Significance
Different elements of a memory, or any mental event, are encoded in locations distributed across the cortex. A prominent hypothesis proposes that widespread networks are integrated with bursts of synchronized high-frequency oscillations called “ripples,” but evidence is limited. Here, using recordings inside the human brain, we show that ripples occur simultaneously in multiple lobes in both cortical hemispheres and the hippocampus, generally during sleep and waking, and especially during memory recall. Ripples phase-lock local cell firing and phase-synchronize with little decay between locations separated by up to 25 cm, enabling long-distance integration. Indeed, corippling sites have increased correlation of very-high-frequency activity which reflects cell firing. Thus, ripples may help bind information across the cortex in memory and other mental events.
Keywords: ripples, cortex, hippocampus, non-rapid eye movement sleep, waking
Abstract
Declarative memory encoding, consolidation, and retrieval require the integration of elements encoded in widespread cortical locations. The mechanism whereby such “binding” of different components of mental events into unified representations occurs is unknown. The “binding-by-synchrony” theory proposes that distributed encoding areas are bound by synchronous oscillations enabling enhanced communication. However, evidence for such oscillations is sparse. Brief high-frequency oscillations (“ripples”) occur in the hippocampus and cortex and help organize memory recall and consolidation. Here, using intracranial recordings in humans, we report that these ∼70-ms-duration, 90-Hz ripples often couple (within ±500 ms), co-occur (≥ 25-ms overlap), and, crucially, phase-lock (have consistent phase lags) between widely distributed focal cortical locations during both sleep and waking, even between hemispheres. Cortical ripple co-occurrence is facilitated through activation across multiple sites, and phase locking increases with more cortical sites corippling. Ripples in all cortical areas co-occur with hippocampal ripples but do not phase-lock with them, further suggesting that cortico-cortical synchrony is mediated by cortico-cortical connections. Ripple phase lags vary across sleep nights, consistent with participation in different networks. During waking, we show that hippocampo-cortical and cortico-cortical coripples increase preceding successful delayed memory recall, when binding between the cue and response is essential. Ripples increase and phase-modulate unit firing, and coripples increase high-frequency correlations between areas, suggesting synchronized unit spiking facilitating information exchange. co-occurrence, phase synchrony, and high-frequency correlation are maintained with little decrement over very long distances (25 cm). Hippocampo-cortico-cortical coripples appear to possess the essential properties necessary to support binding by synchrony during memory retrieval and perhaps generally in cognition.
Ripples are brief high-frequency oscillations that have been well-studied in the rodent hippocampus during non-rapid eye movement sleep (NREM), when they mark the replay of events from the previous waking period, and are critical for memory consolidation in the cortex (1–4). Recently, ripples were found in rat association cortex but not primary sensory or motor cortices during sleep, with increased coupling to hippocampal ripples in sleep following learning (5). An earlier study reported ripples in waking and sleeping cat cortex, especially NREM (6). In humans, cortical ripples have recently been identified during waking and were more frequently found in lateral temporal than in rolandic cortex. Hippocampal sharpwave-ripple occurrence and ripple coupling between parahippocampal gyrus and temporal association cortex increase preceding memory recall in humans (7, 8), possibly facilitating replay of cortical neuron firing sequences established during encoding (9). In rats, ripples co-occur between hippocampus and ∼1 mm2 of parietal cortex in sleep following learning (5), in mice, ripples propagate from the hippocampus to retrosplenial cortex (10), and in cats, ripple co-occurrence is reportedly limited to short distances (6).
We recently reported, using human intracranial recordings, that ∼70-ms-long, ∼90-Hz ripples are ubiquitous in all regions of the cortex during NREM as well as waking (11). During waking, cortical ripples occur on local high-frequency activity peaks. During sleep, cortical ripples typically occur on the cortical down-to-upstate transition, often with 10- to 16-Hz cortical sleep spindles, and local unit firing patterns consistent with generation by pyramidal-interneuron feedback. We found that cortical ripples group cofiring within the window of spike-timing-dependent plasticity. These findings are consistent with cortical ripples contributing to memory consolidation during NREM in humans.
While there is thus an emerging appreciation that hippocampal and cortical ripples have an important role in human and rodent memory, nothing is known of the network properties of cortical ripples. Specifically, it is not known if ripples co-occur or phase-synchronize between cortical sites and, if so, whether this is affected by distance or correlated with the reconstruction of declarative memories. These would be critical properties for cortical ripples to participate in the binding of different elements of memories that are represented in disparate cortical areas, the essence of hippocampus-dependent memory (12).
The binding of disparate elements of a memory is a specific case of a more general problem of how the various contents of a mental event are united into a single experience. Most often addressed is how different visual qualities of an object (e.g., color, shape, location, and texture) are associated with each other (13), but the “binding problem” generalizes to how the contents of awareness are unified in a single stream of consciousness (14). Modern accounts often rely on hierarchical and multimodal convergence. However, cortical processing is distributed, and it would be difficult to represent the combinatorial possibilities contained in all potential experiences with convergence, leading to the suggestion that temporal synchrony binds cortical areas (15). This hypothesis was first supported by phase-locked unit firing and local field potentials (LFPs) at 40 to 60 Hz evoked by simple visual stimuli in the anesthetized cat primary visual cortex at distances <7 mm (16). Although some further studies found similar results in other cortical areas, behaviors, and species, as would be expected under the binding-by-synchrony hypothesis (17, 18), others have been less successful (19). Synchronous high-gamma oscillations have also been criticized as providing no mechanism for neuronal interaction beyond generic activation (19, 20).
Here, using human intracranial stereoelectroencephalography (SEEG) recordings, we find that ripples co-occur and, remarkably, phase-synchronize across all lobes and between both hemispheres, with little decrement, even at long distances. Furthermore, ripple co-occurrence is enhanced between cortical sites as well as between the cortex and hippocampus preceding successful delayed recall. Corippling was progressively above that expected as it involved a larger proportion of sites, and this led to progressively stronger phase locking. Single-unit firing increased during, and phase-locked to, cortical ripples, providing a basic requirement for ripples to enhance communication via gain modulation and coincidence detection. Enhanced communication was supported by our finding of increased high-frequency correlation between even distant corippling regions. These characteristics suggest that distributed, phase-locked cortical ripples possess the properties that may allow them to help integrate different components of a memory. More generally, ripples may help to “bind” different aspects of a mental event encoded in widespread cortical areas into a coherent representation.
Results
Ripple Detection during NREM and Waking.
Ripples were detected using intracranial cortical and hippocampal recordings in 17 patients (SI Appendix, Table 1) undergoing monitoring to localize seizure foci during NREM and waking. Bipolar contact derivations were used to measure LFPs. Ripples were detected only on channels in nonlesional, nonepileptogenic areas. Ripples were required to have three or more cycles of increased peak 70 to 100 Hz analytic amplitude that did not contain epileptiform activity or artifacts. Ripples were also required not to occur at the time of putative interictal spikes detected on any other channel to exclude events that could be spuriously coupled due to epileptiform activity.
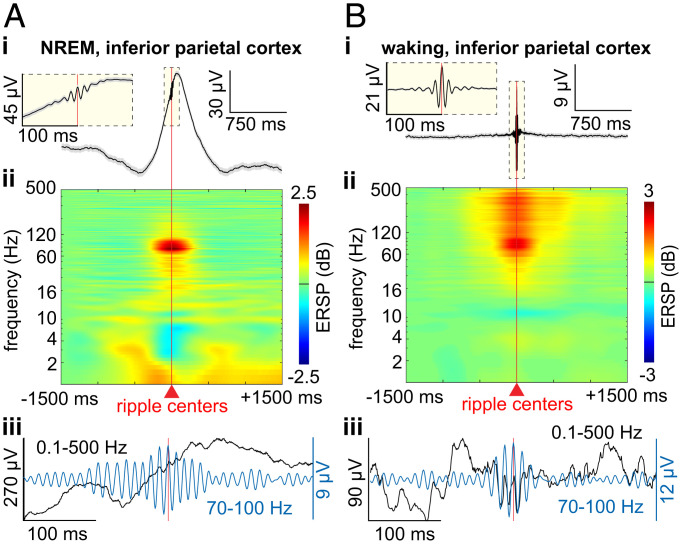
Cortical ripples were detected in all lobes of both hemispheres during NREM and waking (SI Appendix, Fig. S1A and B; n = 273 channels). Cortical and hippocampal ripples were consistently ∼70- to 85-ms-long, ∼90-Hz oscillations. Specifically, cortical ripples during NREM had an average and SD (across channel averages) frequency of 89.1 ± 0.8 Hz, density of 8.4 ± 2.7 min−1, amplitude of 5.1 ± 2.4 µV, and duration of 76.5 ± 10.1 ms. During waking, cortical ripples had a frequency of 89.5 ± 0.7 Hz, density of 5.8 ± 3.4 min−1, amplitude of 7.2 ± 3.9 µV, and duration of 66.6 ± 7.2 ms (Fig. 1A and B; n = 273 channels). Hippocampal ripples during NREM had an average and SD frequency of 85.7 ± 2.0 Hz, density of 7.0 ± 4.8 min−1, amplitude of 17.4 ± 7.8 µV, and duration of 87.5 ± 9.0 ms and during waking had a frequency of 88.2 ± 1.4 Hz, density of 7.4 ± 5.5 min−1, amplitude of 18.0 ± 9.6 µV, and duration of 69.7 ± 7.9 ms (n = 28 channels). An SD of ≤2 Hz in ripple frequency in all circumstances suggests the possibility that local channel and network properties underlie a consistent resonant frequency.
Fig. 1.
Cortical ripple generation during NREM and waking. (A) Average broadband LFP (i) and time-frequency (ii) across cortical ripples and example broadband ripple (iii) unfiltered single sweep (black) and 70- to 100-Hz bandpass (blue) during NREM, in inferior parietal cortex. (B) Same as A except during waking. Error shows SEM. ERSP, event-related spectral power.
Cortical Ripples Couple and Co-occur across Widespread Regions.
