurements
The plate was spun at 1300 rpm for 5 minutes, and liquid discarded. Cells were incubated with 20 µL Fc Receptor Blocking Solution (422302, Biolegend, San Diego, CA, USA) 1:200 in FACS buffer for 10 minutes and after removal with 20 µL CD antibodies (APC/cyanine7 anti-human CD3 antibody (300425, RRID: AB_830754), Alexa Fluor® 700 anti-human CD4 antibody (317425, RRID: AB_571942), Brilliant Violet 421™ anti-human CD14 antibody (325628, RRID: AB_2563296), APC anti-human CD19 antibody (302211, RRID: AB_314241); all Biolegend) 1:100 in FACS buffer for 30 minutes. After washing with 100 µL FACS buffer, cells were fixed with 100 µL fixation buffer (420801, Biolegend) for 10 minutes. After 2 washes with 100 µL FACS buffer, cells were stained with 20 µL of Alexa Fluor® 488 anti-H2A.X phospho (Ser139) antibody (613406, Biolegend, RRID: AB_2248011) 1:100 in permeabilization buffer (421002, Biolegend) for 30 minutes. Afterward, cells were washed twice with 100 µL permeabilization buffer and once with 100 µL FACS buffer and stored in 100 µL FACS buffer at 4°C. All steps were carried out at 4°C. Unstained and single stains for all antibodies were included in each run. Alexa Fluor® 488 Mouse IgG1, κ-Isotype Ctrl (FC) antibody (400132, Biolegend, RRID: AB_2890263) was used as isotype control for Alexa Fluor® 488 anti-H2A.X phospho (Ser139) antibody.
The stained and analyzed cells were measured as frequent of population for all WBC, lymphocytes, monocytes, and T cells using Becton Dickinson LSR II flow cytometer (Heidelberg, Germany) and FlowJo version xV0.7 (Oregon, USA).
Statistical Analysis
Analyses were performed in the intention-to-treat population, of which at least the baseline and 3 and/or 6 months data were available. Missing values were not imputed. Available case analysis was performed, and the respective sample size is reported. Treatment effects between study groups were evaluated using analysis of covariance adjusted for age, sex, weight loss, and the respective baseline value of the outcome. Hierarchical test strategy was applied to adjust for multiple comparison analysis. Between-diet comparisons are presented as estimated marginal means (95% CIs), unless otherwise stated. Descriptive data are shown as mean ± SE of the mean for normally distributed variables, median (25th, 75th percentile) for log-normally distributed variables, and frequencies for categorical variables. Distribution assumption was assessed visually and evaluated by the Kolmogorov-Smirnov test. Statistical computing was performed using R (version 3.6.1 2019-07-05). Visualization of the data was performed employing R core packages tiduverse and pheatmap and GraphPad Prism version 5.00 for Windows (GraphPad Software, San Diego California USA).
Results
Study Flow and Participants
Between March 2018 and May 2020, 41 patients were randomized to FMD group (n = 21) or M-Diet group (n = 20). One patient of the M-Diet group discontinued immediately after randomization due to erysipelas and is not included in the analysis. Thirty-eight (95%) patients completed 3 diet cycles, and 33 (83%) completed the study. Thirty-one (78%) were seen at follow-up (follow-up time was 112 ± 31 days). The main reason for withdrawal after the third diet cycle and at follow-up were the restrictions related to the Covid-19 pandemic. CONSORT study flow diagram is shown in Figure 1B.
Baseline anthropometric and metabolic measures were comparable between groups (Table 1). Health-related physical activity assessed from 12-Item Short-form Health Survey and daily calorie intake, assessed from self-reported diet protocols, did not differ at baseline, and during the study, there was no change between the study groups after 5 days of FMD or M-Diet (data not shown). All patients had normal thyroid function at baseline and during the study, including the patients of the FMD group who had previously had a thyroidectomy.
Table 1.
Patient characteristics at baseline
| M-Diet (n = 19) | FMD (n = 21) | |
|---|---|---|
| Age, year | 66.8 ± 1.4 | 64.9 ± 1.6 |
| Sex, females/males | 5/14 | 6/15 |
| BMI, kg/m² | 30.2 ± 1.0 | 30.9 ± 0.9 |
| WHR | 1.00 ± 0.01 | 1.03 ± 0.02 |
| Diabetes duration, years | 13.6 ± 1.9 | 14.7 ± 2.0 |
| Diabetes complication | ||
| Nephropathy | 19 (100) | 21 (100) |
| Neuropathy | 12 (63) | 10 (48) |
| Retinopathy | 5 (26) | 3 (14) |
| History of: | ||
| Hypertension | 16 (84) | 20 (95) |
| Coronary heart disease | 8 (42) | 7 (33) |
| Myocardial infarction | 5 (26) | 2 (10) |
| OSAS | 1 (5) | 3 (14) |
| Arthrosis | 9 (47) | 10 (47) |
| Thyroid nodules | 7 (37) | 2 (10) |
| Thyroidectomy | 0 | 3 (14) |
| Diabetes therapy | ||
| Dietary measurements | 2 (11) | 1 (5) |
| Metformin | 15 (79) | 16 (76) |
| DPP-4 | 4 (21) | 6 (29) |
| SGLT-2 | 2 (11) | 5 (24) |
| GLP-1 agonist | 3 (16) | 0 |
| Sulfonylureas | 2 (11) | 1 (5) |
| Glinide | 0 (0) | 1 (5) |
| Short-acting insulin | 4 (21) | 4 (19) |
| Long-acting insulin | 7 (37) | 7 (33) |
| Other medication | ||
| RAAS-inhibitors | 17 (89) | 19 (90) |
| Beta-blockers | 11 (58) | 12 (57) |
| Thiazide diuretics | 5 (26) | 7 (33) |
| Loop diuretics | 4 (21) | 7 (33) |
| Calcium-antagonist | 10 (53) | 11 (52) |
| Statin | 13 (68) | 11 (52) |
| Ezetimibe | 1 (5) | 2 (10) |
| Acetyl-salicylic acid | 11 (58) | 8 (38) |
| Glycemic control | ||
| HbA1c, % | 7.7 ± 0.3 | 8.1 ± 0.4 |
| FPG, mg/dL | 158.4 ± 10.6 | 162.1 ± 9.7 |
| Blood pressure | ||
| Systolic, mmHg | 142.0 ± 3.8 | 142.9 ± 3.1 |
| Diastolic, mmHg | 81.8 ± 1.7 | 85.3 ± 1.8 |
| Renal function | ||
| Serum creatinine, mg/dL | 0.84 ± 0.07 | 0.88 ± 0.06 |
| Cystatin C, mg/L | 1.01 ± 0.06 | 1.10 ± 0.09 |
| eGFR CKD-EPI creatinine, mL/min*1.73 m² | 86.97 ± 4.32 | 84.59 ± 4.27 |
| eGFR from cystatin C, mL/min*1.73m² | 84.56 ± 4.52 | 79.47 ± 4.63 |
| Albumin-to-creatinine ratio | ||
| All | 73.4 (205.6) | 51.3 (116.0) |
| Microalbuminuria, mg/g, n | 44.8 (49.2) (15) | 43.7 (39.6) (18) |
| Macroalbuminuria, mg/g, n | 359.3 (1888.5) (4) | 555.2 (593.2) (3) |
| Liver transaminases | ||
| ALT, U/L | 31.3 ± 2.8 | 30.7 ± 3.1 |
| AST, U/L | 27.5 ± 1.7 | 27.5 ± 3.3 |
| Serum lipids | ||
| Triglycerides, mg/dL | 165 (101) | 163 (171) |
| Total cholesterol, mg/dL | 174.8 ± 10.4 | 184.6 ± 18.3 |
| HDL, mg/dL | 43.0 ± 2.5 | 44.3 ± 2.8 |
| LDL, mg/dL | 94.3 ± 8.9 | 87.8 ± 8.5 |
Data represents mean ± SE of the mean for normally distributed variables, median (interquartile range) for log-normally distributed variables, or frequencies n (%) for categorical variables.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; DPP-4, dipeptidyl peptidase 4; eGFR, estimated glomerular filtration rate; FMD, fasting-mimicking diet; FPG, fasting plasma glucose; GLP-1, glucagon-like peptide-1; HDL, high-density lipoprotein; LDL, low-density lipoprotein; M-Diet, Mediterranean diet; OSAS, obstructive sleep apnea syndrome; RAAS, renin-angiotensin-aldosterone system; SGLT-2, sodium-glucose cotransporter 2; WHR, waist-hip ratio,
Albuminuria and Renal Function
After 6 diet cycles, ACR did not change compared to respective baseline in both groups, and change in ACR was comparable between study groups [110.3 (99.2, 121.5) mg/g; P = 0.45] (Table 2). In the prespecified subgroup analysis, when compared to the control group, FMD reduced ACR [−30.3 (−35.7, −24.9) mg/g; P ≤ 0.05] in patients with microalbuminuria levels at baseline (n = 15 in the M-Diet group and n = 18 in the FMD group) (Fig. 2A, Table 2), whereas change in ACR between groups was similar [434.0 (404.7, 463.4) mg/g; P = 0.23] in patients with macroalbuminuria levels at baseline (n = 4 in the M-Diet group and n = 3 in the FMD group) (Table 2). After 3 diet cycles, change in ACR between groups was comparable in all patients [−62.5 (−71.3, −53.7) mg/g; P = 0.45] and in patients with macroalbuminuria levels at baseline [−176.2 (−203.1, −149.4) mg/g; P = 0.47], whereas ACR reduced significantly in patients of the FMD group with microalbuminuria levels at baseline [−23.7 (−28.4, −18.9) mg/g; P ≤ 0.05] (Fig. 2A, Table 2). Compared to control diet, FMD resulted in a reduction of urea levels after 3 [−11.8 (−14.0, −9.7) mg/dL; P ≤ 0.01] and after 6 diet cycles [−17.9 (−21.2, −14.6) mg/dL; P ≤ 0.05] (Table 2). FMD led to less decline in eGFR based on cystatin C after 6 diet cycles [8.3 (5.9, 10.6) mL/min/1.73 m²; P ≤ 0.05] between study groups, whereas no difference was observed in eGFR based on creatinine (Table 2).