We hypothesized that ripples couple (occur within ±500 ms of each other) and especially co-occur between cortical sites, which would be crucial for widespread information integration. We discovered that cortical ripples frequently and strongly couple during both NREM (n = 4,487/4,550 significant channel pairs, randomization test, post-false discovery rate [FDR] P < 0.05) and waking (n = 4,478/4,550), between all cortical areas sampled (Fig. 2A and Table 1), including between hemispheres. The proportion of cortico-cortical channel pairs that were significantly coupled during NREM (98.6%) was not significantly different from that during waking (98.4%; P = 0.44, χ2 = 0.61, degrees of freedom [df] = 1). See SI Appendix, Fig. S2A and B for individual patients.
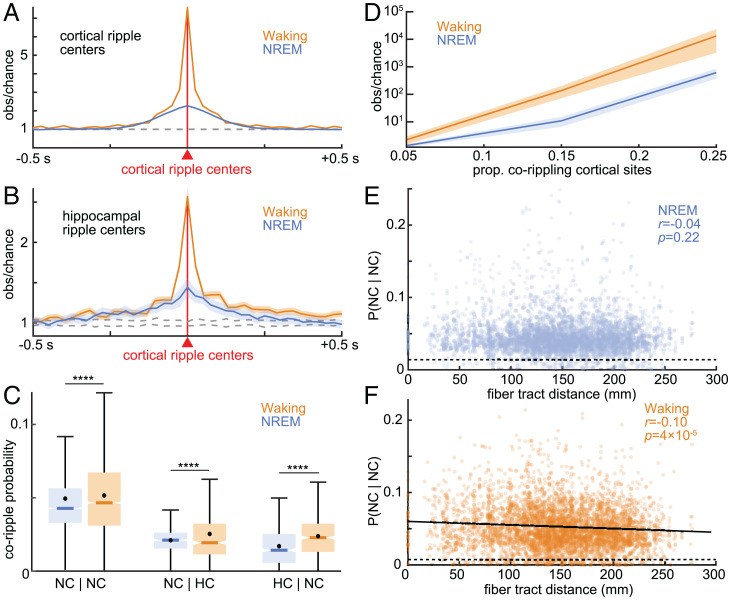
Fig. 2.
Cortico-cortical and hippocampo-cortical ripples couple and co-occur during waking and NREM. (A) Cross-correlograms of ripples between all possible cortical sites reveals strong ripple coupling (occurring within 500 ms of each other without necessarily overlapping) between nearly all sites during NREM (n = 4,487/4,550 significant channel pairs) and waking (n = 4,478/4,550; post-FDR P < 0.05, randomization test). Dashed lines show 99% confidence interval of the null distribution (200 shuffles per channel pair). (B) Same as A except cross-correlograms of hippocampal ripples relative to cortical ripples (NREM: n = 133/461; waking: n = 401/461). (C) Conditional co-occurrence probabilities of cortico-cortical and hippocampo-cortical ripples overlapping for at least 25 ms (i.e., probability of a ripple in a particular channel given a ripple in another particular channel) are greater during waking than NREM (****post-FDR P < 0.0001, two-sided paired t test). (D) Observed over chance cortical ripple co-occurrence (logarithmic scale) increases with the number of sites corippling. (E) Cortical ripple co-occurrence probabilities during NREM are stable with increasing intervening fiber tract distance (linear mixed effects with patient as random effect). Dashed line indicates chance. When two channels were in the same parcel the fiber tract distance was defined as 0. (F) Same as E except for waking. Fit is linear least-squares regression. Error shows SEM.
Table 1.
Cortical ripple coupling with cortical or hippocampal ripples: frequency and order
| Ripple | ripple | Significant modulation | Significant Sidedness | Cortical ripple leading |
|---|---|---|---|
| NREM | |||
| Cort-R | Cort-R | 98.6% (4,487/4,550) | 3.5% (157/4,487) | N/A |
| Hipp-R | Cort-R | 28.9% (133/461) | 31.6% (42/133) | 33.3% (14/42)* |
| Cort-R | Hipp-R | 29.3% (135/461) | 31.1% (42/135) | 33.3% (14/42)* |
| Hipp-SWR | Cort-R | 19.7% (91/461) | 45.1% (41/91) | 24.4% (10/41)* |
| Cort-R | Hipp-SWR | 20.6% (95/461) | 44.2% (42/95) | 21.4% (9/42)* |
| Hipp-SSR | Cort-R | 12.2% (56/461) | 17.9% (10/56) | 50.0% (5/10) |
| Cort-R | Hipp-SSR | 11.7% (54/461) | 22.2% (11/54) | 41.7% (5/12) |
| Waking | |||
| Cort-R | Cort-R | 98.4% (4,478/4,550) | 8.1% (364/4,478) | N/A |
| Hipp-R | Cort-R | 87.0% (401/461) | 22.4% (90/401) | 84.4% (76/90)* |
| Cort-R | Hipp-R | 87.4% (403/461) | 22.3% (90/403) | 84.4% (76/90)* |
Significant modulation: Proportion of channel pairs with a significant increase in the conditional probability of a ripple occurring in the first channel given that one occurred within ±500 ms in the second (e.g., Hipp-R | Cort-R refers to hippocampal ripples occurring within ±500 ms relative to cortical ripples at t = 0; one-sided randomization test, 200 shuffles, 25-ms nonoverlapping bins, three consecutive bins each with post-FDR P < 0.05 required for significance). During NREM, conditional probabilities are shown separately for those hippocampal ripples associated with sharpwaves (Hipp-SWR) and sleep spindles (Hipp-SSR) as well as all ripples (Hipp-R). Significant sidedness: Those with significant modulations that had significant sidedness preference around t = 0 (post-FDR P < 0.05, two-sided binomial test, expected = 0.5, −500 to −1 ms vs. 1 to 500 ms). Cortical ripple leading: Those with significant sidedness around 0 that had cortical ripples leading (according to counts within −500 to −1 ms vs. 1 to 500 ms). During NREM, Hipp-R and Hipp-SWR led Cort-R, and during waking, Cort-R led Hipp-R (*P < 0.05, two-sided binomial test, expected value = 0.5), resulting in a significant preference for cortical ripples to lead hippocampal ripples in waking vs. NREM (P < 0.00001, χ2 = 51.59, df = 1). See SI Appendix, Table 3 for results from individual patients. N/A, not applicable since both channels are cortical.
Critically, we found that short latency coupling led to co-occurrence (≥25-ms overlap) of these ∼70-ms-long ripples, with slightly but significantly higher probabilities during waking than NREM (Fig. 2C). The percent of cortico-cortical channel pairs with significant ripple co-occurrence was very high and nearly equal during NREM (n = 2,218/2,275, 97.5%) vs. waking (n = 2,225/2,275, 97.8%). We also found that the co-occurrence probabilities of cortico-cortical ripples across channels were correlated between NREM and waking (SI Appendix, Fig. S3A; r = 0.41, P = 7 × 10−180, significance of r). In sum, nearly all cortico-cortical channel pairs couple and co-occur above chance during both NREM and waking.
Hippocampal and Cortical Ripples Couple and Co-occur, Especially during Waking, but at Lower Rates than Cortico-Cortical.
As previously found (8), ripples in many hippocampo-cortical channel pairs were also significantly coupled (Fig. 2B and Table 1) during both NREM (n = 133/461) and waking (n = 401/461), but at a significantly lower rate than cortico-cortical pairs in both states (NREM: P < 0.00001, χ2 = 2,832.0, df = 1; waking: P < 0.00001, χ2 = 213.3, df = 1). Unlike cortico-cortical pairs, the proportion of significant hippocampo-cortical pairs was higher during waking (87.0%) than NREM (28.9%, P < 0.00001, χ2 = 319.6, df = 1). See SI Appendix, Fig. S2C and D for individual patients. These findings of cortico-cortical and hippocampo-cortical couplings were maintained when only cortical and hippocampal channels that were free of interictal spikes at any time were analyzed (SI Appendix, Table 2).
Overlapping co-occurrence of human ripples between cortex and hippocampus does not appear to have been studied per se but would also be expected to occur given their coupling. Indeed, we found significant co-occurrence of ripples in 81.1% (n = 374/461) of hippocampo-cortical site pairs during waking and 34.7% (n = 160/461) during NREM. Higher hippocampo-cortical co-occurrence during waking compared to NREM was significant (P < 0.00001, χ2 = 203.8, df = 1). In addition, these percentages for hippocampo-cortical co-occurrences (81.1% waking; 34.7% NREM) are significantly lower than for cortico-cortical site pairs (97.5% waking; 97.8% NREM) (NREM: P < 0.00001, χ2 = 1,328.8, df = 1; waking: P < 0.00001, χ2 = 1,250.1, df = 1). Thus, ripples in hippocampal and cortical sites do couple and co-occur, but at a substantially lower rate than between cortical sites.
Cortical Ripples Lead Hippocampal Ripples during Waking.
Some memory models posit that information is transferred during waking from cortex to hippocampus for memory encoding. During waking, 22.4% (90/401) of channel pairs had a significant order preference (Table 1; post-FDR P < 0.05, two-sided binomial test, expected value = 0.05). Among these significant pairs, cortical ripples led hippocampal ripples in 84.4% (76/90). During NREM, 31.6% (42/133) of the hippocampo-cortical pairs that were significantly coupled had a significant sidedness preference, where one channel’s ripples led the other’s. While hippocampal ripples led cortical ripples in 66.7% (28/42) of these significant pairs during NREM, this effect was largely driven by one patient (SI Appendix, Table 3), unlike the effect of cortical ripples leading hippocampal ripples during waking, which was consistent across the majority of patients (SI Appendix, Table 3). The overall preference for cortical ripples to lead hippocampal ripples during waking compared to NREM was highly significant (P = 4 × 10−9, χ2 = 34.5, df = 1).
Hippocampal Sharpwave Ripples Lead Cortical Ripples during NREM.
While the results above show that cortical ripples precede hippocampal ripples during waking, their order during NREM appears to be a mixed picture, which we hypothesized depends on whether the hippocampal ripples occur in the context of a sharpwave or spindle (2, 21, 22). We found that hippocampal sharpwave ripples significantly preceded cortical ripples by ∼250 ms (SI Appendix, Fig. S4A; P = 0.002, two-sided binomial test, expected value = 0.5), whereas spindle ripples were concurrent (SI Appendix, Fig. S4B; P = 1). These results reinforce a previous suggestion that sharpwave and spindle ripples make sequential contributions to consolidation. Overall, as predicted by models of hippocampo-cortical interaction for memory, hippocampal ripples usually lead cortical during sleep, and cortical usually lead hippocampal during waking.
Cortical Ripple Co-occurrence Is Facilitated through Activation across Multiple Sites.
In each patient, we found that when two cortical sites corippled, one or more additional sites may join them (SI Appendix, Fig. S5). To test if two cortical sites corippling made it more likely for other sites also to coripple, we computed a χ2 test of proportions for all possible groups of three cortical channels under the null hypothesis that the co-occurrence of channels A and B has no relation to the co-occurrence of A and C. We found significantly increased co-occurrence of the third site in an average of 14.1% of triplets during NREM and 38.8% during waking (patient specific results reported in SI Appendix, Table 4, χ2 test of proportions, FDR-corrected P values across channel triplets within patients).
In further support that corippling is facilitated through activation across multiple sites, we found that the number of ripple co-occurrences relative to chance increased with the number of locations corippling (>2) (Fig. 2D). The increase was pronounced, such that the observed number of co-occurrences relative to baseline of 25% of all channels was increased by a factor of ∼104 during waking and ∼5 × 103 during NREM. Thus, corippling appears to beget more corippling, suggesting the possibility of self-reinforcing spread.
Cortical Ripples Co-occur Robustly across Distance.
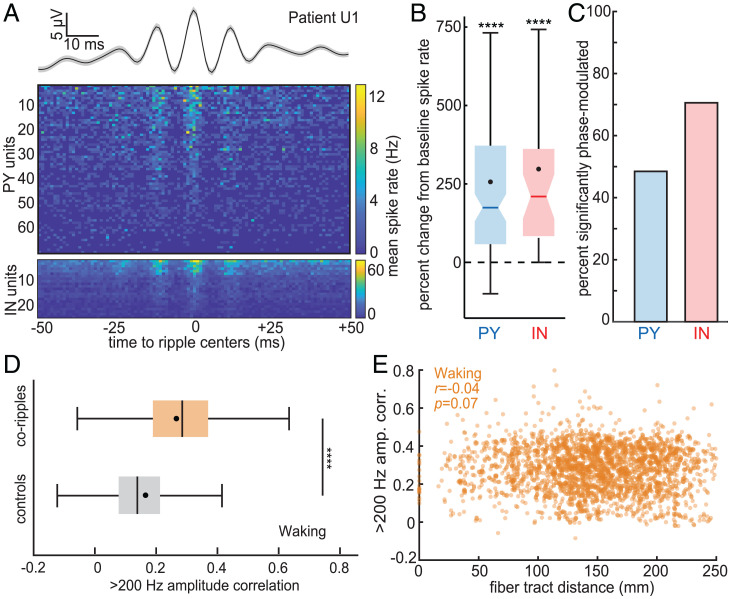
Binding by ripples requires that they co-occur across widespread cortical areas. We compared conditional probabilities of cortico-cortical ripple co-occurrences (i.e., the probability of a ripple in one cortical site given a ripple in another cortical site, requiring ≥25-ms overlap) against white-matter streamline distances between cortical sites. Streamline distances were computed using the 360 parcels of the HCP-MMP1.0 atlas (23), as determined by probabilistic diffusion MRI tractography (24), and are population averages (25). We found that cortico-cortical corippling probability did not decrease with fiber tract distance during NREM (Fig. 2E, r = −0.04, P = 0.22, linear mixed effects with patient as random effect) but rather was maintained up to 25-cm separation, across lobes and between hemispheres. During waking, coripple probabilities were also maintained across this distance range, albeit with a weak but significant negative linear relationship (Fig. 2F, r = −0.10, P = 4 × 10−5). See SI Appendix, Fig. S6A and B for individual patients.
Ripples in All Cortical Areas Co-occur with Hippocampal Ripples.
It was previously reported that ripple coupling occurs between the parahippocampal gyrus and 16.4% of lateral temporal electrodes but only 3.3% of rolandic (8), perhaps reflecting the anatomical location of the hippocampus at the apex of the cortical hierararchy (26). However, we found that hippocampal ripples co-occurred with ripples in all cortical areas at approximately equal rates in both NREM and waking. Taking the myelination index as a measure of position in the cortical hierarchy [association areas are less myelinated (27)], we found a weak but significant effect during NREM but not waking. During NREM, hippocampo-cortical co-occurrence was positively correlated with myelination (SI Appendix, Fig. S7; r = 0.15, P = 0.01), indicating that hippocampal ripples are slightly more likely to co-occur with ripples in primary cortical areas.
Cortico-Cortical and Hippocampo-Cortical Ripple Co-occurrence Precedes Recall.
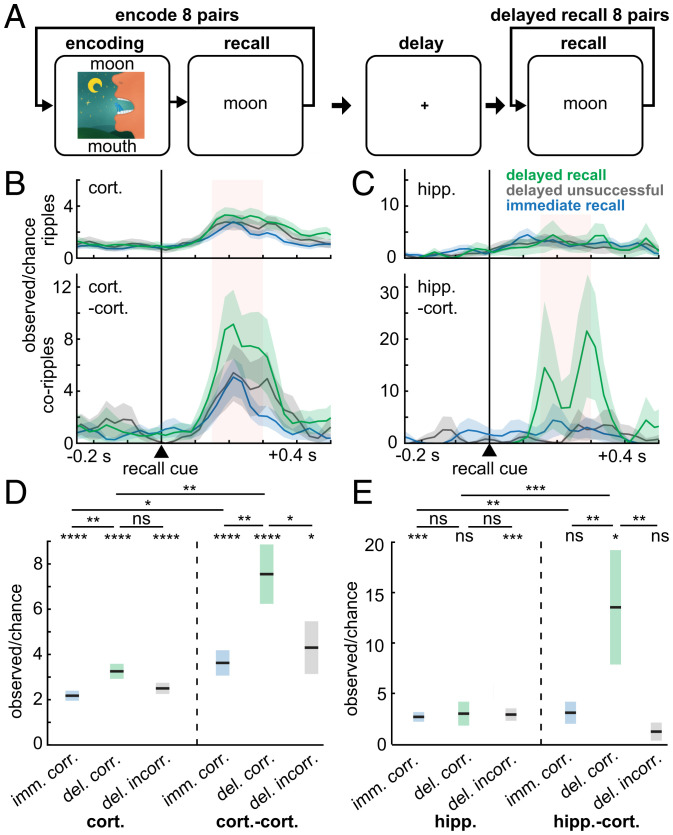
If ripples in different cortical regions bind the elements of a memory, it would be expected that cortical ripples co-occur preceding cued recall, which requires that those elements be coactivated. To test this hypothesis, we analyzed paired-associates memory task data from five SEEG patients (Fig. 3A and SI Appendix, Tables 1 and 5). Preceding delayed cued recall, there was a significant increase in cortical ripple occurrence (P = 1 × 10−11; linear mixed-effects models with patient as random effect) of 330% of chance (computed on a trialwise basis, thus chance was determined separately for immediate and delayed recall), and an even greater increase in cortico-cortical ripple co-occurrence of 758% compared to chance (P = 0.0004; Fig. 3B–E). Furthermore, cortical ripple occurrence (P = 0.002) and cortico-cortical (P = 0.002) and hippocampo-cortical (P = 0.008) ripple co-occurrence modulations were greater preceding delayed vs. immediate recall, which shared the same stimuli and responses. Finally, cortico-cortical (P = 0.04) and hippocampo-cortical (P = 0.004) corippling was enhanced preceding correct vs. incorrect delayed recall, which was not the case for cortical (P = 0.08) or hippocampal (P = 0.94) ripples generally. Notably, delayed but not immediate recall of paired associates is severely impaired by hippocampal damage (12). These data demonstrate increased corippling between cortical sites and between the hippocampus and cortex during hippocampal-dependent retrieval of novel combinations of previously unrelated items. This finding supports the hypothesis that hippocampo-cortical and cortico-cortical corippling may contribute to the reconstruction of declarative memories in humans.
Fig. 3.
Cortico-cortical and hippocampo-cortical ripple co-occurrences increase preceding recall. (A) Schematic of paired-associates memory task. Patients learned word pair associations and were subsequently cued with the first word to recall the second immediately following learning and then after a ∼60-s delay with distraction. (B) Cortical and cortico-cortical corippling increases following the stimulus cue triggering recall (delayed n = 365 trials, immediate n = 698 trials, patients S18–22). (C) Same as B except for hippocampal ripples and hippocampo-cortical coripples (delayed n = 90 trials, immediate n = 304 trials, patients S19, 22). (D and E) Quantification of B and C during the pink shaded 150- to 300-ms interval following stimulus cue onset preceding correct recall in the immediate or delayed condition, or incorrect recall (no attempt or incorrect response) in the delayed condition. Note that cortico-cortical and hippocampo-cortical co-occurrences preceding correct delayed recall have the greatest increases. Errors show SEM. Post-FDR *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001, linear mixed-effects models with patient as random effect. ns, not statistically significant.
Since brain state could affect recall performance, we tested whether there was a difference in alpha (7 to 13 Hz) analytic amplitude between correct and incorrect responses. We found no significant difference in the mean alpha analytic amplitude across cortical channels within a ±1-s window around response times for correct vs. incorrect for any of the five patients with paired-associates task data (P = 0.24 to 0.64 and t = 0.36 to 1.6, one-sided two-sample t test, FDR-corrected P values for multiple patients).
Cortical Ripples Phase-Lock across Widespread Regions.