Table 2.
Metabolic and anthropometric parameters at baseline, after 3 months, after 6 months, and at follow-up
| Parameter | Group | V0 | V3 | V6 | V7 |
|---|---|---|---|---|---|
| Glycemia | |||||
| FPG, mg/dL | M-Diet | 158.4 ± 10.6 (19) | 149.1 ± 11.4 (18) | 151.2 ± 12.1 (16) | 167.5 ± 18.2 (15) |
| FMD | 162.1 ± 9.7 (21) | 146.7 ± 13.8 (20) | 137.4 ± 12.7 (17) | 151.9 ± 8.5 (16) | |
| HbA1c, % | M-Diet | 7.7 ± 0.3 (19) | 7.2 ± 0.3 (18) | 7.7 ± 0.3 (16) | 8.0 ± 0.4 (15) |
| FMD | 8.1 ± 0.4 (21) | 7.2 ± 0.3 (20) | 6.7 ± 0.3 (17)* | 7.6 ± 0.4 (16) | |
| C-peptid, ng/mL | M-Diet | 3.3 ± 0.4 (19) | 2.9 ± 0.4 (18) | 3.2 ± 0.6 (16) | 3 ± 0.5 (14) |
| FMD | 3.1 ± 0.4 (21) | 2.3 ± 0.4 (20) | 2.0 ± 0.3 (17) | 2.7 ± 0.3 (15) | |
| HOMA-IR | M-Diet | 6.1 ± 0.9 (13) | 6.0 ± 0.9 (13) | 5.7 ± 1.0 (11) | 6.1 ± 0.9 (10) |
| FMD | 6.4 ± 1.9 (14) | 4.9 ± 2.7 (14) | 2.6 ± 0.6 (12)** | 5.1 ± 1.7 (10)* | |
| Blood pressure | |||||
| Systolic BP | M-Diet | 142.0 ± 3.8 (19) | 144.6 ± 5.9 (18) | 142.5 ± 4.1 (16) | 136.6 ± 2.9 (14) |
| FMD | 142.9 ± 3.1 (21) | 135.9 ± 3.2 (19) | 141.3 ± 3.2 (16) | 142.6 ± 5.3 (14) | |
| Diastolic BP | M-Diet | 81.8 ± 1.7 (19) | 81.3 ± 2.1 (18) | 81.8 ± 2.4 (16) | 80.5 ± 2.3 (14) |
| FMD | 85.3 ± 1.8 (21) | 81.6 ± 1.5 (19) | 83.2 ± 1.8 (16) | 85.6 ± 2.3 (14) | |
| Lipidemia | |||||
| Cholesterol, mg/dL | M-Diet | 174.8 ± 10.4 (19) | 171.7 ± 9.9 (18) | 184.2 ± 11.2 (16) | 188.7 ± 13.4 (15) |
| FMD | 184.6 ± 18.3 (21) | 164.7 ± 13.1 (20) | 160.2 ± 10.3 (17) | 177.8 ± 11.9 (16) | |
| LDL, mg/dL | M-Diet | 94.3 ± 8.9 (18) | 90.2 ± 7.5 (18) | 96.6 ± 8.4 (16) | 103.8 ± 12.3 (13) |
| FMD | 87.8 ± 8.5 (20) | 95.2 ± 11.5 (19) | 87.2 ± 9.6 (17) | 84.6 ± 10.0 (14) | |
| HDL, mg/dL | M-Diet | 43.0 ± 2.5 (19) | 44.3 ± 2.7 (18) | 46.8 ± 2.6 (16) | 45.7 ± 3.7 (15) |
| FMD | 44.3 ± 2.8 (21) | 43.3 ± 2.6 (20) | 48.8 ± 3.3 (17) | 51.7 ± 3.8 (16) | |
| TG, mg/dL | M-Diet | 205.6 ± 25.8 (19) | 185.5 ± 20.5 (18) | 204.8 ± 24.6 (16) | 214.7 ± 35.2 (15) |
| FMD | 277.2 ± 75.6 (21) | 166.8 ± 37.5 (20) | 121.2 ± 12.4 (17) | 209.4 ± 35.8 (16) | |
| Lp(a), mg/dL | M-Diet | 30.3 ± 8.7 (19) | 35.6 ± 10.5 (17) | 33.6 ± 9.7 (16) | 34.6 ± 10.7 (14) |
| FMD | 16.3 ± 3.5 (21) | 20.6 ± 4.4 (20) | 17.5 ± 2.1 (17) | 14.3 ± 1.7 (15) | |
| Liver parameters | |||||
| AST, U/L | M-Diet | 27.5 ± 1.7 (19) | 31.1 ± 3.0 (18) | 27.2 ± 1.6 (16) | 26.3 ± 1.5 (15) |
| FMD | 27.5 ± 3.3 (21) | 29.9 ± 2.4 (20) | 25.5 ± 1.4 (17) | 23.0 ± 1.6 (16) | |
| ALT, U/L | M-Diet | 31.3 ± 2.8 (19) | 32.8 ± 4.4 (18) | 30.4 ± 2.8 (16) | 30.7 ± 2.7 (15) |
| FMD | 30.6 ± 3.1 (21) | 34.7 ± 3.5 (20) | 25.6 ± 1.9 (17) | 23.3 ± 2.4 (16) | |
| Liver stiffness, kPa | M-Diet | 6.9 ± 0.8 (19) | 6.4 ± 0.6 (18) | 6.0 ± 0.6 (16) | 7.2 ± 1.2 (14) |
| FMD | 8.1 ± 1 (21) | 6.2 ± 0.6 (19) | 6.4 ± 0.7 (17) | 7.1 ± 0.8 (15) | |
| Protein level | |||||
| Plasma-albumin, g/L | M-Diet | 44.3 ± 0.7 (19) | 44.8 ± 0.6 (18) | 44.9 ± 0.7 (16) | 44.6 ± 0.7 (15) |
| FMD | 45.9 ± 0.4 (21) | 46.7 ± 0.5 (20) | 47.3 ± 0.6 (17) | 45.5 ± 0.5 (16) | |
| Plasma-total protein, g/L | M-Diet | 72.9 ± 1.4 (19) | 72.8 ± 1.4 (18) | 72.4 ± 1.5 (16) | 71.5 ± 1.4 (15) |
| FMD | 73.3 ± 0.9 (21) | 74.6 ± 1.1 (20) | 72.9 ± 0.9 (17) | 69.5 ± 0.8 (16) | |
| Renal parameters | |||||
| uACR, mg/g | M-Diet | 73.4 (205.6) (19) | 72.7 (125.8) (18) | 63.1 (353.6) (16) | 76.0 (149.9) (15) |
| FMD | 51.3 (116.0) (21) | 34.8 (43.6) (20) | 25.6 (40.3) (17) | 36.5 (137.8) (16) | |
| Micro.uACR, mg/g | M-Diet | 44.8 (49.2) (14) | 35.8 (58.0) (13) | 37.2 (50.5) (11) | 22.9 (65.6) (11) |
| FMD | 43.7 (39.6) (17) | 29.1 (36.1) (16)* | 23.4 (30.3) (14)* | 27.4 (27.1) (13) | |
| Macro.uACR, mg/g | M-Diet | 359.3 (1888.5) (4) | 249.2 (2533.6) (4) | 430.3 (592.5) (3) | 399.8 (143.4) (3) |
| FMD | 555.2 (593.2) (3) | 666.1 (575.0) (3) | 1034.7 (591.1) (3) | 915.4 (905.8) (3) | |
| Urea, mg/dL | M-Diet | 39.1 ± 3.2 (19) | 38.1 ± 2.9 (18) | 48.8 ± 8.7 (16) | 35.9 ± 2.3 (15) |
| FMD | 38.8 ± 4 (21) | 25.6 ± 1.9 (20)** | 26.2 ± 2.1 (17)* | 38.2 ± 2.9 (16) | |
| Creatinine, mg/dL | M-Diet | 0.8 ± 0.1 (19) | 0.9 ± 0.1 (18) | 0.8 ± 0.1 (16) | 0.8 ± 0.1 (15) |
| FMD | 0.9 ± 0.1 (21) | 0.9 ± 0.1 (20) | 0.9 ± 0.1 (17) | 0.9 ± 0.1 (16) | |
| eGFR CKD-EPI (creatinine), mL/min/1.73 m² | M-Diet | 87 ± 4.3 (19) | 85 ± 4.5 (18) | 86.4 ± 4.7 (16) | 87.7 ± 4.3 (15) |
| FMD | 84.6 ± 4.3 (21) | 83.2 ± 4.2 (20) | 81.8 ± 4.8 (17) | 82.3 ± 4.7 (16) | |