Phase-locked oscillations in widespread locations have been hypothesized to underlie integration (“binding”) of different components of events across the cortical surface. For each channel pair, we calculated the phase-locking value (PLV), a measure of the consistency of 70- to 100-Hz phases between sites, independent of amplitude (28), across all of their coripple events in either NREM or waking. Please note that consistency is measured between different coripples, at each 1-ms bin relative to coripple center, not within coripples. We found significant PLV of co-occurring ripples between all sampled cortical regions, including between hemispheres, in both states (Fig. 4A–C). There were more channel pairs with significant PLV modulations during NREM than waking (Fig. 4C and SI Appendix, Table 6; post-FDR P < 0.05, randomization test; nonsignificant results in SI Appendix, Fig. S8A and B). An example of consistent phase between two cortical locations across two coripples is shown in Fig. 4A. For each channel pair, PLV was measured at each latency relative to the center of their coripple (Fig. 4B), and these time courses were averaged across all significant channel pairs (Fig. 4C). The increased PLV lasted for the entire period when the sites were corippling and arose abruptly from baseline. PLV time course was remarkably similar across channel pairs. During sleep 2,106/2,275 cortico-cortical channel pairs had more than 40 co-occurring ripples, required for a reliable PLV estimate. Of these, 26.3% (554/2,106) had significant PLV modulations. During waking, 1,939/2,275 had more than 40 co-occurring ripples, and 13.9% (269/1,939) of these had significant PLV modulations (SI Appendix, Table 6). Like what we found for co-occurrences (SI Appendix, Fig. S3A), cortico-cortical coripple peak PLVs across channel pairs were positively correlated between NREM and waking (SI Appendix, Fig. S3B; r = 0.20, P = 4 × 10−22, significance of r). In summary, distant pairs of sites throughout the cortex were found to have consistent phases during coripples in both NREM and waking.
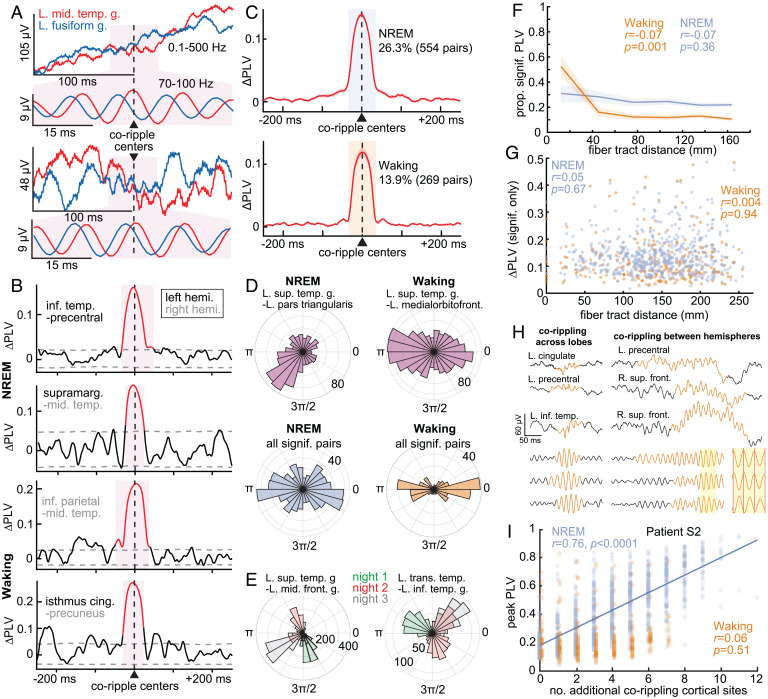
Fig. 4.
Ripples phase-lock across wide separations in the cortex. (A) Two example pairs of co-occurring ripples in broadband LFP and 70- to 100-Hz bandpass. A consistent phase lag from the left middle temporal gyrus (red) to left fusiform gyrus (blue) is evident in the expanded band-passed (70- to 100-Hz) 50-ms-long traces centered on the coripple (pink background). Similar phase lags were also present across other coripples between these sites, resulting in a significant PLV. (B) Example 70- to 100-Hz ΔPLV time courses calculated between ripples co-occurring between ipsilateral and contralateral cortical sites (≥25-ms overlap) in NREM and waking. Red shows significant modulation (post-FDR P < 0.05, randomization test, 200 shuffles per channel pair). (C) Average ΔPLVs (relative to −500 to −250 ms) for cortical channel pairs with significant PLV modulations. A greater percentage of pairs had significant coripple PLV modulations during NREM (26.3%) than waking (13.9%). (D) Polar histograms of waking and NREM 70- to 100-Hz phase lags across coripples for two example cortico-cortical pairs with significant PLV modulations (Top) and across channel pair circular means (Bottom). The magnitude of each pie wedge corresponds to the number of coripples (Top) or number of channel pairs (Bottom) with the indicated phase lag. cortico-cortical phase lags had a significant preference for ∼0 or ∼π during waking compared to NREM based on the counts within 0 ± π/6 or π ± π/6 vs. outside these ranges (P = 5 × 10−8, χ2 = 29.8, df = 1). (E) Example coripple phase-lag distributions for different sleep nights. The dominant phase lag for coripples changes across nights (color-coded). (F) Proportion of channel pairs with significant PLVs has a weak, nonsignificant decrease over distance during NREM but a significant decrease, notably at shorter distances, during waking (linear mixed effects with patient as random effect). See SI Appendix, Fig. S6 C and D for individual patients. (G) ΔPLV does not decrement with intervening fiber tract distance for channel pairs with significant coripple PLV modulations (linear mixed effects with patient as random effect). See SI Appendix, Fig. S6 E and F for results from individual patients. (H) Single-sweep broadband LFP and normalized 70- to 100-Hz bandpass show waking ripples (orange) co-occurring across lobes (Left) and between hemispheres (Right). Note the consistency of ∼0 or ∼π phases in the shaded inset. (I) Peak PLV correlates with the number of additional cortical sites corippling during NREM in a sample patient (n = 10/17 patients significant, post-FDR P < 0.05, significance of r) more often than waking (n = 3/17). Fit is linear least-squares regression. The PLVs between the indicated channels measure the consistency of phase between those channels at each latency relative to ripple peak across all instances of ripples co-occurring between those channels in NREM or waking.
Phase-Locked Coripples Have a Broader Range of Phase Lags in NREM Compared to Waking and May Vary across Nights.
Having demonstrated significant phase locking between cortical corippling sites, we evaluated the distribution of the circular mean phase angles across site pairs. We found that during NREM the average phase angles for different cortical site pairs that had significant coripple phase locking had a fairly even distribution from 0 to 2π radians (Fig. 4D, Bottom Left). However, during waking, coripple phase lags across pairs tended to be ∼0 or ∼π (Fig. 4D, Bottom Right). This difference was significant (P = 5 × 10−8, χ2 = 29.8, df = 1; using counts within 0 ± π/6 or π ± π/6 vs. outside these ranges for NREM vs. waking). This observation suggests a greater tendency toward zero phase lag during waking. We provide evidence below that the ∼0 and ∼π lags are functionally equivalent and may be due to a variability in fine-scale electrode contact placement relative to cortical layers. This may be related to the greater tendency of ripples to couple at near-zero latency during waking (Fig. 2A).
We also tested whether phase lags during NREM varied across nights. For each patient with multiple sleep nights (n = 16), we compared the coripple phase lags between all possible pairs of sleep nights within channel pairs that had significant PLV modulations. We found that 51.8% (1,256/2,426) of such pairs had significantly different phase lags between nights (Fig. 4E; post-FDR P < 0.05, Watson–Williams test; minimum 30 coripples per night per channel pair). These differences in coripple phase lags between particular cortical sites suggest that they may participate in different networks across nights, as may happen, for example, when reactivating cortical representations associated with different memories. Similar phenomena have been noted in large-scale cortical models (29).
Ripples Phase-Lock Robustly across Long Distances.
Since declarative memories characteristically unite disparate elements that are encoded in widespread cortical locations in both hemispheres, any neurophysiological process supporting their encoding, consolidation, or retrieval must likewise function across those distances. Thus, given that cortico-cortical ripple co-occurrences have little to no decrement with distance (tested up to 200 mm; Fig. 2E and F), we hypothesized that cortico-cortical coripple phase locking also does not decrement with distance. Indeed, we found that, like ripple co-occurrences, the proportion of channel pairs with significant PLV modulations (Fig. 4F; r = −0.07, P = 0.36, linear mixed effects with patient as random effect) and the magnitude of these PLV modulations (Fig. 4G; r = 0.05, P = 0.67) did not significantly decrement with intervening fiber tract distance during NREM. During waking, there was a significant decrement in the proportion of channel pairs with significant PLV modulations with distance, especially at shorter distances (Fig. 4F; r = −0.07, P = 0.001), but no decrement in the magnitude of significant channel pair PLV modulations with distance (Fig. 4G; r = 0.004, P = 0.94). Thus, the physiological action supported by corippling may span the entire cortical surface.
Phase Locking Increases with More Cortical Sites Corippling.
Ripples often phase-locked across multiple sites, including between hemispheres (Fig. 4H), and as described above we found that two sites corippling made it more likely that a third site was corippling. These findings raise the question of whether activation of widespread corippling networks facilitates greater network synchronization. We found a strong positive linear correlation between the number of sites corippling and the cortico-cortical coripple phase-locking peak amplitude (Fig. 4I). These results were again found when the ΔPLV (peak PLV minus baseline PLV) was used instead of the peak PLV (ΔPLV: n = 7/17 patients significant during NREM, n = 1/17 significant during waking, post-FDR P < 0.05, significance of r). To test whether more corippling was simply due to greater ripple amplitude, we measured the average 70- to 100-Hz analytic amplitude of the coripples and found that only 2/17 patients in NREM and 0/17 patients in waking had significant correlations (post-FDR P < 0.05, significance of r), and the significant correlations were both negative, demonstrating that more corippling was not due to greater amplitude. In sum, the co-occurrence of ripples promotes further co-occurrence, which enhances phase locking.