| Cystatin C, mg/L | M-Diet | 1 ± 0.1 (19) | 1.1 ± 0.1 (18) | 1.2 ± 0.1 (16) | 1.1 ± 0.1 (15) |
| FMD | 1.1 ± 0.1 (21) | 1.1 ± 0.1 (20) | 1.1 ± 0.1 (17)* | 1.1 ± 0.1 (16) | |
| eGFR (cystatin C), mL/min/1.73 m² | M-Diet | 84.6 ± 4.5 (19) | 80 ± 4.3 (18) | 72.9 ± 5.6 (16) | 73.3 ± 4.9 (15) |
| FMD | 79.5 ± 4.6 (21) | 77.2 ± 4.5 (20) | 77.4 ± 5.1 (17)* | 71.2 ± 4.9 (16) | |
| sUPAR, pg/mL | M-Diet | 3040.5 ± 218.0 (19) | 3106.0 ± 335.6 (17) | 2975.4 ± 187.6 (16) | 3052.8 ± 197.0 (14) |
| FMD | 3122 ± 375 (21) | 2998.6 ± 216.9 (20)* | 2737.1 ± 117.8 (17)* | 3036.7 ± 180.4 (15) | |
| Ketogenesis | |||||
| Blood ketones, mmol/L | M-Diet | 0.1 ± 0.0 (19) | 0.2 ± 0.0 (18) | 0.1 ± 0.0 (16) | 0.1 ± 0.0 (14) |
| FMD | 0.2 ± 0.0 (21) | 0.5 ± 0.1 (20)** | 0.5 ± 0.1 (17)** | 0.1 ± 0.0 (15) | |
| Urine ketones, mg/dL | M-Diet | 0.3 ± 0.3 (19) | 2.2 ± 0.9 (18) | 2.5 ± 1.3 (16) | 1.8 ± 1.1 (14) |
| FMD | 1.2 ± 0.8 (21) | 5.8 ± 1.5 (20) | 7.9 ± 3.2 (17) | 1.0 ± 0.5 (15) | |
| Other parameters | |||||
| Uric acid, mg/dL | M-Diet | 6.0 ± 0.3 (19) | 6.1 ± 0.3 (18) | 6.0 ± 0.4 (16) | 5.9 ± 0.3 (14) |
| FMD | 6.6 ± 0.4 (21) | 6.4 ± 0.3 (20) | 6.8 ± 0.4 (17) | 6.4 ± 0.5 (15) | |
| hsCRP, mg/L | M-Diet | 1.9 ± 0.4 (19) | 2.1 ± 0.5 (18) | 2.2 ± 0.6 (16) | 3.2 ± 1.0 (15) |
| FMD | 5.1 ± 1.6 (21) | 3.2 ± 1.0 (20) | 2.6 ± 1.2 (17) | 2.3 ± 0.9 (16) | |
| hsTNT, pg/mL | M-Diet | 15.9 ± 2.2 (19) | 17.0 ± 2.5 (18) | 18.3 ± 3.6 (16) | 17.1 ± 3.1 (15) |
| FMD | 12.6 ± 1.5 (21) | 12.7 ± 1.6 (20) | 13.0 ± 1.3 (17) | 15.9 ± 2.7 (16) | |
| IGF-1, ng/mL | M-Diet | 111.9 ± 8.5 (19) | 117.2 ± 9.8 (18) | 115.1 ± 10.3 (16) | 123.7 ± 12.6 (14) |
| FMD | 135.1 ± 8.7 (21) | 127.8 ± 7.0 (20) | 127.9 ± 9.1 (16) | 137.2 ± 7.7 (15) | |
| IL-6, pg/mL | M-Diet | 3.0 ± 0.6 (19) | 3.8 ± 0.6 (18) | 3.2 ± 0.8 (15) | 2.9 ± 0.4 (14) |
| FMD | 2.9 ± 0.4 (21) | 3.5 ± 0.6 (20) | 2.3 ± 0.1 (17) | 2.3 ± 0.2 (14) | |
| NT-proBNP, ng/L | M-Diet | 149.7 ± 39.1 (19) | 169.2 ± 34.1 (17) | 129.0 ± 32.2 (16) | 140.4 ± 41.4 (15) |
| FMD | 198.5 ± 100.3 (21) | 202.2 ± 70.0 (19) | 173.5 ± 57.4 (17) | 252.8 ± 111.2 (16) | |
| Plasma MG-H1, nM | M-Diet | 217.9 ± 36.0 (19) | 132.7 ± 15.4 (18) | 246.0 ± 27.9 (16) | 281.1 ± 37.6 (15) |
| FMD | 213.3 ± 32.0 (21) | 81.7 ± 10.7 (20)*** | 297.4 ± 72.0 (17) | 294.7 ± 50.31 (15) | |
| Body weight | |||||
| Body weight, kg | M-Diet | 93.3 ± 3.6 (19) | 93.0 ± 3.9 (18) | 93.1 ± 4.4 (16) | 90.5 ± 4.5 (14) |
| FMD | 95.7 ± 3.6 (21) | 90.0 ± 3.8 (20)*** | 85.8 ± 3.6 (17)*** | 91.4 ± 3.7 (15) | |
| BMI, kg/m2 | M-Diet | 30.2 ± 1.0 (19) | 30.1 ± 1.1 (18) | 30.2 ± 1.2 (16) | 29.8 ± 1.3 (14) |
| FMD | 31 ± 0.9 (21) | 29.1 ± 1.1 (20)*** | 27.7 ± 1.0 (17)*** | 29.4 ± 1.1 (15) | |
| Body composition (BIA) | |||||
| Fat mass, % | M-Diet | 29.9 ± 1.9 (18) | 30.1 ± 1.9 (17) | 30.2 ± 2.1 (15) | 30.3 ± 2.2 (14) |
| FMD | 31.9 ± 1.8 (21) | 31.1 ± 1.9 (20) | 29.6 ± 2.0 (16) | 30.1 ± 2.2 (15) | |
| Fat mass, kg | M-Diet | 28.4 ± 2.7 (18) | 28.4 ± 2.8 (17) | 28.5 ± 2.9 (15) | 27.9 ± 3.0 (14) |
| FMD | 30.7 ± 2.1 (21) | 28.4 ± 2.4 (20) | 25.3 ± 2.2 (16) | 27.7 ± 2.4 (15) | |
| Fat-free mass, % | M-Diet | 70.1 ± 1.9 (18) | 69.9 ± 1.9 (17) | 69.7 ± 2.1 (15) | 70.4 ± 2.6 (14) |
| FMD | 68.1 ± 1.8 (21) | 68.9 ± 1.9 (20) | 70.5 ± 2.1 (16) | 69.9 ± 2.2 (15) | |
| Fat-free mass, kg | M-Diet | 64.8 ± 2.4 (18) | 63.8 ± 2.4 (17) | 63.7 ± 2.8 (15) | 62.7 ± 3.0 (14) |
| FMD | 64.9 ± 2.7 (21) | 61.5 ± 2.6 (20) | 59.4 ± 2.7 (16) | 63.7 ± 3.1 (15) | |
| Body liquid, % | M-Diet | 53.2 ± 1.6 (18) | 52.9 ± 1.5 (17) | 52.5 ± 1.6 (15) | 52.4 ± 1.8 (14) |
| FMD | 51 ± 1.4 (21) | 51.2 ± 1.4 (20) | 51.7 ± 1.5 (16) | 52.7 ± 1.5 (15) | |
| Body liquid, L | M-Diet | 49.2 ± 1.9 (18) | 48.3 ± 1.8 (17) | 48.0 ± 2.2 (15) | 47.2 ± 2.4 (14) |
| FMD | 48.8 ± 2.4 (21) | 46.0 ± 2.3 (20) | 43.8 ± 2.3 (16) | 48.2 ± 2.5 (15) | |
| Phase angle | M-Diet | 5.8 ± 0.1 (18) | 5.8 ± 0.1 (17) | 5.8 ± 0.1 (15) | 5.6 ± 0.1 (14) |
| FMD | 5.8 ± 0.2 (21) | 5.7 ± 0.2 (20) | 5.7 ± 0.2 (16) | 5.5 ± 0.2 (15) |
Data are shown as mean ± SE of the mean of unadjusted values of variable for normally distributed variables or as median (interquartile range) for log-normally distributed variables. Statistically significant values are written in bold and indicate significance level of intervention effect on change of parameter compared to baseline and are based on M-Diet corrected analysis of covariance with adjustment for age, sex, and weight loss. ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05.
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BIA, bioimpedance analysis; BMI, body mass index; BP, blood pressure; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; eGFR, estimated glomerular filtration rate; FMD, fasting-mimicking diet; FPG, fasting plasma glucose; HDL, high-density lipoprotein; HOMA-IR, homeostatic model assessment of insulin resistance; hsCRP, high-sensitivity C-reactive protein; hsTNT, high-sensitivity troponin T; IL-6, interleukin 6; Lp(a), lipoprotein (a); LDL, low-density lipoprotein; Macro.uACR, macroalbuminuria; M-Diet, Mediterranean diet; Micro.uACR, microalbuminuria; NT-proBNP, N-terminal prohormone of brain natriuretic peptide; sUPAR, soluble urokinase-type plasminogen activator receptor; TG, triglyceride; uACR, urinary albumin-to-creatinine ratio; V0, baseline; V3, after 3 diet cycles; V6, after 6 diet cycles; V7, follow-up.
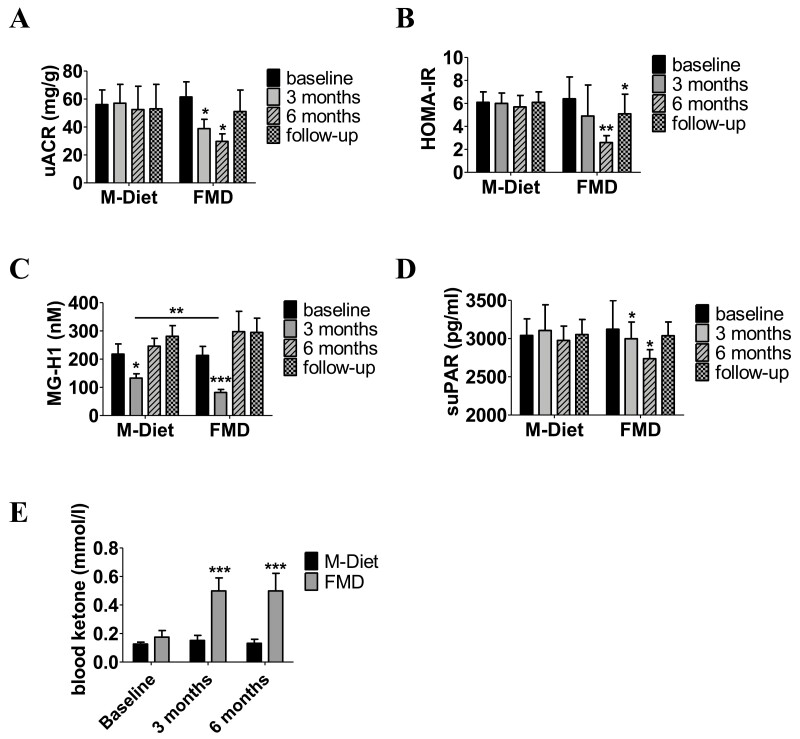
Figure 2.
Effects of fasting on microalbuminuria (A), HOMA-IR (B), MG-H1 (C), suPAR (D), and blood ketone bodies (E) after 3 months, after 6 months, and at follow-up. Data are shown as mean ± SE of the mean of unadjusted values of parameter. P-values indicate significance level of intervention effect on change compared to baseline and are based on M-Diet–corrected analysis of covariance with adjustment for age, sex, and weight loss. ***P ≤ 0.001, **P ≤ 0.01, *P ≤ 0.05. Abbreviations: FMD, fasting-mimicking diet; HOMA-IR, homeostatic model assessment of insulin resistance; M-Diet, Mediterranean diet; MG-H1, methylglyoxal-derived hydroimidazolone 1; suPAR, soluble urokinase plasminogen activator receptor.
Risk Factors for DKD
For the exploratory outcomes, we found that HOMA-IR decreased after 6 diet cycles [−3.8 −5.6, −2.0); P ≤ 0.05] in the noninsulin treated participants of FMD group compared to M-Diet (Fig. 2B, Table 2). After 3 diet cycles MG-H1 reduced in both study groups, and the reduction in the FMD group was significantly higher than the reduction in the M-Diet group leading to a between-groups change in MG-H1 of −39.4 (−46.7, −32.1) nM (P ≤ 0.01) (Fig. 2C, Table 2). After 6 FMD cycles, change in MG-H1 was comparable between study groups [39.5 (−91.3, 170.3) nM; P = 0.13] (Fig. 2C, Table 2). Change in MG-H1 did not associate with change in ACR, either in the M-Diet group or in the FMD group (data not shown). suPAR decreased after 3 [−214.8 (−238.4, −191.3) pg/mL; P ≤ 0.05] and after 6 diet cycles [−156.6 (−172.9, −140.4) pg/mL; P ≤ 0.05] (Fig. 2D, Table 2). Blood ketones increased 3-fold in the FMD group after 3 diet cycles [0.50 mmol (0.32, 0.68), P ≤ 0.001] and after 6 diet cycles [0.50 mmol (0.25, 0.75), P ≤ 0.001] (Fig. 2E, Table 2). A high MDS in the M-Diet group was reported after 3 [6.1 MDS (5.5, 6.7)] and after 6 diet cycles [6.1 MDS (5.4, 6.7)] as sign of good compliance.
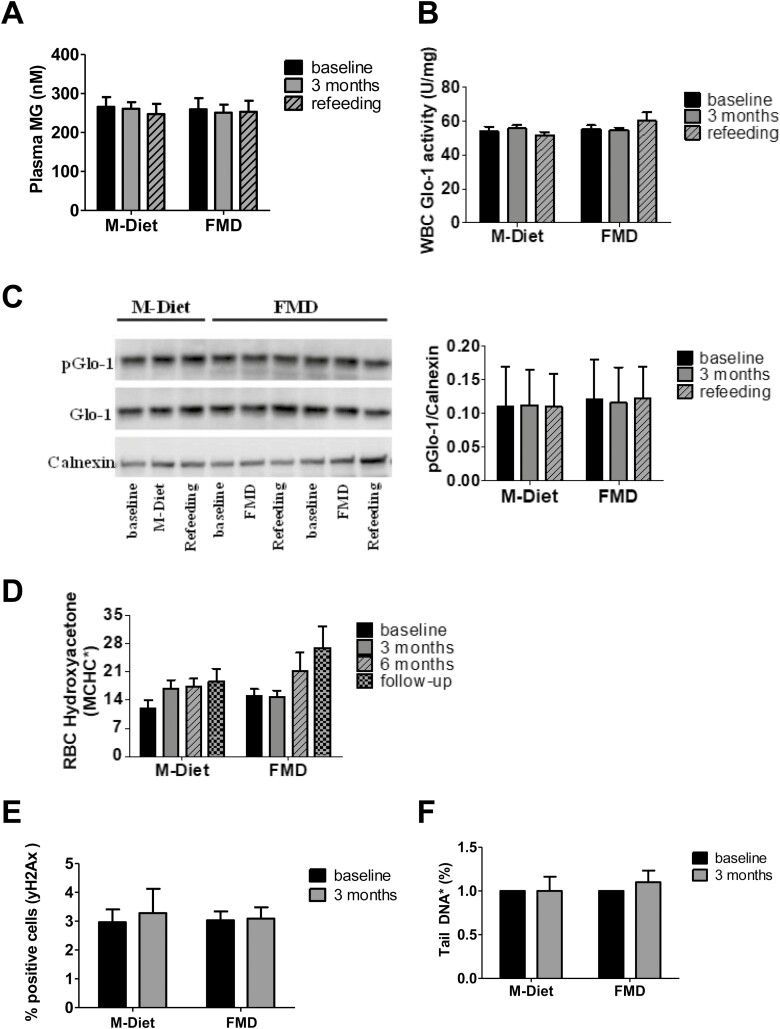
Plasma MG, Glo-1 activity, and pGlo-1 expression in WBC, hydroxyacetone levels in red blood cells (Fig. 3A-D), and yH2Ax levels as well percentage of comet tail in WBC (Fig. 3E and 3F) did not change in either diet. Both study groups were mainly comprised of male participants (74% of the M-Diet group and 71% of the FMD group), and no differences in primary or secondary endpoints were found between female and male participants (data not shown).
Figure 3.
Plasma methylglyoxal level (A), glyoxalase 1 activity in white blood cells (B), phosphorylated glyoxalase-1 expression in white blood cells (C), hydroxyacetone concentration in red blood cells (D), yH2Ax in white blood cells (E), and comet assay with white blood cells (F). Data are shown as mean ± SE of the mean of unadjusted values of parameter. Total Glo-1 immunoblotting is a rehybridization of the pGlo-1 in (C). Representative participants were analyzed in (A) M-Diet, n = 11, FMD, n = 11; (B) M-Diet n = 11, FMD n = 12; (C) M-Diet n = 6, FMD n = 12; (D) M-Diet n = 19, FMD n = 21; (E) M-Diet n = 6, FMD n = 9; and (F) M-Diet n = 6, FMD n = 12. Abbreviations: FMD, fasting-mimicking diet; Glo-1, glyoxalase 1; MCHC, mean corpuscular hemoglobin concentration M-Diet, Mediterranean diet; MG, methylglyoxal; pGlo-1, phosphorylated glyoxalase 1; RBC, red blood cells; WBC, white blood cell; yH2Ax phosphorylated histone H2AX.