Cortico-Cortical Coripples Have Consistent Phase Lags across Successive Cycles.
We hypothesized that a site pair would be effectively “phase-locked” across successive coripple cycles because the ripple oscillation frequency of ∼90 Hz is so similar across ripples and locations. For each coripple from each cortico-cortical channel pair we computed the PLV across the lags between the two ripples using their five peaks closest to the coripple time center and found significant within-ripple phase locking for 98.3% of the 807,213 coripples during NREM and 98.0% of the 1,348,696 coripples during waking (P < 0.05; randomization test, n = 1,000 random phase lags, FDR correction across coripples). Thus, within-ripple phase locking is present in nearly all cortico-cortical coripples.
Hippocampo-Cortical Pairs Rarely, If Ever, Phase-Lock.
Cortico-cortical phase locking could be driven through a network of coupled cortical oscillators, a central driving mechanism, or a combination. Since hippocampal ripples strongly co-occur with cortical ripples (Fig. 2B), we investigated whether the hippocampus drives phase locking of ripples in the cortex by testing if there is phase locking between hippocampo-cortical coripples. For hippocampo-cortical pairs during sleep, 277/461 had more than 40 co-occurring ripples, and 1.4% (4/277) of these had significant PLV modulations. During waking, 333/461 of the hippocampo-cortical channel pairs had more than 40 co-occurring ripples, and 0.3% (1/333) of these had a significant PLV modulation (SI Appendix, Fig. S8C–F and Table S6). Examination of electrode trajectories suggested that the hippocampal contacts with significant PLVs with cortical sites were probably located in the subicular complex rather than the hippocampus proper, consistent with the finding in mice that ripple propagation from hippocampus to retrosplenial cortex is via the subiculum (10). Thus, it is unlikely that cortico-cortical ripple phase locking is driven by common inputs from a hippocampal ripple, which supports the hypothesis that cortico-cortical coripple phase locking is driven intracortically.
Cortical Ripples Are Associated with Increased and Phase-Modulated Single-Unit Firing.
For ripples to have a role in the communication and integration of information between distant cortical sites requires that neuronal action potentials coordinate with ripple occurrence and phase. We analyzed microarray recordings from human lateral temporal cortex granular/supragranular layers in three patients (SI Appendix, Table 7) during NREM to test whether ripples modulate local single-unit spiking. We detected spikes and sorted them into putative pyramidal (PY) and interneuron (IN) units based on waveform shapes and spike-timing characteristics according to previously established methods (30) and verified that the units had large peak signal-to-noise ratios (PY: 9.1 ± 3.4; IN: 5.2 ± 2.9), had minimal interspike intervals that were <3 ms (PY: 0.2 ± 0.3%; IN: 0.3 ± 0.6%; low percentages indicate minimal contamination by other units), and were well-isolated from one another based on the projection test (31) (PY: 95.4 ± 86.0 SD; IN: 82.8 ± 83.4 SD). We also detected ripples recorded by the array’s microcontacts with the average spike waveform of each unit subtracted at the times of the spikes on the unit’s channel to prevent unit spike contamination of the LFP. We found that cortical ripples were associated with increased single-unit firing (Fig. 5A and B). PY had a 255% increase and IN had a 297% increase in spike rates during ripples compared to baseline (randomly selected epochs in between ripples that were matched in number and duration to the ripples). Furthermore, unit firing was strongly phase-modulated by the ripples (Fig. 5A), with 49% (32/66) of PY and 71% (24/34) of IN having significant 70- to 100-Hz phase modulations during local ripples (Fig. 5C; post-FDR P < 0.05, binomial test between phases within 0 ± π/2 vs. π ± π/2, expected value = 0.5, minimum 30 spikes per unit across ripples). Thus, since unit spiking is coupled to the local ripple phase, and since ripple phases are synchronized at long distances, these data suggest that ripples coordinate unit spike timing between wide separations in the cortex, which could enable phase selection, coincidence detection, reentrant processing, and spike-timing-dependent plasticity. These basic neurophysiological processes would influence the cooperative selection of cell assemblies between cortical areas, the essence of binding.
Fig. 5.
Cortical ripples modulate local single-unit spiking and synchronize high-frequency activity between distant regions. (A) Average cortical ripple broadband LFP and associated raster plot of PY and IN mean spike rates across ripples during NREM. Note the phase coupling of both PY and IN spiking to the ripple peaks. (B) Single-unit spike rates increase during cortical ripples compared to baseline, which was composed of randomly selected epochs in between ripples matched in number and duration (PY: n = 127, mean = 255%; IN: n = 38, mean = 297%; patients U1–3). (C) Single units are significantly phase-modulated by cortical ripples (PY: n = 32/66 significant; IN: n = 24/34 significant; binomial test between phases within 0 ± π/2 vs. π ± π/2, expected value = 0.5, minimum 30 spikes per unit across ripples). (D) Correlation of the >200-Hz analytic amplitude, a proxy for unit spiking, increases during waking coripples compared to randomly selected preceding control periods (within −10 to −2 s) matched in number and duration (n = 2,275 SEEG channel pairs from patients S1–17). ****P < 0.0001, two-sided paired t test. (E) Average >200-Hz amplitude correlation between coripples for each cortical channel pair does not decrement with fiber tract distance. PY, putative pyramidal unit; IN, putative interneuron unit.
Corippling Increases Putative Unit-Activity Correlation between Distant Cortical Sites.
Our findings that coripples are often phase-locked across distant sites and that unit firing is phase-locked to local ripples together imply that unit firing is also correlated across distant sites. We were unable to test this directly because we did not perform microelectrode recordings of units in multiple locations separated by more than ∼5 mm. Rather, as an indirect test, we used >200-Hz analytic amplitude from SEEG recordings as a proxy for unit firing (32). During waking, but not NREM, this measure was significantly more correlated when cortical sites were corippling vs. when they were not (Fig. 5D, P = 8 × 10−303, two-sided paired t test). Correlation between corippling sites did not significantly decrement with increasing distance between the sites (Fig. 5E).
As described above, coripple phase lags between channels tended to be near 0 or π, especially during waking. This could indicate that corippling sometimes represents a state of inhibited communication, or simply that our bipolar SEEG derivations had variable relationships to local ripple generating dipoles. Indeed, the 3-mm bipolar SEEG contact separation is large enough to record from multiple ripple dipoles with different orientations. In rats, ripple generators occupy ∼1 mm2 of cortical surface (5), and cellular generators are located in multiple layers separated by ∼1 mm (10). To test these hypotheses, we compared the correlations of the analytic amplitudes of the 200-Hz high-passed signals between sites, when the sites had coripple phase lags of 0 ± π/6 vs. π ± π/6 (averaged within these ranges for each channel pair). No significant difference was found for NREM or waking (NREM: P = 0.07; waking: P = 0.17; two-sided paired t test, n = 2,275 channel pairs), thus suggesting that the lags near 0 and π are functionally equivalent, as would be expected if they are due to slight differences in electrode location relative to cortical lamina.
Discussion
Here, using human intracortical recordings, we show that ripples couple, co-occur, and phase-synchronize across all lobes and between both hemispheres, with little decrement, even at long distances. The rate of corippling above chance increased exponentially with the proportion of corippling sites, which was correlated with stronger phase locking. Cortical neurons increased firing during ripples and phase-locked to them, a requisite for ripples to enhance interaction via gain modulation and coincidence detection. Enhanced long-distance interaction during corippling between sites was supported by increased high-frequency correlation. Ripples co-occurred between cortical sites and between the cortex and hippocampus during both spontaneous waking and NREM, and there were more co-occurrences preceding successful delayed recall. Overall, our results suggest that distributed, phase-locked cortical ripples possess the properties that may allow them to facilitate integration of the different elements comprising a particular declarative memory, or more generally, to help “bind” different aspects of a mental event encoded in widespread cortical areas into a coherent representation.
Previous work showed that coupling of anterolateral temporal and parahippocampal ripples in humans increases prior to correct recall in paired-associates learning (8). We show that such coupling also occurs between hippocampal and cortical ripples. Further, we show that immediate recall with no intervening distractor, a task with identical sensory and motor stimulation but that does not require the hippocampus (12), is not associated with increased hippocampo-cortical ripple coupling. Critically, given the importance of transcortical connections between sites encoding previously unrelated elements in declarative memory, we show that cortico-cortical ripple coupling also strongly increases prior to correct recall following a delay.
Transient medial temporal inactivation disrupts both memory formation and retrieval, implying a contribution to both (33), in addition to the hippocampal role in consolidation during NREM (1–4). We found that hippocampo-cortical ripple coupling occurred spontaneously in both waking and NREM, but with different order preferences—cortex leading during waking and hippocampus during NREM, possibly reflecting different overall flow of information during memory formation versus consolidation. The order effect during NREM was only found for hippocampal sharpwave ripples (21), not spindle ripples (22). Sharpwave ripples have stronger associations with prefrontal areas supporting contextual aspects of episodic memory and spindle ripples with parietal areas supporting detailed autobiographical recollection. These results thus reinforce a previous suggestion that ripples make sequential contributions to consolidation (22).
A finding of this study is that spontaneous cortical ripples couple (within ±500 ms) co-occur (≥25-ms overlap) and often phase-lock (synchronize) across large areas of both hemispheres during both sleep and waking. The minimal decrement with distance in co-occurrence or phase locking suggests either a central driving oscillator or a population origin, such as a web of coupled oscillators (Fig. 6A). The hippocampus is unlikely to act as a central driving oscillator because hippocampo-cortical coupling and corippling is rarer than cortico-cortical, and hippocampo-cortical phase locking is essentially absent. A preponderance of cortico-cortical influences might be expected given that each cortical pyramidal cell receives input from thousands of other pyramids, whereas the fan-out from hippocampus (CA1 plus subiculum) to cortex is ∼1:500, so each cortical pyramid gets on average less than 10 hippocampal synapses (34, 35). The thalamus is another possible source of synchronization of multiple cortical locations. However, thalamocortical connections are also very rare compared to cortico-cortical (36). Given the strong relationship of cortical ripples to upstates, and less strongly to sleep spindles (11), and the role of thalamocortical interactions in the generation of upstates and spindles (37), it is possible that thalamocortical modulation via sleep waves may contribute to cortico-cortical ripple synchronization.