The changes in fasting plasma glucose, low-density lipoprotein, high-density lipoprotein, or triglyceride levels were similar between study groups throughout the study (Table 2). Weight loss of −5.6kg [−5.9% (−6.5, −4.6), P ≤ 0.001] after 3 diet cycles and of −7.2 kg [−7.5% (−9.4, −4.9), P ≤ 0.001] after 6 diet cycles was observed in the FMD group, compared to a weight loss of −0.7kg [−0.8% (−2.1, 0.8)] after 3 diet cycles and −1.1 kg [−1.2% (−2.9, 0.7)] after 6 diet cycles in the M-Diet group. However, when adjusted for weight loss, body composition analysis revealed no change between the 2 study groups, neither in fat nor in fat-free mass (Table 2).
Fatty Acid Oxidation and Amino Acid Metabolism
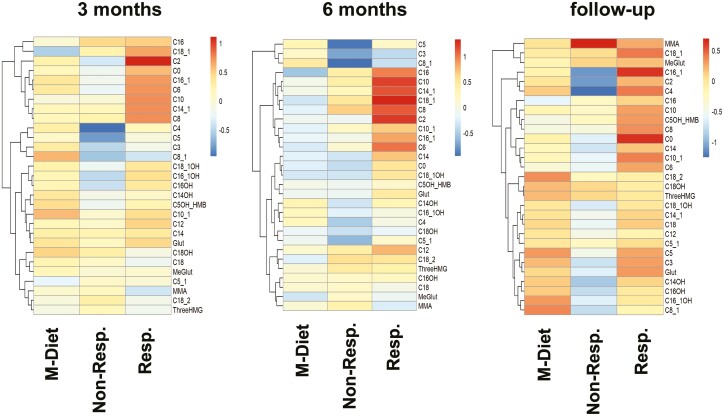
Post hoc exploratory analysis of participants of the FMD group who showed at least a 30% improvement in ACR (referred to as “responders”) elicited a distinct response in AC profile compared to the nonresponders, especially in acetylcarnitine levels (C2) (Fig. 4). Notably, C2 levels increased after 3 diet cycles in responders [4.2 µmol/L (0.2, 8.2), P ≤ 0.01] compared to nonresponders [−1.9 µmol/L (−4.5, 0.8)] and to the M-Diet group [0.7µmol/L (−0.7, 2.1)]. A similar increase was observed also after 6 diet cycles (P ≤ 0.01). Alanine concentration in responders after 6 diet cycles was reduced [−48.4 µmol/L (−78.8, −18.1), P ≤ 0.05], whereas glycine concentration was increased [39.6 µmol/L (18.8, 60.4), P ≤ 0.05]. When exploring for parameters that could explain the distinct metabolic response between responders and nonresponders in both study groups, we found no differences in anthropometric and metabolic parameters or body weight (data not shown). Moreover, no differences in medication at baseline and throughout the study was observed between responders and nonresponders (data not shown). Of note, responders of both groups showed lower ACR levels already at baseline [FMD responders 85.7 mg/g (46.7, 124.7)], compared to FMD nonresponders [271 mg/g (20, 541.2), P ≤ 0.05] and M-Diet responders [55.5 mg/g (23.3, 87.7)] compared to M-Diet nonresponders [563.0 mg/g (6, 1120), P ≤ 0.05].
Figure 4.
Effects of fasting on acylcarnitines after 3 months, after 6 months, and at follow-up. Heat map analysis of acylcarnitine profile reveal differences between the study groups dependent on change in albuminuria. Patients in the intervention group that show at least a 30% decrease in albuminuria level after 3 and after 6 diet cycles compared to the respective baseline are referred as “responders,” while the rest are referred as “nonresponders.” Groups in the analysis: M-Diet, nonresponders, and responders. Each row displays a metabolite and each column represent the absolute change of the annotated metabolite after 3 diet cycles (left panel), after 6 diet cycles (middle panel), and at follow-up (right panel) compared to baseline of the respective group and is displayed as range-scaled Z-score. Metabolites increased are displayed in red while metabolites decreased are displayed in blue. Abbreviations: 3HMG, 3-hydroxy-3-methylglutarylcarnitin; C0, carnitine; C2, acetylcarnitine; C3, propionylcarnitine; C4, butyrylcarnitine; isobutyrylcarnitine; C5, valerylcarnitine, isovalerylcarnitine, methylbutyrylcarnitine; C5_1, tiglylcarnitine, methylcrotonylcarnitine; C6, hexanoylcarnitine; C5OH + HMB, hydroxyvalerylcarnitine + 2-OH-3-methyl-butyrylcarnitin; C8, octanoylcarnitine; C8_1, octenoylcarnitine, C10, decanoylcarnitine; C10_1, decenoylcarnitine; C12, dodecanoylcarnitine; C14, tetradecanoylcarnitine; C14_1, tetradecenoylcarnitine; C14OH, hydroxytetradecanoylcarnitin; C16, hexadecanoylcarnitine; C16_1, hexadecenoylcarnitine; C16_1OH, hydroxyhexadecenoylcarnitine; C16OH, hydroxyhexadecanoylcarnitine; C18, octadecanoylcarnitine; C18_1, octadecenoylcarnitine; C18_1OH, hydroxyoctadecenoylcarnitine; C18_2, octadecadienylcarnitine; C18OH, hydroxyoctadecanoylcarnitin; glut glutarylcarnitine; M-Diet, Mediterranean diet; MeGlut, methylglutarylcarnitine; MMA, methylmalonylcarnitin, Non-Resp., nonresponders; Resp., responders.
Partially Sustained Changes at Follow-up
At follow-up, only the reduction in HOMA-IR was sustained [−1.9 (−3.7, −0.1), P ≤ 0.05], (Fig. 2B, Table 2), whereas no change in body weight or body composition was reported compared to baseline (Table 2). Apart from 3 participants of the FMD group, all other participants had to some degree increased the antihyperglycemic medication (data not shown). At follow-up the changes reported during FMD on ACR (Fig. 2A, Table 2), MG-H1 plasma levels (Fig. 2C), suPAR (Fig. 2D), and AC plasma profile (Fig. 4) were reversed to baseline values.
Safety of FMD and Effect on Antihyperglycemic and Antihypertensive Medication
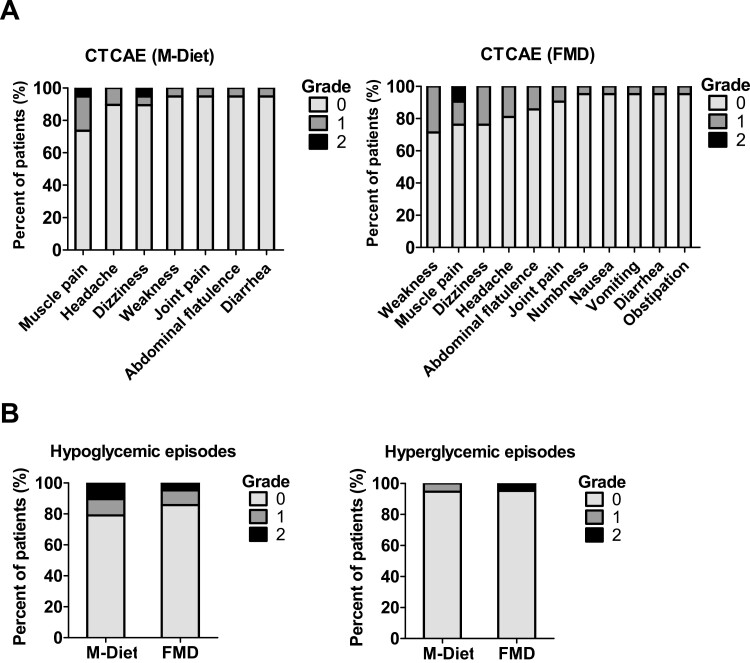
FMD was well tolerated with 71% to 95% of the participants (depending on the adverse event) reporting no adverse effects. The most common self-reported grade 1 (mild) or grade 2 (moderate) symptoms experienced were weakness, muscle pain, dizziness, and headache. No adverse effects of grade 3 or higher were reported (Fig. 5A). Hypoglycemic episodes experienced during diet intervention did not differ between the study groups [grade 1 hypoglycemic episodes: 10% (FMD) vs 11% (M-Diet), grade 2 hypoglycemic episodes 5% (FMD) vs 11% (M-Diet)] (Fig. 5B).
Figure 5.
Subject self-reported adverse effects based on Common Terminology Criteria for Adverse Events (A). Hypoglycemic and hyperglycemic episodes in both study groups reported during the study (B). 1 = mild, 2 = moderate, 3 = severe, 4 = life-threatening, 5 = death. Percentage of subjects reporting no adverse effect (grade 0), grade 1, or grade 2 adverse effects; grades 3 to 5 were not reported. Abbreviations: FMD, fasting-mimicking diet; M-Diet, Mediterranean diet.
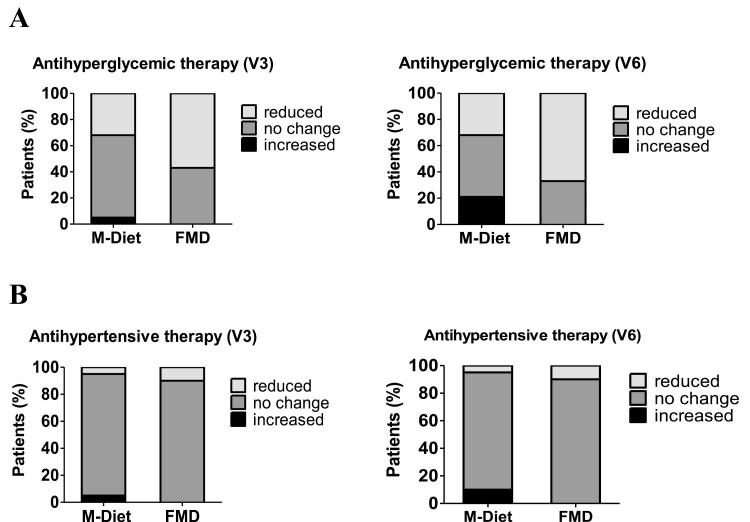
Glycemic and blood pressure control were comparable at baseline (Table 2). After 3 diet cycles, antihyperglycemic medication could be reduced in 57% of participants in the FMD group compared to 32% of the M-Diet group. In 5% of the participants of the M-Diet group, antihyperglycemic medication had to be increased (Fig. 6A). After 6 diet cycles, antihyperglycemic medication could be reduced in 67% of participants in the FMD group compared to baseline, whereas in 21% of the participants of the M-Diet group antihyperglycemic medication had to be increased compared to baseline (Fig. 6A). Antihypertensive medication could also be reduced in 10% of the participants of the FMD group compared to 5% of the participants of the M-Diet group after 3 and after 6 diet cycles. In contrast, in 5 % of the participants of the M-Diet group after 3 diet cycles and 10% after 6 diet cycles, antihypertensive medication had to be increased (Fig. 6B). Subjective health status, as well as somatic and depression symptoms, did not differ between the groups and throughout the study (data not shown).
Figure 6.
Change of antihyperglycemic (A) and antihypertensive medication (B) after 3 diet cycles (V3) and after 6 diet cycles (V6). Abbreviations: FMD, fasting-mimicking diet; M-Diet, Mediterranean diet.
Discussion
Our study explored for the first time in a randomized controlled design the clinical impact of periodic fasting in type 2 diabetes patients and showed that FMD is safe and well tolerated when accompanied by intensive diabetes care. No episodes of severe hypoglycemia or hypotension were reported, and the intensive medical control the study participants received in this study makes, on one hand, this study exceptional and, on the other hand, points out the complexity of a fasting intervention in type 2 diabetes patients—a message we believe will be very helpful in implementing such dietary recommendations in diabetes care. There were no significant differences in change of albuminuria between the study groups in the overall study population. The small size of the study did not provide adequate power to assess diet efficacy against the primary endpoint in the subgroup of patients with macroalbuminuria at baseline. However, the significant reduction of ACR in the FMD group in patients with microalbuminuria levels at baseline is important, since baseline albuminuria levels have been shown to be predictors of the development of diabetic nephropathy in type 2 diabetes (38). Moreover, the reduction in microalbuminuria observed in this study is comparable to the reduction reported in placebo-controlled studies on SGLT-2 inhibitors (39, 40). On the other hand, the effects of fasting and ketogenesis on renal function in macroalbuminuria phase and overt diabetic nephropathy should be carefully addressed in future studies, since worsening of macroalbuminuria under fasting conditions cannot be excluded. Although improvement and reversibility of microalbuminuria has been reported in studies using angiotensin-converting-enzyme inhibitors or angiotensin II receptor blockers (2, 3), the evidence on therapies improving or resolving macroalbuminuria remains vague (41). These findings suggest for distinct mechanisms in the natural course of DKD and future studies addressing the effect of fasting on kidney-specific disease mechanisms are required (42). Acknowledging the limitation of a small sample size, future studies should address the effect of fasting regimes in albuminuria in type 2 diabetes patients in larger cohorts, also addressing generalizability of such dietary interventions in type 2 diabetes. Albuminuria is thought to be the result of pathogenic events targeting the vasculature, the glomerulus, and tubular cells (43-45). The changes observed during FMD and at follow-up point toward dynamic changes in the cellular events controlling albumin excretion. Of note, this study shows that FMD has no sustained effect on albuminuria but cannot forecast any effects on parameters determining renal function over longer time.
The control group received an isocaloric diet in contrast to the caloric restriction of the FMD. Although, the control group showed a mild weight loss, weight loss in the FMD group was higher and significant when compared to baseline and to the control group. Being aware of this limitation, we adjusted all statistical analyses of the study endpoints with a correction for body weight loss. Moreover, we believe that reduction of albuminuria is less likely affected by the weight loss reported in this study since such weight loss is still persistent at refeeding (1 week after FMD), whereas albuminuria levels increase (data not shown). It is previously reported that improved glycemia and blood pressure, as well as weight loss, cannot completely explain the antialbuminuric effect, and other independent pathways could be involved (33). Few short-term controlled studies on fasting have revealed positive effects on glucose metabolism independent of weight loss (46, 47). On the other hand, glycemia and blood pressure, 2 important factors that could affect albuminuria, were strictly controlled in this study, as reflected in the comparable values between the study groups throughout the study. However, also when analyzing the possible effect of HbA1c or HOMA-IR values on the change in albuminuria, a Pearson correlation analysis between the change in HbA1c and the change in HOMA-IR after 6 months with the change of microalbuminuria after 6 months showed no significant correlation (β = 0.001, P = 0.95 for HbA1c and β = 0.131, P = 0.47 for HOMA-IR). The relationship between glycemia and change in albuminuria is beyond the scope of this manuscript and should be addressed in future studies. Comparable glycemic and blood pressure control between both groups was achieved by a strong reduction in antihyperglycemic and antihypertensive medication over time in the FMD group. This reduction is attributed to the diet intervention, since no other change took place, and reflects an improved metabolic control in these patients.
Our study provides a robust exploratory analysis for potential protective mechanisms of periodic fasting in type 2 diabetes. The acylcarnitine profile and 2 amino acids discriminated between responders and nonresponders with respect to improvement in ACR. While the specific increase in acetylcarnitine levels results most likely from pronounced activation of lipolysis during prolonged fasting periods, it is yet unclear whether acetylcarnitine is just a marker of high compliance to FMD or serves as a substrate for renal metabolism and thereby affects the response of microalbuminuria to FMD (48). This finding is also in line with a previous report associating circulating short-chain fatty acids with normoalbuminuria in diabetes (49). The nephroprotective effects of fatty acid oxidation and ketogenesis are probably explained by the fact that kidney metabolism relies highly on lipolysis and ketone bodies. Several studies on SGLT-2 inhibitors in diabetic patients and animal models of diabetes have shown that it is the induction of a mild ketogenesis that orchestrates the nephroprotective effects of SGLT-2 inhibitors, by shifting energy metabolism to ketolysis (23, 24).
The increase in glycine concentration observed in the responders is in line with protective effects of glycine on renal tubular injury as previously described in experimental models of kidney injury (50). Although we could not show a change in Glo-1 activity in WBC, previous studies have reported that glycine may enhance the function of Glo-1 and restore antioxidant defense in kidney of streptozotocin-induced diabetic rats, thus protecting against renal oxidative stress (51).
We could show that at follow-up, apart from a sustained reduction of HOMA-IR, all other observed changes were reversed to values comparable to baseline. We excluded insulin-treated patients when calculating HOMA-IR values. Nevertheless, also when excluding patients treated with insulin secretagogues, the results on HOMA-IR were comparable to the ones reported in Table 2 (data not shown). The mechanisms behind this sustained reduction of HOMA-IR at follow-up in the patients of the fasting group remain unknown and should be addressed in future studies, although possible effect of the antidiabetic medication during the study and at follow-up cannot be excluded.
ACR values were comparable at follow-up between study groups. The patients with microalbuminuria had nonsignificantly decreased ACR values at follow-up compared to respective baseline values in both study groups. These might be due to the high inter- and intraindividual variability of albuminuria as a biomarker itself. However, other factors could have played a role during the follow-up period—for instance, change of medication, as well as change in blood pressure and glucose control, factors that were strictly controlled during the study intervention. Future studies should explore change of albuminuria under fasting interventions in larger study cohort and should also investigate whether the classical albuminuric DKD and the new-emerging nonalbuminuric DKD are driven from distinct pathophysiological mechanisms. There is growing epidemiological evidence on the heterogeneity in the clinical presentation and course of DKD with divergence between albuminuria and GFR decline (52, 53), challenging, on one hand, the albuminuria-centric model of the natural course of DKD and, on the other hand, suggesting that albuminuria is a dynamic process (42, 54).