Fig. 6.
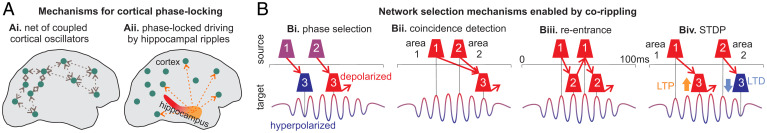
Mechanisms of phase-locked networks of cortical ripples and their selection. (A) Potential mechanisms for ripple phase locking across broad cortical regions: (A, i) multiple cortico-cortical reentrant connections between the cortical oscillators; (A, ii) distributed driving oscillations from a subcortical location (e.g., hippocampal ripples). (B) Multiple synergistic mechanisms enabled by cortico-cortical corippling could select a spatiotemporal neural network. (B, i) Spikes arriving at the hyperpolarized phase (e.g., cell #1→3) will be ineffective relative to those arriving at the depolarized phase (2→3) in eliciting spikes in the rippling target area. (B, ii) Coincident spikes arriving from two source areas that are phase-locked with the target area are more likely to trigger an action potential, especially if they arrive in the depolarized phase. Note that conduction times between areas do not need to match the phase lag but could equal the phase lag plus multiples of the cycle time (e.g., 1→3 vs. 2→3). (B, iii) Reentrant activation across cycles could also result from reciprocal connections. Initial (1→2) and return (2→1) distances are equal and so their conduction delays should be similar. (B, iv) The synapses effectively evoking spikes (1→3) would be strengthened with spike-timing-dependent plasticity, whereas those arriving from a non-phase-locked area (2→3) would arrive after the target cell fires and thus be weakened. Whereas the mechanisms in B, i–iii would act synergistically to reinforce in-phase firing between corippling sites within a given ripple, the mechanism in B, iv would act across ripples to reinforce a particular network of corippling sites.
Conversely, a cortico-cortical network of coupled oscillators is consistent with the finding in cats that section of the corpus callosum disrupts gamma synchrony between V1 in the two hemispheres (17). Furthermore, the highly uniform ripple frequency observed across sites, cortical regions, and individual ripples suggests that local cellular and network mechanisms set the resonant oscillation frequency of each cortical module to the same 90-Hz frequency, which are thereby prone to co-oscillate when excited and connected. A major role of cortico-cortical interactions in ripple co-occurrence and phase locking is also suggested by the strong positive feedback we observed in the spread of cortico-cortical co-occurrence and in the intensity of cortico-cortical phase locking. Specifically, cortico-cortical co-occurrence probability during waking increases from about twice chance levels for two co-occurring sites to about 20,000 times chance for co-occurrence in 25% of the sites. Similarly, during NREM, peak cortical ripple PLV between sites increases linearly with the number of additional sites corippling, from ∼0.2 with no additional sites to ∼0.9 with 12. Note that a PLV of 1.0 would indicate perfect consistency of phase between sites over all of their co-occurring ripples.
The mechanism whereby cortico-cortical interactions could support our finding of zero-lag phase locking at long distances, with minimal decrement up to 250 mm, is unclear. Fast cortico-cortical fibers conduct ∼10 m/s, traveling 111 mm between successive peaks of a 90-Hz ripple (38). However, synchronizing projections can still be effective at multiples of the cycle time, especially with recurrent connectivity (Fig. 6B). Since long-range fibers are quite rare in human cortex, whereas local connectivity and U-fibers are dense, an astronomical number of possible multisynaptic routes exist between any two cortical locations (36). Indeed, modeling studies show that phase-locked oscillations at ∼90 Hz can occur in extended cortical networks, even at zero lag, provided that the neurons have multiple-path recurrent connectivity (39, 40). Such “polychronous” models (41) spontaneously select paths involving multiple relays with consistent sums, and the same pair of locations can display multiple phase lags depending on the network they are participating in, as we observed over multiple nights of sleep. Thus, although direct evidence is lacking, the most likely possibility given our findings is that ripples co-occur and phase-lock due to emergent cortico-cortical interactions.
We found that cortico-cortical corippling and phase locking show consistent differences between states. Although spontaneous cortical ripple occurrence density is higher in NREM, co-occurrence density is higher in waking. Accordingly, the likelihood of multiple sites corippling relative to chance grows more rapidly with number of sites during waking than NREM, the increase being about 25 times larger when 25% of sites are corippling. Overall, the proportion of ripple peaks that occur within 25 ms of each other is about five times greater in waking than in NREM. Furthermore, when coripples phase-lock they are more likely to have near 0 phase lag in waking than NREM. Higher activation is indicated by the 10 times higher increase in >200-Hz amplitude during waking vs. NREM ripples. In contrast, NREM ripples tend to recruit sequentially across the cortex (as indicated by nonzero phase-lags), with a smaller but significant increase in >200-Hz amplitude. These properties may be consistent with a rapid highly synchronous recruitment of multiple cortical sites by a high activation during waking ripples and, by contrast, more sparsely activated NREM ripples. Conversely, zero-lag recruitment could be the cause rather than the effect of the greater >200-Hz activation during waking.
A mean of about 16 widely distributed cortical locations were sampled per patient. Assuming that a ripple-generating module spans ∼1 mm2 of cortical surface (5), then we recorded from ∼1/10,000th of the ripple modules. Since corippling is not increased at short interelectrode distances, the proportion of recorded sites can be used as an estimate of the proportion of the cortex that is rippling. If so, then at times as much as 25% of the cortex is corippling. Similarly, the usual proportion of the cortex corippling can be roughly estimated from the probability, given a ripple, that another site is corippling, ∼8% (assuming that the ripple’s lead field is comparable to its module size).
Given the strong ripple phase modulation of cell firing we demonstrated, ripples can be expected to strongly modulate the effectiveness of arriving synaptic input, with spikes arriving on the depolarized phase being far more likely to trigger postsynaptic firing (18). Thus, there may be a strong selection for cells that project (even multisynaptically) between corippling sites with a latency matching the phase lag (possibly plus multiples of the cycle time; Fig. 6B, i). Additionally, when multiple sites coripple, then spikes that arrive together at a third location may have greatly enhanced effectiveness (i.e., the postsynaptic site acts as a coincidence detector; Fig. 6B, ii) (42). Synaptic phase selection and coincidence detection are synergistic and would further imply positive feedback due to recurrence (Fig. 6B, iii). Connectivity between corippling locations supporting in-phase interactions would be progressive because the pre- and postsynaptic activation these mechanisms induce would strengthen in-phase connections via spike-timing-dependent plasticity (Fig. 6B, iv).
Note the requisite precondition for this progressive strengthening of in-phase connections is that there is consistency of phase lags across waking or NREM coripples of a site pair, which is what our criterion for phase locking required. However, in addition to consistent phase locking across coripples, phase locking is virtually universal within coripples because the frequencies of ripples are so similar, across ripples and locations, which would enable phase selection, coincidence detection, and reentrance mechanisms for selection of neural networks connecting the corippling sites provided that the two sites are connected either mono- or polysynaptically.
These mechanisms would function to select the members of the neural network including both sites, which is the core of the “binding-by-synchrony” hypothesis. In neural modeling studies of such “polychrnmous” networks (41), any two sites typically participate in multiple networks, but with a different latency for each network. Conversely, consistent phase lag across multiple occurrences of coripples indicates that the two sites participate in a consistent network. In psychological binding, combinatorial associations power vast encoding spaces (e.g., letters into words) which nonetheless include some consistently associated elements (e.g., <u> usually follows <q>). Thus, the finding that phase locking can be either consistent or inconsistent across coripples between two sites would be expected under the hypothesis that coripples help implement binding.
Regardless of whether phase locking is within or between coripples, it is only a statistical relationship which, like “functional connectivity” based on functional MRI, does not prove a physical cortico-cortical interaction. For example, both within-ripple and across-ripple phase locking could arise if the corippling sites had a shared abrupt depolarizing input and an intrinsic tendency to oscillate at 90 Hz to such an input. However, it is much easier to construct scenarios wherein within-ripple phase locking would arise with only shared modulations, because it only requires that the shared modulations overlap in timing by ∼30 ms, and no consistency across coripples in that timing. In contrast, across-coripple phase locking would further require an extremely consistent across-coripple timing of the modulatory input if that were the only synchronizing influence. For example, for the site pairs in Fig. 4E such modulation on a given night of NREM would need to arrive with an accuracy of about ±1 ms (the full circle represents ∼11 ms). Furthermore, this modulation latency could not be hard-wired because it changes from night to night. Given the strong association of NREM ripples with upstates and waking ripples with increased very high gamma, it seems likely that shared modulation plays a role in both within-ripple and across-ripple phase locking. However, the consistent precision of the across-coripple phase locking seems to require additional synchronizing mechanisms which, given our data, we believe are likely cortico-cortical.
Through these mechanisms, cortical ripples could activate and couple with distant but related encoding areas through progressive activation of a web of cortico-cortical positive feedback connections. The strongly increased probability of coactivation and phase locking with increasing numbers of sites already coactivating suggests positive feedback recruitment of the most interconnected and coactivated cells into a brief synchronous ripple. When locations join the rippling network and resonate with other members, their activity levels increase. The function of the ripple could thus be to amplify through resonance, leading to the rapid assembly of a network encoding the various aspects of an event, or in the case of recall or consolidation, the network encoding the various aspects of the memory.
A potential role of coripples in facilitating the communication and integration of information between distant cortical sites requires both the synchronized oscillations in transmembrane currents generating the coripples per se and coordinated neuronal action potentials. We show here that single putative pyramidal cells and interneurons in lateral temporal cortex increase their firing during ripples in a manner that is strongly modulated by the phase of the individual ripple cycles. Therefore, the cell firing patterns necessary to support selection of activated neurons within the corippling locations using gain modulation and coincidence detection are in place. While we did not simultaneously record single units in multiple distant locations to directly confirm that they are cofiring, we obtained some indication that this may be the case by examining the amplitude envelope of >200-Hz LFPs. We found that the correlation between these envelopes in distant locations is greater when they are corippling and, furthermore, this increase in correlation does not decrease with distance, even if the sites are in different lobes or hemispheres.
The principal alternative proposed mechanism for binding relies on hierarchical convergence in multiattribute, multisensory areas (19, 20). This mechanism seems inconsistent with the highly distributed nature of cortical processing and poses the difficulty of how to prerepresent in a small cortical module each of the combinatorial possibilities of all elements contained in all potential experiences. However, the hippocampus seems to do this, albeit temporarily, with receptive fields that combine indicators of position from multiple modalities, as well as valence, history, and context (43). Hippocampal assembly of the different elements of an event does not require integration across the hippocampus because these elements are available locally, having been premixed by the entorhinal cortex and dentate gyrus. Thus, hippocampal ripples are for communication with the cortex rather than with other hippocampal sites, and indeed they are typically local within the hippocampus (22, 44); rather, their crucial co-occurrences may be with widespread cortical ripples. In this view, binding of cortical elements can occur in the absence of hippocampal input if they are previously consolidated, because cortico-cortical corippling and phase locking are dependent on intracortical processes.
In summary, the characteristics of co-occurring and phase-locked cortical ripples found in the current study appear to fulfill the central requirements for a neurophysiological process that could facilitate binding by synchrony. First, ripples occur in all regions of the cortex in both hemispheres (required by binding because the elements of experience are encoded throughout the cortex). Second, ripples occur spontaneously throughout waking and NREM (required since binding is a ubiquitous process) and are elevated during task periods when binding may be useful (such as reassembling the components of a memory). Third, ripples last ∼70 ms, a duration considered in the range of the “psychological moment” (45). Fourth, ripples strongly increase local firing and firing-proxy >200-Hz amplitude (required for interareal communication). Fifth, ripples strongly phase-modulate local putative pyramidal and interneuron firing in a manner consistent with input modulation (a primary hypothesized mechanism whereby oscillations selectively amplify particular neural assemblies). Sixth, ripples strongly co-occur and phase-lock between cortical sites indicating active cortico-cortical communication (required because without communication there cannot be integration). This strong phase locking occurs between cortical sites but not between cortex and hippocampus, indicating that transcortical synchrony is probably intrinsic, i.e., not projected from elsewhere. Furthermore, co-occurrence and phase locking are minimally affected by distance (required for integration of diverse elements). Finally, >200-Hz amplitude is more correlated between sites when they are corippling, further suggesting interareal integration of unit firing. Additional evidence from simultaneous distributed cellular level recordings as well as direct interventions in model systems will be necessary to confirm that cortical coripples provide the neural substrate for binding.
Methods
Patient Selection.
Data from a total of 25 patients (13 female, 31 ± 11 y old) with pharmacoresistant epilepsy undergoing intracranial recording for seizure onset localization preceding surgical treatment were included in this study (SI Appendix, Tables 1 and 7). Patients whose SEEG recordings were analyzed were only included in the study if they had no prior brain surgery; background EEG (with the exception of epileptiform transients) in the normal range; and electrodes implanted in what was eventually found to be nonlesional, nonepileptogenic cortex, as well as nonlesional, nonepileptogenic hippocampus (such areas were suspected to be part of the focus prior to implantation or were necessary to pass through to reach suspected epileptogenic areas). Based on these criteria, 25 patients were included in this study out of a total of 83. All patients gave fully informed written consent for their data to be used for research. This study was approved by the local Institutional Review Boards at Cleveland Clinic, University of California San Diego, Oregon Health & Science University, and Partners HealthCare (including Massachusetts General Hospital).
Ripple Detection.
Ripple detection was performed in the same way for all structures and states, based on a previously described hippocampal ripple detection method (21, 22). Requirements for inclusion and criteria for rejection were determined using an iterative process across patients, structures, and states. Data were band-passed with a Butterworth filter at 60 to 120 Hz (forward and reverse for zero-phase shift, sixth order) and the top 20% of 20-ms moving root-mean-squared peaks were detected. It was further required that the maximum z-score of the analytic amplitude of the 70- to 100-Hz bandpass (sixth order zero-phase shift Butterworth) be greater than 3 and that there be at least three distinct oscillation cycles in the 120-Hz low-passed signal, determined by shifting a 40-ms window in increments of 5 ms across ±50 ms relative to the ripple midpoint and requiring that at least one window have at least three peaks. Adjacent ripples within 25 ms were merged. Ripple centers were determined as the maximum positive peak in the 70- to 100-Hz bandpass. Ripple onsets and offsets were marked when the 70- to 100-Hz amplitude envelope fell below a z-score of 0.75. To reject epileptiform activities or artifacts, ripples were excluded if the absolute value of the 100-Hz high-pass z-score exceeded 7 or they occurred within 2 s of a ≥3 mV/ms LFP change. Ripples were also excluded if they fell within ±500 ms of putative interictal spikes, detected as described in SI Appendix. To exclude events that could be coupled across channels due to epileptiform activity, we excluded ripples that coincided with a putative interictal spike on any cortical or hippocampal channel. Events that had only one prominent cycle or deflection were excluded if the largest valley-to-peak amplitude in the broadband LFP was 2.5 times greater than the third-largest. For each channel, the mean ripple-locked LFP was visually examined to confirm that there were multiple prominent cycles at ripple frequency (70 to 100 Hz), and the mean time-frequency plot was examined to confirm there was a distinct increase in power within the 70- to 100-Hz band. In addition, multiple individual ripples in the broadband LFP and 70- to 100-Hz bandpass from each channel were visually examined to confirm that there were multiple cycles at ripple frequency without contamination by artifacts or epileptiform activity. Channels that did not contain ripples that met these criteria were excluded from the study.
Ripple Temporal Relationships.
Ripple cross-correlograms (peri-cortical ripple time histograms of cortical or hippocampal ripples on a different channel) were computed to assess for ripple coupling between sites. Gaussian smoothed (window = 250 ms, σ = 50 ms) ripple center counts in channel B were computed in 25-ms bins within ±1,500-ms relative to ripple centers at t = 0 in channel A. A null distribution was generated by shuffling (n = 200 times) the times of ripple centers on channel B relative to the ripple centers on channel A (at t = 0) within the ±1,500 window. Pre-FDR P values were calculated by comparing the observed and null distributions for each bin over ±500 ms. P values were then FDR-corrected for the number of channel pairs across patients multiplied by the number of bins per channel pair (46). A channel pair was determined to have a significant modulation if there were at least three consecutive bins each with FDR-corrected P < 0.05 in order to minimize the possibility of false positive significance. Whether cortical ripples were leading or lagging was determined using a two-sided binomial test with expected value of 0.5, using event counts in the 500 ms before vs. 500 ms after t = 0. For plots, 50-ms Gaussian smoothed (σ = 10 ms) event counts with 50-ms bins were used.
Cortical Ripple Co-occurrences.
Ripple co-occurrences between channel pairs were identified by finding ripples that overlapped for at least 25 ms. The center of the co-occurring ripple event was determined by finding the temporal center of the ripple overlap. Conditional probabilities of ripple co-occurrence were computed by finding the probability of co-occurrence (minimum 25-ms overlap) between two channels given that there was a ripple in one of the channels, separately for each channel. This was done for P(NC|NC) (both orders), P(NC|HC), and P(HC|NC). To estimate the extent of corippling across the cortex at any moment, the probability that a given proportion of channels was corippling at any time point for each patient was computed.
Observed over chance cortical ripple co-occurrence was computed as a function of the number of sites corippling. Ripple co-occurrence of a given number of sites required that all of those sites had at least 25-ms ripple overlap. Chance was computed for each patient by randomly shuffling the ripple epochs and interripple epochs of all sites 200 times and calculating the mean number of co-occurrences for each proportion of corippling sites (i.e., the number of channels corippling divided by the total number of channels, assessed for the minimum value of two or more channels corippling).
Cortical ripple co-occurrence significance for each channel pair was computed by comparing the number of observed co-occurrences (25-ms minimum overlap) for each channel pair with a null co-occurrence distribution derived from shuffling ripples and interripple intervals 200 times in a moving nonoverlapping 5-min window and counting co-occurrences.
Ripple Phase-Locking Analyses.
To determine if co-occurring ripples at different sites were synchronized, we used the PLV, an instantaneous measure of phase locking (28). PLV time courses were computed using the analytic angle of the Hilbert transformed 70- to 100-Hz band-passed (zero-phase shift) signals of each channel pair when there were at least 40 coripples with a minimum of 25-ms overlap for each. PLVs were computed at 1-ms timepoints across all such coripples for each channel pair within a ±500-ms window relative to the coripple temporal centers. A null distribution was generated by selecting 200 random times within −10 to −2 s relative to each coripple center. Pre-FDR P values were determined by comparing the observed and null distributions in 5-ms duration bins (averaged across five 1-ms time points) within ±50 ms around the coripple centers. These distributions for each channel pair were across coripples at each 5-ms bin relative to the coripple center, not within coripples. These P values were then FDR-corrected across bins and channel pairs of all patients. A channel pair was considered to have significant phase locking if it had two consecutive 5-ms bins with post-FDR P < 0.05 to minimize the possibility of false positives. Phase-locking modulation was computed for each channel pair as the difference from the average baseline PLV within −500 to −250 ms to the peak PLV within ±50 ms around the coripple center. Separate calculations were made for NREM and waking. For plots, PLV traces were smoothed with a 10-ms Gaussian window.