We interpret the lack of improvement of dicarbonyl detoxification and lack of reconstitution of DNA damage/repair during FMD as a potential explanation for the lack of change in ACR. This finding is supported also by the fast increase of suPAR (Table 2) after FMD, a marker of accelerated aging and senescence (55). Thus, neither of 2 important defense pathways able to control nephropathy was activated or reconstituted in this study, and longer period of FMD may be needed for nephropathy reversal. However, the different response in MG-H1 reduction after 3 but not after 6 FMD cycles suggests that the changes seen in this study are unlikely due only to reduced energy intake during FMD and that instead a sustained redirection of metabolism is taking place during FMD to some degree.
While diabetic retinopathy was not the focus of this study, the rate of diabetic retinopathy reported at baseline (Table 1) may be underestimated, since we evaluated the presence of diabetic retinopathy with only a funduscopic examination of the undilated pupil. Future studies addressing change of diabetic retinopathy under fasting conditions should use a comprehensive dilated eye exam (including fluorescein angiography and optical coherence tomography).
Despite the robust exploratory analysis, we are aware of the risk for spurious findings, and future studies should address mechanistic explanations of the effect of periodic fasting on albuminuria and kidney function.
In conclusion, this proof-of-concept study showed that when accompanied by intensive diabetes care, FMD cycles can be well integrated into clinical practice, complementary to current guidelines. While not changing albuminuria in the overall study population, FMD could lead to a reduction in albuminuria of patients with microalbuminuria at baseline, leading to an improvement of glycemic and blood pressure control and to a sustained reduction in insulin resistance when adjusted for weight loss. Improvement of microalbuminuria and of markers of insulin resistance, lipid oxidation, and senescence suggests the potential beneficial effects of periodic fasting in type 2 diabetes and should be explored in future studies.
Acknowledgments
The authors thank all study participants and staff members of the Study Ambulance for Diabetes Research Heidelberg involved in the conduct of the study. We thank you Marietta Kirchner (Institute of Medical Biometry, Heidelberg) for her assistance in the statistical analysis.
Glossary
Abbreviations
- AC
acylcarnitines
- ACR
albumin-to-creatinine ratio
- C2
acetylcarnitine
- DKD
diabetic kidney disease
- eGFR
estimated glomerular filtration rate
- FMD
fasting-mimicking diet
- Glo-1
glyoxalase-1
- HOMA-IR
homeostatic model assessment of insulin resistance
- M-Diet
Mediterranean diet
- MDS
Mediterranean diet score
- MG
methylglyoxal
- MG-H1
methylglyoxal-derived hydroimidazolone 1
- SGLT-2
sodium-glucose cotransporter-2
- suPAR
soluble urokinase plasminogen activator receptor
- pGlo-1
phosphorylated glyoxalase-1
- WBC
white blood cells
- yH2Ax
phosphorylated histone H2AX
Contributor Information
Alba Sulaj, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Stefan Kopf, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Ekaterina von Rauchhaupt, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Elisabeth Kliemank, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Maik Brune, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; Joint Heidelberg-IDC Translational Diabetes Program, Helmholtz Center Munich, Neuherberg, Germany.
Zoltan Kender, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Hannelore Bartl, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany.
Fabiola Garcia Cortizo, German Cancer Research Center (DKFZ), Division of Signal Transduction in Cancer and Metabolism, Heidelberg, Germany.
Katarina Klepac, Institute for Diabetes and Cancer, Helmholtz Center Munich, Neuherberg, Germany.
Zhe Han, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany.
Varun Kumar, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany.
Valter Longo, Longevity Institute, School of Gerontology, and Department of Biological Sciences, University of Southern California, Los Angeles, CA, USA; FIRC Institute of Molecular Oncology, Italian Foundation for Cancer Research Institute of Molecular Oncology, Milan, Italy.
Aurelio Teleman, German Cancer Research Center (DKFZ), Division of Signal Transduction in Cancer and Metabolism, Heidelberg, Germany.
Jürgen G Okun, Department of General Pediatrics, Division of Neuropediatrics and Metabolic Medicine, Centre for Pediatric and Adolescent Medicine, University Hospital Heidelberg, Heidelberg, Germany.
Jakob Morgenstern, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Thomas Fleming, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany.
Julia Szendroedi, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany; Joint Heidelberg-IDC Translational Diabetes Program, Helmholtz Center Munich, Neuherberg, Germany.
Stephan Herzig, German Center of Diabetes Research (DZD), Neuherberg, Germany; Institute for Diabetes and Cancer, Helmholtz Center Munich, Neuherberg, Germany; Joint Heidelberg-IDC Translational Diabetes Program, Internal Medicine 1, Heidelberg University Hospital, Heidelberg, Germany; Chair Molecular Metabolic Control, Technical University Munich, Munich, Germany.
Peter P Nawroth, Department of Endocrinology, Diabetology, Metabolism and Clinical Chemistry (Internal Medicine 1), Heidelberg University Hospital, Heidelberg, Germany; German Center of Diabetes Research (DZD), Neuherberg, Germany; Joint Heidelberg-IDC Translational Diabetes Program, Helmholtz Center Munich, Neuherberg, Germany.
Author Contributions
A.S., E.R., E.K., Z.K., H.B., M.B., F.G.C., Z.H., V.K., J.G.O., J.M., and T.F. contributed to the acquisition and interpretation of data. A.S., S.H., and P.N. contributed to the study design and interpretation of data. A.S., J.S., and P.N. drafted the report. All authors contributed to the review of the report and approved the final version for submission. A.S. and P.N. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Financial Support
This study was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation; project number 236360313–SFB 1118 and project number 255156212–SFB 1158) as well as by the Deutsches Zentrum für Diabetesforschung (DZD; German Center for Diabetes Research; project number 82DZD07C2G). The fasting-mimicking diet used in this study was funded by L-Nutra. All funding sources had no influence on design and conduct of this study, collection, analysis, and interpretation of the data or on the preparation, review, or approval of this article.
Clinical Trial Registration
German Clinical Trials Register (Deutsches Register Klinischer Studien DRKS), DRKS-ID: DRKS00014287.
Disclosures
The authors declare that there are no relationships or activities that might bias, or be perceived to bias, their work. L-Nutra, as funder of the fasting-mimicking diet used in this study, has no role in the design or conduct of the study nor in the preparation, review, or approval of the manuscript. V.L. is founder and shareholder of L-Nutra; his shares are destined to the Create Cures Foundation and other charitable and research organizations.
Data Availability
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, the data sets generated from the current study are available from the corresponding author on reasonable request.
Prior Presentation
Preliminary data of this study were presented as an abstract at the German Diabetes Congress in 2019 and 2021, at the Annual Meeting of the European Diabetic Nephropathy Study Group in 2021 and at the Annual Meeting of the European Association for the Study of Diabetes in 2021.