Phase locking as a function of the proportion of additional sites corippling was computed by identifying coripples on the two cortical channels of interest then sorting these events into groups based on what proportion of additional sites had a ripple that overlapped with the coripple by any amount of time and computing the peak PLV and ΔPLV around the coripple centers. Plots of the peak PLV for channel pairs as a function of the number of additional sites co-occurring (e.g., 3 on the x-axis means 2 + 3 = 5 total sites corippling) are for channel pairs with significant PLV modulations.
Intraripple phase locking was tested by first generating a null distribution by computing the PLV of five random phase lags 1,000 times. Next, the observed PLV was computed using phase lags between the five peaks of each of the two ripples in a coripple that were closest to the coripple’s time center. A one-tailed P value for each coripple was then computed as the proportion of null PLVs that were equal to or exceeded the observed PLV. The data were then FDR-corrected across all coripples from all cortico-cortical channel pairs from all patients.
Supplementary Material
Acknowledgments
We thank Adam Niese, Christine Smith, Christopher Gonzalez, Daniel Cleary, Eran Mukamel, Erik Kaestner, Jacob Garrett, Maxim Bazhenov, Terrence Sejnowski, and Zarek Siegel for their support. This work was supported by the National Institute of Mental Health (1RF1MH117155-01 and T32 MH020002) and the Office of Naval Research Multidisciplinary University Research Initiative (N00014-16-1-2829). Portions of this article were developed from the original doctoral dissertation by C.W.D.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2107797119/-/DCSupplemental.
Data Availability
Data and code have been deposited in Zenodo (https://zenodo.org/record/6270017) (47).
References
- 1.Wilson M. A., McNaughton B. L., Reactivation of hippocampal ensemble memories during sleep. Science 265, 676–679 (1994). [DOI] [PubMed] [Google Scholar]
- 2.Buzsáki G., Hippocampal sharp wave-ripple: A cognitive biomarker for episodic memory and planning. Hippocampus 25, 1073–1188 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maingret N., Girardeau G., Todorova R., Goutierre M., Zugaro M., Hippocampo-cortical coupling mediates memory consolidation during sleep. Nat. Neurosci. 19, 959–964 (2016). [DOI] [PubMed] [Google Scholar]
- 4.Ego-Stengel V., Wilson M. A., Disruption of ripple-associated hippocampal activity during rest impairs spatial learning in the rat. Hippocampus 20, 1–10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khodagholy D., Gelinas J. N., Buzsáki G., Learning-enhanced coupling between ripple oscillations in association cortices and hippocampus. Science 358, 369–372 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grenier F., Timofeev I., Steriade M., Focal synchronization of ripples (80-200 Hz) in neocortex and their neuronal correlates. J. Neurophysiol. 86, 1884–1898 (2001). [DOI] [PubMed] [Google Scholar]
- 7.Norman Y., et al. , Hippocampal sharp-wave ripples linked to visual episodic recollection in humans. Science 365, eaax1030 (2019). [DOI] [PubMed] [Google Scholar]
- 8.Vaz A. P., Inati S. K., Brunel N., Zaghloul K. A., Coupled ripple oscillations between the medial temporal lobe and neocortex retrieve human memory. Science 363, 975–978 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz A. P., J. H. Wittig, Jr, Inati S. K., Zaghloul K. A., Replay of cortical spiking sequences during human memory retrieval. Science 367, 1131–1134 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitzan N., et al. , Propagation of hippocampal ripples to the neocortex by way of a subiculum-retrosplenial pathway. Nat. Commun. 11, 1947 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickey C. W., et al. , Cortical ripples during NREM sleep and waking in humans. bioRxiv [Preprint] (2022). 10.1101/2021.05.11.443637 (Accessed 28 February 2022). [DOI] [PMC free article] [PubMed]
- 12.Squire L. R., Memory and the hippocampus: A synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 99, 195–231 (1992). [DOI] [PubMed] [Google Scholar]
- 13.Treisman A., Solutions to the binding problem: Progress through controversy and convergence. Neuron 24, 105–110, 111–125 (1999). [DOI] [PubMed] [Google Scholar]
- 14.Revonsuo A., Newman J., Binding and consciousness. Conscious. Cogn. 8, 123–127 (1999). [DOI] [PubMed] [Google Scholar]
- 15.von der Malsburg C., The what and why of binding: The modeler’s perspective. Neuron 24, 95–104, 111–125 (1999). [DOI] [PubMed] [Google Scholar]
- 16.Gray C. M., König P., Engel A. K., Singer W., Oscillatory responses in cat visual cortex exhibit inter-columnar synchronization which reflects global stimulus properties. Nature 338, 334–337 (1989). [DOI] [PubMed] [Google Scholar]
- 17.Uhlhaas P. J., et al. , Neural synchrony in cortical networks: History, concept and current status. Front. Integr. Nuerosci. 3, 17 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fries P., Neuronal gamma-band synchronization as a fundamental process in cortical computation. Annu. Rev. Neurosci. 32, 209–224 (2009). [DOI] [PubMed] [Google Scholar]
- 19.Shadlen M. N., Movshon J. A., Synchrony unbound: A critical evaluation of the temporal binding hypothesis. Neuron 24, 67–77, 111–125 (1999). [DOI] [PubMed] [Google Scholar]
- 20.Merker B., The efference cascade, consciousness, and its self: Naturalizing the first person pivot of action control. Front. Psychol. 4, 501 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jiang X., Gonzalez-Martinez J., Halgren E., Coordination of human hippocampal sharpwave ripples during NREM sleep with cortical theta bursts, spindles, downstates, and upstates. J. Neurosci. 39, 8744–8761 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiang X., Gonzalez-Martinez J., Halgren E., Posterior hippocampal spindle-ripples co-occur with neocortical theta-bursts and down-upstates, and phase-lock with parietal spindles during NREM sleep in humans. J. Neurosci. 38, 8949–8968 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Glasser M. F., et al. , A multi-modal parcellation of human cerebral cortex. Nature 536, 171–178 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Behrens T. E. J., Berg H. J., Jbabdi S., Rushworth M. F. S., Woolrich M. W., Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage 34, 144–155 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosen B. Q., Halgren E., A whole-cortex probabilistic diffusion tractography connectome. eNeuro 8, ENEURO.0416-20.2020 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Felleman D. J., Van Essen D. C., Distributed hierarchical processing in the primate cerebral cortex. Cereb. Cortex 1, 1–47 (1991). [DOI] [PubMed] [Google Scholar]
- 27.Glasser M. F., Van Essen D. C., Mapping human cortical areas in vivo based on myelin content as revealed by T1- and T2-weighted MRI. J. Neurosci. 31, 11597–11616 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lachaux J. P., Rodriguez E., Martinerie J., Varela F. J., Measuring phase synchrony in brain signals. Hum. Brain Mapp. 8, 194–208 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izhikevich E. M., Kuramoto Y., “Weakly coupled oscillators” in Encyclopedia of Mathematical Physics, Françoise J.-P., Naber G. L., Tsun T. S., Eds. (Academic Press, Oxford, 2006), pp. 448–453. [Google Scholar]
- 30.Dickey C. W., et al. , Travelling spindles create necessary conditions for spike-timing-dependent plasticity in humans. Nat. Commun. 12, 1027 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pouzat C., Mazor O., Laurent G., Using noise signature to optimize spike-sorting and to assess neuronal classification quality. J. Neurosci. Methods 122, 43–57 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Mukamel R., et al. , Coupling between neuronal firing, field potentials, and FMRI in human auditory cortex. Science 309, 951–954 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Halgren E., “Insights from evoked potentials into the neuropsychological mechanisms of reading” in Neurobiology of Cognition, Scheibel A., Weschsler A., Eds. (Guilford, New York, 1990), pp. 103–150. [Google Scholar]
- 34.Simić G., Kostović I., Winblad B., Bogdanović N., Volume and number of neurons of the human hippocampal formation in normal aging and Alzheimer’s disease. J. Comp. Neurol. 379, 482–494 (1997). [DOI] [PubMed] [Google Scholar]
- 35.Azevedo F. A., et al. , Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 513, 532–541 (2009). [DOI] [PubMed] [Google Scholar]
- 36.Rosen B. Q., Halgren E., An estimation of the absolute number of axons indicates that human cortical areas are sparsely connected. PLoS Biol. 20, e3001575 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mak-McCully R. A., et al. , Coordination of cortical and thalamic activity during non-REM sleep in humans. Nat. Commun. 8, 15499 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waxman S. G., Swadlow H. A., The conduction properties of axons in central white matter. Prog. Neurobiol. 8, 297–324 (1977). [DOI] [PubMed] [Google Scholar]
- 39.Bazhenov M., Rulkov N. F., Timofeev I., Effect of synaptic connectivity on long-range synchronization of fast cortical oscillations. J. Neurophysiol. 100, 1562–1575 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vicente R., Gollo L. L., Mirasso C. R., Fischer I., Pipa G., Dynamical relaying can yield zero time lag neuronal synchrony despite long conduction delays. Proc. Natl. Acad. Sci. U.S.A. 105, 17157–17162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Izhikevich E. M., Polychronization: Computation with spikes. Neural Comput. 18, 245–282 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Salinas E., Sejnowski T. J., Correlated neuronal activity and the flow of neural information. Nat. Rev. Neurosci. 2, 539–550 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Eichenbaum H., Time cells in the hippocampus: A new dimension for mapping memories. Nat. Rev. Neurosci. 15, 732–744 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel J., Schomburg E. W., Berényi A., Fujisawa S., Buzsáki G., Local generation and propagation of ripples along the septotemporal axis of the hippocampus. J. Neurosci. 33, 17029–17041 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wittmann M., Wenn die Zeit stehen bleibt. Kleine Psychologie der Grenzerfahrungen (Verlag C.H. Beck, ed. 1, 2015). [Google Scholar]
- 46.Benjamini Y., Hochberg Y., Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B 57, 289–300 (1995). [Google Scholar]
- 47.Dickey C. W., et al., Widespread ripples synchronize human cortical activity during sleep, waking, and memory recall. Zenodo. https://zenodo.org/record/6270017. Deposited 27 February 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data and code have been deposited in Zenodo (https://zenodo.org/record/6270017) (47).