References
- 1. Brenner B, Parving H-H, Mauer M, Ritz E. Diabetic nephropathy. In: Brenner BM, ed. Brenner and Rector’sThe Kidney, 8th ed. WB Saunders; 2006:1265-1298. [Google Scholar]
- 2. Wanner C, Inzucchi SE, Lachin JM, et al. . Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334. [DOI] [PubMed] [Google Scholar]
- 3. Ruggenenti P, Cravedi P, Remuzzi G. The RAAS in the pathogenesis and treatment of diabetic nephropathy. Nat Rev Nephrol. 2010;6(6):319-330. [DOI] [PubMed] [Google Scholar]
- 4. Zoja C, Xinaris C, Macconi D. Diabetic nephropathy: novel molecular mechanisms and therapeutic targets. Front Pharmacol. 2020;11:586892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Brownlee M. The pathobiology of diabetic complications: a unifying mechanism. Diabetes. 2005;54(6):1615-1625. [DOI] [PubMed] [Google Scholar]
- 6. Schleicher E, Nerlich A. The role of hyperglycemia in the development of diabetic complications. Horm Metab Res. 1996;28(08):367-373. [DOI] [PubMed] [Google Scholar]
- 7. Kolm-Litty V, Sauer U, Nerlich A, Lehmann R, Schleicher E. High glucose-induced transforming growth factor beta1 production is mediated by the hexosamine pathway in porcine glomerular mesangial cells. J Clin Invest. 1998;101(1):160-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brownlee M, Cerami A, Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988;318(20):1315-1321. [DOI] [PubMed] [Google Scholar]
- 9. Bierhaus A, Narwoth PP. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52(11):2251-2263. [DOI] [PubMed] [Google Scholar]
- 10. Rabbani N, Thornalley PJ. Advanced glycation end products in the pathogenesis of chronic kidney disease. Kidney Int. 2018;93(4):803-813. [DOI] [PubMed] [Google Scholar]
- 11. Bierhaus A, Nawroth P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia. 2009;52(11):2251-2263. [DOI] [PubMed] [Google Scholar]
- 12. Fleming T, Cuny J, Nawroth G, et al. . Is diabetes an acquired disorder of reactive glucose metabolites and their intermediates? Diabetologia. 2012;55(4):1151-1155. [DOI] [PubMed] [Google Scholar]
- 13. Blasiak J, Arabski M, Krupa R, et al. . DNA damage and repair in type 2 diabetes mellitus. Mutat Res. 2004;554(1-2):297-304. [DOI] [PubMed] [Google Scholar]
- 14. Burton D, Faragher R. Obesity and type-2 diabetes as inducers of premature cellular senescence and ageing. Biogerontology. 2018;19(6):447-459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tatsch E, Bochi GV, Piva SJ, et al. . Association between DNA strand breakage and oxidative, inflammatory and endothelial biomarkers in type 2 diabetes. Mutat Res. 2012;732(1-2):16-20. [DOI] [PubMed] [Google Scholar]
- 16. Palmer AK, Tchkonia T, LeBrasseur NK, Chini EN, Xu M, Kirkland JL. Cellular senescence in type 2 diabetes: a therapeutic opportunity. Diabetes. 2015;64(7):2289-2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kumar V, Agrawal R, Pandey A, et al. . Compromised DNA repair is responsible for diabetes-associated fibrosis. EMBO J. 2020;39(11):e103477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Longo VD, Mattson MP. Fasting: molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stranahan AM, Mattson MP. Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci. 2012;13(3):209-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Longo VD, Panda S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016;23(6):1048-1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wei M, Brandhorst S, Shelehchi M, et al. . Fasting-mimicking diet and markers/risk factors for aging, diabetes, cancer, and cardiovascular disease. Sci Transl Med. 2017;9(377):eaai8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brandhorst S, Choi IY, Wei M, et al. . A periodic diet that mimics fasting promotes multi-system regeneration, enhanced cognitive performance, and healthspan. Cell Metab. 2015;22(1):86-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrannini E, Mark M, Mayoux E. CV protection in the EMPA-REG OUTCOME trial: a “thrifty substrate” hypothesis. Diabetes Care. 2016;39(7):1108-1114. [DOI] [PubMed] [Google Scholar]
- 24. Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Diabetes Care. 2016;39(7):1115-1122. [DOI] [PubMed] [Google Scholar]
- 25. Ferrannini E, Baldi S, Frascerra S, et al. . Shift to fatty substrate utilization in response to sodium–glucose cotransporter 2 inhibition in subjects without diabetes and patients with type 2 diabetes. Diabetes. 2016;65(5):1190-1195. [DOI] [PubMed] [Google Scholar]
- 26. Verma S, McMurray JJ. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61(10):2108-2117. [DOI] [PubMed] [Google Scholar]
- 27. Diabetes AA. Updates to the standards of medical care in diabetes—2018. Diabetes Care. 2018;41(9):2045-2047. [DOI] [PubMed] [Google Scholar]
- 28. Molitch ME, Adler AI, Flyvbjerg A, et al. . Diabetic kidney disease: a clinical update from kidney disease: improving global outcomes. Kidney Int. 2015;87(1):20-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Estruch R, Ros E, Salas-Salvadó J, et al. . Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N Engl J Med. 2018;378(25):e34. [DOI] [PubMed] [Google Scholar]
- 30. Ware J Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. [DOI] [PubMed] [Google Scholar]
- 31. Gräfe K, Zipfel S, Herzog W, Löwe B. Screening psychischer Störungen mit dem “Gesundheitsfragebogen für Patienten (PHQ-D)”: Ergebnisse der deutschen Validierungsstudie [Screening for psychiatric disorders with the Patient Health Questionnaire (PHQ). Results from the German validation study]. Diagnostica; 2004;50(4):171-181. [Google Scholar]
- 32. Wanner C, Inzucchi SE, Lachin JM, et al. ; EMPA-REG OUTCOME Investigators. Empagliflozin and progression of kidney disease in type 2 diabetes. N Engl J Med. 2016;375(4):323-334. [DOI] [PubMed] [Google Scholar]
- 33. Heerspink HJ, Perkins BA, Fitchett DH, Husain M, Cherney DZ. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes mellitus: cardiovascular and kidney effects, potential mechanisms, and clinical applications. Circulation. 2016;134(10):752-772. [DOI] [PubMed] [Google Scholar]
- 34. Sauer SW, Okun JG, Fricker G, et al. . Intracerebral accumulation of glutaric and 3-hydroxyglutaric acids secondary to limited flux across the blood–brain barrier constitute a biochemical risk factor for neurodegeneration in glutaryl-CoA dehydrogenase deficiency. J Neurochem. 2006;97(3):899-910. [DOI] [PubMed] [Google Scholar]
- 35. Rabbani N, Thornalley PJ. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat Protocols. 2014;9(8):1969-1979. [DOI] [PubMed] [Google Scholar]
- 36. Schumacher D, Morgenstern J, Oguchi Y, et al. . Compensatory mechanisms for methylglyoxal detoxification in experimental & clinical diabetes. Mol Metab. 2018;18:143-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72(1-2):248-254. [DOI] [PubMed] [Google Scholar]
- 38. Murussi M, Baglio P, Gross JL, Silveiro SP. Risk factors for microalbuminuria and macroalbuminuria in type 2 diabetic patients: a 9-year follow-up study. Diabetes Care. 2002;25(6):1101-1103. [DOI] [PubMed] [Google Scholar]
- 39. Cherney D, Lund SS, Perkins BA, et al. . The effect of sodium glucose cotransporter 2 inhibition with empagliflozin on microalbuminuria and macroalbuminuria in patients with type 2 diabetes. Diabetologia. 2016;59(9):1860-1870. [DOI] [PubMed] [Google Scholar]
- 40. Heerspink H, Johnsson E, Gause-Nilsson I, Cain V, Sjöström C. Dapagliflozin reduces albuminuria in patients with diabetes and hypertension receiving renin-angiotensin blockers. Diabetes Obes Metab. 2016;18(6):590-597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brenner BM, Cooper ME, De Zeeuw D, et al. . Effects of losartan on renal and cardiovascular outcomes in patients with type 2 diabetes and nephropathy. N Engl J Med. 2001;345(12):861-869. [DOI] [PubMed] [Google Scholar]
- 42. Alicic RZ, Rooney MT, Tuttle KR. Diabetic kidney disease: challenges, progress, and possibilities. Clin J Am Soc Nephrol. 2017;12(12):2032-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Raja P, Maxwell AP, Brazil DP. The potential of albuminuria as a biomarker of diabetic complications. Cardiovasc Drugs Ther. 2021;35(3):455-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Benzing T, Salant D. Insights into Glomerular Filtration and Albuminuria. N Engl J Med. 2021;384(15):1437-1446. [DOI] [PubMed] [Google Scholar]
- 45. Sulaj A, Kopf S, Gröne E, et al. . ALCAM a novel biomarker in patients with type 2 diabetes mellitus complicated with diabetic nephropathy. J Diabetes Complications. 2017;31(6):1058-1065. [DOI] [PubMed] [Google Scholar]
- 46. Parr EB, Devlin BL, Lim K, et al. . Time-restricted eating as a nutrition strategy for individuals with type 2 diabetes: a feasibility study. Nutrients. 2020;12(11):3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212-1221.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Koves TR, Ussher JR, Noland RC, et al. . Mitochondrial overload and incomplete fatty acid oxidation contribute to skeletal muscle insulin resistance. Cell Metab. 2008;7(1):45-56. [DOI] [PubMed] [Google Scholar]
- 49. Moon S, Tsay JJ, Lampert H, et al. . Circulating short and medium chain fatty acids are associated with normoalbuminuria in type 1 diabetes of long duration. Sci Rep. 2021;11(1):8592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yin M, Zhong Z, Connor HD, et al. . Protective effect of glycine on renal injury induced by ischemia-reperfusion in vivo. Am J Physiol Renal Physiol. 2002;282(3):F417-F423. [DOI] [PubMed] [Google Scholar]
- 51. Wang Z, Zhao D, Chen L, et al. . Glycine increases glyoxalase-1 function by promoting nuclear factor erythroid 2-related factor 2 translocation into the nucleus of kidney cells of streptozotocin-induced diabetic rats. J Diabetes Investig. 2019;10(5):1189-1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gregg EW, Li Y, Wang J, et al. . Changes in diabetes-related complications in the United States, 1990–2010. N Engl J Med. 2014;370(16):1514-1523. [DOI] [PubMed] [Google Scholar]
- 53. Kume S, Maegawa H. A new era of diabetic kidney disease treatment with sodium–glucose cotransporter-2 inhibitors. J Diabetes Investig. Published online January 13, 2022. Doi: 10.1111/jdi.13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pugliese G, Penno G, Natali A, et al. . Diabetic kidney disease: new clinical and therapeutic issues. Joint position statement of the Italian Diabetes Society and the Italian Society of Nephrology on “The natural history of diabetic kidney disease and treatment of hyperglycemia in patients with type 2 diabetes and impaired renal function.” Nutr Metab Cardiovasc Dis. 2019;29(11):1127-1150. [DOI] [PubMed] [Google Scholar]
- 55. Hartmann Rasmussen L, Caspi A, Ambler A, et al. . Association between elevated suPAR, a new biomarker of inflammation, and accelerated aging. J Gerontol A Biol Sci Med Sci. 2021;76(2):318-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data are subject to national data protection laws. Therefore, data cannot be made freely available in a public repository. However, the data sets generated from the current study are available from the corresponding author on reasonable request.